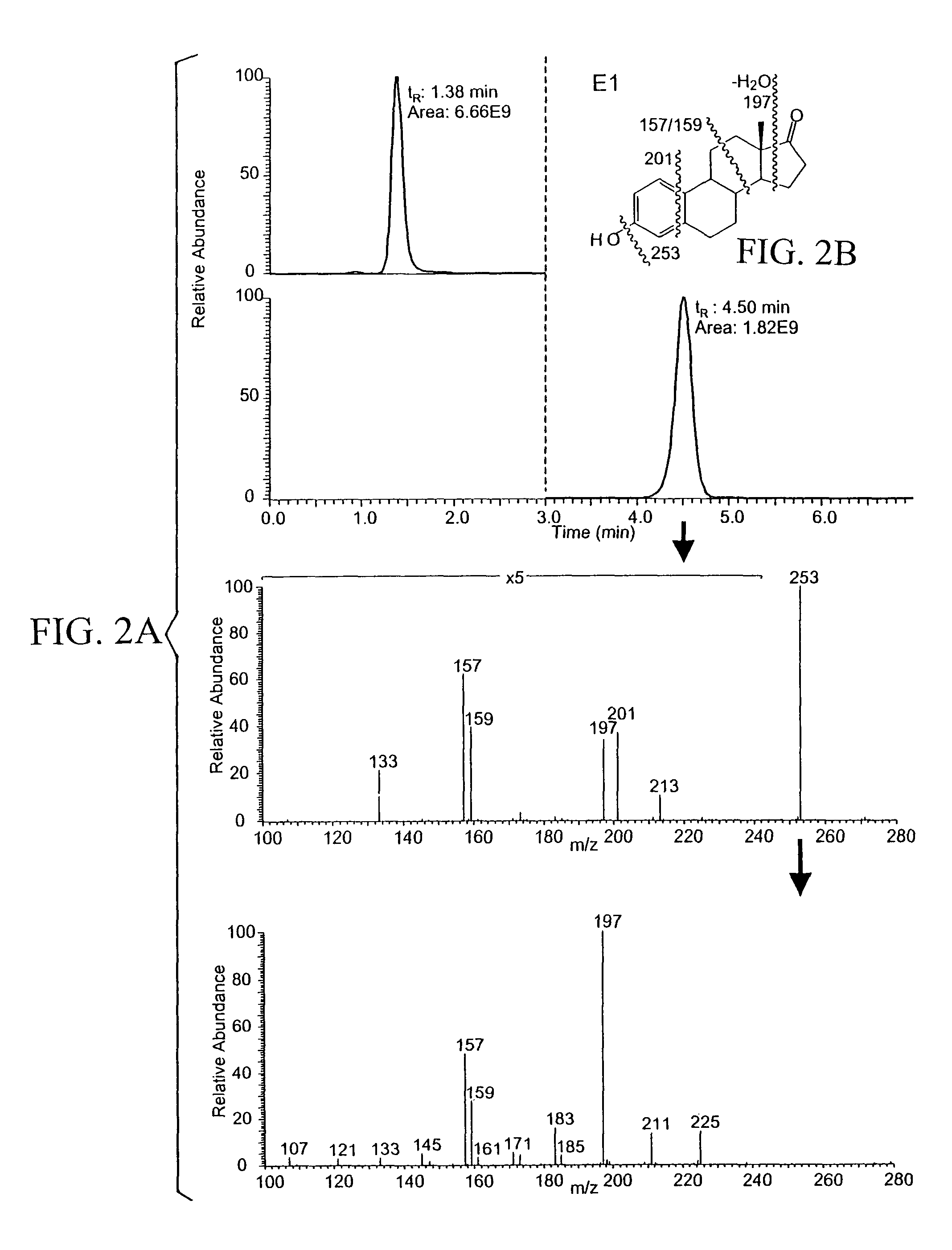
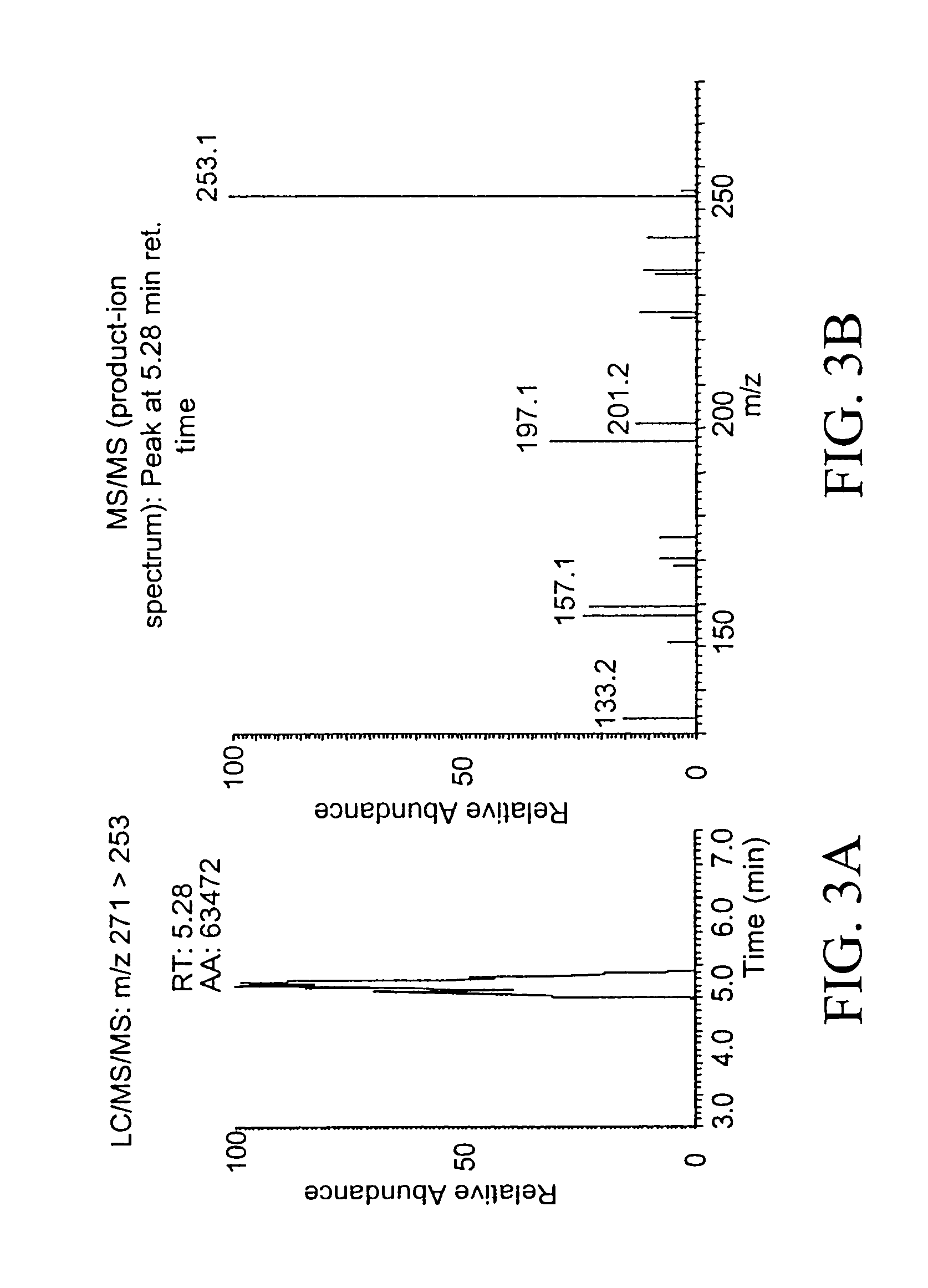
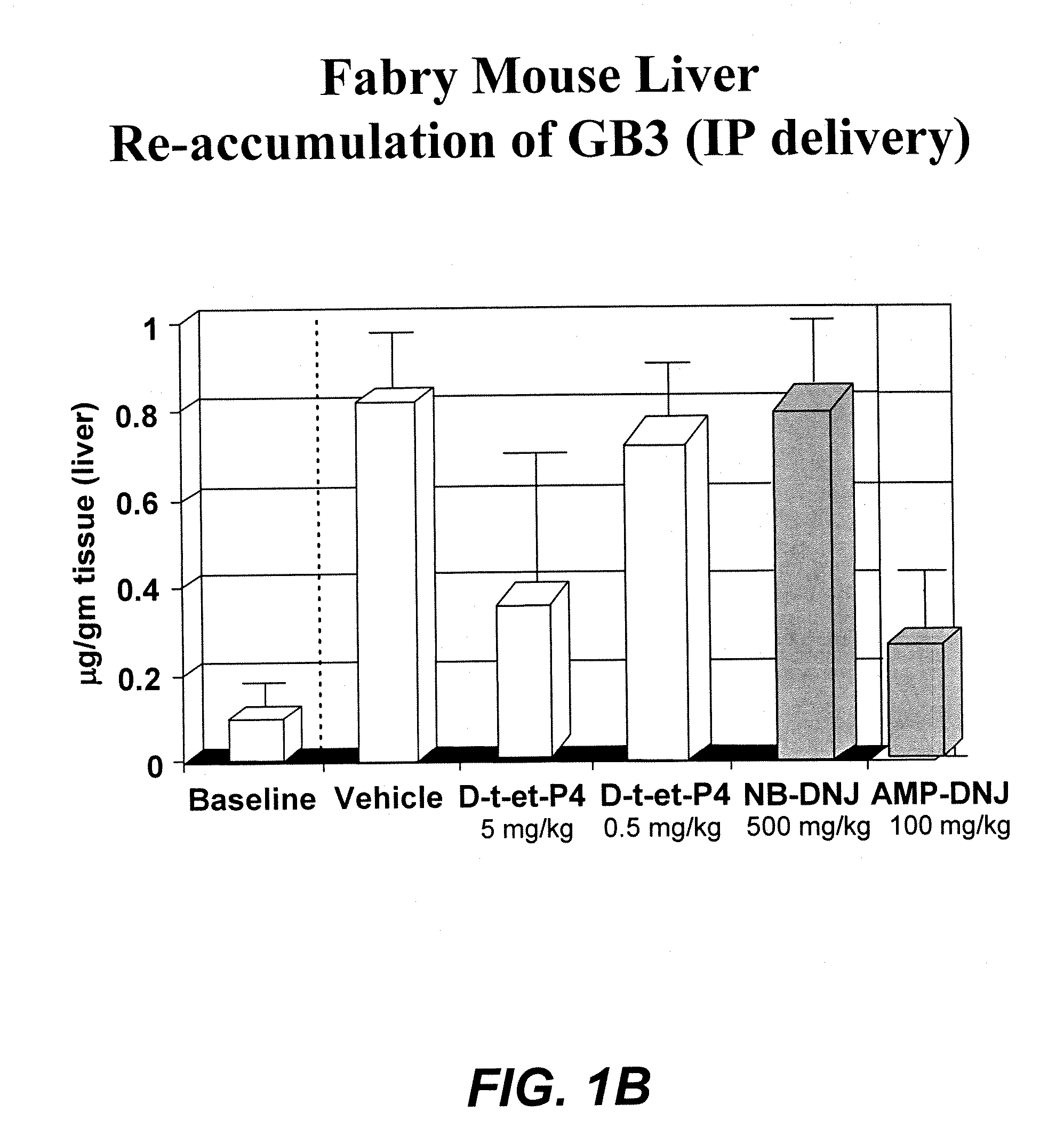
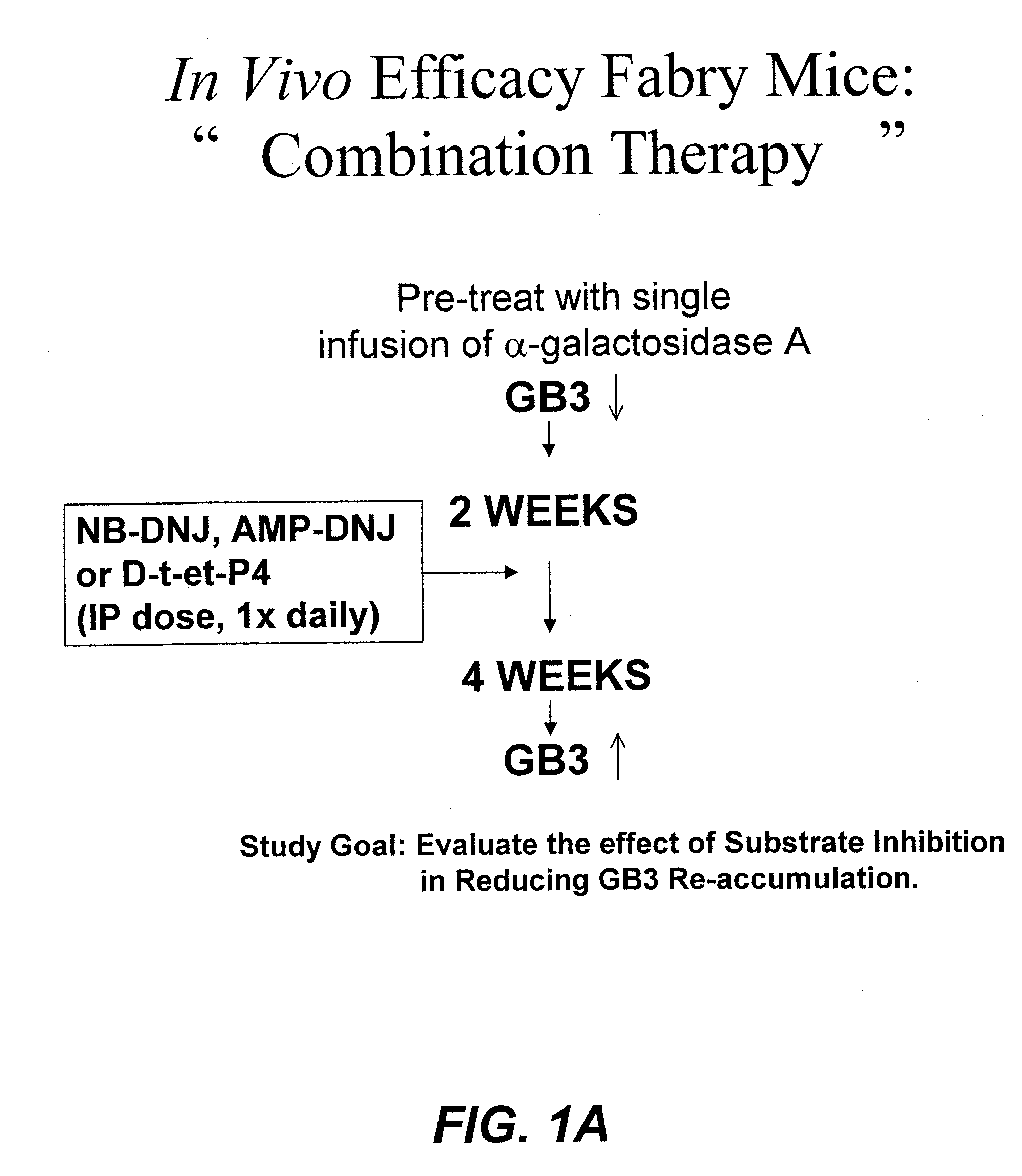Patents
Literature
114 results about "Enzyme replacement therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enzyme replacement therapy (ERT) is a medical treatment which replaces an enzyme that is deficient or absent in the body. Usually, this is done by giving the patient an intravenous (IV) infusion of a solution containing the enzyme.
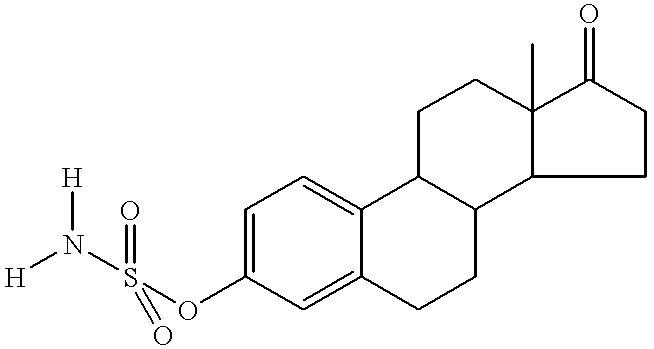
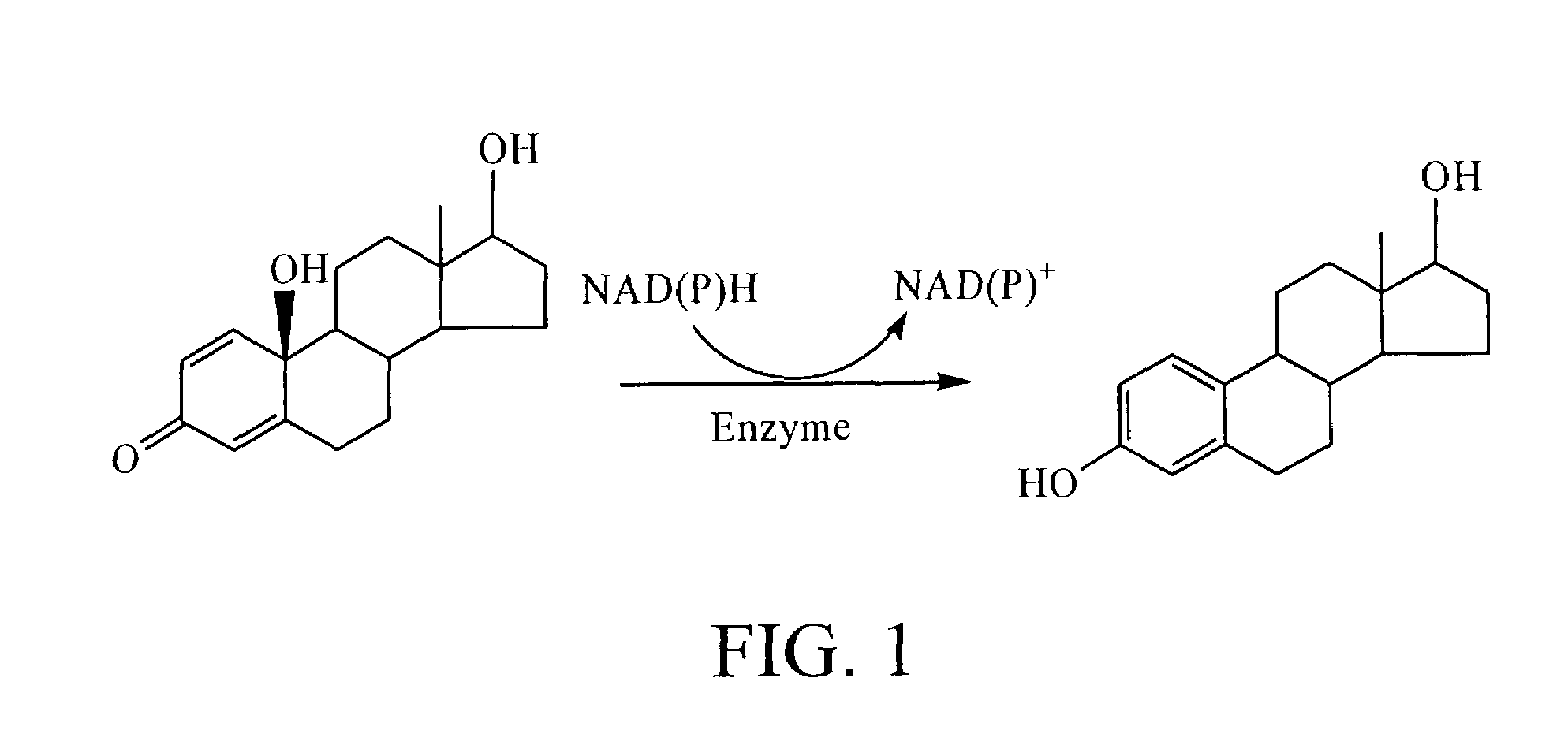
Drospirenone for hormone replacement therapy
InactiveUS20020132801A1Dissolve fastImprove bioavailabilityOrganic active ingredientsBiocideDrospirenoneBULK ACTIVE INGREDIENT
Drospirenone for Hormone Replacement Therapy A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
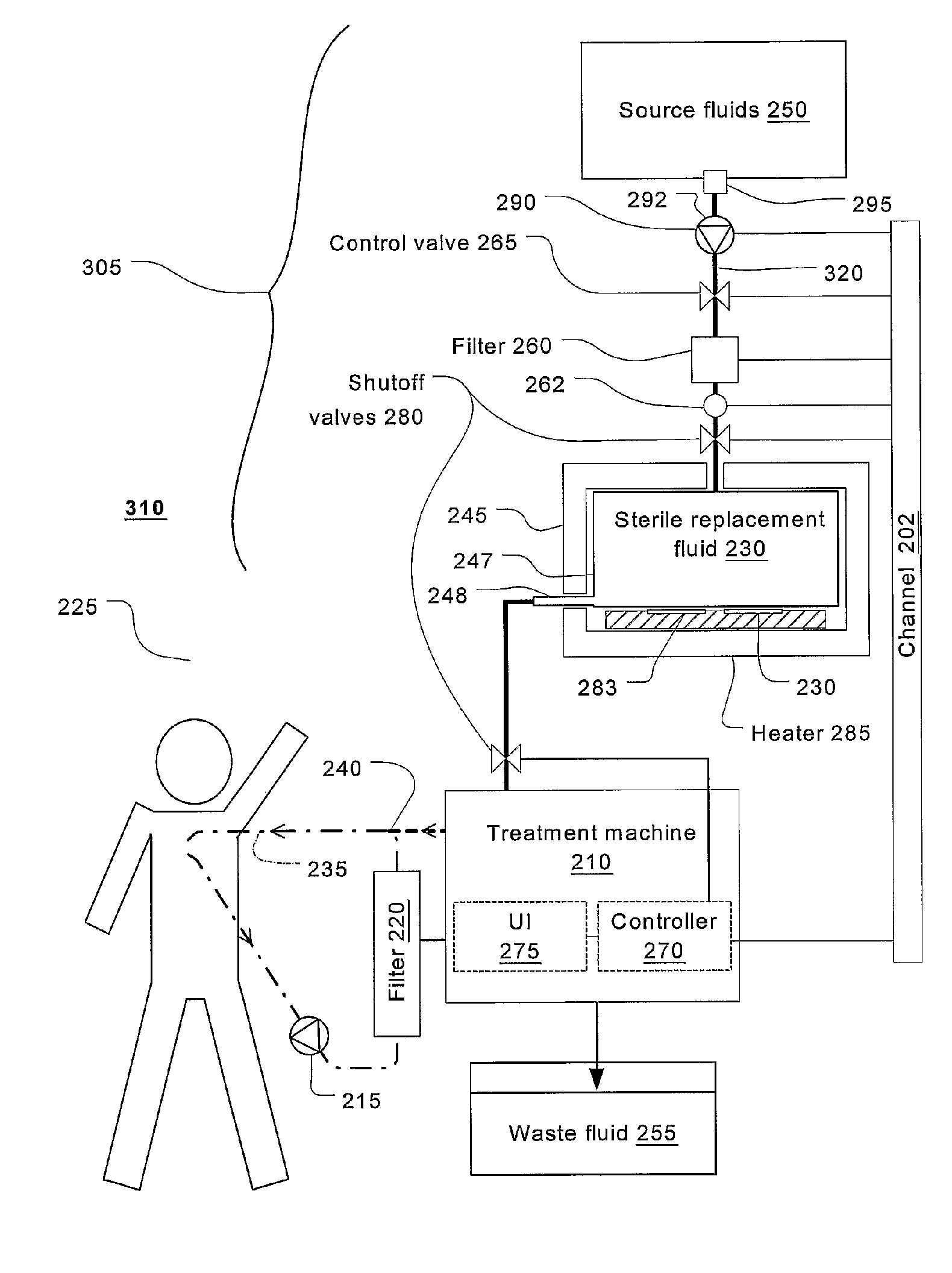
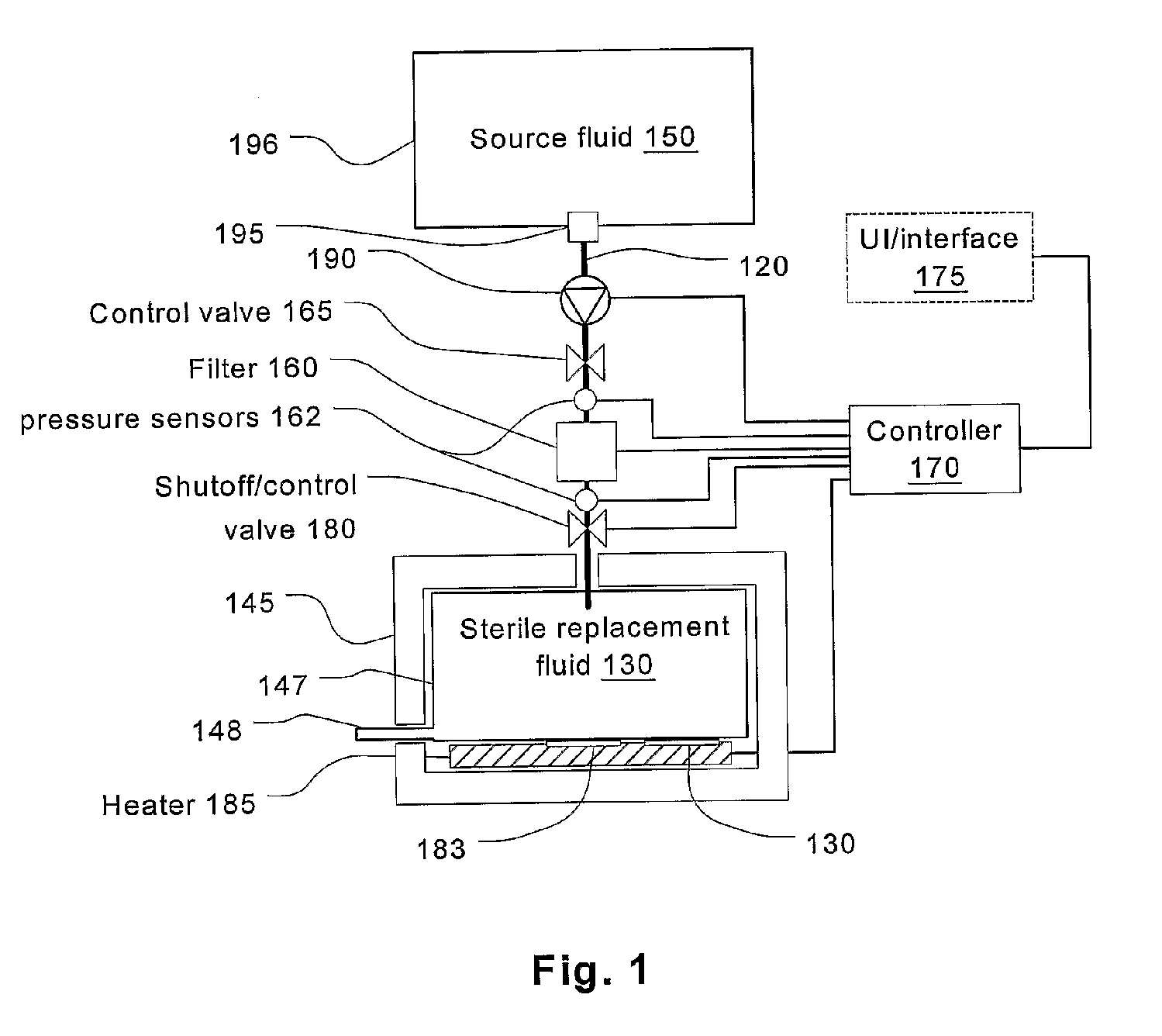
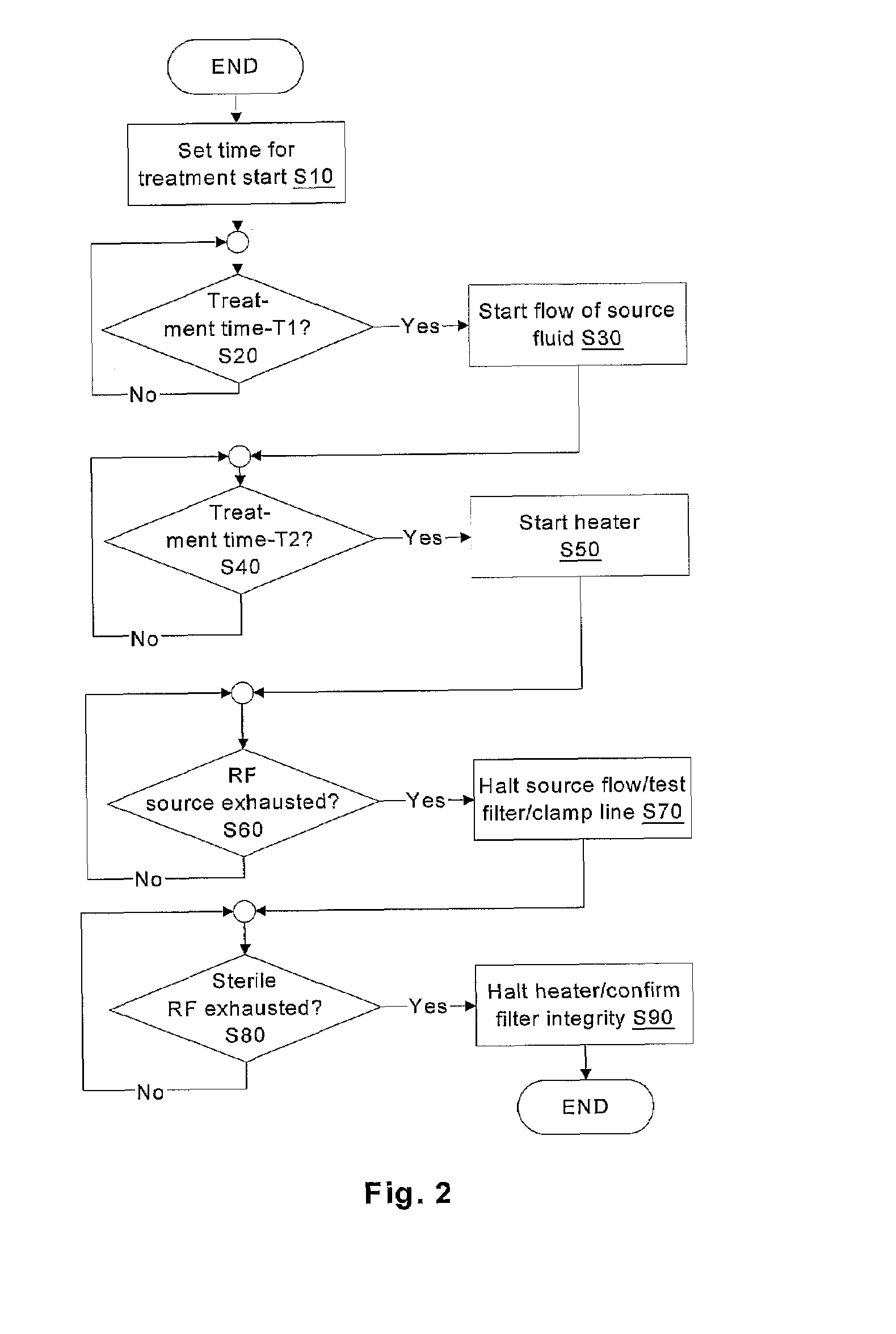
Batch filtration system for preparation of sterile fluid for renal replacement therapy
InactiveUS7544300B2Solve insufficient capacityPrevent heat lossSolvent extractionSettling tanks feed/dischargeBlood treatmentsDialysis fluid
A method and device for blood treatments that use fluids such as dialysate and replacement fluid for renal replacement therapy. In an embodiment, fluid is passed either by pump or passively by gravity feed, through a microporous sterilization filter from a fluid source to a replacement fluid container. The latter forms a batch that may be used during treatment. The advantage of forming the batch before treatment is that the rate of filtering needn't match the rate of consumption during treatment. As a result, the sterilization filter can have a small capacity. In another embodiment, a filter is placed immediately prior to the point at which the sterile fluid is consumed by the treatment process. The latter may be used in combination with the former embodiment as a last-chance guarantee of sterility and / or that the fluid is free of air bubbles. It may also be used as the primary means of sterile-filtration.
