Patents
Literature
68 results about "Dystrophin gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
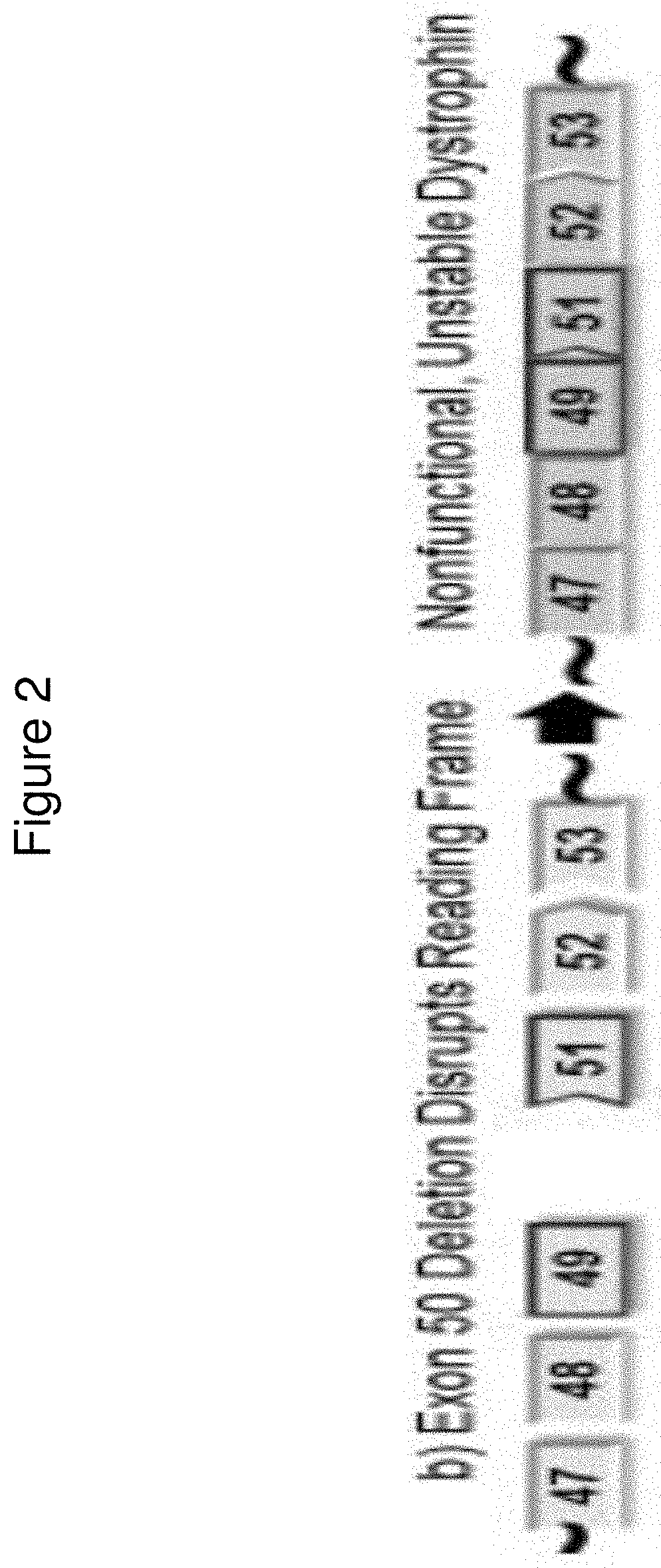
Dystrophin is a very large protein having 3685 amino acids. Duchenne muscular dystrophy is caused by mutations in the very large dystrophin gene, which is too large to transfer when incorporated into the tiny adeno-associated virus.
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 202.
Owner:UNIV OF WESTERN AUSTRALIA
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 202.
Owner:UNIV OF WESTERN AUSTRALIA
Meganuclease variants cleaving a DNA target sequence from the dystrophin gene and uses thereof
InactiveUS20130145487A1Expression is sufficient and stableNo impact on the expression of other genesSugar derivativesBacteriaA-DNANuclease
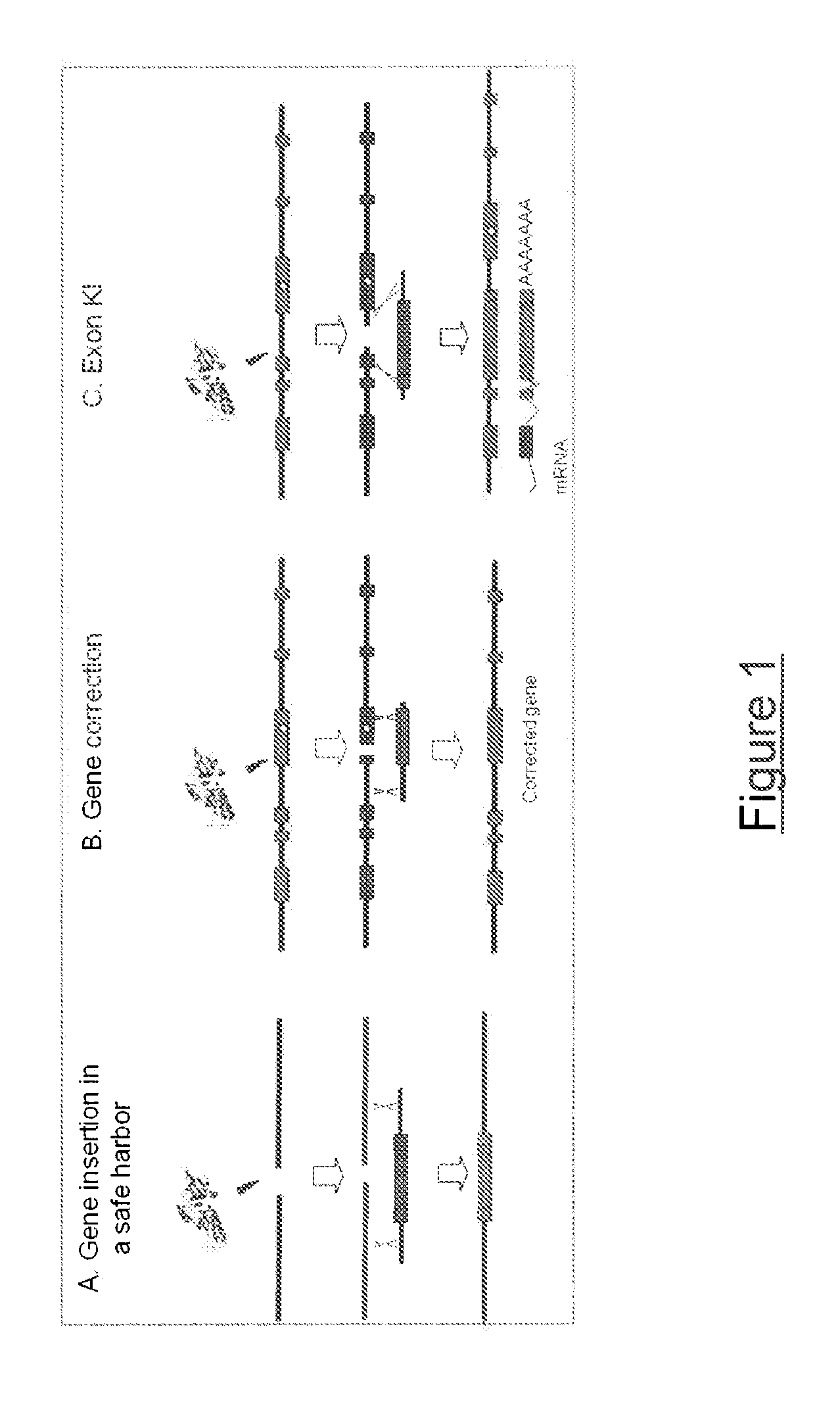
The invention relates to meganuclease variants which cleave a DNA target sequence from the human dystrophin gene (DMD), to vectors encoding such variants, to a cell, an animal or a plant modified by such vectors and to the use of these meganuclease variants and products derived therefrom for genome therapy, ex vivo (gene cell therapy) and genome engineering including therapeutic applications and cell line engineering. The invention also relates to the use of meganuclease variants for inserting therapeutic transgenes other than DMD at the dystrophin gene locus, using this locus as a safe harbor locus. The invention also relates to the use of meganuclease variants for using the dystrophin gene locus as a landing pad to insert and express genes of interest.
Owner:CELLECTIS SA
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 202.
Owner:UNIV OF WESTERN AUSTRALIA
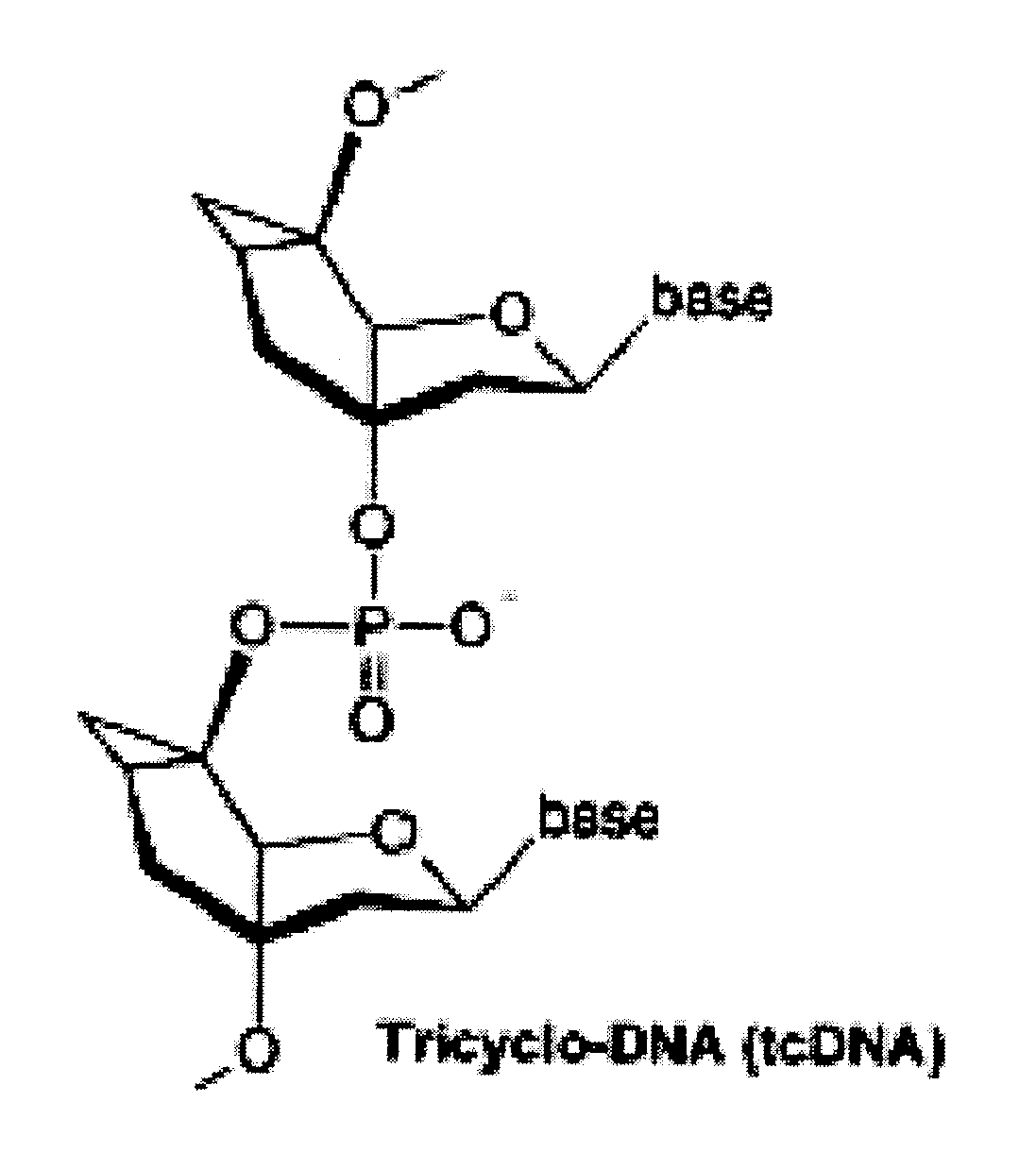
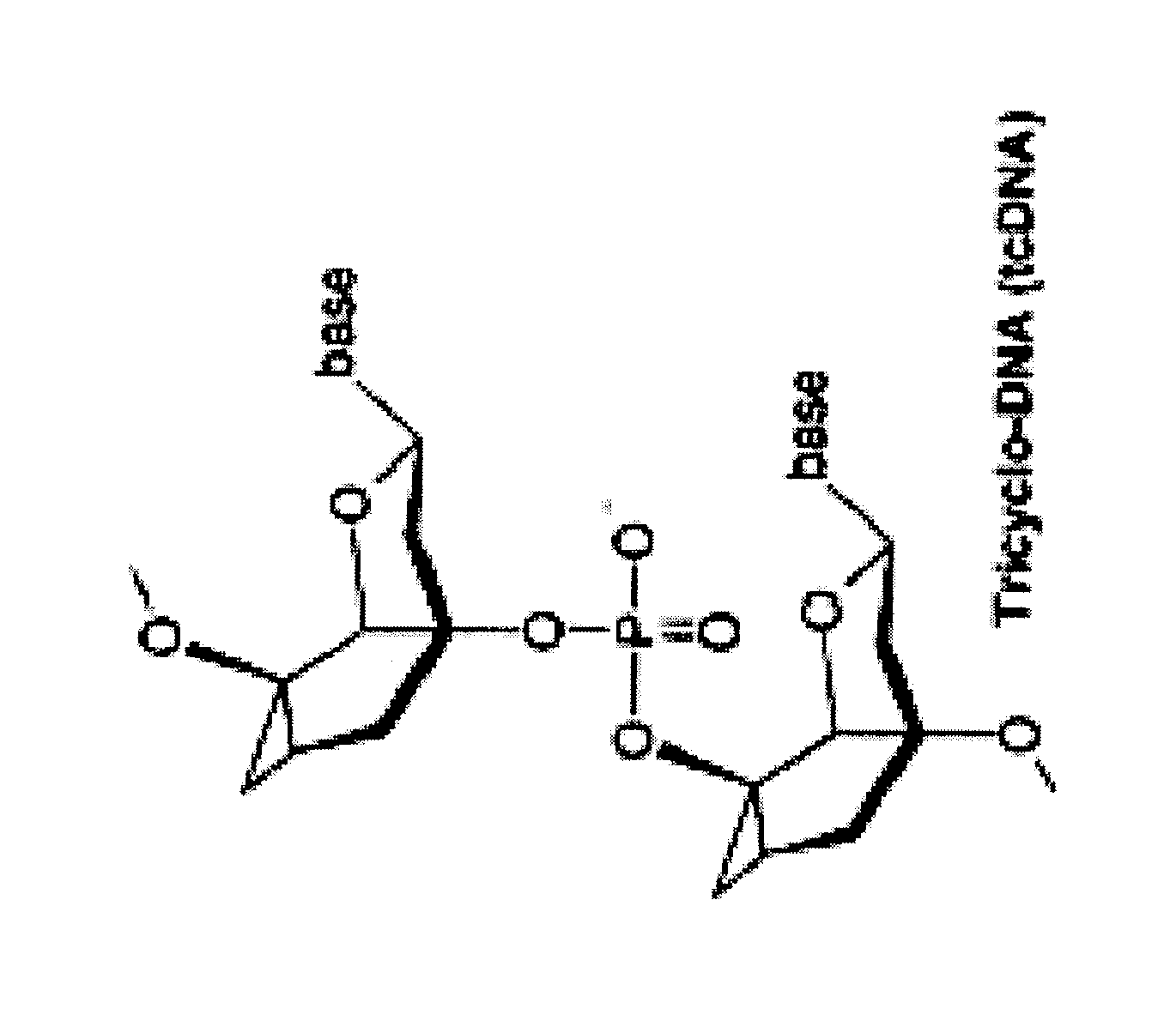
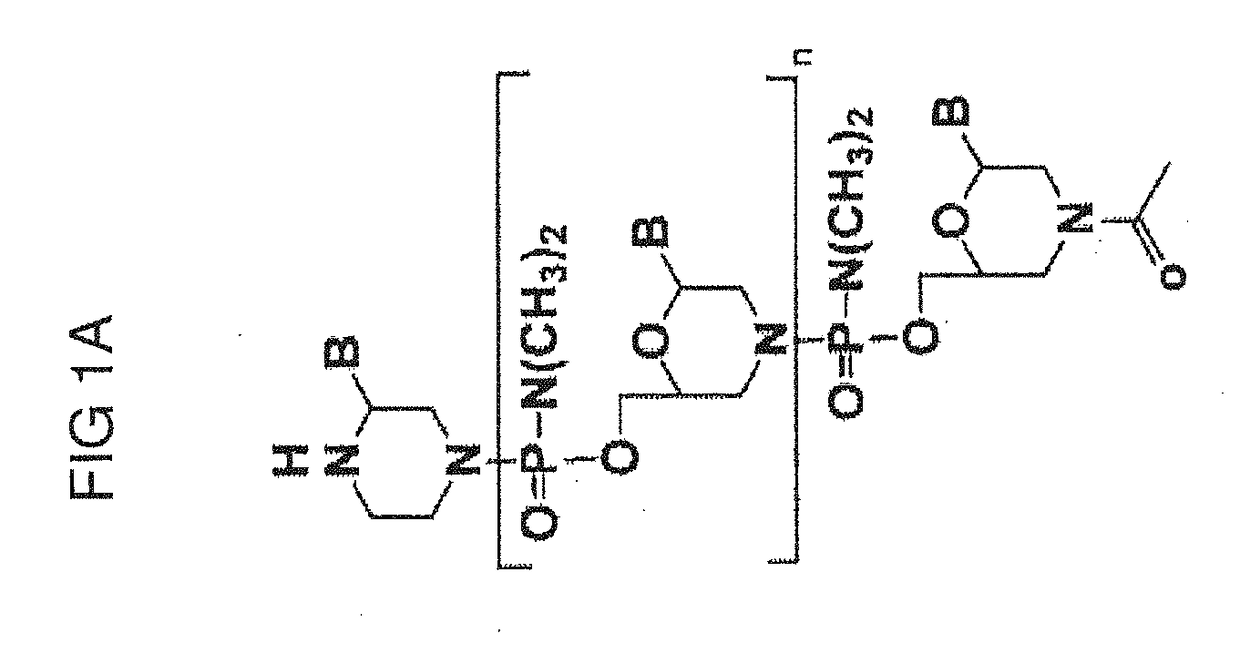
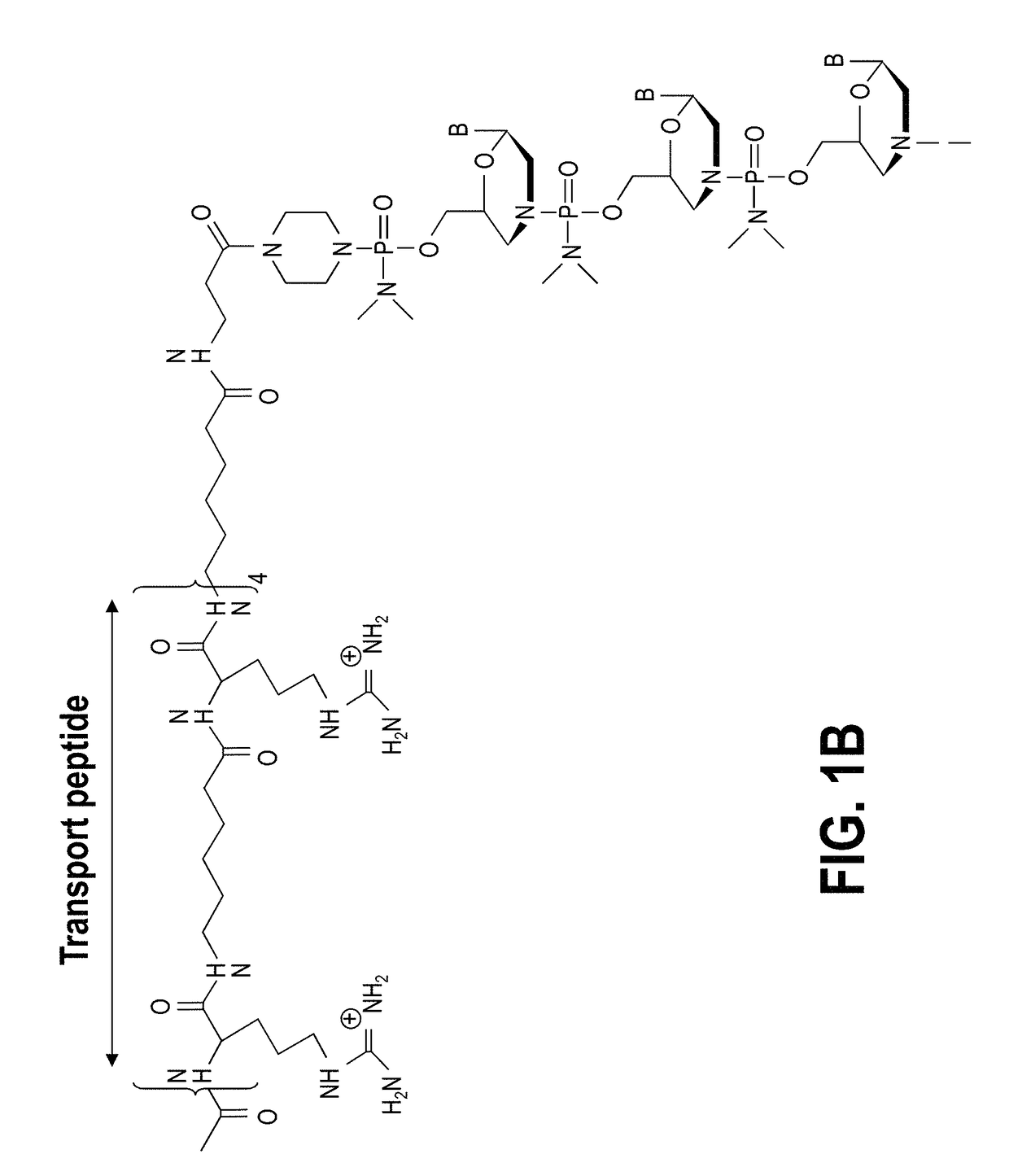
Tricyclo-dna antisense oligonucleotides, compositions, and methods for the treatment of disease
InactiveUS20120149756A1Find utilityFacilitates inclusionOrganic active ingredientsSplicing alterationDiseasePre mrna processing
Provided are tricyclo-DNA (tc-DNA) AON and methods employing tc-DNA AON for modifying splicing events that occur during pre-mRNA processing. Tricyclo-DNA (tc-DNA) AON are described that may be used to facilitate exon skipping or to mask intronic silencer sequences and / or terminal stem-loop sequences during pre-mRNA processing and to target RNase-mediated destruction of processed mRNA. Tc-DNA AON described herein may be used in methods for the treatment of