Patents
Literature
96 results about "Neuromuscular junction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
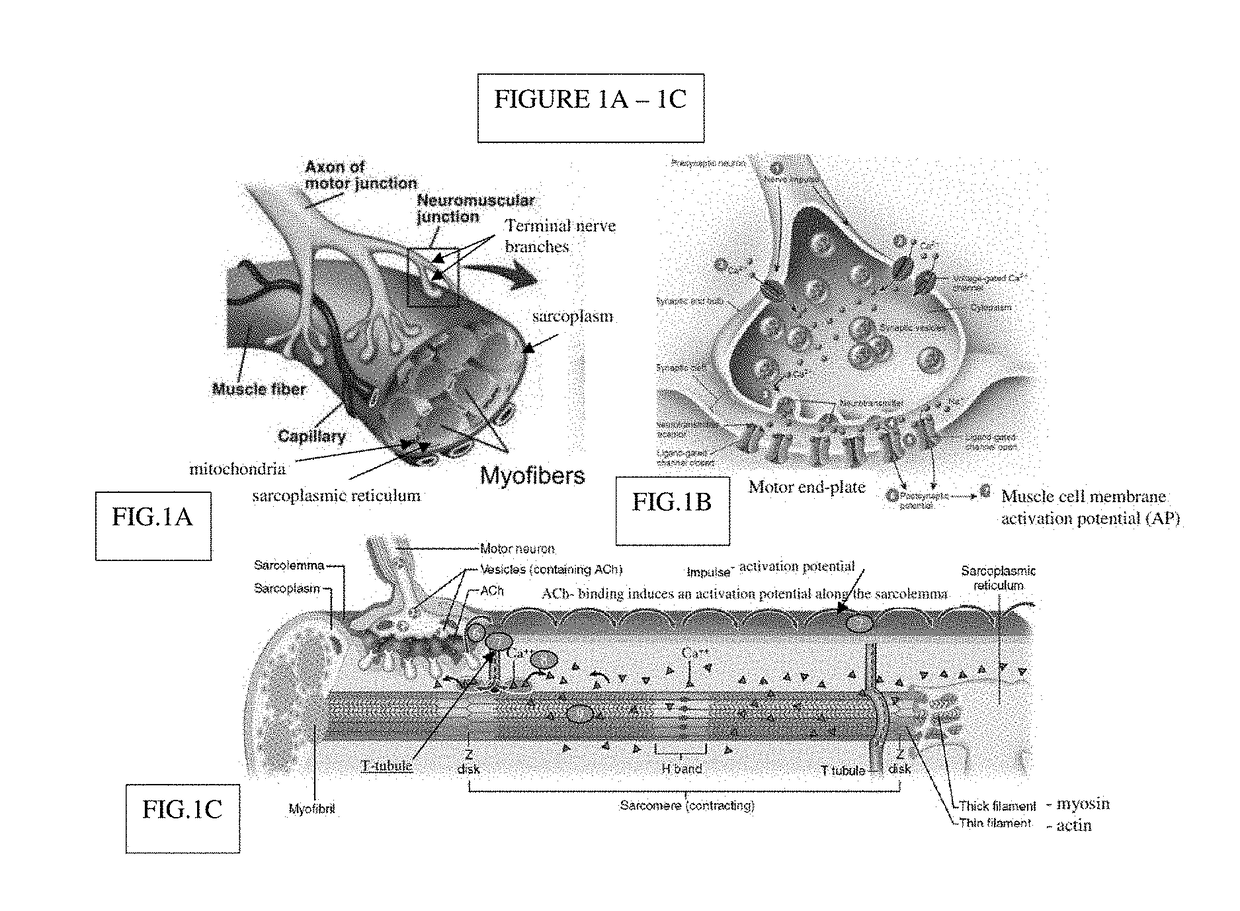
A neuromuscular junction (or myoneural junction) is a chemical synapse formed by the contact between a motor neuron and a muscle fiber. It is at the neuromuscular junction that a motor neuron is able to transmit a signal to the muscle fiber, causing muscle contraction.
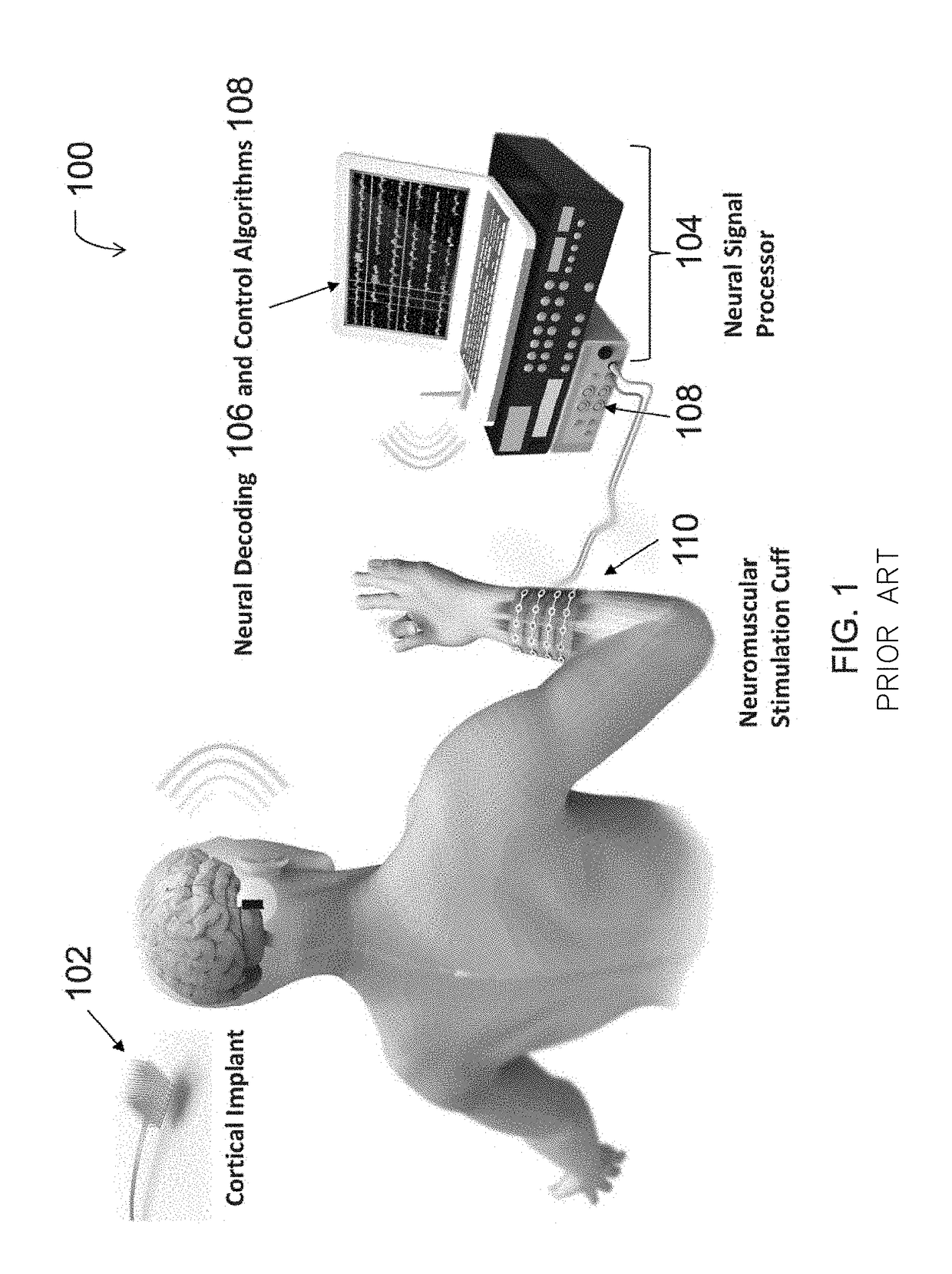
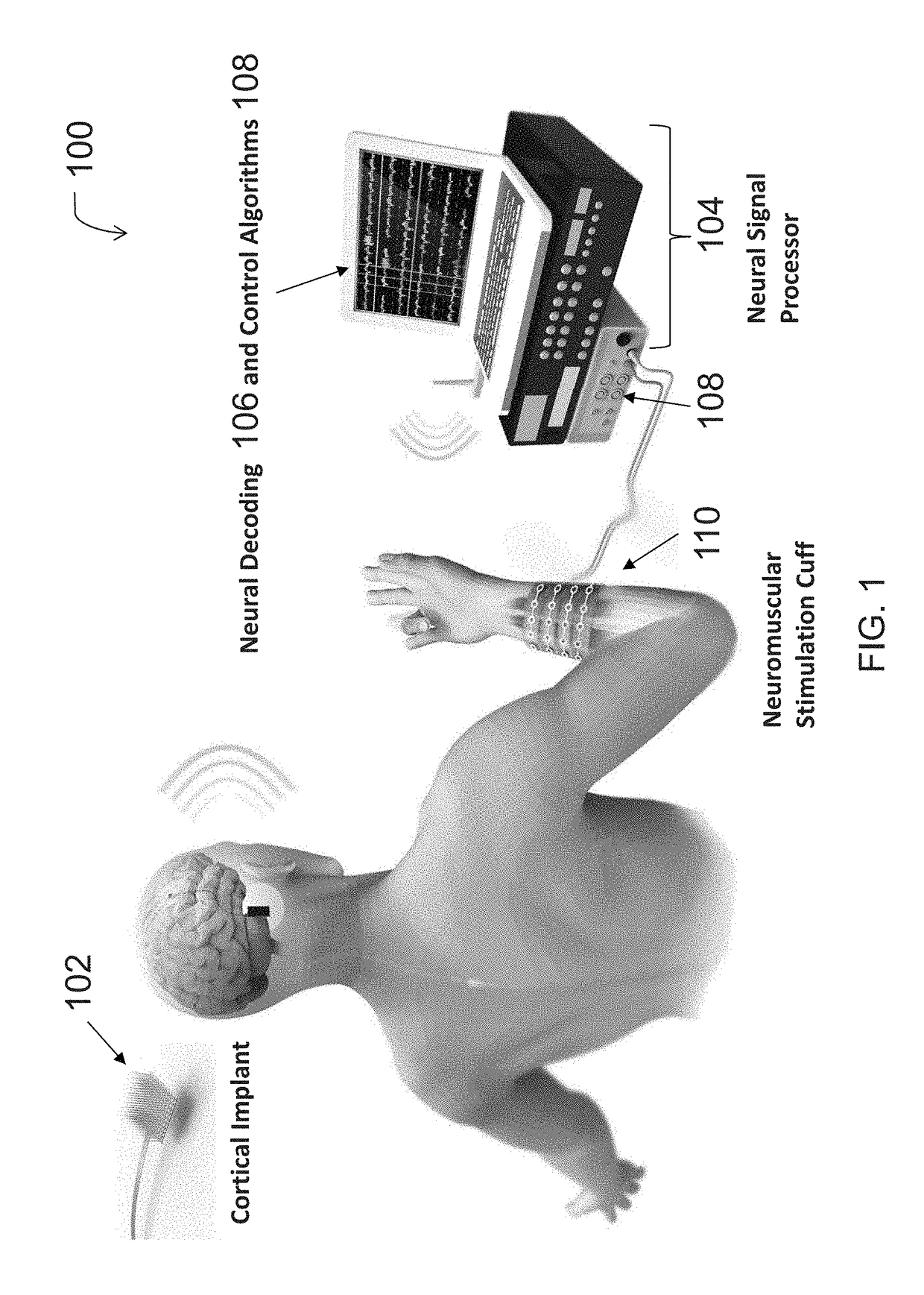
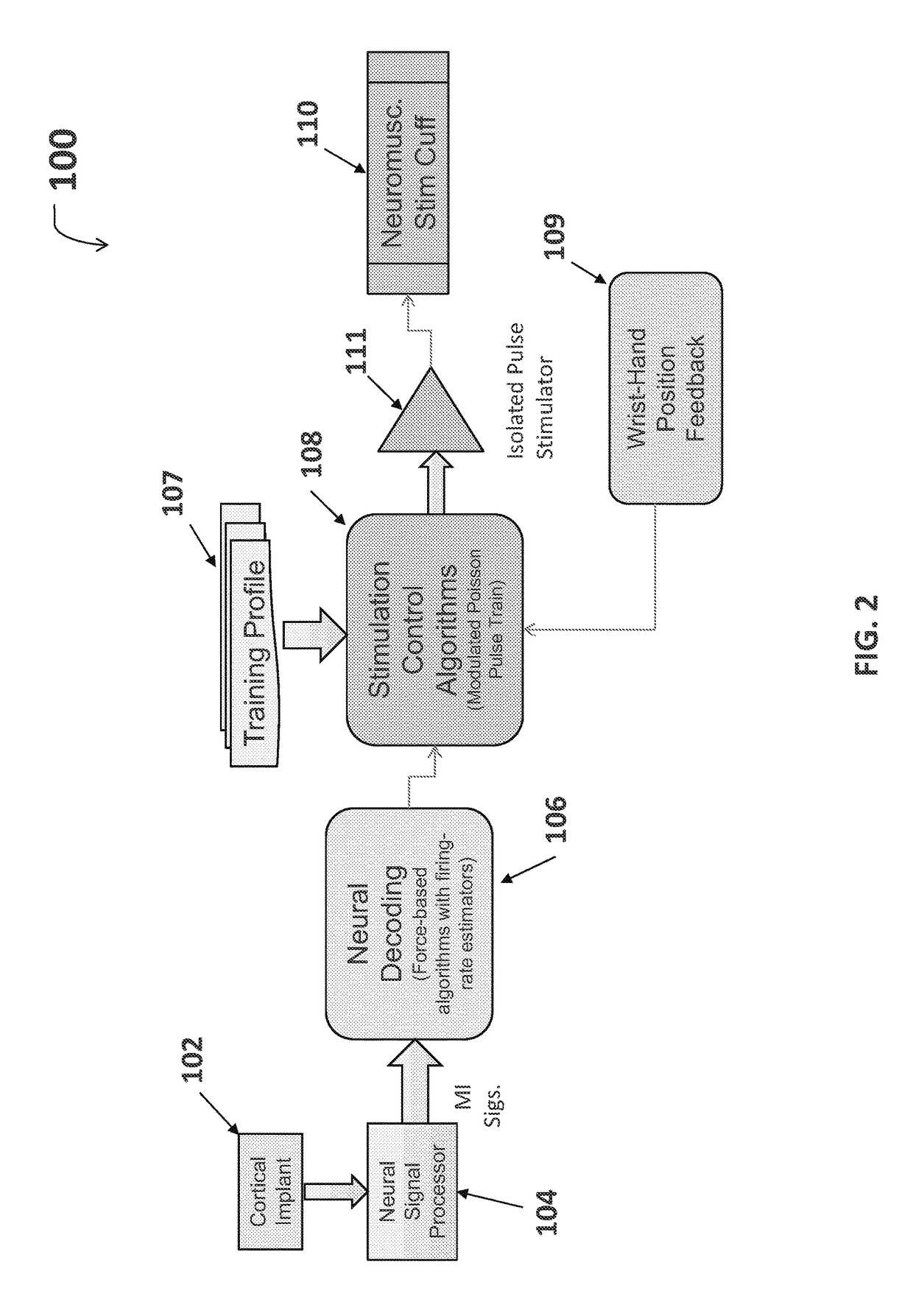
Neuromuscular Stimulation
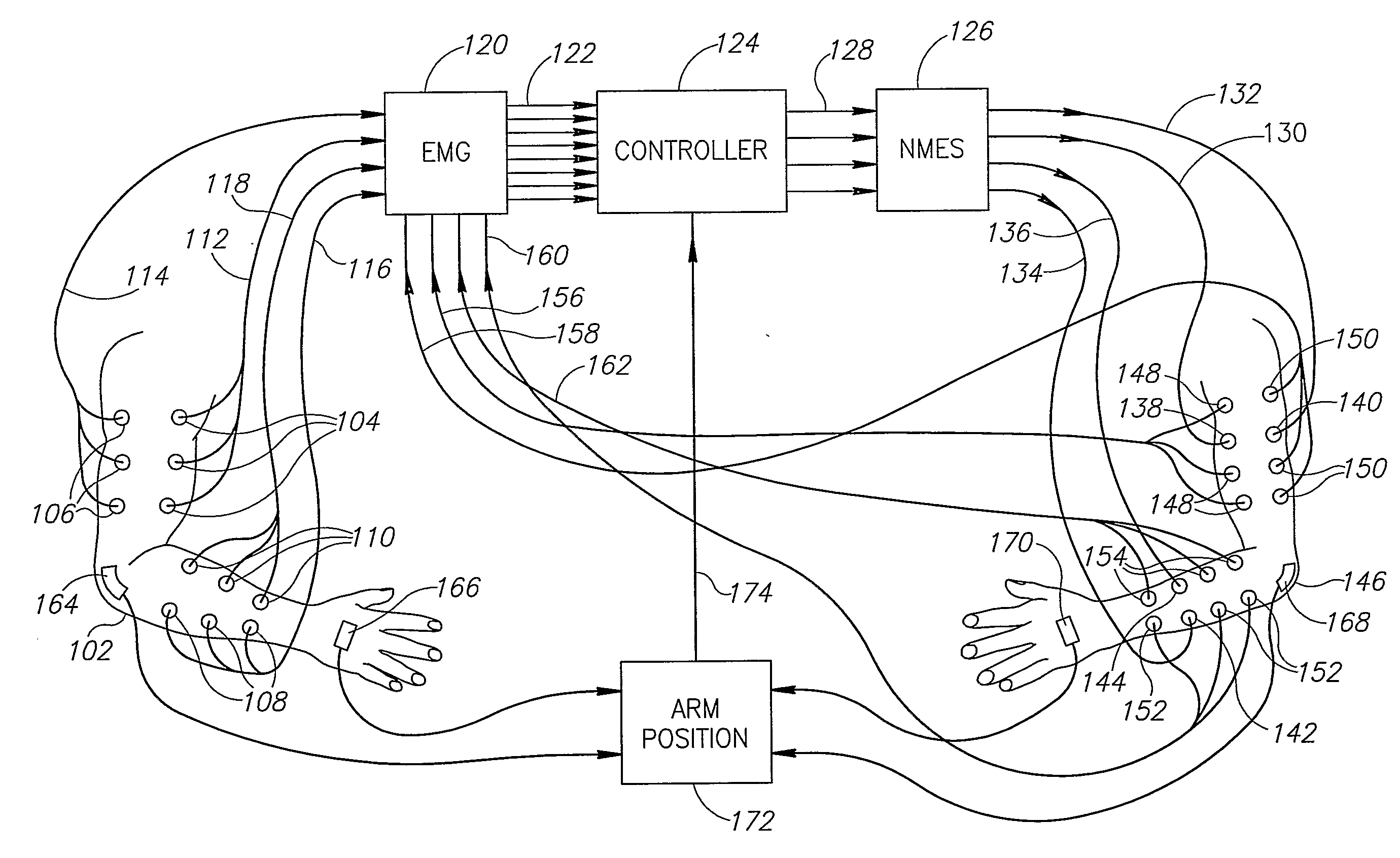
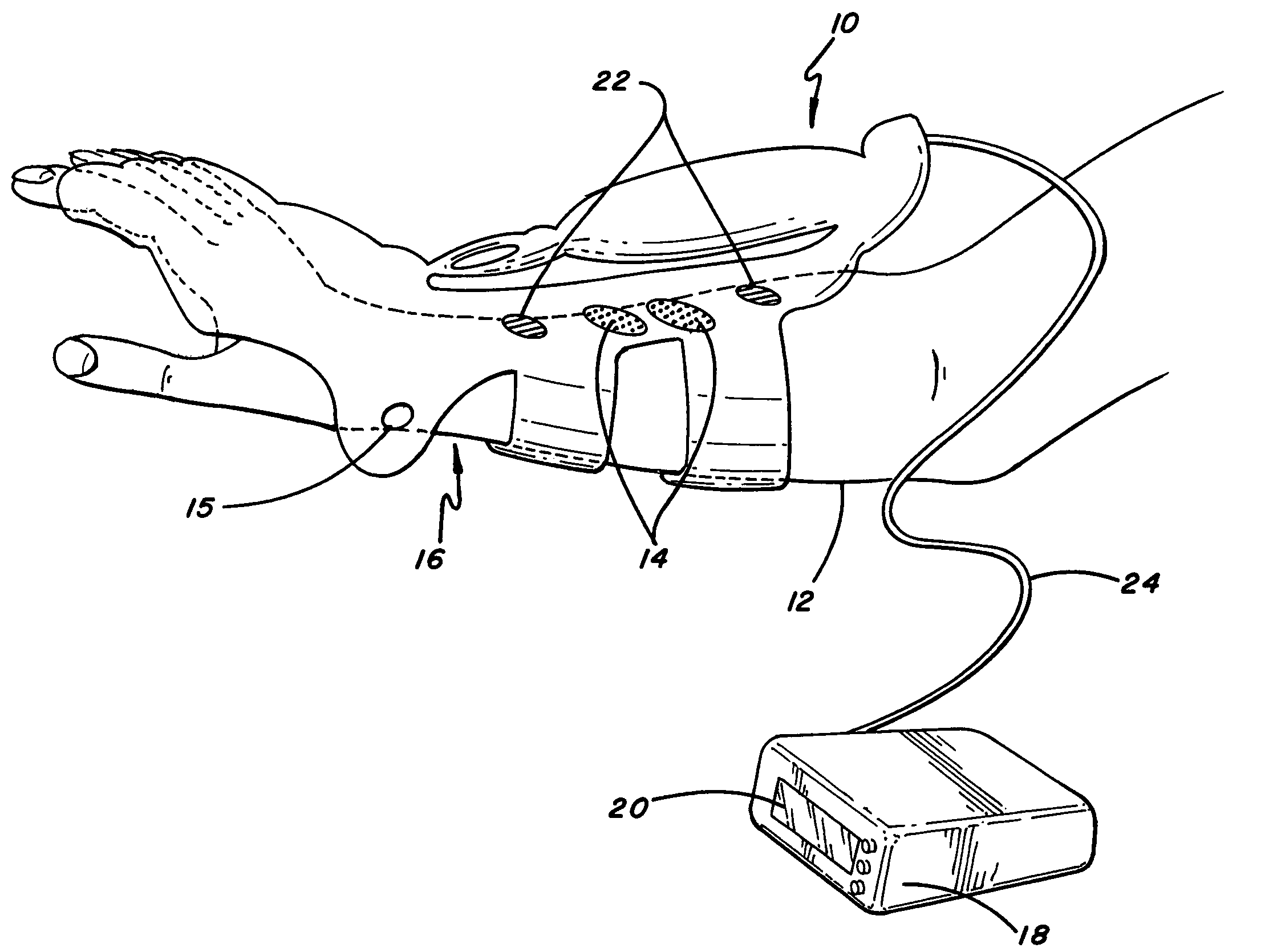
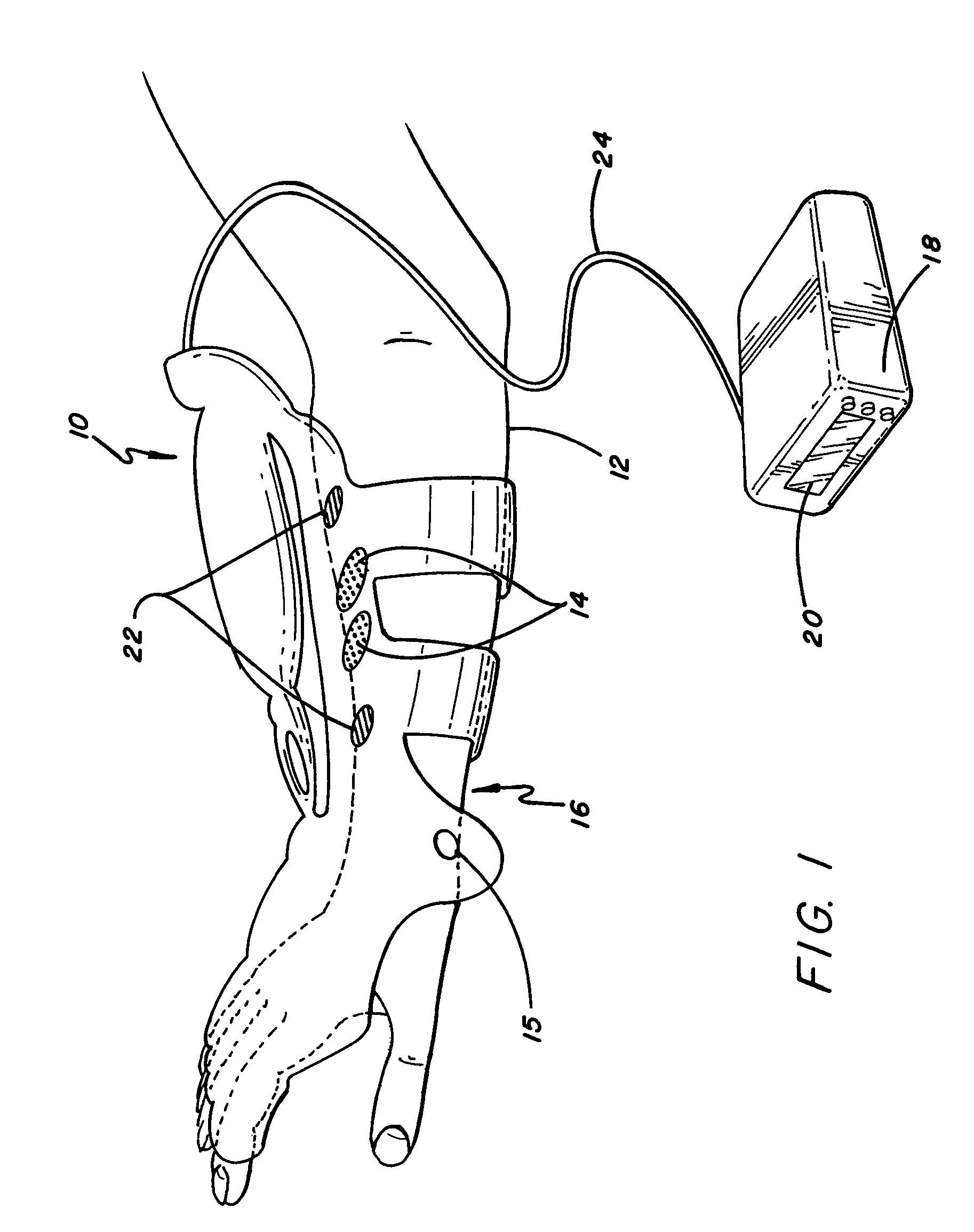
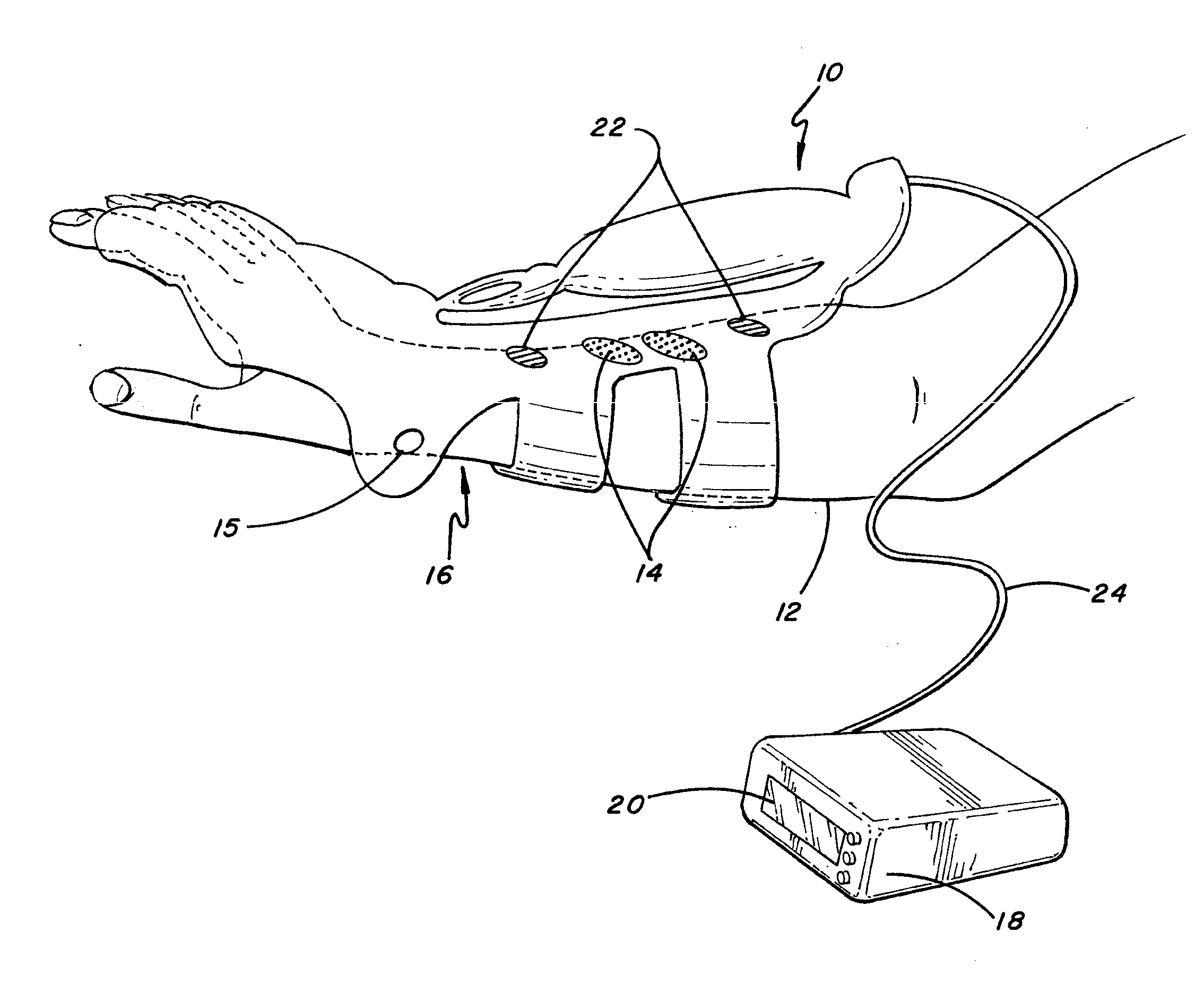
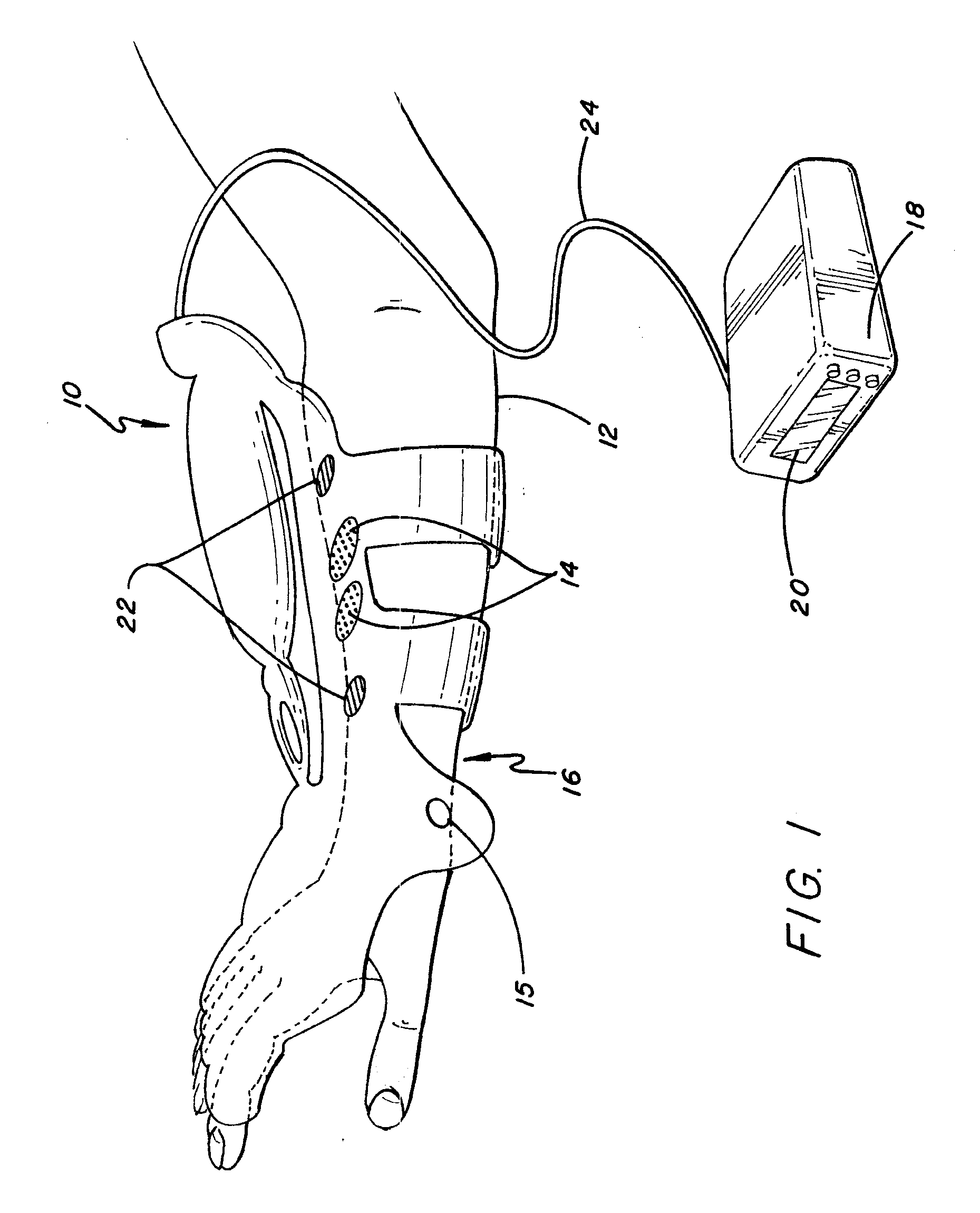
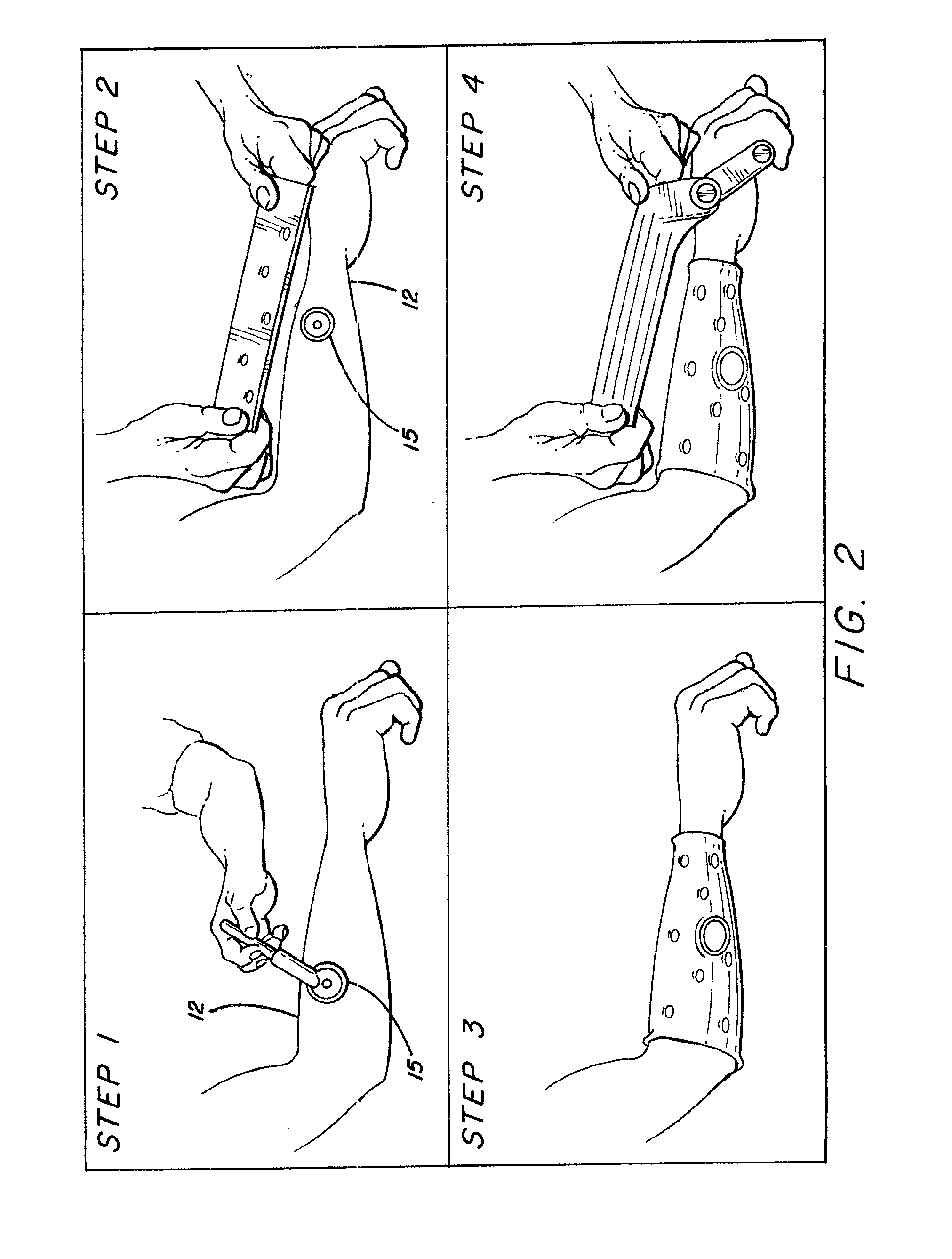
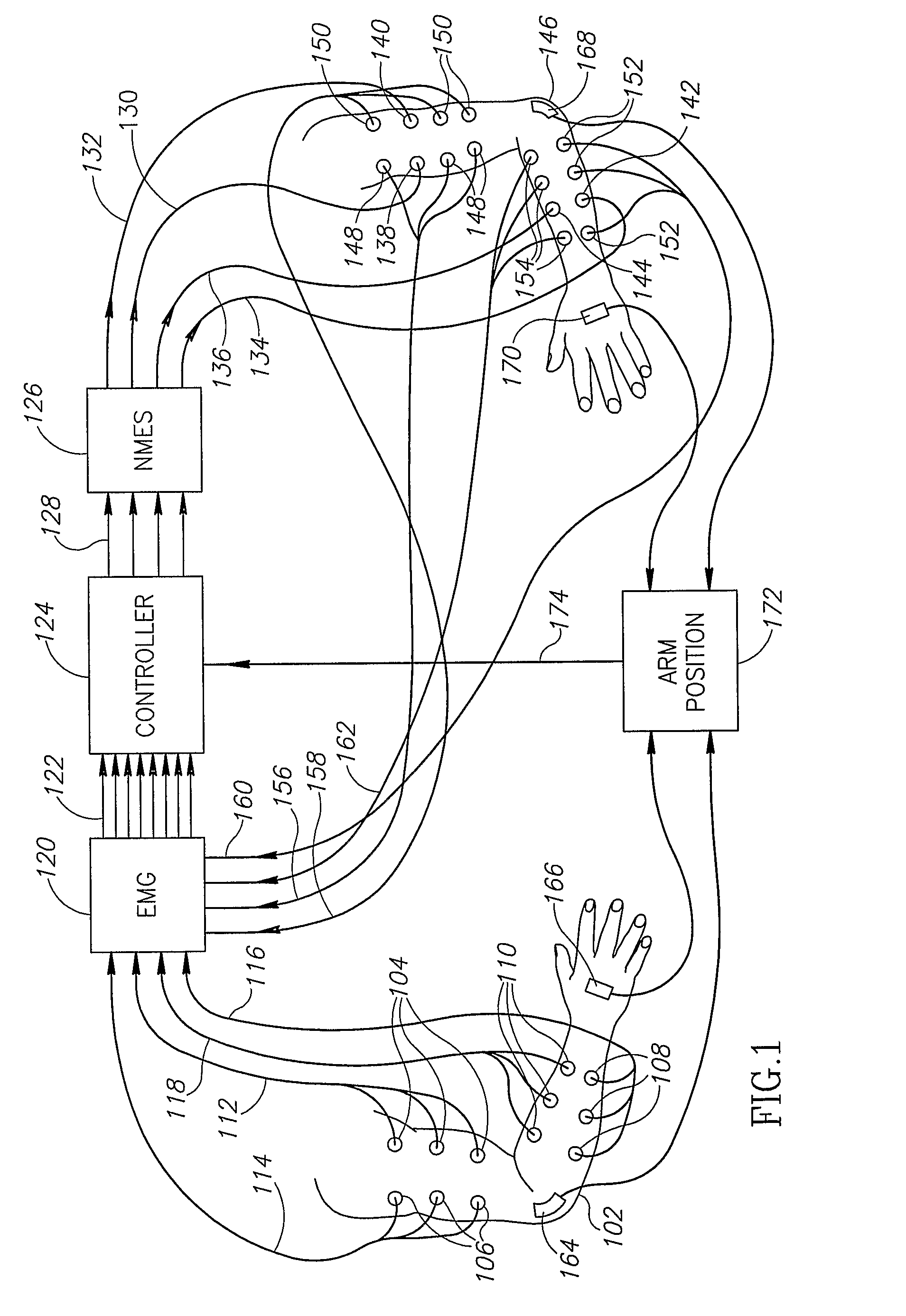
ActiveUS20080288020A1Move his arm more effectivelyElectrotherapyElectromyographyPhysical medicine and rehabilitationNeuromuscular stimulation
Apparatus for muscle activation, comprising: at least one electrode (138, 140, 142, 144) adapted to deliver a neuromuscular stimulation (NMES) signal to a body portion (146); at least one controller (124) adapted to provide a NMES signal comprising a sequence of stimulation signals to said at least one electrode; and a mechanical motion element (300) coupled to at least one of said body portion and a mirror body portion, wherein said mechanical motion element is operatively coupled to said at least one controller and wherein said at least one controller controls said NMES signal in conjunction with said mechanical motion element.
Owner:MOTORIKA
Compositions and delivery methods for the treatment of wrinkles, fine lines and hyperhidrosis
The present invention describes compositions and methods for treating, preventing and improving the appearance of skin, particularly, treating, preventing, ameliorating, reducing and / or eliminating fine lines and / or wrinkles of skin, wherein the compositions include limonoid constituents which inhibit acetylcholine release at neuromuscular junctions of skeletal muscle so as to relax the muscles involved with wrinkling, folding and creasing of skin, e.g., facial movement and expression. The limonoids preferably include the plant alkaloids toosendanin and azadirachtin. The compositions, which also are used to treat hyperhidrosis, are preferably applied to the skin, or are delivered by directed means to a site in need thereof.
