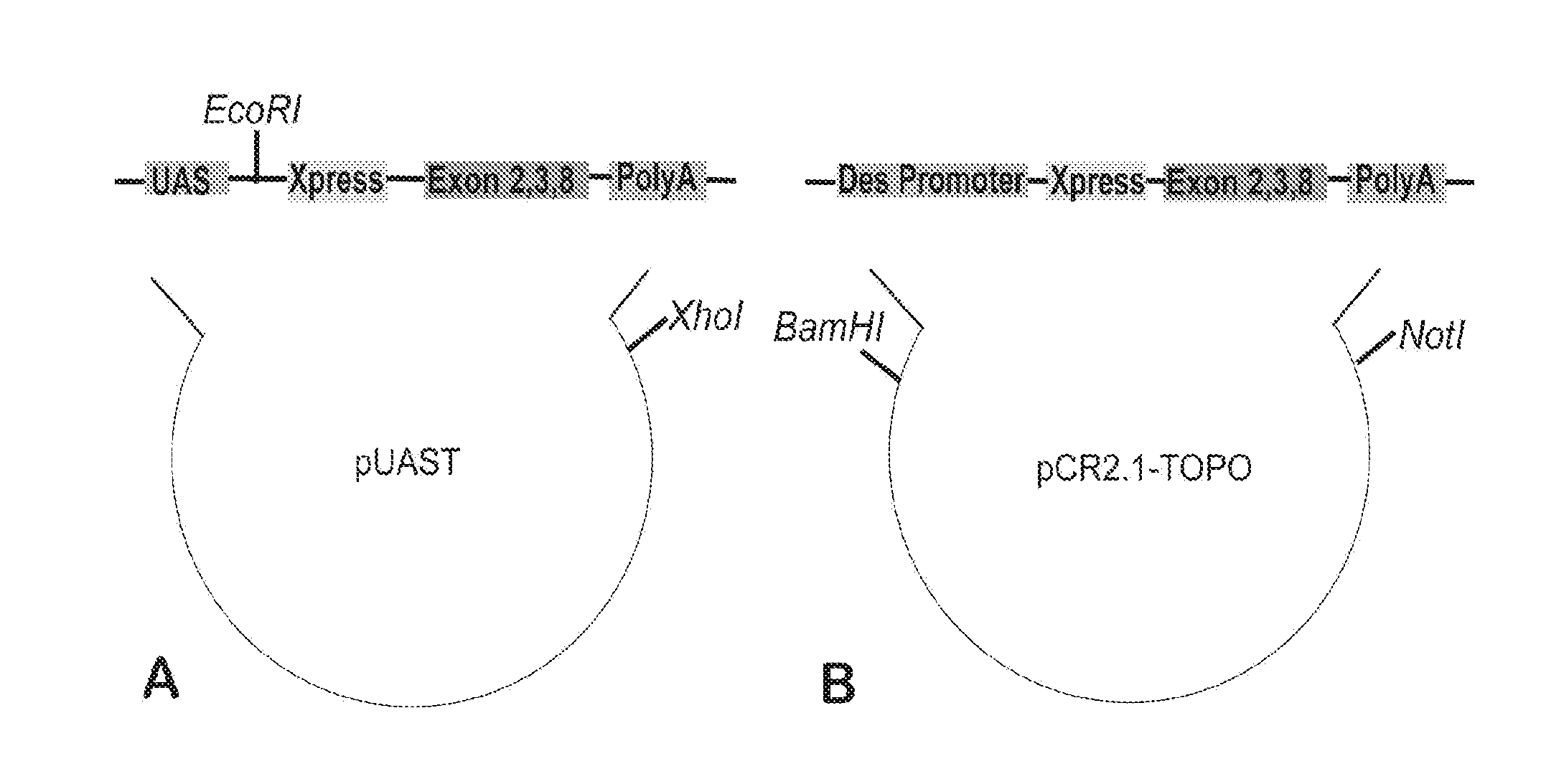
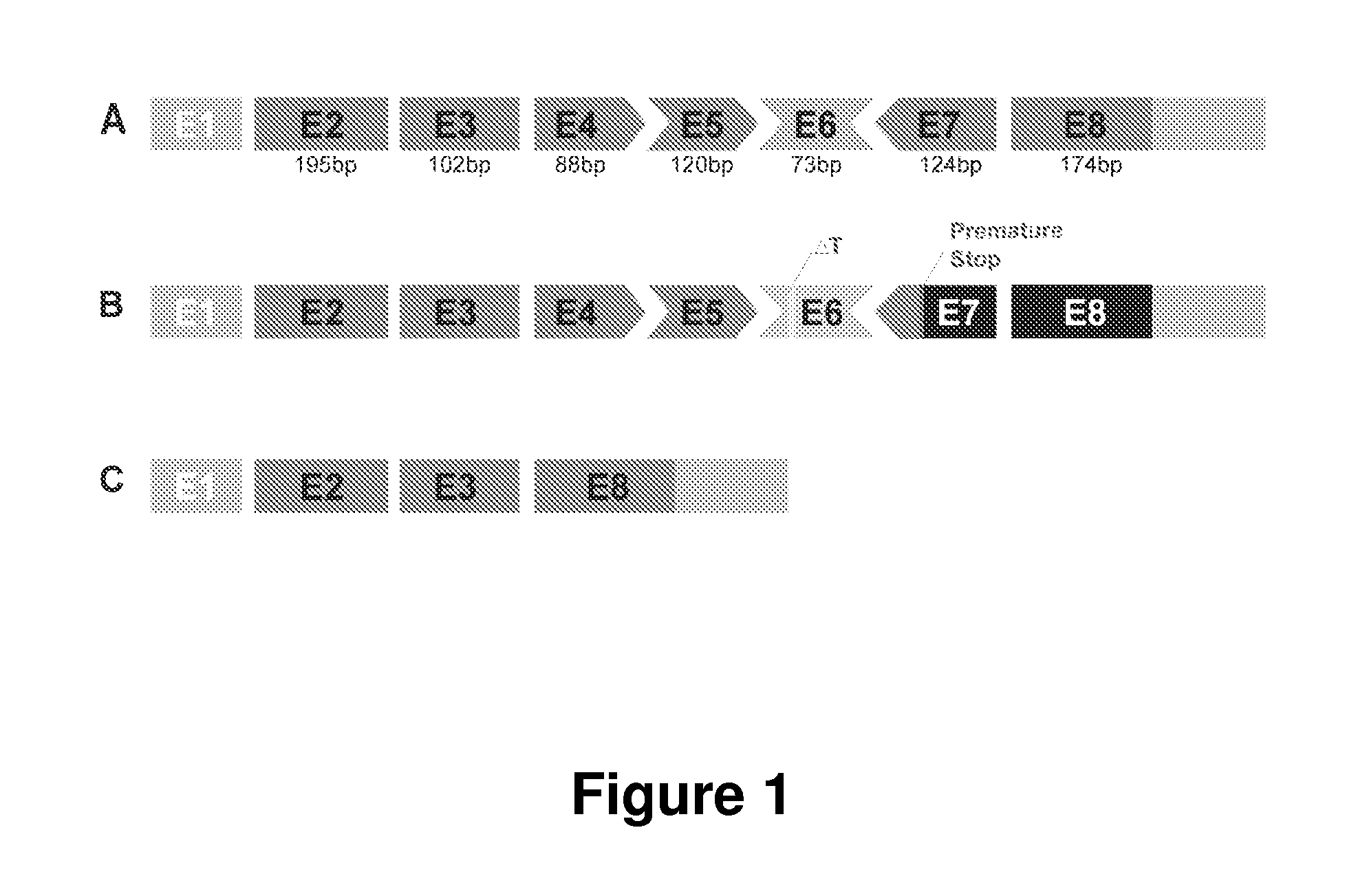
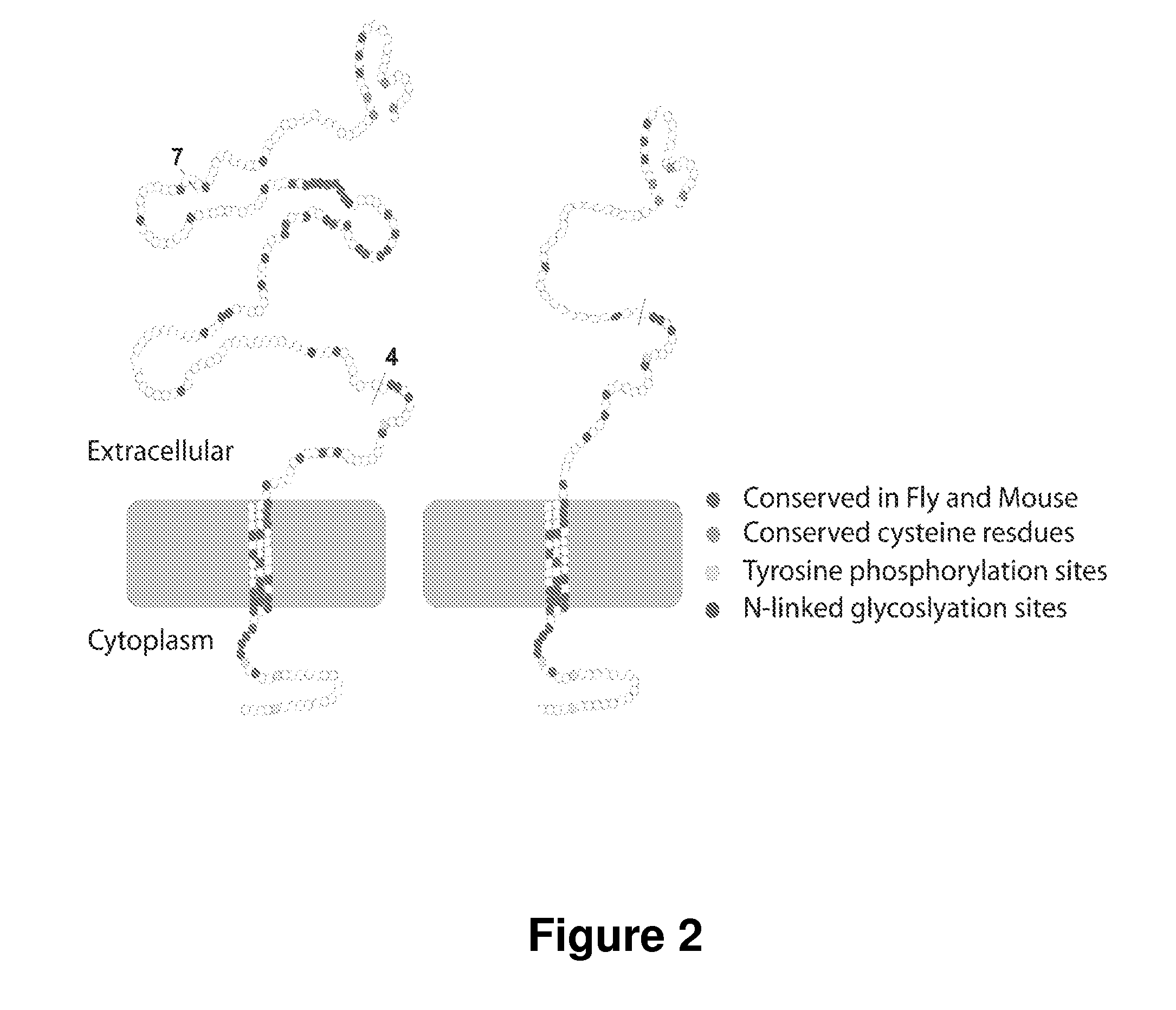
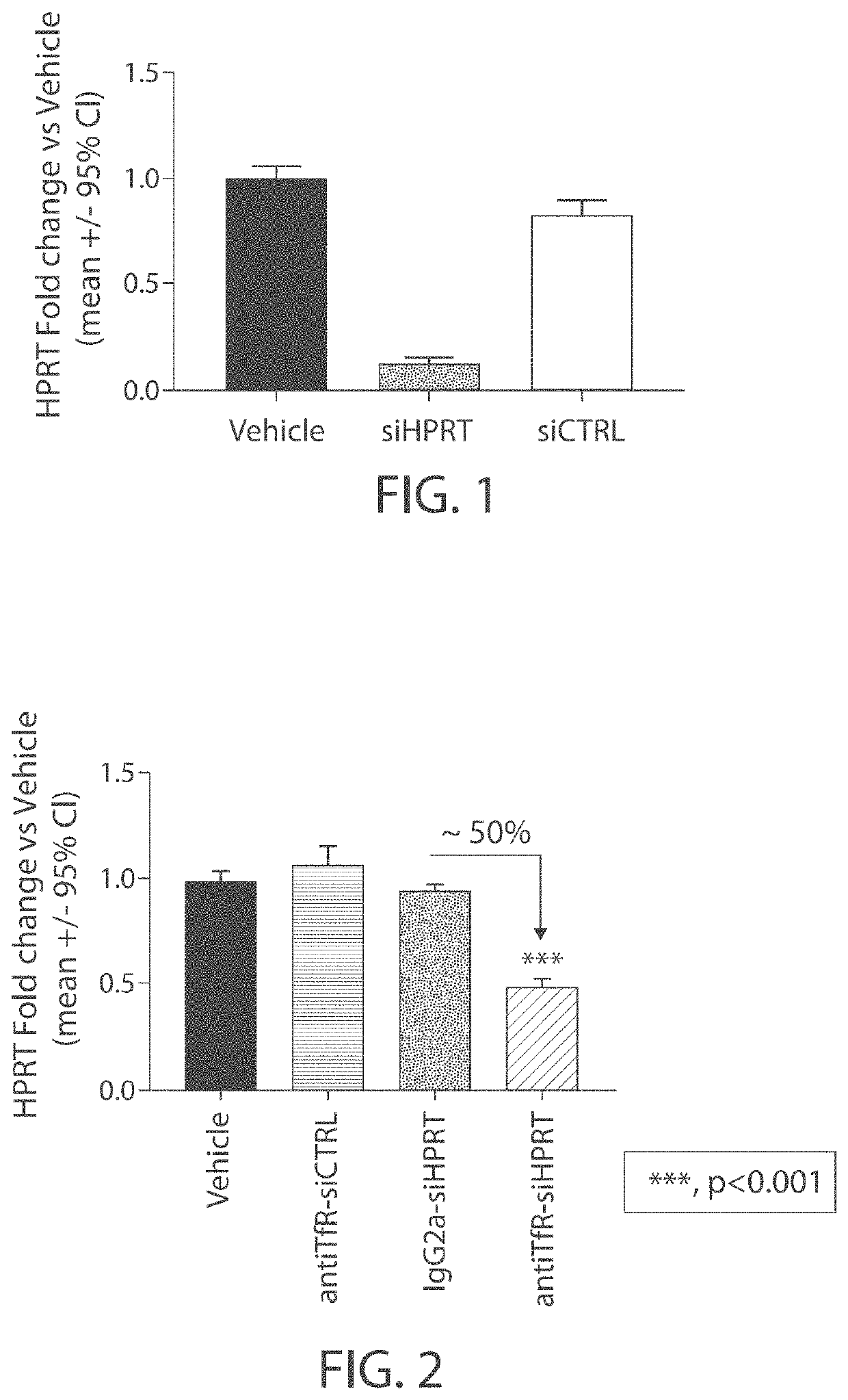
Patents
Literature
107 results about "Exon skipping" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
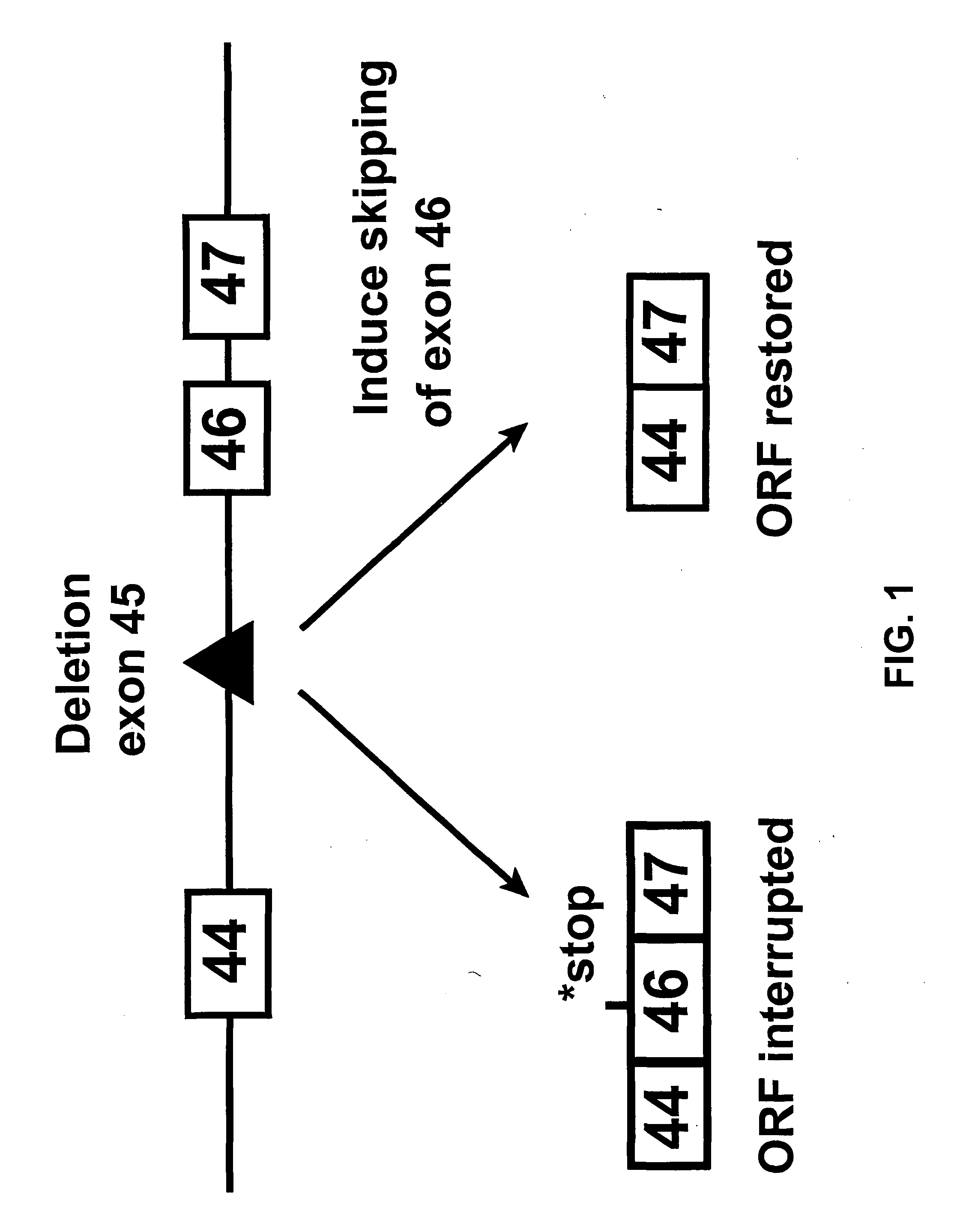
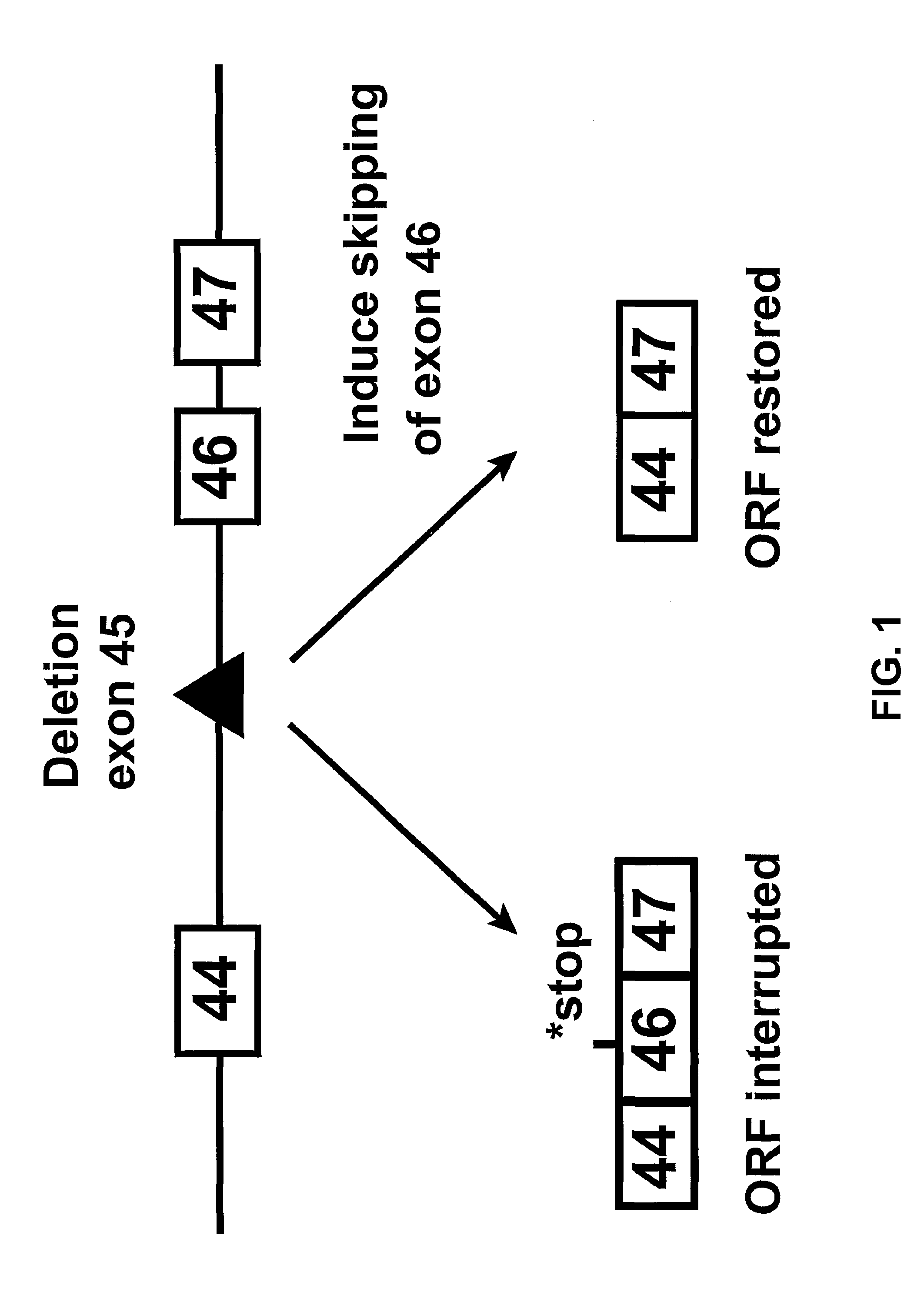
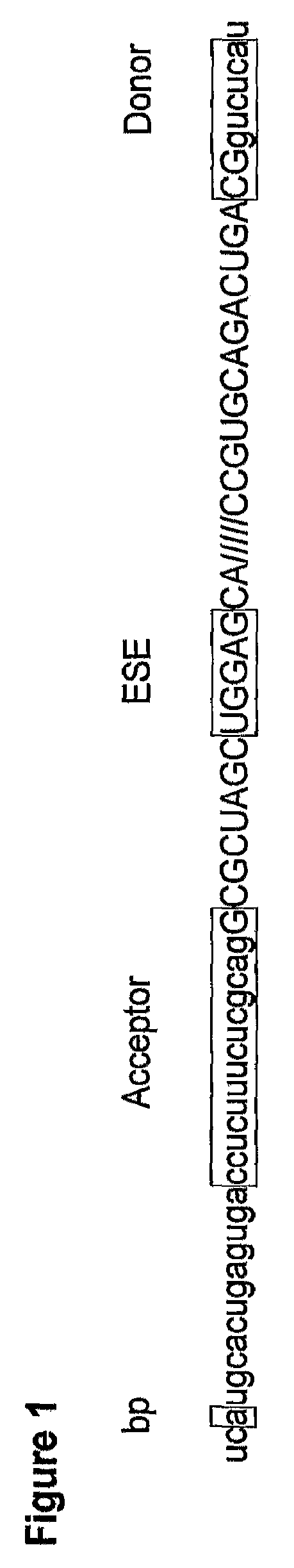
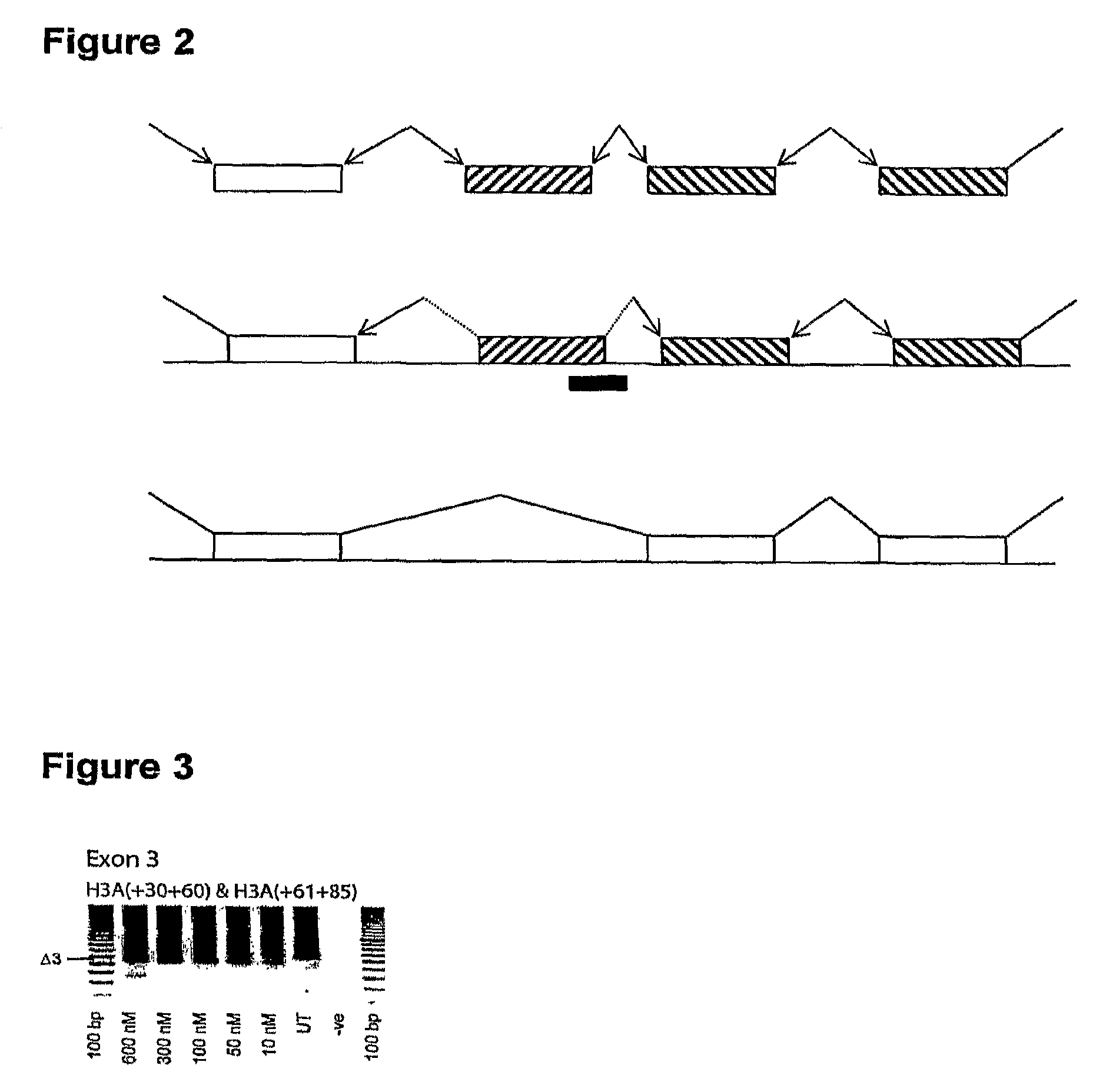
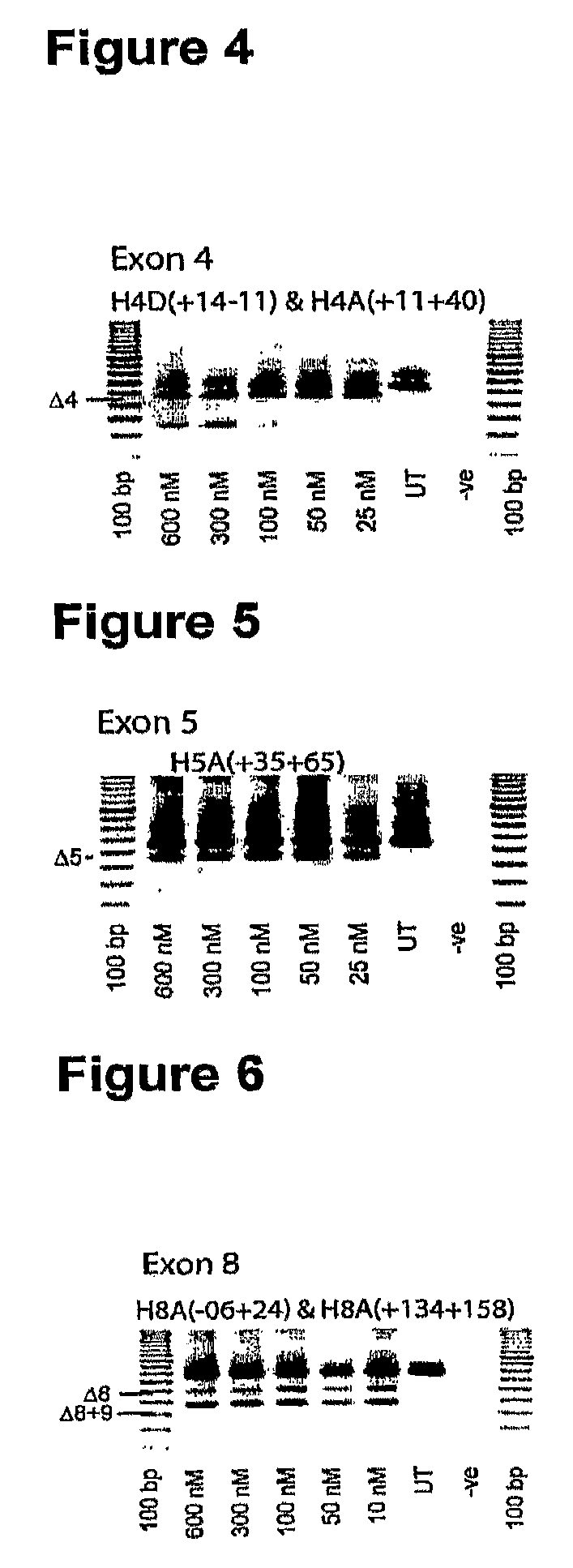
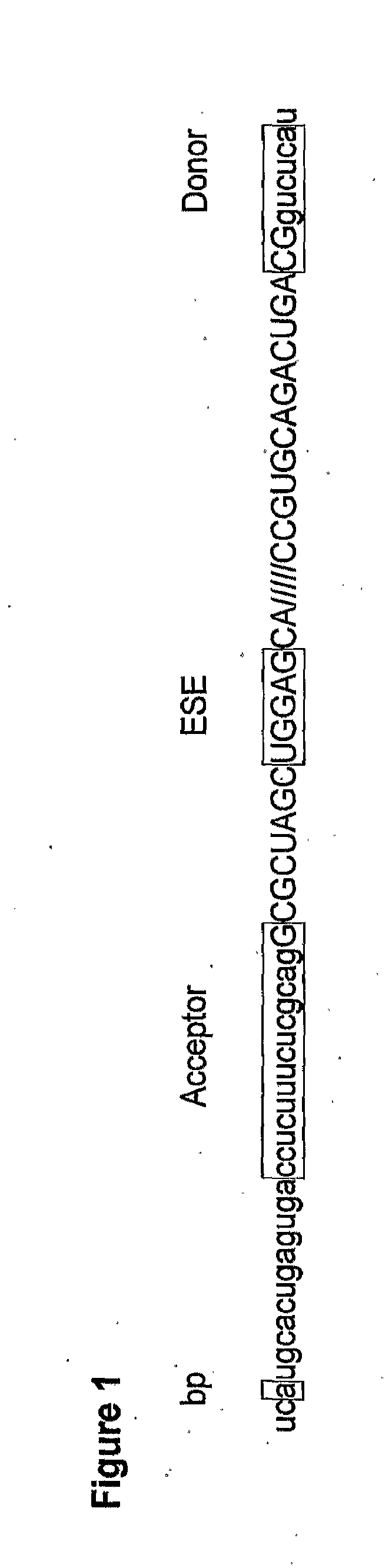
In molecular biology, exon skipping is a form of RNA splicing used to cause cells to “skip” over faulty or misaligned sections of genetic code, leading to a truncated but still functional protein despite the genetic mutation.
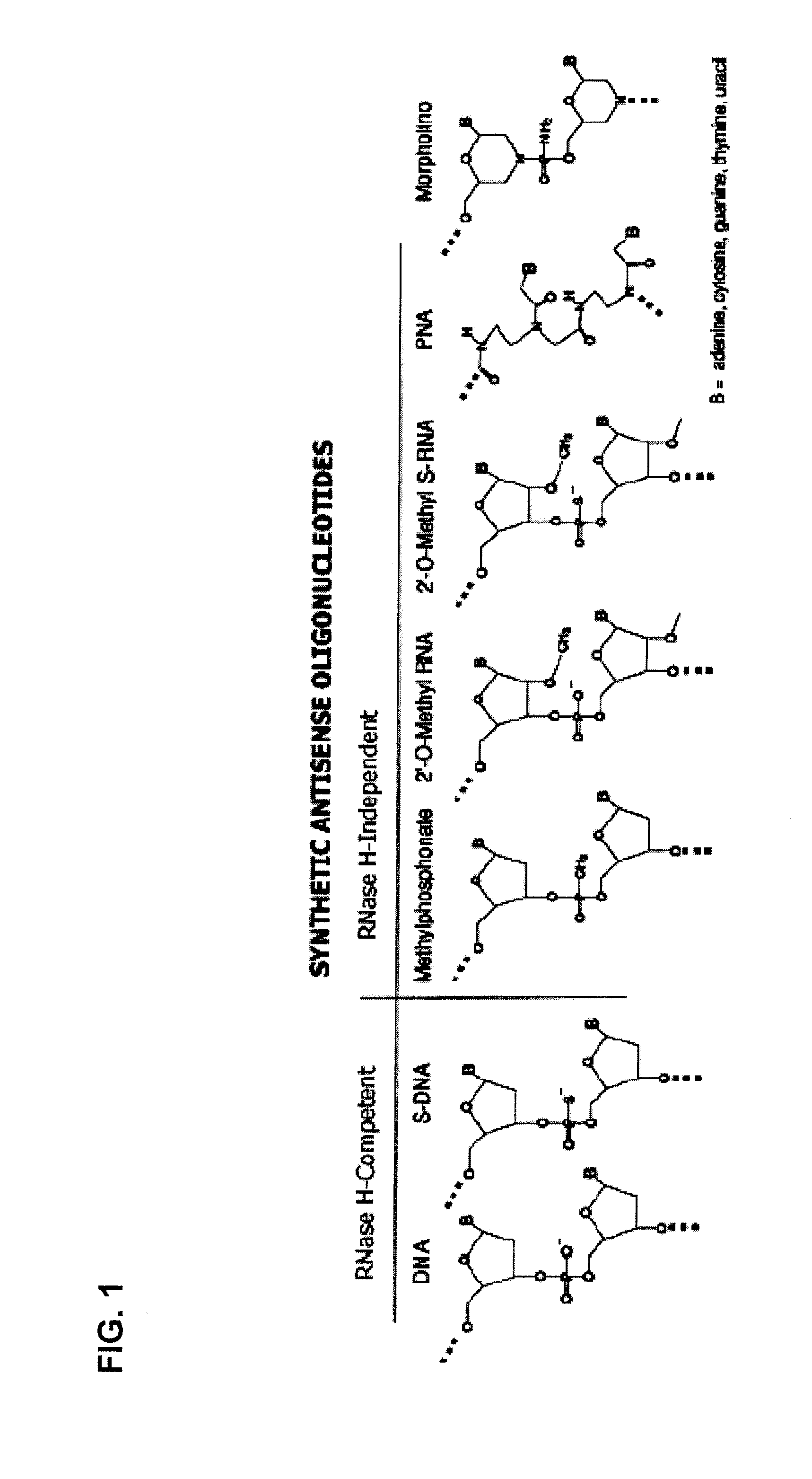
Oligomers
ActiveUS20100168212A1High degreeOrganic active ingredientsSugar derivativesMuscular dystrophyOligomer
Molecules are provided for inducing or facilitating exon skipping in forming spliced mRNA products from pre-mRNA molecules in cells. The molecules may be provided directly as oligonucleotides or expression products of vectors that are administered to a subject. High rates of skipping can be achieved. High rates of skipping reduce the severity of a disease like Duchene Muscular Dystrophy so that the disease is more like Becker Muscular Dystrophy. This is a severe reduction in symptom severity and mortality.
Owner:ROYAL HOLLOWAY & BEDFORD NEW COLLEGE
Induction of exon skipping in eukaryotic cells
InactiveUS7973015B2Efficiently maskedPotential for manipulationOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