Duchenne Muscular Dystrophy by skipping a mutated exon 23 or exon 51 within a dystrophin gene to restore functionality of a dystrophin protein; in methods for the treatment of Spinal Muscular Atrophy by masking an intronic silencing sequence and / or a terminal stem-loop sequence within an SMN2 gene to yield modified functional SMN2 protein, including an amino acid sequence encoded by exon 7, which is capable of at least partially complementing a non-functional SMN1 protein; and in methods for the treatment of Steinert's Myotonic Dystrophy by targeting the destruction of a mutated DM1 mRNA comprising 3′-terminal CUG repeats.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Antisense nucleic acids
ActiveUS20130211062A1Improve efficiencyOrganic active ingredientsSplicing alterationOligomerAntisense nucleic acid
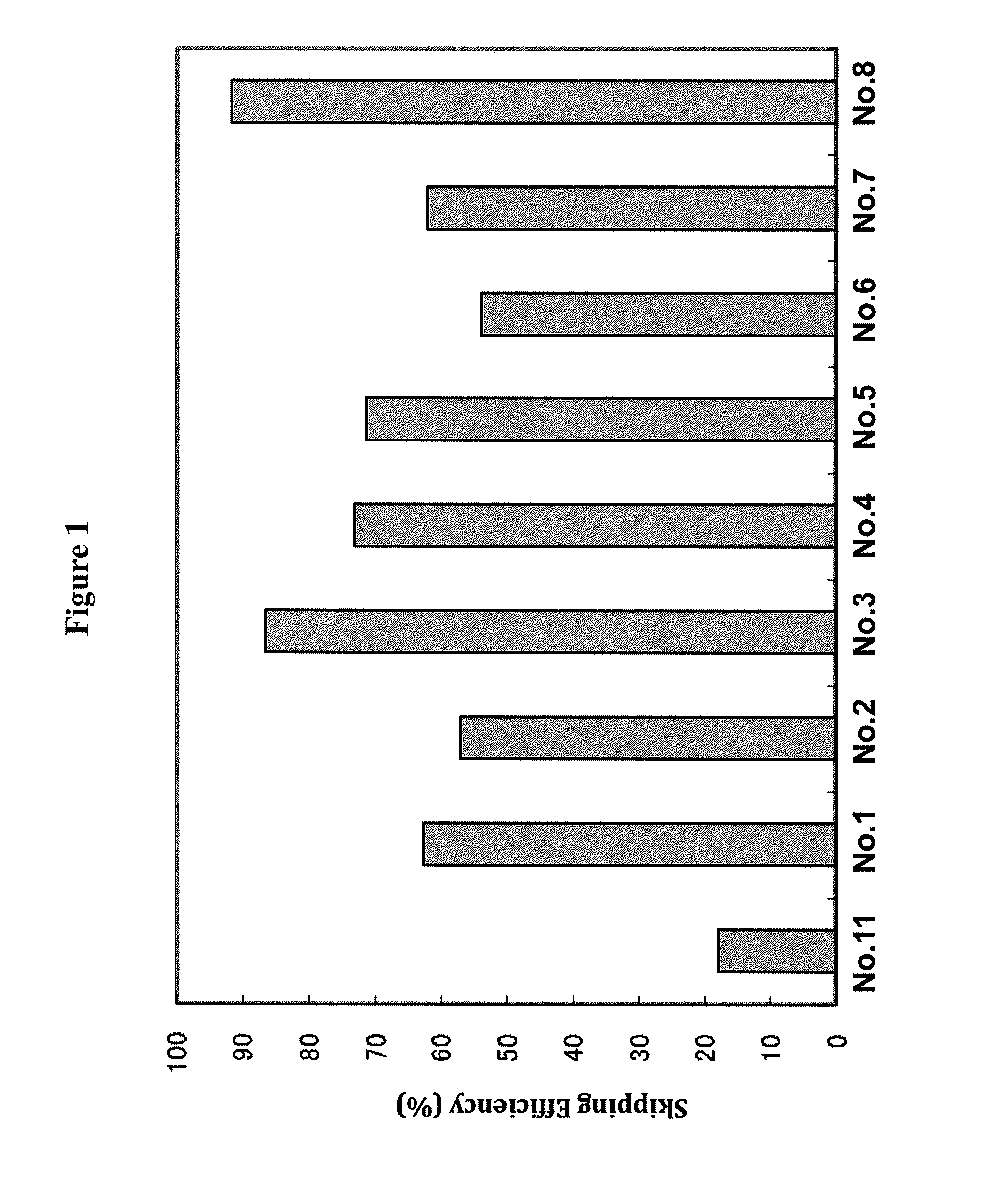
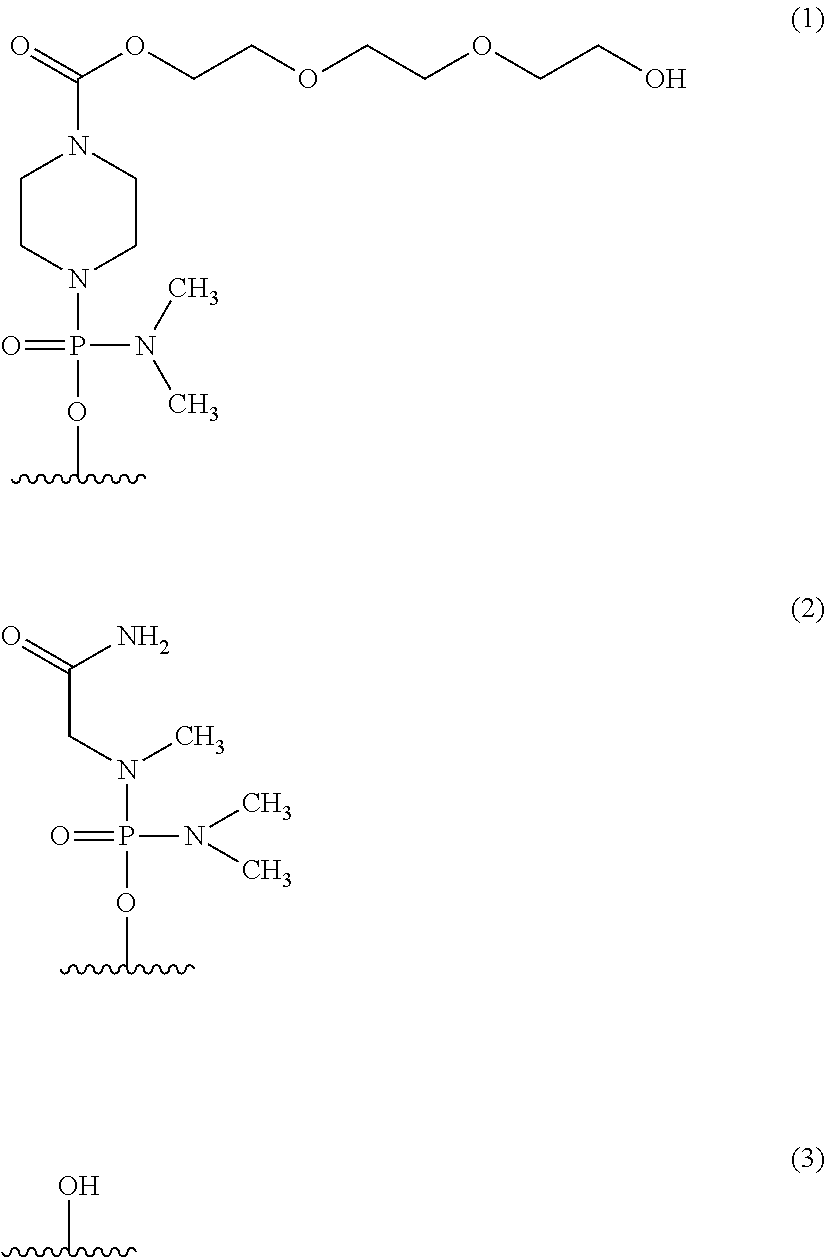
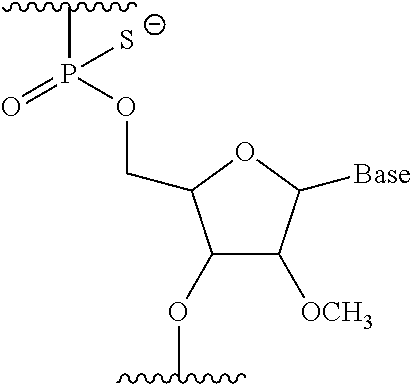
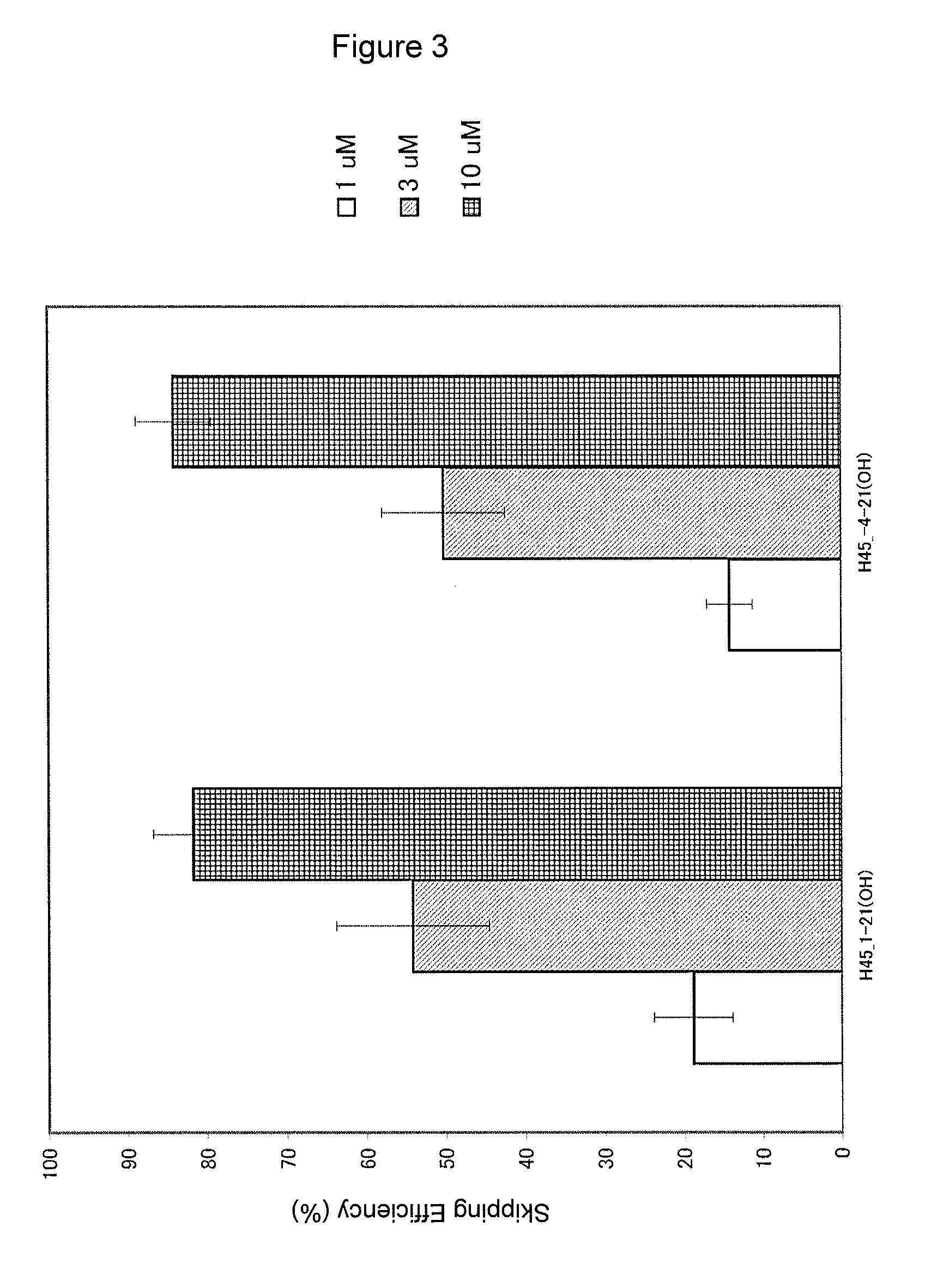
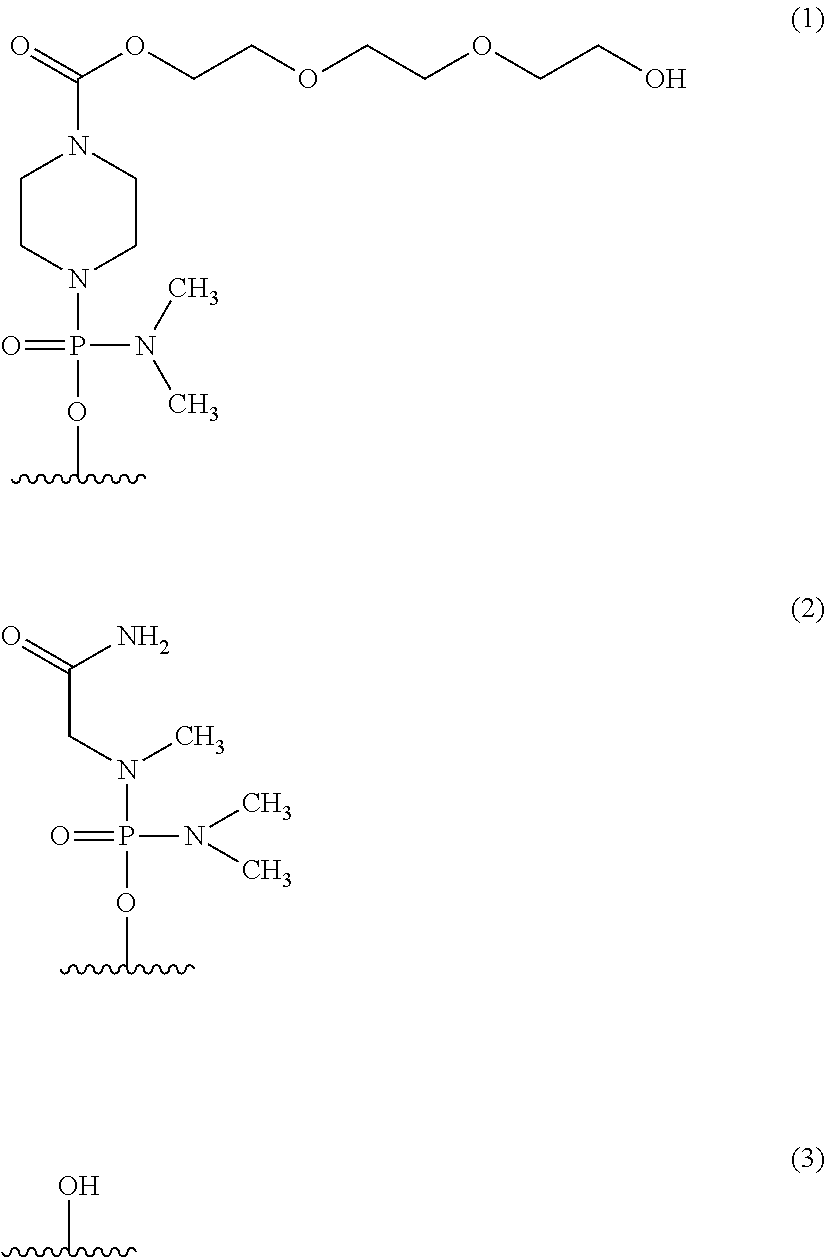
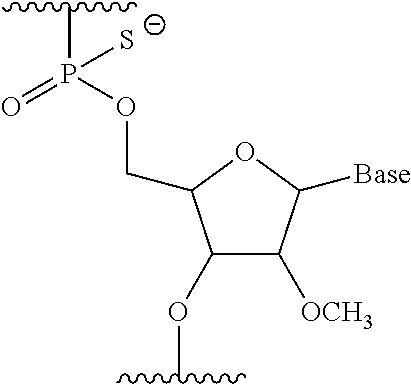
The present invention provides a pharmaceutical composition which causes skipping of the 53rd exon in the human dystrophin gene with a high efficiency.The present invention provides an oligomer which efficiently enables to cause skipping of the 53rd exon in the human dystrophin gene.
Owner:NIPPON SHINYAKU CO LTD +1
Antisense nucleic acids
ActiveUS20140343266A1Improve efficiencyExcellent skipping efficiencyOrganic active ingredientsSplicing alterationAntisense nucleic acidGene
The present invention provides a pharmaceutical agent which causes skipping of the 55th, 45th, 50th or 44th exon in the human dystrophin gene with a high efficiency.The present invention provides an oligomer which efficiently enables to cause skipping of the 55th, 45th, 50th or 44th exon in the human dystrophin gene.
Owner:NIPPON SHINYAKU CO LTD +1
Antisense molecules and methods for treating pathologies
ActiveUS20120270925A1Specific and efficient exon skippingOrganic active ingredientsSplicing alterationExon skippingCell biology
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 59.
Owner:UNIV OF WESTERN AUSTRALIA
Exon skipping compositions for treating muscular dystrophy
InactiveUS20140315977A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationDuchenne muscular dystrophyMuscular dystrophy
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 53 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Exon skipping compositions for treating muscular dystrophy
