Compound and method for treating myotonic dystrophy
a myotonic dystrophy and compound technology, applied in the direction of drug compositions, specific cell targeting, peptide/protein ingredients, etc., can solve the problems of often hindering the practical utility of many drugs with potentially useful biological activity, and achieve the effect of blocking the sequestration of muscleblind-like 1 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of Cell Penetrating Peptide Conjugated PMOs in the EGFP-654 Transgenic Mouse Model
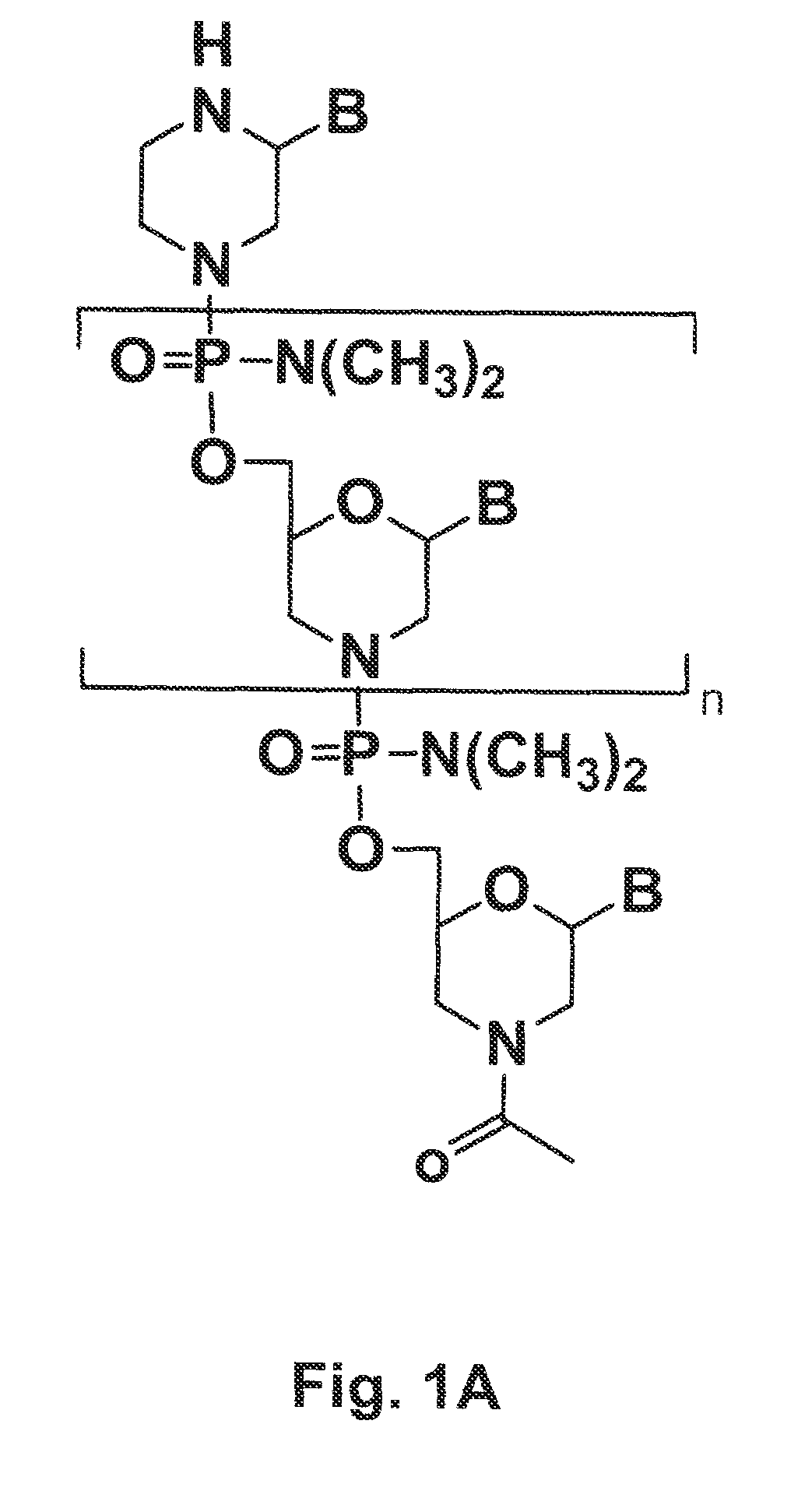
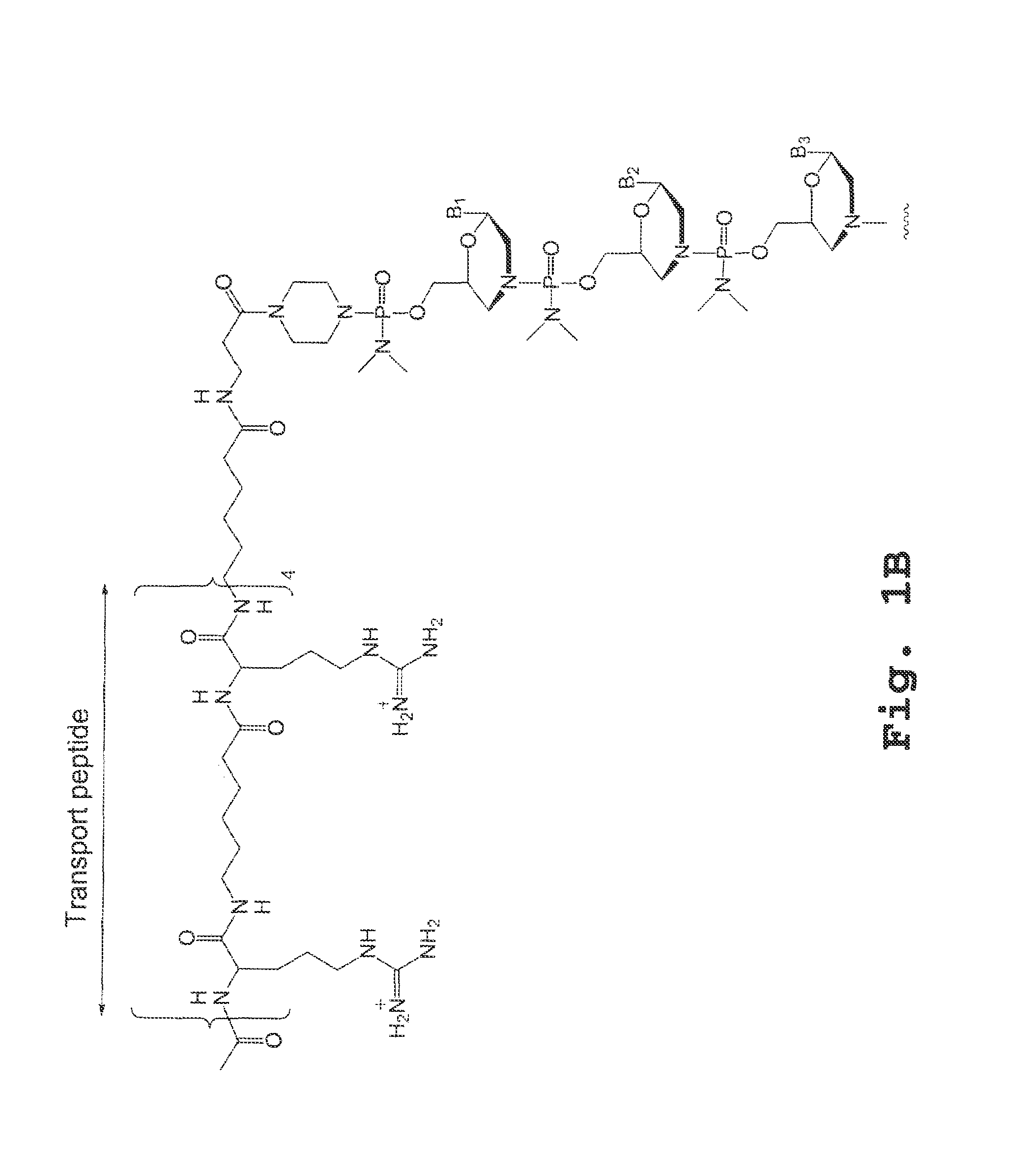
[0168]A PMO (designated 654; 5′-GCT ATT ACC TTA ACC CAG-3′; SEQ ID NO: 2) designed to restore correct splicing in the enhanced green fluorescent protein (EGFP) gene was conjugated to various cell penetrating peptides (SEQ ID NOS: 2, 3, 6, 11, 13-14, 19, 20-27) to produce P-PMOs (peptide-conjugated PMOs), which were evaluated in vivo for their splice-correction activity and toxicity in the EGFP-654 transgenic mouse model (Sazani, Gemignani et al. 2002). In this model, the EGFP-654 gene encoding for functional EGFP is interrupted by an aberrantly-spliced mutated intron, and cellular uptake of EGFP-654 targeted P-PMOs can be evaluated by RT-PCR detection of the restored EGFP-654 splice product in tissues.
[0169]Female EGFP-654 transgenic mice were injected IP once daily for 4 consecutive days with saline or a 12.5 mg / kg dose of P-PMO. Post treatment on day 4, the heart, muscles, liver, kidney, l...