Owner:AVON PROD INC
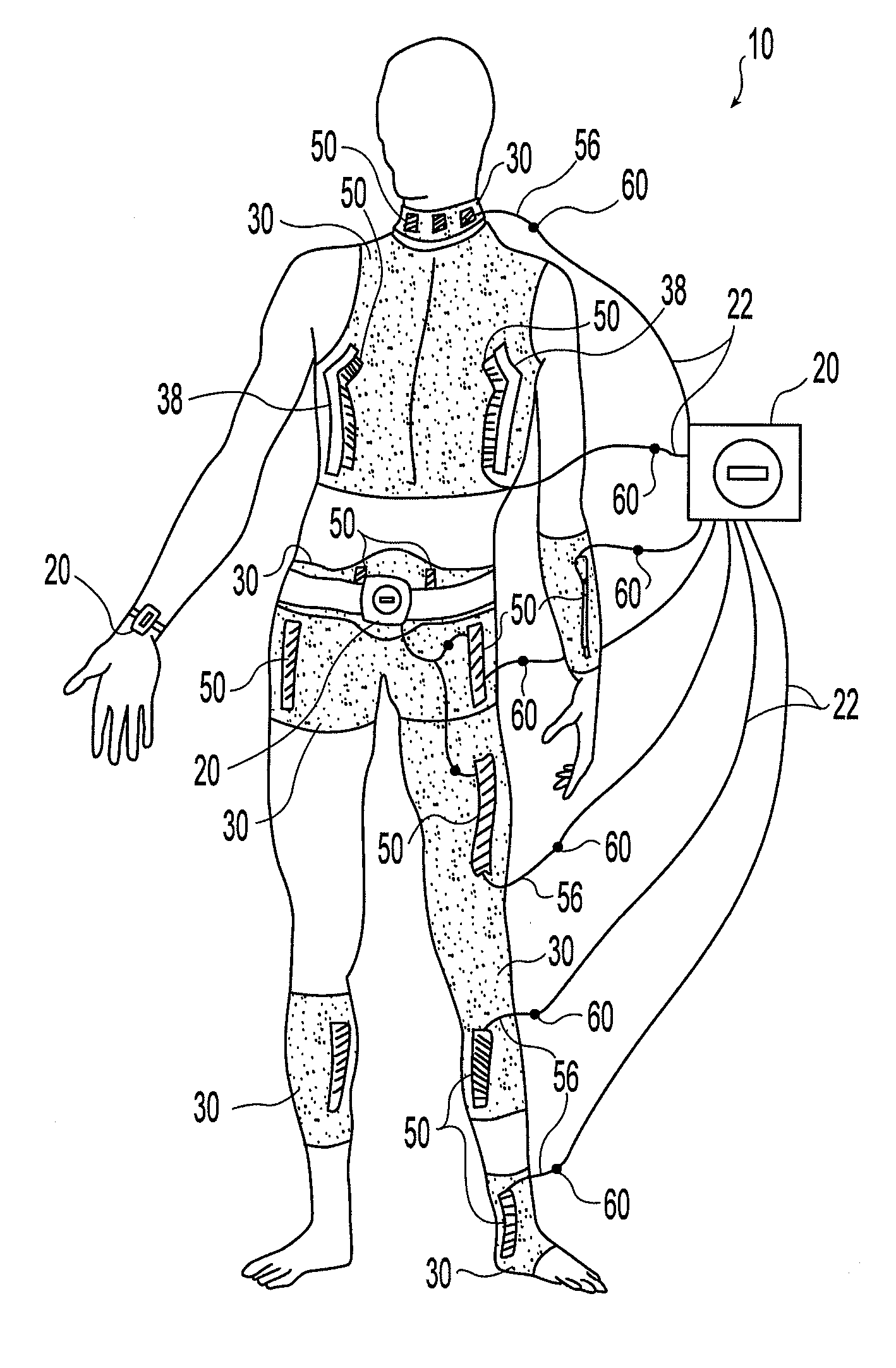
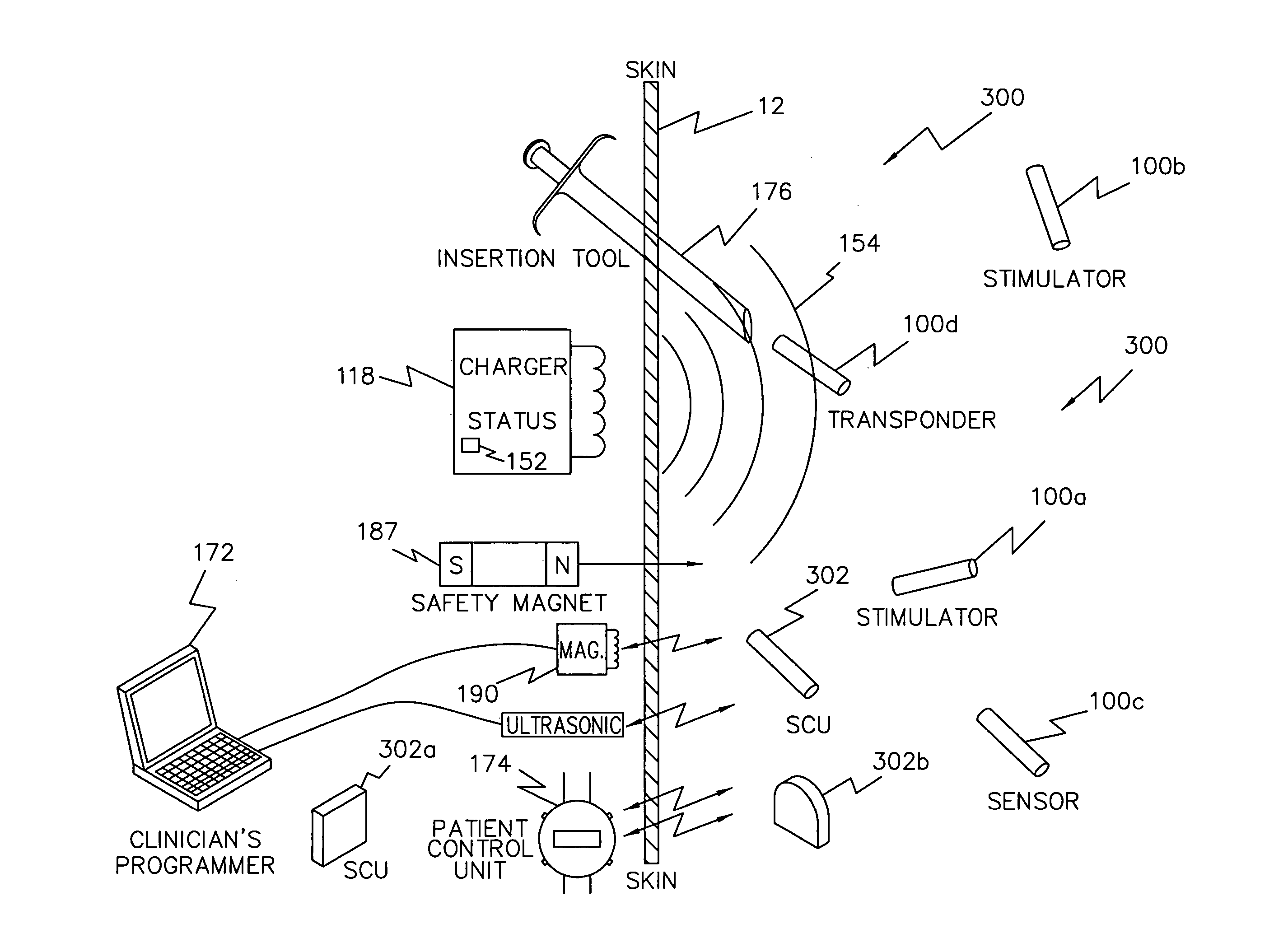
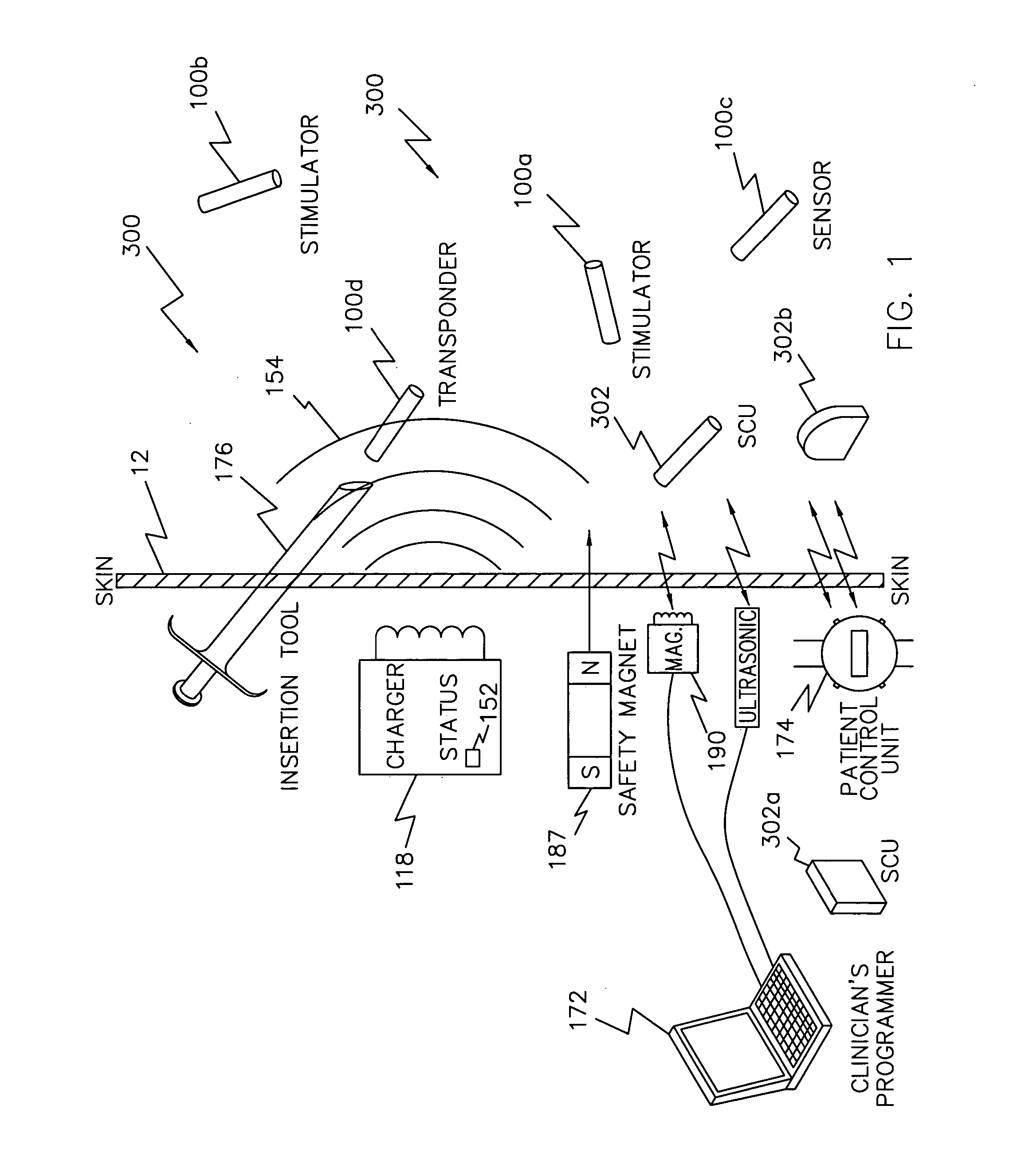
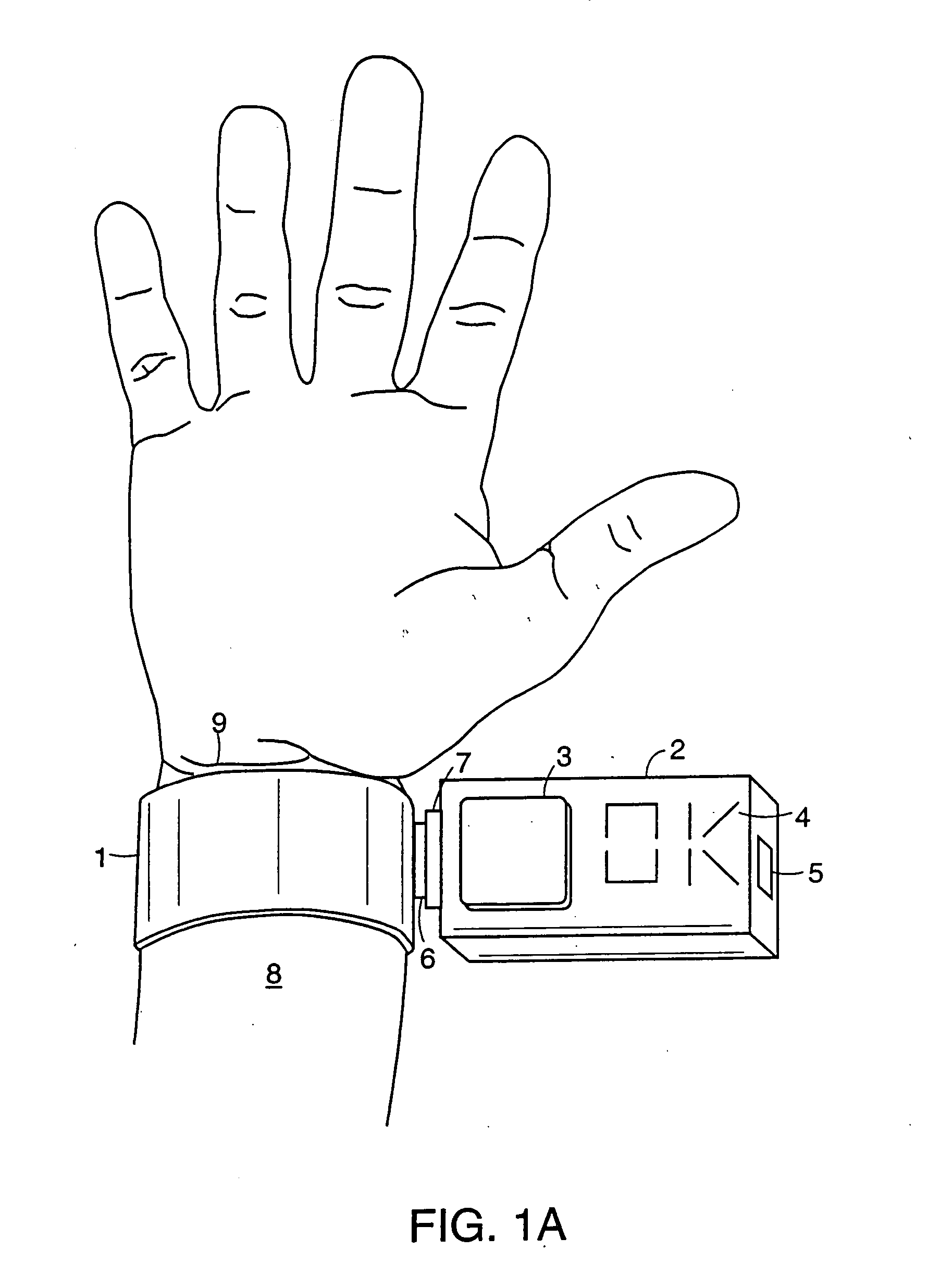
System and device for neuromuscular stimulation
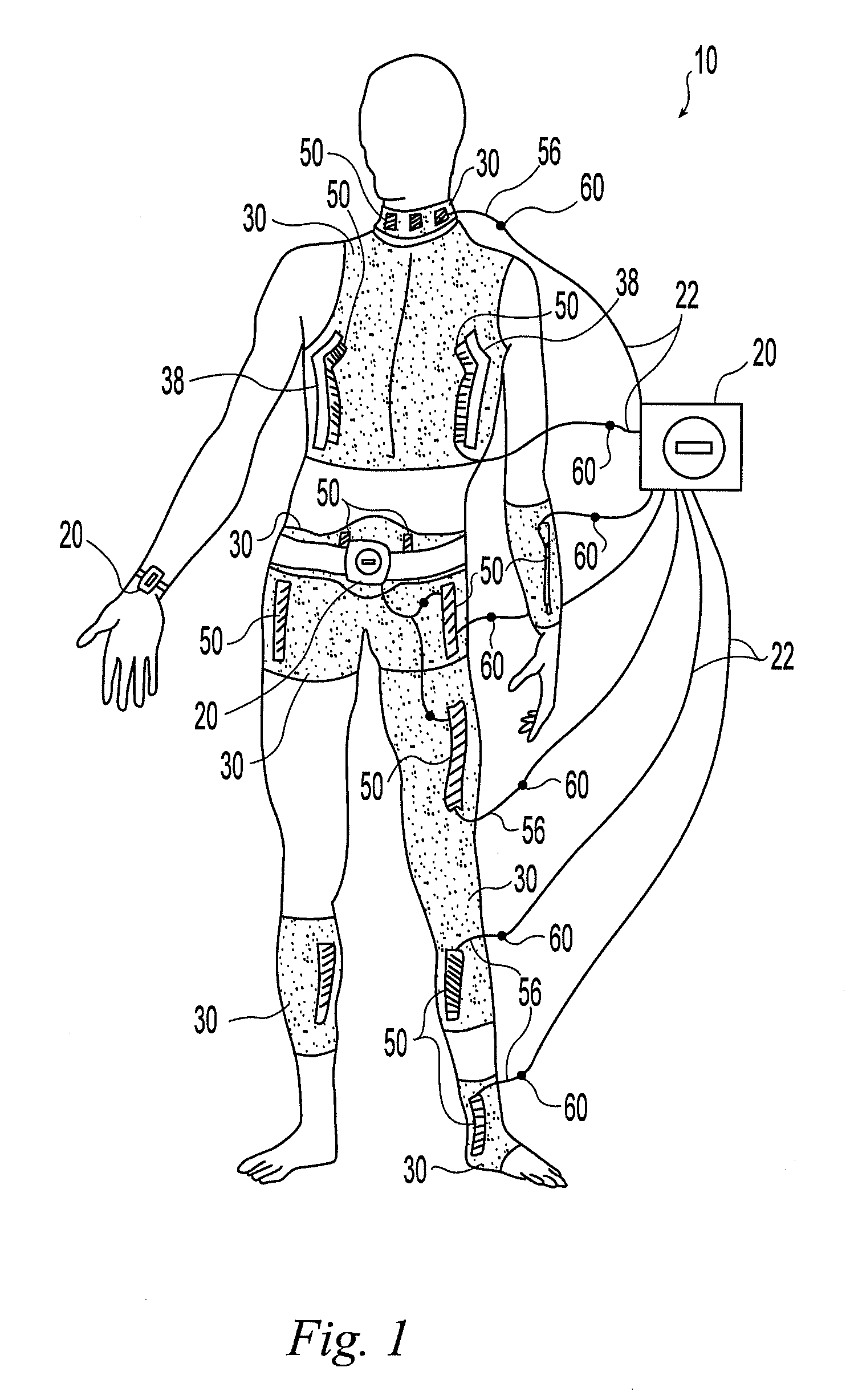
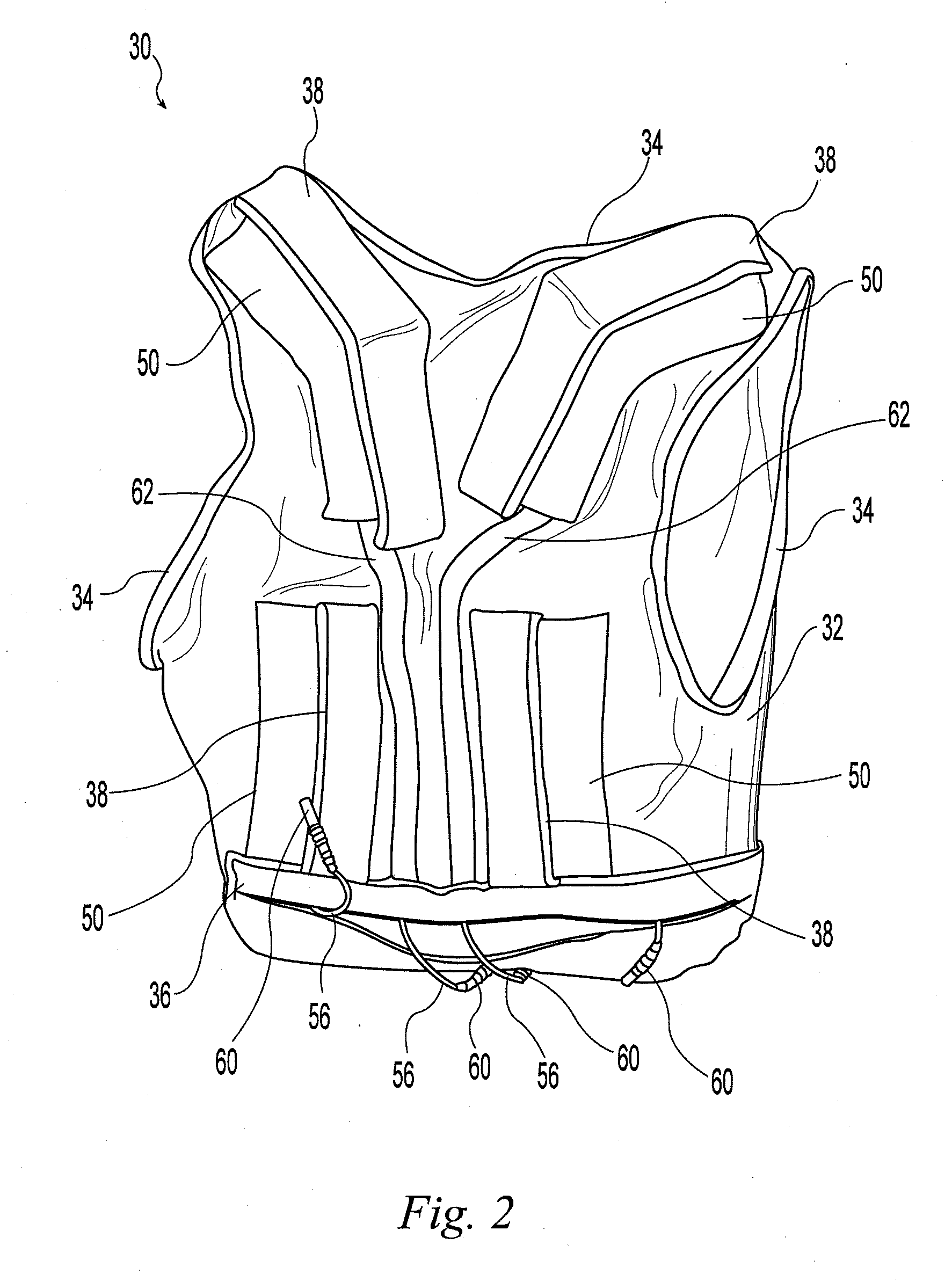
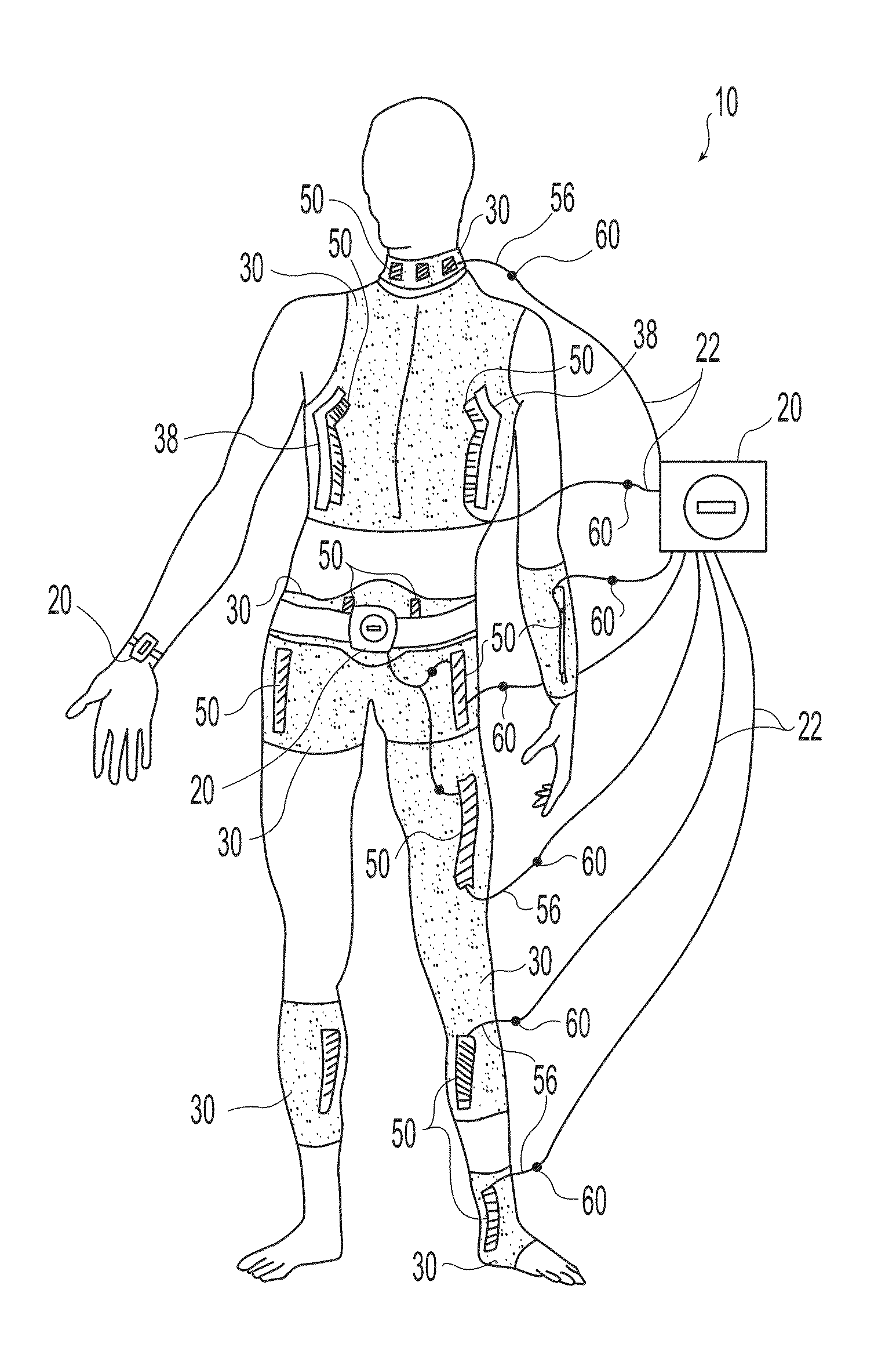
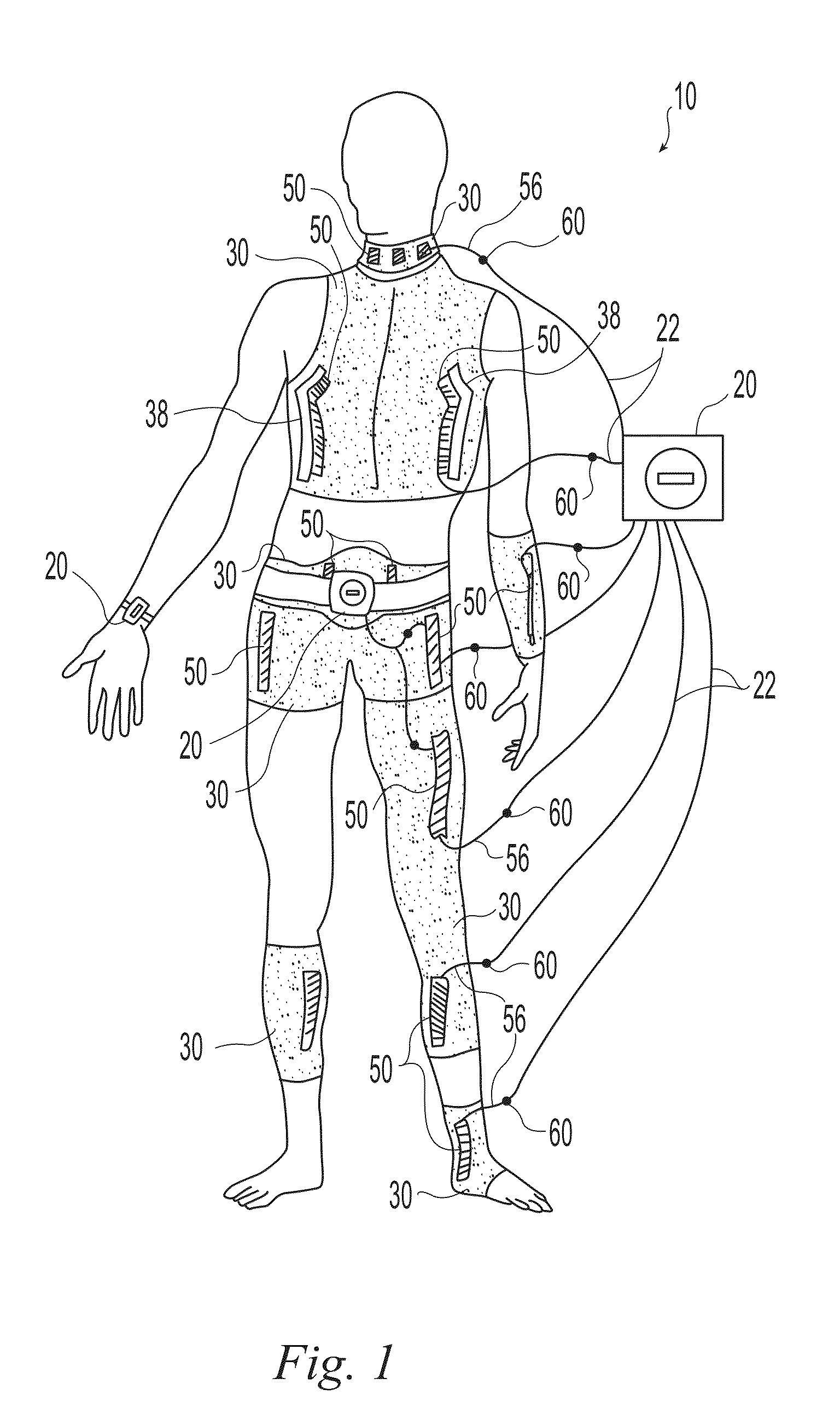
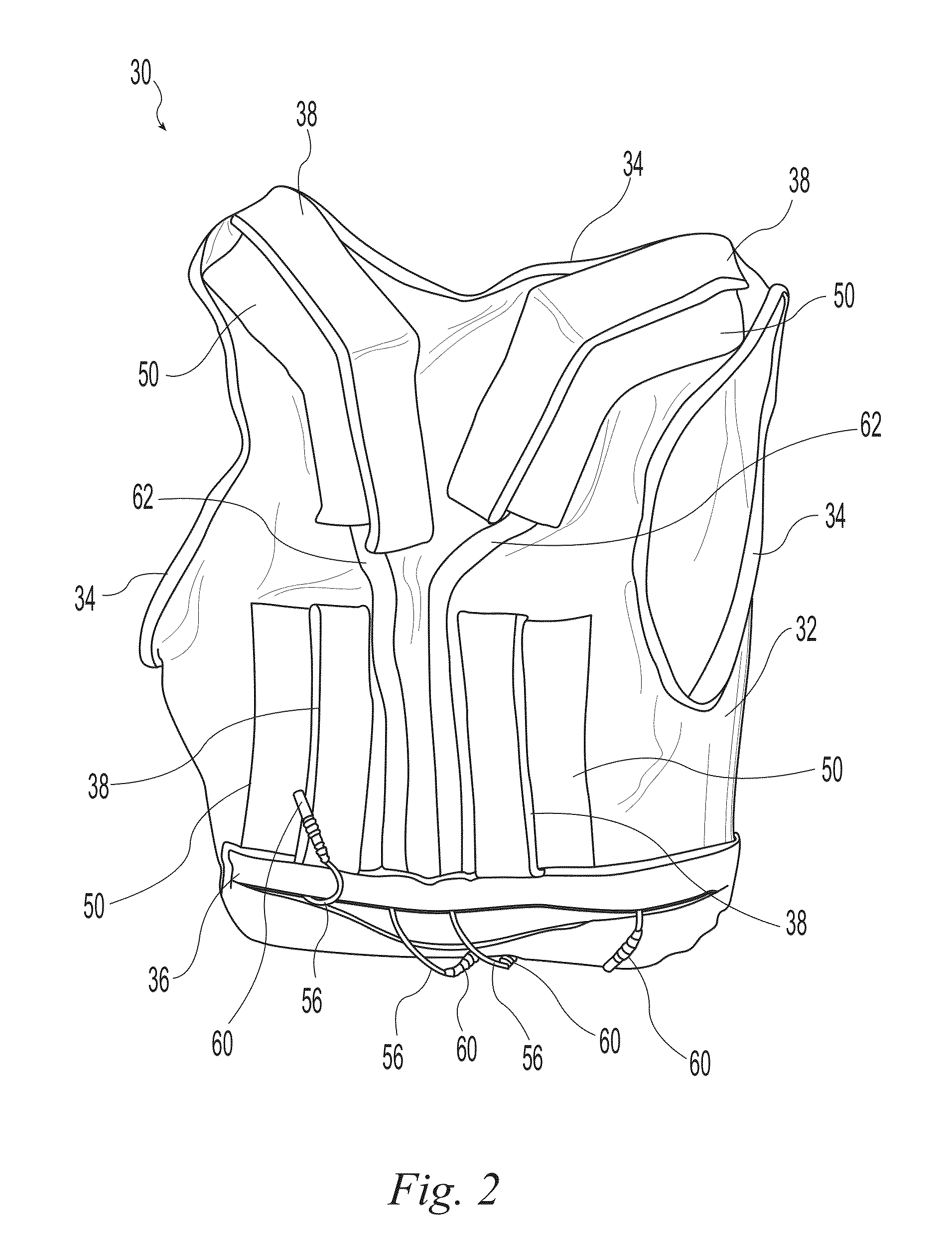
A wearable neuromuscular stimulation and neuroprosthetic system and device for treating spinal cord injury, stroke, and other neurological conditions; and for the management of chronic pain. This invention provides a system for transcutaneous neuromuscular stimulation and typically comprises a wearable item that further includes a flexible, non-conductive material; at least one flexible, generally flat electrode attachable to or embedded within the wearable item; and a programmable electrical stimulation device connectable to the electrode(s). Each electrode typically includes a silver-impregnated or silver-treated material.