InactiveUS20160040162A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationDuchenne muscular dystrophyMuscular dystrophy
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 53 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Exon skipping compositions for treating muscular dystrophy
ActiveUS20140323544A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationMedicineDystrophin
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 44 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Exon skipping compositions for treating muscular dystrophy
InactiveUS20140329881A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationDuchenne muscular dystrophyMuscular dystrophy
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 44 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Exon skipping compositions for treating muscular dystrophy
InactiveUS20150361428A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideSplicing alterationSugar derivativesDuchenne muscular dystrophyMuscular dystrophy
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 53 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Antisense nucleic acids
ActiveUS9988629B2Improve efficiencyOrganic active ingredientsSplicing alterationAntisense nucleic acidGene
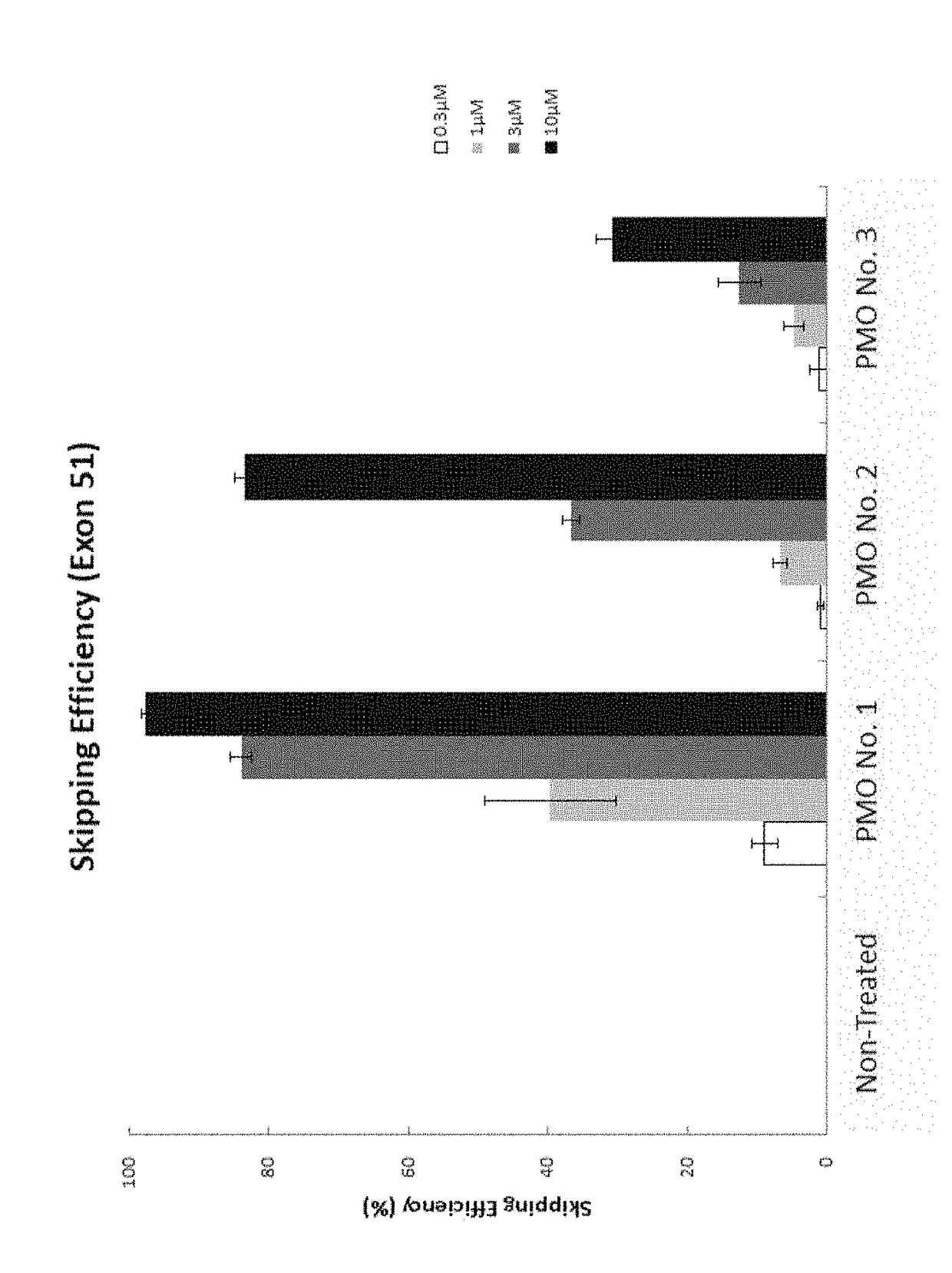
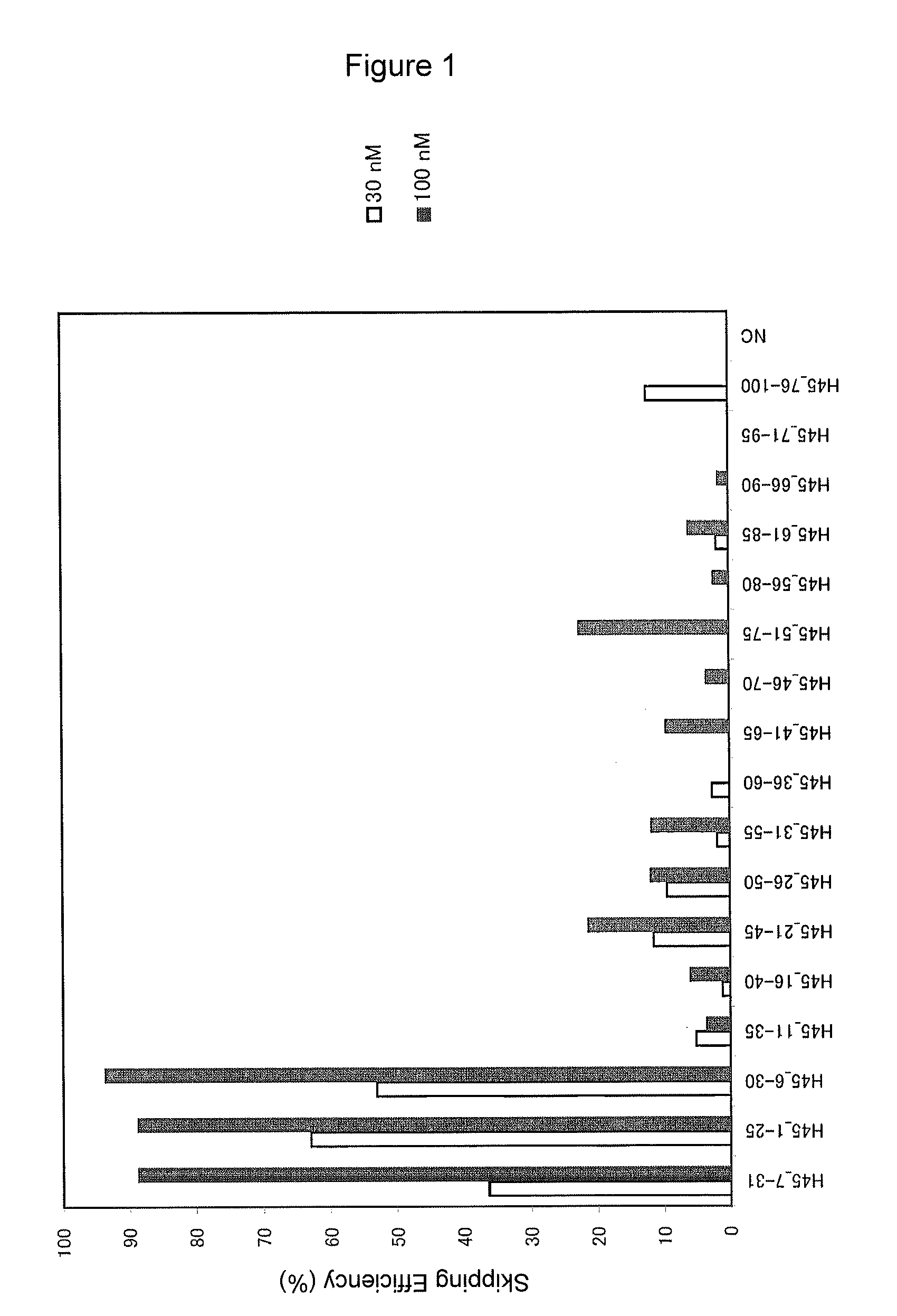
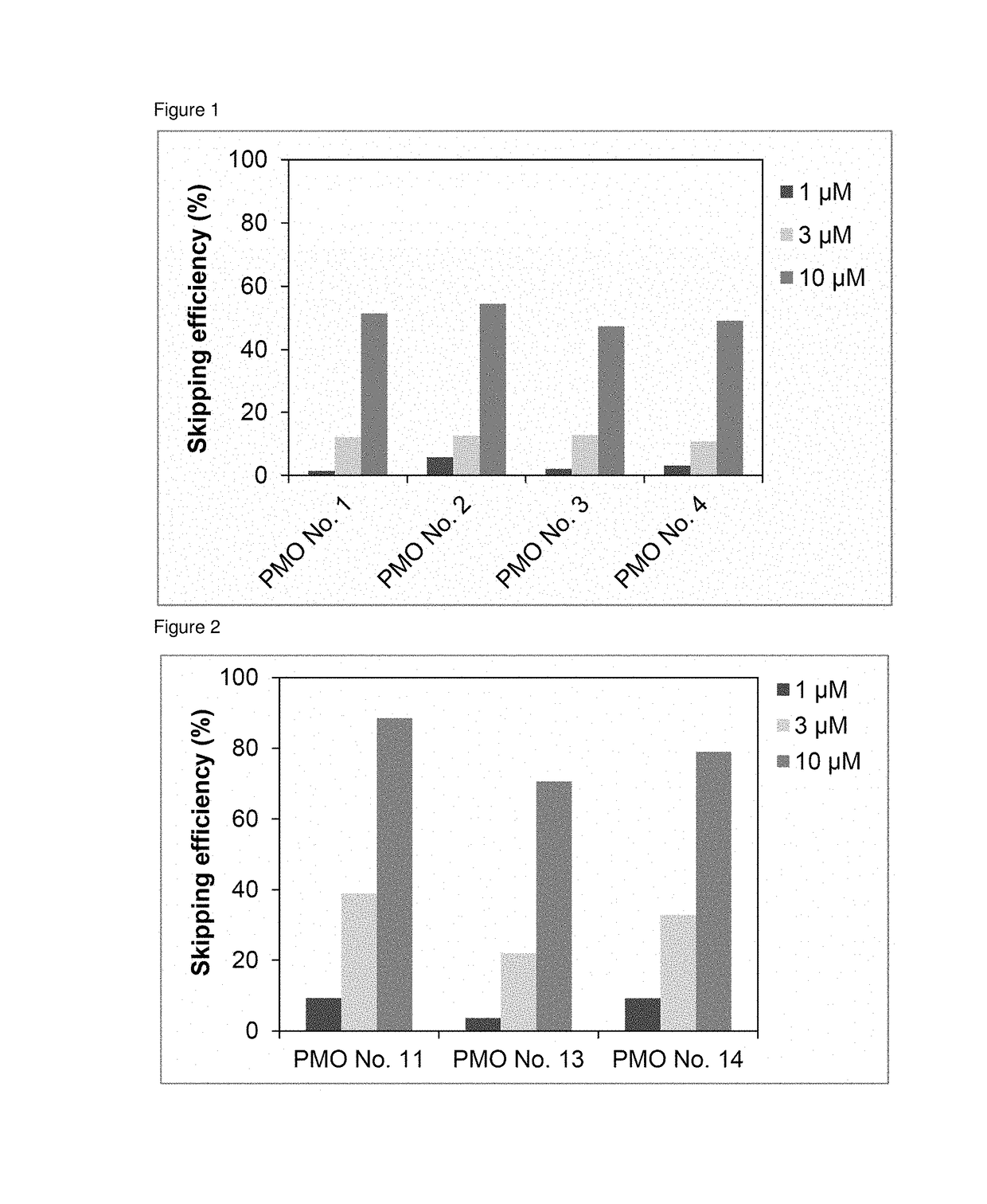
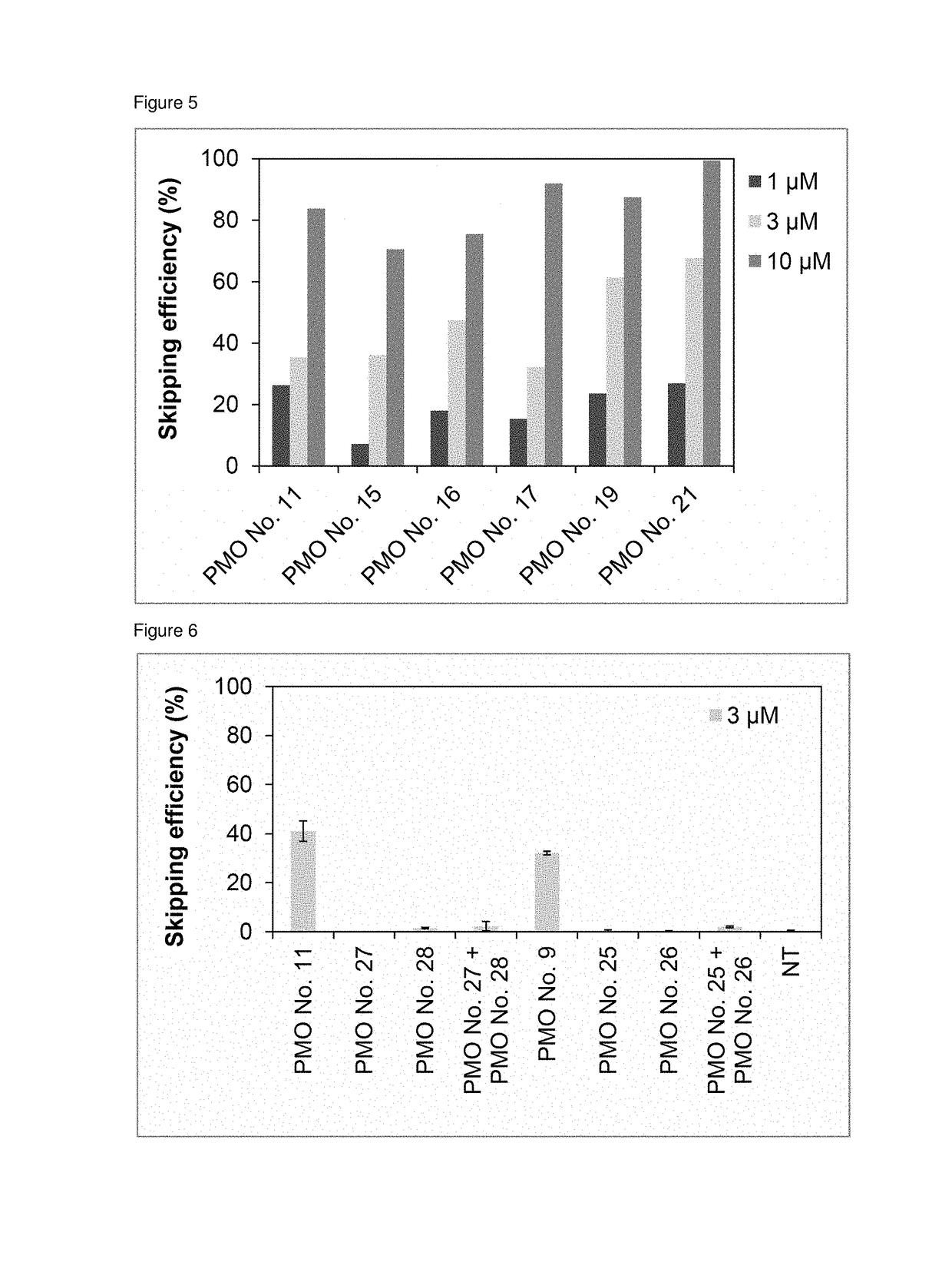
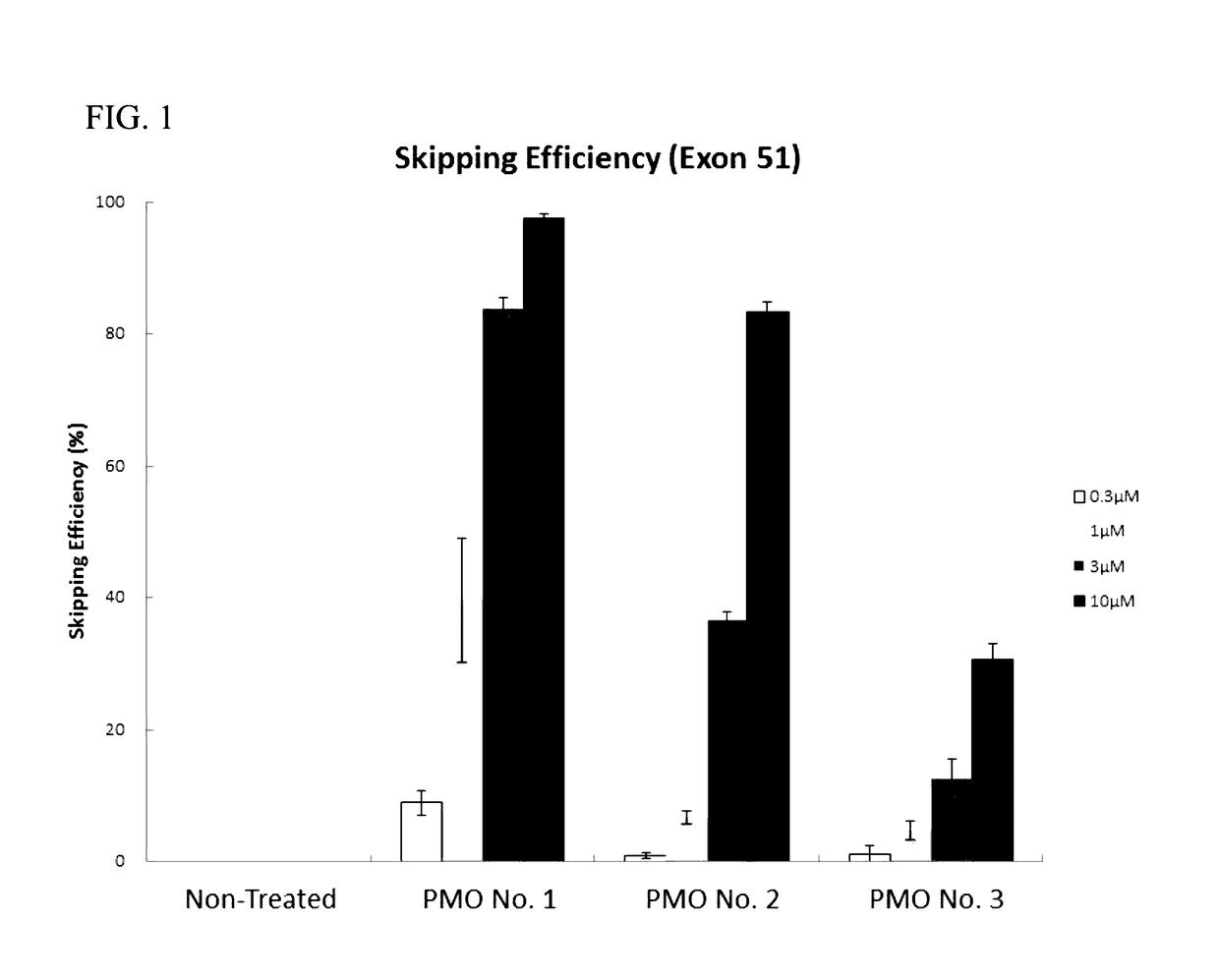
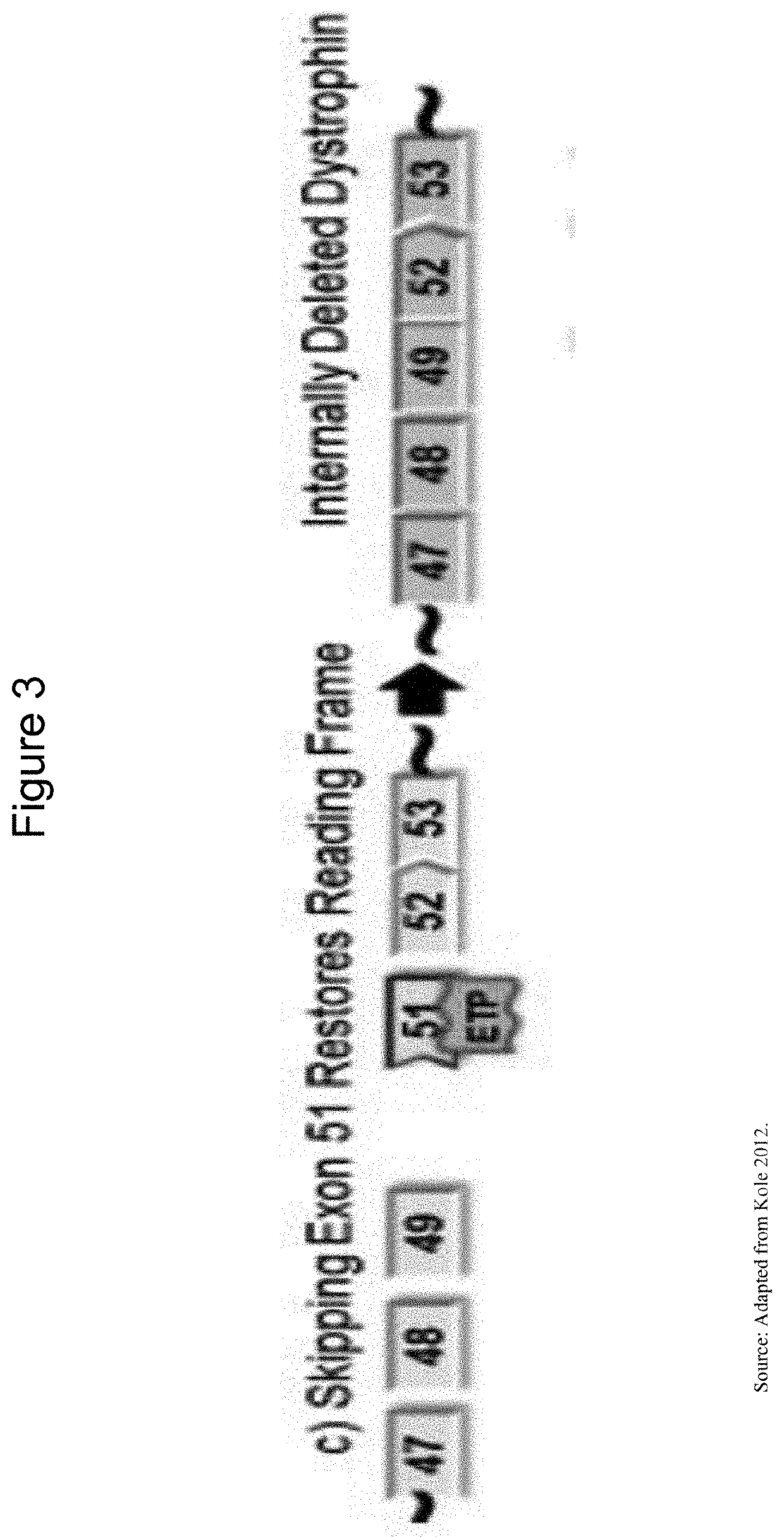
Provided is a drug that allows highly-efficient skipping of exon 51 in the human dystrophin gene. The present invention provides an antisense oligomer which enables exon 51 in the human dystrophin gene to be skipped.
Owner:NIPPON SHINYAKU CO LTD +1
Antisense nucleic acids
ActiveUS9512424B2Improve efficiencyShorten the lengthOrganic active ingredientsSplicing alterationOligomerAntisense nucleic acid
The present invention provides a pharmaceutical agent which causes skipping of the 55th, 45th, 50th or 44th exon in the human dystrophin gene with a high efficiency. The present invention provides an oligomer which efficiently enables to cause skipping of the 55th, 45th, 50th or 44th exon in the human dystrophin gene.
Owner:NIPPON SHINYAKU CO LTD +1
Antisense nucleic acids
ActiveUS10144931B2Effectively alleviatedEfficient inductionSugar derivativesGenetic material ingredientsGeneBioinformatics
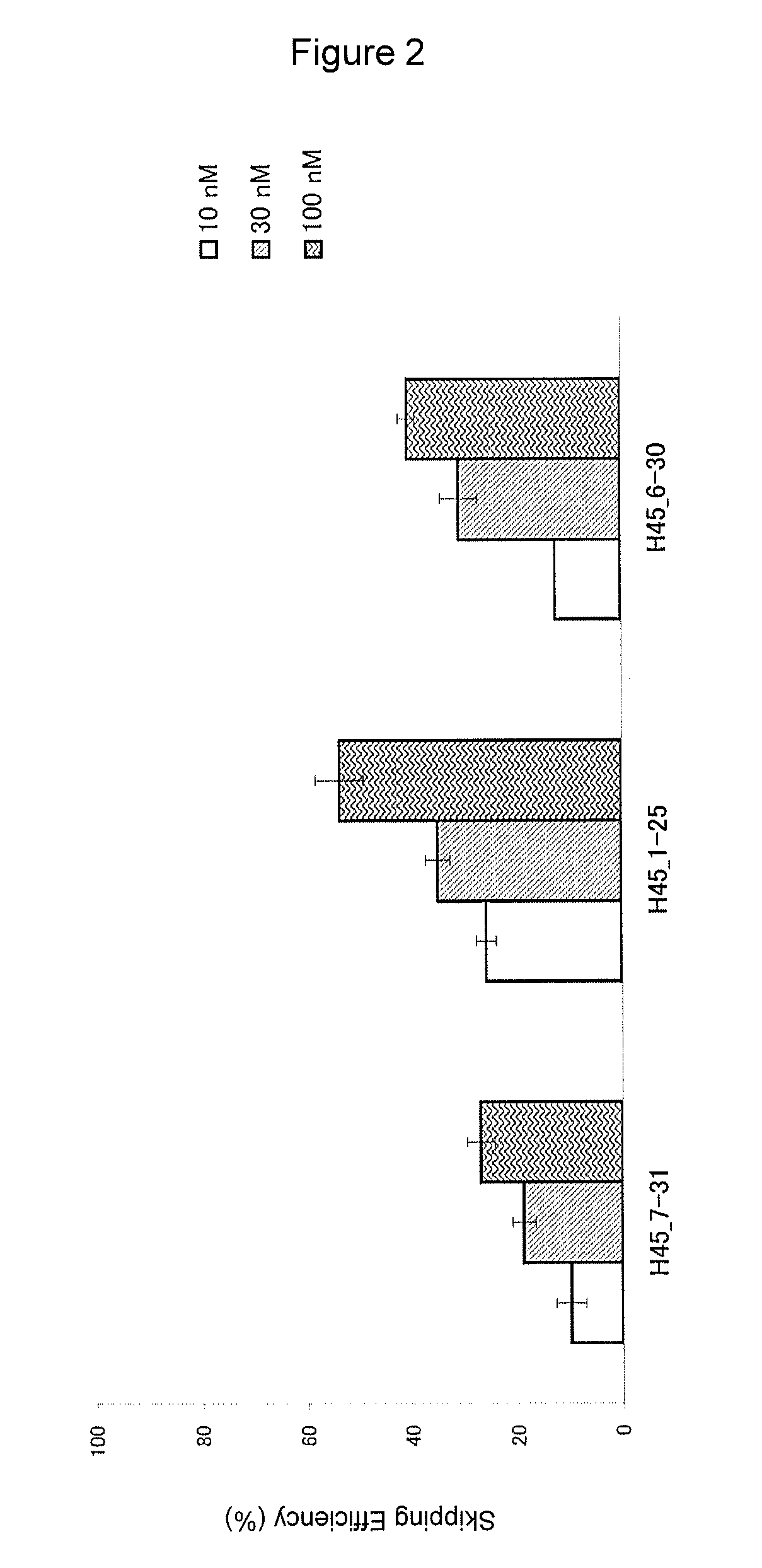
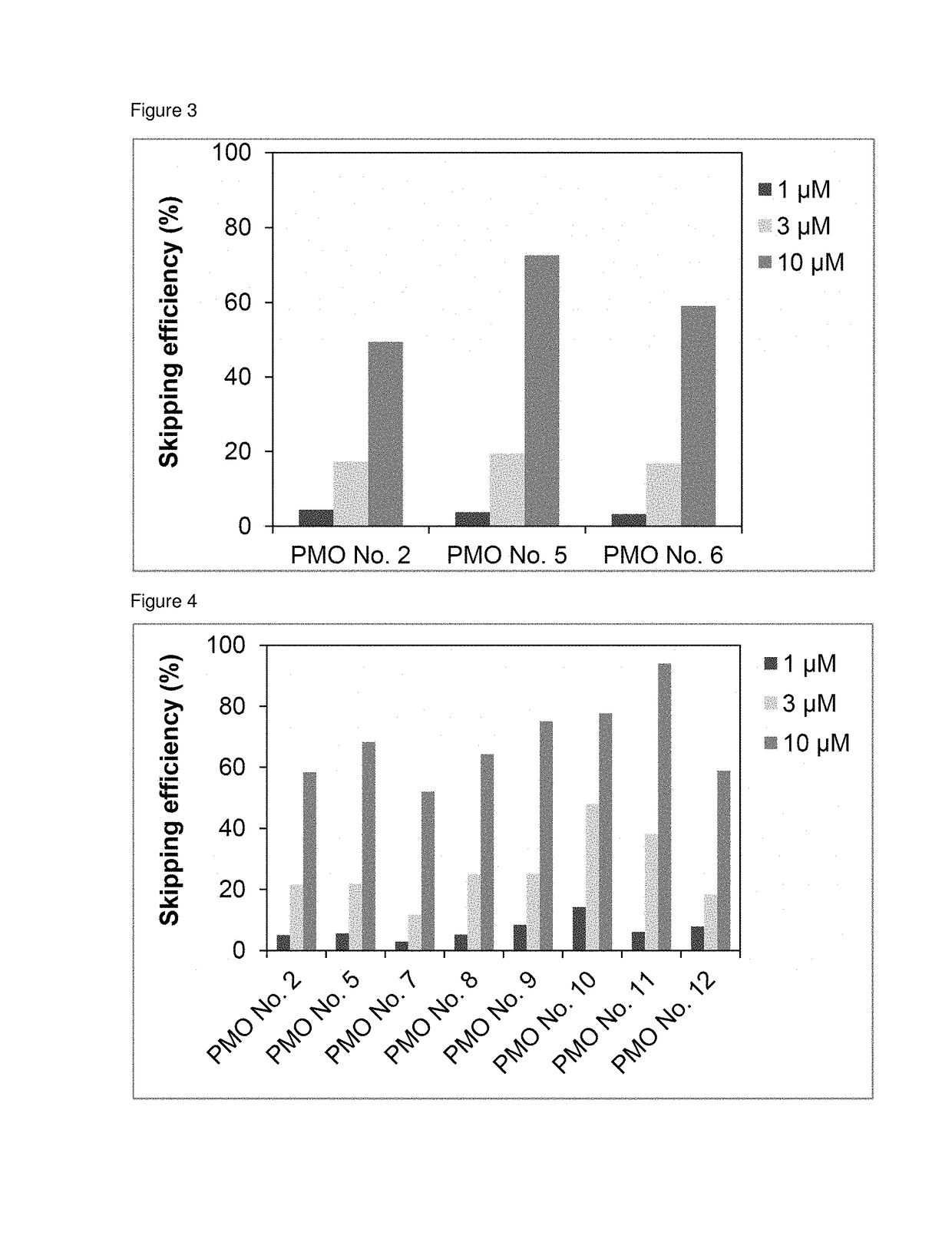
The present invention provides an oligomer which allows exon 45 skipping in the human dystrophin gene.
