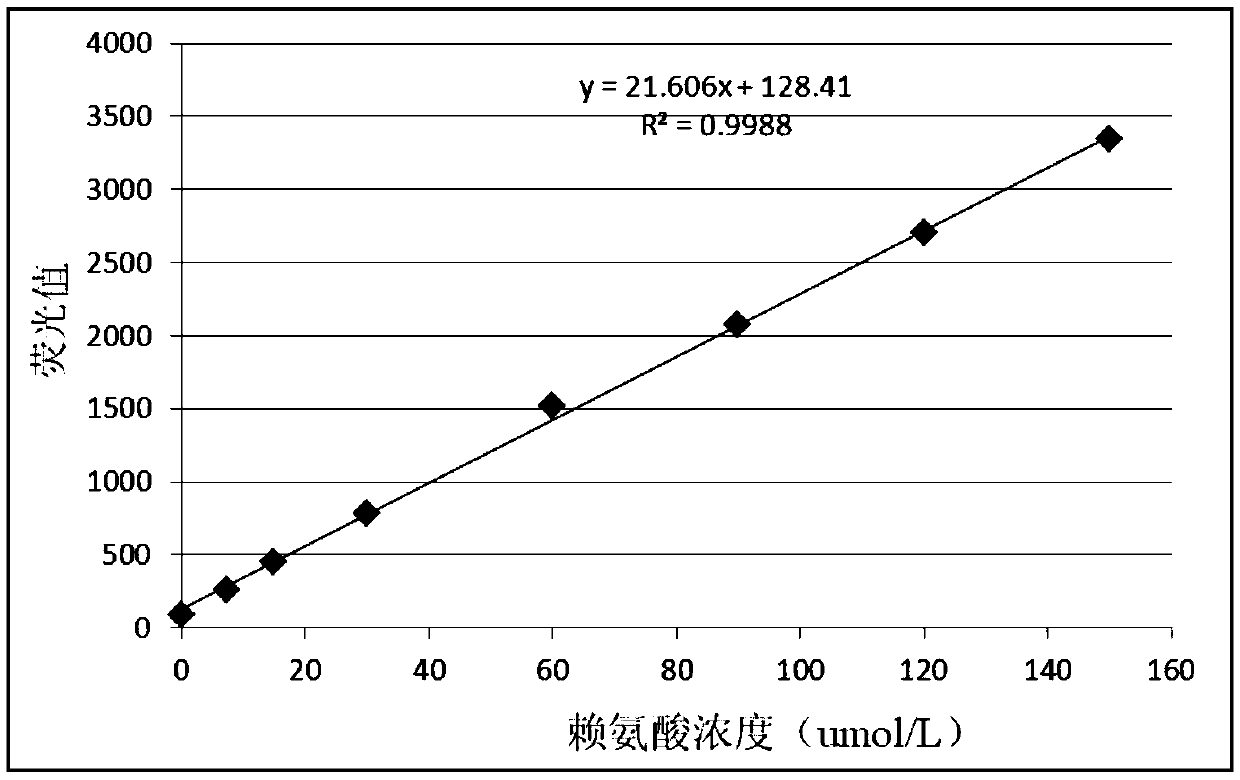
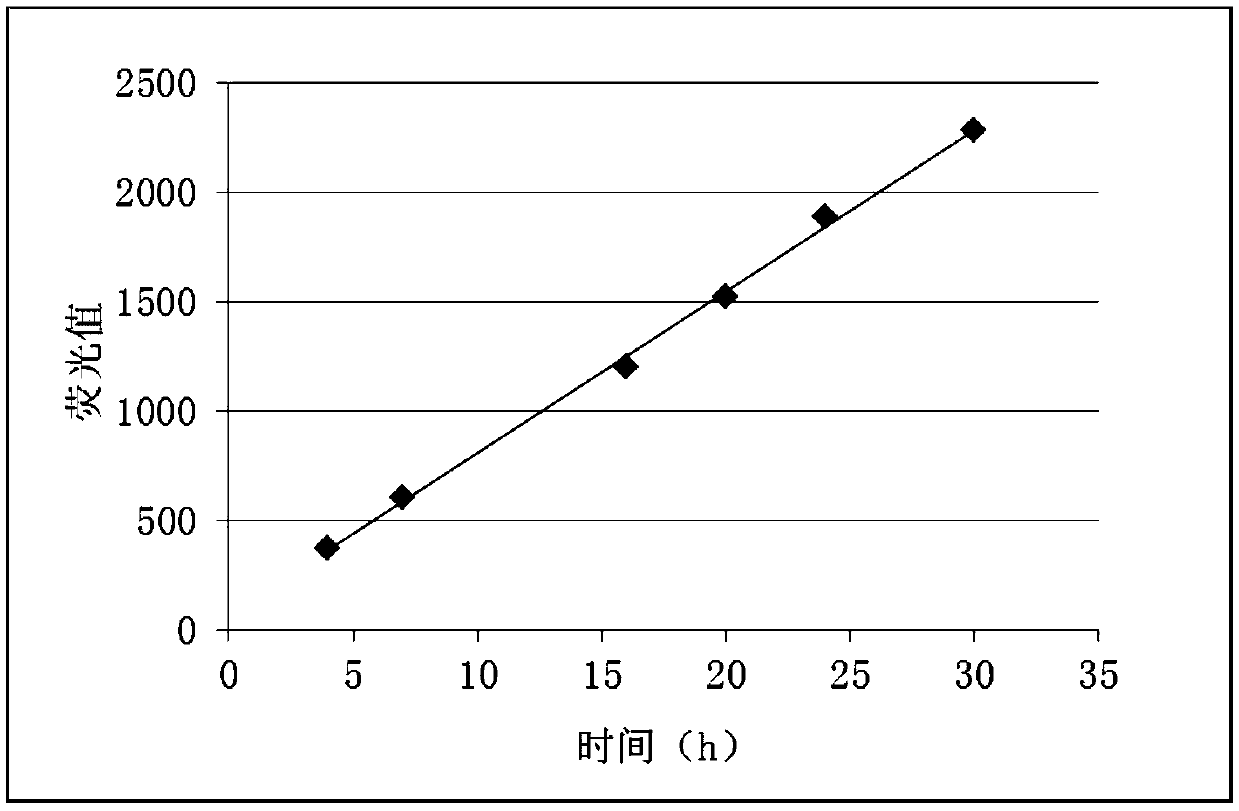
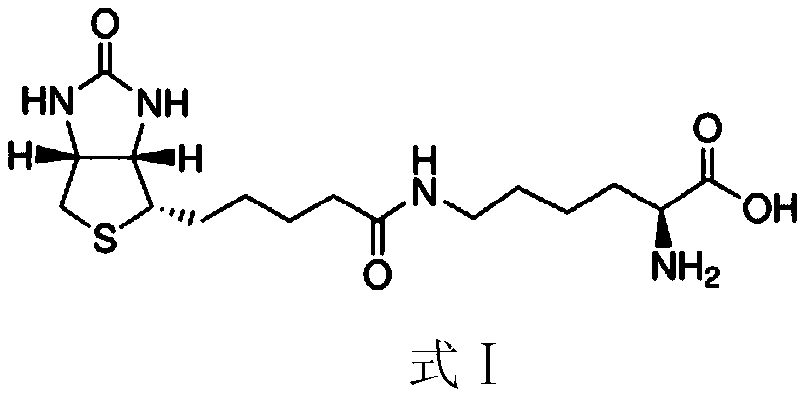
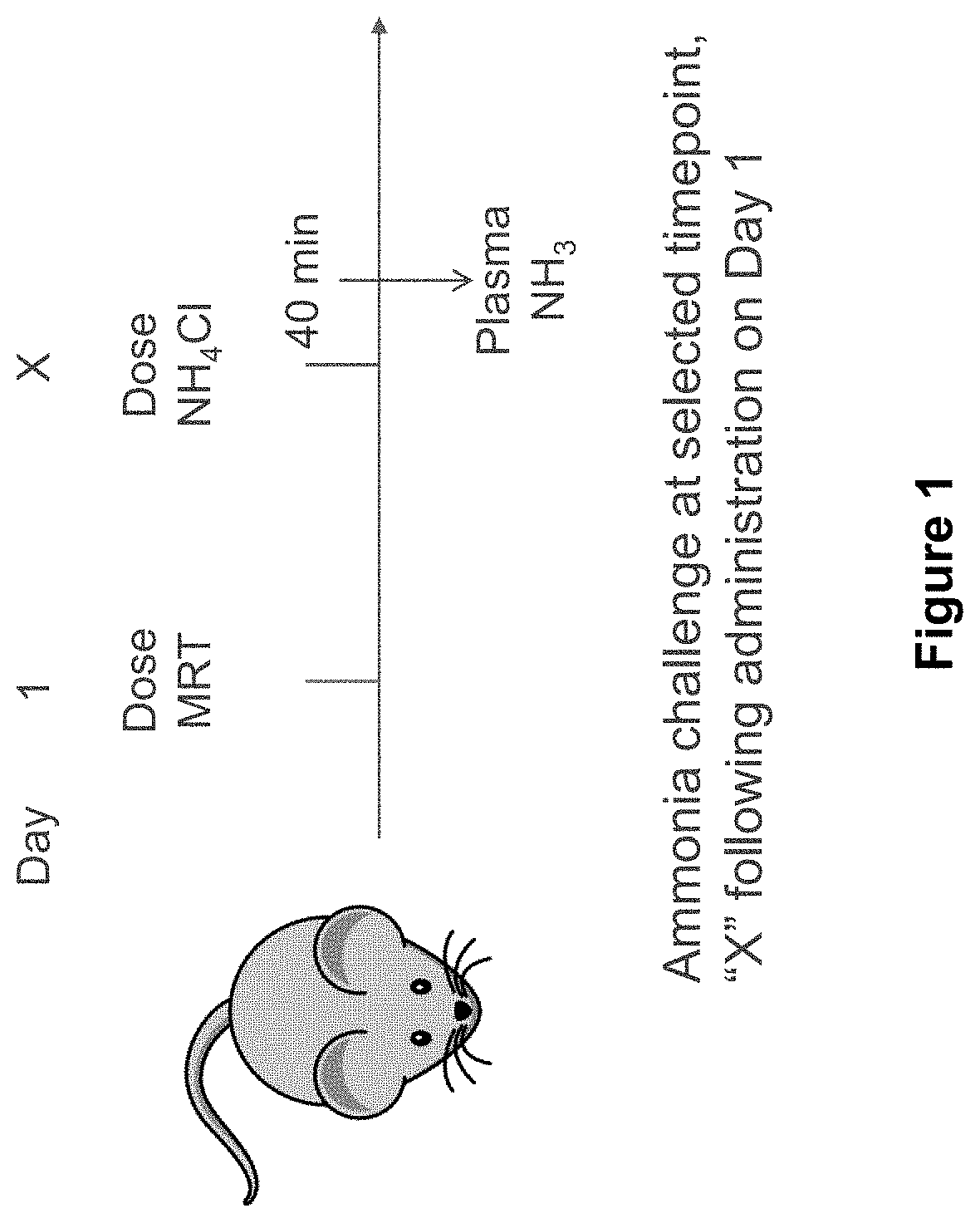
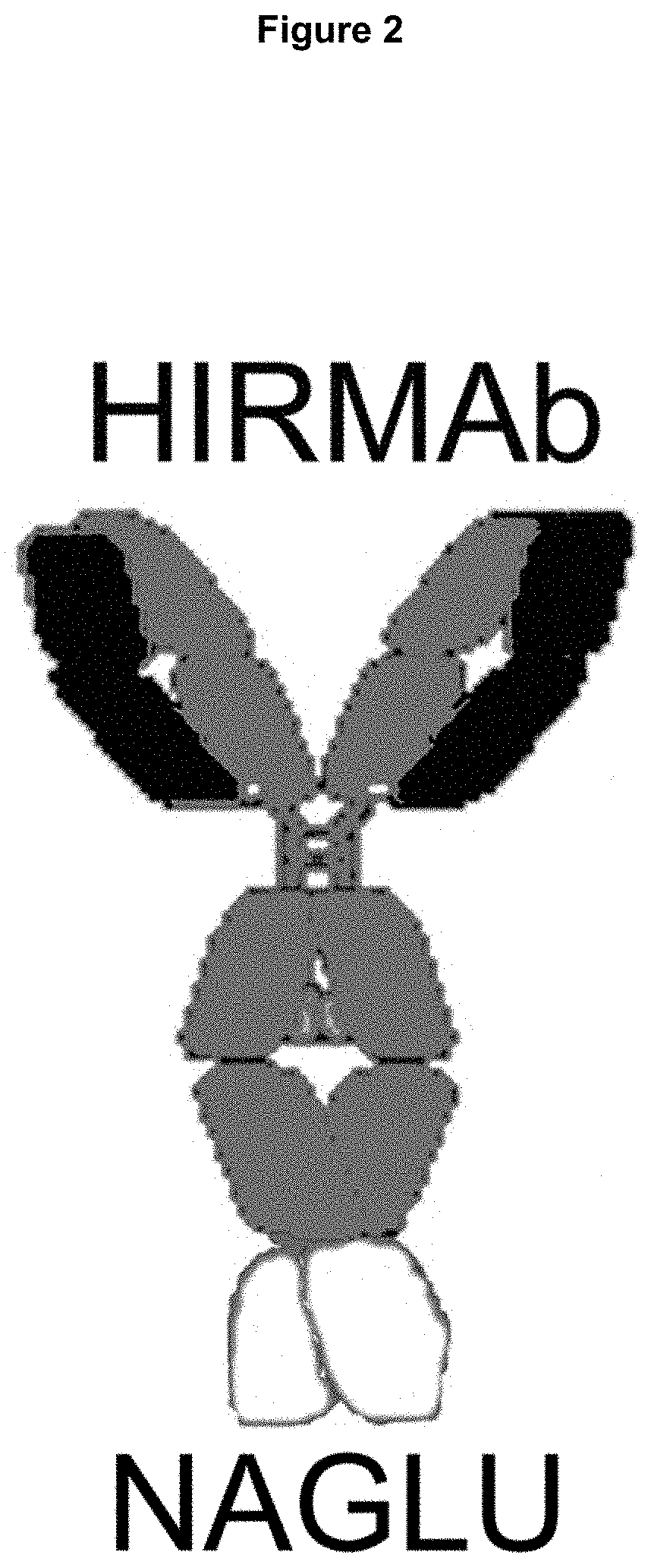Patents
Literature
38 results about "Enzyme deficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An enzyme deficiency is often caused by a genetic error. Enzymes are involved in the gross breakdown of chemical compounds processed in the liver.