example 2
Evaluation of PMOs Conjugated to a Cell Penetrating Peptide (CPP) and / or a Muscle Specific Homing Peptide (HP) in the MDX Murine Model of Duschenes Muscular Dystrophy
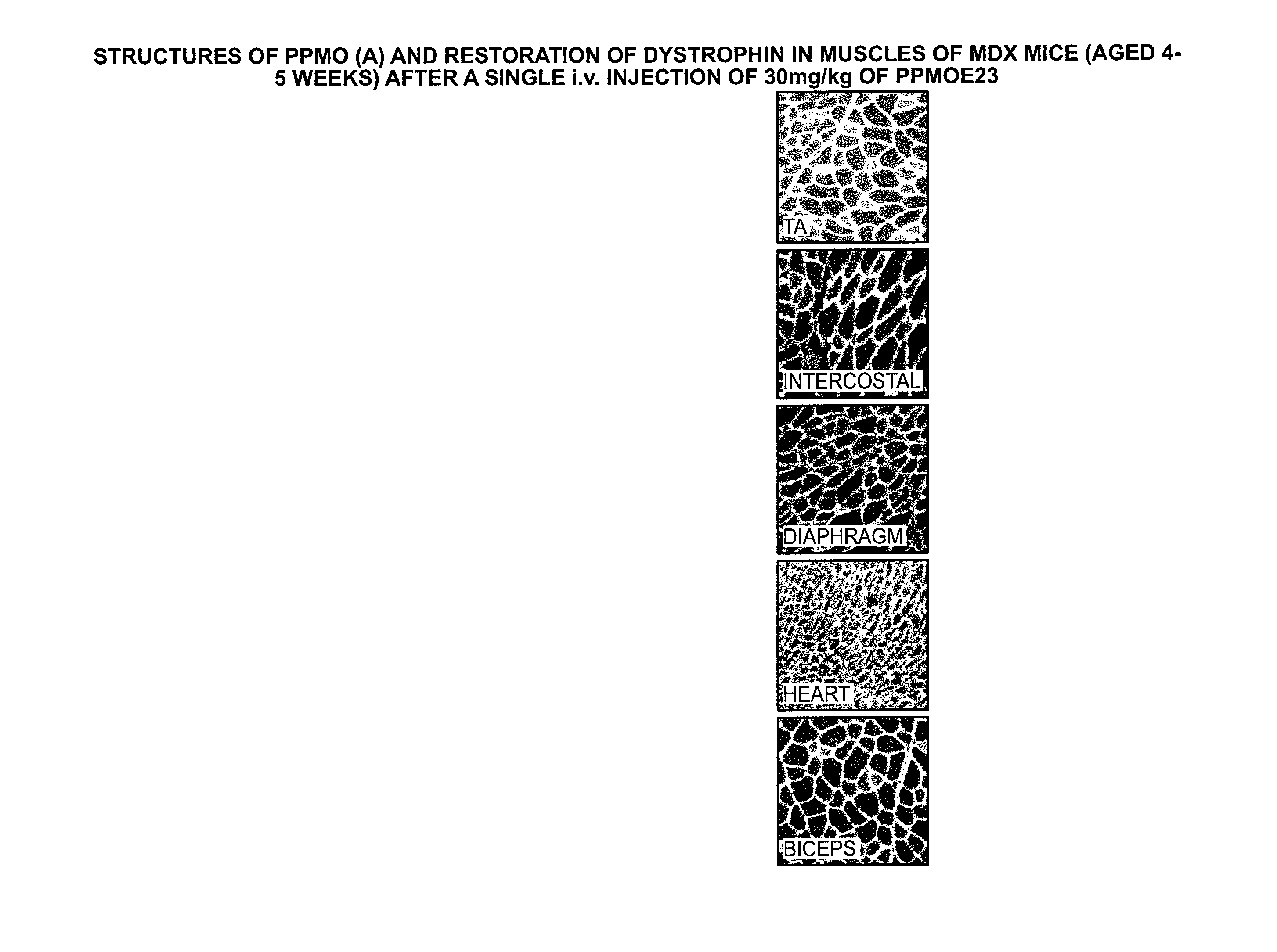
[0171]MDX mice were treated with a series of P-PMO (peptide-conjugated PMOs) containing various combinations of muscle-specific CPPs and HPs conjugated to the M23d antisense PMO. The muscle specific CPP used was the “B peptide”, also designated CP06062 (SEQ ID NO: 19), and the muscle specific homing peptide, designated SMP1, was SEQ ID NO: 51. Four combinations were tested including CP06062-PMO, MSP-PMO, CP06062-MSP-PMO and MSP-CP06062-PMO, whose compositions are shown in the appended Sequence Table. The M23d antisense PMO (SEQ ID NO: 77) has a sequence targeted to induce an exon 23 skip in the murine dystrophin gene and restores functional dystrophin.
[0172]The mice received six weekly intravenous injections of a 3 mg / kg dose. The treated mice were sacrificed and various muscle tissues were removed and stained for full-...
example 3
Improved Cardiac Function in Dystrophin-Deficient Mice by a (RXRRBR)2XB-Conjugated PMO
[0176]It has been demonstrated that a PMO (M23d; SEQ ID NO: 37) targeting the junction of exon 23 and intron 23 of mouse dystrophin (referred to as M23d hereafter), was able to induce up to functional levels of dystrophin expression in some skeletal muscles by regular i.v. injections in mdx mice (Alter, J., F. Lou et al. (2006)). However, dystrophin expression induced by PMO required high doses and was highly variable between muscles and myofibers in terms of observed efficacy. Of greater concern, cardiac muscle seemed to be refractory to the antisense therapy, failing to produce detectable dystrophin even after repeated treatment (seven times at ≈60 mg / kg PMO per injection; Alter, J., F. Lou et al. (2006)). Both potency and cardiac delivery represent major limitations to antisense therapy as an effective treatment for muscle-specific diseases such like DMD, DM1 and DM2. Because DMD patients live l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| conduction properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com