Owner:NIPPON SHINYAKU CO LTD +1
Retinal dystrophin transgene and methods of use thereof
InactiveUS20080044393A1Alleviation of muscular dystrophy symptomReduce the possibilityBiocideNervous disorderBehavioral studyTransgene
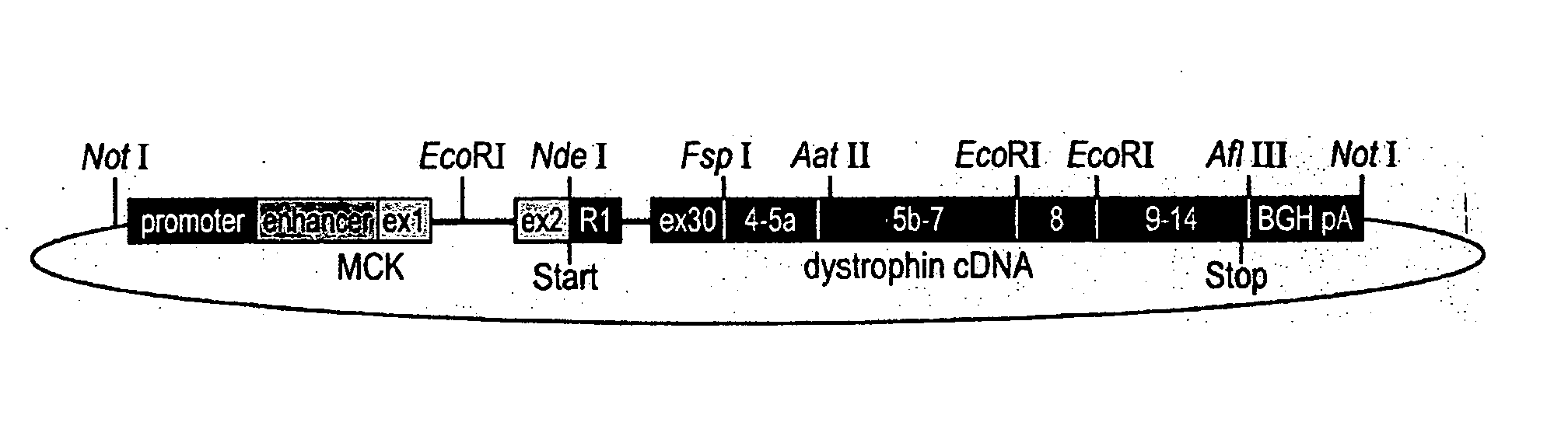
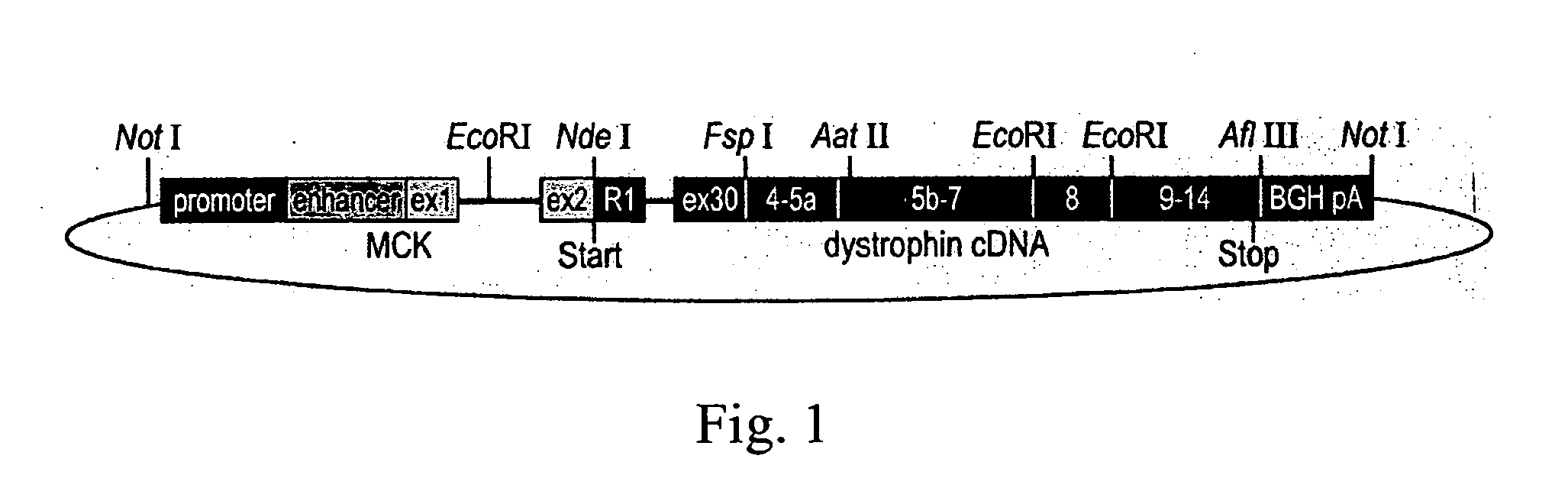
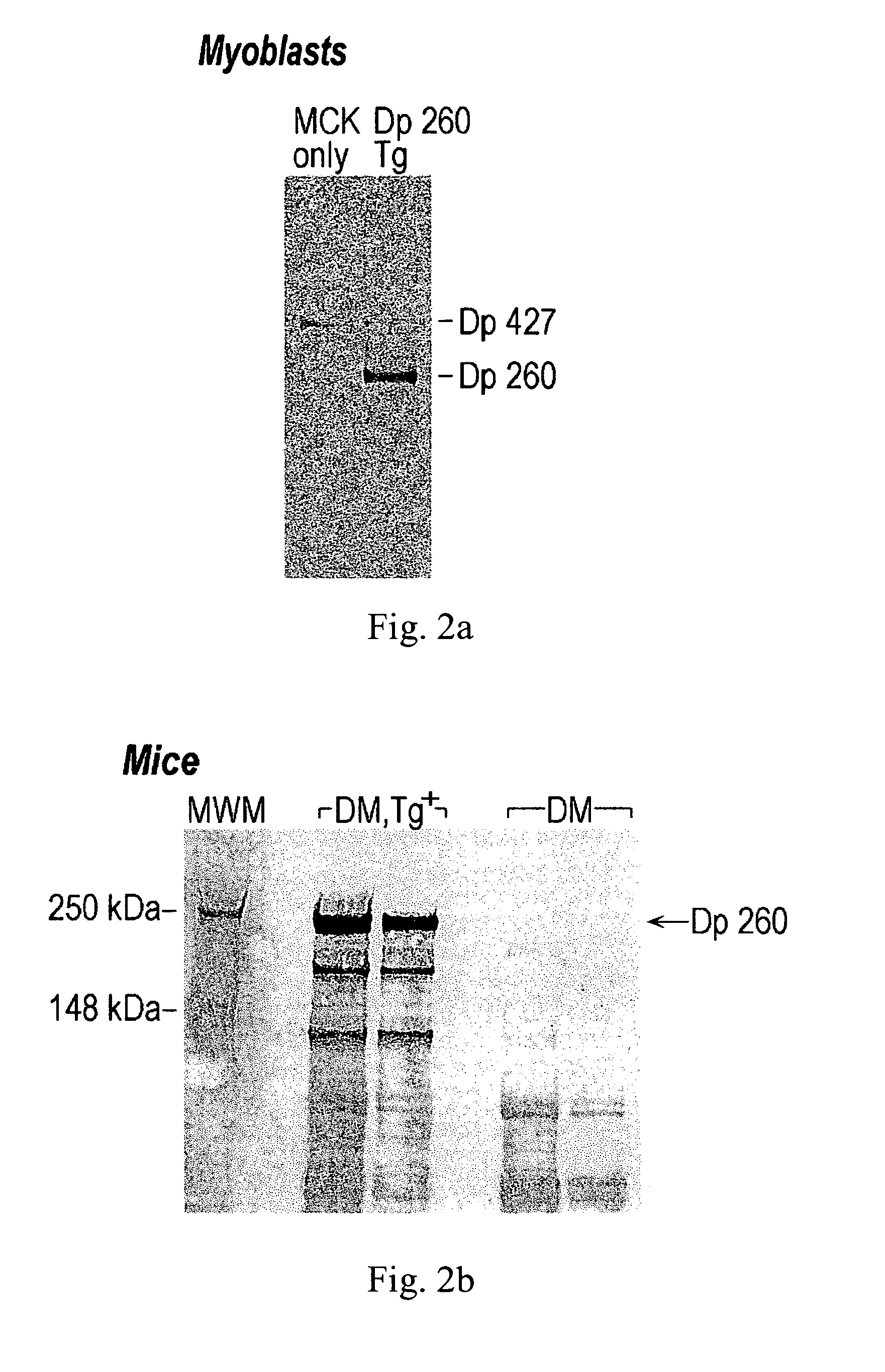
Duchenne muscular dystrophy (DMD) is a progressive muscle disease that is caused by severe defects in the dystrophin gene and results in the patient's death by the third decade. The present invention utilizes the Double Mutant mice (DM) as an appropriate human model for DMD as these mice are deficient for both dystrophin and utrophin (mdx / +, utrn − / −), die at 3 months of age and suffer from severe muscle weakness, pronounced growth retardation, kyphosis, weight loss, slack posture, and immobility. Expression from a transgene of novel human retinal dystrophin Dp260 was shown to prevent premature death and reduce the severe muscular dystrophy phenotype to a mild clinical myopathy. Electromyography, histology, radiography, magnetic resonance imaging, and behavior studies concluded that DM transgenic mice grew normally, had normal spinal curvature and mobility, and had reduced muscle pathology. EMG and histologic data from transgenic DM mice showed decreased abnormalities to levels typical of mild myopathy, while the DM mice exhibited severe abnormalities commonly seen in human dystrophinopathies. The transgenic DM mice also had measurable movement levels comparable to those of untreated mdx mice and controls.
Owner:WHITE ROBERT L +2
Modification of the dystrophin gene and uses thereof
InactiveUS20180265859A1Restoring correct reading frameAvoid large deletionsOrganic active ingredientsSugar derivativesMuscular dystrophyFrameshift mutation
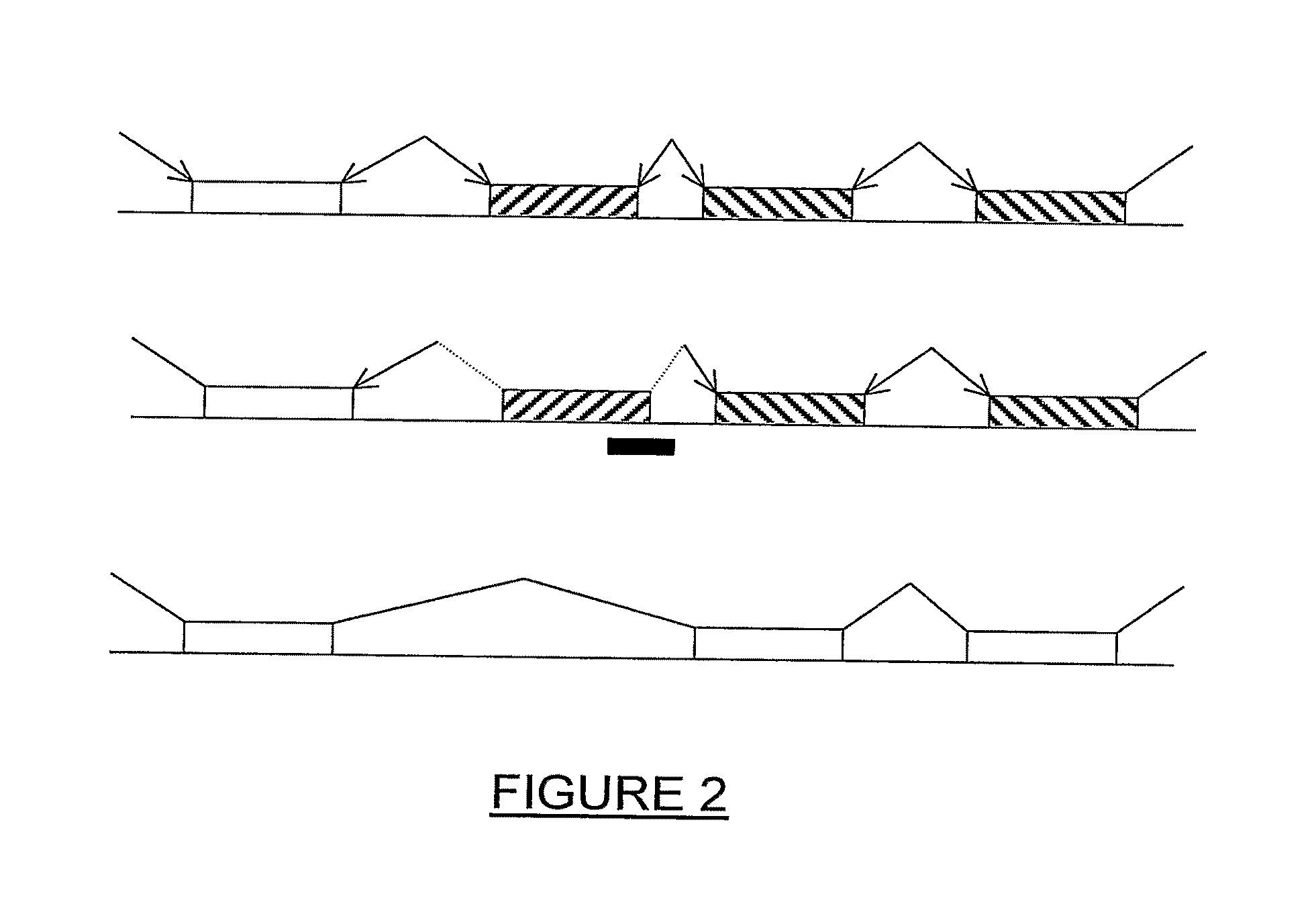
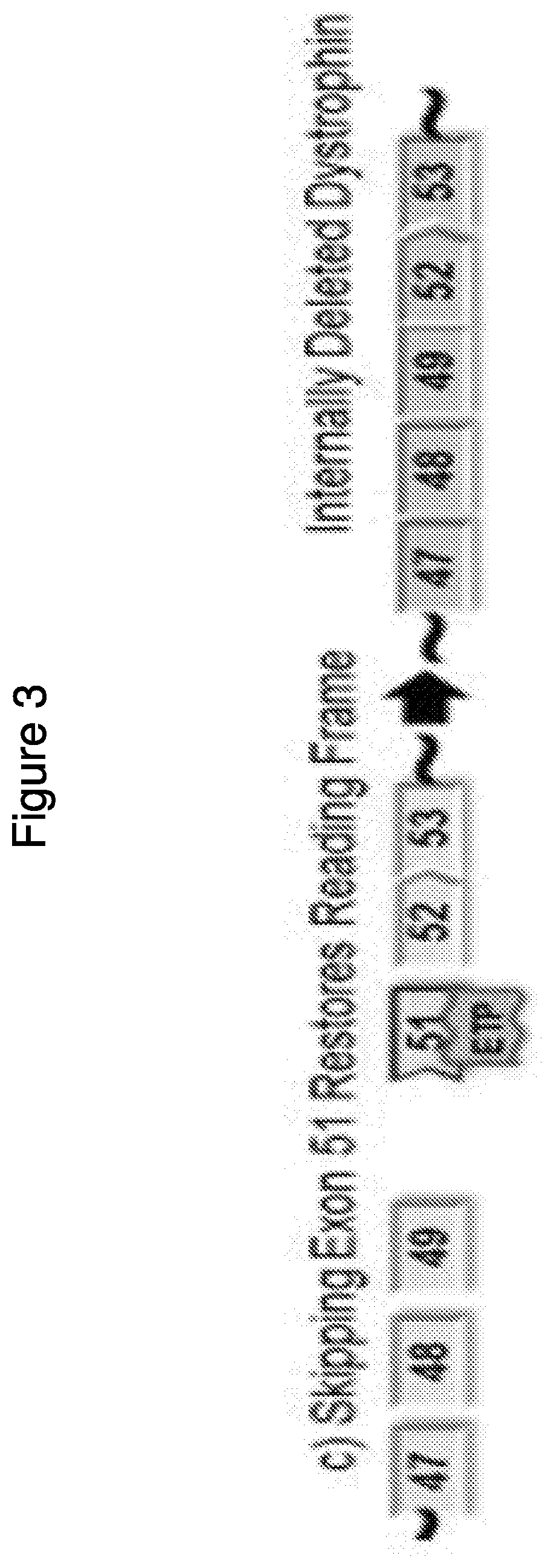
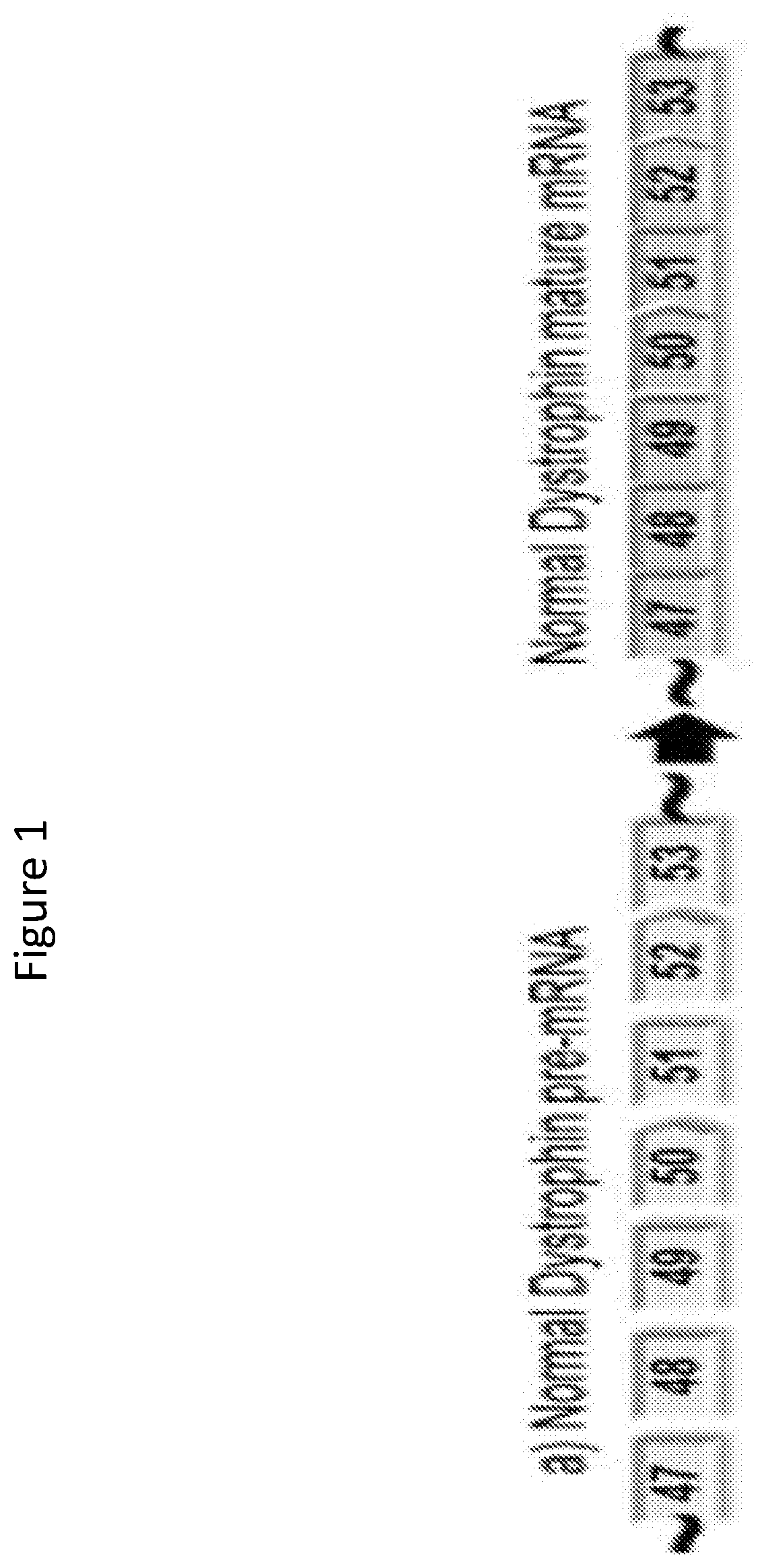
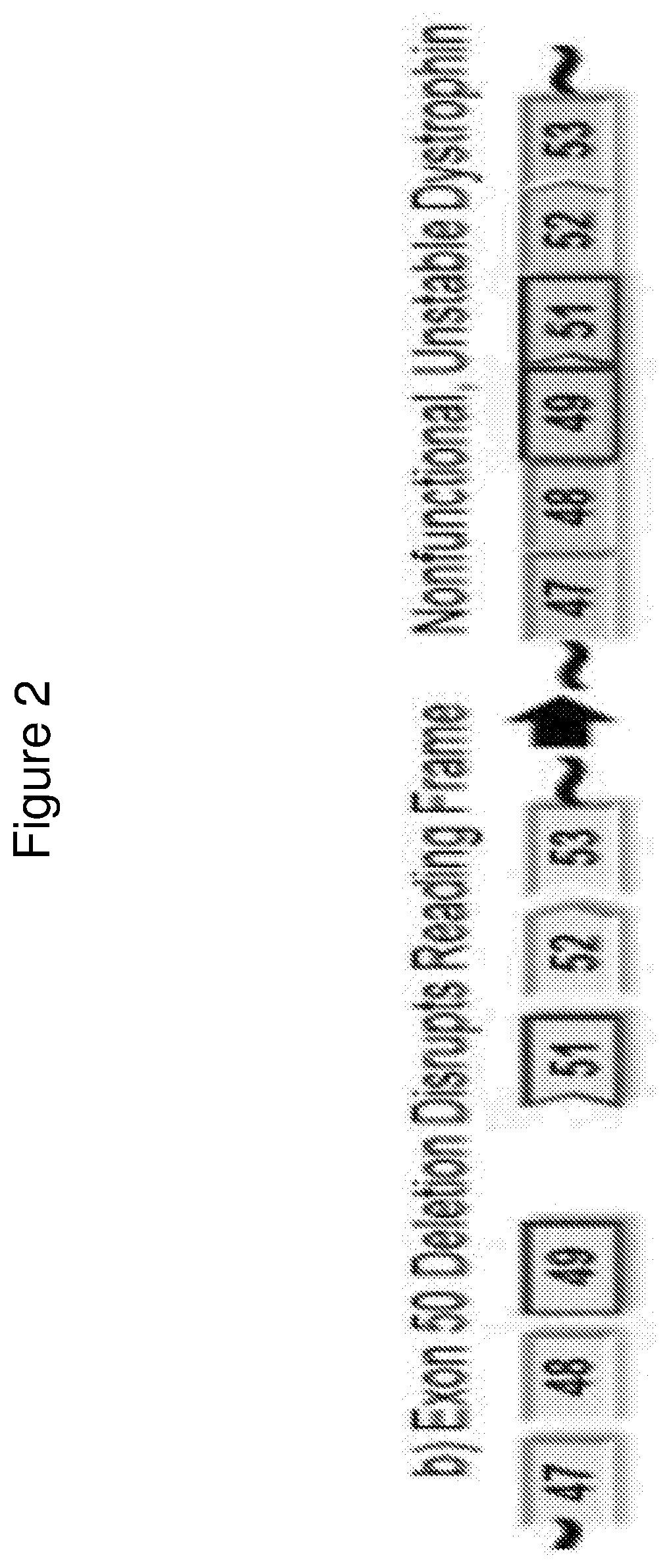
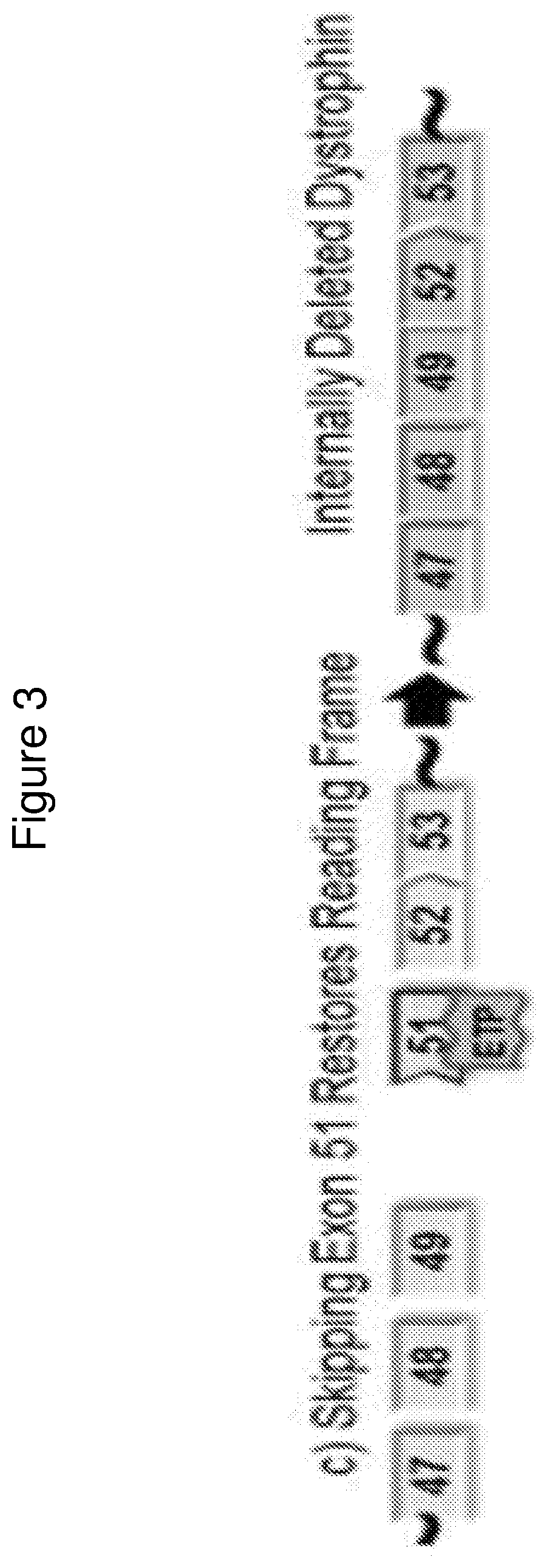
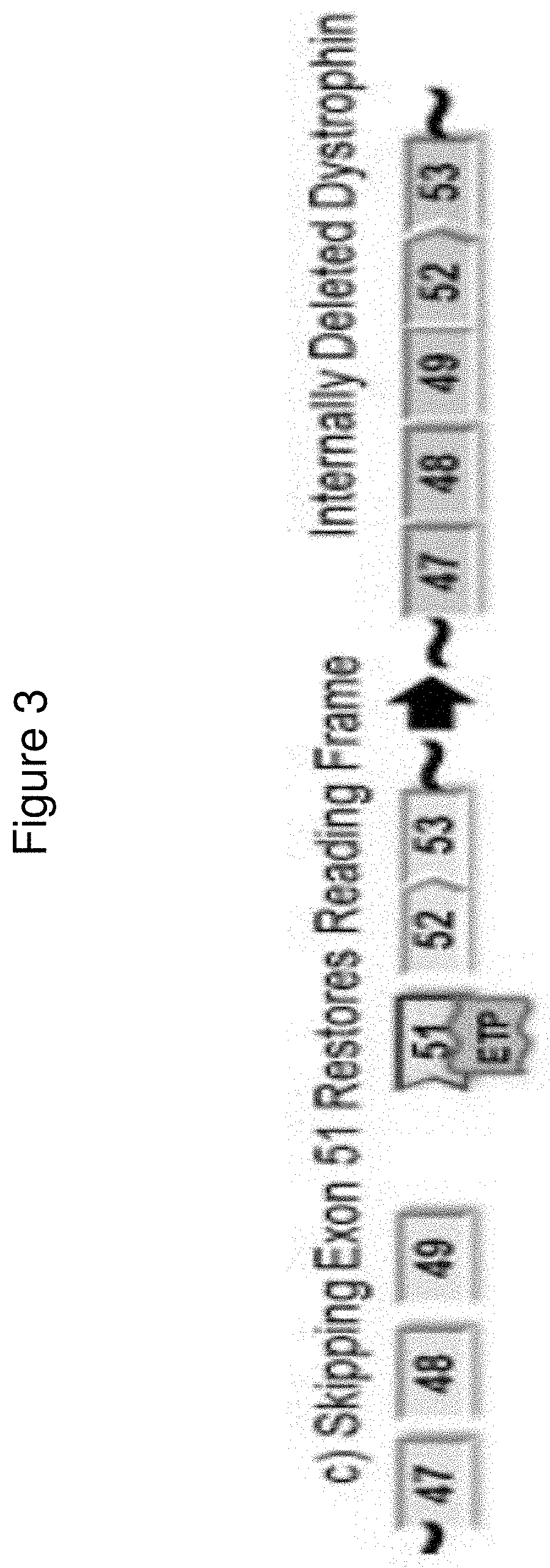
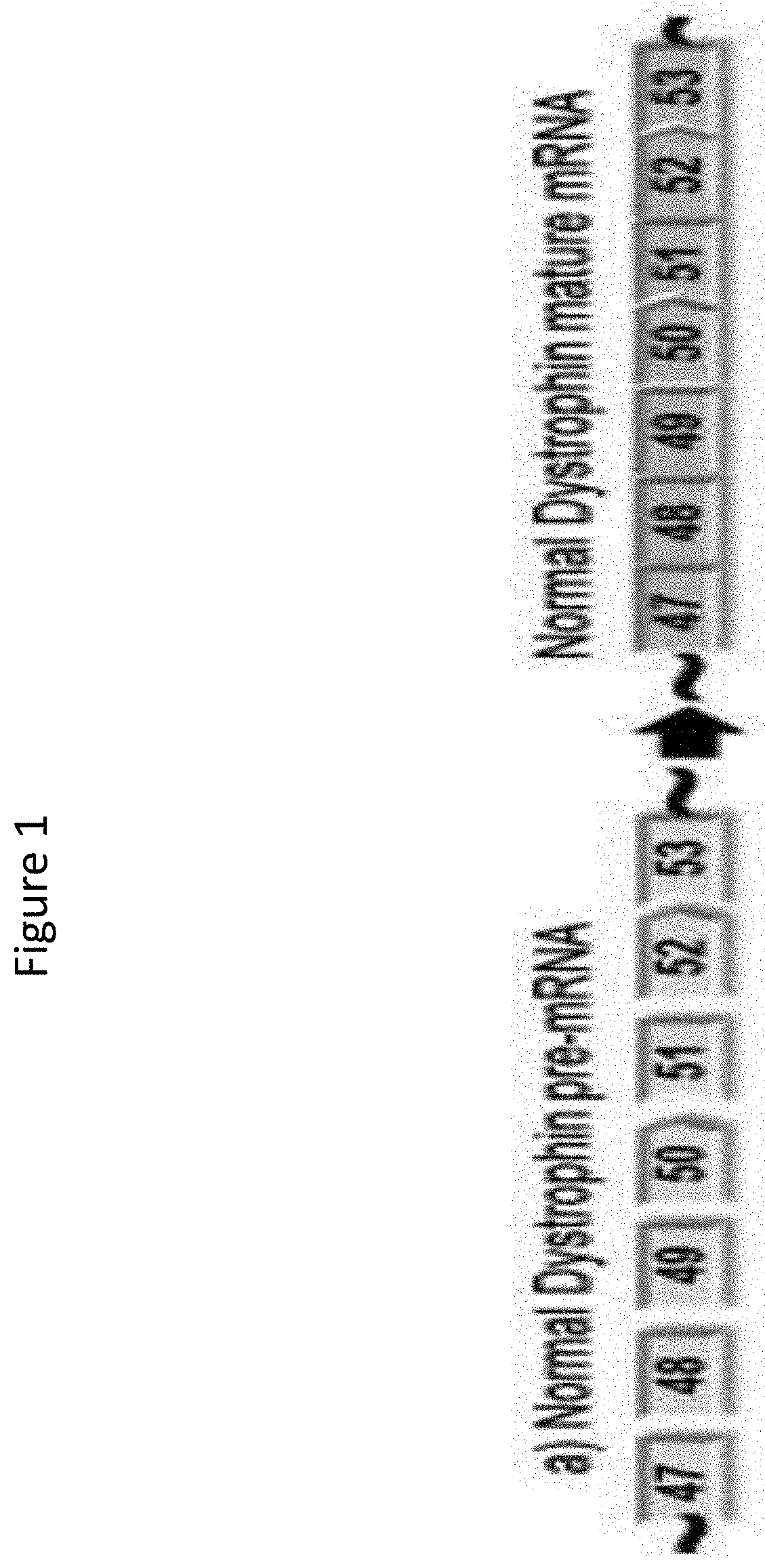
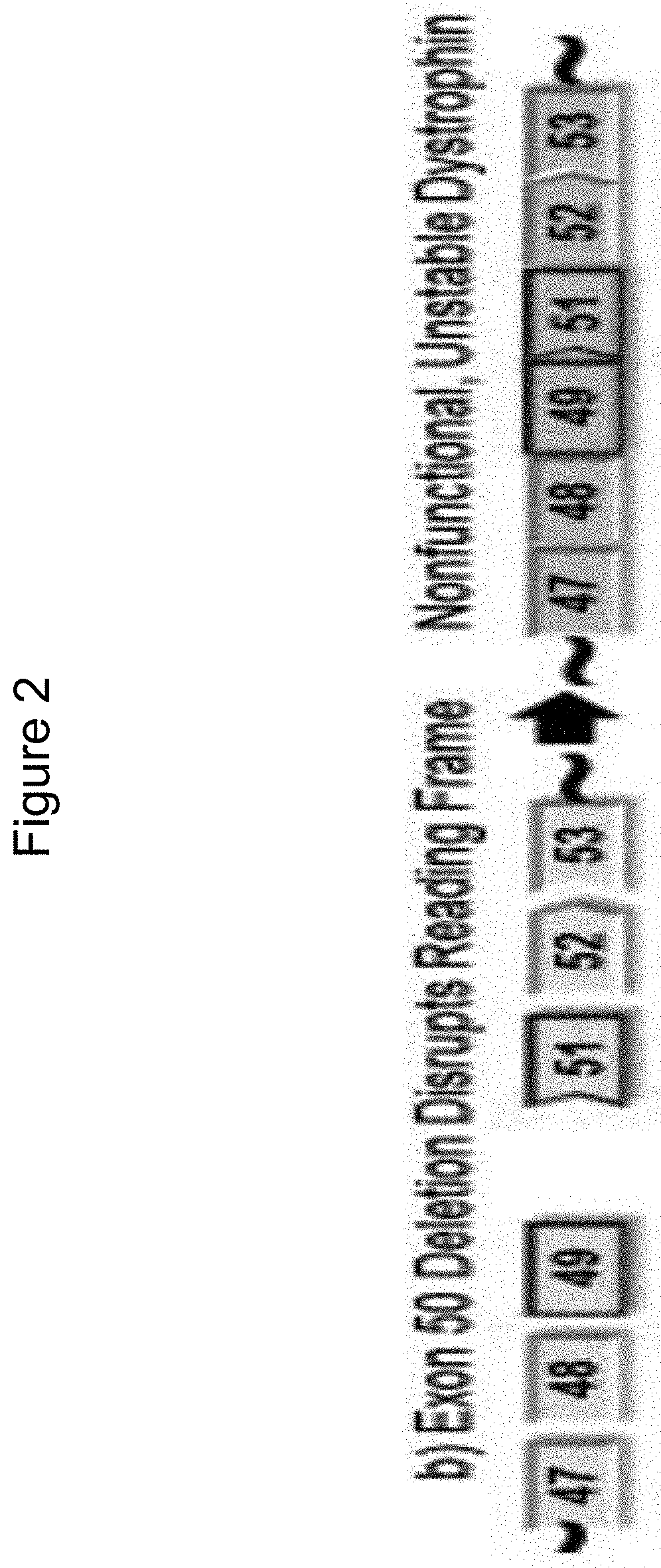