Owner:MUCCIO PHILIP E
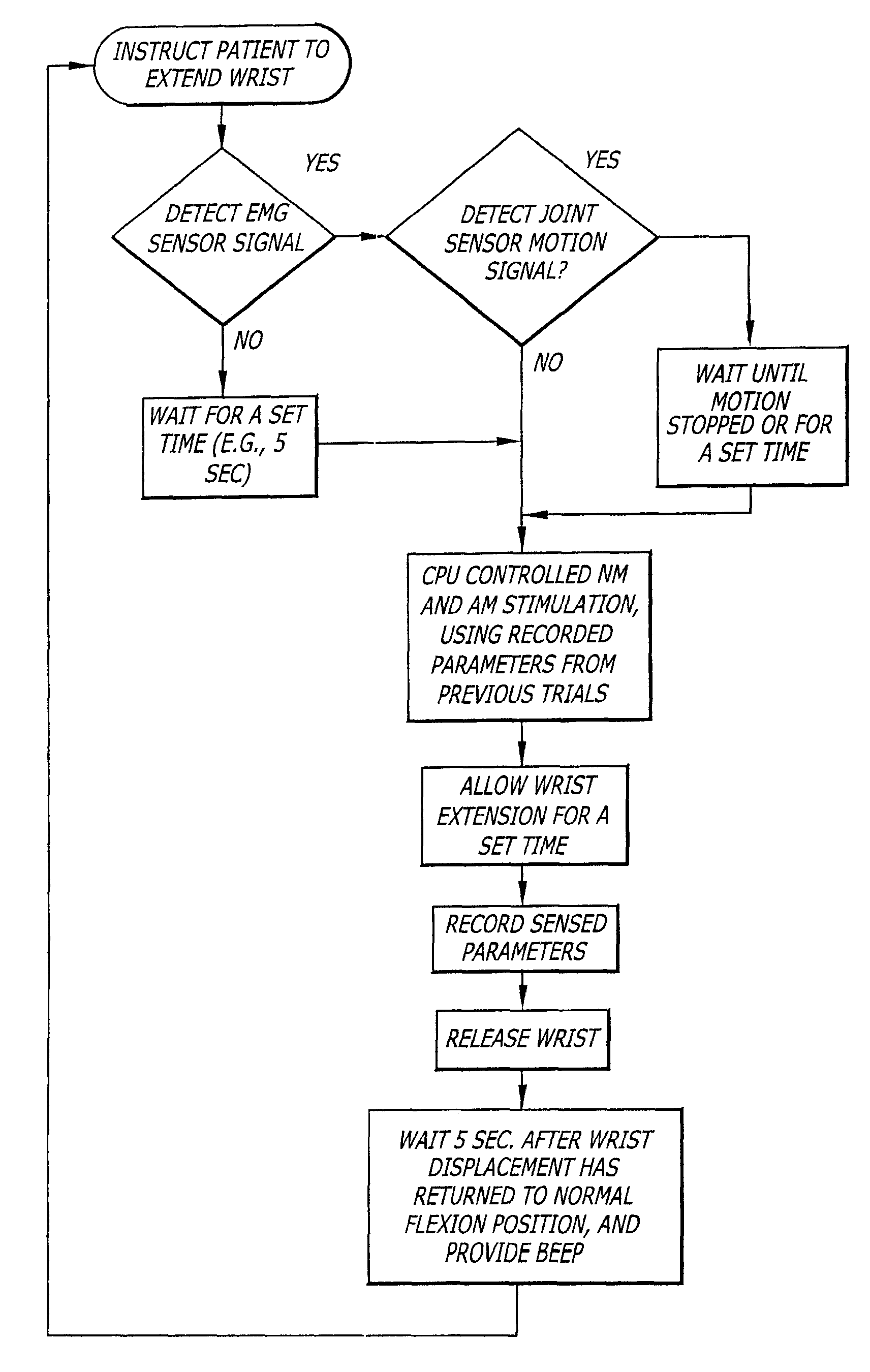
System and method for neuromuscular reeducation
ActiveUS7725175B2Increase volumeReduce the amount requiredElectrotherapyChiropractic devicesDisplay deviceTreatment modality
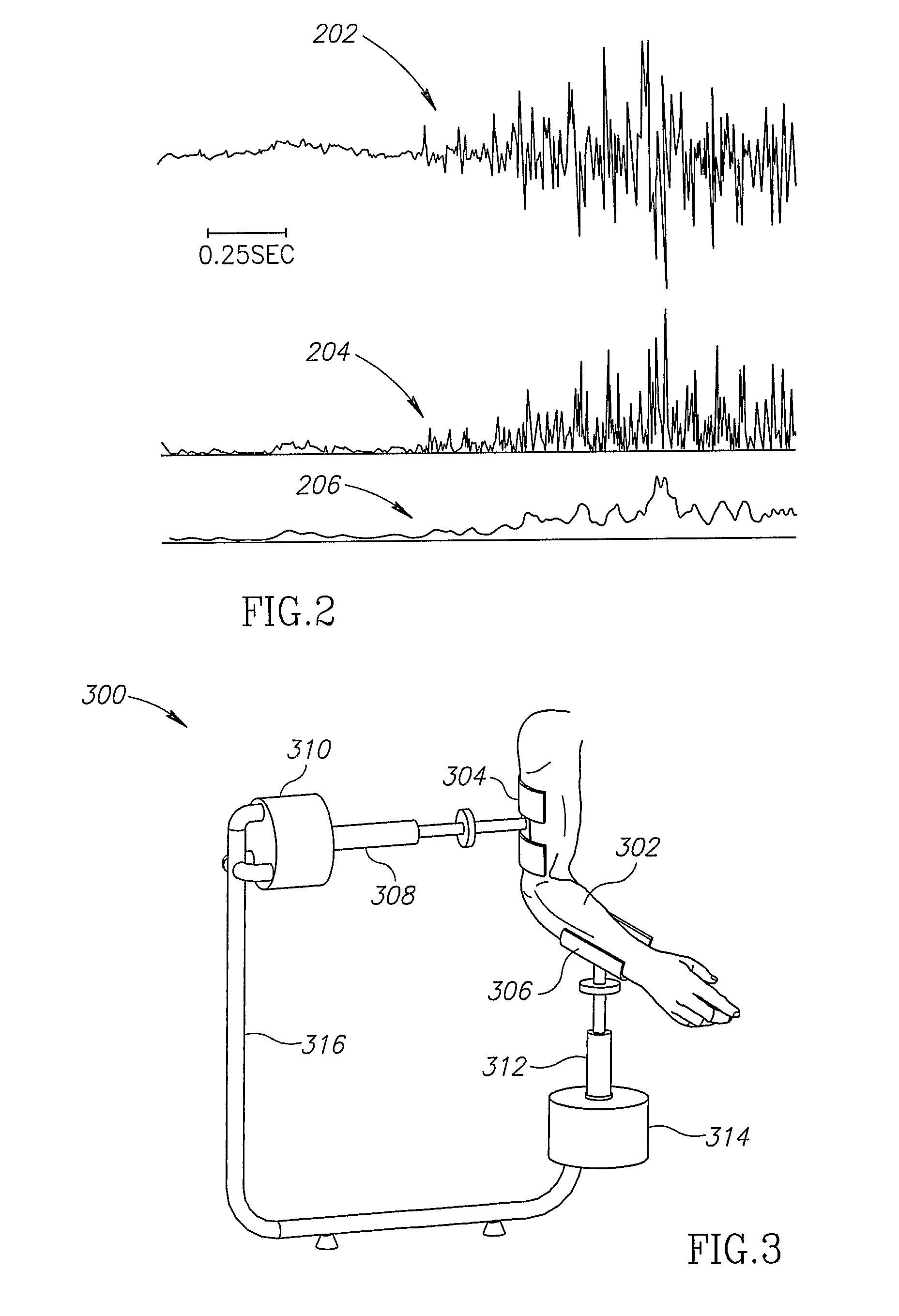
A system and a method that promotes the restoration of physical functions of the neuromuscular system by incorporating into one device the treatment modalities of biofeedback based repetitive practice, includes an actuator, a joint position measurement system, a force sensing measurement system, an EMG measurement system, a neuromuscular low-level stimulation system, a controller, and a display device.
Owner:KINETIC MUSCLES INC
Flexible sheet for neuromuscular stimulation
A flexible sheet for neurostimulation is described having a flexible non-conductive substrate matrix in which electrodes are embedded along a lower surface. Electrically conductive wires extend from the electrodes through the flexible substrate to another exterior surface of the substrate. Methods of making the flexible sheet and making a device using the flexible sheet are also disclosed.
Owner:BATTELLE MEMORIAL INST
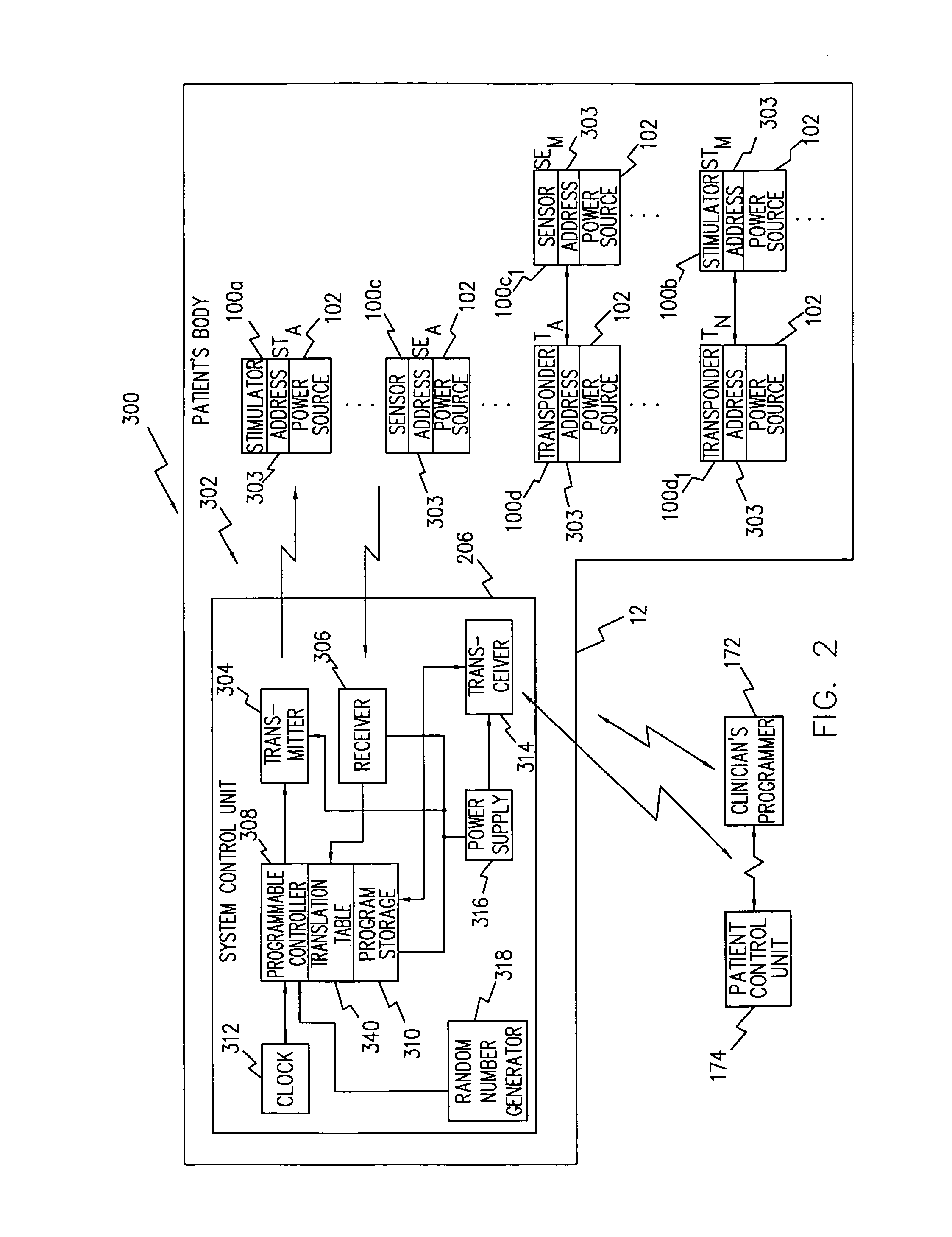
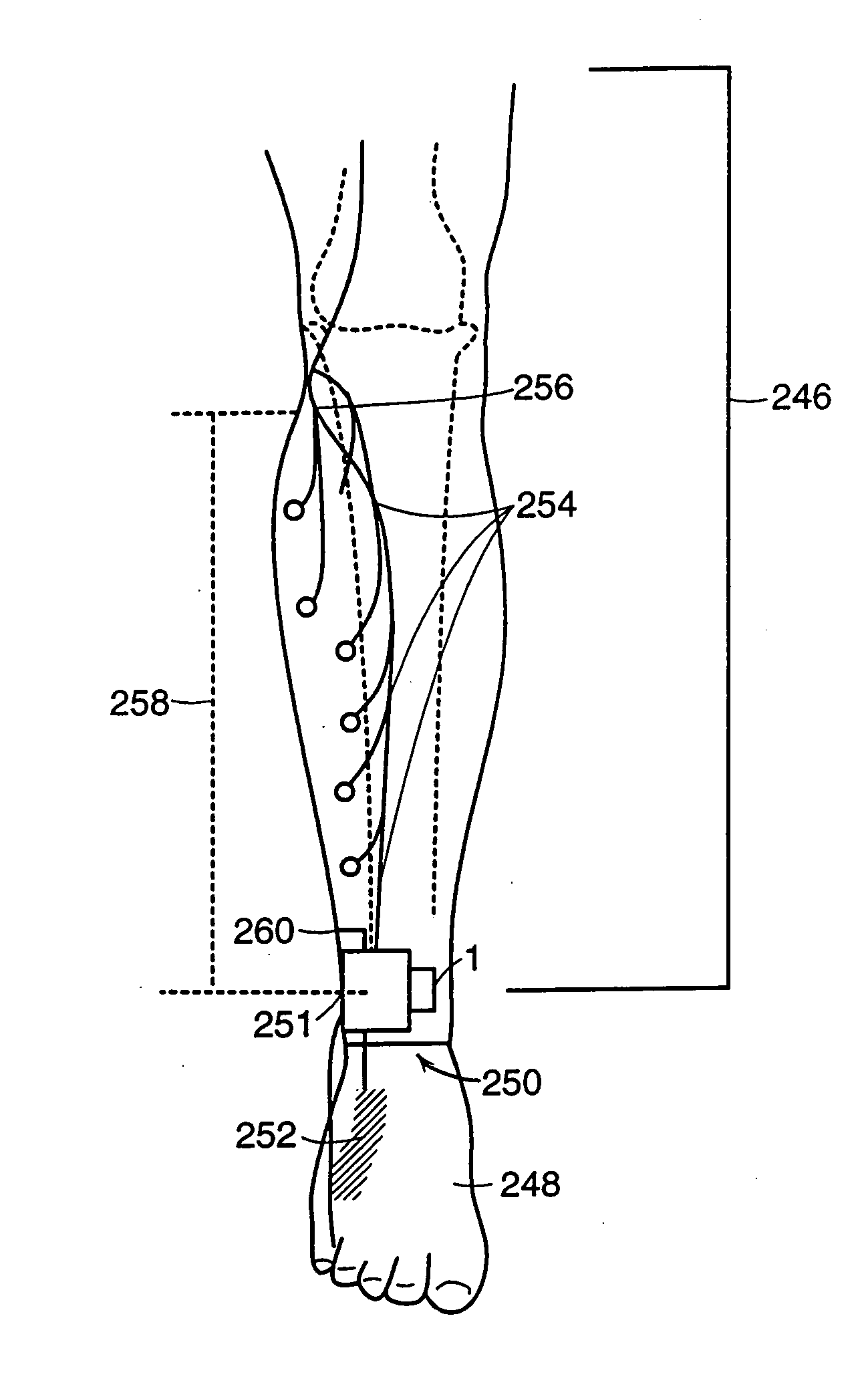
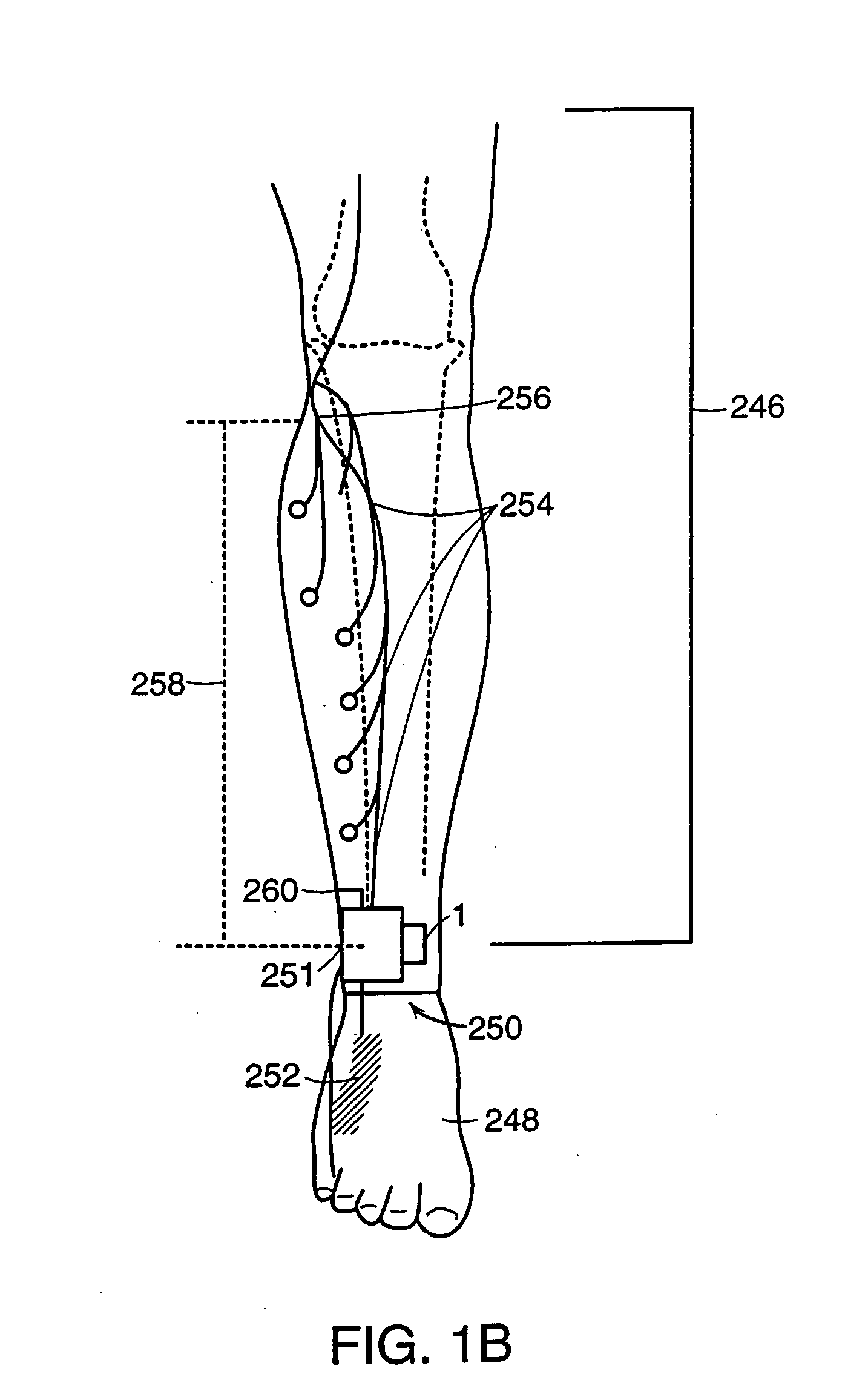
Neuromuscular stimulation to avoid pulmonary embolisms
ActiveUS20060122663A1Avoid it happening againPerson identificationImplantable neurostimulatorsAccelerometerMedicine
A system and method that provide a prophylactic treatment to a person, e.g., a patient, to avoid the occurrence of pulmonary embolisms by the system providing neuromuscular stimulation to a person's lower extremities, e.g., the person's legs, when the system senses that a person has been immobile for an extended period of time. An implantable neuromuscular pacer, such as that described in U.S. Pat. Nos. 5,193,539; 5,193,540; 6,164,284; 6,185,452; 6,208,894; 6,315,721; 6,564,807; and their progeny, may be used to provide to selectively provide such stimulation. Preferably, such a device may be battery powered so that it can operate independent of an external apparatus. In particular, systems and devices of the present invention preferably additionally include an activity monitor, e.g., an accelerometer or the like, that disables or limits stimulation to pronged time periods in which there is limited activity.