The present invention provides a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature MRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:LEIDEN ACADEMISCH ZIEKENHUIS
Oligomers
Molecules are provided for inducing or facilitating exon skipping in forming spliced mRNA products from pre-mRNA molecules in cells. The molecules may be provided directly as oligonucleotides or expression products of vectors that are administered to a subject. High rates of skipping can be achieved. High rates of skipping reduce the severity of a disease like Duchene Muscular Dystrophy so that the disease is more like Becker Muscular Dystrophy. This is a severe reduction in symptom severity and mortality.
Owner:ROYAL HOLLOWAY & BEDFORD NEW COLLEGE
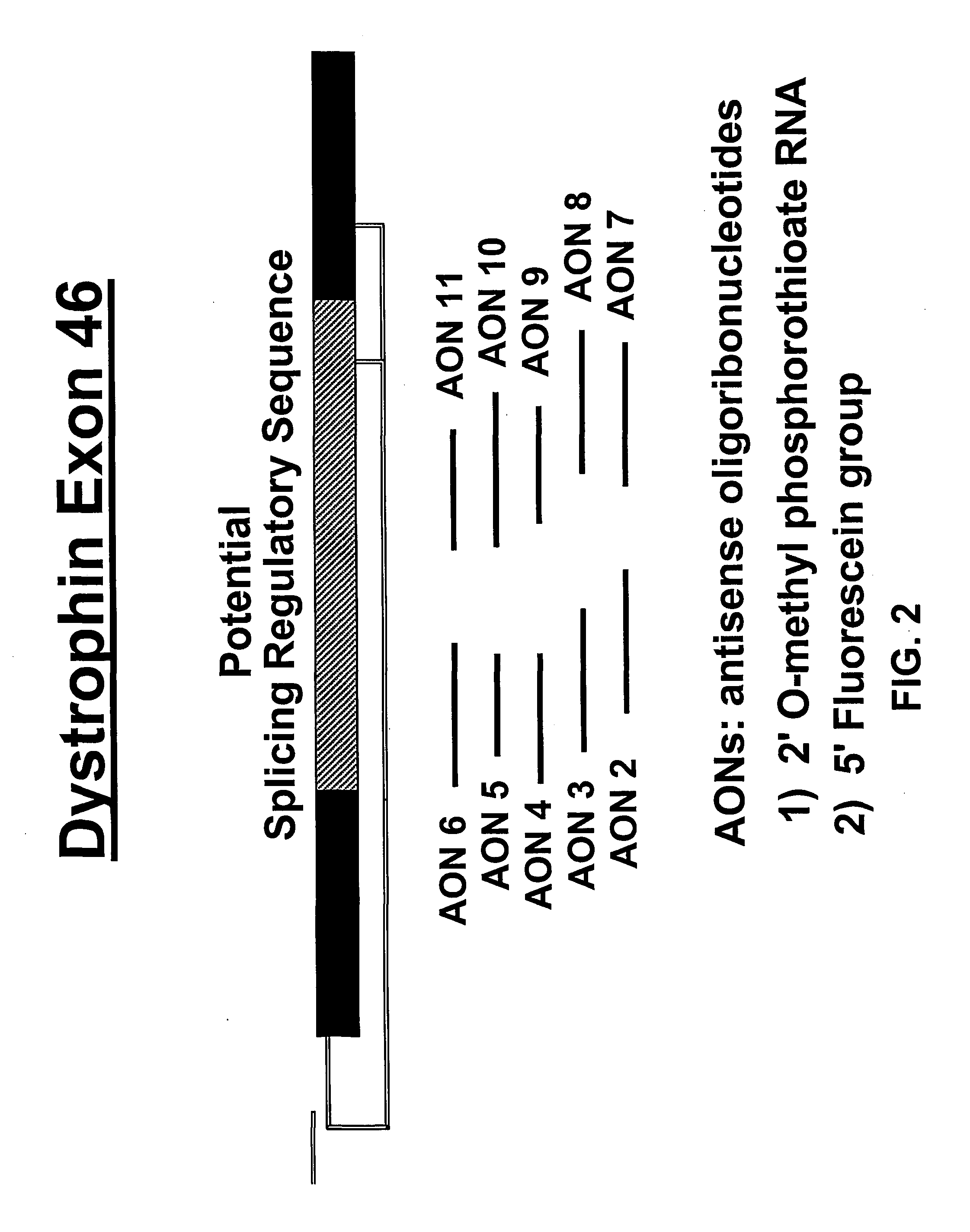
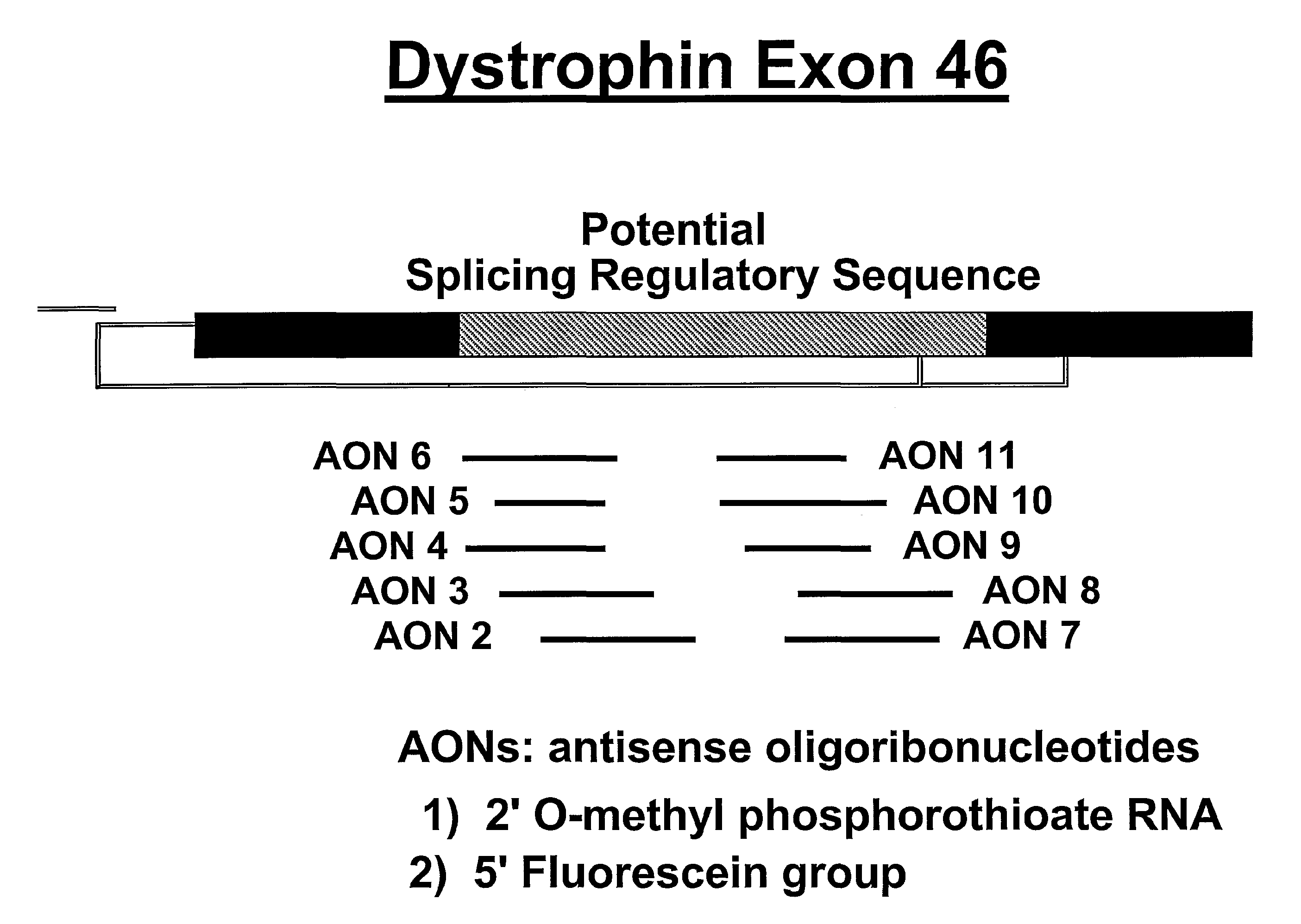
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 202.
Owner:UNIV OF WESTERN AUSTRALIA
Induction of exon skipping in eukaryotic cells
InactiveUS20090228998A1Efficiently maskedPotential for manipulationOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
The present invention provides a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature mRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Induction of exon skipping in eukaryotic cells
InactiveUS20080209581A1Reduce productionReduce amountOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
Described is a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature mRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 202.
Owner:UNIV OF WESTERN AUSTRALIA
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 202.