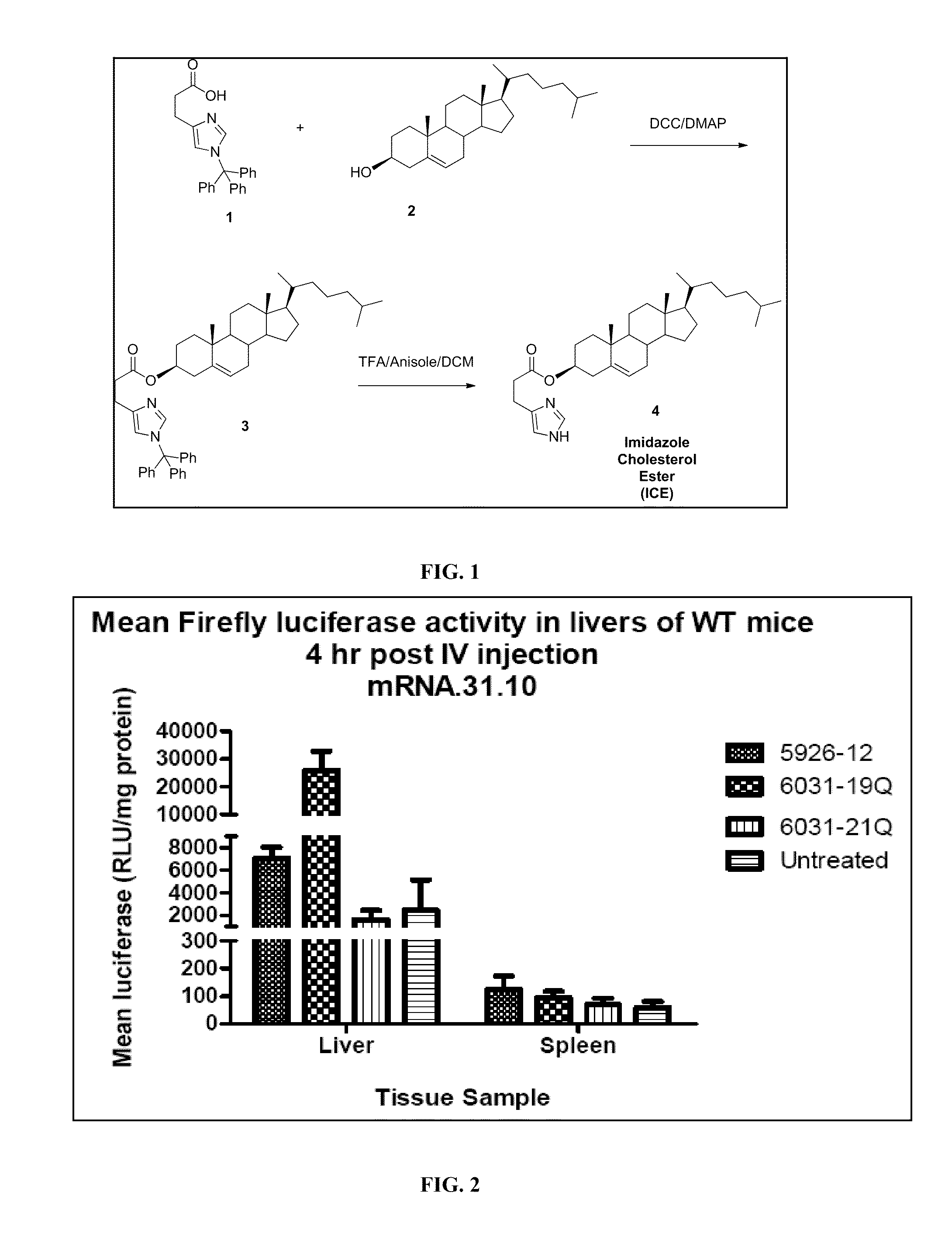
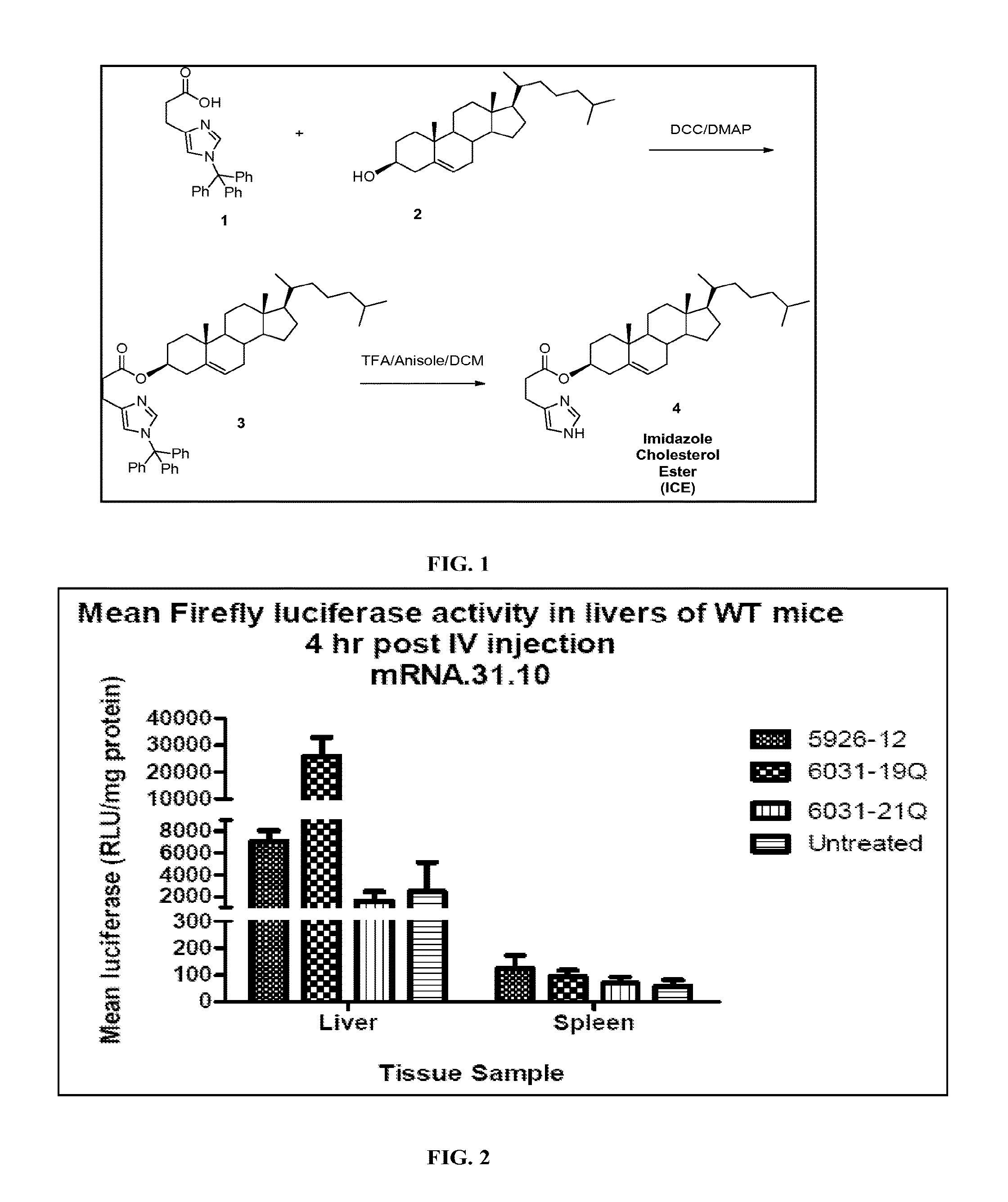
Delivery of mRNA for the augmentation of proteins and enzymes in human genetic diseases
InactiveUS20110244026A1Facilitating transfectionReduce deliveryOrganic active ingredientsDigestive systemDiseaseADAMTS Proteins
Disclosed herein are compositions and methods of modulating the expression of gene or the production of a protein by transfecting target cells with nucleic acids. The compositions disclosed herein demonstrate a high transfection efficacy and are capable of ameliorating diseases associated with protein or enzyme deficiencies.
Owner:TRANSLATE BIO INC
Liver specific delivery of messenger RNA
Disclosed herein are compositions and methods of modulating the expression of gene or the production of a protein by transfecting target cells with nucleic acids. The compositions disclosed herein demonstrate a high transfection efficacy and are capable of ameliorating diseases associated with protein or enzyme deficiencies.
Owner:TRANSLATE BIO INC
Pluripotent therapeutic compositions and uses thereof
InactiveUS20090274660A1Reduce riskReduce significant riskBiocideOrganic active ingredientsSelf-healingAnticarcinogen
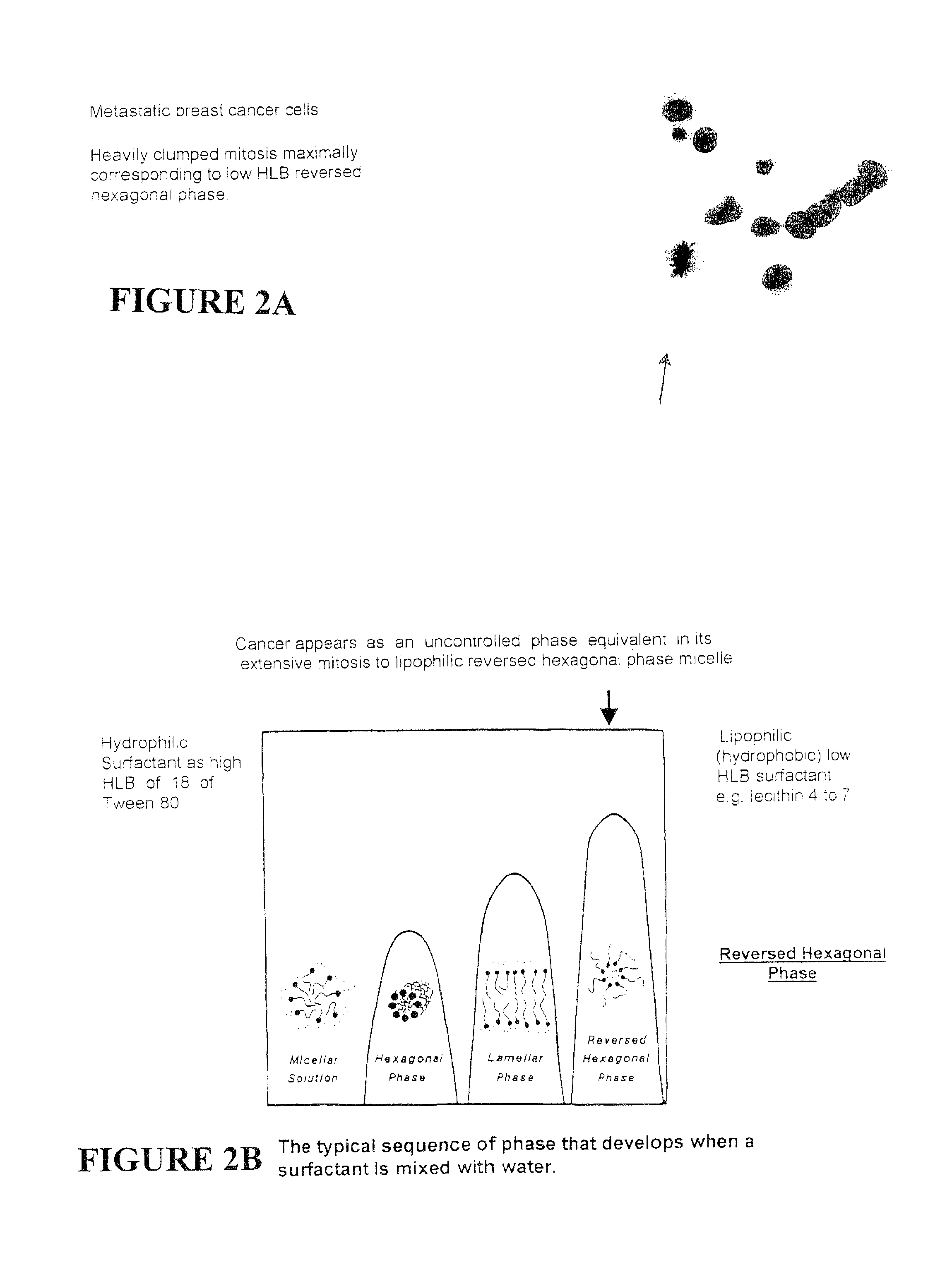
Synthetic Stem Cell-like Tissue Healing and Regeneration Medication with Anti-inflammatory, Protein Synthesis, Enzyme Deficiency Activation and Genetic Therapy, and Anti-cancer Agent derived from a series of inventions that include these products of Biomolecular Engineering, Drug Discovery from a Biologic Periodic Table of Applied Biochemistry and Biophysics. Tissue has a self healing effect promoting tissue healing and tissue regeneration. Not only does it maintain good health but also it has been observed that the patient's blood is withdrawn from the patient and applied to the ulcer has healing qualities. Cartilage placed in a wound promotes and accelerates wound healing. The anabolic biochemical and biophysical equivalent of tissue has been found in these embodiments to have the same pharmacologic qualities, when devoid of genetic DNA mismatch and other catabolic factors including the catabolic effects of microorganism overgrowth that lacks pro-biotic qualities. The healing efficacy of these tissue components gives us further appreciation of the protective action of human tissue over and above and other than the immune protective system or perhaps an integral component part of the immune system.
