Patents
Literature
77 results about "Dystrophin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
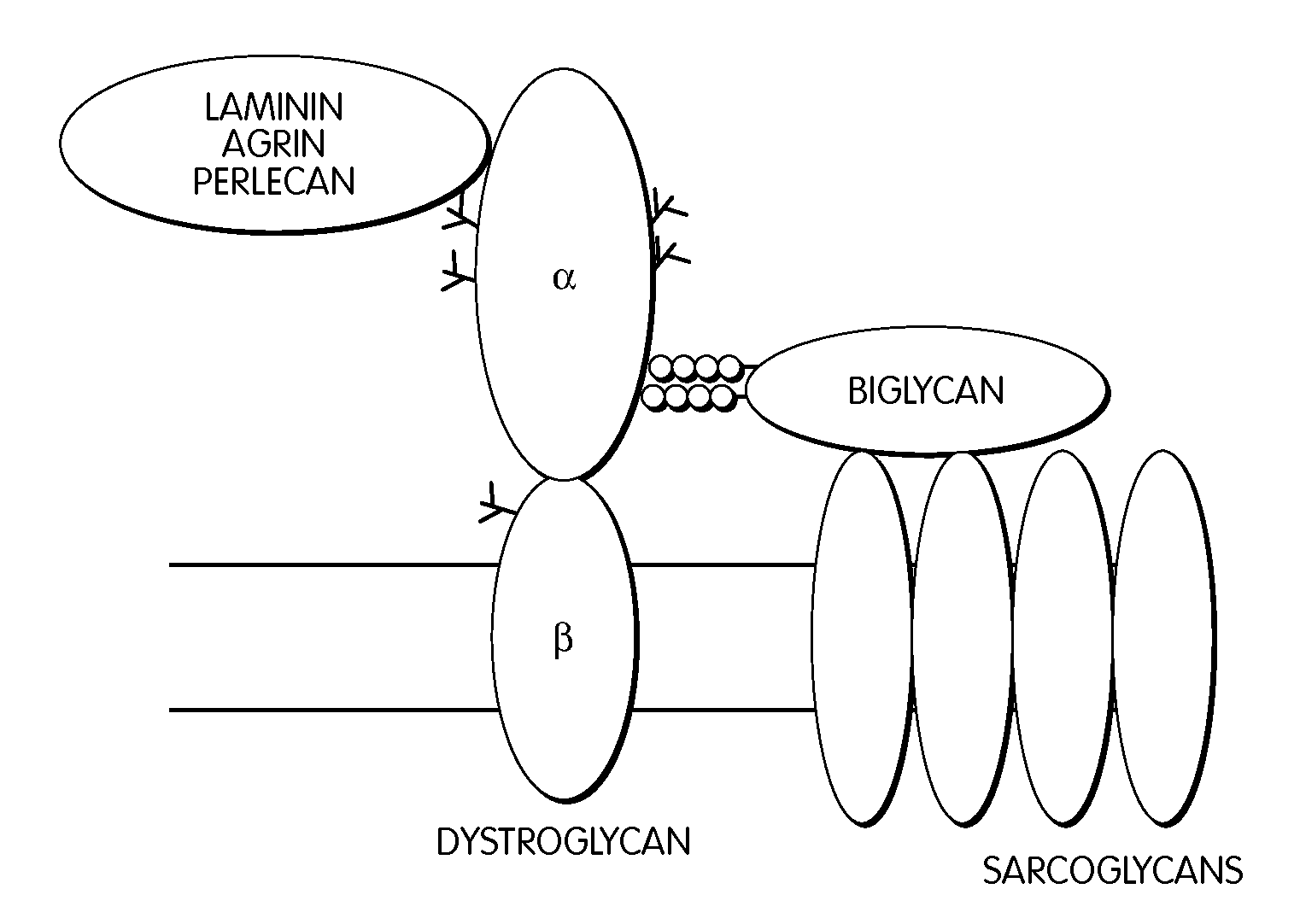
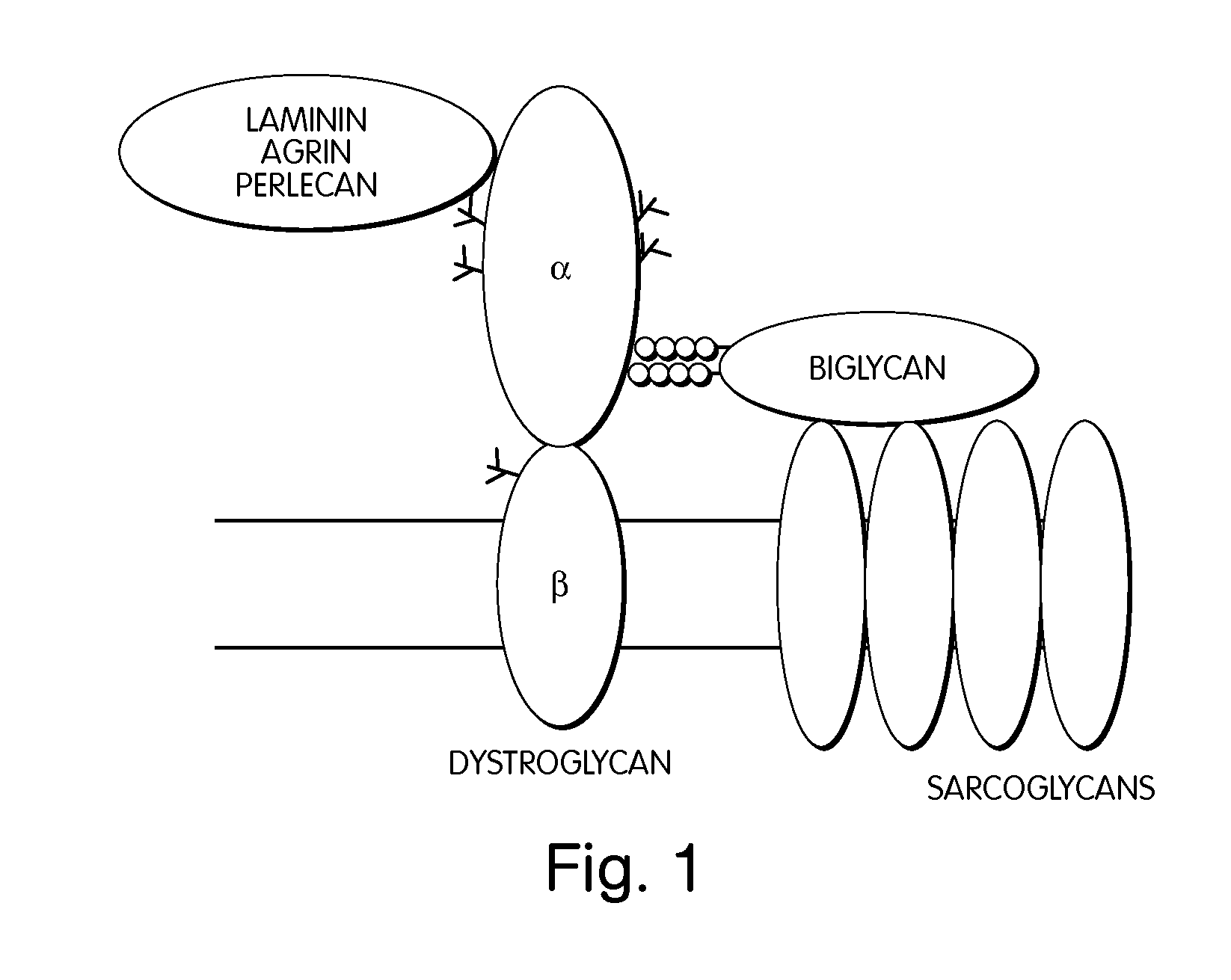
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere.
Compound and method for treating myotonic dystrophy
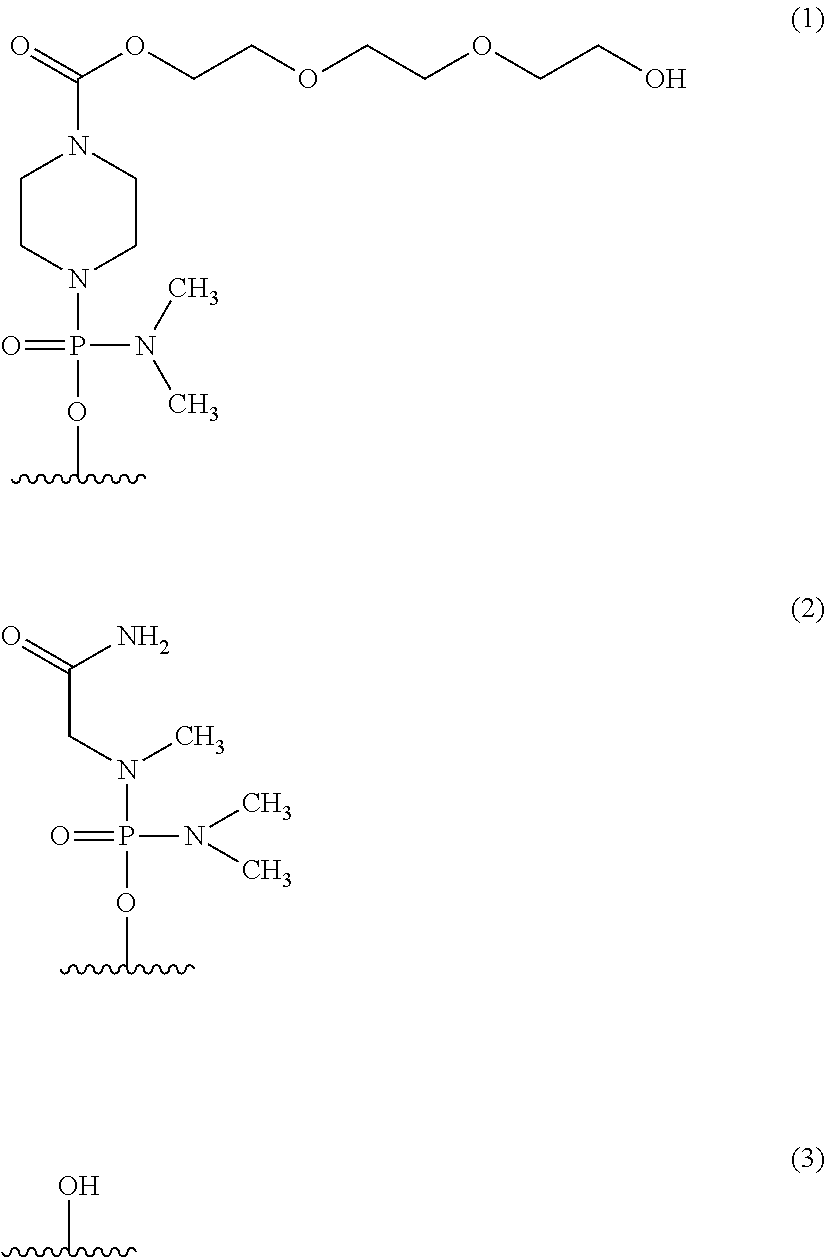
An antisense compound for use in treating myotonic dystrophy DM1 or DM2, a method of enhancing antisense targeting to heart and quadricep muscles, and a method for treating DM1 or DM2 in a mammalian subject are disclosed. The oligonucleotide has 8-30 bases, with at least 8 contiguous bases being complementary to the polyCUG or polyCCUG repeats in the 3′UTR region of dystrophia myotonica protein kinase (DMPK) mRNA in DM1 or DM2, respectively. Conjugated to the oligonucleotide is a cell-penetrating peptide having the sequence (RXRR(B / X)R)2XB, where R is arginine; B is β-alanine; and each X is —C(O)—(CH2)n—NH—, where n is 4-6. The antisense compound is effective to selectively block the sequestration of muscleblind-like 1 protein (MBNL1) and / or CUGBP, in heart and quadricep muscle in a myotonic dystrophy animal model.
Owner:SAREPTA THERAPEUTICS INC
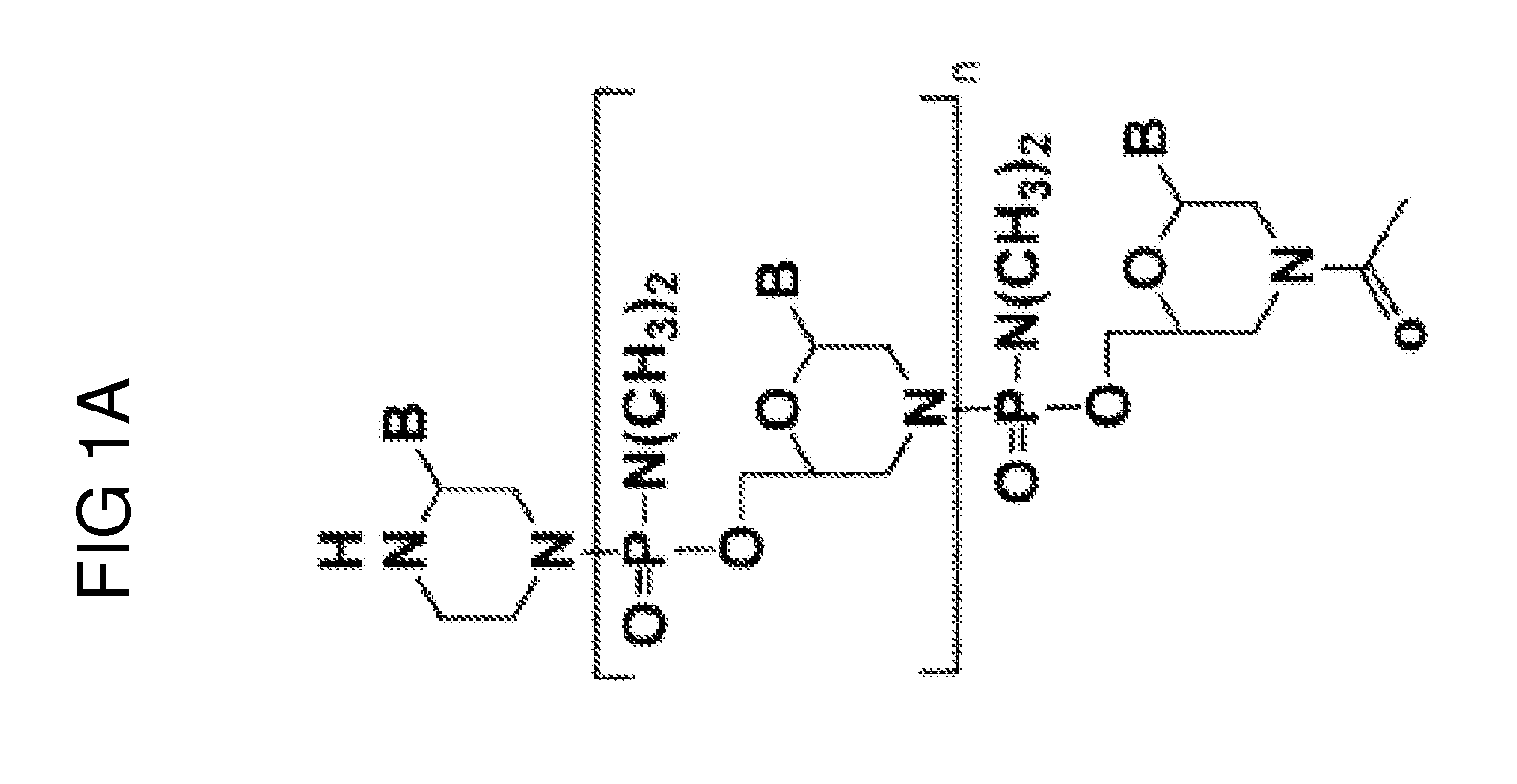
Ena nucleic acid drugs modifying splicing in mrna precursor
ActiveUS20070082861A1Efficient processImprove bindingSplicing alterationSugar derivativesGene bankDystrophin
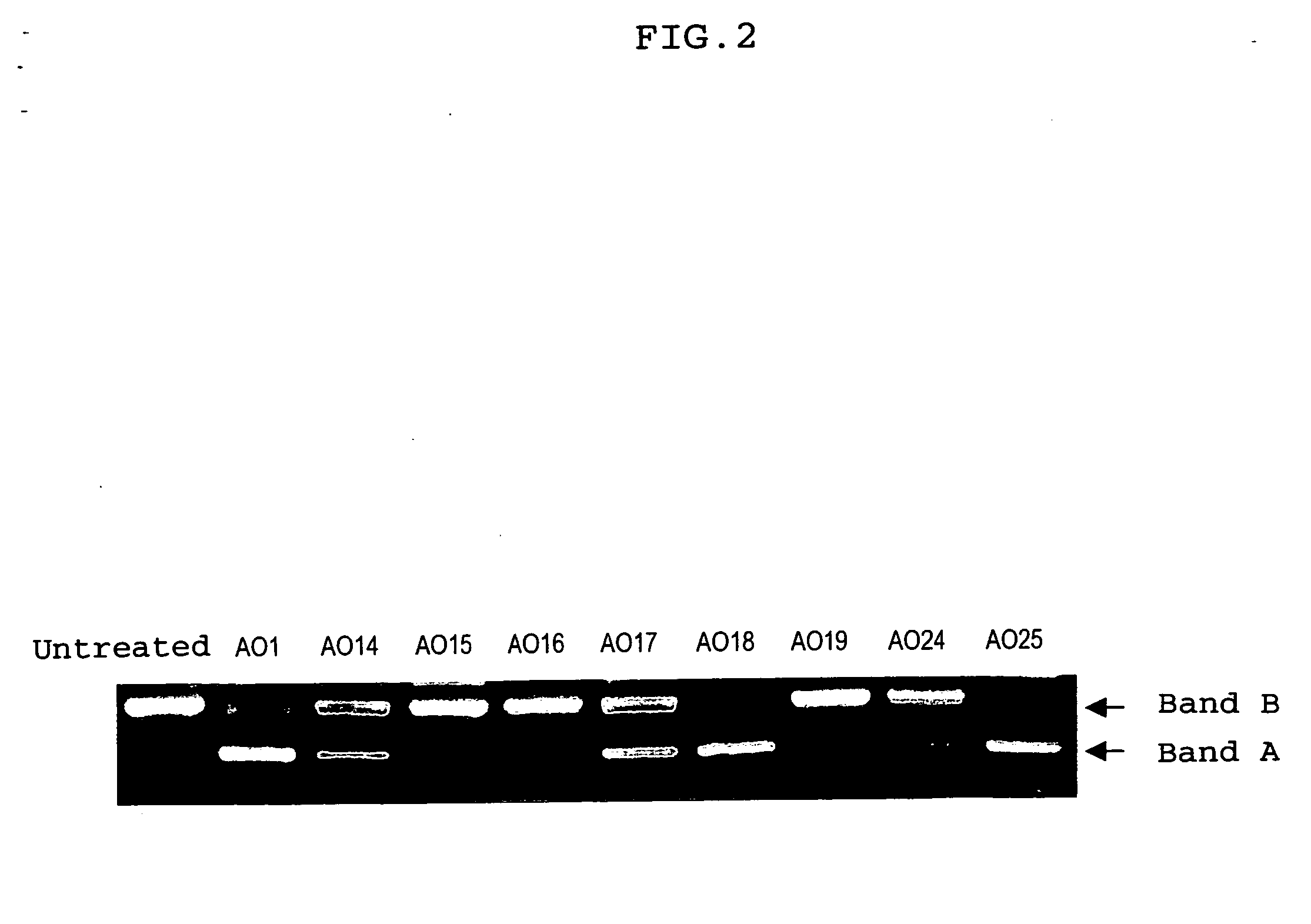
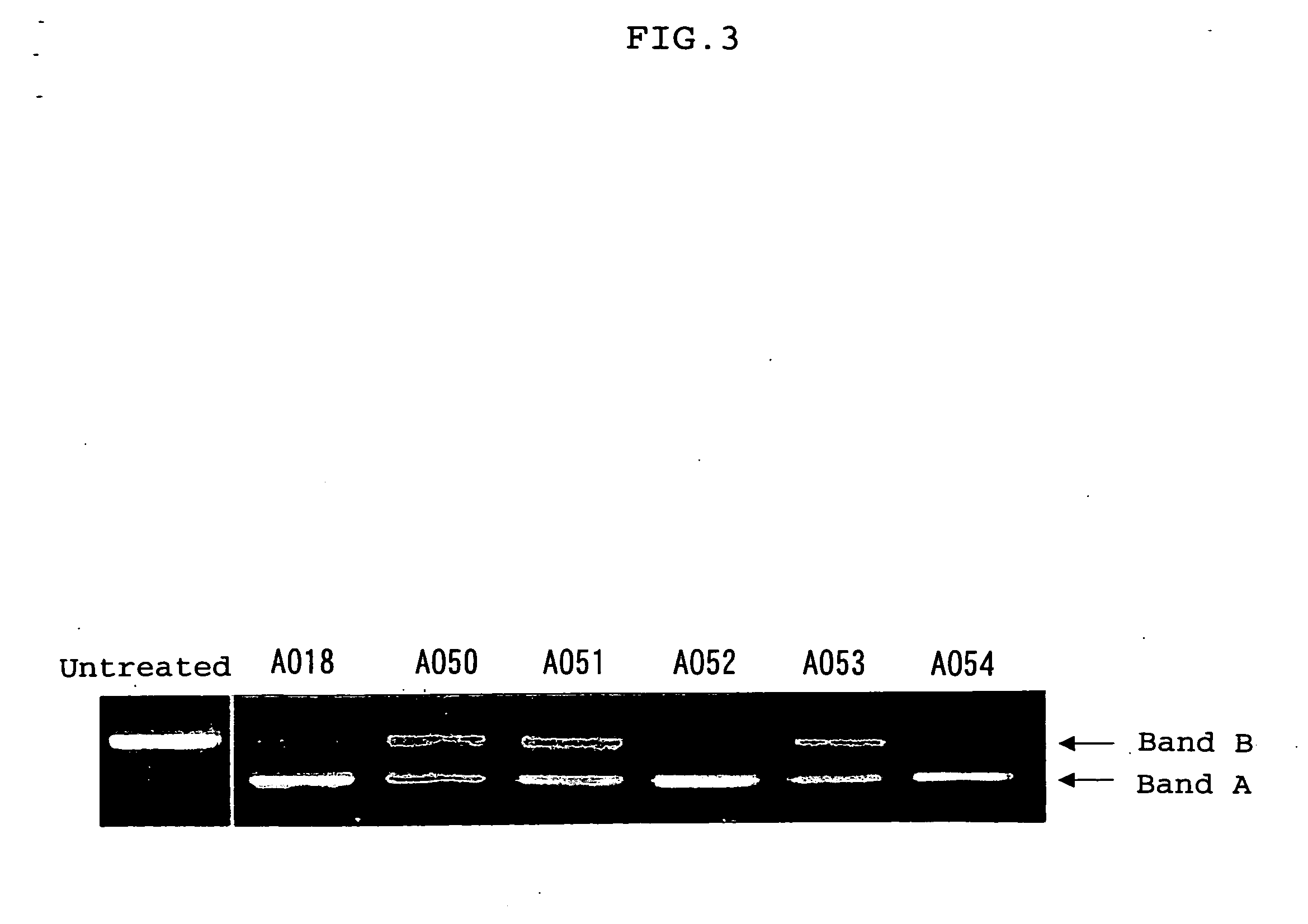
Oligonucleotides having a nucleotide sequence complementary to nucleotide numbers such as 2571-2607, 2578-2592, 2571-2592, 2573-2592, 2578-2596, 2578-2601 or 2575-2592 of the dystrophin cDNA (Gene Bank accession No. NM_004006.1) and therapeutic agents for muscular dystrophy comprising such oligonucleotides.