Owner:UNIV OF WESTERN AUSTRALIA
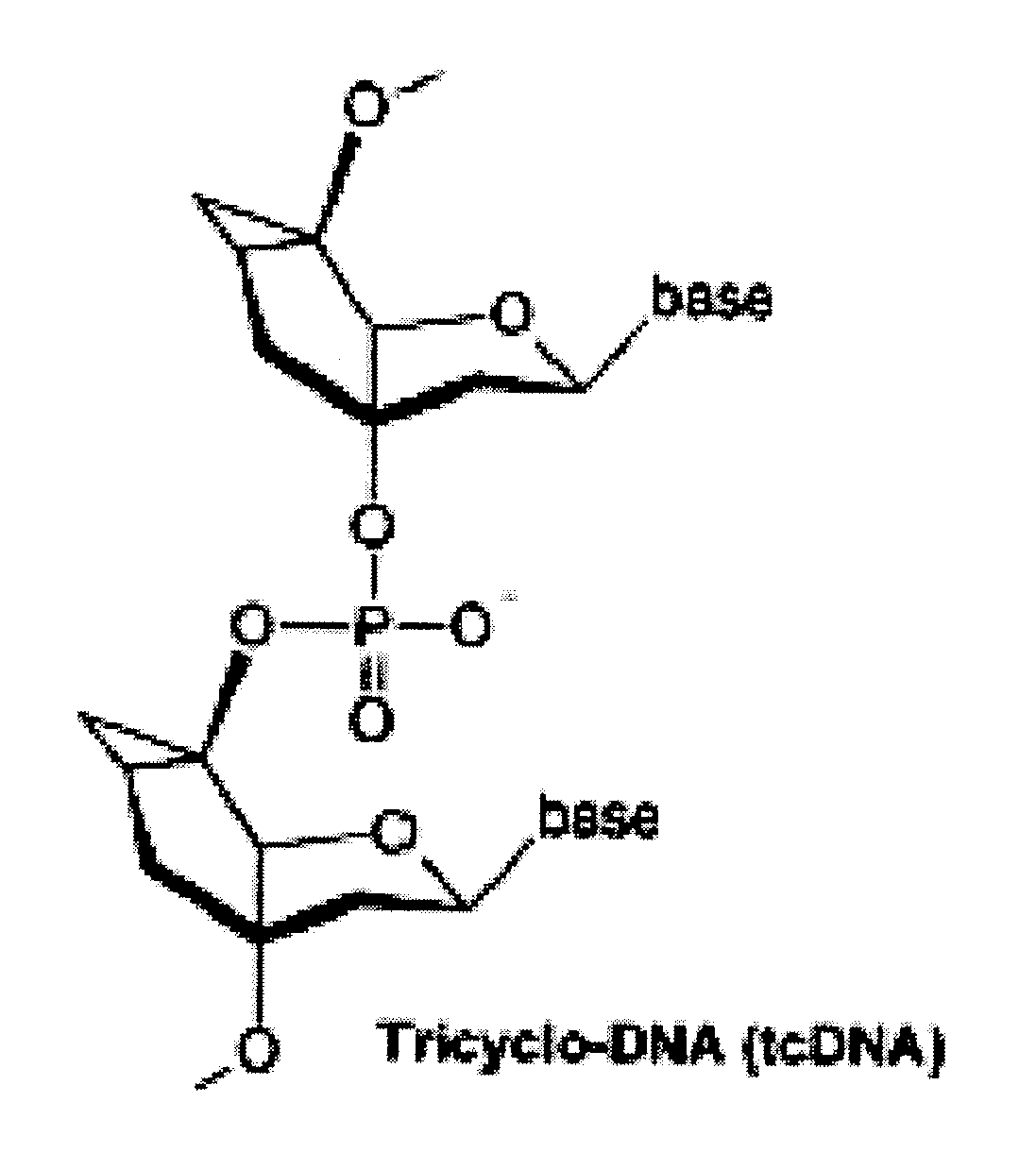
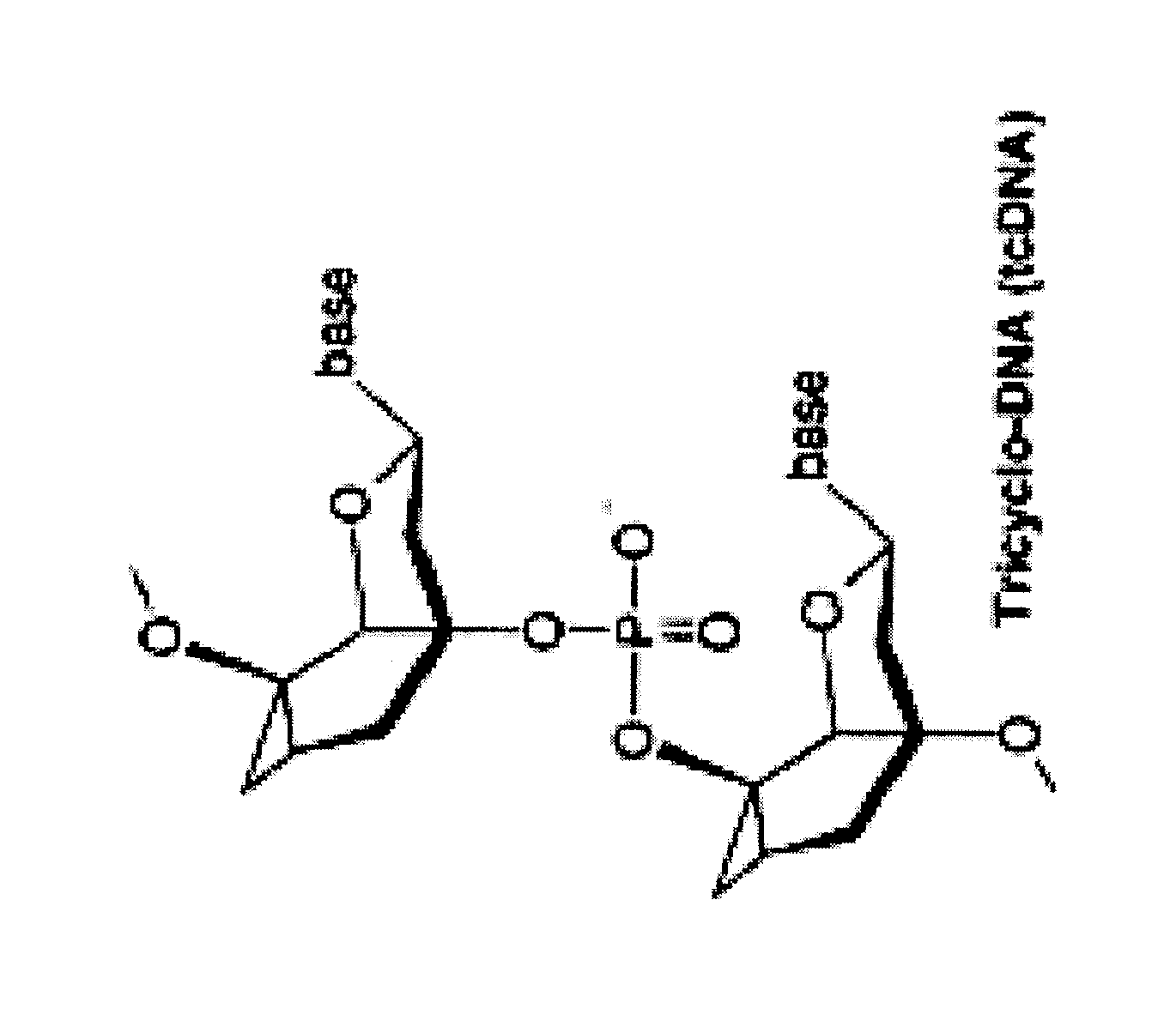
Tricyclo-dna antisense oligonucleotides, compositions, and methods for the treatment of disease
InactiveUS20120149756A1Find utilityFacilitates inclusionOrganic active ingredientsSplicing alterationDiseasePre mrna processing
Provided are tricyclo-DNA (tc-DNA) AON and methods employing tc-DNA AON for modifying splicing events that occur during pre-mRNA processing. Tricyclo-DNA (tc-DNA) AON are described that may be used to facilitate exon skipping or to mask intronic silencer sequences and / or terminal stem-loop sequences during pre-mRNA processing and to target RNase-mediated destruction of processed mRNA. Tc-DNA AON described herein may be used in methods for the treatment of Duchenne Muscular Dystrophy by skipping a mutated exon 23 or exon 51 within a dystrophin gene to restore functionality of a dystrophin protein; in methods for the treatment of Spinal Muscular Atrophy by masking an intronic silencing sequence and / or a terminal stem-loop sequence within an SMN2 gene to yield modified functional SMN2 protein, including an amino acid sequence encoded by exon 7, which is capable of at least partially complementing a non-functional SMN1 protein; and in methods for the treatment of Steinert's Myotonic Dystrophy by targeting the destruction of a mutated DM1 mRNA comprising 3′-terminal CUG repeats.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
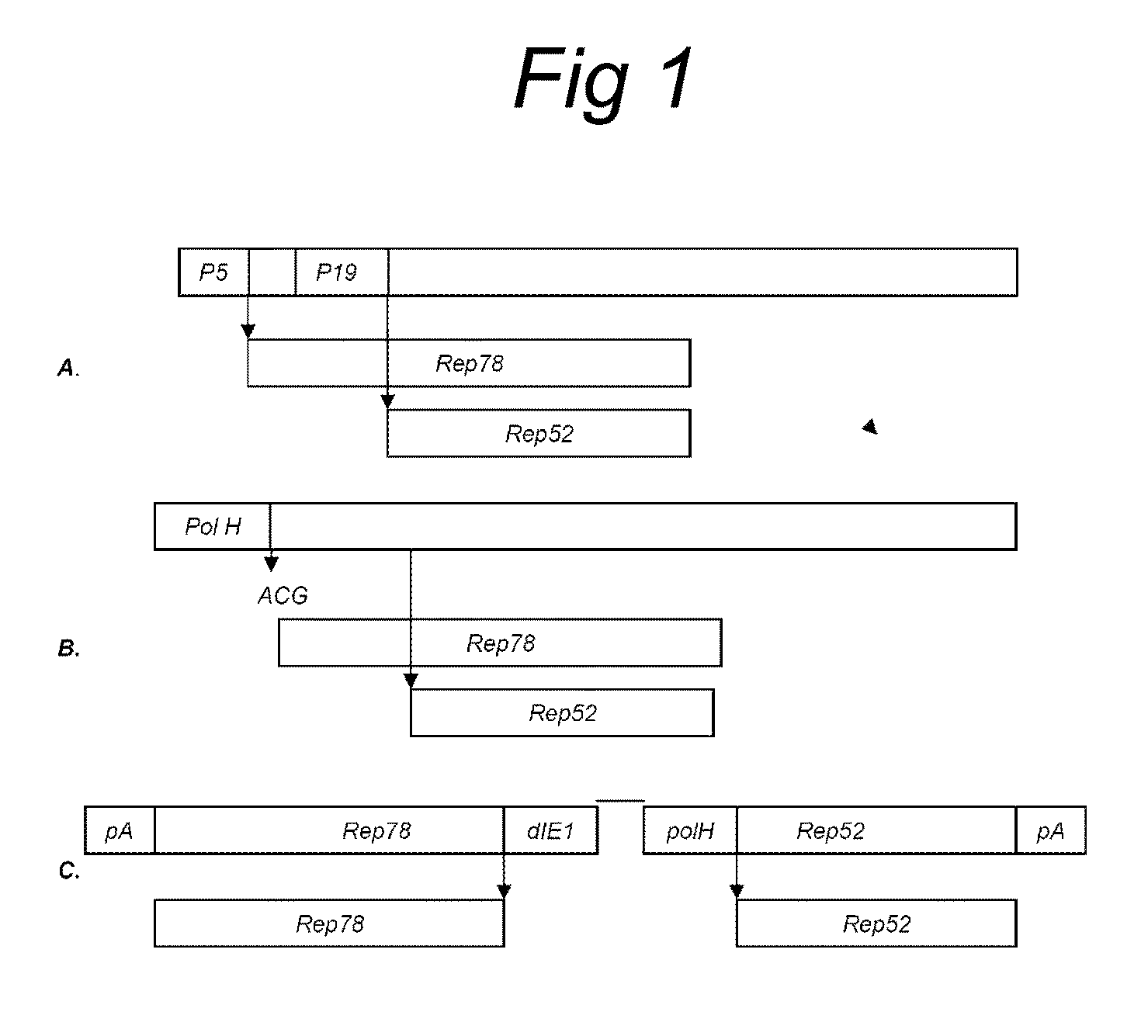
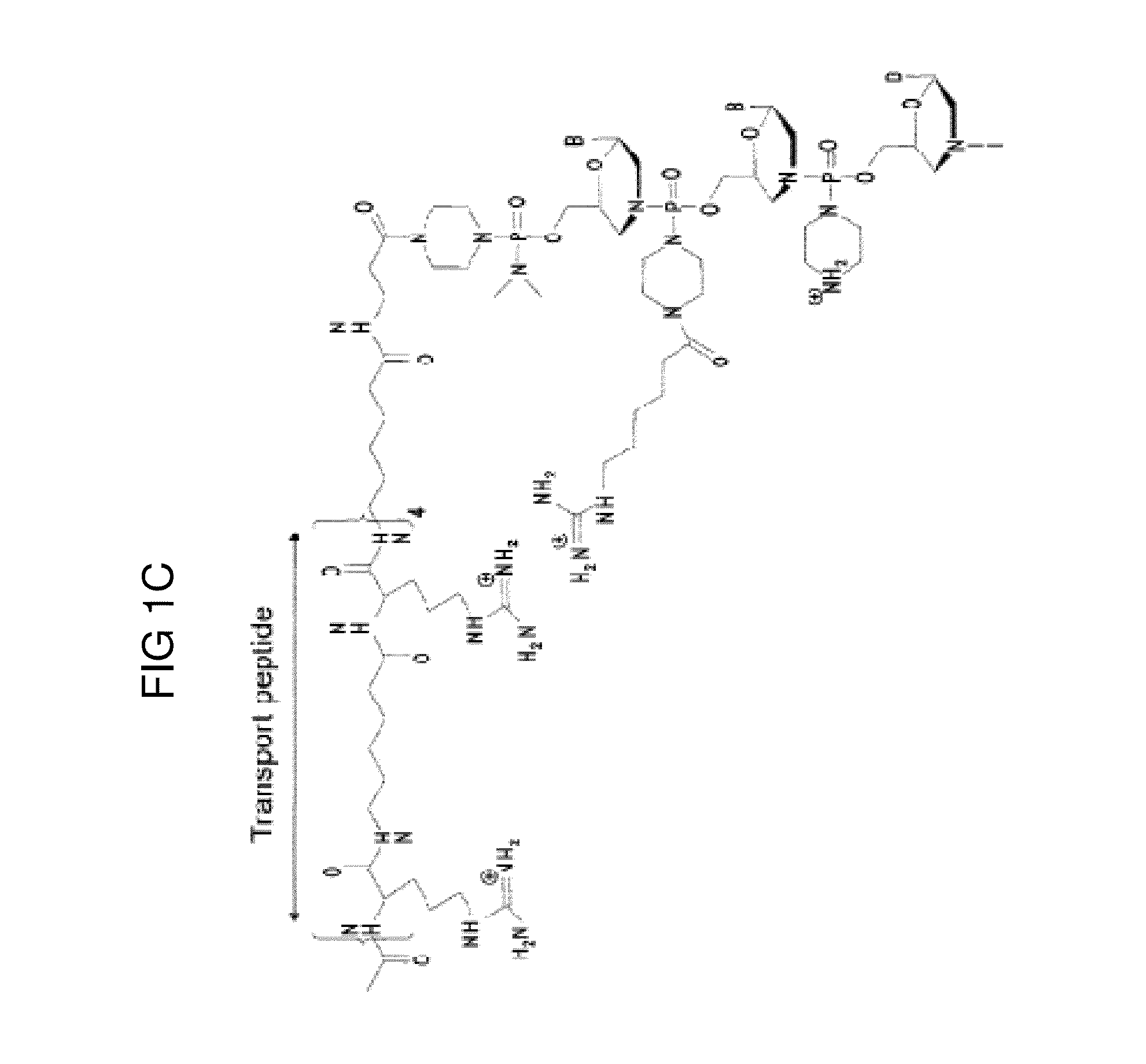
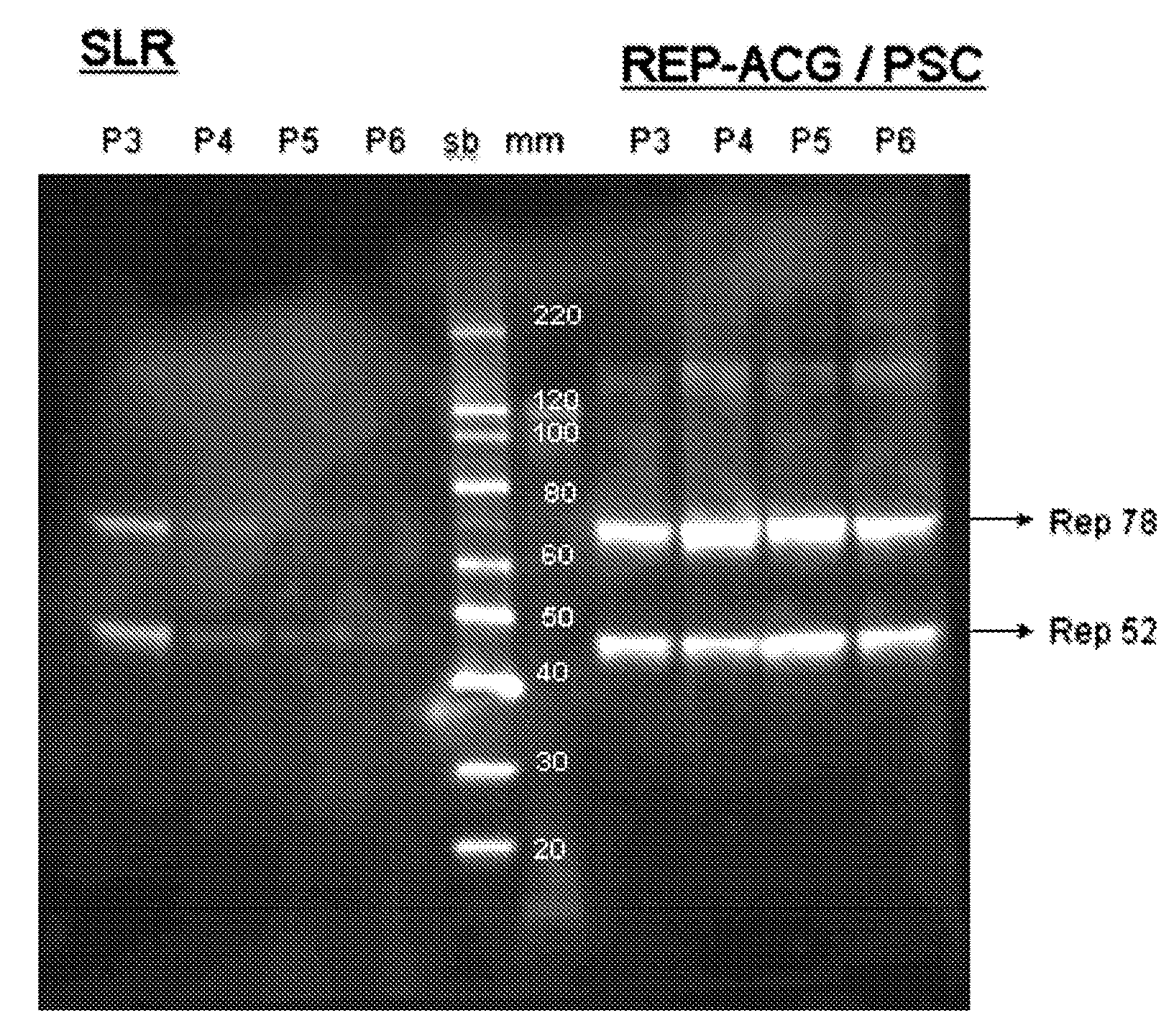
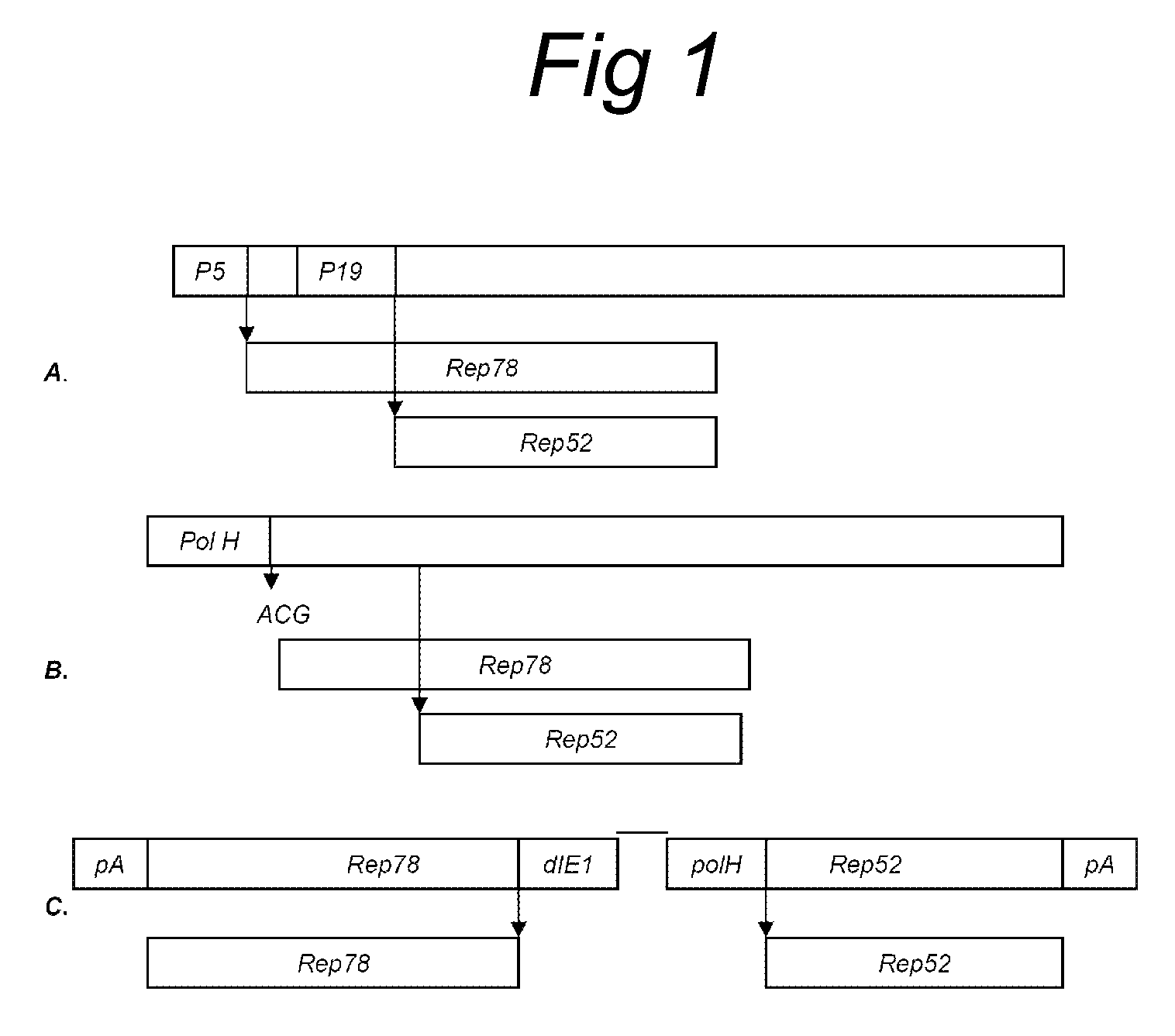
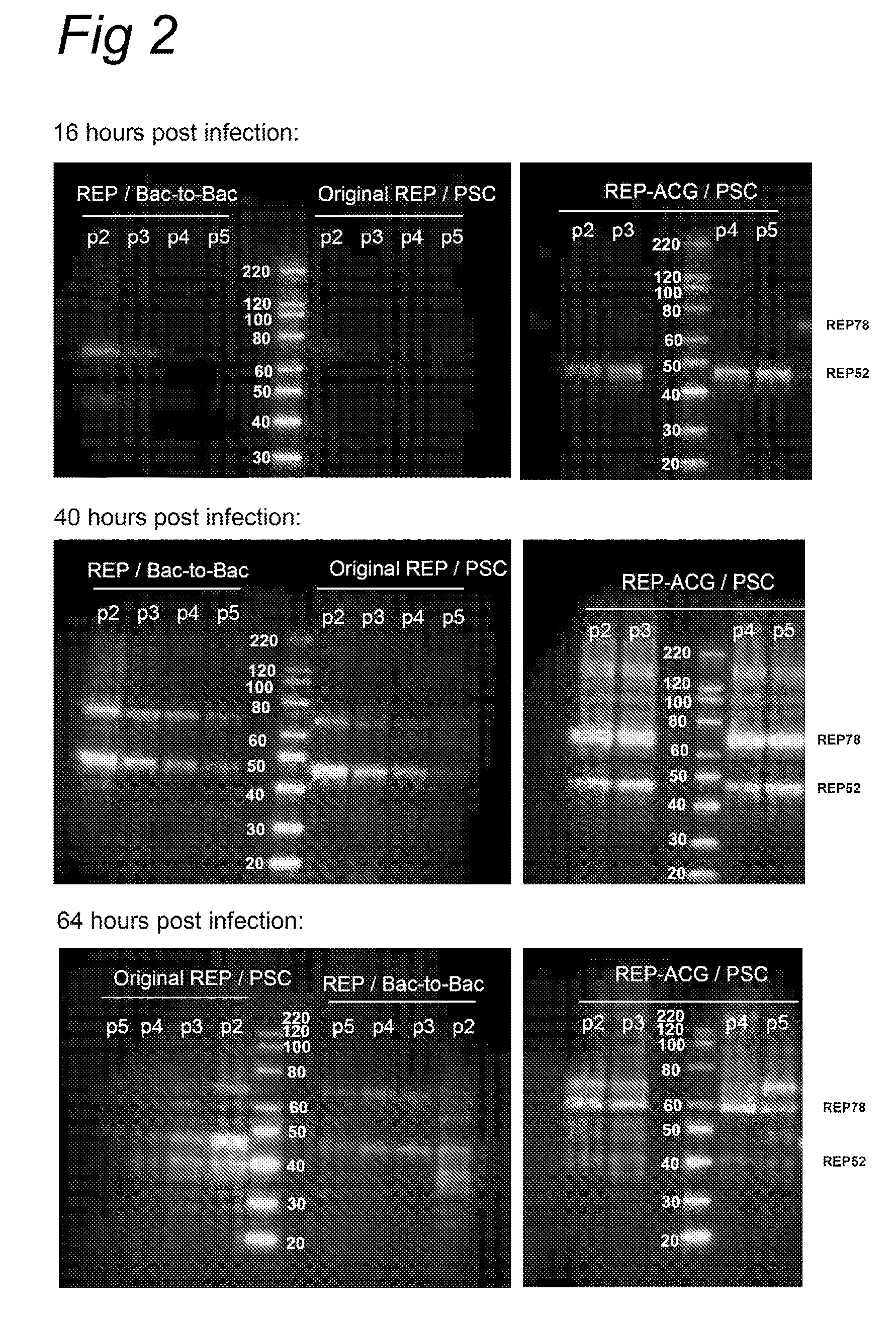
Vectors with modified initiation codon for the translation of AAV-Rep78 useful for production of AAV
ActiveUS8512981B2Improve stabilityReduced expression levelVirus peptidesNucleic acid vectorStart codonNucleotide
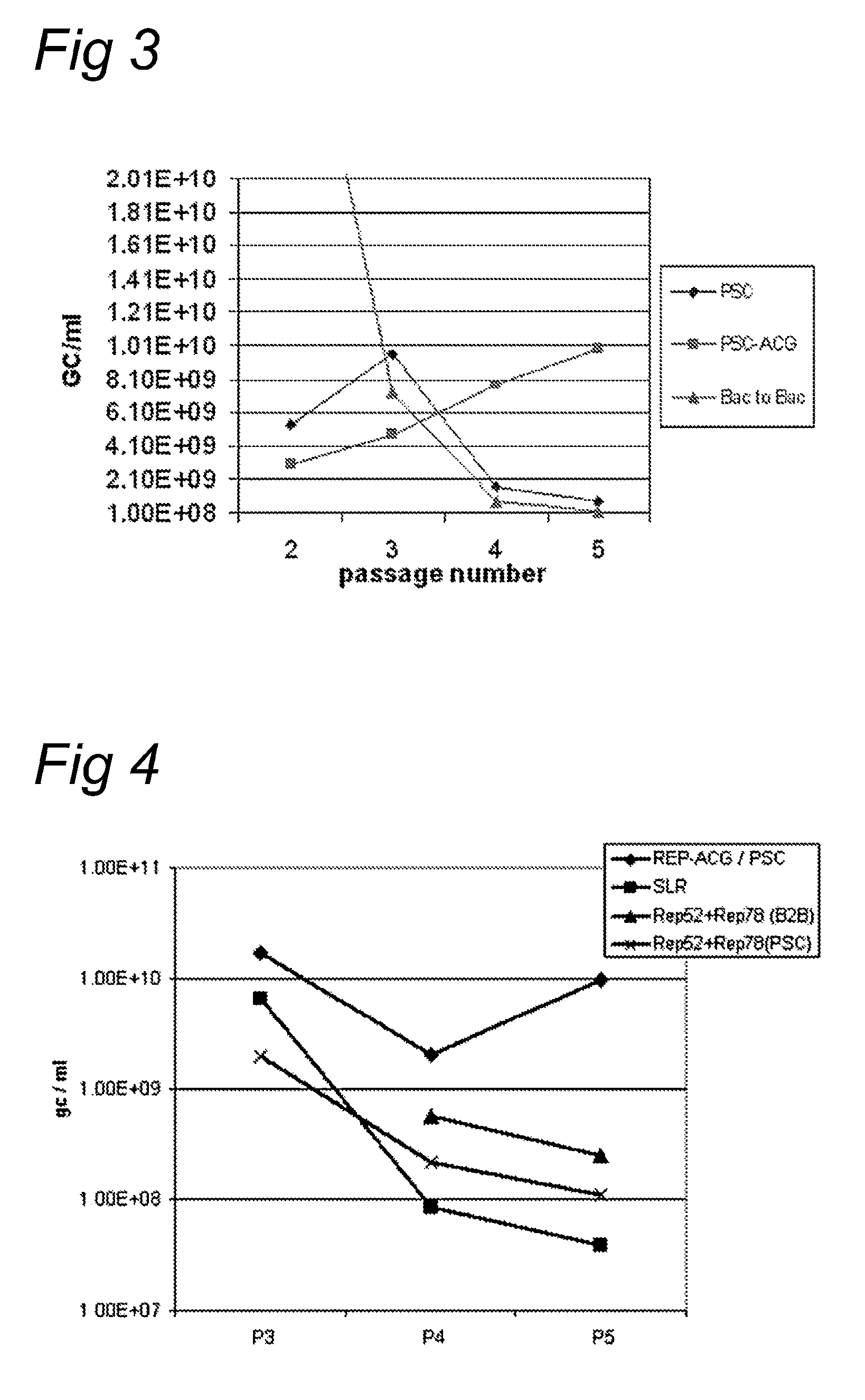
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS +1
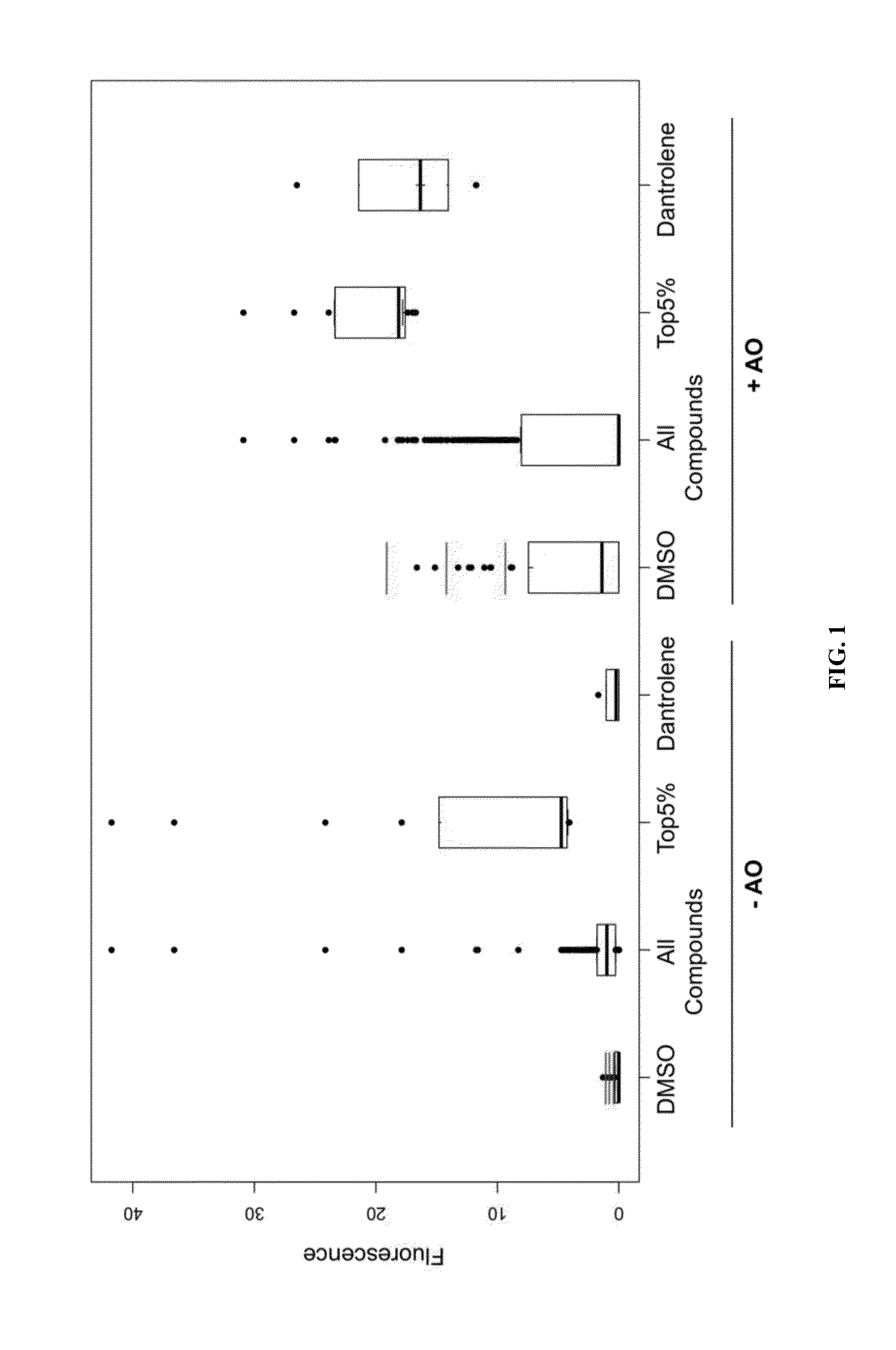
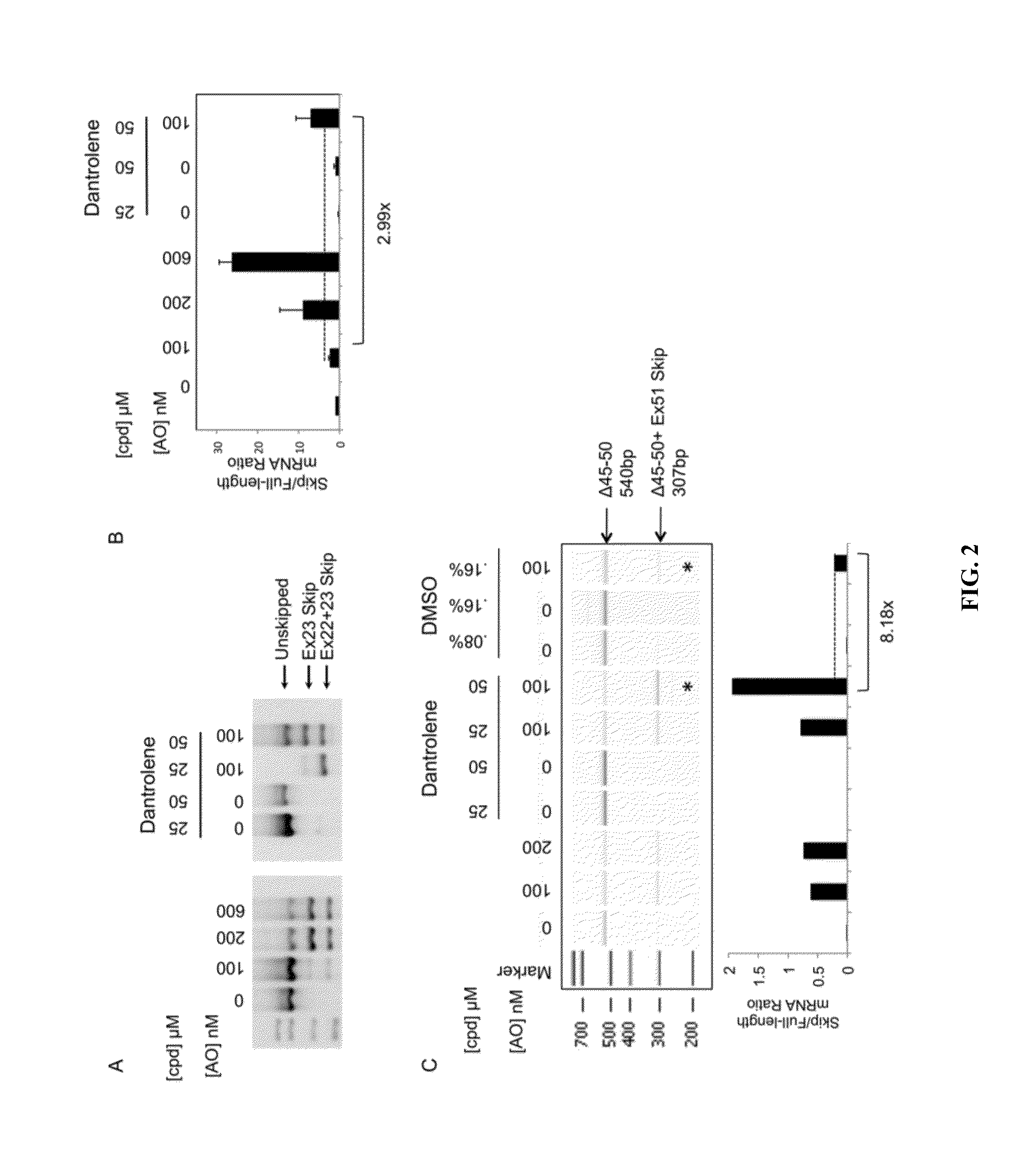
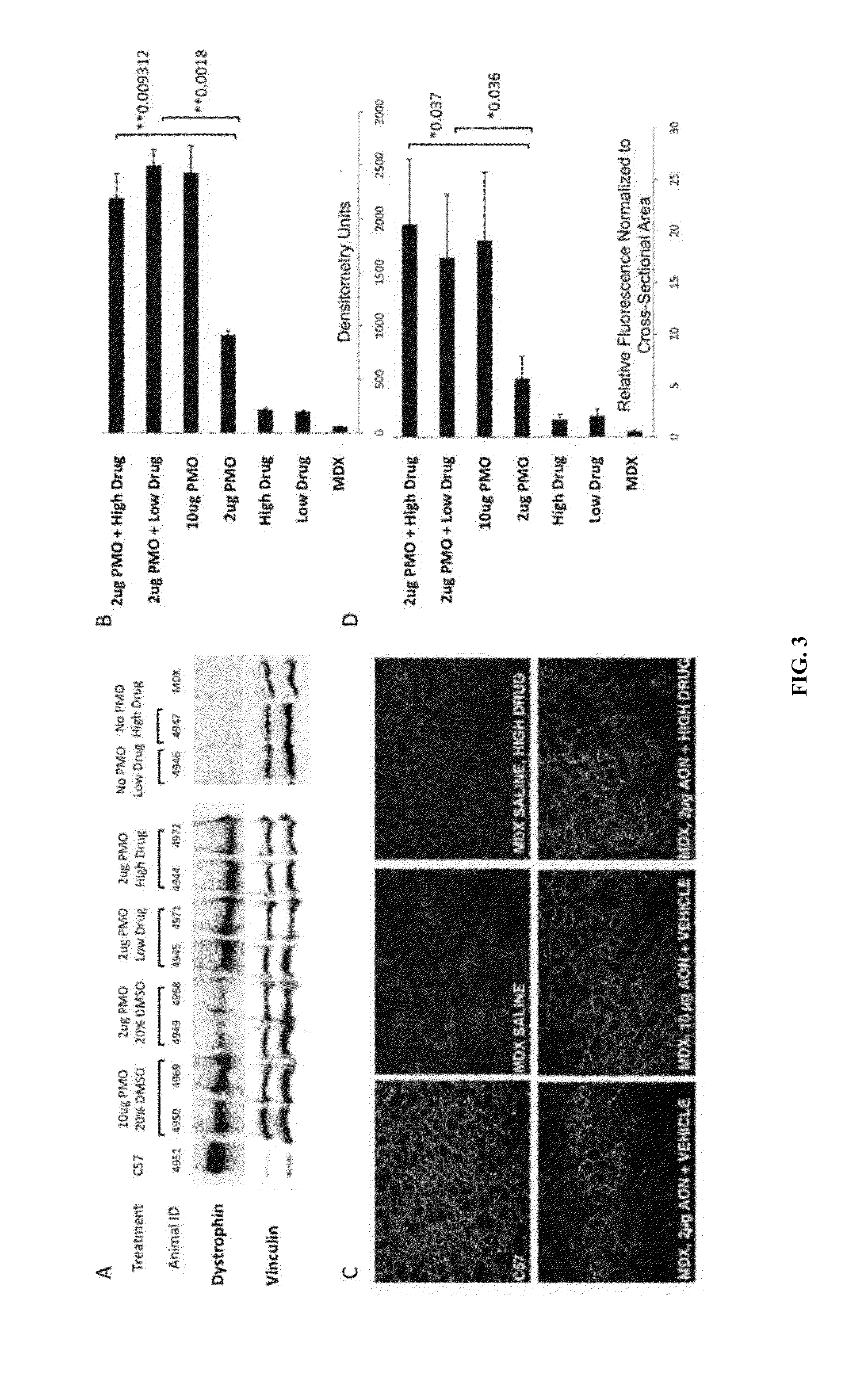
Identification of small molecules that facilitate therapeutic exon skipping