Owner:NXSTAGE MEDICAL INC +1
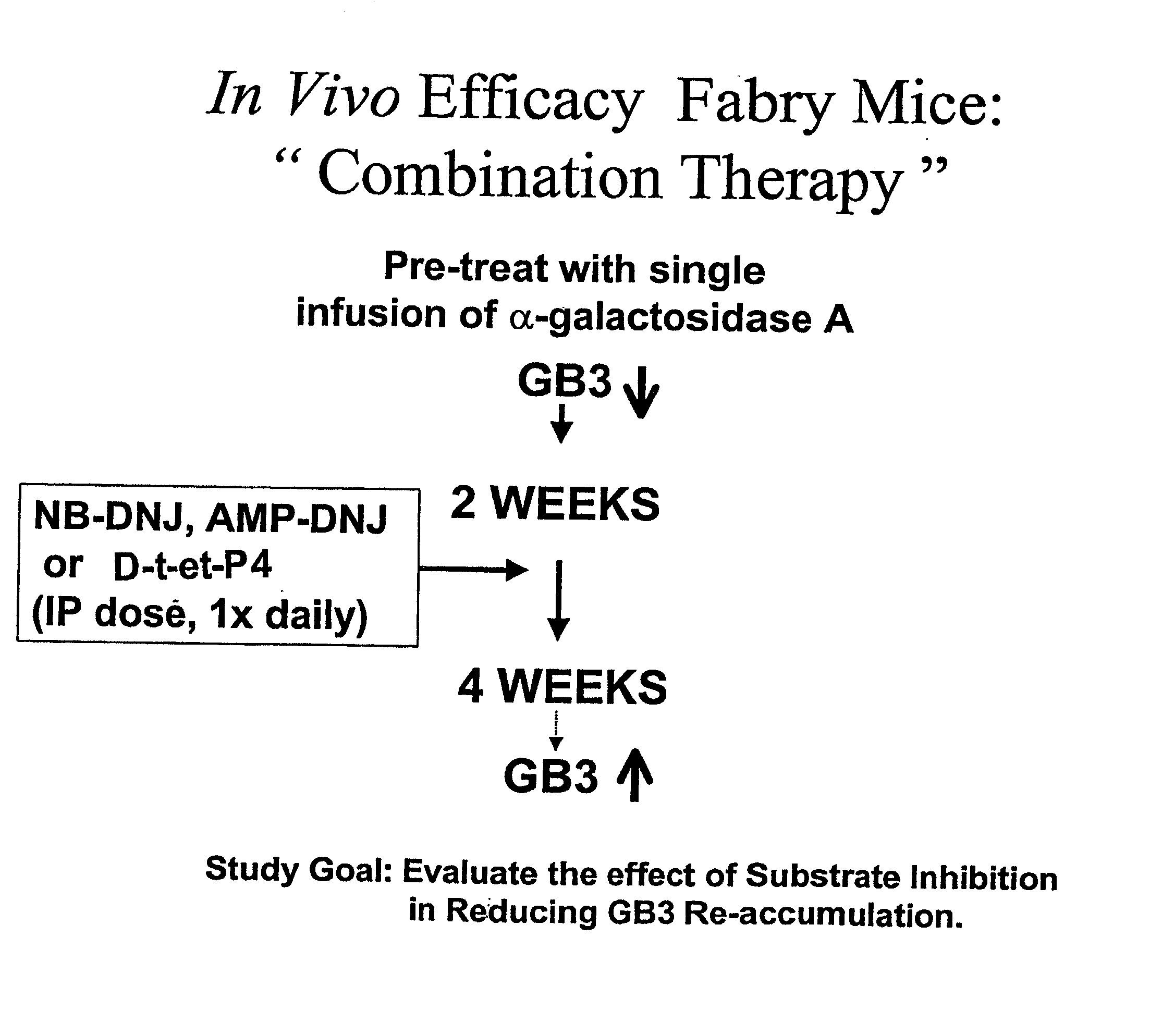
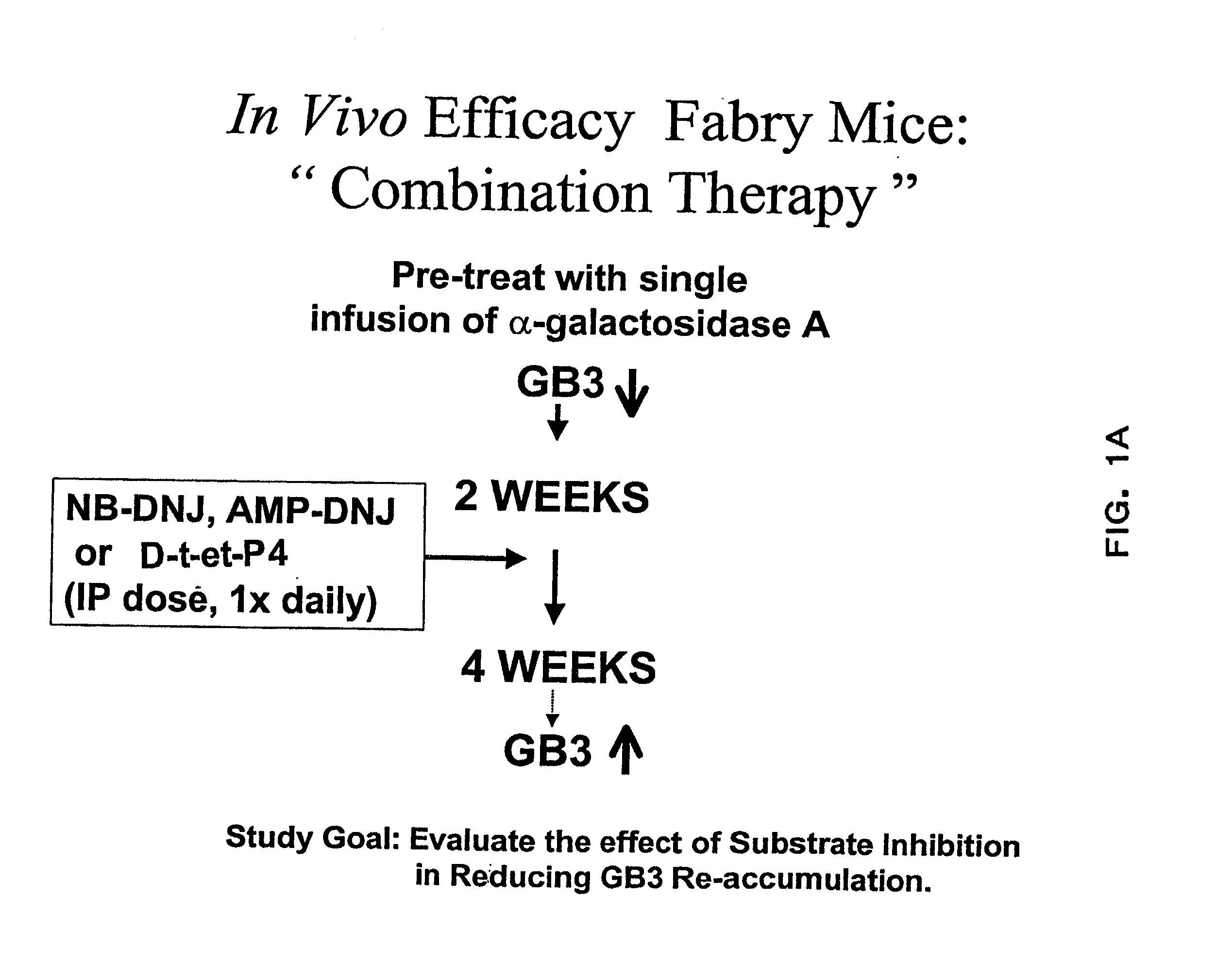
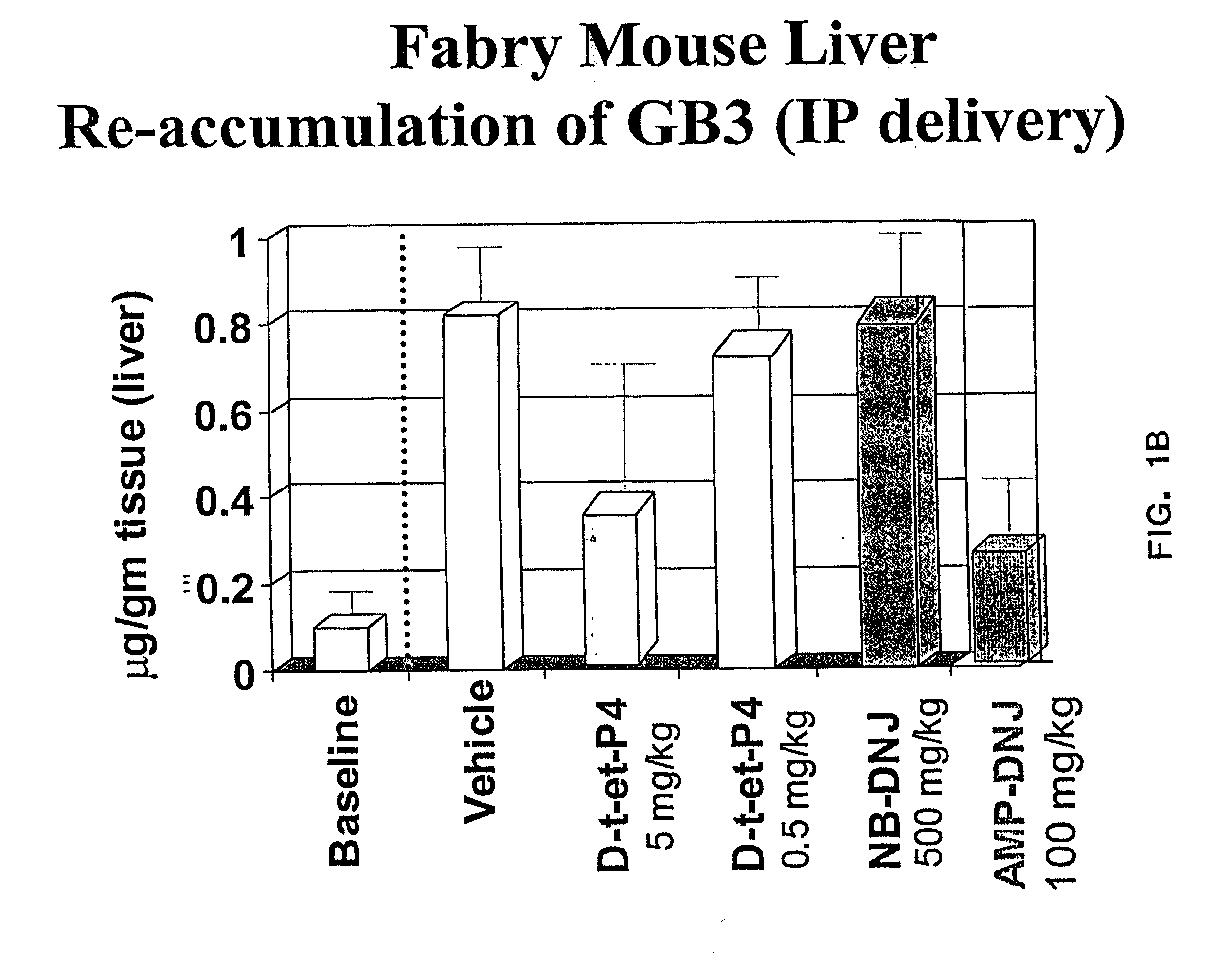
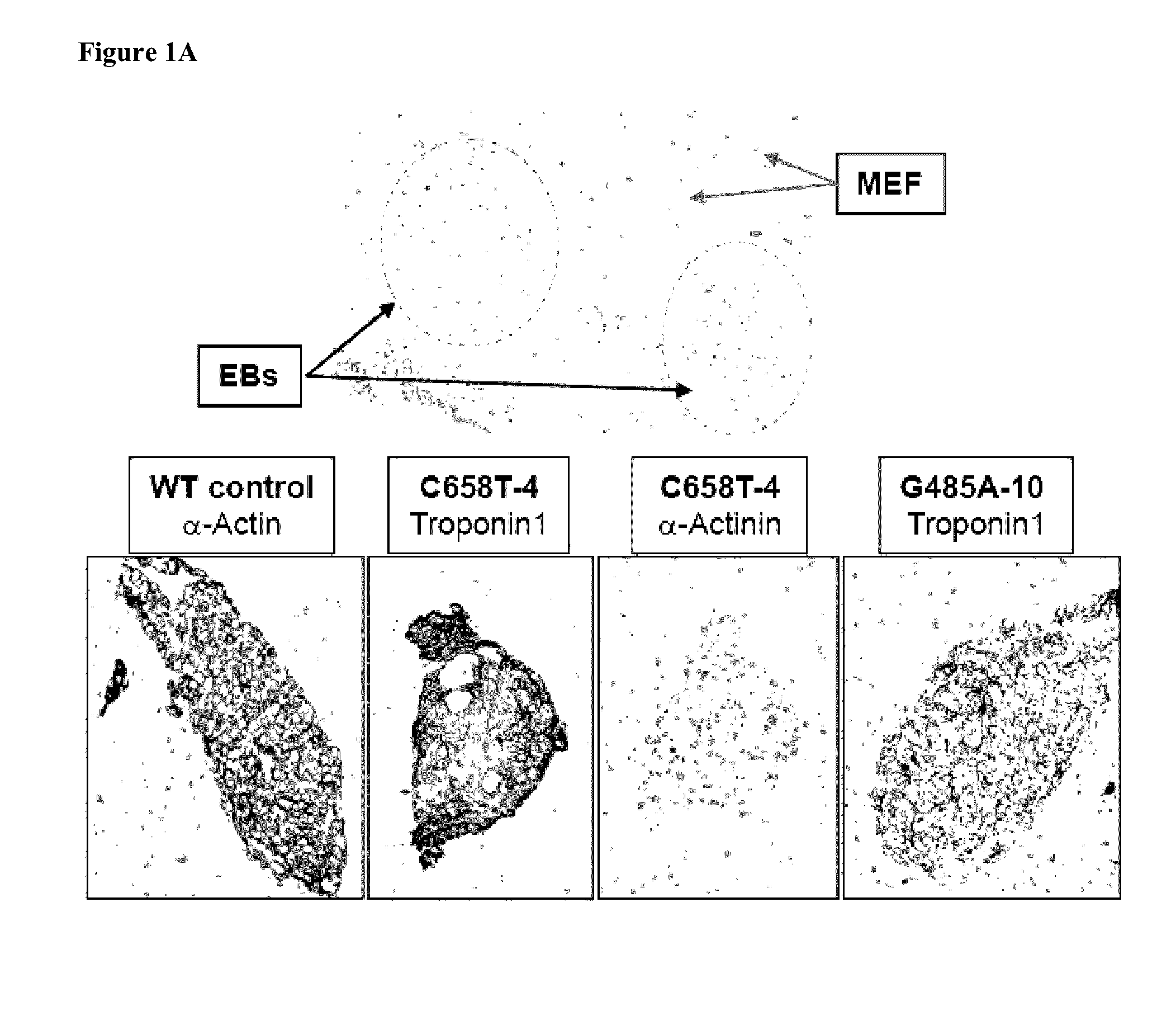
Combination enzyme replacement, gene therapy and small molecule therapy for lysosomal storage diseases
InactiveUS20020095135A1Optimizes clinical benefitReduce disadvantagesPeptide/protein ingredientsMetabolism disorderLysosomal enzyme defectGene
This invention provides various combinations of enzyme replacement therapy, gene therapy, and small molecule therapy for the treatment of lysosomal storage diseases.
Owner:MEEKER DAVID +1
Drospirenone for hormone replacement therapy
InactiveUS20030144258A1Dissolve fastImprove bioavailabilityBiocideOrganic active ingredientsDrospirenoneBULK ACTIVE INGREDIENT
A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:SCHERING AG
Composition
InactiveUS6653298B2Low hepatic metabolismImprove bioavailabilityOrganic active ingredientsBiocideHormone replacementHormone
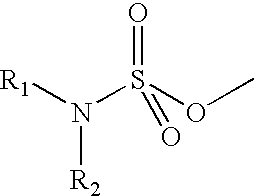
Disclosed and claimed are methods for oral contraception, or a hormone replacement therapy, or for treating breast cancer, in a patient in need thereof involving administering to the patient, at a dosage of no greater than 200 mug / day per 70 kg subject, a compound having Formula (I):wherein X in combination with K form a steroidal ring and R5 is a sulphamate group that has of the formula:wherein each of R1 and R2 is H.
Owner:SCHERING AG +1
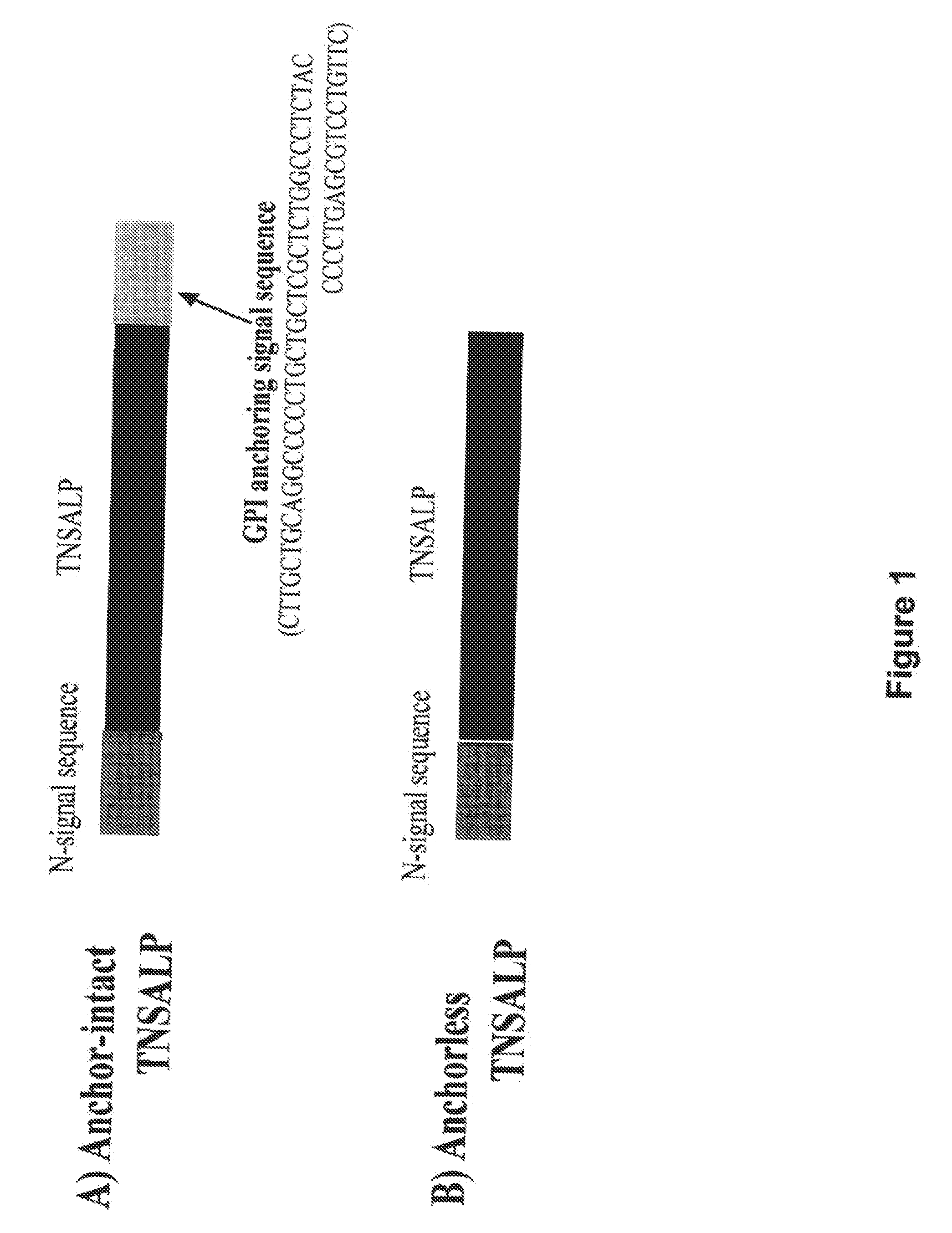
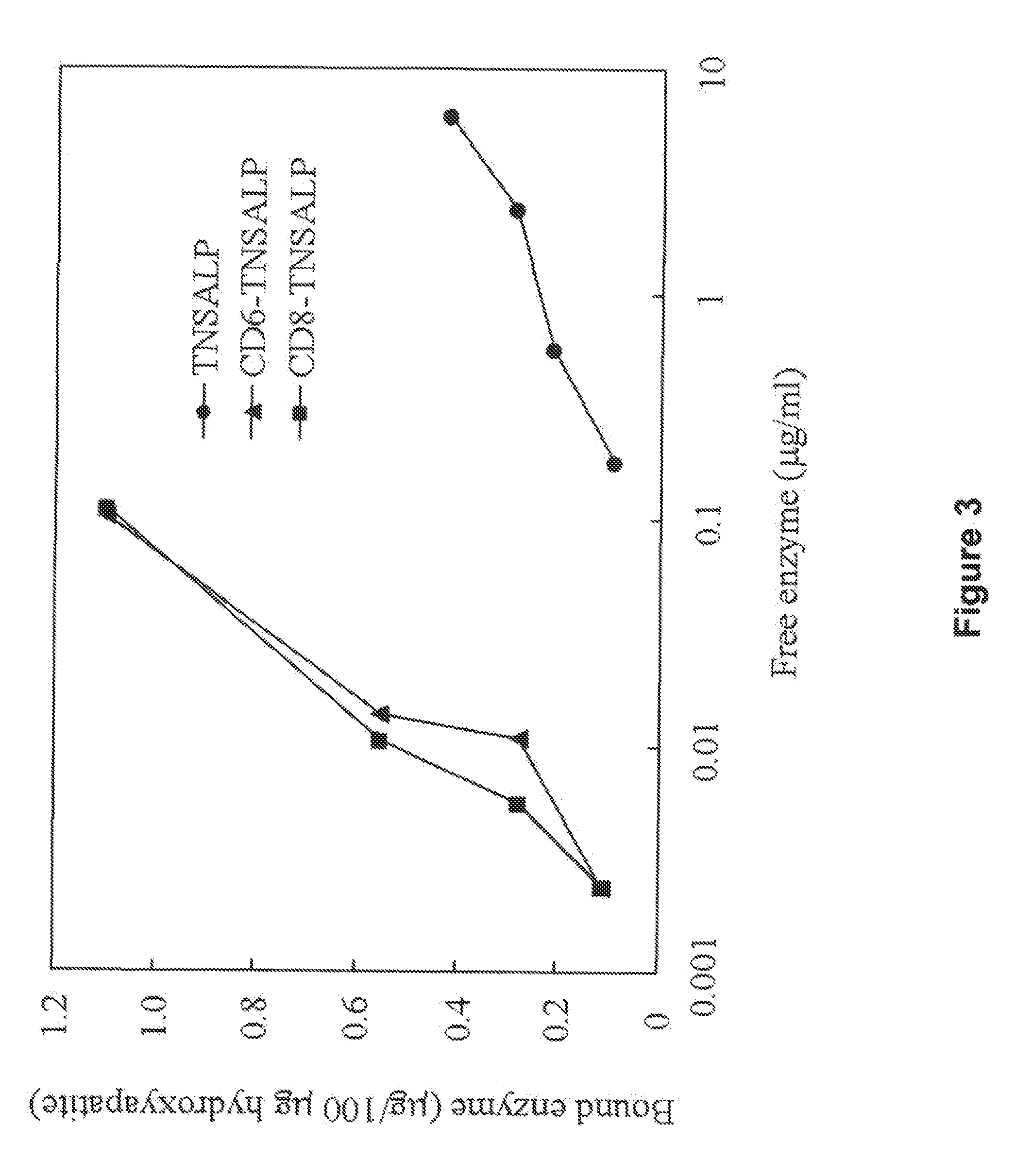
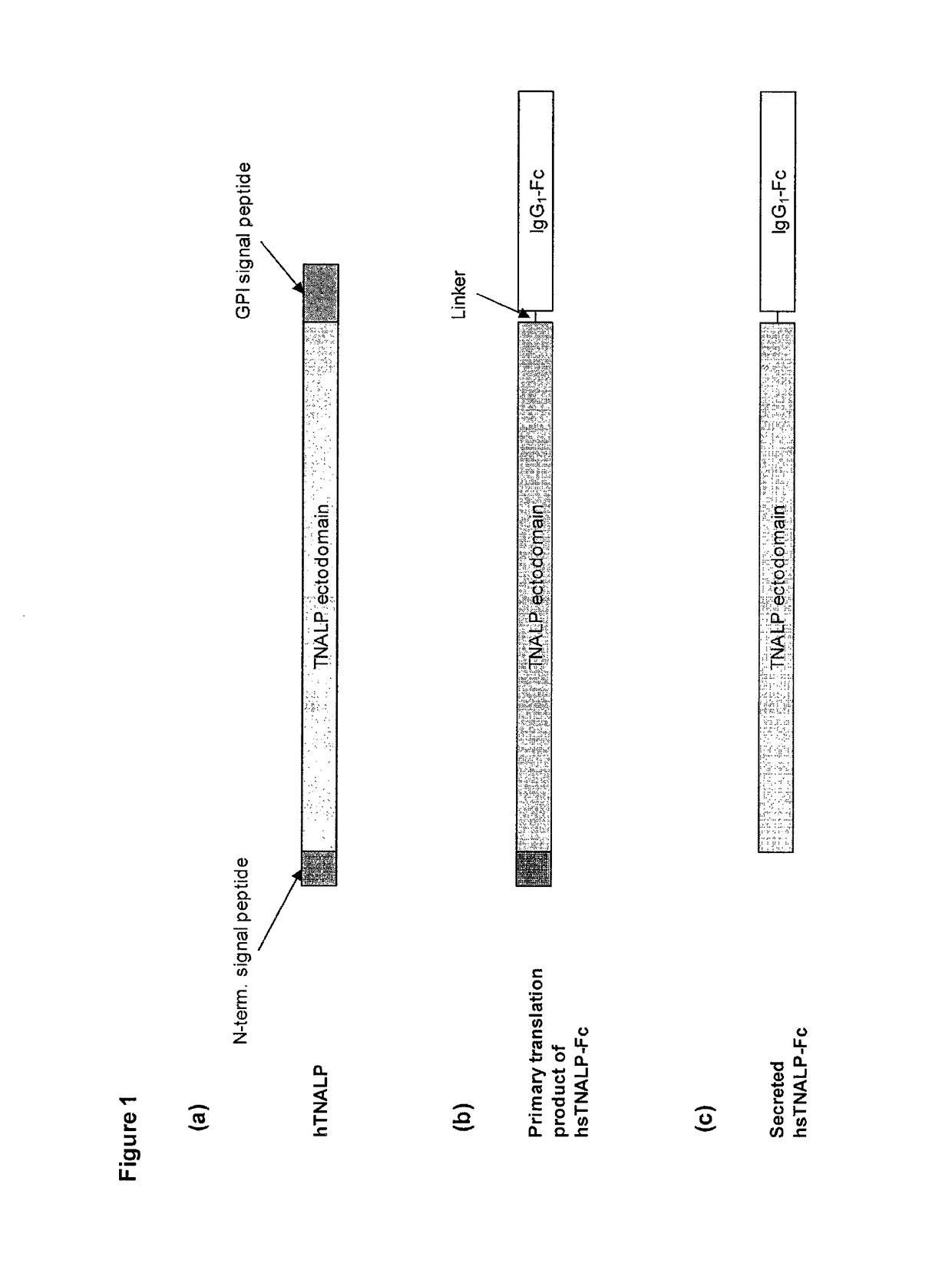
Compositions and methods for treating hypophosphatasia
InactiveUS20070081984A1Extend your lifeWeight increaseHydrolasesPeptide/protein ingredientsBone tissueMembrane bound enzyme
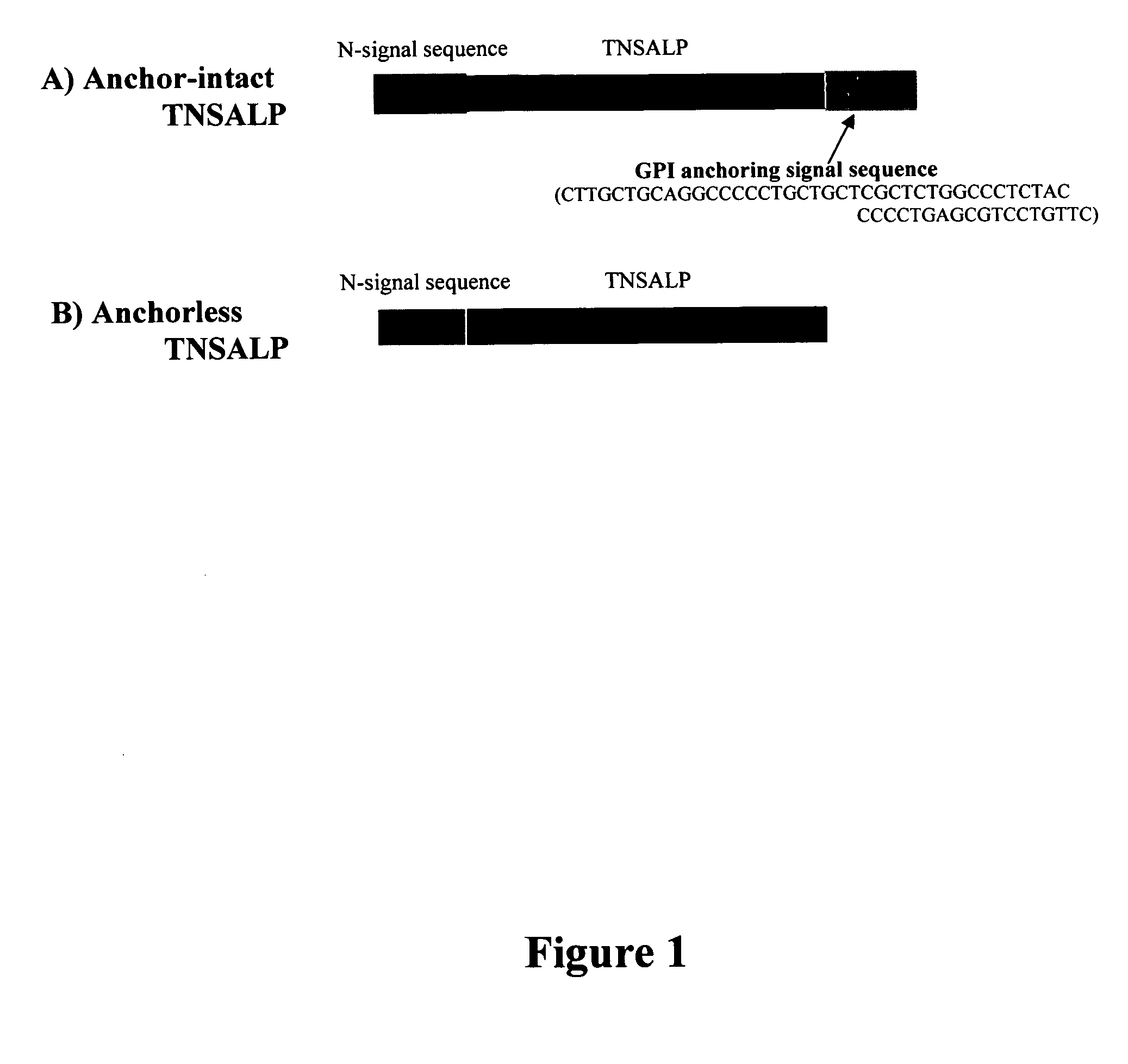
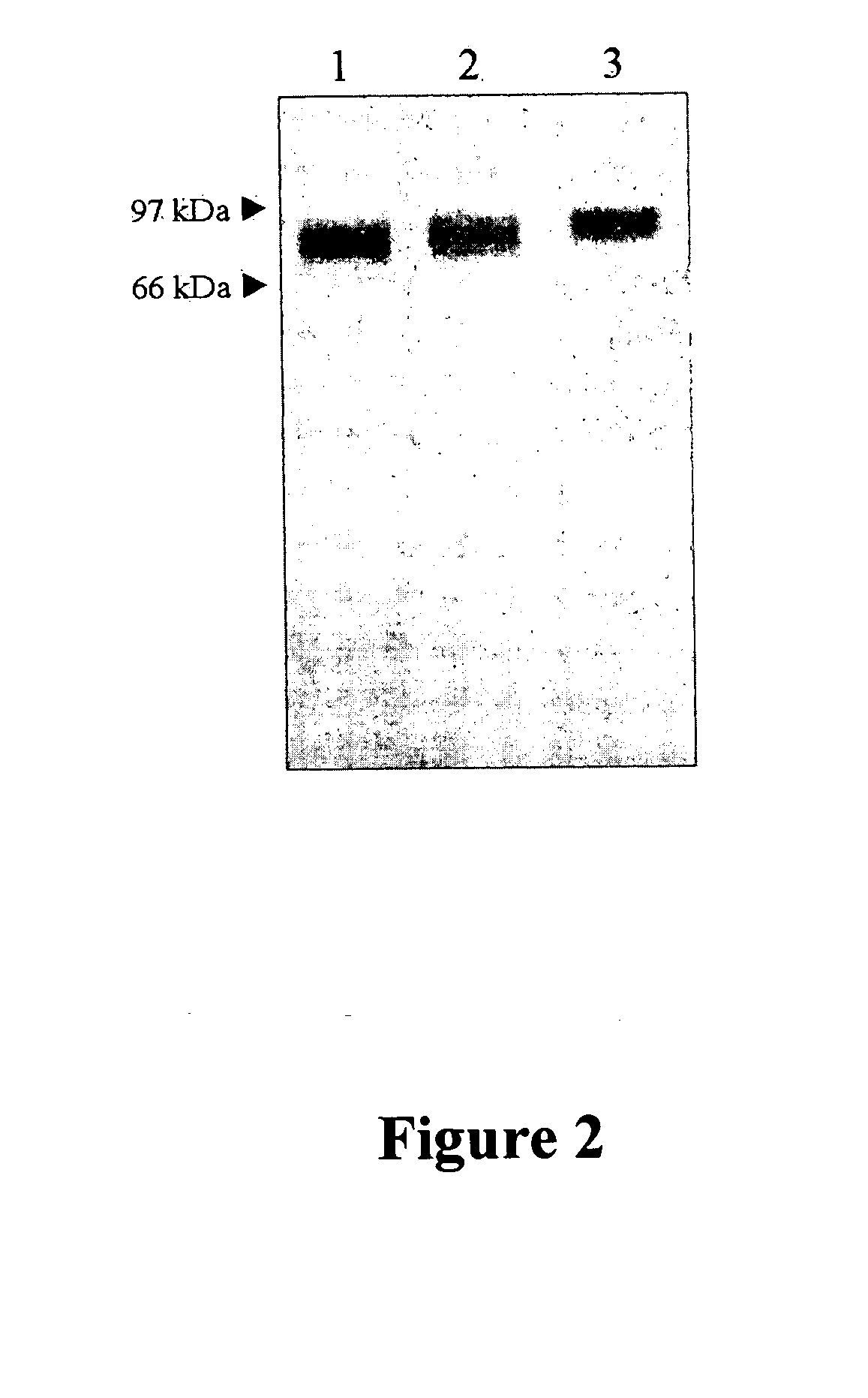
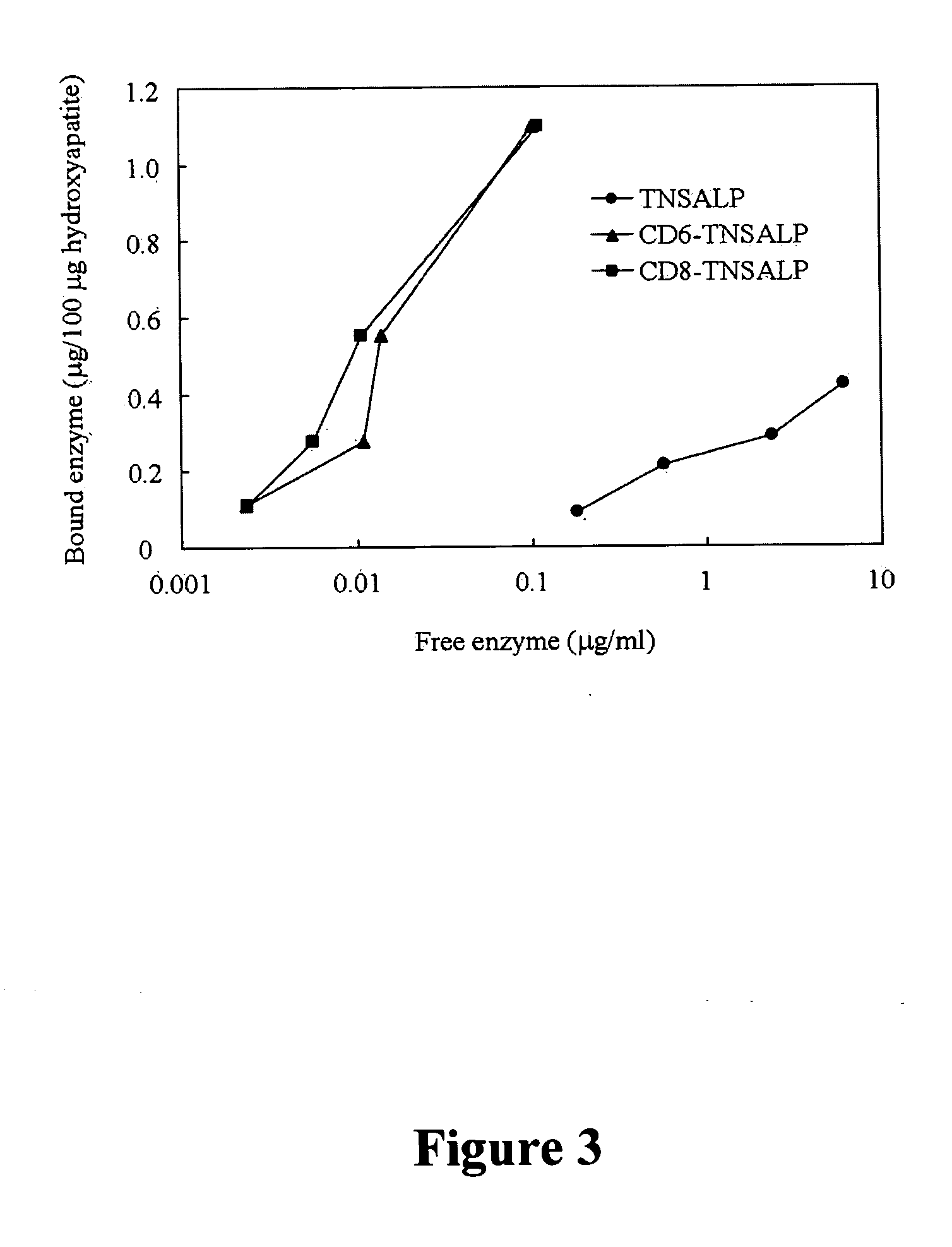
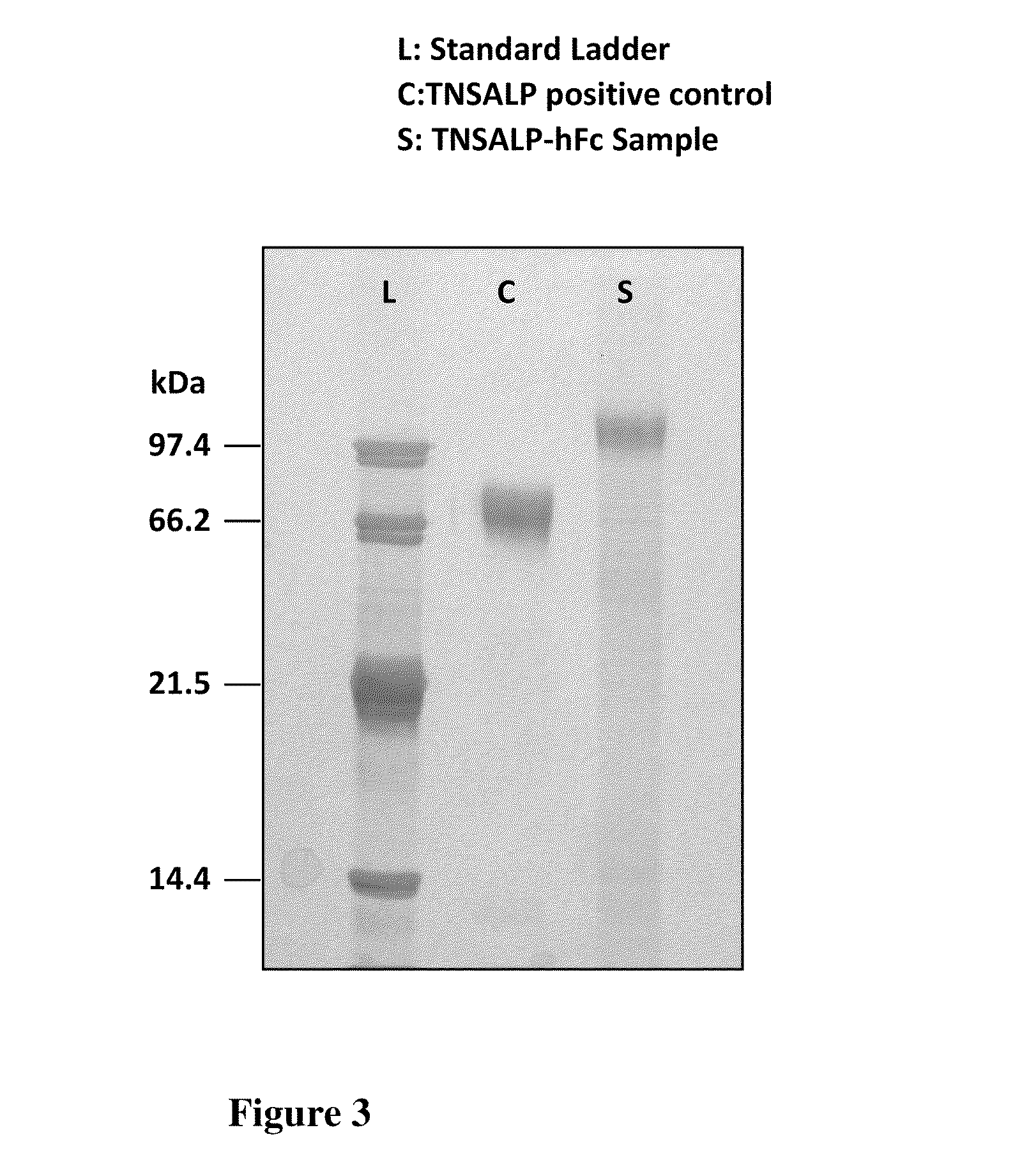
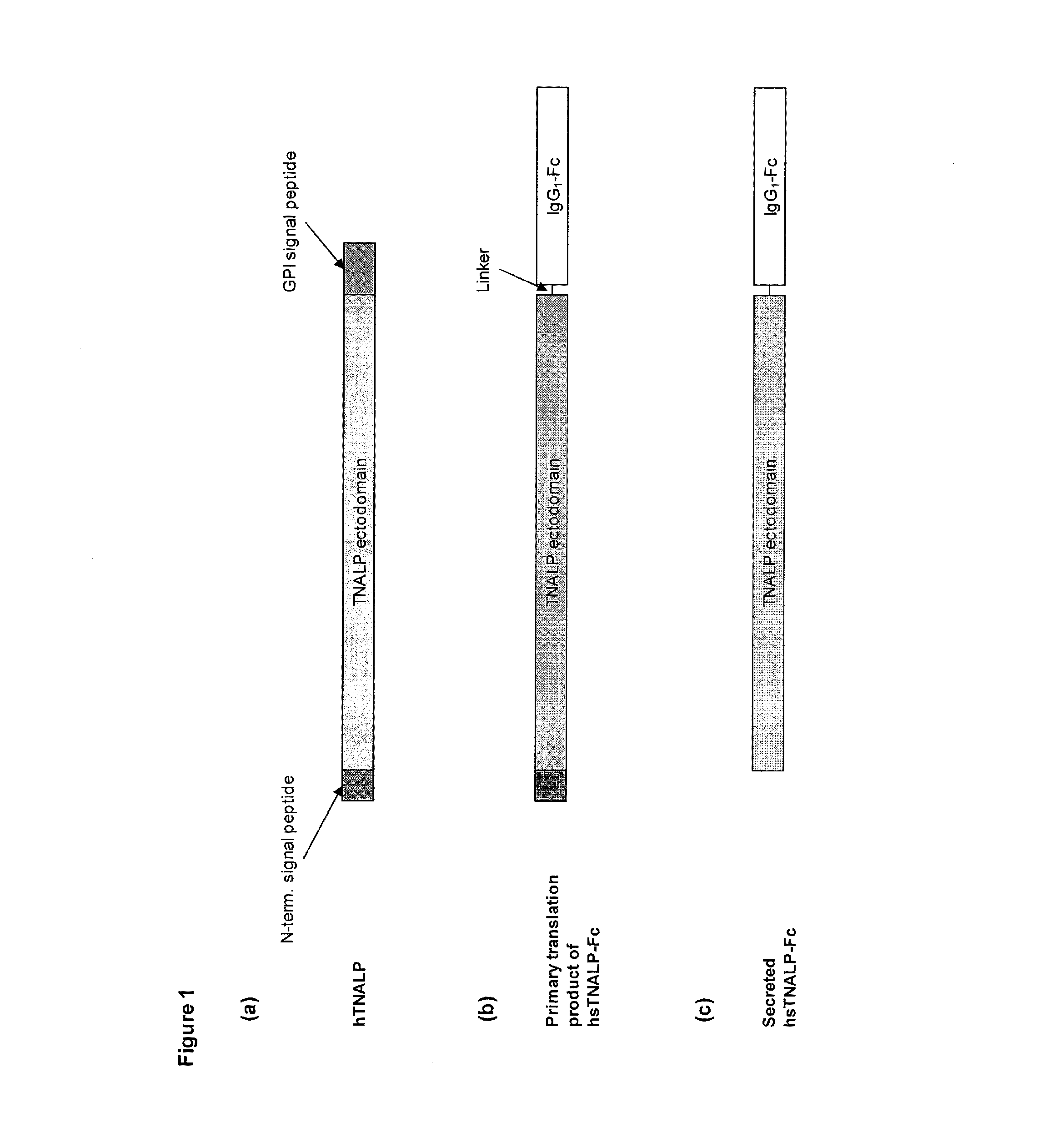
