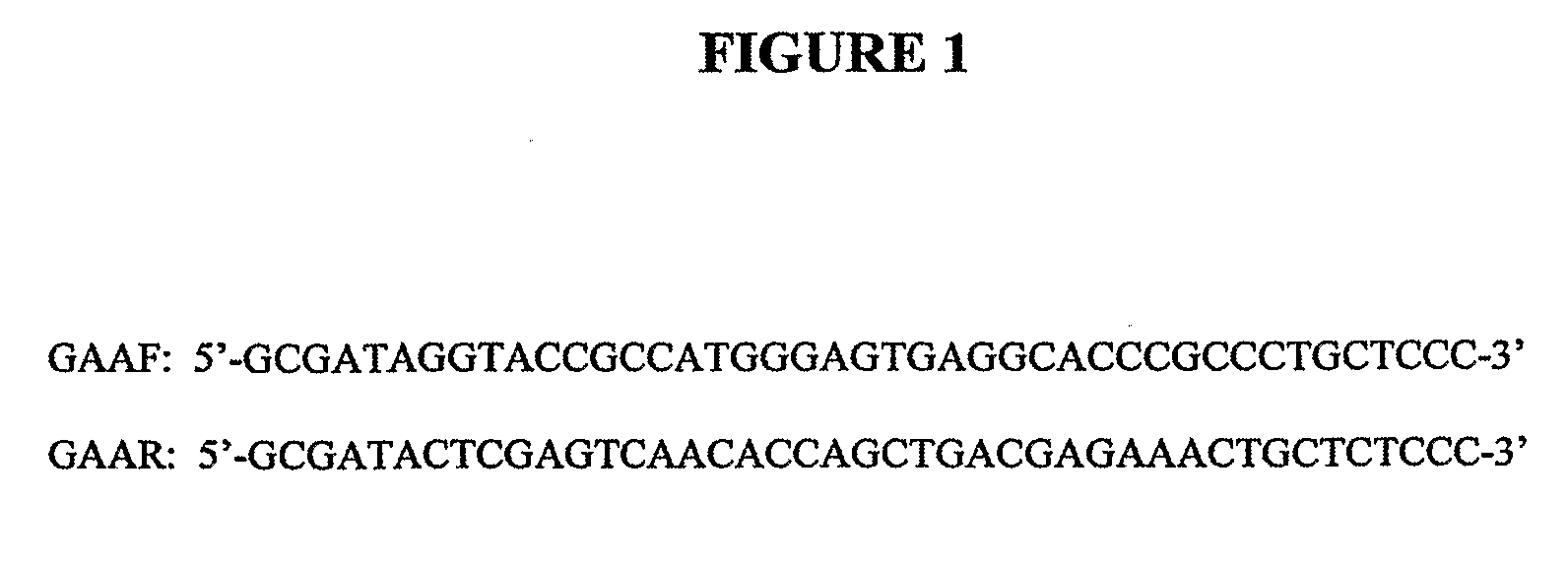
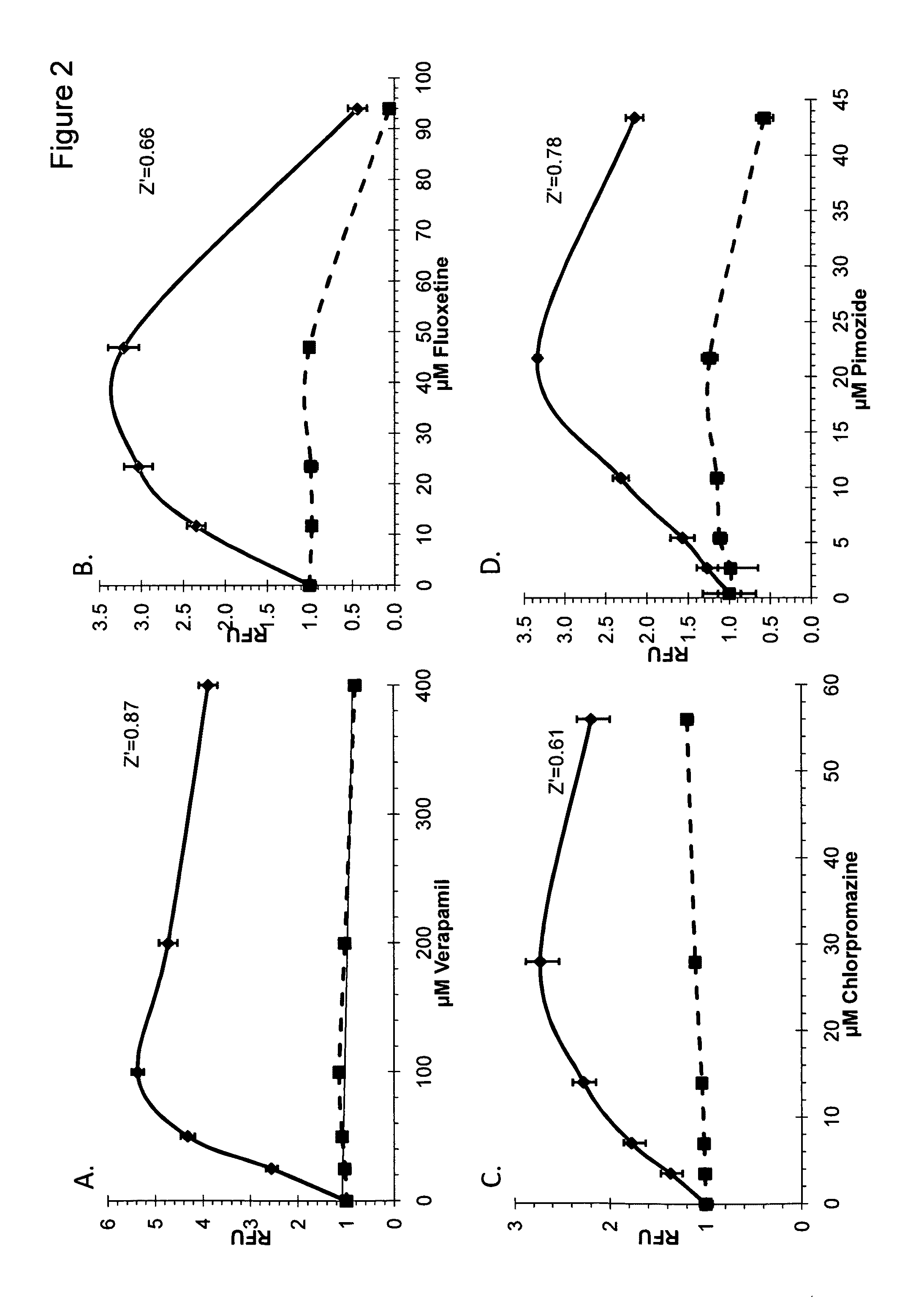
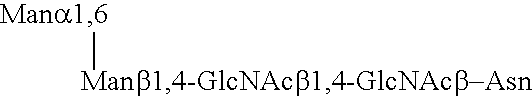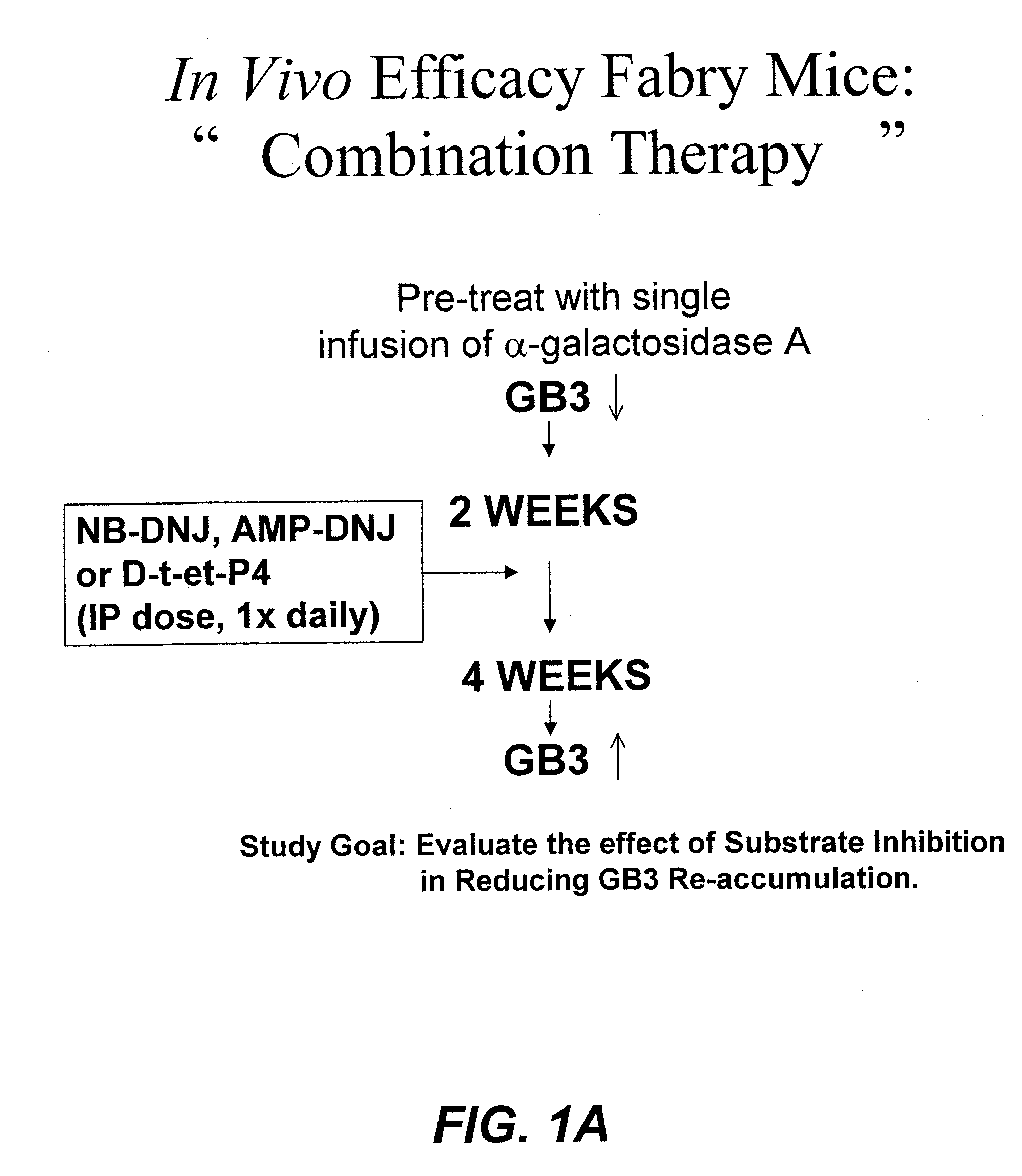Patents
Literature
172 results about "Lysosomal enzyme defect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
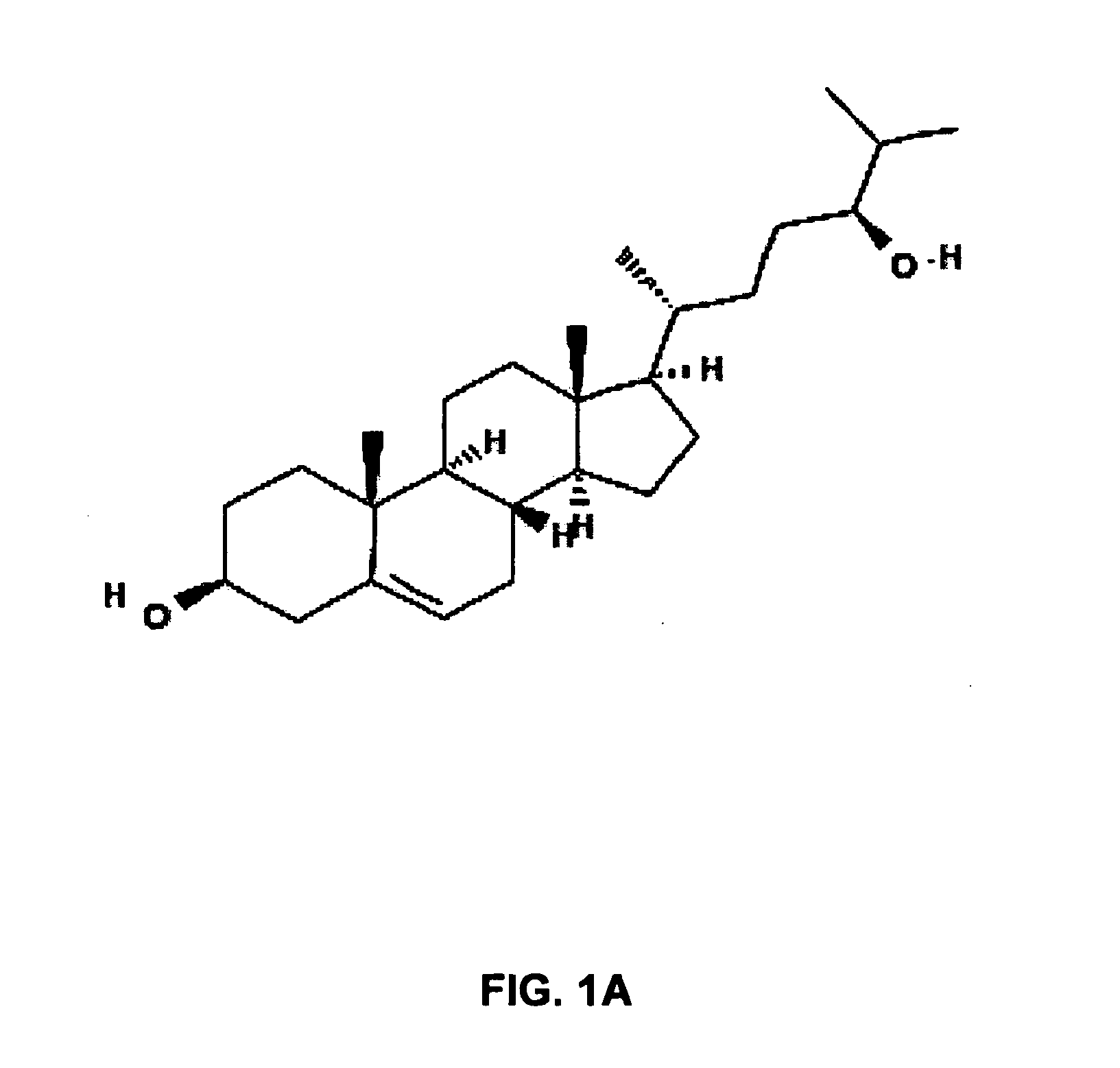
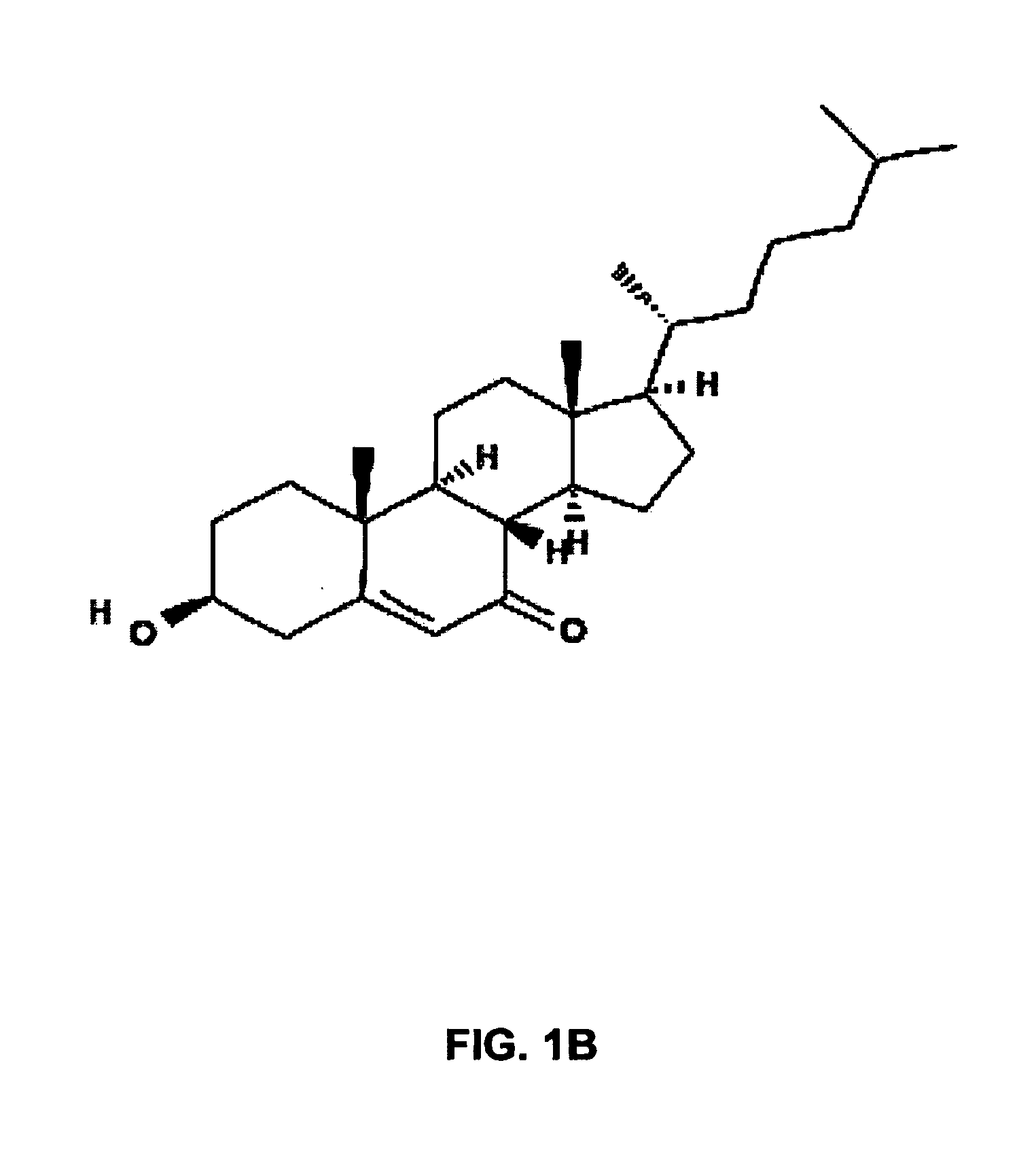
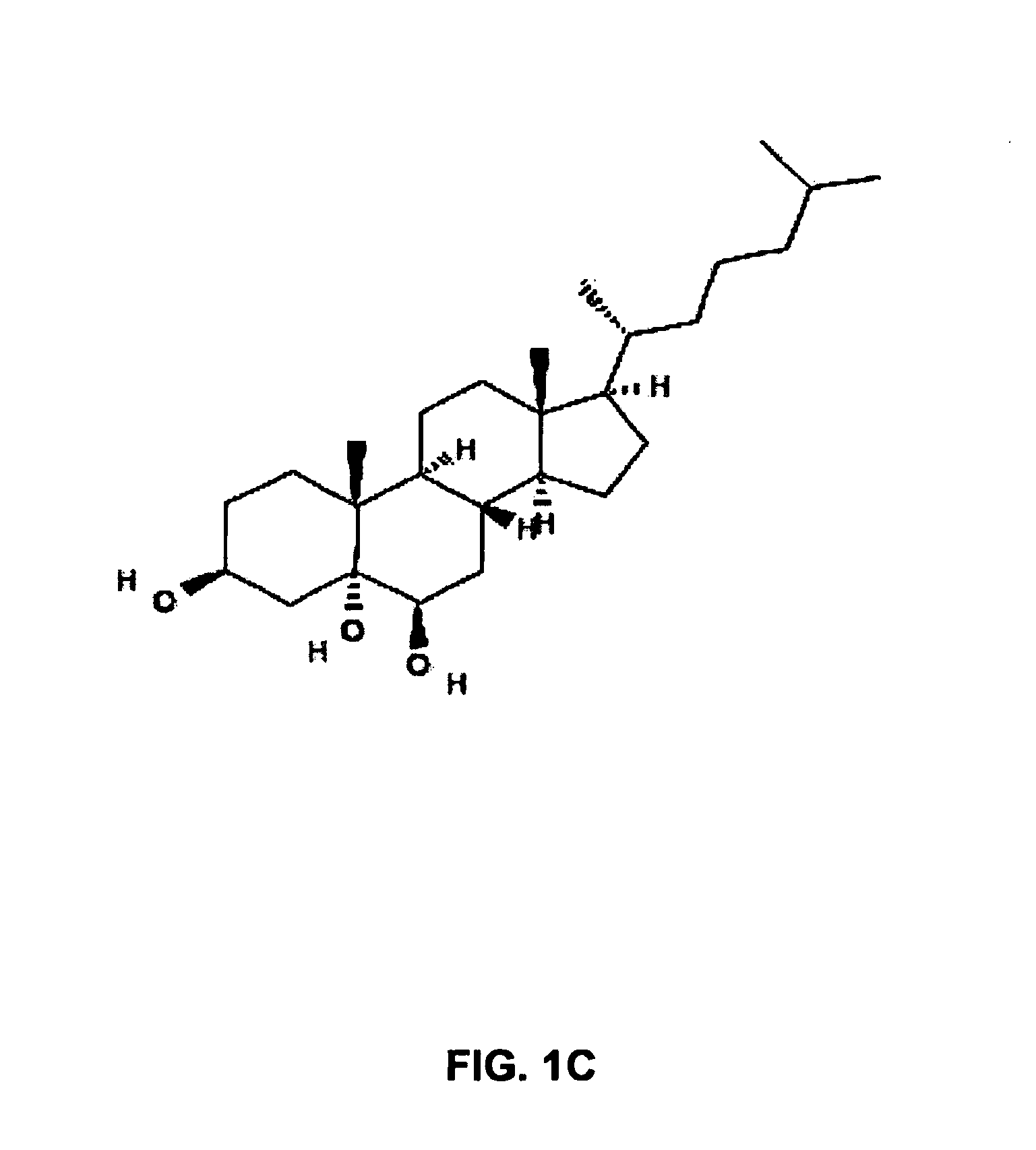
Lysosomal storage diseases (LSDs; /ˌlaɪsəˈsoʊməl/) are a group of about 50 rare inherited metabolic disorders that result from defects in lysosomal function. Lysosomes are sacs of enzymes within cells that digest large molecules and pass the fragments on to other parts of the cell for recycling.