This invention relates, e.g., to a method for enhancing exon skipping in a pre-mRNA of interest, comprising contacting the pre-mRNA with an effective amount of a small molecule selected from the compounds shown in Table 1, or a pharmaceutically acceptable salt, hydrate, solvate, or isomer thereof, and, optionally, with an antisense oligonucleotide that is specific for a splicing sequence in the pre-mRNA Methods for treating Duchenne muscular dystrophy (DMD) are disclosed.
Owner:RGT UNIV OF CALIFORNIA
Antisense molecules and methods for treating pathologies
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 59.
Owner:UNIV OF WESTERN AUSTRALIA
Antisense molecules and methods for treating pathologies
ActiveUS20120270925A1Specific and efficient exon skippingOrganic active ingredientsSplicing alterationExon skippingCell biology
An antisense molecule capable of binding to a selected target site to induce exon skipping in the dystrophin gene, as set forth in SEQ ID NO: 1 to 59.
Owner:UNIV OF WESTERN AUSTRALIA
Exon skipping compositions for treating muscular dystrophy
InactiveUS20140315977A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationDuchenne muscular dystrophyMuscular dystrophy
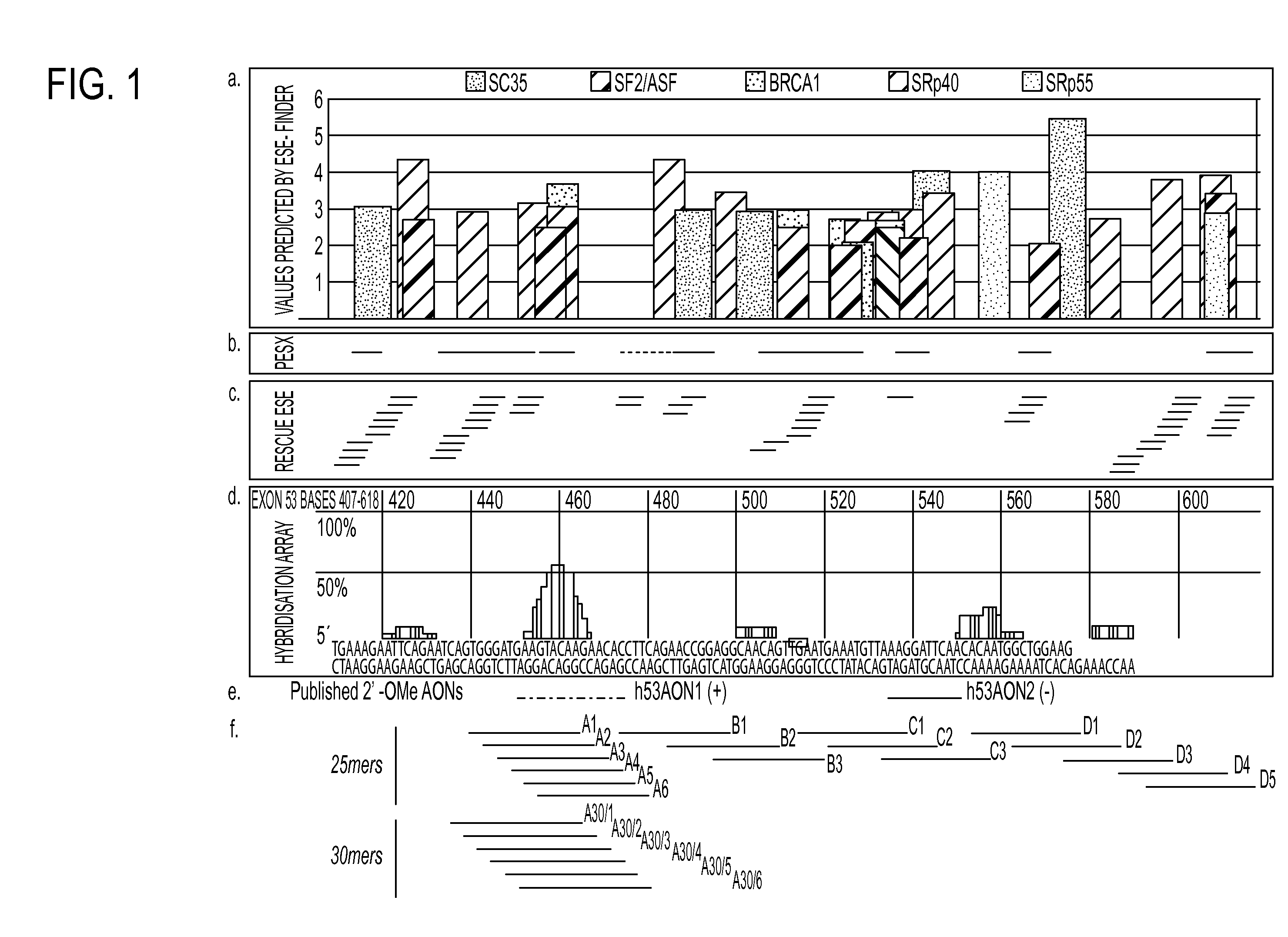
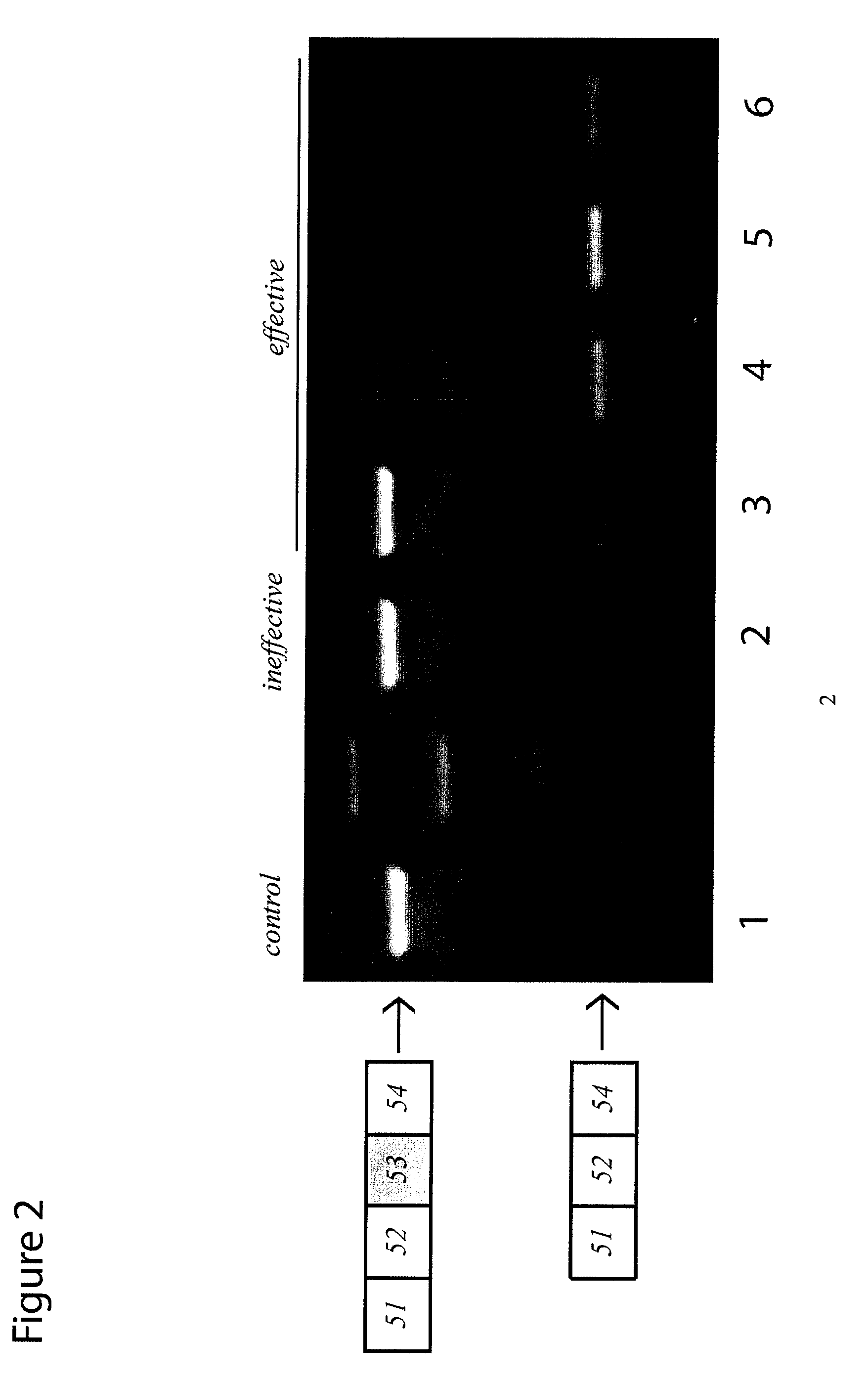
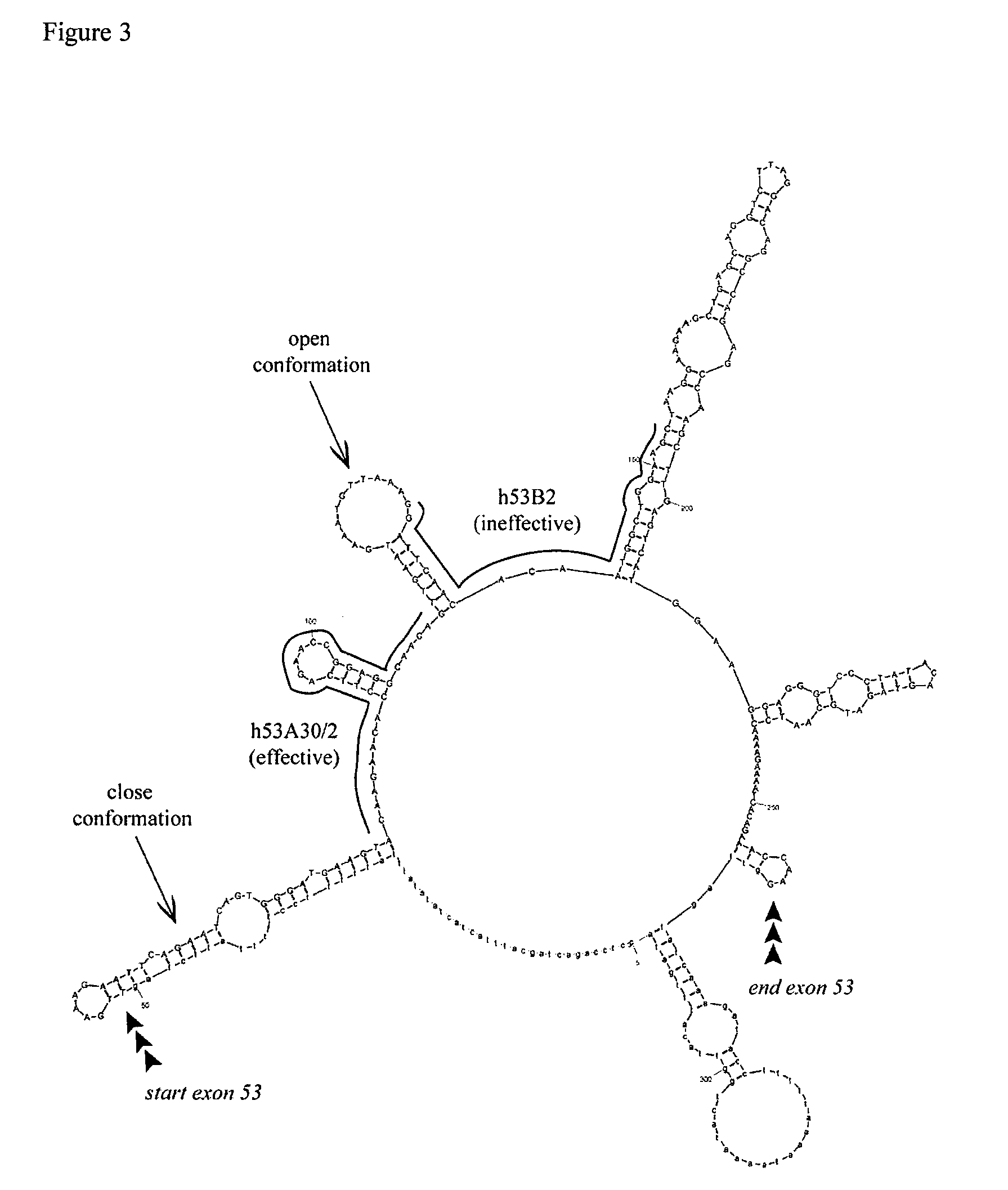
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 53 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Vectors with modified initiation codon for the translation of aav-rep78 useful for production of aav
ActiveUS20130296532A1Improve stabilityReduced expression levelVirus peptidesNucleic acid vectorNucleotideCapsid
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:UNIQURE IP BV
Exon skipping compositions for treating muscular dystrophy
InactiveUS20160040162A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationDuchenne muscular dystrophyMuscular dystrophy
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 53 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
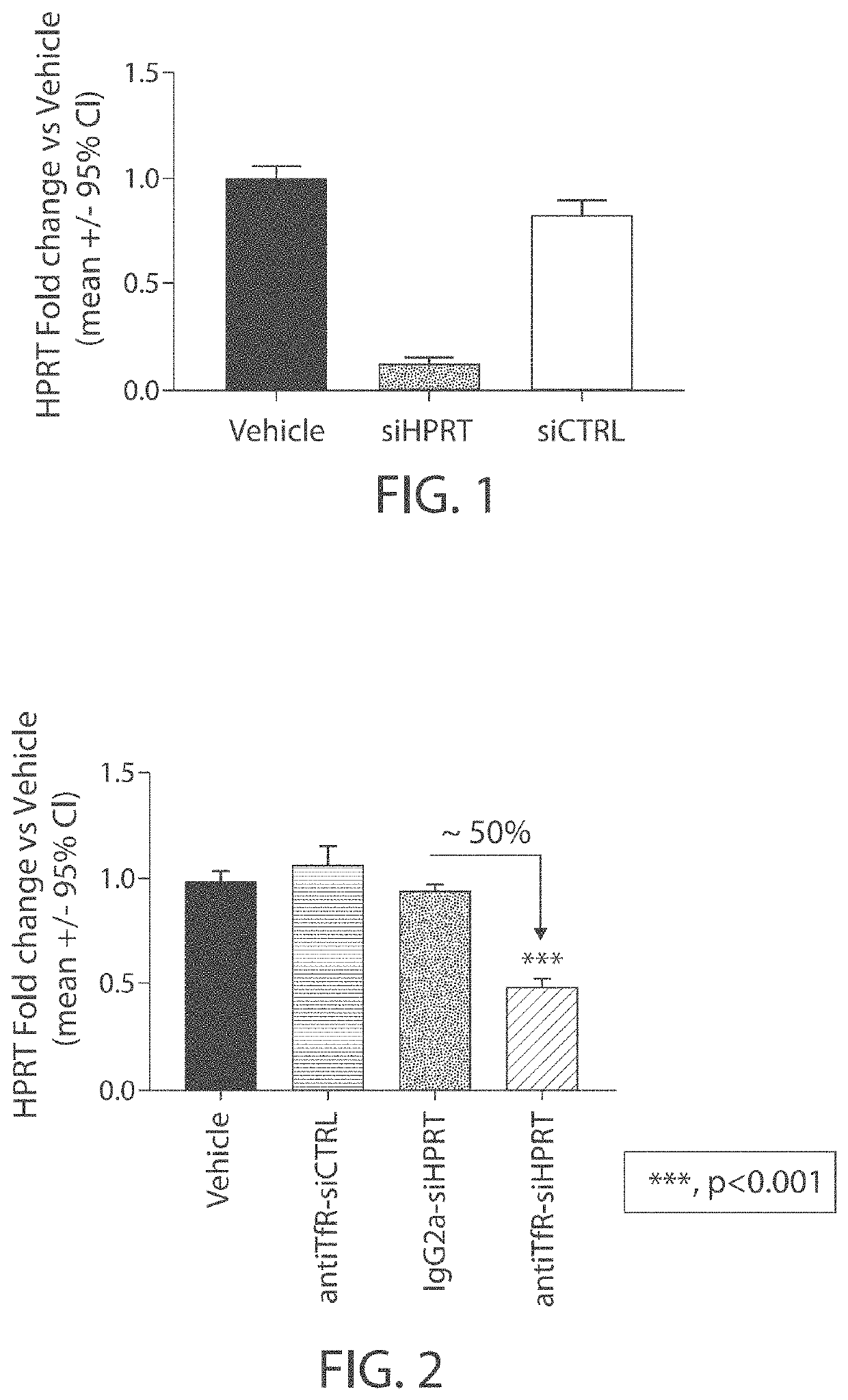
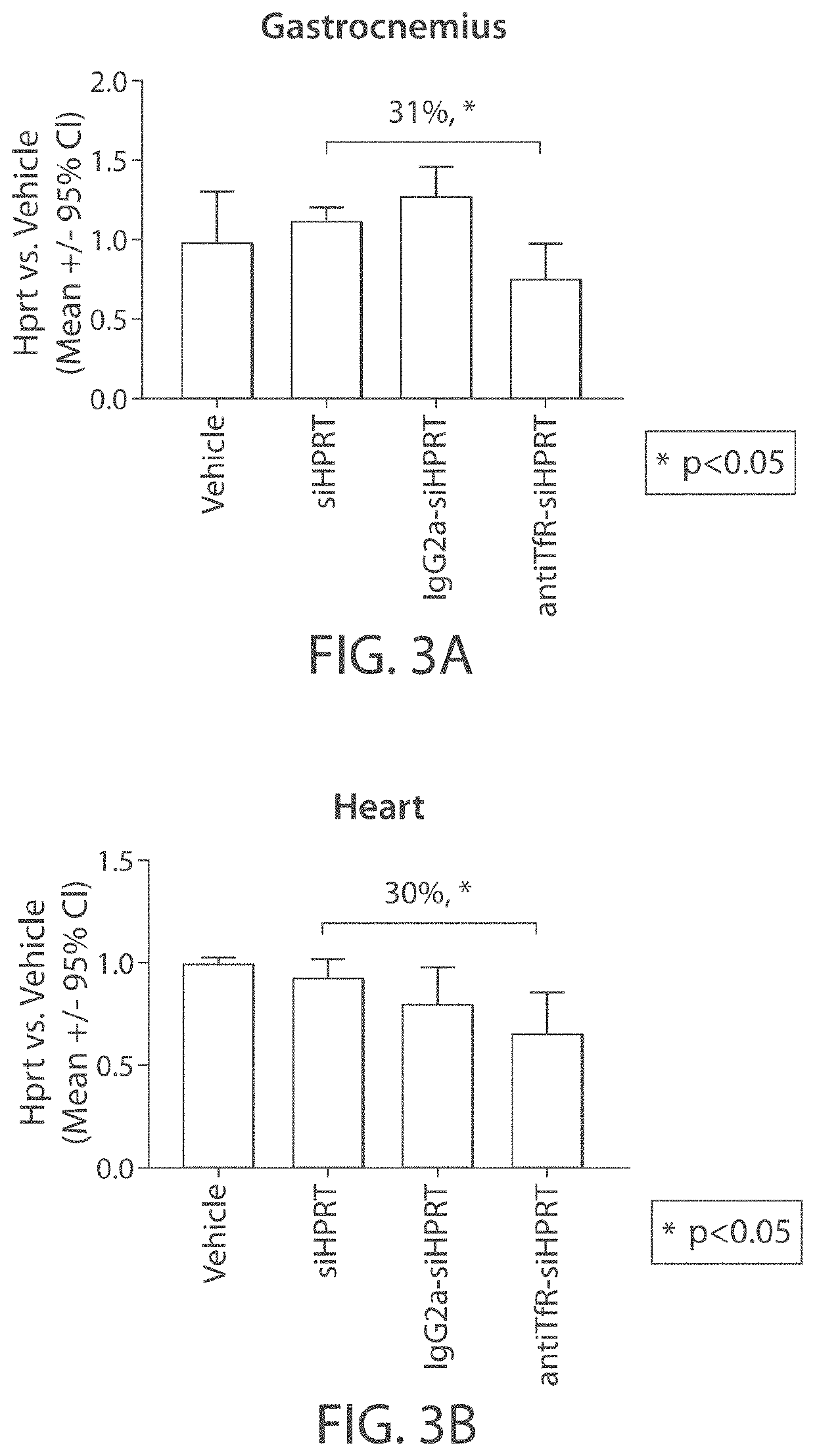
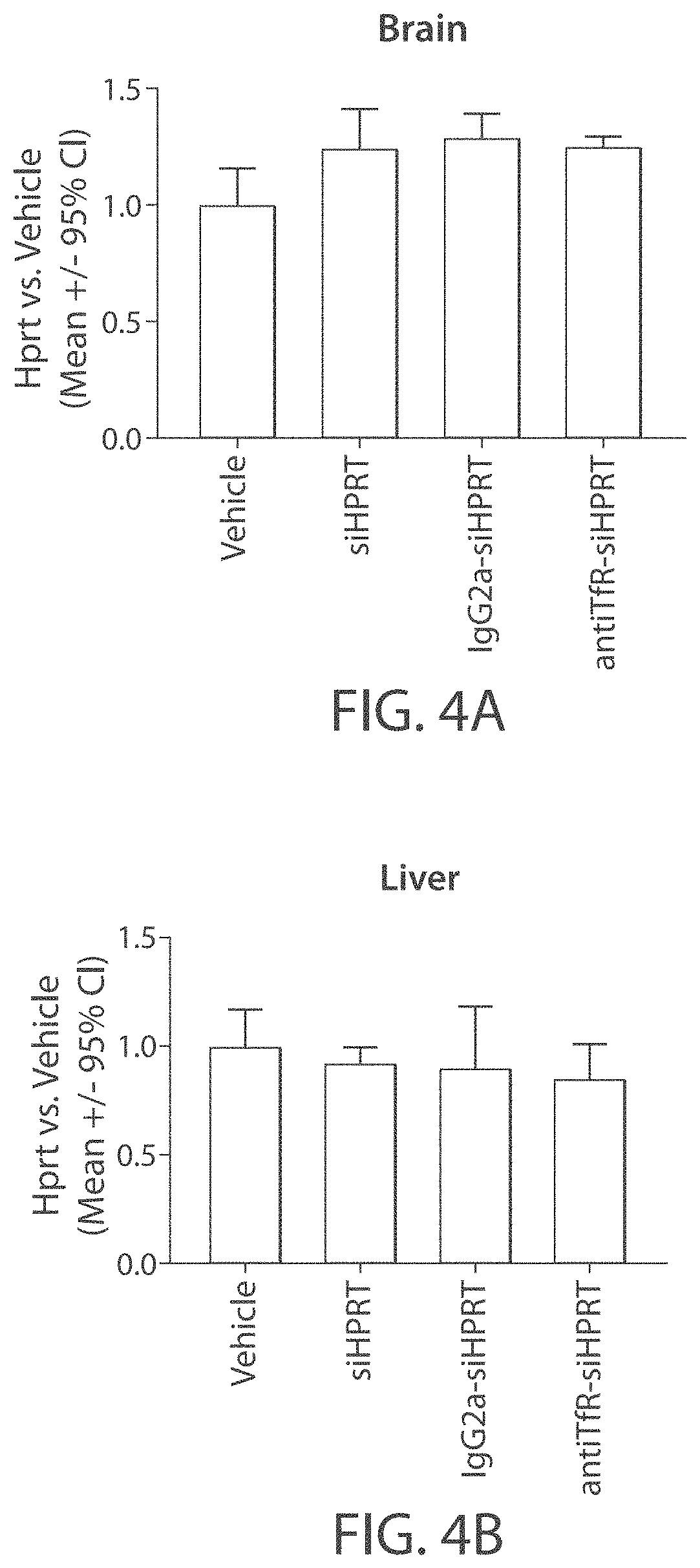
Mannose-6-phosphate receptor mediated gene transfer into muscle cells
InactiveUS20110110960A1Organic active ingredientsSpecial deliveryDystrophinMannose 6-phosphate receptor