The present invention provides compositions and methods for use in enzyme replacement therapy. The inventors disclose a method of producing membrane bound enzymes in an active soluble form by eliminating the glycosylphosphatidylinositol (GPI) membrane anchor. In particular the inventors disclose a soluble active form of the membrane bound enzyme TNSALP which they produced by deleting the GPI anchor single peptide sequence. They have further shown that this composition is useful for treatment of hypophosphatasia. The inventors also disclose oligo acid amino acid variants thereof which specifically target bone tissue.
Owner:SAINT LOUIS UNIVERSITY +2
Steroidal quinols and their use for estrogen replacement therapy
ActiveUS7300926B2Optimize allocationImprove permeabilityOrganic active ingredientsSteroidsUterusBioavailability
The present invention relates to novel estrogen-related steroidal quinols and their use as drugs for estrogen replacement therapy. The quinols of the present invention provide improved physicochemical properties, increased bioavailability, and improved distribution into tissues, bone, in the cardiovascular system, and in the CNS (central nervous system) with only a slight estrogenic action or no estrogenic action in the uterus. The compounds are suitable for the production of pharmaceutical agents for use in numerous indications (for example, estrogen replacement therapy, prevention and treatment of osteoporosis).
Owner:UNIV OF FLORIDA RES FOUNDATION INC +2
Combination enzyme replacement, gene therapy and small molecule therapy for lysosomal storage diseases
InactiveUS20070280925A1Benefit optimizationReduce disadvantagesBiocidePeptide/protein ingredientsLysosomal enzyme defectGene
This invention provides various combinations of enzyme replacement therapy, gene therapy, and small molecule therapy for the treatment of lysosomal storage diseases.
Owner:GENZYME CORP
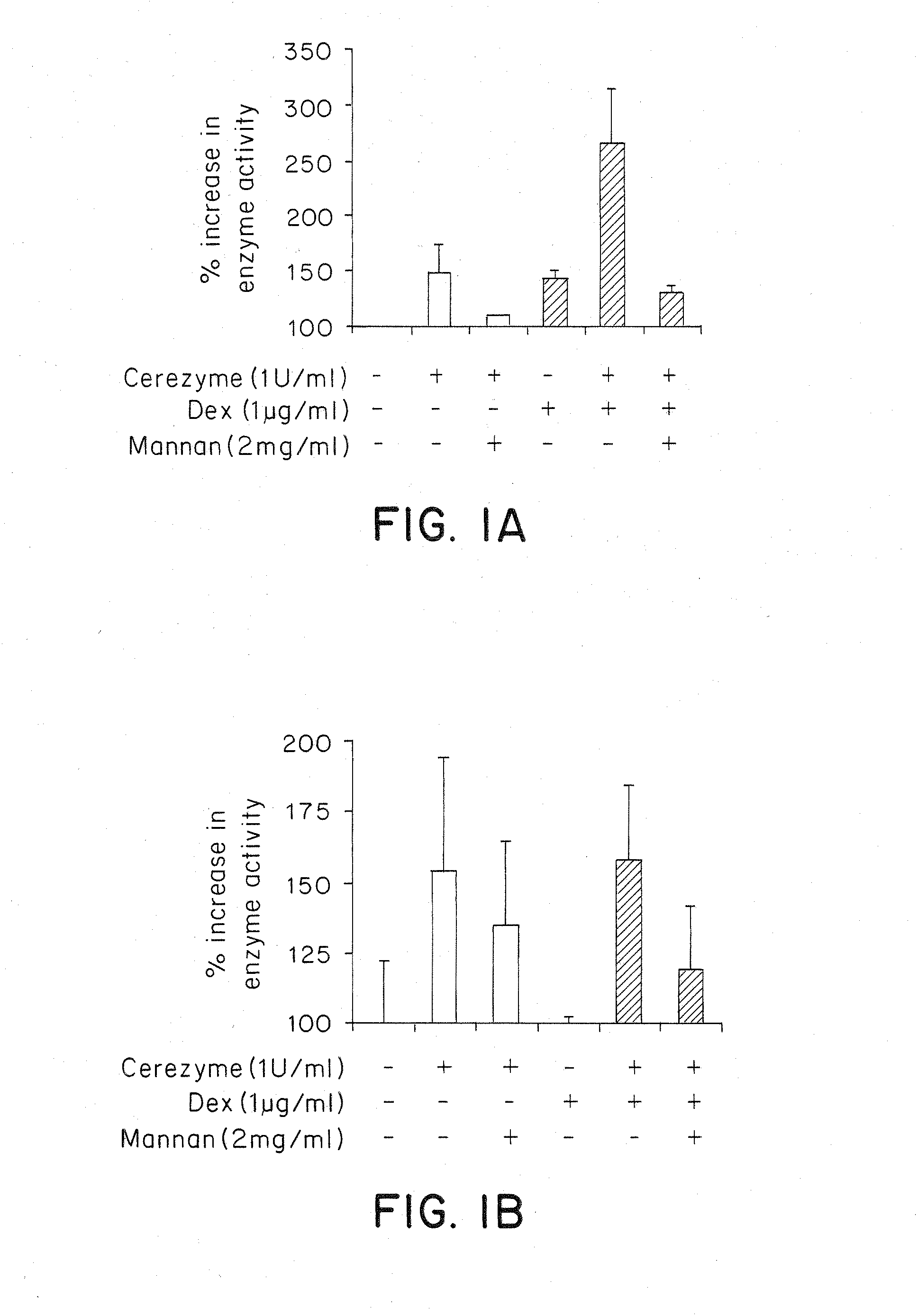
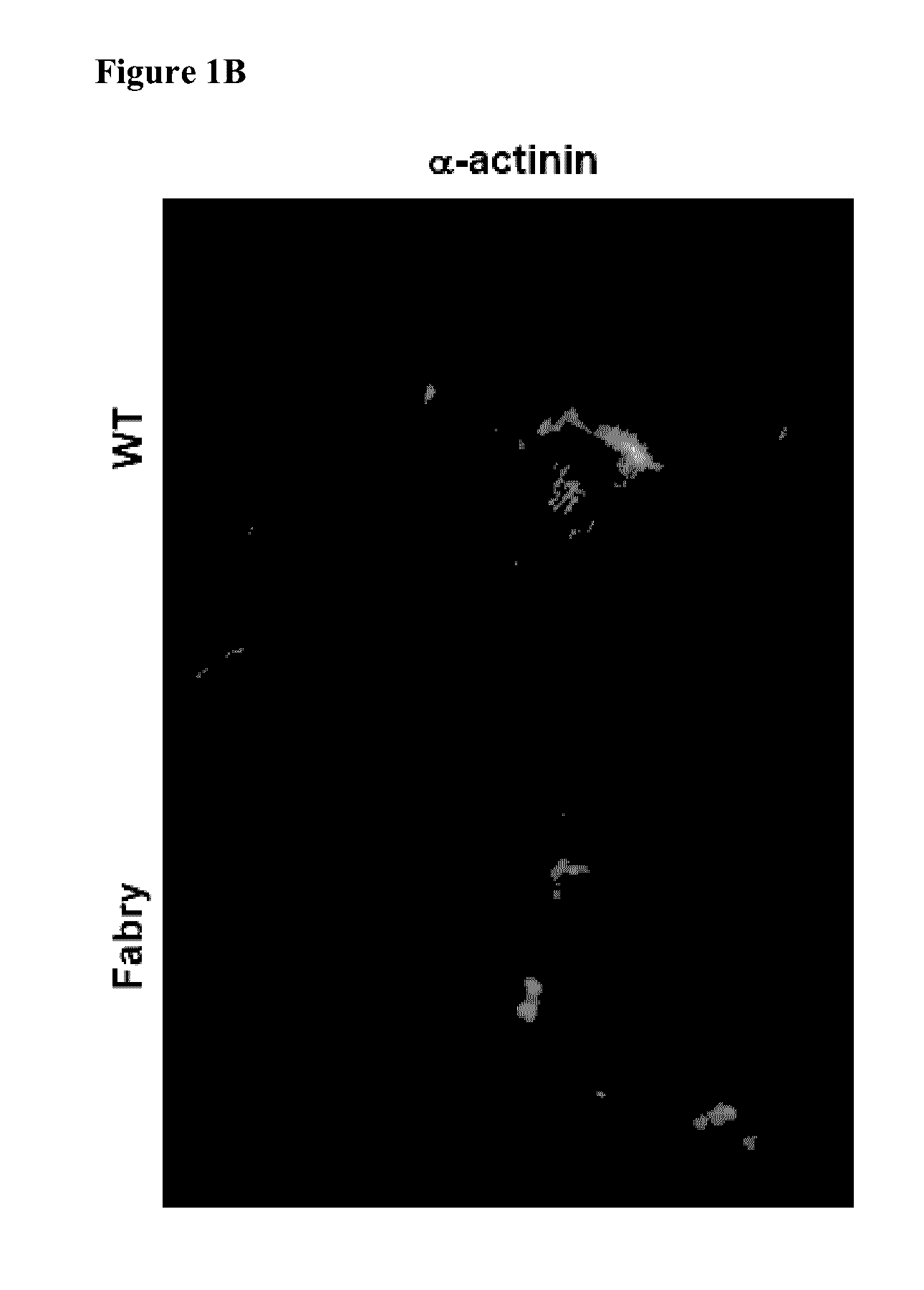
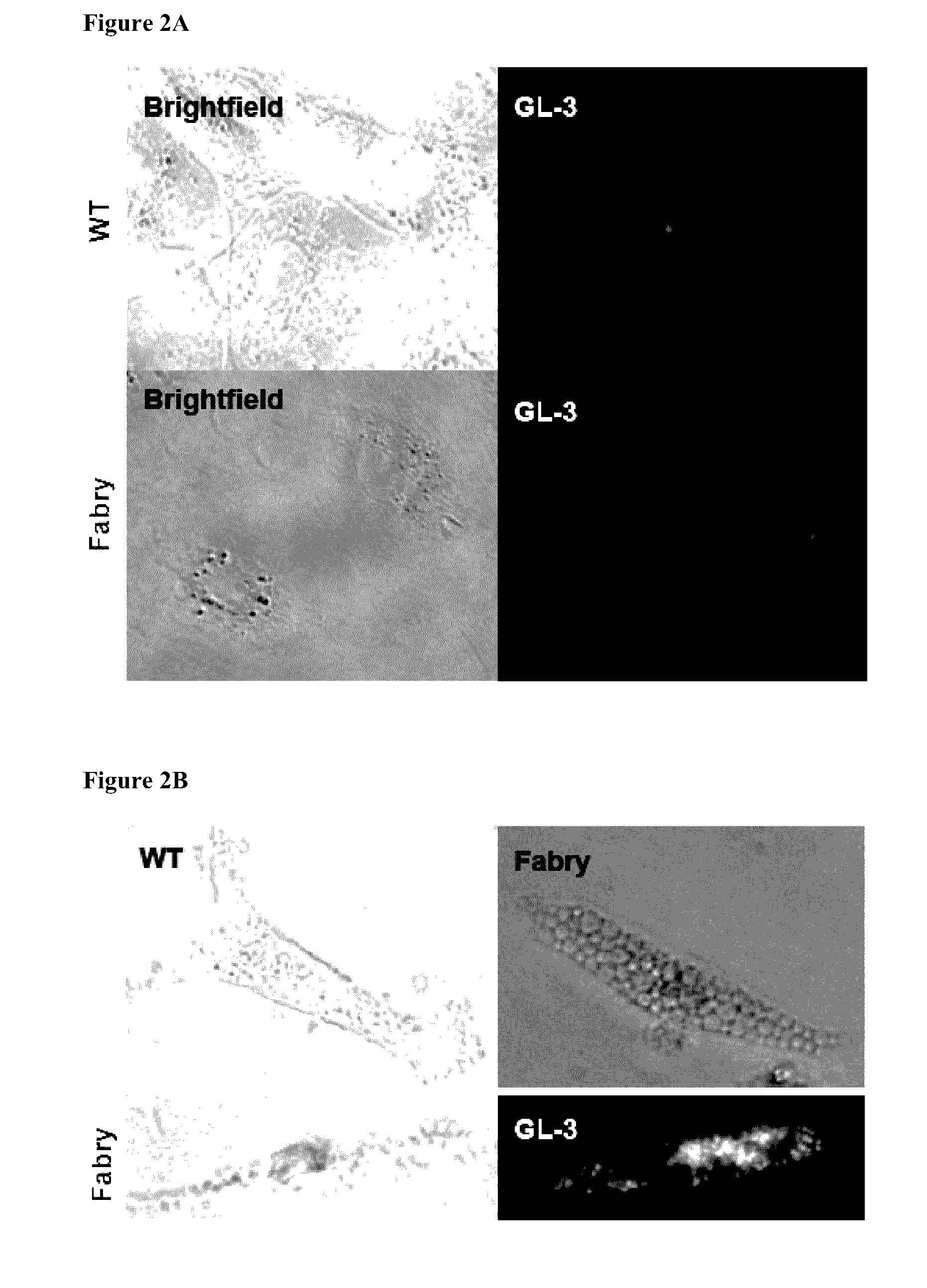
Methods of enhancing lysosomal storage disease therapy by modulation of cell surface receptor density