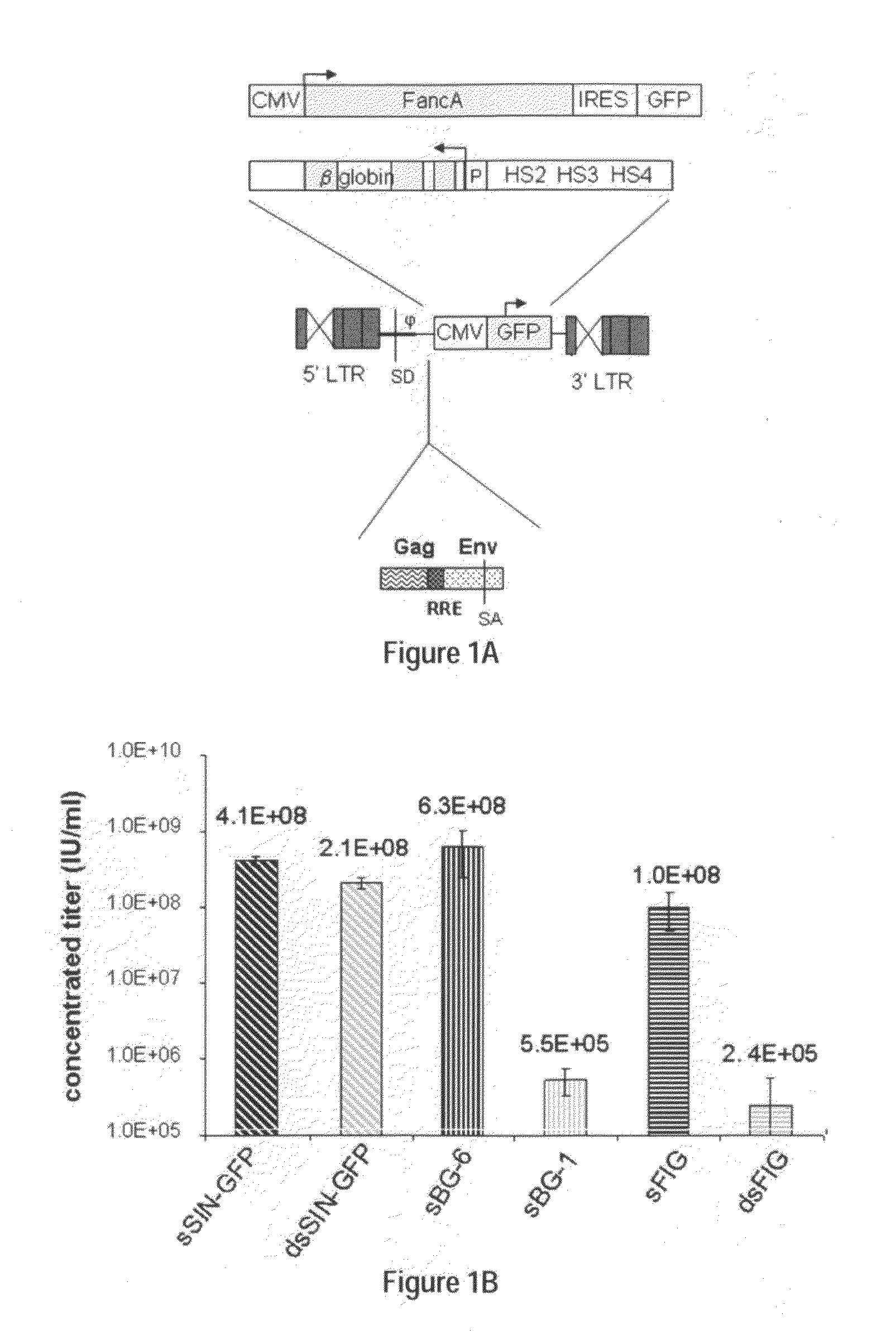
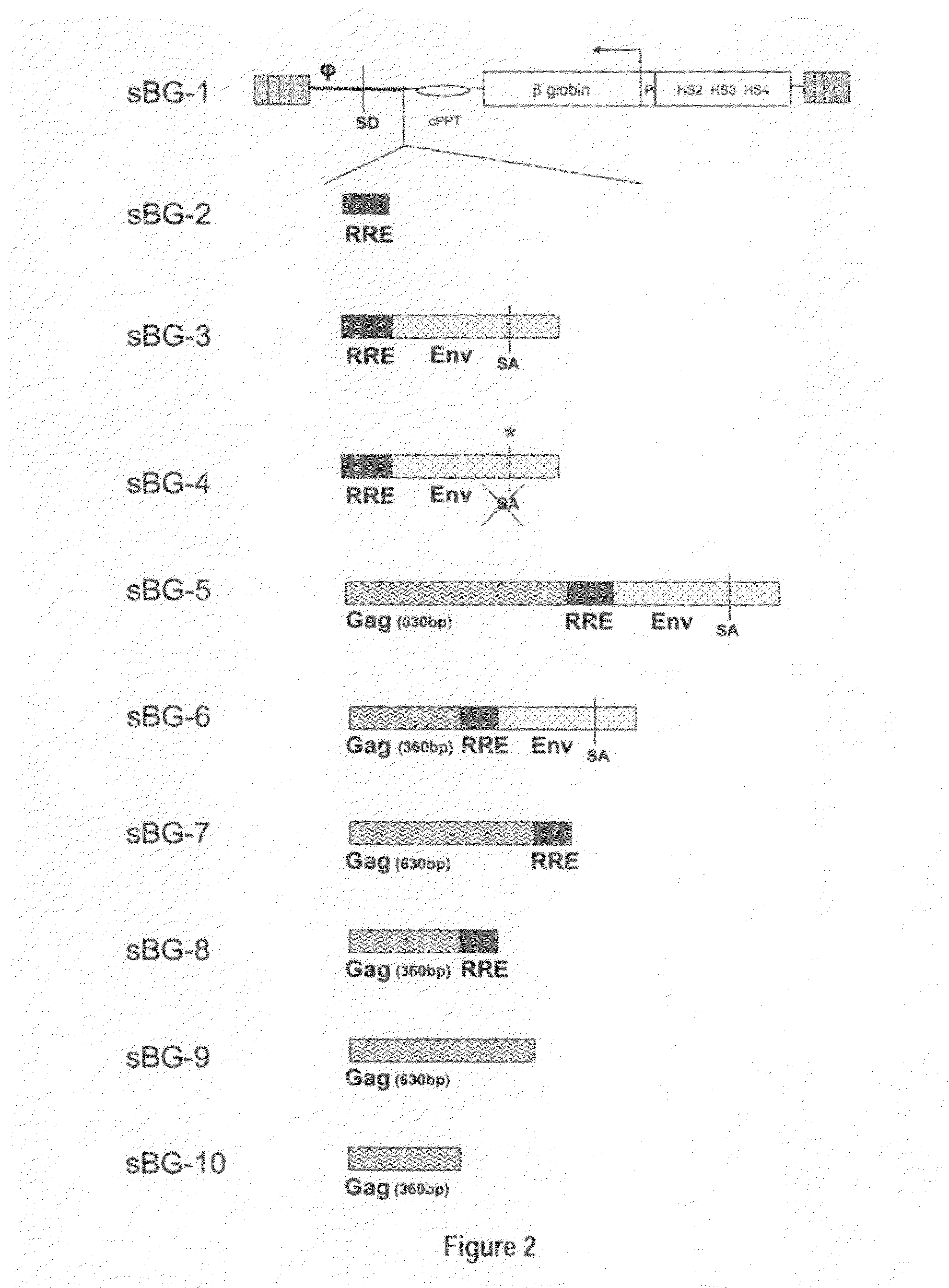
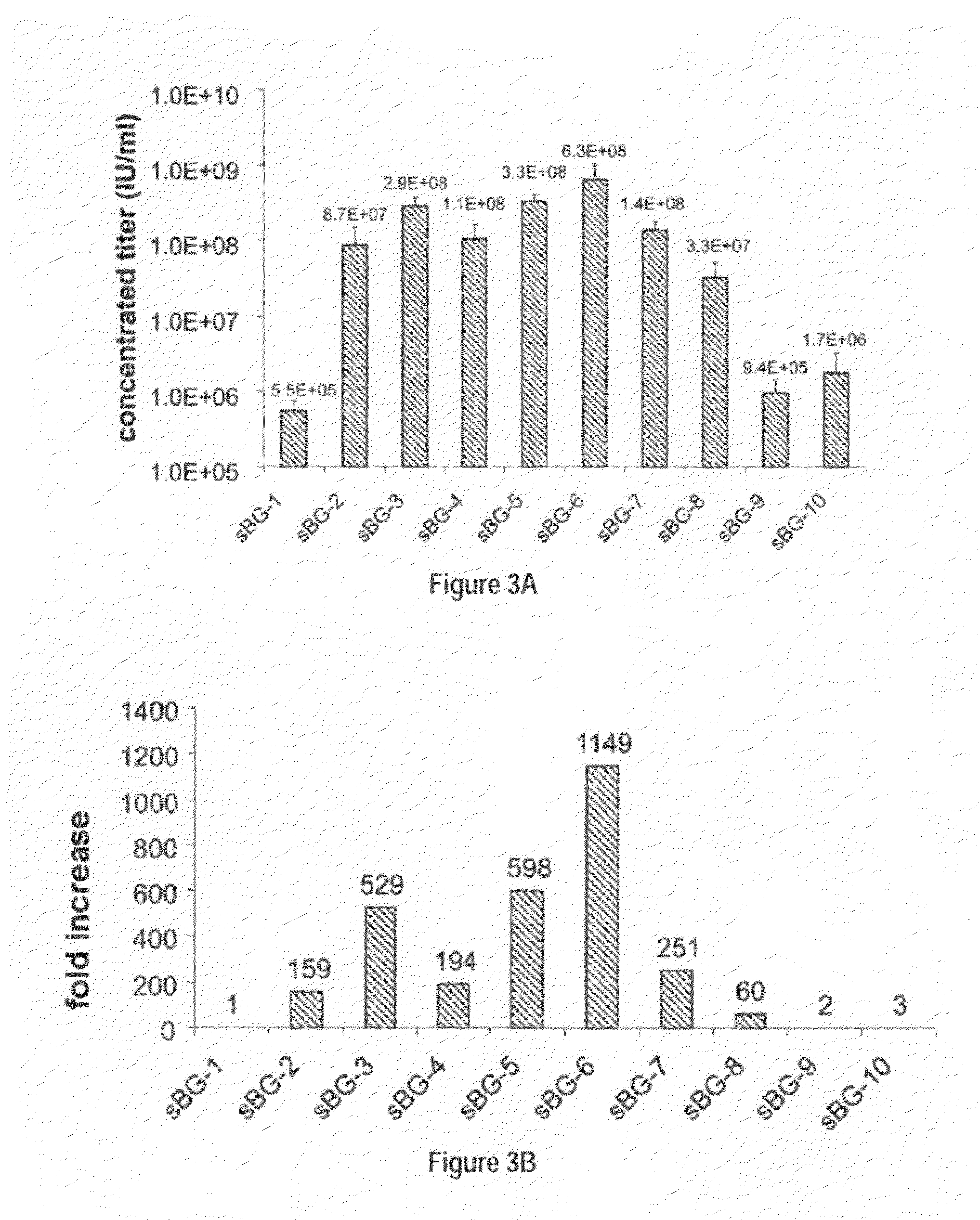
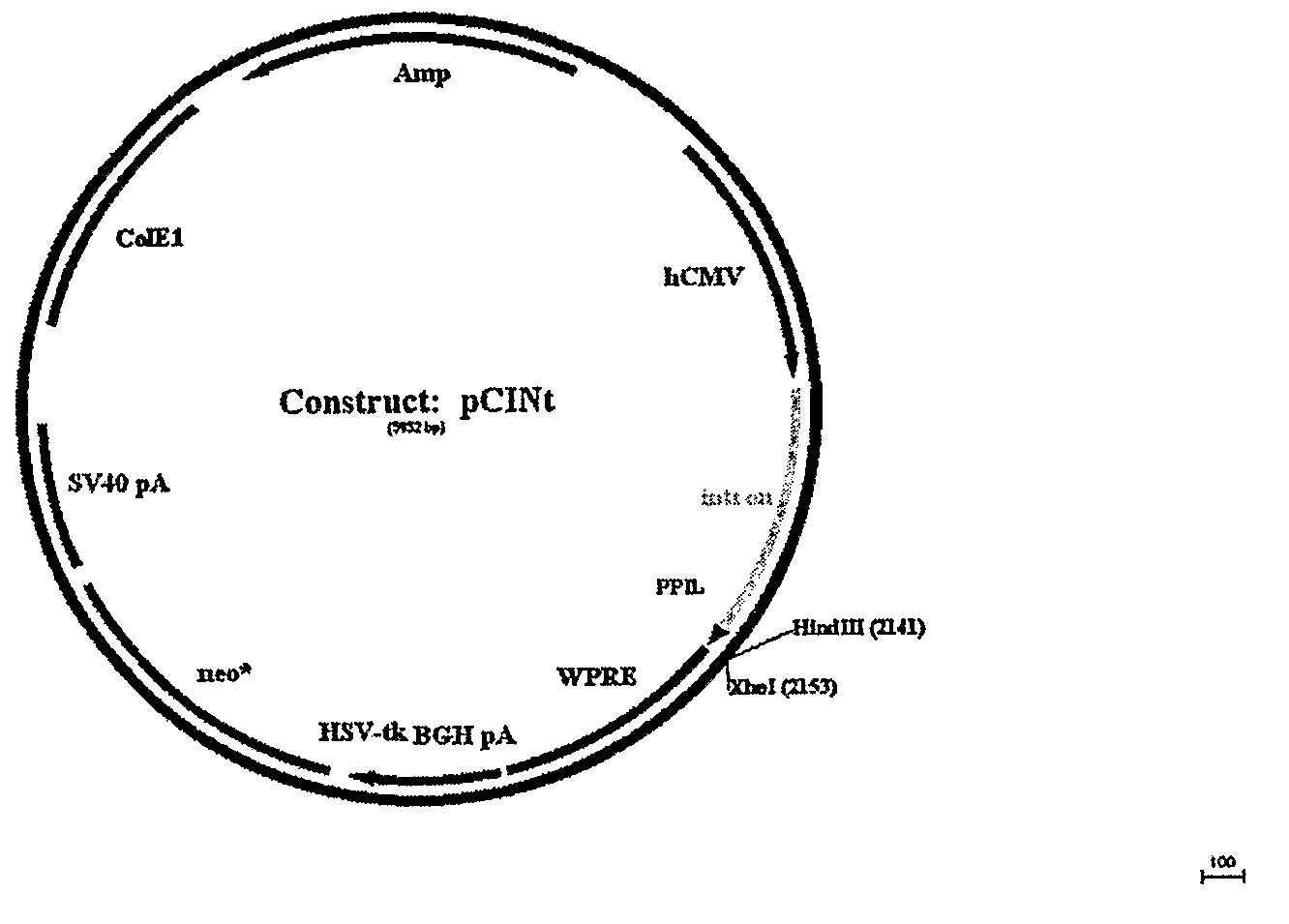
Optimization of determinants for successful genetic correction of diseases, mediated by hematopoietic stem cells
InactiveUS20110294114A1Improve stabilityImprove securityVectorsSugar derivativesNervous systemSickle cell anemia
Methods and compositions disclosed herein generally relates to methods of determining minimum hematopoietic stem cell (HSC) chimerism and gene dosage for correction of a hematopoietic disease; in particular, in in vivo models. The invention also relates to modified lentiviral expression vectors for increase a viral titer and various methods for increasing such titers as well as expression vectors capable of enhancing such titers. The invention also relates to CHS4 chromatin insulator-derived functional insulator sequences. The invention further relates to methods for genetic correction of diseases or reducing symptoms thereof, such as sickle cell anemia, a lysosomal storage disease. The invention further relates to a method of improving and / or correcting one or more central nervous system (CNS) abnormalities caused by one or more lysosomal storage disease. The invention further relates to methods of improving titer in transfection-based bioreactor culture production or transfection-based production systems using eukaryotic cells.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
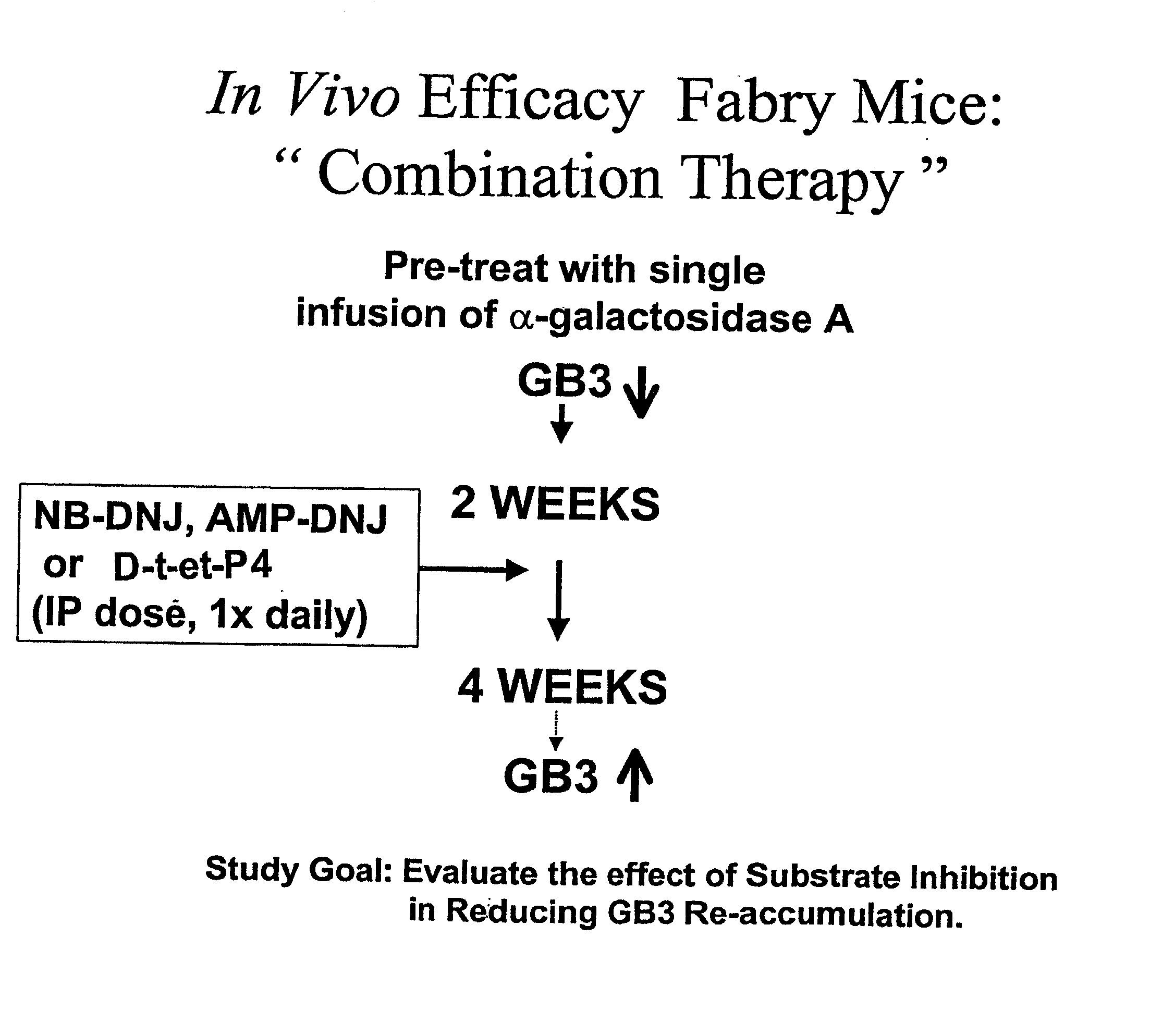
Combination enzyme replacement, gene therapy and small molecule therapy for lysosomal storage diseases
InactiveUS20020095135A1Optimizes clinical benefitReduce disadvantagesPeptide/protein ingredientsMetabolism disorderLysosomal enzyme defectGene
This invention provides various combinations of enzyme replacement therapy, gene therapy, and small molecule therapy for the treatment of lysosomal storage diseases.