Owner:DAIICHI SANKYO CO LTD
Meganuclease variants cleaving a DNA target sequence from the dystrophin gene and uses thereof
InactiveUS20130145487A1Expression is sufficient and stableNo impact on the expression of other genesSugar derivativesBacteriaA-DNANuclease
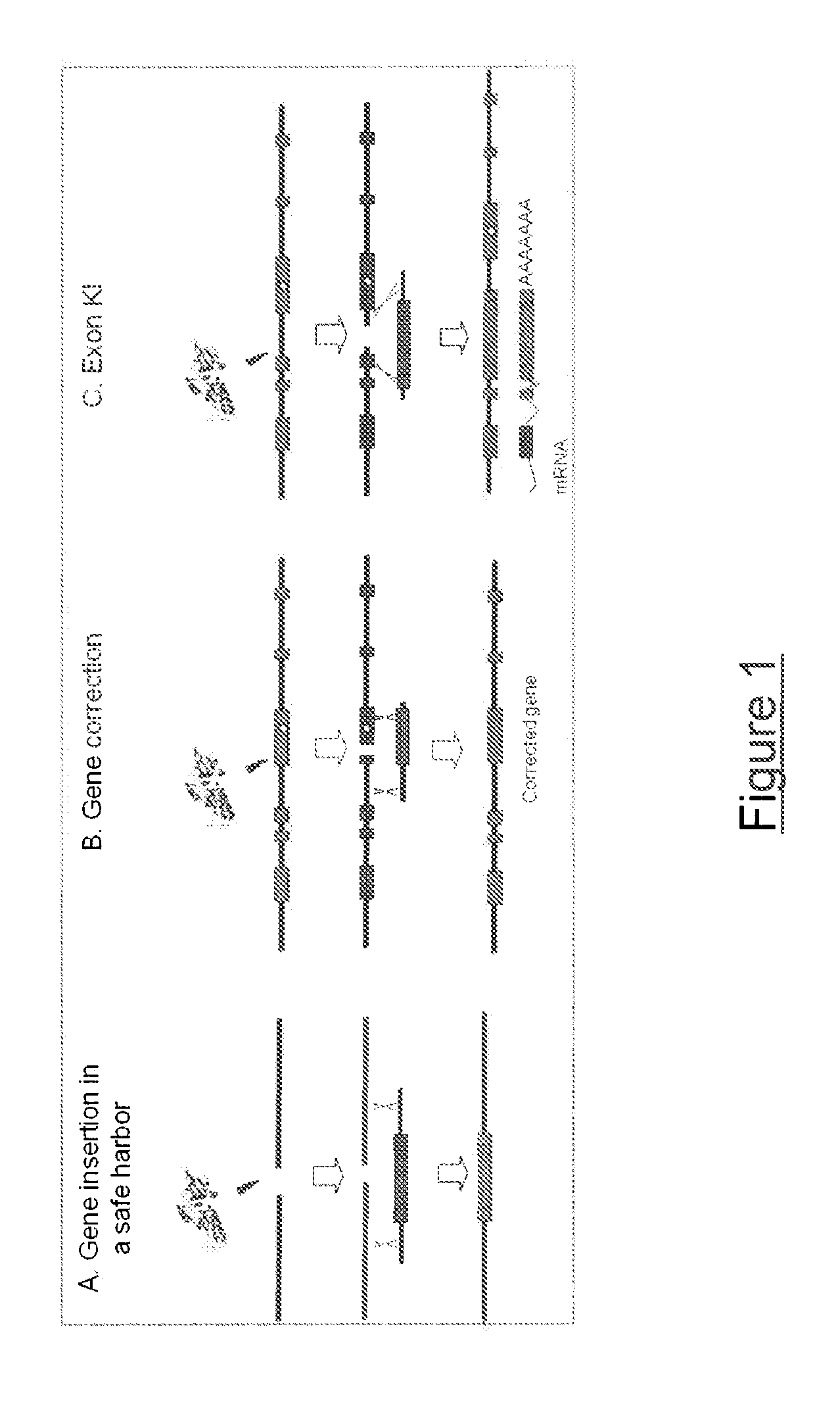
The invention relates to meganuclease variants which cleave a DNA target sequence from the human dystrophin gene (DMD), to vectors encoding such variants, to a cell, an animal or a plant modified by such vectors and to the use of these meganuclease variants and products derived therefrom for genome therapy, ex vivo (gene cell therapy) and genome engineering including therapeutic applications and cell line engineering. The invention also relates to the use of meganuclease variants for inserting therapeutic transgenes other than DMD at the dystrophin gene locus, using this locus as a safe harbor locus. The invention also relates to the use of meganuclease variants for using the dystrophin gene locus as a landing pad to insert and express genes of interest.
Owner:CELLECTIS SA
Mannose-6-phosphate receptor mediated gene transfer into muscle cells
InactiveUS20120122801A1Organic active ingredientsSpecial deliveryGlycoside formationMannose 6-phosphate receptor
The invention relates to glycoside-compound conjugates for use in antisense strategies and / or gene therapy. The conjugates comprise a glycoside linked to a compound, in which the glycoside is a ligand capable of binding to a mannose-6-phosphate receptor of a muscle cell. For example the cells are muscle cells of a Duchenne Muscular Dystrophy (DMD) patient and the conjugate comprises an antisense oligonucleotide which causes ex on skipping and induces or restores the synthesis of dystrophin or variants thereof.
Owner:PROSENSA THERAPEUTICS
ENA nucleic acid drugs modifying splicing in mRNA precursor
ActiveUS7902160B2Efficient processImprove bindingSplicing alterationSugar derivativesNucleotideDystrophin
Oligonucleotides having a nucleotide sequence complementary to nucleotide numbers such as 2571-2607, 2578-2592, 2571-2592, 2573-2592, 2578-2596, 2578-2601 or 2575-2592 of the dystrophin cDNA (Gene Bank accession No. NM_004006.1) and therapeutic agents for muscular dystrophy comprising such oligonucleotides.
Owner:DAIICHI SANKYO CO LTD
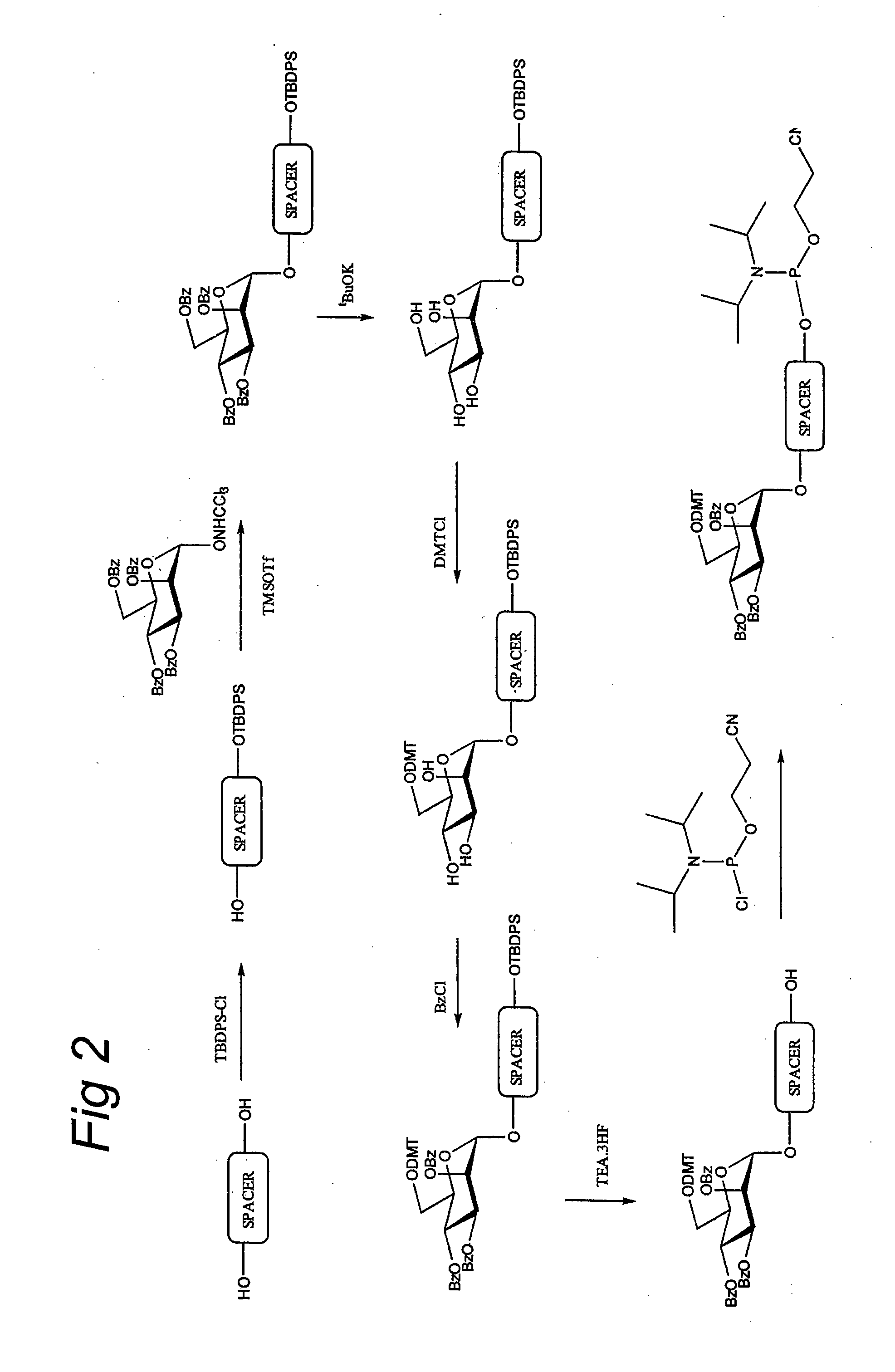
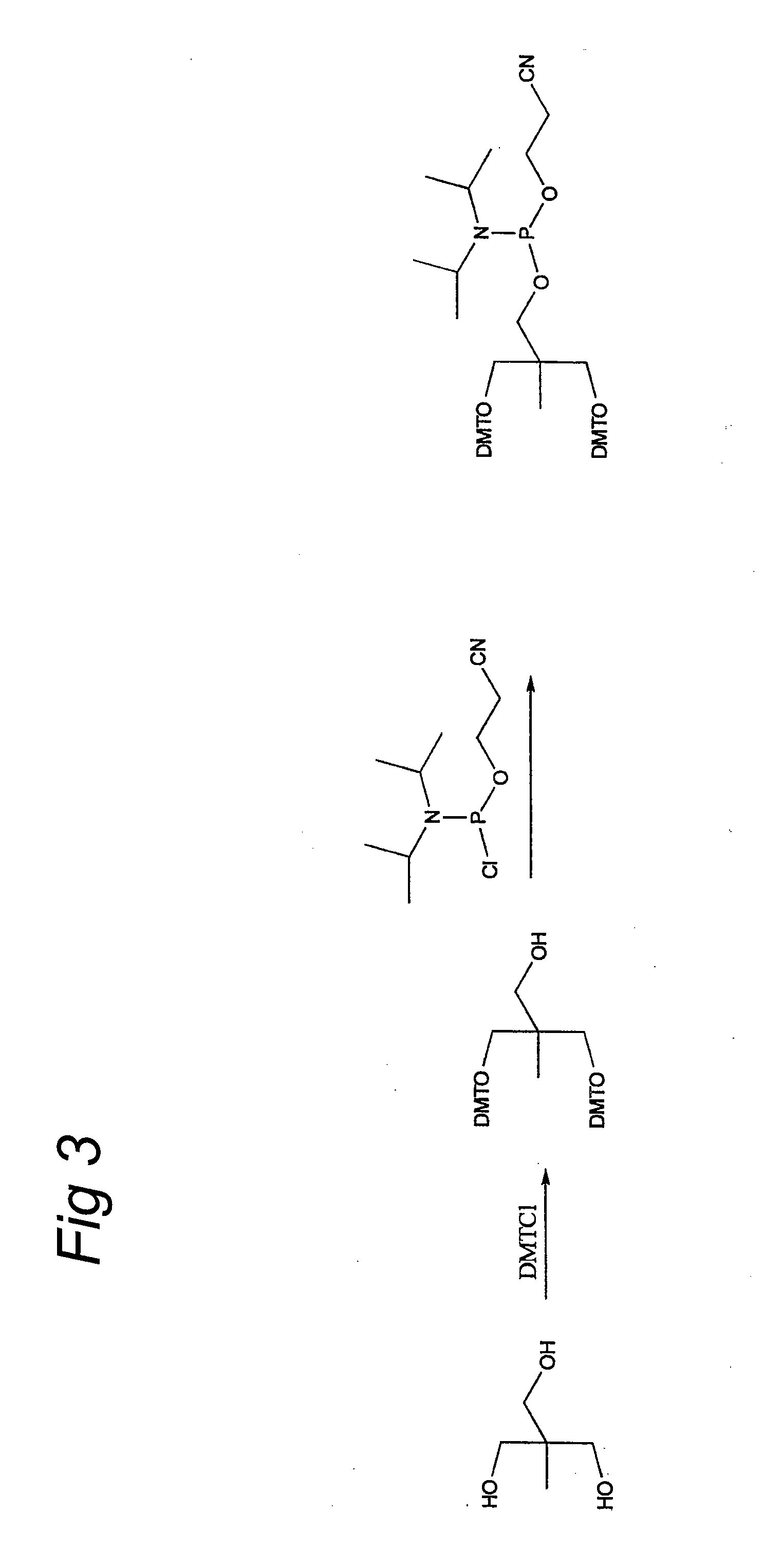
DNA sequences comprising dystrophin minigenes and methods of use thereof
InactiveUS7001761B2Small sizeEfficient and stable correctionVirusesSugar derivativesPoly-A RNAPolyadenylation
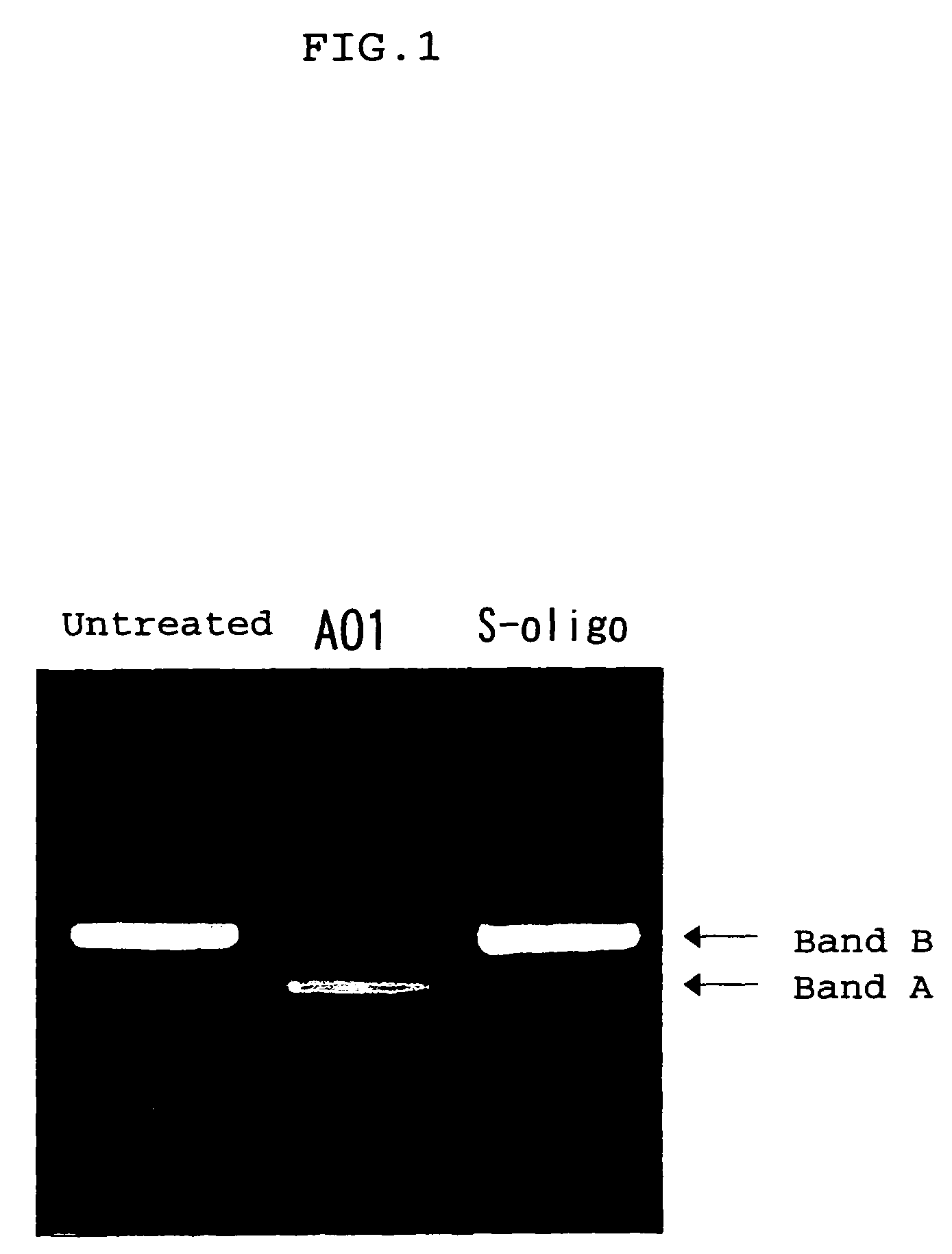
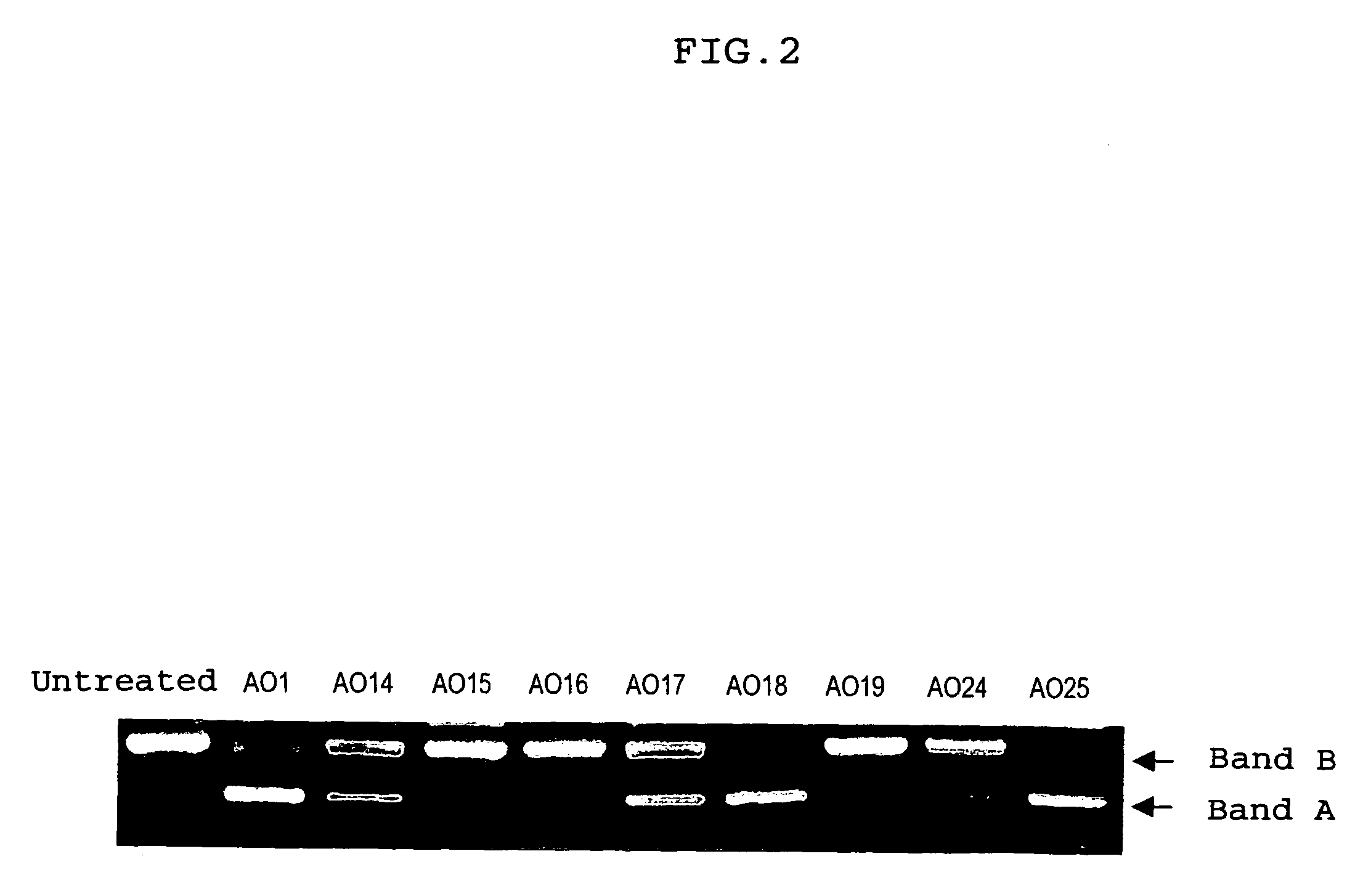
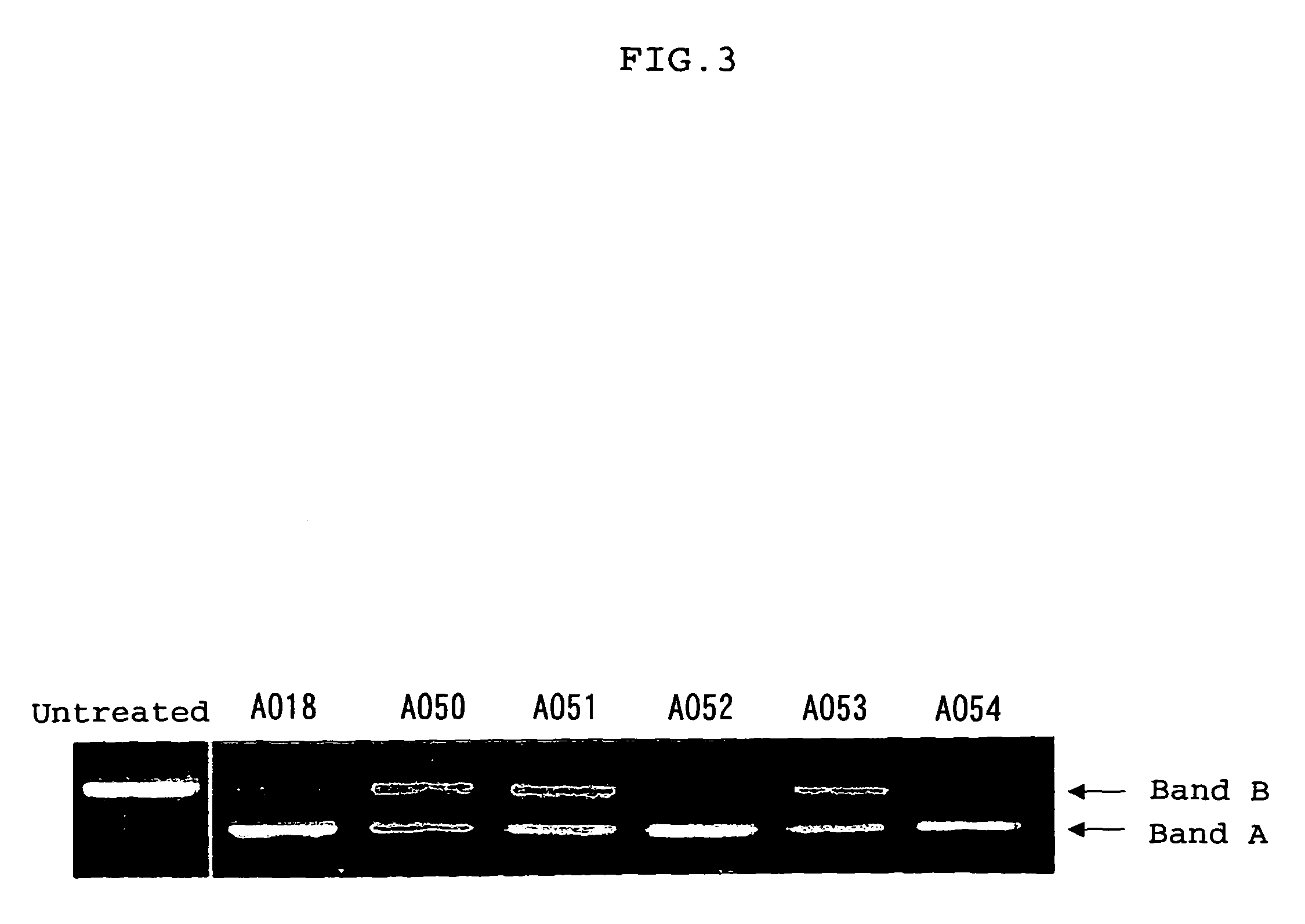
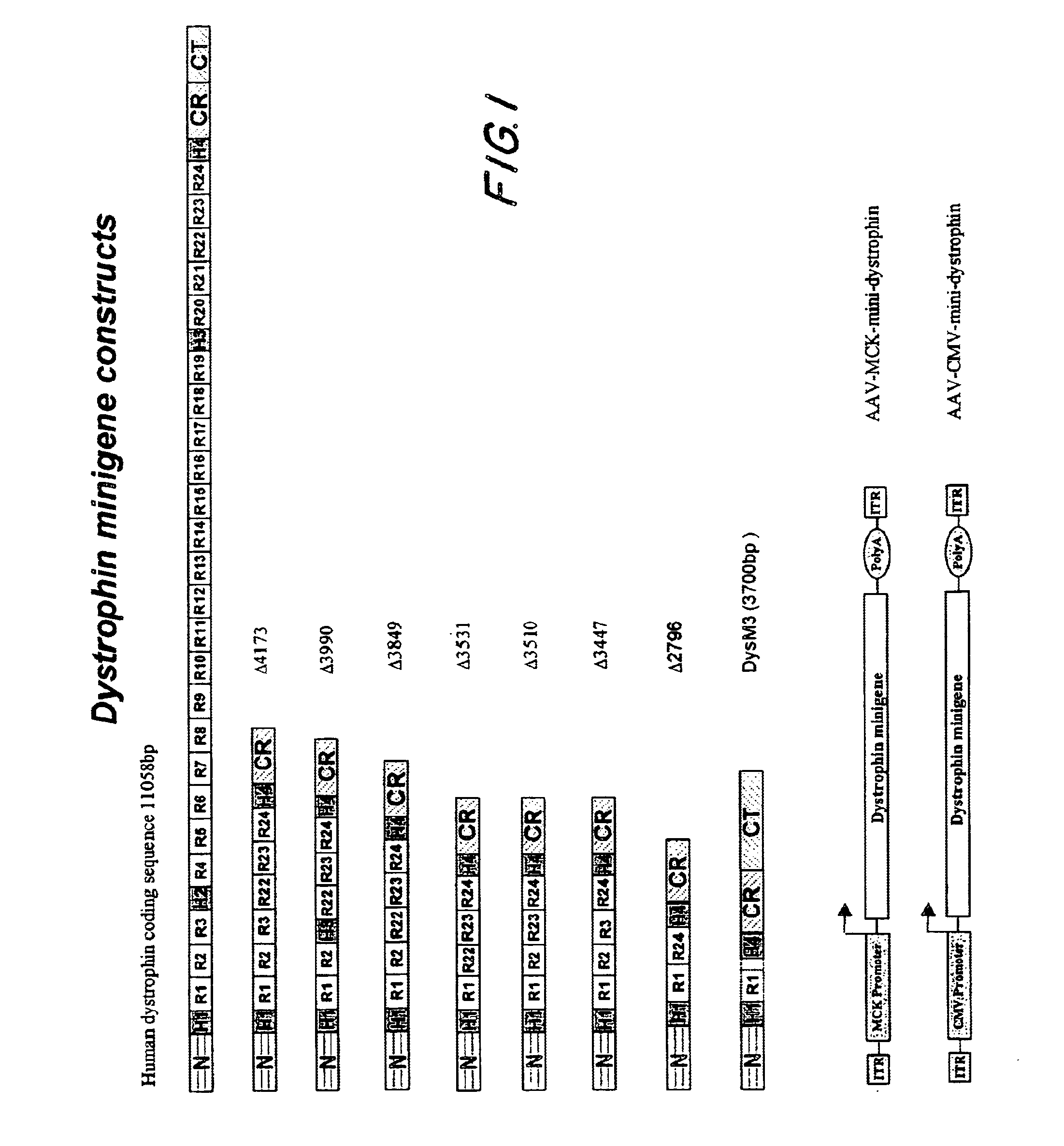
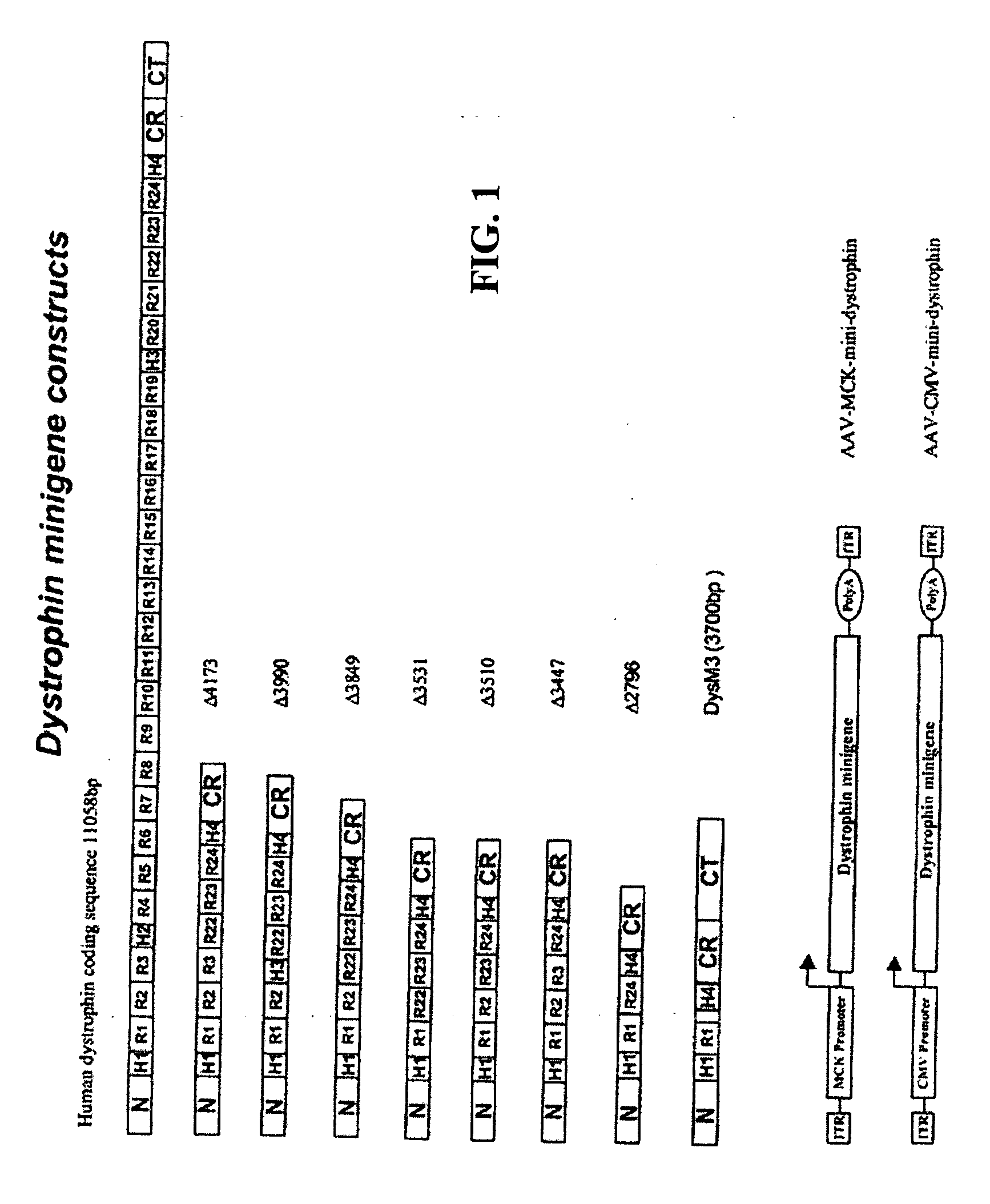
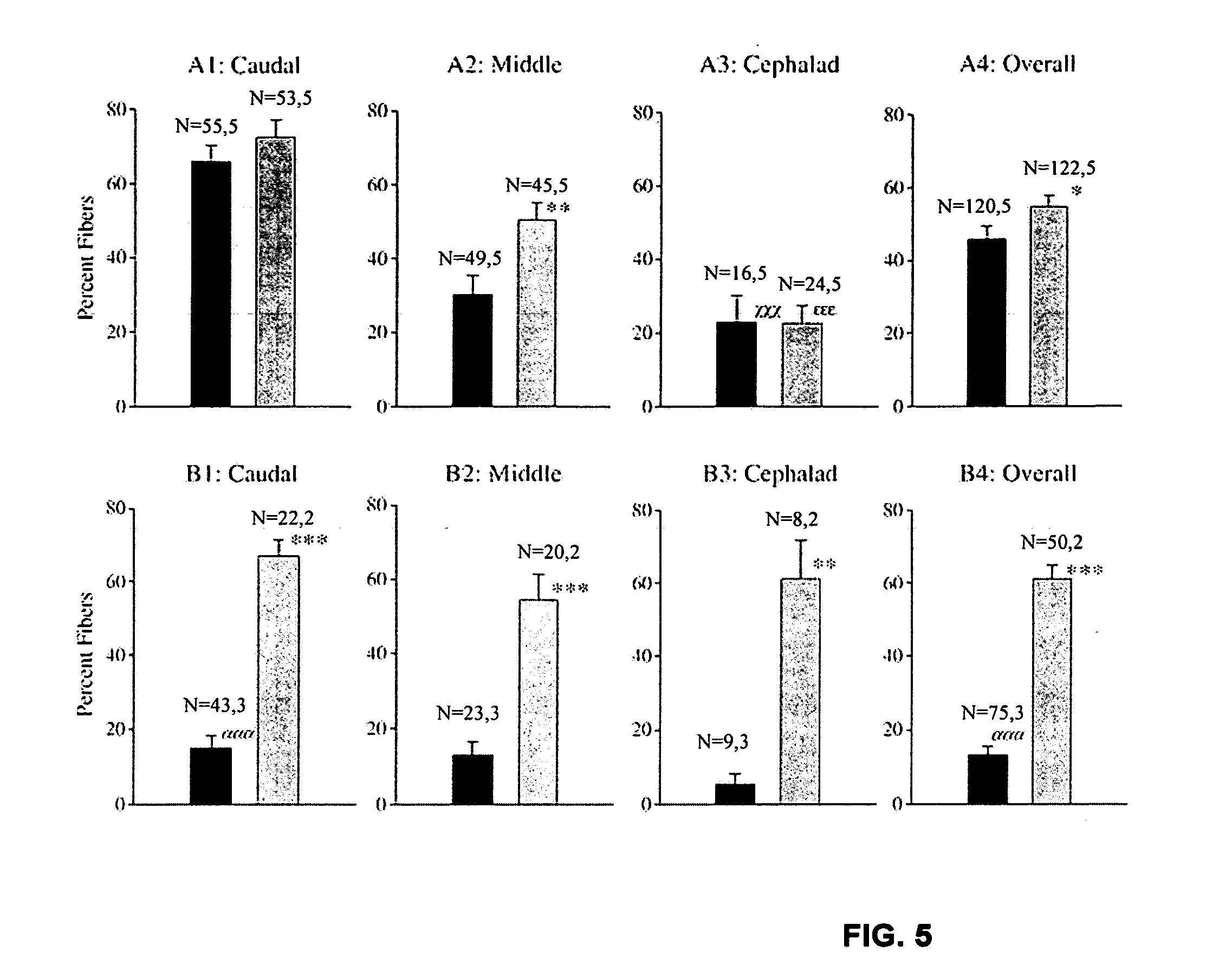
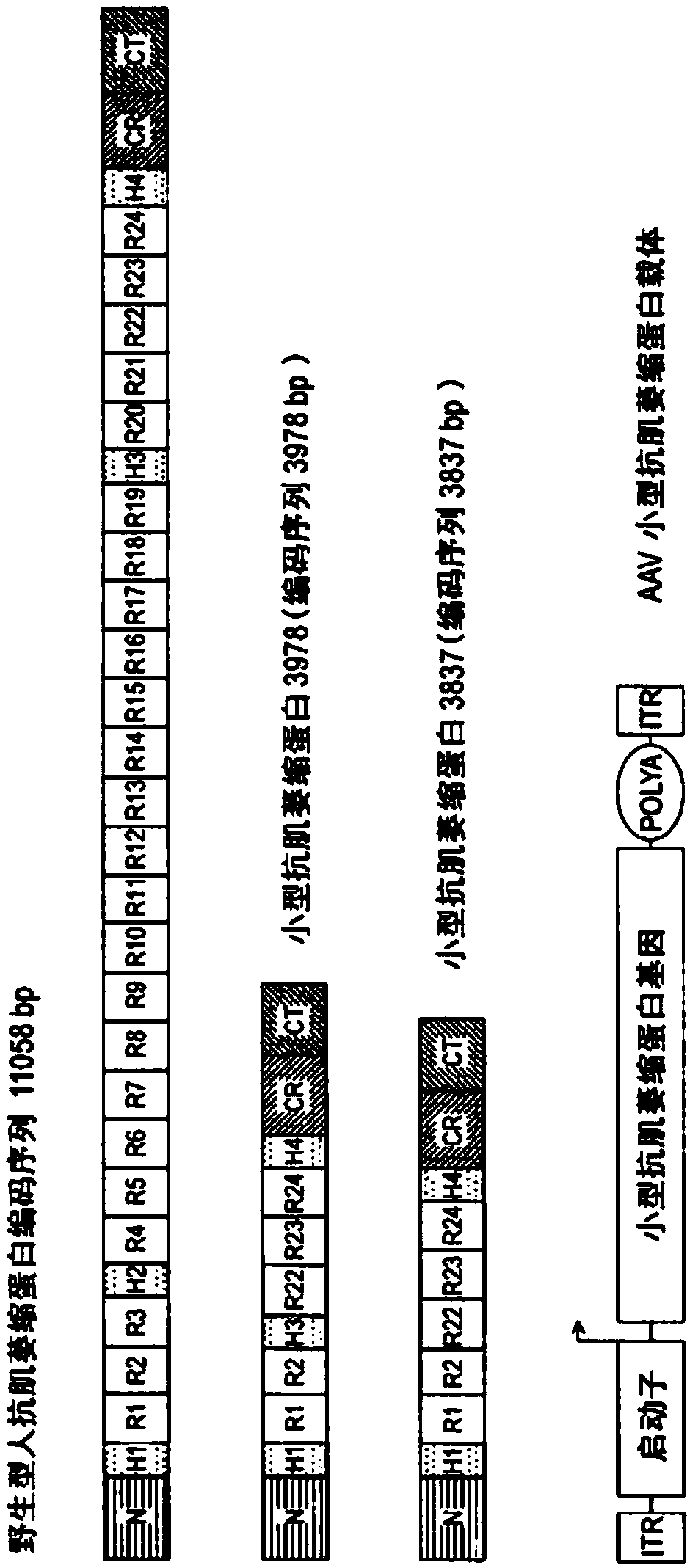
