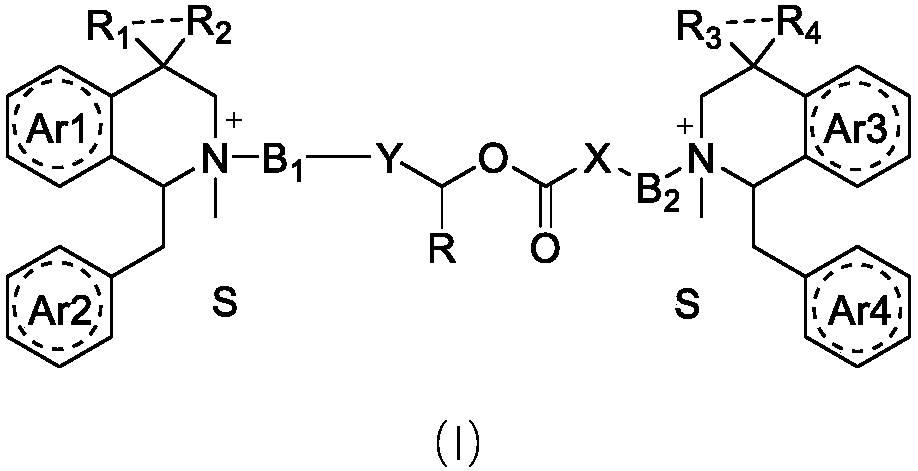
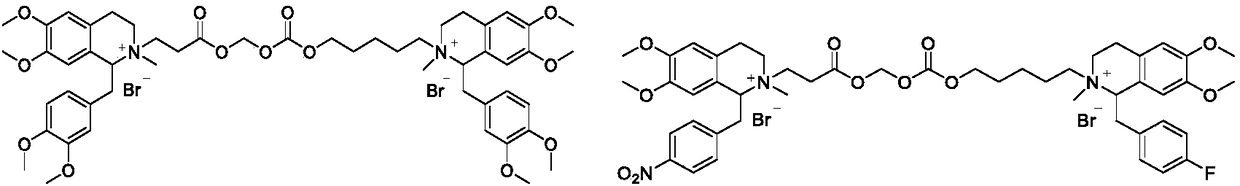
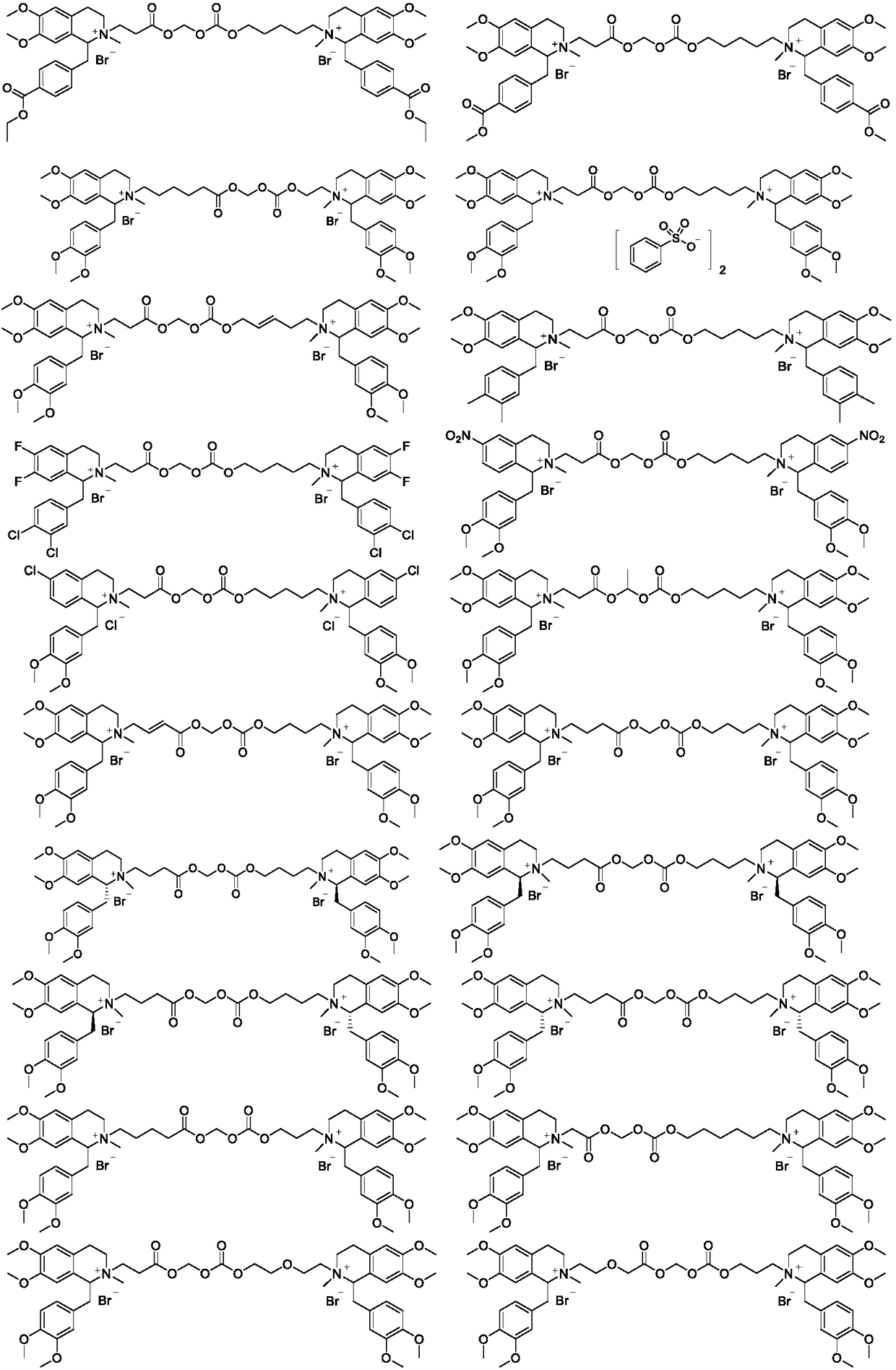
Benzylisoquinoline compound, preparation method and application
A technology of benzylisoquinolines and compounds, applied in the fields of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve severe allergies and other problems, and achieve the effect of short duration of muscle relaxation and rapid disappearance of muscle relaxation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] Put 715 mg of labdansu (CAS: 1699-51-0) and 334 mg of methyl 3-bromopropionate in 30 mL of acetonitrile, stir at 55°C for 24 hours, evaporate the acetonitrile to dryness under reduced pressure, and perform column chromatography (dichloromethane / methanol) =10 / 1) to obtain 590mg of N-methyl-N-propionic acid methyl ester labdansin quaternary ammonium salt, add 2N aqueous sodium hydroxide solution 20mL, hydrolyze at room temperature for 2 hours, adjust the Ph value to 9 with hydrobromic acid aqueous solution, reduce The solvent was evaporated to dryness under pressure, and the crude product was subjected to column chromatography (dichloromethane / methanol=10 / 1) to obtain 280 mg of N-methyl-N-propionyllaudansin quaternary ammonium bromide sodium salt, namely Fragment a.
[0045] Put 715 mg of labdansu (CAS: 1699-51-0) and 334 mg of 5-bromopentanol in 30 mL of acetonitrile, stir at 55°C for 24 hours, evaporate the acetonitrile to dryness under reduced pressure, and ...