The invention relates to glycoside-compound conjugates for use in antisense strategies and / or gene therapy. The conjugates comprise a glycoside linked to a compound, in which the glycoside is a ligand capable of binding to a mannose-6-phosphate receptor of a muscle cell. For example the cells are muscle cells of a Duchenne Muscular Dystrophy (DMD) patient and the conjugate comprises an antisense oligonucleotide which causes exon skipping and induces or restores the synthesis of dystrophin or variants thereof.
Owner:PROSENSA
Exon skipping compositions for treating muscular dystrophy
ActiveUS20140323544A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationMedicineDystrophin
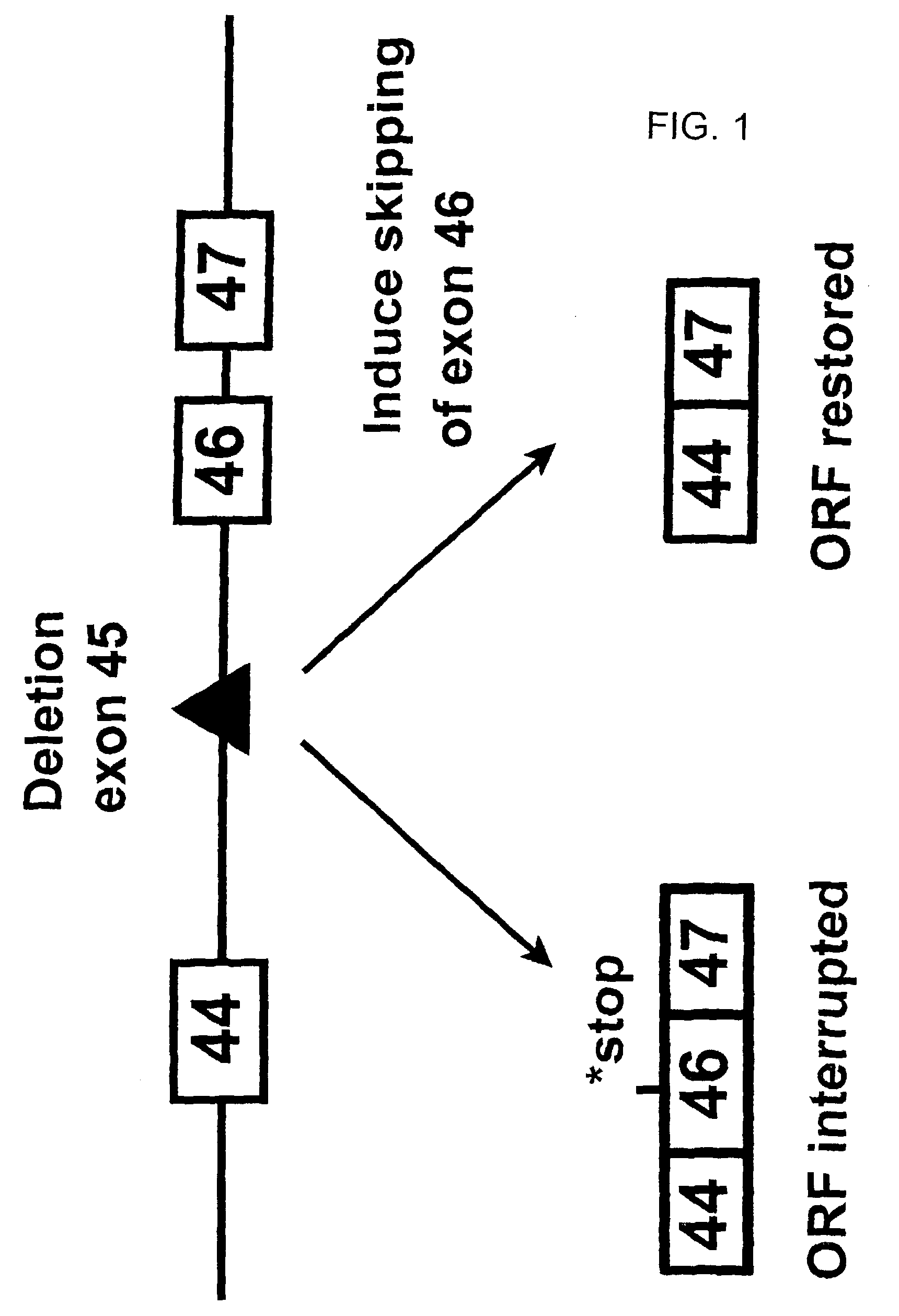
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 44 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Exon skipping compositions for treating muscular dystrophy
InactiveUS20140329881A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideOrganic active ingredientsSplicing alterationDuchenne muscular dystrophyMuscular dystrophy
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 44 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Exon skipping compositions for treating muscular dystrophy
InactiveUS20150361428A1Enhance the activity, cellular distribution, or cellular uptake of the antisense oligonucleotideSplicing alterationSugar derivativesDuchenne muscular dystrophyMuscular dystrophy
Antisense molecules capable of binding to a selected target site in the human dystrophin gene to induce exon 53 skipping are described.
Owner:SAREPTA THERAPEUTICS INC
Enhanced Production of Functional Proteins From Defective Genes
InactiveUS20080207538A1Increase productionIncrease transcriptionBiocideAntibioticsPremature Stop CodonADAMTS Proteins
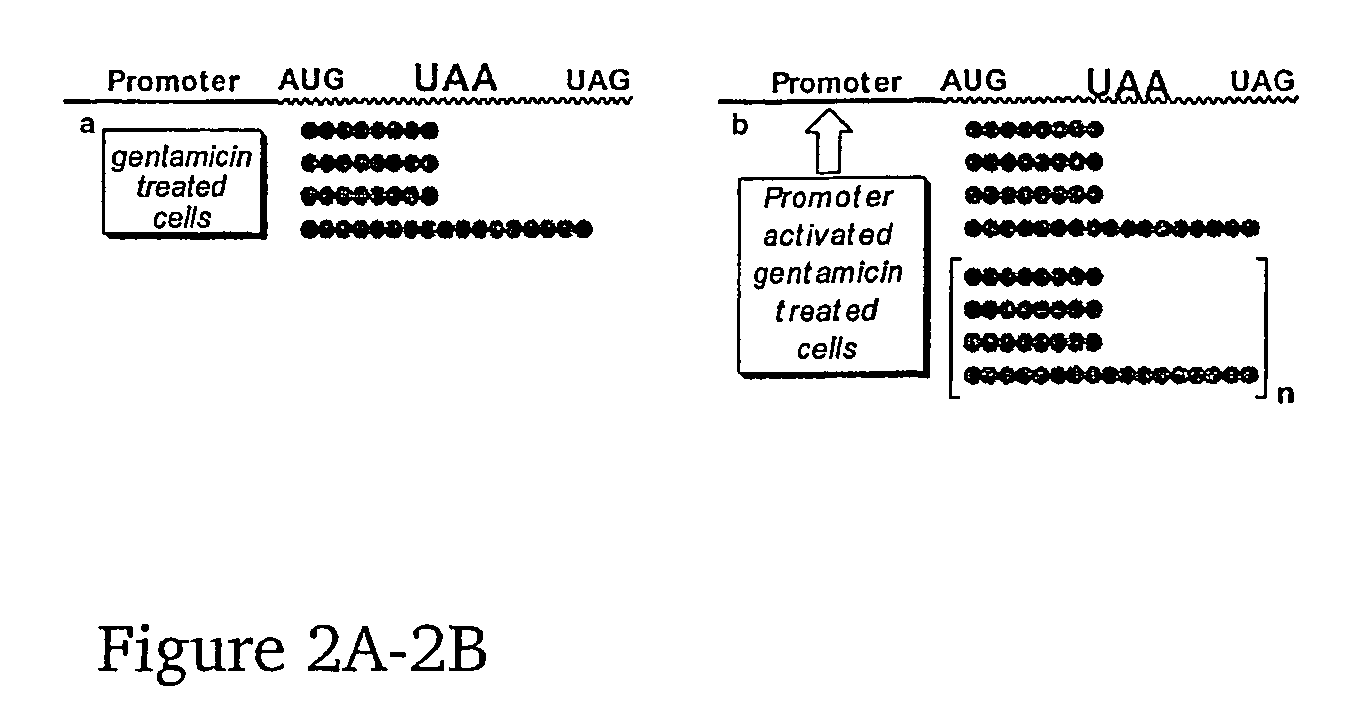
This invention provides methods for enhancing production in a subject of a functional protein from a gene disrupted by a mutation, for example by the presence of a premature stop codon or by an exon skipping mutation, comprising administering to the subject an amount of an agent effective to suppress the mutation and an amount of an agent effective to increase transcription of the gene.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
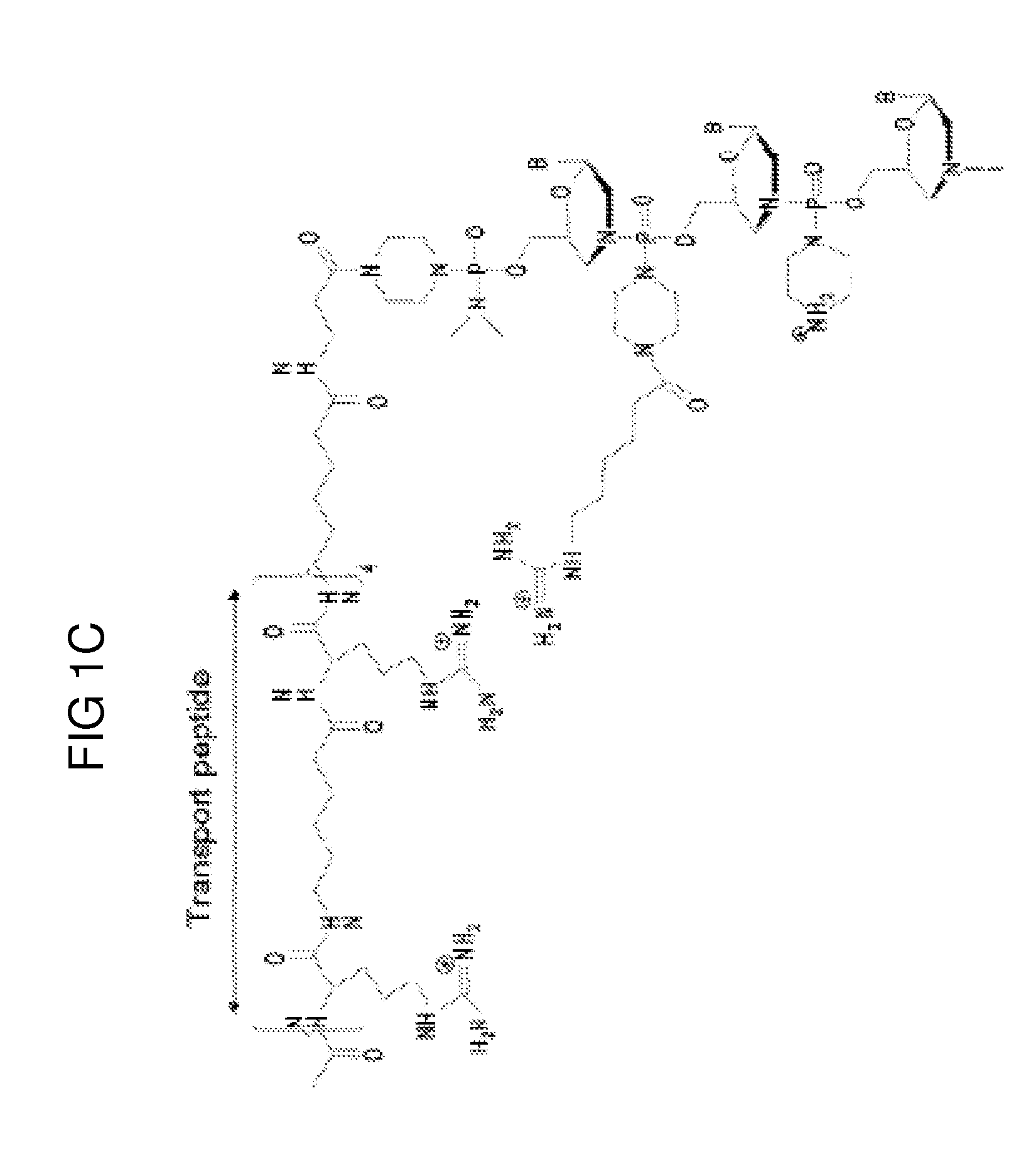
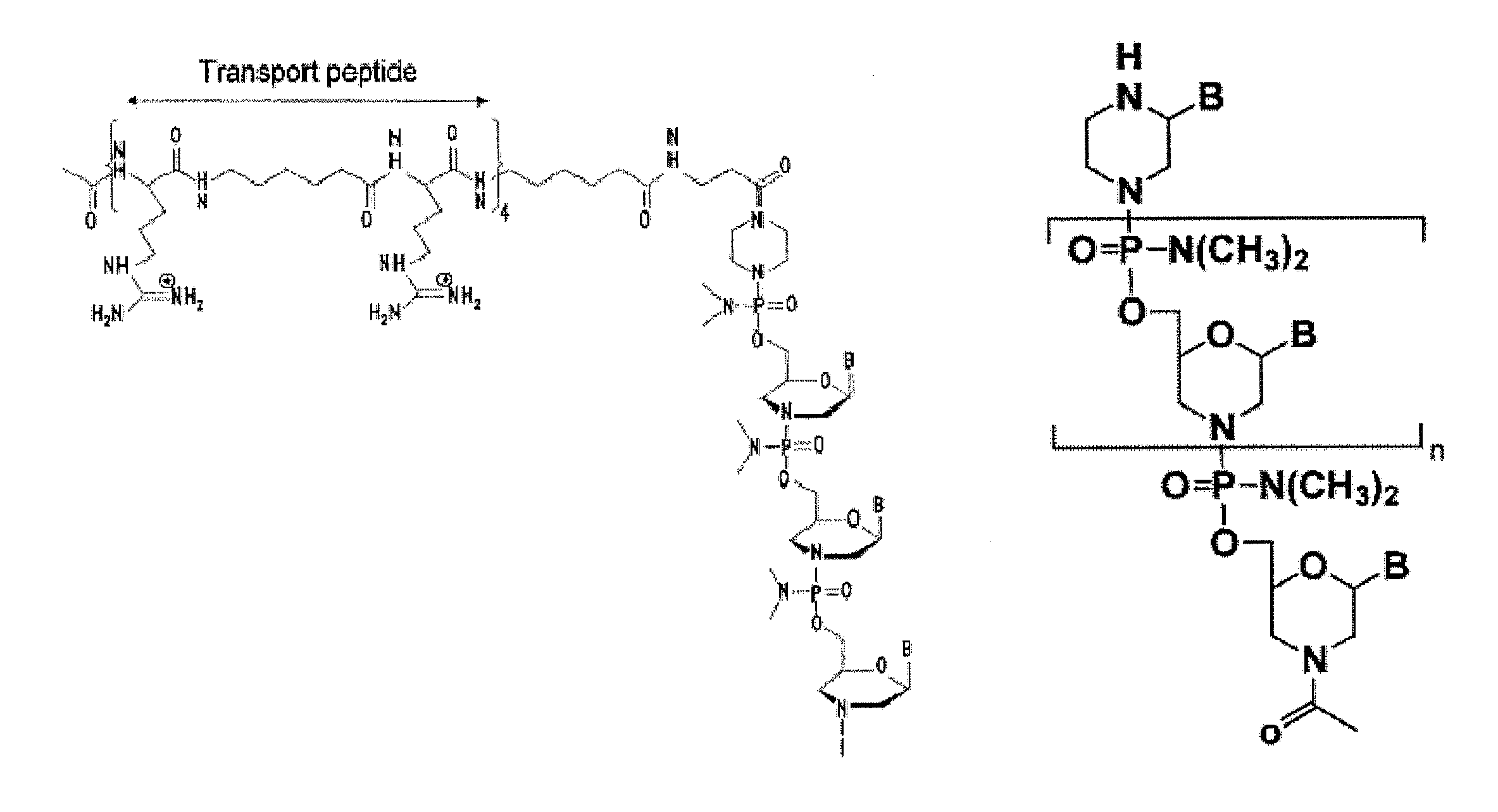
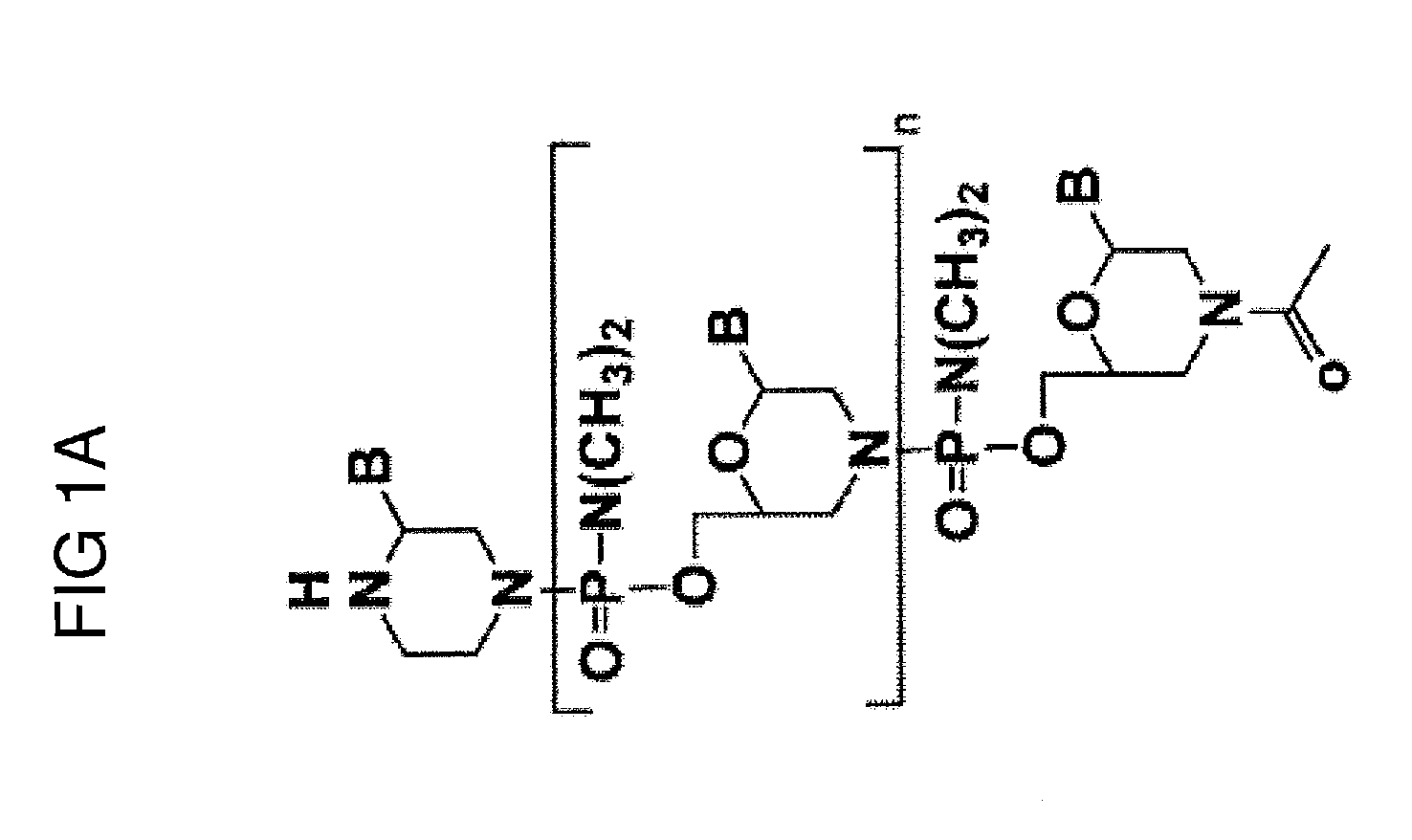