The present invention provides a series of novel dystrophin minigenes that retain the essential biological functions. The expression of the dystrophin minigenes may be controlled by a regulatory element along with a small polyadenylation signal. The entire gene expression cassettes may be readily packaged into a viral vector, preferably an AAV vector. The present invention further defines the minimal functional domains of dystrophin and provides ways to optimize and create new versions of dystrophin minigenes. Finally, the present invention provides a method of treatment for Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD).
Owner:ASKLEPIOS BIOPHARMACEUTICAL INC
Compound and method for treating myotonic dystrophy
ActiveUS8741863B2Organic active ingredientsPeptide/protein ingredientsArginineMyotonic dystrophy gene
An antisense compound for use in treating myotonic dystrophy DM1 or DM2, a method of enhancing antisense targeting to heart and quadricep muscles, and a method for treating DM1 or DM2 in a mammalian subject are disclosed. The oligonucleotide has 8-30 bases, with at least 8 contiguous bases being complementary to the polyCUG or polyCCUG repeats in the 3′UTR region of dystrophia myotonica protein kinase (DMPK) mRNA in DM1 or DM2, respectively. Conjugated to the oligonucleotide is a cell-penetrating peptide having the sequence (RXRR(B / X)R)2XB, where R is arginine; B is β-alanine; and each X is —C(O)—(CH2)n—NH—, where n is 4-6. The antisense compound is effective to selectively block the sequestration of muscleblind-like 1 protein (MBNL1) and / or CUGBP, in heart and quadricep muscle in a myotonic dystrophy animal model.
Owner:SAREPTA THERAPEUTICS INC
Oligonucleotide comprising an inosine for treating dmd
InactiveUS20120046342A1Avoid or decrease a potential multimerisation or aggregation of oligonucleotidesLow efficiencyOrganic active ingredientsSplicing alterationInosineNucleotide
The invention provides an oligonucleotide comprising an inosine, and / or a nucleotide containing a base able to form a wobble base pair or a functional equivalent thereof, wherein the oligonucleotide, or a functional equivalent thereof, comprises a sequence which is complementary to at least part of a dystrophin pre-m RNA exon or at least part of a non-exon region of a dystrophin pre-m RNA said part being a contiguous stretch comprising at least 8 nucleotides. The invention further provides the use of said oligonucleotide for preventing or treating DMD or BMD.
Owner:BIOMARIN TECH BV
Mannose-6-phosphate receptor mediated gene transfer into muscle cells
InactiveUS20110110960A1Organic active ingredientsSpecial deliveryDystrophinMannose 6-phosphate receptor
The invention relates to glycoside-compound conjugates for use in antisense strategies and / or gene therapy. The conjugates comprise a glycoside linked to a compound, in which the glycoside is a ligand capable of binding to a mannose-6-phosphate receptor of a muscle cell. For example the cells are muscle cells of a Duchenne Muscular Dystrophy (DMD) patient and the conjugate comprises an antisense oligonucleotide which causes exon skipping and induces or restores the synthesis of dystrophin or variants thereof.