Owner:MEEKER DAVID +1
Lysosomal enzymes and lysosomal enzyme activators
InactiveUS20020127219A1Improve in vivo bioactivityConvenient treatmentPeptide/protein ingredientsAntibody mimetics/scaffoldsLysosomeEnzyme activator
Owner:MAXYGEN +1
Methods and systems for treatment of neurological diseases of the central nervous system
InactiveUS20050208090A1Reduce degradationAdequate transportNervous disorderPeptide/protein ingredientsSystems designActive enzyme
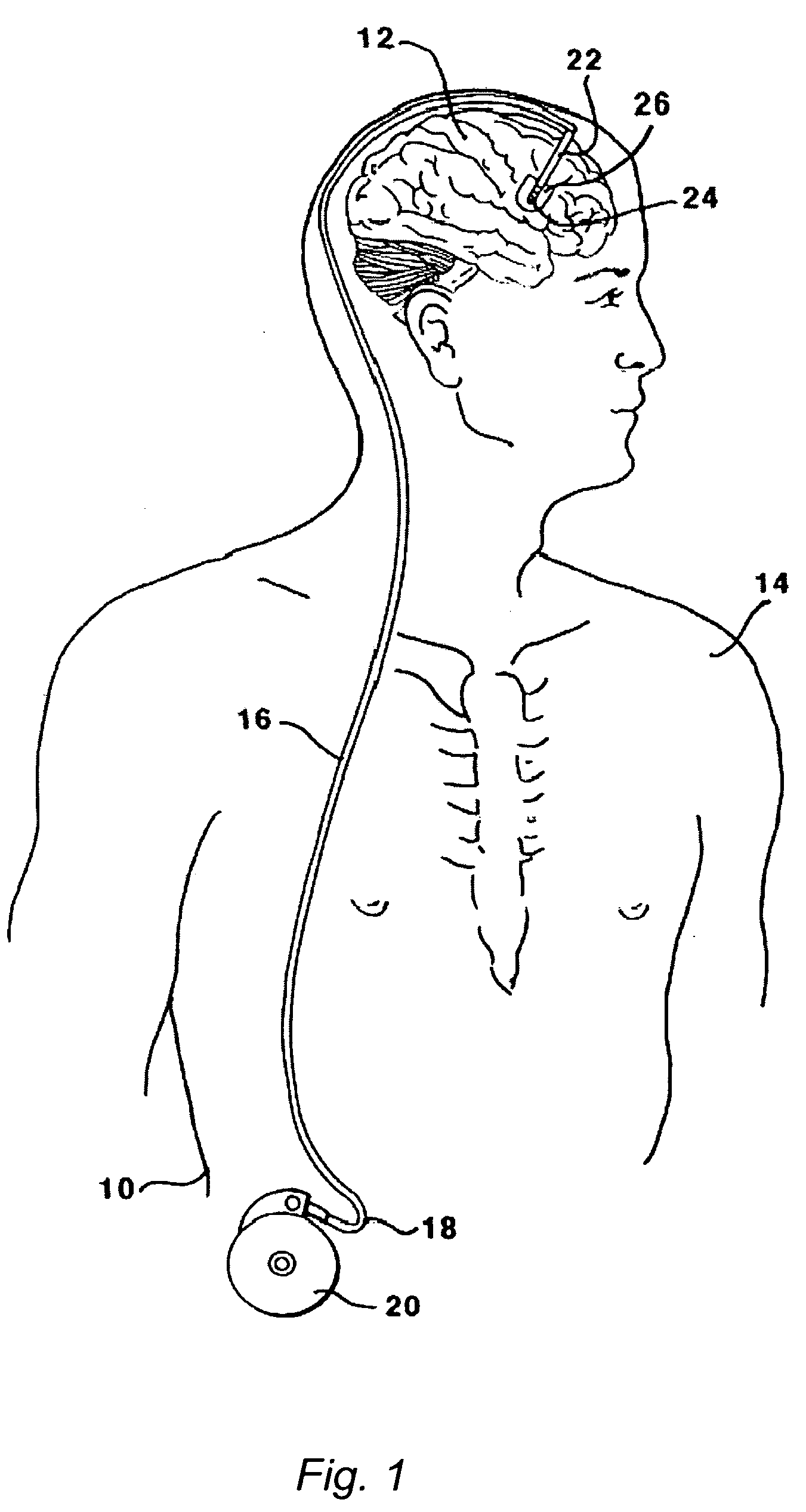
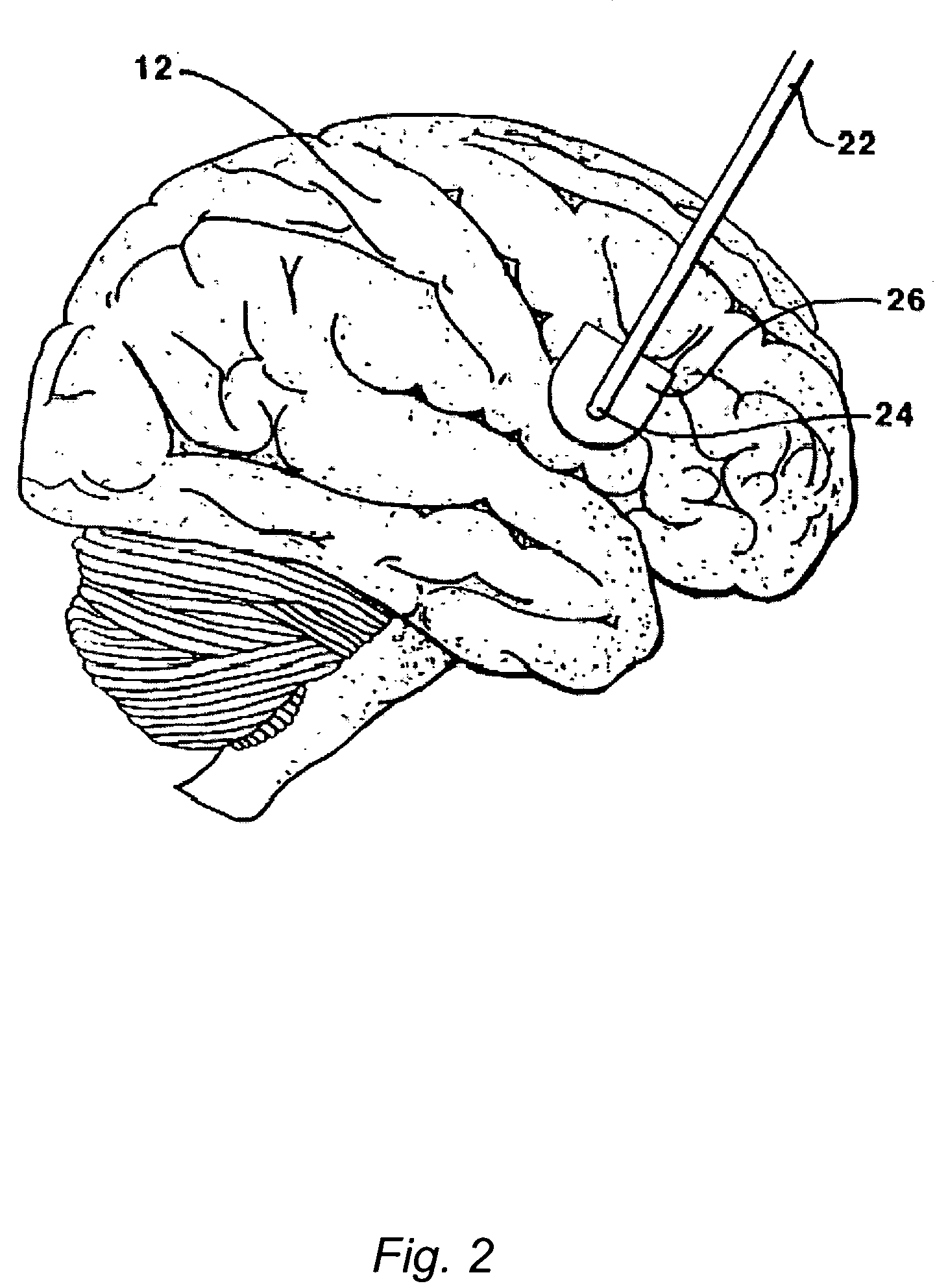
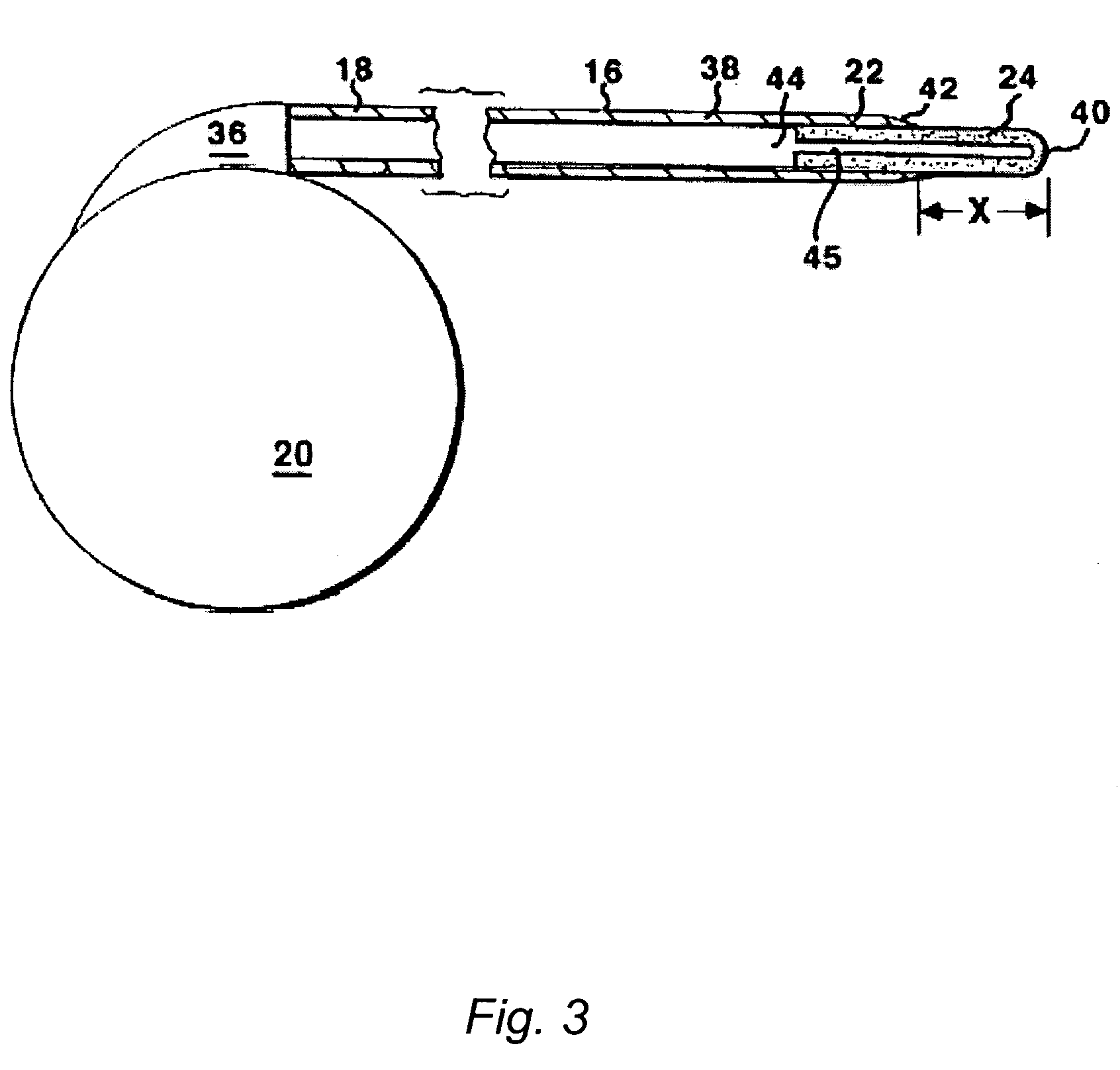
The present invention is directed to methods and systems for the treatment of inborn genetic errors or other defects that cause deficiencies of active enzymes or proteins within the cells of the central nervous system. Such methods and systems generally comprise an implantable catheter system designed for the chronic delivery of specially formulated proteins to intrathecal, intracerebroventricular, and / or intraparenchymal regions of the central nervous system. The invention has application in the neuropathic aspects of the broad category of lysosomal storage diseases. These genetic based diseases are the result of insufficient enzyme activity to catabolize specific substances, which thereby accumulate in the cellular lysosomes.
Owner:MEDTRONIC INC
Production of high mannose proteins in plant culture
ActiveUS20090208477A1High glycosylationEfficient targetingBioreactor/fermenter combinationsBiological substance pretreatmentsHigh mannosePlant roots
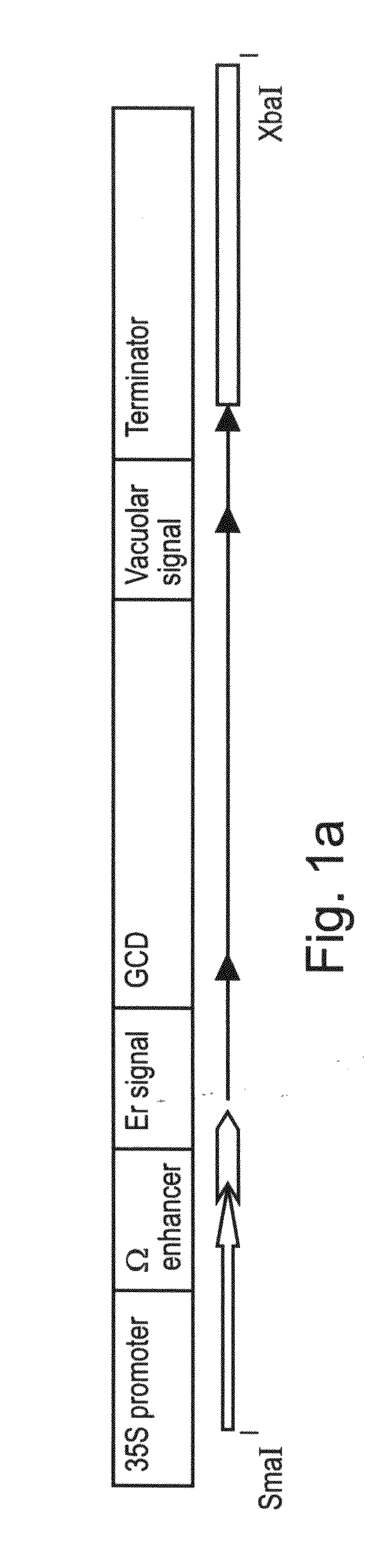
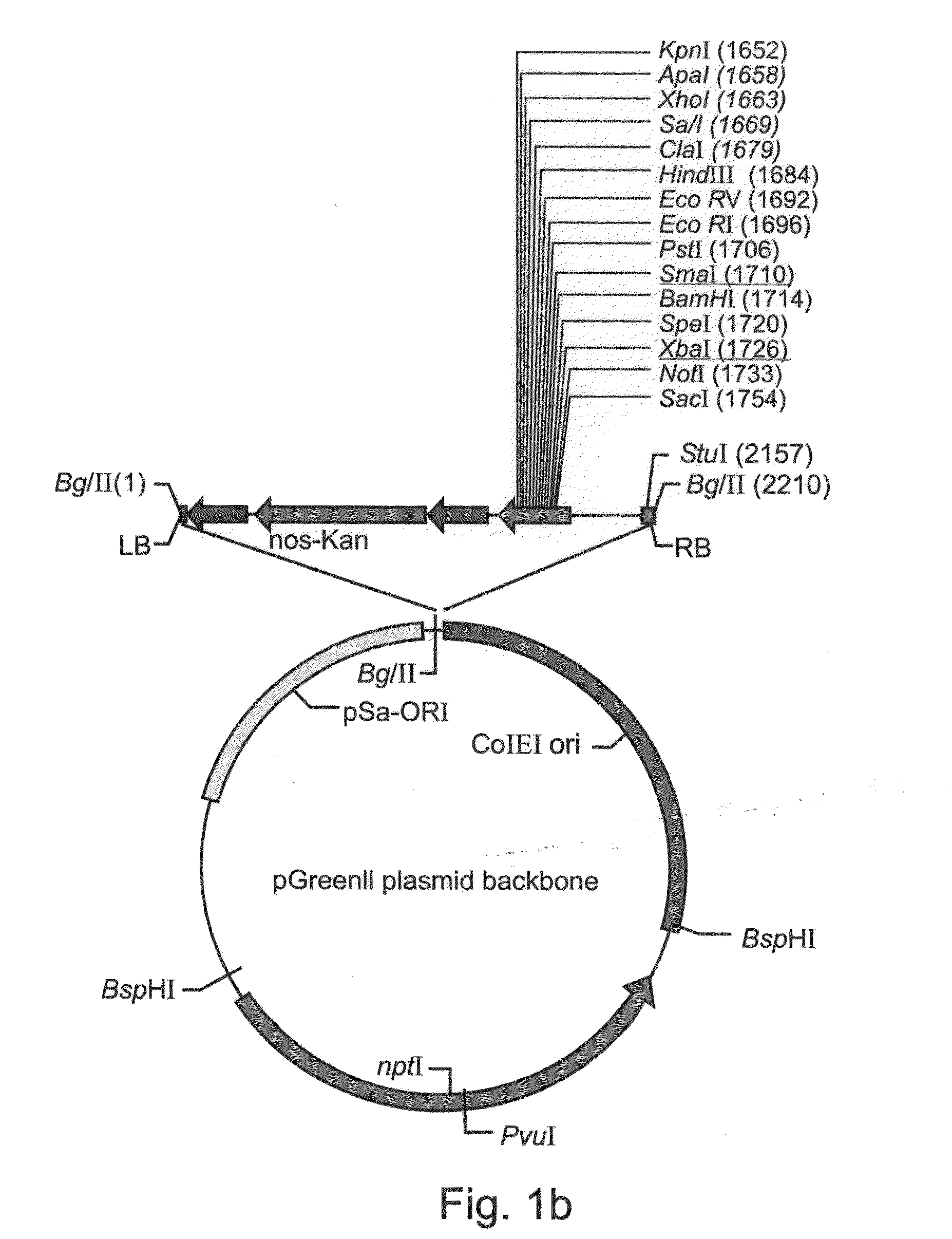
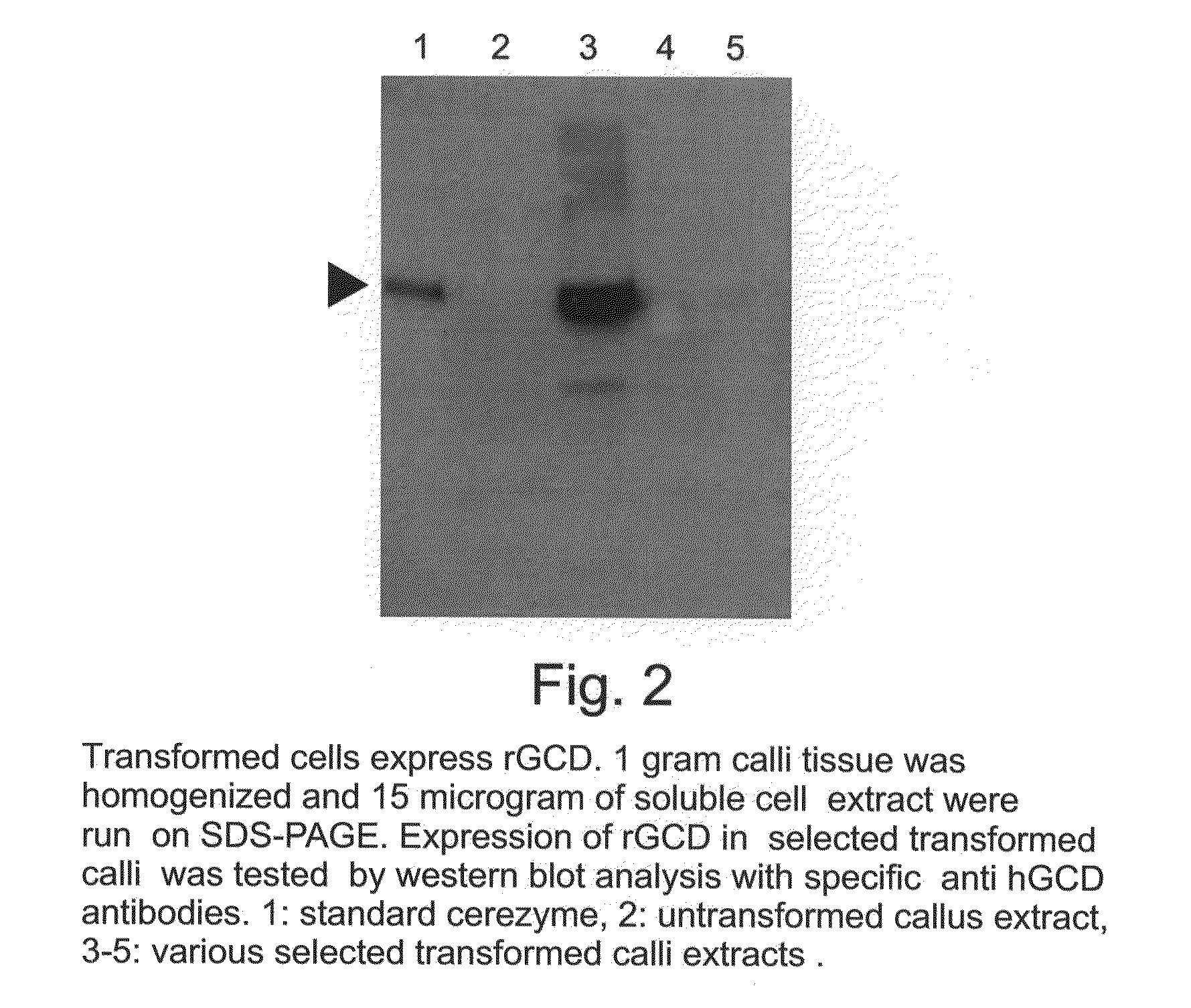
A device, system and method for producing glycosylated proteins in plant culture, particularly proteins having a high mannose glycosylation, while targeting such proteins with an ER signal and / or by-passing the Golgi. The invention further relates to vectors and methods for expression and production of enzymatically active high mannose lysosomal enzymes using transgenic plant root, particularly carrot cells. More particularly, the invention relates to host cells, particularly transgenic suspended carrot cells, vectors and methods for high yield expression and production of biologically active high mannose Glucocerebrosidase (GCD). The invention further provides for compositions and methods for the treatment of lysosomal storage diseases.