Methods of modifying a dystrophin gene are disclosed, for restoring dystrophin expression within a cell having an endogenous frameshift mutation within the dystrophin gene. The methods comprising introducing a first cut within an exon of the dystrophin gene creating a first exon end, wherein said first cut is located upstream of the endogenous frameshift mutation; and introducing a second cut within an exon of the dystrophin gene creating a second exon end, wherein said second cut is located downstream of the frameshift mutation. Upon joining / ligation of said first and second exon ends dystrophin expression is restored, as the correct reading frame is restored. Reagents and uses of the method are also disclosed, for example to treat a subject suffering from muscular dystrophy.
Owner:UNIV LAVAL
Exon skipping compositions for treating muscular dystrophy
InactiveUS20170369875A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideSplicing alterationMuscular disorderDuchenne muscular dystrophyMuscular dystrophy
Owner:SAREPTA THERAPEUTICS INC
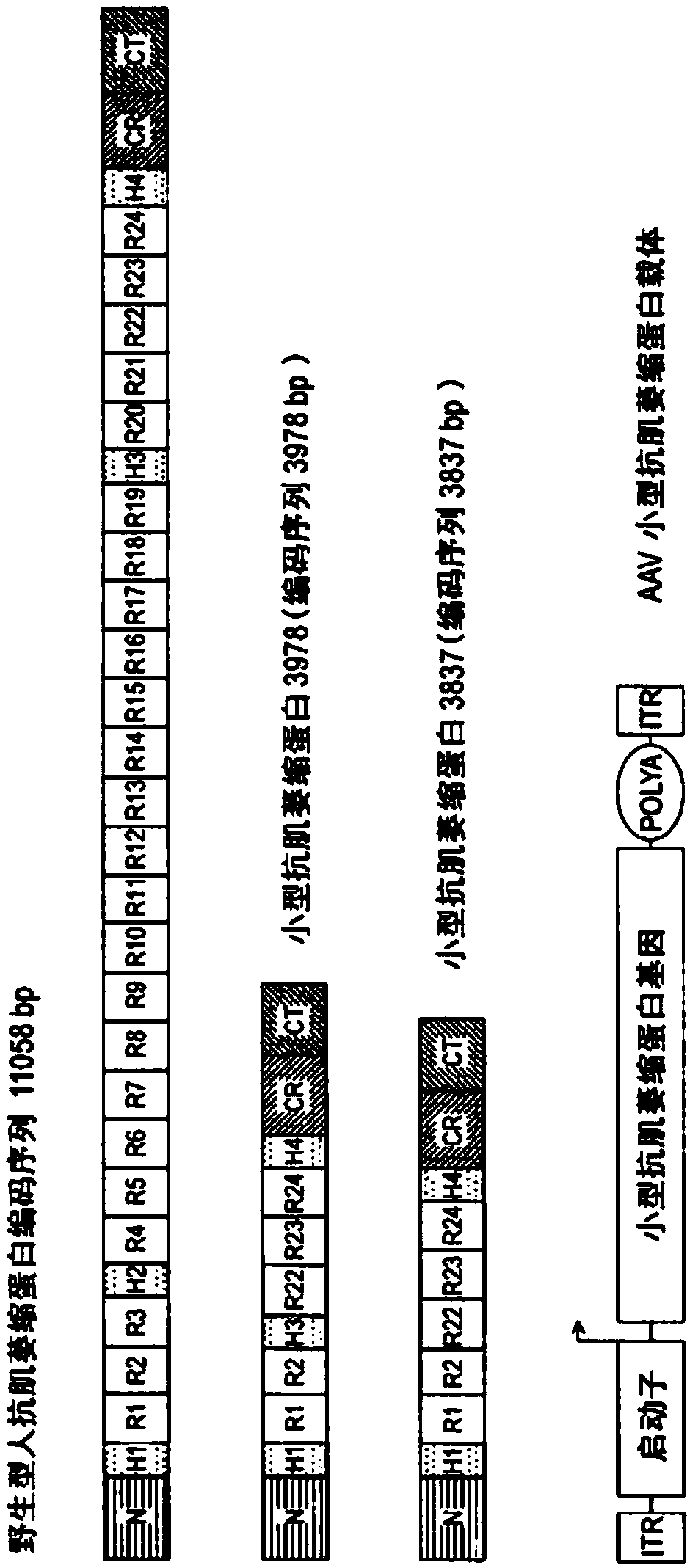
Micro-dystrophins and related methods of use
ActiveUS10479821B2Reduce pathological effect and symptomIncrease the diameterPeptide/protein ingredientsAntibody mimetics/scaffoldsDuchenne muscular dystrophyMuscular dystrophy
Nucleotide sequences including a micro-dystrophin gene are provided. The micro-dystrophin genes may be operatively linked to a regulatory cassette. Methods of treating a subject having, or at risk of developing, muscular dystrophy, sarcopenia, heart disease, or cachexia are also provided. The methods may include administering a pharmaceutical composition including the micro-dystrophin gene and a delivery vehicle to a subject. Further, the methods may include administering the pharmaceutical composition a subject having Duchenne muscular dystrophy or Becker muscular dystrophy.
Owner:UNIV OF WASHINGTON
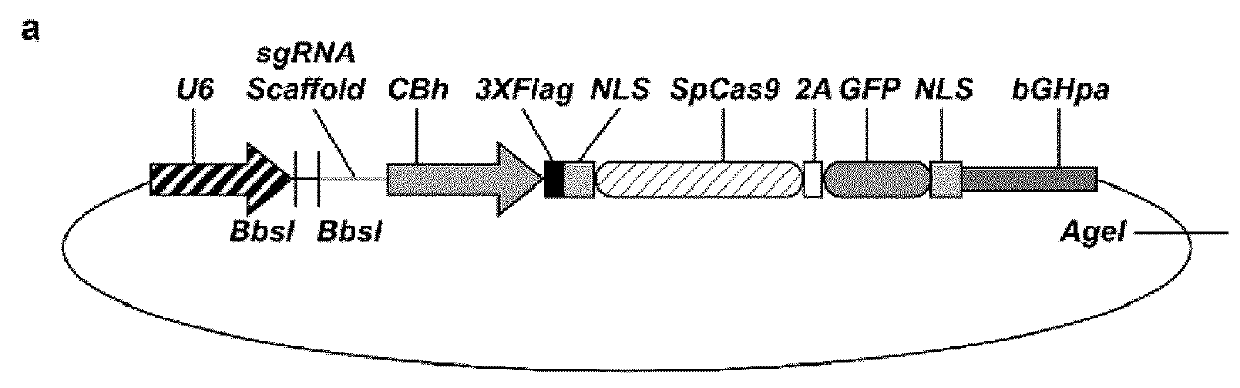
Crispr/cas-related methods and compositions for treating duchenne muscular dystrophy
Disclosed herein are vectors that targets a dystrophin gene, encoding at least one Cas9 molecule or a Cas9 fusion protein, and at least one gRNA molecule (e.g., two gRNA molecules), and compositions and cells comprising such vectors. Also provided are methods for using the vectors, compositions and cells for genome engineering (e.g., correcting a mutant dystrophin gene), and for treating DMD.