Owner:ALFRED E MANN FOUND FOR SCI RES
Neural sleeve for neuromuscular stimulation, sensing and recording
ActiveUS9884179B2Increased electrode positioning choiceGood electrical contactInput/output for user-computer interactionExternal electrodesFlexible circuitsNeuromuscular stimulation
Owner:BATTELLE MEMORIAL INST
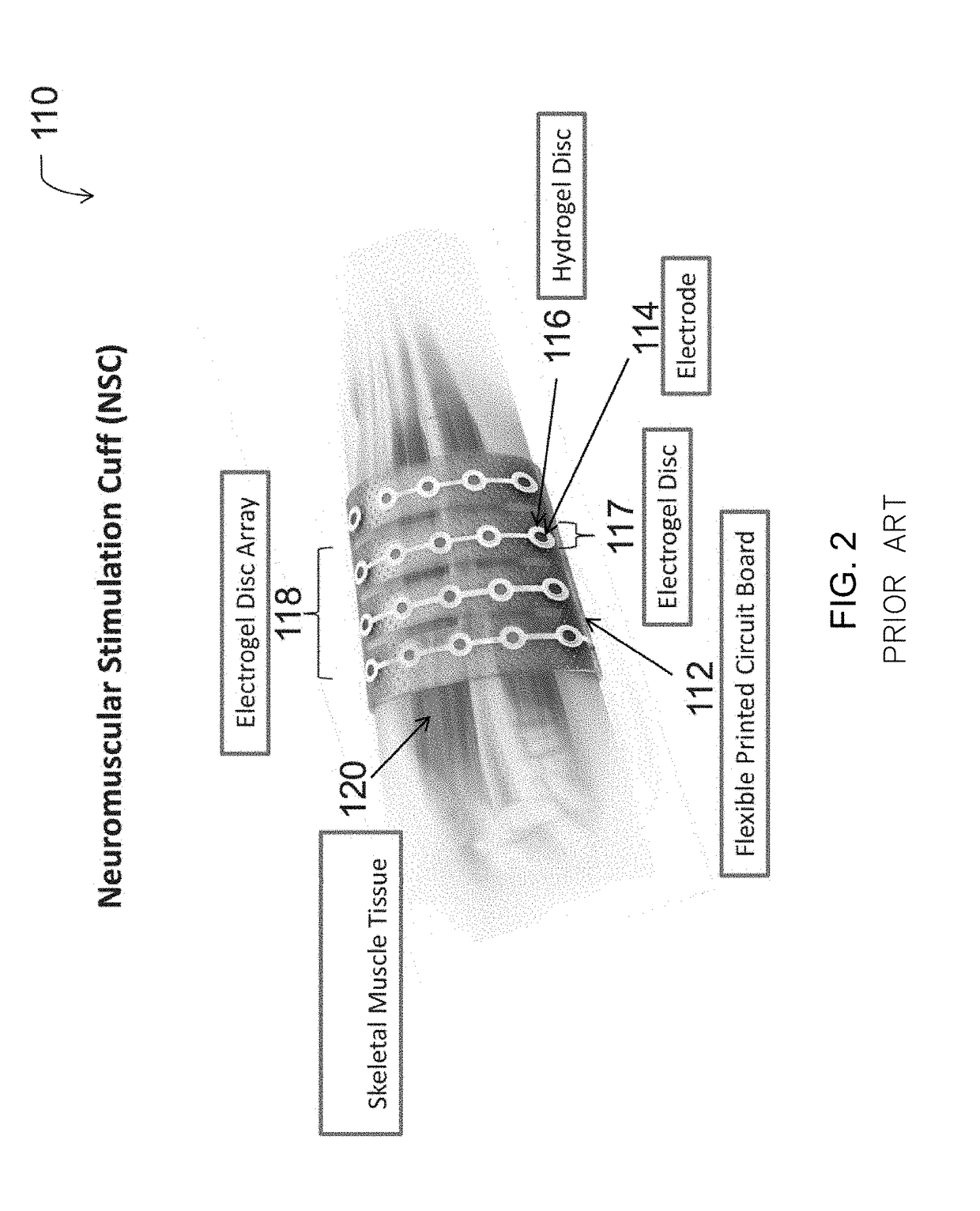
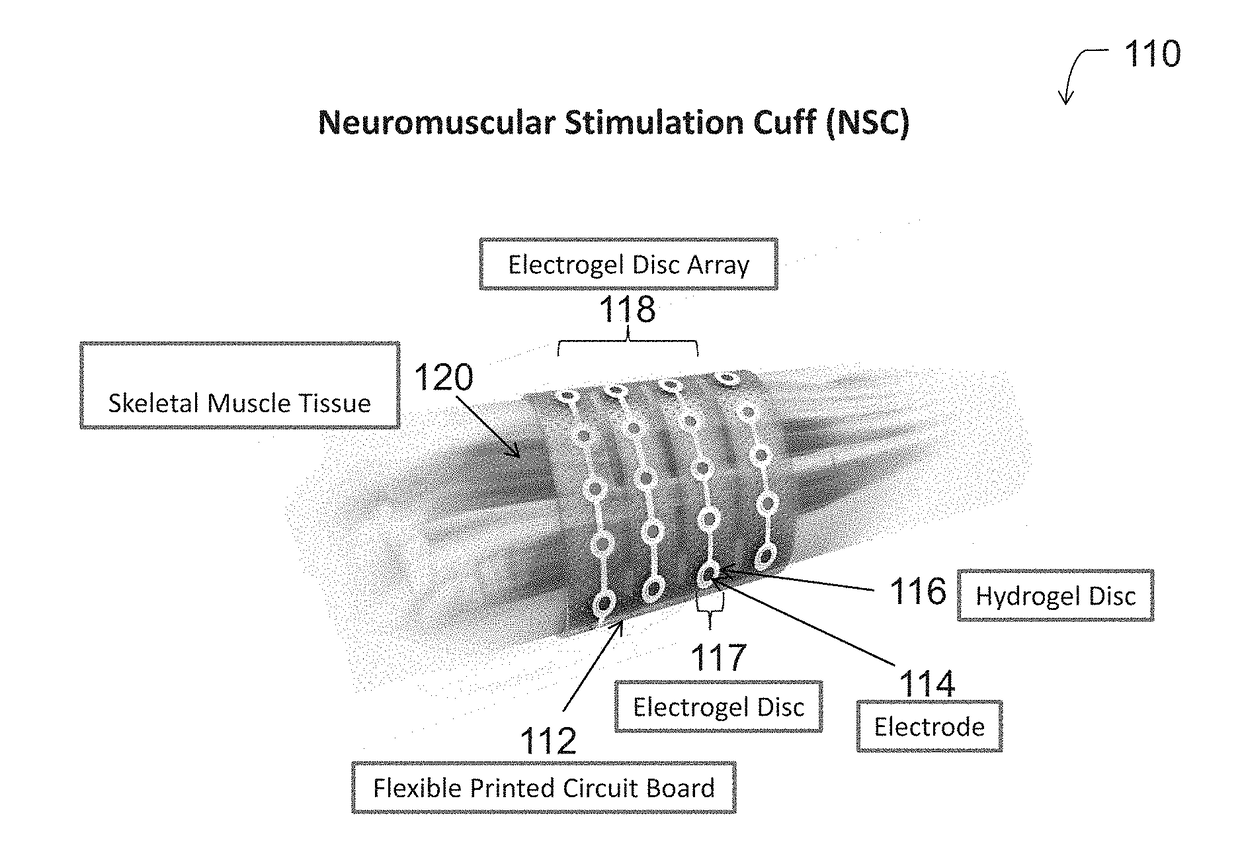
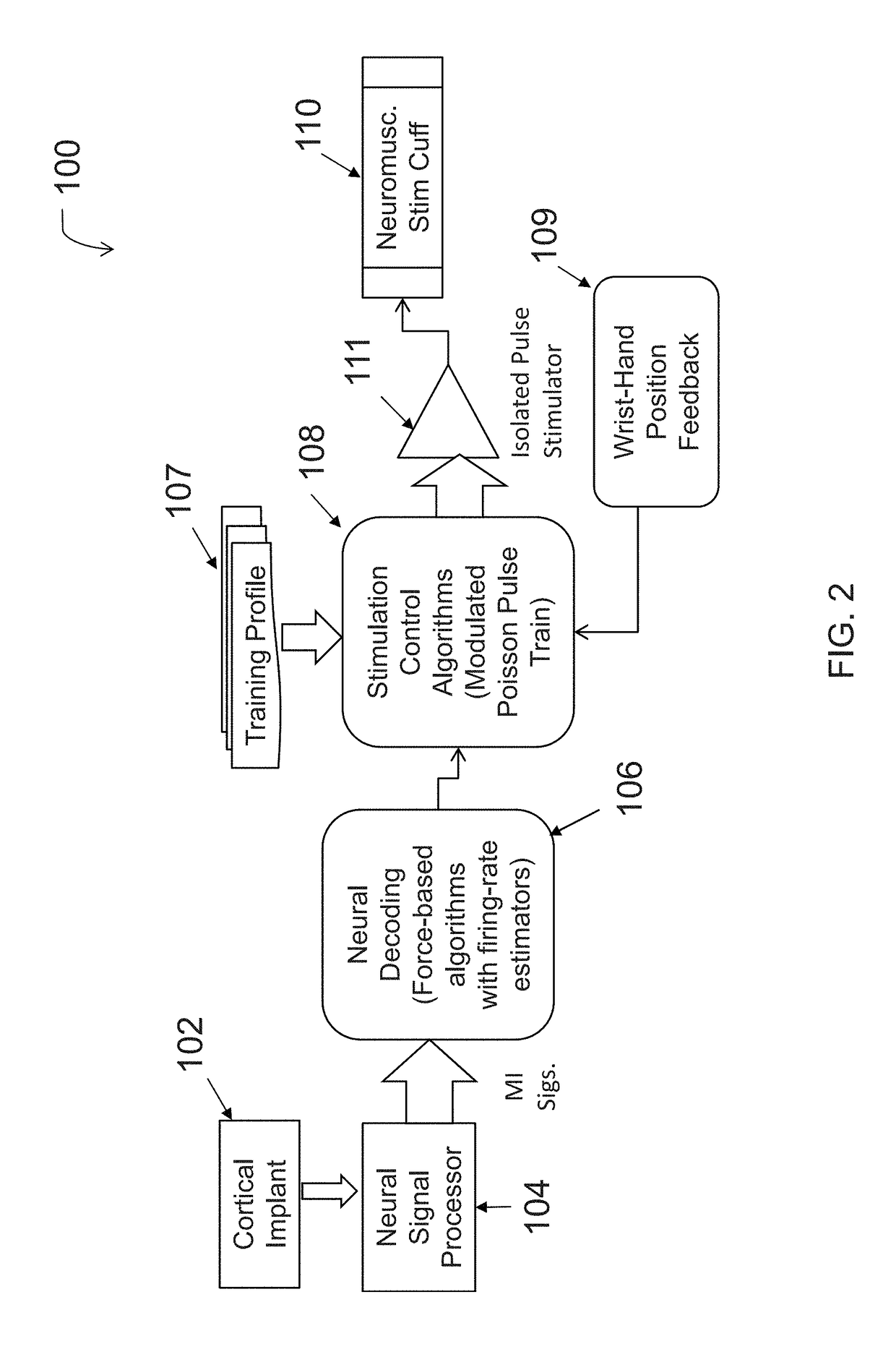
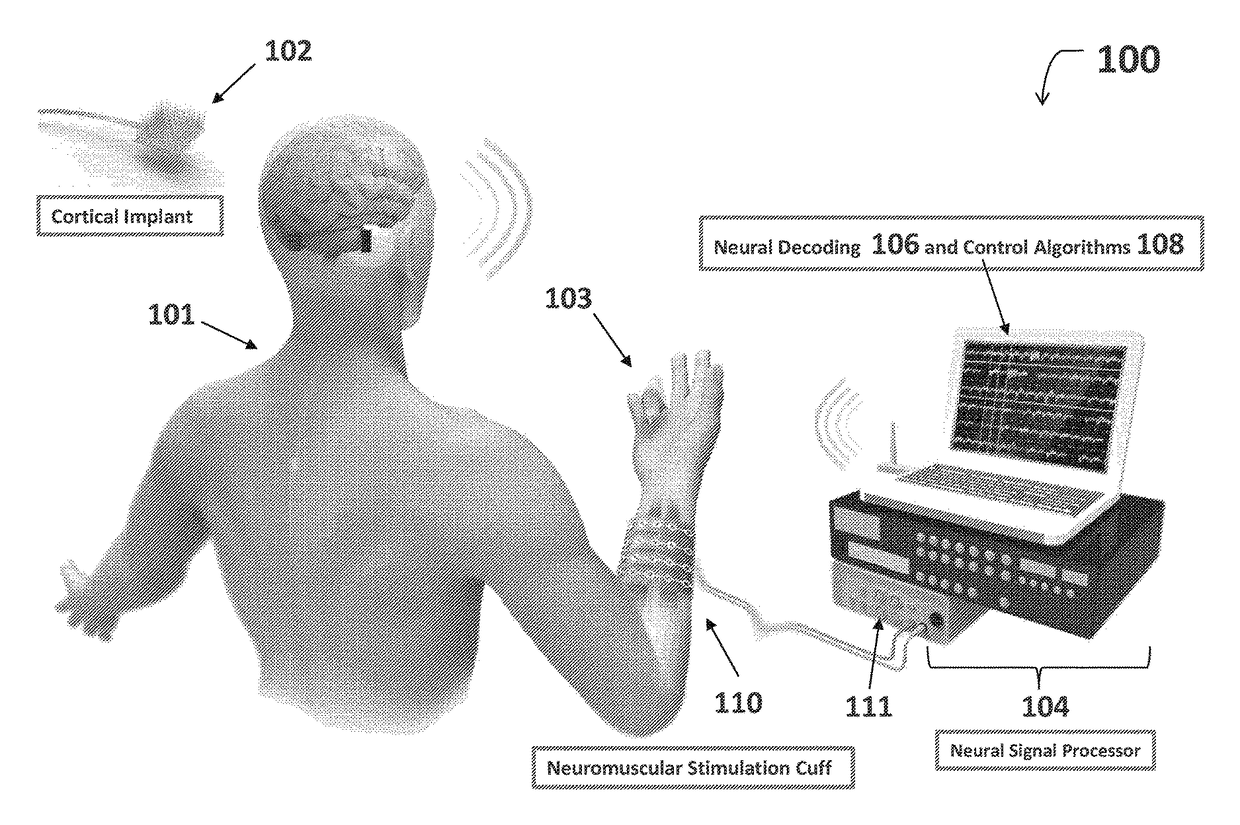
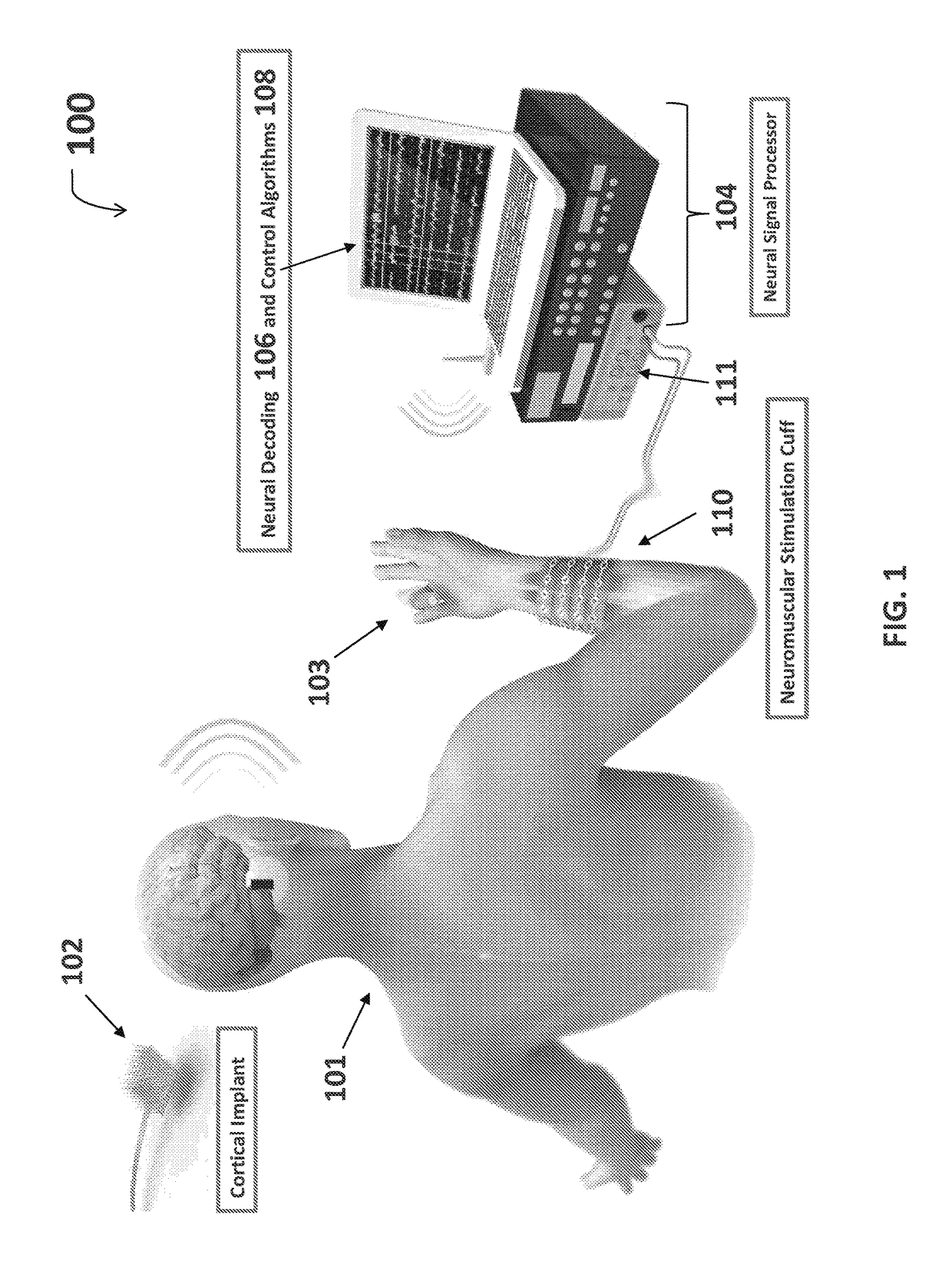
Neuromuscular stimulation cuff
ActiveUS9884178B2Increased electrode positioning choiceGood electrical contactDiagnostic recording/measuringSensorsPhysical therapyNeuromuscular stimulation
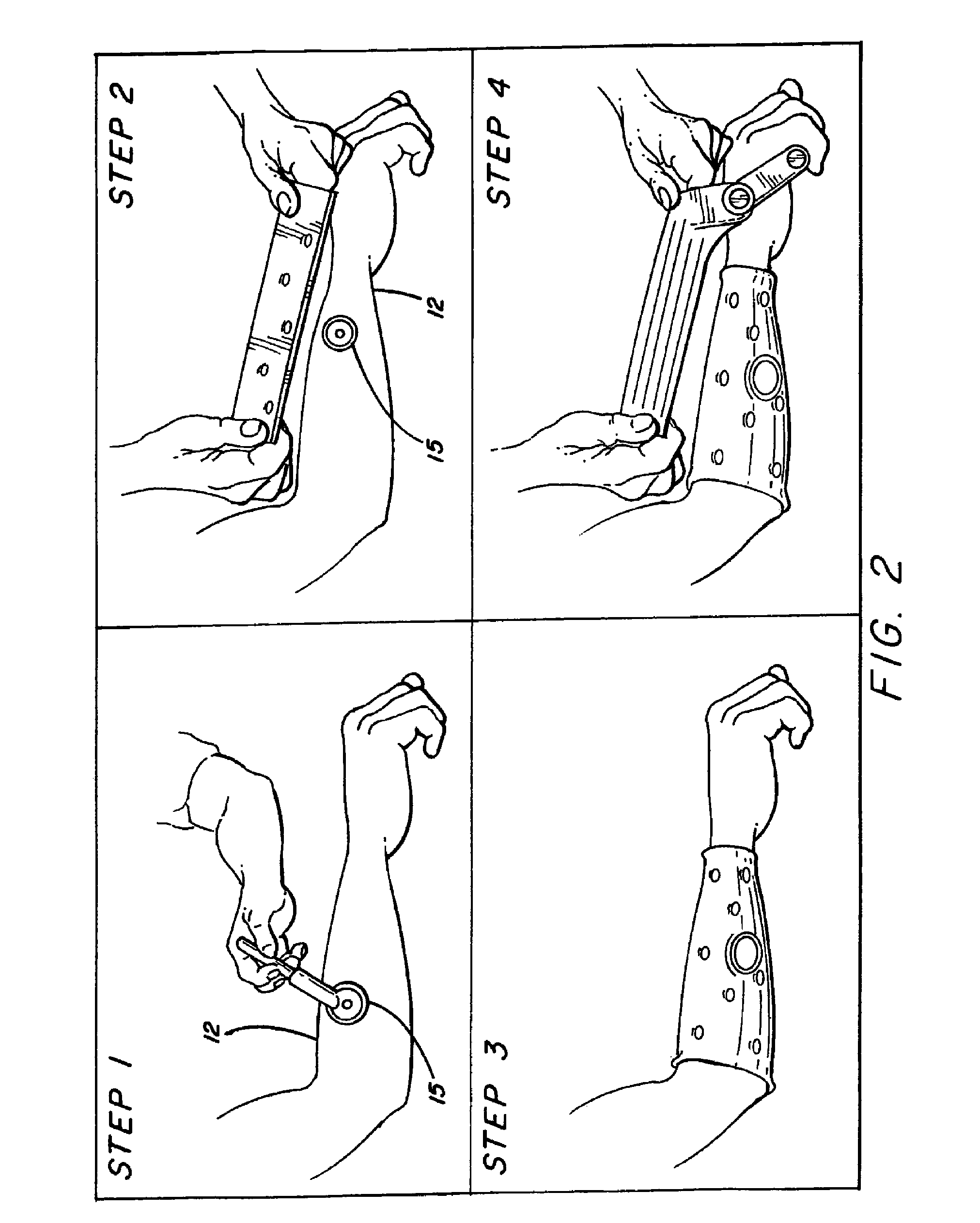
The present disclosure describes systems, methods, and devices for performing thought-controlled neuromuscular stimulation. Also described are methods for producing a neuromuscular stimulation cuff. The systems and methods generally relate to receiving and processing thought signals indicative of an intended action, and then delivering stimulation to effectuate the intended action through a neuromuscular stimulation cuff. The neuromuscular stimulation cuff includes a flexible printed circuit board having at least one finger and a plurality of electrogel discs disposed on the at least one finger. The neuromuscular stimulation cuff may be produced as described herein. The neuromuscular stimulation cuff employs a flexible multi-electrode design which allows for reanimation of complex muscle movements in a patient, including individual finger movement.
