Optimized strategy for exon skipping modifications using crispr/cas9 with triple guide sequences
A sequence, target sequence technology, applied in the composition of genome editing and in the field of correcting in vivo mutations using exon skipping method, can solve problems such as loss of dystrophin expression, muscle membrane fragility, muscle atrophy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0505] Example 1 - Materials and methods
[0506] Research Approval. All experimental procedures involving animals in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
[0507] CRISPR / Cas9-mediated exon 50 deletion in mice. Two single guide RNAs (sgRNAs) specific for the inner region surrounding the exon 50 sequence of the mouse Dmd locus were cloned into vector px330 using the following primers:
[0508]
[0509] For in vitro transcription of sgRNAs, add the T7 promoter sequence to the sgRNA template by PCR using the following primers:
[0510]
[0511] Gel-purified PCR products were used as templates for in vitro transcription using the MEGAshortscript T7 Kit (Life Technologies). sgRNA was purified by MEGAclear kit (Life Technologies) and eluted with nuclease-free water (Ambion). The concentration of guide RNA was measured by NanoDrop instrument (Thermo Scientific).
[0...
Embodiment 2
[0522] Example 2 - Results
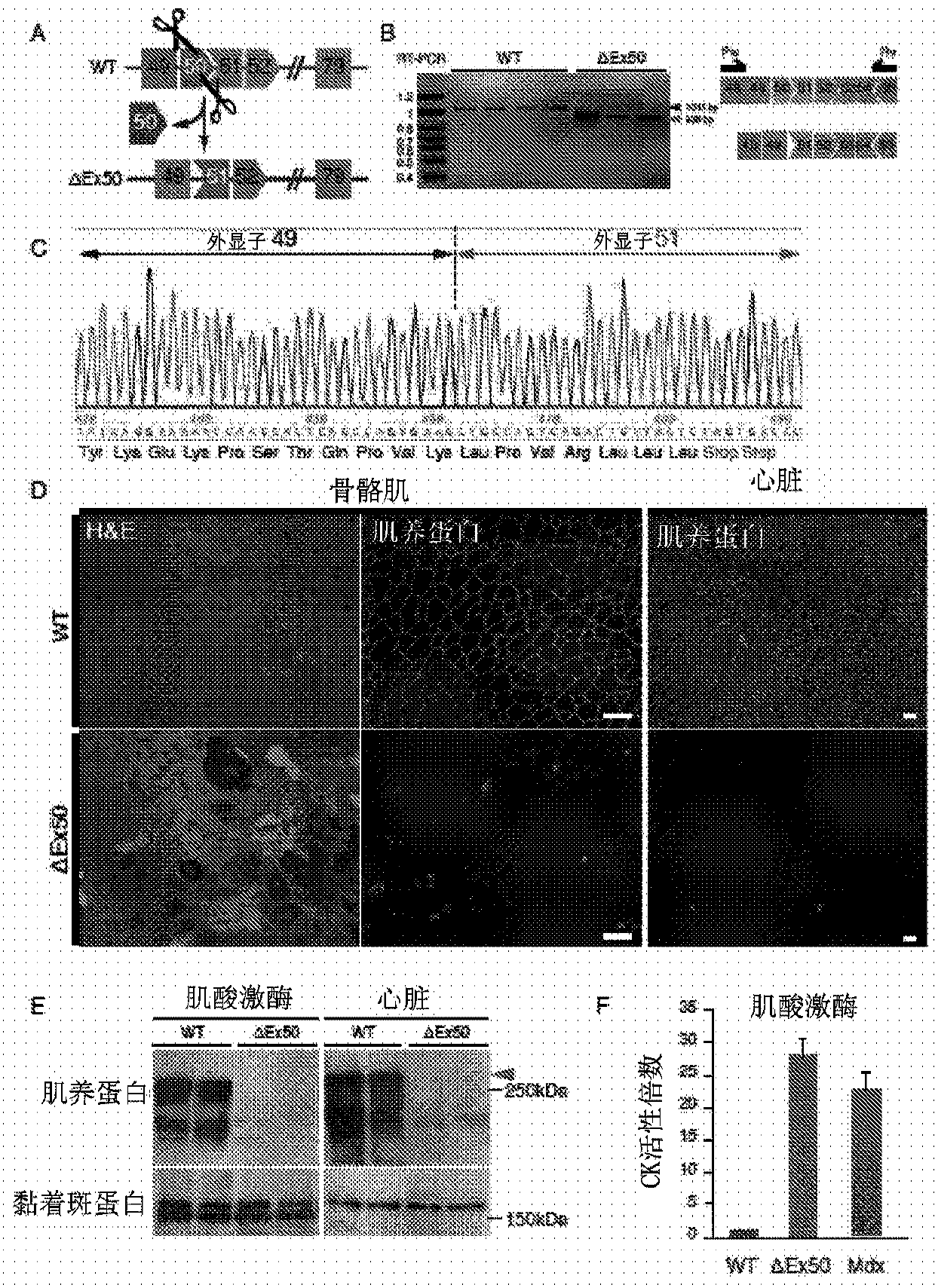
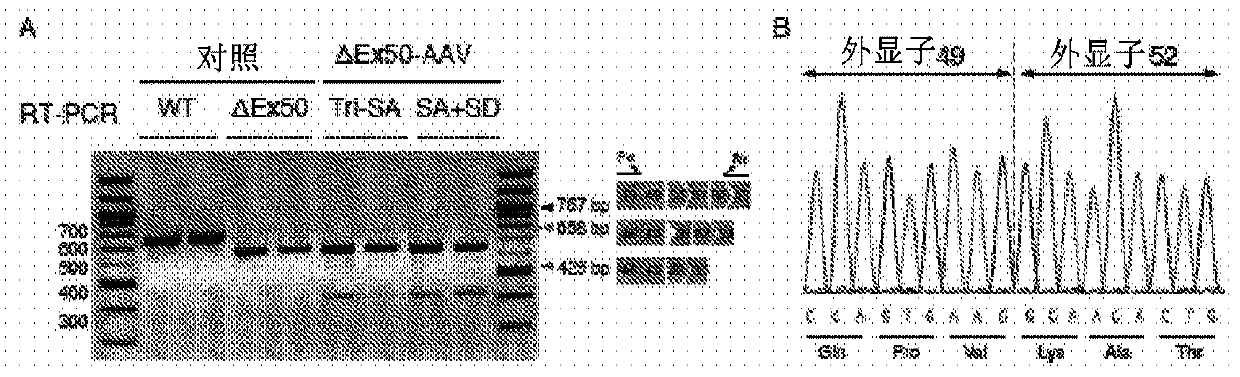
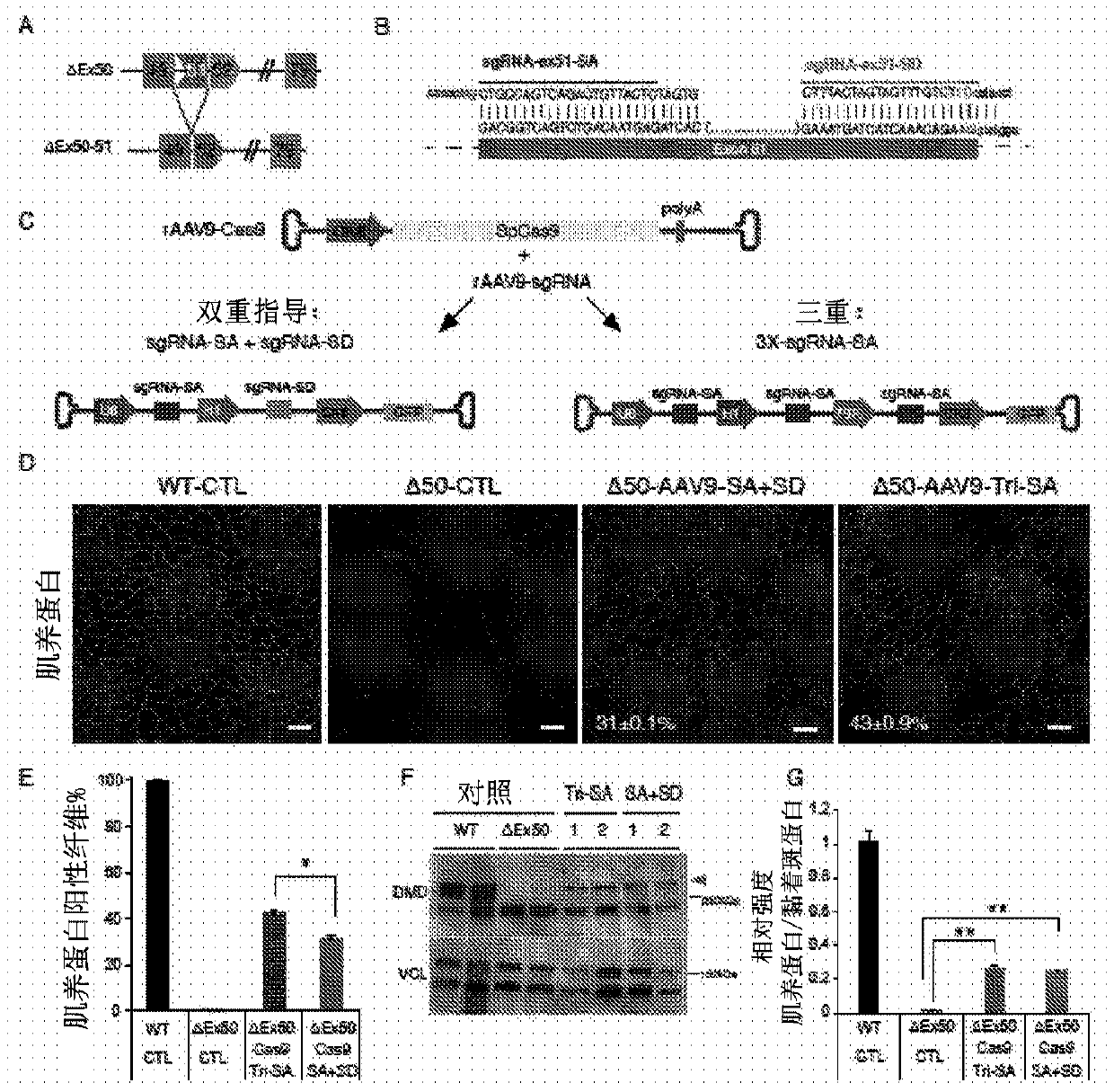
[0523] Humanized model of DMD. The most common hotspot mutation region in DMD patients was the region between exons 45 to 51, and skipping of exon 51 was available for treatment in the largest group (13-14%) of patients. To study CRISPR / Cas9-mediated exon 51 skipping in vivo, the inventors generated a mouse model mimicking the human "hotspot" region by deleting exon 50 using the CRISPR / Cas9 system guided by 2 sgRNAs (Fig. 1A). The deletion of exon 50 was confirmed by DNA sequencing (Fig. 1B). Deletion of exon 50 puts the dystrophin gene out of frame, resulting in dystrophin deficiency in skeletal muscle and heart (Fig. 1C–E). Mice lacking exon 50 showed marked dystrophic muscle changes at 2 months of age (Fig. 1E). Serum analysis of delta exon 50 mice revealed significantly increased levels of creatine kinase (CK), indicative of muscle damage (Fig. 1F). In conclusion, dystrophin expression, muscle histology, and serum CK levels confirmed the d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com