InactiveUS7658916B2Promote absorptionUptake of extracellular lysosomal enzymes by cells can be increasedBiocidePeptide/protein ingredientsLysosomeFabry disease
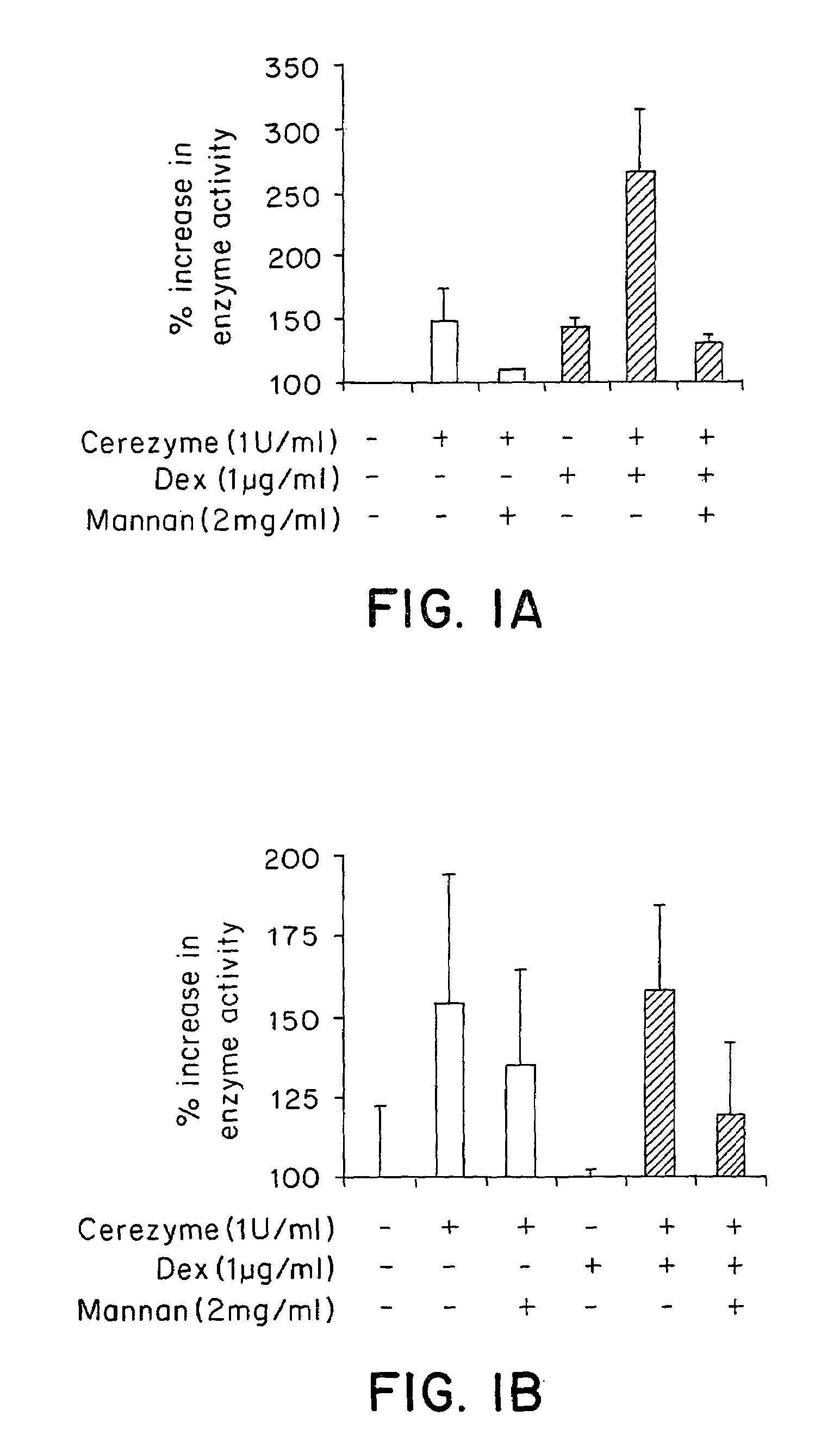
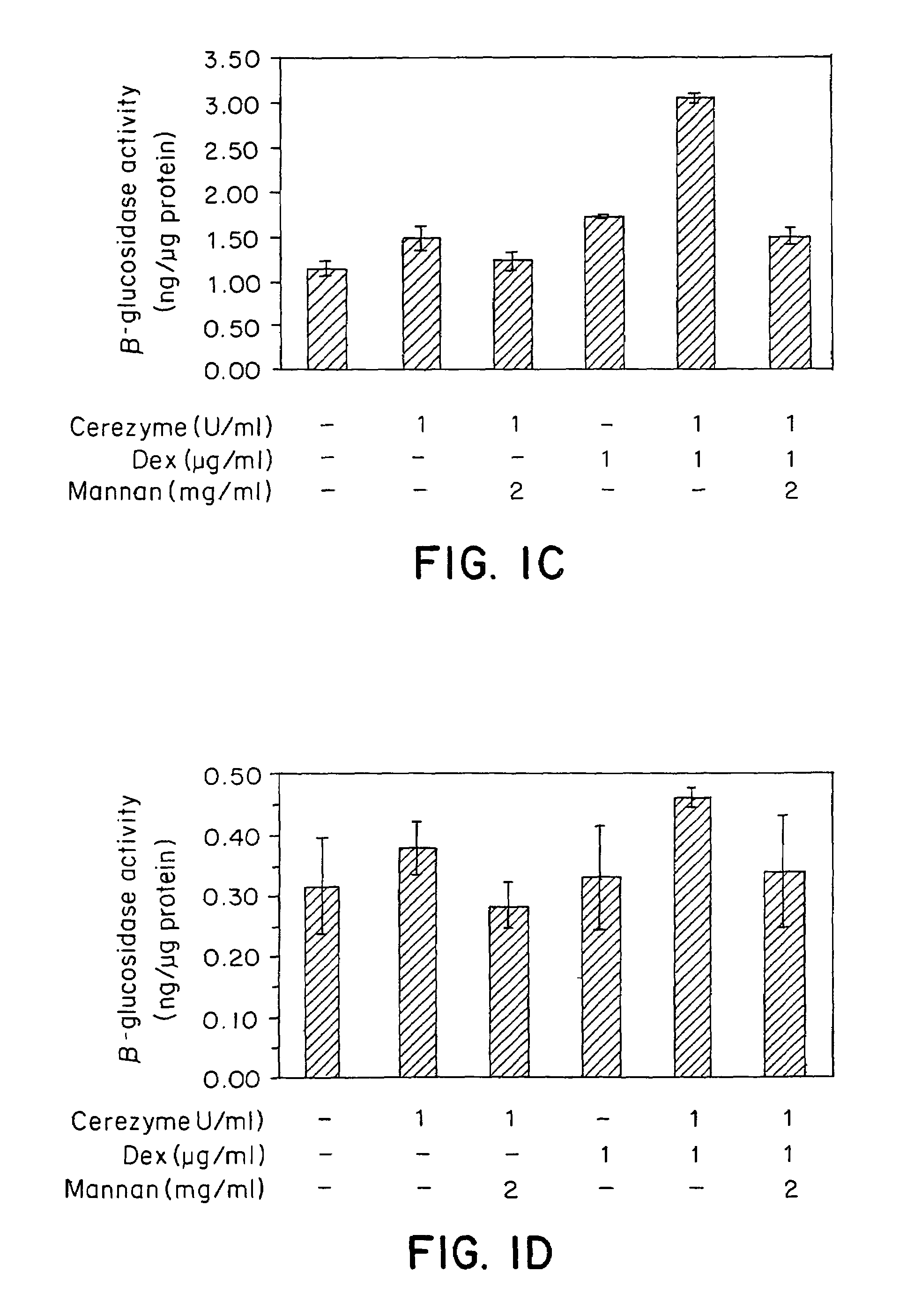
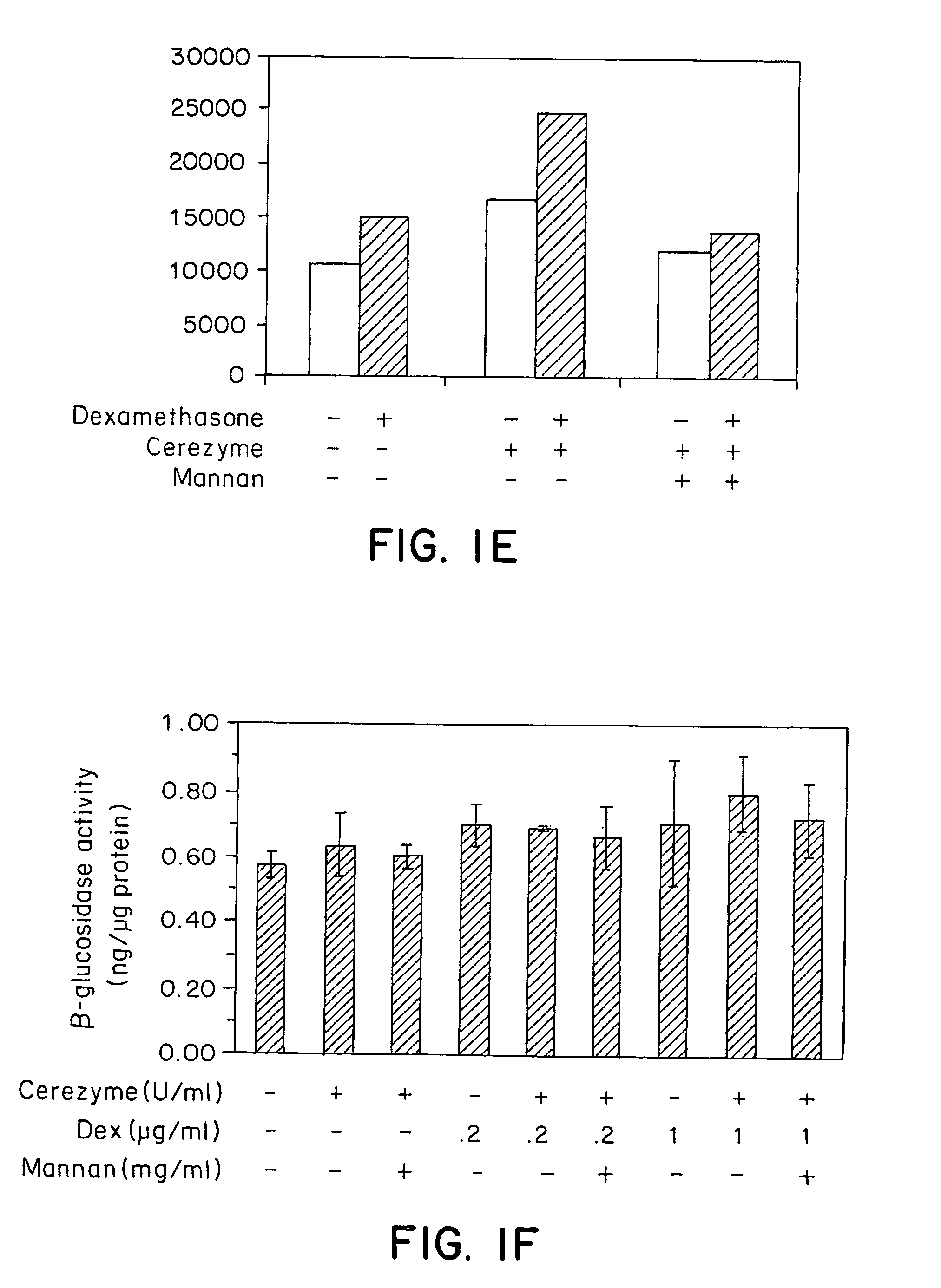
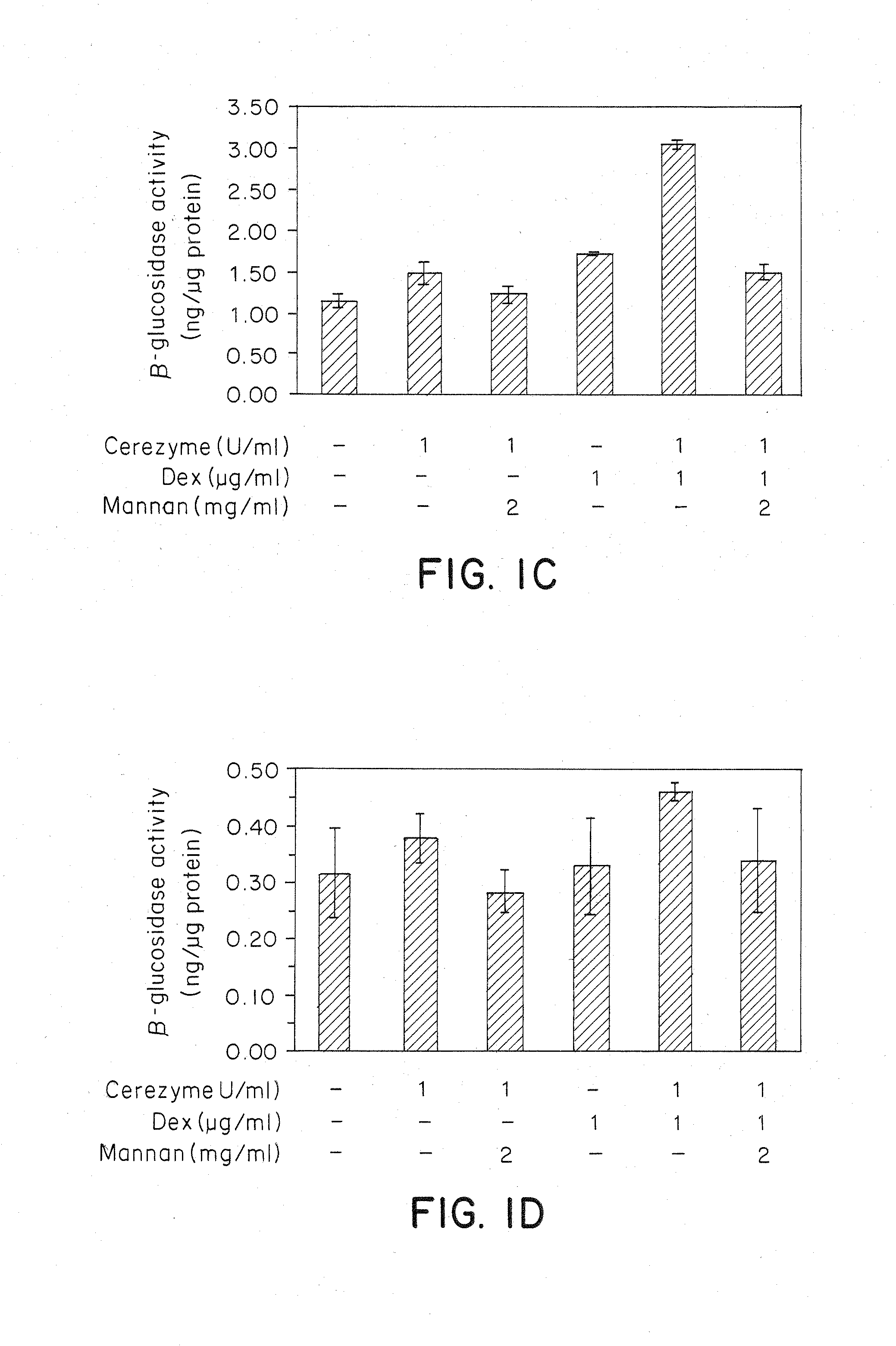
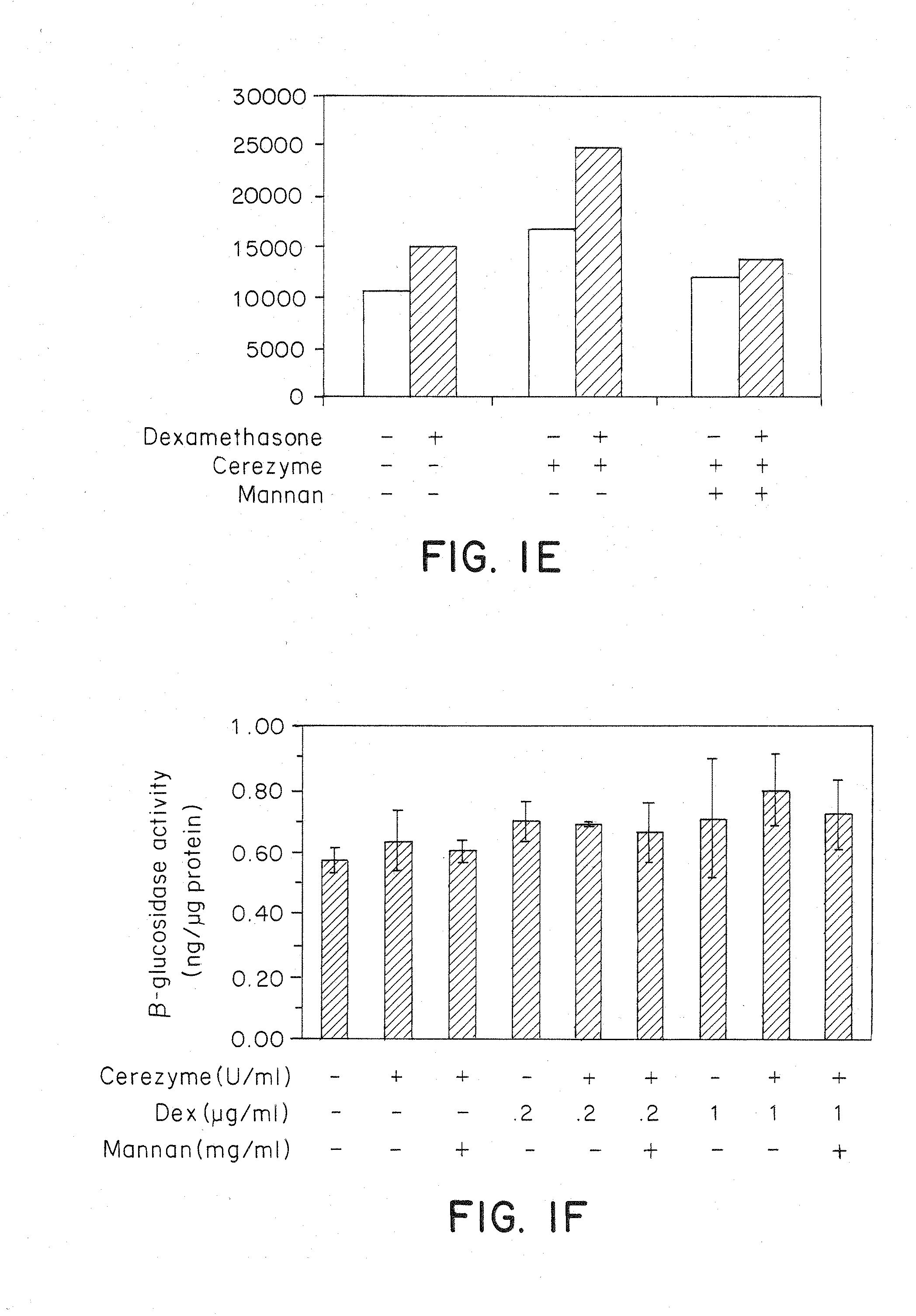
Methods of modulating uptake of extracellular lysosomal enzymes by administering a pharmaceutical agent and methods of treating a lysosomal storage disease (such as Gaucher disease, Pompe disease, Fabry disease or Niemann-Pick disease) or enhancing enzyme replacement therapy or gene therapy, comprising administering a pharmaceutical agent such as dexamethasone, glucose or insulin, are provided.
Owner:GENZYME CORP
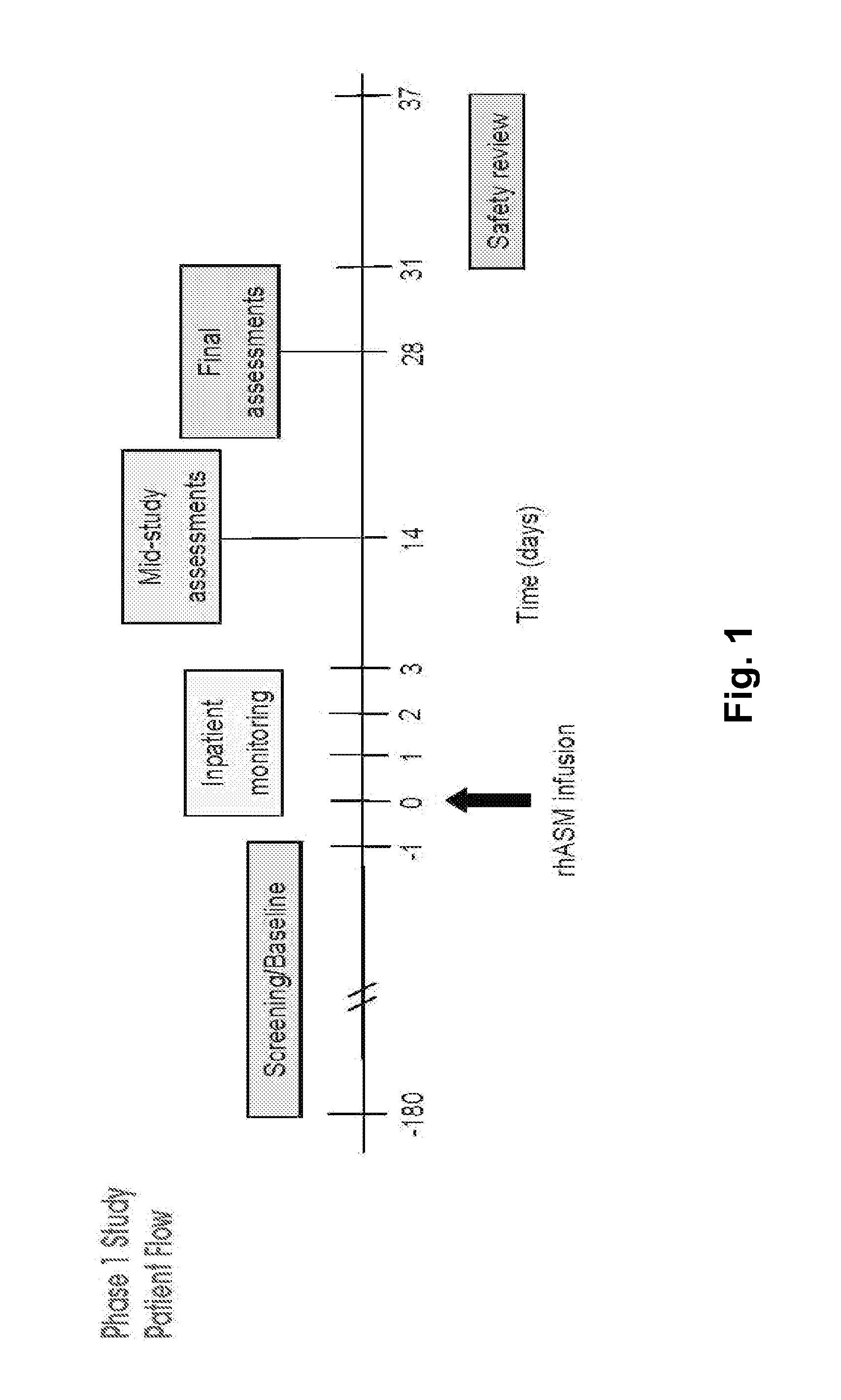
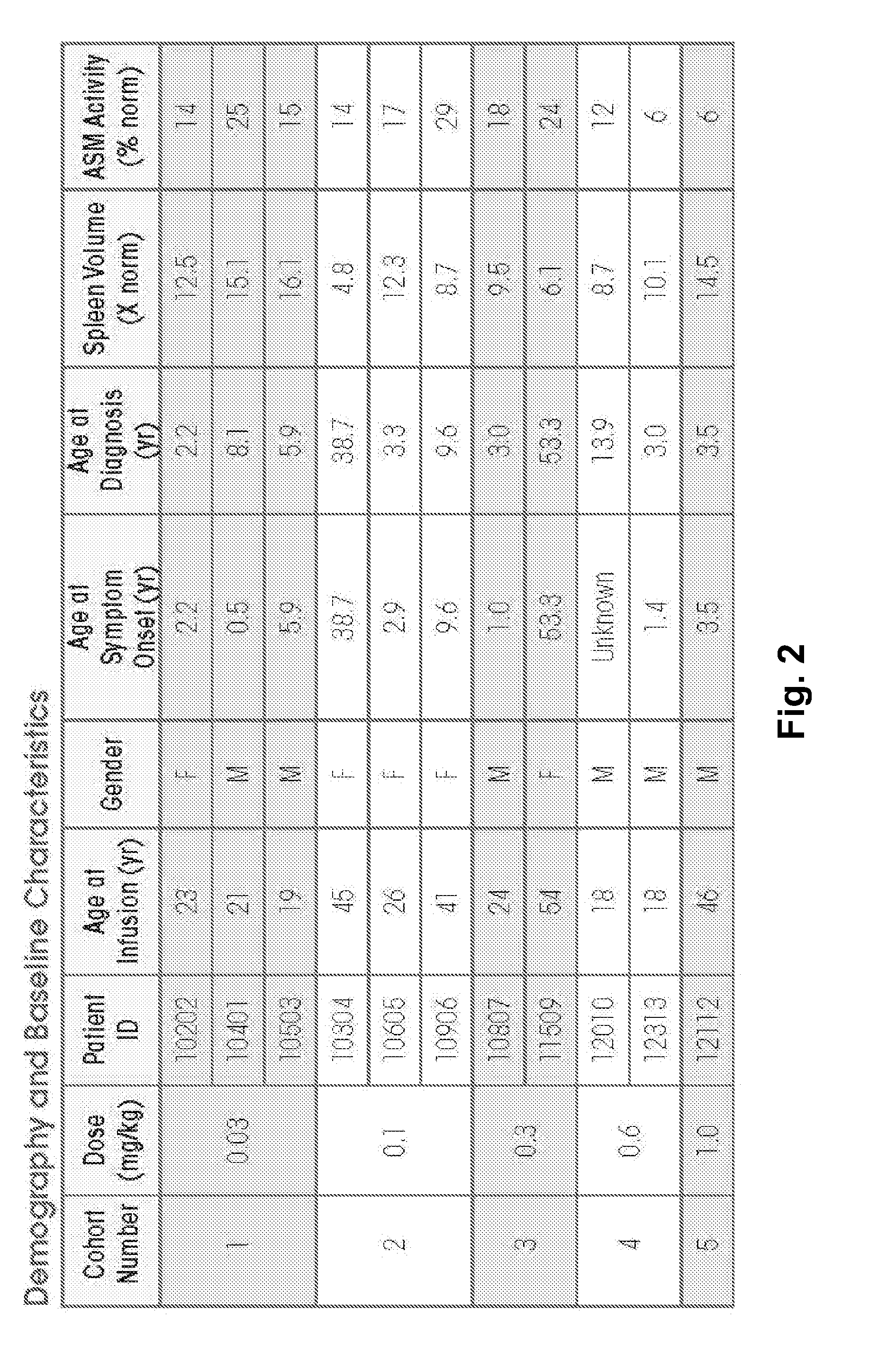
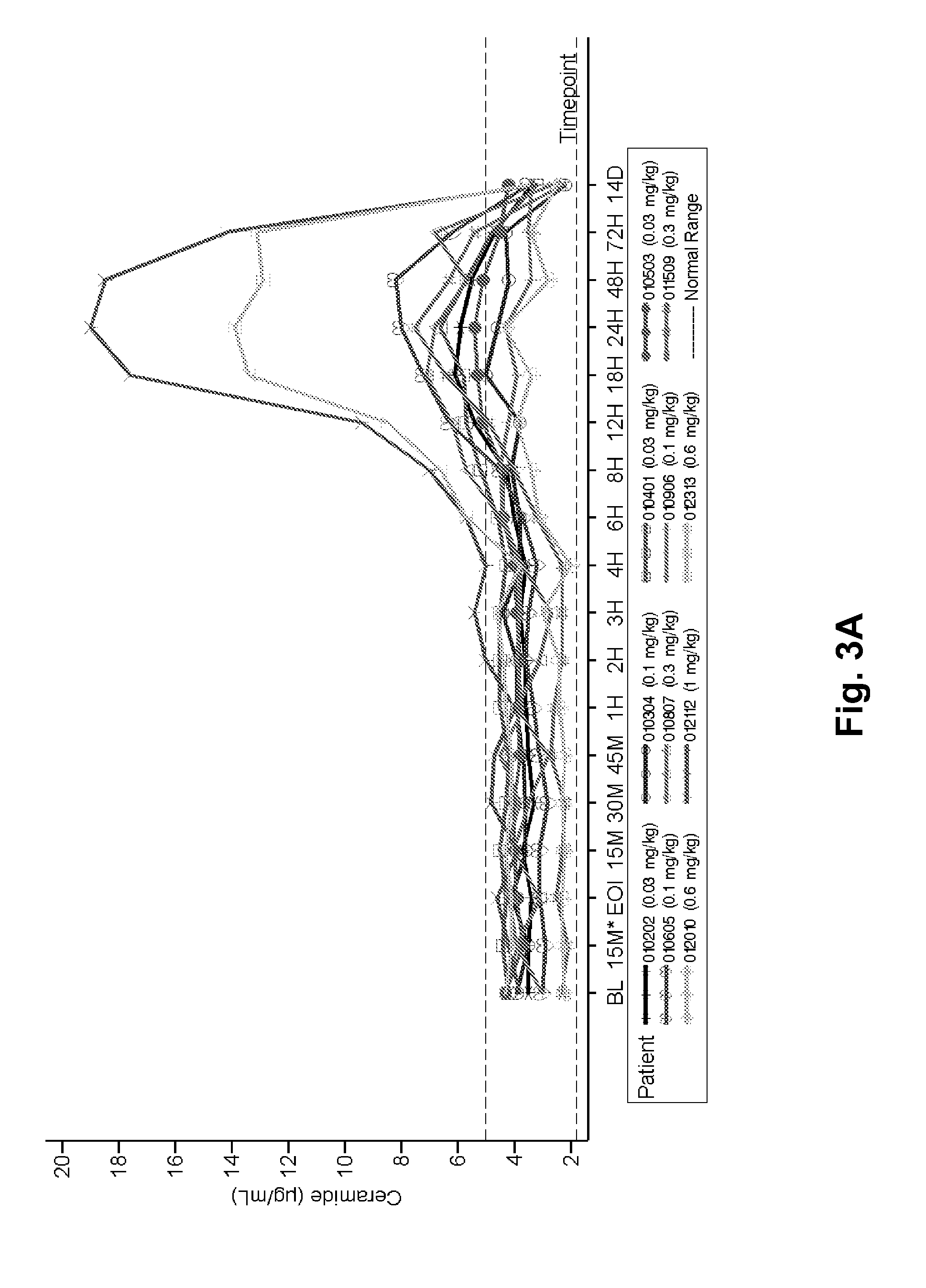
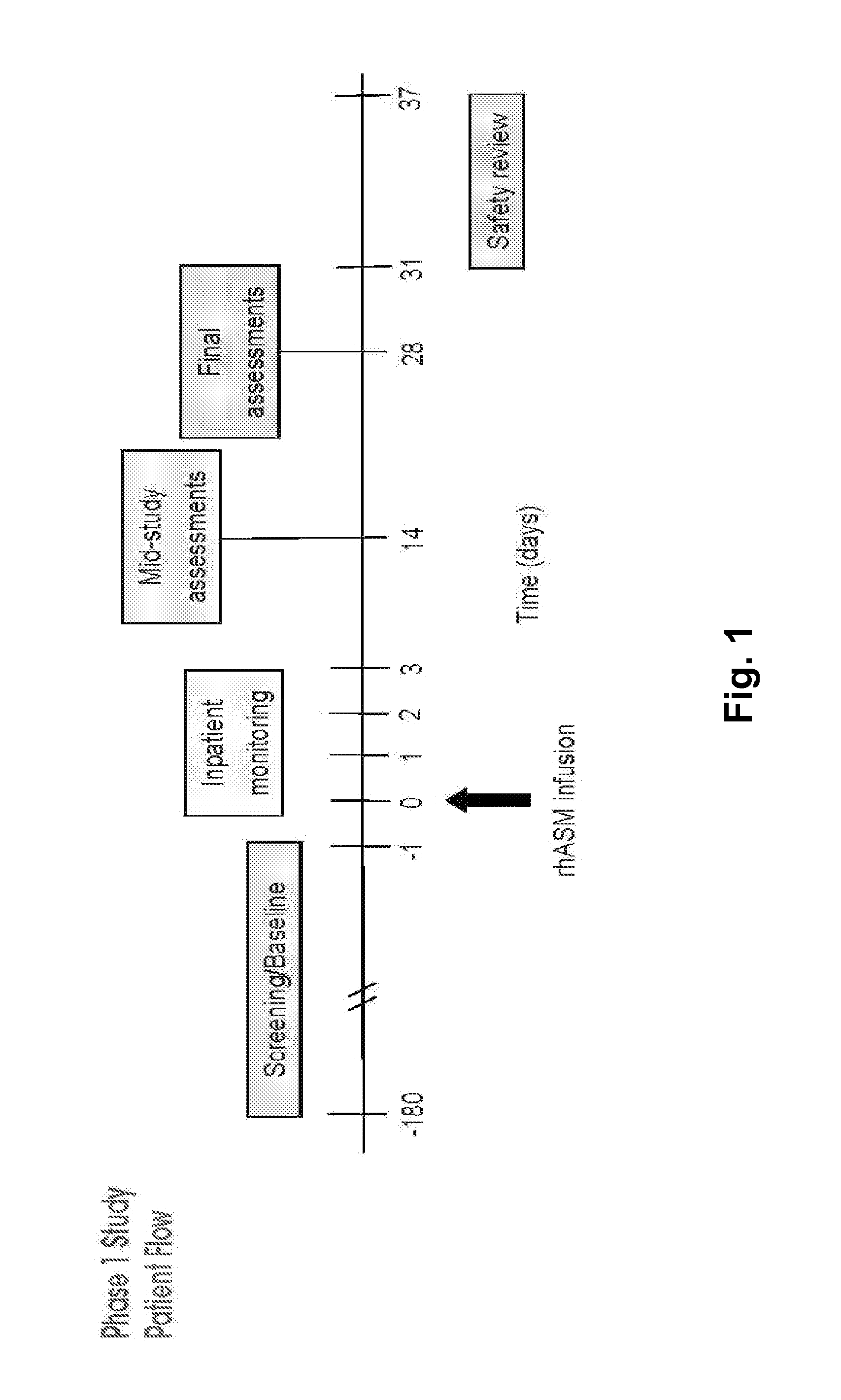
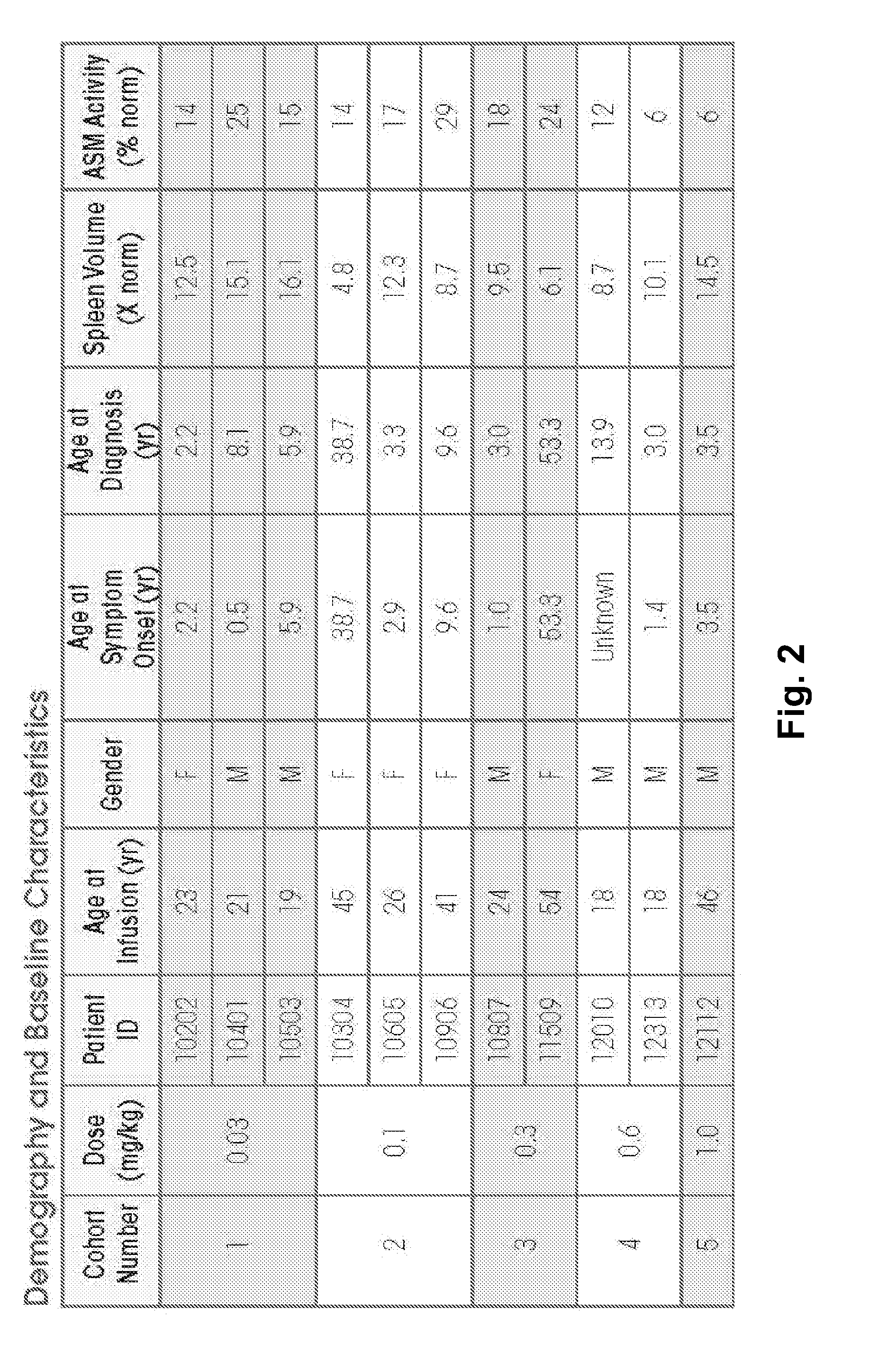
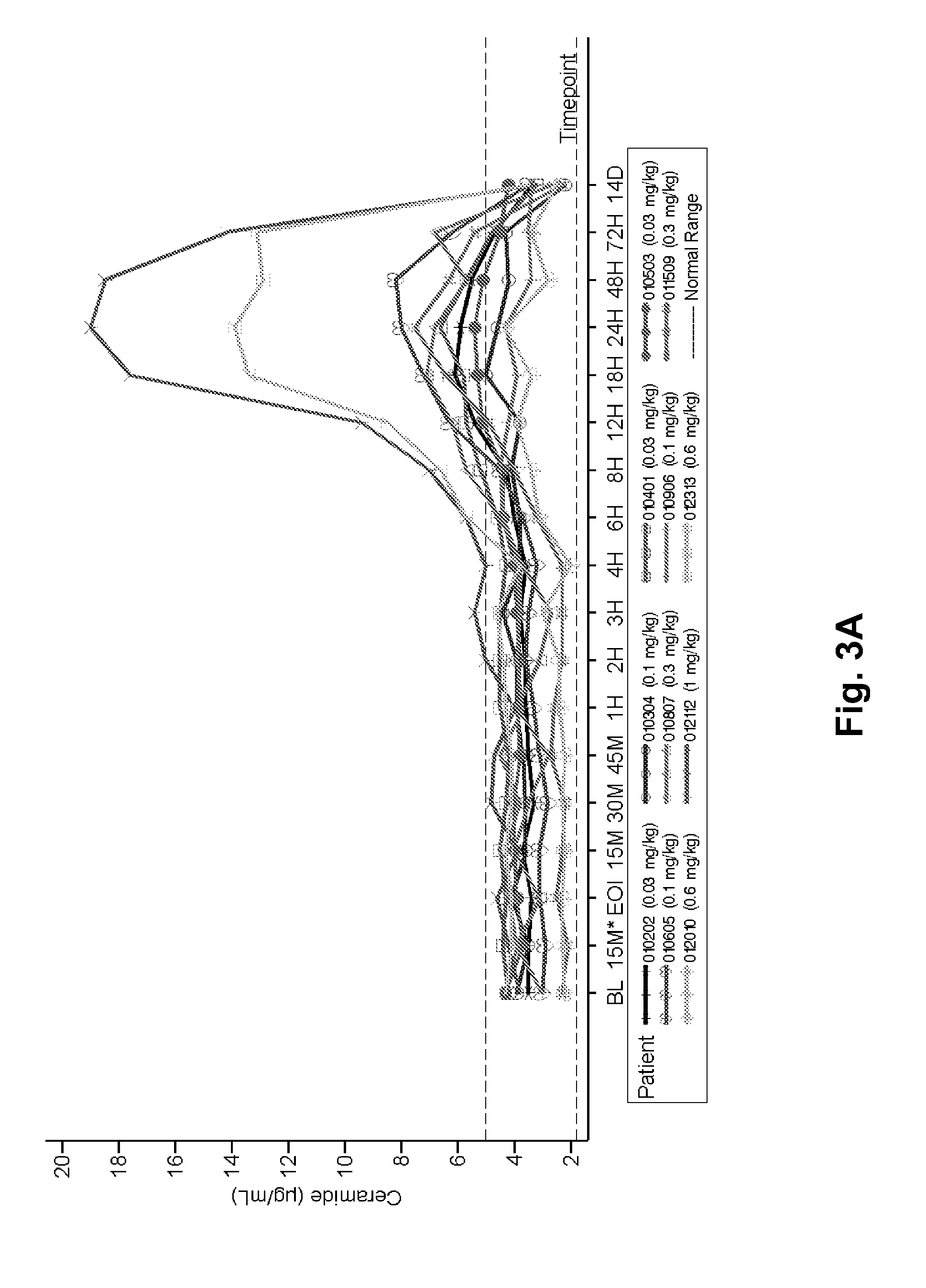
Dose escalation enzyme replacement therapy for treating acid sphingomyelinase deficiency
ActiveUS20110052559A1Avoid rapid degradationLess side effectsNervous disorderHydrolasesNeurological manifestationDose escalation
The invention relates to dose escalation enzyme replacement therapy using acid sphingomyelinase (ASM) for the treatment of human subjects having acid sphingomyelinase deficiency (ASMD), and, in particular, patients with non-neurological manifestations of Niemann-Pick Disease (NPD), and in certain embodiments, NPD type B.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Formulations for Lysosomal Enzymes
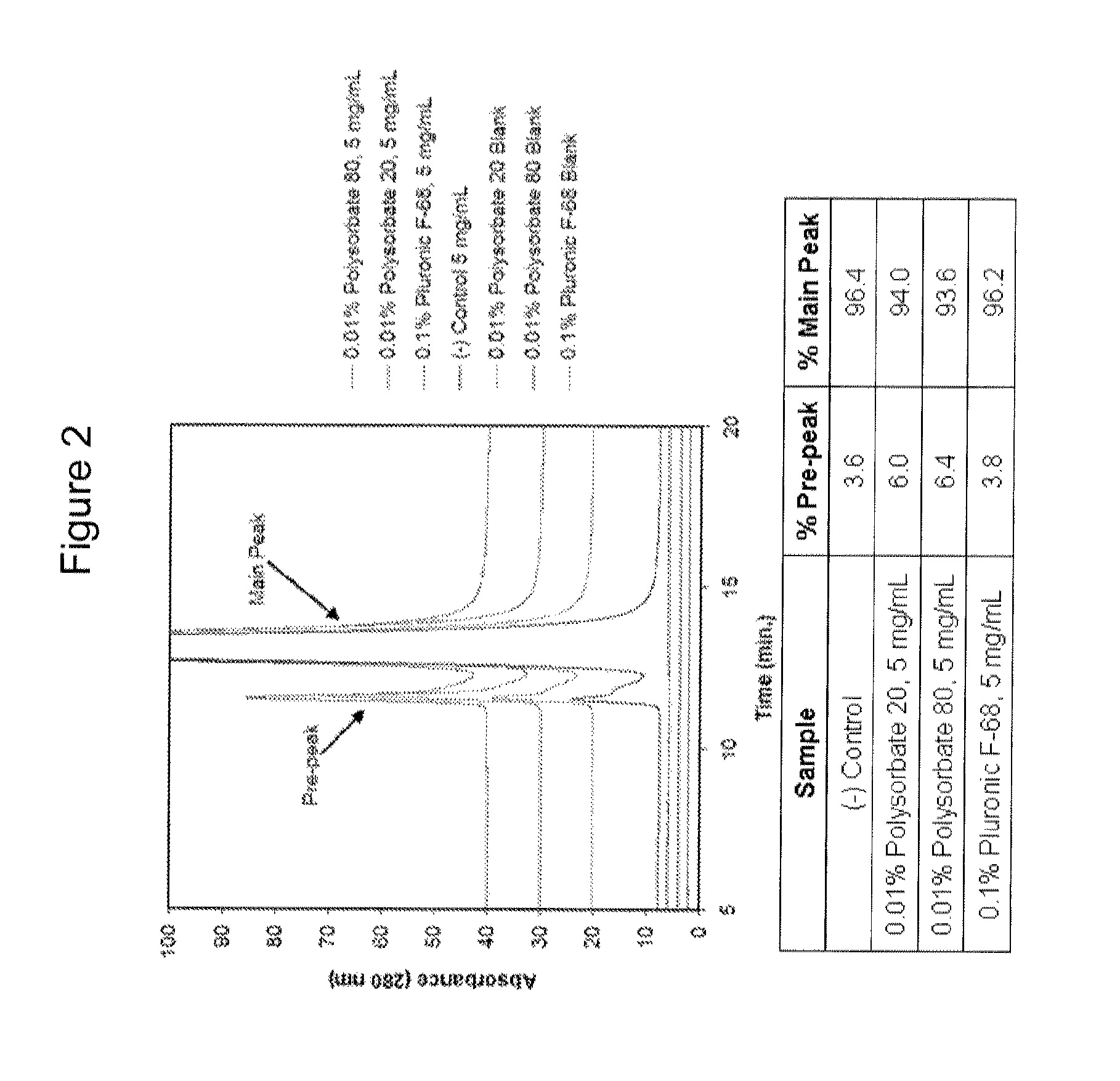
ActiveUS20120148556A1Preserve and enhance stabilityPreserve and enhance and efficacyPeptide/protein ingredientsMetabolism disorderAcid alpha-glucosidaseLysosome
The present invention provides improved formulations for lysosomal enzymes useful for enzyme replacement therapy. Among other things, the present invention provides formulations that preserve or enhance the stability and / or efficacy of a lysosomal enzyme such as acid alpha-glucosidase.
Owner:BIOMARIN PHARMA INC +1
