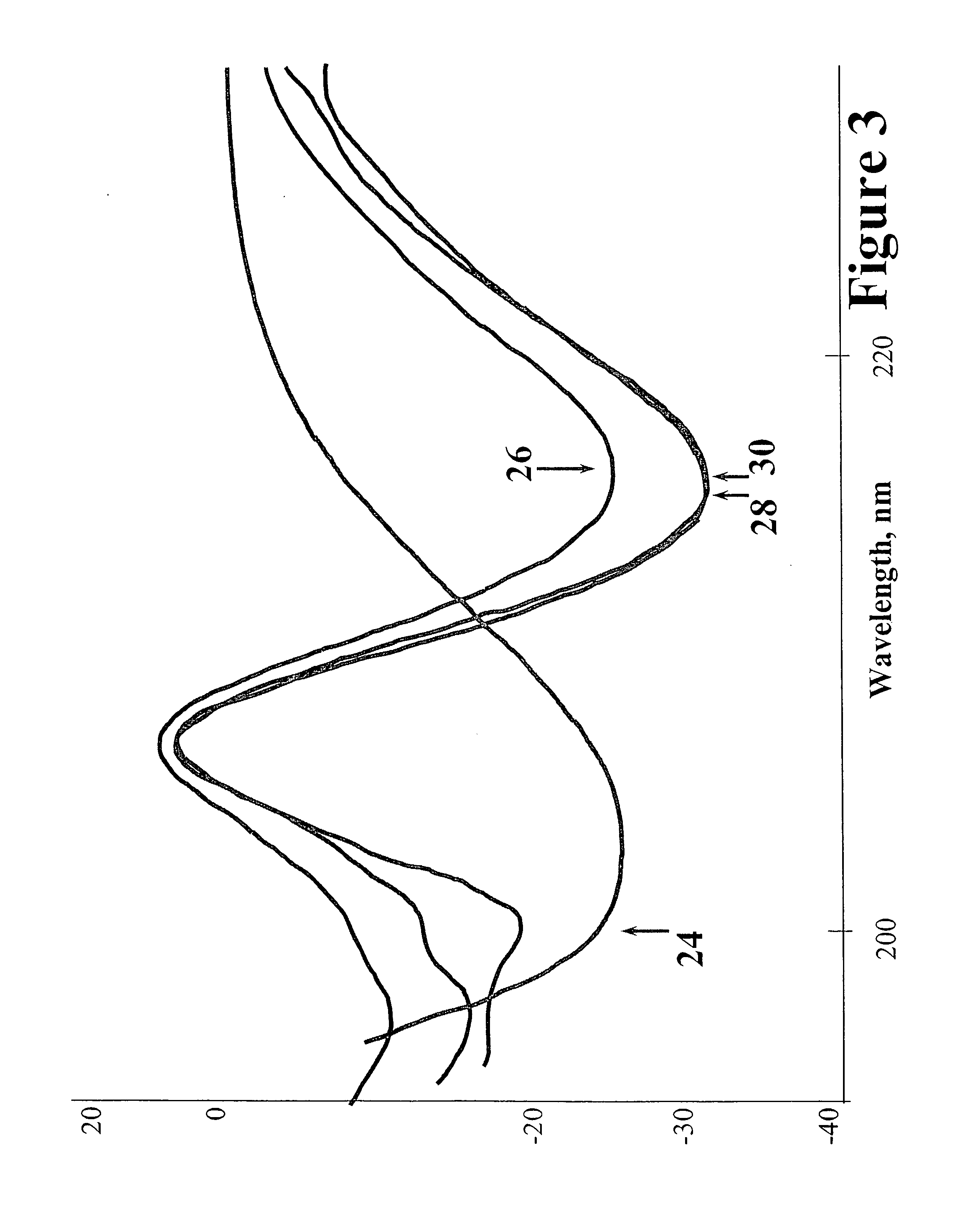
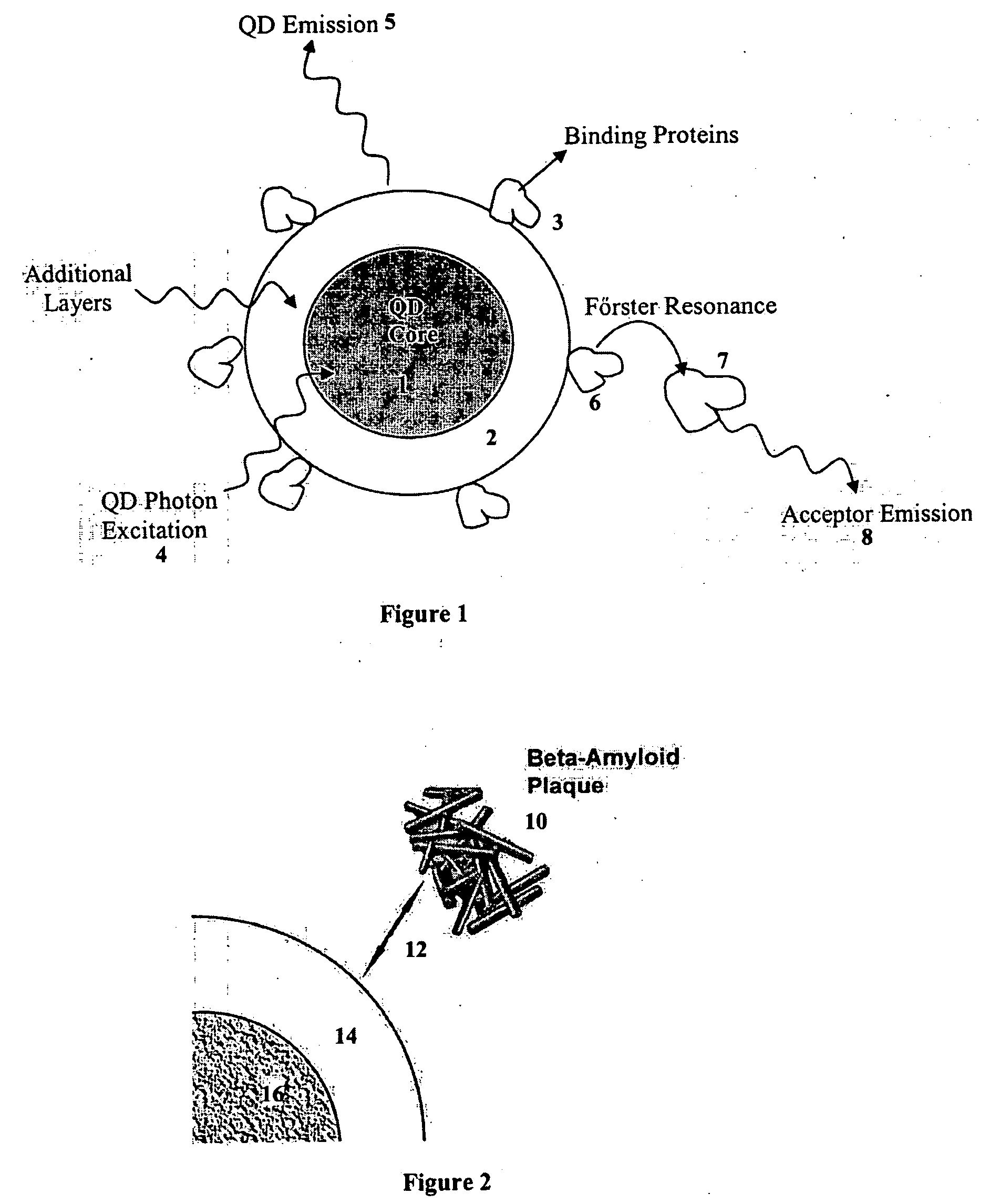
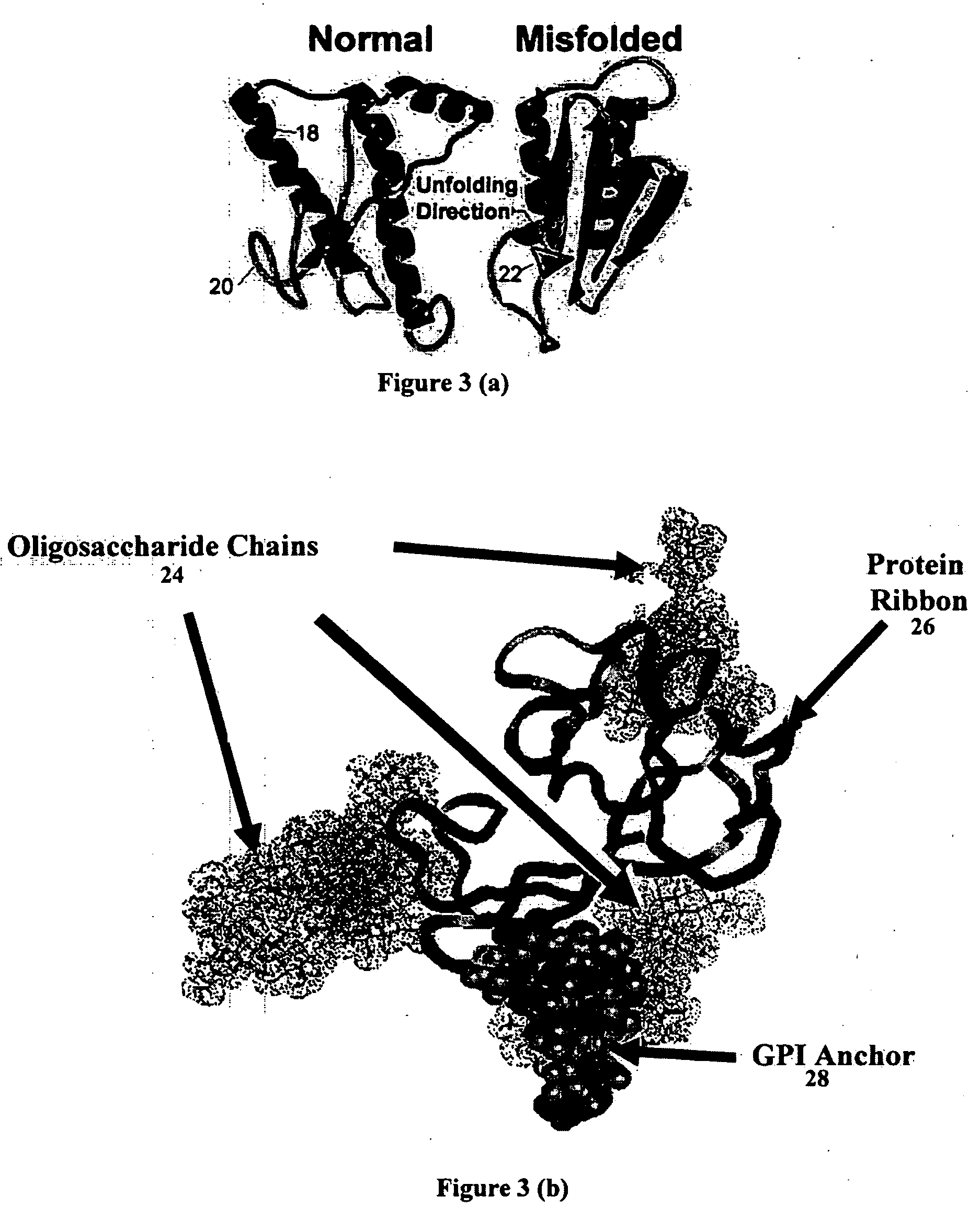
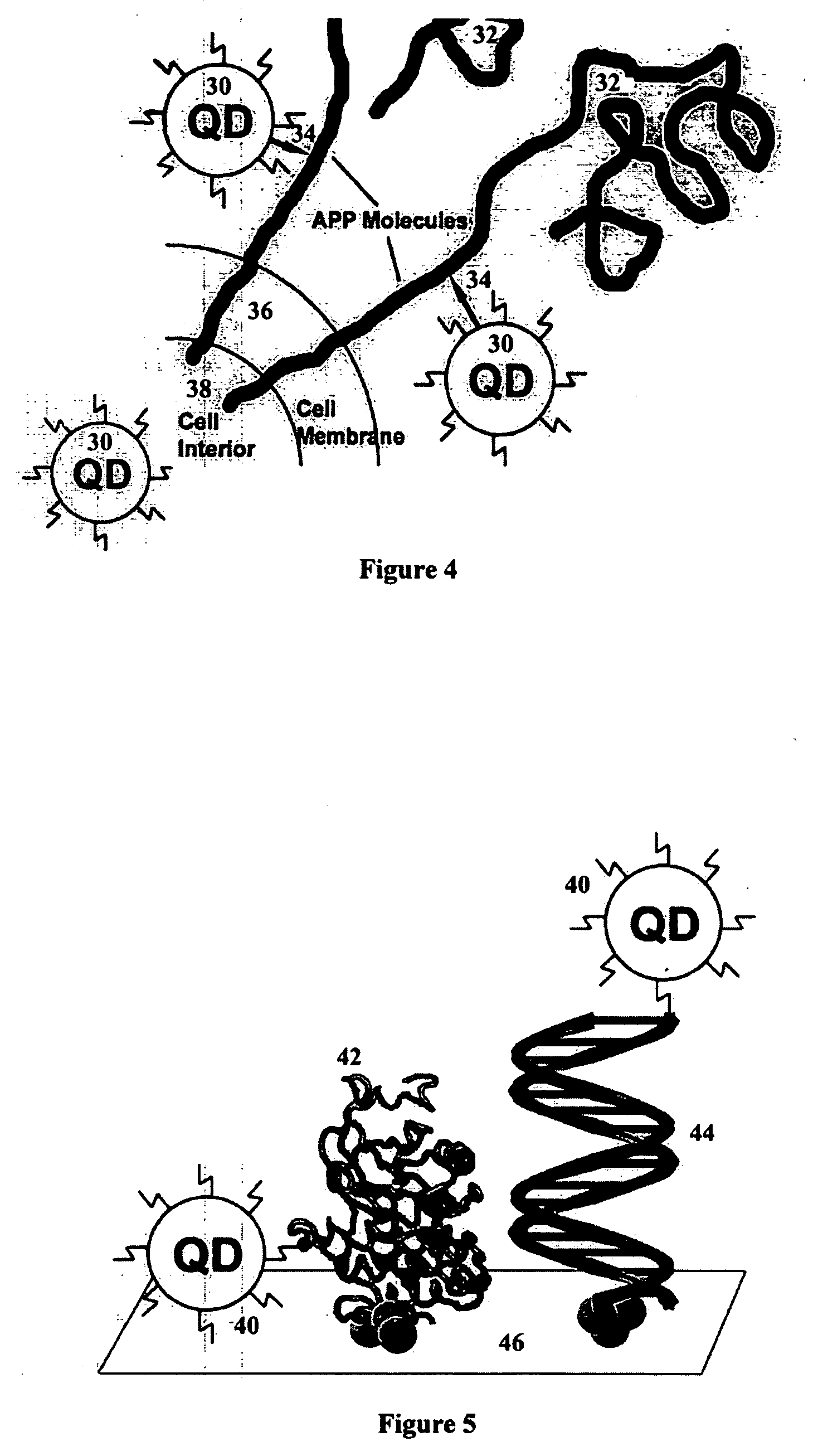
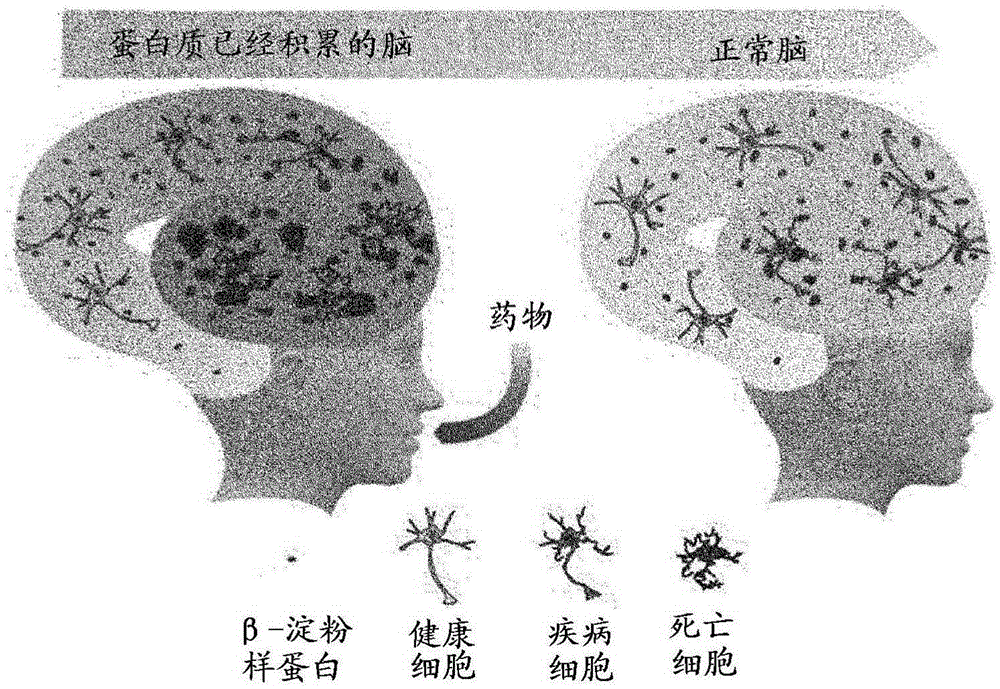
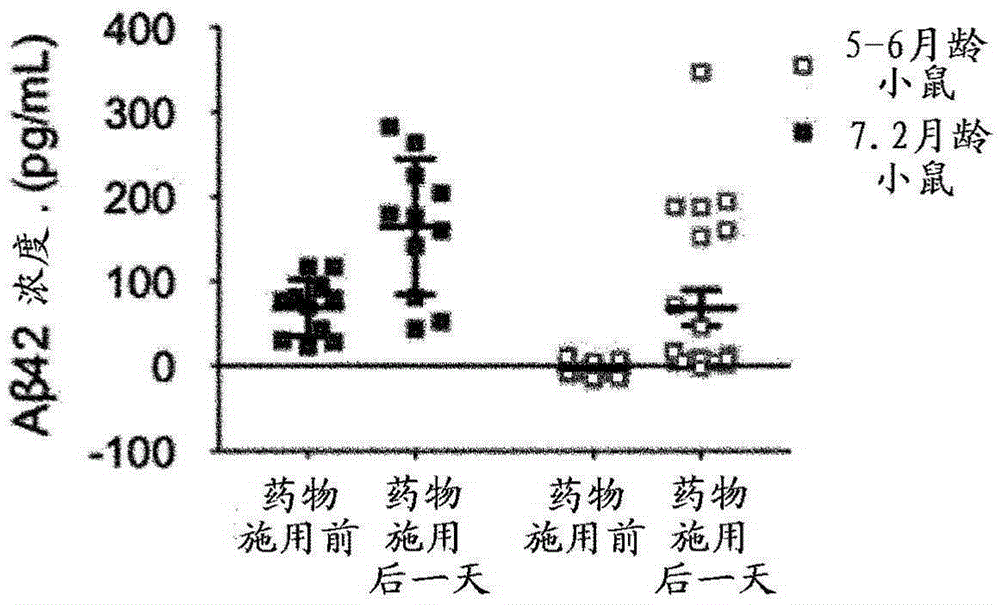
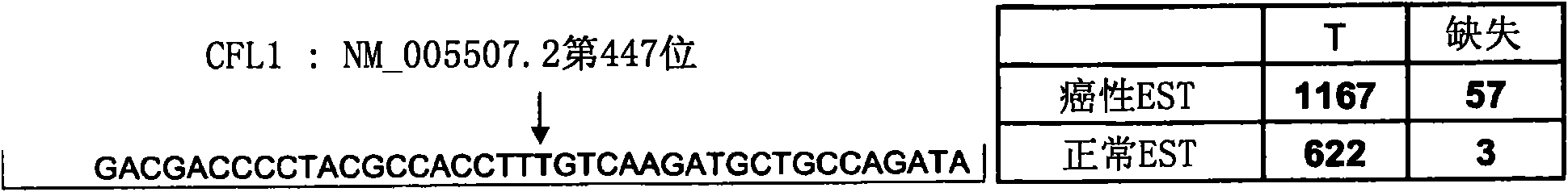Patents
Literature
66 results about "Aberrant protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aberrant protein prevents self-cleaning of nerve cells in ALS. Scientists have discovered that TDP-43, a nuclear RNA-binding protein that accumulates in amyotrophic lateral sclerosis (ALS), interferes with lysosomal pathways responsible for its clearance and induces autophagy.
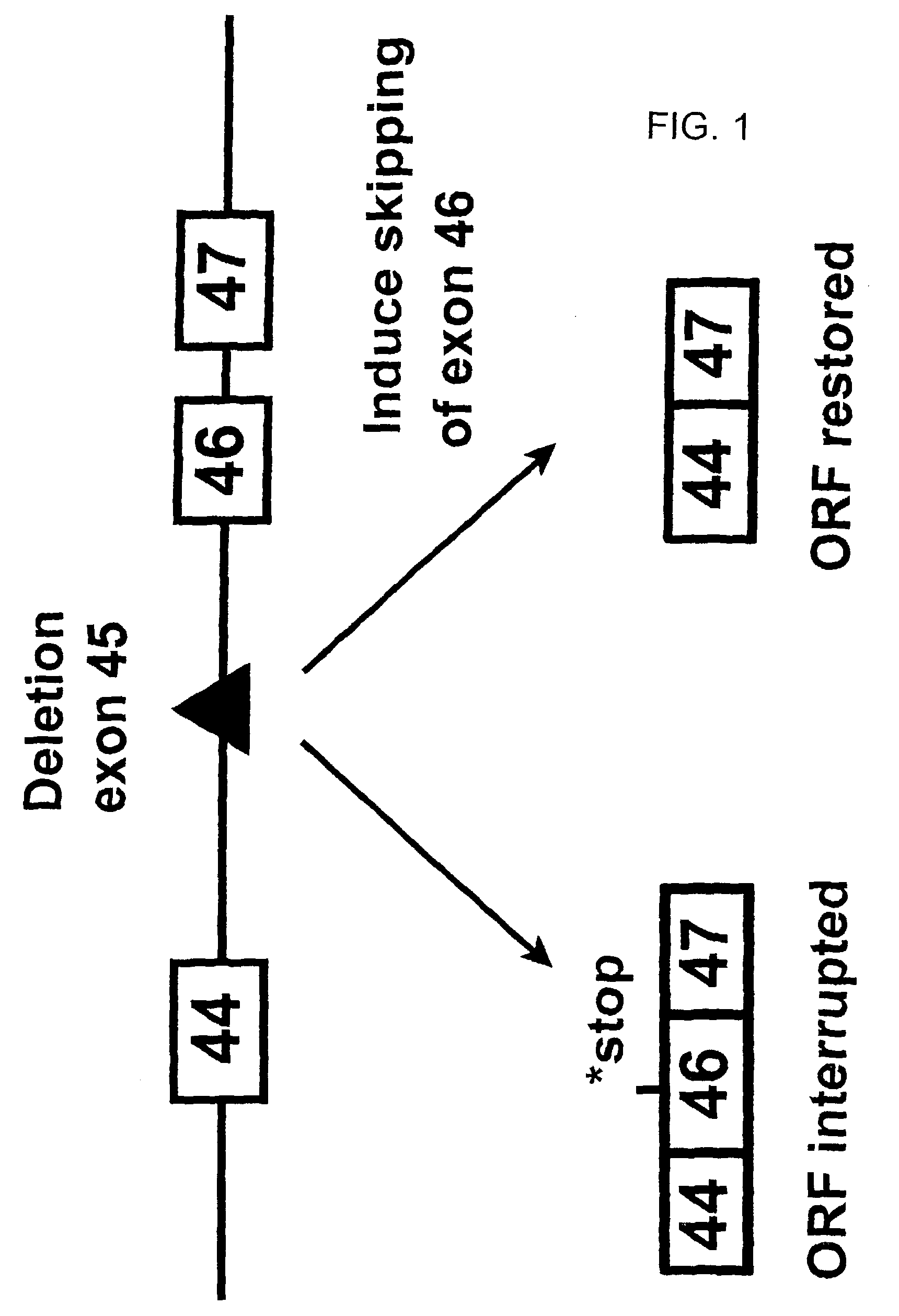
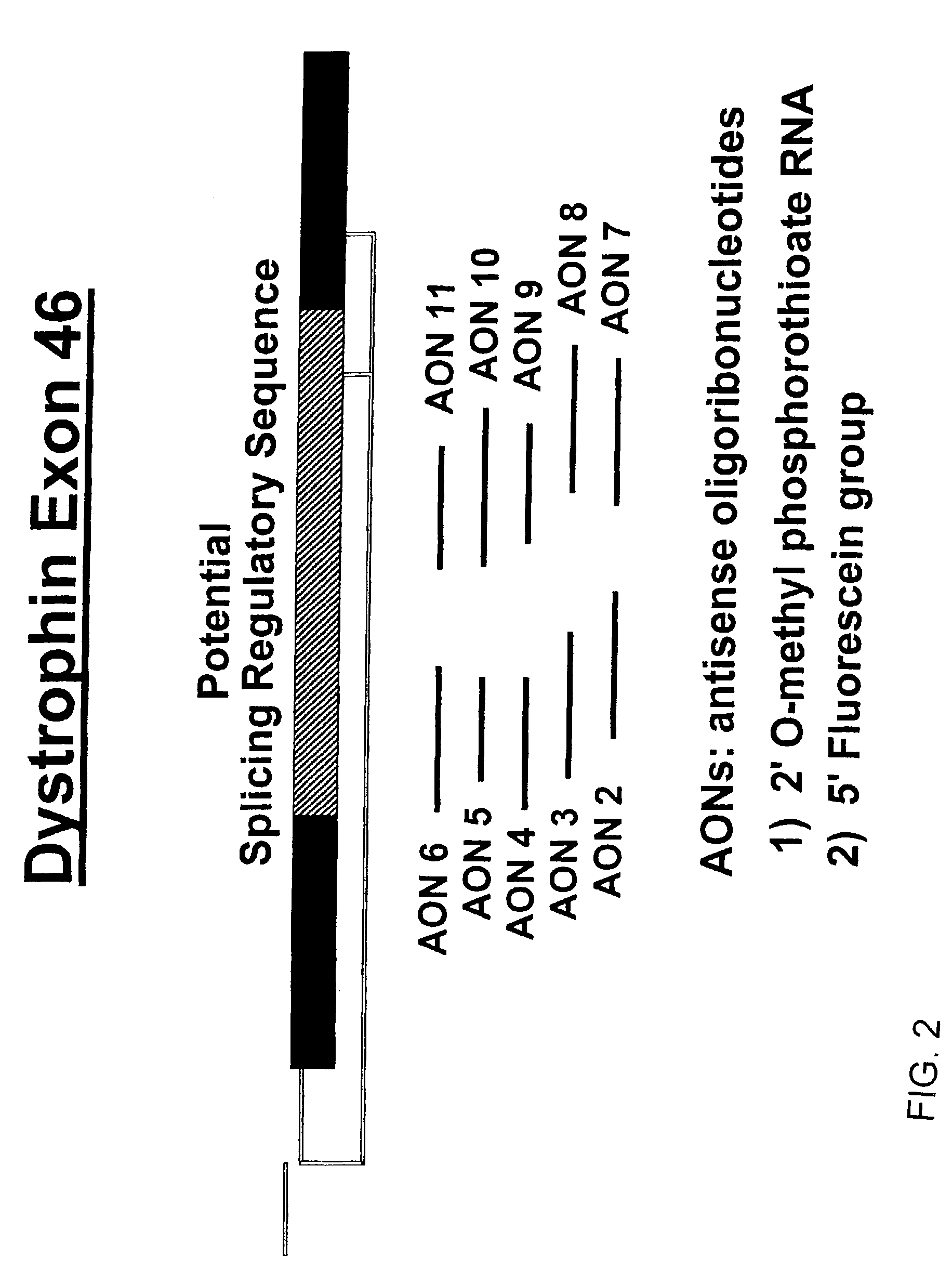
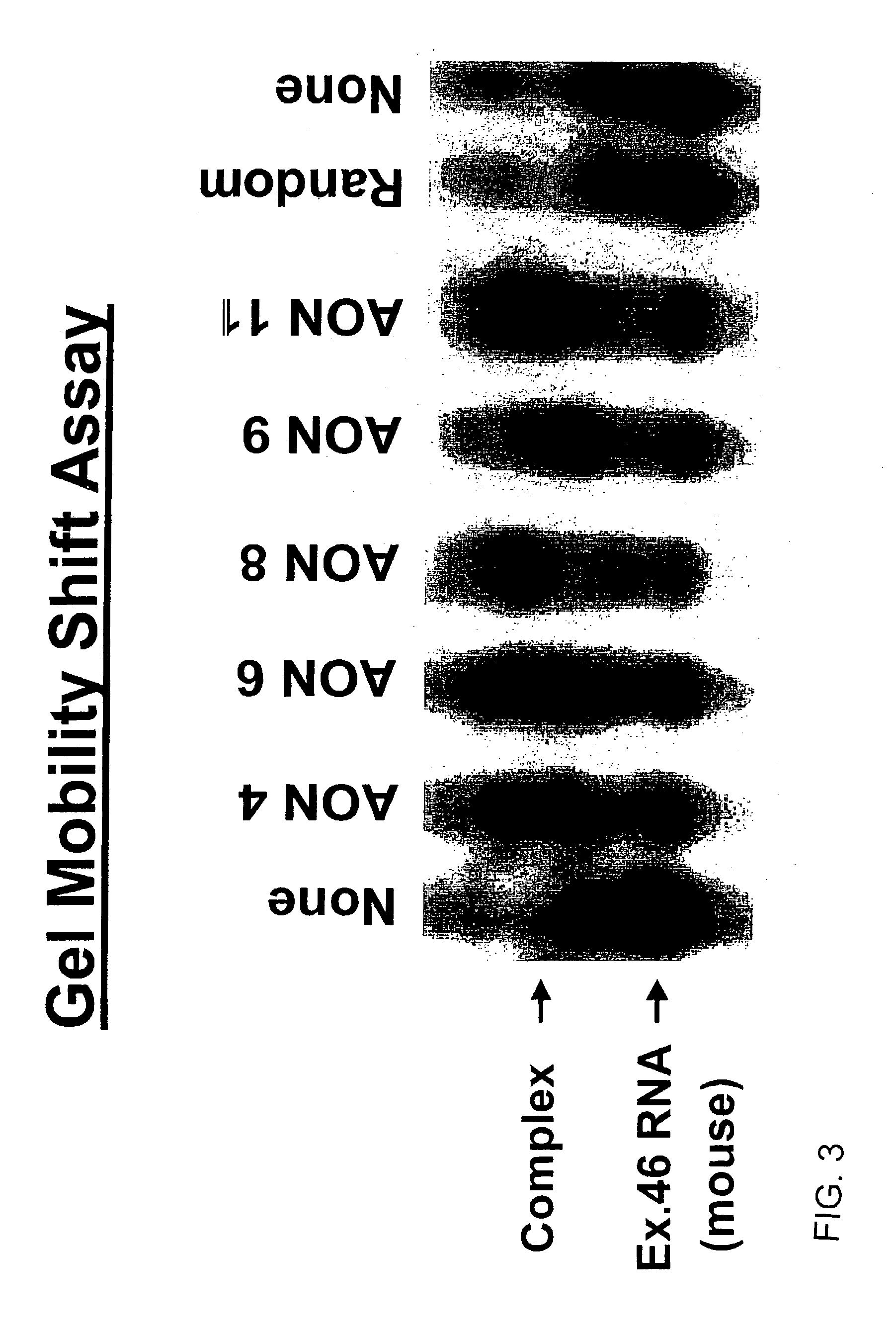
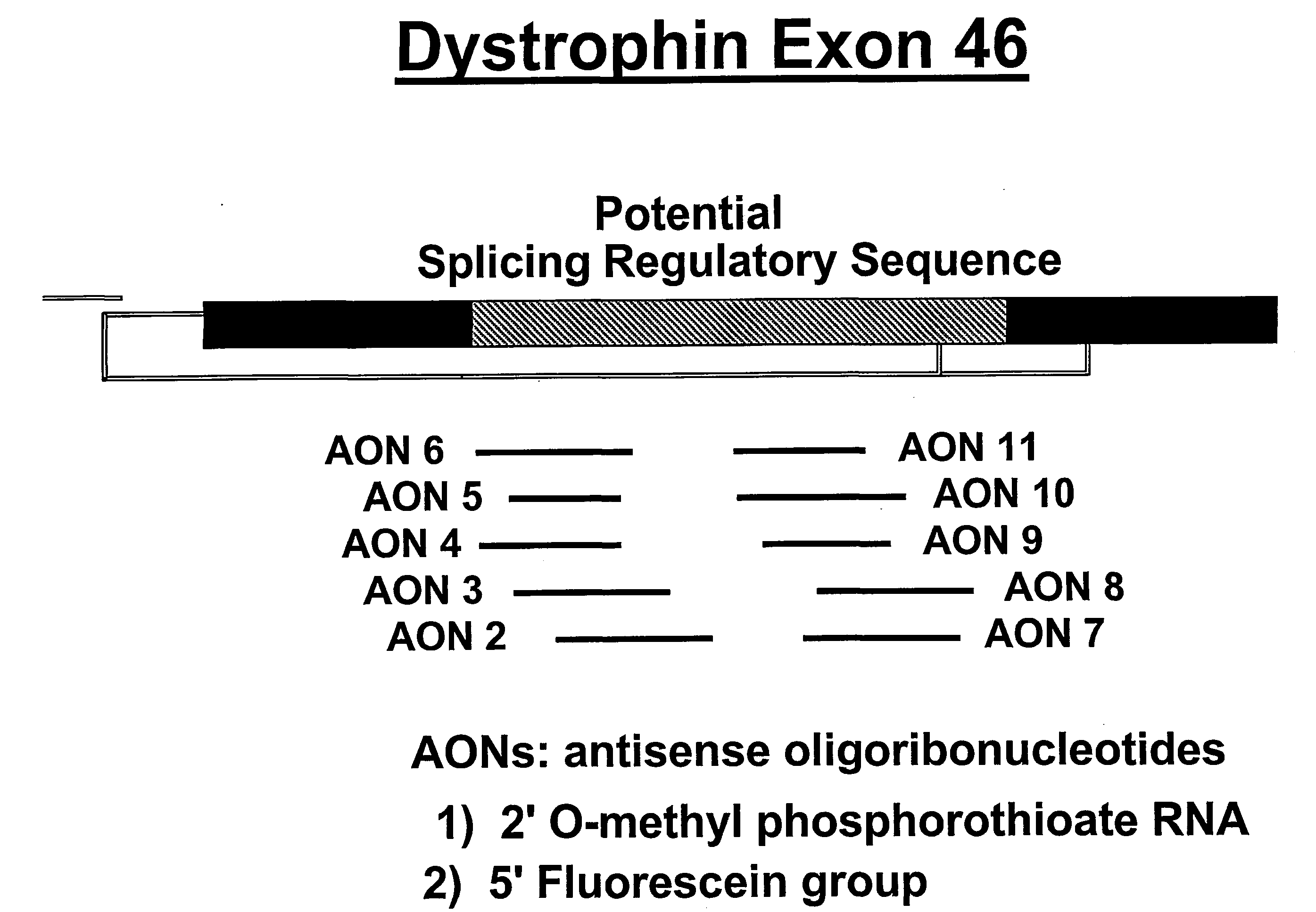
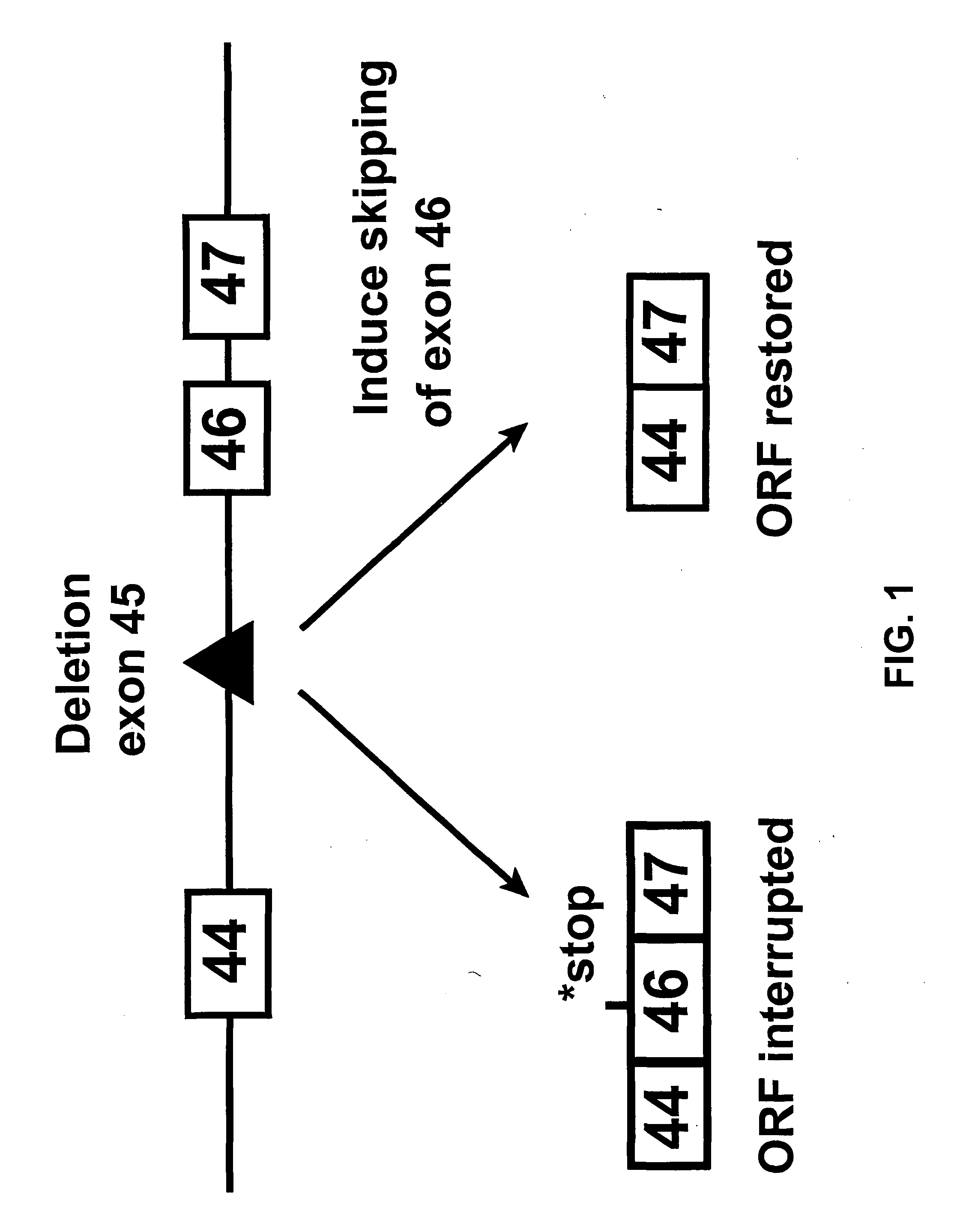
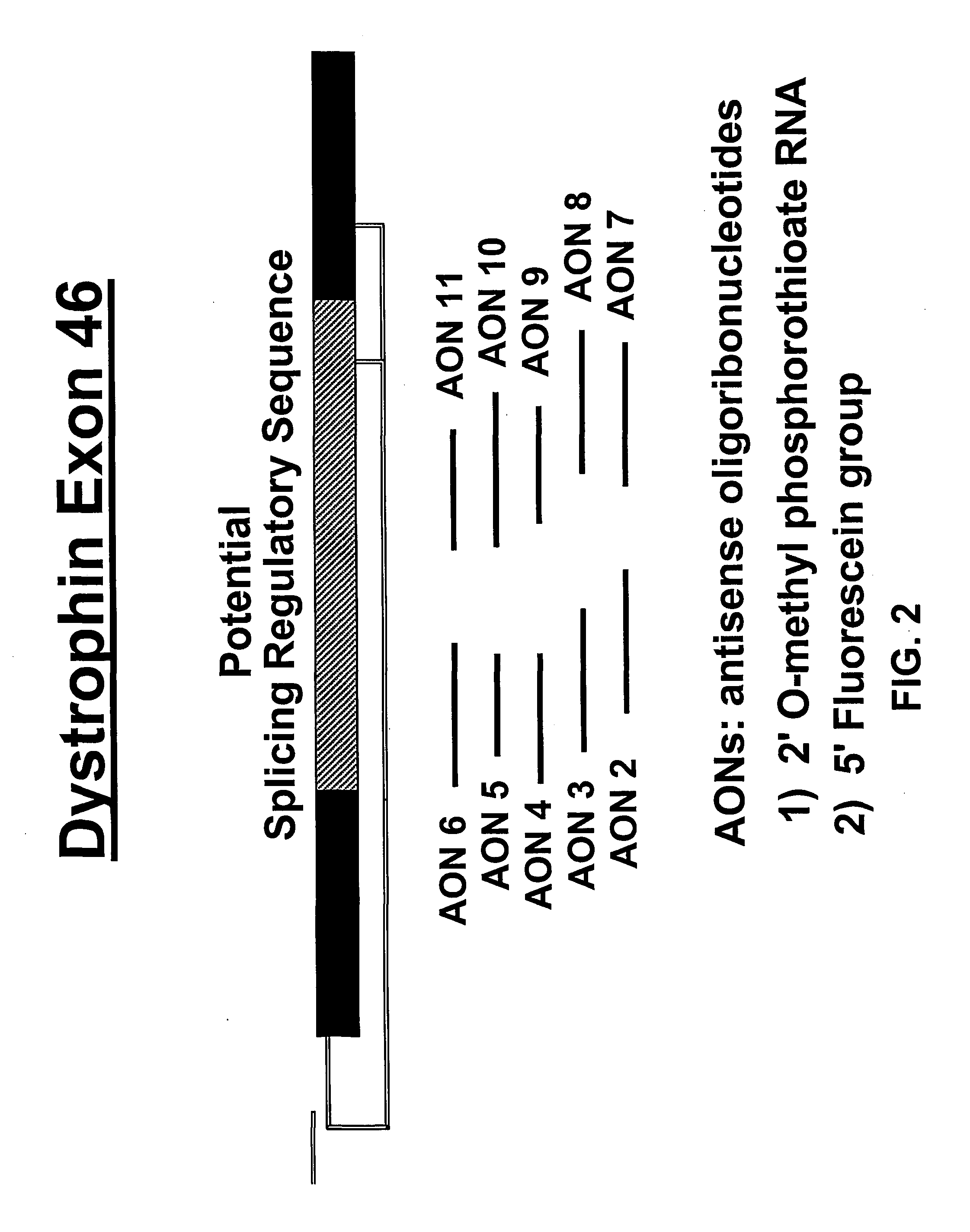
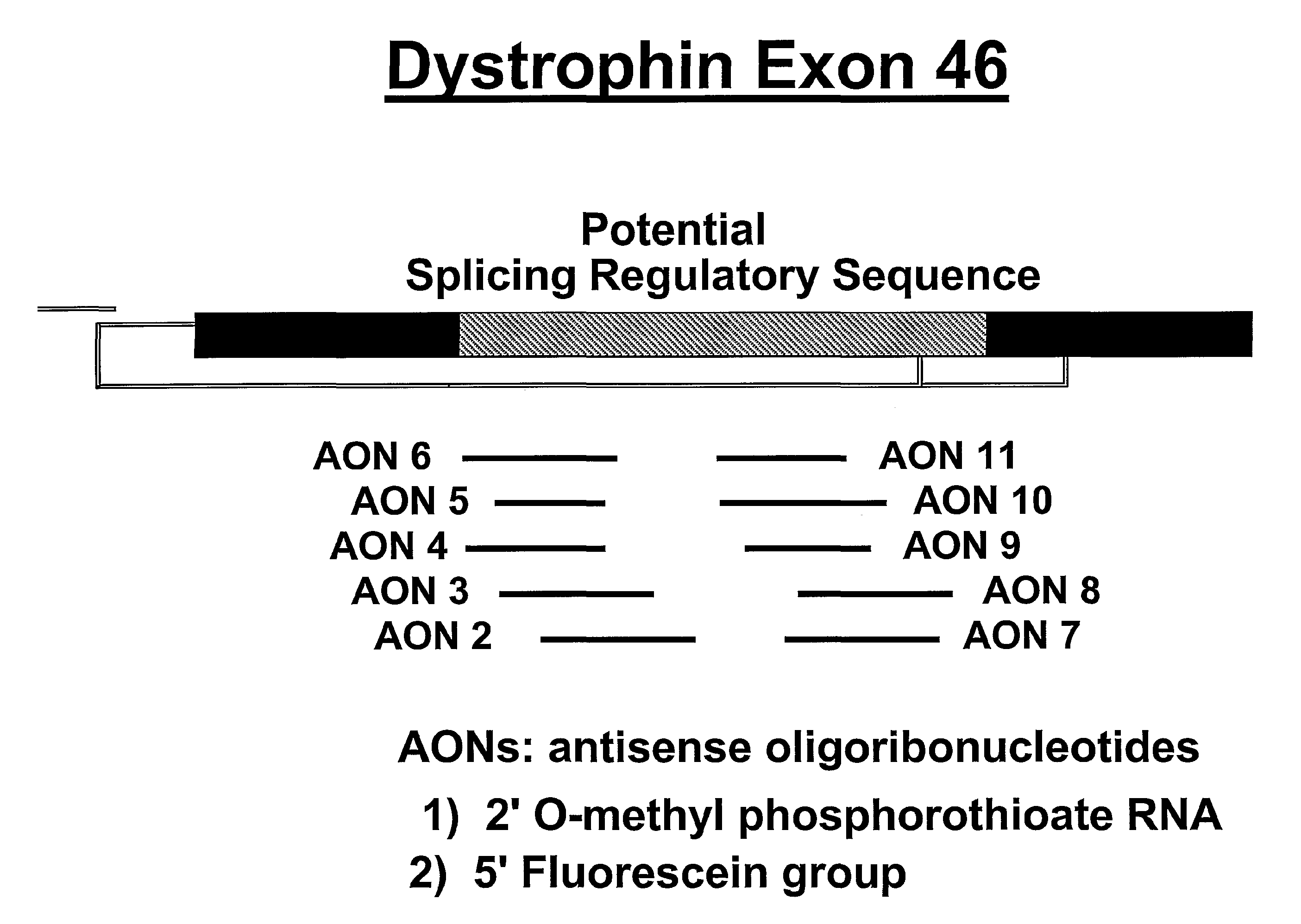
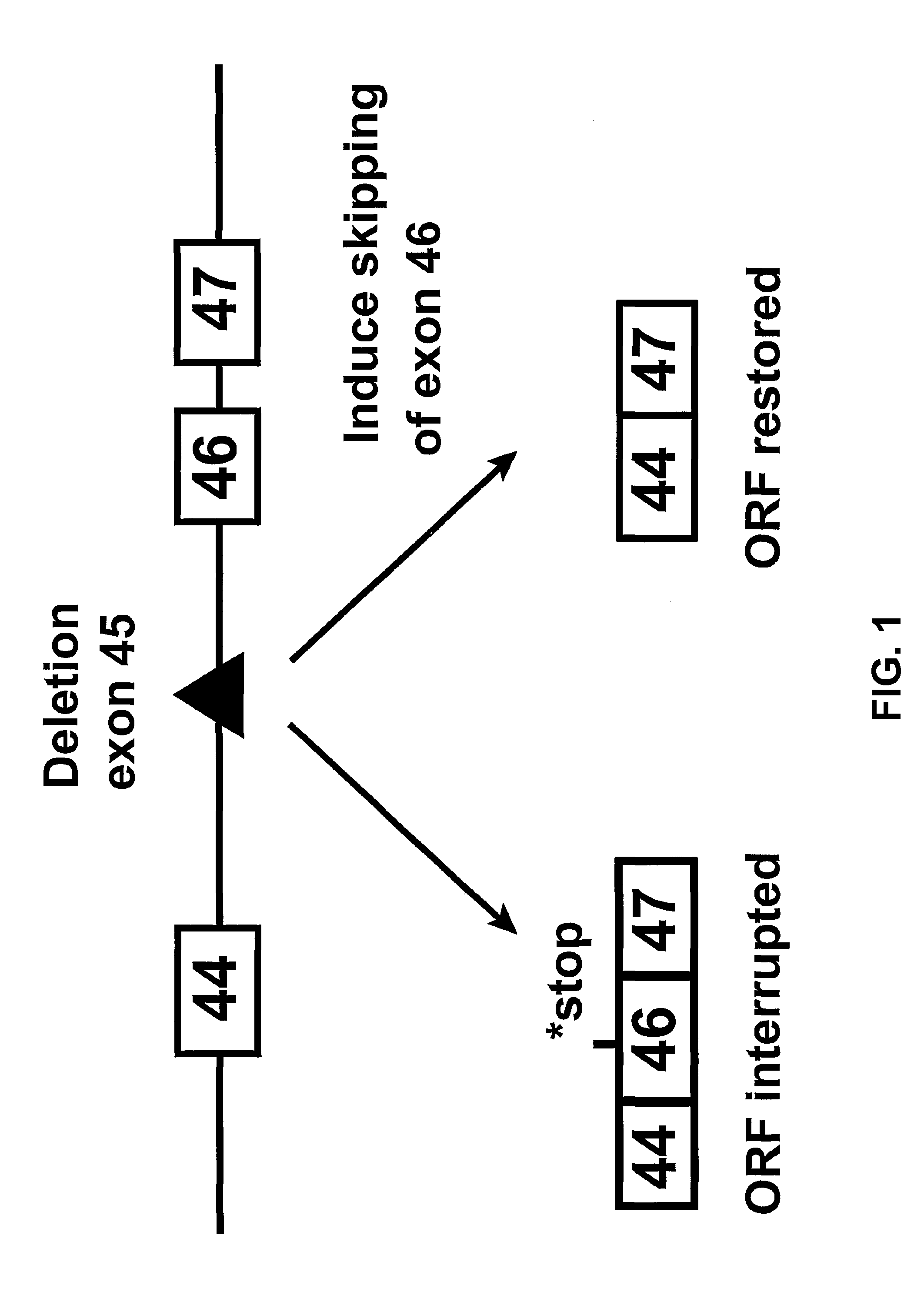
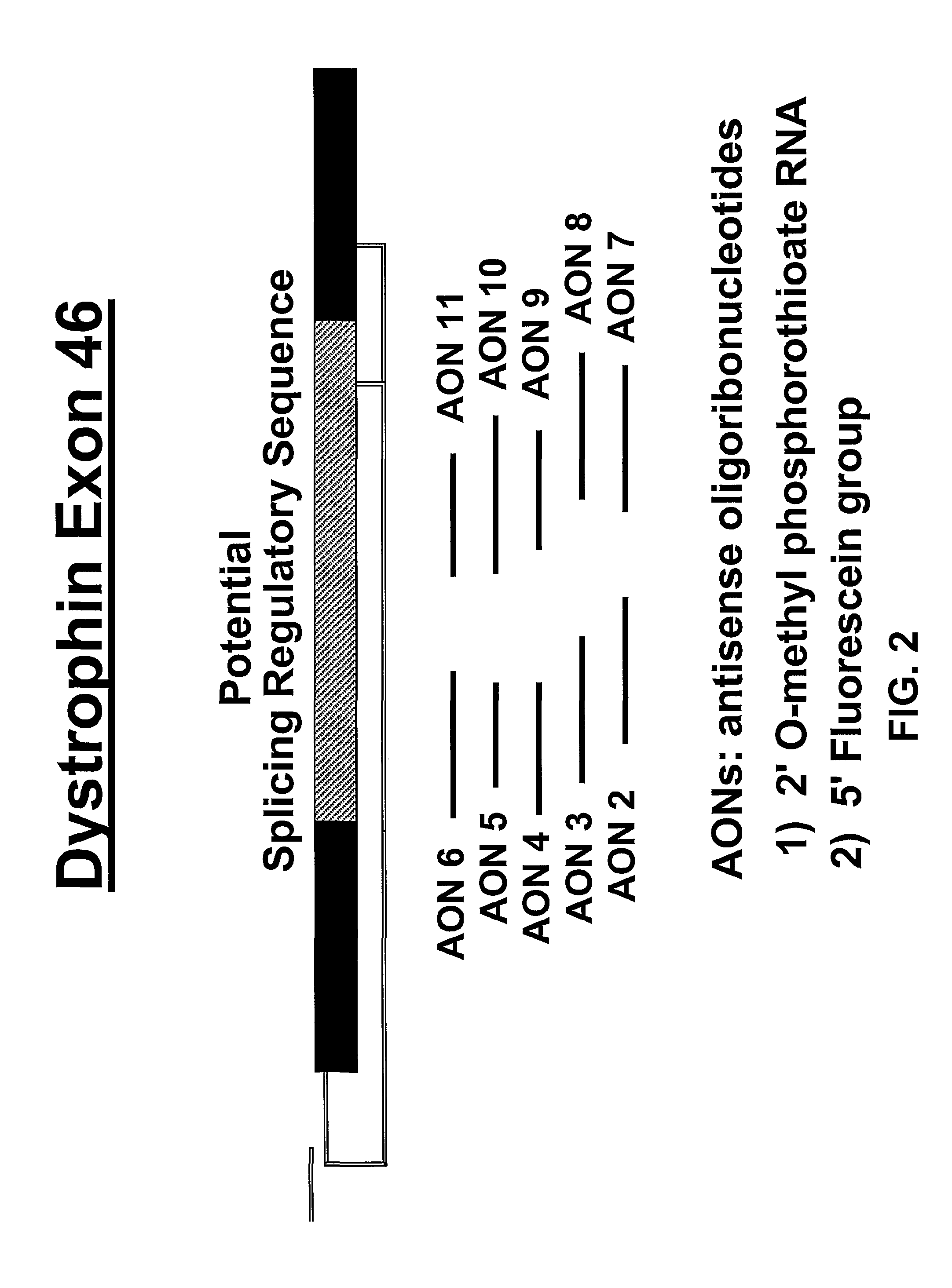
Induction of exon skipping in eukaryotic cells
InactiveUS7973015B2Efficiently maskedPotential for manipulationOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
The present invention provides a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature MRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:LEIDEN ACADEMISCH ZIEKENHUIS
Induction of exon skipping in eukaryotic cells
InactiveUS20090228998A1Efficiently maskedPotential for manipulationOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
The present invention provides a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature mRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Induction of exon skipping in eukaryotic cells
InactiveUS20080209581A1Reduce productionReduce amountOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
Described is a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature mRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Modulation of neurodegenerative disease by modulating xbp-1 activity
InactiveUS20110142799A1Extend your lifeReduces motor neuron apoptosisBiocideNervous disorderCell biologyAberrant protein
The invention provides methods for treating and preventing a neurodegenerative disease associated with aberrant protein aggregation by modulating the expression and / or activity of XBP-1, IRE-1 alpha, and / or EDEM. The present invention also pertains to methods for identifying compounds that modulate the expression and / or activity of XBP-1, 1RE-1 alpha, and / or EDEM. The present invention further provides methods for determining whether a subject is at risk of developing or has developed a neurodegenerative disease associated with aberrant protein aggregation.