Owner:PROSENSA
Exon skipping compositions for treating muscular dystrophy
ActiveUS20140323544A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationMedicineDystrophin
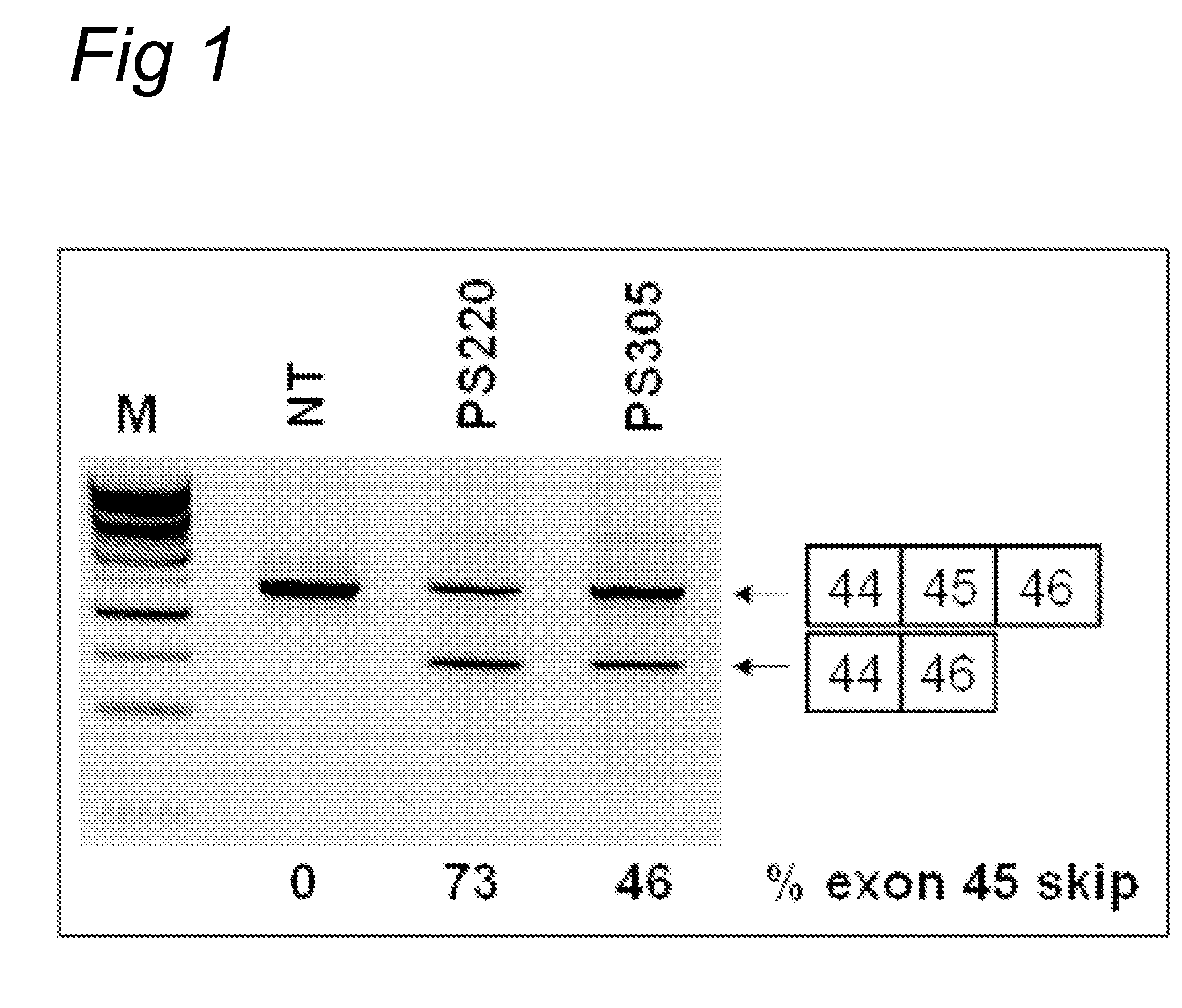
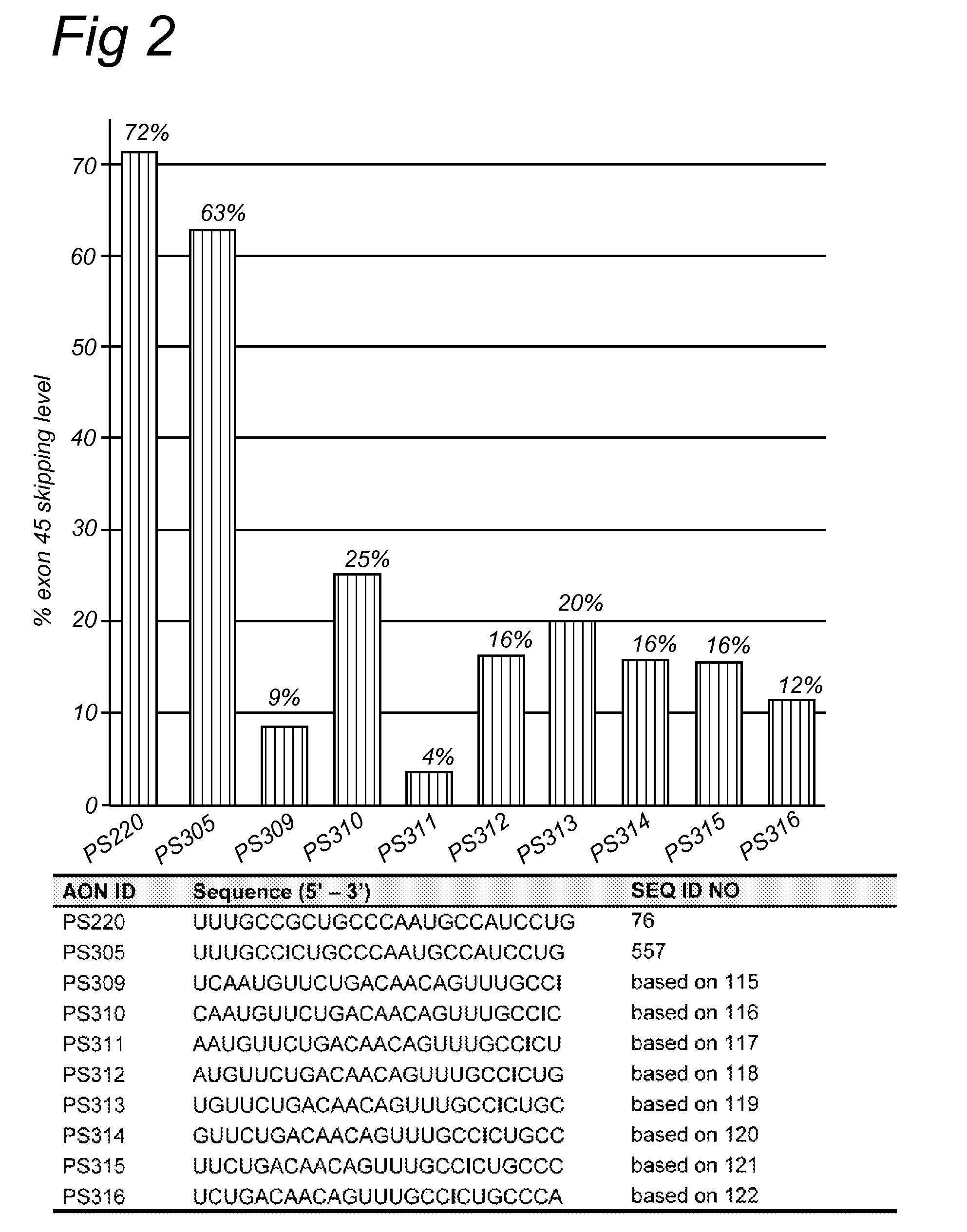
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 44 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Treatment of dystrophin family related diseases by inhibition of natural antisense transcript to dmd family
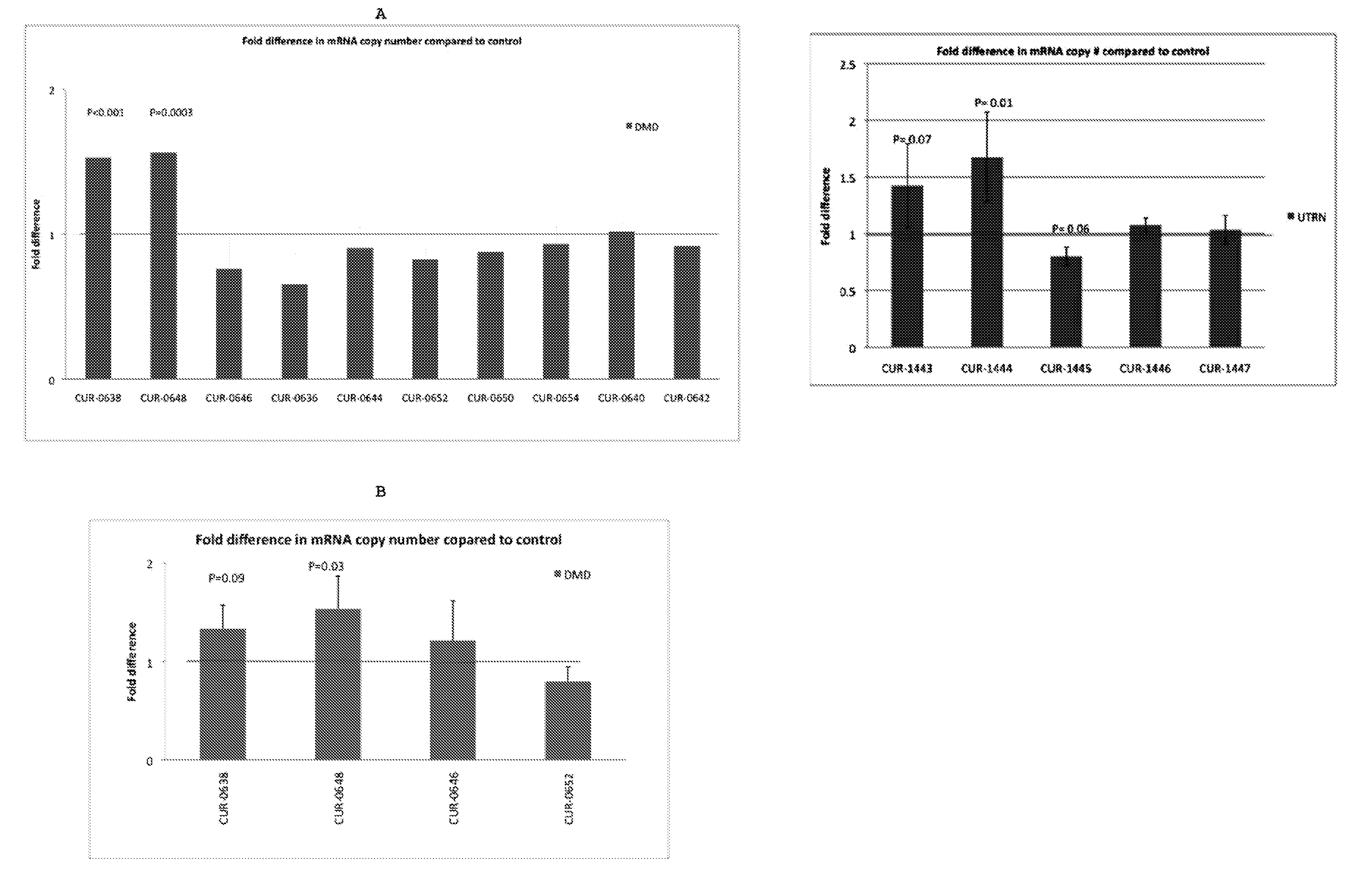
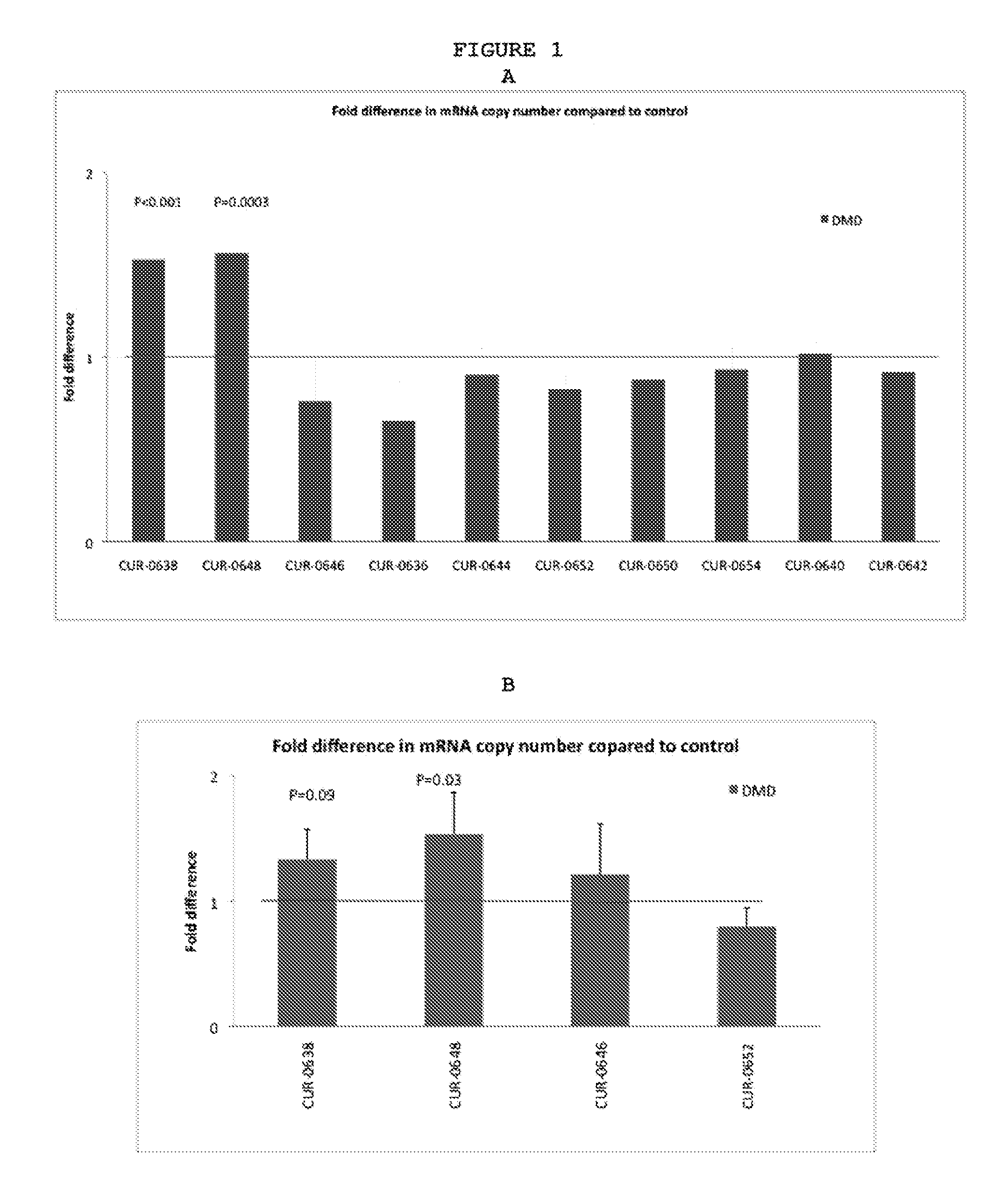
ActiveUS20120046345A1Modulate its functionOrganic active ingredientsSenses disorderDiseaseDystrophin
The present invention relates to antisense oligonucleotides that modulate the expression of and / or function of Dystrophin family, in particular, by targeting natural antisense polynucleotides of Dystrophin family. The invention also relates to the identification of these antisense oligonucleotides and their use in treating diseases and disorders associated with the expression of DMD family.
Owner:CURNA INC
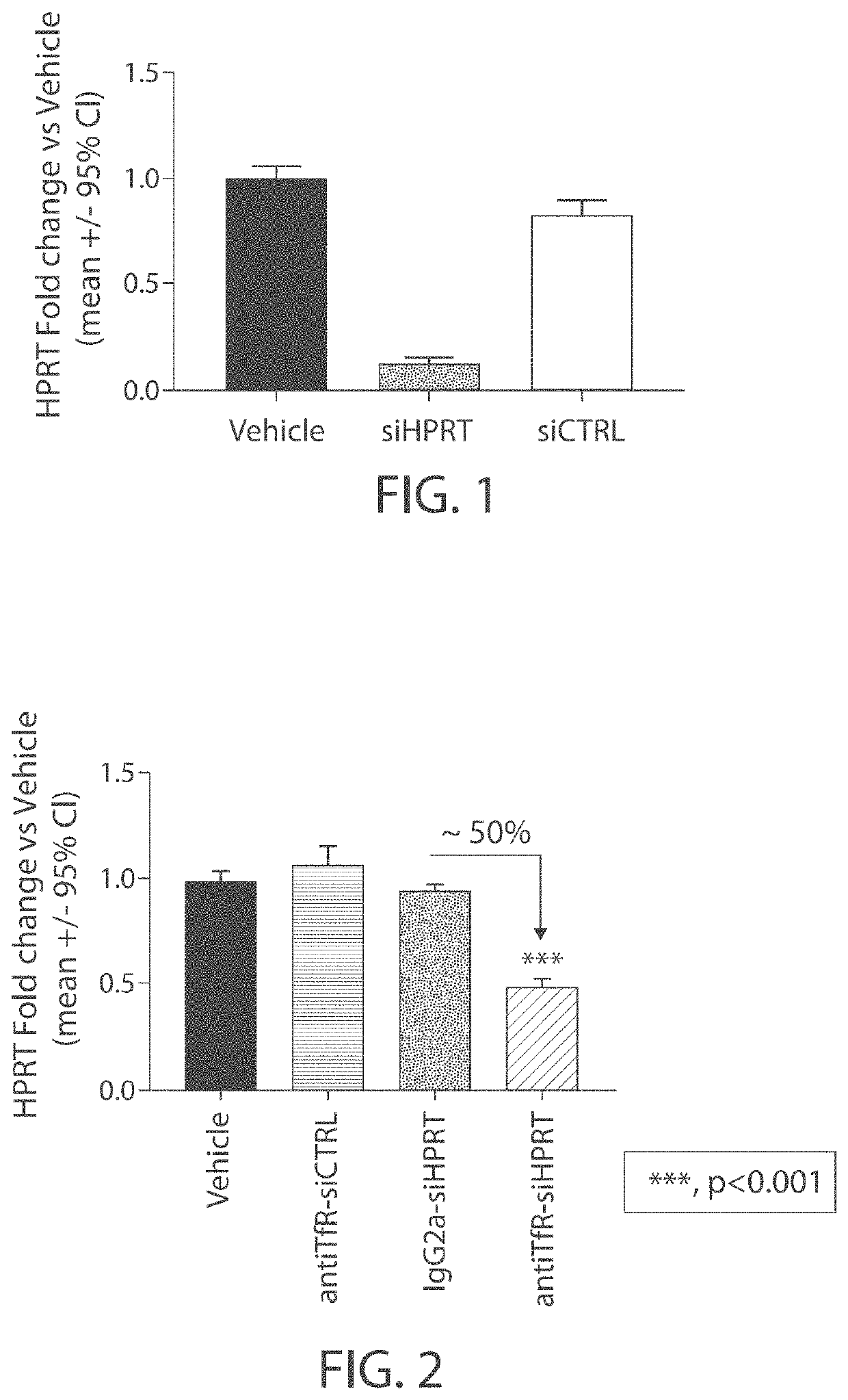
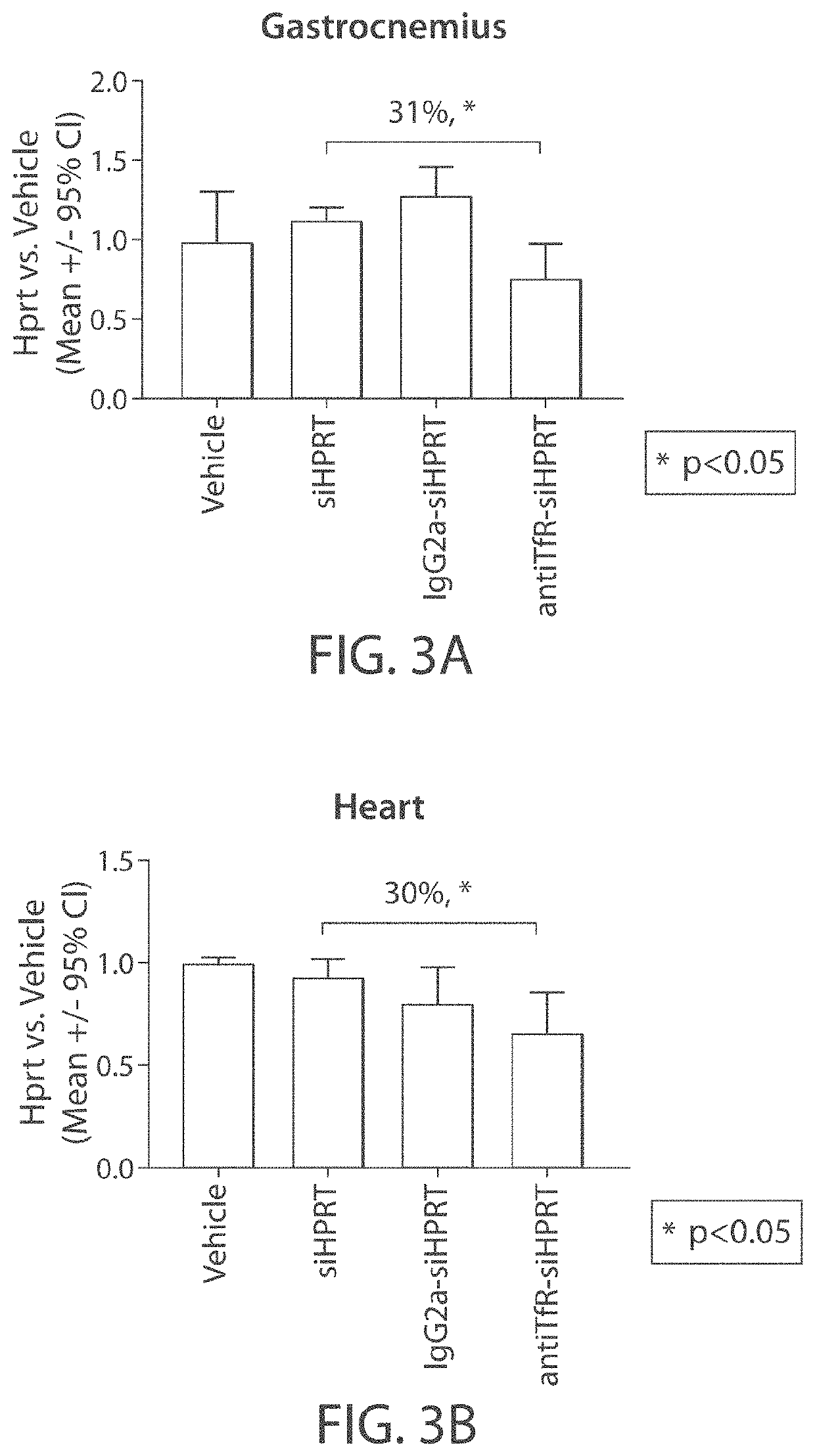
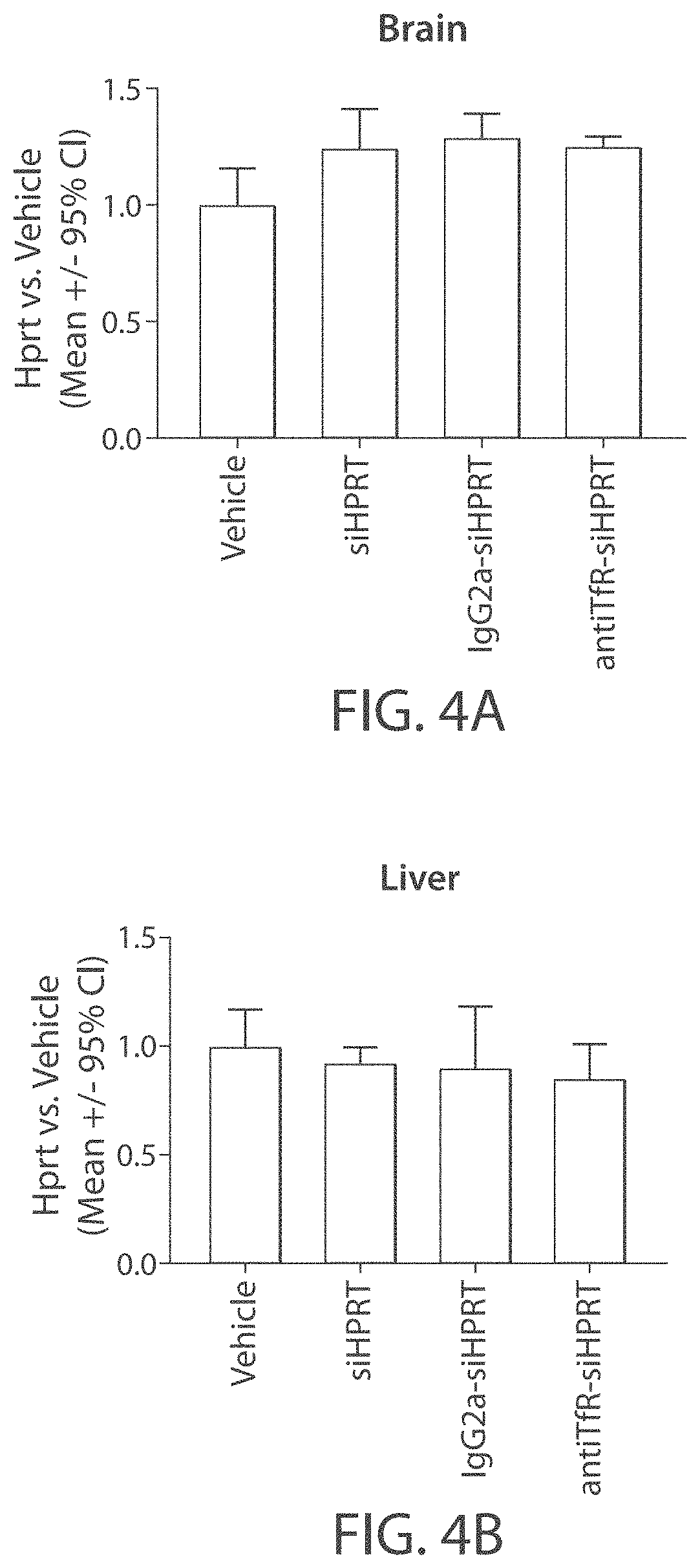
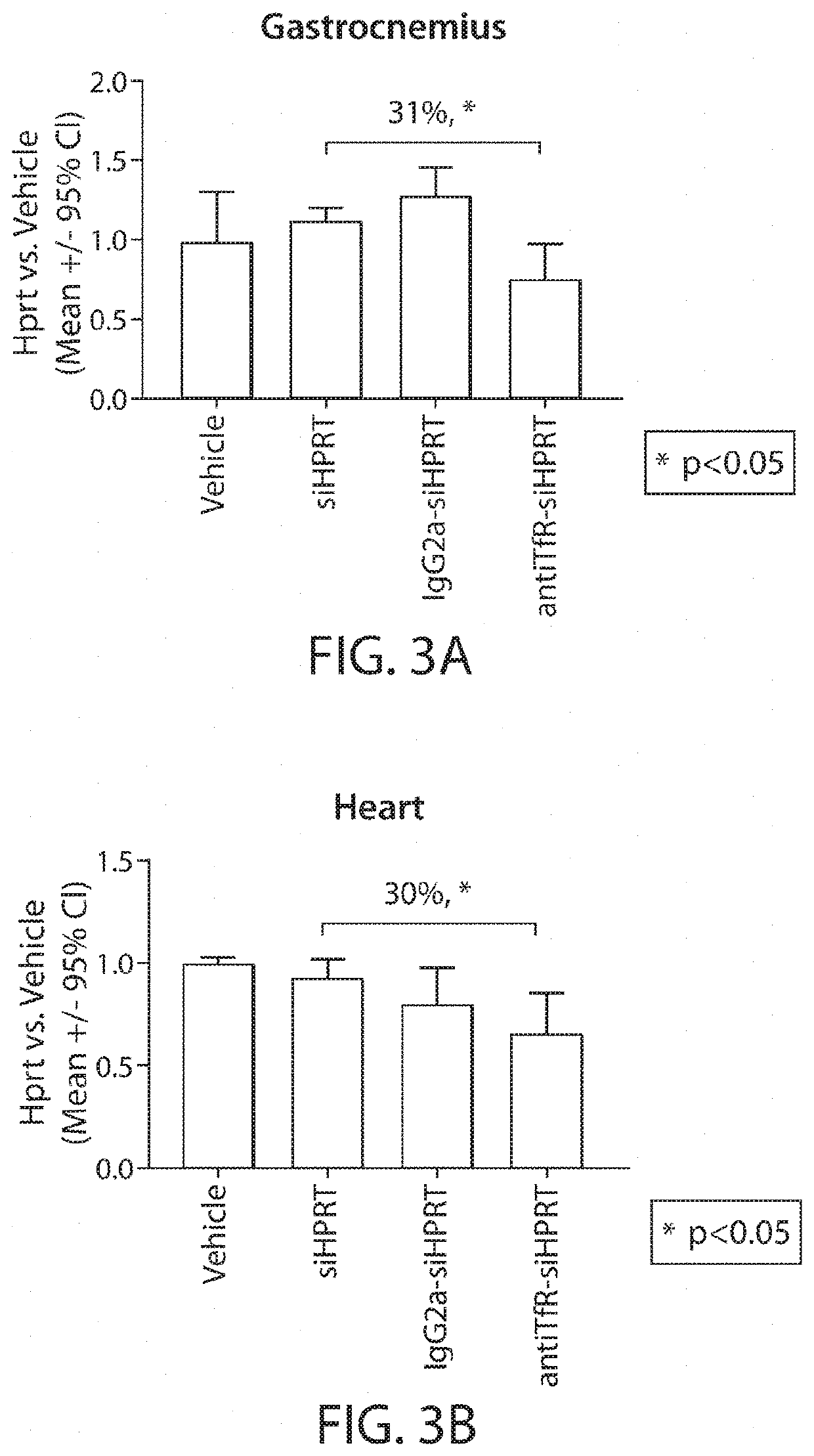
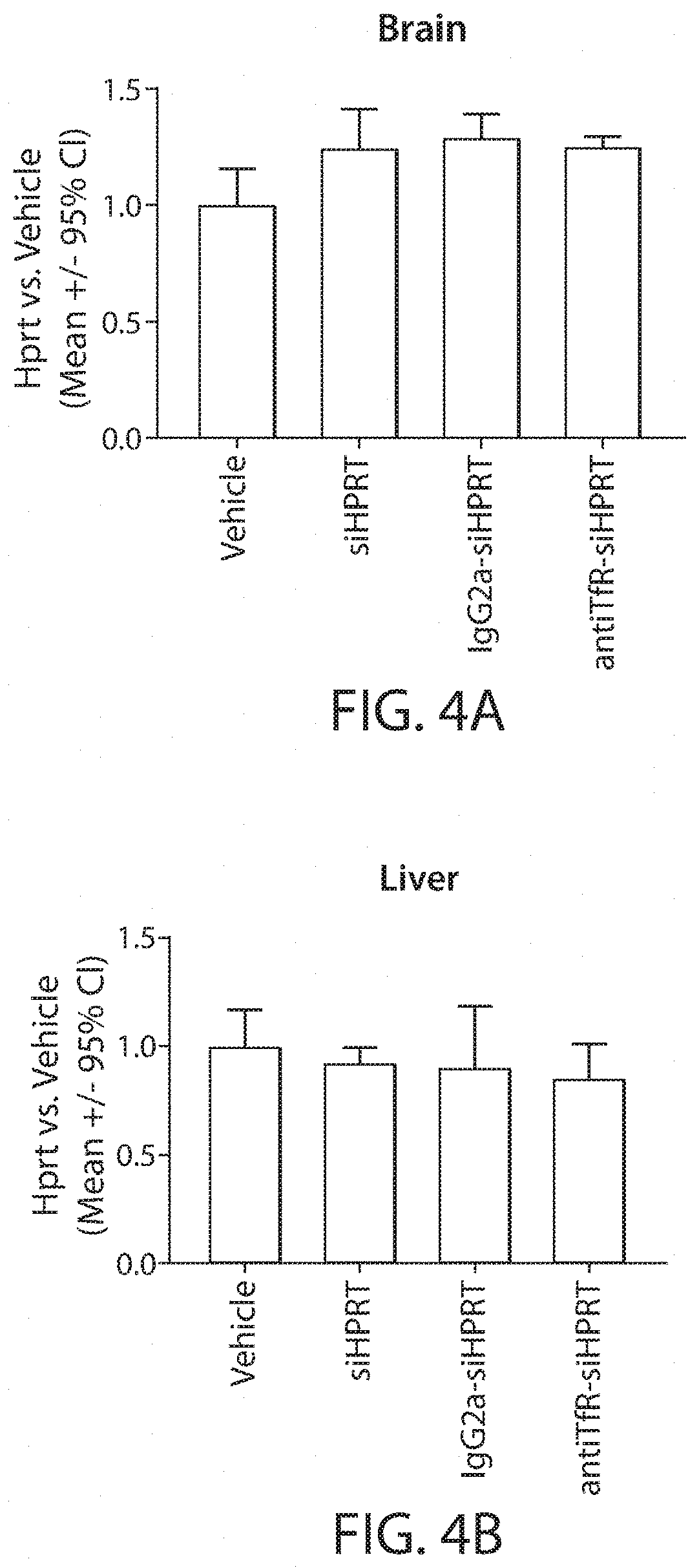
Muscle targeting complexes and uses thereof for treating dystrophinopathies
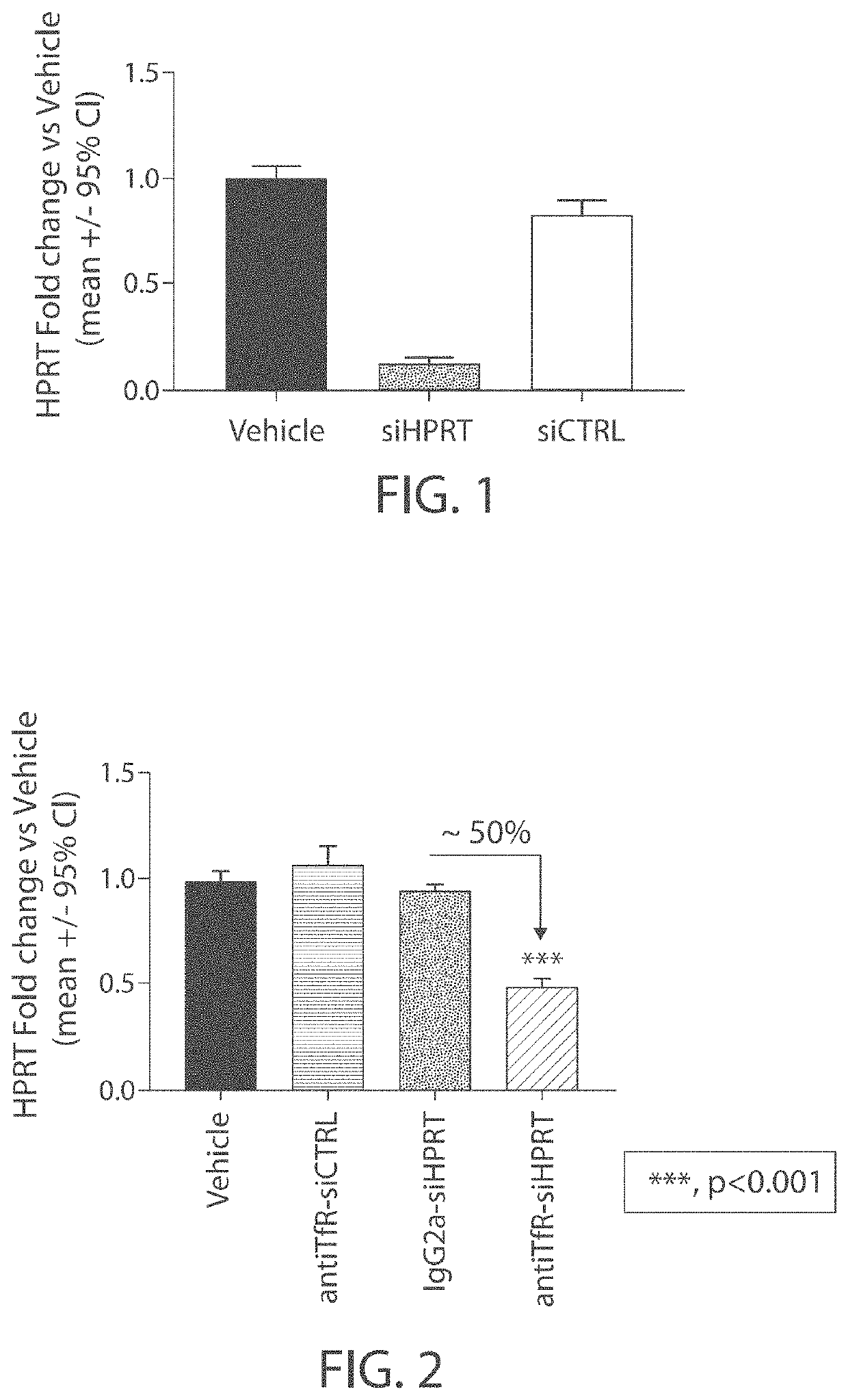
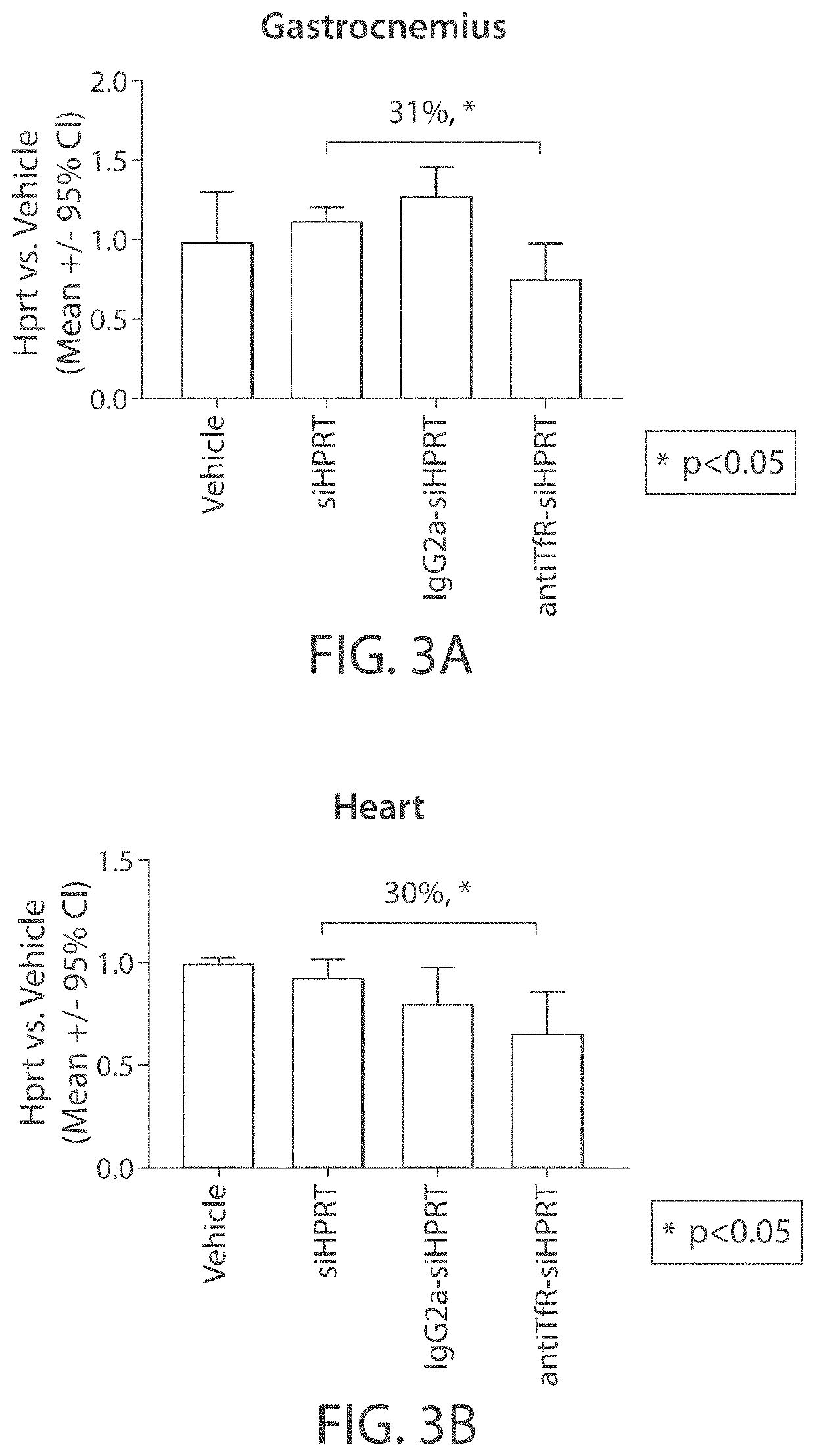
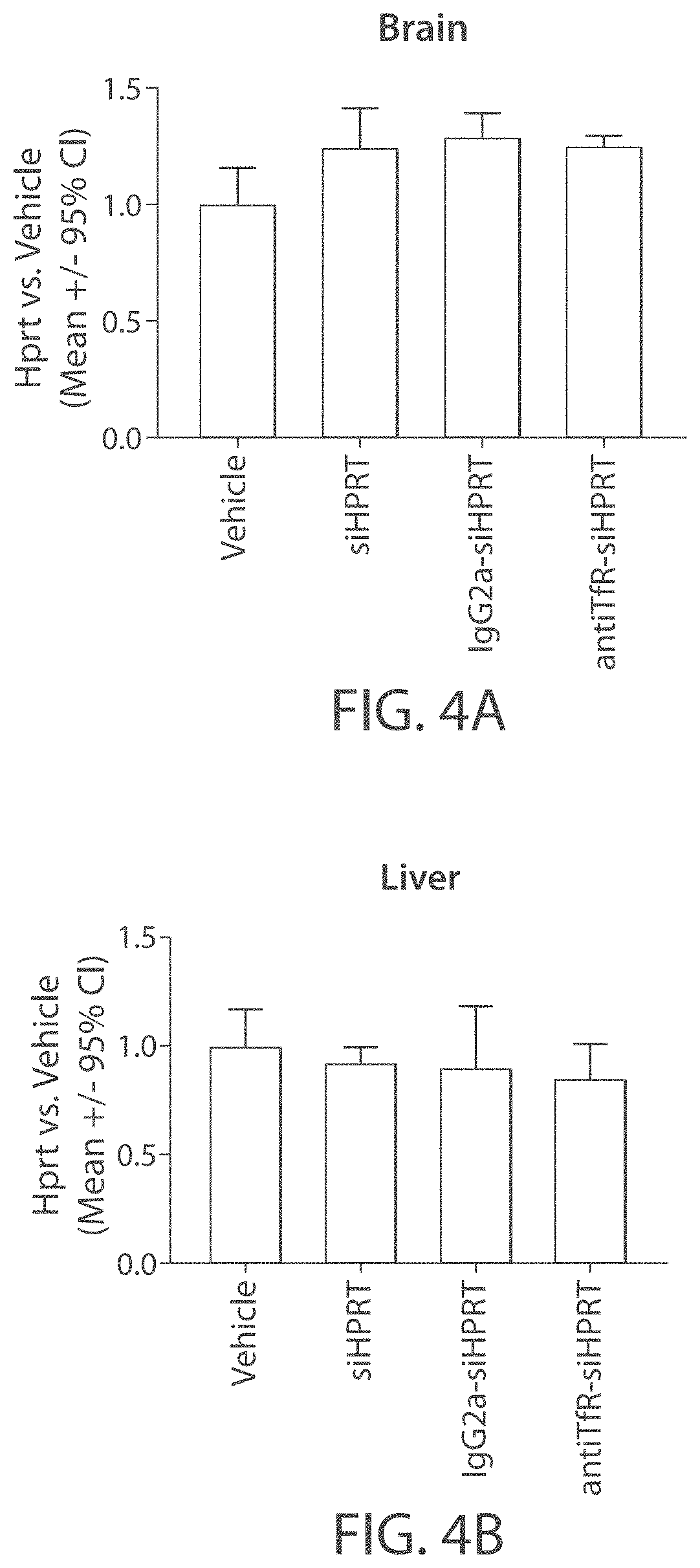
Aspects of the disclosure relate to complexes comprising a muscle-targeting agent covalently linked to a molecular payload. In some embodiments, the muscle-targeting agent specifically binds to an internalizing cell surface receptor on muscle cells. In some embodiments, the