Owner:IMMUNOPATH PROFILE INC A CORP OF PA
Methods and compositions for increasing enzyme activity in the CNS
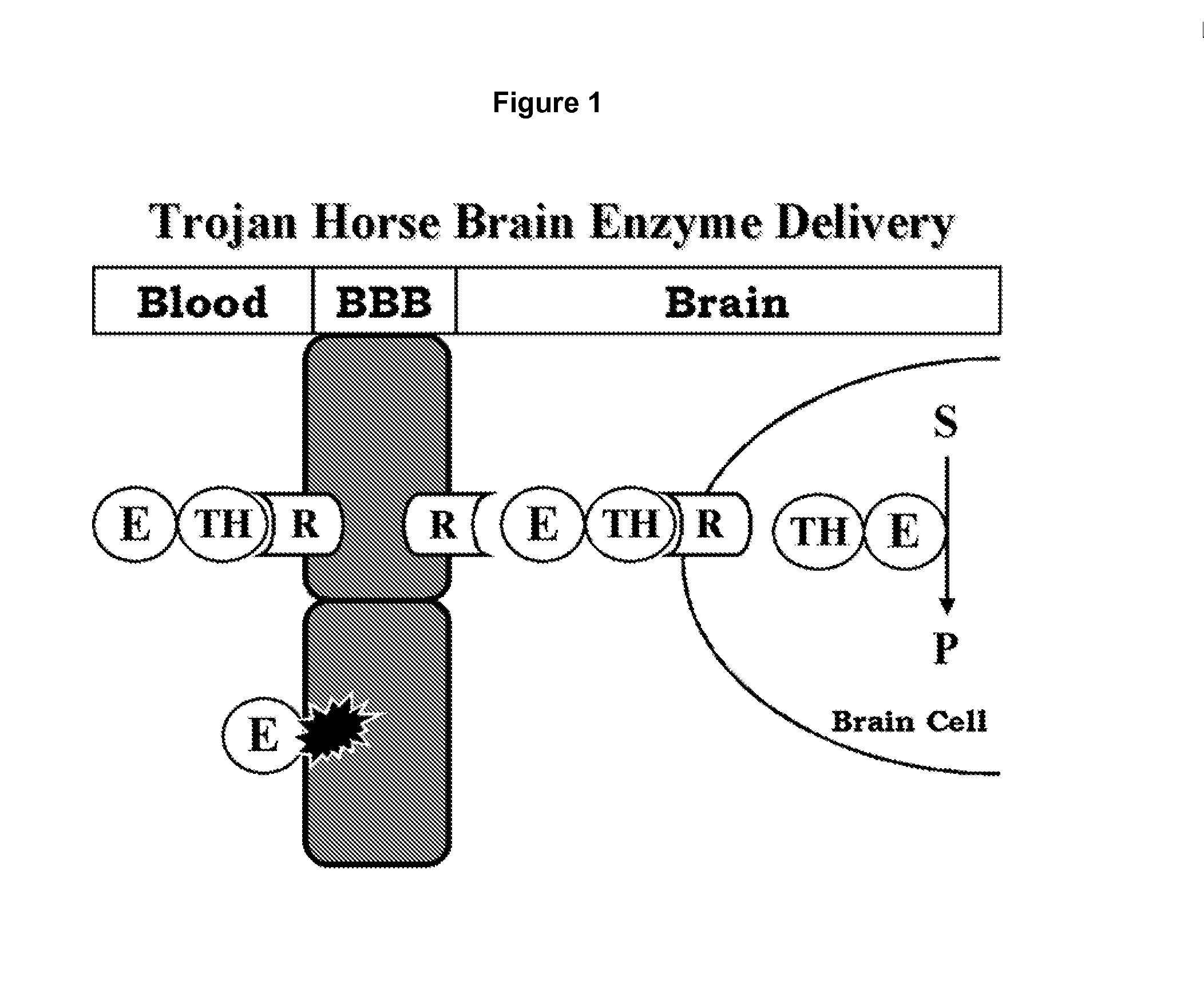
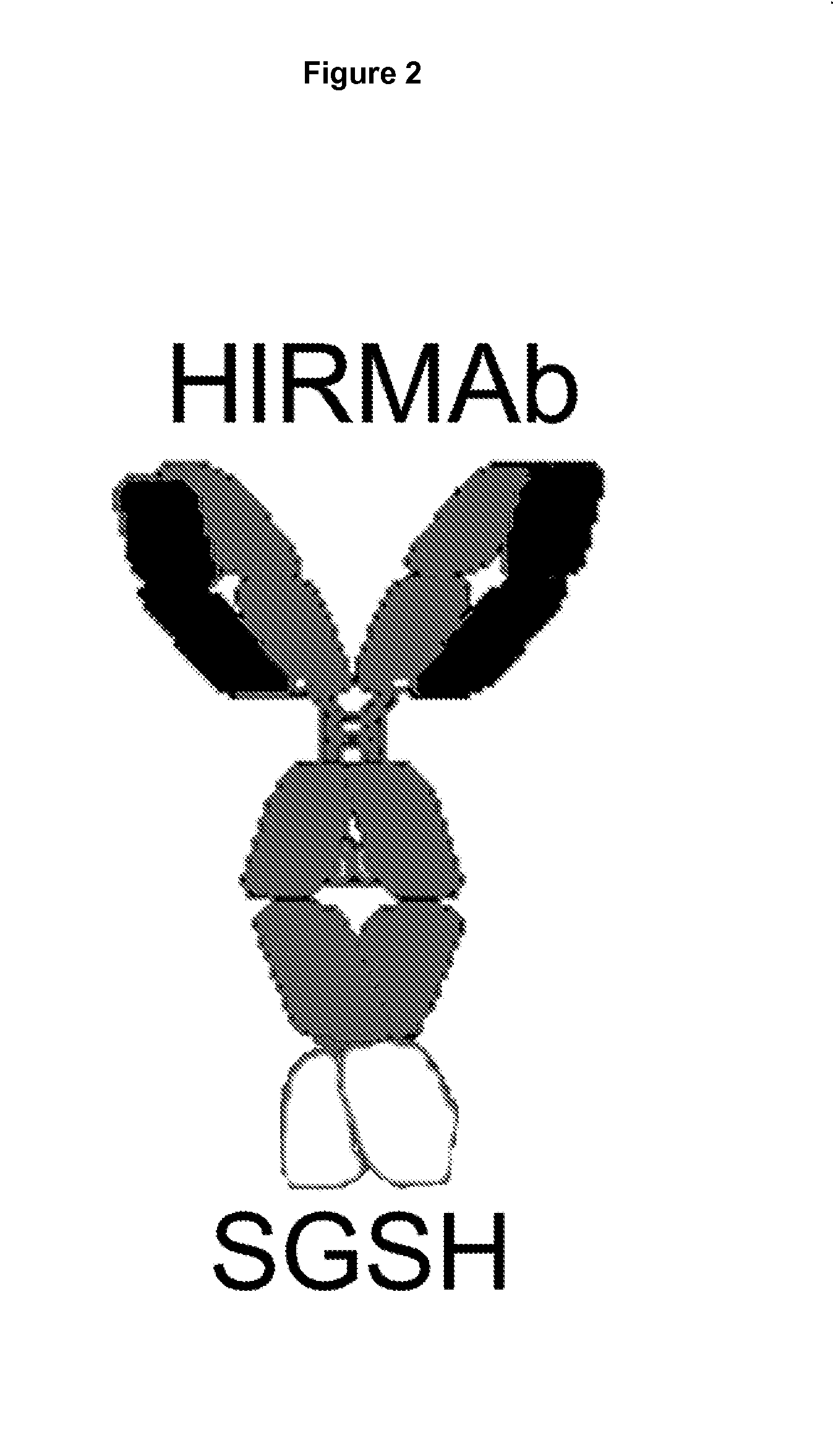
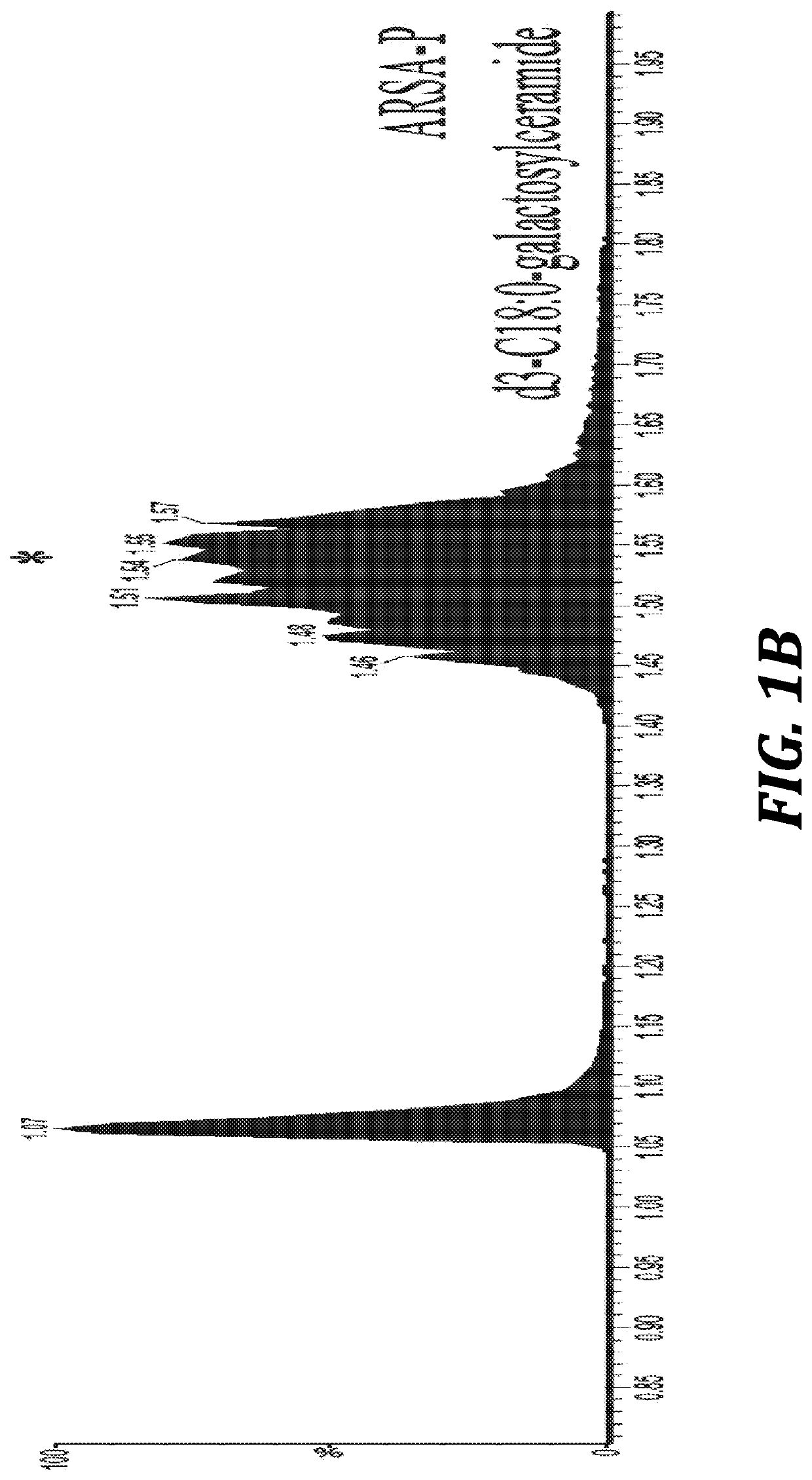
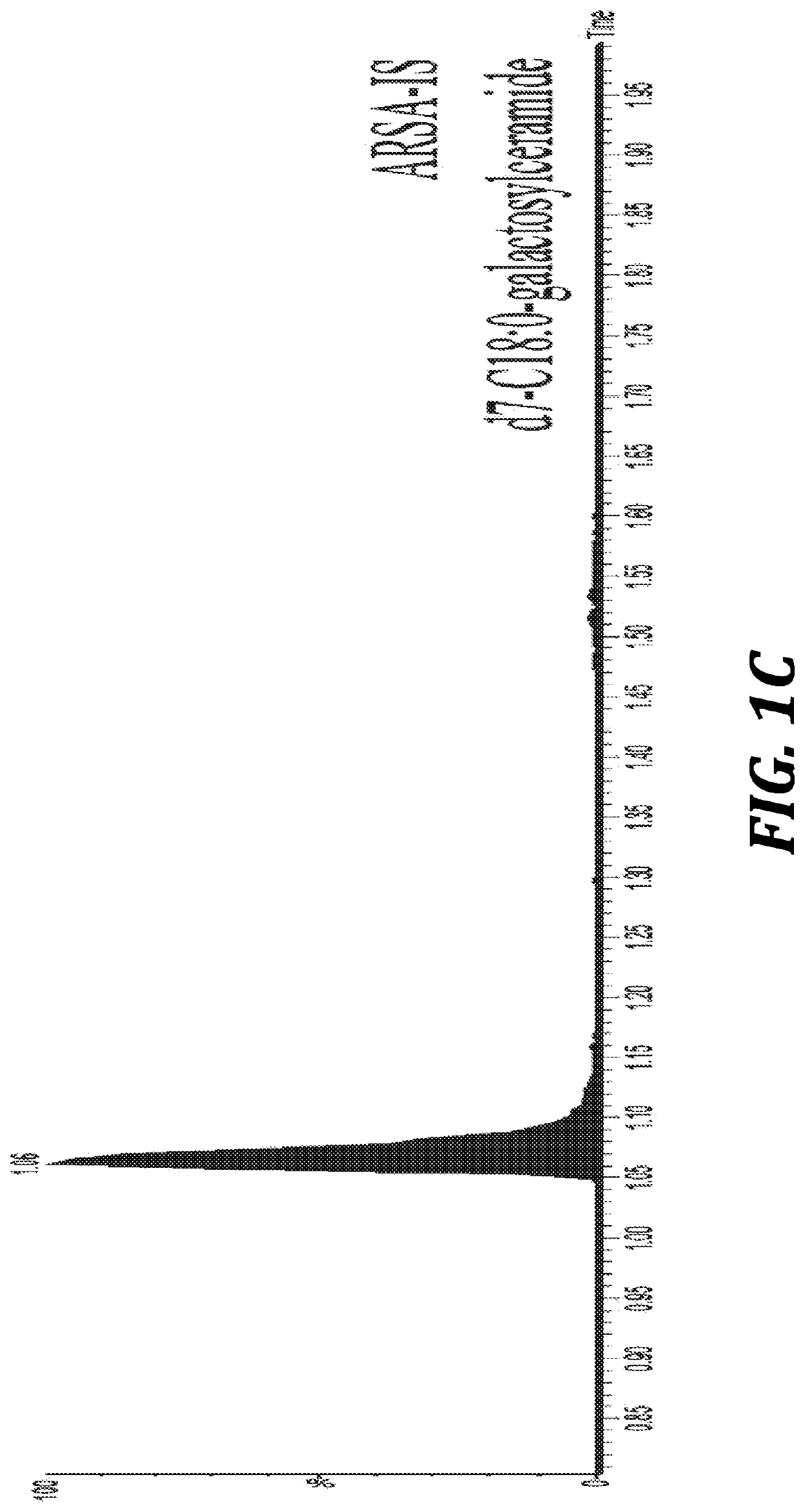
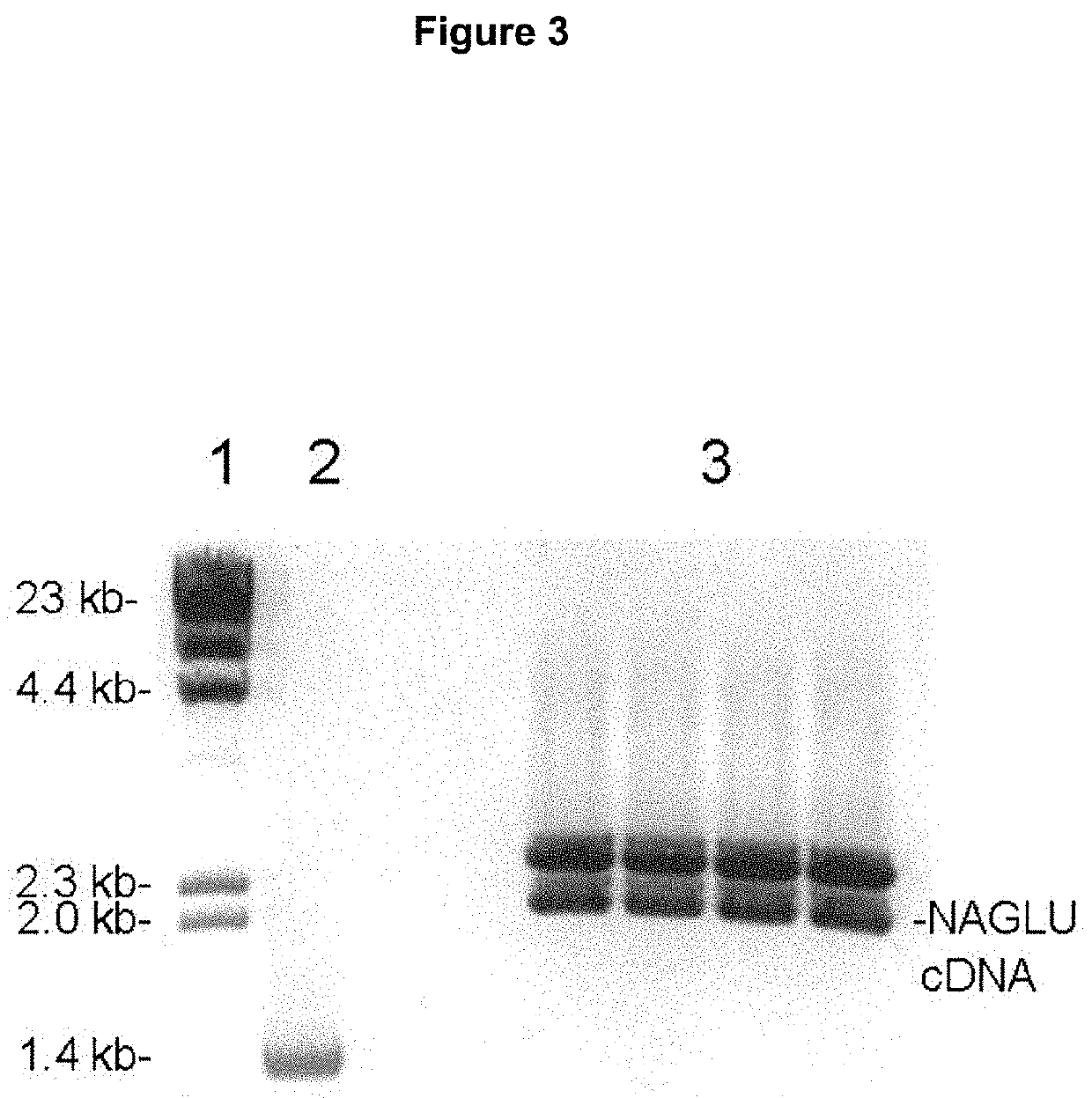
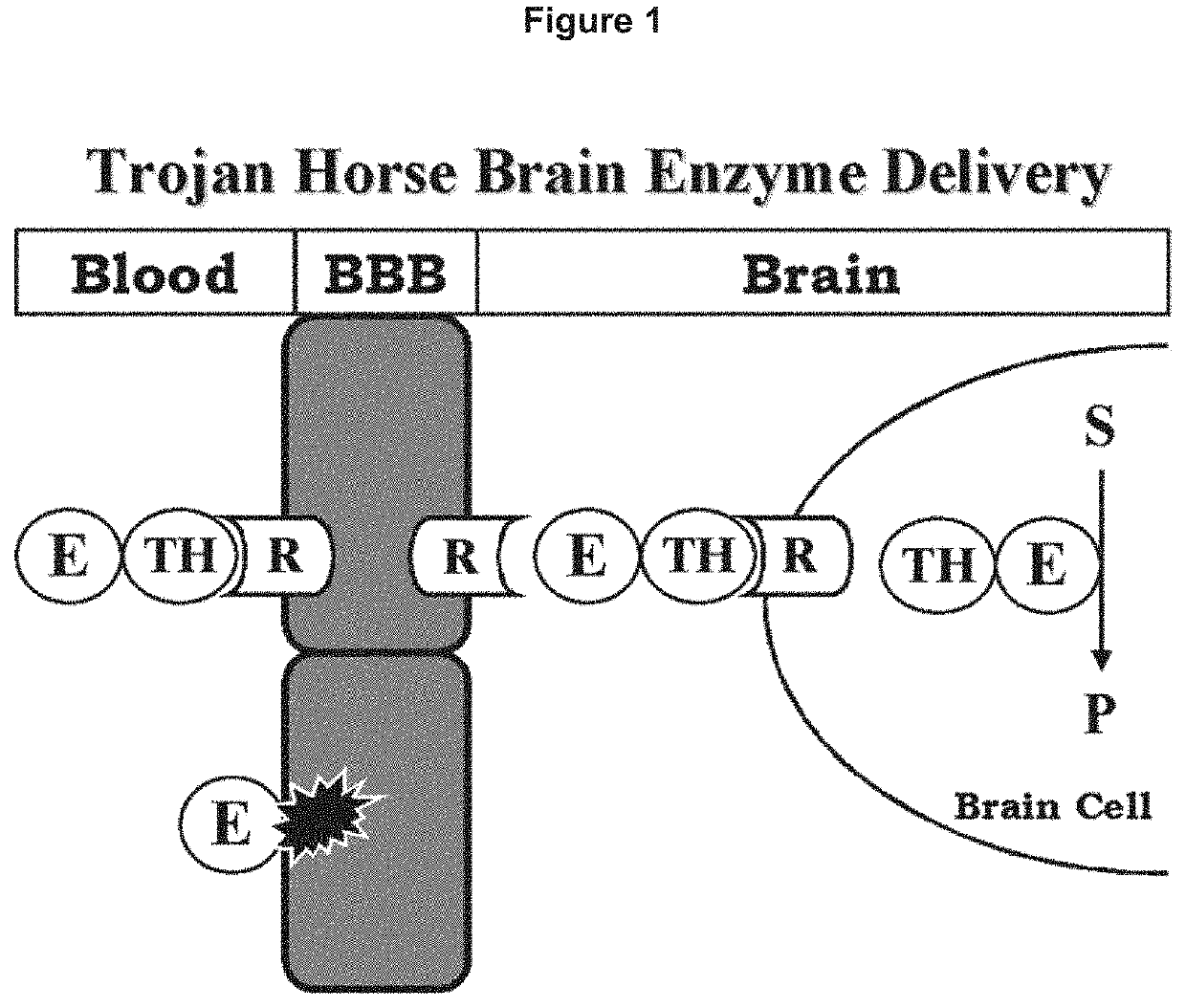
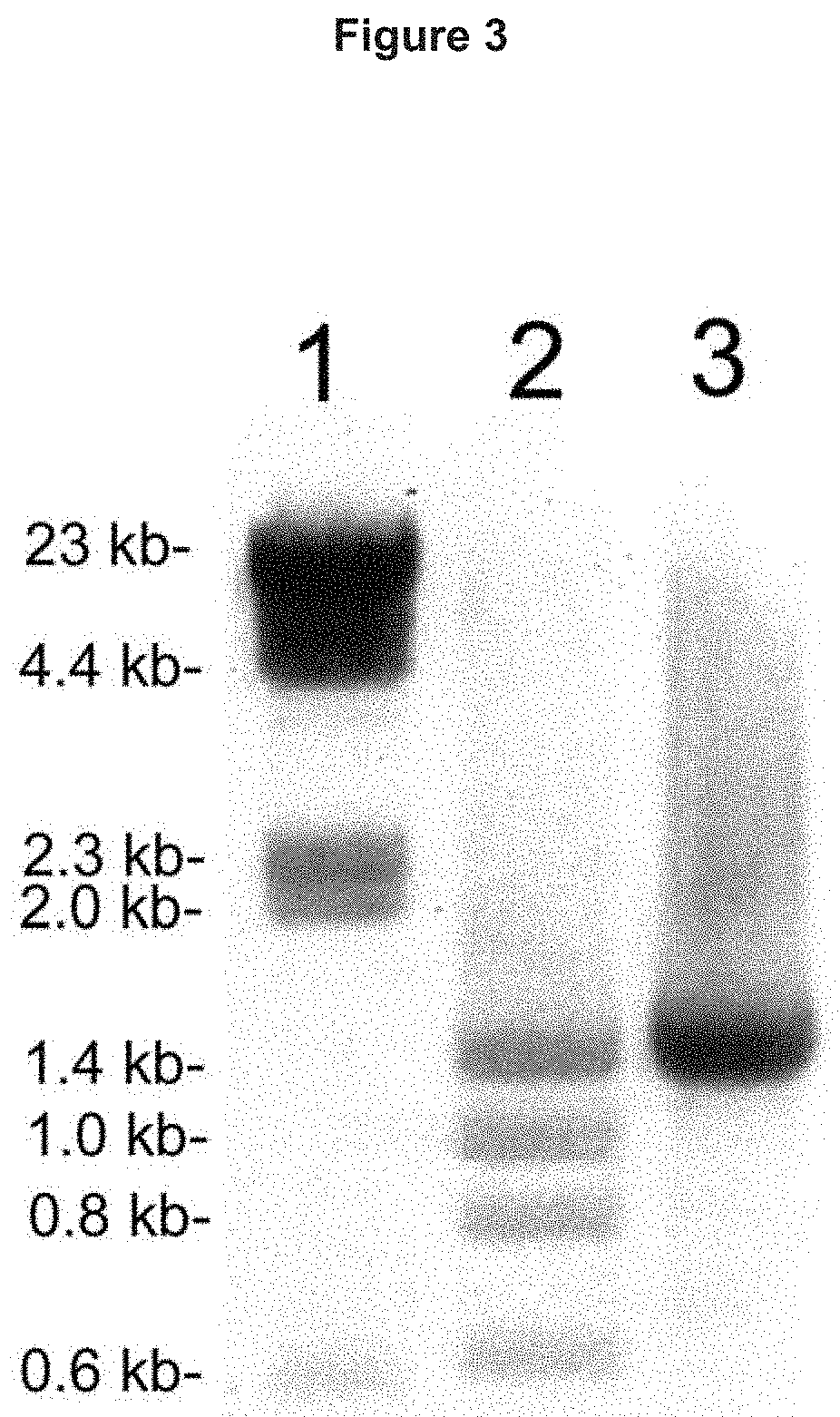
Provided herein are methods and compositions for treating a subject suffering from an enzyme deficiency in the central nervous system (CNS). The bifunctional fusion antibodies provided herein comprise an antibody to an endogenous blood brain barrier (BBB) receptor and an enzyme deficient in mucopolysaccharidosis III (MPS-III). The fusion antibodies provided herein comprise N-sulfoglucosamine sulfohydrolase (SGSH), alpha-N-acetylgulcosaminidase (NAGLU), heparin-alpha-glucosaminide N-acetyltransferase (HGSNAT), or N-acetylglucosamine-6-sulfatase (GNS). The methods of treating an enzyme deficiency in the CNS comprise systemic administration of a fusion antibody provided herein.
Owner:JCR PHARMA +1
Treatment of α-galactosidase A deficiency
InactiveUS7833742B2Minimize frequencyCost-effectiveNervous disorderMammal material medical ingredientsΑ galactosidase aEndocrinology
Owner:TAKEDA PHARMA CO LTD
Manufacture of active highly phosphorylated human n-acetylgalactosamine-6-sulfatase and uses thereof
This invention provides compositions of active highly phosphorylated human N-acetylgalactosamine-6-sulfatase (GALNS), and pharmaceutical compositions and formulations thereof, methods of producing and purifying GALNS, and its use in the diagnosis, prophylaxis, or treatment of diseases and conditions, including particularly lysosomal storage diseases that are caused by, or associated with, a deficiency in the GALNS enzyme, e.g., Mucopolysaccharidosis IVa (MPS IVa or Morquio A syndrome).