Owner:CORNELL UNIVERSITY
Compositions and methods for treating neurological disorders
InactiveUS20060229233A1Preventing new amyloid plaqueMaintaining current amyloid plaque levelOrganic active ingredientsBiocideEmulsionCell Aggregations
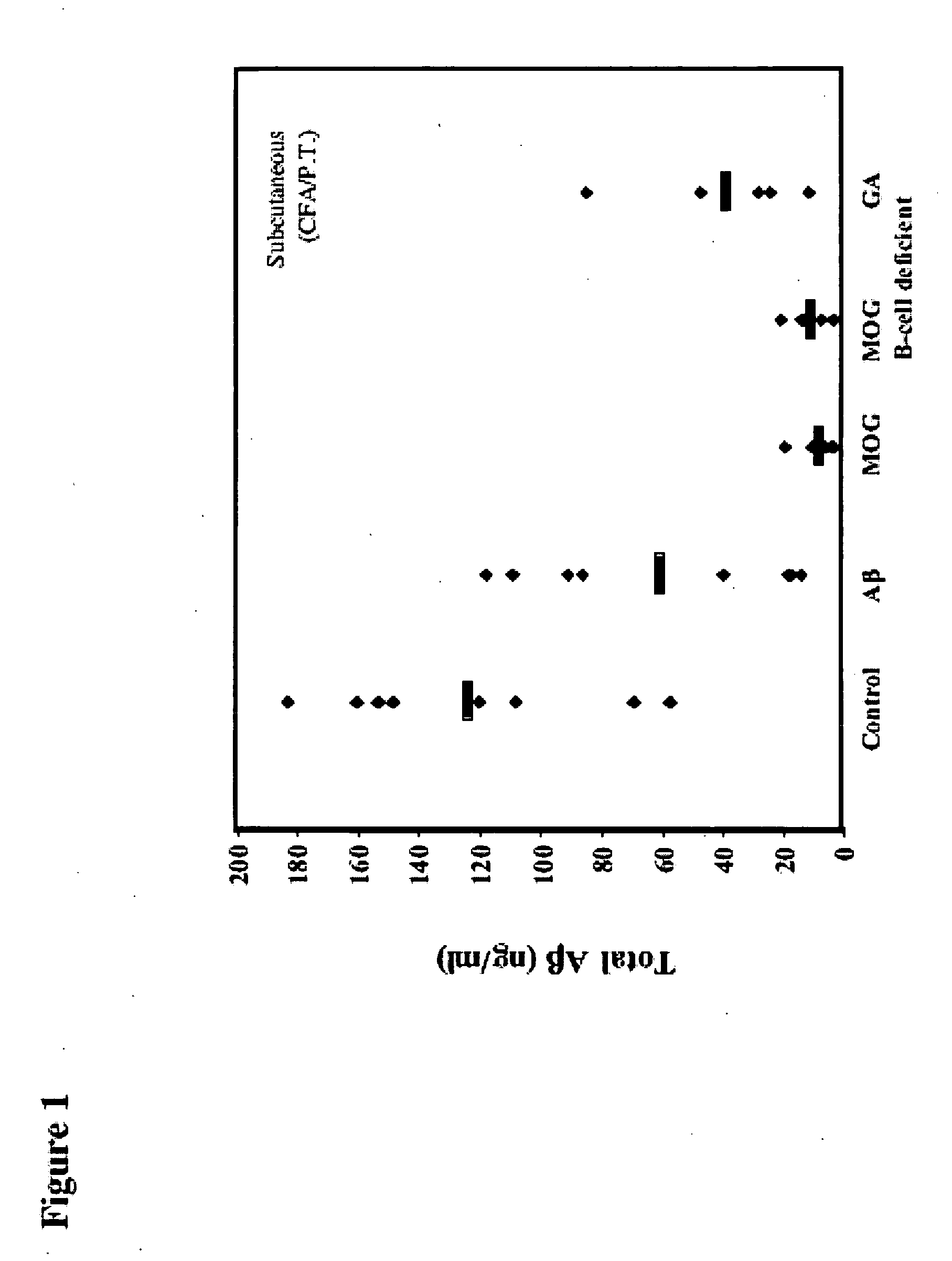
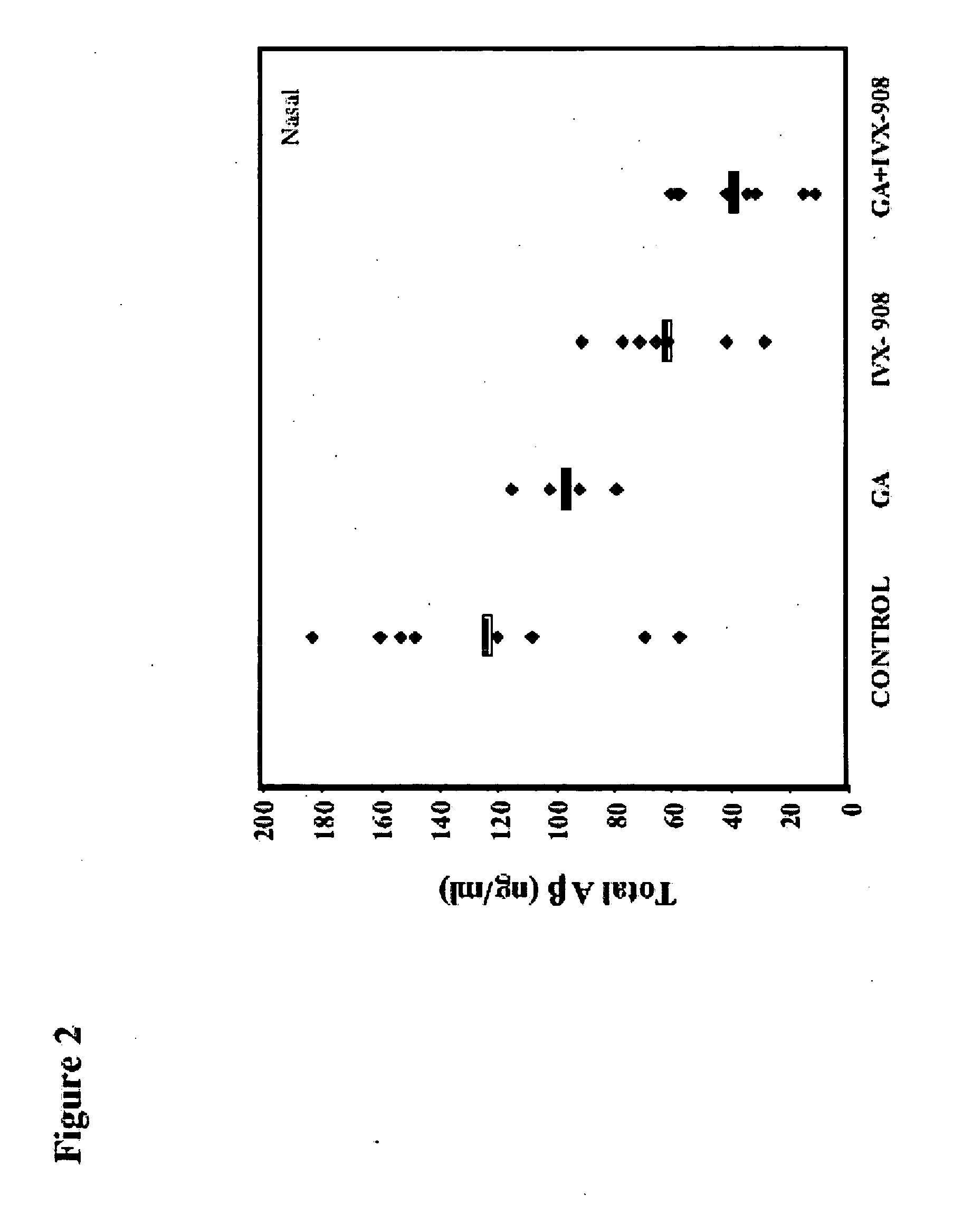
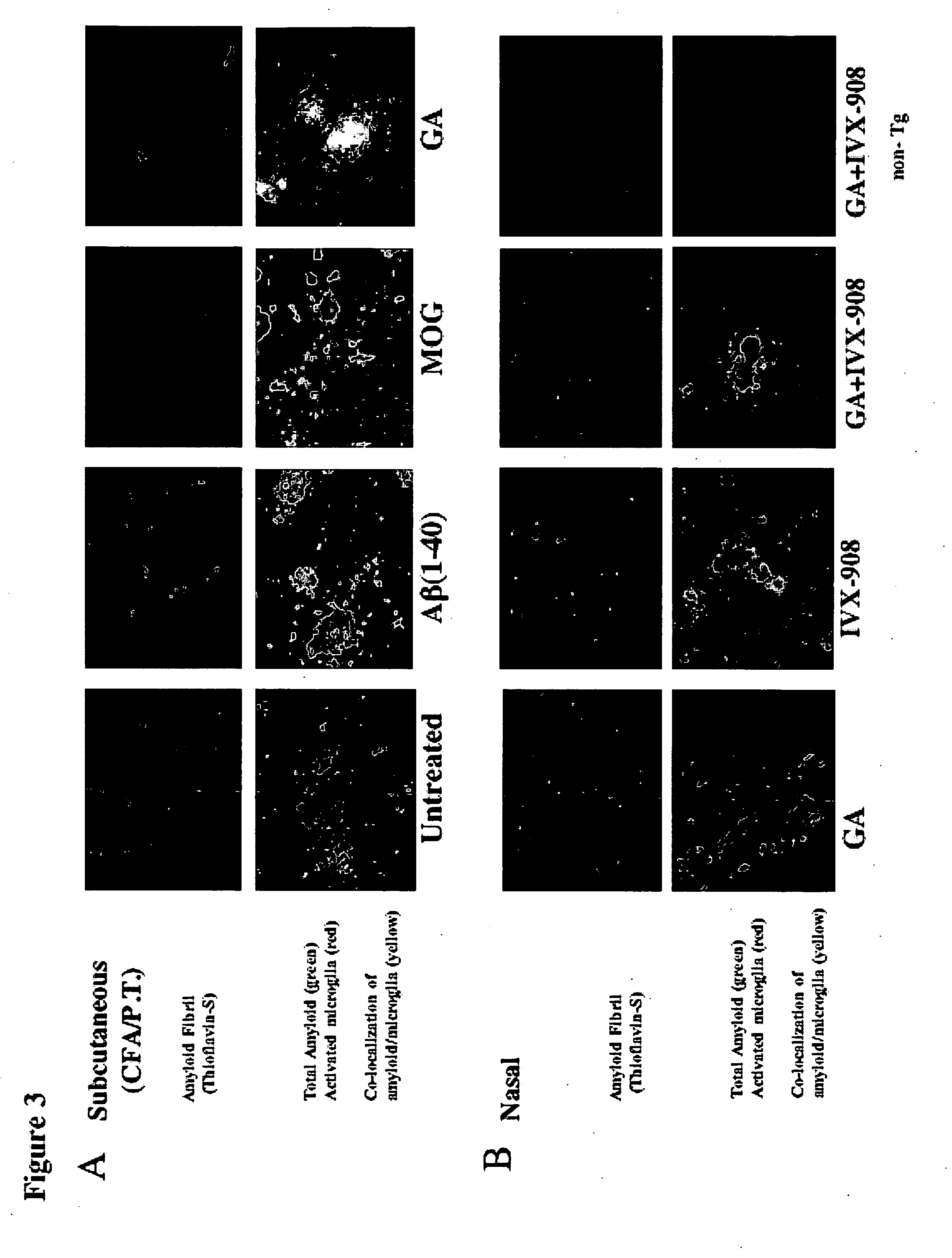
Compositions useful for treating neurological disorders including neurodegenerative disorders associated with deleterious protein aggregation, aberrant protein folding, such as brain amylogenic diseases, and / or neurodegenerative autoimmune disorders are described. Methods of using said compositions also are described. In particular, methods to treat a neurodegenerative disorder such as Alzheimer's disease and a neurodegenerative autoimmune disorder such as Multiple Sclerosis are contemplated utilizing proteosomes and / or Glatiramer Acetate, wherein the GA is in a submicron emulsion or a nanoemulsion.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Abnormal protein removing composition
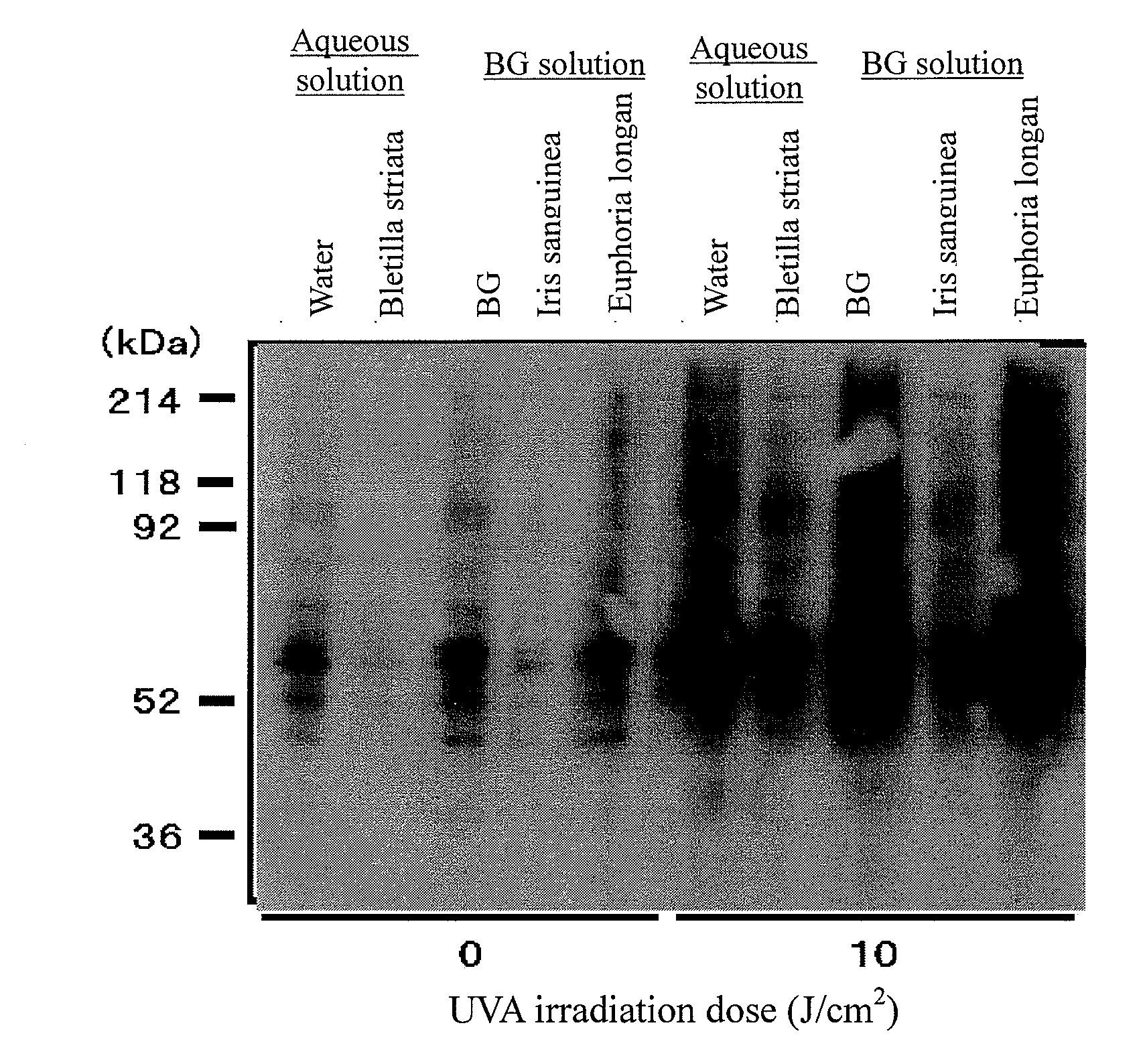
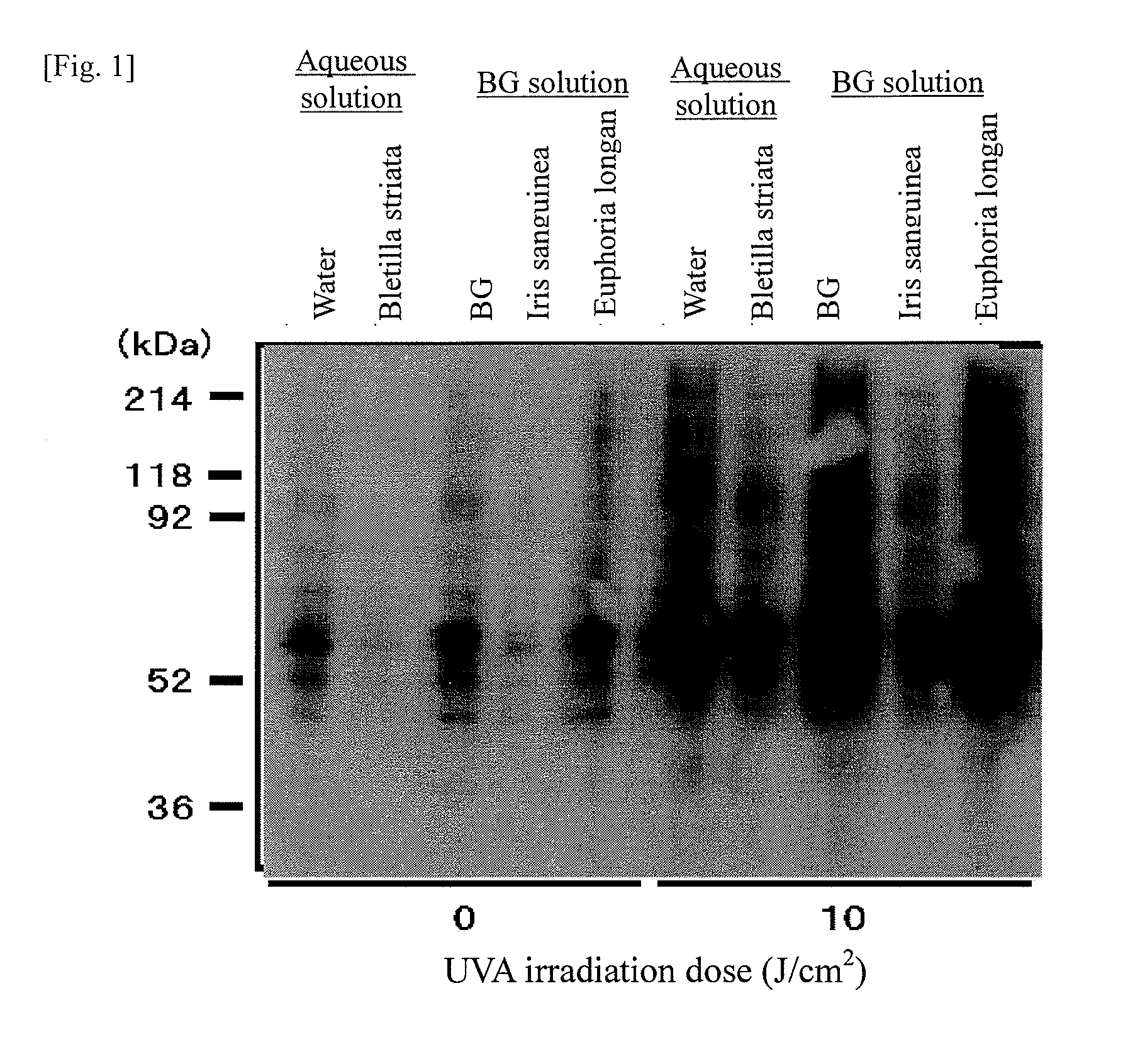
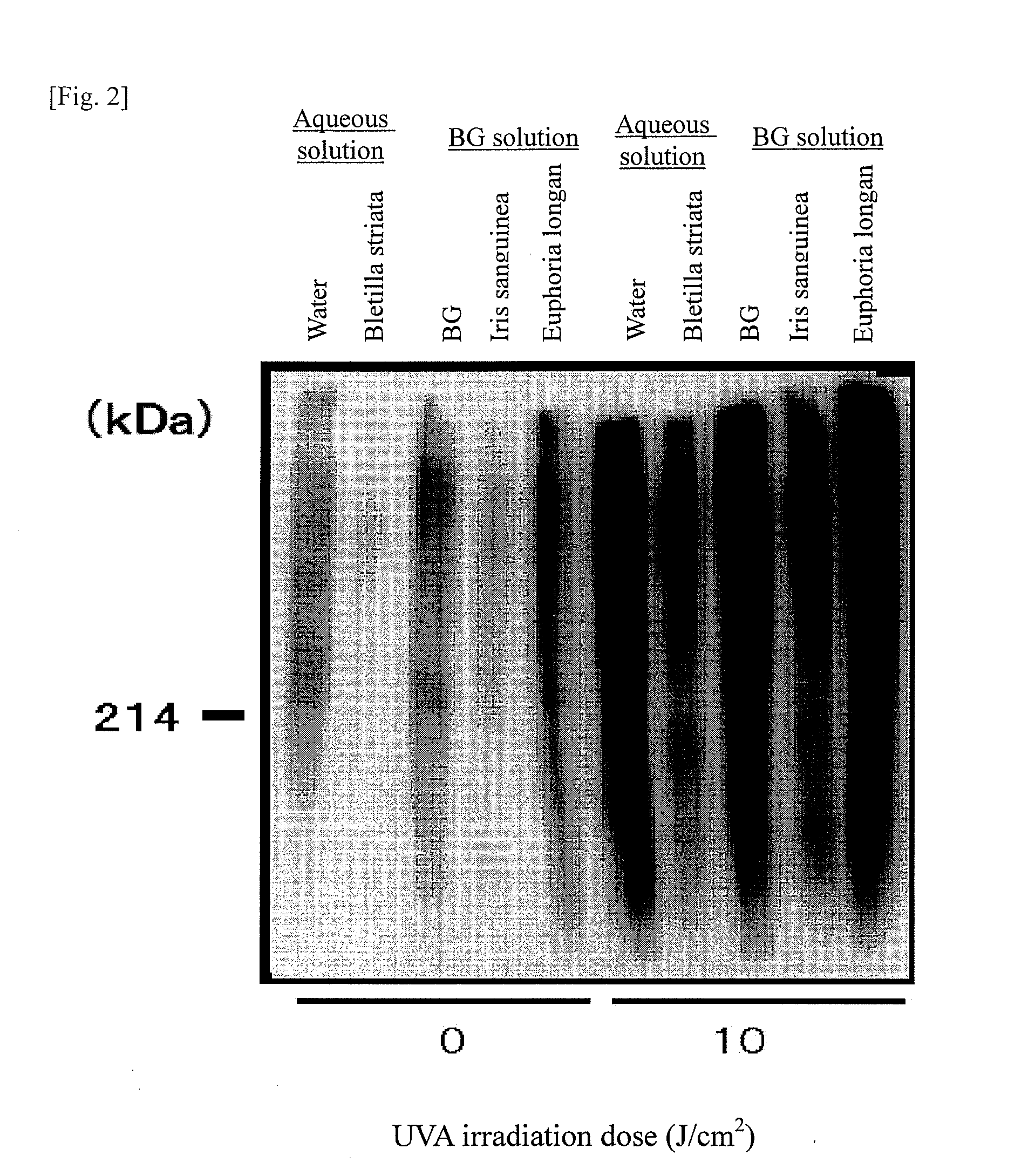
InactiveUS20090041866A1Abnormal protein can be removed or suppressedEase complexityBiocideCosmetic preparationsBletilla striataIris sanguinea
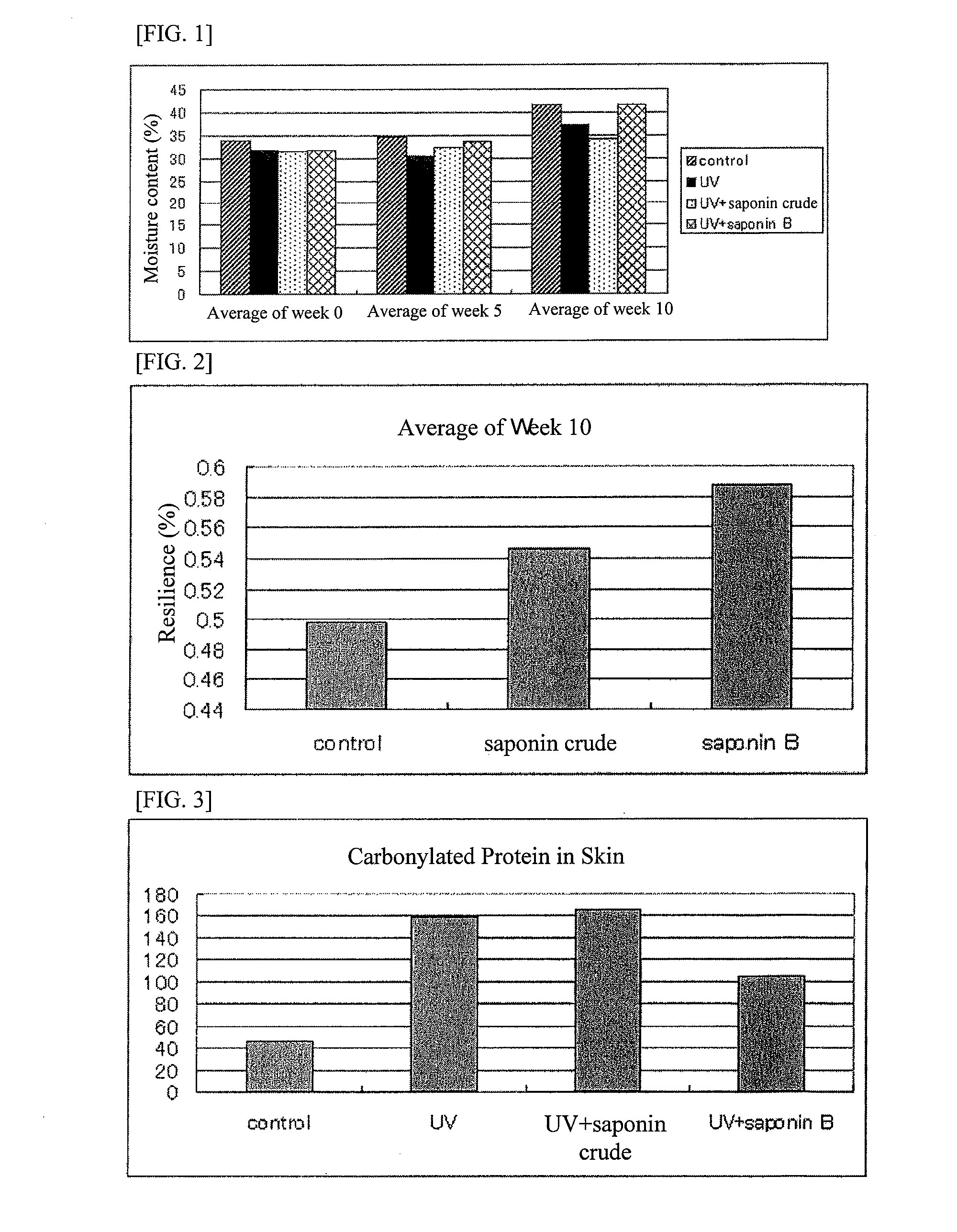
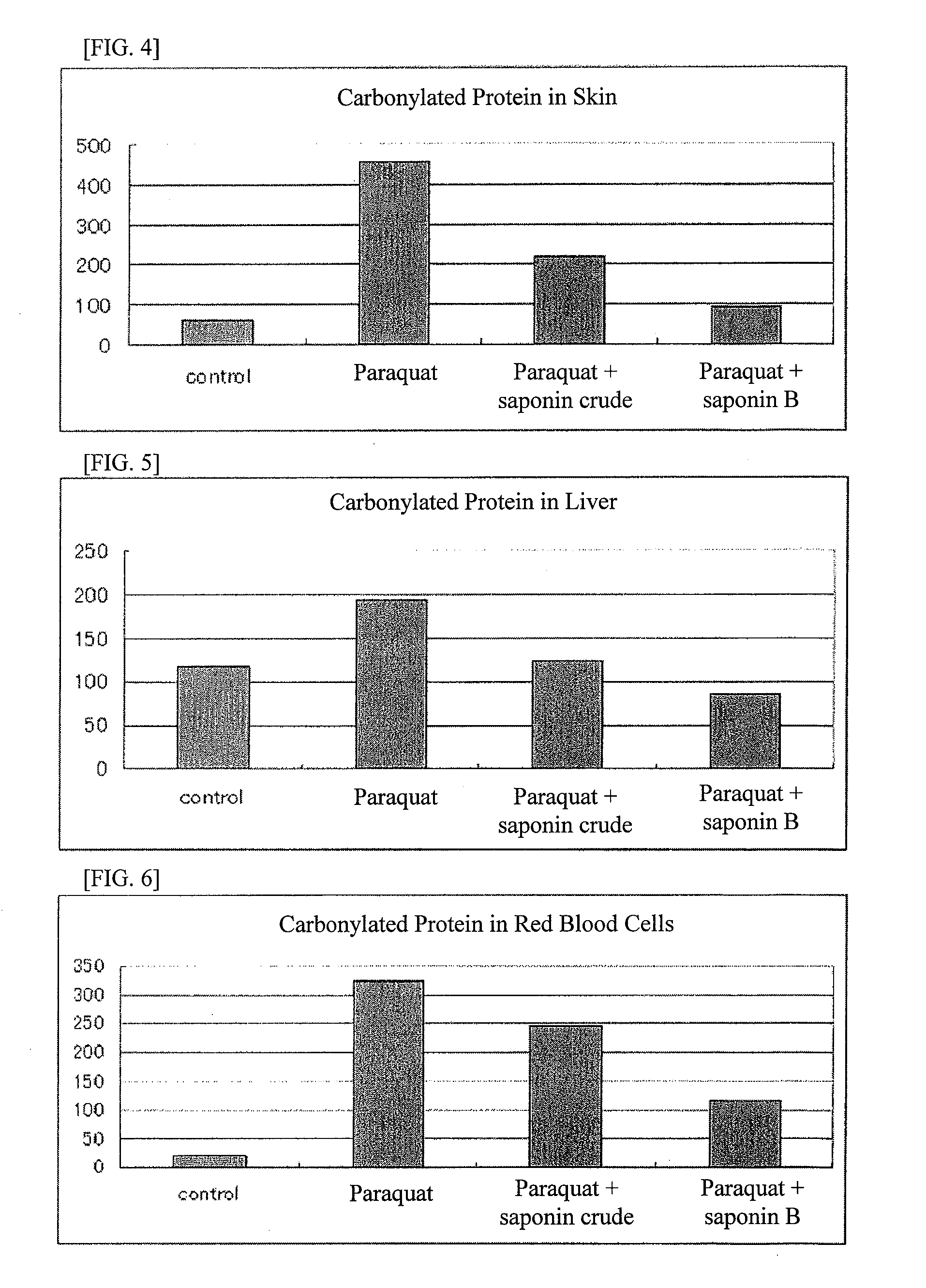
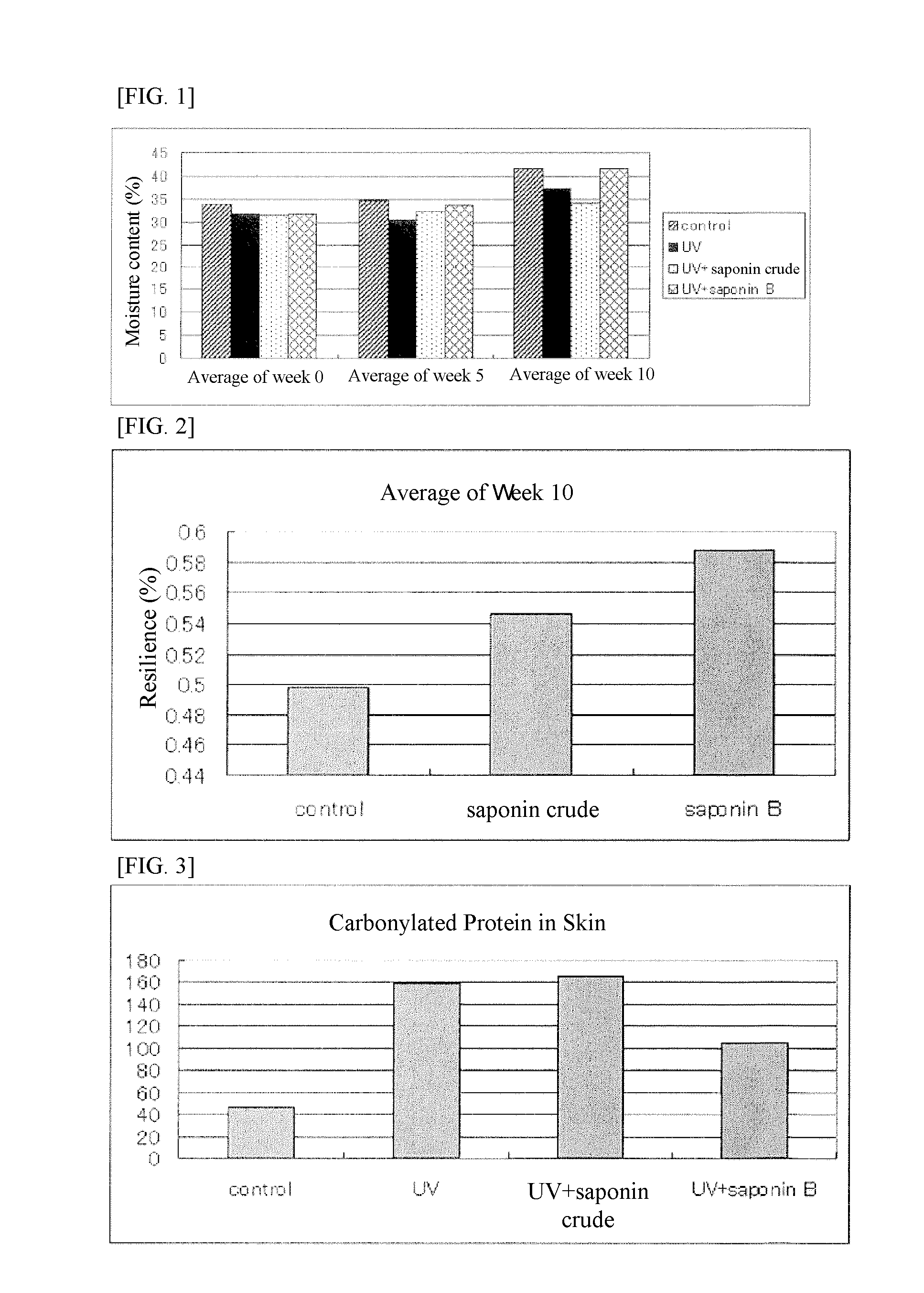
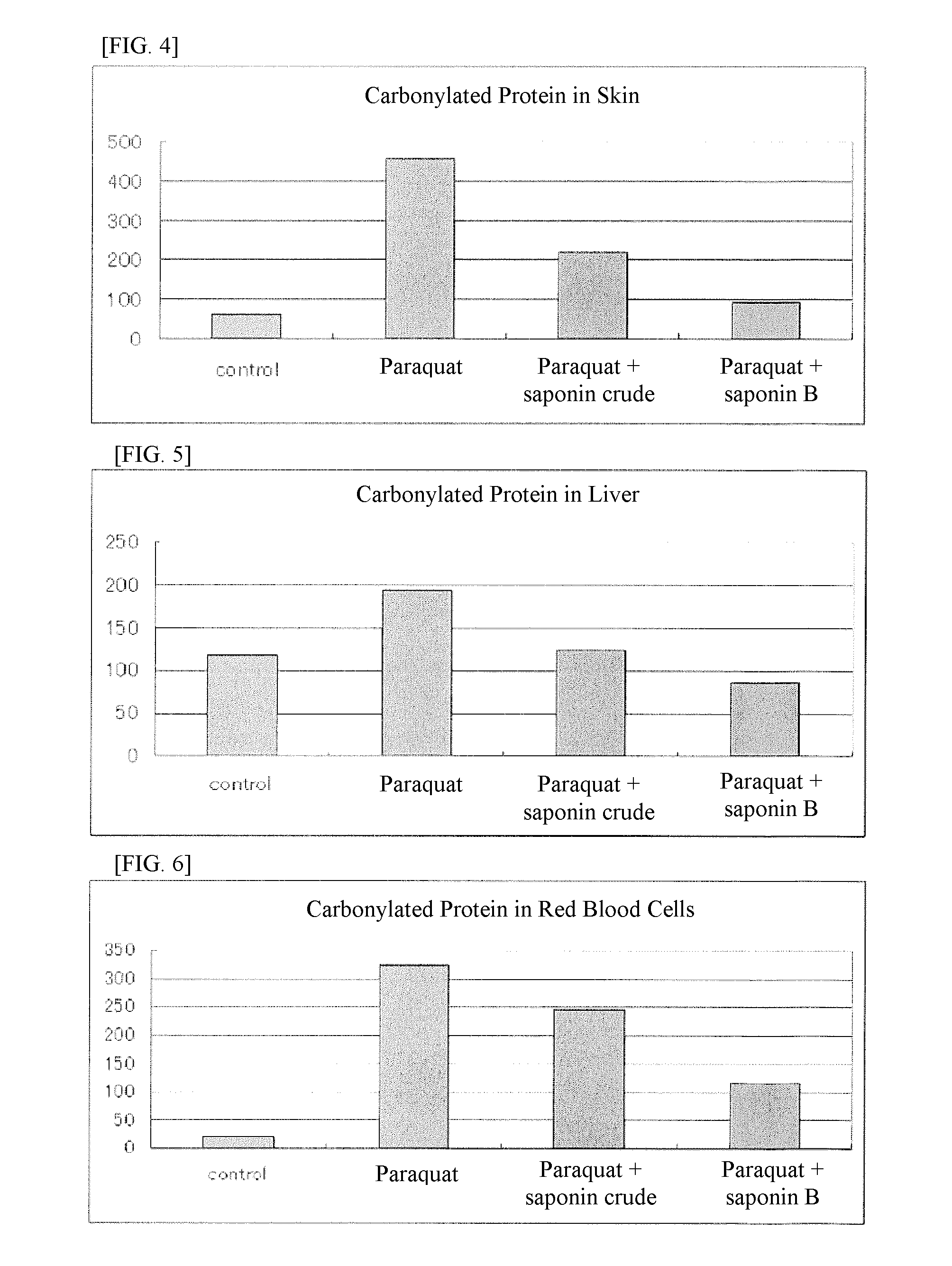
It is an object of the present invention to efficiently remove abnormal protein produced in living tissues, especially skin, due to exposure to UV light, etc.A composition for removing abnormal protein containing one or two or more substances selected from silybin, Bletilla striata extract and Iris sanguinea extract.