molecular payload promotes the expression or activity of a functional dystrophin protein. In some embodiments, the molecular payload is an oligonucleotide, such as an antisense oligonucleotide, e.g., an oligonucleotide that causes exon skipping in a mRNA expressed from a mutant DMD allele.
Owner:DYNE THERAPEUTICS INC
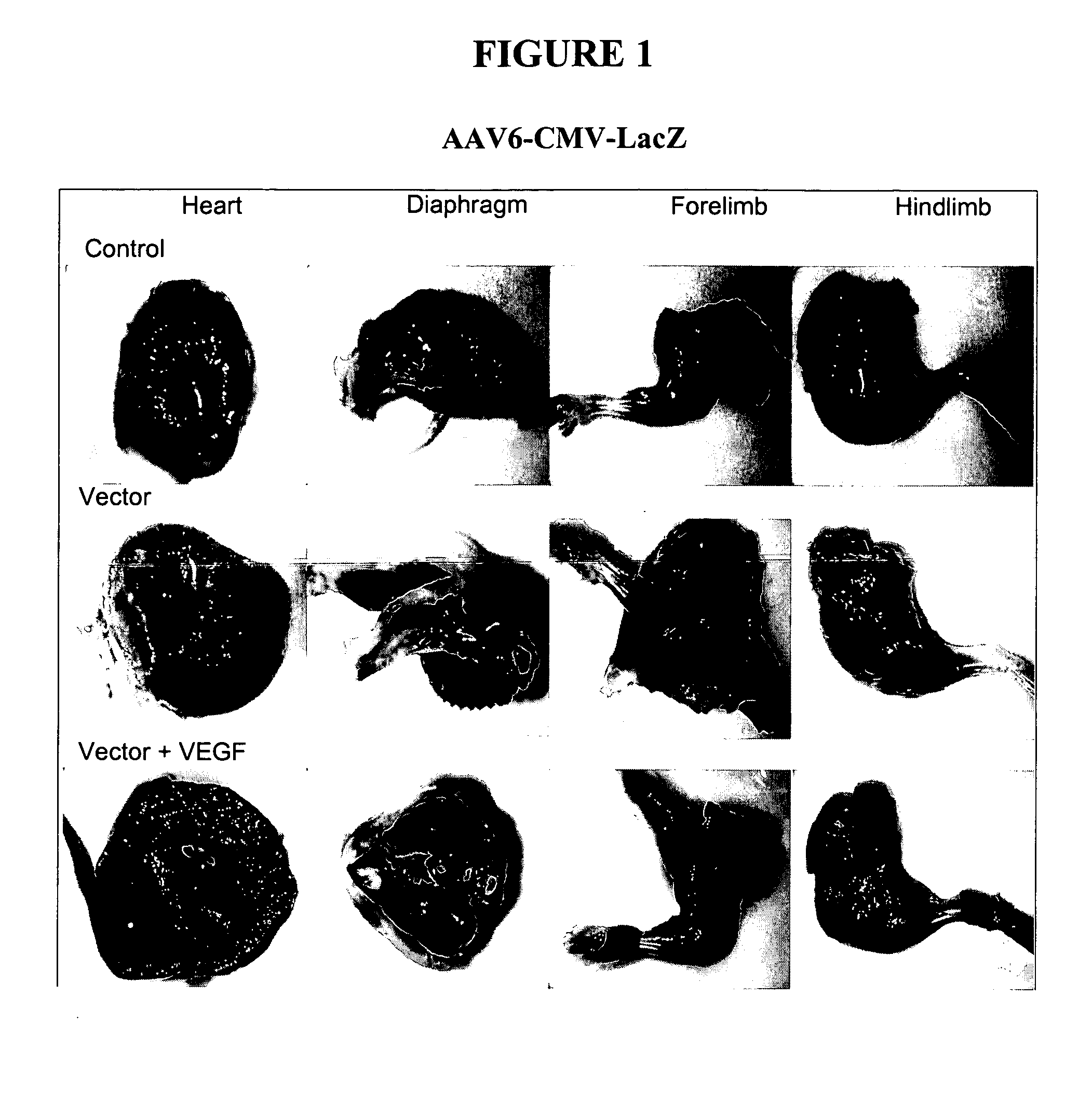
Compositions and methods for systemic nucleic acid sequence delivery
The present invention provides systemic nucleic acid sequence delivery without conventional systemic administration aids (SAAs). In certain embodiments, vascular permeability agents (VPAs), such as VEGF, are used in conjunction with nucleic acid viral vectors, such as adeno-associated virus (AAV). The present invention also provides methods of treating disease by co-administration of nucleic cid sequences encoding Igf-1 and dystrophin or dystrophin-like proteins.
Owner:UNIV OF WASHINGTON
Muscle targeting complexes and uses thereof for treating dystrophinopathies
Owner:DYNE THERAPEUTICS INC
Oligonucleotide for the Treatment of Muscular Dystrophy Patients
InactiveUS20150191725A1Avoid or decrease a potential multimerisation or aggregationSplicing alterationSugar derivativesDystrophinExon
The invention relates to an oligonucleotide and to a pharmaceutical composition comprising said oligonucleotide. This oligonucleotide is able to bind to a region of a first exon from a dystrophin pre-mRNA and to a region of a second exon within the same pre-mRNA, wherein said region of said second exon has at least 50% identity with said region of said first exon, wherein said oligonucleotide is suitable for the skipping of said first and second exons of said pre-mRNA, and preferably the entire stretch of exons in between.
