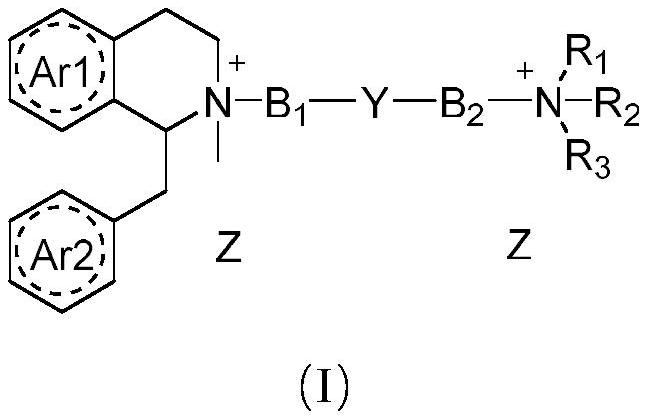
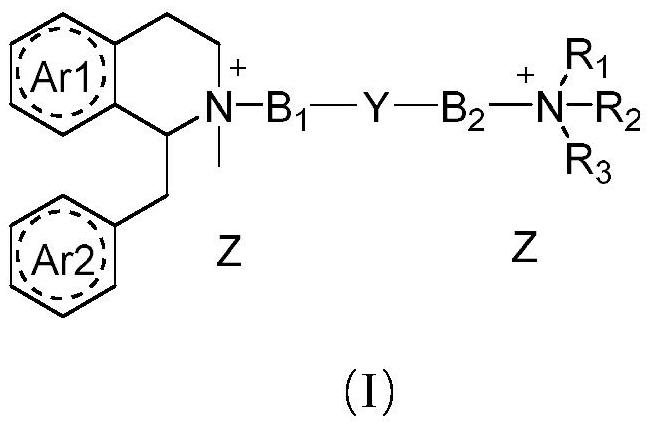
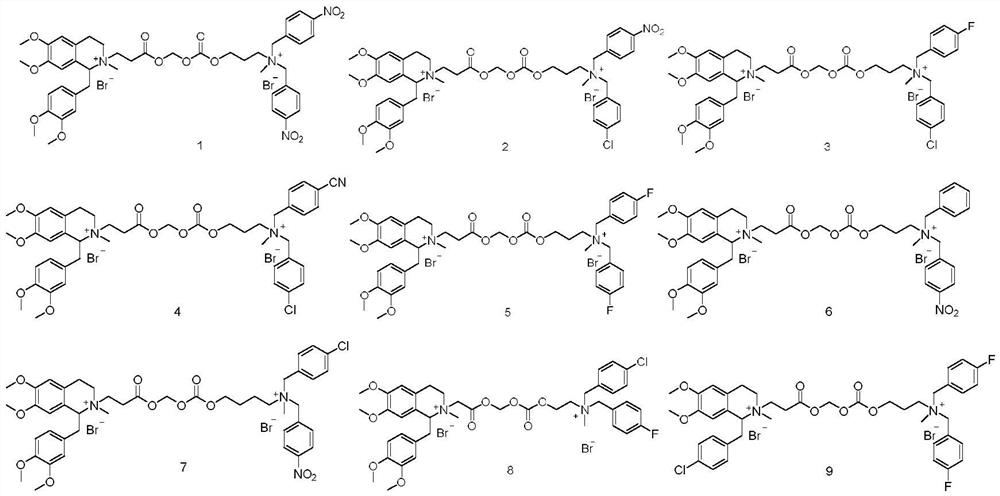
Benzyl isoquinoline compounds, preparation method and application thereof
A technology of benzylisoquinolines and compounds, which is applied in the fields of sulfonate preparation, organic chemistry, drug combination, etc., can solve problems such as severe allergies, and achieve the effect of avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Put 715 mg of labdansu (CAS: 1699-51-0) and 334 mg of methyl 3-bromopropionate in 30 mL of acetonitrile, stir at 55°C for 24 hours, evaporate the acetonitrile to dryness under reduced pressure, and perform column chromatography (dichloromethane / methanol) =10 / 1) to obtain 590mg of N-methyl-N-propionic acid methyl ester labdansin quaternary ammonium salt, add 2N aqueous sodium hydroxide solution 20mL, hydrolyze at room temperature for 2 hours, adjust the Ph value to 9 with hydrobromic acid aqueous solution, reduce The solvent was evaporated to dryness under pressure, and the crude product was subjected to column chromatography (dichloromethane / methanol=10 / 1) to obtain 280 mg of N-methyl-N-propionyllaudansin quaternary ammonium bromide sodium salt, namely Fragment a.
[0037] 3-(Methylamino)-1-propanol (CAS: 42055-15-2) 178 mg and 4-nitrobenzyl bromide (CAS: 100-11-8) 648 mg were dissolved in 30 mL of acetonitrile, stirred at 55°C for 24 hours, Acetonitrile was...
Embodiment 2
[0041]
[0042] Fragment a was prepared according to the method described in Example 1.
[0043] 178mg of 3-(methylamino)-1-propanol (CAS: 42055-15-2) and 410mg of 4-chlorobenzyl bromide were dissolved in 30mL of acetonitrile, stirred at 50°C for 3 hours, then 450mg of 4-nitrobenzyl bromide was added, Stir at 55°C for 24 hours, evaporate acetonitrile to dryness under reduced pressure, and obtain brominated N-methyl-N-hydroxypropyl-N-p-chlorobenzyl-N- 435 mg of p-nitrobenzyl quaternary ammonium salt, add 20 mL of dichloromethane, 200 mg of chloromethyl chloroformate and 350 mg of pyridine, stir at room temperature for 5 hours, and obtain brominated N-methyl-N-chloromethyl carbonate propyl group by column chromatography -415 mg of N-p-chlorobenzyl-N-p-nitrobenzyl quaternary ammonium salt, namely fragment b.
[0044] Dissolve 261 mg of Fragment b and 266 mg of Fragment a in 20 mL of DMF, stir and react at room temperature for 36 hours, evaporate the DMF to dryness under reduc...
Embodiment 3
[0047]
[0048] Put 715 mg of labdansu (CAS: 1699-51-0) and 304 mg of methyl 2-bromoacetate in 30 mL of acetonitrile, stir at 55°C for 24 hours, evaporate the acetonitrile to dryness under reduced pressure, and perform column chromatography (dichloromethane / methanol = 10 / 1) to obtain 510mg of N-methyl-N-methyl acetate labdansin quaternary ammonium salt, add 2N aqueous sodium hydroxide solution 20mL, hydrolyze at room temperature for 2 hours, adjust the pH value to 9 with hydrobromic acid aqueous solution, evaporate under reduced pressure The solvent was dried, and the crude product was subjected to column chromatography (dichloromethane / methanol=10 / 1) to obtain 280 mg of N-methyl-N-acetoxylaudansin quaternary ammonium bromide sodium salt, namely Fragment a.
[0049] Add 150 mg of 2-(methylamino)-ethanol (CAS: 109-83-1) and 410 mg of 4-chlorobenzyl bromide in 30 mL of acetonitrile, stir at 50°C for 3 hours, then add 378 mg of 4-fluorobenzyl bromide, and stir at 55°C for 24 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com