Owner:FUAN KERU
Misfolded protein sensor method
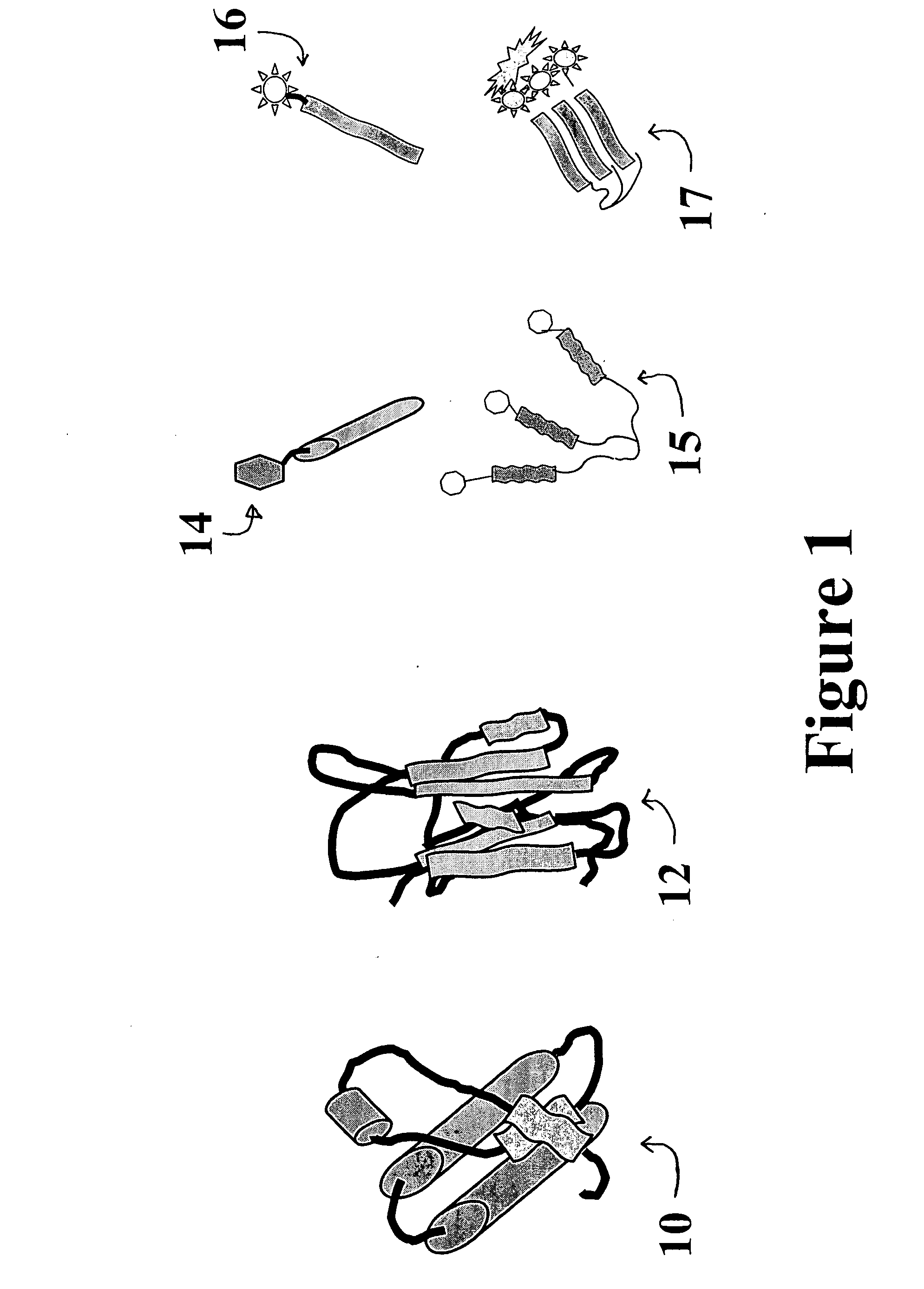
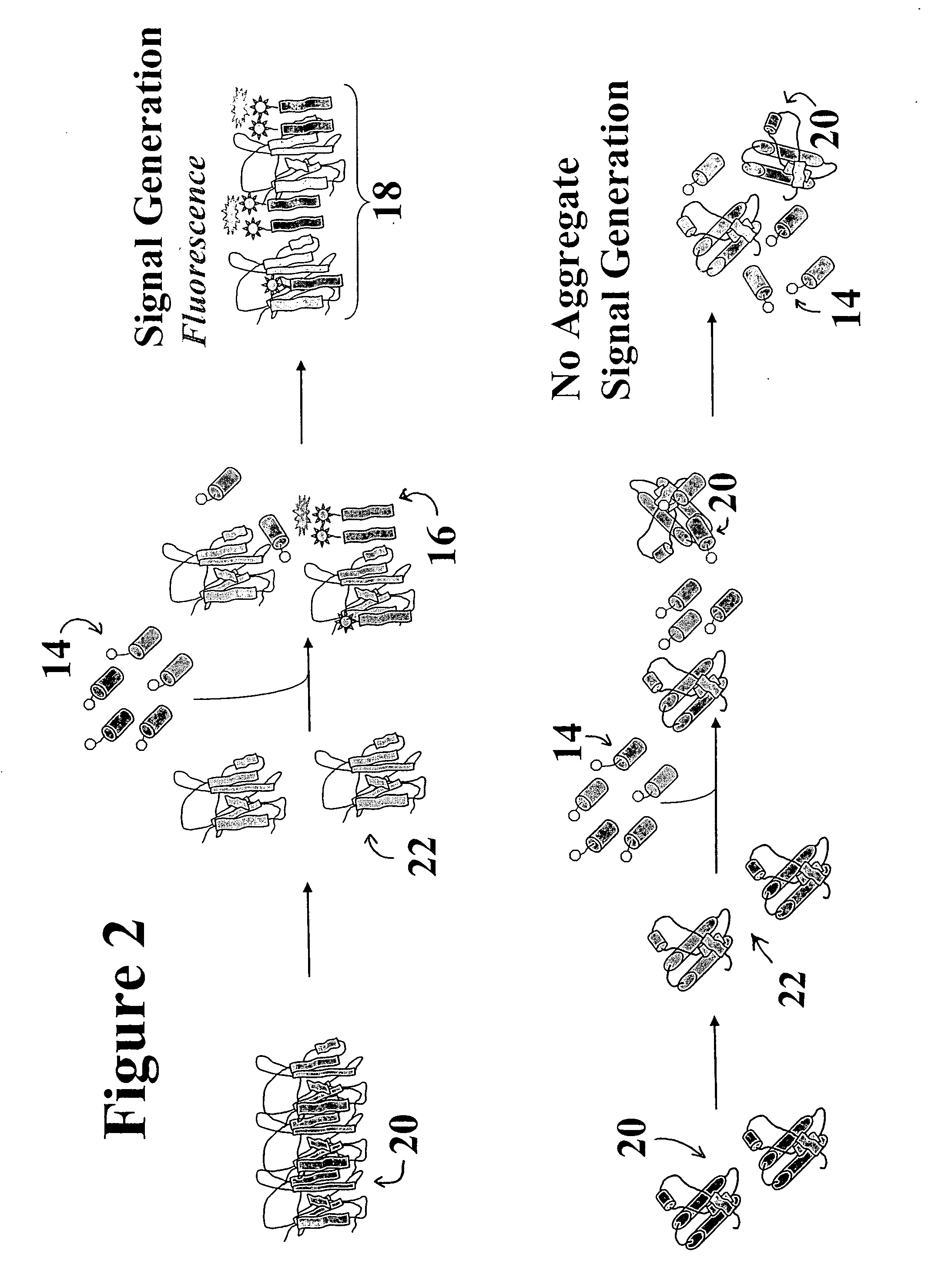
ActiveUS20060286672A1Easy to detectRapid and cost-effectivePeptide preparation methodsDepsipeptidesCrystallographyDendrimer
A catalytic conformational sensor method for detecting abnormal proteins and proteinaceous particles. The method is based on the interaction of a peptide fragment or probe with an abnormal proteinaceous particle. The interaction catalyzes transformation of the probe to a predominately beta sheet conformation and allows the probe to bind to the abnormal proteinaceous particle. This in turn, catalyzes propagation of a signal associated with the test sample-bound probe. As a result signals can be propagated even from samples containing very low concentrations of abnormal proteinaceous particles. The peptide probes can be designed to bind to a desired peptide sequence or can even be based on dendrimer structure to control further aggregate propagation.
Owner:PRESYMPTO INC
Nanobioprocessor for protein and cell therapy
InactiveUS20050214380A1Increase relative motionSuperior and stable diagnostic and treatment agentHeavy metal active ingredientsBiocideSelective excitationQuantum dot
A nanobioprocessor for protein and cell therapy comprises a selectively coated quantum dot having selected band gap energies, characteristic absorption, emission spectra and outer coatings for therapy and diagnostic purposes in biophotonics and nanomedicine, and an electromagnetic radiation and detector source configured to remotely heat and / or selectively excite the quantum dot to associate with target specific misfolded or anomalous proteins, diseased cells and tissue.
Owner:APPLIED PHOTONICS WORLDWIDE
Treatment of protein folding disorders
InactiveUS20100317690A1Enhance normal foldingBiocideOrganic chemistryImino SugarsLysosomal enzyme defect
Described are various compounds and methods for the treatment of disorders arising from aberrant protein folding, including in particular lysosomal storage diseases. In particular, polyhydroxylated alkaloids and imino sugars which are pharmacoperones of an enzyme and which do not bind to a catalytic site of said enzyme are described.
Owner:SUMMIT
Agents for suppressing neural fibrotic degeneration
InactiveUS20090202515A1Suppress neural fibrotic degenerationGreat medical and industrial significanceOrganic active ingredientsNervous disorderSide chainNeural cell
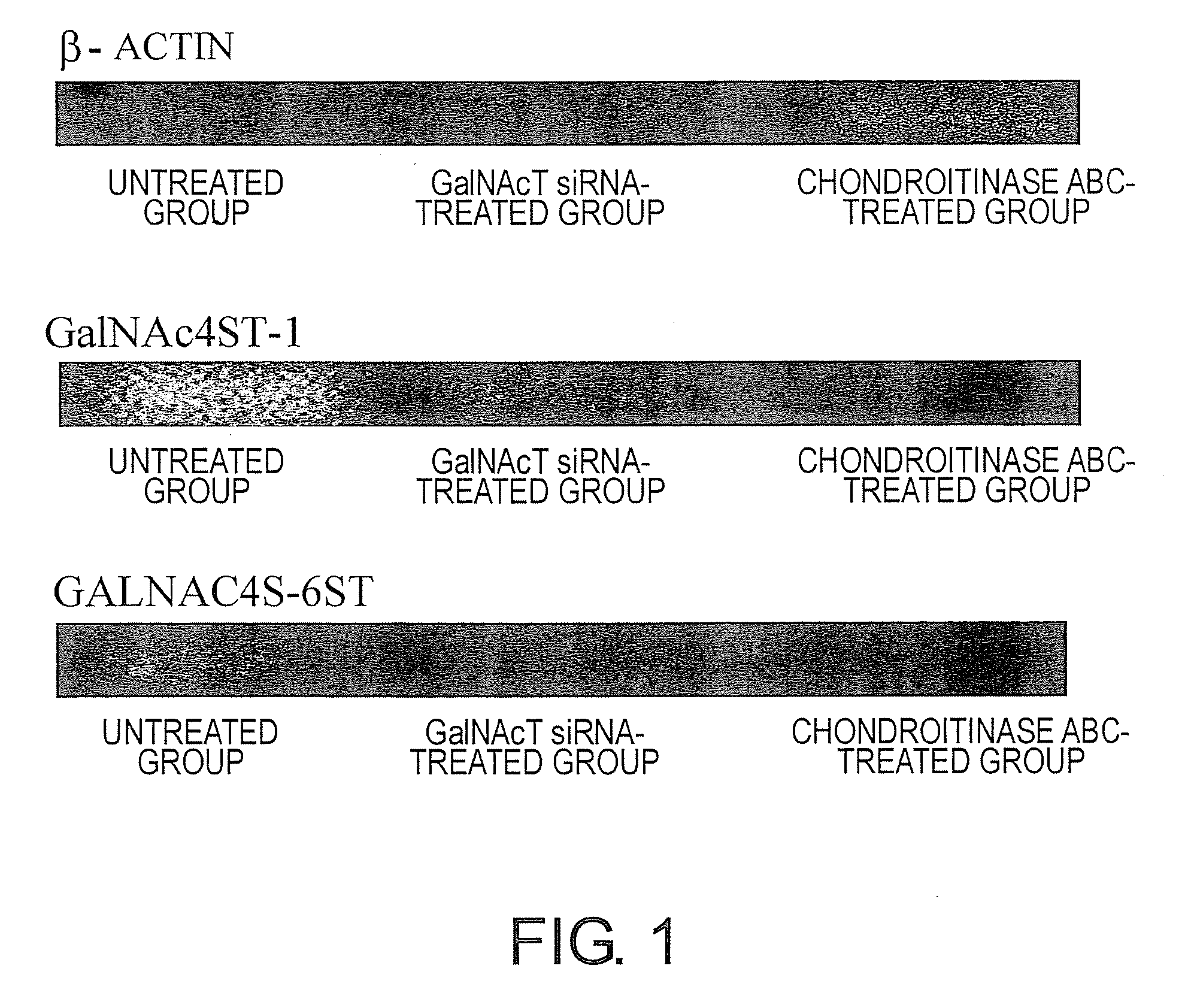
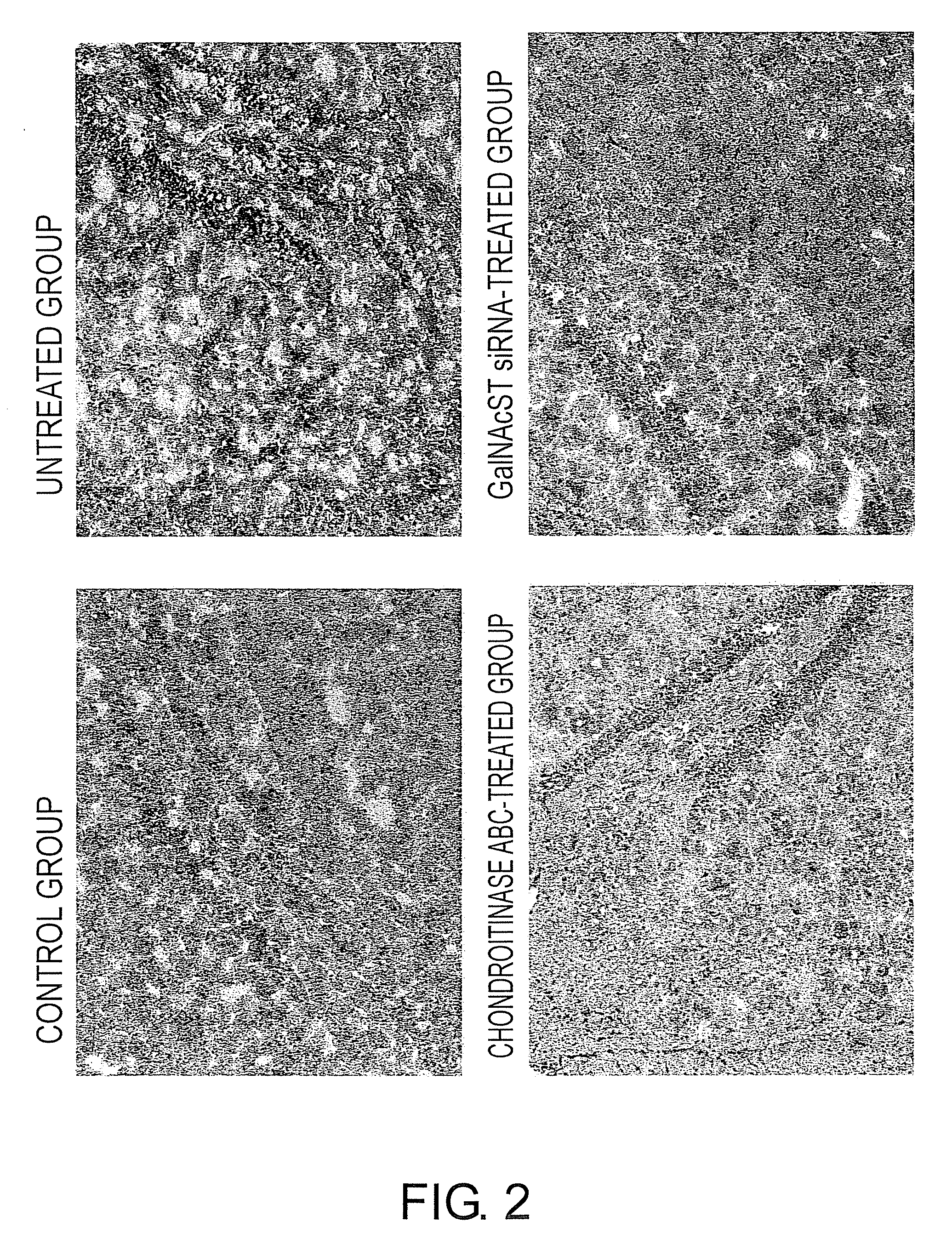
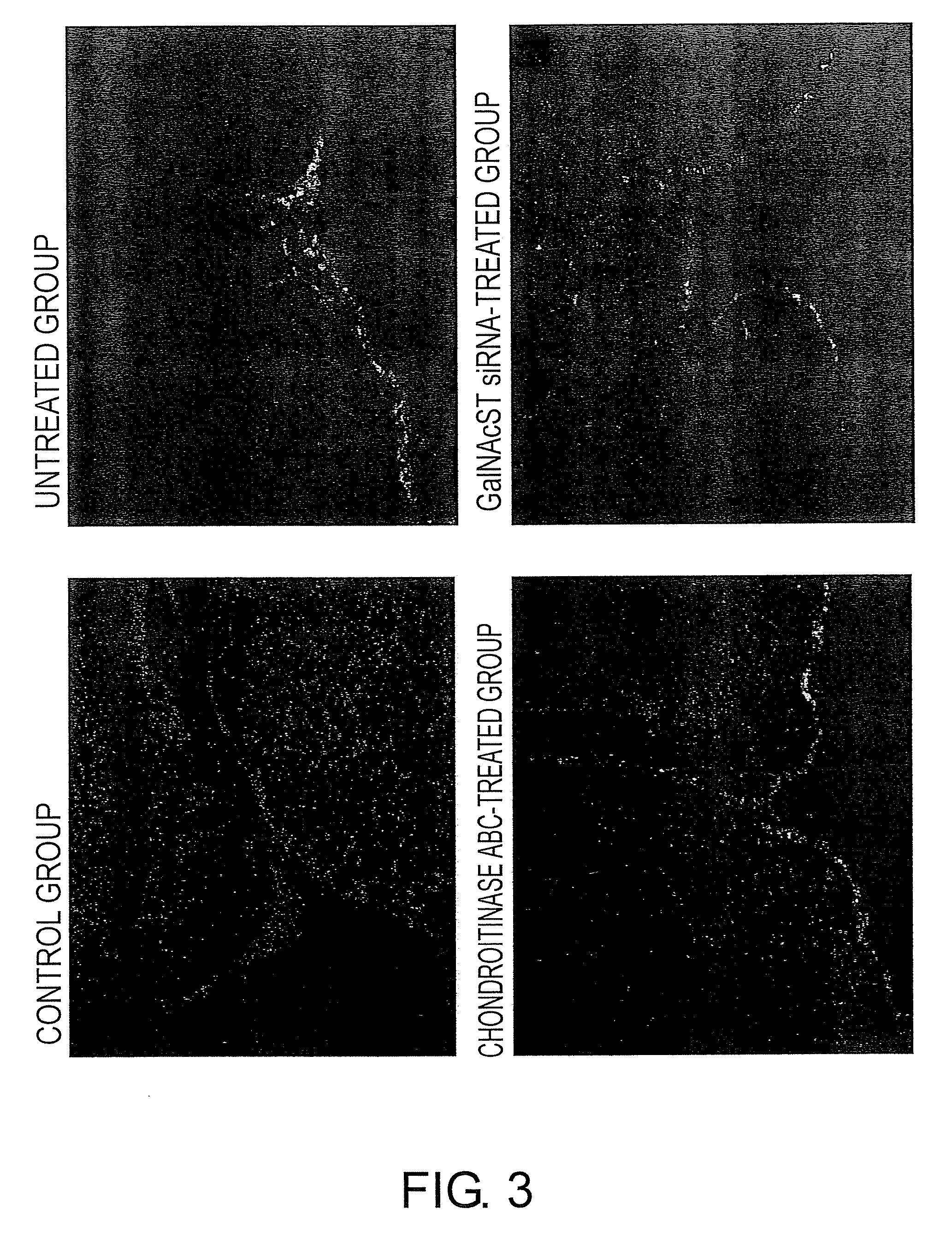
The present invention examined the accumulation of chondroitin sulfate proteoglycans (CSPGs). The present invention relates to neurodegeneration-suppressing agents that are suitable for gene therapy or prevention of neural fibrotic degenerative diseases which induce neural cell death due to an accumulation of abnormal proteins, where the therapies are based on siRNAs against N-acetylgalactosamine-4-O-sulfotransferases (N-acetylgalactosamine-4-O-sulfotransferase-1, N-acetylgalactosamine-4-O-sulfotransferase-2, and N-acetylgalactosamine-4-sulfate 6-O-sulfotransferase (GalNAc4ST-1, GalNAc4ST-2, and GALNAC4S-6ST, respectively)), which are sulfotransferases for acetylgalactosamine, a CSPG side chain, and chondroitinase ABC, an enzyme that degrades chondroitin sulfate, another CSPG side chain.