Owner:BIOMARIN TECH BV
Compound and method for treating myotonic dystrophy
ActiveUS20120058946A1Organic active ingredientsPeptide/protein ingredientsArginineMyotonic dystrophy gene
An antisense compound for use in treating myotonic dystrophy DM1 or DM2, a method of enhancing antisense targeting to heart and quadricep muscles, and a method for treating DM1 or DM2 in a mammalian subject are disclosed. The oligonucleotide has 8-30 bases, with at least 8 contiguous bases being complementary to the polyCUG or polyCCUG repeats in the 3′UTR region of dystrophia myotonica protein kinase (DMPK) mRNA in DM1 or DM2, respectively. Conjugated to the oligonucleotide is a cell-penetrating peptide having the sequence (RXRR(B / X)R)2XB, where R is arginine; B is β-alanine; and each X is —C(O)—(CH2)n—NH—, where n is 4-6. The antisense compound is effective to selectively block the sequestration of muscleblind-like 1 protein (MBNL1) and / or CUGBP, in heart and quadricep muscle in a myotonic dystrophy animal model.
Owner:SAREPTA THERAPEUTICS INC
Modification of the dystrophin gene and uses thereof
InactiveUS20180265859A1Restoring correct reading frameAvoid large deletionsOrganic active ingredientsSugar derivativesMuscular dystrophyFrameshift mutation
Methods of modifying a dystrophin gene are disclosed, for restoring dystrophin expression within a cell having an endogenous frameshift mutation within the dystrophin gene. The methods comprising introducing a first cut within an exon of the dystrophin gene creating a first exon end, wherein said first cut is located upstream of the endogenous frameshift mutation; and introducing a second cut within an exon of the dystrophin gene creating a second exon end, wherein said second cut is located downstream of the frameshift mutation. Upon joining / ligation of said first and second exon ends dystrophin expression is restored, as the correct reading frame is restored. Reagents and uses of the method are also disclosed, for example to treat a subject suffering from muscular dystrophy.
Owner:UNIV LAVAL
Muscle targeting complexes and uses thereof for treating dystrophinopathies
Aspects of the disclosure relate to complexes comprising a muscle-targeting agent covalently linked to a molecular payload. In some embodiments, the muscle-targeting agent specifically binds to an internalizing cell surface receptor on muscle cells. In some embodiments, the molecular payload promotes the expression or activity of a functional dystrophin protein. In some embodiments, the molecular payload is an oligonucleotide, such as an antisense oligonucleotide, e.g., an oligonucleotide that causes exon skipping in a mRNA expressed from a mutant DMD allele.
Owner:DYNE THERAPEUTICS INC
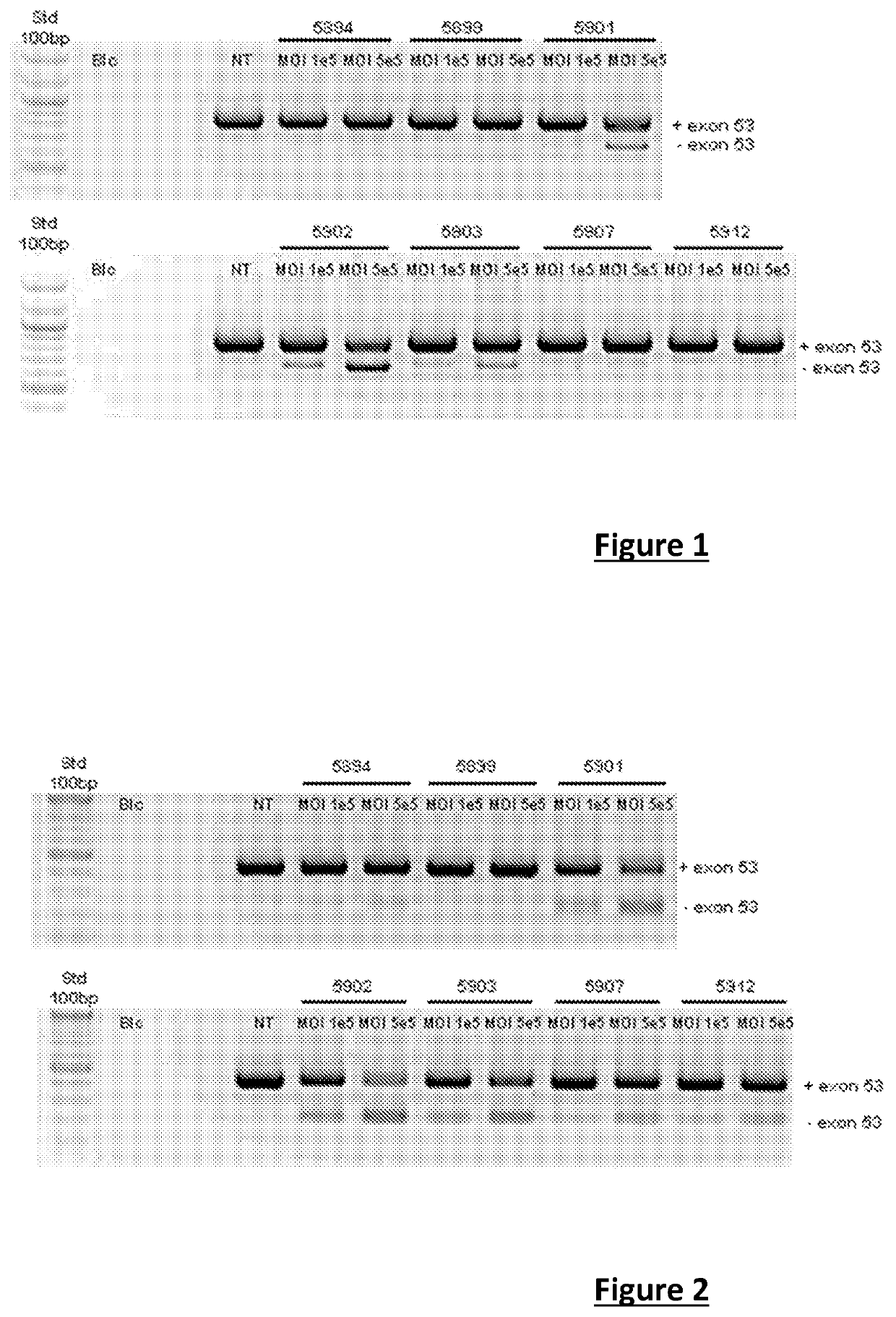
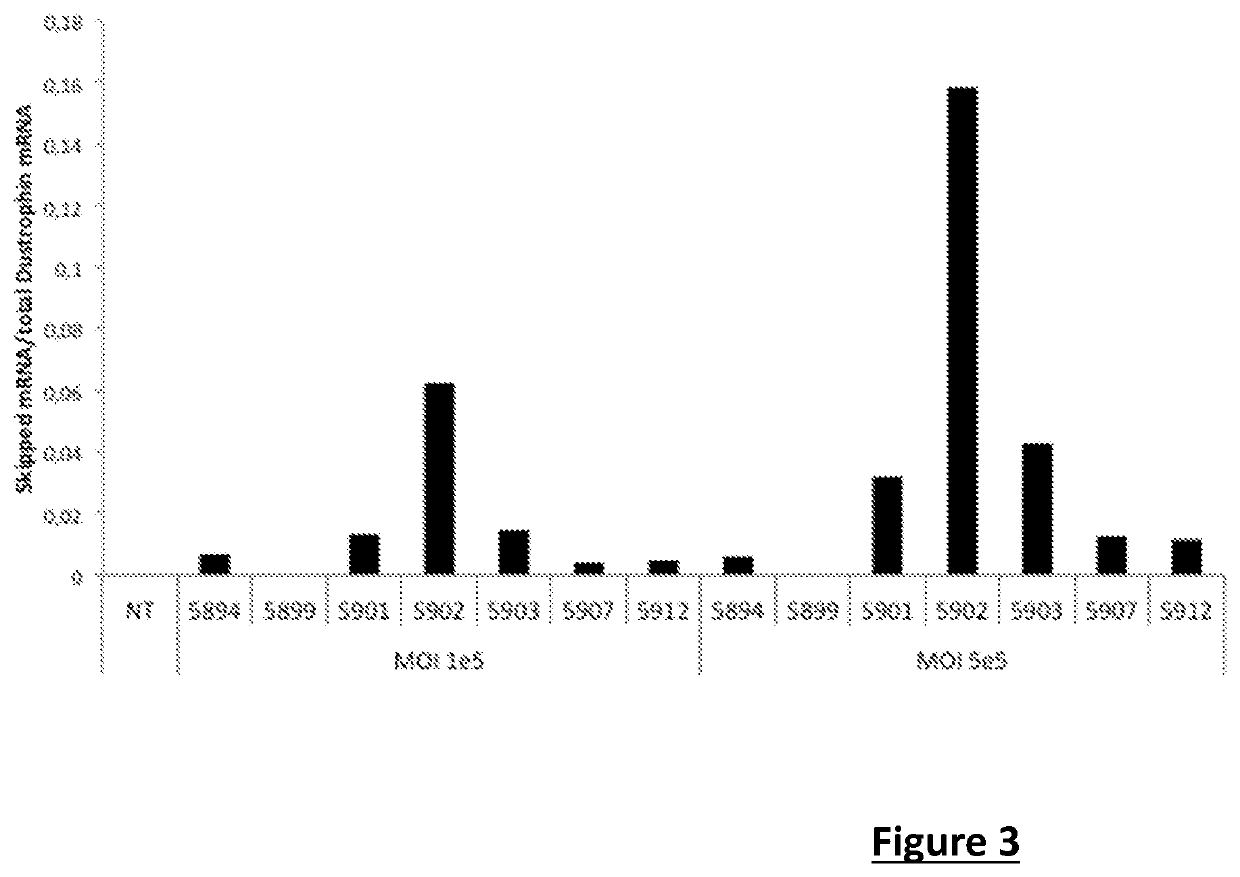
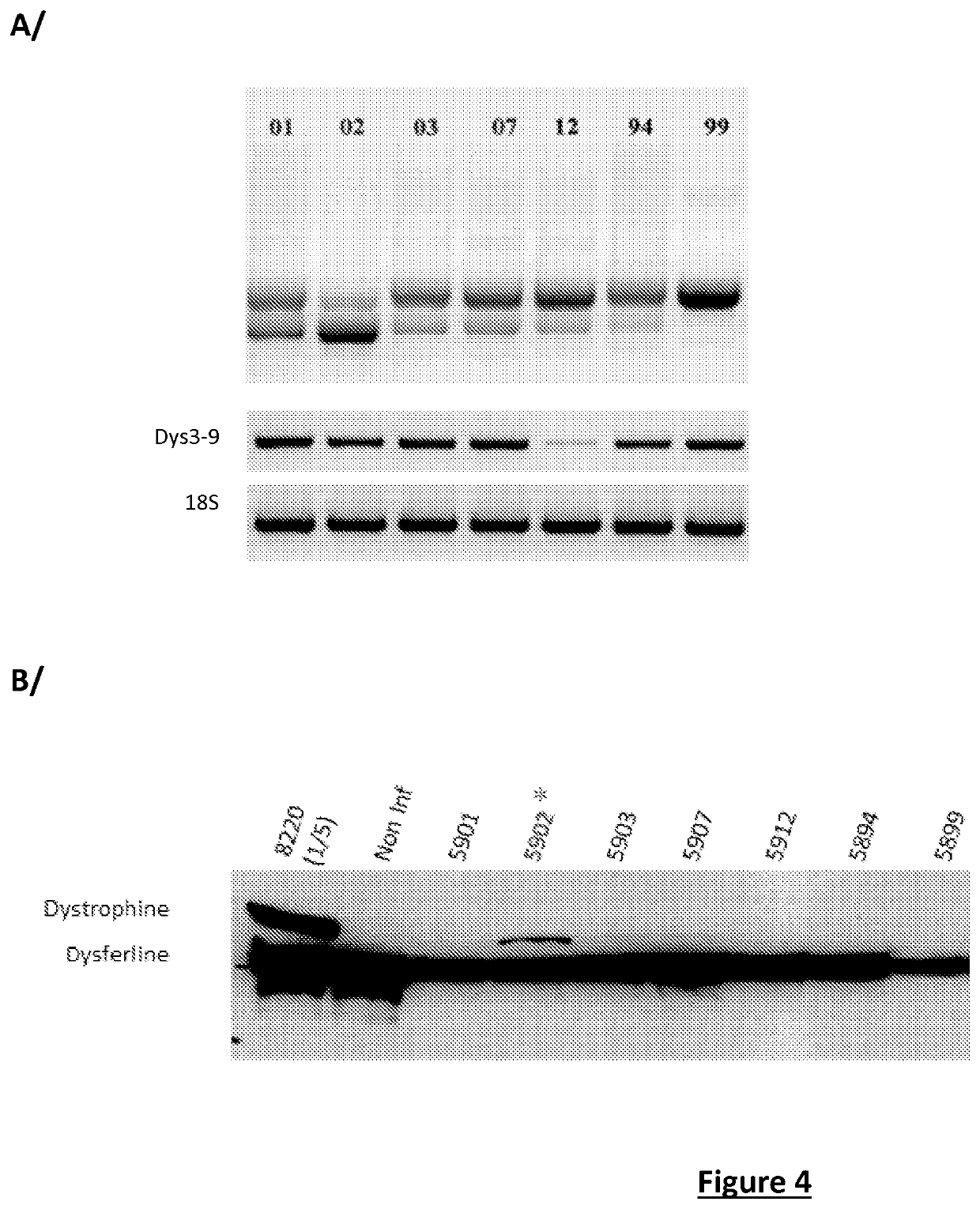
Effective gene therapy tools for dystrophin exon 53 skipping
The invention relates to a recombinant adeno-associated viral vector (rAAV) comprising a sequence encoding an antisense oligonucleotide (AON) directed against a segment of at least 33 bases from the +30 to +69 region of exon 53 of the pre-messenger RNA (pre-mRNA) of dystrophin, advantageously of human origin, and to the use thereof as a drug, in particular for the treatment of Duchenne muscular dystrophy (DMD).
Owner:GENETHON
Muscle targeting complexes and uses thereof for treating dystrophinopathies
PendingUS20210308273A1High activityHigh expressionOrganic active ingredientsSplicing alterationMutant alleleInternalization
Aspects of the disclosure relate to complexes comprising a muscle-targeting agent covalently linked to a molecular payload. In some embodiments, the muscle-targeting agent specifically binds to an internalizing cell surface receptor on muscle cells. In some embodiments, the molecular payload promotes the expression or activity of a functional dystrophin protein. In some embodiments, the molecular payload is an oligonucleotide, such as an antisense oligonucleotide, e.g., an oligonucleotide that causes exon skipping in a mRNA expressed from a mutant DMD allele.
Owner:DYNE THERAPEUTICS INC
Treatment of muscular dystrophies and related disorders
InactiveUS20110053854A1Less “ leaky ”Extend your lifePeptide/protein ingredientsMuscular disorderSynapseDisease
The invention provides, among other aspects, compositions and methods for treating, preventing, and diagnosing diseases or conditions associated with an abnormal level or activity of biglycan; diseases or conditions associated with an abnormal level or activity of collagen VI; disorders associated with an unstable cytoplasmic membrane, due, e.g., to an unstable dystrophin associated protein complex (DAPC); and disorders associated with abnormal synapses or neuromuscular junctions, including those resulting from an abnormal MuSK activation or acetylcholine receptor (AChR) aggregation.
Owner:BROWN UNIVERSITY
Muscle targeting complexes and uses thereof for treating dystrophinopathies
ActiveUS20220025066A1High activityHigh expressionOrganic active ingredientsSplicing alterationMutant alleleInternalization
Aspects of the disclosure relate to complexes comprising a muscle-targeting agent covalently linked to a molecular payload. In some embodiments, the muscle-targeting agent specifically binds to an internalizing cell surface receptor on muscle cells. In some embodiments, the molecular payload promotes the expression or activity of a functional dystrophin protein. In some embodiments, the molecular payload is an oligonucleotide, such as an antisense oligonucleotide, e.g., an oligonucleotide that causes exon skipping in a mRNA expressed from a mutant DMD allele.
Owner:DYNE THERAPEUTICS INC
Compositions And Methods For The Treatment Of Muscular Dystrophy
InactiveUS20100292306A1Long-term treatmentLess efficaciousBiocideMuscular disorderDystrophinMuscle damage
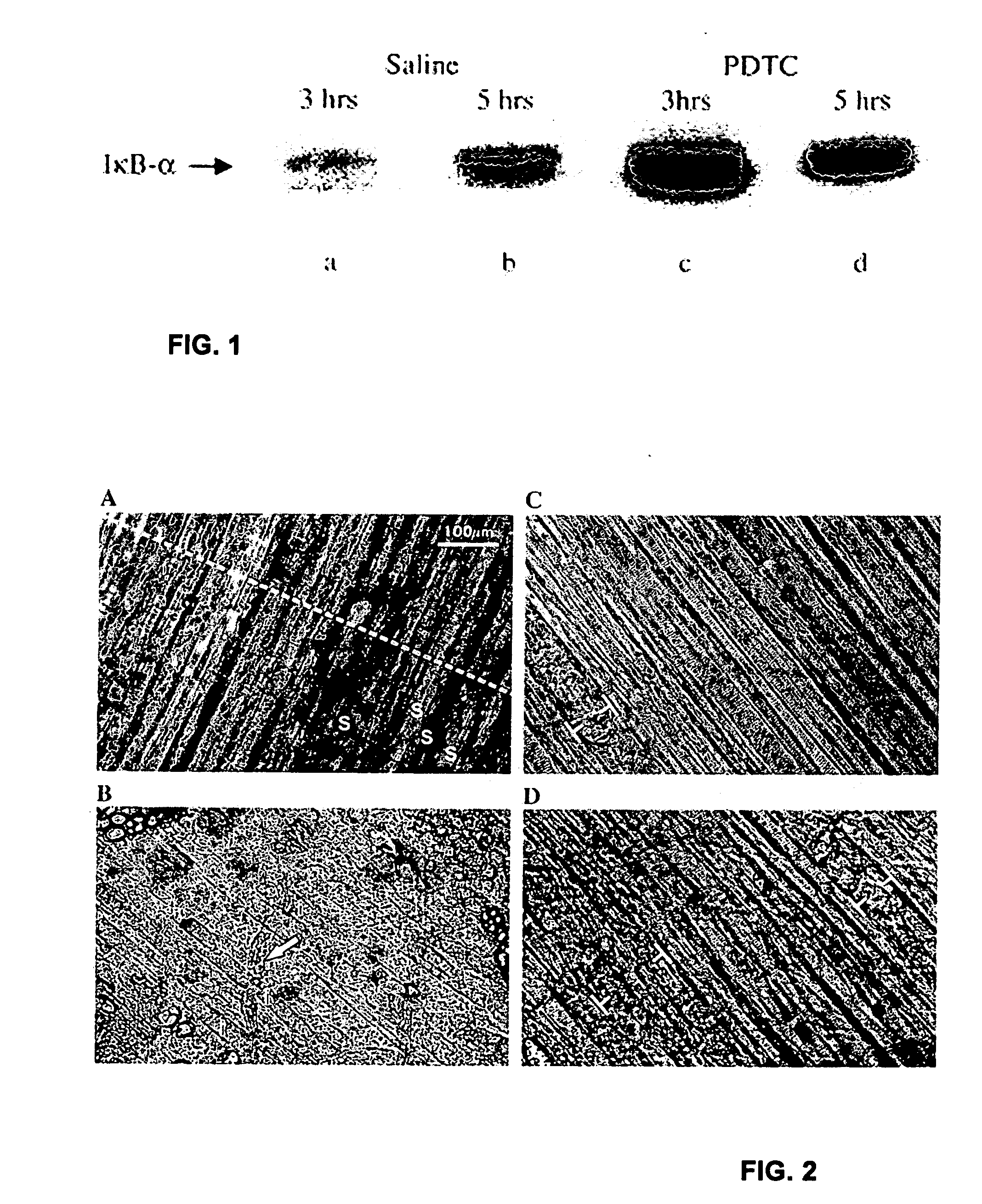
Compositions and methods for treatment of individuals diagnosed with a dystrophin deficiency are disclosed. In particular, inhibitors of NFκB transactivation and / or inhibitors that suppress p65 expression are used to prevent and / or reverse muscle damage in animals or humans lacking dystrophin. Such compositions and methods are useful in the treatment of individuals with muscular dystrophy.
Owner:A T STILL UNIV
DNA sequences encoding dystrophin minigenes and methods of use thereof
InactiveUS20060073586A1Small sizeEfficient and stable correctionVirusesSugar derivativesPolyadenylationMyodystrophies
The present invention provides a series of novel dystrophin minigenes that retain the essential biological functions. The expression of the dystrophin minigenes may be controlled by a regulatory element along with a small polyadenylation signal. The entire gene expression cassettes may be readily packaged into a viral vector, preferably an AAV vector. The present invention further defines the minimal functional domains of dystrophin and provides ways to optimize and create new versions of dystrophin minigenes. Finally, the present invention provides a method of treatment for Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD).
Owner:ASKLEPIOS BIOPHARMACEUTICAL INC
Oligonucleotide comprising an inosine for treating dmd
InactiveUS20160264967A1Avoid or decrease a potential multimerisation or aggregation of oligonucleotidesLow efficiencySplicing alterationAntipyreticInosineDystrophin
The invention provides an oligonucleotide comprising an inosine, and / or a nucleotide containing a base able to form a wobble base pair or a functional equivalent thereof, wherein the oligonucleotide, or a functional equivalent thereof, comprises a sequence which is complementary to at least part of a dystrophin pre-m RNA exon or at least part of a non-exon region of a dystrophin pre-m RNA said part being a contiguous stretch comprising at least 8 nucleotides. The invention further provides the use of said oligonucleotide for preventing or treating DMD or BMD.
