Combination enzyme replacement, gene therapy and small molecule therapy for lysosomal storage diseases
a technology of lysosomal storage and enzyme replacement, applied in the field of therapeutics for lysosomal storage diseases, can solve the problems of frequency of administration, and achieve the effect of optimizing clinical benefit and minimizing disadvantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
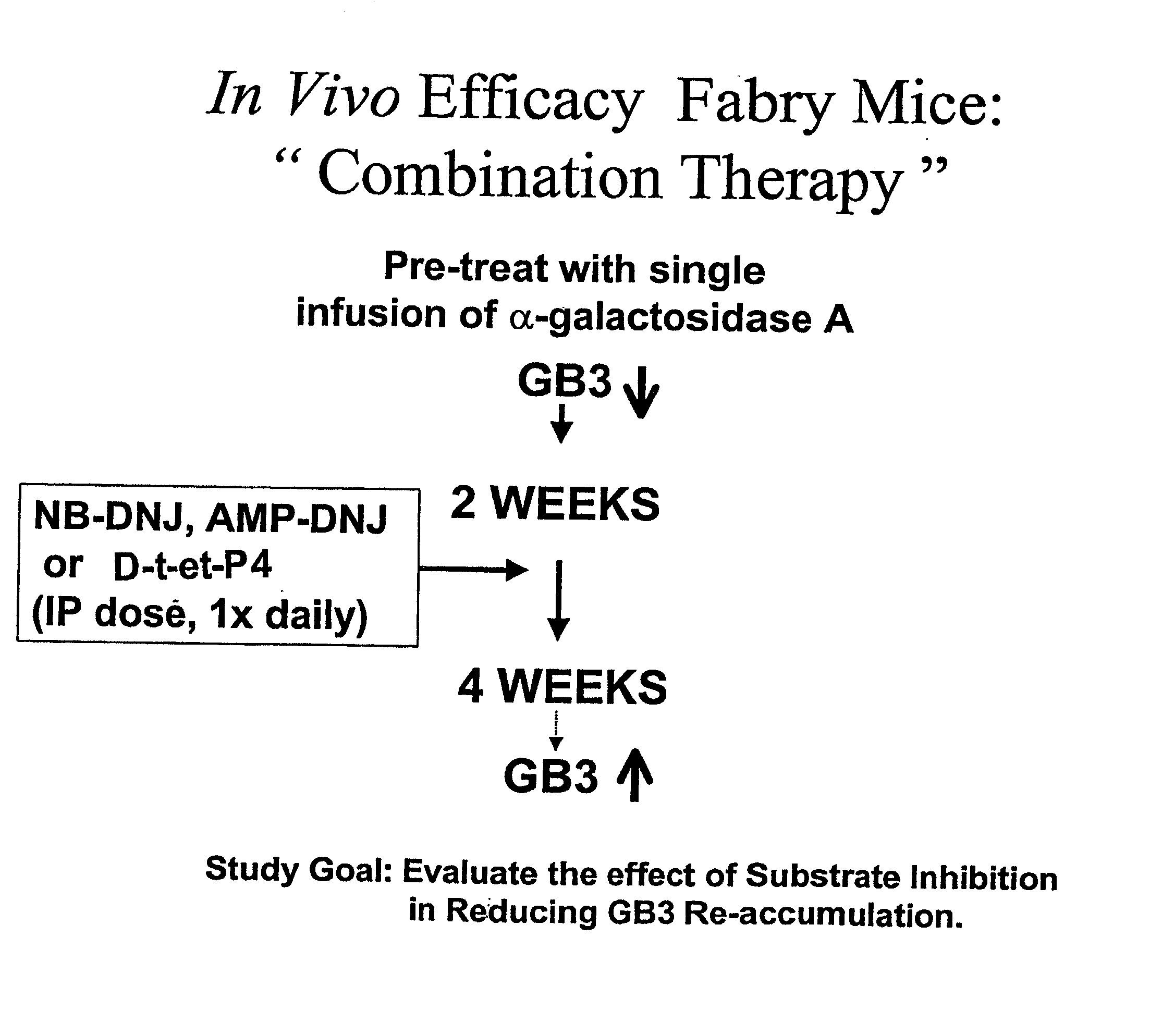
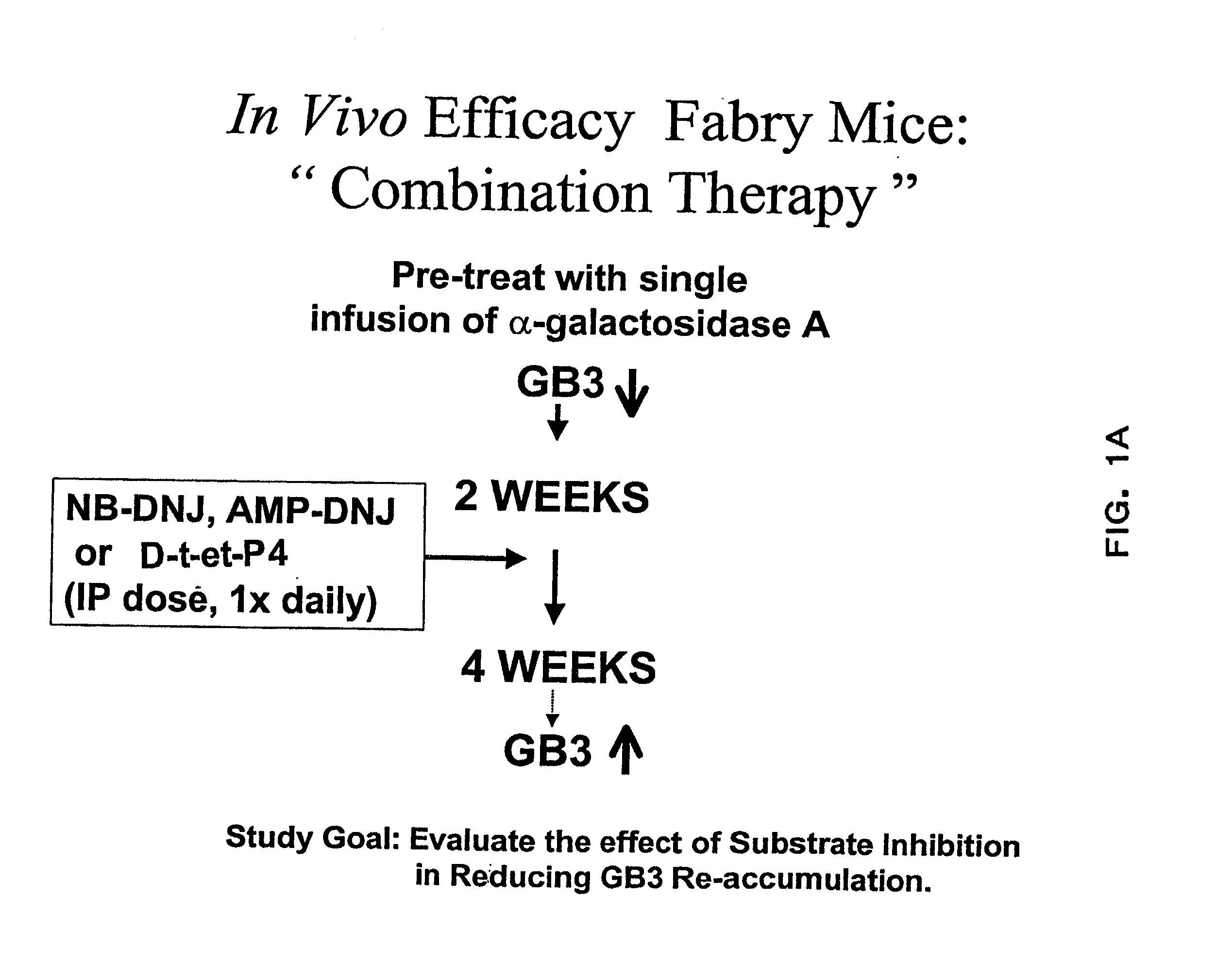
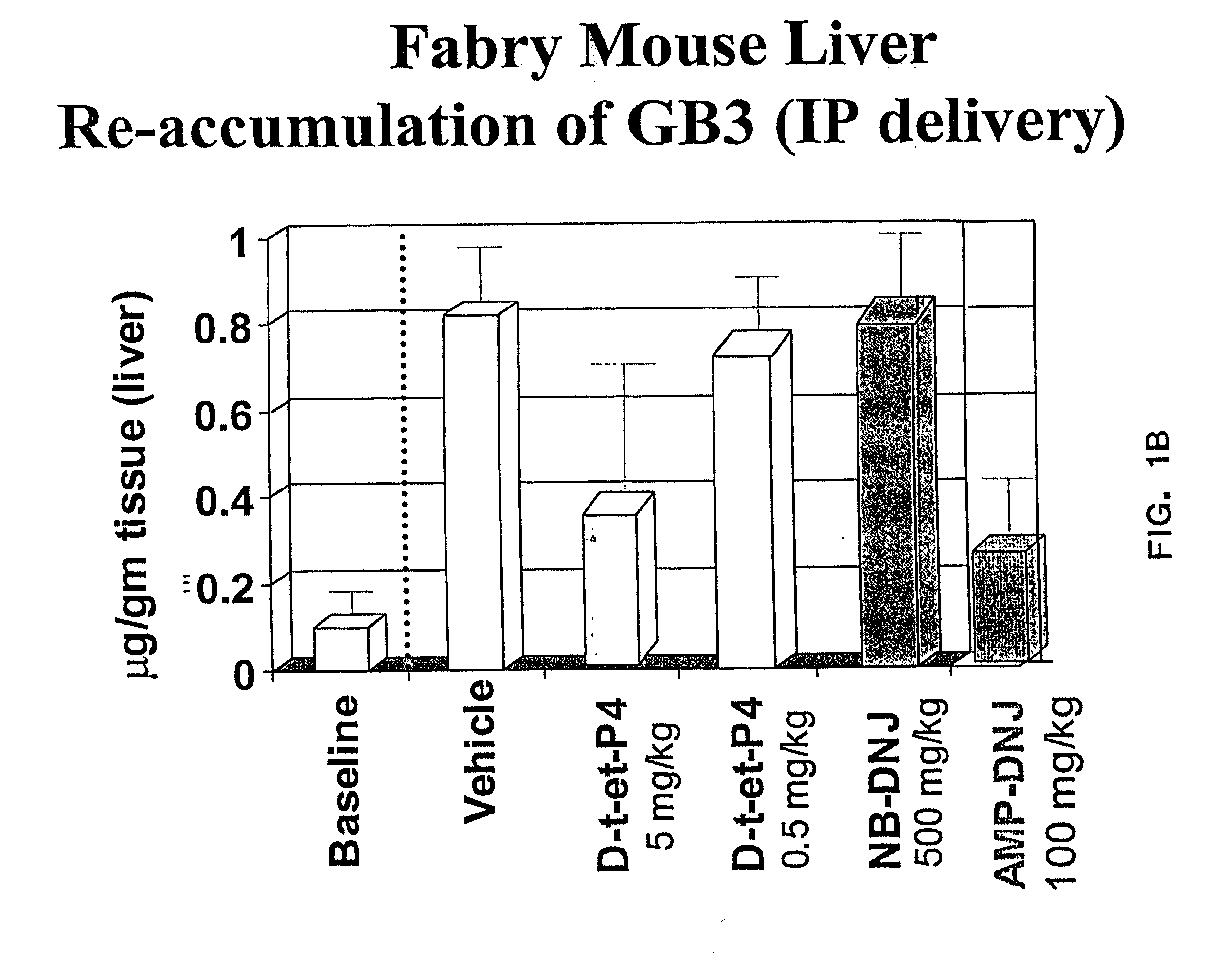
[0075] The therapeutic methods of the invention described herein provide treatment options for the practitioner faced with management of various lysosomal storage diseases, as described in detail below. More specifically, the invention relates to various combinations of enzyme replacement therapy and gene therapy for the treatment of lysosomal storage diseases.
[0076] A partial list of known lysosomal storage diseases that can be treated in accordance with the invention is set forth in Table 1, including common disease name, material stored, and corresponding enzyme deficiency (adapted from Table 38-4 of Kolodny et al., 1998, Id.).
1TABLE 1 Lysosomal Storage Diseases Disease Material Stored Enzyme Deficiency Sphingolipidoses Gaucher Glucocerebroside Glucocerebrosidase Niemann-Pick Sphingomyelin Sphingomyelinase Farber Ceramide Ceramidase G.sub.M1-gangliosidosis G.sub.M1-ganglioside, G.sub.M1-ganglioside-.beta.-- glycoprotein galactosidase G.sub.M2-gangliosidosis G.sub.M2-ganglioside, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com