Owner:EDITAS MEDICINE +1
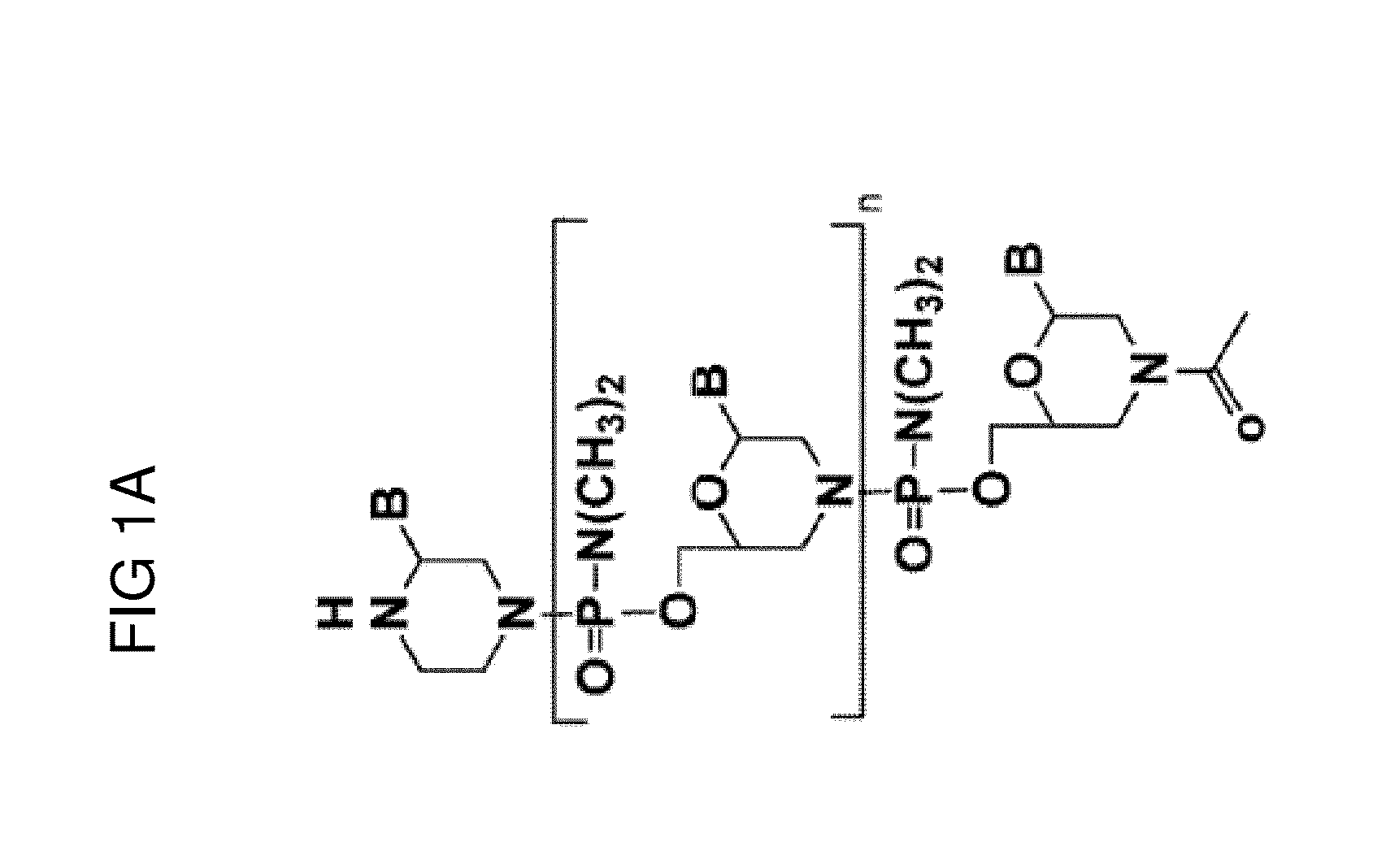
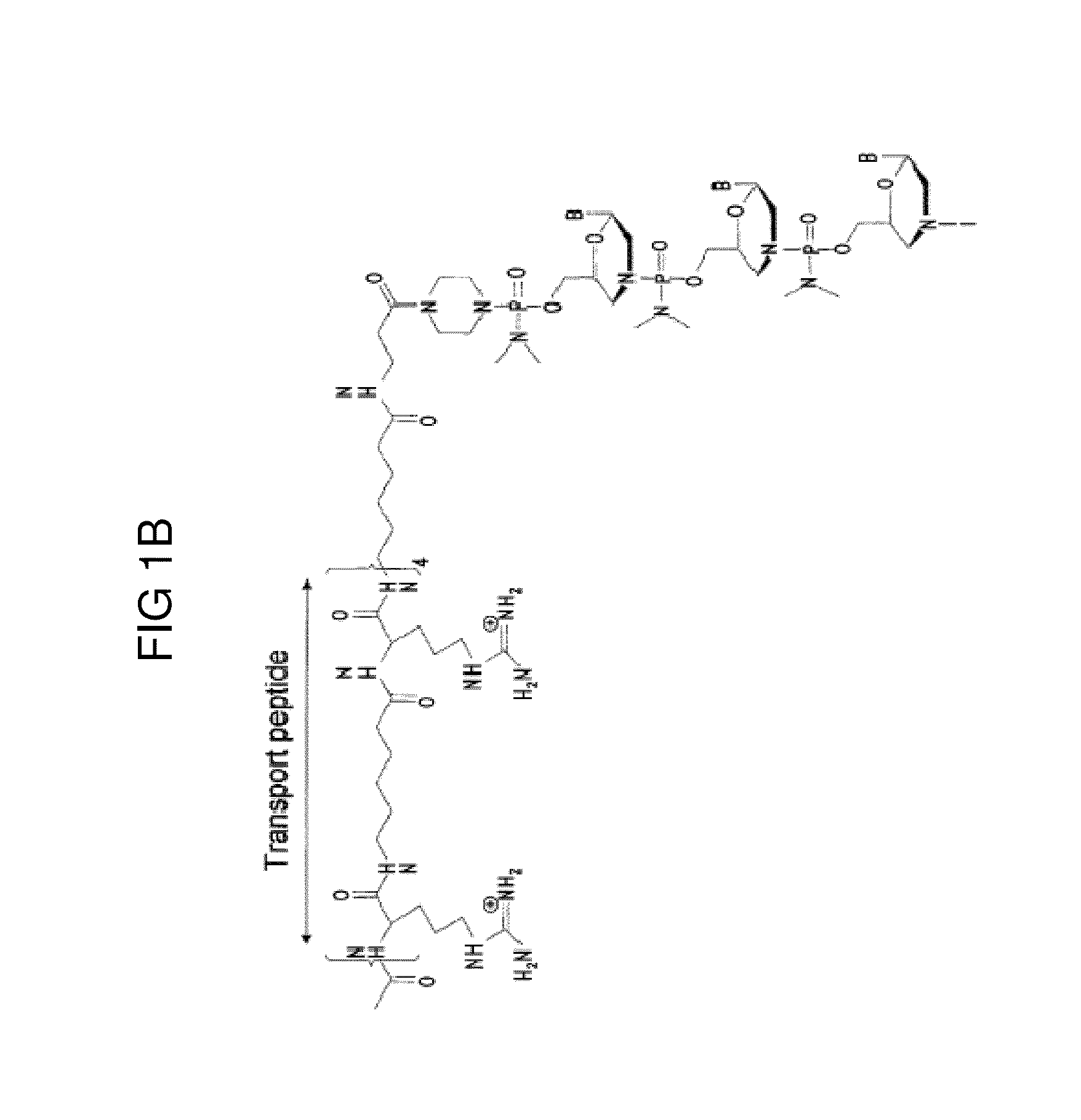
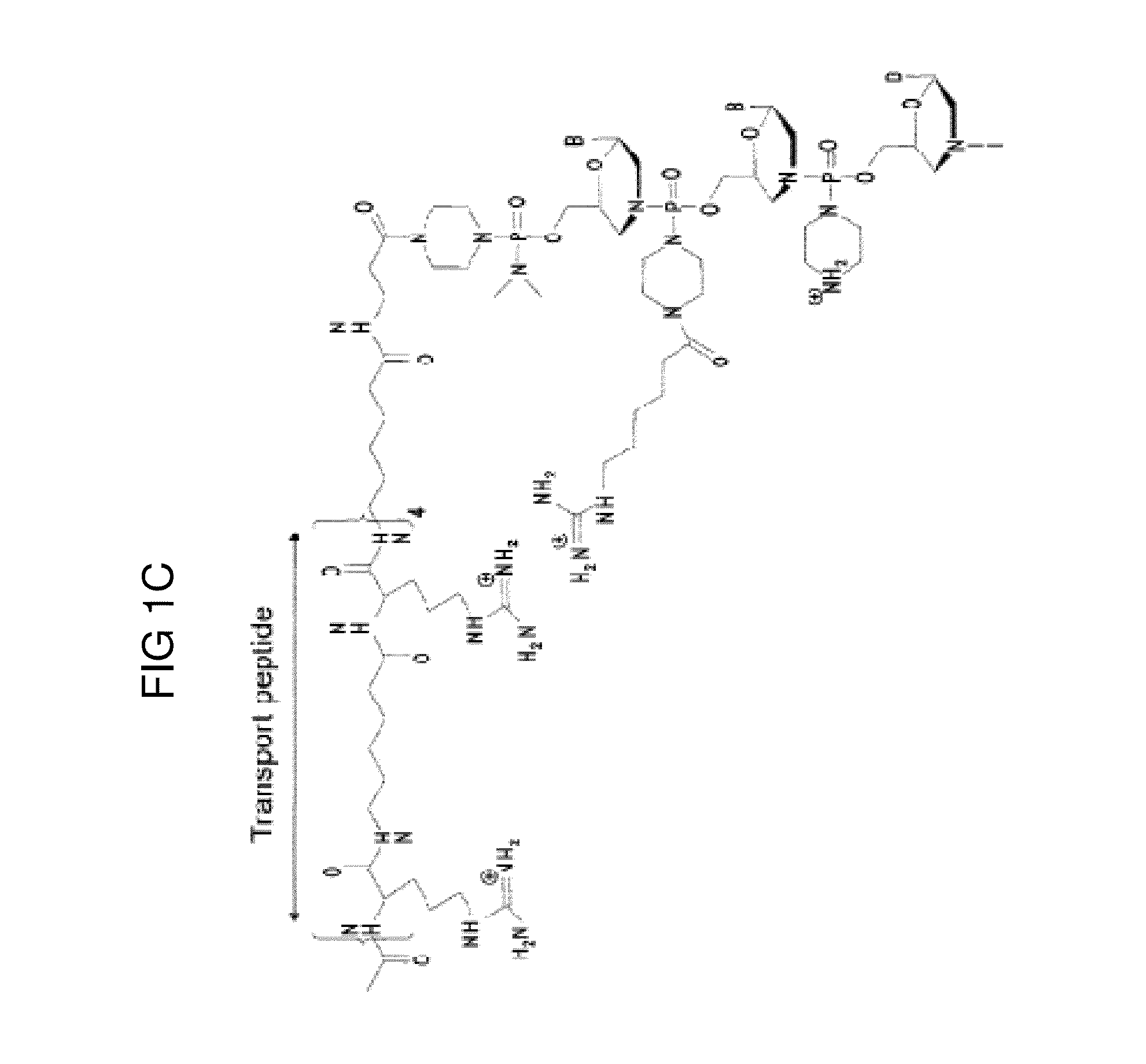
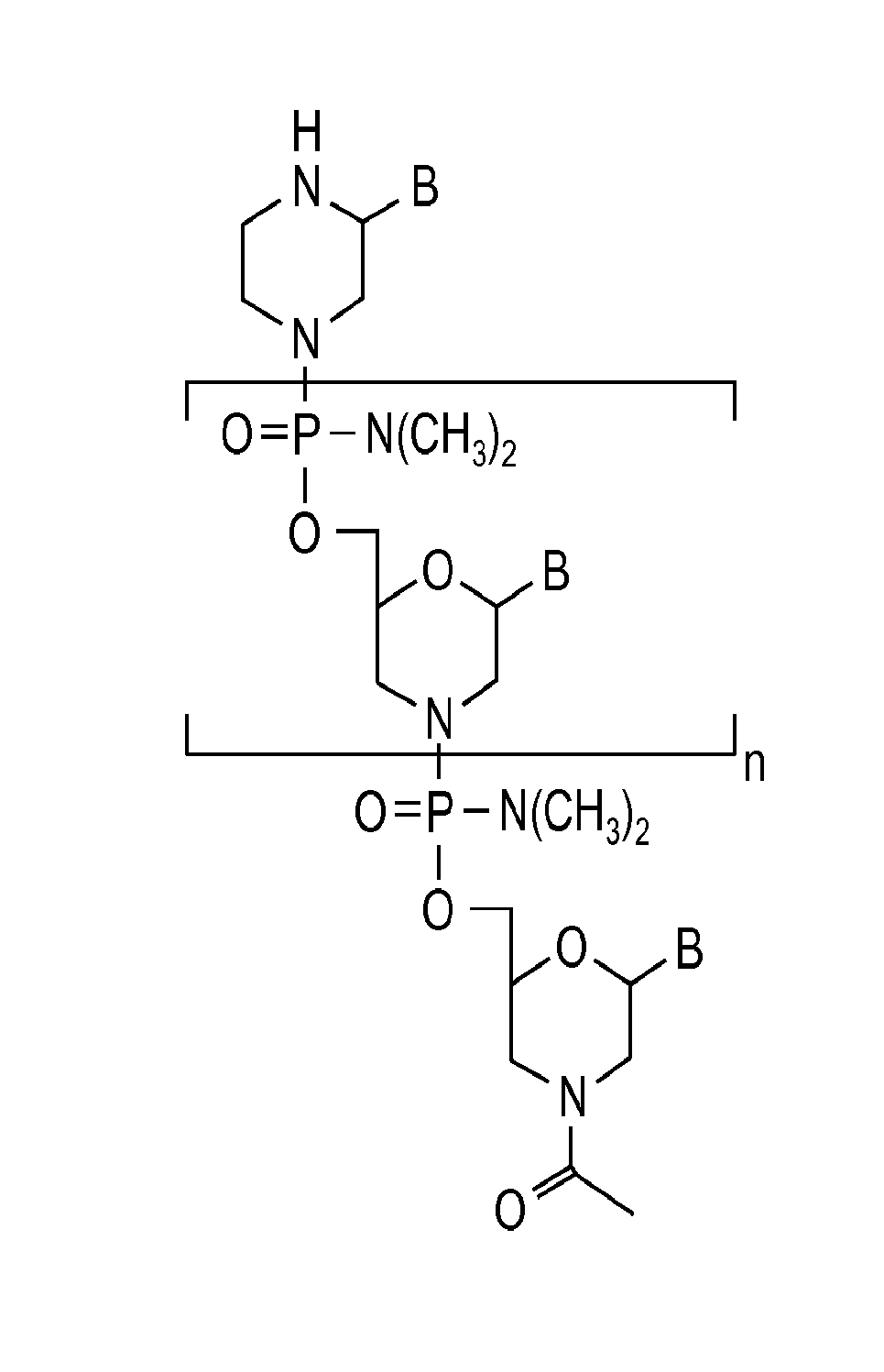
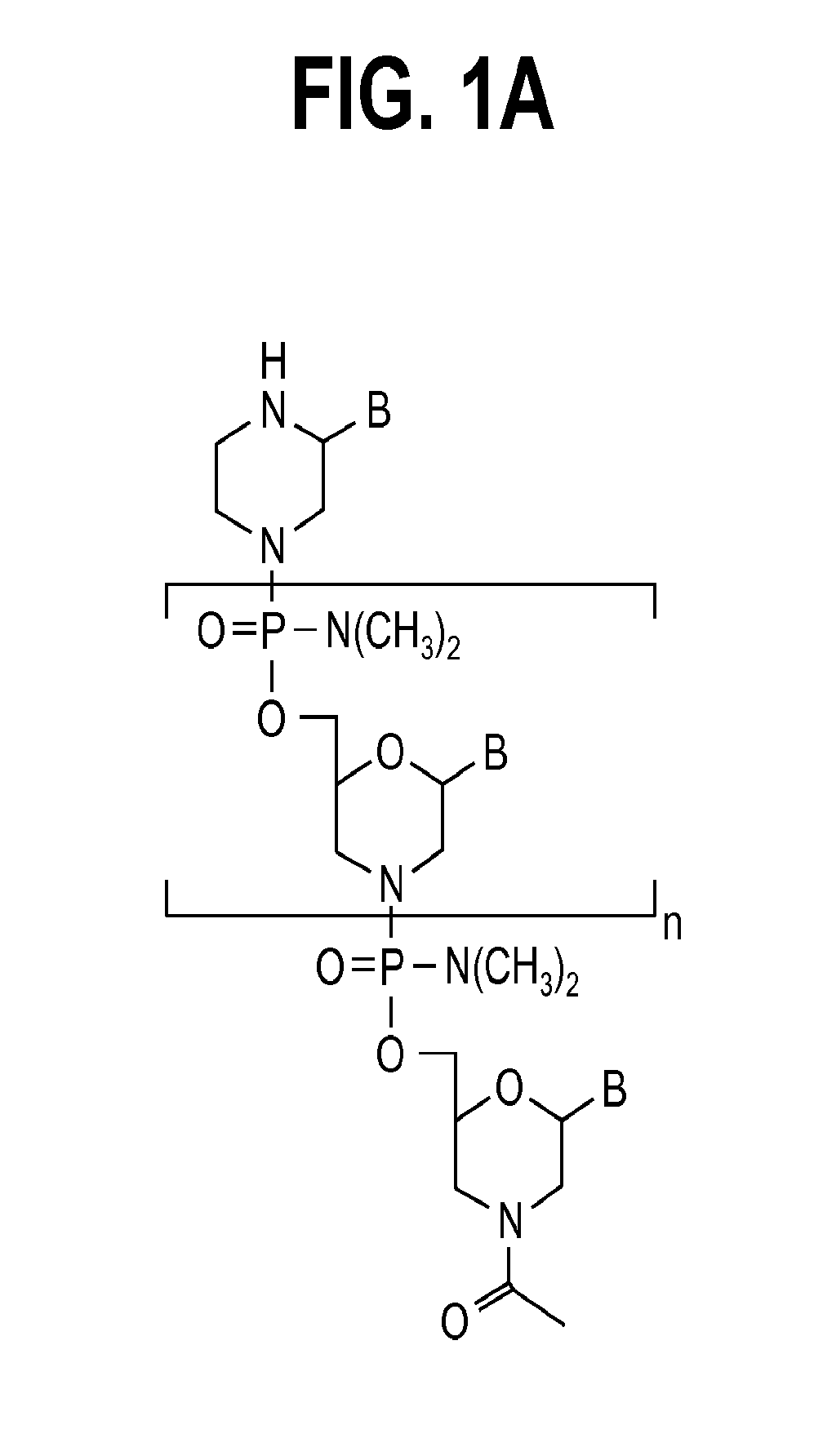
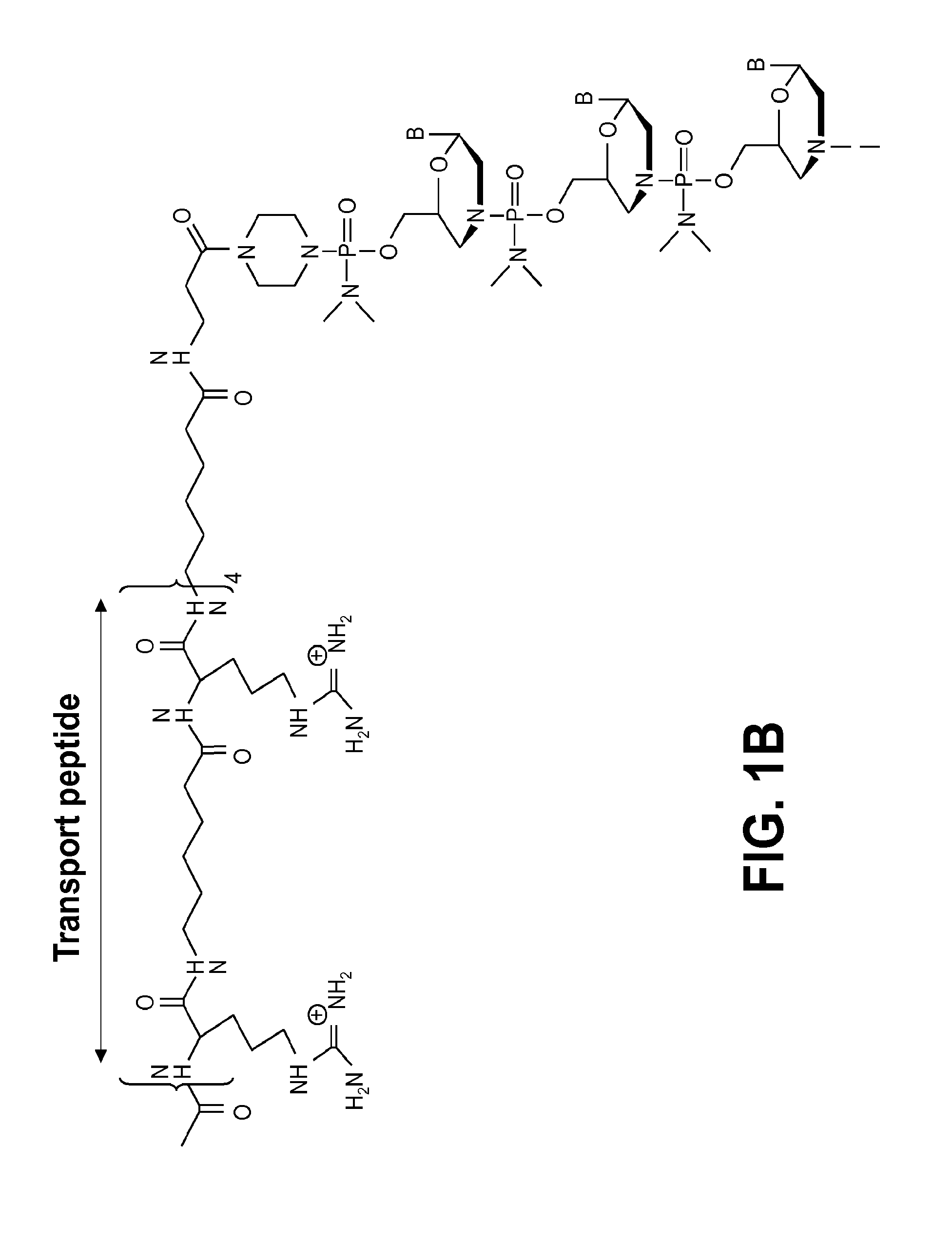
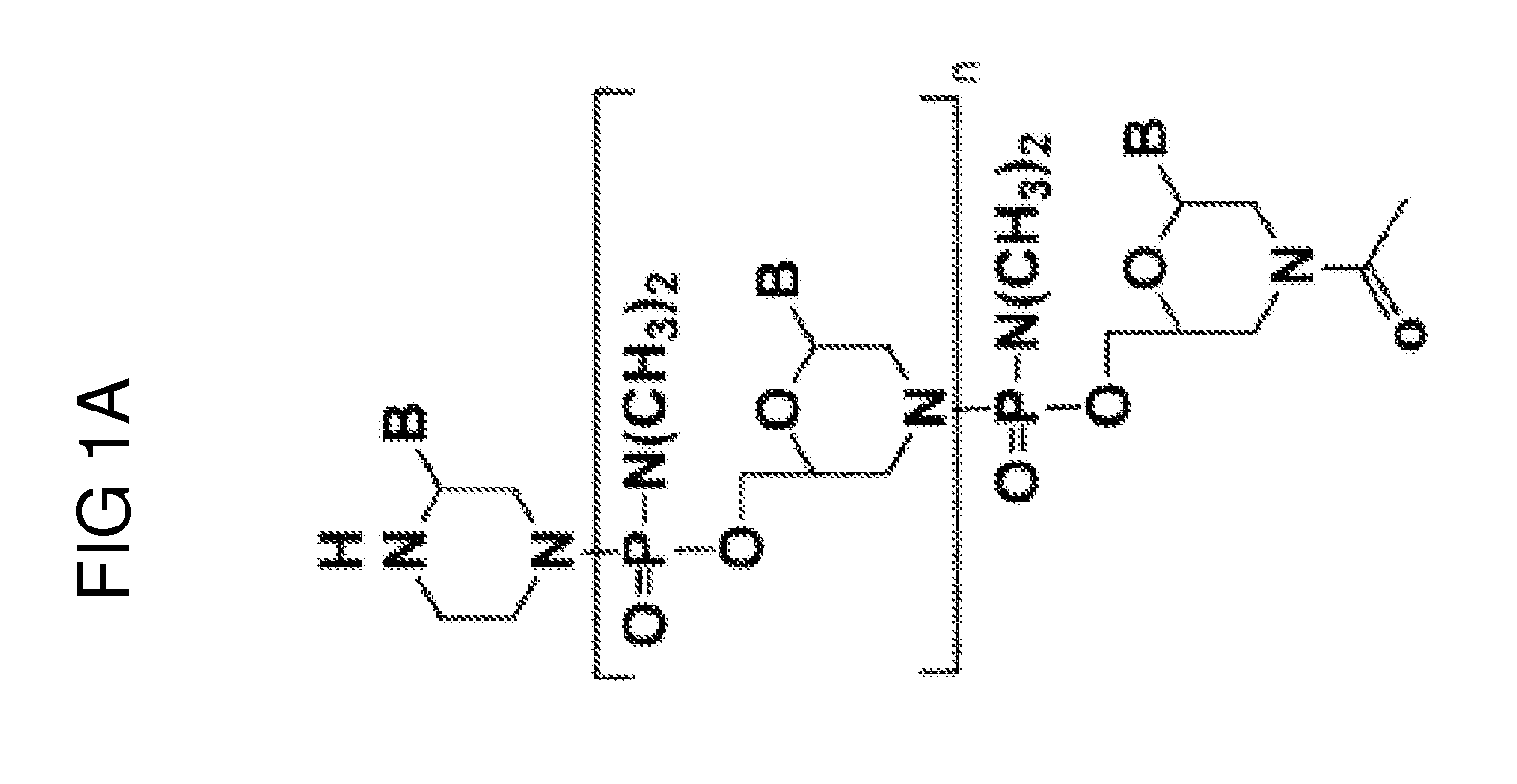
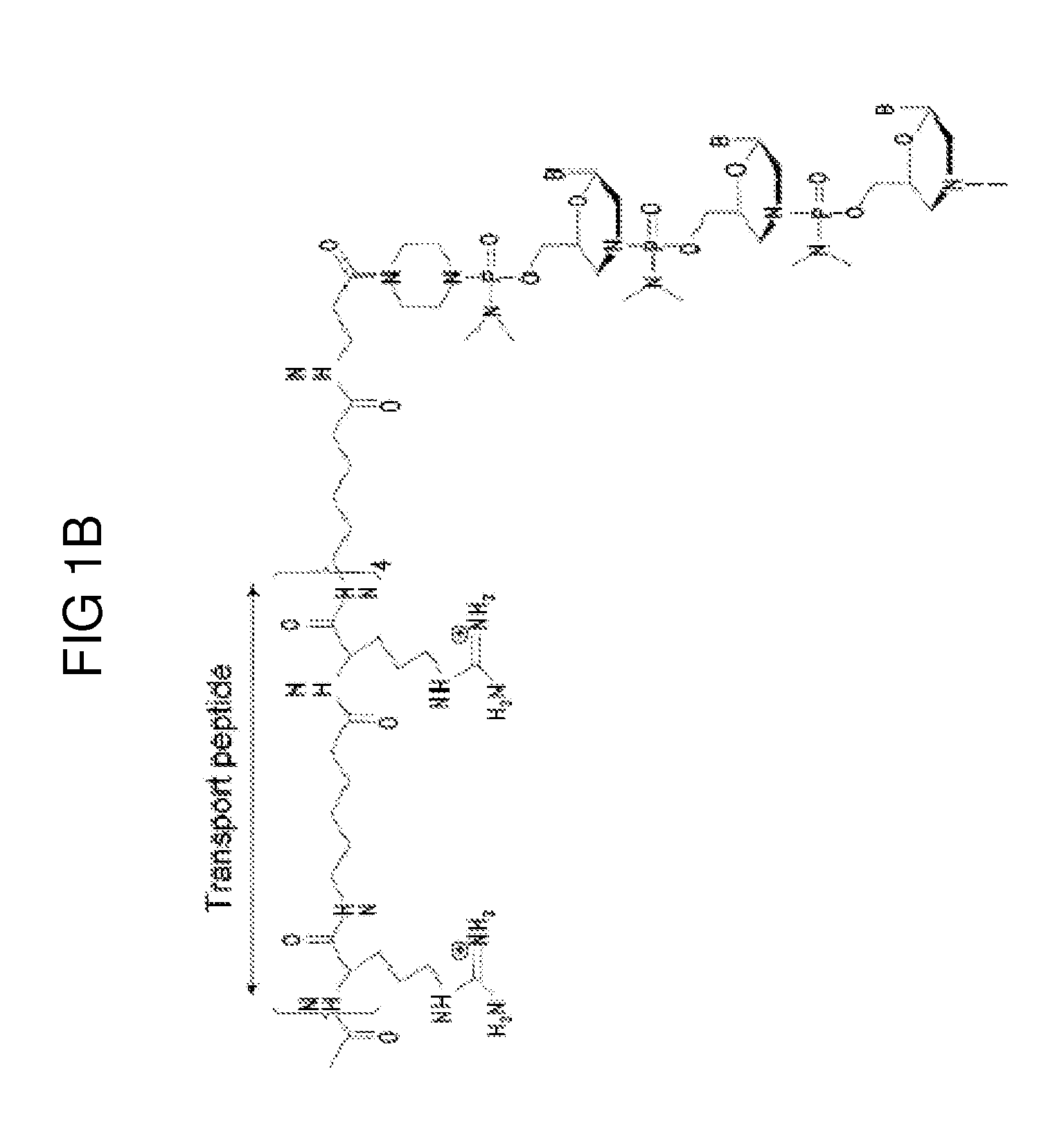
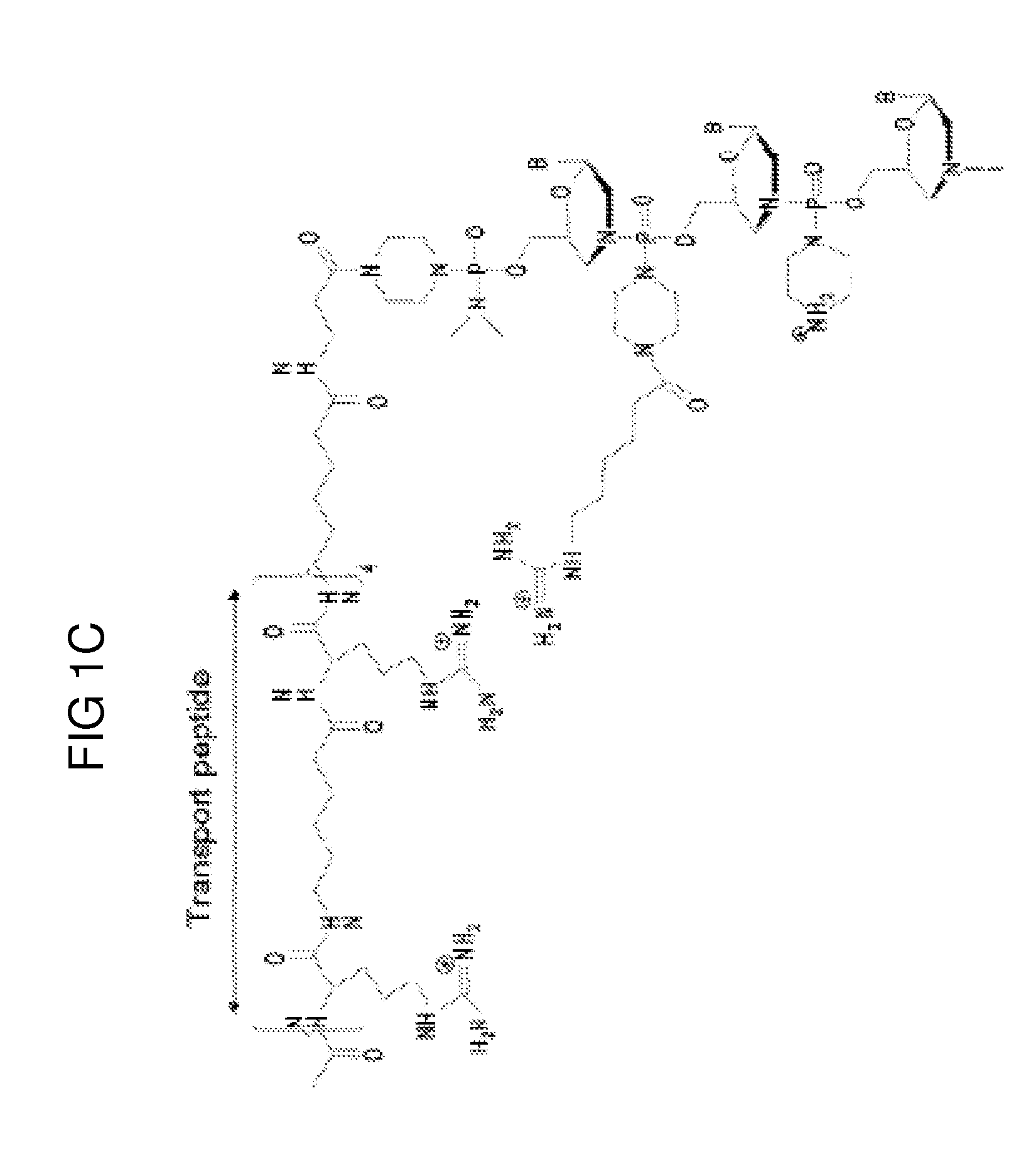
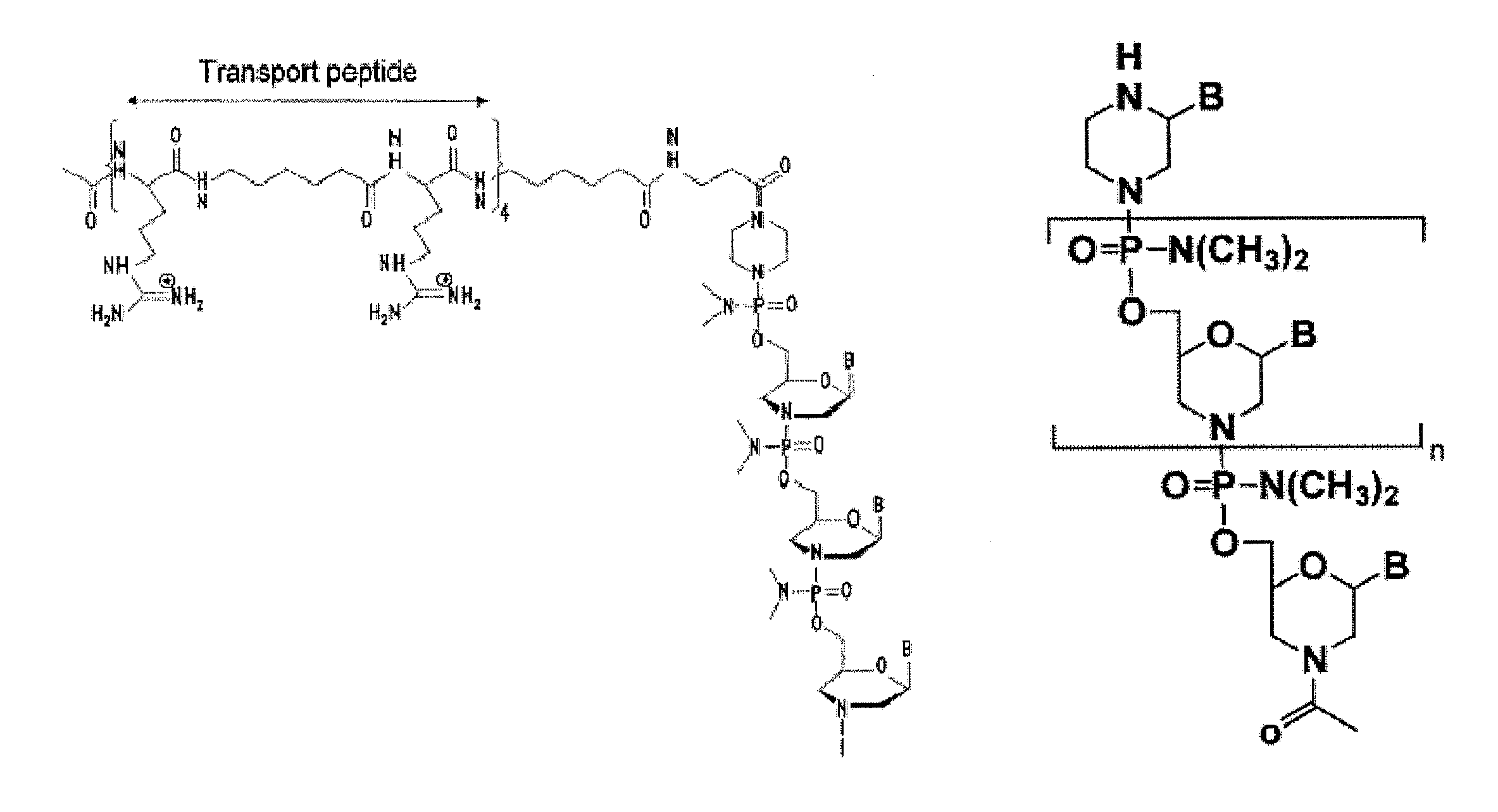
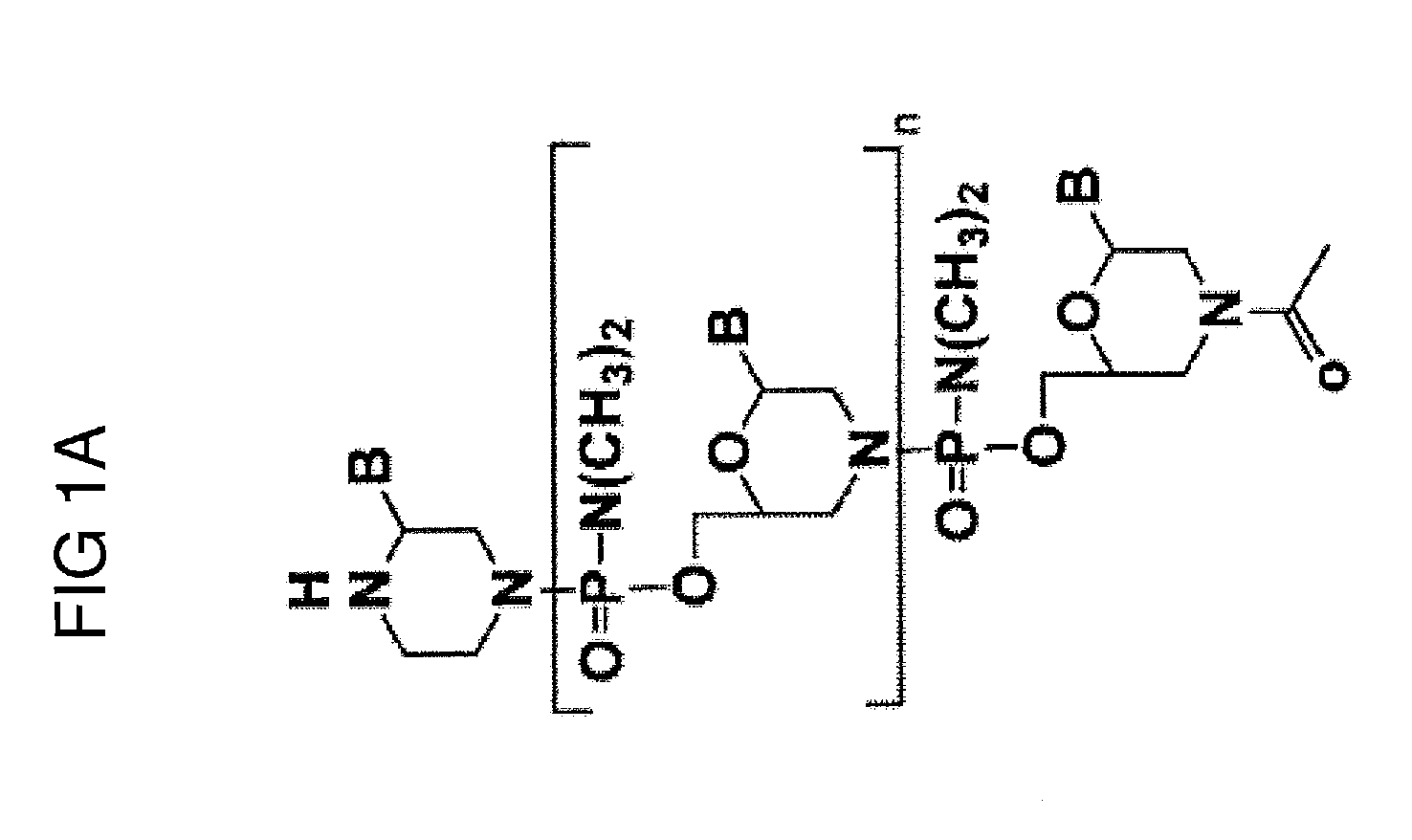
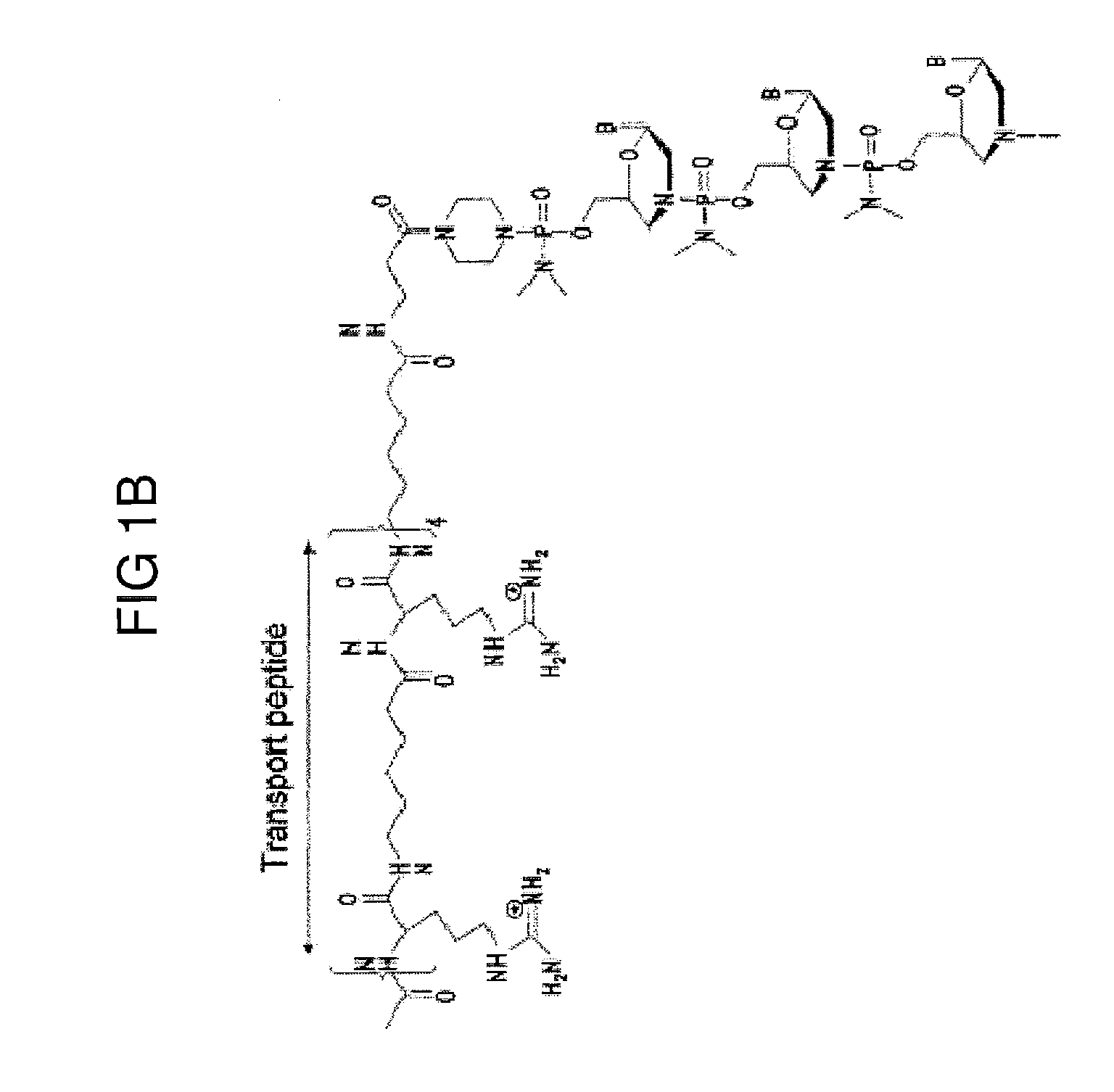
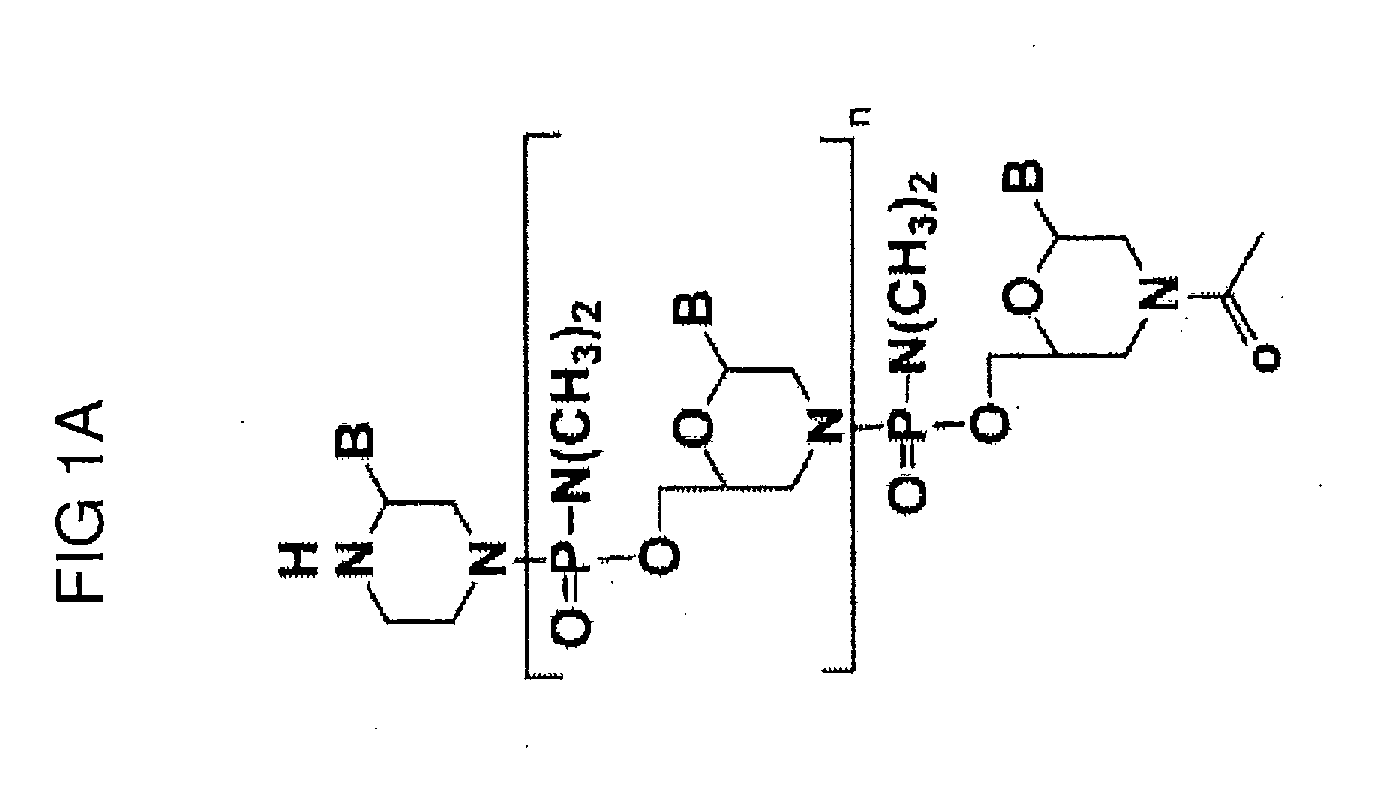
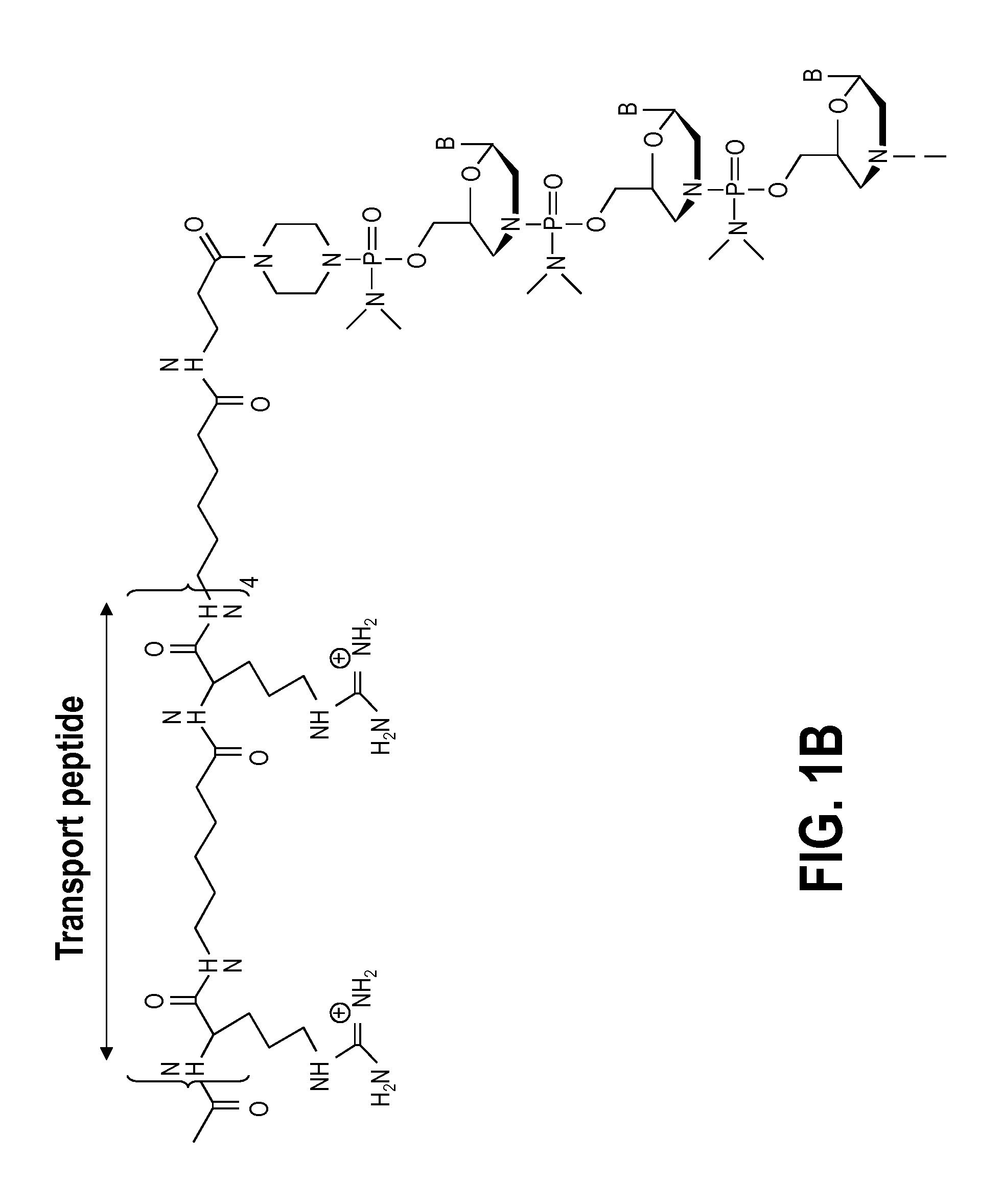