Owner:PROTALIX
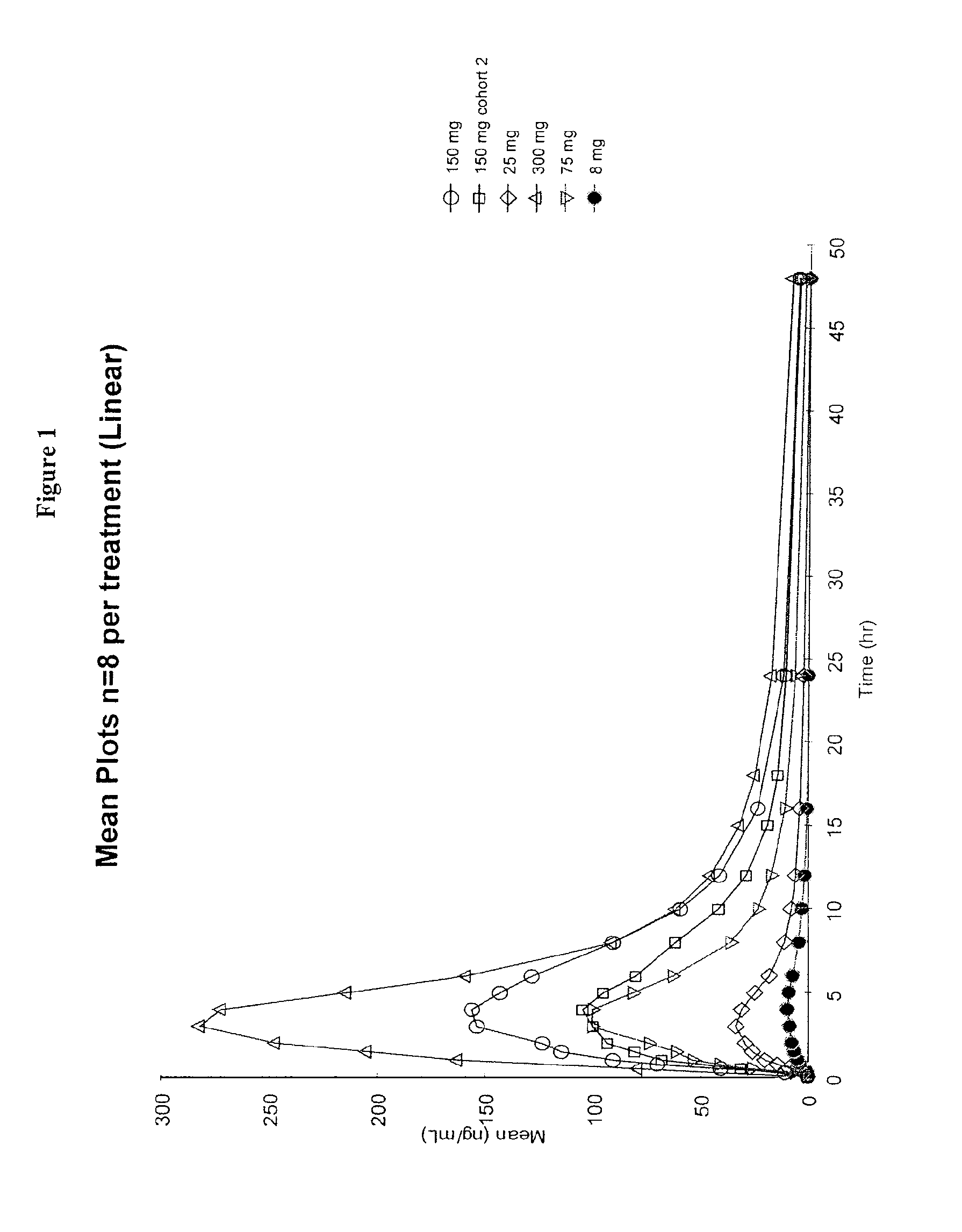
Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones
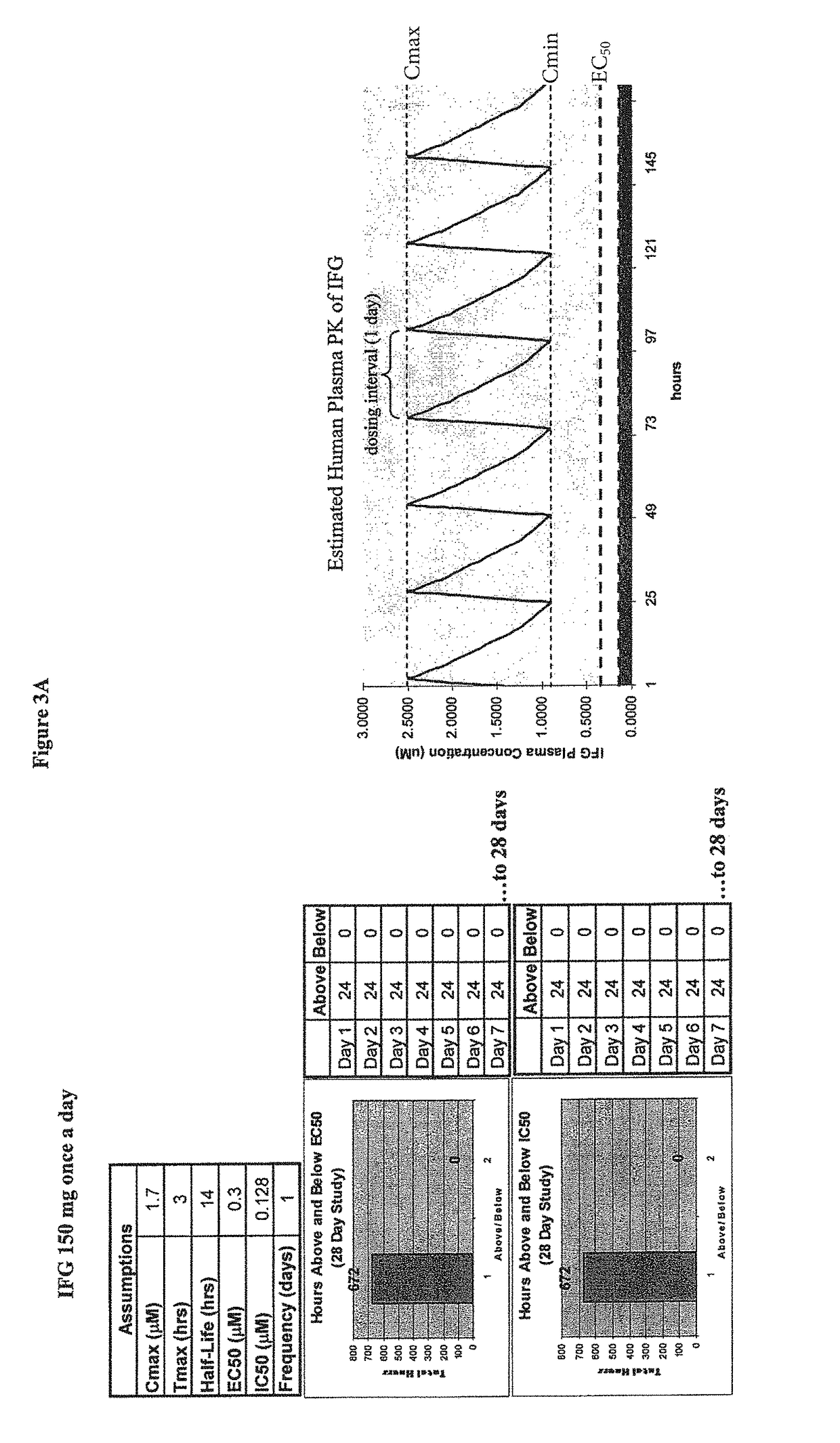
The present invention provides dosing regimens for administering pharmacological chaperones to a subject in need thereof. The dosing regimens can be used to treat disorders caused by improper protein misfolding, such as lysosomal storage disorders.
Owner:AMICUS THERAPEUTICS INC
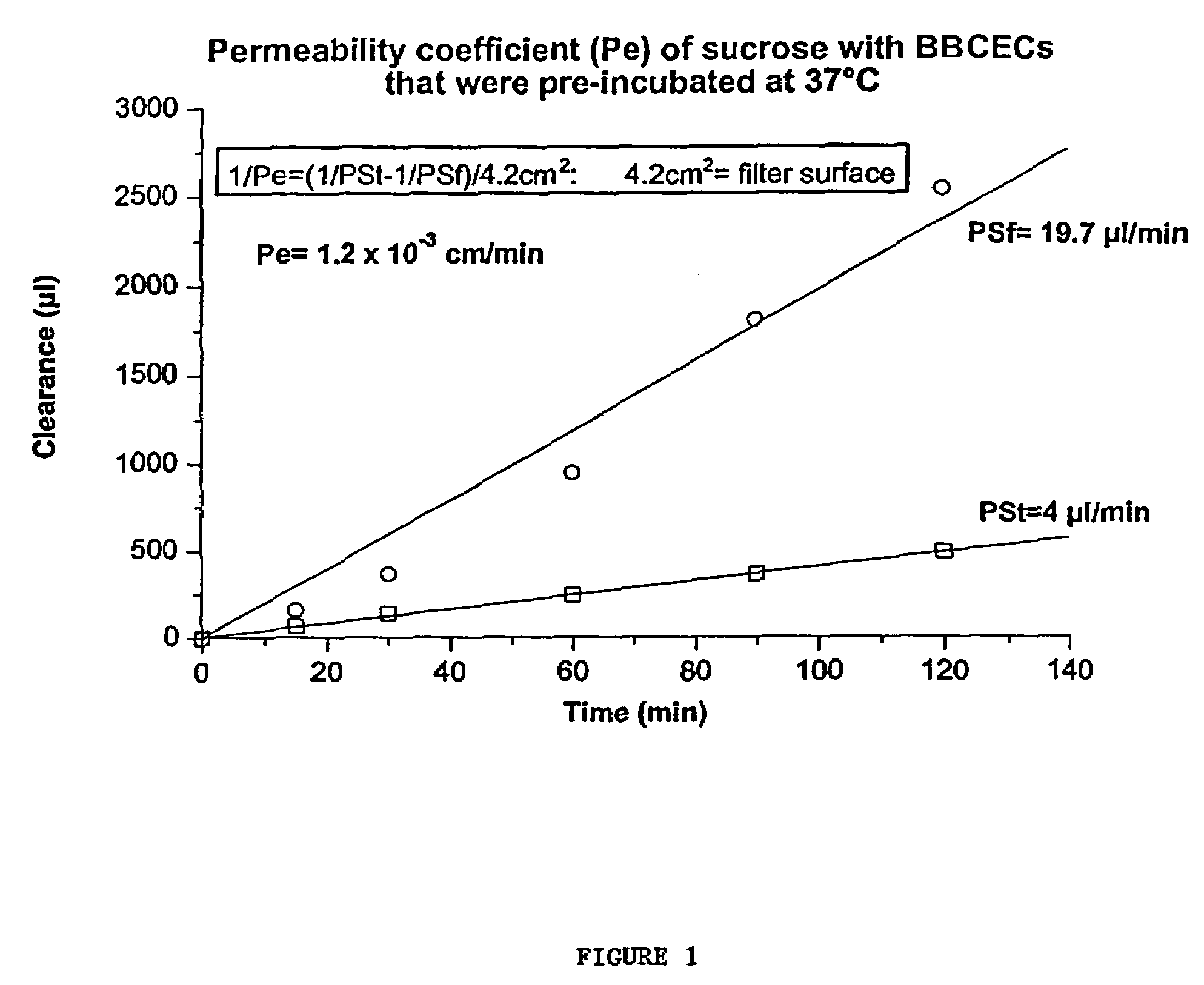
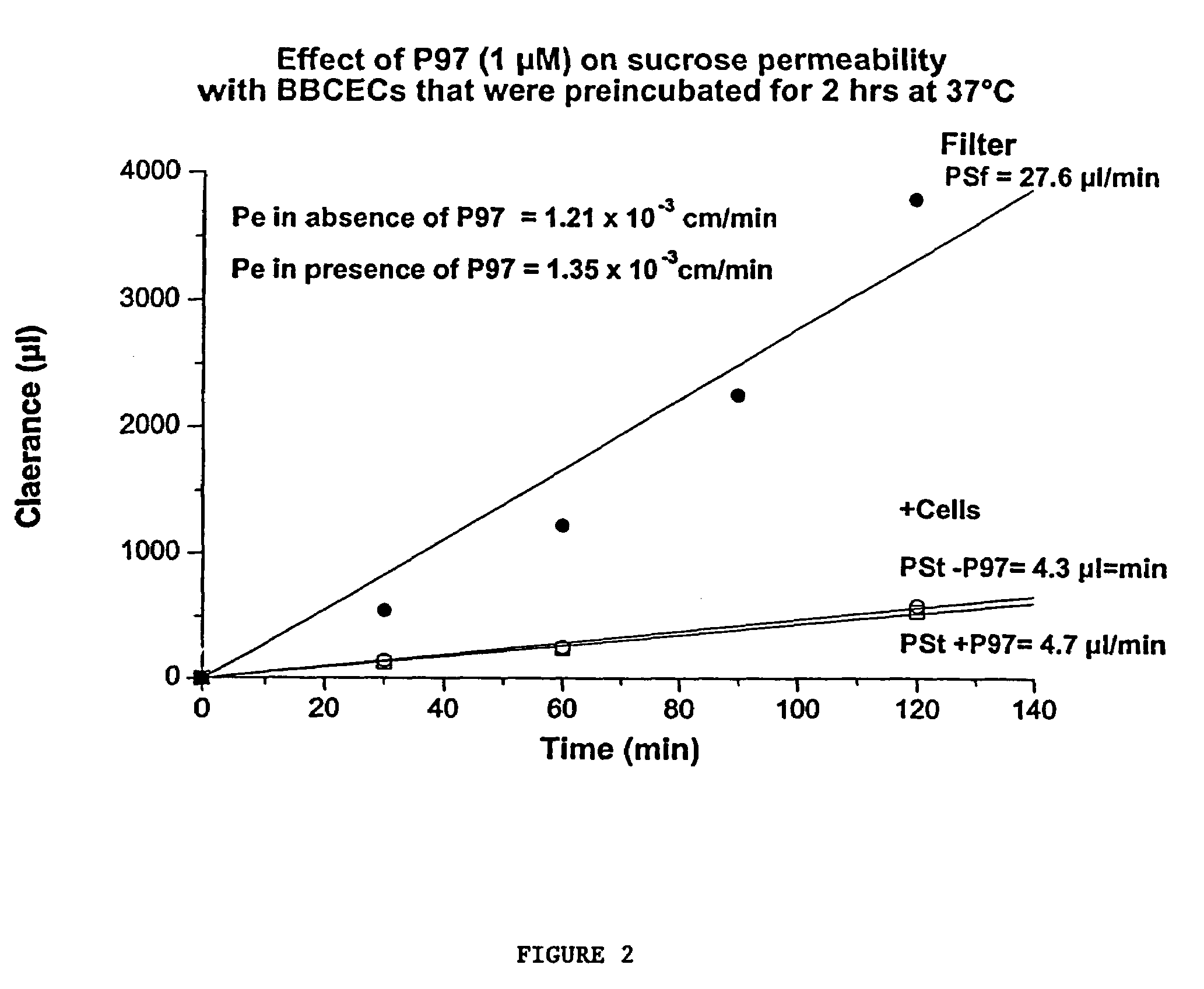
Intraventricular enzyme delivery for lysosomal storage diseases
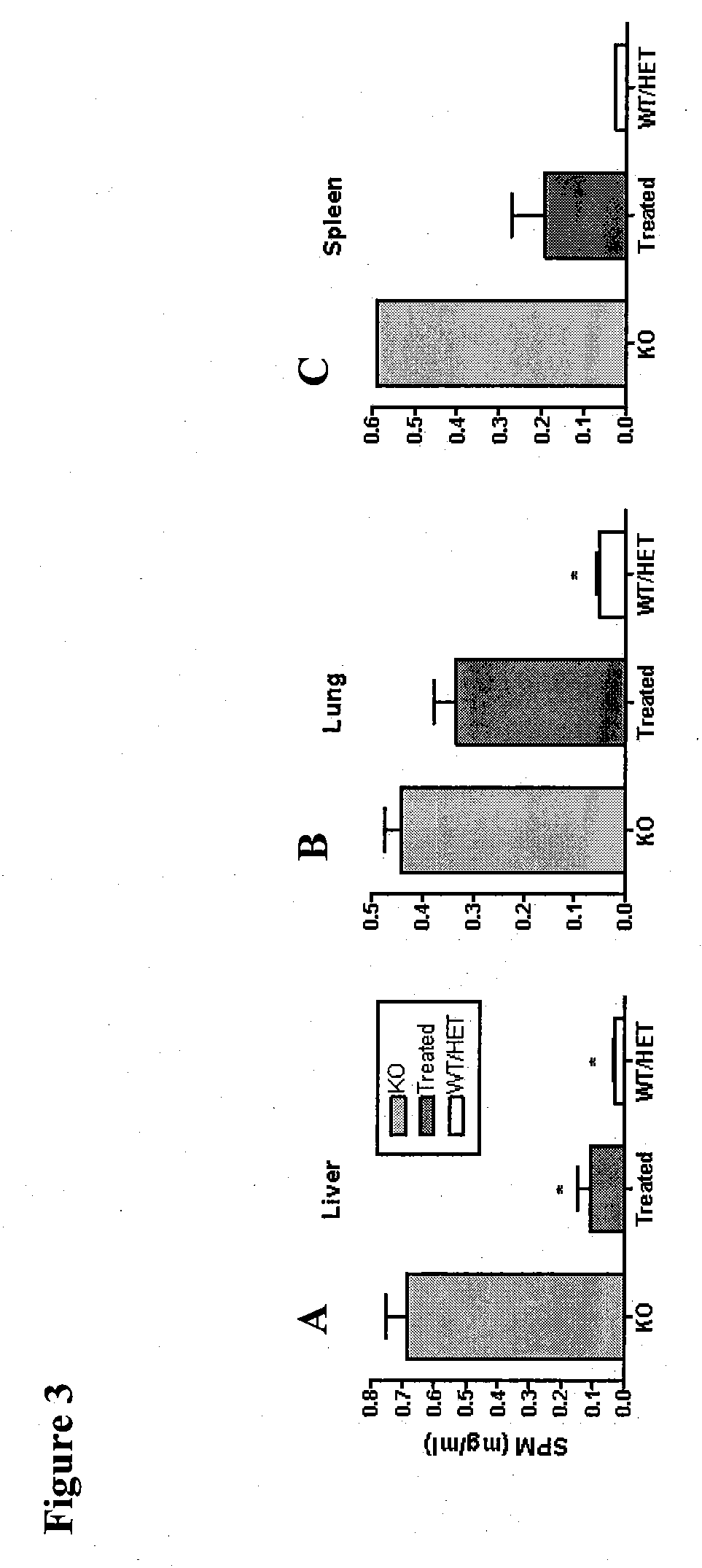
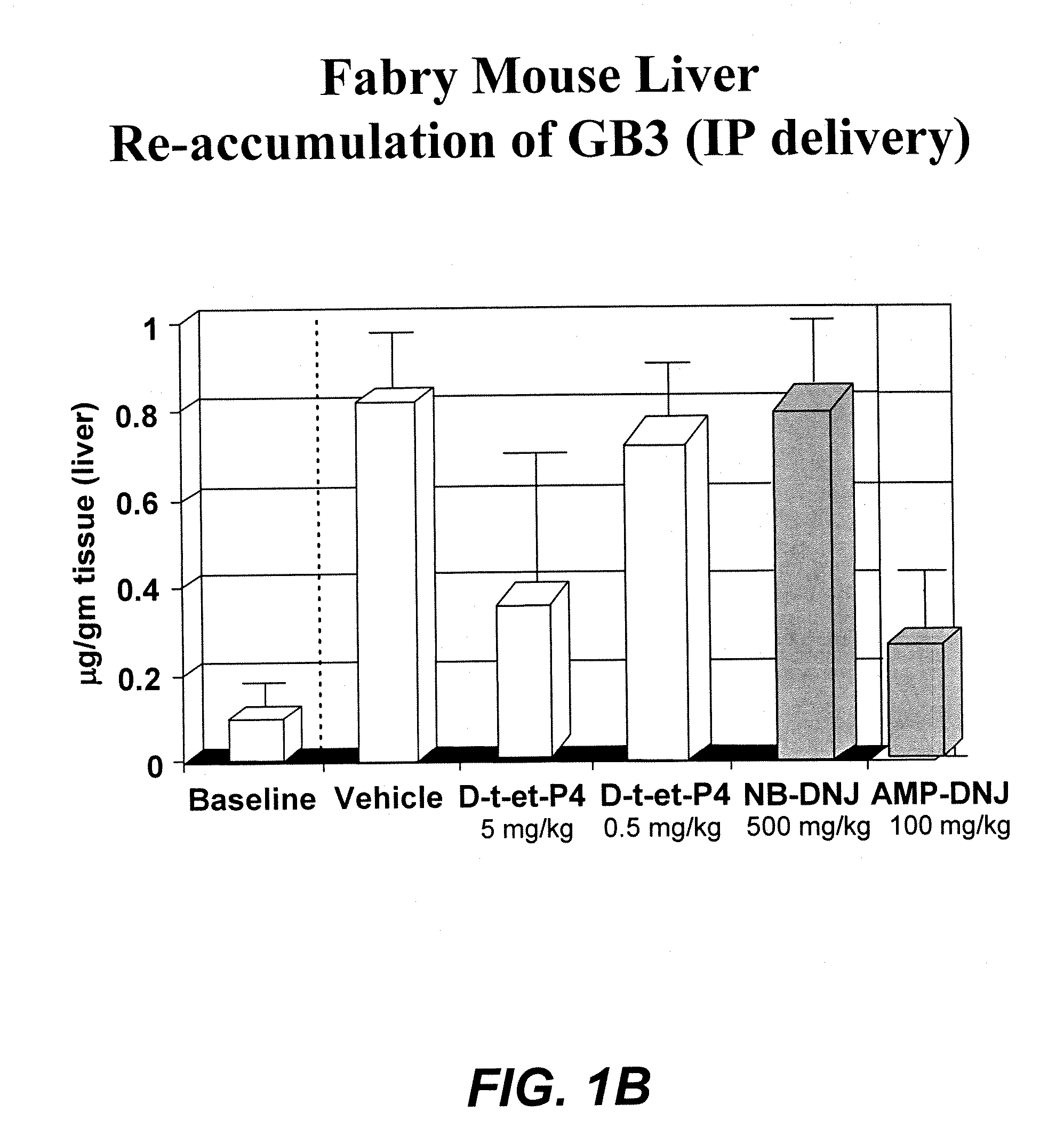
ActiveUS20090130079A1Reduction in substrate accumulated in the brain, lungs, spleen, kidney, and/or liver may be dramaticNervous disorderPeptide/protein ingredientsLysosomal enzyme defectVisceral organ
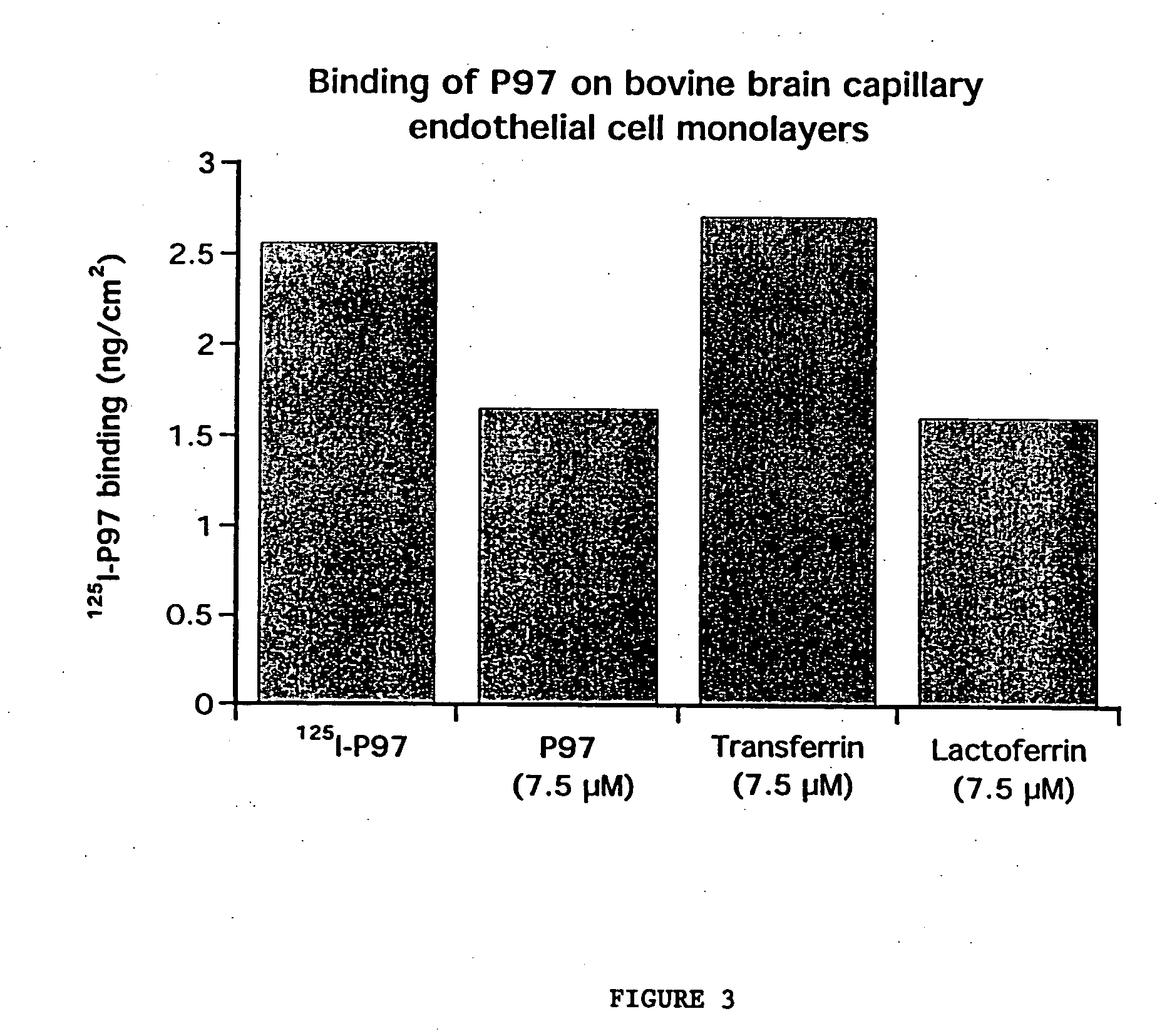
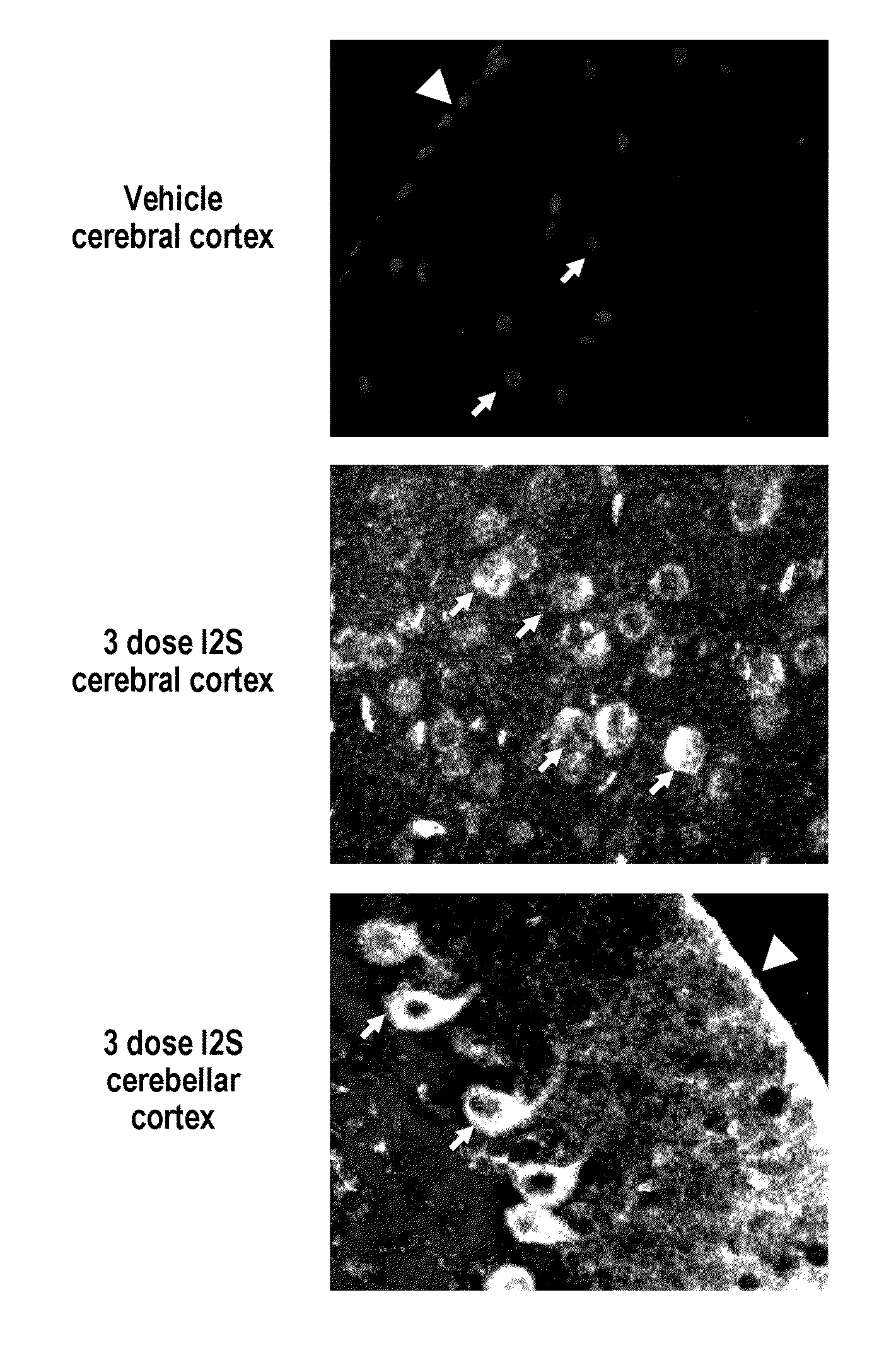
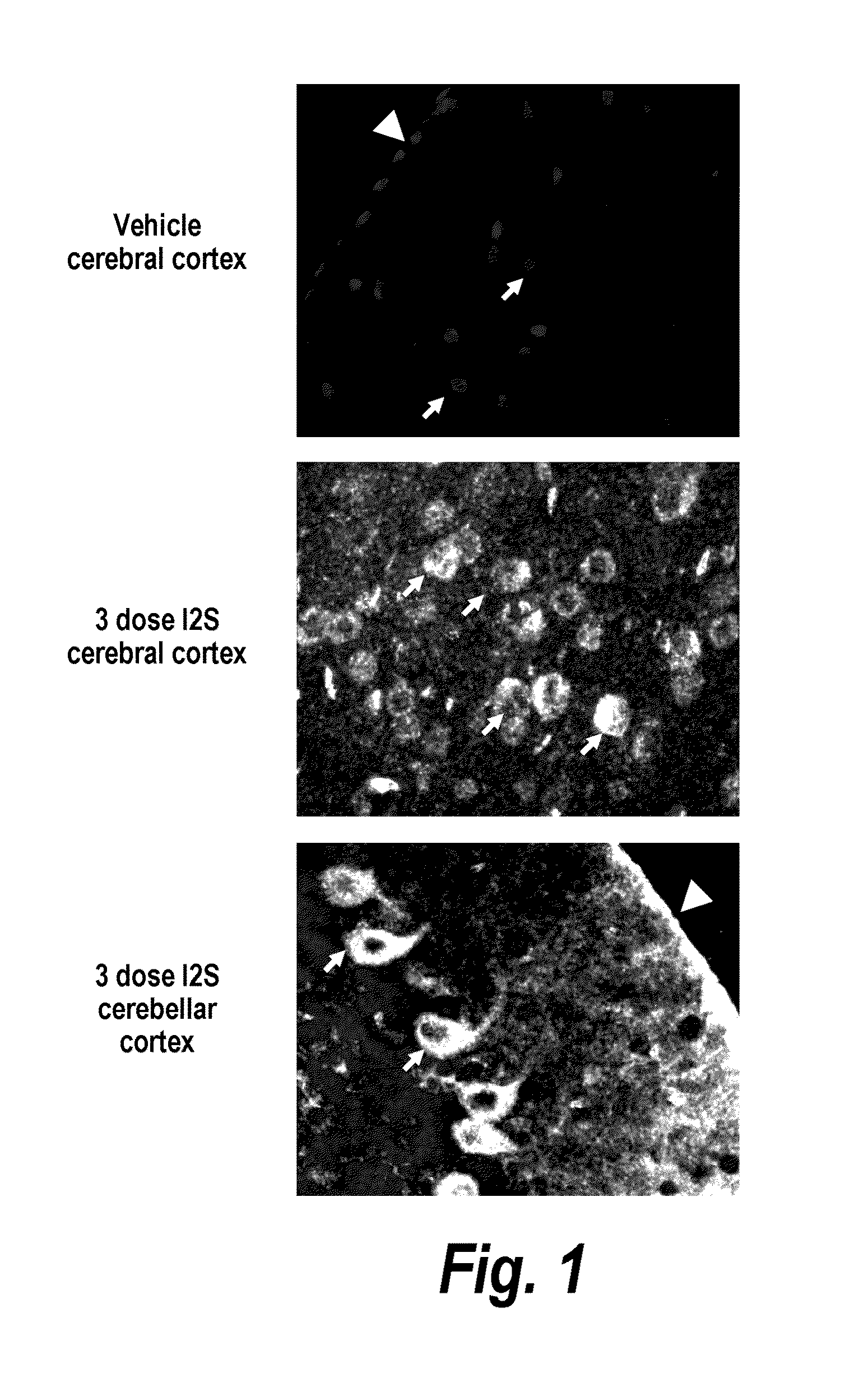
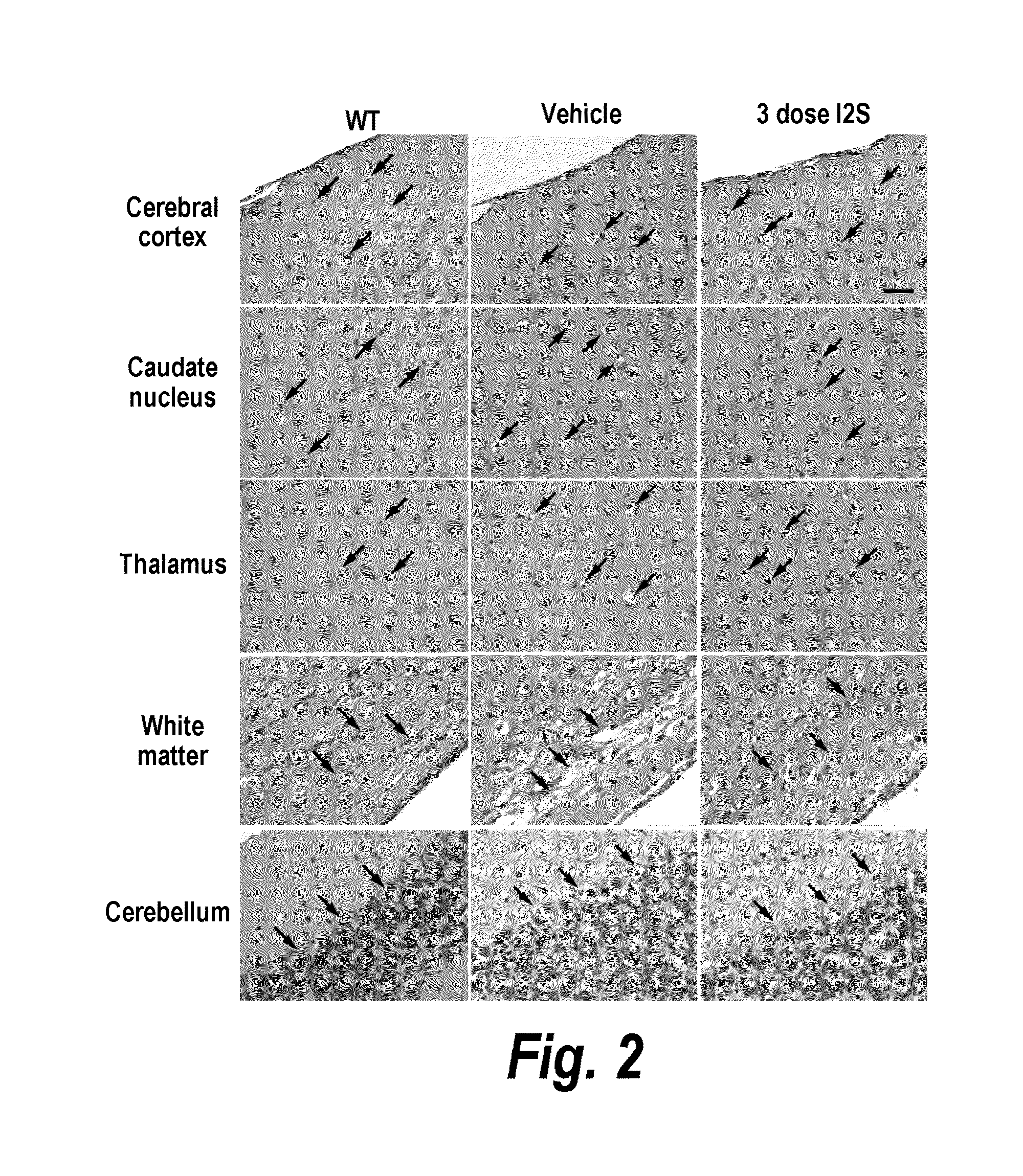
Lysosomal storage diseases can be successfully treated using intraventricular delivery of the enzyme which is etiologically deficient in the disease. The administration can be performed slowly to achieve maximum effect. Surprisingly, effects are seen on both sides of the blood-brain barrier, making this an ideal delivery means for lysosomal storage diseases which affect both brain and visceral organs.
Owner:GENZYME CORP
Combination enzyme replacement, gene therapy and small molecule therapy for lysosomal storage diseases
InactiveUS20070280925A1Benefit optimizationReduce disadvantagesBiocidePeptide/protein ingredientsLysosomal enzyme defectGene