Owner:STELIC INST OF REGENERATIVE MEDICINE STELIC INST
Sulfide and disulfide compounds and compositions for cholesterol management and related uses
InactiveUS20050020694A1Increase weightImprove the level ofBiocideOrganic chemistryDysproteinemiasDisease cause
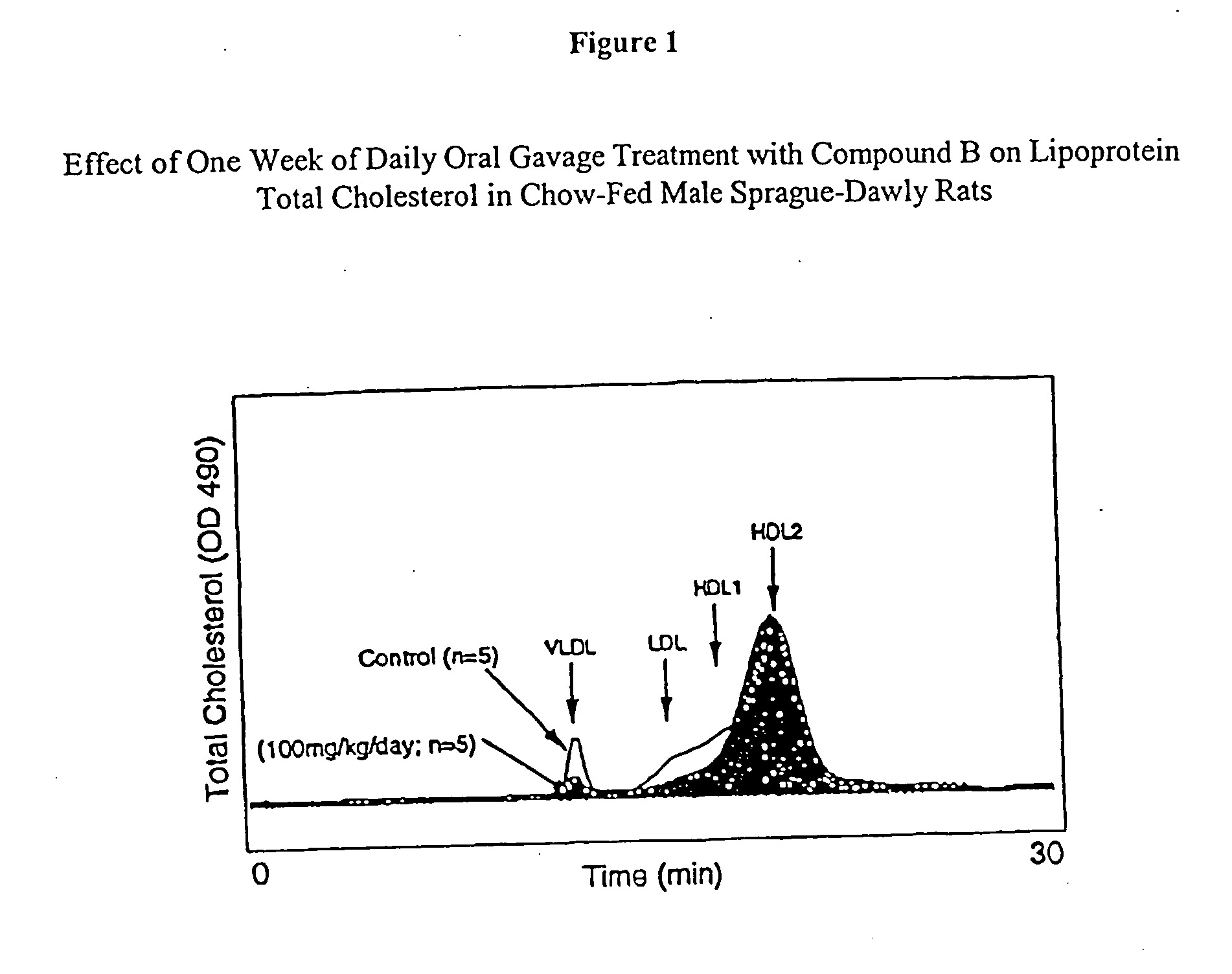
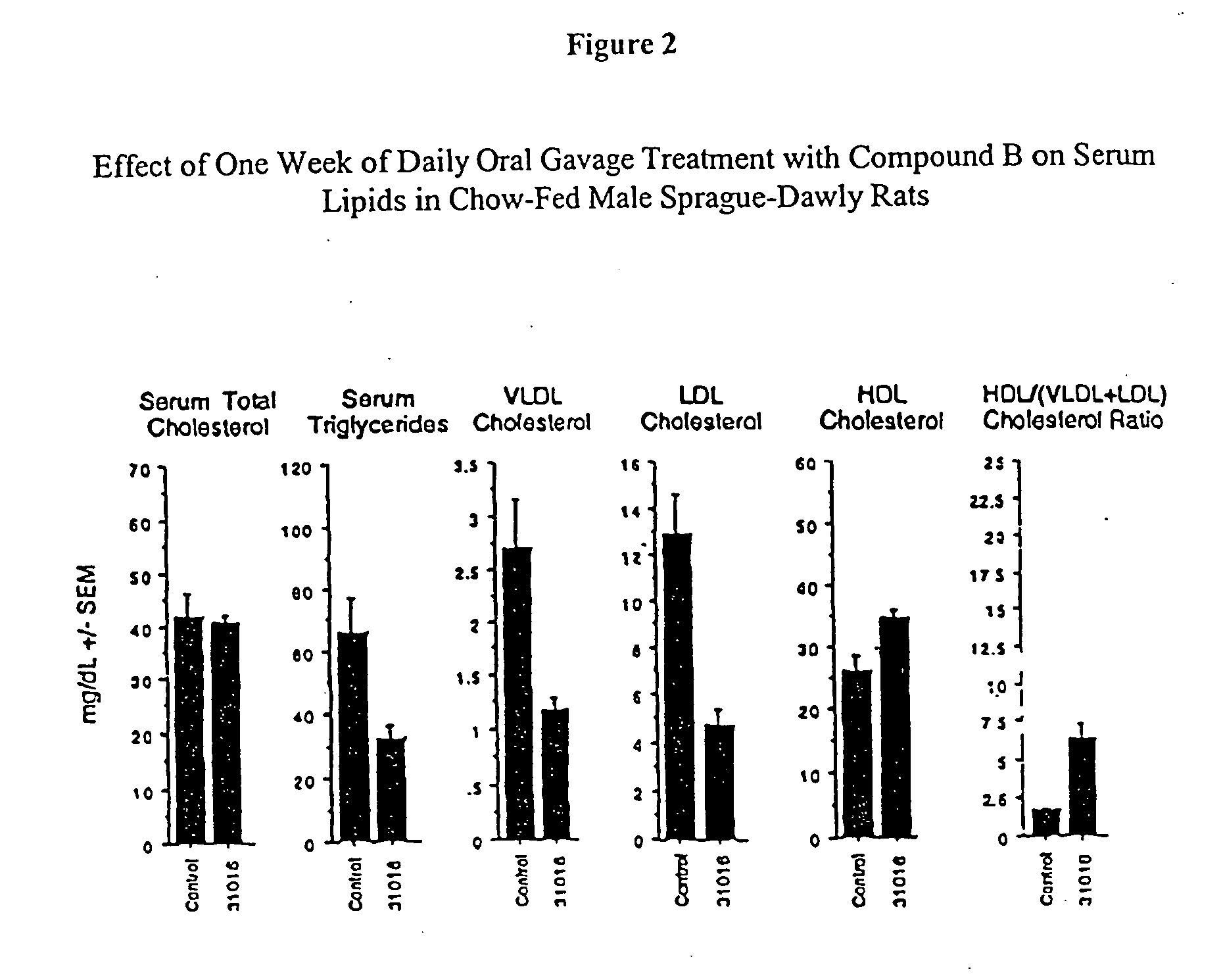
The invention encompasses novel sulfide and disulfide compounds, compositions comprising sulfide and disulfide compounds, and methods useful for treating and preventing cardiovascular diseases, dyslipidemias, dysproteinemias, and glucose metabolism disorders comprising administering a composition comprising an ether compound. The compounds, compositions, and methods of the invention are also useful for treating and preventing Alzheimer's Disease, Syndrome X, peroxisome proliferator activated receptor-related disorders, septicemia, thrombotic disorders, obesity, pancreatitis, hypertension, renal disease, cancer, inflammation, and impotence. In certain embodiments, the compounds, compositions, and methods of the invention are useful in combination therapy with other therapeutics, such as hypocholesterolemic and hypoglycemic agents.
Owner:ESPERION THERAPEUTICS
Misfolded protein sensor method in body fluids
InactiveUS20060275910A1Easy to detectRapid and cost-effective analyticalDisease diagnosisBiological testingCrystallographyTest sample
A catalytic conformational sensor method for detecting abnormal proteins and proteinaceous particles. The method is based on the interaction of a peptide fragment or probe with an abnormal proteinaceous particle. The interaction catalyzes transformation of the probe to a predominately beta sheet conformation and allows the probe to bind to the abnormal proteinaceous particle. This in turn, catalyzes propagation of a signal associated with the test sample-bound probe. As a result signals can be propagated even from samples containing very low concentrations of abnormal proteinaceous particles as is the case in many body-fluid derived samples.
Owner:ADLYFE INC
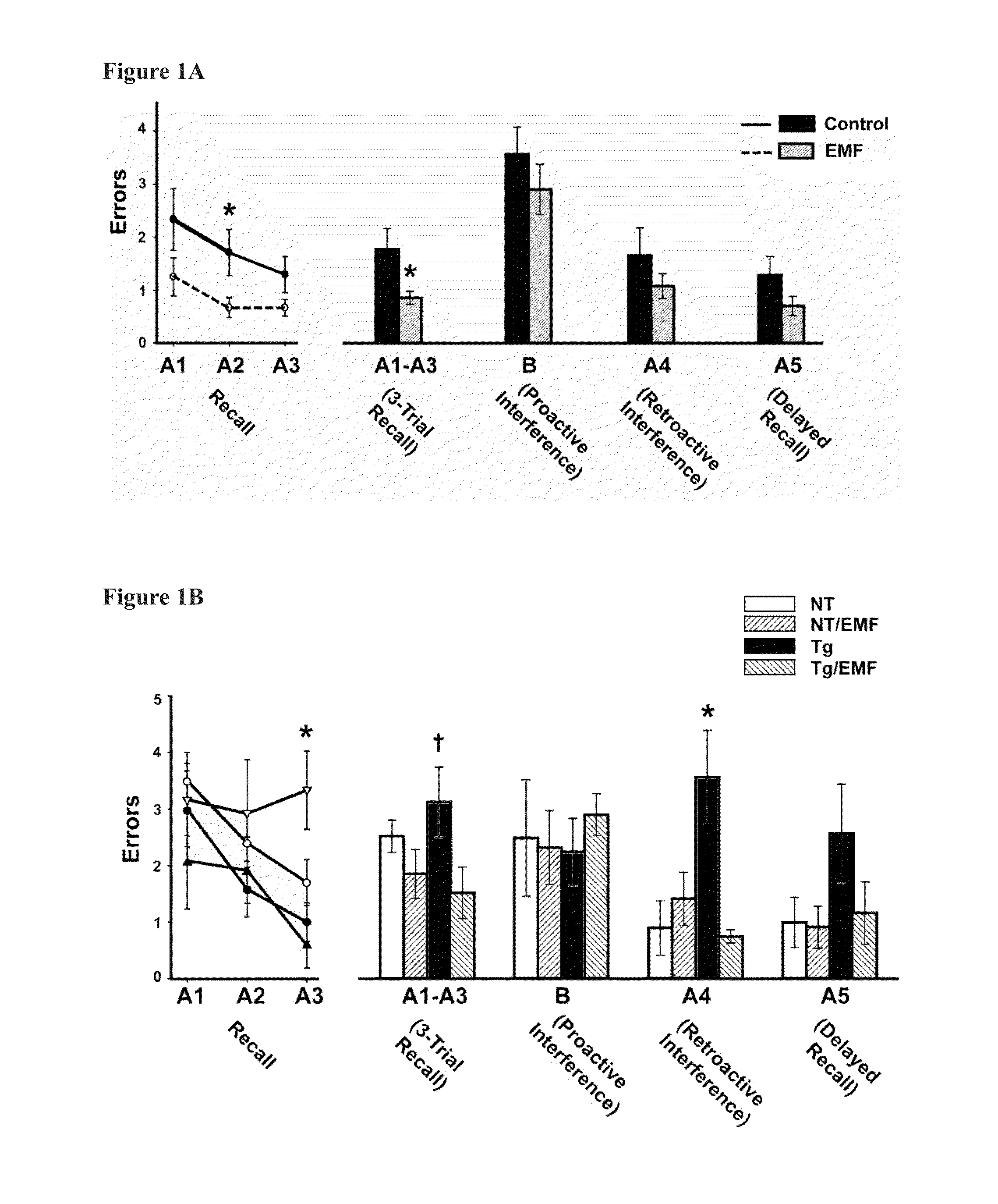
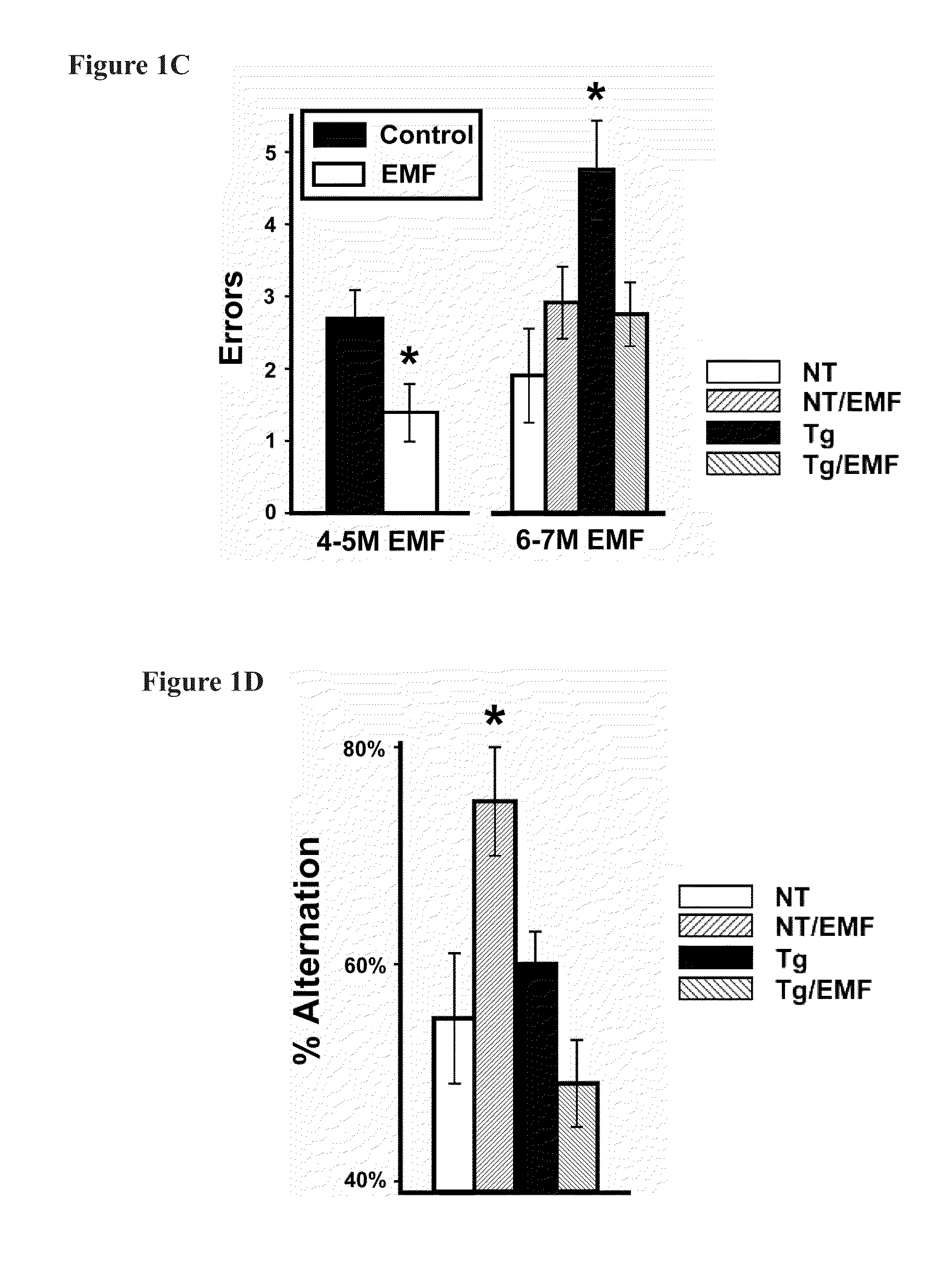
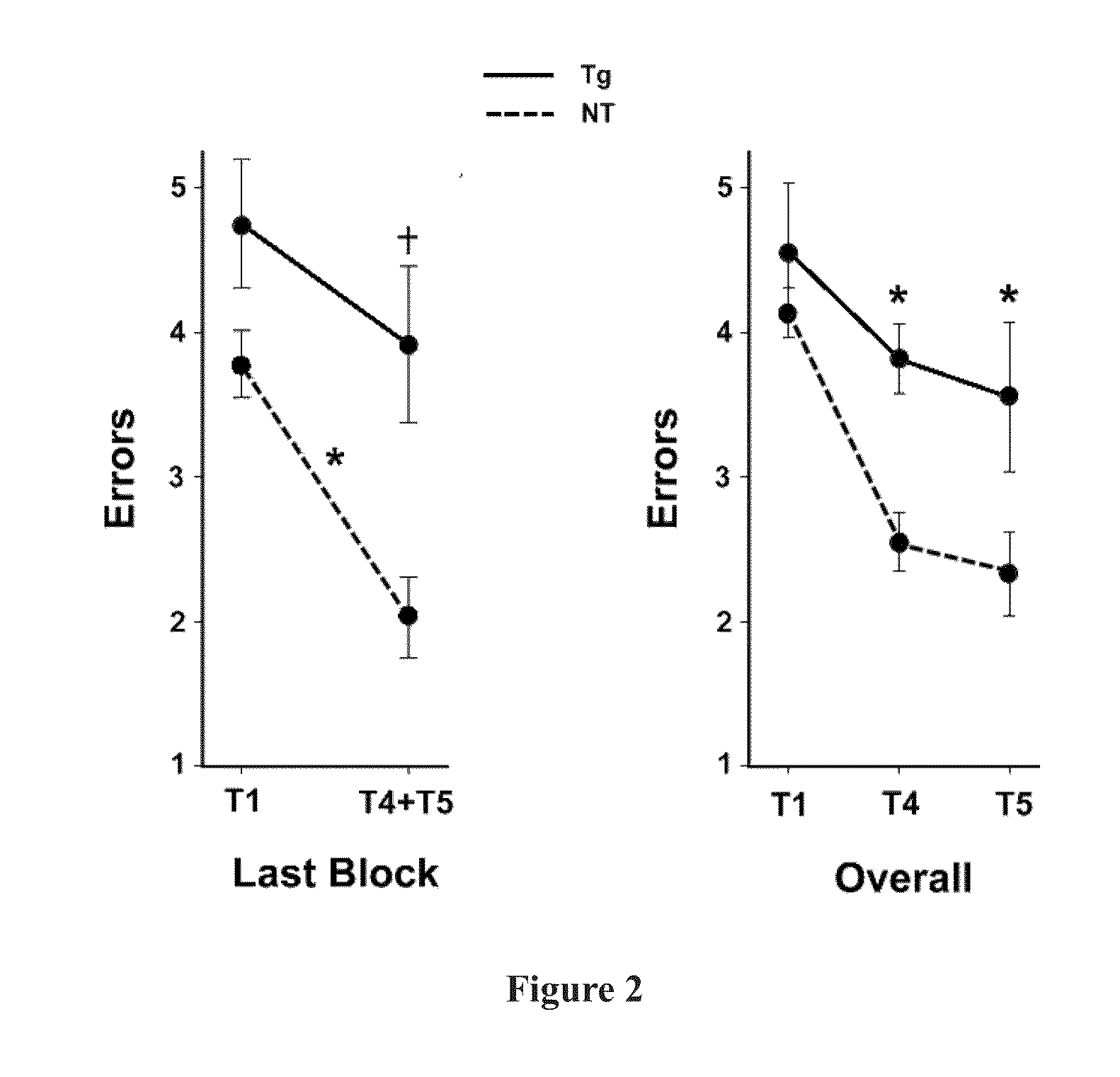
Prevention and treatment of brain diseases and disorders related to abnormal protein aggregation through electromagnetic field treatment
ActiveUS20160106997A1Improve cognitive functionReduce depositionElectrotherapyMagnetotherapy using coils/electromagnetsCognitionProtein aggregation
A method of treating and preventing a neurological disorder, such as Alzheimer's disease, in a subject in need thereof by positioning an electromagnetic field emitting source proximal to the subject and exposing the subject to an electromagnetic field having a predetermined frequency (preferably ≈300-3,000 MHz) for a predetermined absorption period (preferably greater than ≈3 days). Each individual treatment (comprising exposure to the predetermined frequency for the predetermined absorption period) is continued at a predetermined schedule for a predetermined treatment period. The EMF can have a specific absorption rate up to about 8 W / kg. The methodology enhances cognition in the subject and / or treats / prevents the underlying neurological disorder or a symptom thereof.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
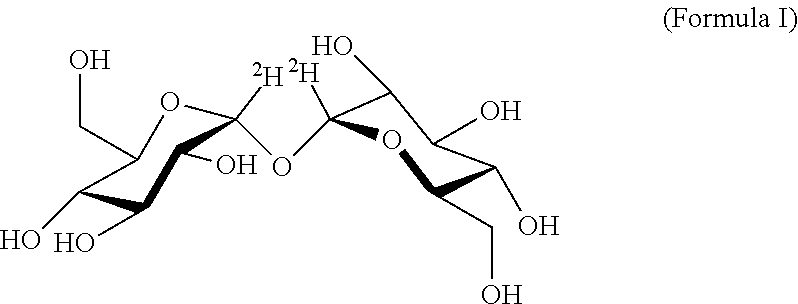
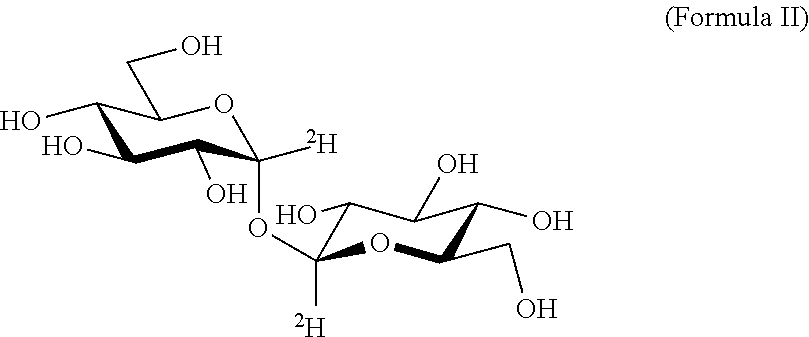
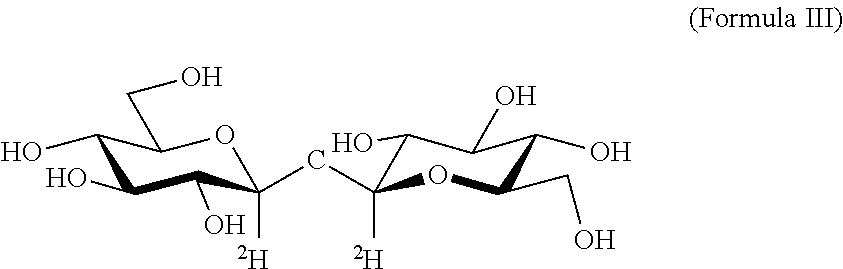
Deuterated trehalose formulations and uses thereof
The presently invention relates to methods, formulations and kits comprising deuterated trehalose for treating myopathies, neurodegenerative disorders, or tauopathies associated abnormal protein aggregation.
Owner:BIOBLAST PHARMA
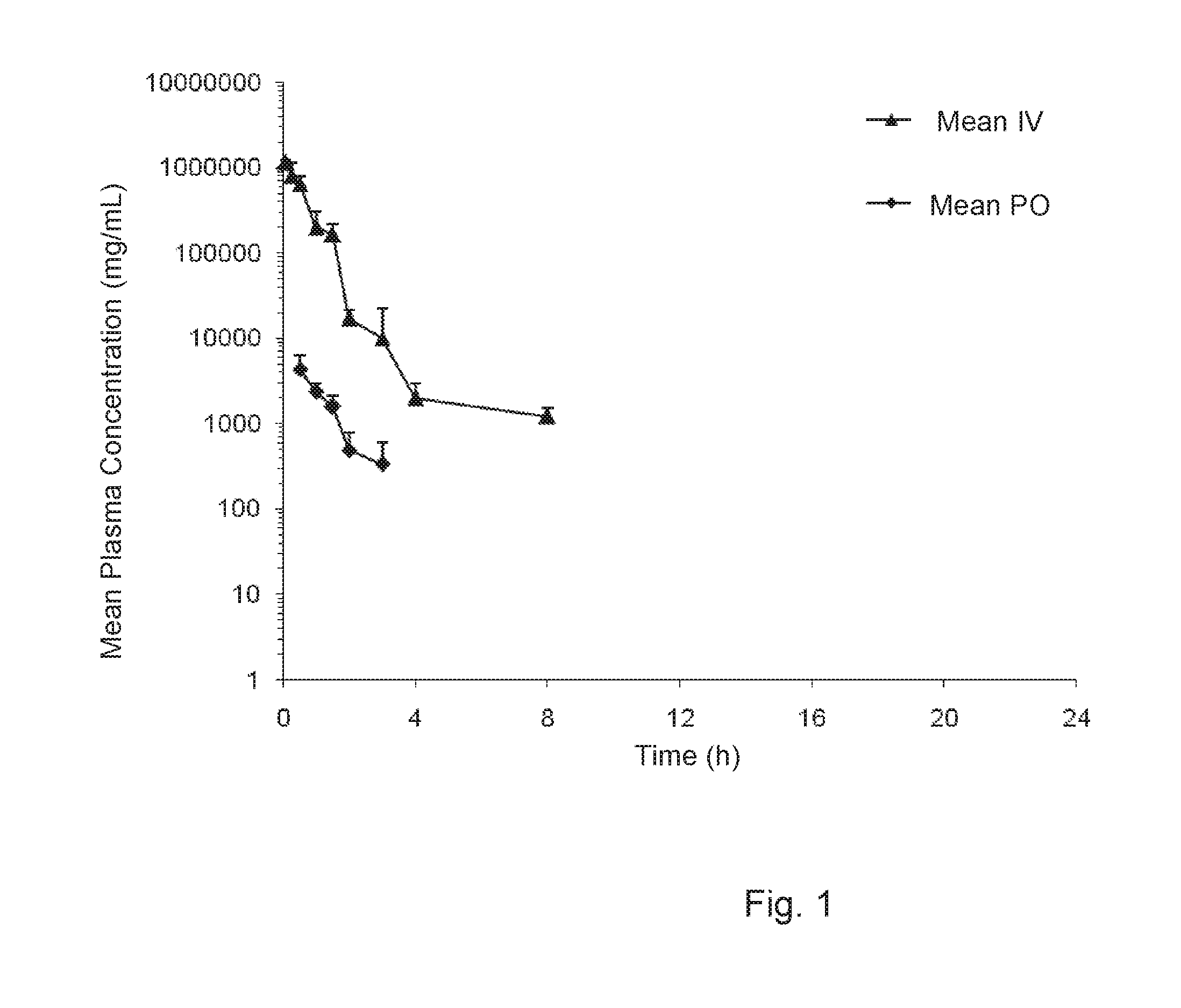
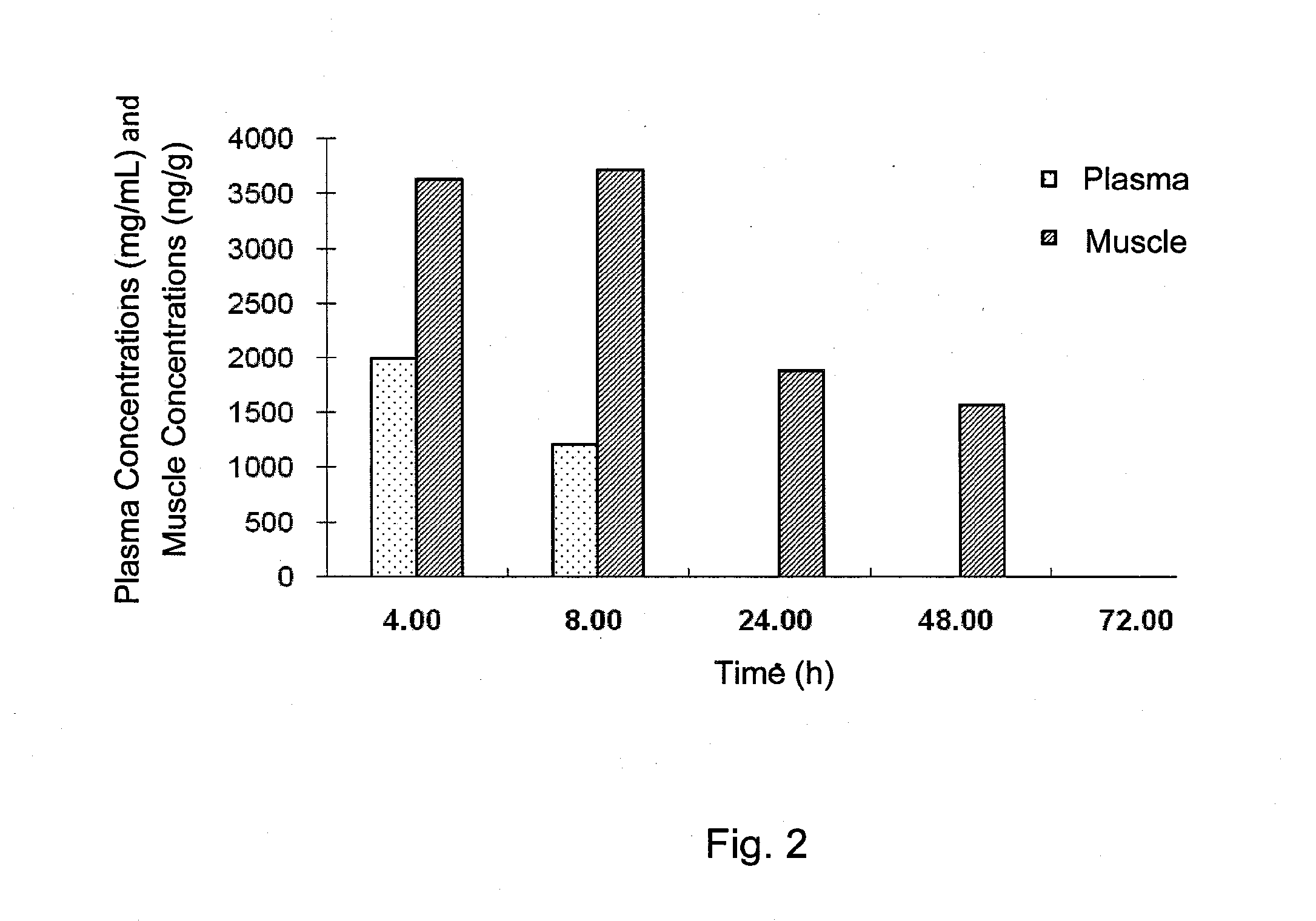
Treatment of protein aggregation myopathic and neurodegenerative diseases by parenteral administration of trehalose
Disclosed is a method of treatment of a disease associated with abnormal protein aggregation comprising parenterally administering pharmaceutical formulations comprising trehalose. Also disclosed is an injectable aqueous pharmaceutical formulation comprising a therapeutically effective amount of trehalose.