Owner:BIOMARIN PHARMA INC
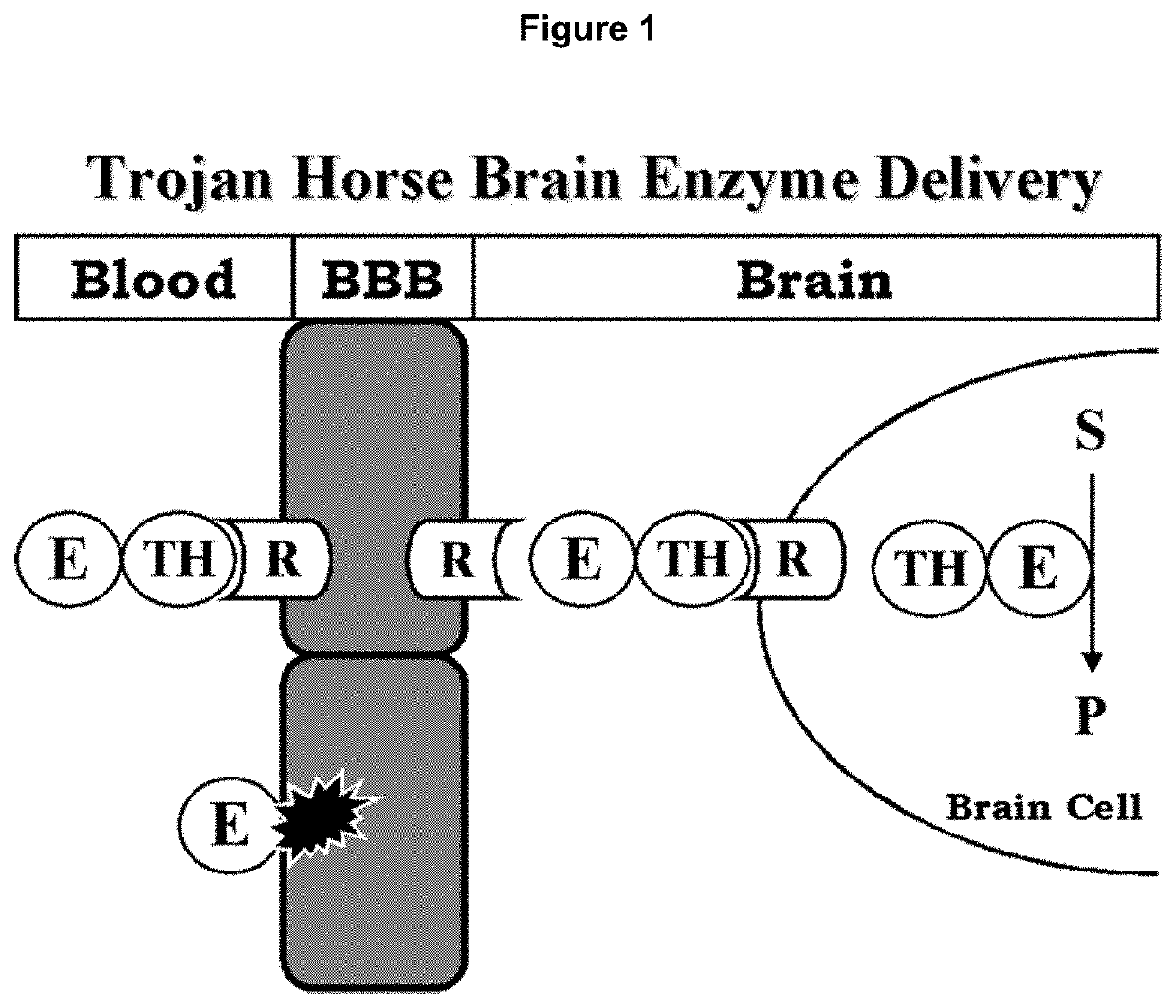
Methods and compositions for delivering enzymes and nucleic acid molecules to brain, bone and other tissues
Disclosed are methods for delivering an enzyme to a subject's brain or bone. The methods include administering a hyaluronidase to the subject and administering the enzyme to the subject. The hyaluronidase and the enzyme are administered to the subject under conditions effective to deliver the enzyme to the subject's brain or bone. Compositions and kits which include hyaluronidase and an enzyme are also disclosed, as are methods for increasing blood-brain barrier permeability in a subject. Also disclosed are methods, compositions, and kits for delivering genes or other nucleic acid molecules to a subject's brain or bone, as well as methods, compositions, and kits for delivering enzymes to a subject's tissues. The methods, compositions, and kits are disclosed as being useful in treating or preventing a variety of enzyme deficiency diseases, such as those affecting brain and / or bone, e.g., as Canavan's disease, Fabry disease, Gaucher's disease, various forms of mucopolysaccharidosis (e.g., Hurler's syndrome, Scheie syndrome, Hurler-Scheie syndrome, Sanfillippo A syndrome, Morquio A syndrome, Morquio B syndrome, etc.), Niemann-Pick disease, Schindler disease, and Pompe disease.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
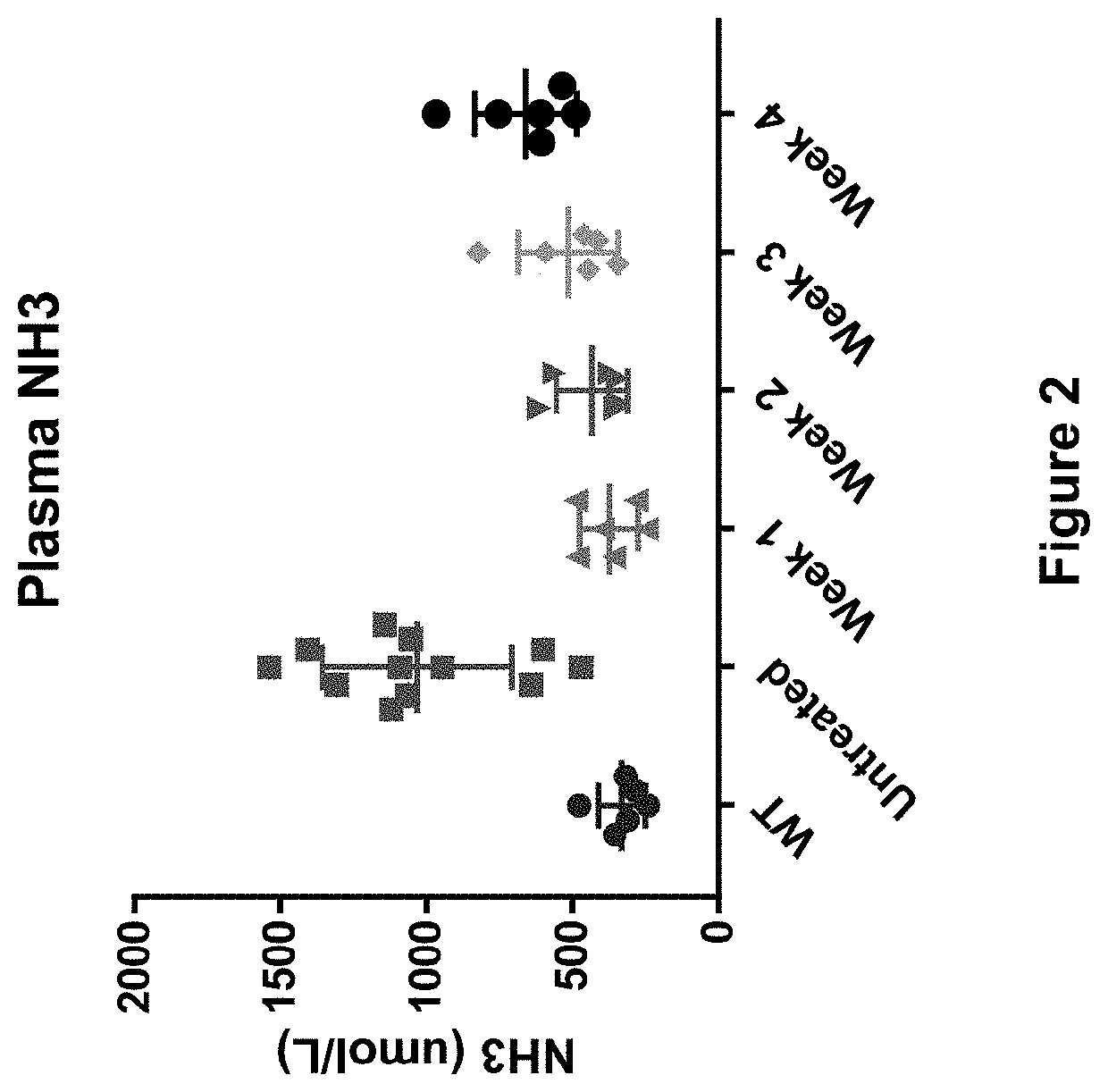
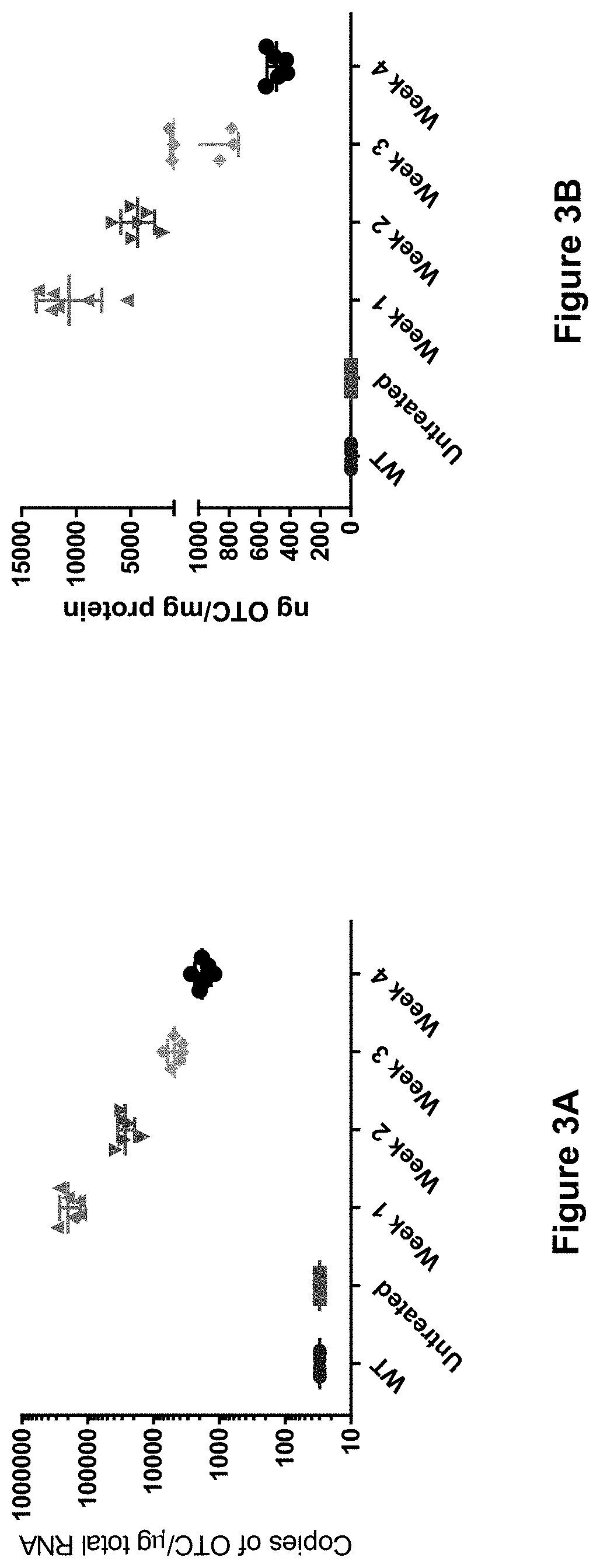
Compositions useful in treatment of ornithine transcarbamylase (OTC) deficiency
Viral vectors comprising engineered hOTC DNA and RNA sequences are provided which when delivered to a subject in need thereof are useful for treating hyperammonemia, ornithine transcarbamylase transcarbamylase deficiency and symptoms associated therewith. Also provided are methods of using hOTC for treatment of liver fibrosis cirrhosis in OTCD patients by administering hOTC.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Therapeutic transplantation using developing, human or porcine, renal or hepatic, grafts
A method of treating a renal, hepatic or enzyme-deficiency disorder in a subject in need thereof is disclosed. The method is effected by transplanting into the subject tissue derived from a human or porcine, kidney or liver, the kidney or liver being at a selected gestational stage.
Owner:YEDA RES & DEV CO LTD
Therapeutic transplantation using developing, human or porcine, renal or hepatic, grafts
A method of treating a renal, hepatic or enzyme-deficiency disorder in a subject in need thereof is disclosed. The method is effected by transplanting into the subject tissue derived from a human or porcine, kidney or liver, the kidney or liver being at a selected gestational stage.