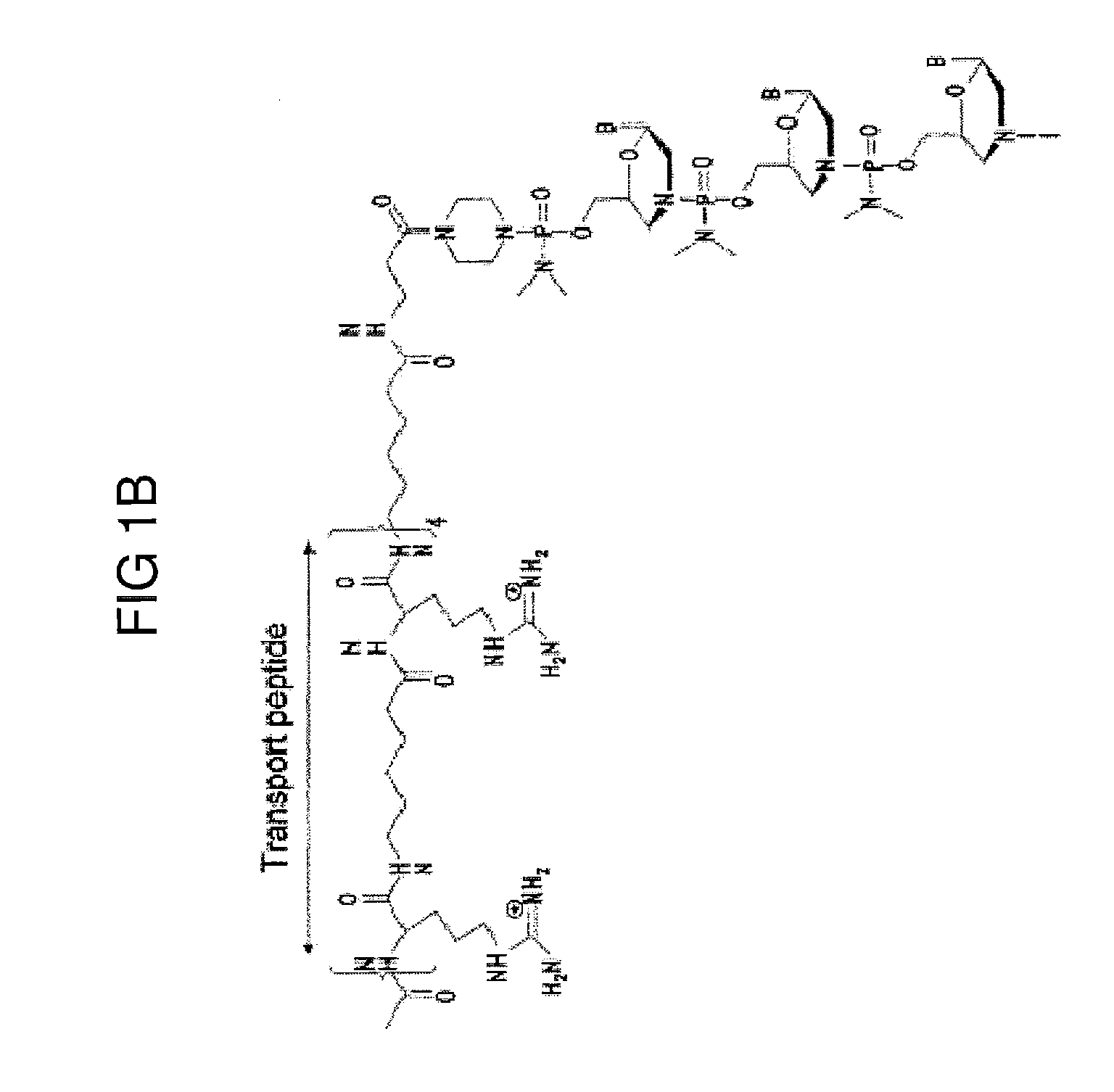
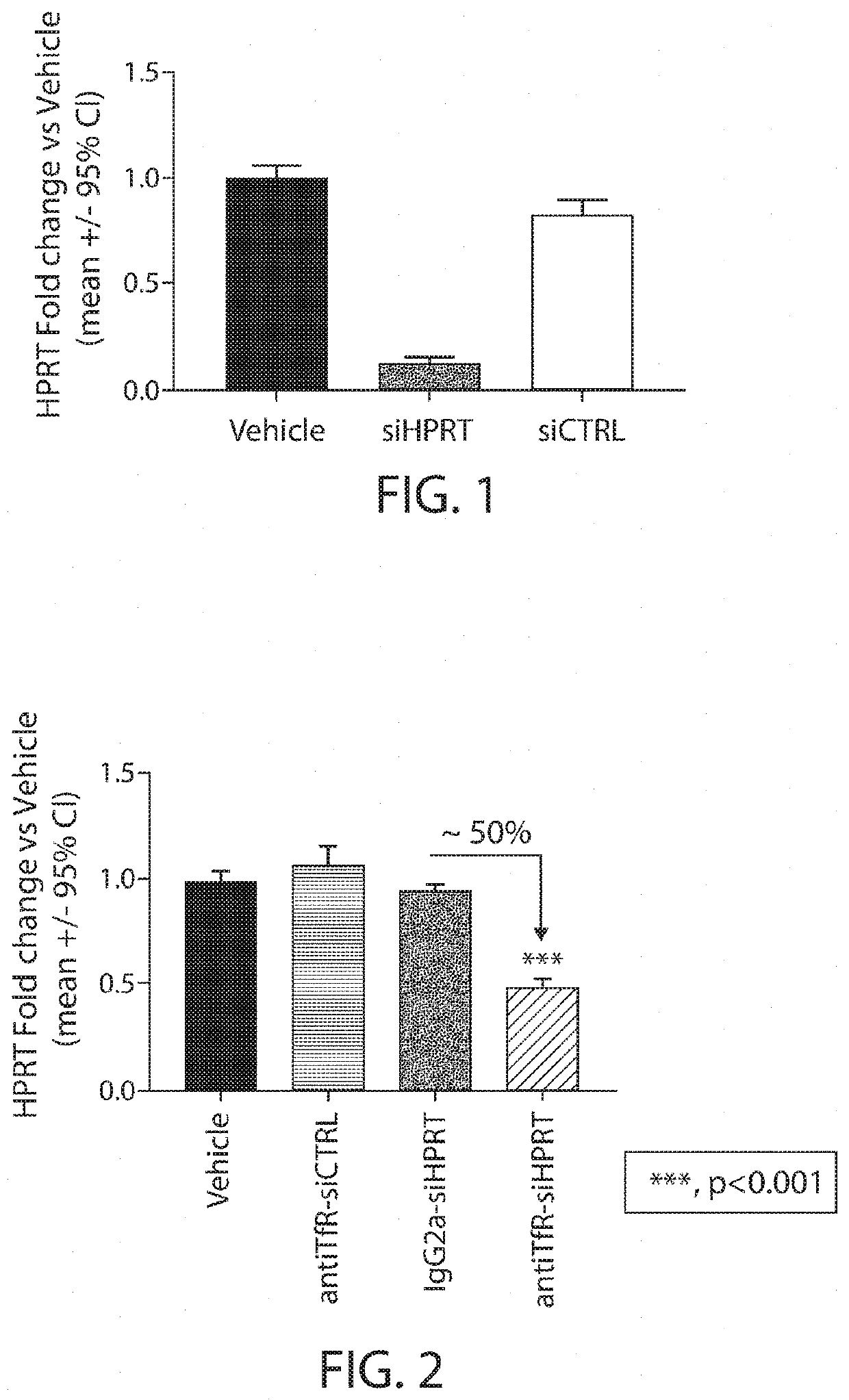
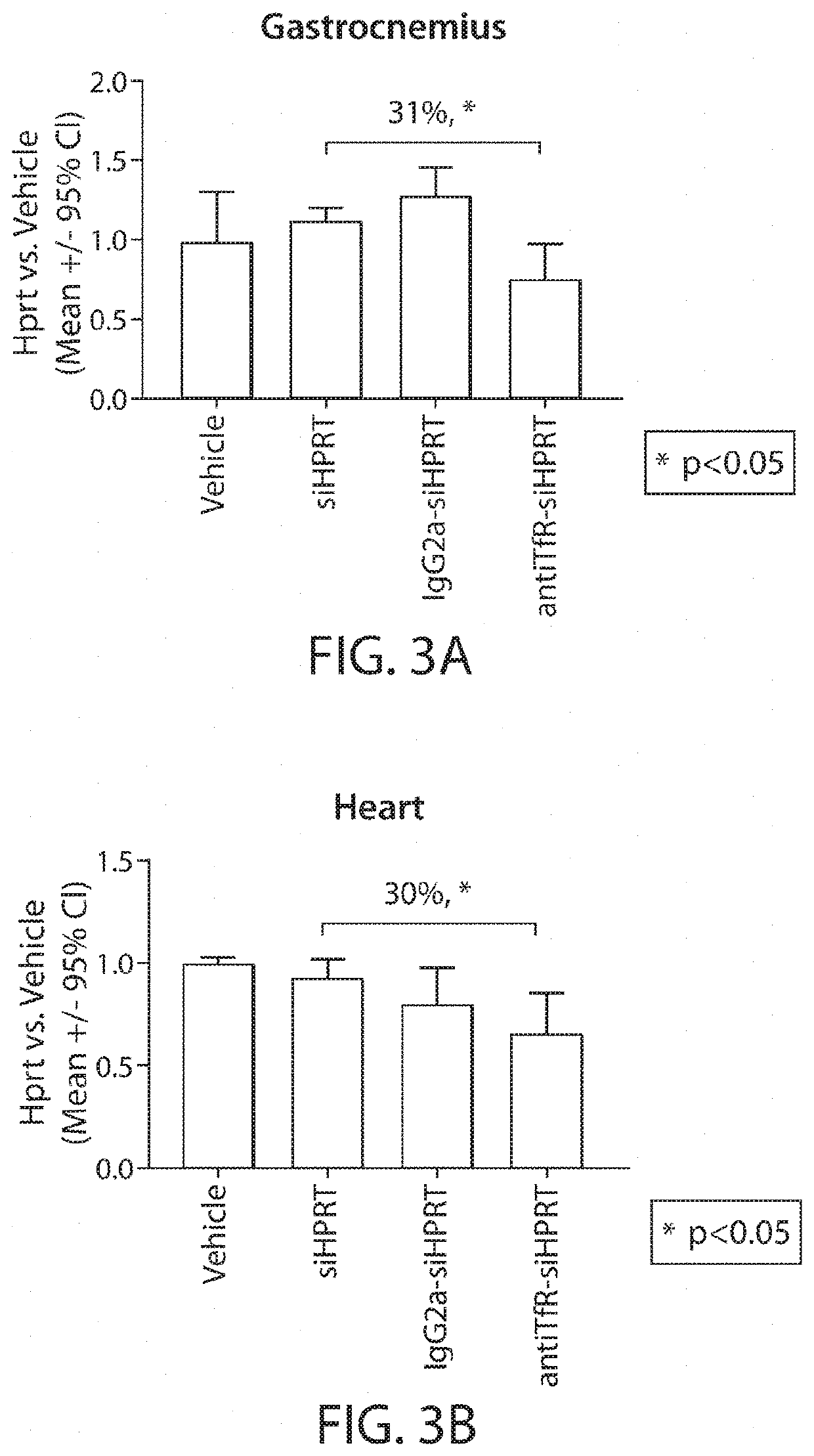
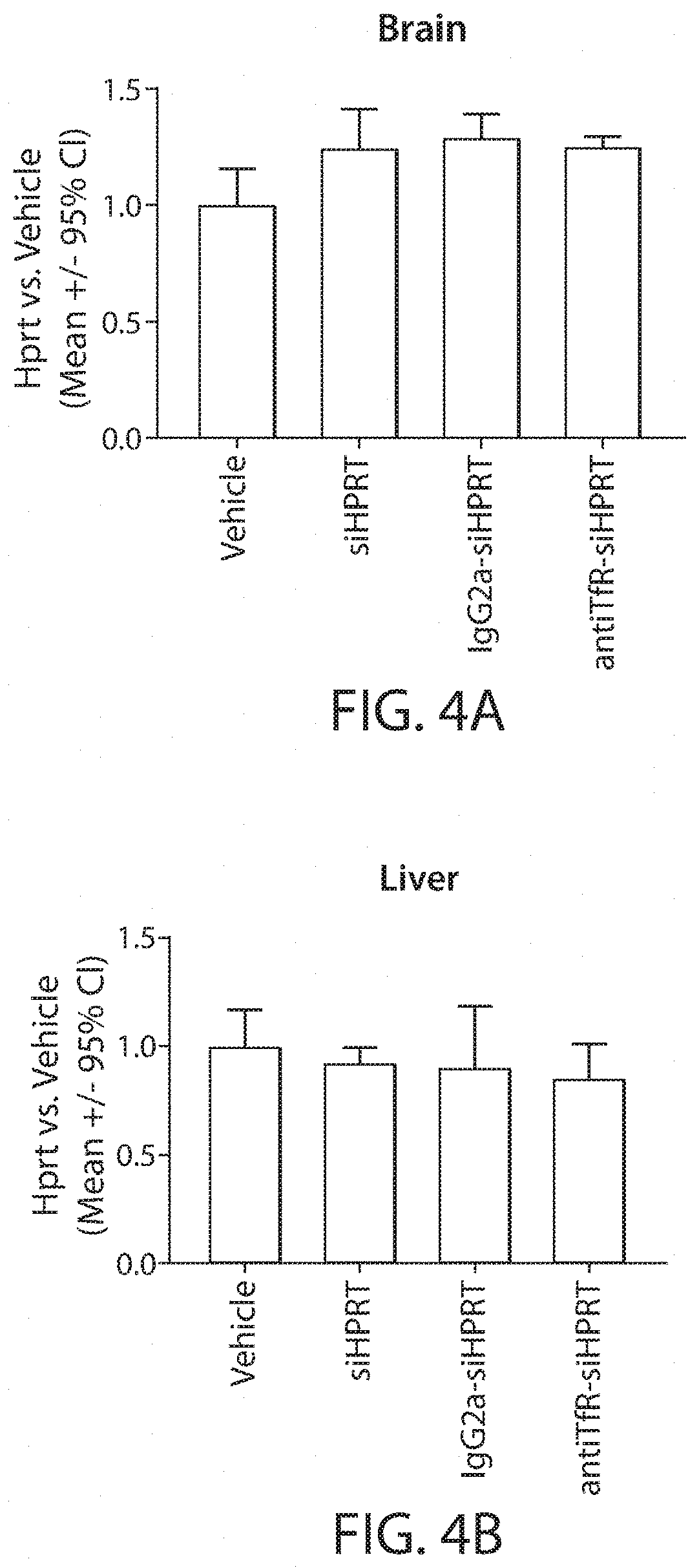
Muscle targeting complexes and uses thereof for treating dystrophinopathies
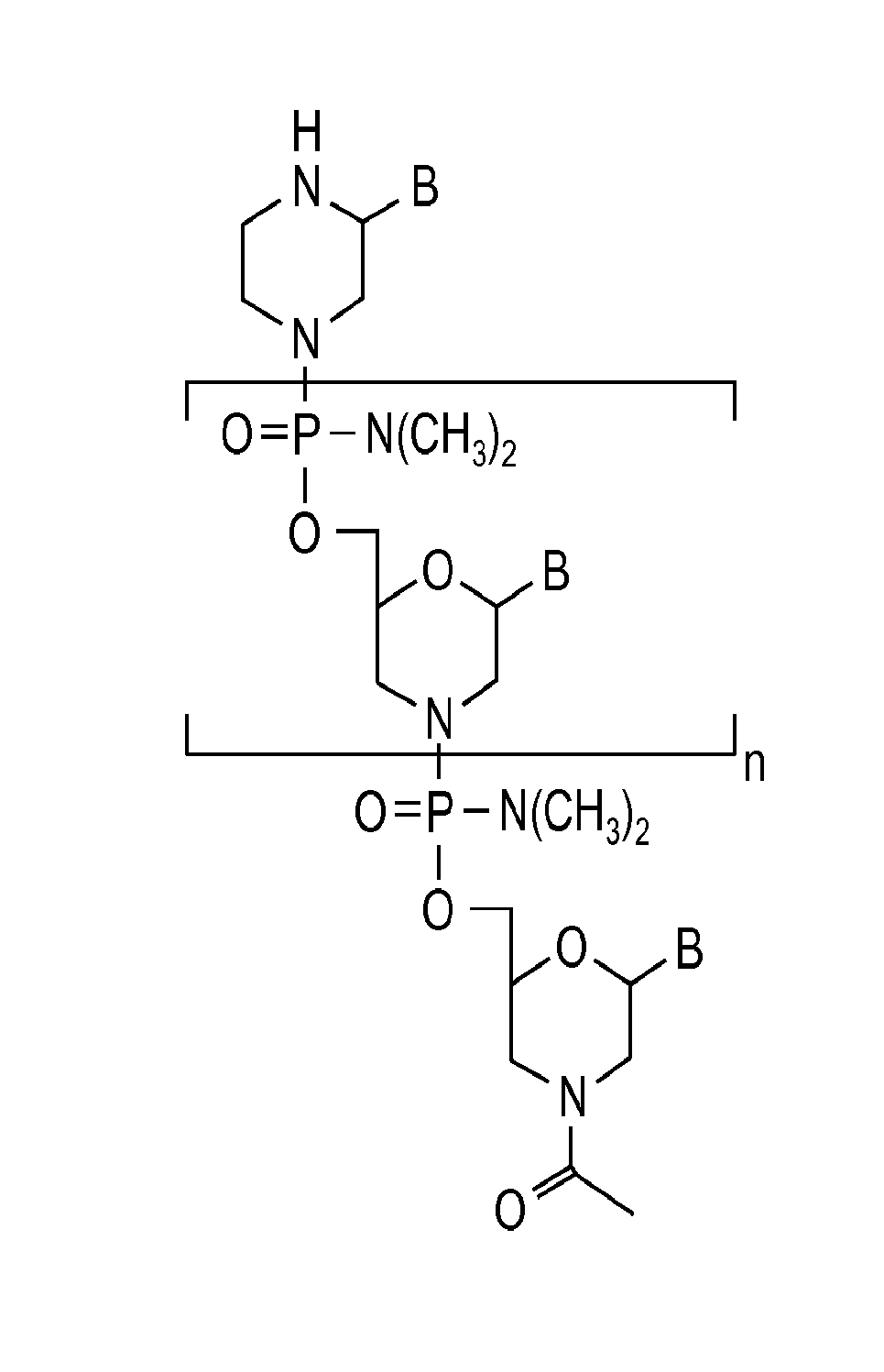
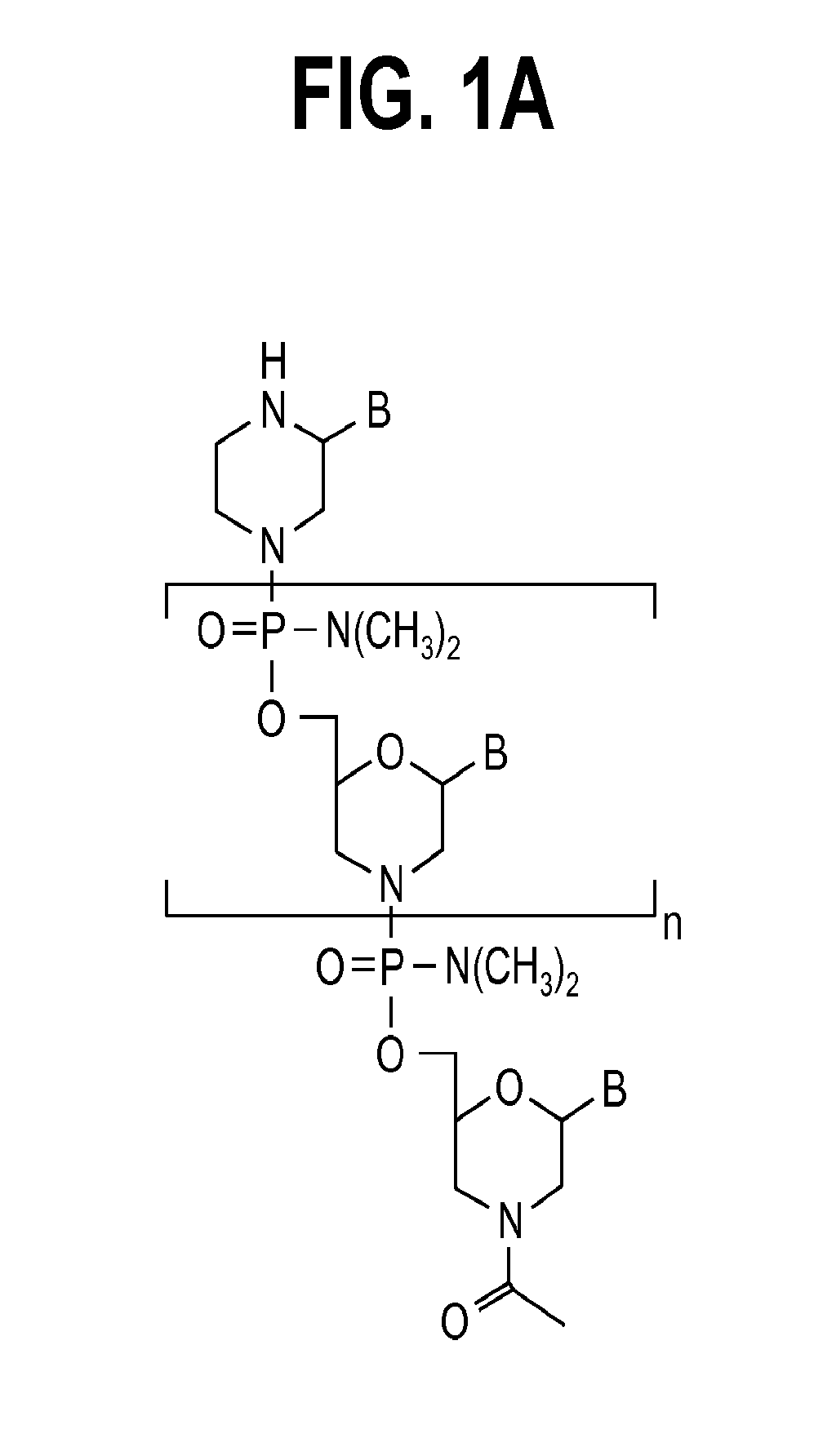
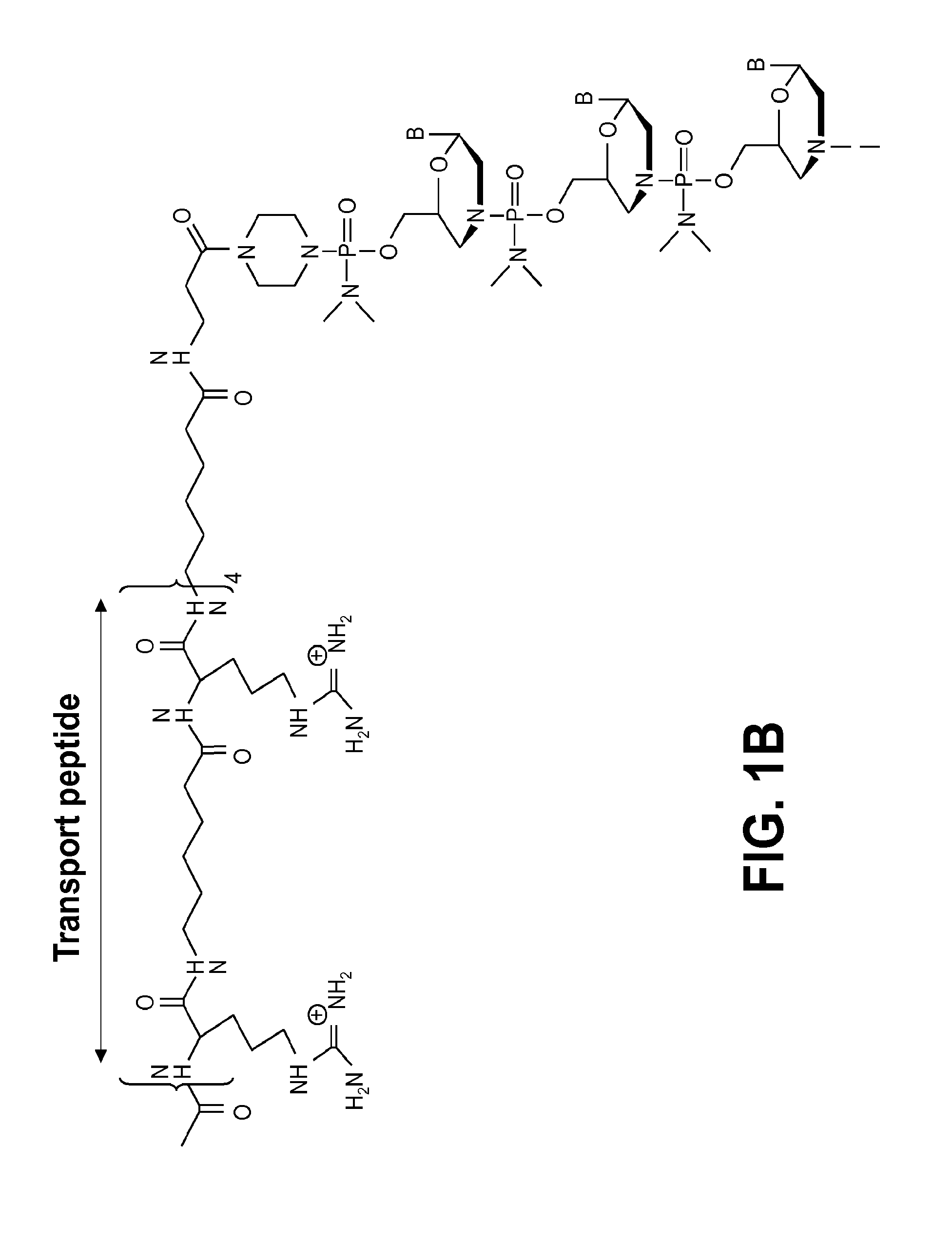
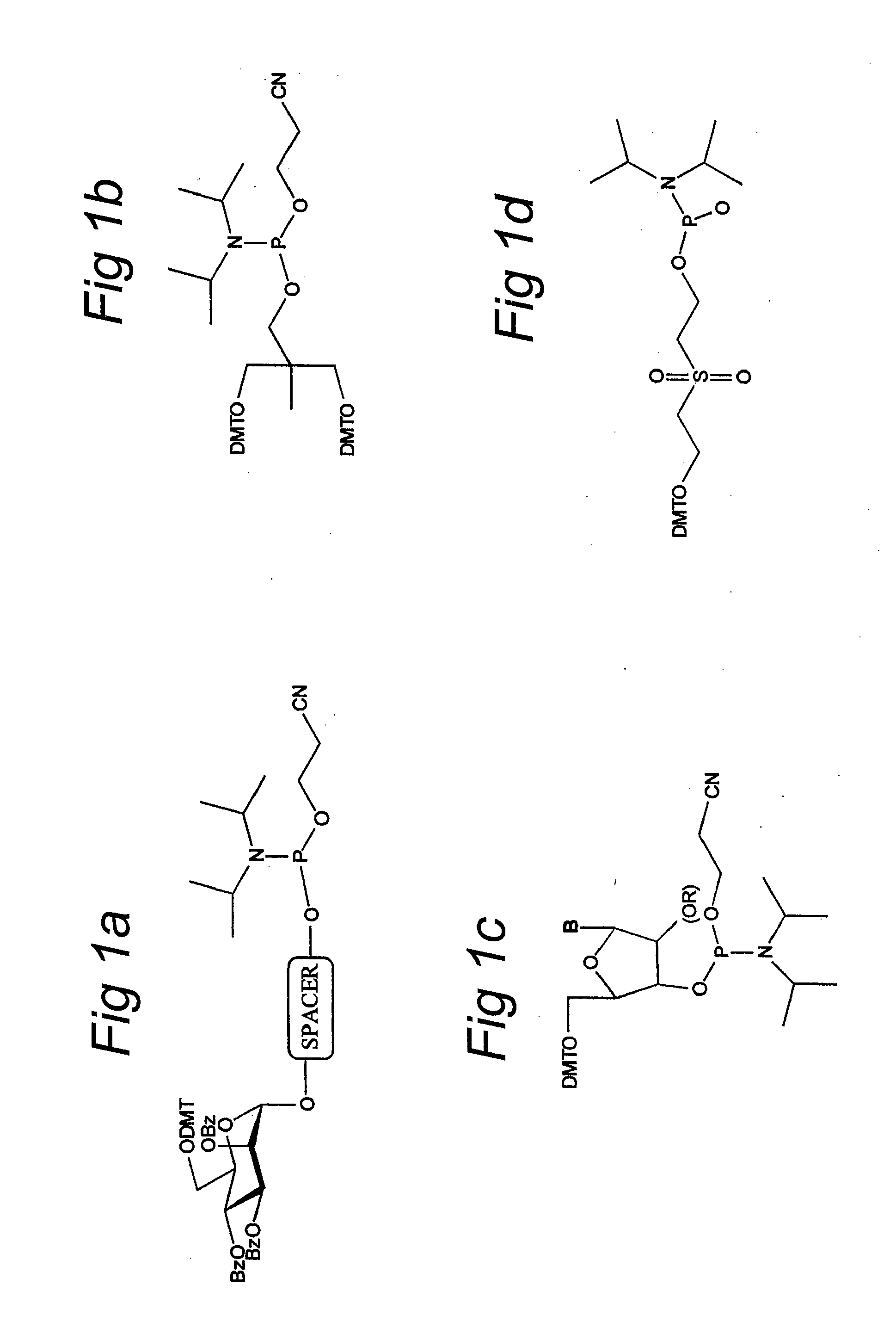
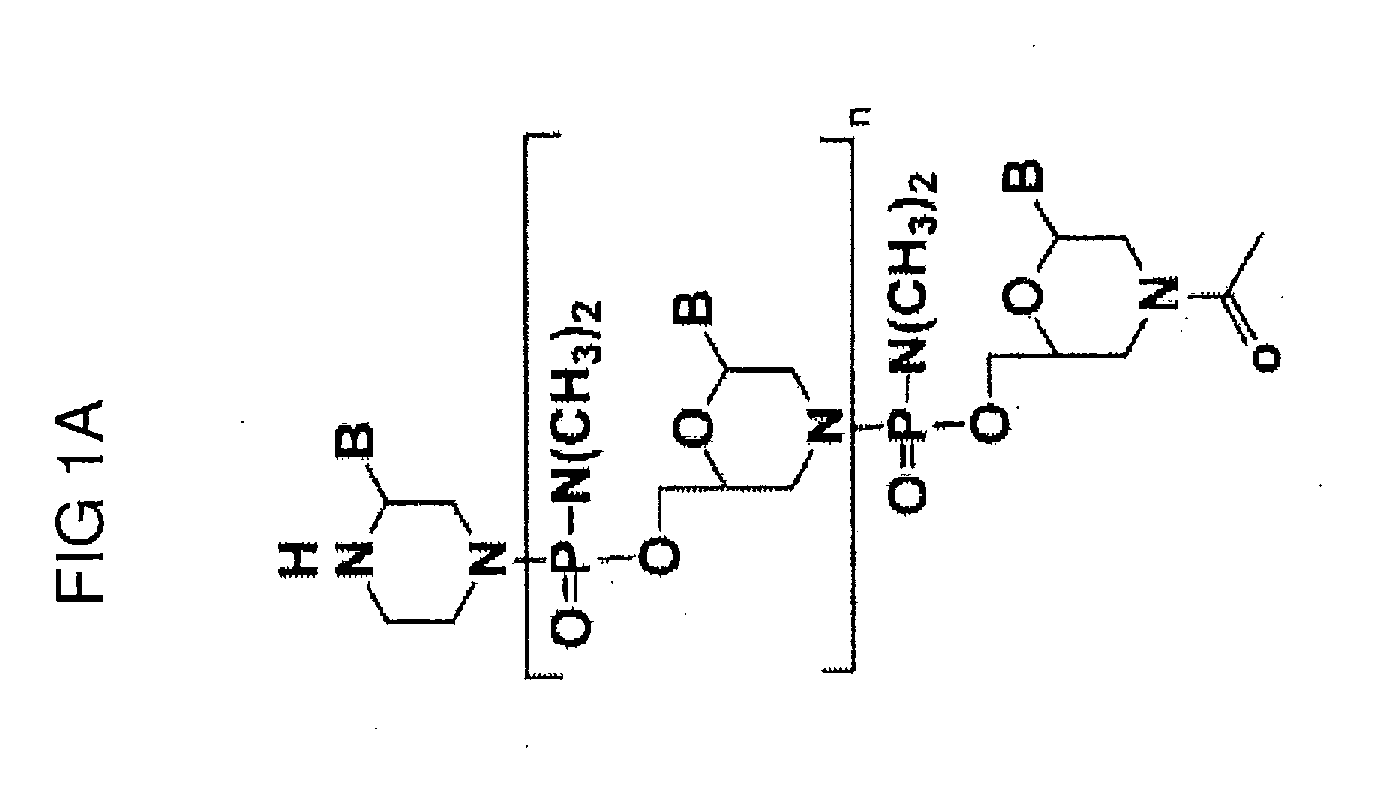
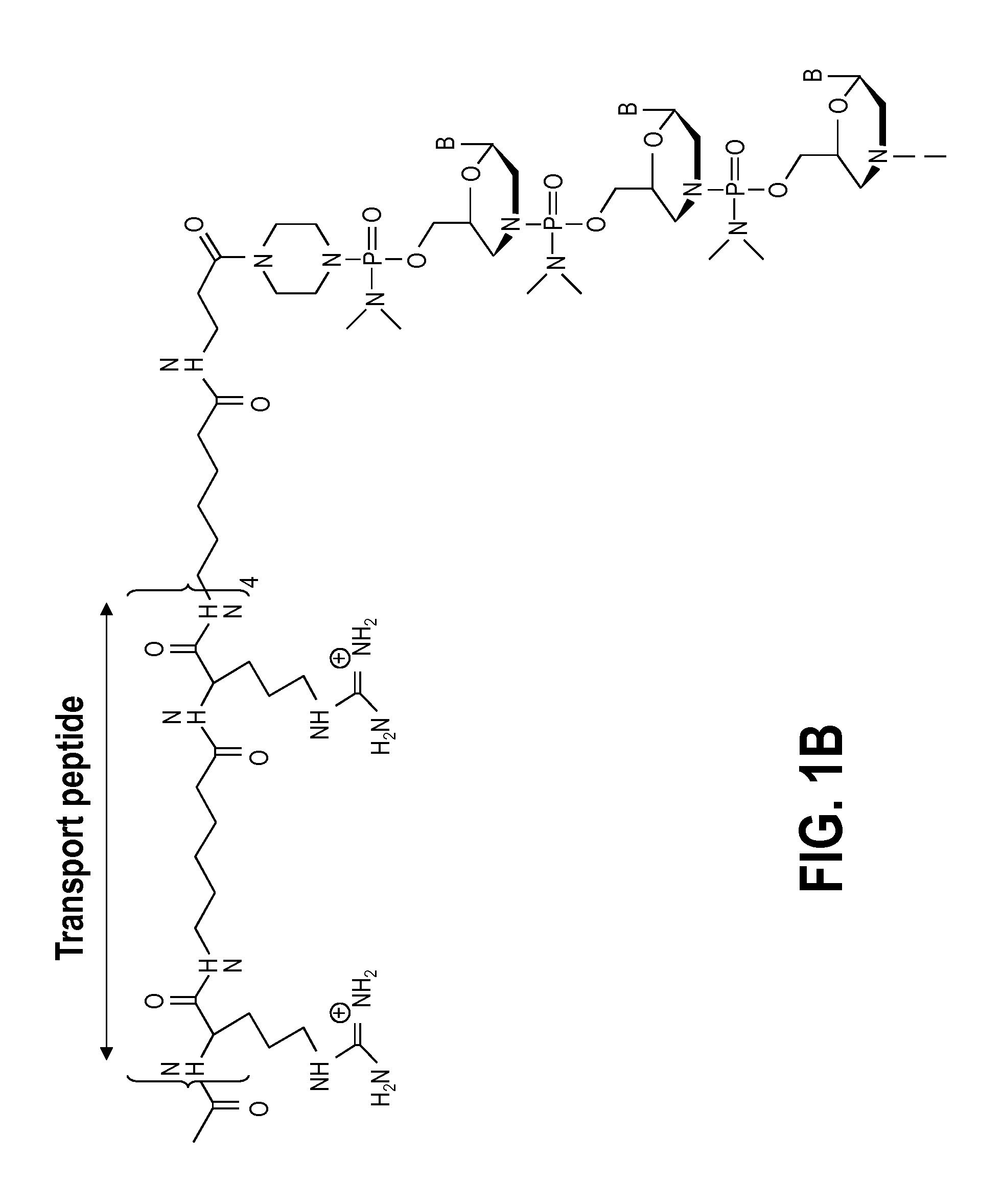
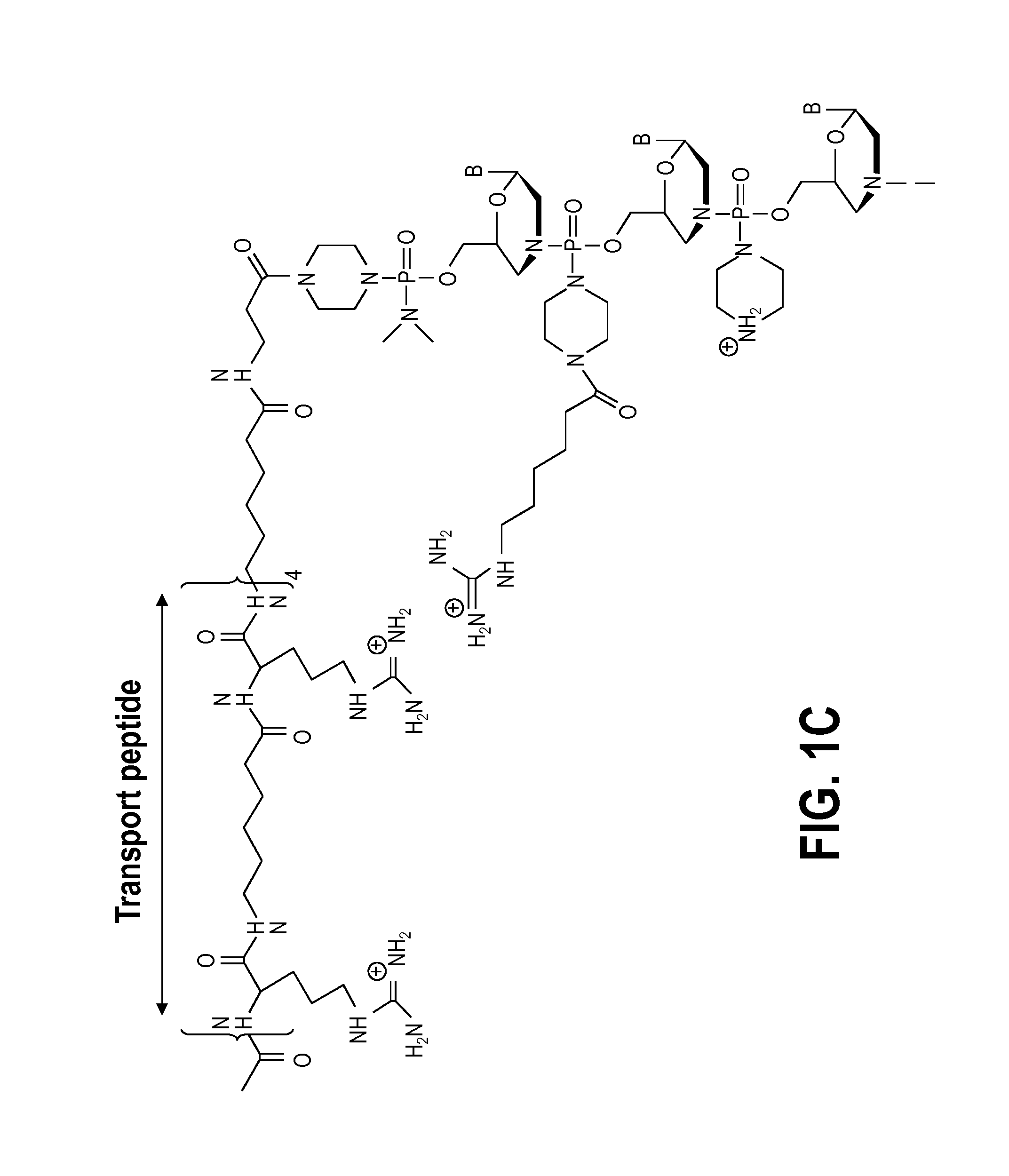
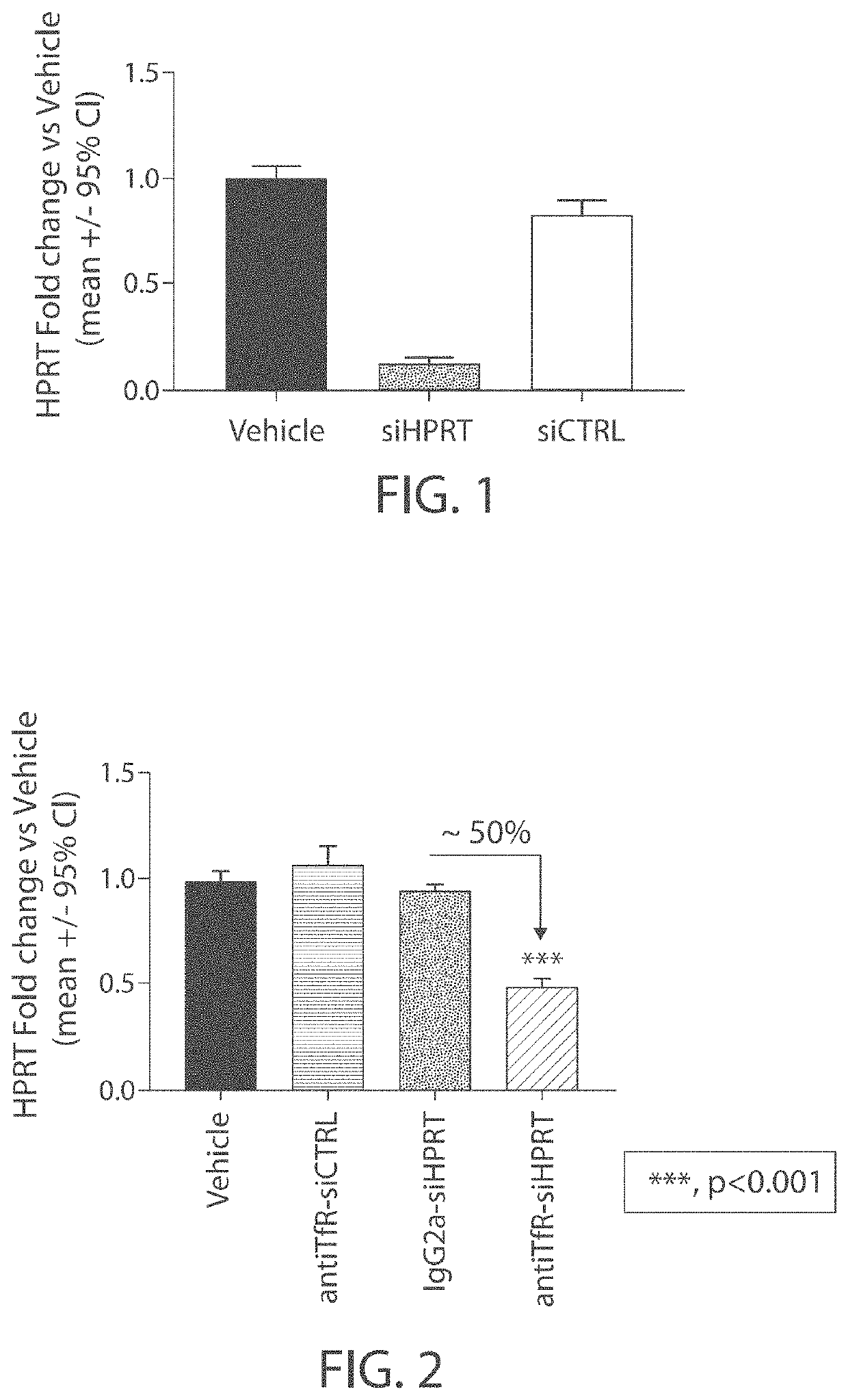
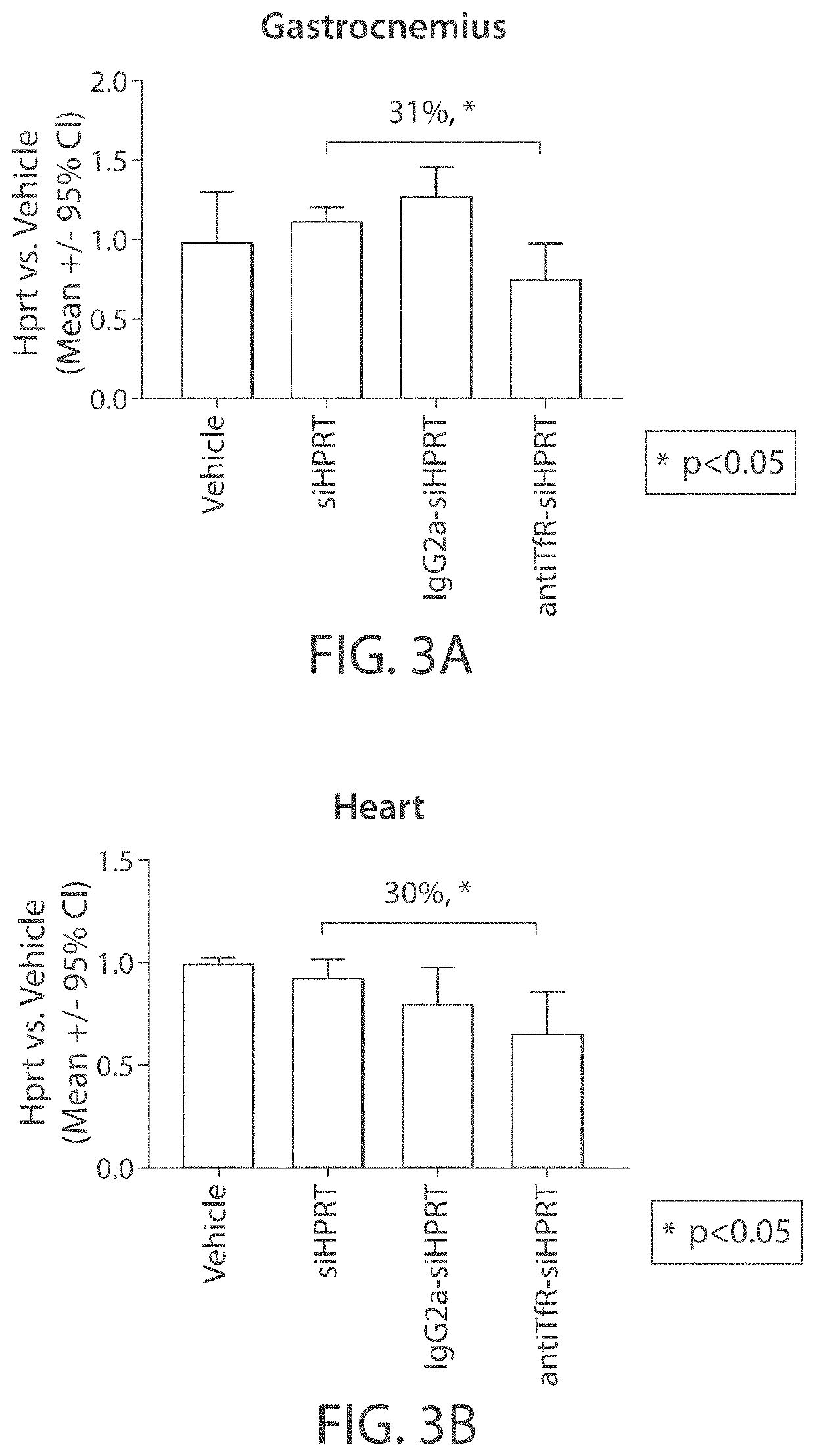
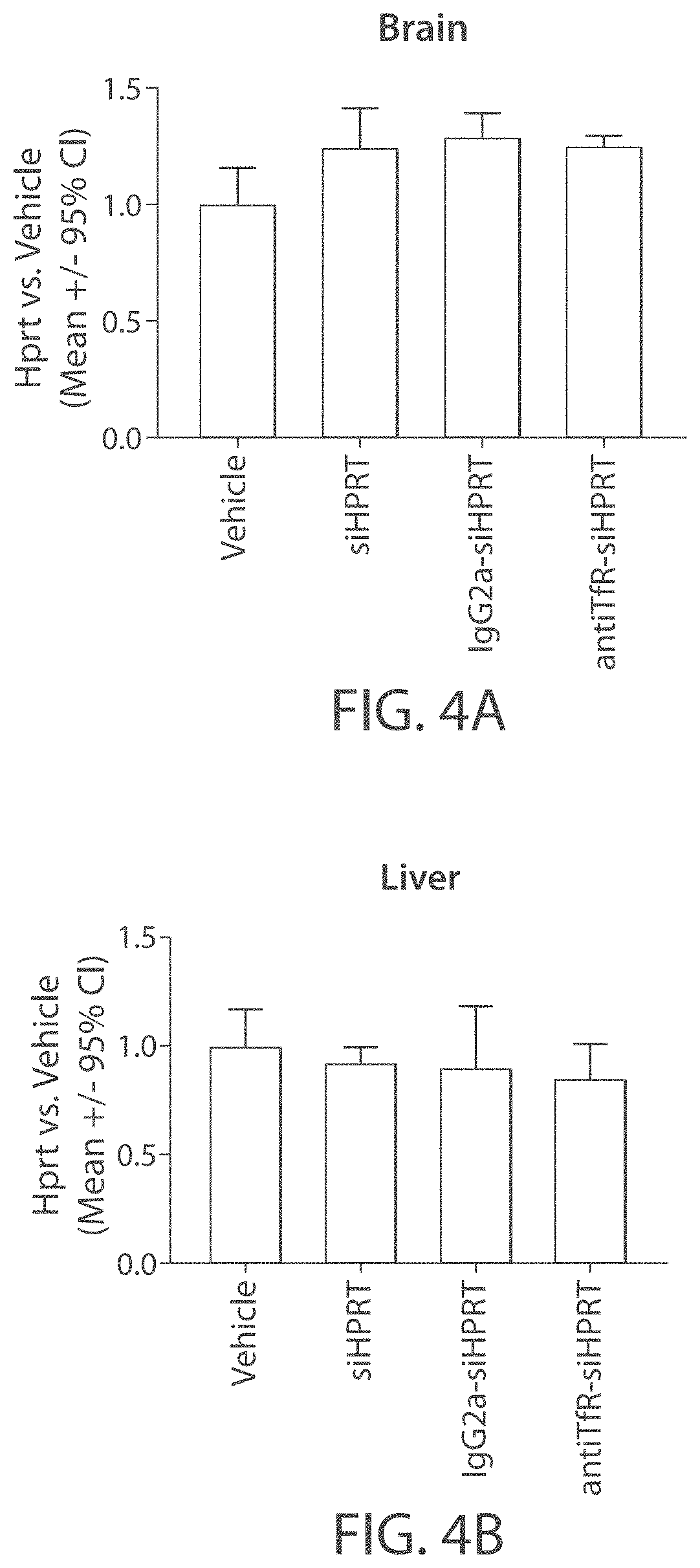
Aspects of the disclosure relate to complexes comprising a muscle-targeting agent covalently linked to a molecular payload. In some embodiments, the muscle-targeting agent specifically binds to an internalizing cell surface receptor on muscle cells. In some embodiments, the molecular payload promotes the expression or activity of a functional dystrophin protein. In some embodiments, the molecular payload is an oligonucleotide, such as an antisense oligonucleotide, e.g., an oligonucleotide that causes exon skipping in a mRNA expressed from a mutant DMD allele.
Owner:DYNE THERAPEUTICS INC
Oligomers
InactiveUS20130085139A1Improve securityLasting effectOrganic active ingredientsSplicing alterationMyostatinPrecursor mRNA
Certain disclosed oligomers induce exon skipping during processing of myostatin pre-mRNA. The oligomers may be in a vector or encoded by the vector. The vector is used for inducing exon skipping during processing of myostatin pre-mRNA. A therapeutically effective amount of the oligomer may be administered to a subject patient such that exon skipping during processing of myostatin pre-mRNA is induced. The administration to a subject may be used in order to increase or maintain muscle mass, or slowing degeneration of muscle mass in the subject. The administration to a subject may ameliorate muscle wasting conditions, such as muscular dystrophy. Examples of such muscular dystrophies which may be so treated include Becker's muscular dystrophy, congenital muscular dystrophy, Duchenne muscular dystrophy, distal muscular dystrophy, Emery-Dreifuss muscular dystrophy, facioscapulohumeral muscular dystrophy (FSHD), limb-girdle muscular dystrophy, myotonic muscular dystrophy, and oculopharyngeal muscular dystrophy