Owner:SEELOS THERAPEUTICS INC
Ketone compounds and compositions for cholesterol management and related uses
InactiveUS20070149615A9Reduce contentLower cholesterol levelsBiocideOrganic compound preparationKetoneDysproteinemias
The present invention relates to novel ketone compounds, compositions comprising ketone compounds, and methods useful for treating and preventing cardiovascular diseases, dyslipidemias, dysproteinemias, and glucose metabolism disorders comprising administering a composition comprising a ketone compound. The compounds, compositions, and methods of the invention are also useful for treating and preventing Alzheimer's Disease, Syndrome X, peroxisome proliferator activated receptor-related disorders, septicemia, thrombotic disorders, obesity, pancreatitis, hypertension, renal disease, cancer, inflammation, and impotence. In certain embodiments, the compounds, compositions, and methods of the invention are useful in combination therapy with other therapeutics, such as hypocholesterolemic and hypoglycemic agents.
Owner:ESPERION THERAPEUTICS
Methods for analyzing blood to detect diseases associated with abnormal protein aggregation
A method of detecting a disease associated with abnormal protein aggregation in a subject is provided, the method comprising (a) contacting leukocytes from the subject with a probe that binds to pathogenic protein aggregates, and (b) detecting the probe bound to the pathogenic protein aggregates, wherein the presence of pathogenic protein aggregates in the leukocytes is indicative that the subject has a disease associated with abnormal protein aggregation. In one embodiment, the disease is Alzheimer's disease, mild cognitive impairment or traumatic brain injury.
Owner:彼德·史戴斯 +1
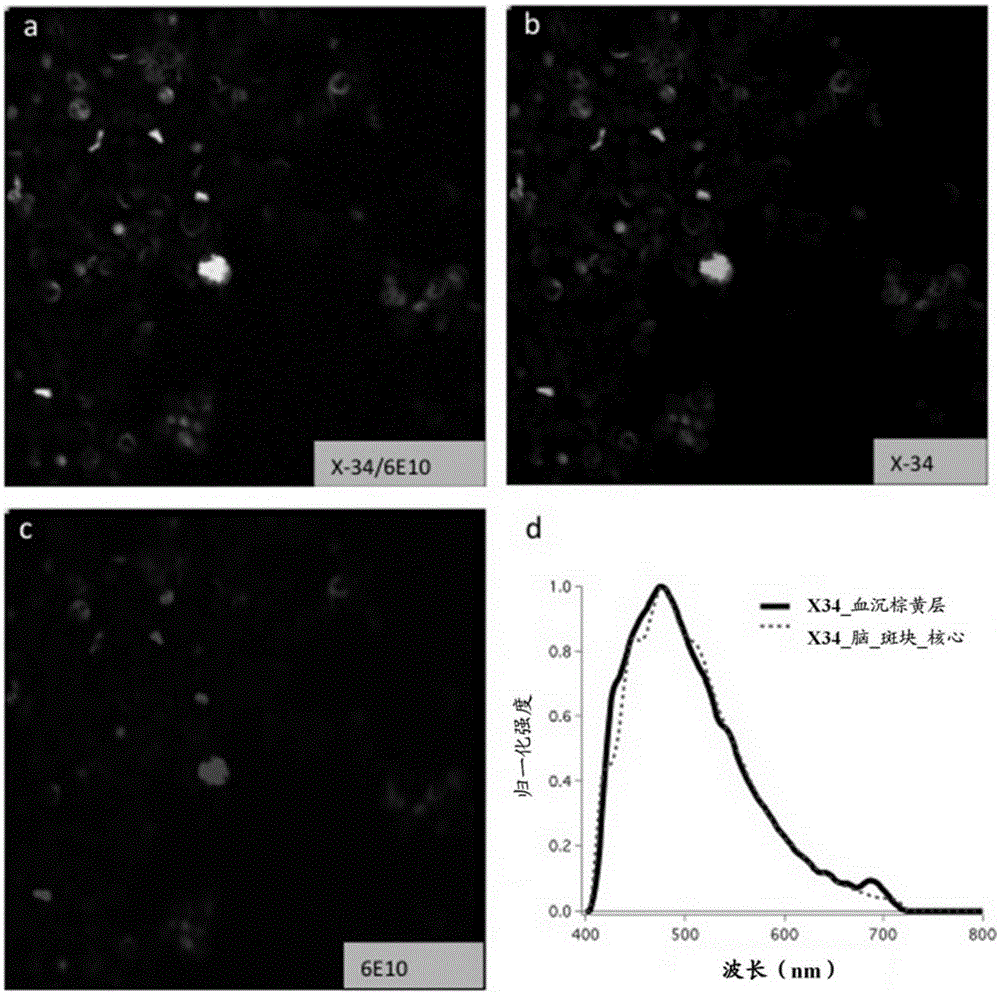
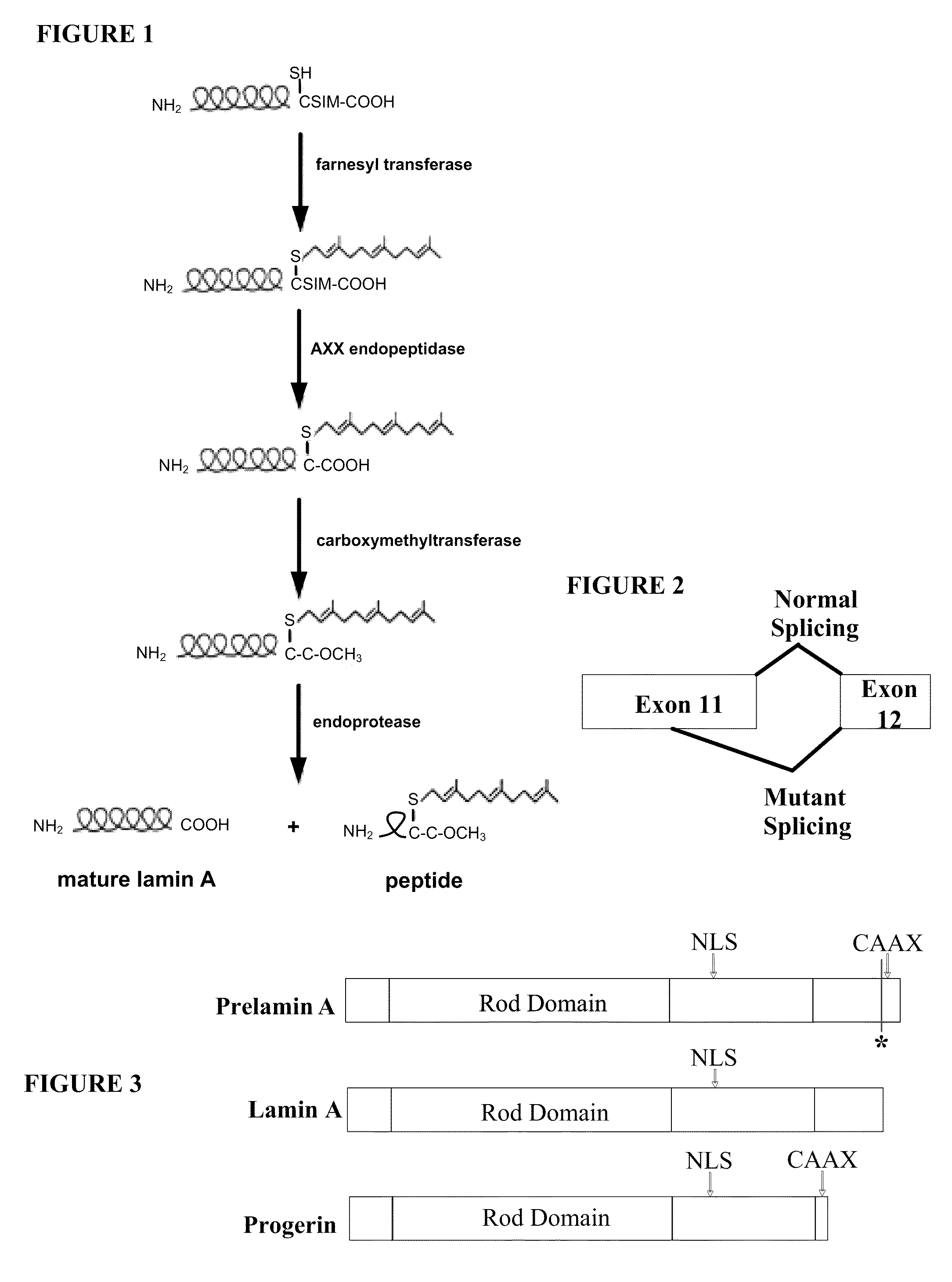
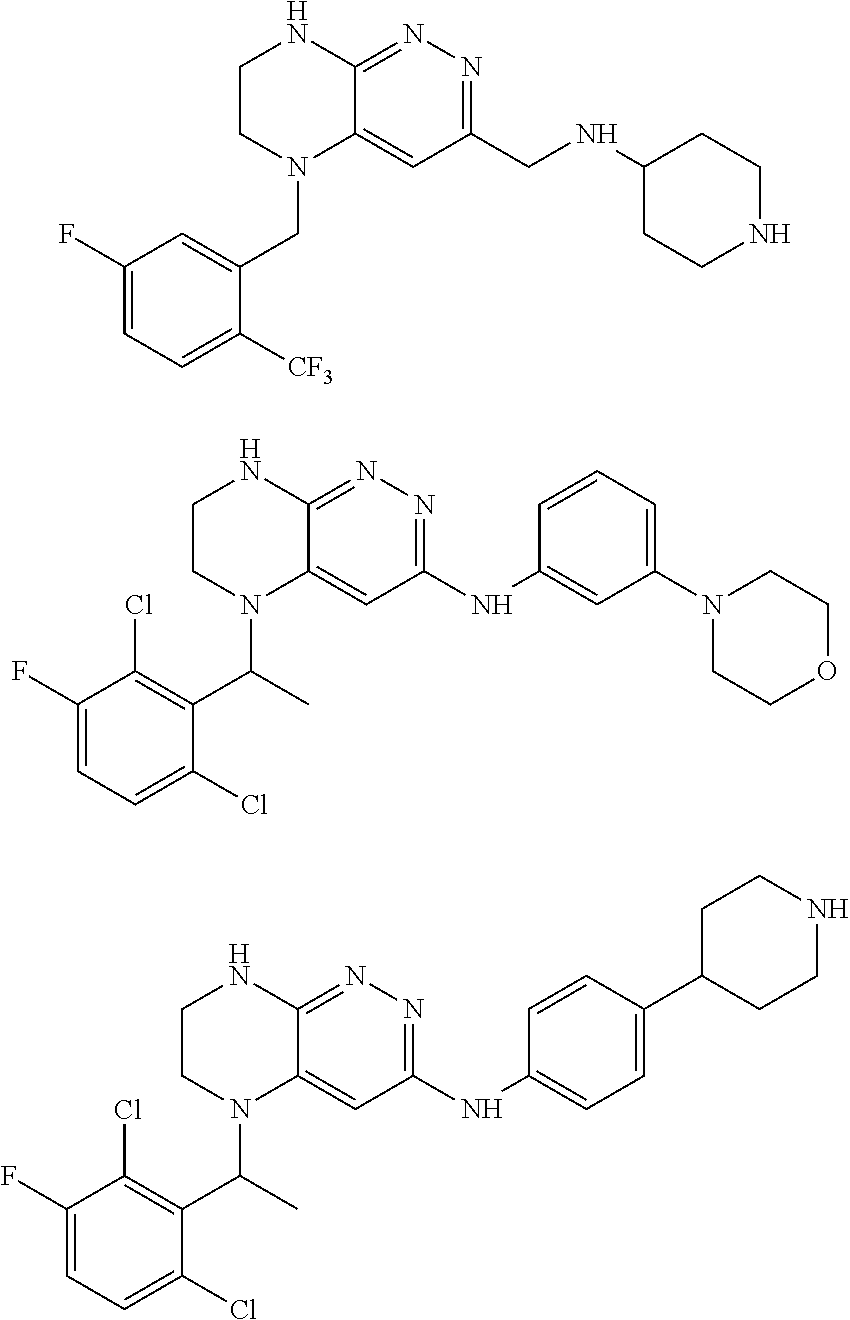
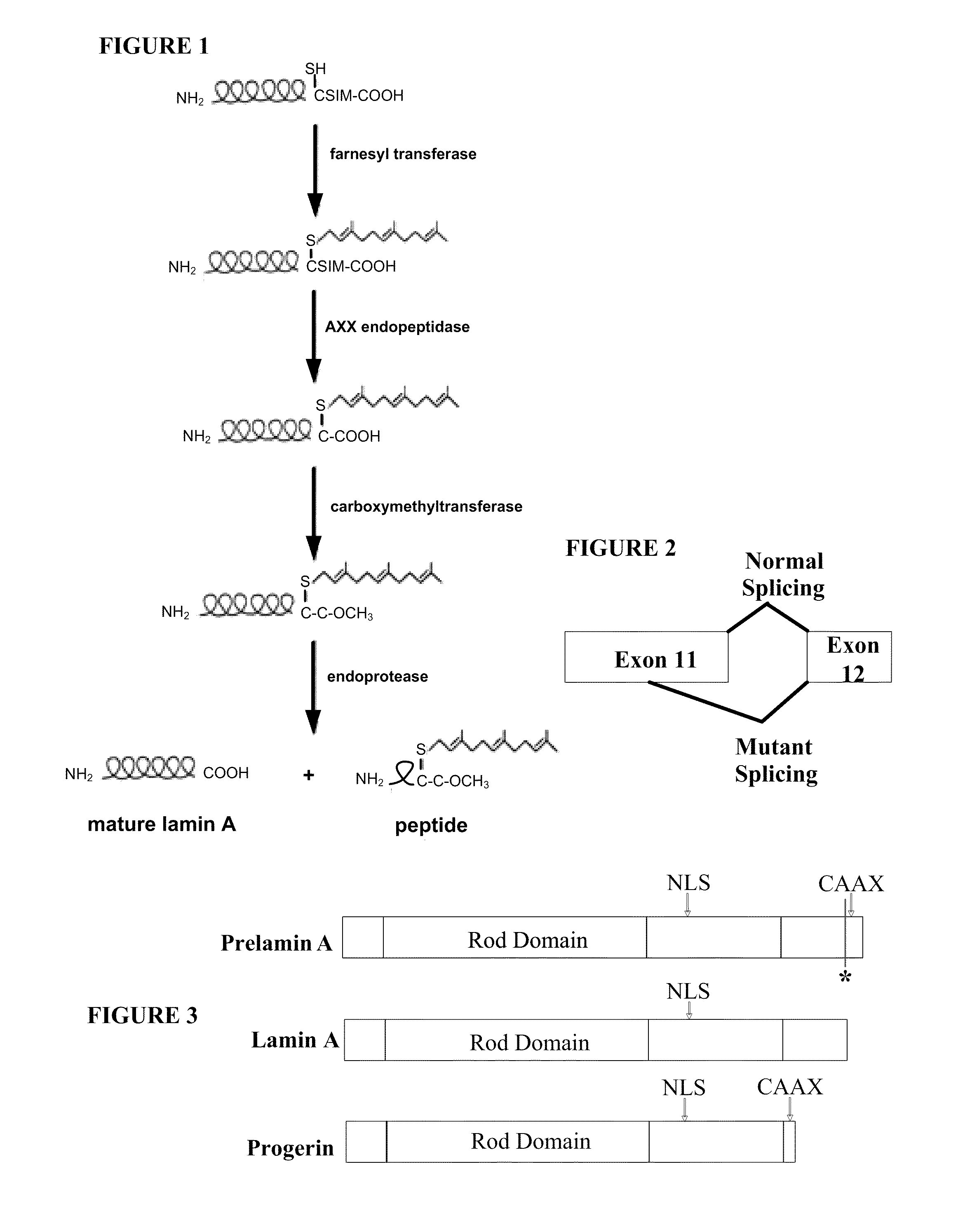
Farnesyltransferase inhibitors for treatment of laminopathies, cellular aging and atherosclerosis
ActiveUS7838531B2Additional elementDecrease in lamin A proteinCompound screeningBiocideNuclear membraneCellular Aging
Although it can be farnesylated, the mutant lamin A protein expressed in Hutchison Gilford Progeria Syndrome (HGPS) cannot be defarnesylated because the characteristic mutation causes deletion of a cleavage site necessary for binding the protease ZMPSTE24 and effecting defarnesylation. The result is an aberrant farnesylated protein (called “progerin”) that alters normal lamin A function as a dominant negative, as well as assuming its own aberrant function through its association with the nuclear membrane. The retention of farnesylation, and potentially other abnormal properties of progerin and other abnormal lamin gene protein products, produces disease. Farnesyltransferase inhibitors (FTIs) (both direct effectors and indirect inhibitors) will inhibit the formation of progerin, cause a decrease in lamin A protein, and / or an increase prelamin A protein. Decreasing the amount of aberrant protein improves cellular effects caused by and progerin expression. Similarly, treatment with FTIs should improve disease status in progeria and other laminopathies. In addition, elements of atherosclerosis and aging in non-laminopathy individuals will improve after treatment with farnesyltransferase inhibitors.
Owner:RGT UNIV OF MICHIGAN +3
Peptides that bind eukaryotic translation initiation factor 4e
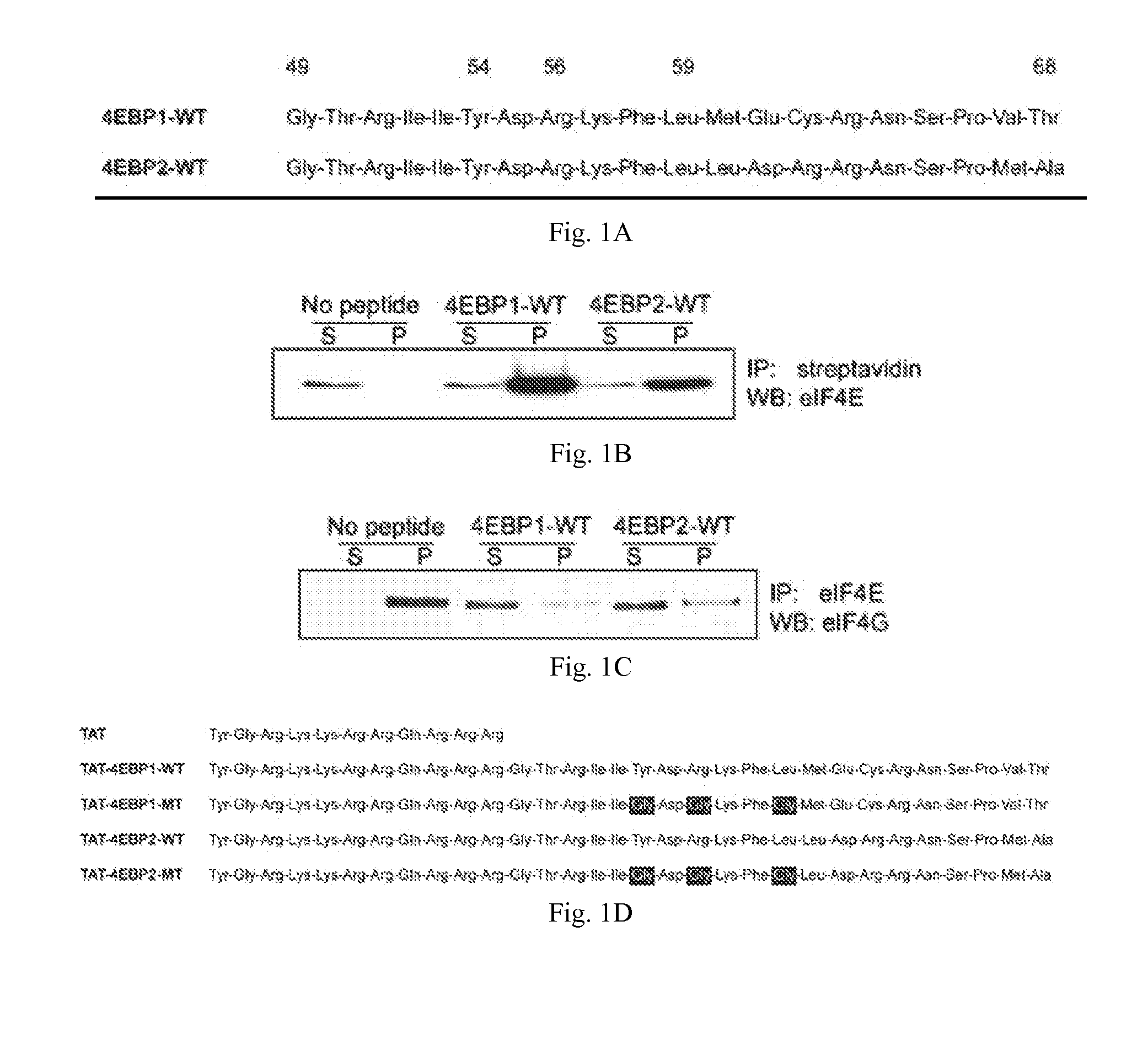
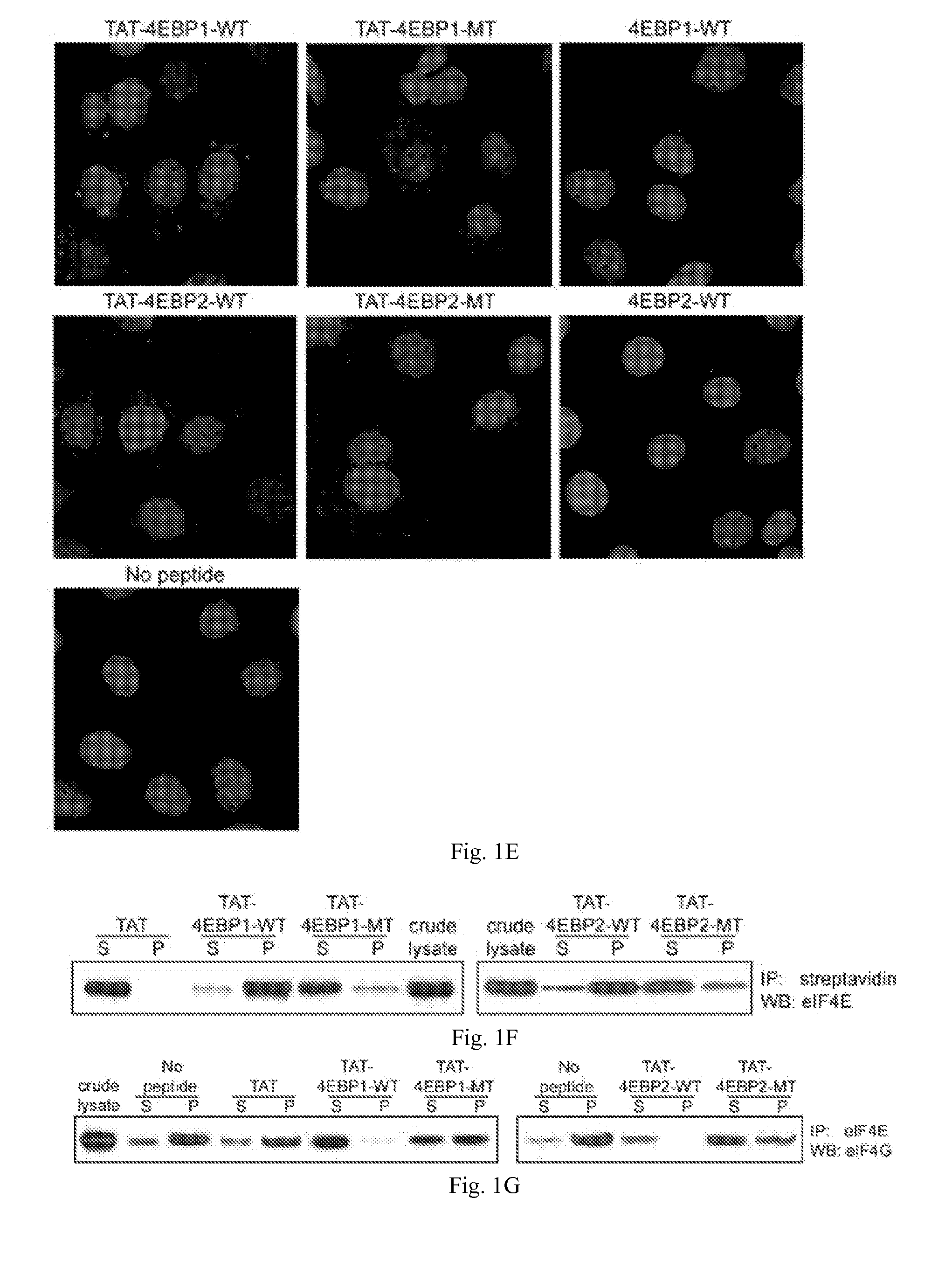
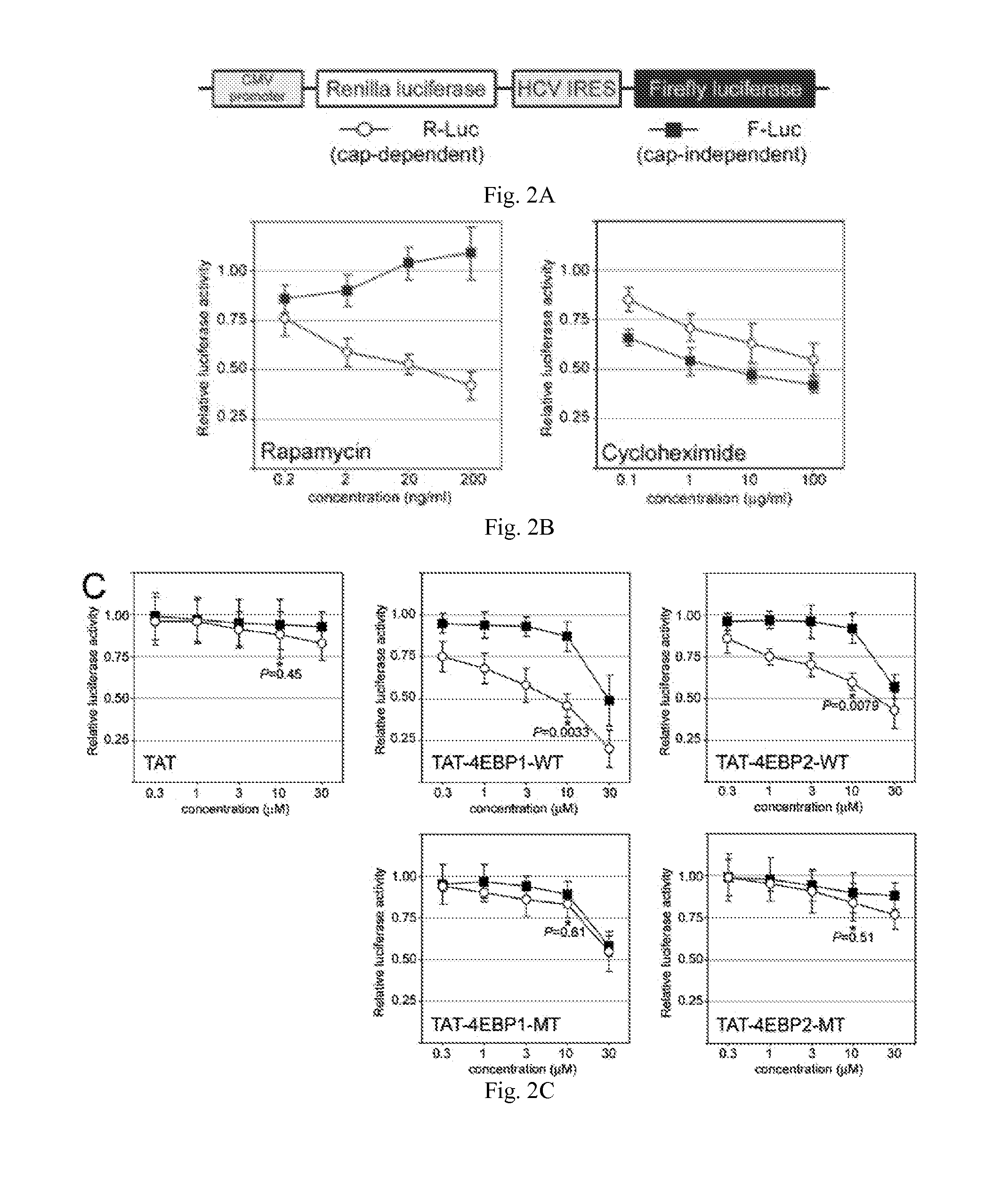
InactiveUS20110319338A1Increase serum stabilityEffective cell penetrationPeptide/protein ingredientsVirus peptidesInitiation factorADAMTS Proteins
Methods, compositions and kits for treating proliferative and non-proliferative diseases associated with abnormal protein synthesis. Chimeric peptide constructs are comprised in compositions and kits for use in the treatment of proliferative diseases, such as ovarian cancer, and for inhibiting protein synthesis in a tumor cell compared to a non-tumor cell.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
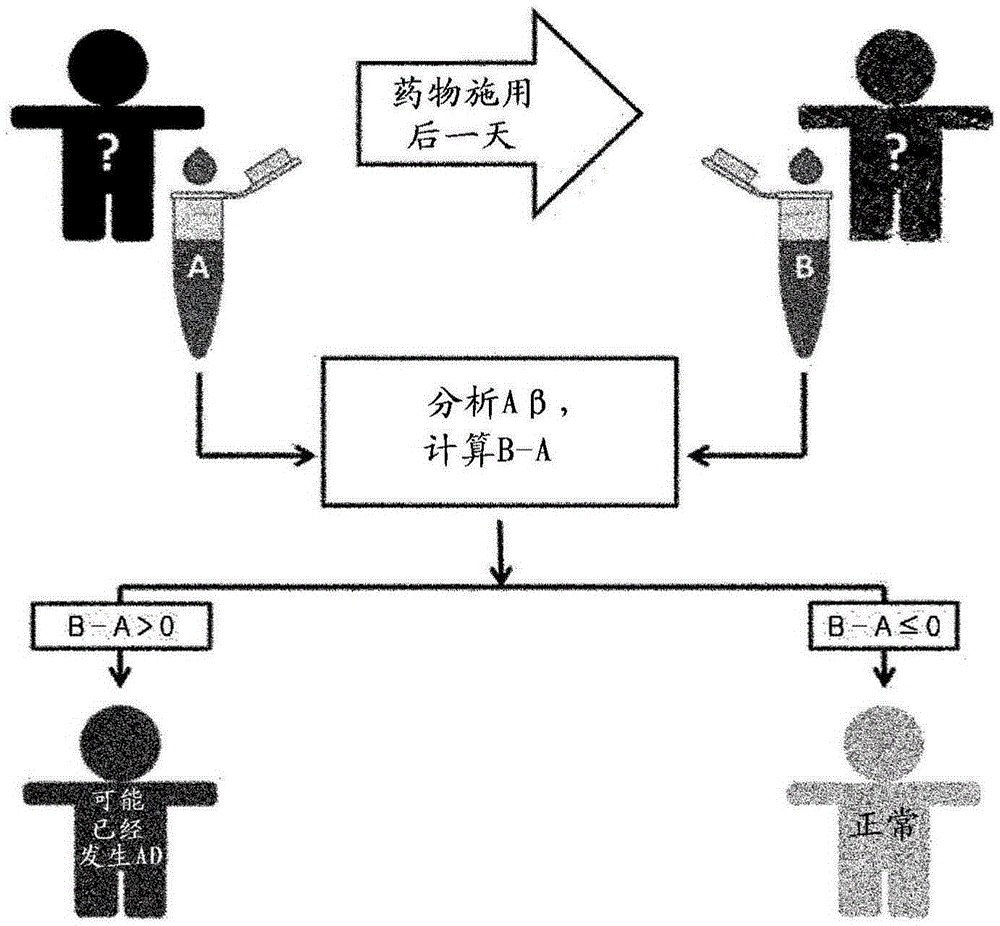
Diagnostic kit for diagnosing disorders or diseases related to abnormal protein aggregation or misfolding of protein using dissolution of protein aggregates
InactiveCN105339798AReduce concentrationLess invasivePeptide/protein ingredientsMicrobiological testing/measurementCell AggregationsMedicine
The present invention relates to a diagnostic kit for diagnosing disorders or diseases related to abnormal protein aggregation or misfolding of protein by using dissolution of protein aggregates in the body, capable of accurately diagnosing disorders or diseases such as Alzheimer's disease caused by aggregation of beta amyloid, as well as disorders and diseases related to abnormal protein aggregation or misfolding of protein by analyzing concentrations through the dissolution of aggregated protein in the blood of a person having other disorders or diseases due to protein aggregation.