Owner:YEDA RES & DEV CO LTD
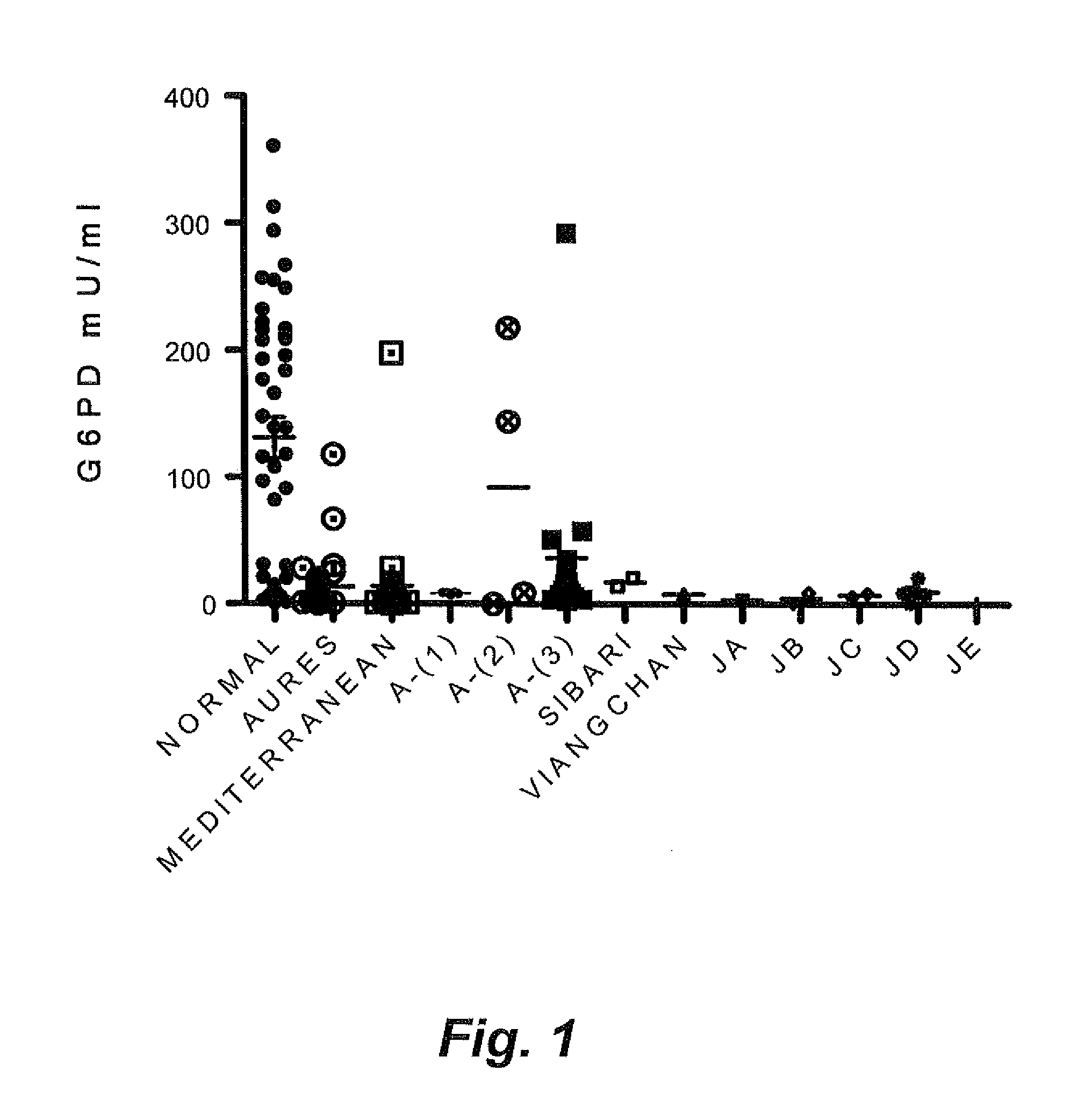
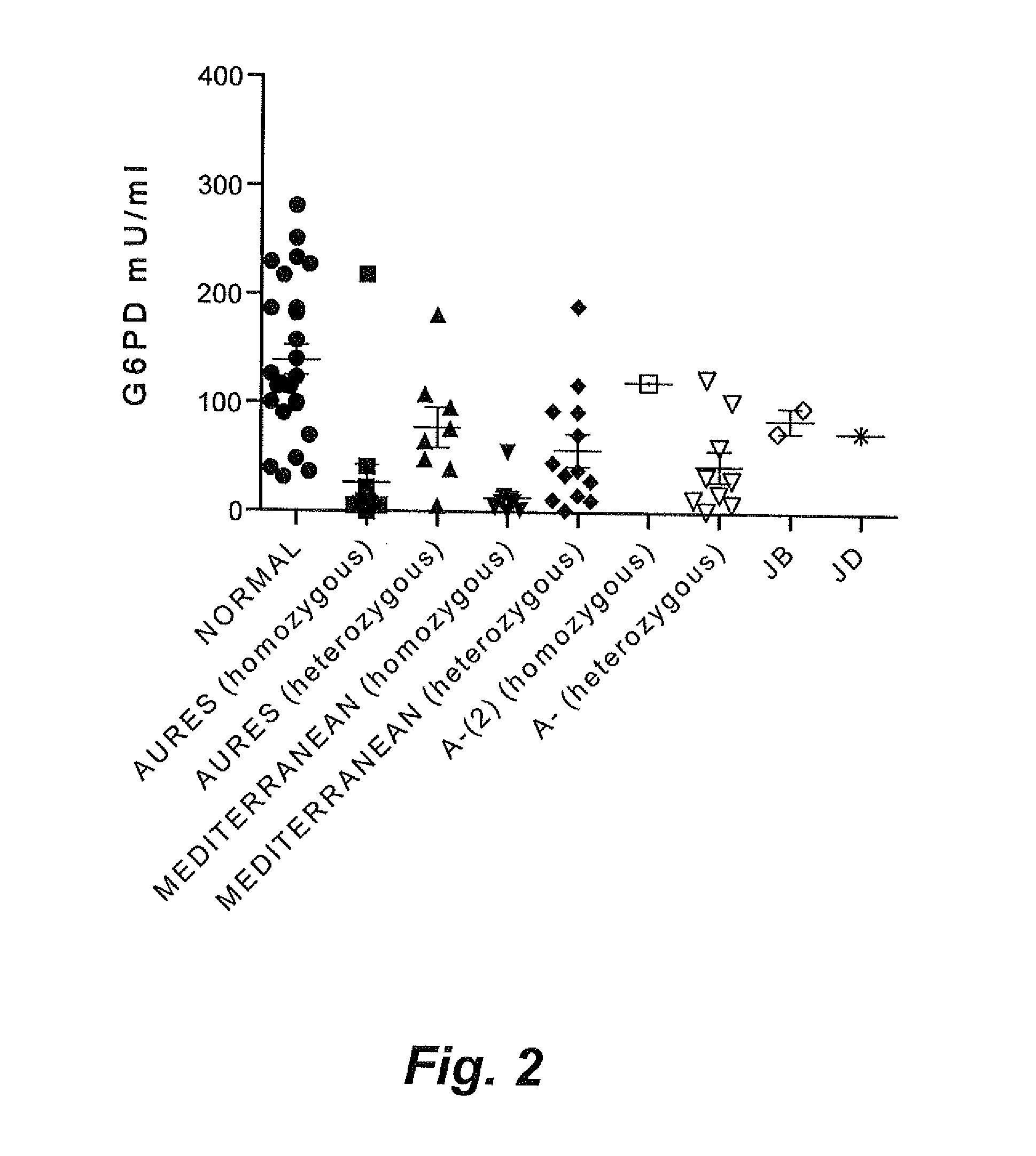
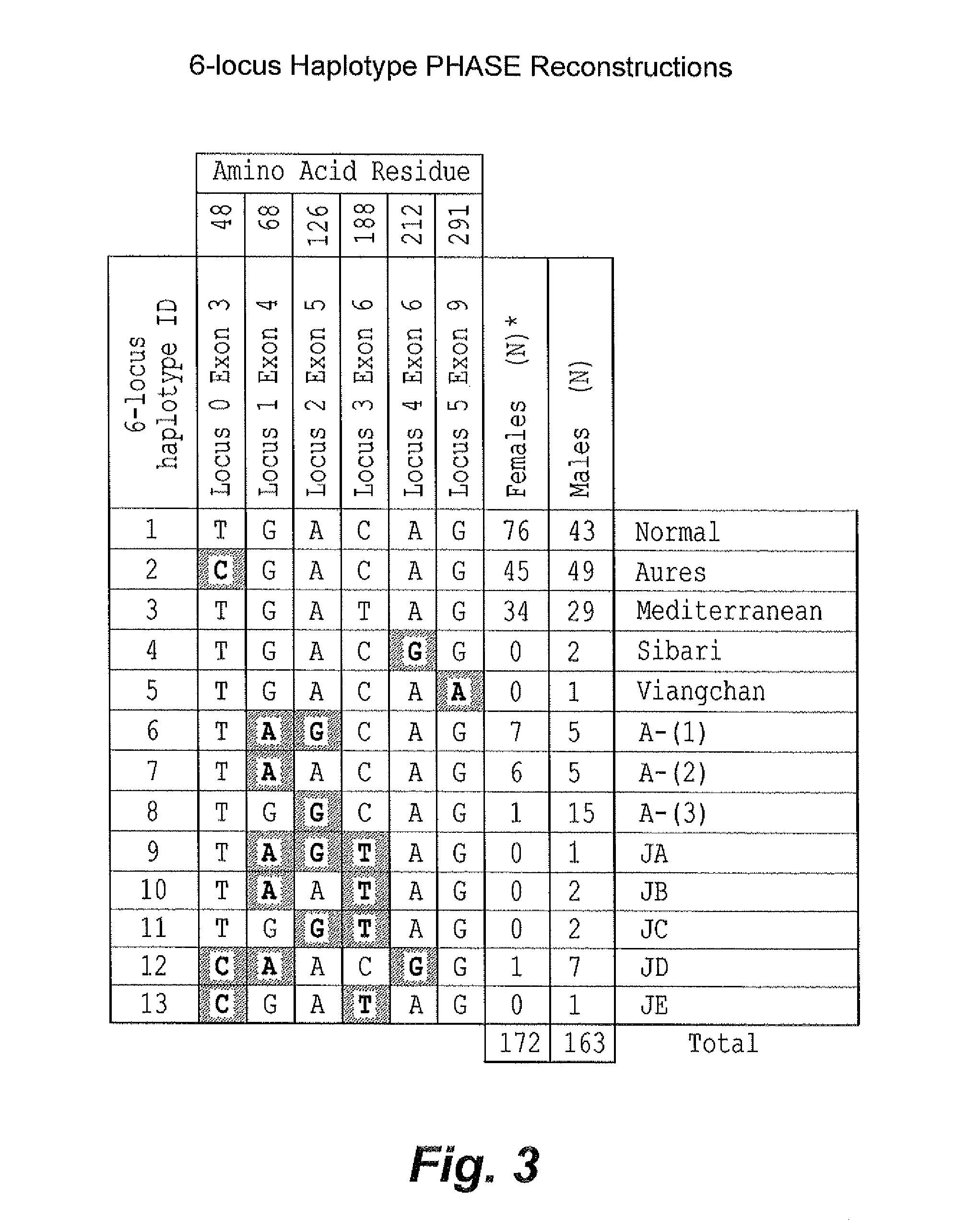
Genetic biomarkers for glucose-6-phosphate dehydrogenase deficiency
InactiveUS20140072966A1Reduce expressionReduced stabilitySugar derivativesMicrobiological testing/measurementGene mutationDisease
The genetic biomarkers for glucose-6-phosphate dehydrogenase (G6PD) deficiency are five haplotypes representing mutations of the G6PD gene on the X chromosome. Each of these haplotypes codes for more than one non-conservative amino acid change. The present inventors have discovered that when mutations of the G6PD gene result in at least two non-conservative amino acid changes in combination, expression of the G6PD enzyme or the stability of the G6PD enzyme is severely decreased, presenting a substantial risk of disease resulting from the deficiency, even in female patients who would normally be considered asymptomatic carriers of the genetic mutation(s).