Aminoglycoside treatment for lysosomal storage diseases
ActiveUS20050153906A1Effective treatmentHigh activityBiocideSugar derivativesLysosomal enzyme defectHurler syndrome
The present invention provides a method of treating lysosomal storage diseases such as Hurler syndrome and Batten disease in individuals in need of such treatment, comprising the step of administering to said individuals a therapeutically effective dose of an aminoglycoside. In addition, this method may further comprise treating the individual with enzyme replacement therapy. Furthermore, the present invention provides method of pharmacologically suppressing premature stop mutations in an individual with these lysosomal storage diseases, comprising the step of administering to said individual a pharmacologically effective dose of an aminoglycoside.
Owner:UAB RES FOUND
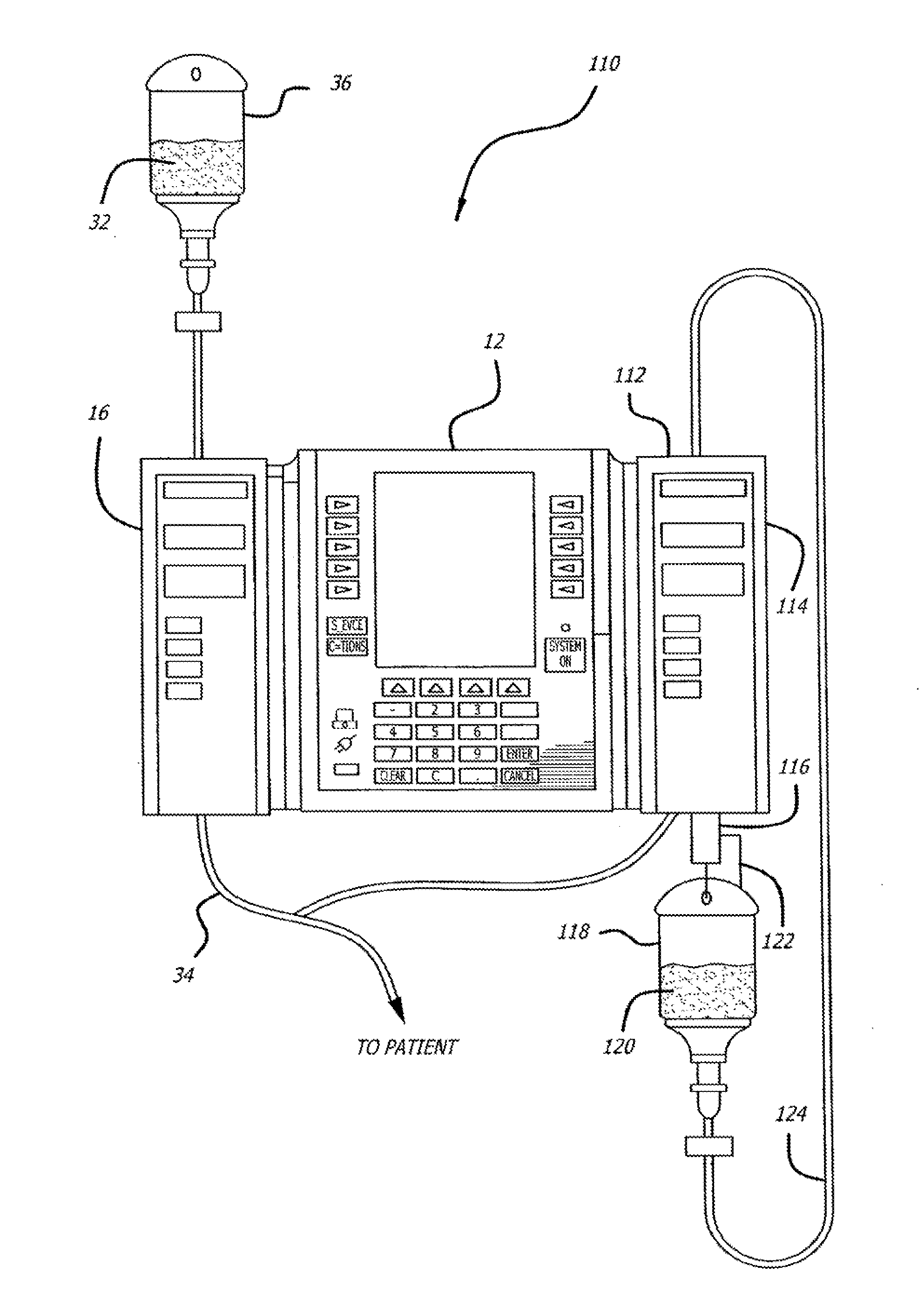
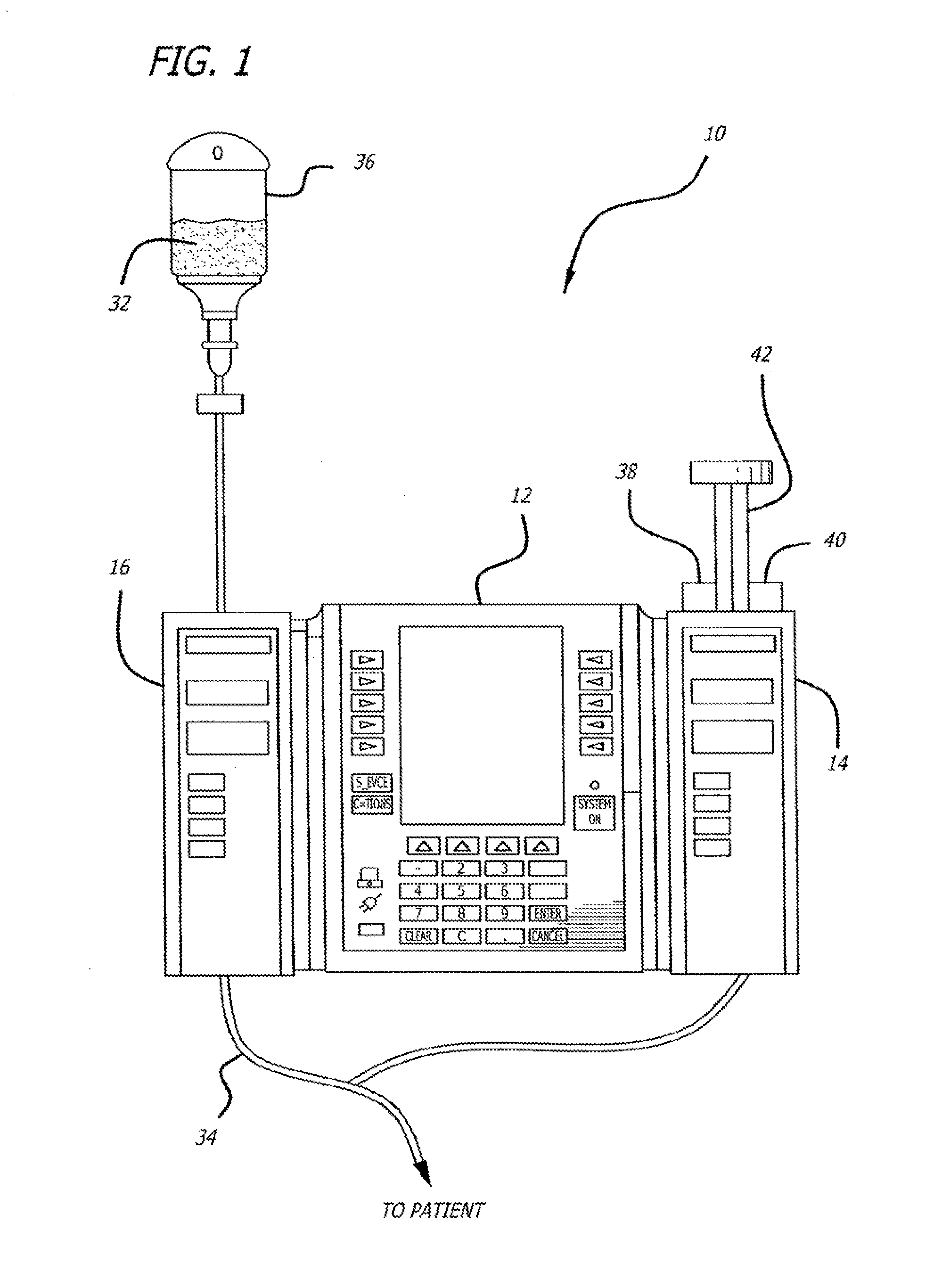
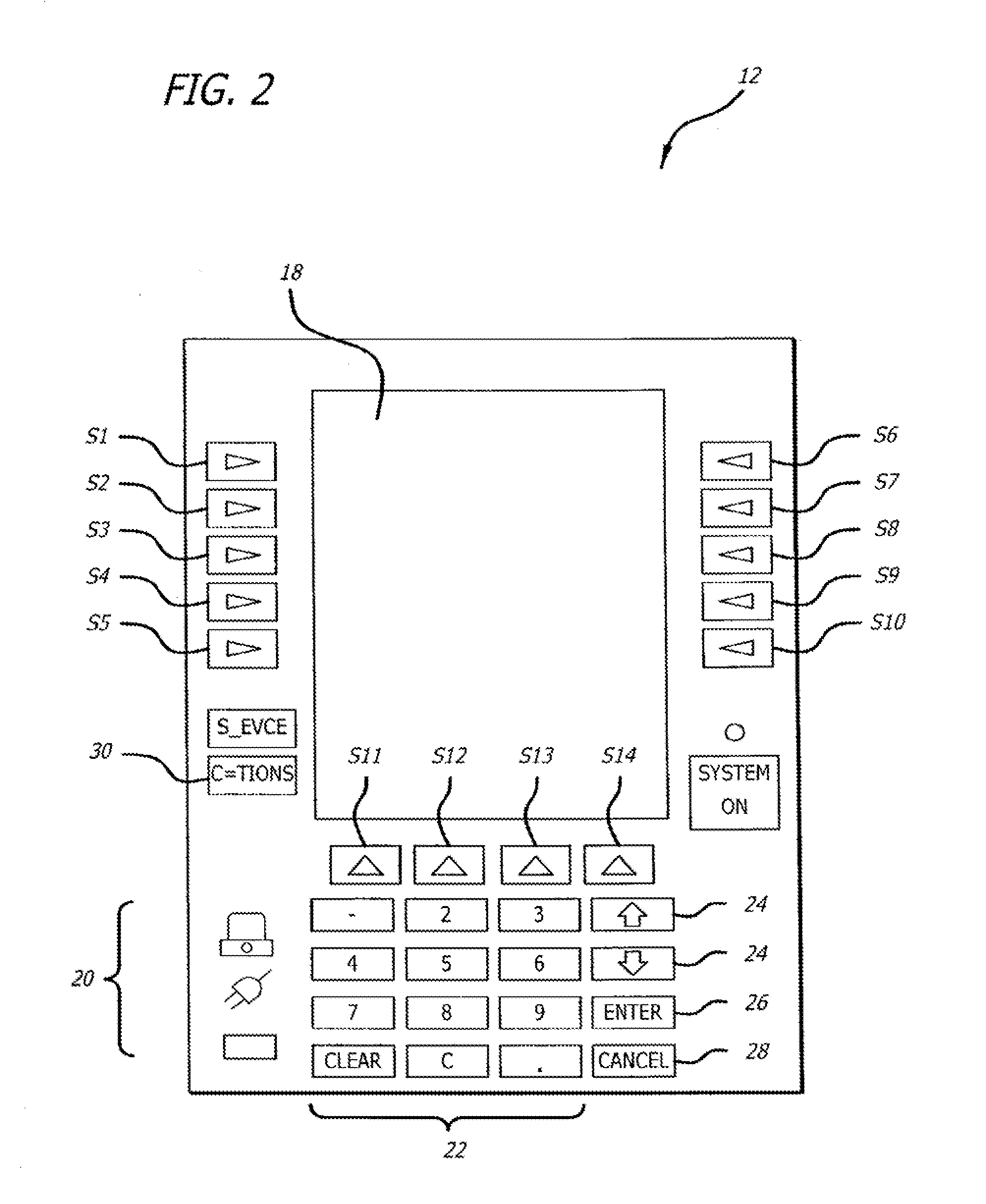
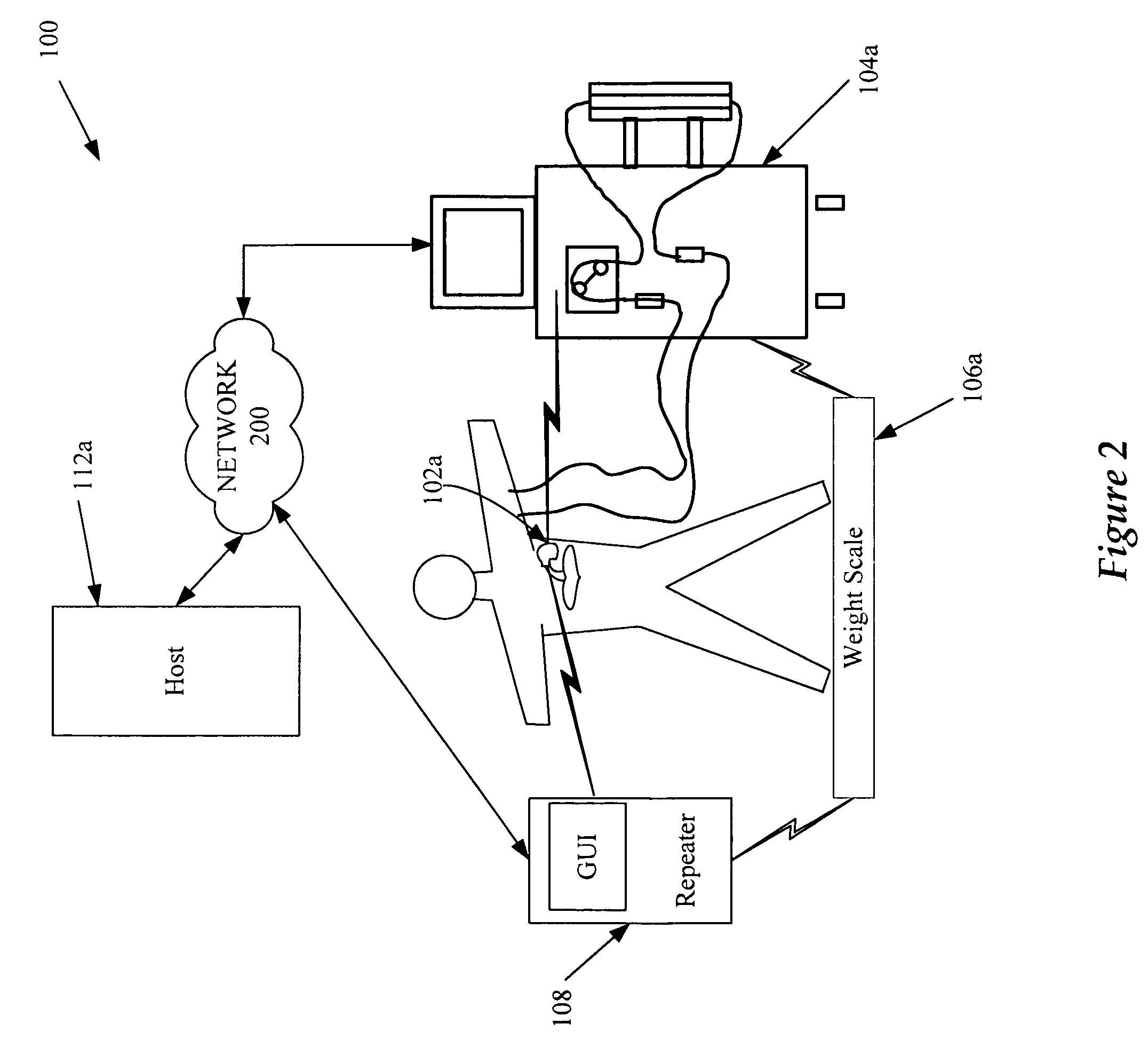
Multichannel infusion system for renal replacement therapy
InactiveUS20130150821A1Other blood circulation devicesDialysis systemsMeasurement deviceControl system
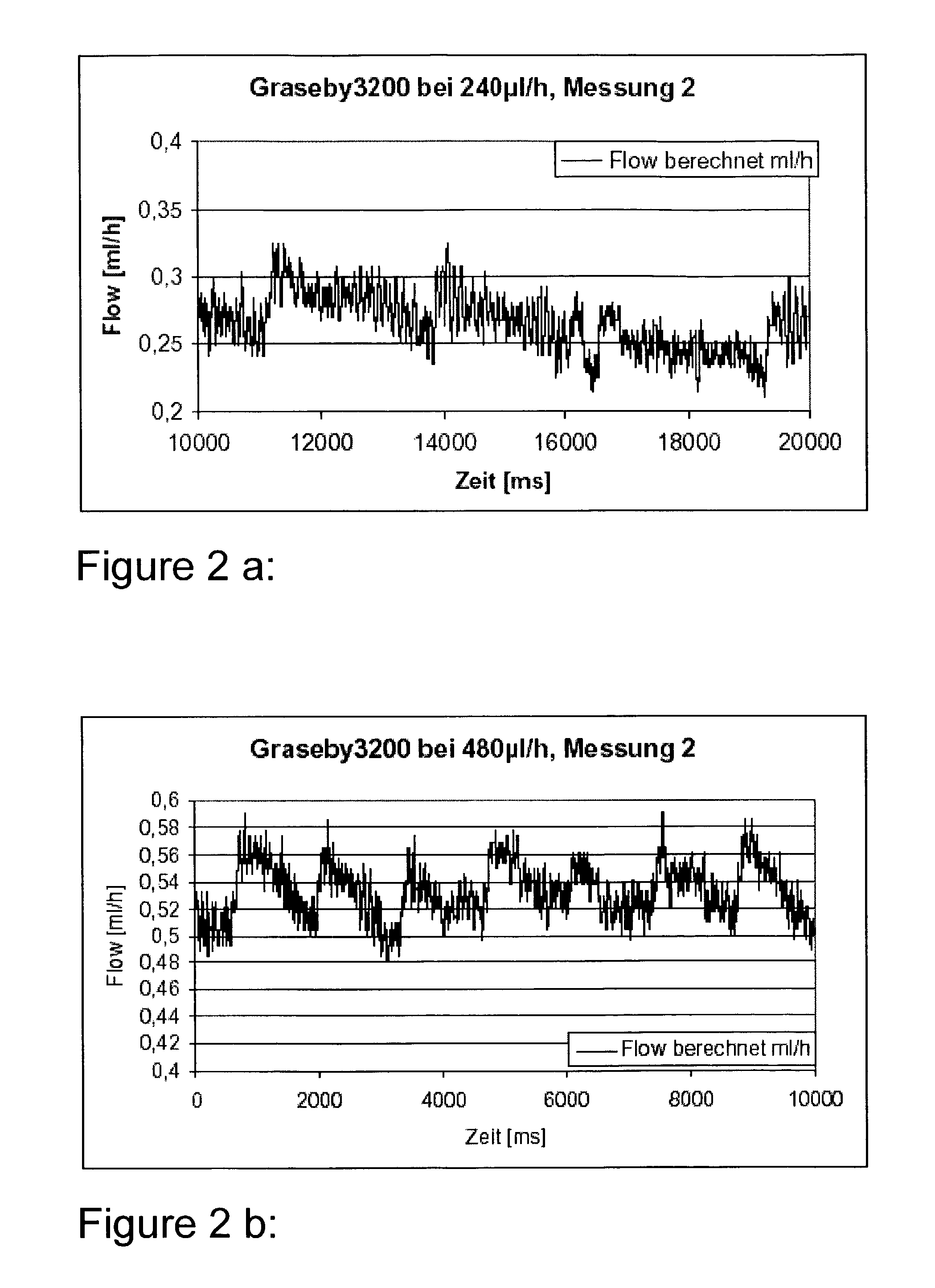
A multichannel infusion system for use with a hemofilter includes first and second measurement devices that measure the weight of a replacement fluid in a first receptacle and an ultrafiltrate in a second receptacle, a first pumping module configured to accept a first desired flow rate and deliver the replacement fluid from the first receptacle to the hemofilter at the accepted first desired flow rate, a second pumping module configured to accept a second desired flow rate and remove the ultrafiltrate from the hemofilter to the second receptacle at the accepted second desired flow rate, and a control system in communication with the first and second measurement units and the first and second pumping modules. The control system is configured to adjust the first and second desired flow rates based on the measurements made by the first and second measurement devices.