This invention provides various combinations of enzyme replacement therapy, gene therapy, and small molecule therapy for the treatment of lysosomal storage diseases.
Owner:GENZYME CORP
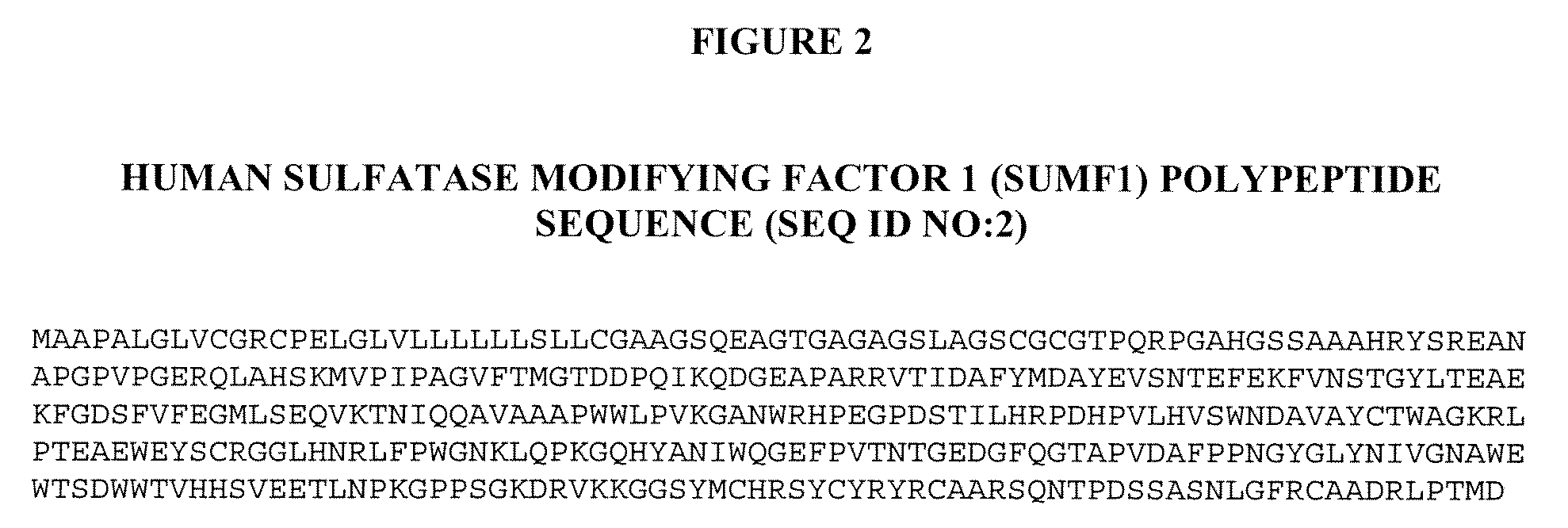
Manufacture of Active Highly Phosphorylated Human Lysosomal Sulfatase Enzymes and Uses Thereof
ActiveUS20090186011A1High yieldAvoid material lossCompound screeningNervous disorderLysosomePhosphorylation
This invention provides compositions of active highly phosphorylated lysosomal sulfatase enzymes, their pharmaceutical compositions, methods of producing and purifying such lysosomal sulfatase enzymes and compositions and their use in the diagnosis, prophylaxis, or treatment of diseases and conditions, including particularly lysosomal storage diseases that are caused by, or associated with, a deficiency in the lysosomal sulfatase enzyme.
Owner:BIOMARIN PHARMA INC
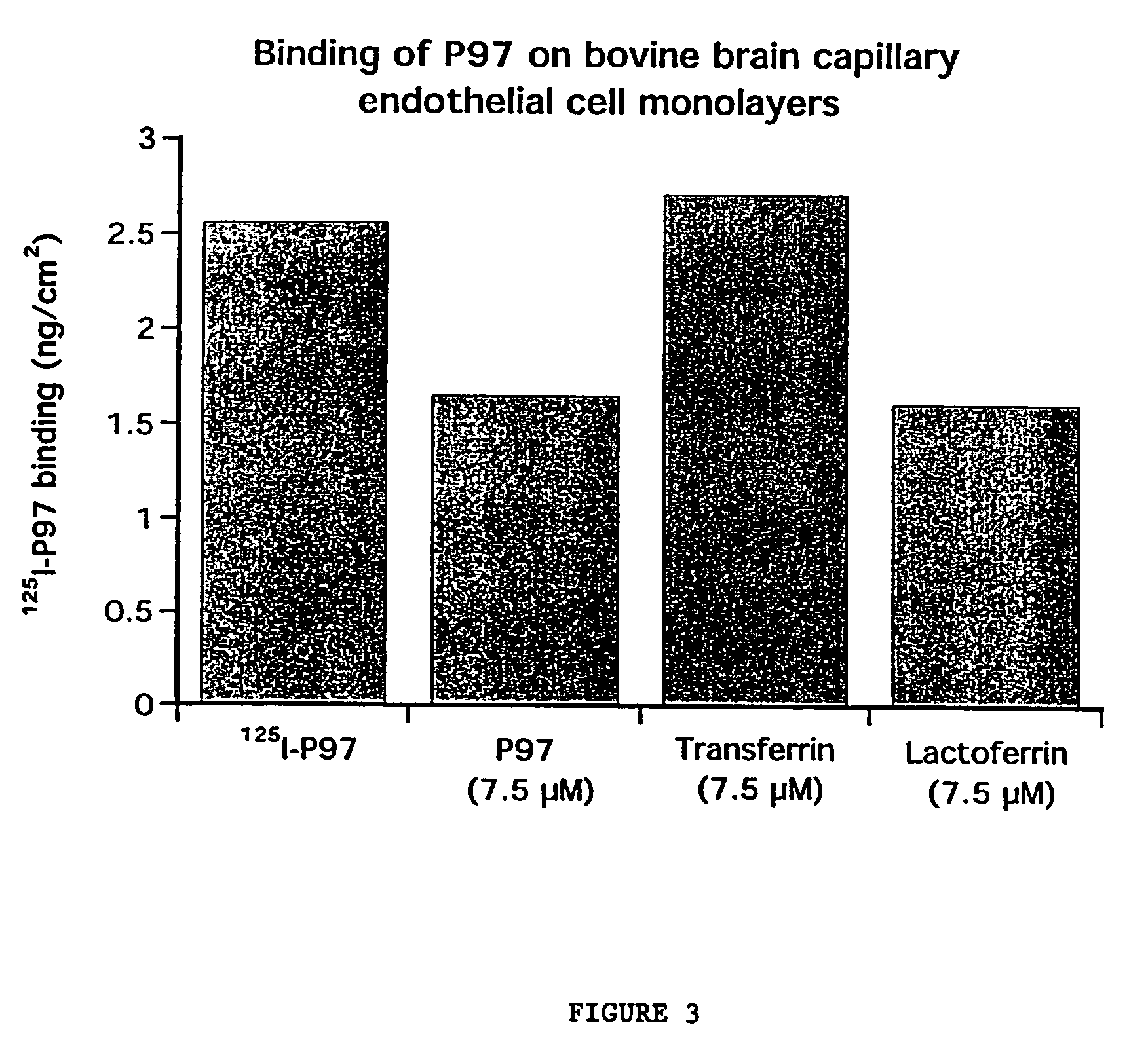
Compositions for modulating blood-brain barrier transport
InactiveUS7700554B2Reducing a neurological side-effectReceive treatment wellOrganic active ingredientsNervous disorderActive agentLysosome
This invention provides conjugates of therapeutic or active agents with melanotransferrin or with other ligands of a melanotransferrin receptor, melanotransferrin receptor modulators, and related compositions and methods for modulating blood-brain barrier transport by providing methods of screening and selecting such conjugates, ligands, and modulators in vitro and in vivo, and methods of use of such conjugates, modulators and ligands in diagnosis and the treatment of diseases, including particularly diseases of the central nervous system or lysosomal storage diseases.