Owner:KING ABDULAZIZ UNIV +1
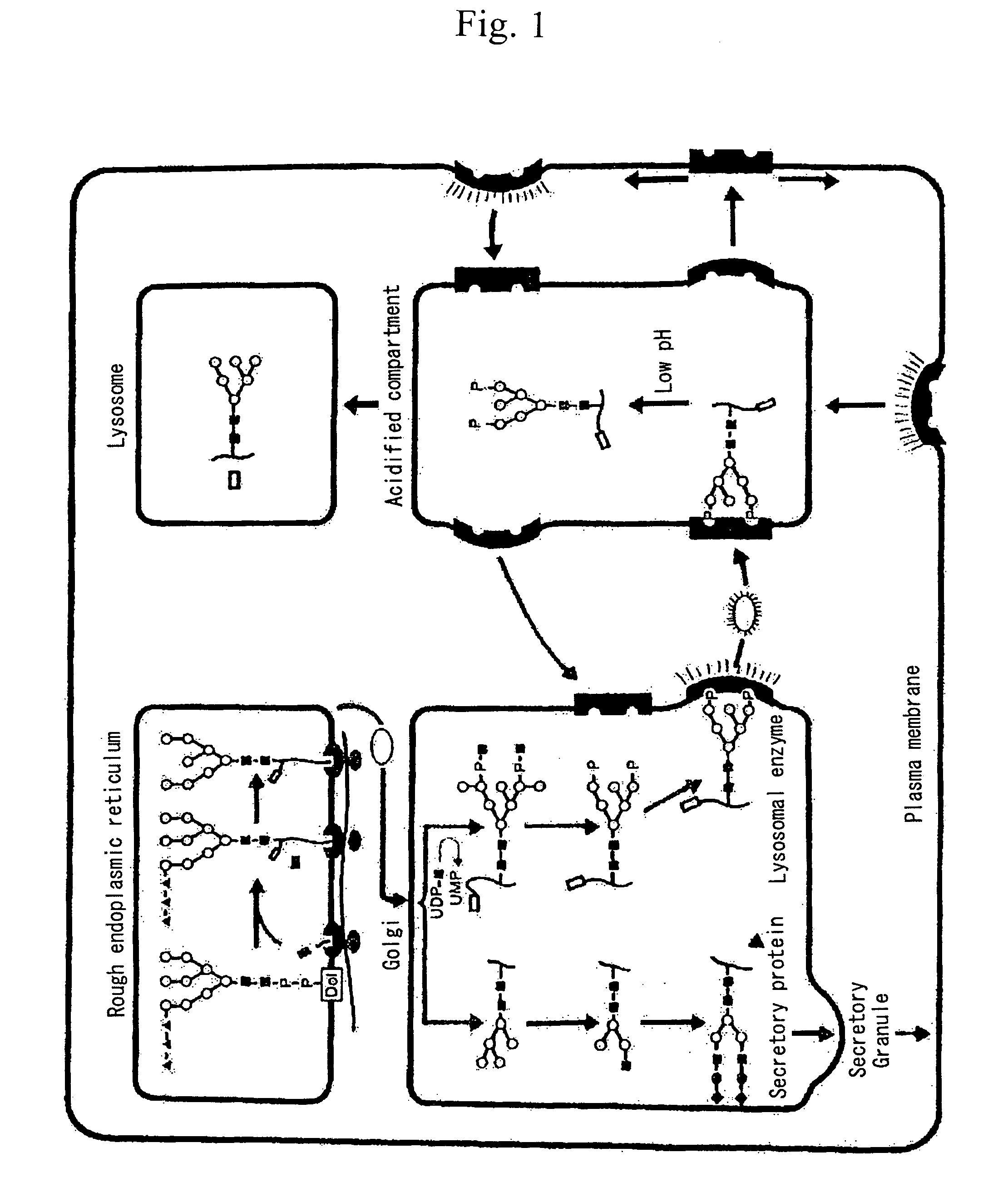
Glycoprotein and process for producing the same
InactiveUS7579166B2High purityMaintain physiological activitySenses disorderPeptide/protein ingredientsBiotechnologyEnzyme Gene
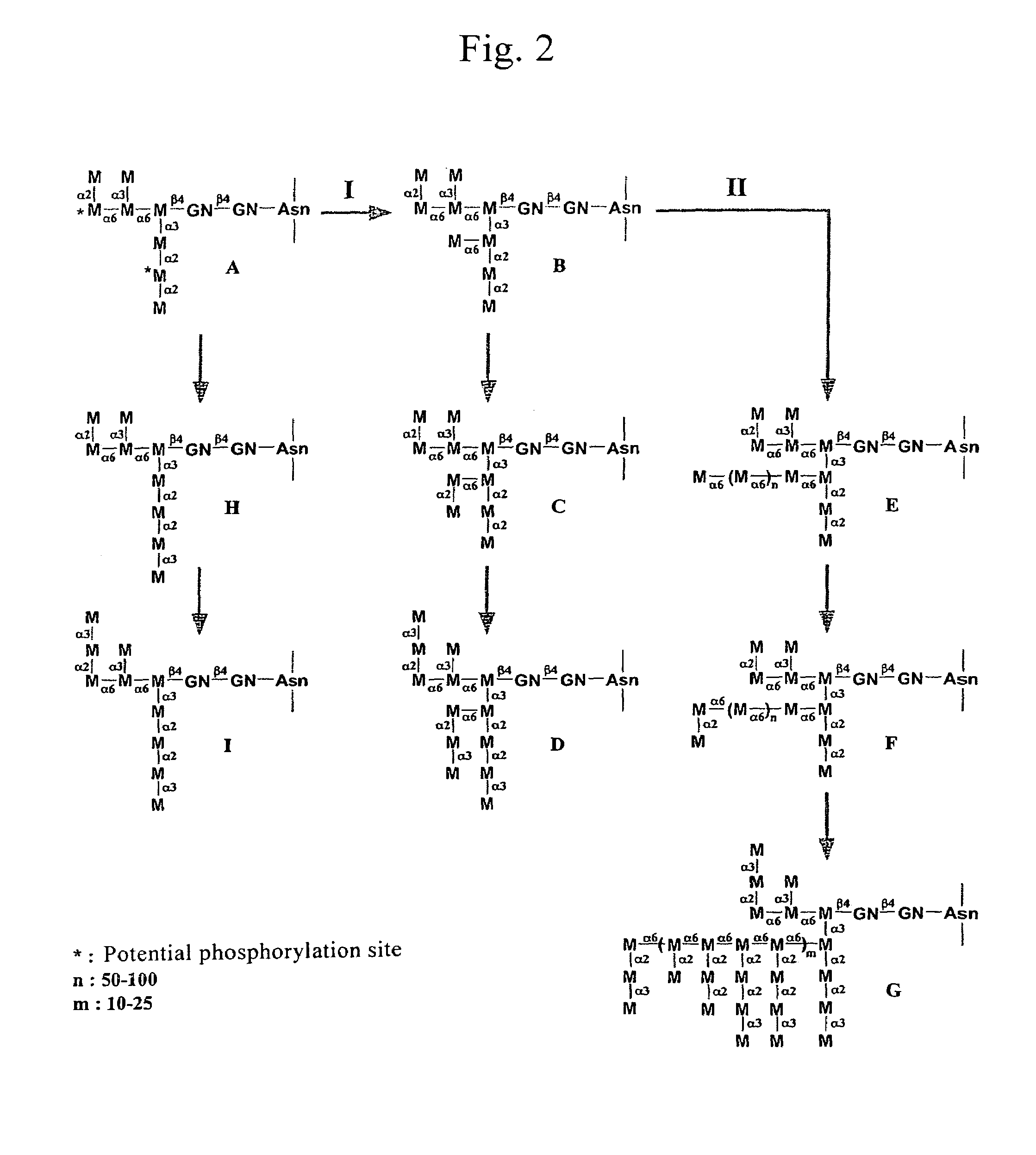
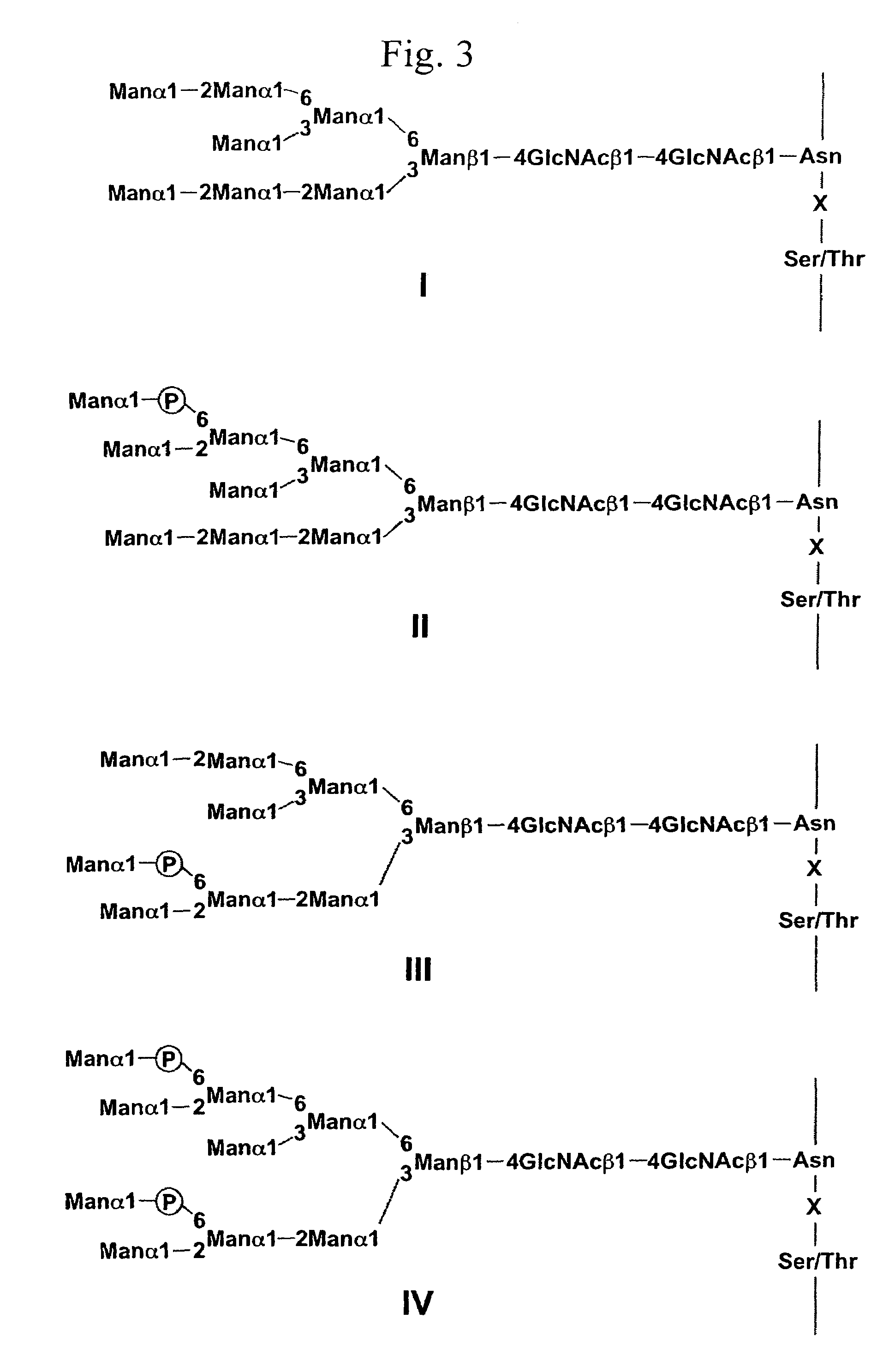
A process for producing a lysosomal enzyme having a mannose-6-phosphate-containing acidic sugar chain, wherein the process comprising: culturing in a medium yeast cells obtained by introducing a lysosomal enzyme gene into a sugar chain biosynthetic enzyme gene mutant strain of yeast, collecting a lysosomal enzyme having a phosphate-containing sugar chain from the culture, and then treating the enzyme with α-mannosidase; and pharmaceutical compositions for treatment of human lysosomal enzyme deficiencies produced by the process. The genetic engineering technique using the yeast according to the present invention allows large-amount and high-purity production of a glycoprotein having a phosphate-containing acidic sugar chain which can serve as a labeling marker for transporting into lysosomes in cells of mammals such as human. The glycoprotein having a phosphate-containing acidic sugar chain according to the invention may be utilized as a drug effective in treatment of human lysosomal enzyme deficiencies, etc.
Owner:TOKYO METROPOLITAN INST OF MEDICAL SCI +1
Primer composition for detecting harmful gene of deficiency of uridine monophosphate synthase of cattle, kit with primer composition and application of kit
ActiveCN103627804AStrong amplification specificityHigh amplification efficiencyMicrobiological testing/measurementDNA/RNA fragmentationNucleotide sequencingUridine monophosphate
The invention discloses a primer composition for detecting a harmful gene of deficiency of uridine monophosphate synthase of cattle, a kit with the primer composition and an application of the kit. The primer composition disclosed by the invention is composed of a primer group A and a primer group B, wherein the primer group A is composed of a primer 1 and a primer 2, the primer group B is composed of a primer 3 and a primer 4, and the nucleotide sequences of the primer 1, the primer 2, the primer 3 and the primer 4 are respectively shown as SEQIDNO. 1-4. The invention also provides the kit with the primer composition. The method for applying the kit disclosed by the invention to the detection of the harmful gene of the deficiency of uridine monophosphate synthase of cattle comprises the steps of extracting the complete set of DNA (Deoxyribonucleic acid) in cattle blood as a template to carry out nested PCR (Polymerase Chain Reaction) amplification to obtain a PCR product, and sequencing the obtained PCR product so as to directly know about the basic group change on a mutation site according to a sequenced result, thereby ensuring the accuracy of the result and meeting the requirements of a detecting technology for characteristics such as high speed, precision, high throughput and the like.
Owner:SOUTH CHINA AGRI UNIV
Recombinant (alpha)-L-iduronidase, methods for producing and purifying the same and methods for treating diseases caused by deficiencies thereof
The present invention provides a recombinant alpha -L-iduronidase and biologically active fragments and mutants thereof, methods to produce and purify this enzyme as well as methods to treat certain genetic disorders including alpha -L-iduronidase deficiency and mucopolysaccharidosis I (MPS I).
Owner:RES & EDUCATION INST HARBOR - UCLA MEDICAL CENT
Kit used for detecting enzyme activity of biotin enzyme
The invention discloses a kit used for detecting enzyme activity of biotin enzyme. The kit comprises a reagent 1, a reagent 2, a reagent 3 and a reagent 4 which are independently packaged. The reagent 1 is a buffer system of a buffer 1, an enzyme activity stabilizing agent and biocytin, a pH value of the reagent 1 is 3-11; the reagent 2 is a stopping solution; the reagent 3 is a neutralization solution; the reagent 4 is a system composed of the buffer 2 and a derivative substrate, and the derivative substrate is any one of 1,2-diacetybenzene, ninhydrin, fluorescamine and o-phthalaldehyde. When the kit is used for detection, dry blood slices can be taken as a detection sample, so that the method can be used for neonatal screening, can satisfy timely diagnosis and treatment requirements of biotin enzyme deficiency, and is easily popularized and used.
Owner:北京中科非凡生物技术有限公司 +2
Composition and methods for treatment of ornithine transcarbamylase deficiency
ActiveUS11167043B2Wide therapeutic indexKeep for a long timePowder deliveryPeptide/protein ingredientsOrnithine transcarbamylase deficiencyEnzyme deficiency
The present invention provides, among other things, methods of treating ornithine transcarbamylase deficiency, including administering to a subject in need of treatment a composition comprising an mRNA encoding an ornithine transcarbamylase protein at a low dose and at an administration interval such that at least one symptom or feature of the OTC deficiency is reduced.