Owner:BATTELLE MEMORIAL INST
Methods for the assessment of neuromuscular function by F-wave latency
Methods are provided for the assessment of neuromuscular function by F-wave latency. Stimuli are applied to a nerve that traverses a wrist or an ankle joint of an individual. Stimulation of the nerve causes a muscle innervated by that nerve to respond, thereby generating a myoelectric potential. One component of the myoelectric potential is the F-wave component. The F-wave latency between application of the stimulus and the detection of the myoelectric potential is used to provide an assessment of a neuromuscular function of the nerve and / or muscle.
Owner:GOZANI SHAI N +1
System and method for neuromuscular reeducation
ActiveUS20100198115A1Increase volumeReduce the amount requiredElectrotherapyPerson identificationDisplay deviceTreatment modality
Owner:KINETIC MUSCLES INC
System and method for neuromuscular reeducation
InactiveUS8214029B2Increase volumeReduce the amount requiredElectrotherapyPerson identificationTreatment modalityDisplay device
A system and a method that promotes the restoration of physical functions of the neuromuscular system by incorporating into one device the treatment modalities of biofeedback based repetitive practice, includes an actuator, a joint position measurement system, a force sensing measurement system, an EMG measurement system, a neuromuscular low-level stimulation system, a controller, and a display device.
Owner:KINETIC MUSCLES INC
Neuromuscular stimulation to avoid pulmonary embolisms
A system and method that provide a prophylactic treatment to a person, e.g., a patient, to avoid the occurrence of pulmonary embolisms by the system providing neuromuscular stimulation to a person's lower extremities, e.g., the person's legs, when the system senses that a person has been immobile for an extended period of time. An implantable neuromuscular pacer, such as that described in U.S. Pat. Nos. 5,193,539; 5,193,540; 6,164,284; 6,185,452; 6,208,894; 6,315,721; 6,564,807; and their progeny, may be used to provide to selectively provide such stimulation. Preferably, such a device may be battery powered so that it can operate independent of an external apparatus. In particular, systems and devices of the present invention preferably additionally include an activity monitor, e.g., an accelerometer or the like, that disables or limits stimulation to pronged time periods in which there is limited activity.
Owner:ALFRED E MANN FOUND FOR SCI RES
Recombinant light chains of botulinum neurotoxins and light chain fusion proteins for use in research and clinical therapy
Botulinum neurotoxins, the most potent of all toxins, induce lethal neuromuscular paralysis by inhibiting exocytosis at the neuromuscular junction. The light chains (LC) of these dichain neurotoxins are a new class of zinc-endopeptidases that specifically cleave the synaptosomal proteins, SNAP-25, VAMP, or syntaxin at discrete sites. The present invention relates to the construction, expression, purification, and use of synthetic or recombinant botulinum neutoroxin genes. For example, a synthetic gene for the LC of the botulinum neurotoxin serotype A (BoNT / A) was constructed and overexpressed in Escherichia coli. The gene product was purified from inclusion bodies. The methods of the invention can provide 1.1 g of the LC per liter of culture. The LC product was stable in solution at 4° C. for at least 6 months. This rBoNT / A LC was proteolytically active, specifically cleaving the Glu-Arg bond in a 17-residue synthetic peptide of SNAP-25, the reported cleavage site of BoNT / A. Its calculated catalytic efficiency kcat / Km was higher than that reported for the native BoNT / A dichain. Treating the rBoNT / A LC with mercuric compounds completely abolished its activity, most probably by modifying the cysteine-164 residue located in the vicinity of the active site. About 70% activity of the LC was restored by adding Zn2+ to a Zn2+-free, apo-LC preparation. The LC was nontoxic to mice and failed to elicit neutralizing epitope(s) when the animals were vaccinated with this protein. In addition, injecting rBoNT / A LC into sea urchin eggs inhibited exocytosis-dependent plasma membrane resealing.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Patterned Cell Network Substrate Interface and Methods and Uses Thereof