Owner:BIOMARIN TECH BV
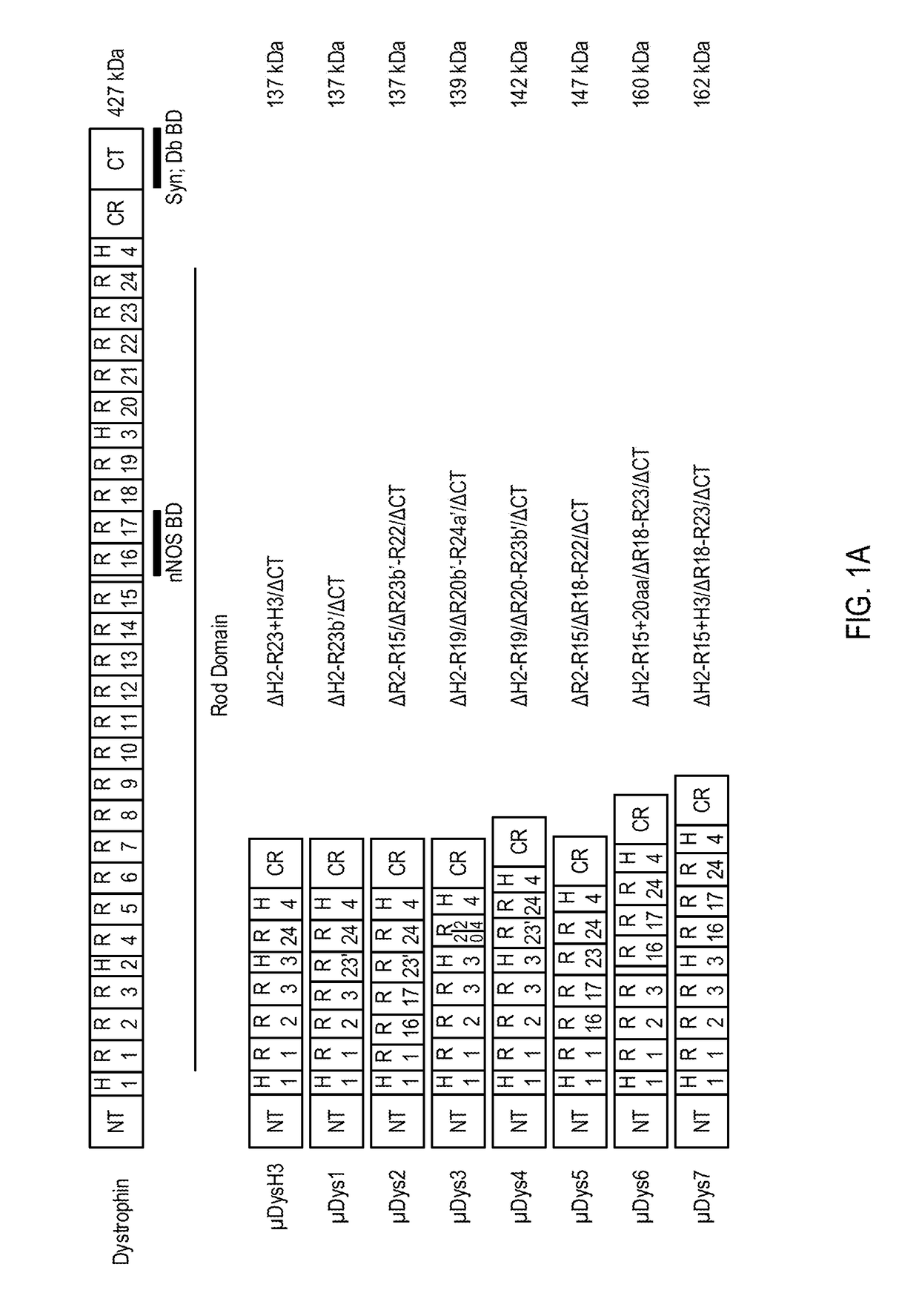
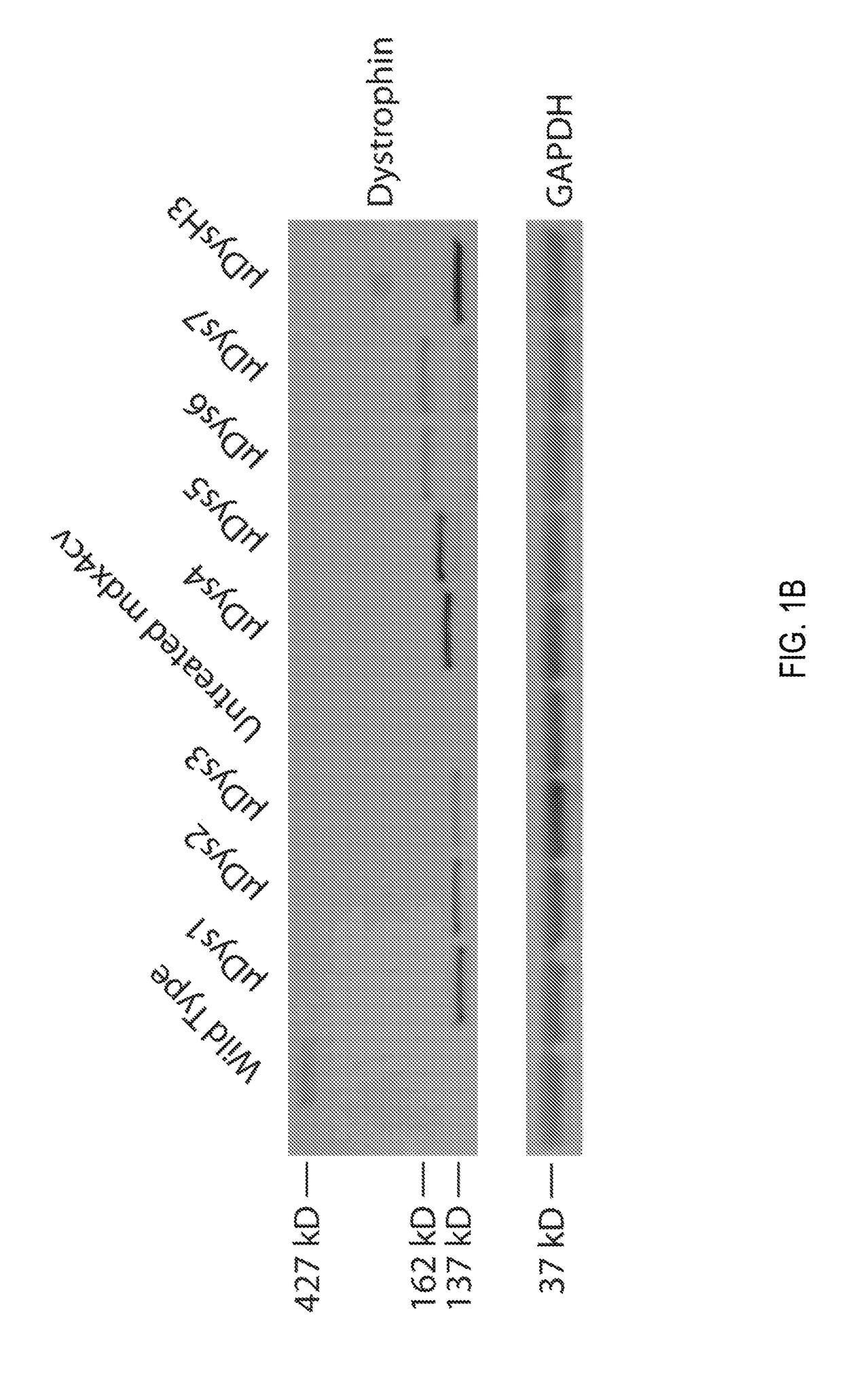
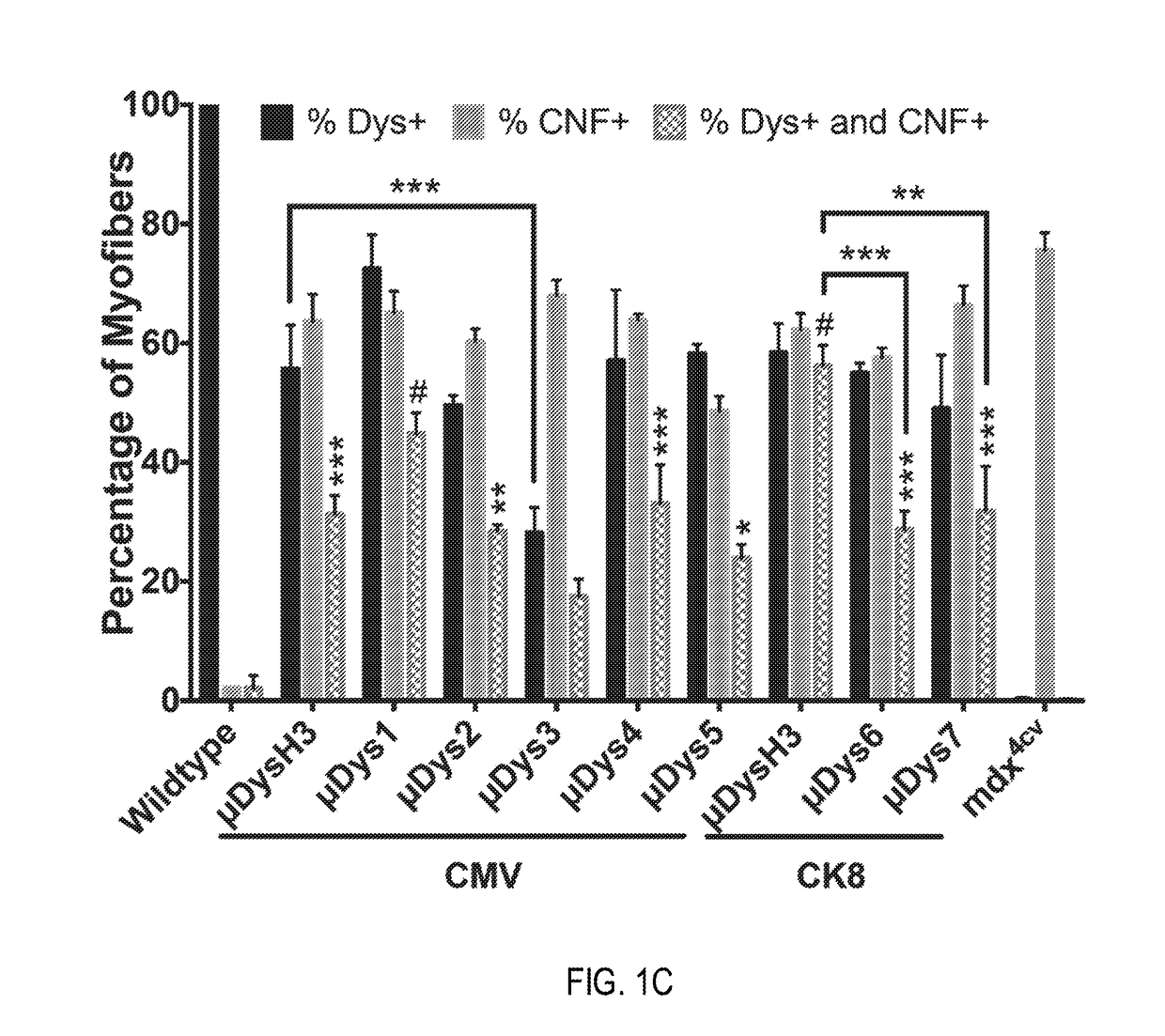
Novel micro-dystrophins and related methods of use
ActiveUS20180148488A1Reduce development riskIncrease in average myofiber diameterPeptide/protein ingredientsAntibody mimetics/scaffoldsDelivery vehicleMedicine
Nucleotide sequences including a micro-dystrophin gene are provided. The micro-dystrophin genes may be operatively linked to a regulatory cassette. Methods of treating a subject having, or at risk of developing, muscular dystrophy, sarcopenia, heart disease, or cachexia are also provided. The methods may include administering a pharmaceutical composition including the micro-dystrophin gene and a delivery vehicle to a subject. Further, the methods may include administering the pharmaceutical composition a subject having Duchenne muscular dystrophy or Becker muscular dystrophy.
Owner:UNIV OF WASHINGTON
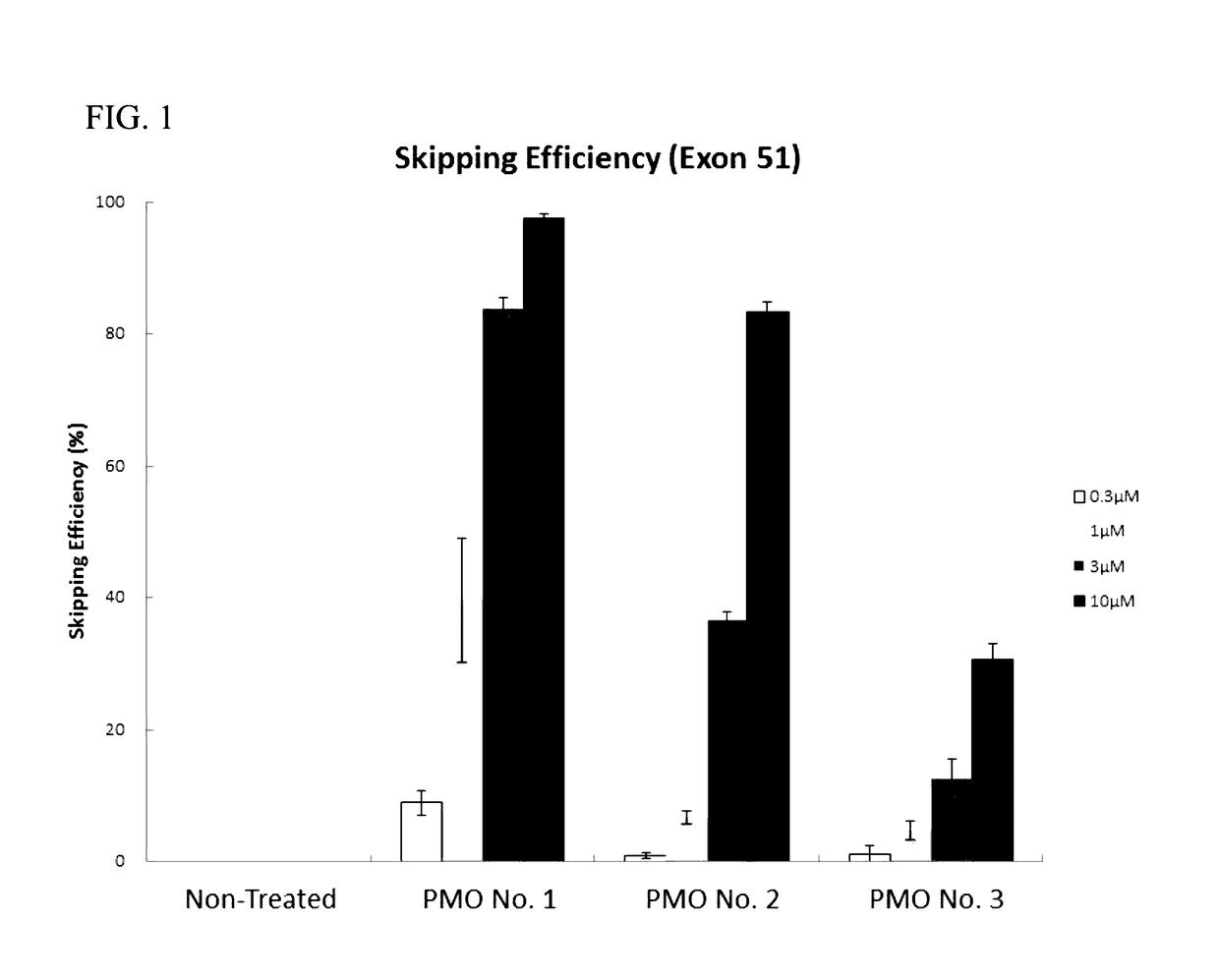
Antisense nucleic acids
ActiveUS20170067048A1Improve efficiencyOrganic active ingredientsSplicing alterationAntisense nucleic acidExon
Provided is a drug that allows highly-efficient skipping of exon 51 in the human dystrophin gene. The present invention provides an antisense oligomer which enables exon 51 in the human dystrophin gene to be skipped.
Owner:NIPPON SHINYAKU CO LTD +1
Compositions and methods for the treatment of muscular dystrophy
InactiveUS20060280812A1Long-term treatmentLess efficaciousBiocidePeptide/protein ingredientsDystrophinPyrrolidine dithiocarbamate
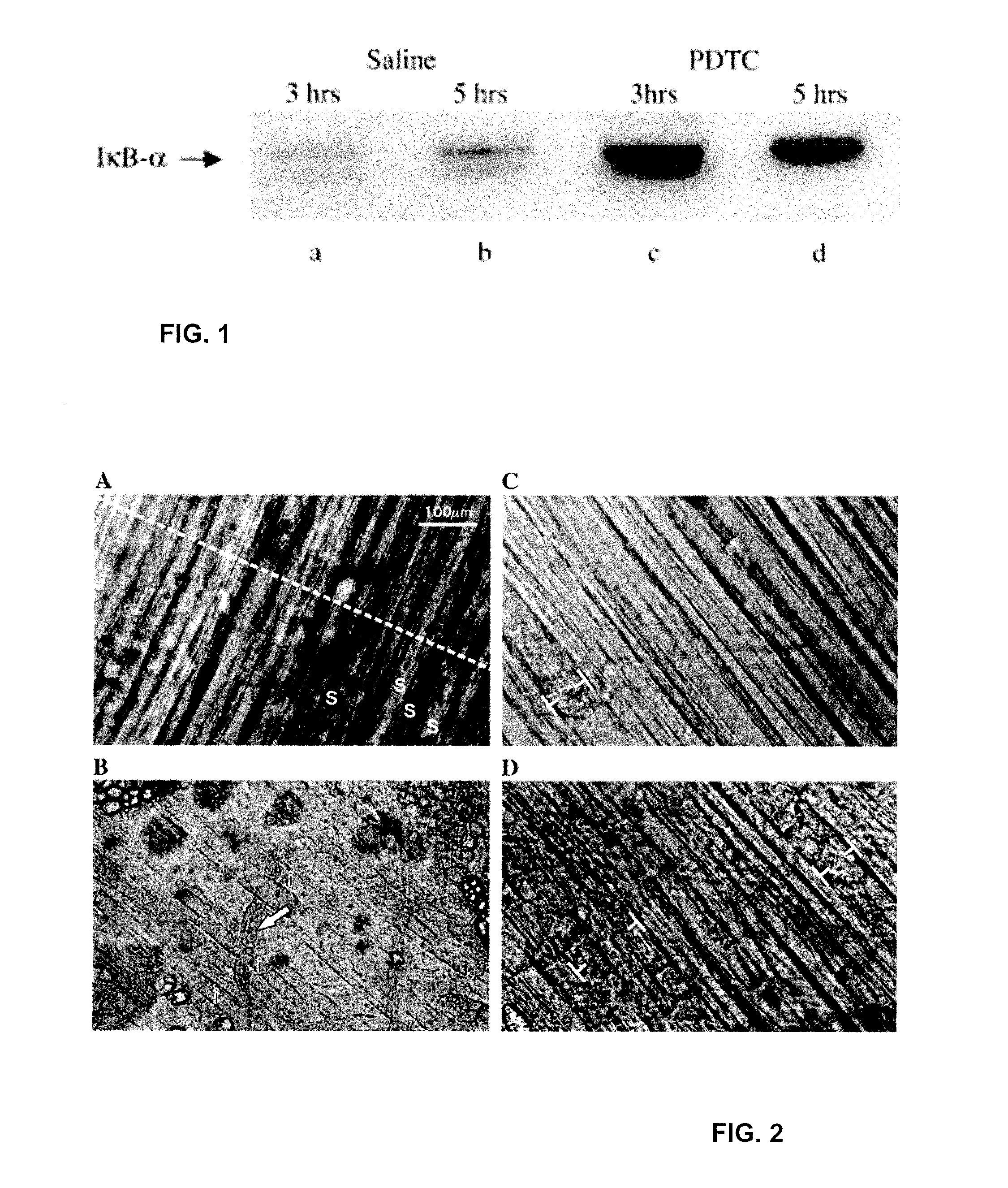
Compositions and methods for treatment of individuals diagnosed with a dystrophin deficiency are disclosed. In particular, inhibitors of NFκB activation, such as pyrrolidine dithiocarbamate (PDTC), have been shown to prevent and reverse muscle damage in animals lacking dystrophin. Such compositions and methods are useful in the treatment of individuals with muscular dystrophy.
Owner:A T STILL UNIV OF HEATH SCI
Optimized mini-dystrophin genes and expression cassettes and their use
This invention relates to polynucleotides encoding mini-dystrophin proteins, viral vectors comprising the same, and methods of using the same for delivery of mini-dystrophin to a cell or a subject.