Embodiment 2
[0049]
[0050] According to the method described in Example 1, using (S)-laudansu (CAS: 2688-77-9) as a starting material, the S, S type isomer of the compound of formula (I) can be obtained.
[0051] HNMR(d-DMSO): 1.21~1.27(2H,m), 1.56~1.73(4H,m), 2.72(2H,t,J=8Hz), 2.88~3.68(46H,m), 4.19(2H,t , J=8Hz), 4.91~4.97(2H,m), 5.87(2H,s), 6.05~6.89(10H,m).
Embodiment 3
[0053] Some of the compounds described in the examples were subjected to rabbit plasma decomposition experiments in vitro. Add DMSO solution (10 μL) containing 200 μg of drug to 4 mL of rabbit plasma to make the plasma drug concentration 50 μg / mL, incubate at 37 °C immediately after mixing, and take 200 μL of drug-containing plasma at 2 min, 5 min, 10 min, and 20 min respectively , add 600 μ L of methanol, after centrifugation, take the supernatant liquid for measurement, and measure the drug content by HPLC, the specific measurement conditions are: 4.6X50mm, 5 μm C18 column, 0.04% trifluoroacetic acid aqueous solution is A mobile phase, 0.02% trifluoroacetic acid acetonitrile solution is B mobile phase, flow rate 1.5mL / min, column temperature 45°C, gradient elution (B: 10% / 2.5min→80% / 1.5min→10% / 2min), diode array detector. The obtained drug concentration is measured, compared with the initial drug concentration at the time of injection, and the remaining percentage of the dru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com