InactiveUS20070231850A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCell membraneCell network
There is provided herein a method and apparatus suitable for use in studying cell membrane related activities. Activities of interest include patch-clamp related studies of networks of cells on a solid substrate. Cells are grown, preferably in a patterned manner, on a substrate having microholes therein. Seals between the cells and the microholes are formed. Each microhole is attached to a channel. In many cases only one hole will be attached to a single channel, allowing examination of effects of a stimulus at a number of different points in a network of one or more cell types. This may be interest, for example, to those wishing to study interactions between neurons or neuromuscular junctions.
Owner:GEOFFREY MEALING +6
Neuromuscular stimulation
ActiveUS8112155B2Move his arm more effectivelyElectromyographySensorsEngineeringNeuromuscular stimulation
An apparatus for muscle activation includes least one electrode adapted to deliver a neuromuscular stimulation (NMES) signal to a body portion. A controller provides a NMES signal comprising a sequence of stimulation signals to the electrode. A mechanical motion element coupled to the body portion and a mirror body portion is operatively coupled to the controller. The controller controls the NMES signal in conjunction with the mechanical motion element.
Owner:MOTORIKA
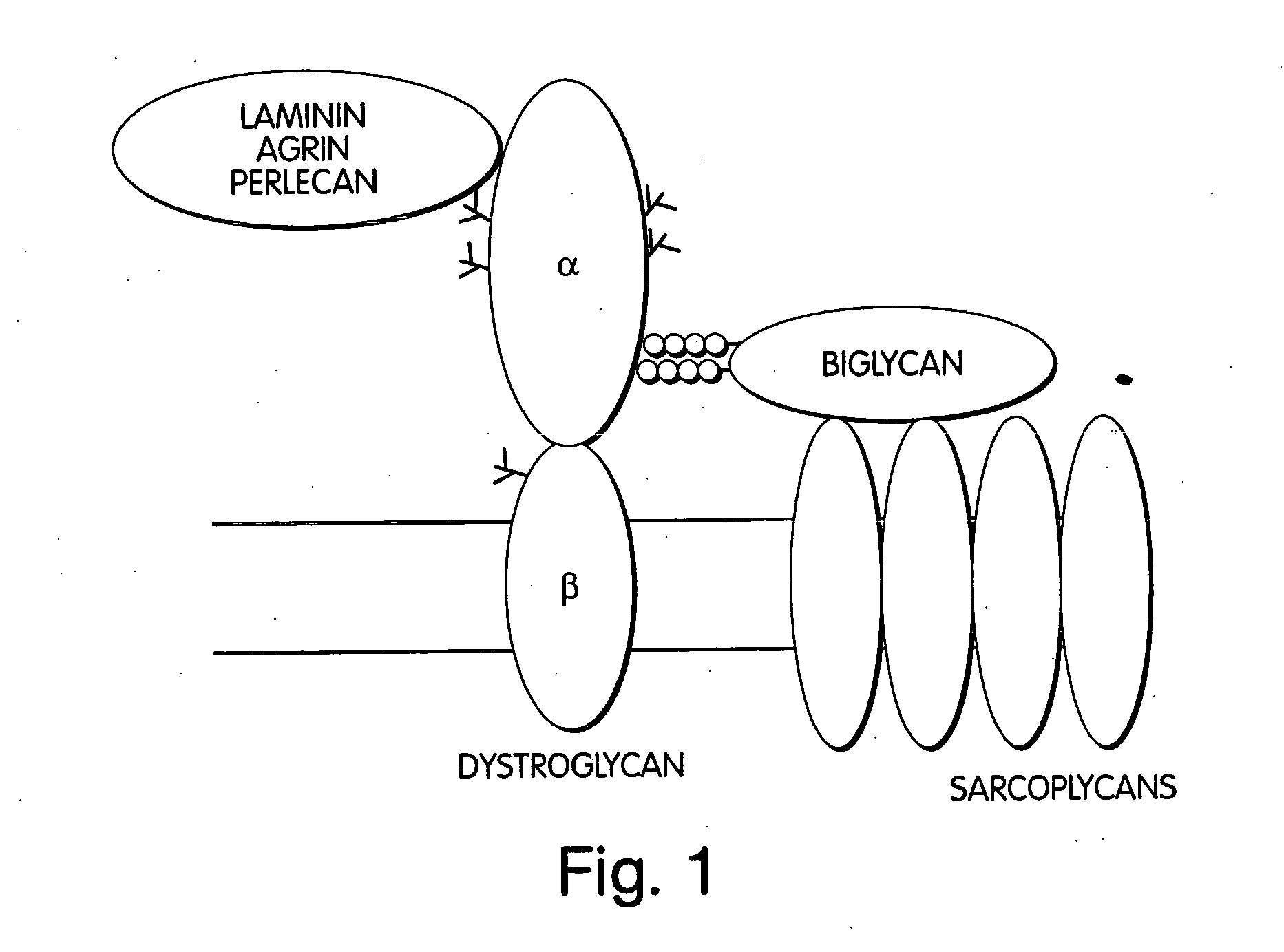
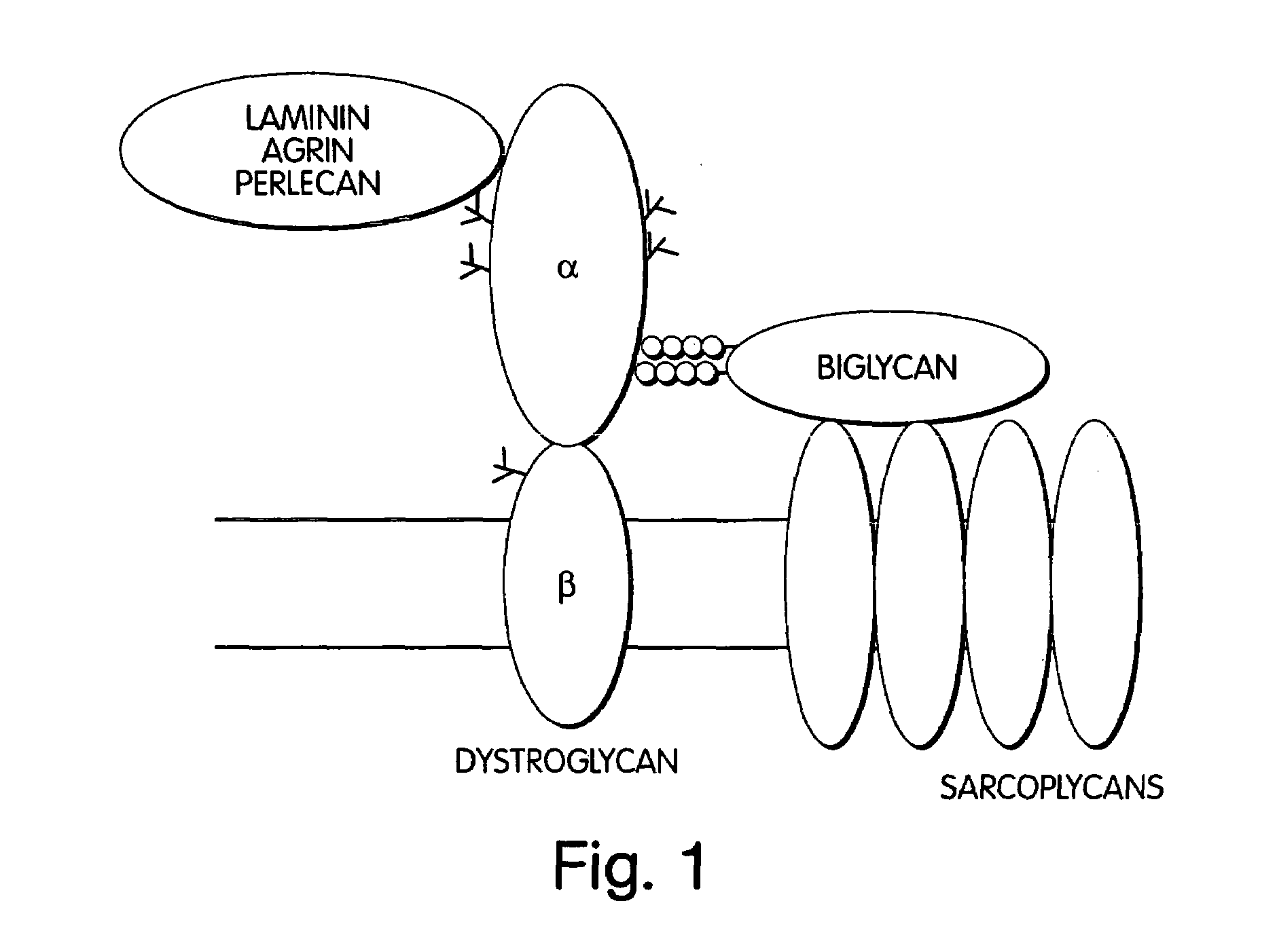
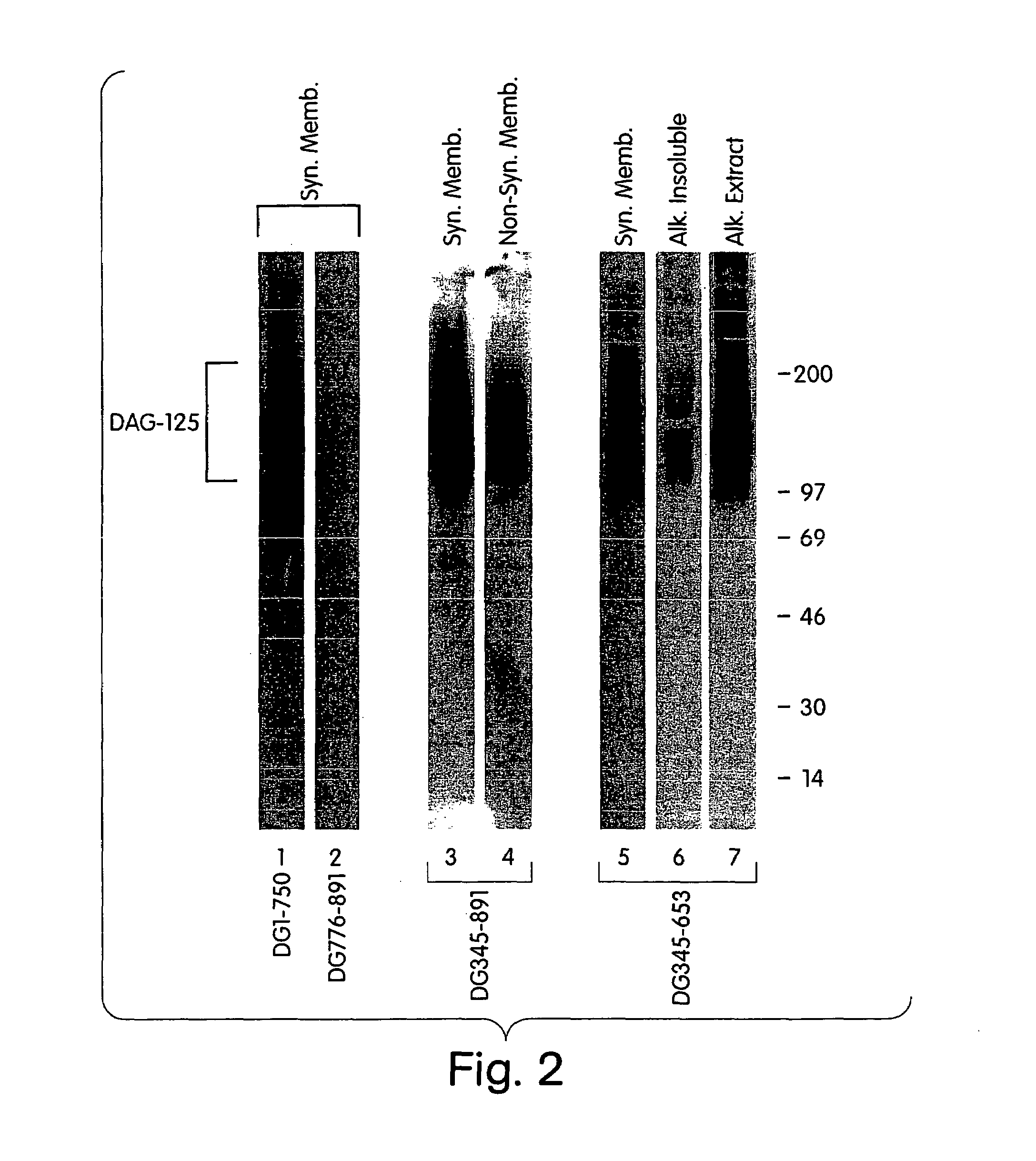
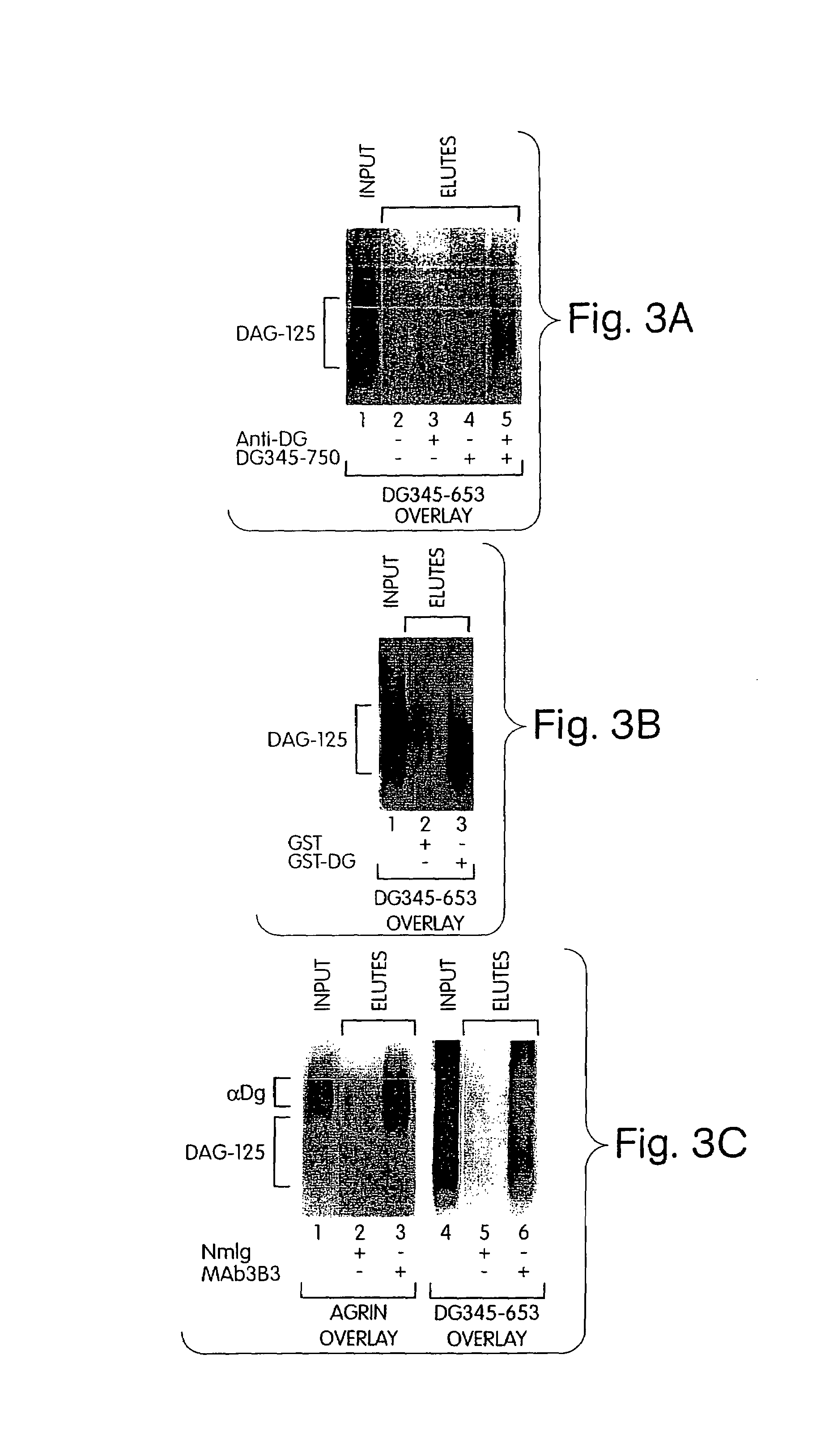
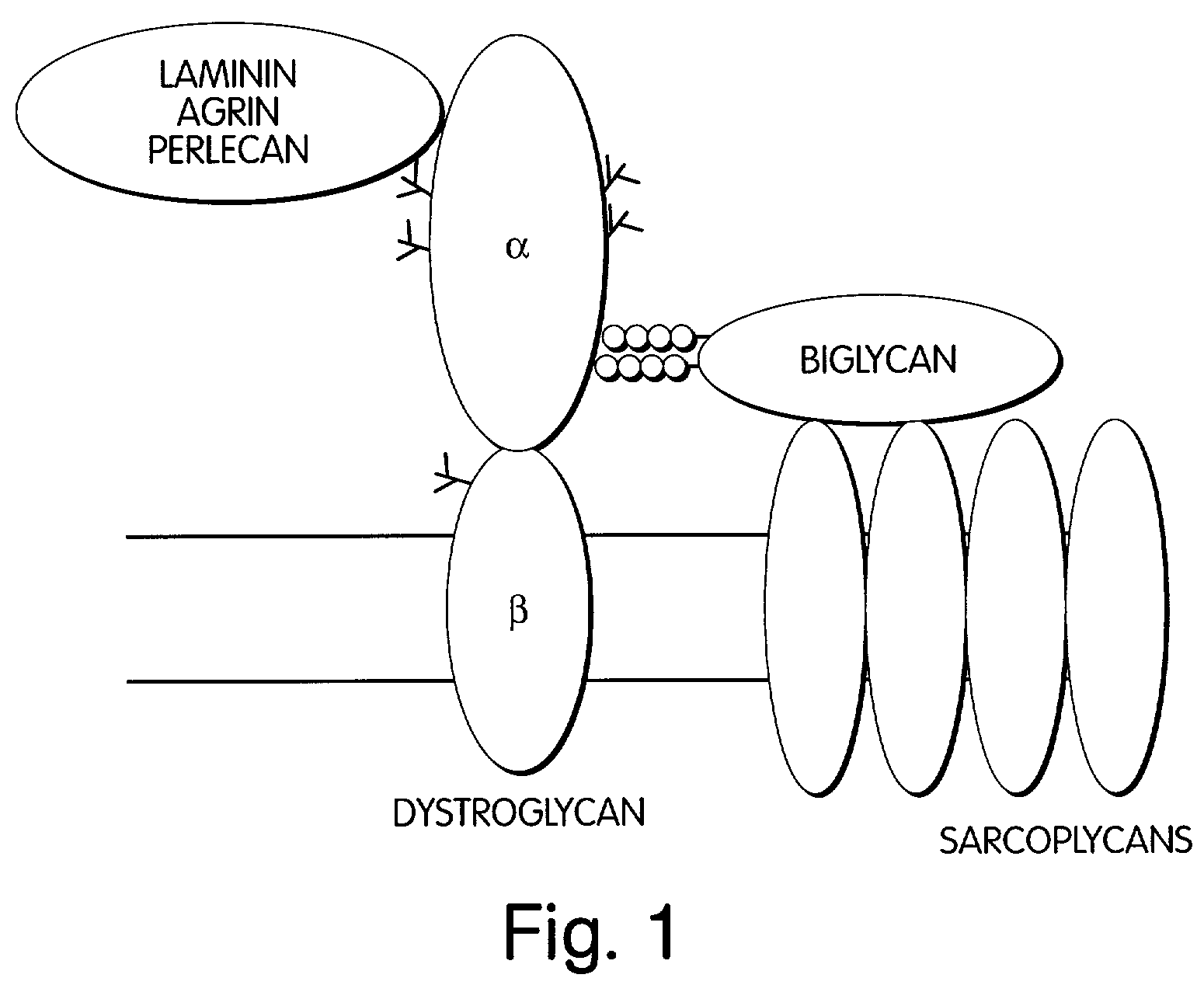
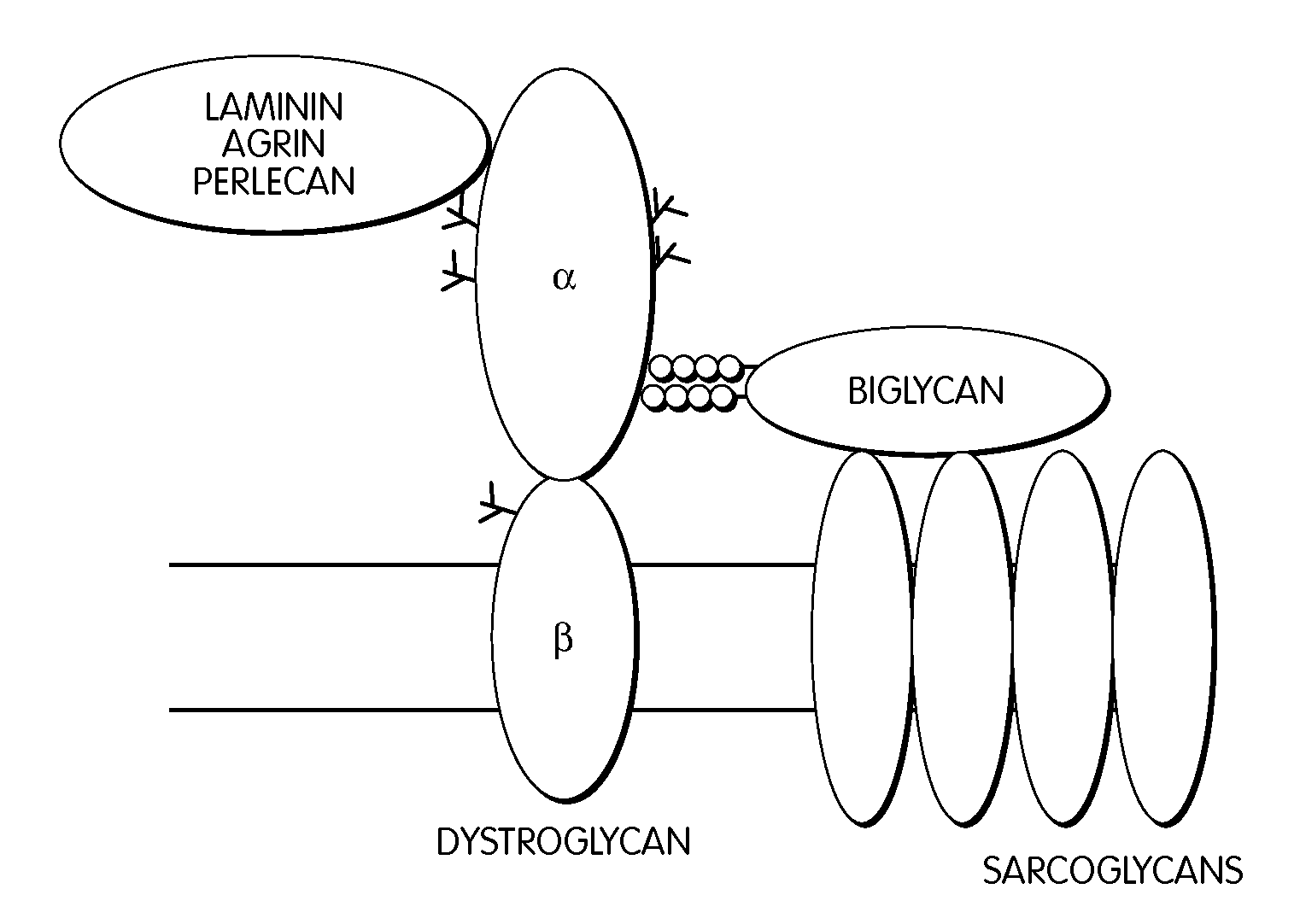
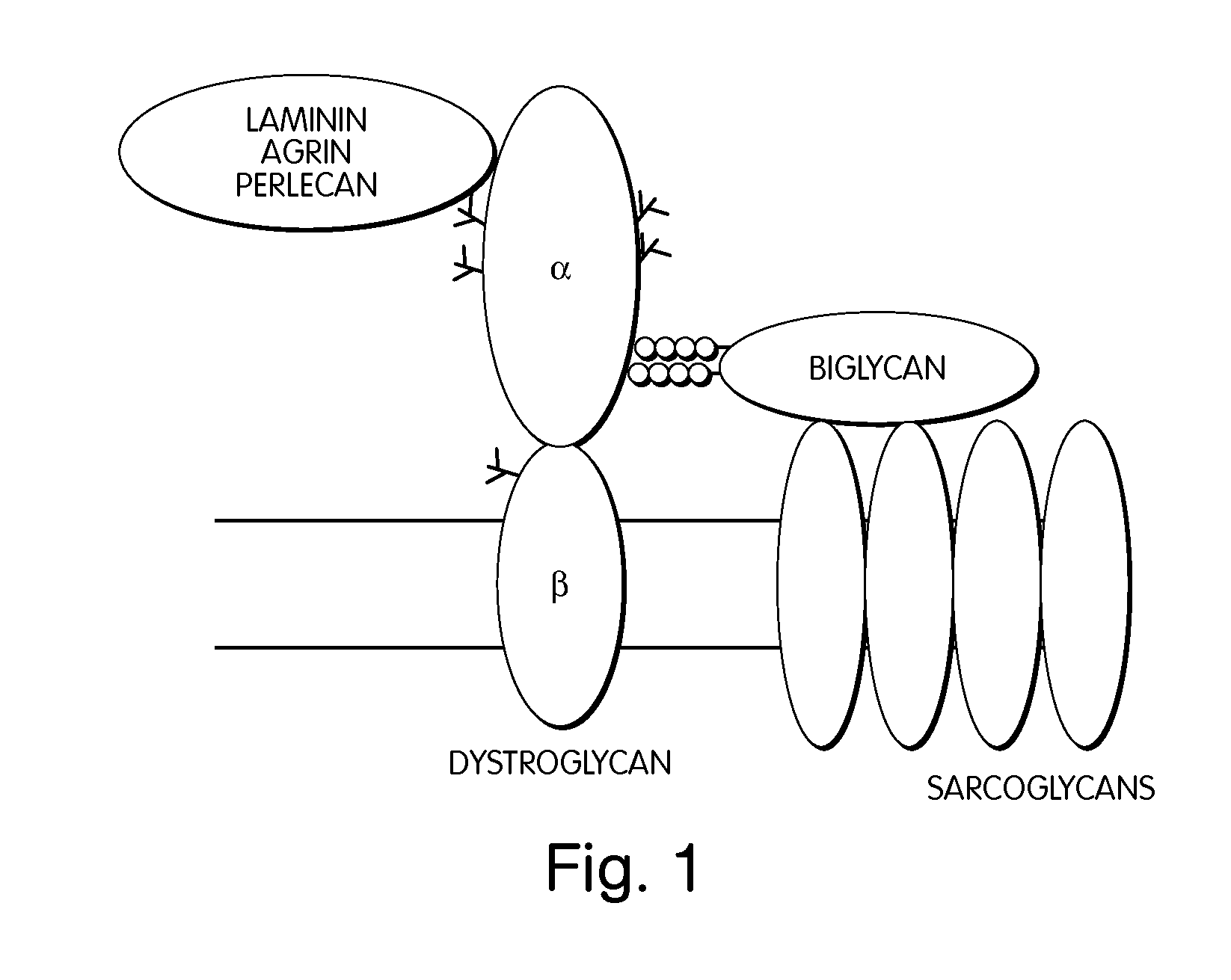
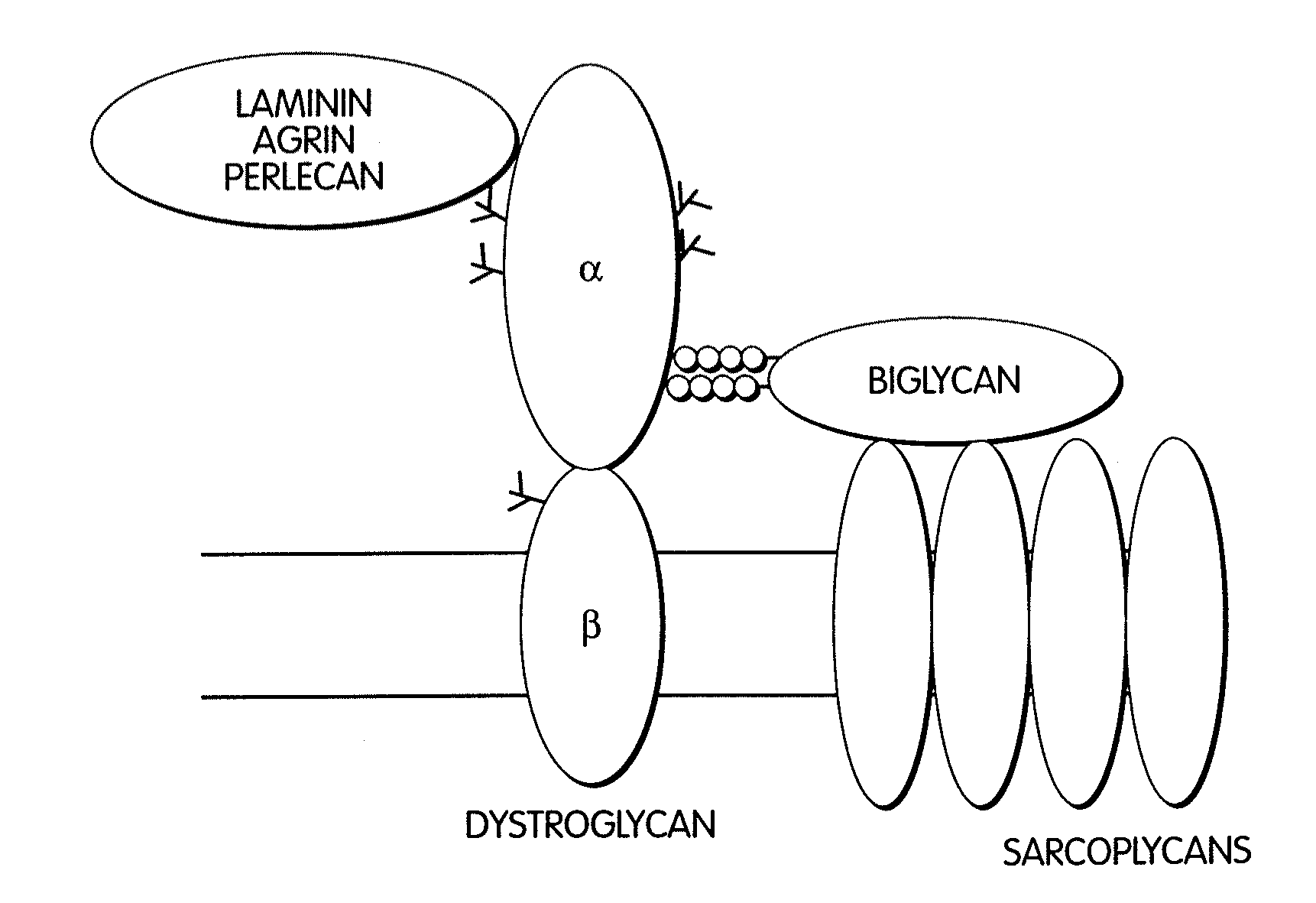
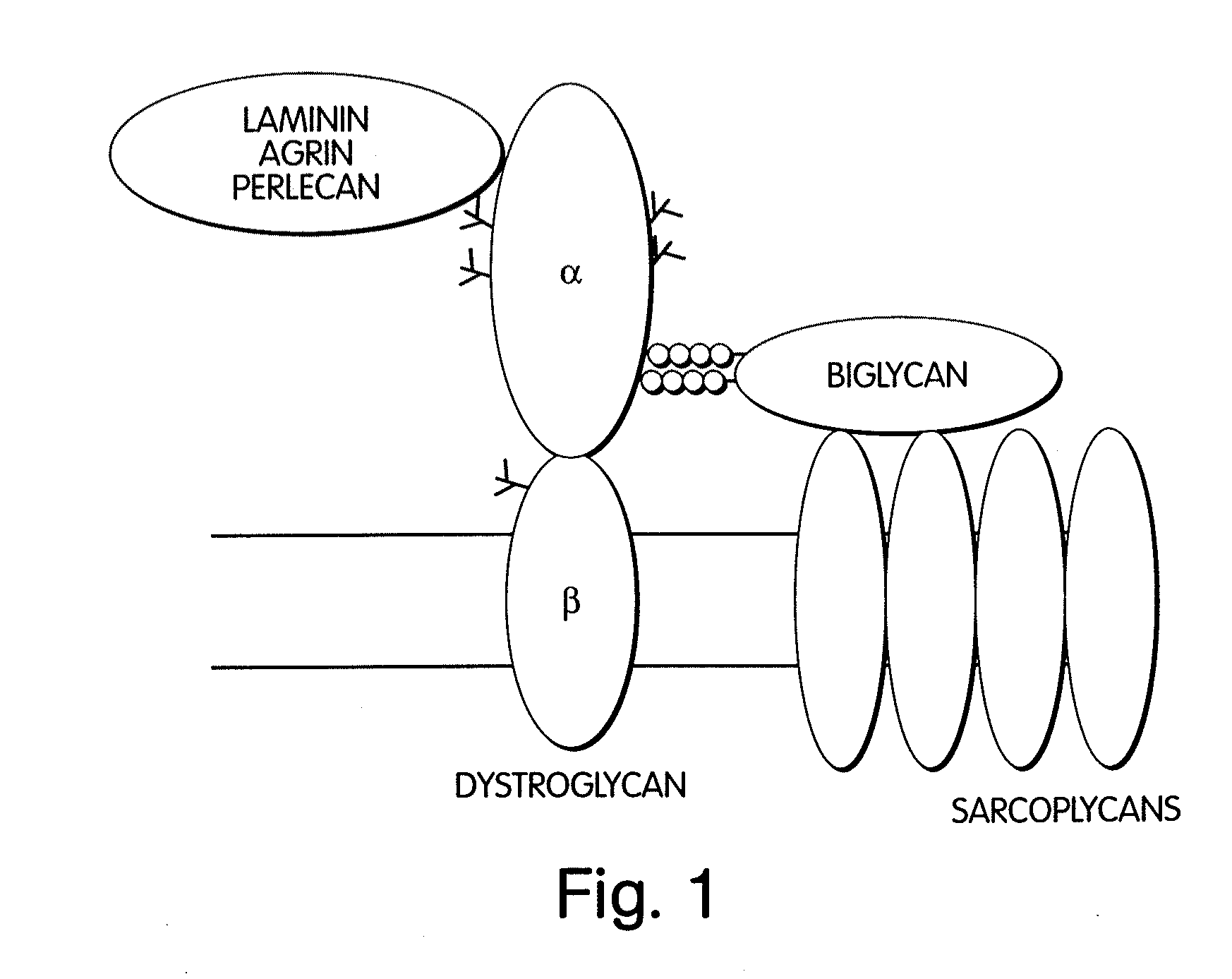
Biglycan and related therapeutics and methods of use
InactiveUS20050059580A1Less “ leaky ”Extend your lifeHormone peptidesSnake antigen ingredientsNervous systemBiglycan
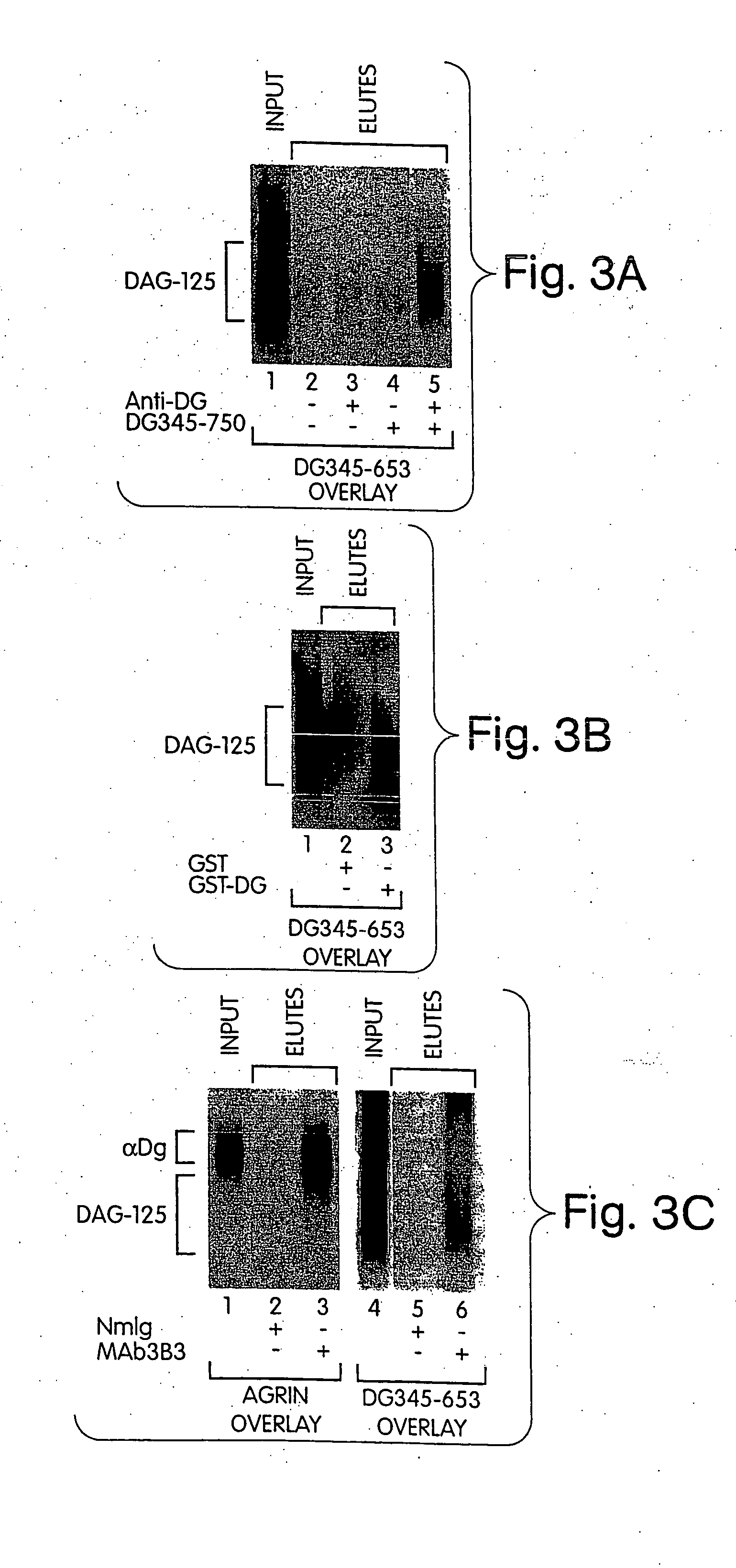
The invention provides compositions and methods for treating, preventing, and diagnosing diseases or conditions associated with an abnormal level or activity of biglycan; disorders associated with an unstable cytoplasmic membrane, due, e.g., to an unstable dystrophin associated protein complex (DAPC); disorders associated with abnormal synapses or neuromuscular junctions, including those resulting from an abnormal MuSK activation or acetylcholine receptor (AChR) aggregation. Examples of diseases include muscular dystrophies, such as Duchenne's Muscular Dystrophy, Becker's Muscular Dystrophy, neuromuscular disorders and neurological disorders.
Owner:BROWN UNIV RES FOUND INC
Neuromuscular stimulation system
A wearable neuromuscular stimulation and neuroprosthetic system and device for treating spinal cord injury, stroke, and other neurological conditions; and for the management of chronic pain. This invention provides a system for transcutaneous neuromuscular stimulation and typically comprises a wearable item that further includes a flexible, non-conductive material; at least one flexible, generally flat electrode attachable to or embedded within the wearable item; and a programmable electrical stimulation device connectable to the electrode(s). Each electrode typically includes a silver-impregnated or silver-treated material.
Owner:MUCCIO PHILIP E
Biglycan and related therapeutics and methods of use
InactiveUS7335637B2Less “ leaky ”Extend your lifeBiocideHormone peptidesAcetylcholine receptorMedicine
The invention provides compositions and methods for treating, preventing, and diagnosing diseases or conditions associated with an abnormal level or activity of biglycan; disorders associated with an unstable cytoplasmic membrane, due, e.g., to an unstable dystrophin associated protein complex (DAPC); disorders associated with abnormal synapses or neuromuscular junctions, including those resulting from an abnormal MuSK activation or acetylcholine receptor (AChR) aggregation. Examples of diseases include muscular dystrophies, such as Duchenne's Muscular Dystrophy, Becker's Muscular Dystrophy, neuromuscular disorders and neurological disorders.