Owner:ROYAL HOLLOWAY & BEDFORD NEW COLLEGE
Methods and compositions for attenuating exon skipping Anti-viral transfer vector immune responses
Owner:SELECTA BIOSCI
Vectors with modified initiation codon for the translation of aav-rep78 useful for production of aav
ActiveUS20090191588A1Improve stabilityReduced expression levelAnimal cellsSugar derivativesStart codonNucleotide
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS +1
Muscle targeting complexes and uses thereof for treating dystrophinopathies
Owner:DYNE THERAPEUTICS INC
Antisense Polynucleotides to Induce Exon Skipping and Methods of Treating Dystrophies
ActiveUS20150225718A1Inhibit progressImprove muscle functionSplicing alterationSugar derivativesDiseasePolynucleotide
Antisense polynucleotides and their use in pharmaceutical compositions to induce exon skipping in targeted exons of the gamma sarcoglycan gene are provided, along with methods of preventing or treating dystrophic diseases such as Limb-Girdle Muscular Dystrophy. One aspect the disclosure provides an isolated antisense polynucleotide wherein the polynucleotide specifically hybridizes to an exon target region of a gamma sarcoglycan RNA, wherein the exon is selected from the group consisting of exon 4 (SEQ ID NO:1), exon 5 (SEQ ID NO: 2), exon 6 (SEQ ID NO: 3), exon 7 (SEQ ID NO: 4) and a combination thereof. In some embodiments, the antisense polynucleotide cannot form an RNase H substrate, and in further embodiments the antisense polynucleotide comprises a modified polynucleotide backbone.
Owner:UNIVERSITY OF CHICAGO
Muscle targeting complexes and uses thereof for treating dystrophinopathies
Aspects of the disclosure relate to complexes comprising a muscle-targeting agent covalently linked to a molecular payload. In some embodiments, the muscle-targeting agent specifically binds to an internalizing cell surface receptor on muscle cells. In some embodiments, the molecular payload promotes the expression or activity of a functional dystrophin protein. In some embodiments, the molecular payload is an oligonucleotide, such as an antisense oligonucleotide, e.g., an oligonucleotide that causes exon skipping in a mRNA expressed from a mutant DMD allele.