Owner:BAMBOO THERAPEUTICS INC +1
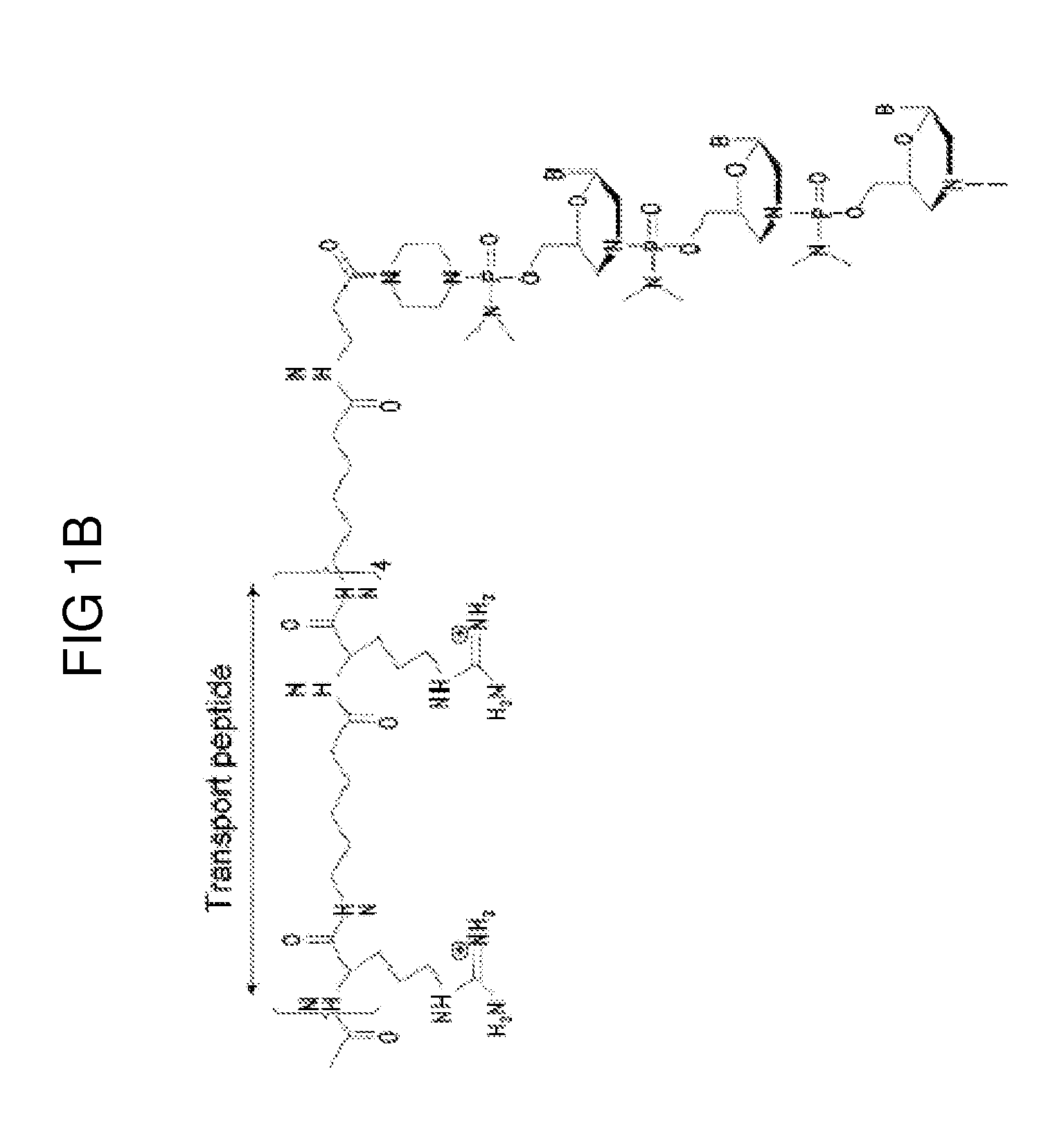
Chimeric dystrophin proteins to treat dystrophinopathies
InactiveUS20150329609A1SsRNA viruses negative-sensePolypeptide with localisation/targeting motifMelanomaMarfan syndrome
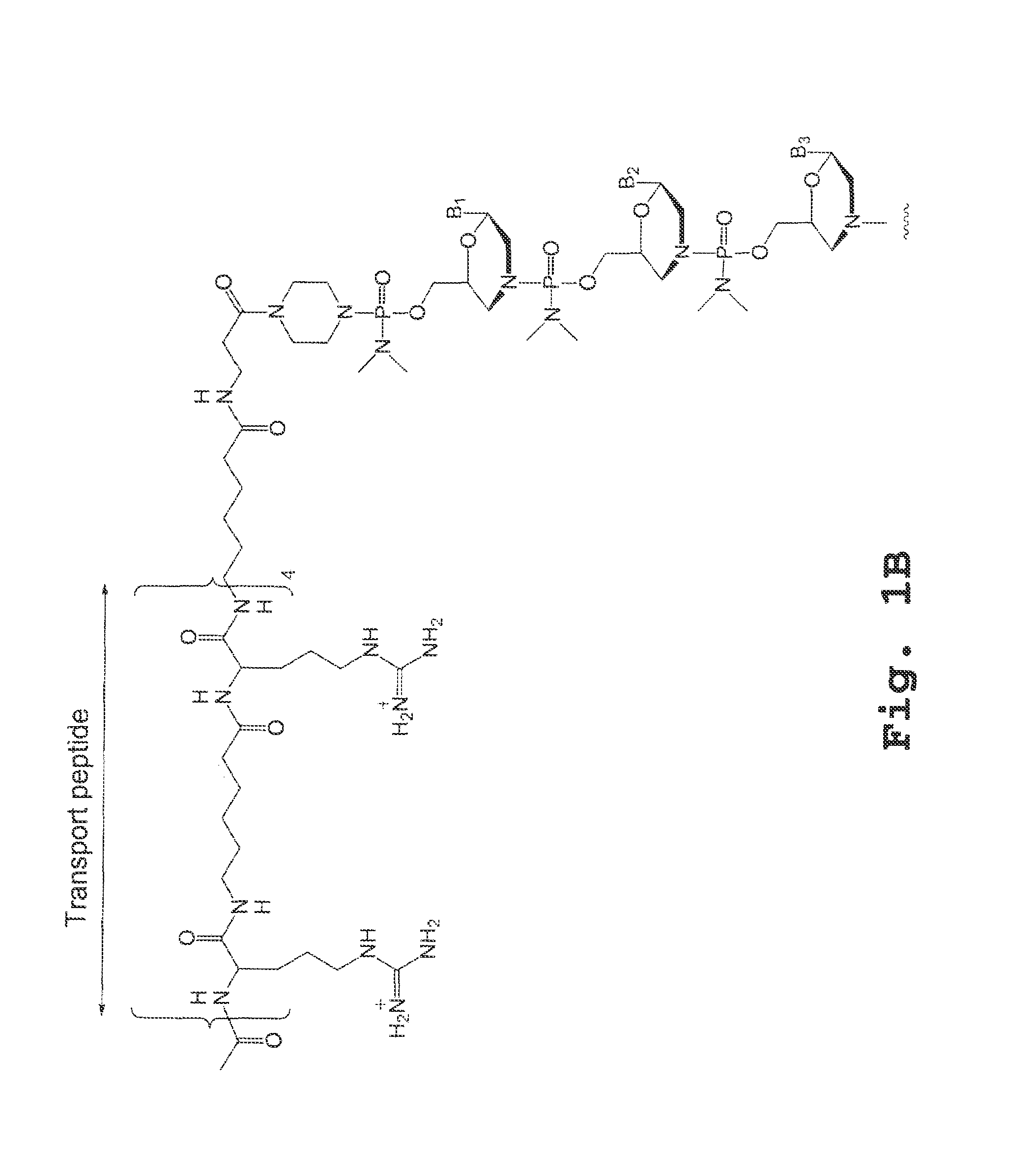
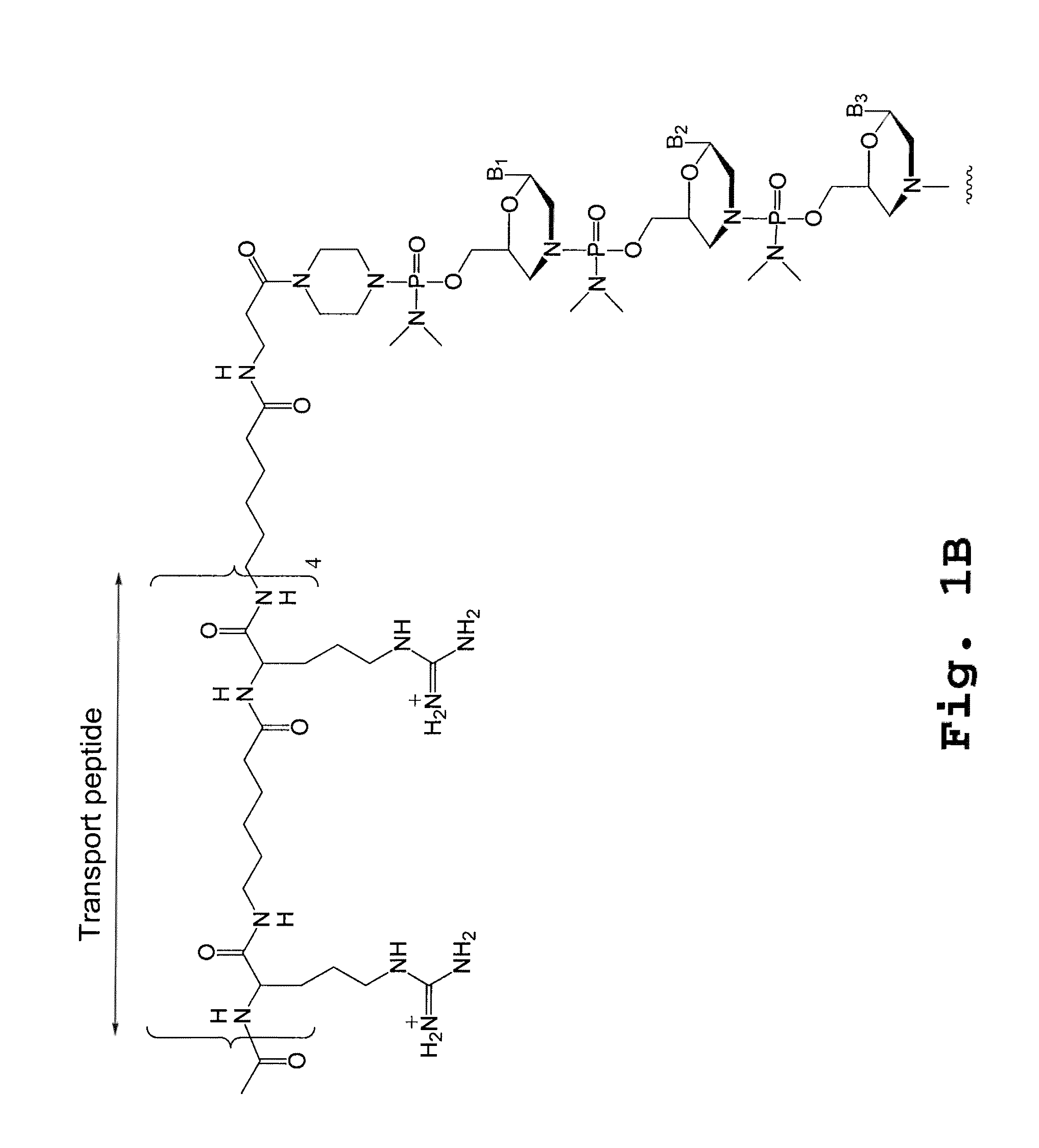
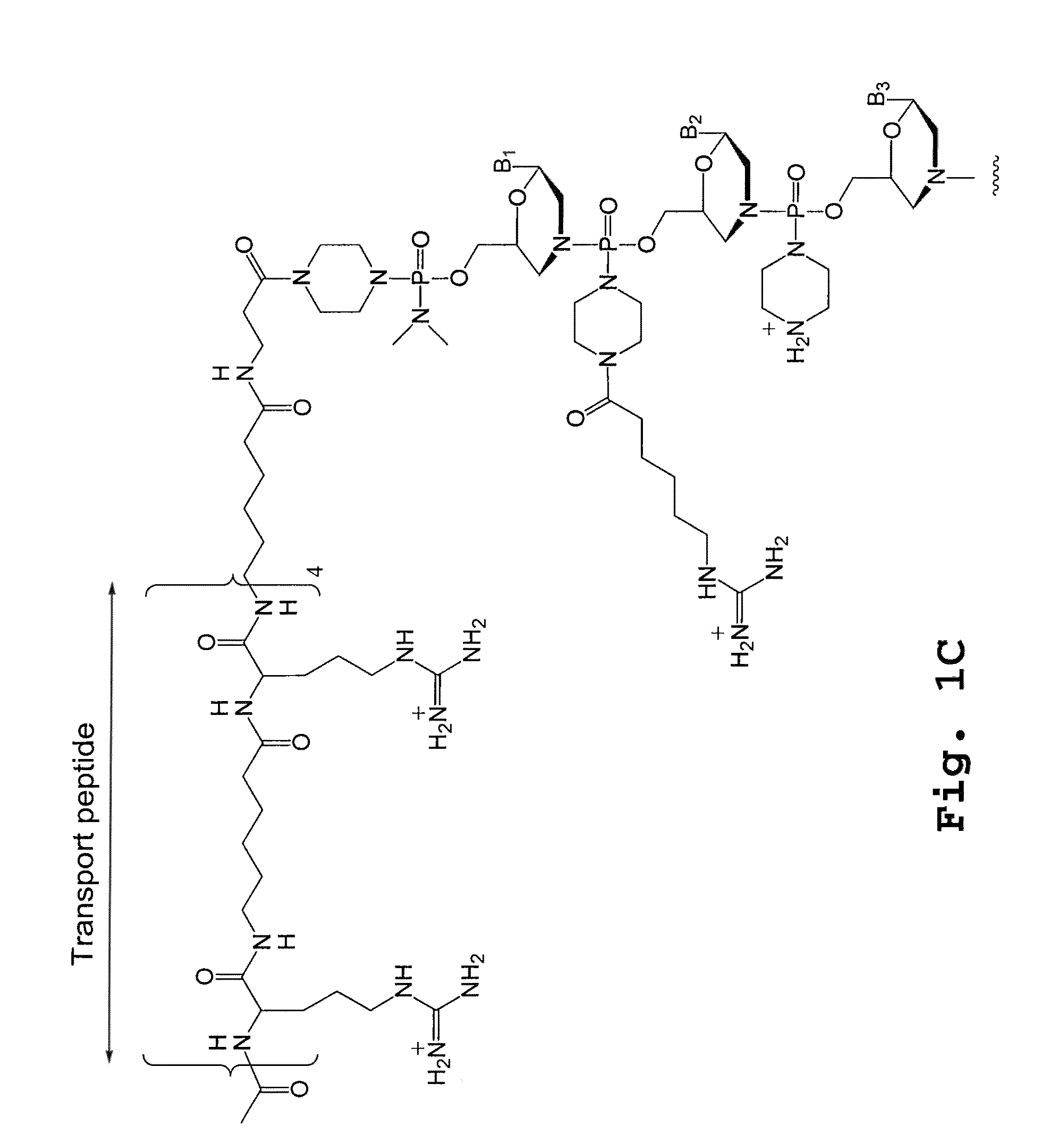
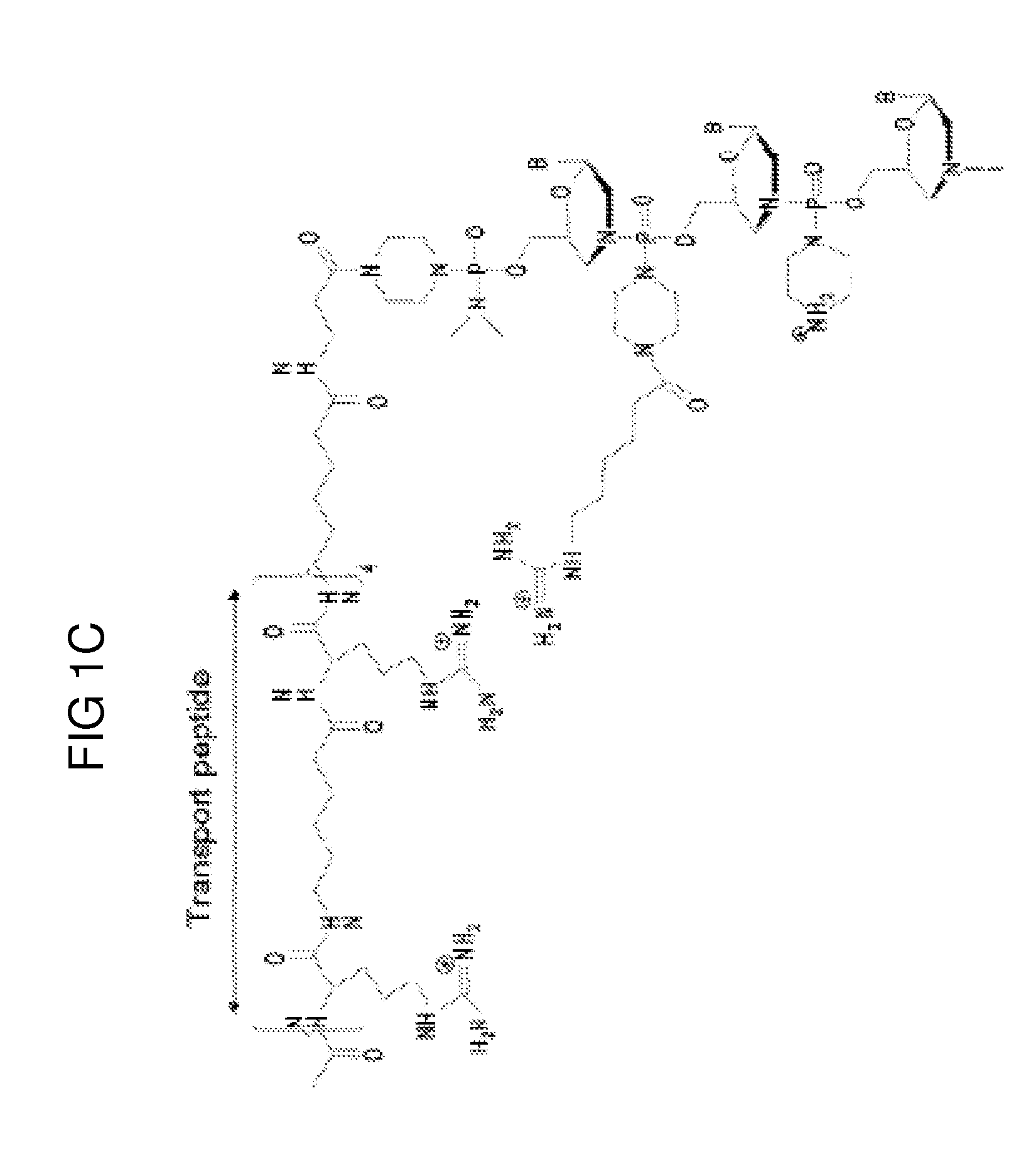
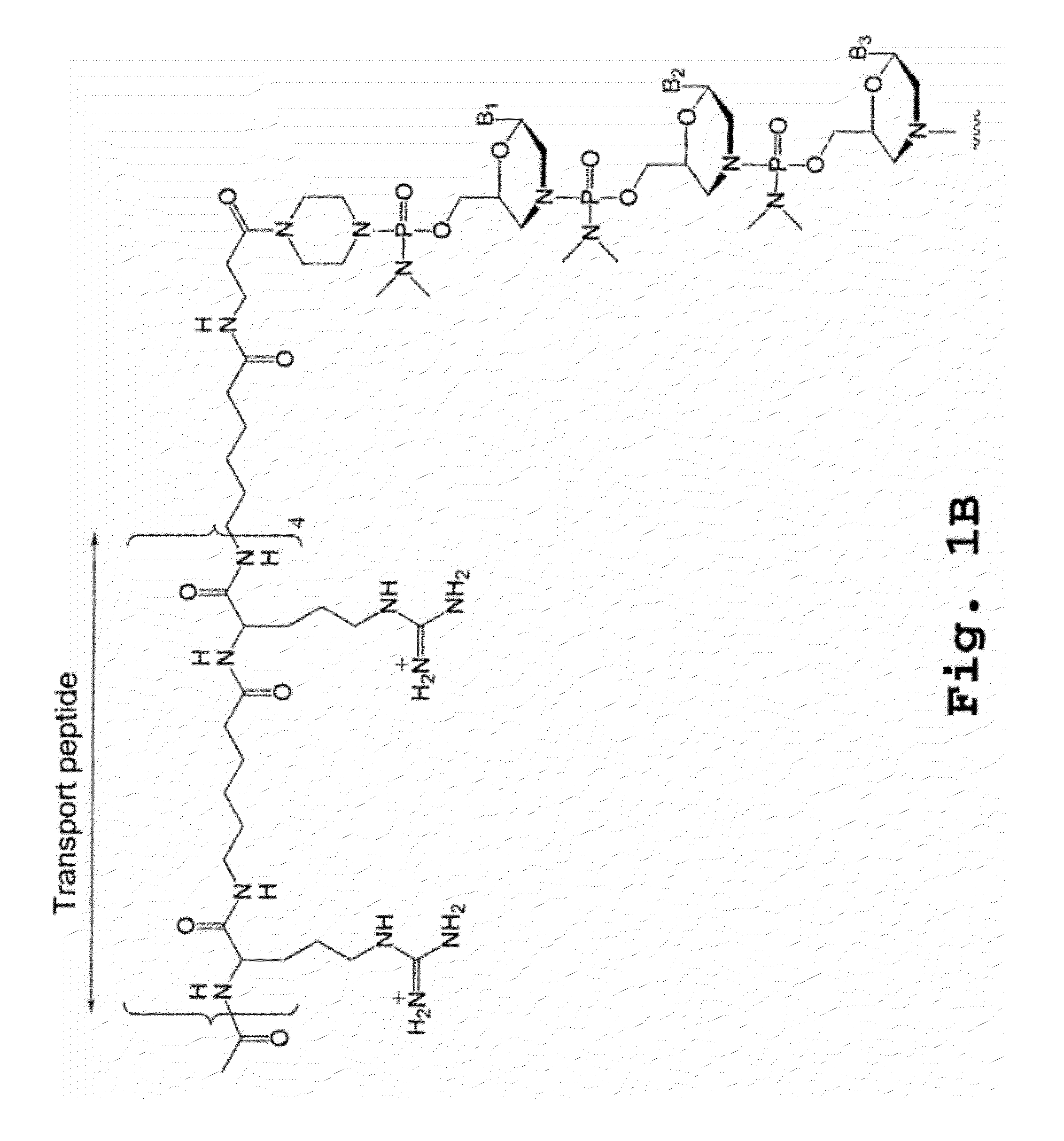
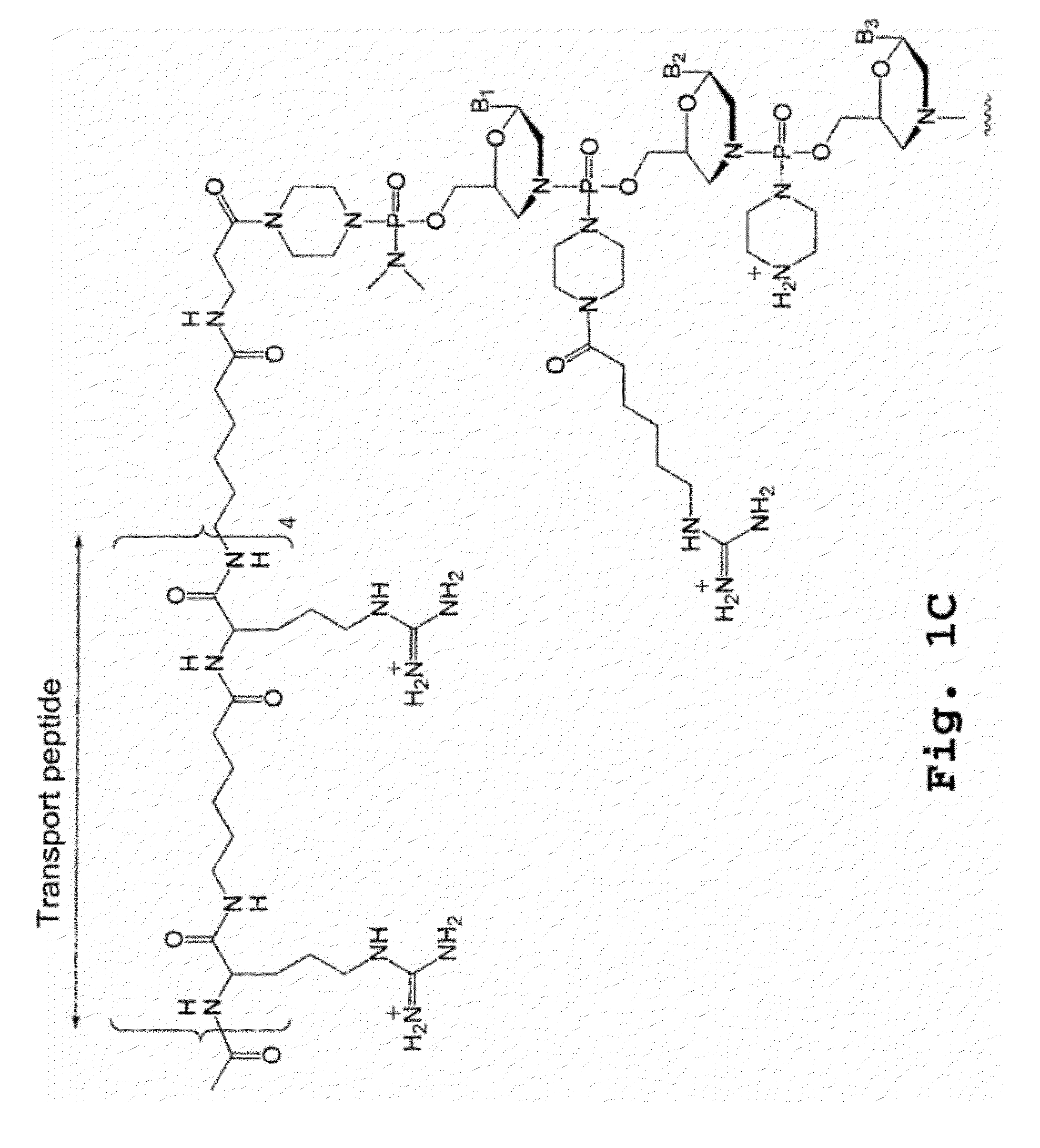
A chimeric protein that is a fusion construct of a series of functional domains is used to deliver a therapeutic agent to a human subject suffering from disease. In some embodiments, the chimeric protein includes a therapeutic region and a transportation region. The transportation region allows the chimeric protein to be moved across a cellular membrane of an affected cell within the subject. The therapeutic region can be effective in the treatment of, for example, muscular dystrophy, diastrophic dysplasia, malignant melanoma, porphyria, alpha-1 antitrypsin deficiency, Aicardi-Goutieres syndrome, cystic fibrosis, progeria, Marfan syndrome, tuberous sclerosis, adrenoleukodystrophy, and the like.
Owner:SERENDIPITY BIOTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com