Owner:KOREA INST OF SCI & TECH
New chemical compounds (derivatives) and their application for the treatment of oncological diseases
InactiveUS20160200729A1Good curative effectImprove bioavailabilityBiocideOrganic chemistryWAS PROTEINKinase activity
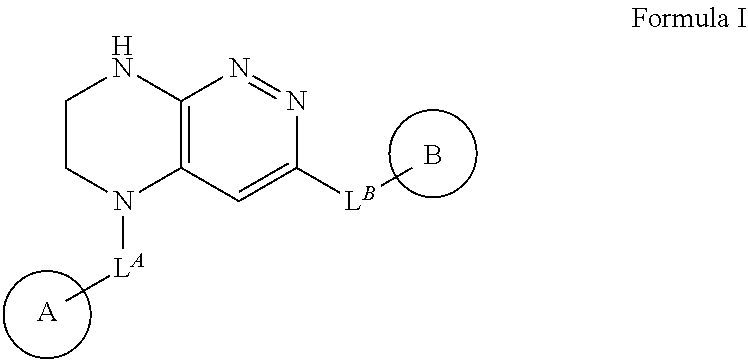
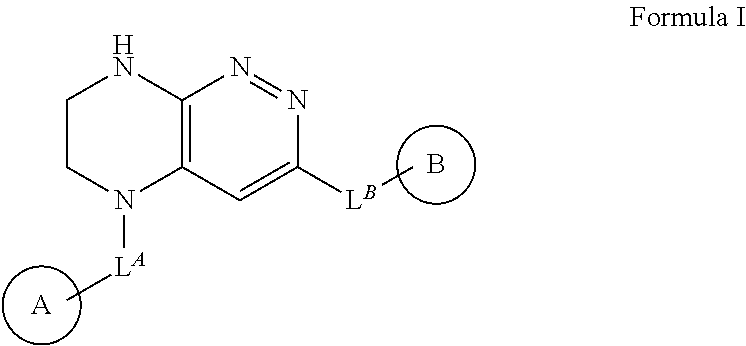
The invention relates to novel chemical compounds of general formula I, in which LA, LB, LC, ring A, ring B, RA, RB, RC, RD, RE and RF have are defined in the description, and which are protein kinases inhibitors. The invention also relates to pharmaceutical compositions containing said compounds and also to the use of said compounds for treatment and / or prevention of diseases related with aberrant protein kinase activity.
Owner:LTD LIABILITY NT
Farnesyltransferase inhibitors for treatment of laminopathies, cellular aging and atherosclerosis
InactiveUS20110027806A1Additional elementDecrease in lamin A proteinCompound screeningOrganic active ingredientsLaminopathyNuclear membrane
Although it can be farnesylated, the mutant lamin A protein expressed in Hutchinson Gilford Progeria Syndrome (HGPS) cannot be defarnesylated because the characteristic mutation causes deletion of a cleavage site necessary for binding the protease ZMPSTE24 and effecting defarnesylation. The result is an aberrant farnesylated protein (called “progerin”) that alters normal lamin A function as a dominant negative, as well as assuming its own aberrant function through its association with the nuclear membrane. The retention of farnesylation, and potentially other abnormal properties of progerin and other abnormal lamin gene protein products, produces disease. Farnesyltransferase inhibitors (FTIs) (both direct effectors and indirect inhibitors) will inhibit the formation of progerin, cause a decrease in lamin A protein, and / or an increase prelamin A protein. Decreasing the amount of aberrant protein improves cellular effects caused by and progerin expression. Similarly, treatment with FTIs should improve disease status in progeria and other laminopathies. In addition, elements of atherosclerosis and aging in non-laminopathy individuals will improve after treatment with farnesyltransferase inhibitors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +3
Abnormal protein removing composition
InactiveUS20090257965A1Good effectPrevent agingCosmetic preparationsBiocideAdditive ingredientProteolysis
Provide an effective composition for removing abnormal protein. Also, a composition for removing abnormal protein whose main ingredient is soyasaponin B group, which is used to provide a composition, agent or food contributing to the prevention or treatment of diseases caused by proteolysis abnormality, among others.
Owner:FUAN KERU
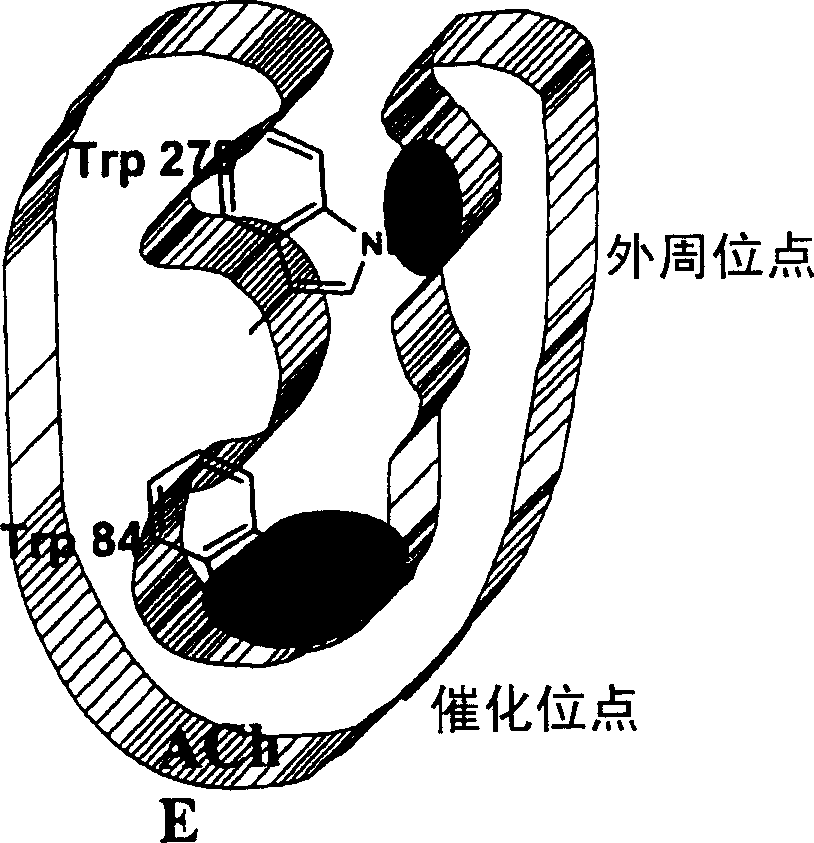
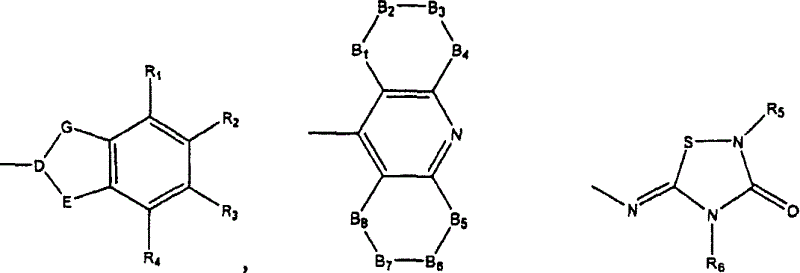
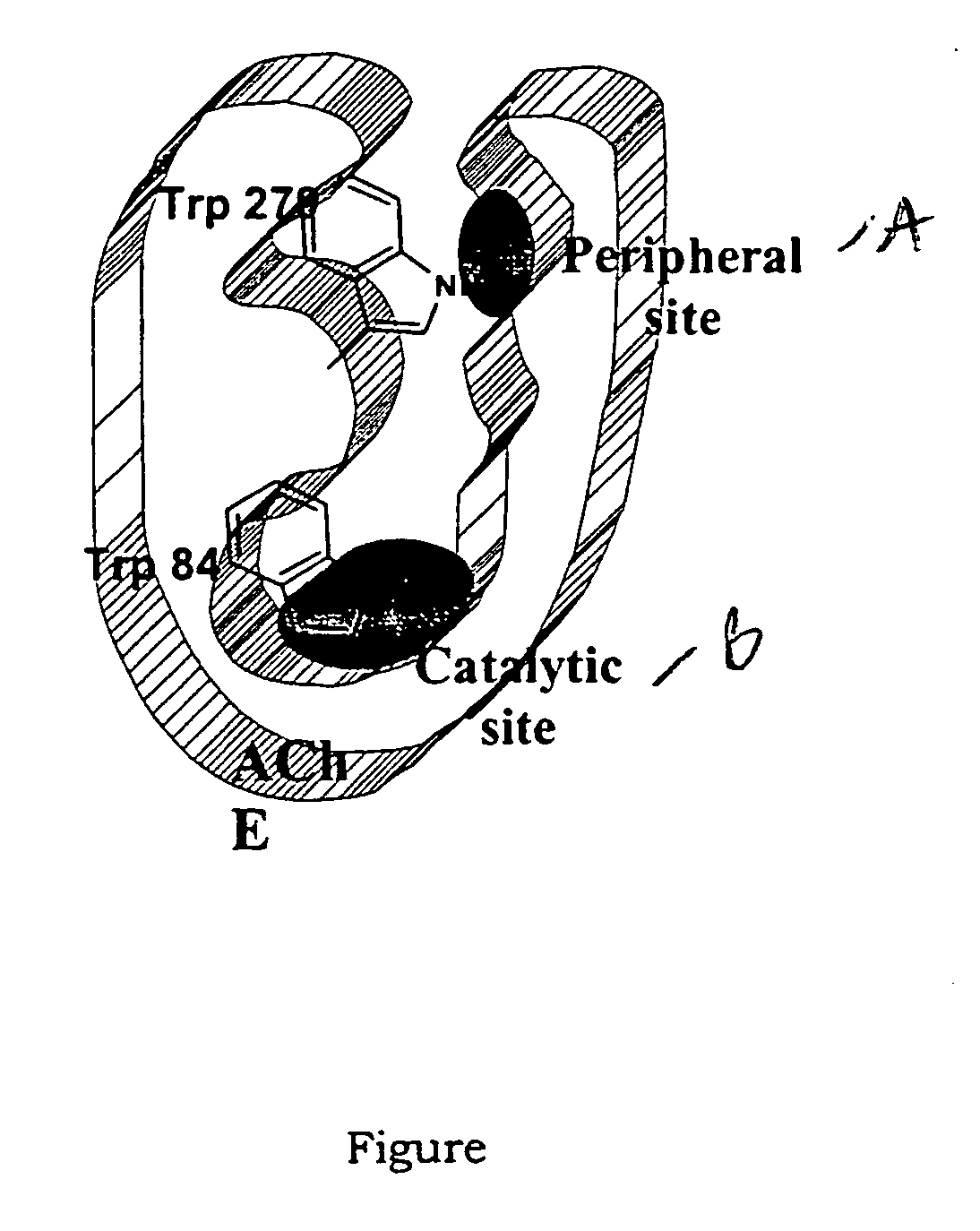
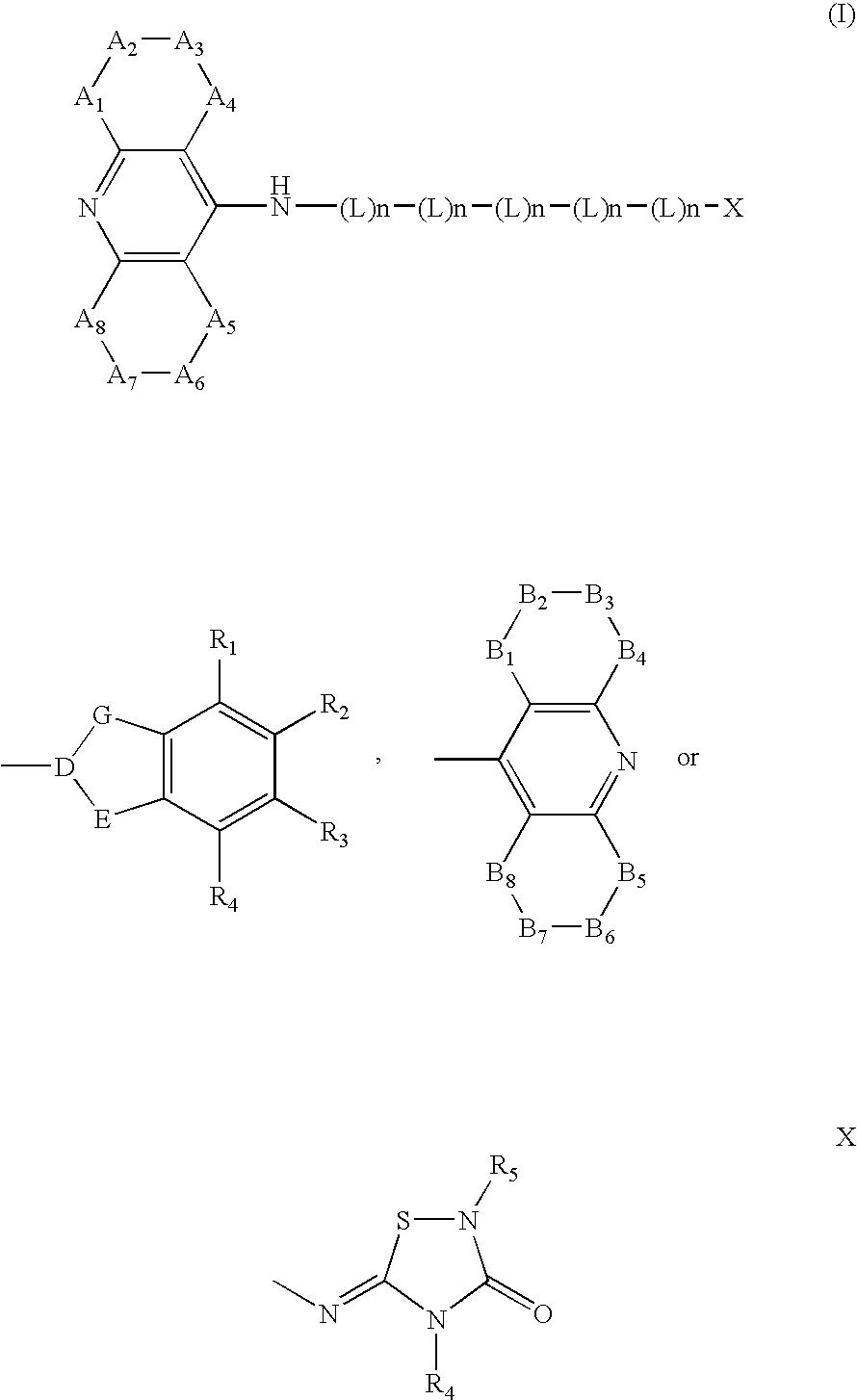
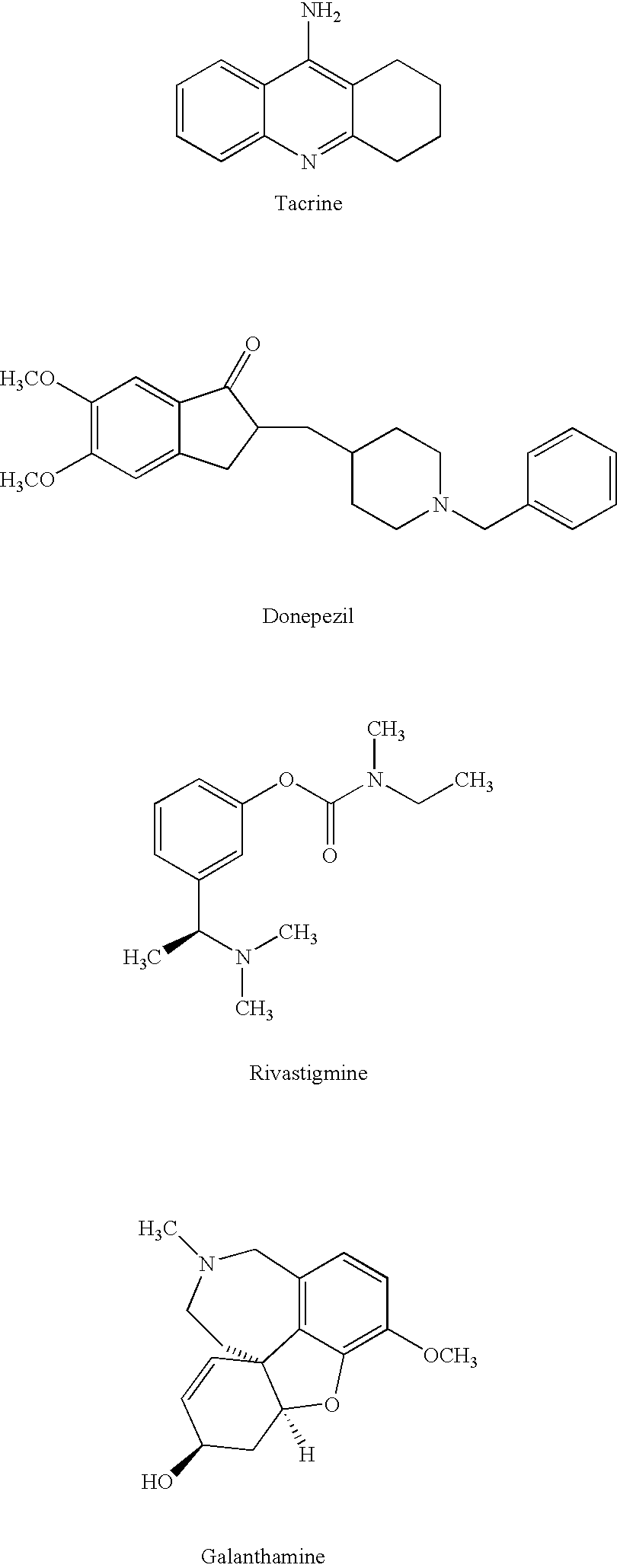
Dual binding site acetylcholinesterase inhibitors for the treatment of alzheimer's disease
Owner:NEUROPHARMA SA (ES)
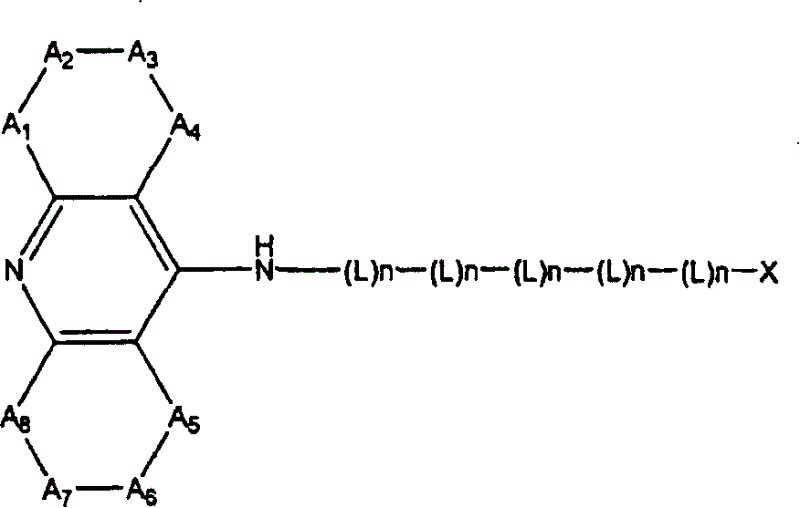
Dual binding site acetylcholinesterase inhibitors for the treatment of alzheimer's disease
A family of compounds of formula (I) wherein: X is one of the following radicals: which behaves as dual site acetyl-cholinesterase inhibitors and which are especially useful for the treatment of cognitive disorders as senile dementia, cerebrovascular dementia, mild cognition impairment, attention deficit disorder, and / or neurodegenerative dementing disease with aberrant protein aggregations as specially Alzheimer's disease, Parkinson disease, ALS, or prion diseases, as Creutzfeldt-Jakob disease or Gerst-mann-Straussler-Scheiner disease.
Owner:NEUROPHARMA SA (ES)
Compositions and methods of detecting tiabs
The present invention relates to novel methods and products for assessing the physiological status of a subject. More particularly, the invention relates to methods of assessing the presence, risk or stage of a cancer in a subject by measuring the levels of antibodies against particular aberrant protein domains in a sample from the subject, or the presence or number of immune cells bearing TCR specific for such aberrant protein domains. The invention is also suitable to assess the responsiveness of a subject to a treatment, as well as to screen candidate drugs and design novel therapies. The invention may be used in any mammalian subject, particularly in human subjects.