Owner:BROWN UNIV RES FOUND INC
Recombinant light chains of botulinum neurotoxins and light chain fusion proteins for use in research and clinical therapy
InactiveUS20070104737A1High A+T rich base compositionFungiBacteriaEndopeptidaseNeuromuscular junction
Botulinum neurotoxins, the most potent of all toxins, induce lethal neuromuscular paralysis by inhibiting exocytosis at the neuromuscular junction. The light chains (LC) of these dichain neurotoxins are a new class of zinc-endopeptidases that specifically cleave the synaptosomal proteins, SNAP-25, VAMP, or syntaxin at discrete sites. The present invention relates to the construction, expression, purification, and use of synthetic or recombinant botulinum neutoroxin genes. For example, a synthetic gene for the LC of the botulinum neurotoxin serotype A (BoNT / A) was constructed and overexpressed in Escherichia coli. The gene product was purified from inclusion bodies. The methods of the invention can provide 1.1 g of the LC per liter of culture. The LC product was stable in solution at 4° C. for at least 6 months. This rBoNT / A LC was proteolytically active, specifically cleaving the Glu-Arg bond in a 17-residue synthetic peptide of SNAP-25, the reported cleavage site of BoNT / A. Its calculated catalytic efficiency kcat / Km was higher than that reported for the native BoNT / A dichain. Treating the rBoNT / A LC with mercuric compounds completely abolished its activity, most probably by modifying the cysteine-164 residue located in the vicinity of the active site. About 70% activity of the LC was restored by adding Zn2+ to a Zn2+-free, apo-LC preparation. The LC was nontoxic to mice and failed to elicit neutralizing epitope(s) when the animals were vaccinated with this protein. In addition, injecting rBoNT / A LC into sea urchin eggs inhibited exocytosis-dependent plasma membrane resealing.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
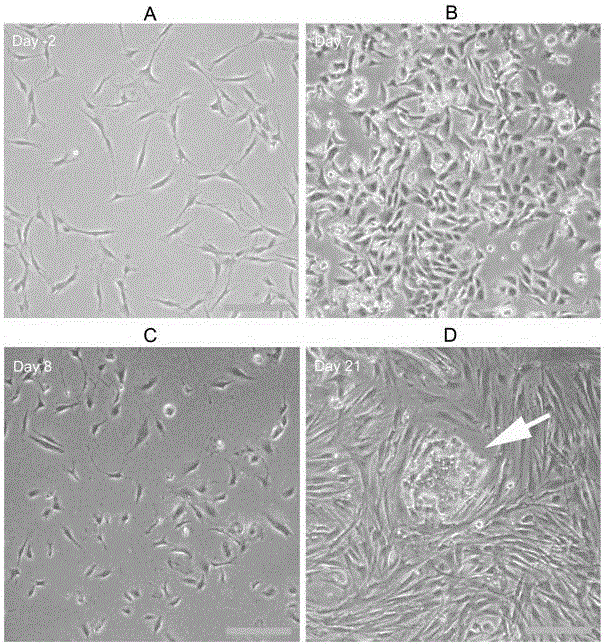
Method for obtaining spinal motoneurons and functional cells thereof based on human iPS (induced pluripotent stem) cells
ActiveCN106701824APromote directed differentiationRapid directed differentiationGenetically modified cellsNervous system cellsDisease phenotypeCells transplantation
The invention belongs to the field of biomedicine, and relates to a safe and reliable reprogrammed method for obtaining human iPS (induced pluripotent stem) cells, a method for simply, rapidly and efficiently inducing and differentiating iPS cells into spinal motoneurons, and a method for economically and efficiently obtaining functional spinal motoneurons and application of the method. Human fibroblasts are infected with Sendai virus carrying a reprogramming factor by using vitronectin as the sole growth medium, and are cultured for 3 weeks to obtain human iPS cells without exogenous serum interference; the iPS cells are efficiently induced and differentiated into spinal motoneurons by a combination of multiple small molecule compounds; and further, an astrocyte conditioned culture medium promotes maturation culture and is applied in the formation of neuromuscular junctions. The functional spinal motoneurons obtained by efficient induction can be applied to simulation of disease phenotype and discussion of pathogenesis, and provides a theoretical basis for the screening of effective therapeutic drugs in the future, and even in the future can be applied to the cell transplantation therapy of diseases.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV
Biglycan and related therapeutics and methods of use
The invention provides compositions and methods for treating, preventing, and diagnosing diseases or conditions associated with an abnormal level or activity of biglycan; disorders associated with an unstable cytoplasmic membrane, due, e.g., to an unstable dystrophin associated protein complex (DAPC); disorders associated with abnormal synapses or neuromuscular junctions, including those resulting from an abnormal MuSK activation or acetylcholine receptor (AChR) aggregation. Example of diseases include muscular dystrophies, such as Duchenne's Muscular Dystrophy, Becker's Muscular Dystrophy, neuromuscular disorders and neurological disorders.
Owner:BROWN UNIV RES FOUND INC
Treatment of muscular dystrophies and related disorders
InactiveUS20110053854A1Less “ leaky ”Extend your lifePeptide/protein ingredientsMuscular disorderSynapseDisease
The invention provides, among other aspects, compositions and methods for treating, preventing, and diagnosing diseases or conditions associated with an abnormal level or activity of biglycan; diseases or conditions associated with an abnormal level or activity of collagen VI; disorders associated with an unstable cytoplasmic membrane, due, e.g., to an unstable dystrophin associated protein complex (DAPC); and disorders associated with abnormal synapses or neuromuscular junctions, including those resulting from an abnormal MuSK activation or acetylcholine receptor (AChR) aggregation.
Owner:BROWN UNIVERSITY
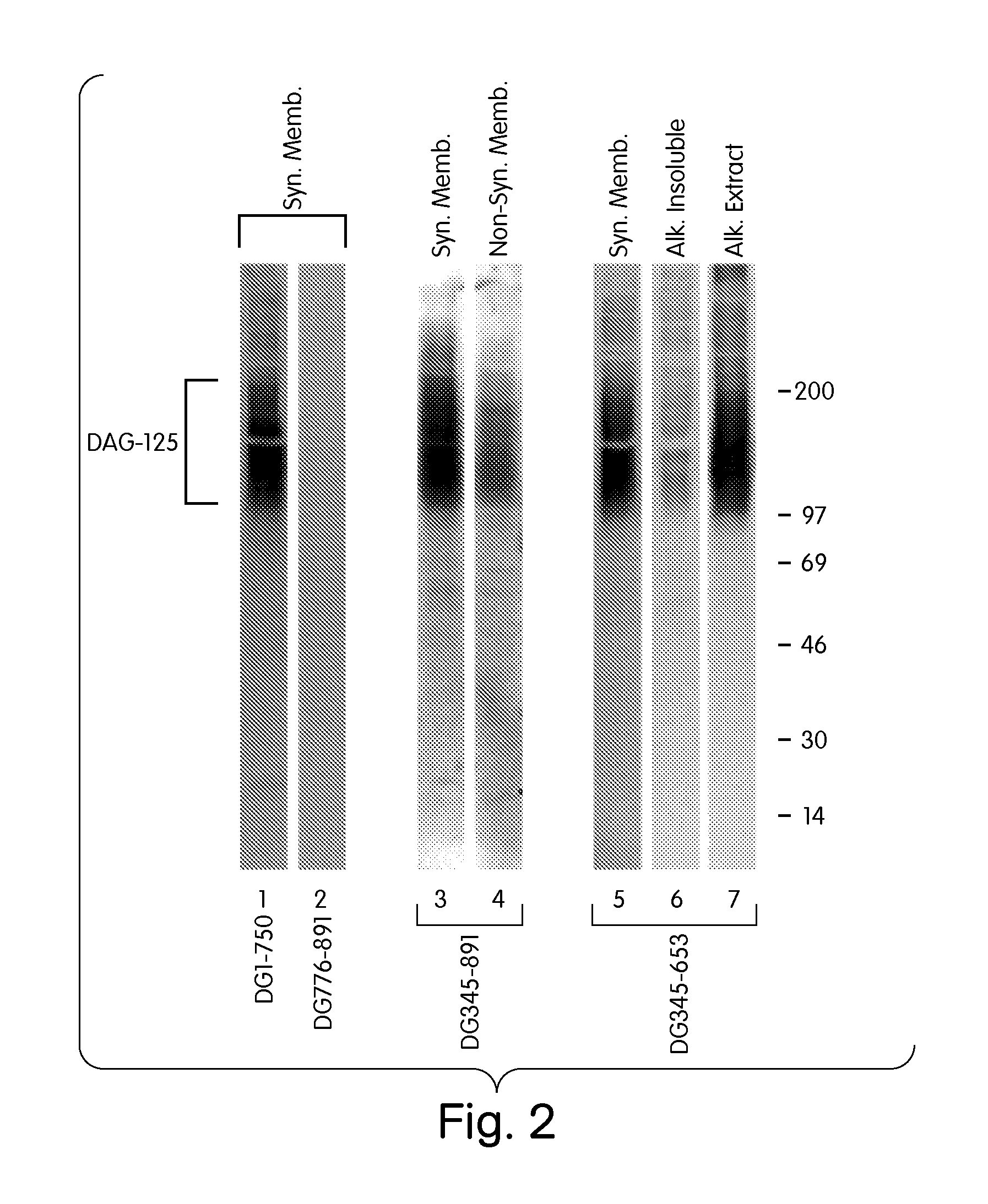
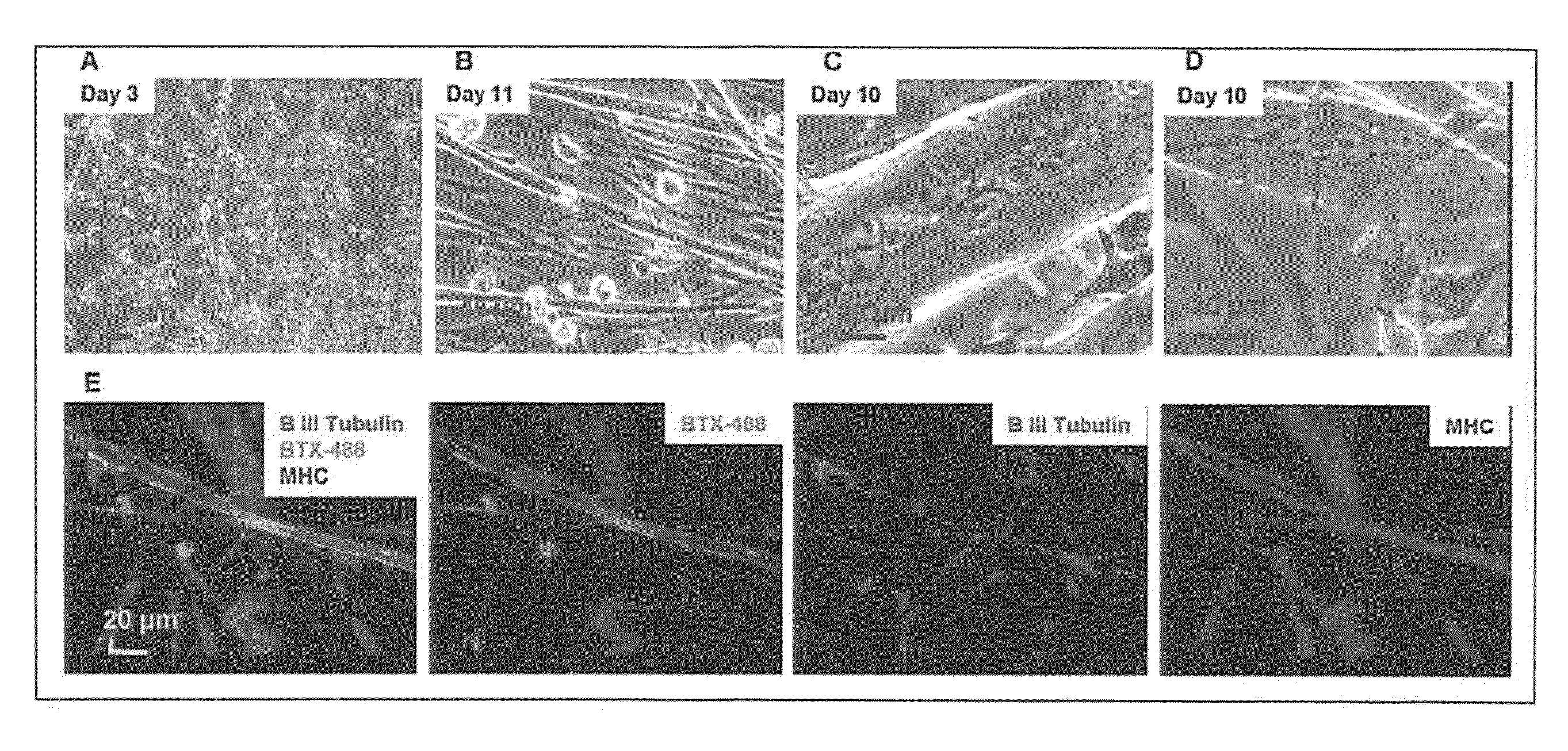
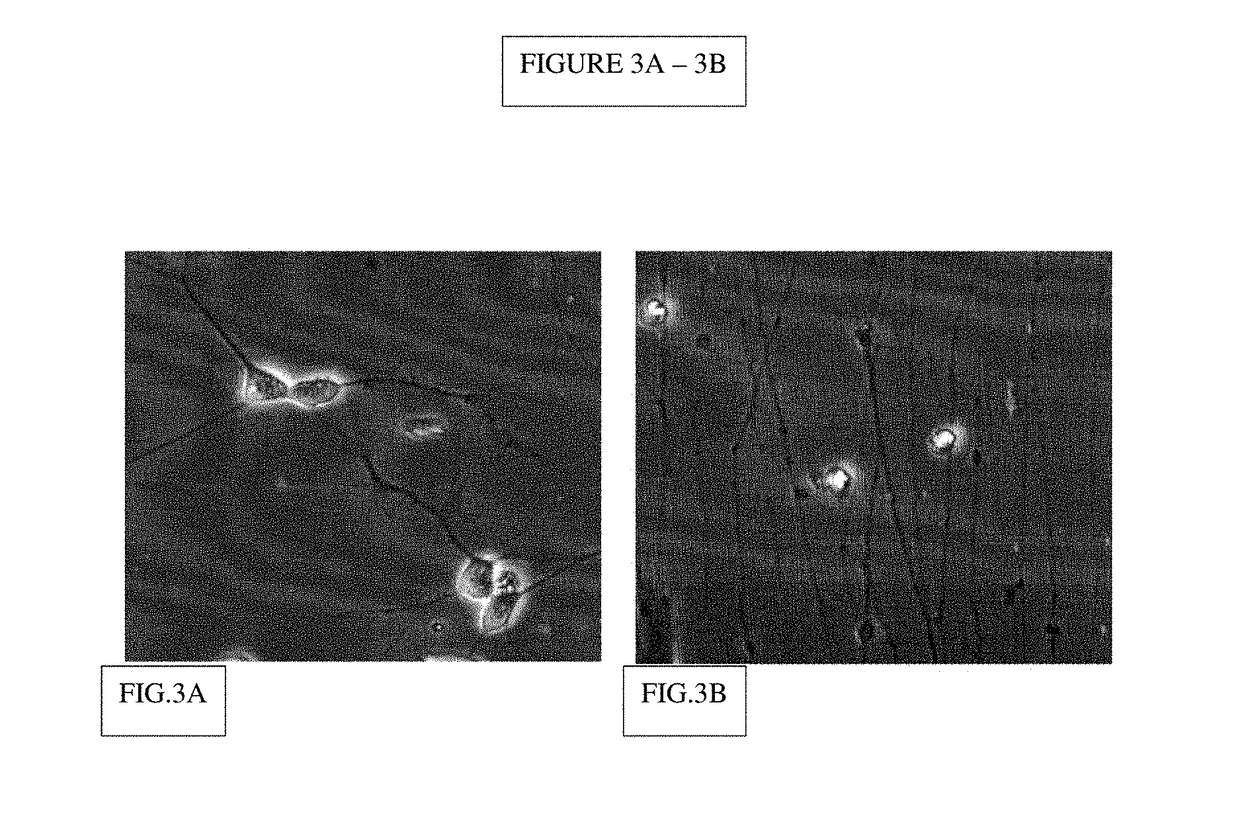
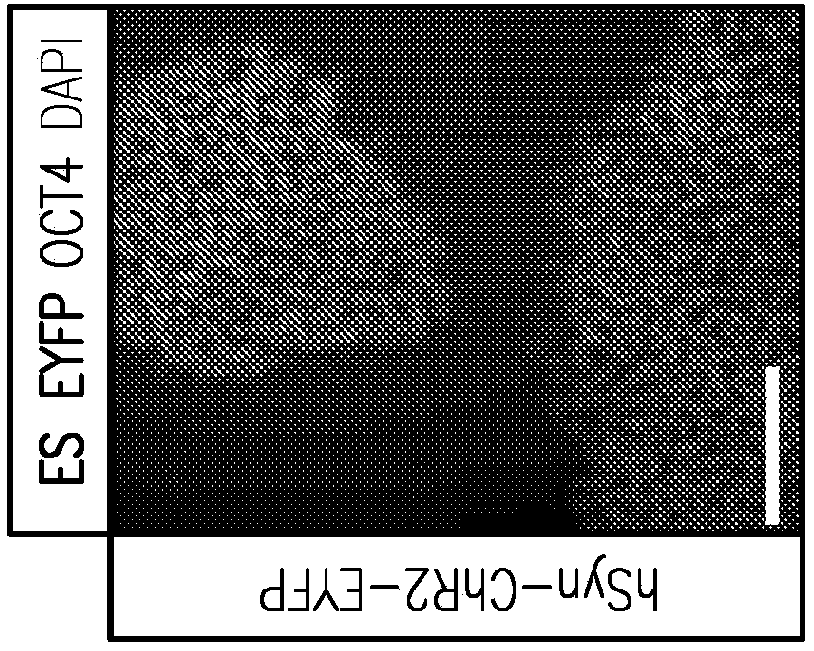
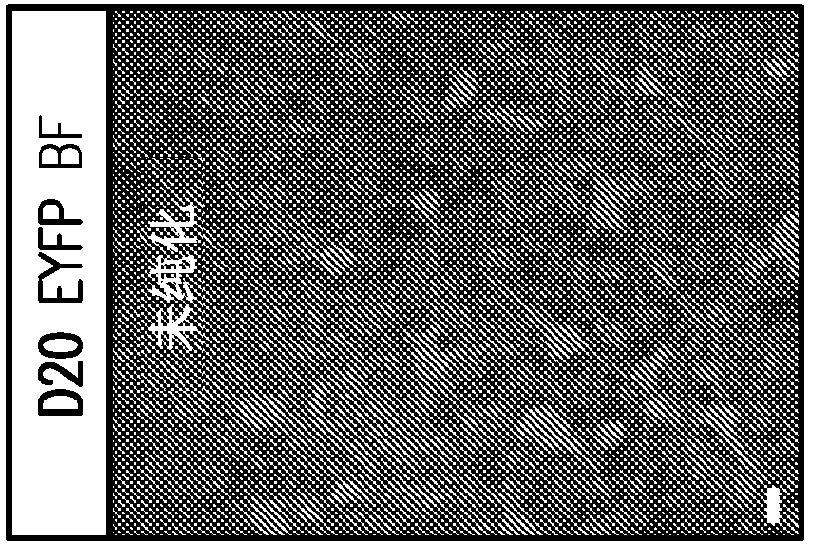
Formation of neuromuscular junctions in a defined system
A method for forming neuromuscular junctions includes forming functional neuromuscular junctions between motoneurons and muscle cells by co-culturing one or more human motoneurons and one or more rat muscle cells in a substantially serum-free medium. A synthetic mammalian neuromuscular junction includes a human motoneuron functionally linked to a rat muscle cell in a substantially serum-free medium. An artificial substrate may be used to support the one or more neuromuscular junctions.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Formation of neuromuscular junctions in a defined system
A method for forming neuromuscular junctions includes forming functional neuromuscular junctions between motoneurons and muscle cells by co-culturing one or more human motoneurons and one or more human muscle cells in a substantially serum-free medium. A synthetic mammalian neuromuscular junction includes a human motoneuron functionally linked to a human muscle cell in a substantially serum-free medium. An artificial substrate may be used to support the one or more neuromuscular junctions.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Biglycan and related therapeutics and methods of use
InactiveUS20100130405A1Less “ leaky ”Extend your lifeCompound screeningNervous disorderBiglycanBecker's muscular dystrophy
The invention provides compositions and methods for treating, preventing, and diagnosing diseases or conditions associated with an abnormal level or activity of biglycan; disorders associated with an unstable cytoplasmic membrane, due, e.g., to an unstable dystrophin associated protein complex (DAPC); disorders associated with abnormal synapses or neuromuscular junctions, including those resulting from an abnormal MuSK activation or acetylcholine receptor (AChR) aggregation. Example of diseases include muscular dystrophies, such as Duchenne's Muscular Dystrophy, Becker's Muscular Dystrophy, neuromuscular disorders and neurological disorders.
Owner:BROWN UNIV RES FOUND INC
Dose, localization, and formulation of botulinum toxins in skin and muscle
InactiveUS20140242110A1Simple and easily with dosingMinimize diffusionPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsDosing regimenSide effect
Formulations of and dosing protocols for the administration of botulinum toxin that maximize efficacy and specificity while minimizing the likelihood of overdosing and undesirable side effects of treatment. The formulations include positively charged carriers, such as cationic peptides, which otherwise have no inherent botulinum-toxin-like activity. The dosing regimen is based on the pattern, quantity, and location of neuromuscular junctions in the target tissue. Because the number of neuromuscular junctions in a target tissue remains generally stable throughout life and because the pharmacological effect of botulinum toxin is localized at the neuromuscular junction, dosing efficacy is unaffected by muscle mass, age of the patient, or body weight.
Owner:DT SCIMED
Botanical pesticide
The invention discloses a botanical pesticide, which consists of matrine, stemonine, nicotine, veratramine, osthole, chamaejasmine, root stem and leaf of hairypetal millettia, fulvic acid, an additive and a preservative. The botanical pesticide can paralyze feelers of pests to ensure that the feelers lose the function so as not to forage and mate, can also stimulate the skin of the pests at the same time to make sensory nerve of skin mucosa show an excitatory state to produce pruritus and burning heat sensation further to anaesthetize the pests so that the pests lose consciousness, and for the activity of neuromuscular junctions and nerve compound potential, stop the conduction of the excitement on nerve endings first, and can also make a nerve stem completely lose excitement and conduction impulse functions to die to ensure that the pests are also in a paralysis state even if the pests are not dead, lose the ability of damaging crops, and are nontoxic to human bodies at the same time.
Owner:赵子库
Neuromuscular Junction: NMJ-ON-CHIP
PendingUS20170226478A1Facilitate wide range of flow rateEliminate wasteCulture processBiochemistry apparatusFiberMuscle Cell Contraction
The invention relates to culturing motor neuron cells together with skeletal muscle cells in a fluidic device under conditions whereby the interaction of these cells mimic the structure and function of the neuromuscular junction (NMJ) providing a NMJ-on-chip. Good viability, formation of myo-fibers and function of skeletal muscle cells on fluidic chips allow for measurements of muscle cell contractions. Embodiments of motor neurons co-cultures with contractile myo-fibers are contemplated for use with modeling diseases affecting NMJ's, e.g. Amyotrophic lateral sclerosis (ALS).
Owner:EMULATE INC +1
In vitro methods of identifying modulators of neuromuscular junction activity
ActiveCN108368486ANervous system cellsSkeletal/connective tissue cellsSkeletal muscle cellNeuromuscular junction
The present invention relates to an in vitro human neuromuscular junction model prepared from a co-culture of human pluripotent stem cell (PSC)-derived spinal motorneurons and human myoblast-derived skeletal muscle cells. The present invention also provides for methods of screening compounds for their ability to modulate neuromuscular junction activity by determining whether a candidate compound increases or decreases the activity of the in vitro human neuromuscular junction model.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Conus lividus neurotoxin and coding sequence and use thereof
InactiveCN102154300AInhibition of contractionBlock transmitter deliveryNervous disorderPeptide/protein ingredientsCDNA libraryNeural biology
The invention provides a Conus lividus neurotoxin gene 1v1.3 and a polypeptide 1v1c coded thereby and use of the polypeptide 1v1c in the preparation of tool medicines for neurobiological study and analgesic medicines. In the invention, a process of constructing a cDNA library is adopted to clone the conotoxin gene 1v1.3 in the toxin canal of Conus lividus, and through analysis on a base sequence with a tool software SEQTOOL, the mature peptide amino acid sequence of the polypeptide 1v1c coded by the Conus lividus neurotoxin gene 1v1.3 is determined to be represented by a sequence 400(2) in a sequence table. The polypeptide 1v1c provided by the invention can block the transmission of a transmitter of neuromuscular junction, and according to a mouse hot-plate method, the polypeptide 1v1c has pain relieving effect and can be used for preparing the tool medicines for neurobiological study and analgesic medicines.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com