Owner:HORIZON ORPHAN LLC
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS20110318323A1Effective and less approachEffectively and extensivelyNervous disorderHydrolasesIduronate-2-sulfataseHunter syndrome
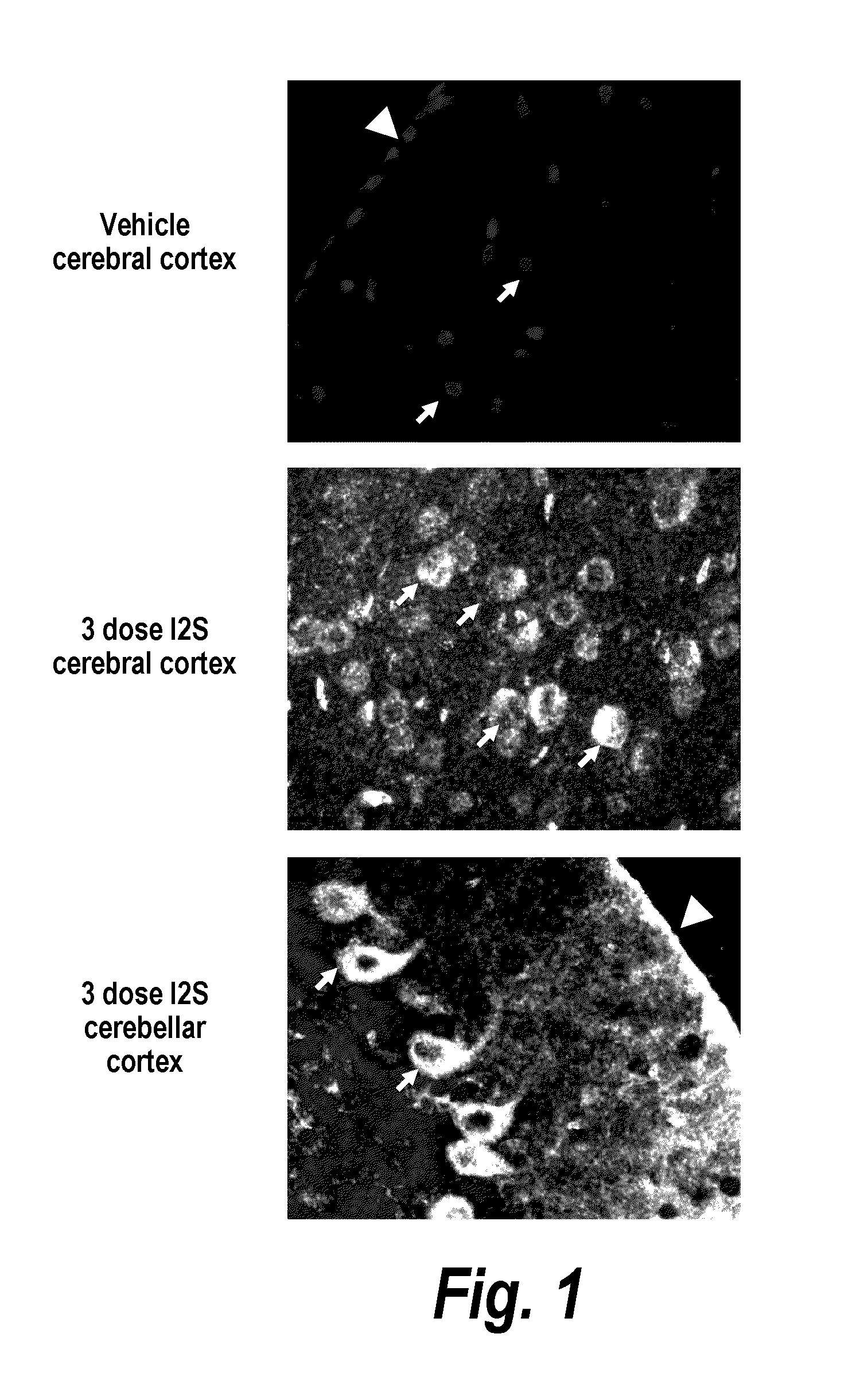
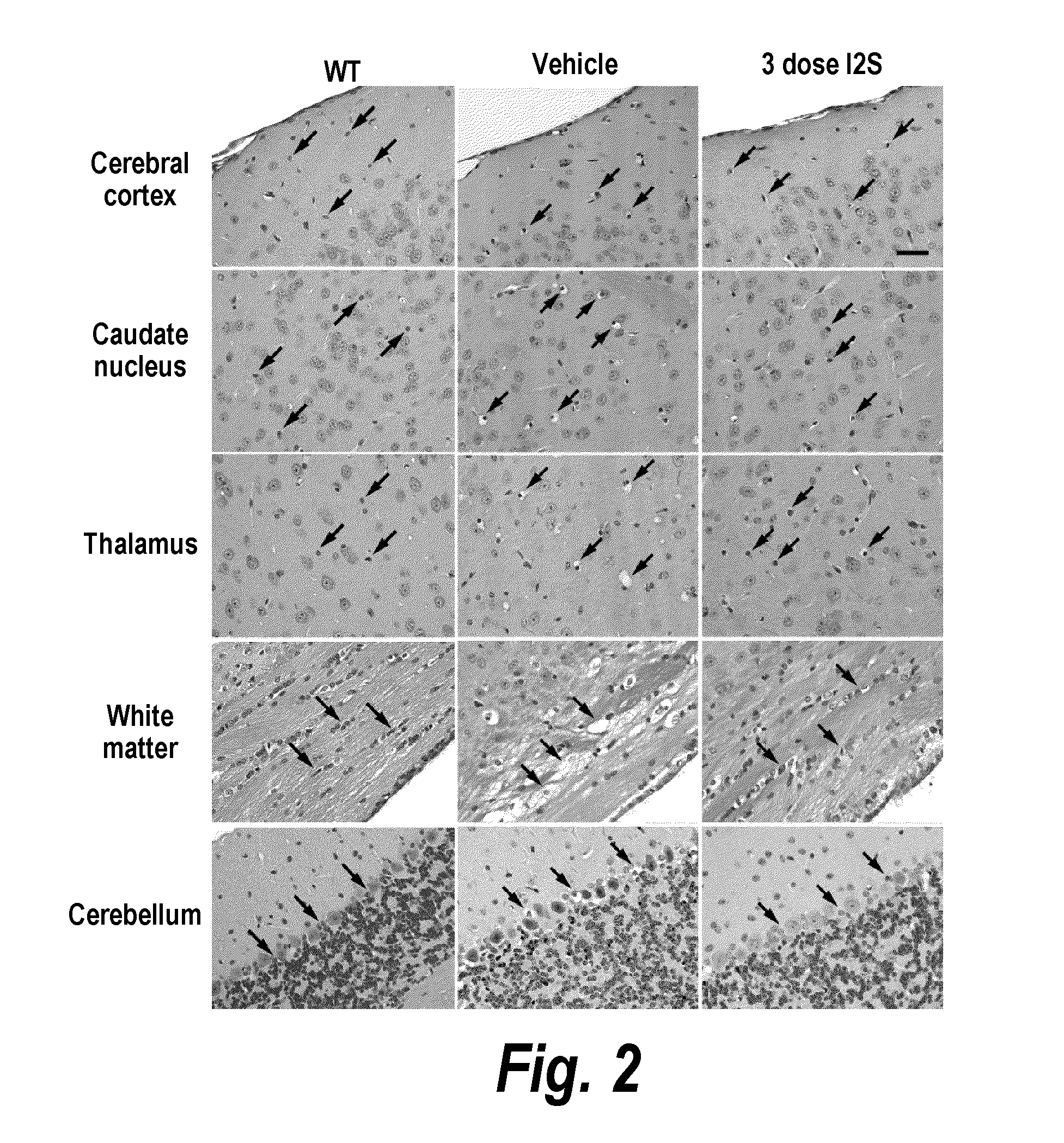
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones
The present invention provides dosing regimens for administering pharmacological chaperones to a subject in need thereof. The dosing regimens can be used to treat disorders caused by improper protein misfolding, such as lysosomal storage disorders.
Owner:AMICUS THERAPEUTICS INC
Methods of enhancing lysosomal storage disease therapy by modulation of cell surface receptor density
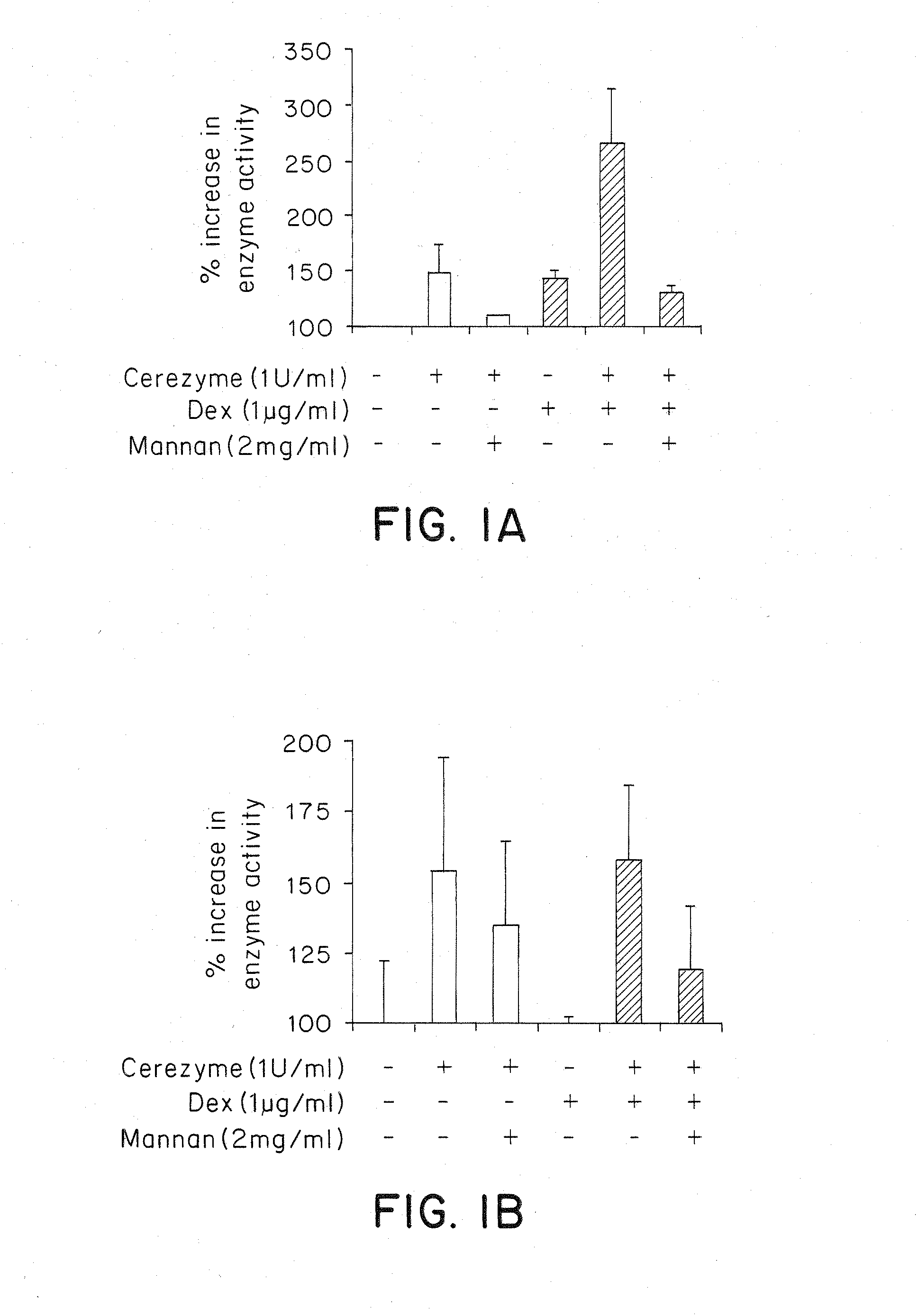
InactiveUS7658916B2Promote absorptionUptake of extracellular lysosomal enzymes by cells can be increasedBiocidePeptide/protein ingredientsLysosomeFabry disease
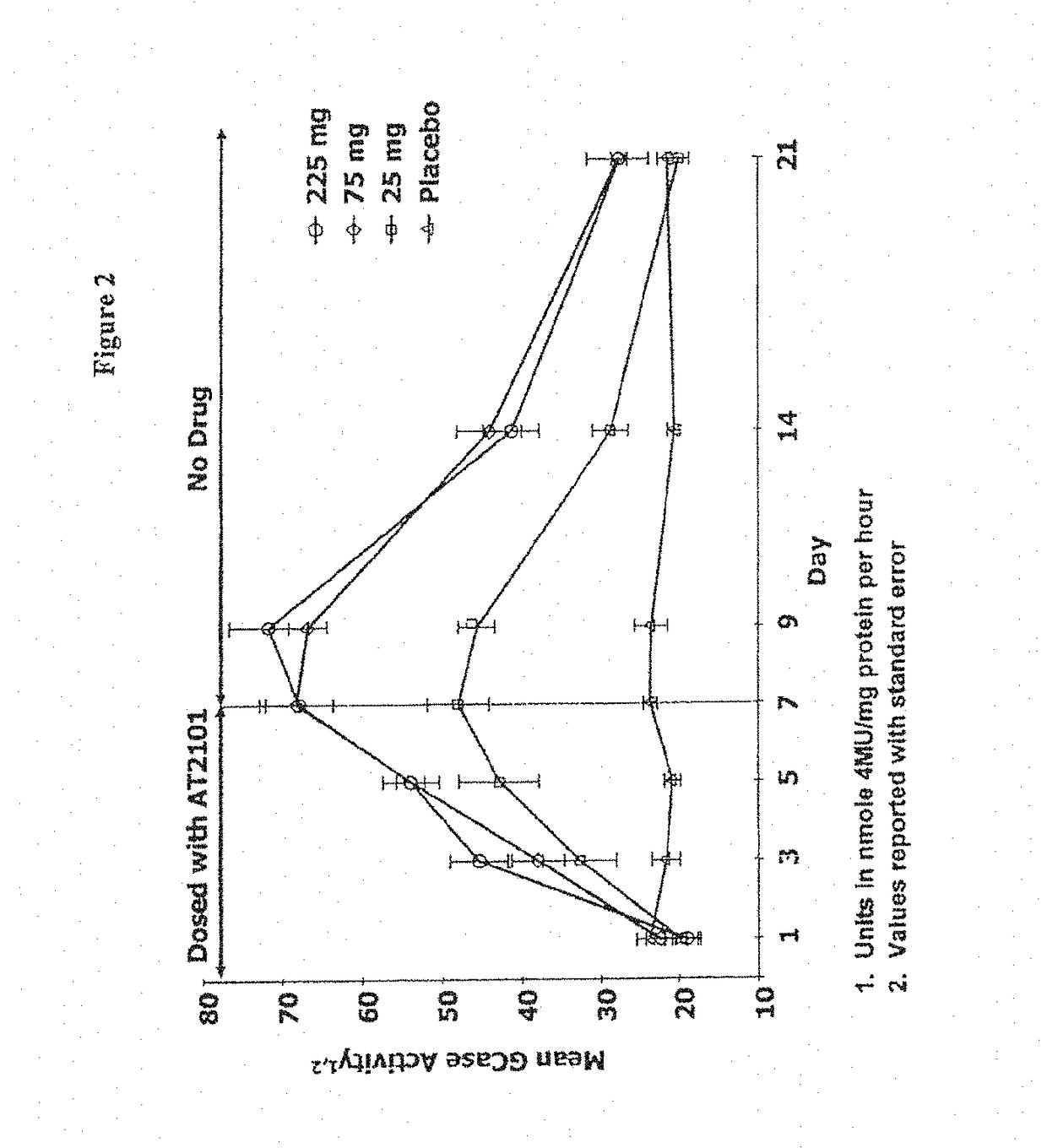
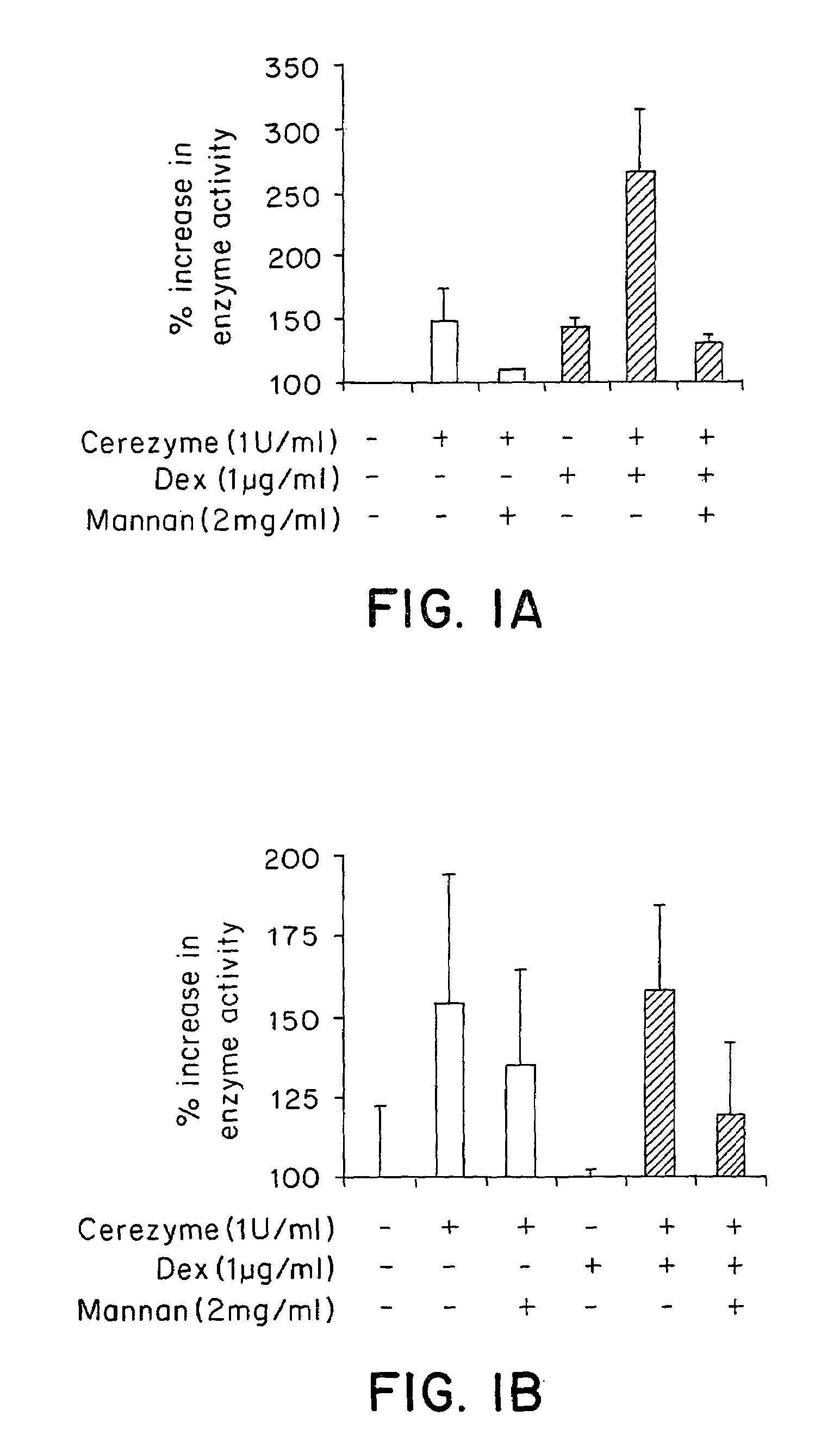
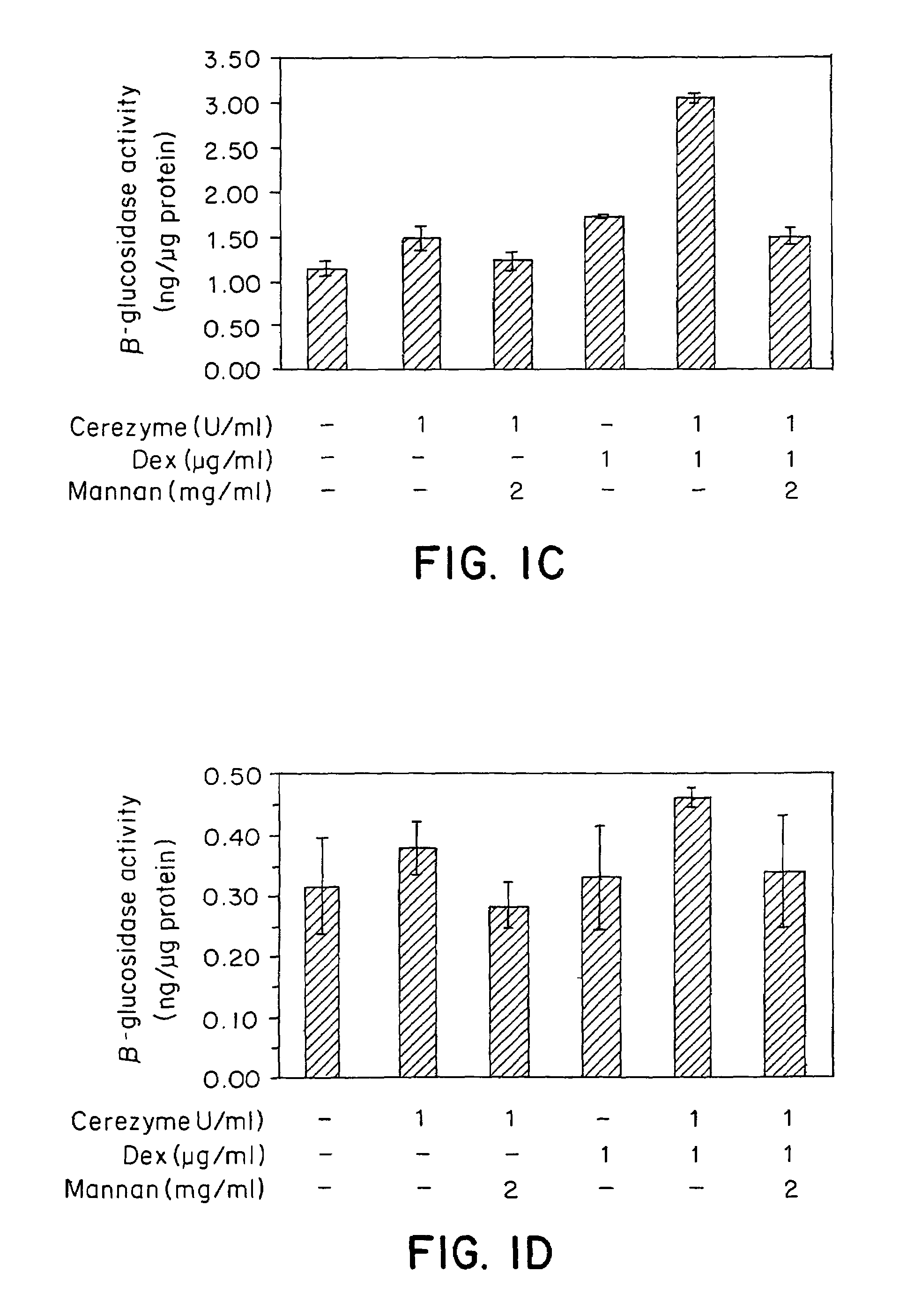
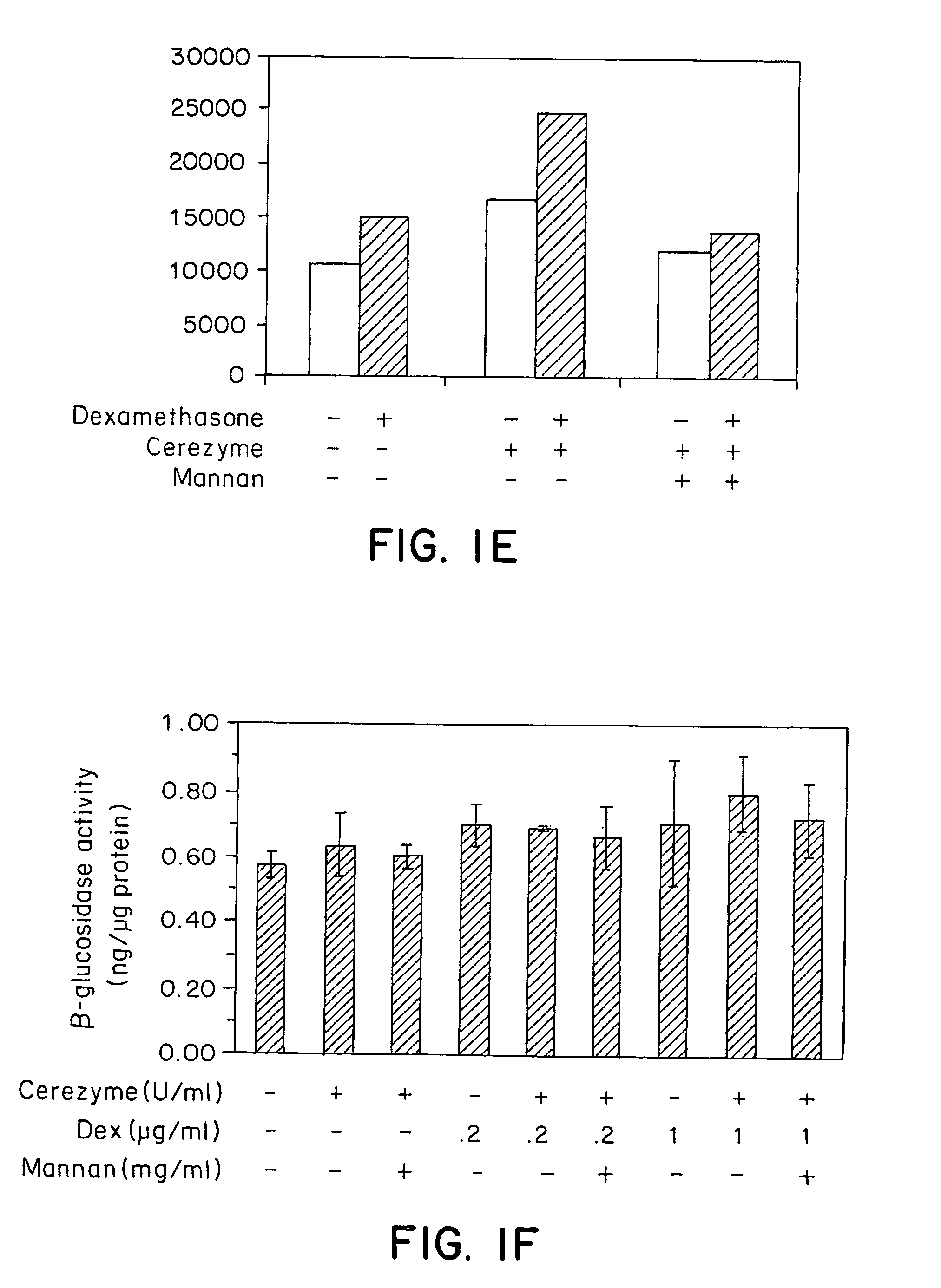
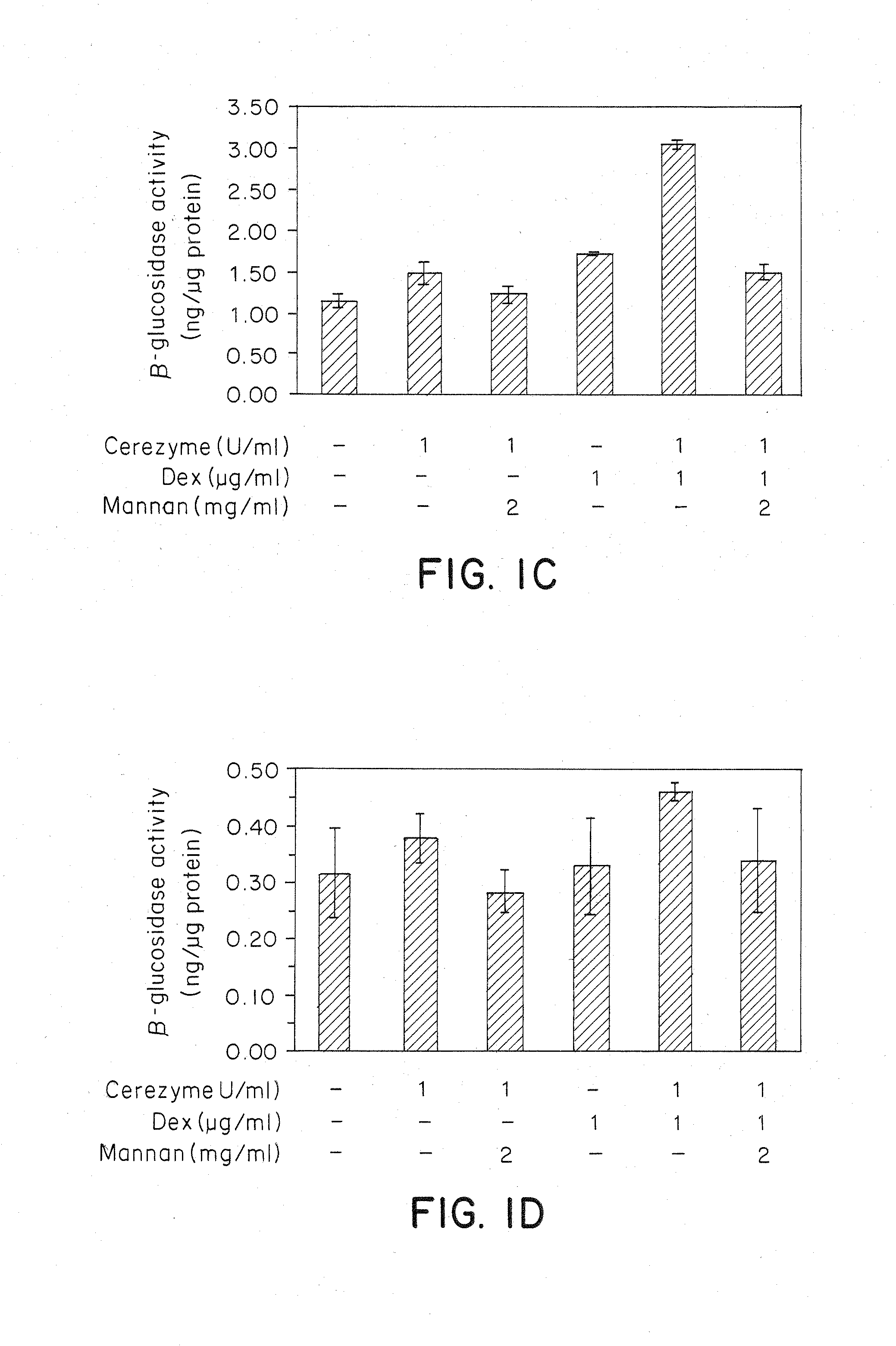
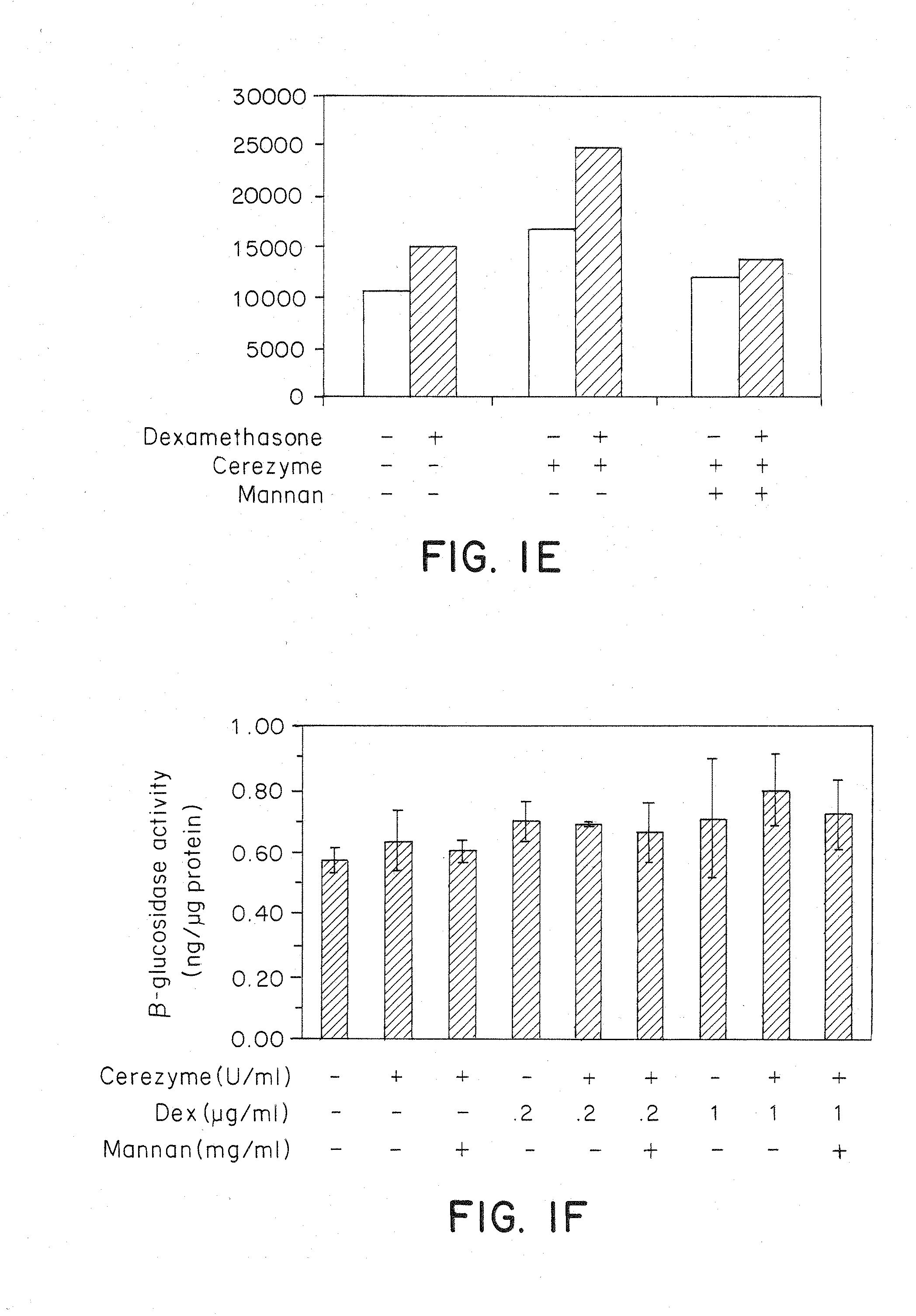
Methods of modulating uptake of extracellular lysosomal enzymes by administering a pharmaceutical agent and methods of treating a lysosomal storage disease (such as Gaucher disease, Pompe disease, Fabry disease or Niemann-Pick disease) or enhancing enzyme replacement therapy or gene therapy, comprising administering a pharmaceutical agent such as dexamethasone, glucose or insulin, are provided.
Owner:GENZYME CORP
Dosing Regimens for the Treatment of Lysosomal Storage Diseases Using Pharmacological Chaperones
The present invention provides dosing regimens for administering pharmacological chaperones to a subject in need thereof. The dosing regimens can be used to treat disorders caused by improper protein misfolding, such as lysosomal storage disorders.