Owner:特兰斯麦迪公司
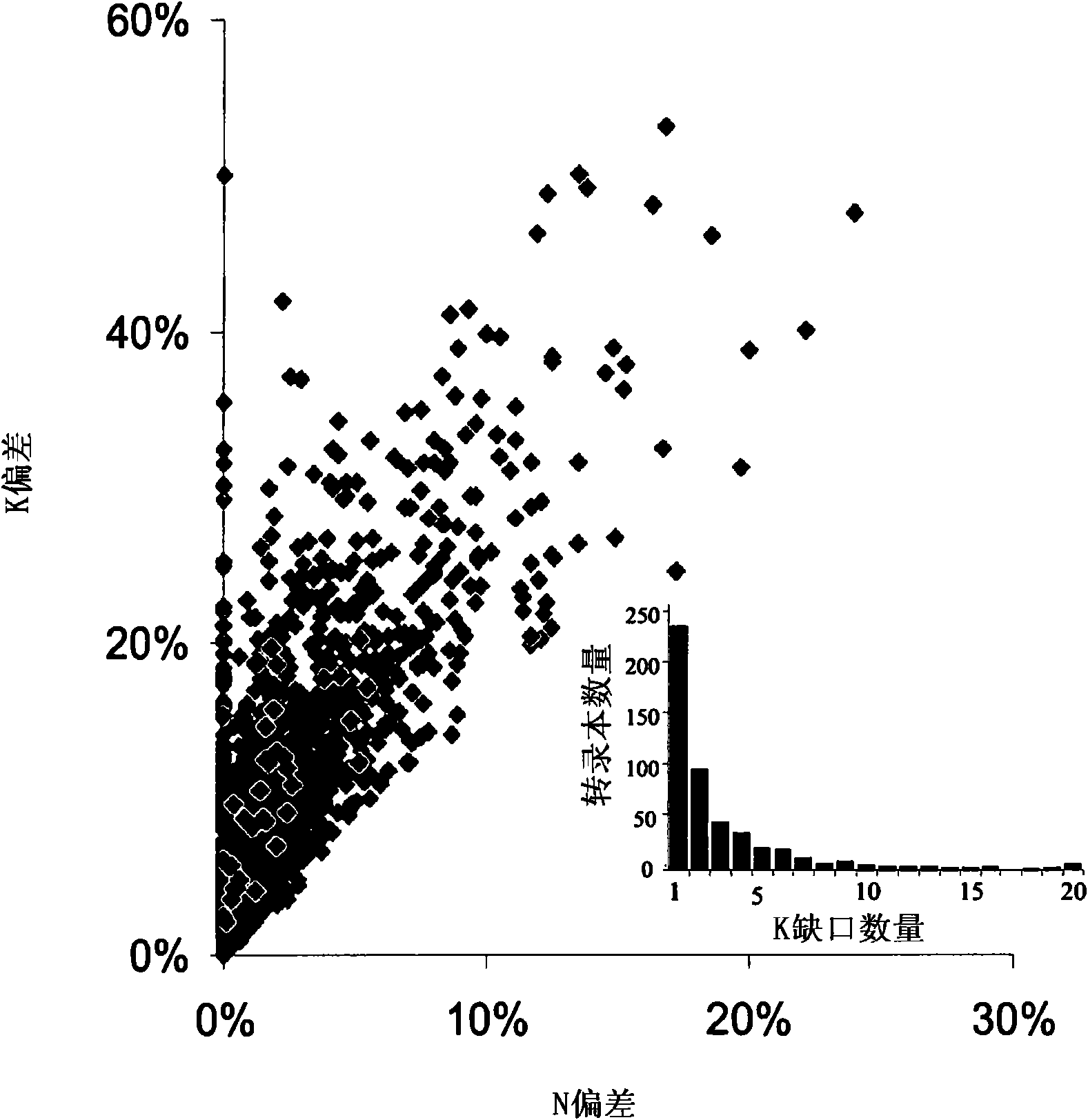
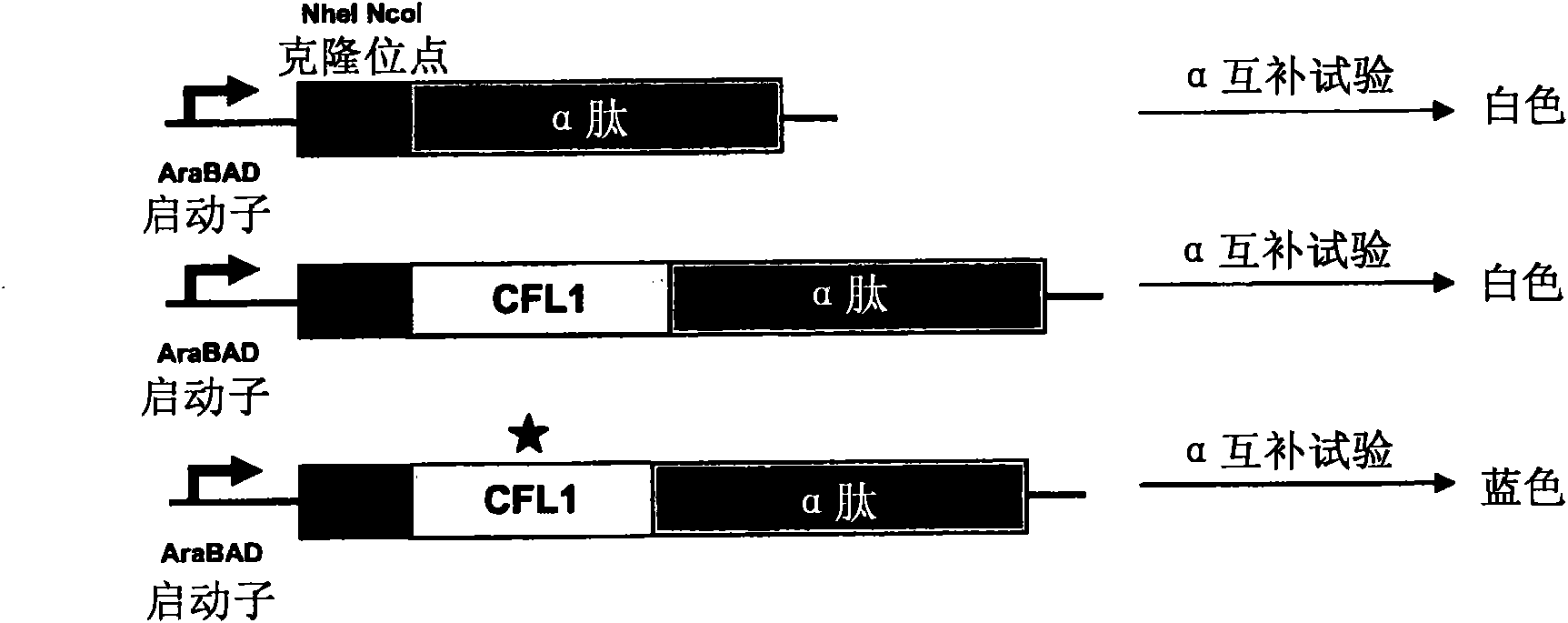
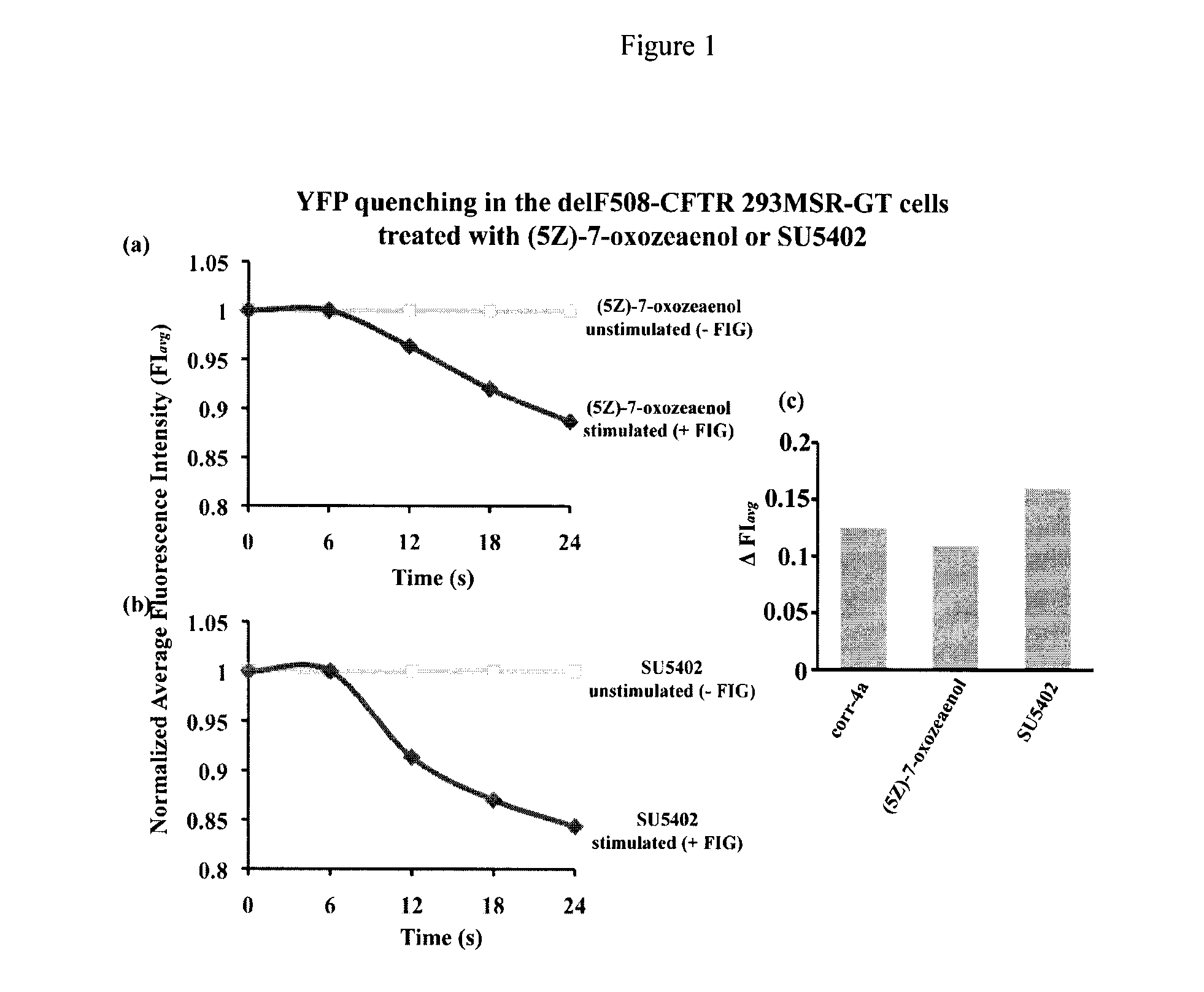
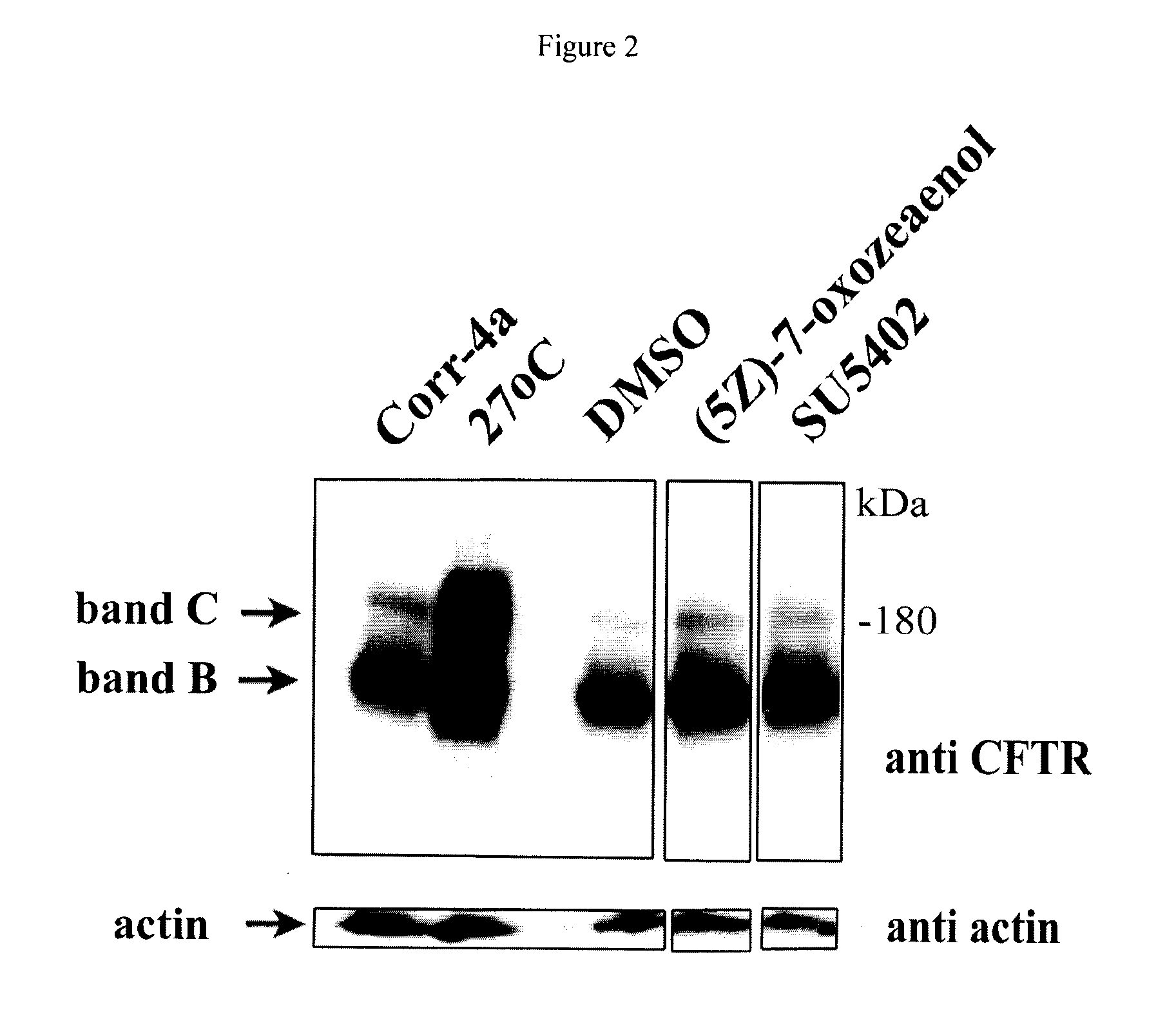
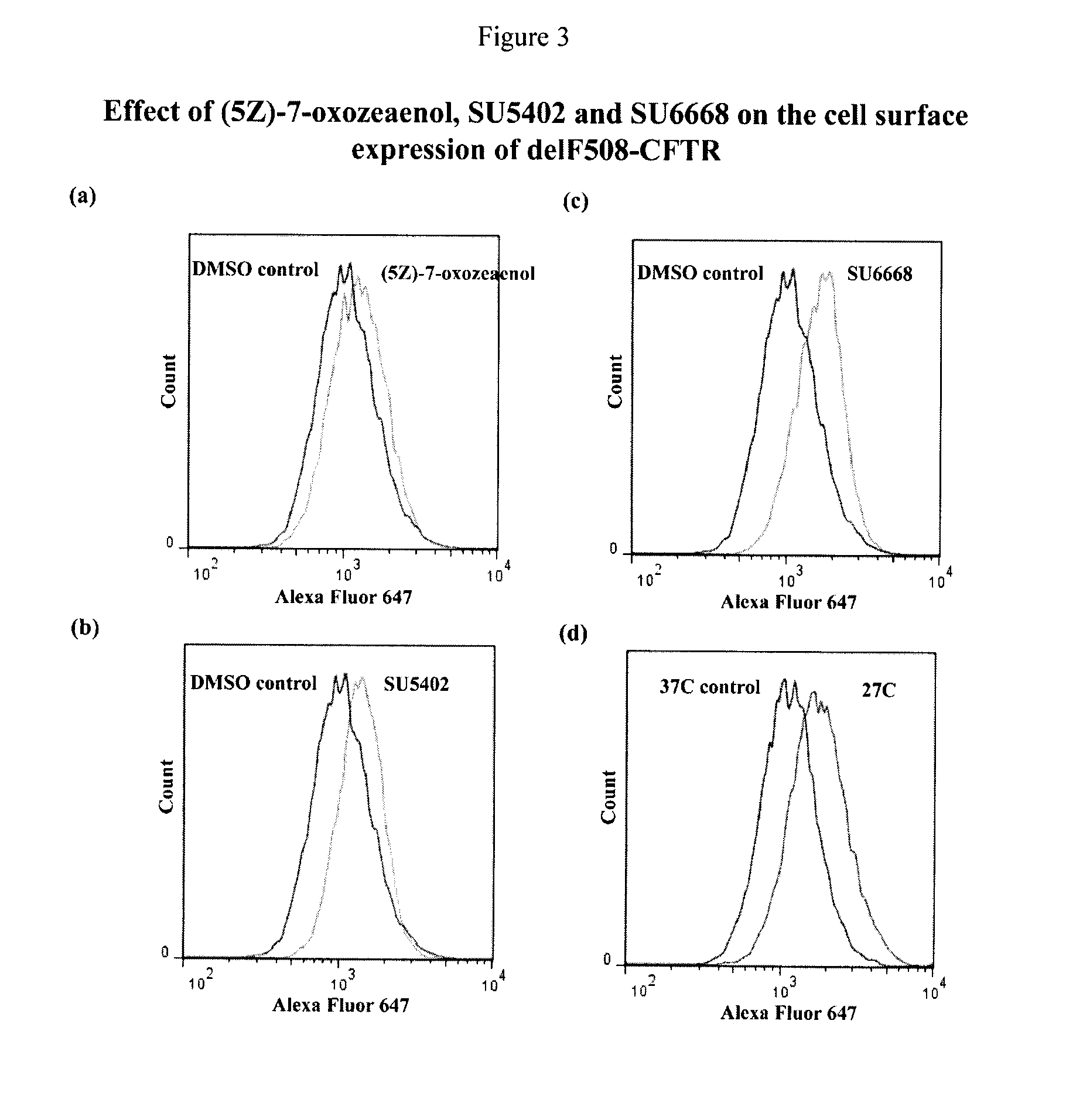
Compositions and methods for treatment of cystic fibrosis and diseases associated with aberrant protein cellular processing
ActiveUS20130150422A1Precise processingRestore trans-membrane transport capacityBiocideOrganic chemistryMedicineCystic fibrosis lungs
The disclosure relates to resorcylic acid lactones and indolinone-containing compounds for use in treatment of diseases associated with aberrant protein processing, such as cystic fibrosis (CF; mucoviscidosis). The disclosure more generally relates to treatment of aberrant protein processing, such as errors in protein folding, trafficking or post-translational modification.
Owner:HOSPITAL FOR SICK CHILDREN
Nisod-like compound and its derivatives for the prevention and treatment of neurodegenerative disorders
ActiveUS20150209370A1Suppress abnormal protein aggregationRelieve pressureOrganic active ingredientsNervous disorderAlzheimer SyndromeBrains tissue
The accumulation of abnormal proteins in neurodegenerative disorders is considered to induce oxidative stress and lead to cell death. Thus suppression of abnormal protein aggregation and reducing oxidative stress or reactive oxygen species (ROS) are expected to inhibit a wide range of harmful downstream events, providing an observation for identifying the potential prevention and treatment of neurodegenerative disorders comprising spinocerebellar ataxia (SCA), Alzheimer's disease (AD) or Parkinson's disease (PD). In the present invention, there are several cell and animal experiment models for SCA, AD and PD established, and NiSOD-like compounds were applied to these experiment models. The results have demonstrated how NiSOD-like compounds are likely to work in suppressing abnormal protein aggregation and reducing oxidative stress; in addition, the results were also confirmed with brain slice culture of SCA17 transgenic mice and AD mice, brain tissues immunostaining of PD mice, and SCA17 transgenic, AD and PD mice.
Owner:NATIONAL TAIWAN NORMAL UNIVERSITY
Compositions and methods of using tyrosine kinase inhibitors
ActiveUS20180271864A1Decrease aberrant levelImprove heart functionOrganic active ingredientsCardiovascular disorderTyrosine-kinase inhibitorHeart disease
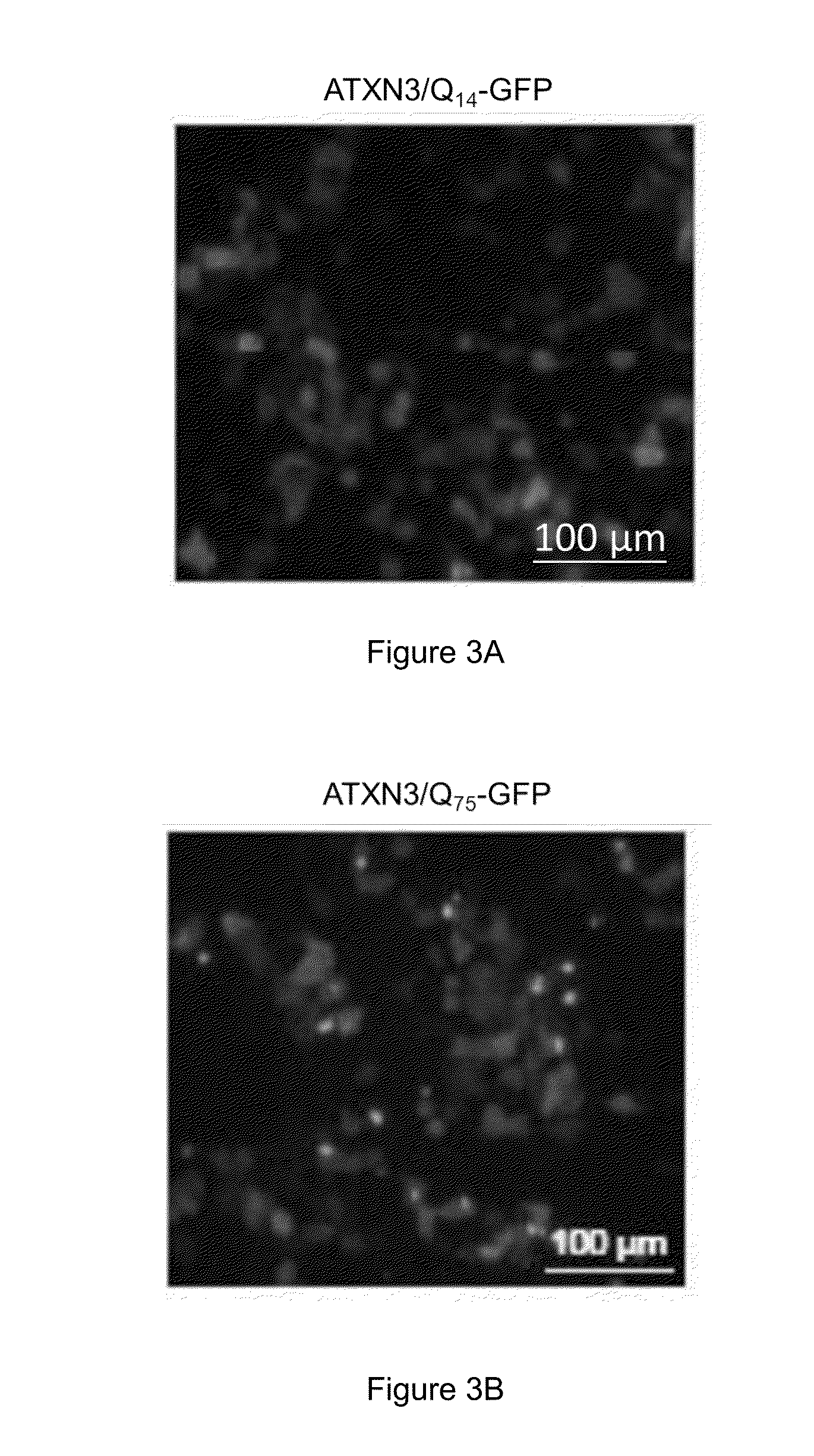
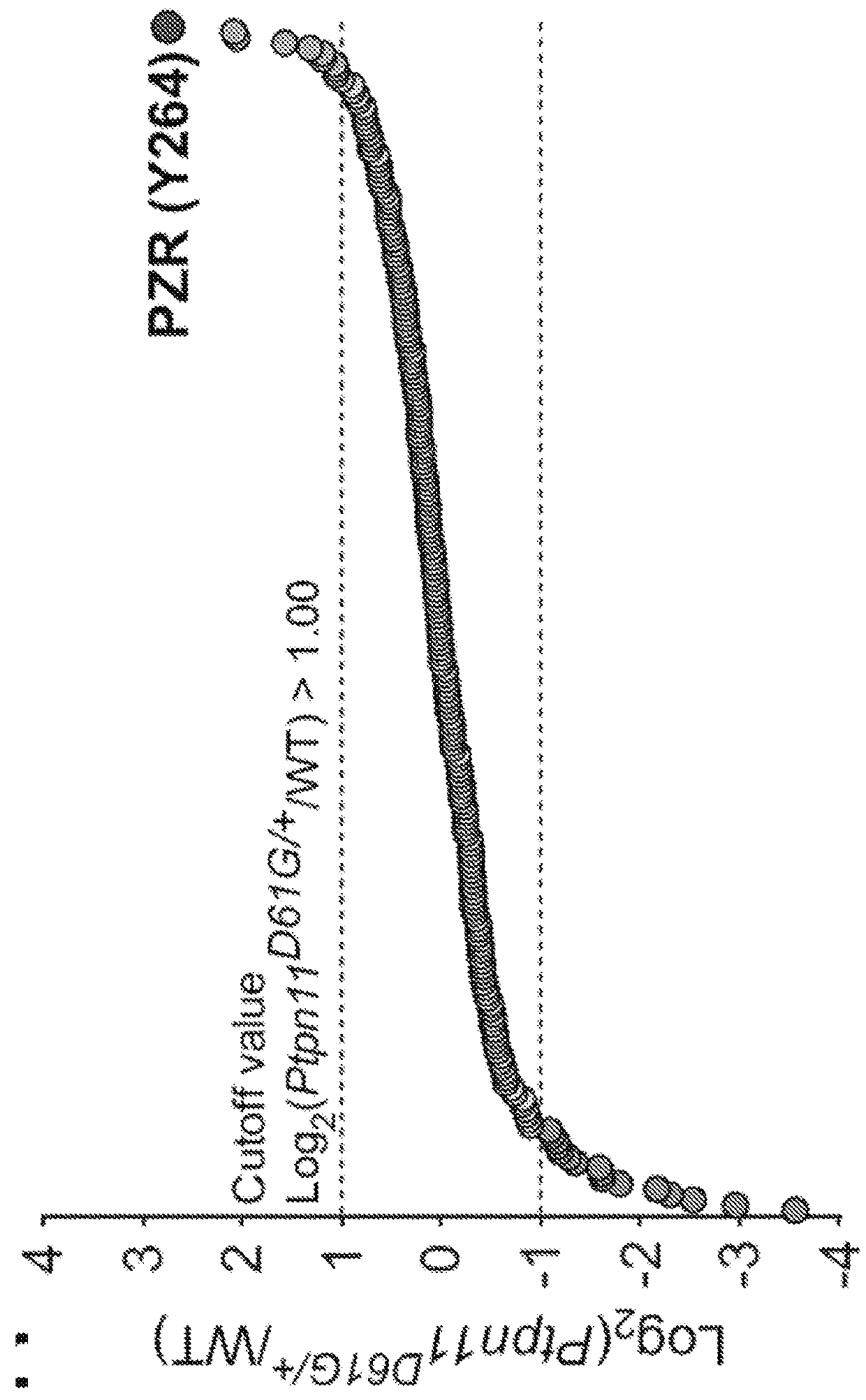
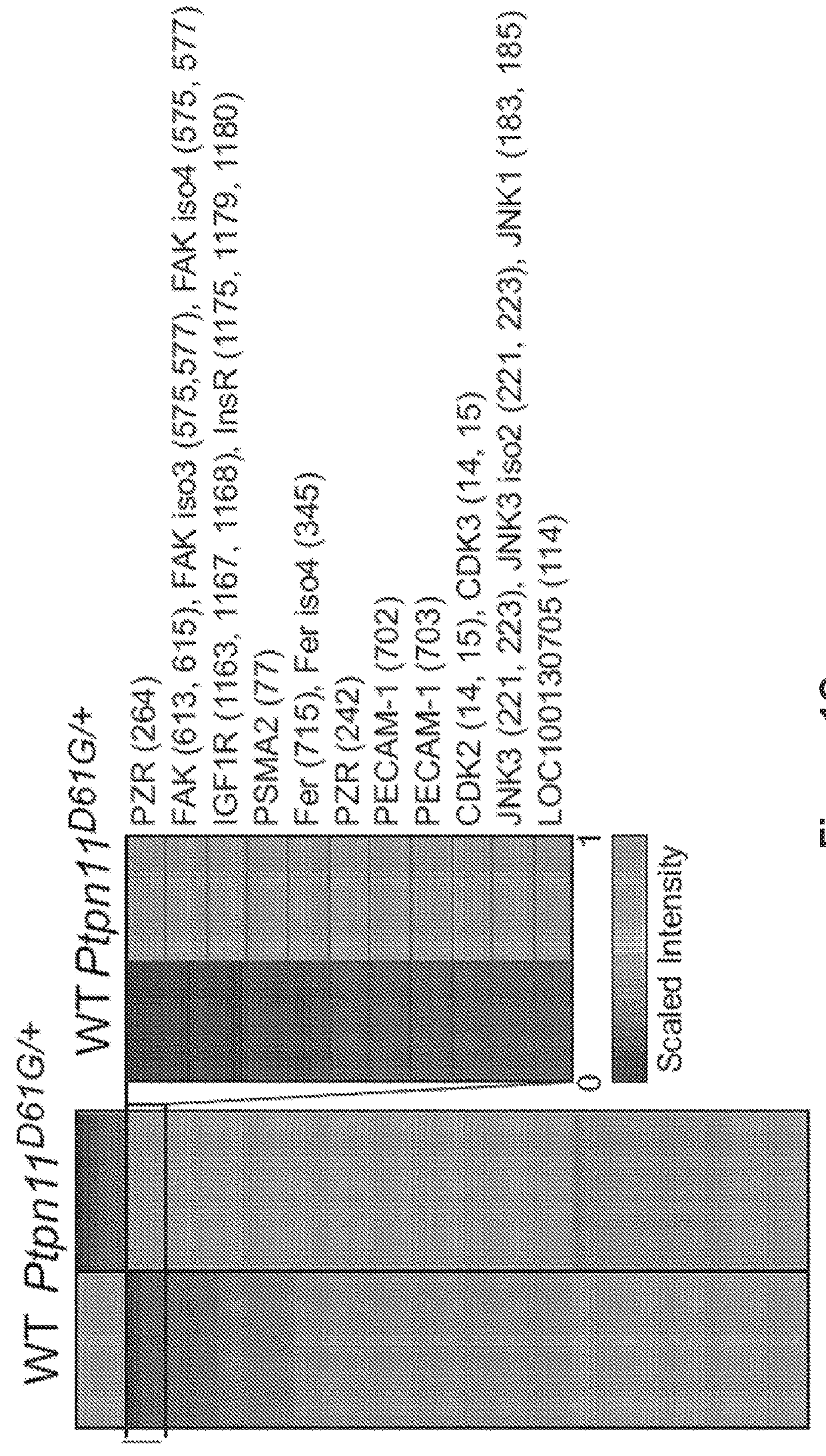
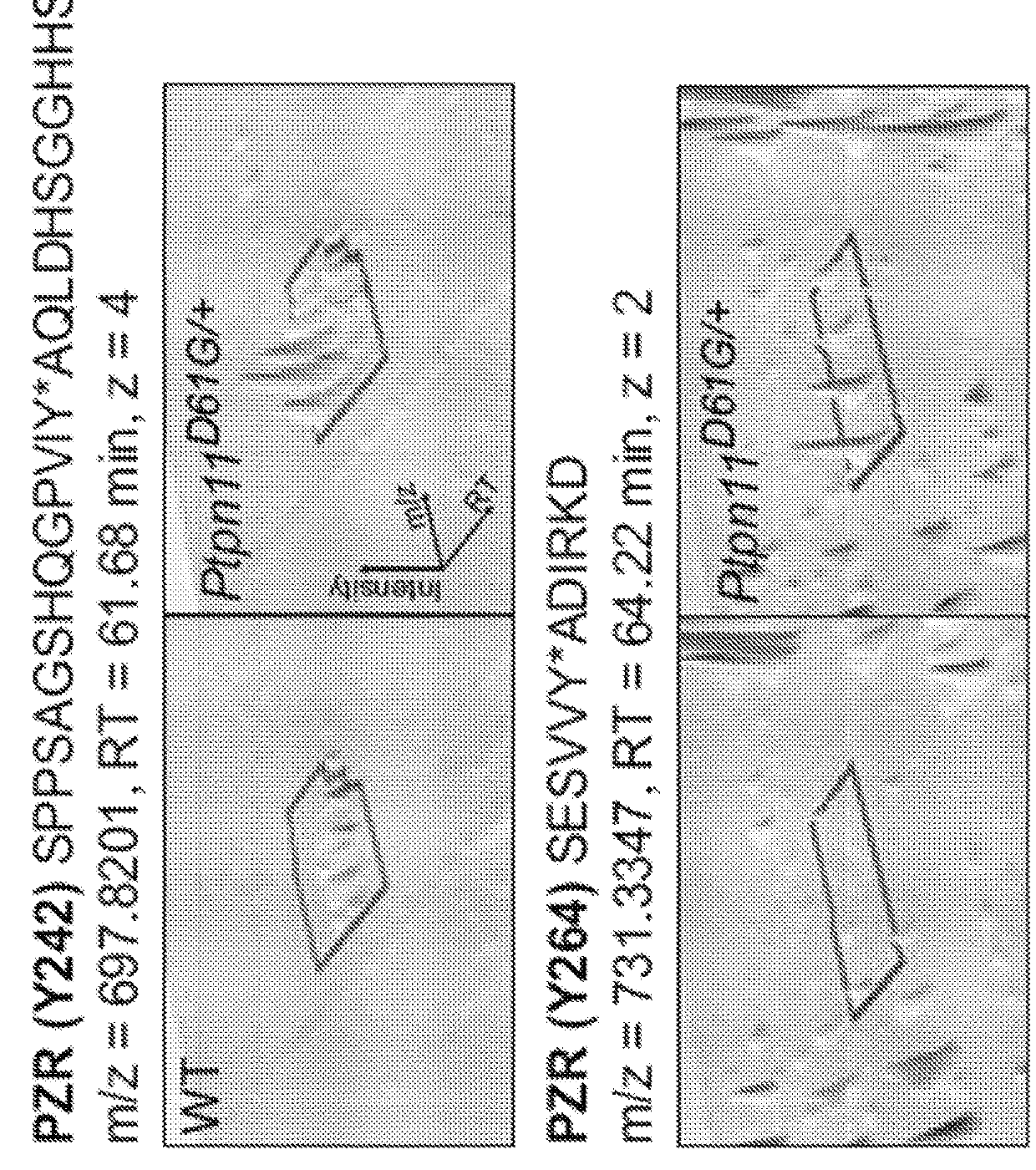
The present invention provides compositions and methods of inhibiting tyrosine phosphorylation. In one aspect, a composition comprising a low-dosage tyrosine kinase inhibitor, where the low-dosage tyrosine kinase inhibitor decreases tyrosine phosphorylation, is provided. In another aspect, a method for treating cardiovascular disease or condition associated with a RASopathy having aberrant protein tyrosine phosphorylation is described. Methods for treating congenital heart disease associated with Noonan or Noonan syndrome with multiple lentigines and decreasing aberrant levels of Protein Zero-Related (PZR) tyrosyl phosphorylation are also described.
Owner:YALE UNIV
Abnormal protein removing method
A method for suppressing dull complexion or wrinkles or providing moisture-keeping effect in skin of a subject by removing abnormal protein includes administering to the subject desiring such suppression and moisture-keeping effect a food composition containing a soybean extract containing 40 to 100 parts by weight of soyasaponin B group relative to 100 parts by weight of the soybean extract in an effective amount.
Owner:FUAN KERU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com