Owner:CAREFUSION 303 INC
Pharmaceutical composition for enzyme replacement therapy
ActiveUS20100291059A1Increase doseEffective absorptionSenses disorderSugar derivativesSide effectWild type
Owner:ALTIF LAB +1
Compositions and methods for treating hypophosphatasia
ActiveUS20090238814A1Extend your lifeWeight increaseHydrolasesPeptide/protein ingredientsMembrane bound enzymeBone tissue
The present invention provides compositions and methods for use in enzyme replacement therapy. The inventors disclose a method of producing membrane bound enzymes in an active soluble form by eliminating the glycosylphosphatidylinositol (GPI) membrane anchor. In particular the inventors disclose a soluble active form of the membrane bound enzyme TNSALP which they produced by deleting the GPI anchor single peptide sequence. They have further shown that this composition is useful for treatment of hypophosphatasia. The inventors also disclose oligo acid amino acid variants thereof which specifically target bone tissue.
Owner:SAINT LOUIS UNIVERSITY +2
Portable equipment for administration of fluids into tissues and tumors by convection enhanced delivery technique
InactiveUS7963956B2Efficient deliveryImprove bioavailabilityMedical devicesPharmaceutical delivery mechanismEngineeringConvection-Enhanced Delivery
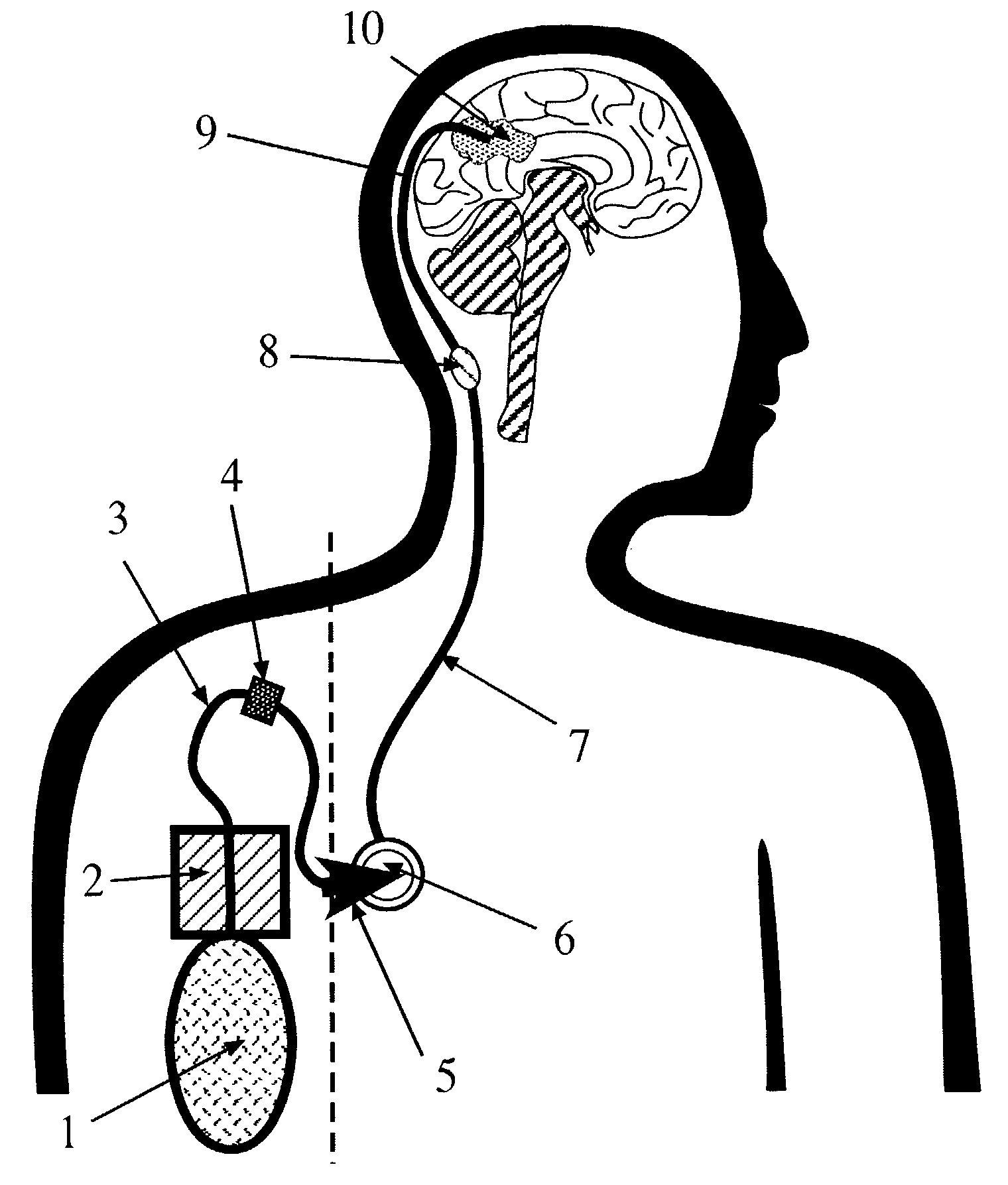
The present invention is directed to a device for a portable convection enhanced delivery system that allows administering liquids to specific locations within the body, especially tissues and tumors also allowing outsubject treatment. The application system comprises a portable extracorporal pump with a fluid reservoir that is connected via an infusion system to an infusion catheter placeable to any tissue or tumor the fluid should be administered to by high flow microperfusion. The system enables administration of fluids of any kind by convection enhanced delivery also in out-patient treatment. The system can be used for delivering various drugs, proteins, protein toxins, antibodies-for treatment or imaging, proteins in enzyme replacement therapy, growth factors and viruses or oligonucleotides in gene therapy etc. The application methods as well as the surgical method to implant this device are enclosed to this invention.
Owner:ANTISENSE PHARMA GMBH
Lipid Vesicle Composition
InactiveUS20090297592A1Excellent in stability in bloodEasy to usePeptide/protein ingredientsMetabolism disorderLipid bilayerOptic vesicle
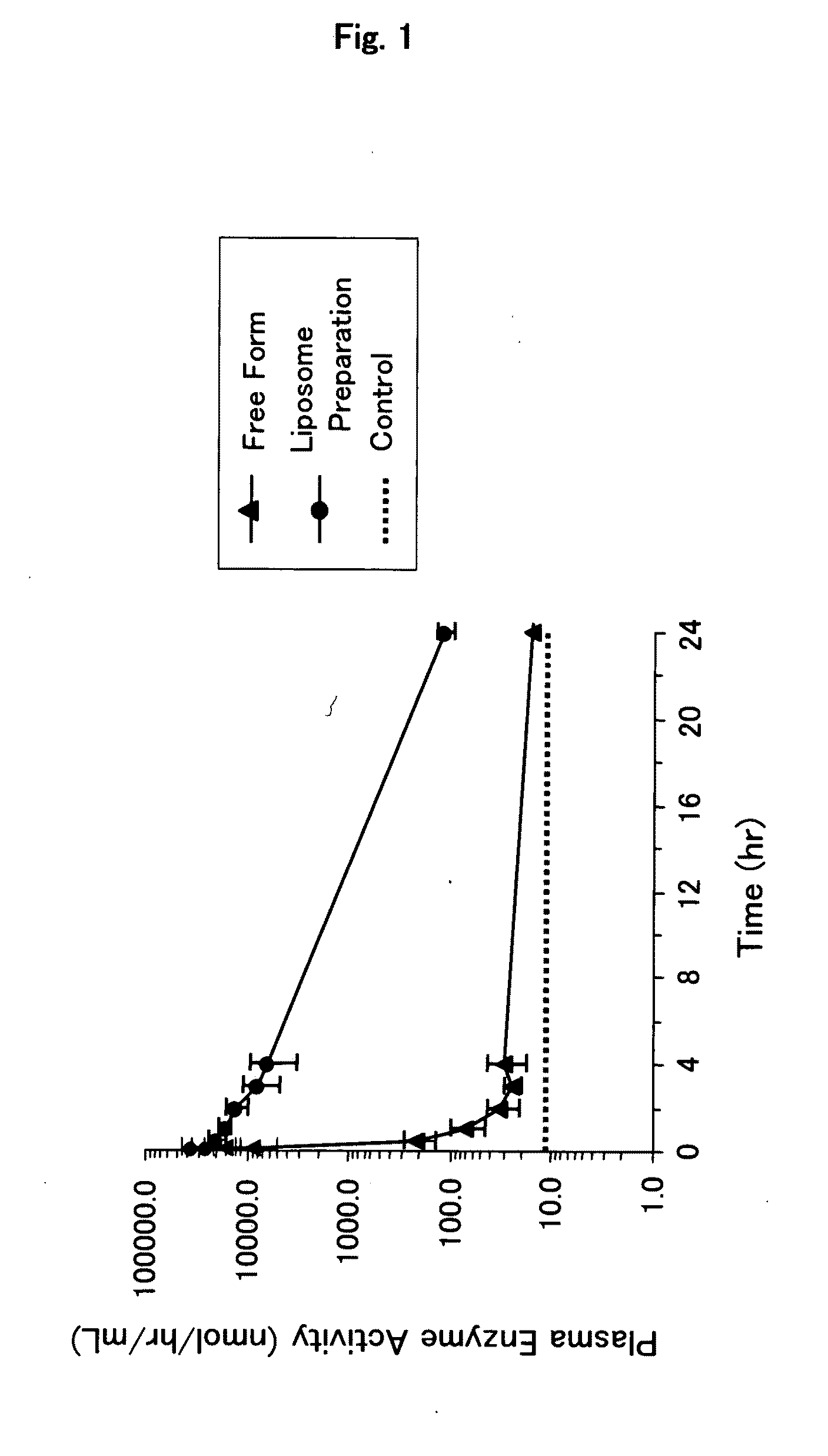
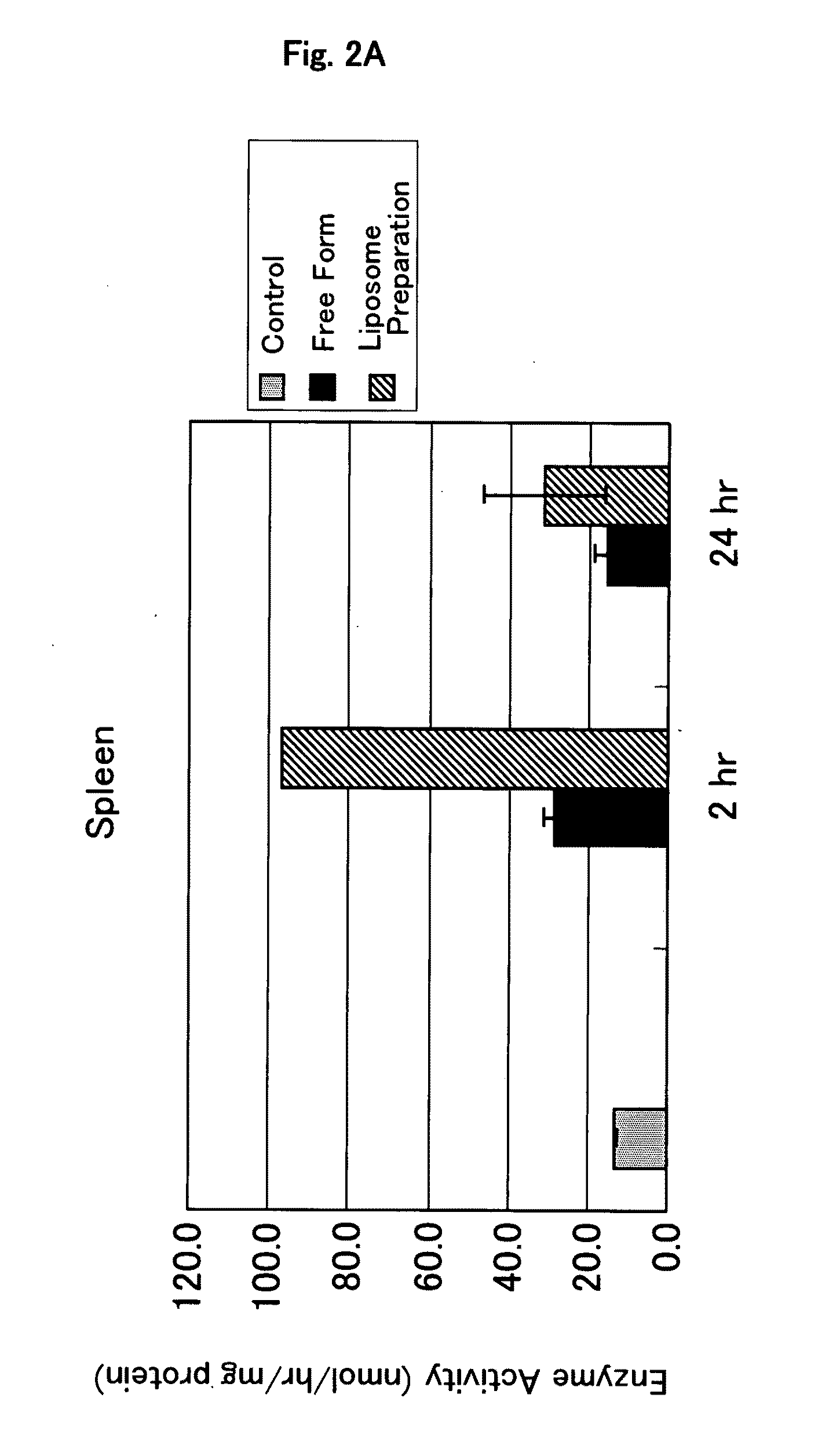
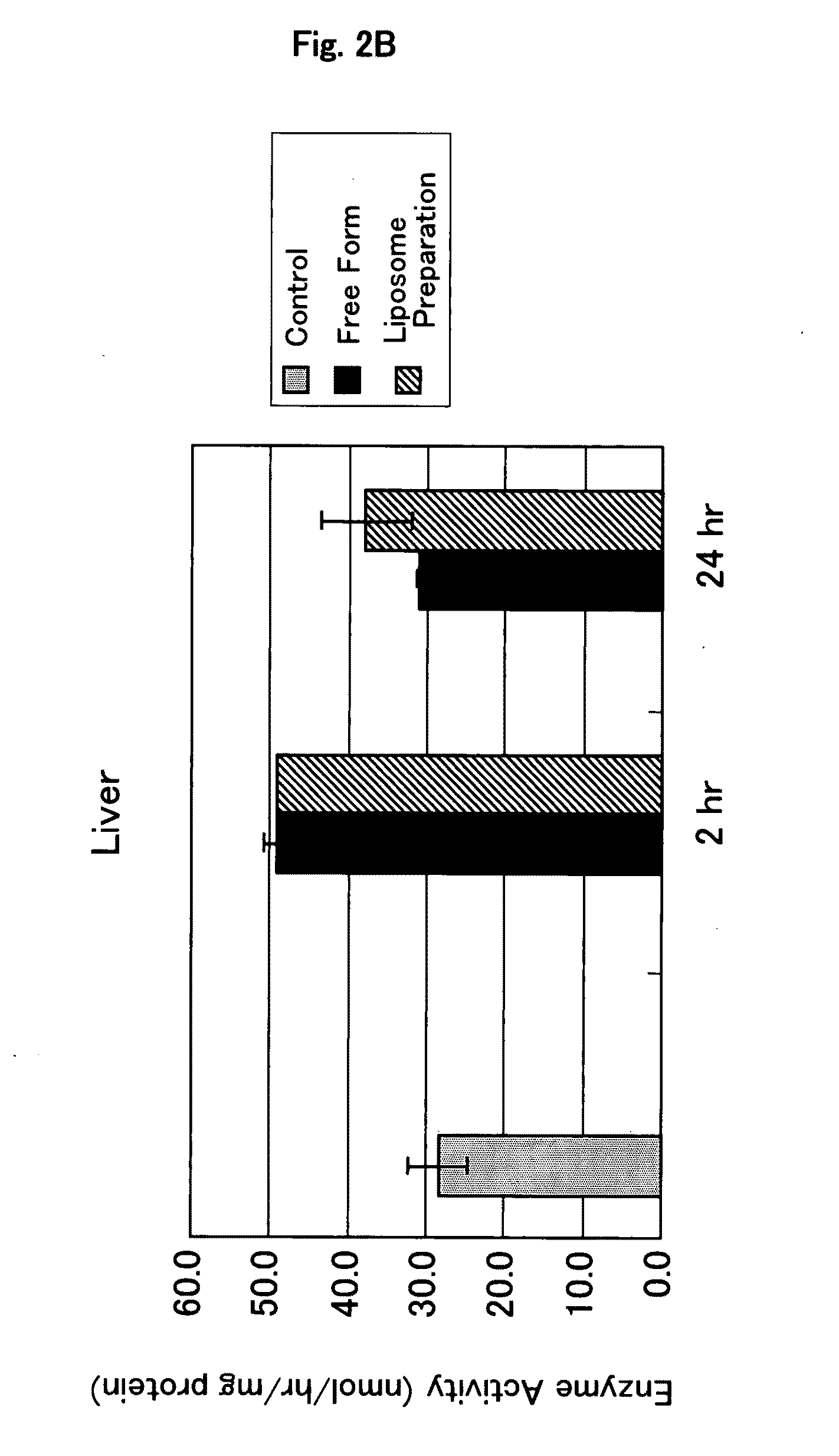
It is an object of the present invention to provide an enzyme preparation which is excellent in stability in blood (blood residence) and in transfer to a target organ (targeting property), and can be used effectively in enzyme replacement therapy or the like.This problem is solved by a lipid vesicle composition wherein vesicles composed of a lipid bilayer membrane are encapsulating an enzyme, the composition being capable of retaining stably the activity of the enzyme even outside the stable pH range of the enzyme.
Owner:JCR PHARMA +1
Dose escalation enzyme replacement therapy for treating acid sphingomyelinase deficiency
ActiveUS8349319B2Avoid rapid degradationLess side effectsNervous disorderHydrolasesNeurological manifestationAcid sphingomyelinase
Owner:MT SINAI SCHOOL OF MEDICINE +1
Methods, compositions, and kits for the treatment of matrix mineralization disorders
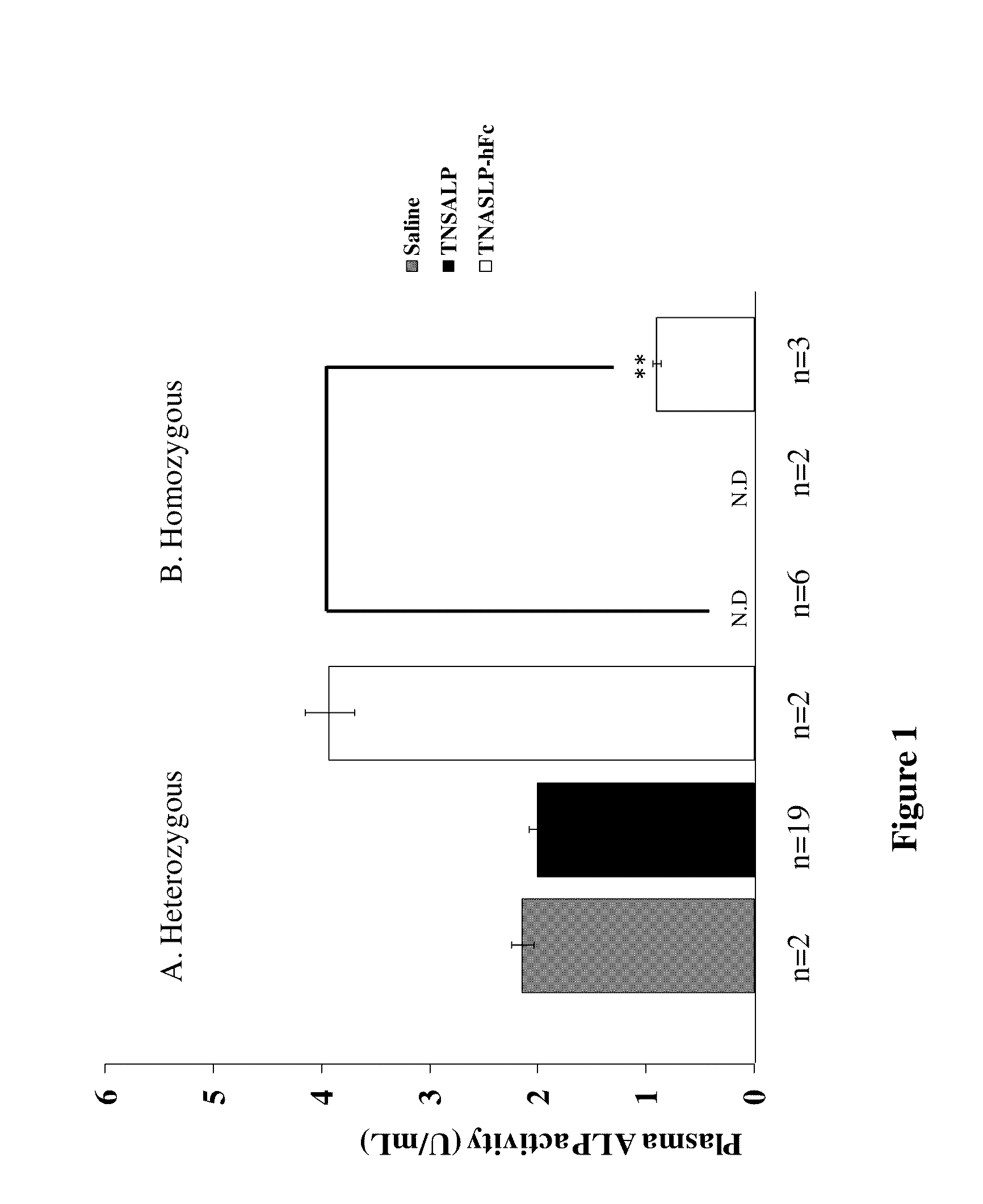
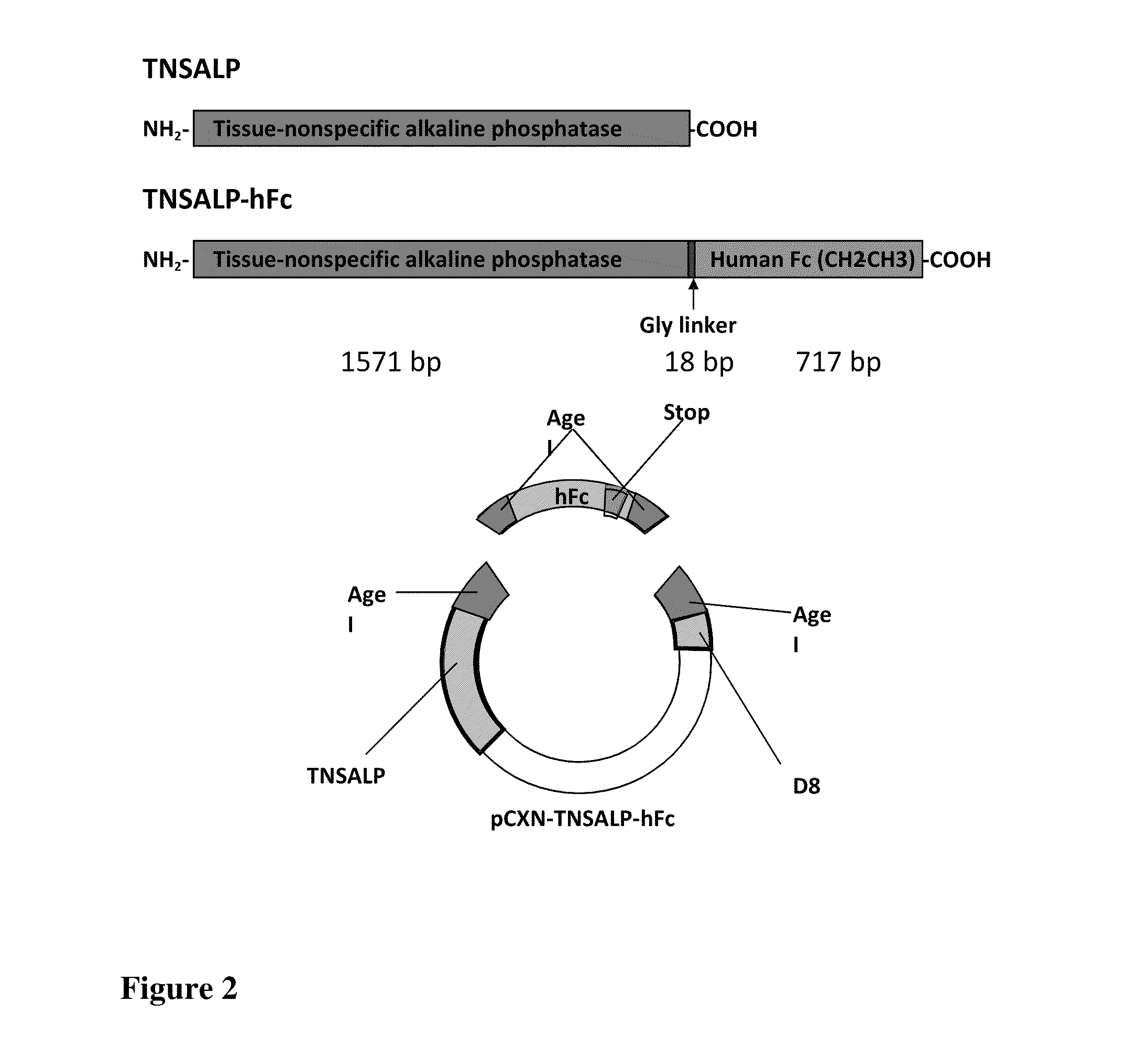
The present invention provides methods, compositions, and kits for the treatment of matrix mineralization disorders such as hypophosphatasia. In particular, the present invention provides polypeptides having a soluble alkaline phosphatase fused to an Fc domain of an immunoglobulin. Such polypeptides can be administered to patients, e.g., subcutaneously, to treat hypophosphatasia using enzyme replacement therapy. The invention also features nucleic acids encoding such polypeptides and the use of the nucleic acids for treating matrix mineralization disorders.
Owner:ALEXION PHARMA INC
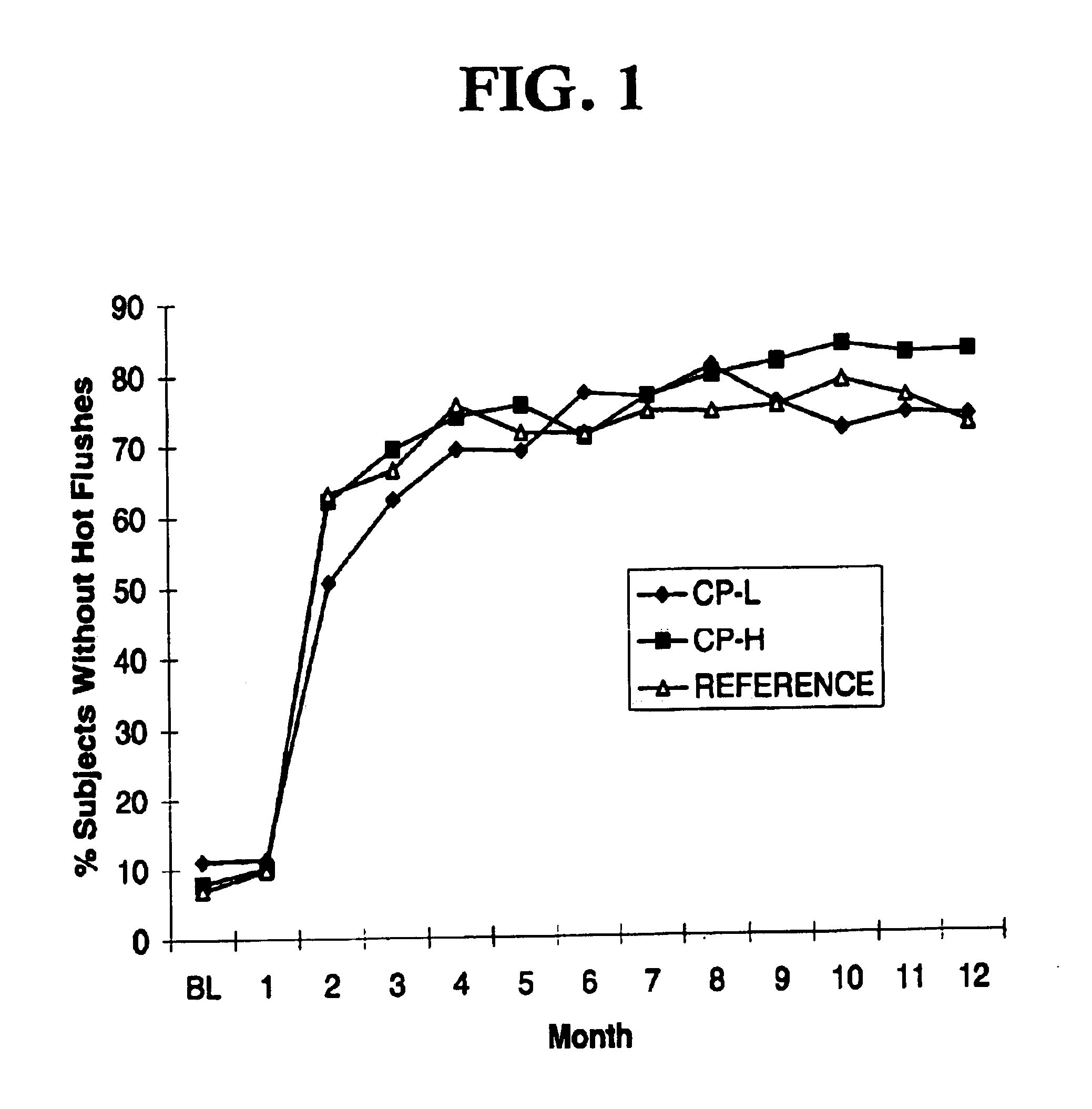
Low dose estrogen interrupted hormone replacement therapy
A hormone replacement therapy, comprising a plurality of daily doses of a pharmaceutical preparation, the doses being administered continuously and consecutively in alternating phases of three daily doses, a relatively dominant estrogenic activity phase comprising three daily doses of a substance exhibiting estrogenic activity equivalent to about 1 mg per day of 17beta-estradiol per day, and a relatively dominant progestagenic activity phase of a combination of a substance exhibiting estrogenic activity equivalent to about 1 mg per day of 17beta-estradiol and a substance exhibiting progestogenic activity equivalent to about 90 mug per day of norgestimate.