Owner:BPCR LLP
Modified enzyme and treatment method
InactiveUS20090041741A1Extended half-lifeConvenient treatmentPeptide/protein ingredientsTransferasesLysosomeCompound (substance)
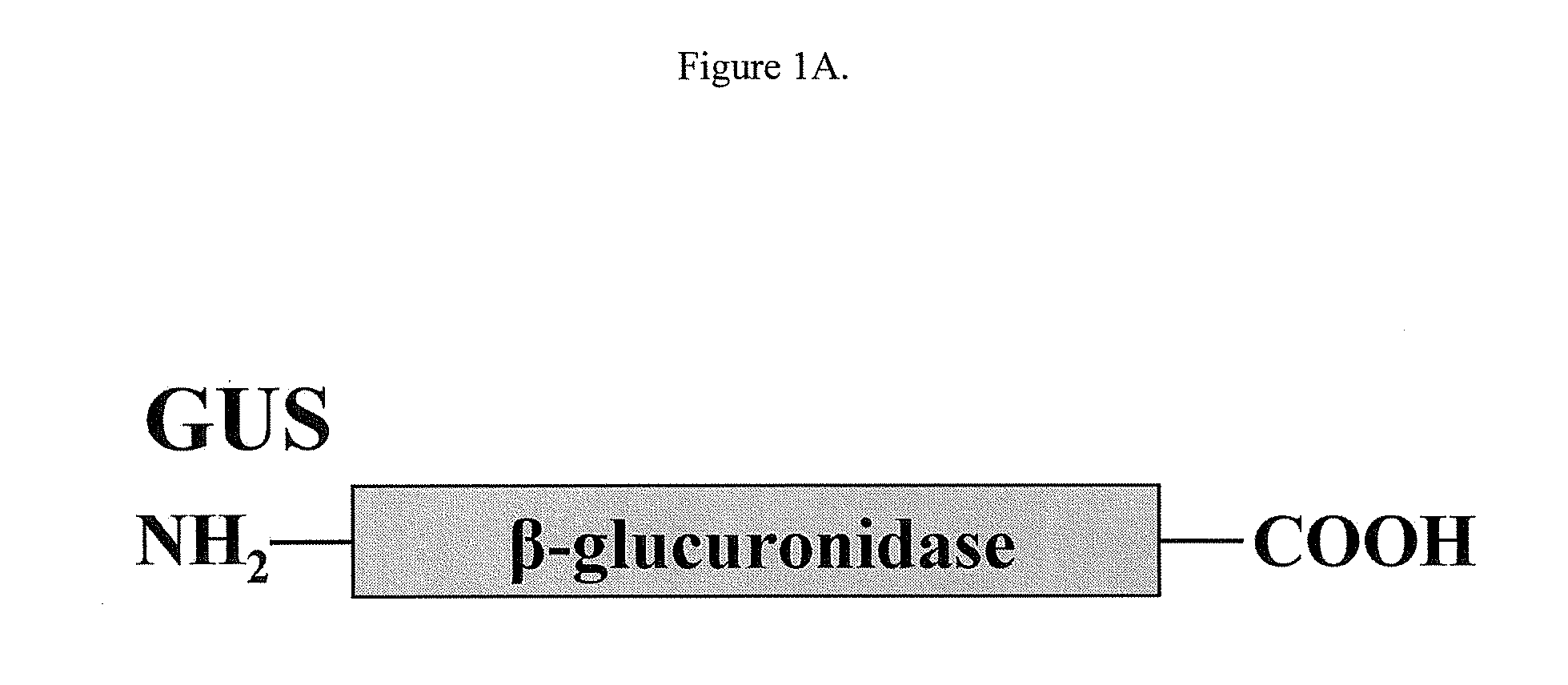
There is disclosed an isolated, modified recombinant β-glucuronidase wherein the modification is having its carbohydrate moeties chemically modified so as to reduce its activity with respect to mannose and mannose 6-phosphate cellular delivery system while retaining enzymatic activity Also disclosed are methods for the treatment of lysosomal storage disease in mammals wherein the mammal is administered a therapeutically effective amount of isolated, modified recombinant β-glucuronidase whereby said storage diseased is relieved in the brain and visceral organs of the mammal. Also disclosed are other lysosomal enzymes within the scope of the invention.
Owner:SAINT LOUIS UNIVERSITY
Compositions and methods for modulating blood-brain barrier transport
InactiveUS20070167365A1Receive treatment wellIncrease the amount addedNervous disorderPeptide/protein ingredientsActive agentLysosomal enzyme defect
This invention provides conjugates of therapeutic or active agents with melanotransferrin or with other ligands of a melanotransferrin receptor, melanotransferrin receptor modulators, and related compositions and methods for modulating blood-brain barrier transport by, providing methods of screening and selecting such conjugates, ligands, and modulators in vitro and in vivo, and methods of use of such conjugates, modulators and ligands in diagnosis and the treatment of diseases, including particularly diseases of the central nervous system or lysosomal storage diseases.