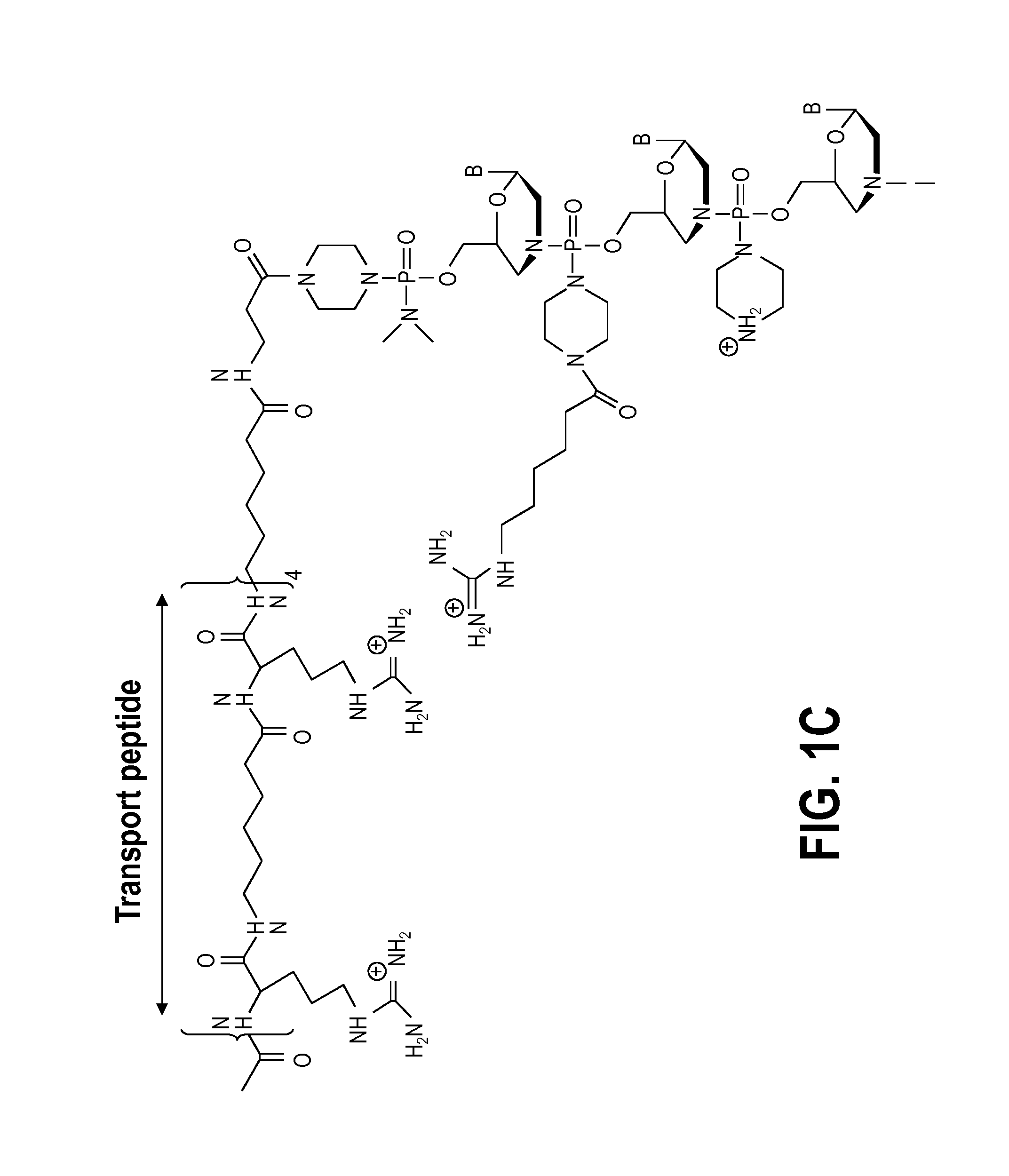
Exon skipping oligomer conjugates for muscular dystrophy
Antisense oligomer conjugates complementary to a selected target site in the human dystrophin gene to induce exon 53 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Antisense nucleic acids
ActiveUS20170067048A1Improve efficiencyOrganic active ingredientsSplicing alterationAntisense nucleic acidExon
Provided is a drug that allows highly-efficient skipping of exon 51 in the human dystrophin gene. The present invention provides an antisense oligomer which enables exon 51 in the human dystrophin gene to be skipped.