Owner:DURAMED PHARMA +1
Methods of enhancing lysosomal storage disease therapy by modulation of cell surface receptor density
InactiveUS20100143297A1Uptake of extracellular lysosomal enzymes by cells can be increasedIncreases the target organ uptake of glucocerebrosidaseBiocideElcosanoid active ingredientsLysosomeFabry disease
Methods of modulating uptake of extracellular lysosomal enzymes by administering a pharmaceutical agent and methods of treating a lysosomal storage disease (such as Gaucher disease, Pompe disease, Fabry disease or Niemann-Pick disease) or enhancing enzyme replacement therapy or gene therapy, comprising administering a pharmaceutical agent such as dexamethasone, glucose or insulin, are provided.
Owner:GENZYME CORP
Prenatal enzyme replacement therapy
The invention contemplates transplacental enzyme replacement therapy (ERT) for deficiency of a polypeptide such as a tissue-nonspecific alkaline phosphatase (TNSALP) by administering a before-described pharmaceutical composition to a pregnant animal whose fetus or embryo is in need of such therapy. The fusion protein of such a composition comprises a water-soluble TNSALP portion, e.g., C-terminus-truncated TNSALP peptide-bonded to an IgG1 antibody Fc portion.The invention also contemplates a method for treating a metabolic disorder, such as HPP, in a fetus or embryo were a protein is administered to a pregnant mother. The fusion protein comprises a Fc fragment of an IgG1 antibody peptide-bonded to TNSALP. The protein crosses the placenta of the mother and enters the fetal blood stream. The protein is taken up into fetal tissue such that the TNSALP restores normal metabolic activity in the fetus.
Owner:SAINT LOUIS UNIVERSITY
Methods, compositions, and kits for the treatment of matrix mineralization disorders
The present invention provides methods, compositions, and kits for the treatment of matrix mineralization disorders such as hypophosphatasia. In particular, the present invention provides polypeptides having a soluble alkaline phosphatase fused to an Fc domain of an immunoglobulin. Such polypeptides can be administered to patients, e.g., subcutaneously, to treat hypophosphatasia using enzyme replacement therapy. The invention also features nucleic acids encoding such polypeptides and the use of the nucleic acids for treating matrix mineralization disorders.
Owner:ALEXION PHARMA INC
Cardiac rhythm management device and sensor-suite for the optimal control of ultrafiltration and renal replacement therapies
ActiveUS8246563B2ElectrotherapyMechanical/radiation/invasive therapiesUltrafiltrationOptimal control
A cardiorenal patient monitoring system comprising, either implanted or non-implanted device(s), remote peripheral device(s), computer network(s), host, and communication means between the device(s), computer network(s), and host. The preferred embodiment shows an advanced patient monitoring system for using an implanted cardiac device and a dialysis machine in renal therapy. In addition, the method of advanced patient monitoring is in conjunction with the advanced patient monitoring system is disclosed.
Owner:CARDIAC PACEMAKERS INC
Glucosylceramide synthase inhibitors
InactiveUS20160361301A1Delay disease progressionReduce intensityOrganic active ingredientsOrganic chemistryLysosomal enzyme defectGLYCOCERAMIDES
The invention relates to inhibitors of glucosylceramide synthase (GCS) useful for the treatment metabolic diseases, such as lysosomal storage diseases, either alone or in combination with enzyme replacement therapy.
Owner:GENZYME CORP
Formulations for lysosomal enzymes
ActiveUS8785168B2Preserve and enhance stabilityPreserve and enhance and efficacyPeptide/protein ingredientsMetabolism disorderAcid alpha-glucosidaseLysosome
Owner:BIOMARIN PHARMA INC +1
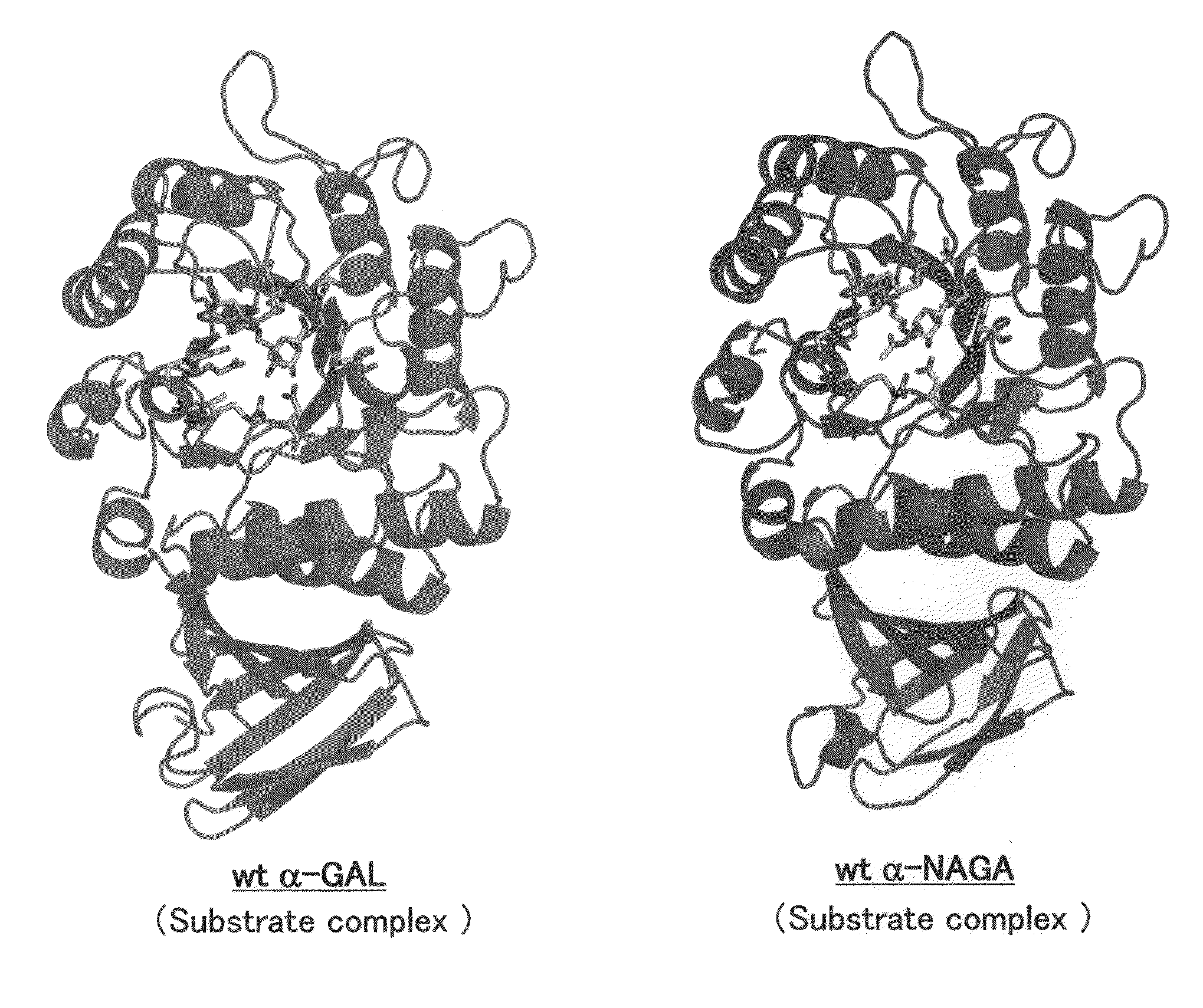
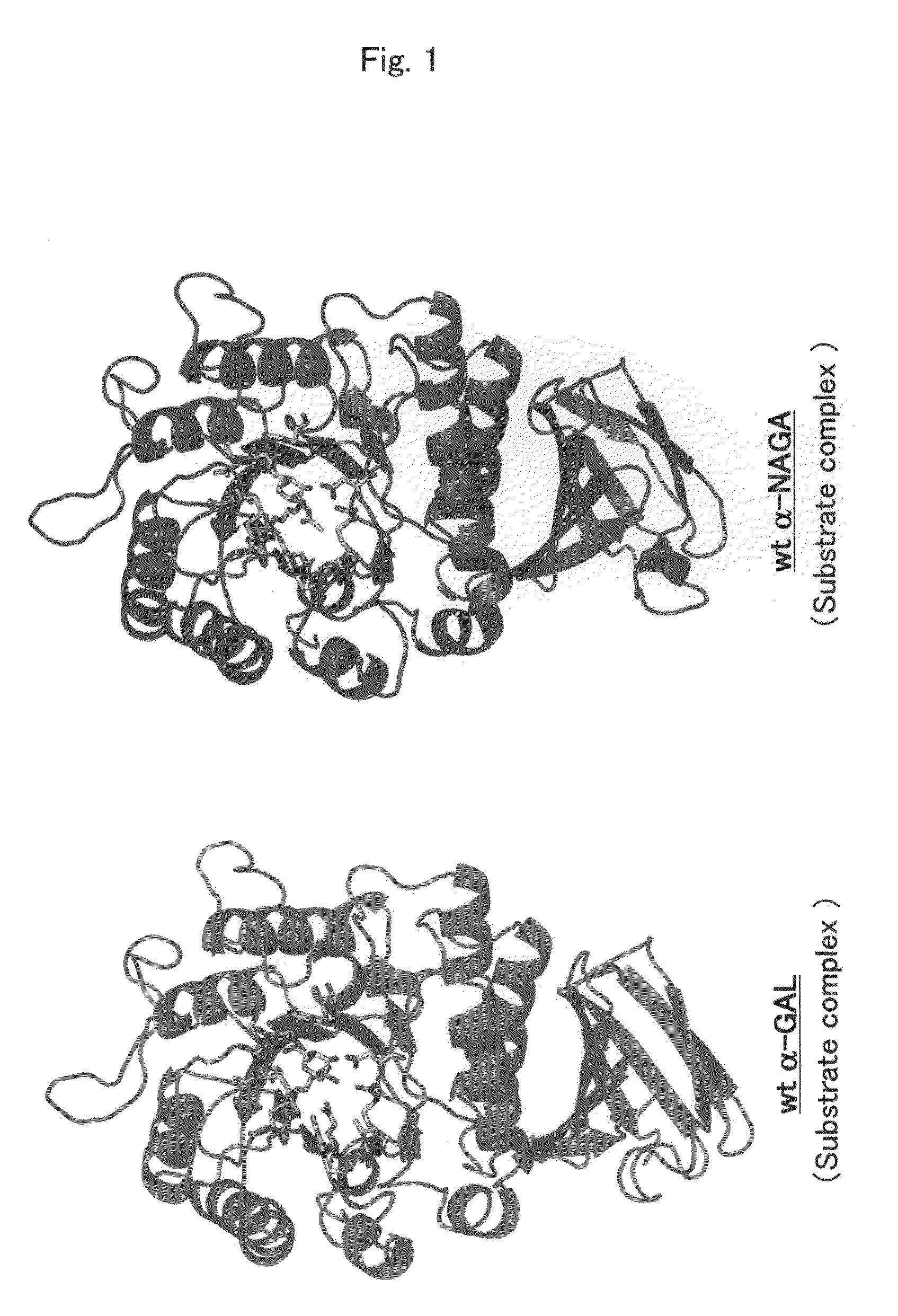
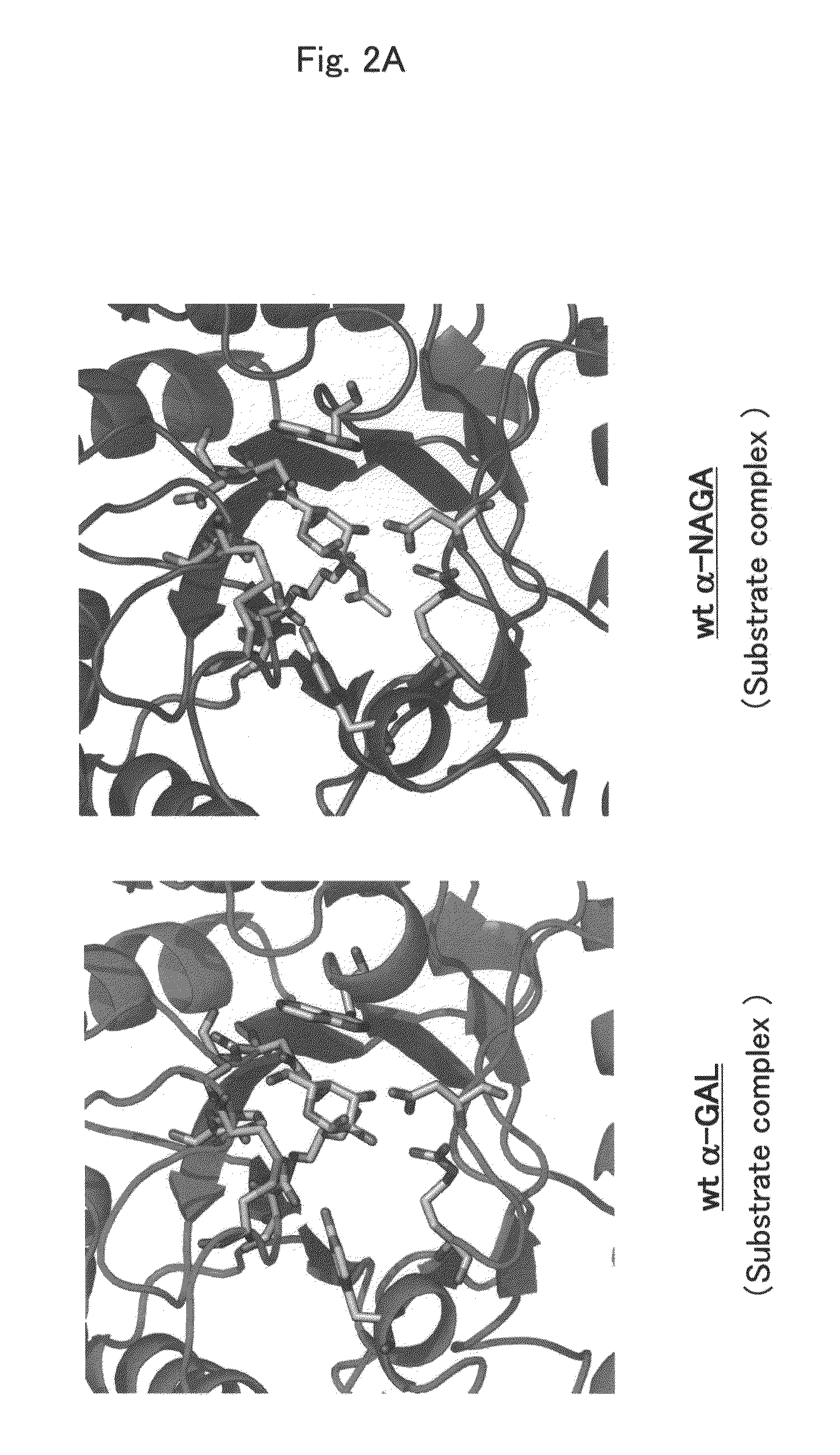
Highly functional enzyme having α-galactosidase activity
ActiveUS7935336B2Improve stabilityEasily incorporated into cellSenses disorderNervous disorderSide effectFabry disease
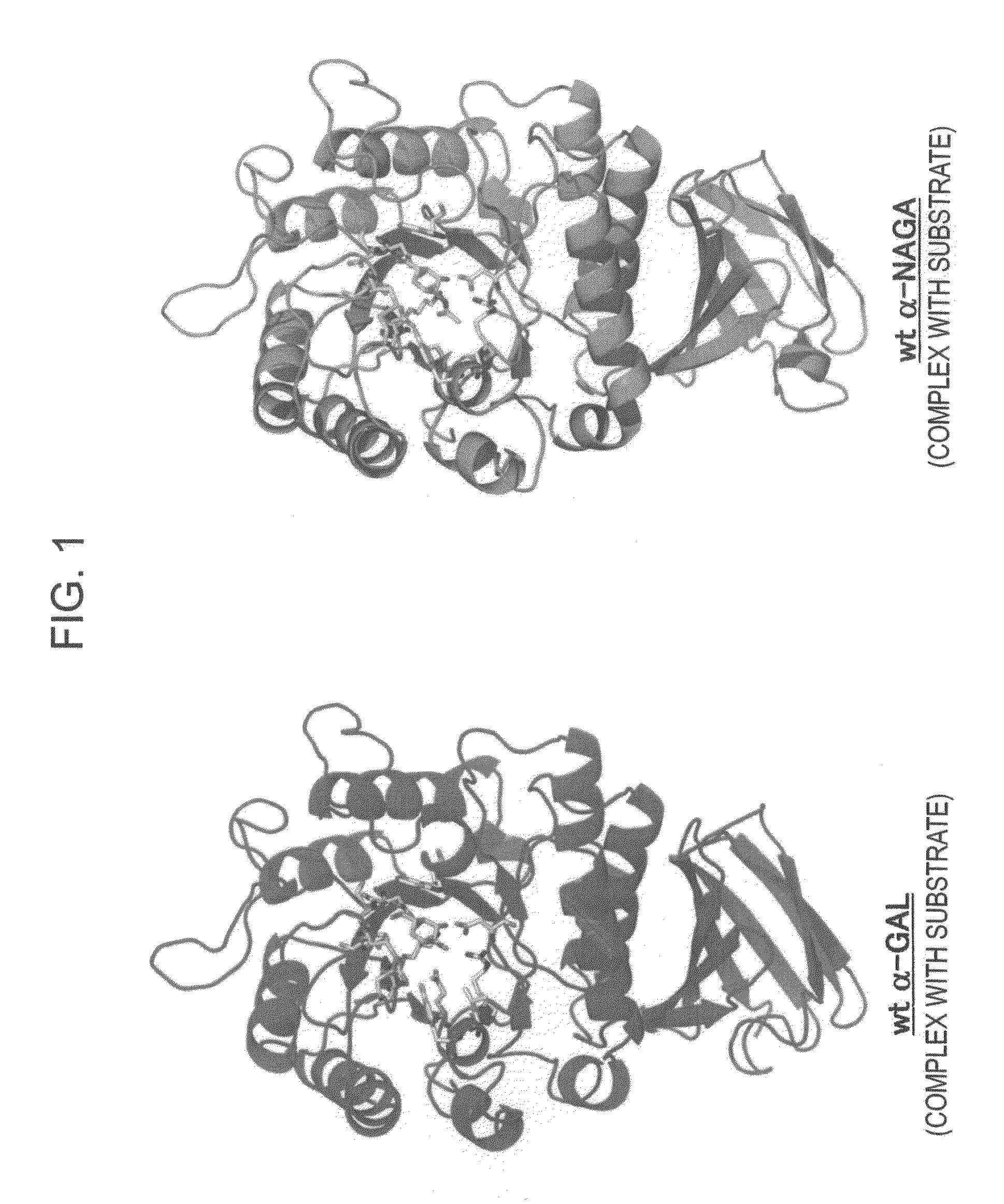
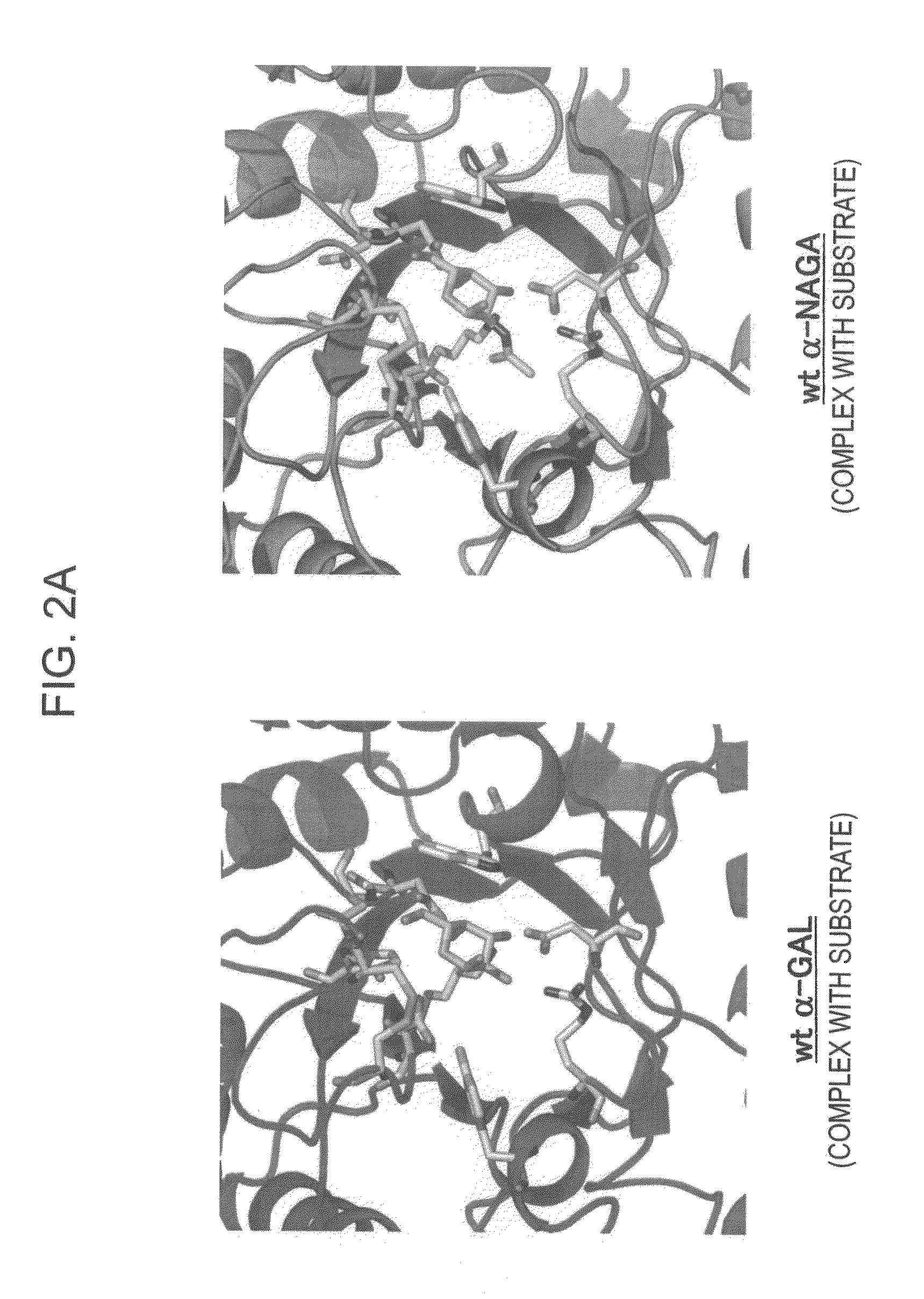
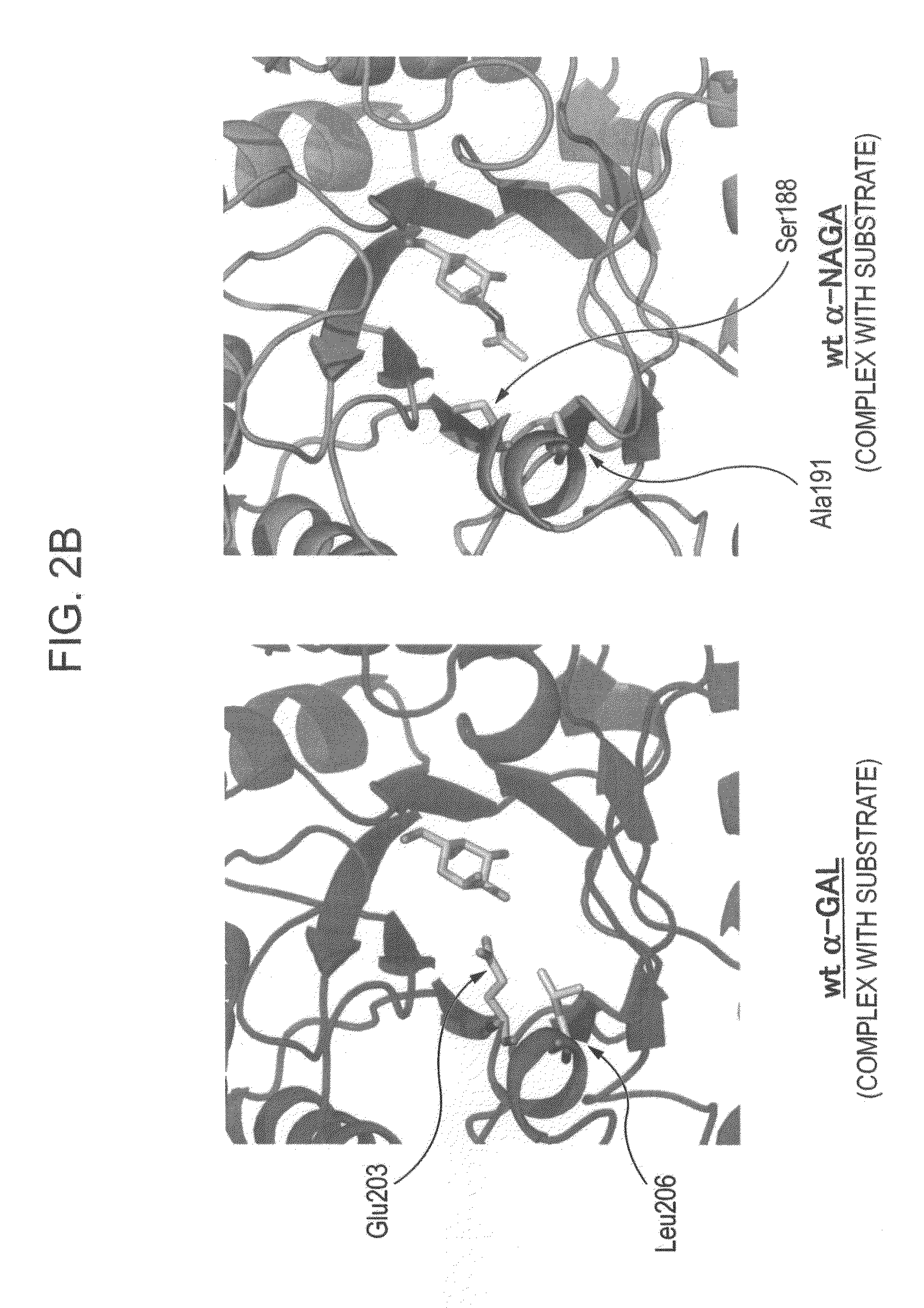
The present invention provides, as an enzyme which can be used for enzyme replacement therapy for Fabry disease, a protein having α-galactosidase activity, which shows no allergic adverse side effect, shows a high stability in blood, and can be easily incorporated into a cell of an affected organ. The protein of the present invention is a protein which has acquired α-galactosidase activity by changing the structure of the active site of wild-type human α-N-acetylgalactosaminidase.