Owner:HORIZON ORPHAN LLC
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS8545837B2Effective and less invasiveEffectively and extensivelyNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseHunter syndrome
Owner:TAKEDA PHARMA CO LTD
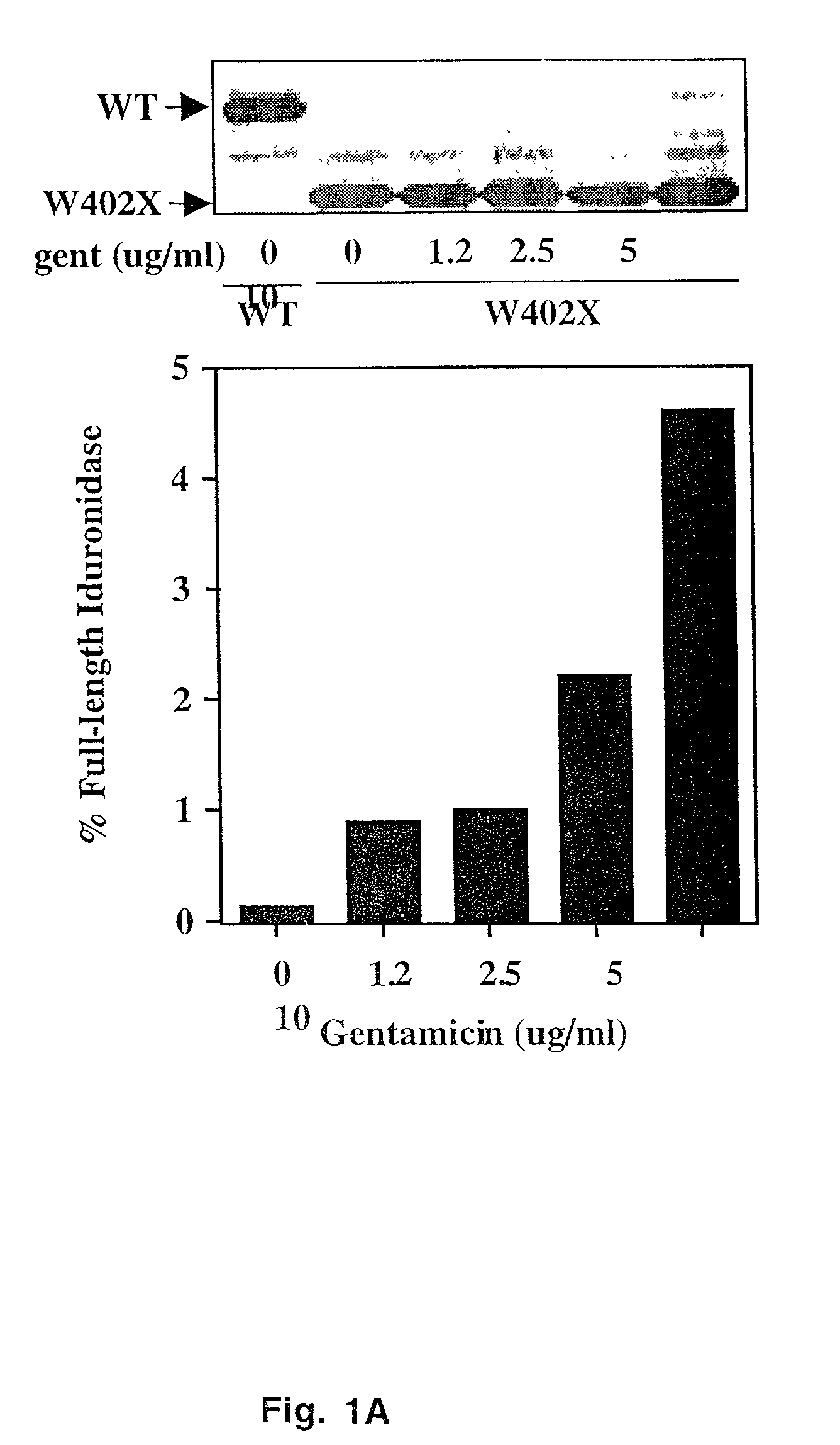
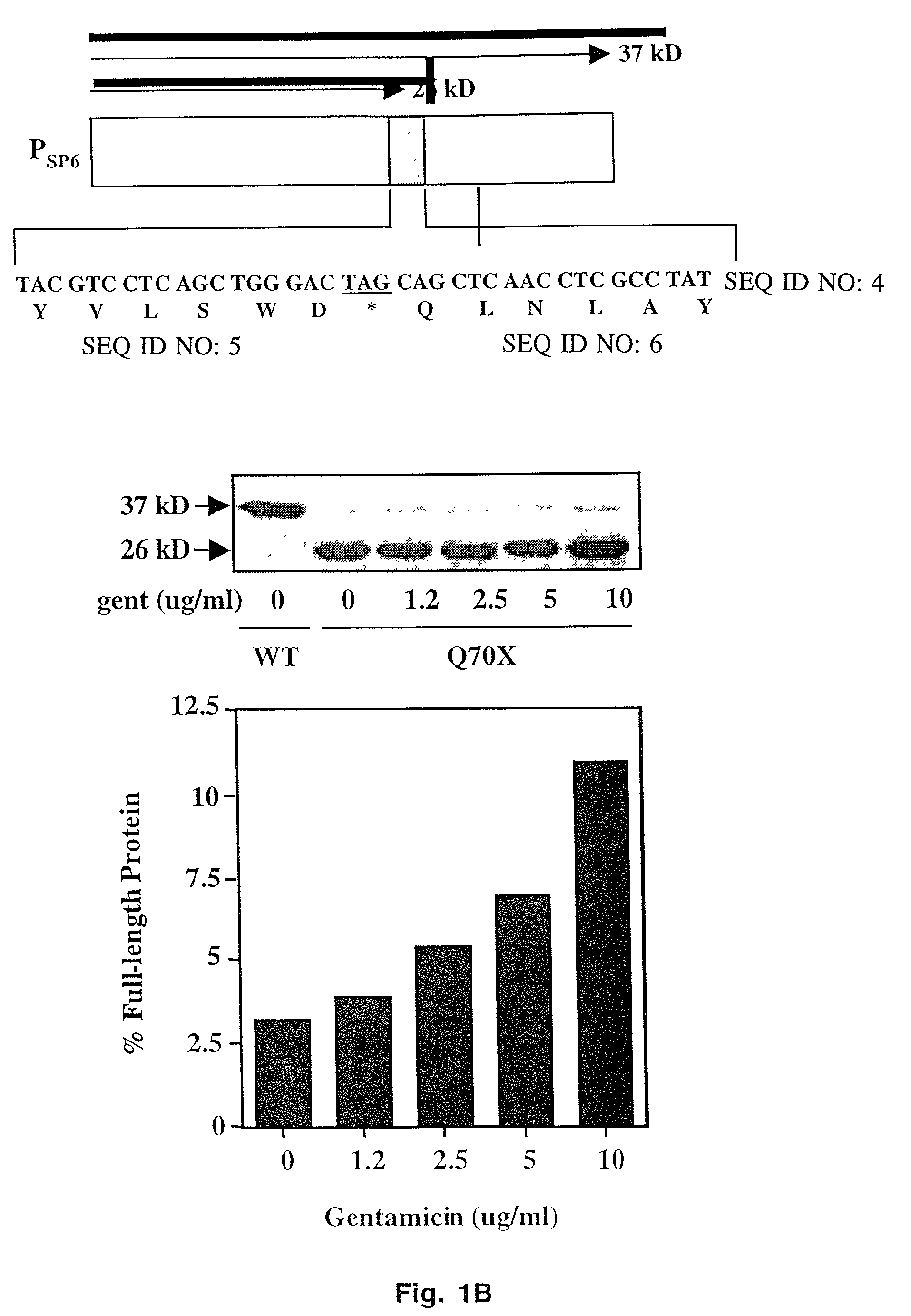
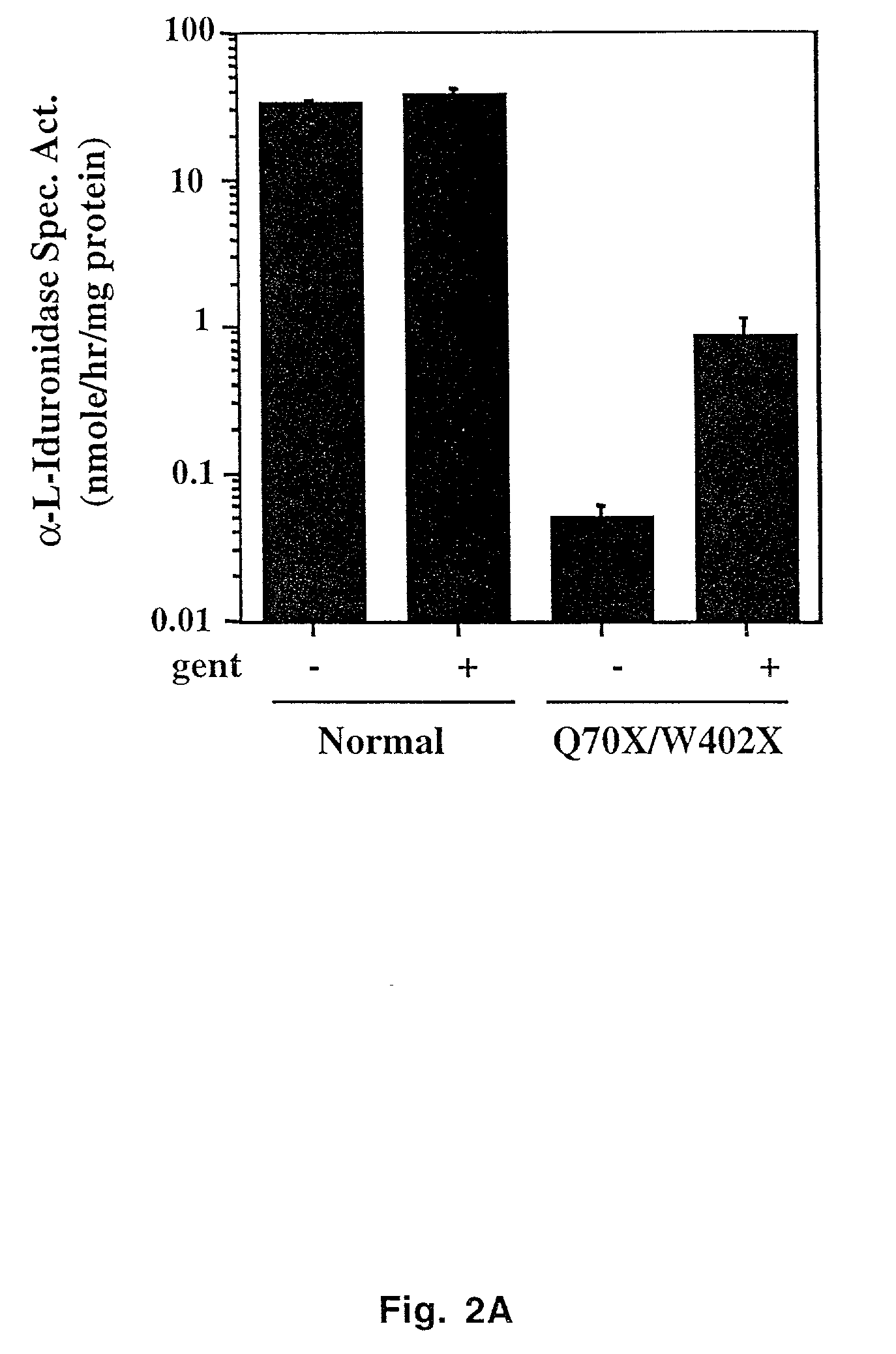
Aminoglycoside treatment for lysosomal storage diseases
ActiveUS20050153906A1Effective treatmentHigh activityBiocideSugar derivativesLysosomal enzyme defectHurler syndrome
The present invention provides a method of treating lysosomal storage diseases such as Hurler syndrome and Batten disease in individuals in need of such treatment, comprising the step of administering to said individuals a therapeutically effective dose of an aminoglycoside. In addition, this method may further comprise treating the individual with enzyme replacement therapy. Furthermore, the present invention provides method of pharmacologically suppressing premature stop mutations in an individual with these lysosomal storage diseases, comprising the step of administering to said individual a pharmacologically effective dose of an aminoglycoside.
Owner:UAB RES FOUND
Manufacture of active highly phosphorylated human lysosomal sulfatase enzymes and uses thereof
ActiveUS8128925B2High yieldAvoid material lossCompound screeningNervous disorderPhosphorylationLysosome
This invention provides compositions of active highly phosphorylated lysosomal sulfatase enzymes, their pharmaceutical compositions, methods of producing and purifying such lysosomal sulfatase enzymes and compositions and their use in the diagnosis, prophylaxis, or treatment of diseases and conditions, including particularly lysosomal storage diseases that are caused by, or associated with, a deficiency in the lysosomal sulfatase enzyme.
Owner:BIOMARIN PHARMA INC
Methods and compositions for CNS delivery of heparan n-sulfatase
ActiveUS20120014936A1Effective and less approachReduce deliverySenses disorderNervous disorderSanfilippo syndrome type aLysosomal enzyme defect
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising a heparan N-sulfatase (HNS) protein, salt, and a polysorbate surfactant for the treatment of Sanfilippo Syndrome Type A.
Owner:TAKEDA PHARMA CO LTD
Manufacture of Highly Phosphorylated Lysosomal Enzymes and Uses Thereof
InactiveUS20090191178A1High yieldAvoid material lossAnimal cellsNervous disorderLysosomePhosphorylation
Owner:BIOMARIN PHARMA INC
Methods for increasing intracellular activity of Hsp70
ActiveUS9662375B2Reduce the amount of solutionFacilitate the uptake of enzymesBiocidePeptide/protein ingredientsGlycoprotein metabolismLysosomal enzyme defect
Owner:KEMPHARM DENMARK AS
Biomarkers for niemann-pick C disease and related disorders
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Manufacture of Highly Phosphorylated Lysosomal Enzymes and Uses Thereof
InactiveUS20080014188A1High yieldLow levelNervous disorderPeptide/protein ingredientsPhosphorylationLysosome
Owner:BIOMARIN PHARMA INC
Autophagy and phospholipidosis pathway assays
Owner:ENZO BIOCHEM
Methods and compositions for targeting proteins across the blood brain barrier
InactiveUS20060121018A1Polypeptide with localisation/targeting motifNervous disorderProtein targetTherapeutic protein
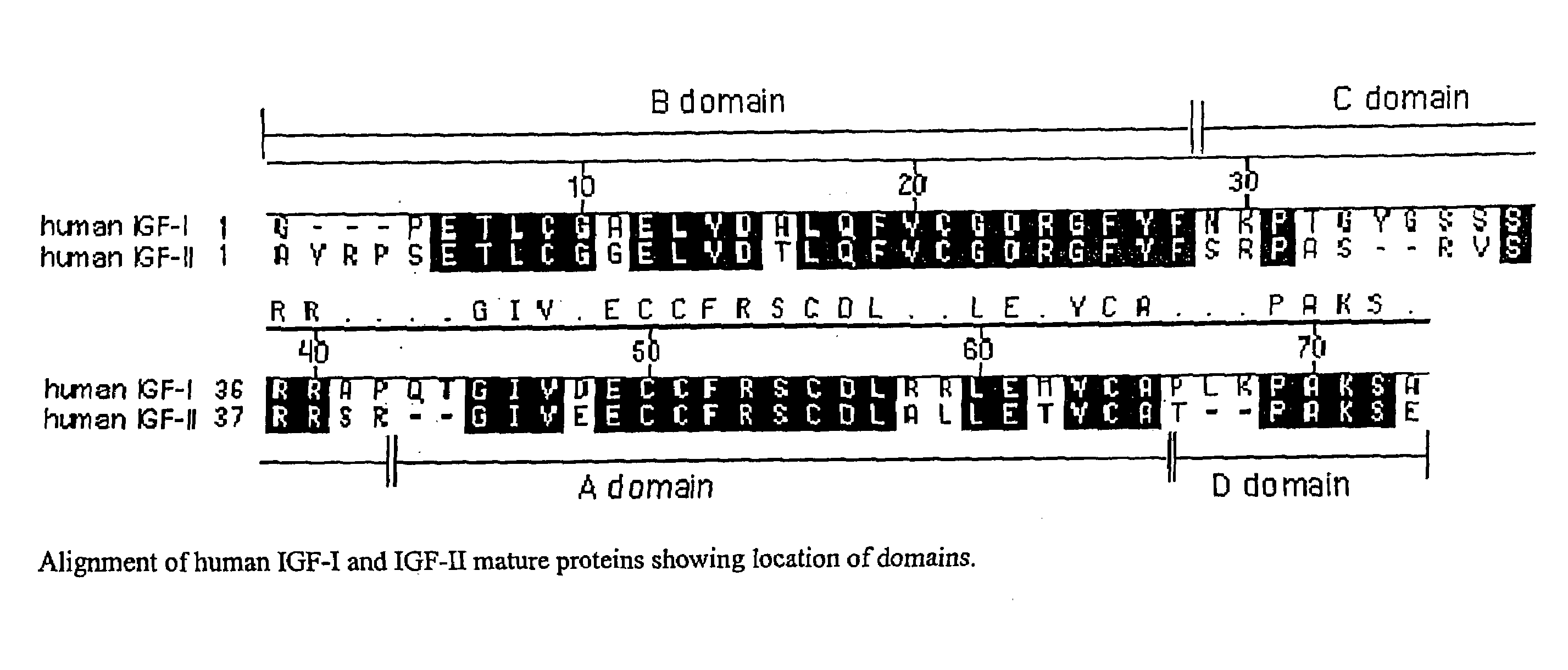
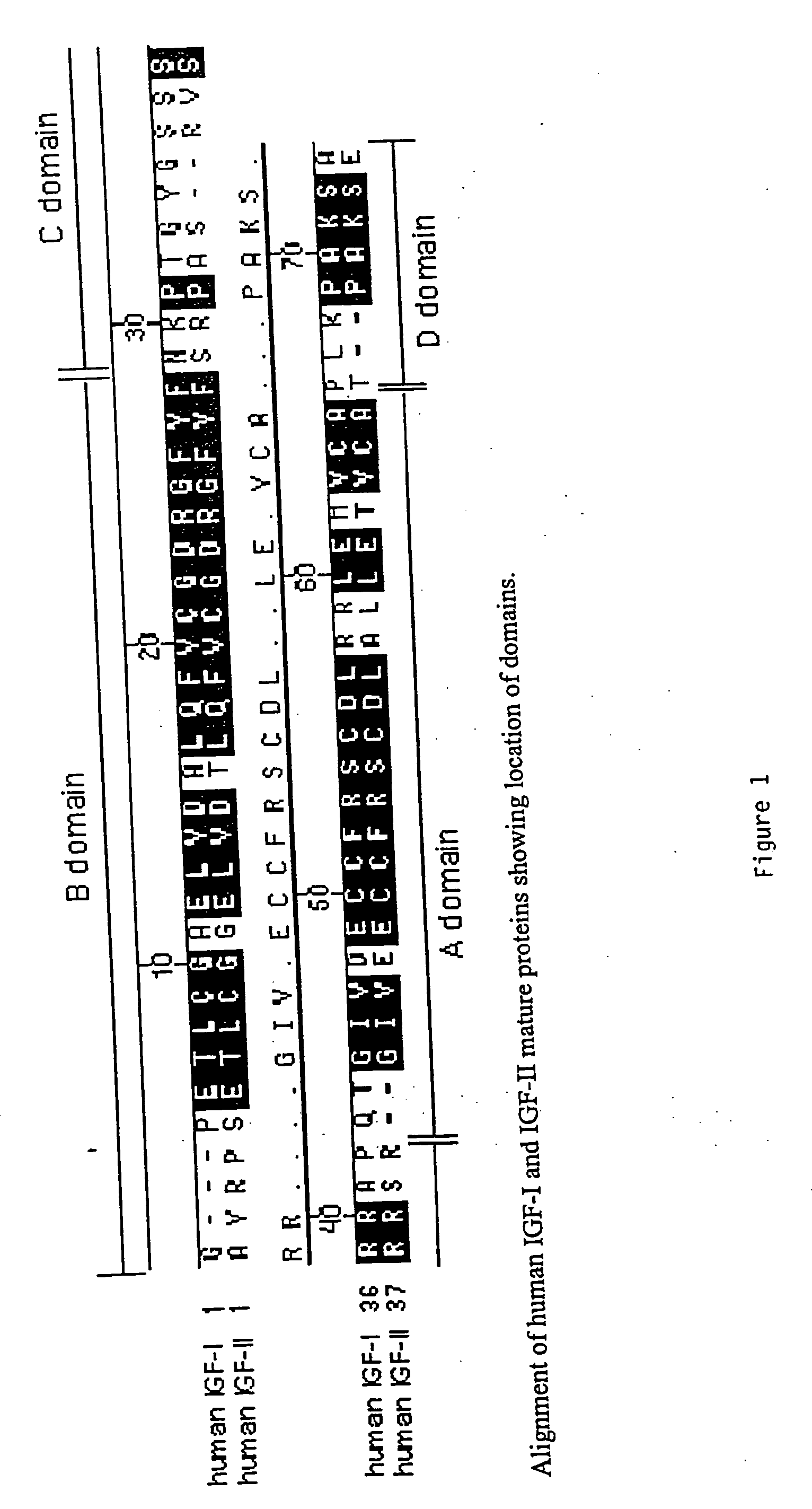
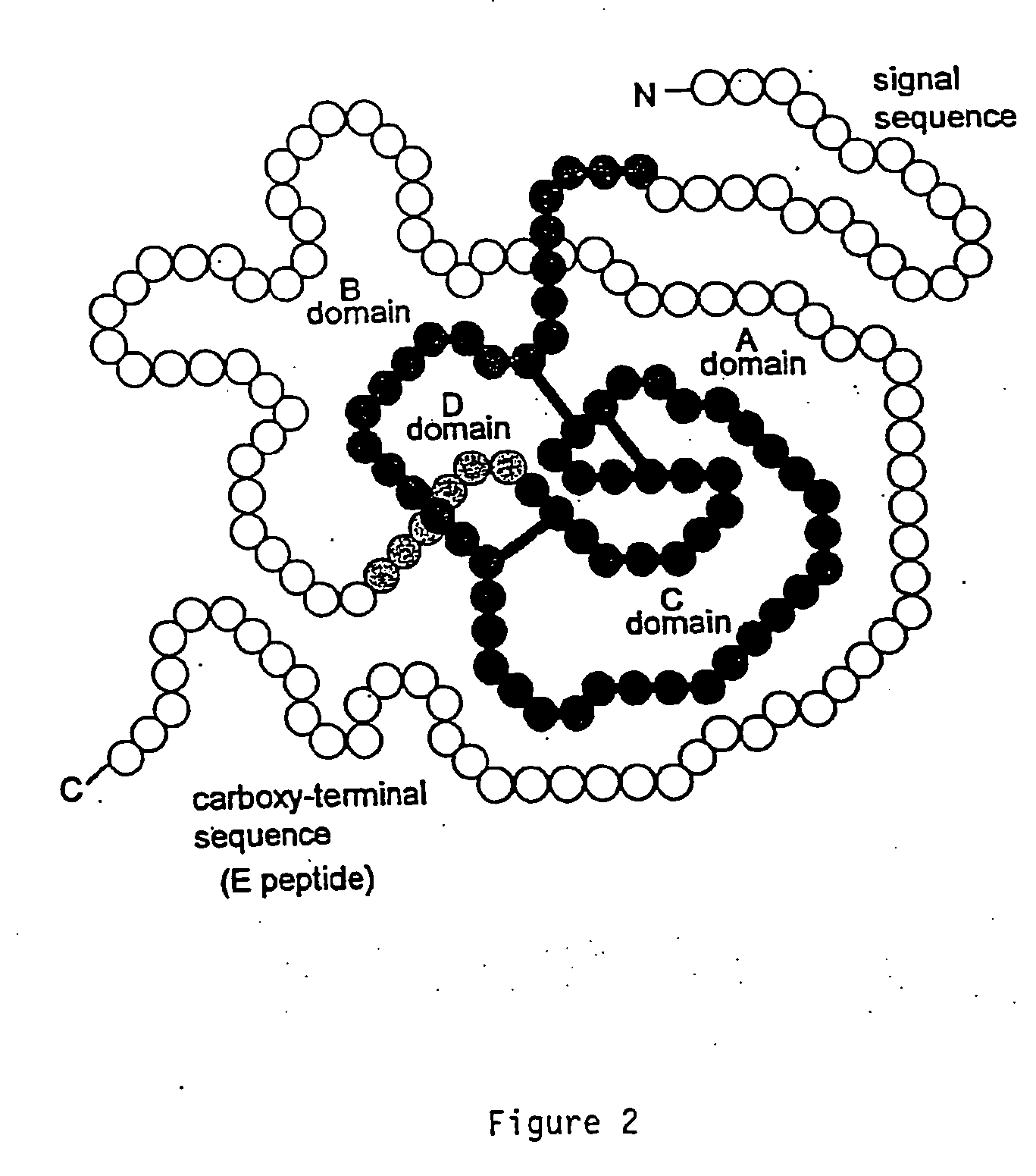
Disclosed are methods and compositions for targeting therapeutic proteins to the brain. Methods and compositions of the invention involve associating an IGF moiety with a therapeutic protein in order to target the therapeutic protein to the brain. Soluble fusion proteins that include an IGF targeting moiety are transported to neural tissue in the brain from blood. Methods and compositions of the invention include therapeutic applications for treating lysosomal storage diseases. The invention also provides nucleic acids and cells for expressing IGF fusion proteins.
Owner:BIOMARIN PHARMA INC
Intranasal delivery of therapeutic enzymes to the central nervous system for the treatment of lysosomal storage diseases
ActiveUS20120288489A1Maximizes deliveryBlock deliveryOrganic active ingredientsNervous disorderOpen reading frameAdeno associate virus
The invention provides a method to prevent, inhibit or treat one or more neurological symptoms associated with a lysosomal storage disease in a mammal in need thereof, which includes intranasally administering to the mammal a composition comprising an effective amount of a lysosomal storage enzyme or a recombinant adeno-associated virus vector comprising an open reading frame encoding a lysosomal storage enzyme. Also provided are compositions and devices useful in the methods.
Owner:RGT UNIV OF MINNESOTA +1
Slow intraventricular delivery
InactiveUS20090123451A1Lower Level RequirementsOrganic active ingredientsNervous disorderAnesthetic AgentDiagnostic agent
Neurological diseases, including lysosomal storage diseases, can be successfully treated using intraventricular delivery of the therapeutic agents to bypass the blood-brain barrier. Similarly, diagnostic agents and anesthetic agents can be delivered to the brain in this manner. The administration can be performed slowly to achieve maximum effect. Such administration permits greater penetration of distal portions of the brain.
Owner:GENZYME CORP
Methods and Compositions for Targeting Proteins Across the Blood Brain Barrier
InactiveUS20080241118A1Polypeptide with localisation/targeting motifSugar derivativesProtein targetTherapeutic protein
Disclosed are methods and compositions for targeting therapeutic proteins to the brain. Methods and compositions of the invention involve associating an IGF moiety with a therapeutic protein in order to target the therapeutic protein to the brain. Soluble fusion proteins that include an IGF targeting moiety are transported to neural tissue in the brain from blood. Methods and compositions of the invention include therapeutic applications for treating lysosomal storage diseases. The invention also provides nucleic acids and cells for expressing IGF fusion proteins.
Owner:BIOMARIN PHARMA INC
Methods of enhancing lysosomal storage disease therapy by modulation of cell surface receptor density
InactiveUS20100143297A1Uptake of extracellular lysosomal enzymes by cells can be increasedIncreases the target organ uptake of glucocerebrosidaseBiocideElcosanoid active ingredientsLysosomeFabry disease
Methods of modulating uptake of extracellular lysosomal enzymes by administering a pharmaceutical agent and methods of treating a lysosomal storage disease (such as Gaucher disease, Pompe disease, Fabry disease or Niemann-Pick disease) or enhancing enzyme replacement therapy or gene therapy, comprising administering a pharmaceutical agent such as dexamethasone, glucose or insulin, are provided.
Owner:GENZYME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com