Owner:NIPPON SHINYAKU CO LTD +1
Optimized mini-dystrophin genes and expression cassettes and their use
This invention relates to polynucleotides encoding mini-dystrophin proteins, viral vectors comprising the same, and methods of using the same for delivery of mini-dystrophin to a cell or a subject.
Owner:BAMBOO THERAPEUTICS INC +1
Exon skipping oligomer conjugates for muscular dystrophy
Antisense oligomer conjugates complementary to a selected target site in the human dystrophin gene to induce exon 45 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Exon skipping oligomer conjugates for muscular dystrophy
Antisense oligomer conjugates complementary to a selected target site in the human dystrophin gene to induce exon 53 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
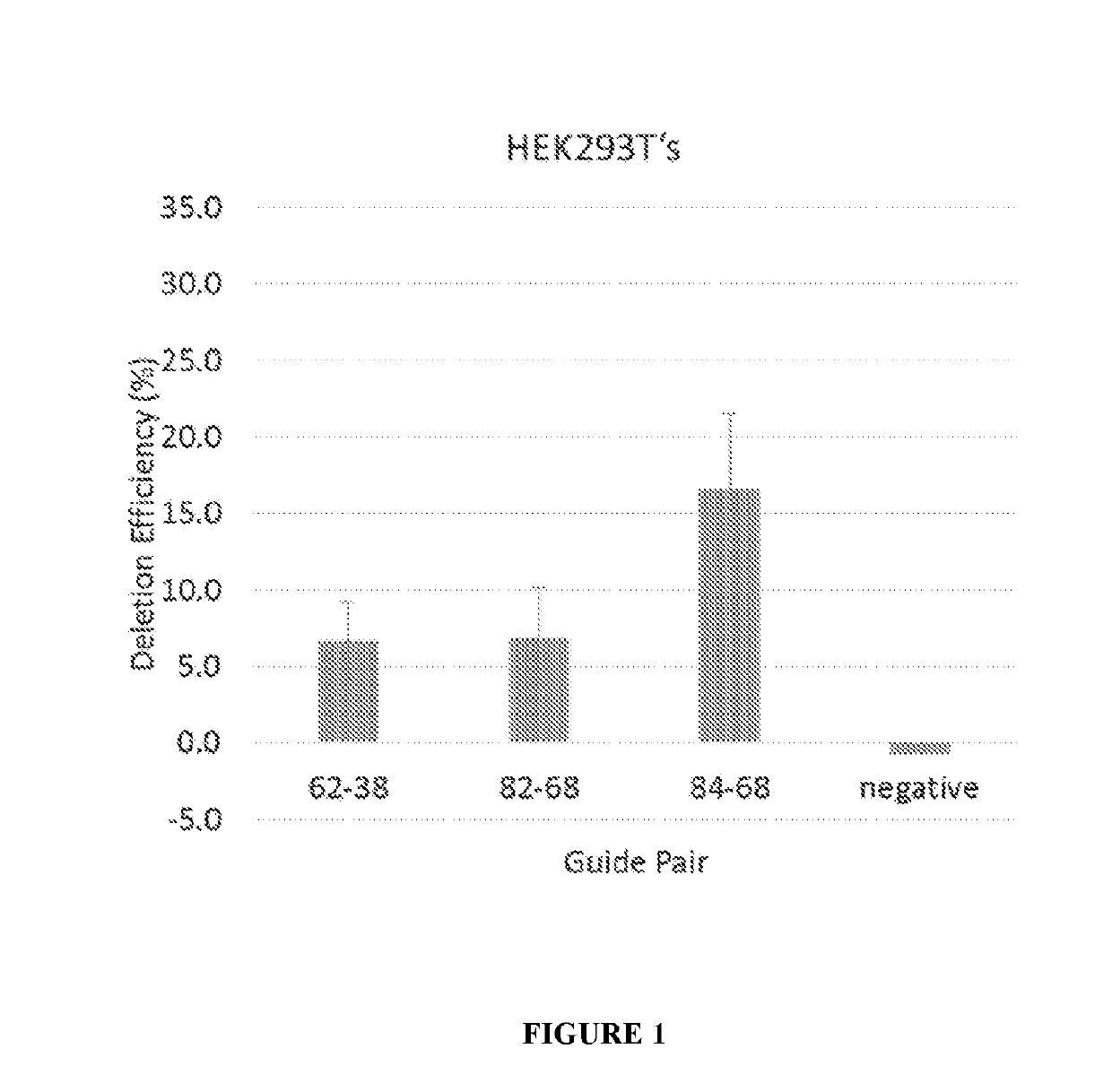
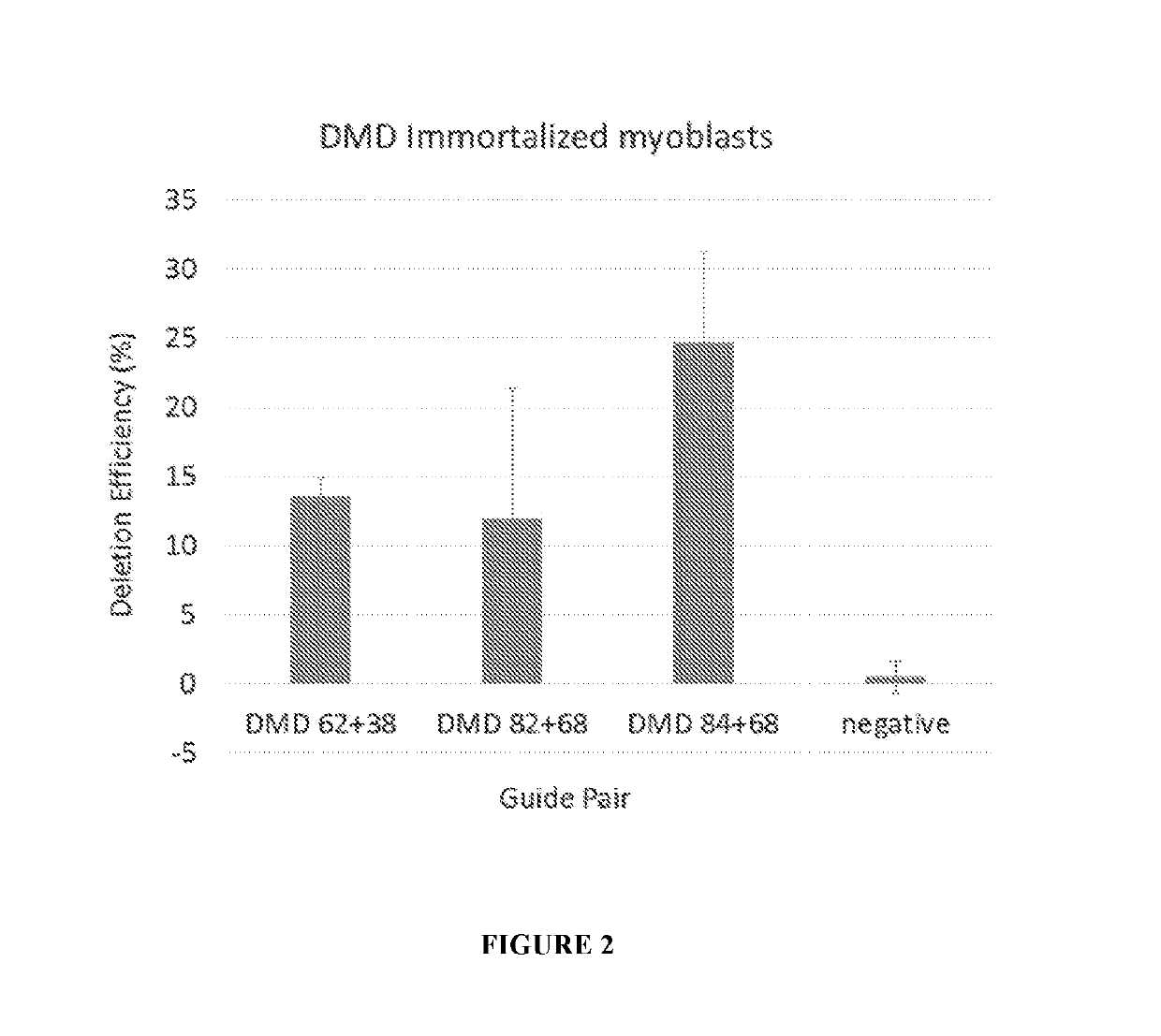
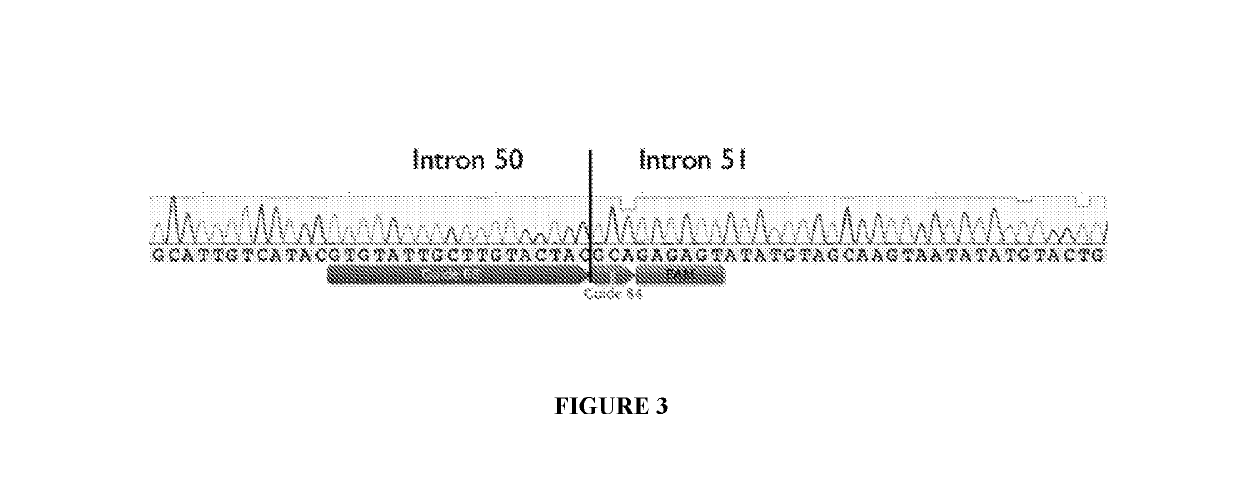
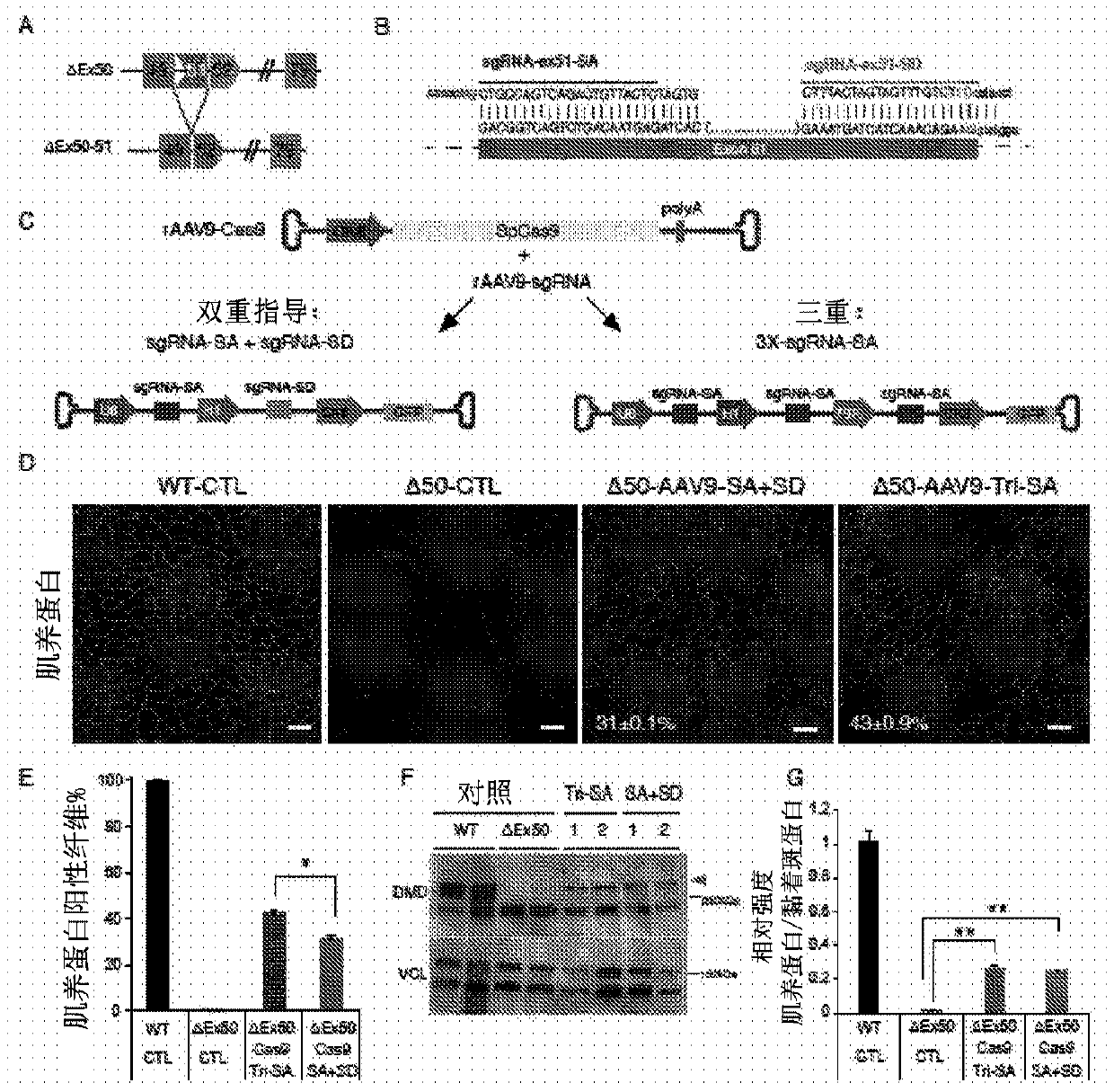
Optimized strategy for exon skipping modifications using crispr/cas9 with triple guide sequences
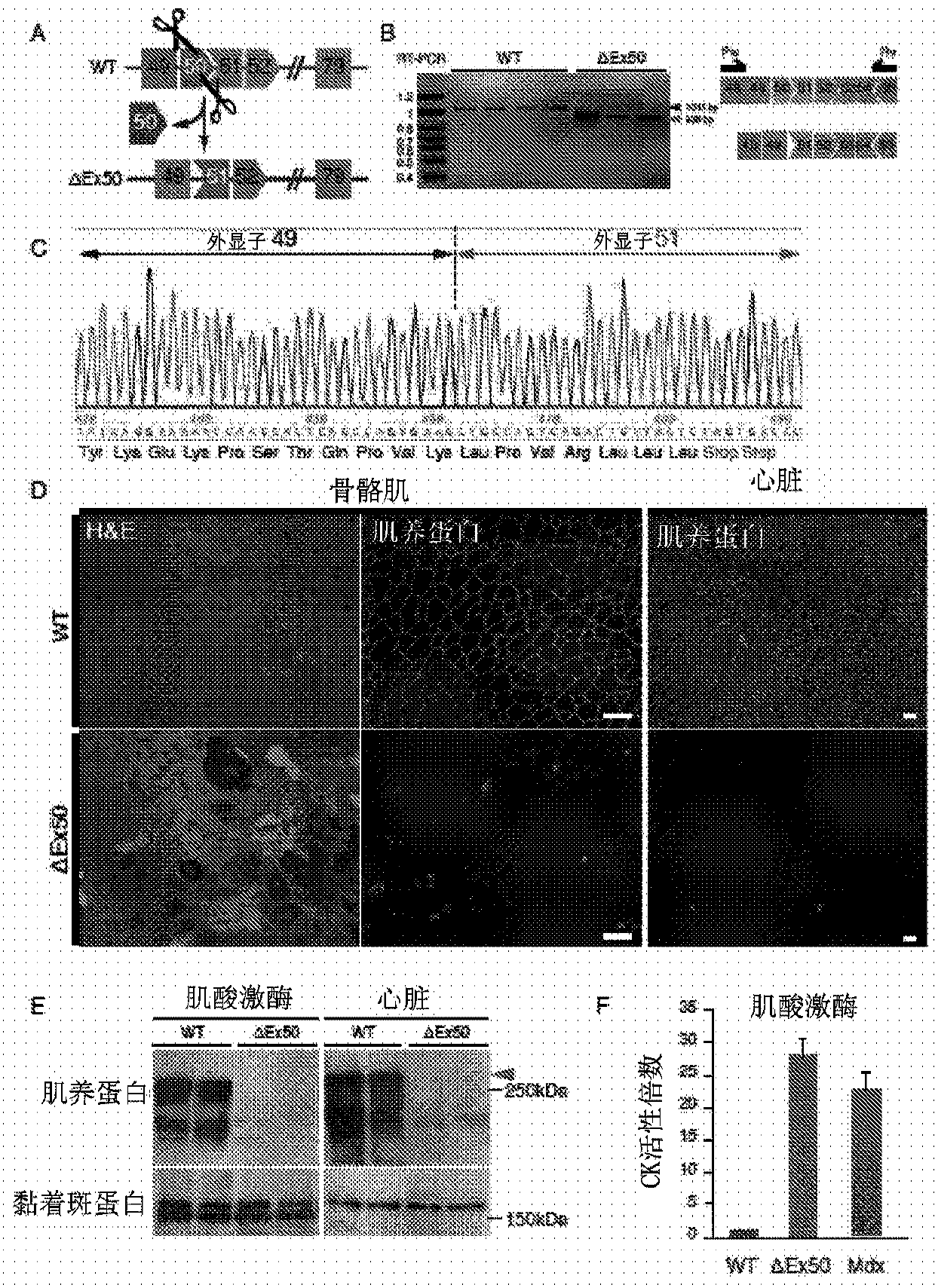
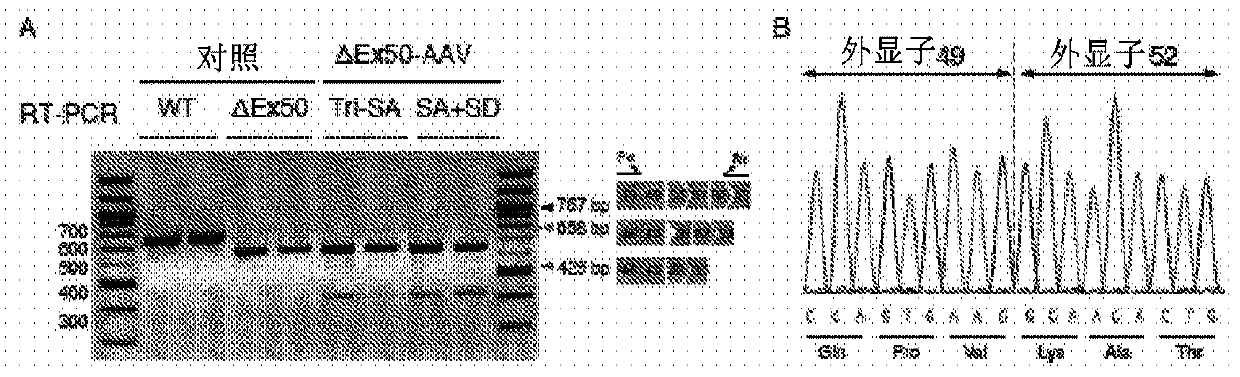
CRISPR / Cas9-mediated genome editing holds clinical potential for treating genetic diseases, such as Duchenne muscular dystrophy (DMD), which is caused by mutations in the dystrophin gene. Here, usingthree promoters to drive expression of the same DMD guide RNA, a more robust and safe form of genome editing was achieved in a humanized mouse model for DMD with a deletion in exon 50, and in a DeltaEx50-MD Dog.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Exon skipping oligomer conjugates for muscular dystrophy
Antisense oligomer conjugates complementary to a selected target site in the human dystrophin gene to induce exon 51 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Exon skipping oligomer conjugates for muscular dystrophy
Owner:SAREPTA THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com