Owner:DYNE THERAPEUTICS INC
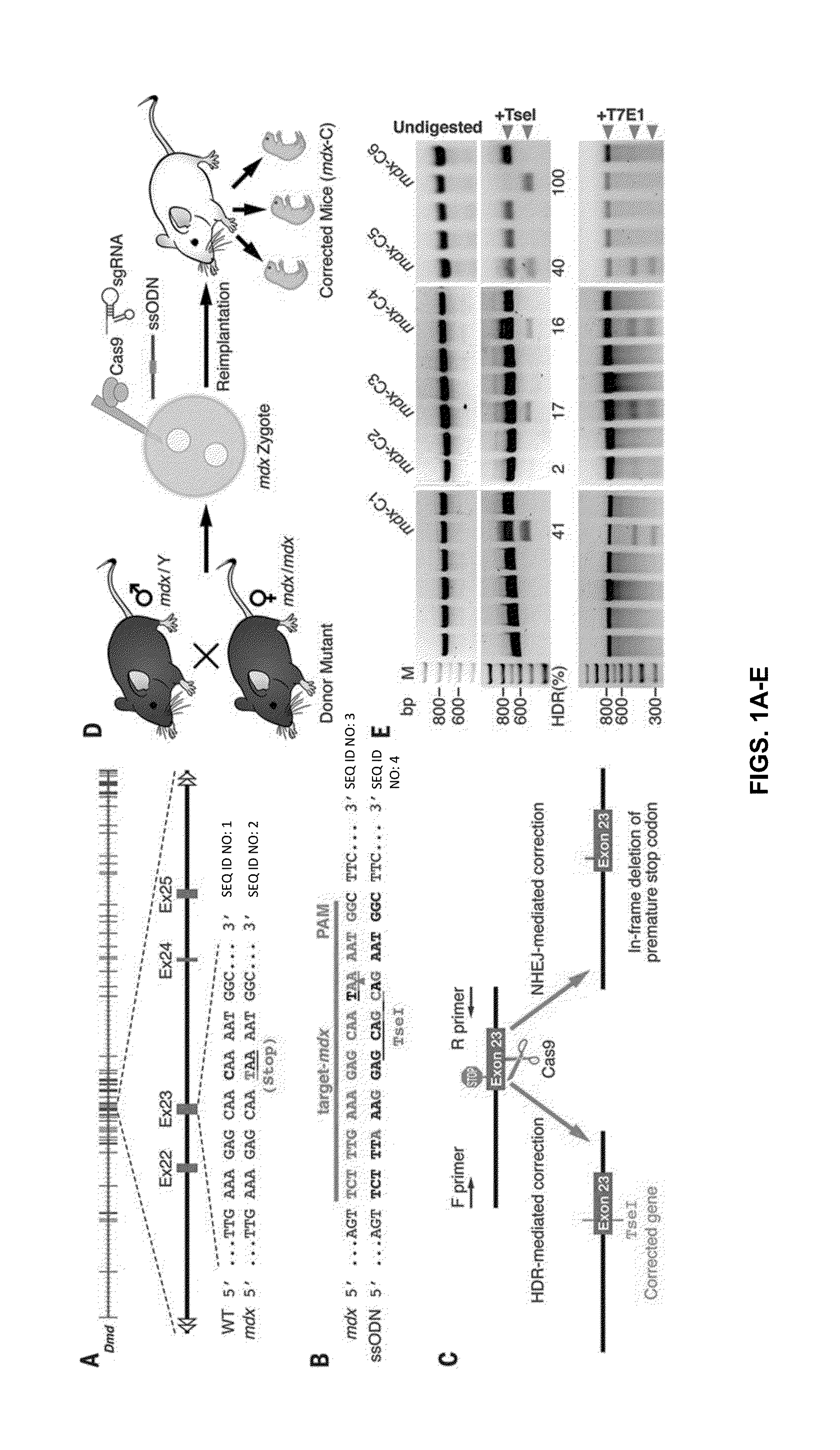
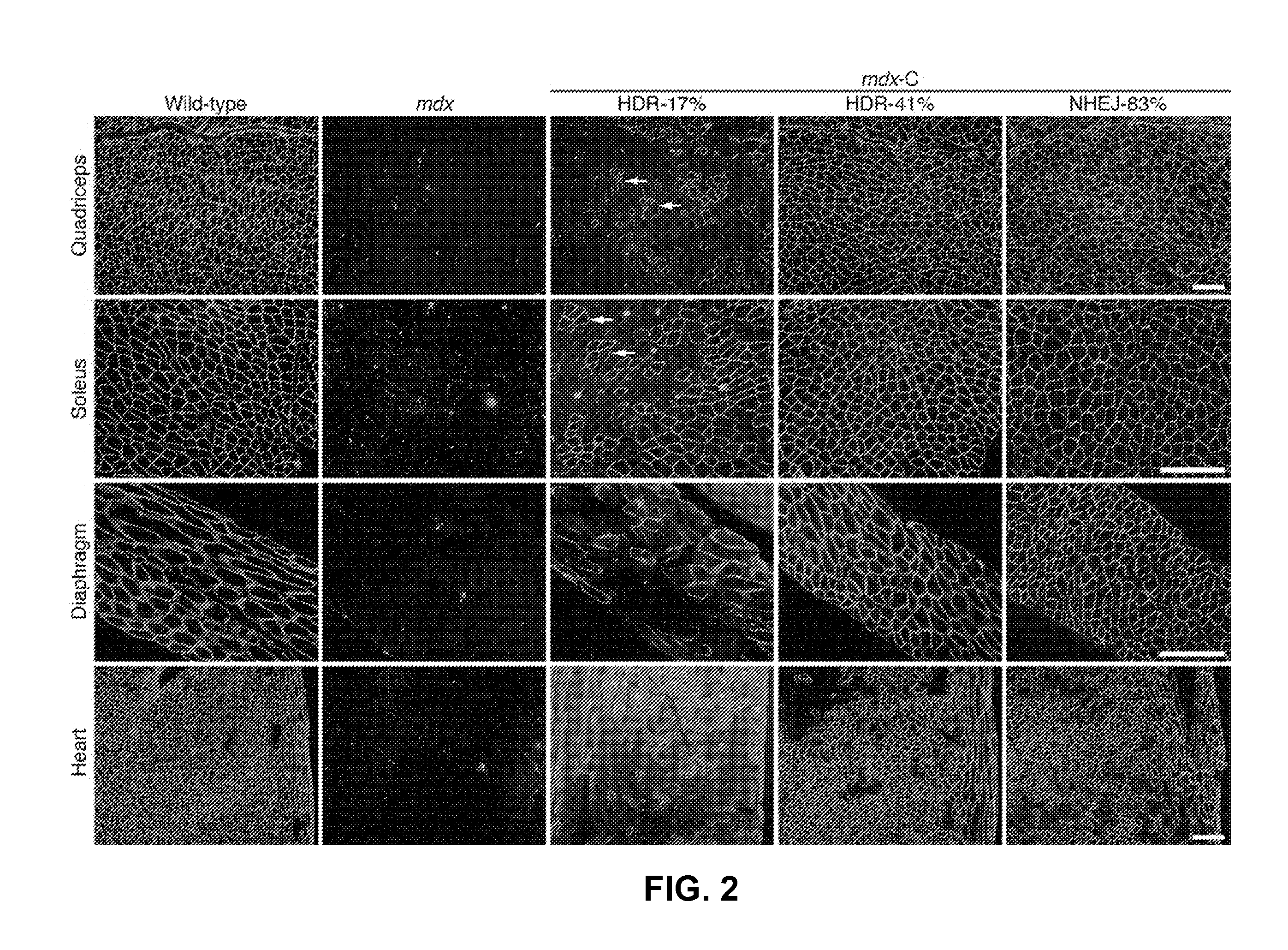
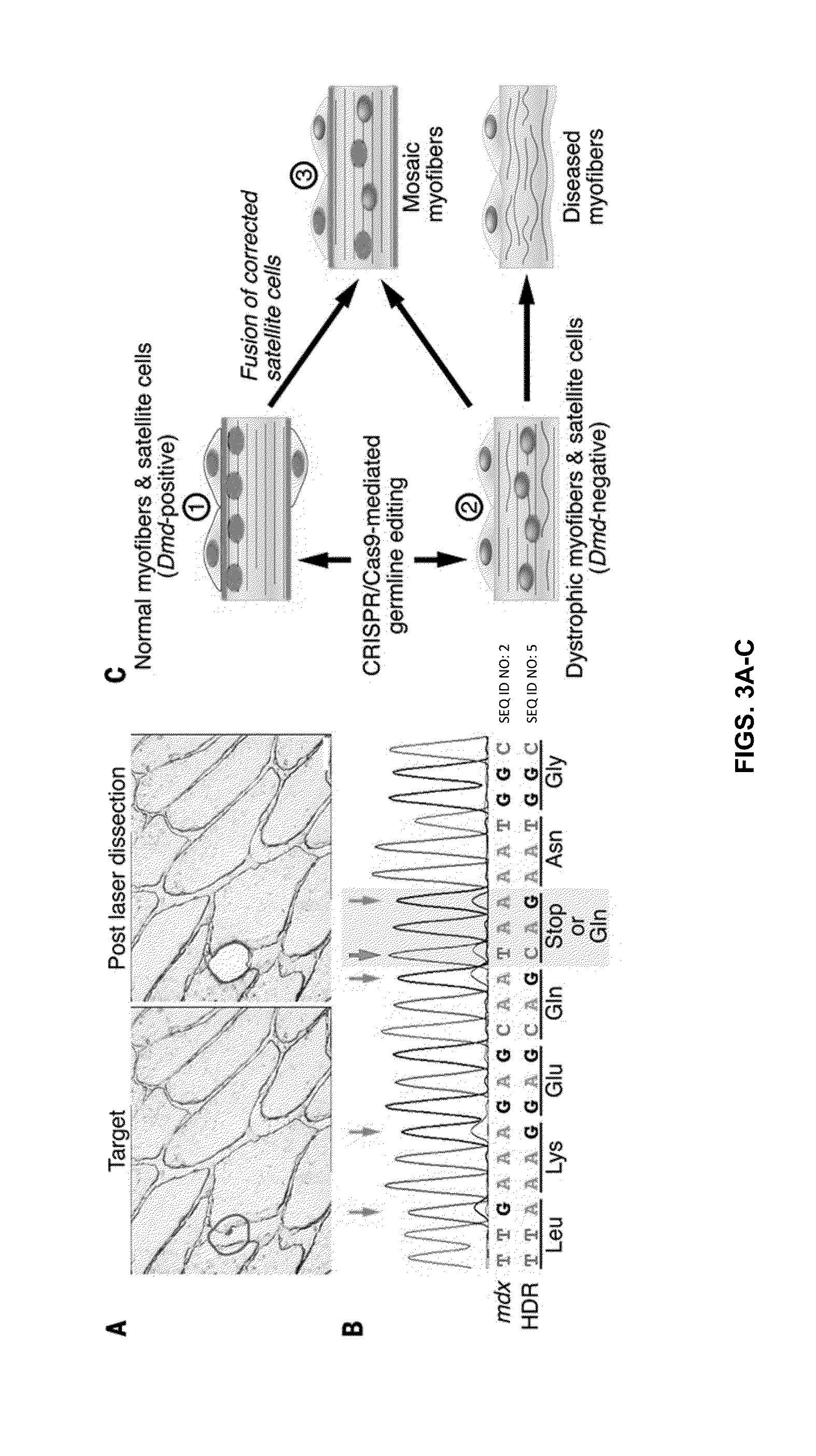
Prevention of muscular dystrophy by crispr/cas9-mediated gene editing
InactiveUS20160058889A1Lower Level RequirementsImprove grip strengthSplicing alterationPeptide/protein ingredientsDiseaseIn vivo
Duchenne muscular dystrophy (DMD) is an inherited X-linked disease caused by mutations in the gene encoding dystrophin, a protein required for muscle fiber integrity. The disclosure reports CRISPR / Cas9-mediated gene editing (Myo-editing) is effective at correcting the dystrophin gene mutation in the mdx mice, a model for DMD. Further, the disclosure reports optimization of germline editing of mdx mice by engineering the permanent skipping of mutant exon (exon 23) and extending exon skipping to also correct the disease by post-natal delivery of adeno-associate virus (AAV). AAV-mediated Myo-editing can efficiently rescue the reading frame of dystrophin in mdx mice in vivo. The disclosure reports means of Myo-editing-mediated exon skipping has been successfully advanced from somatic tissues in mice to human DMD patients-derived iPSCs (induced pluripotent stem cells). Custom Myo-editing was performed on iPSCs from patients with differing mutations and successfully restored dystrophin protein expression for all mutations in iPSCs-derived cardiomyocytes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Muscle targeting complexes and uses thereof for treating dystrophinopathies
PendingUS20210308273A1High activityHigh expressionOrganic active ingredientsSplicing alterationMutant alleleInternalization
Aspects of the disclosure relate to complexes comprising a muscle-targeting agent covalently linked to a molecular payload. In some embodiments, the muscle-targeting agent specifically binds to an internalizing cell surface receptor on muscle cells. In some embodiments, the molecular payload promotes the expression or activity of a functional dystrophin protein. In some embodiments, the molecular payload is an oligonucleotide, such as an antisense oligonucleotide, e.g., an oligonucleotide that causes exon skipping in a mRNA expressed from a mutant DMD allele.
Owner:DYNE THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com