Owner:ALTIF LAB
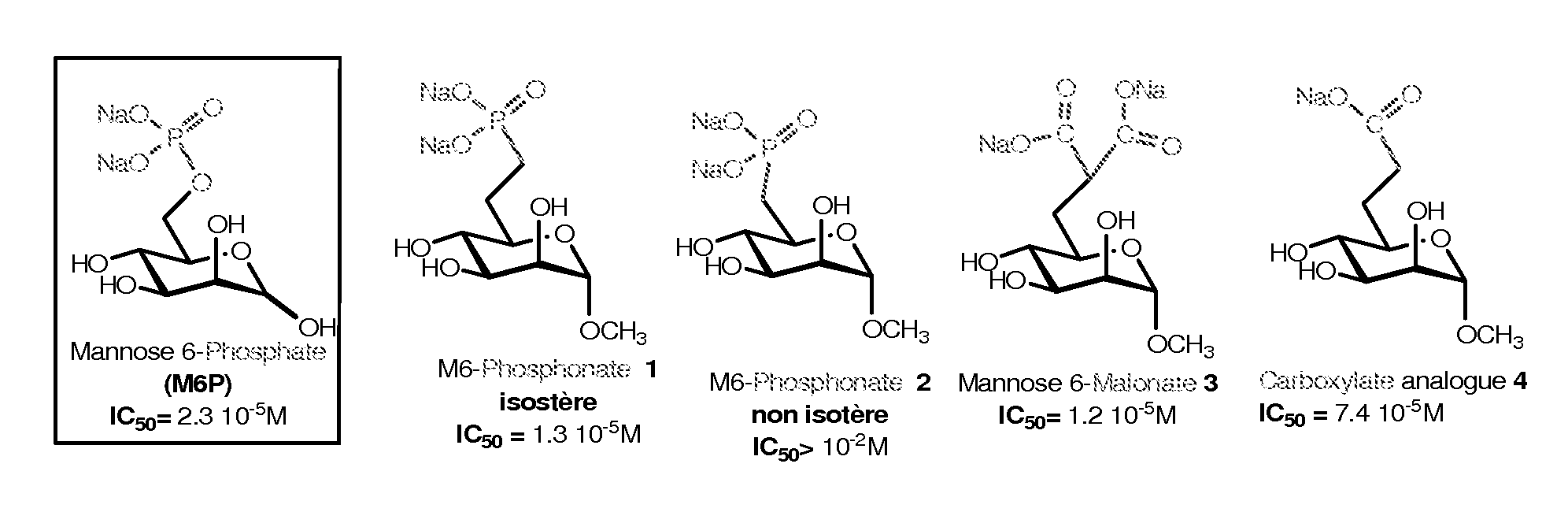
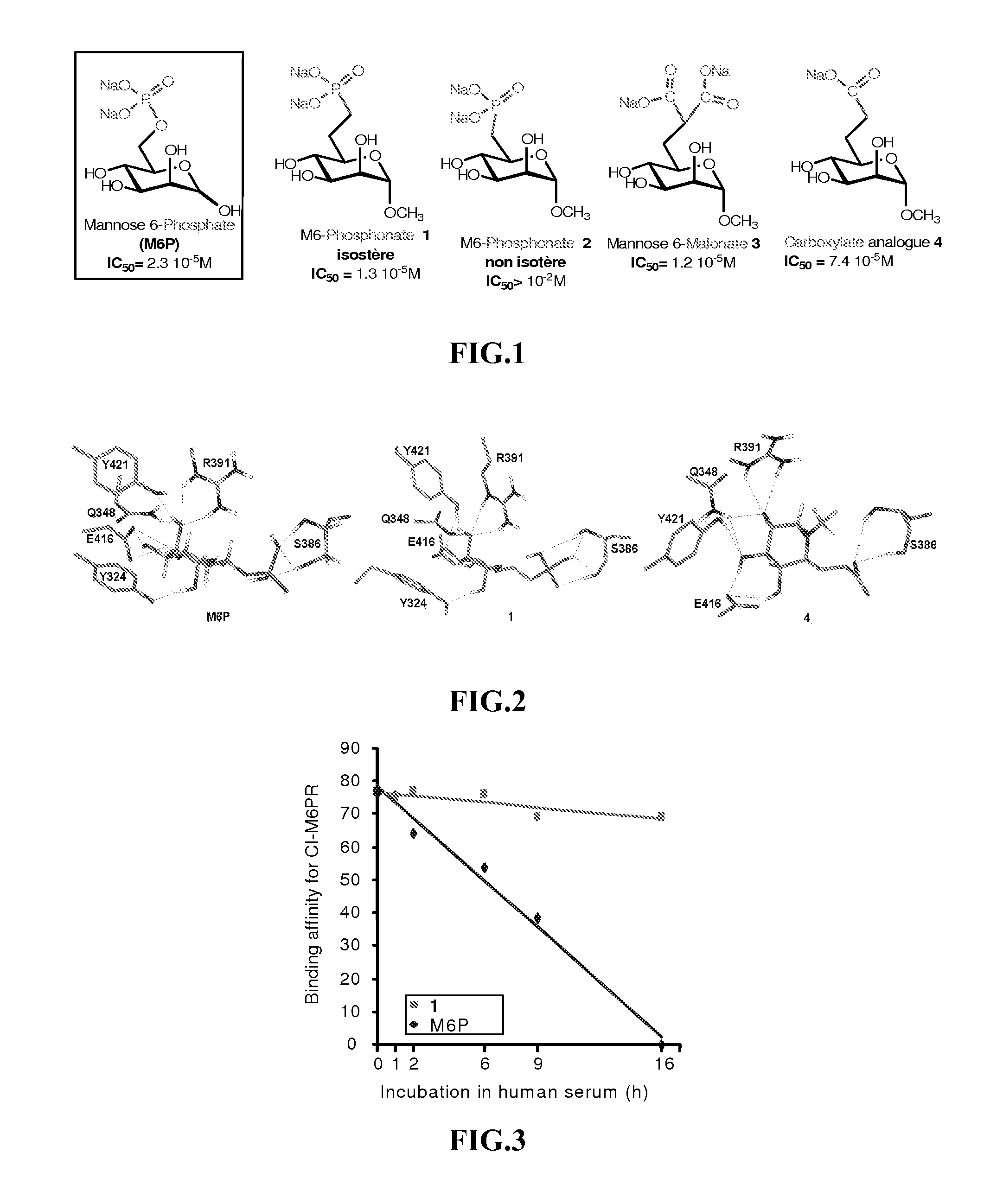
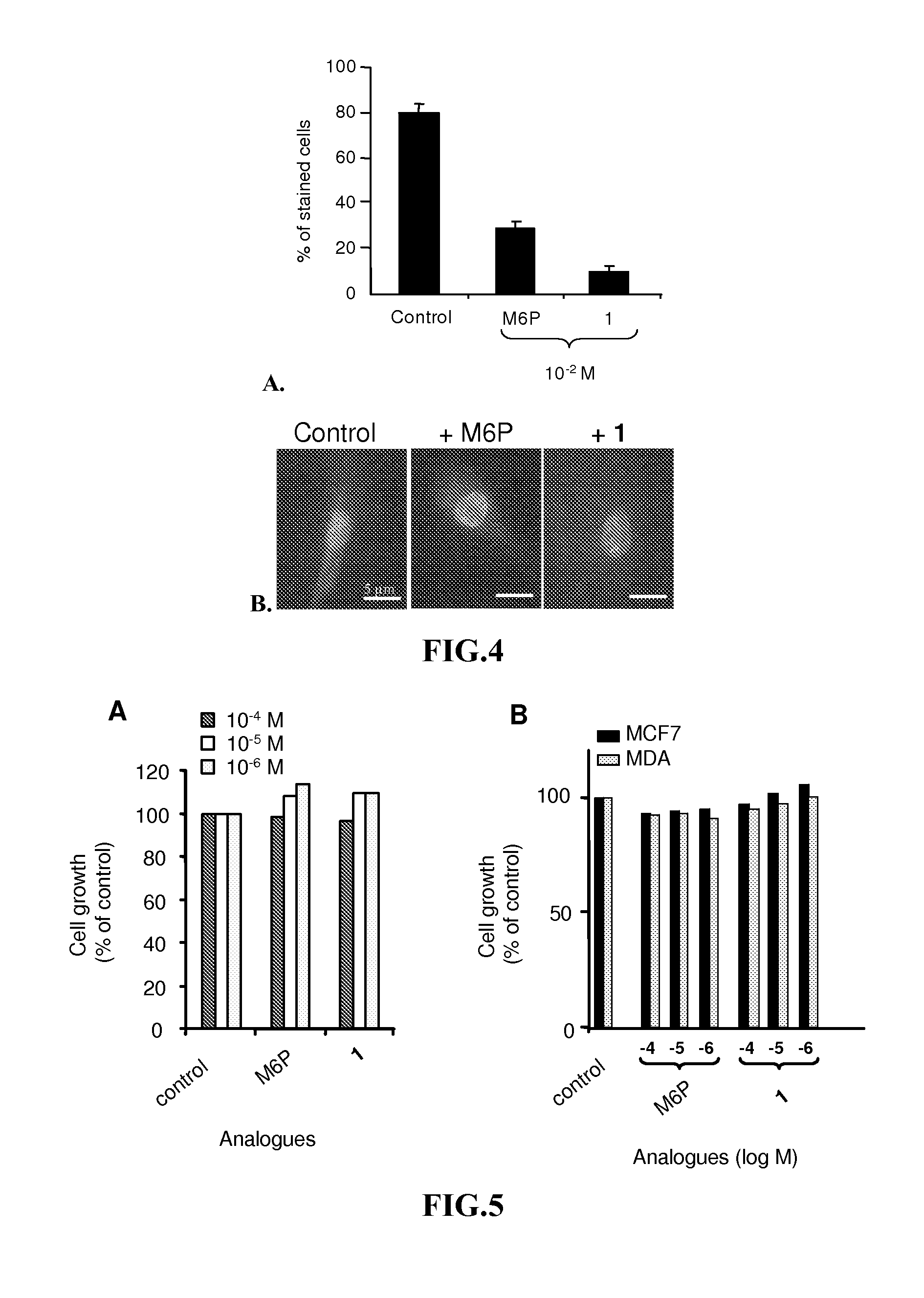
Compounds targeting the cation-independent mannose 6-phosphate receptor
ActiveUS20120093795A1High affinityMany applicationsSenses disorderNervous disorderCation-independent mannose-6 phosphate receptorEnzyme replacement therapy
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
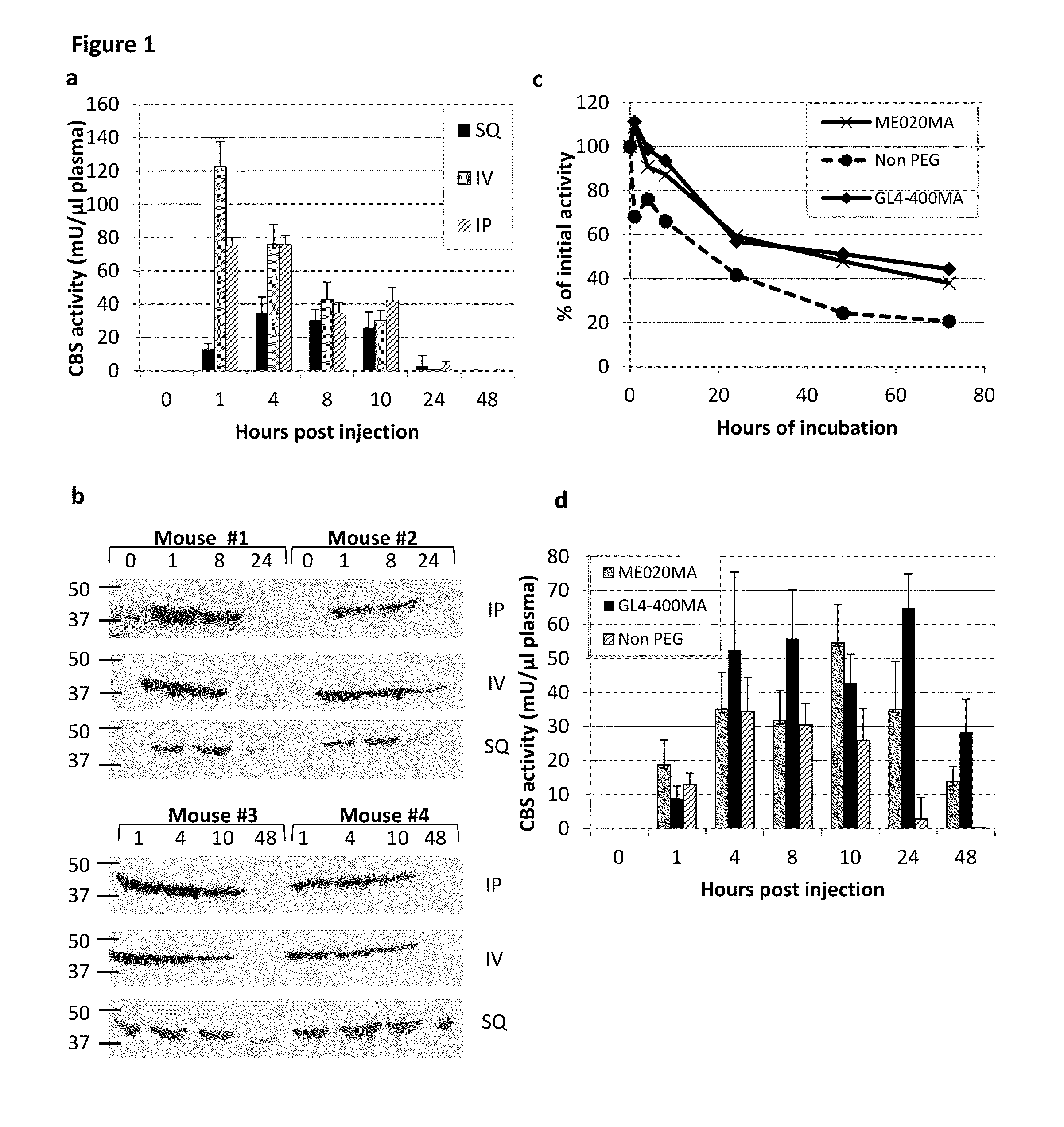
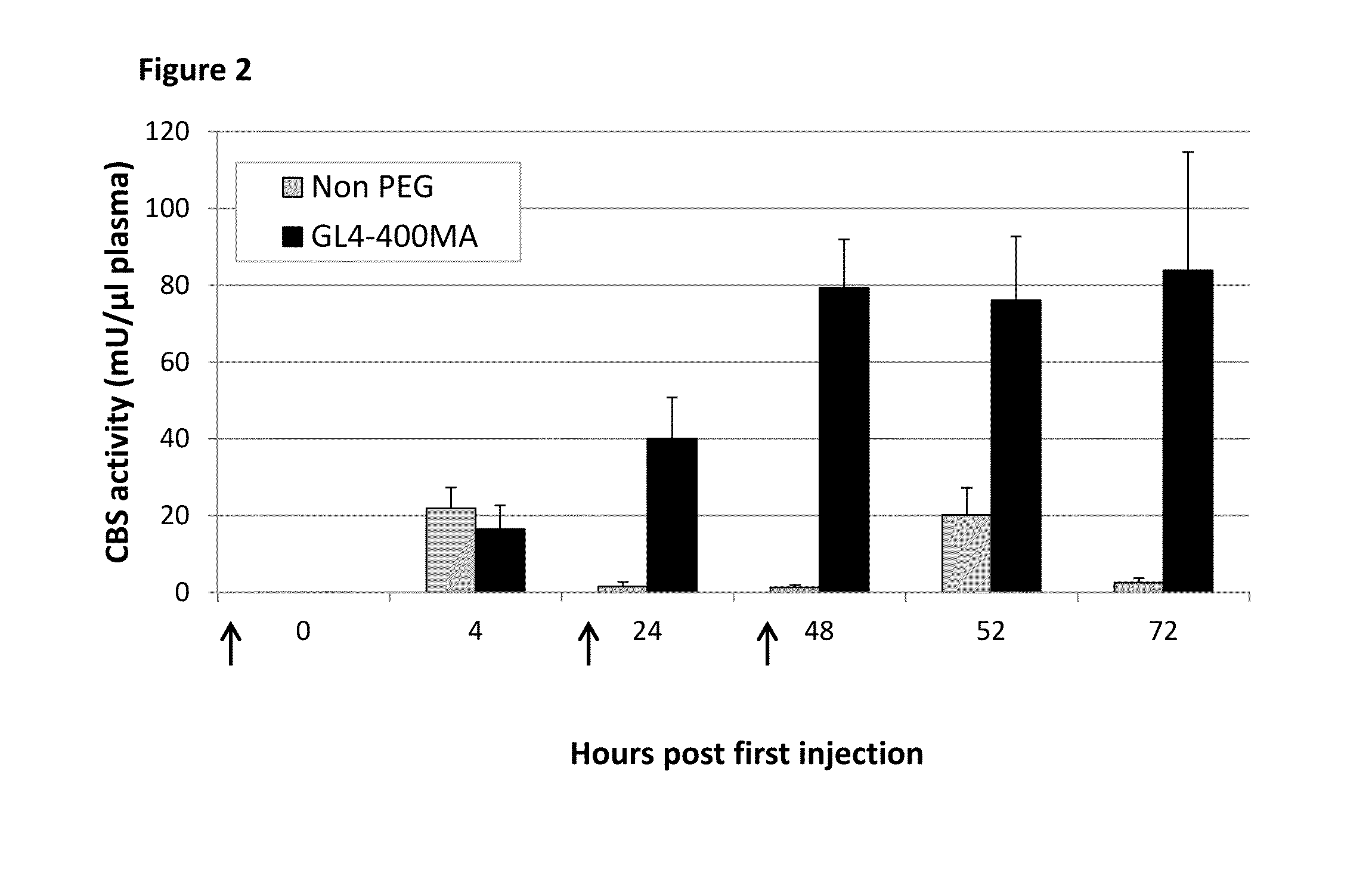
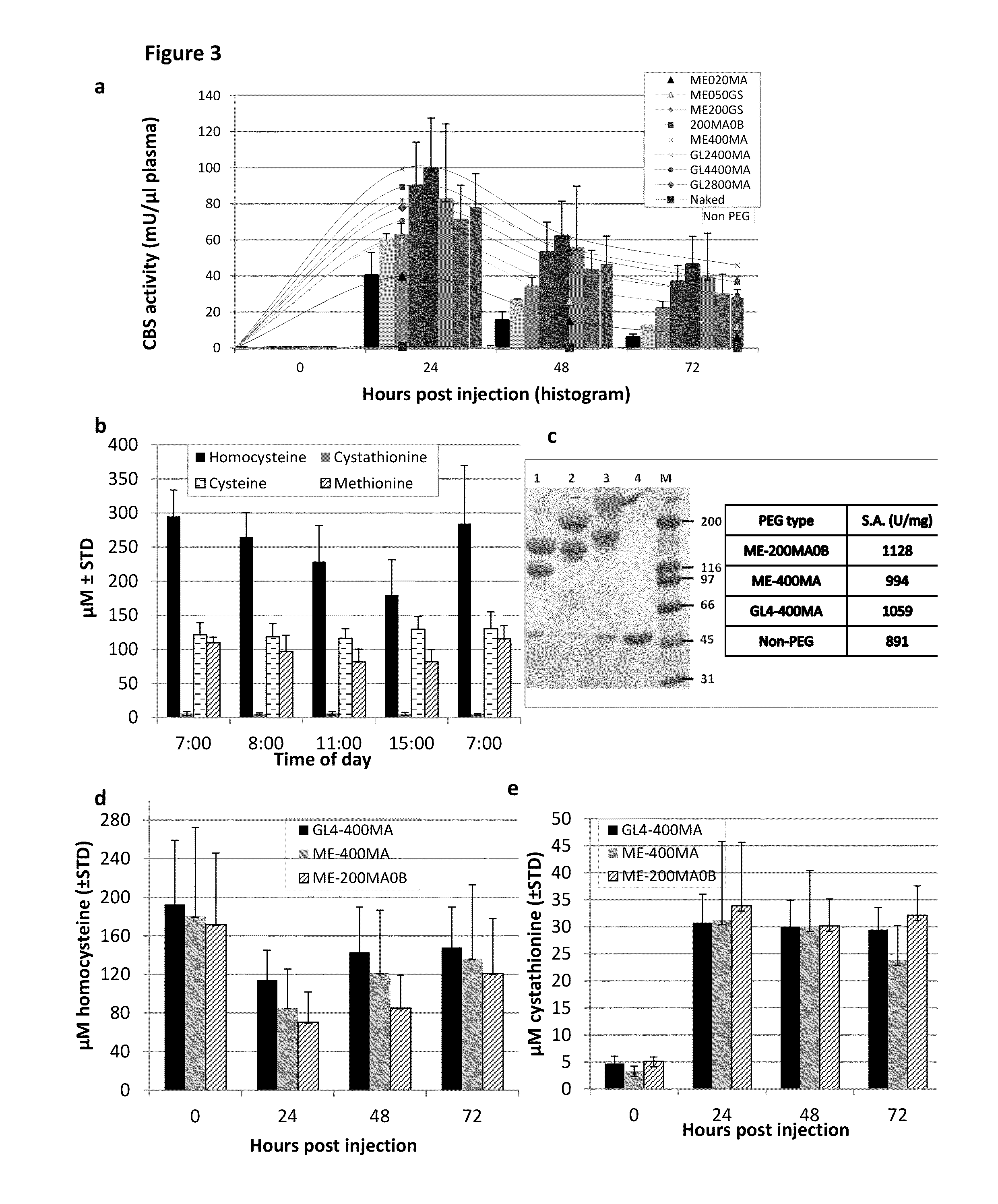
Chemically modified cystathionine beta-synthase enzyme for treatment of homocystinuria
ActiveUS9034318B2Reducing serum HcyHigh activityOrganic active ingredientsSenses disorderMedicineCystinuria
The invention provides reagents and methods for enzyme replacement therapy using chemically modified species of human cystathionine β-synthase (CBS) to treat homocystinuria and other related diseases and disorders.
Owner:UNIV OF COLORADO THE REGENTS OF
Scavenger receptor uptake for fabry disease enzyme replacement therapy
ActiveUS20140050666A1Ultrasonic/sonic/infrasonic diagnosticsBiocideScavenger receptor ligandLysosome
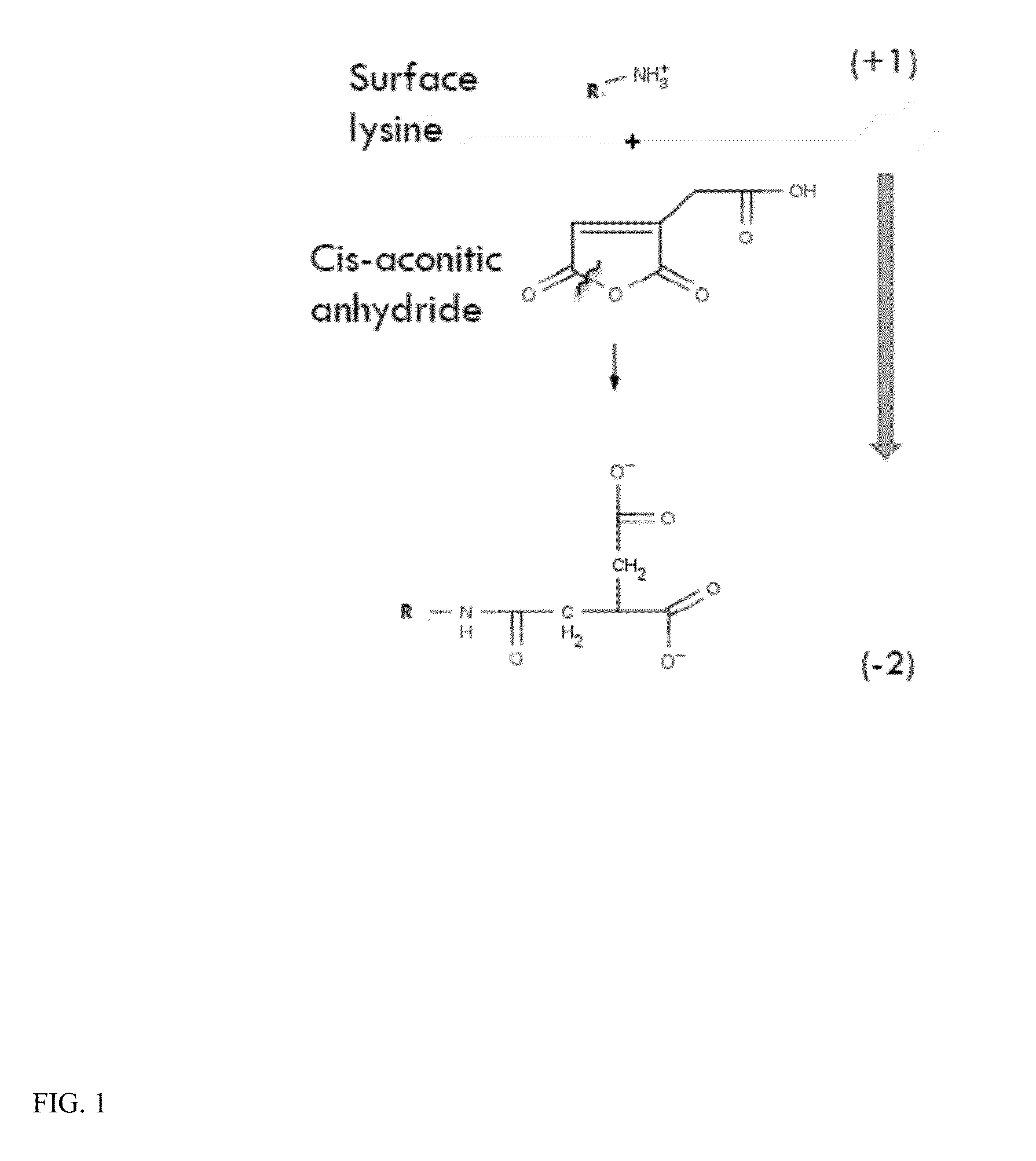
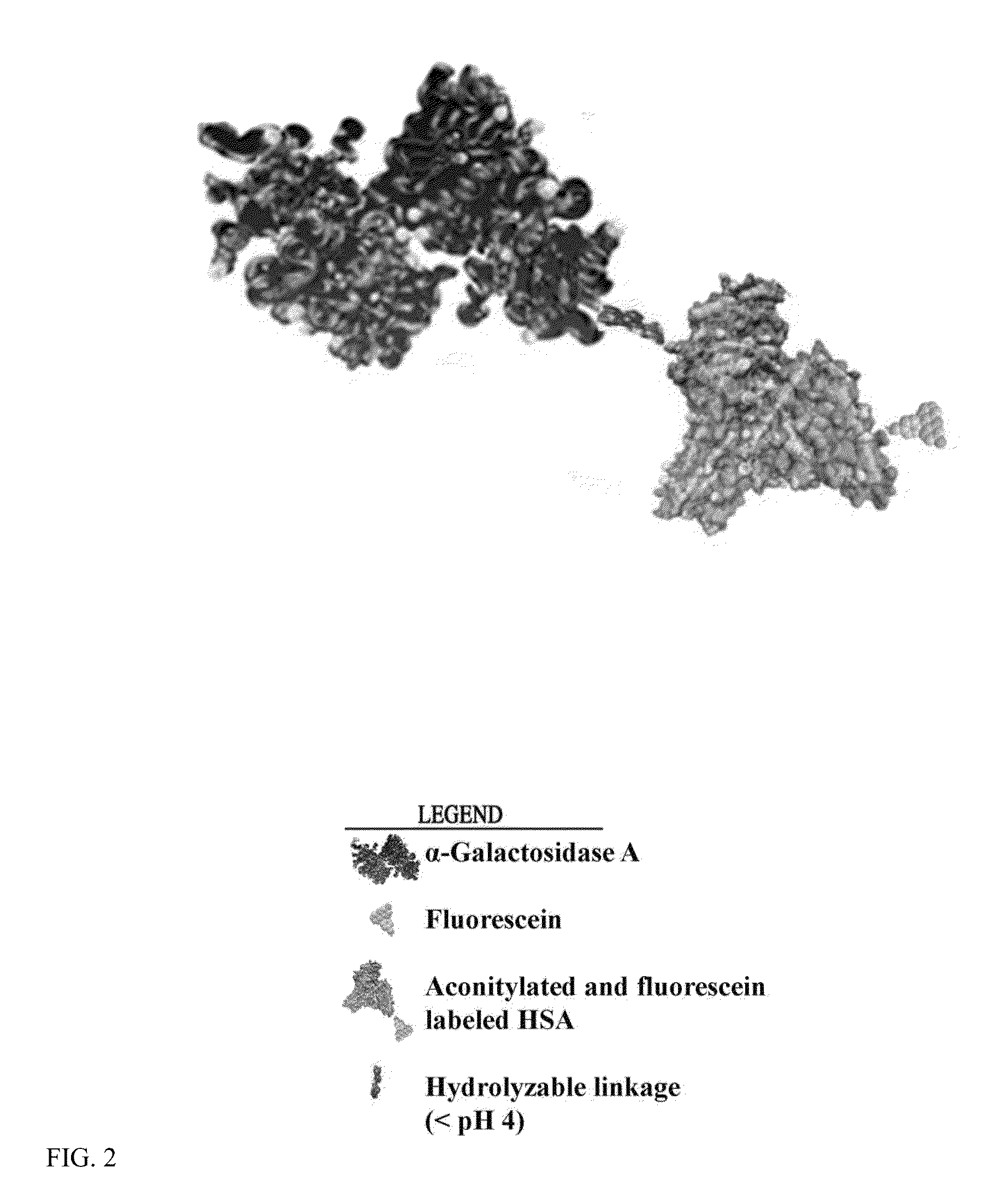
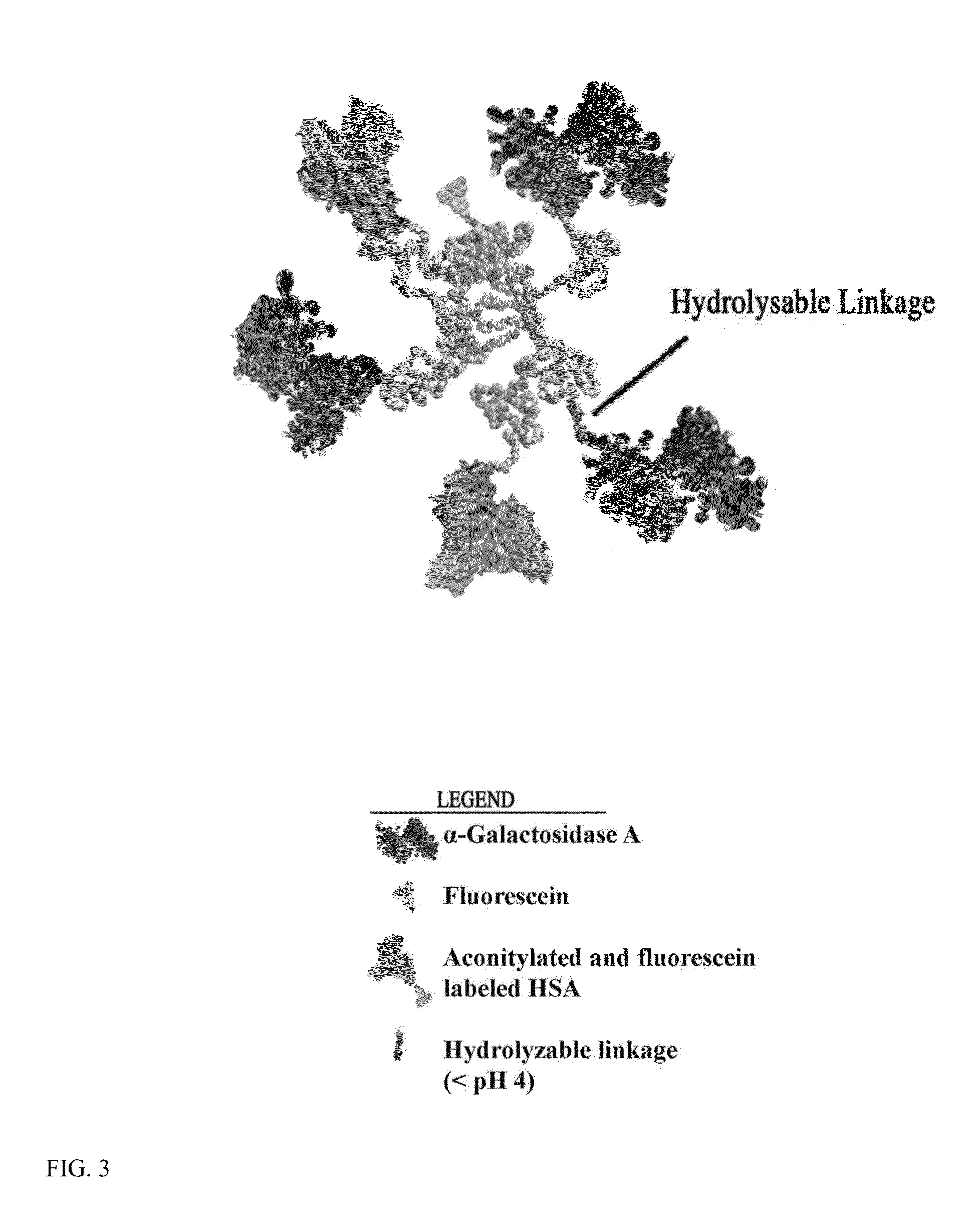
The present invention relates to a composition comprising a lysosomal enzyme conjugated to a negatively charged scavenger receptor ligand. In some embodiments, the lysosomal enzyme is conjugated to the scavenger receptor ligand by way of a linker. The present invention also relates to a composition comprising lysosomal enzyme encapsulated by a liposome, said liposome externally comprising a negatively charged scavenger receptor ligand. The invention further encompasses a method of treating a lysosomal storage disease with the compositions listed above. The invention further encompasses a method of treating a lysosomal storage disease with an acylated, acetylated, or aconitylated lysosomal enzyme.
Owner:RES FOUND THE CITY UNIV OF NEW YORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com