Owner:TRANSLATE BIO INC
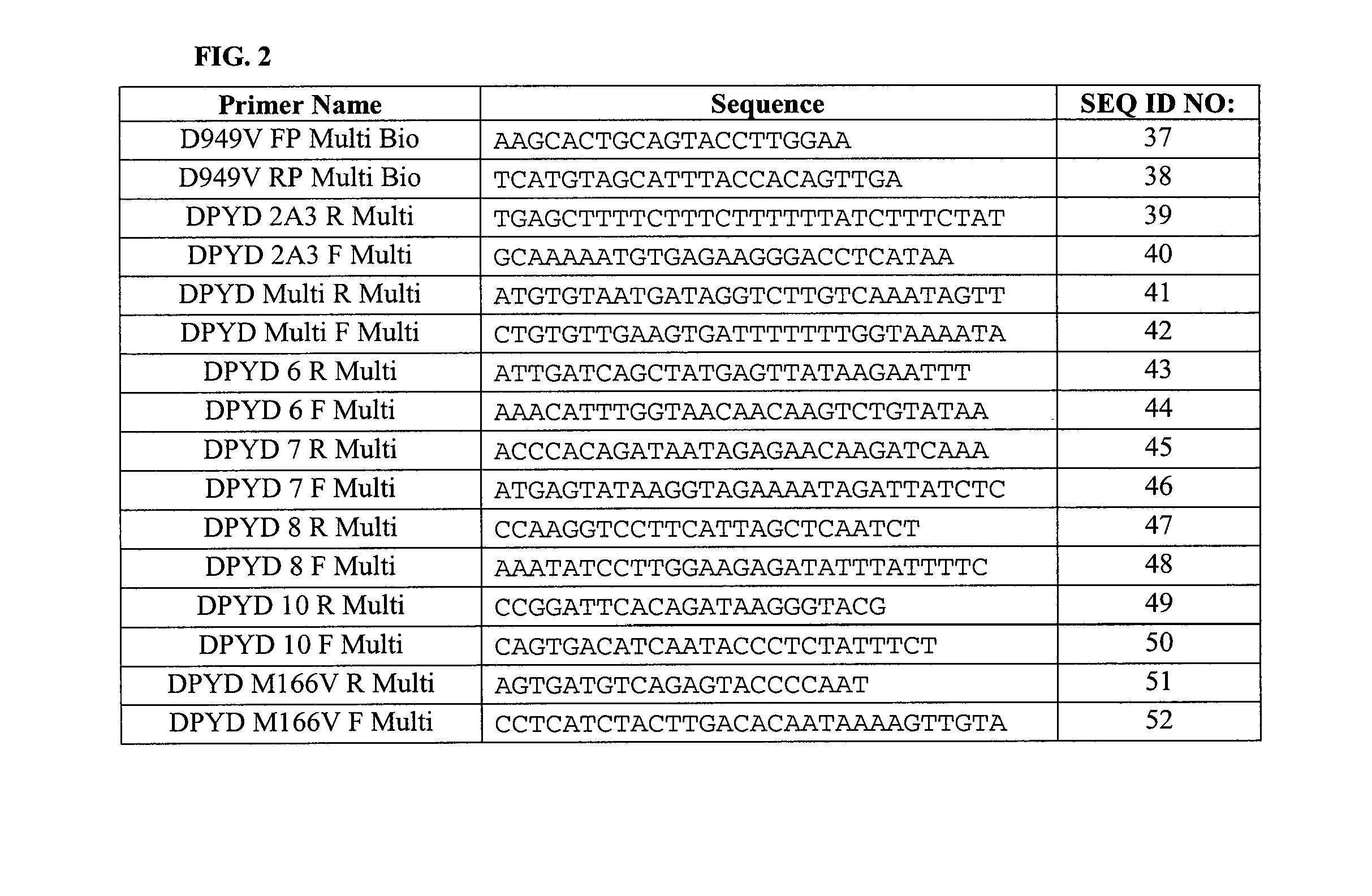
Genotyping Dihydropyrimidine Dehydrogenase Deficiency
InactiveUS20100240548A1Accurate profileRisk minimizationNucleotide librariesMicrobiological testing/measurementMultiplexGenetic risk
The invention provides compositions and methods relating to a multiplex test which detects all relevant genetic risk markers associated with DPD deficiency in one single reaction test.
Owner:PHARMGENOMICS
Composition with Anti-Inflammatory, Protein Synthesizing, Enzyme Deficiency Activating Genetic Therapy and Anti-Cancer Activity and Methods of Use
InactiveUS20100323030A1Improve hydrophilicityReduce their significant risk and dosageHeavy metal active ingredientsBiocideLipid formationDamages tissue
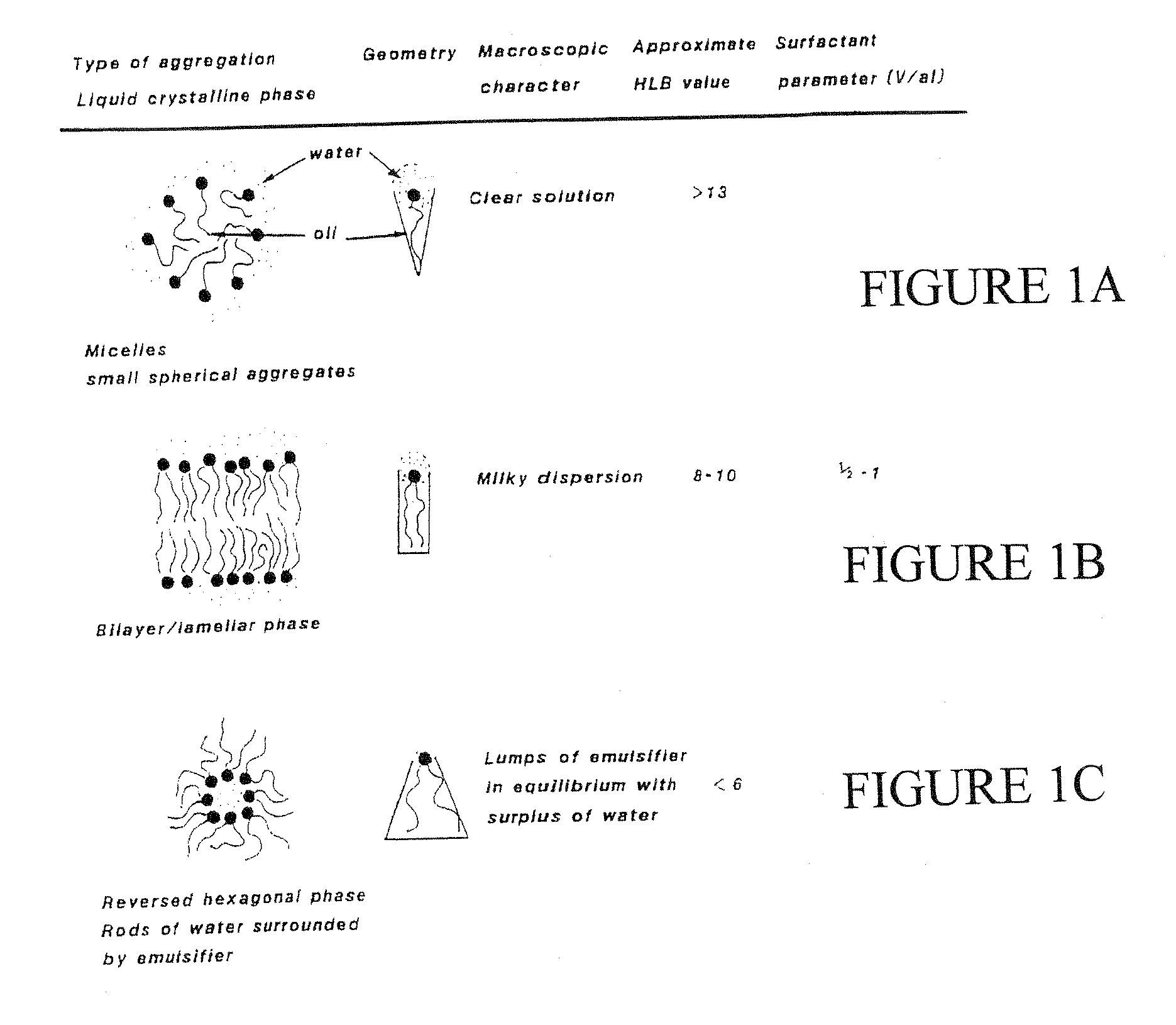
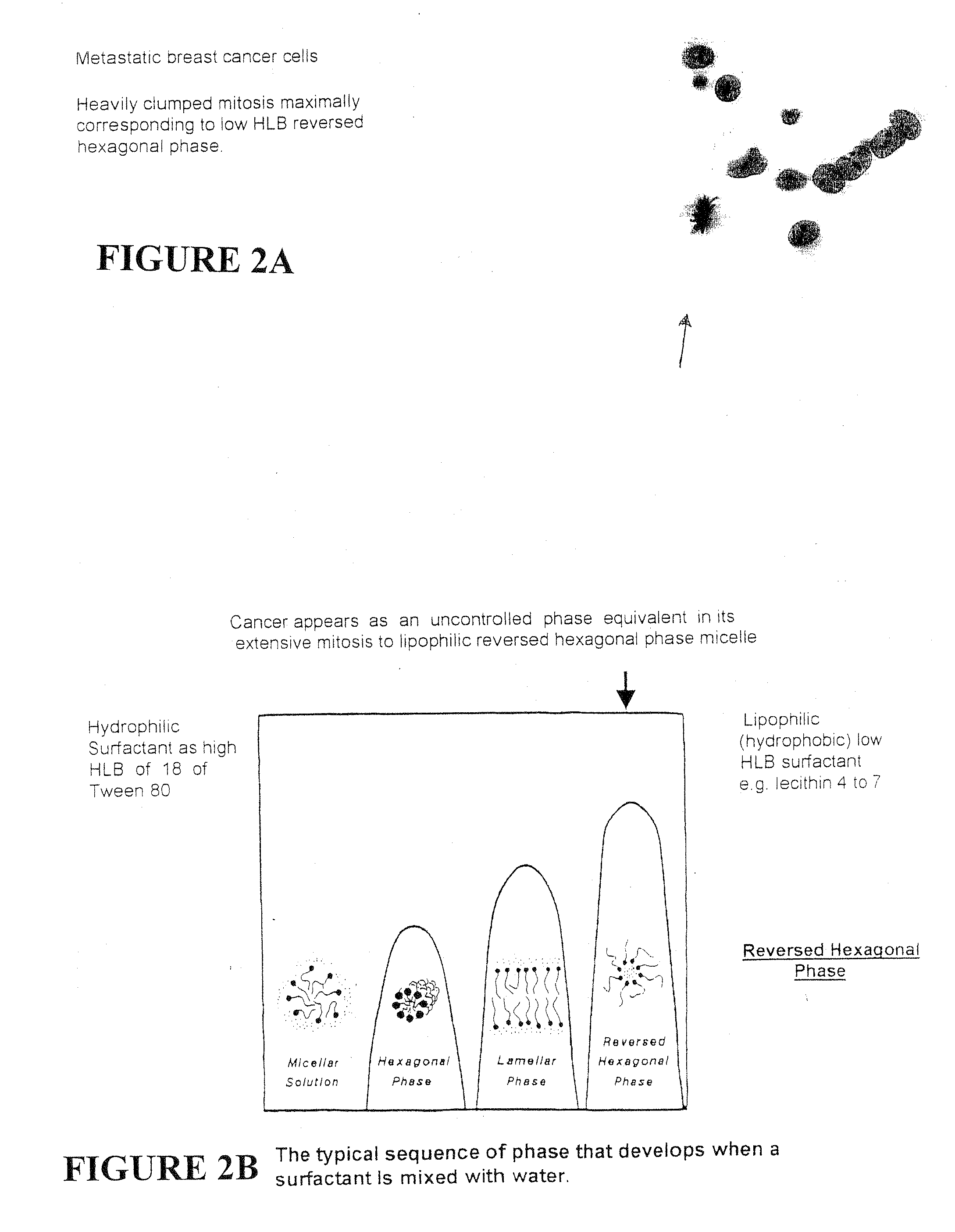
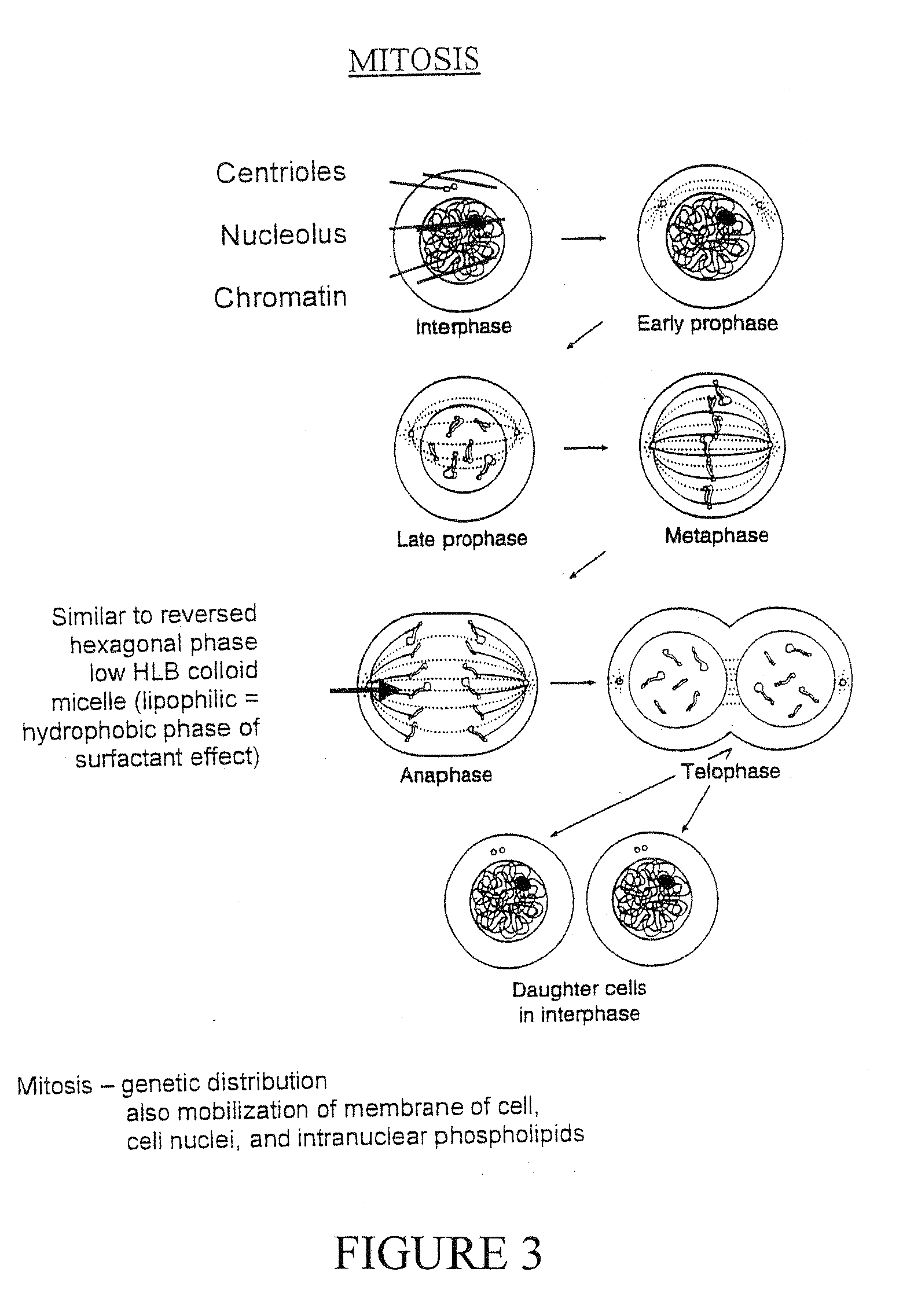
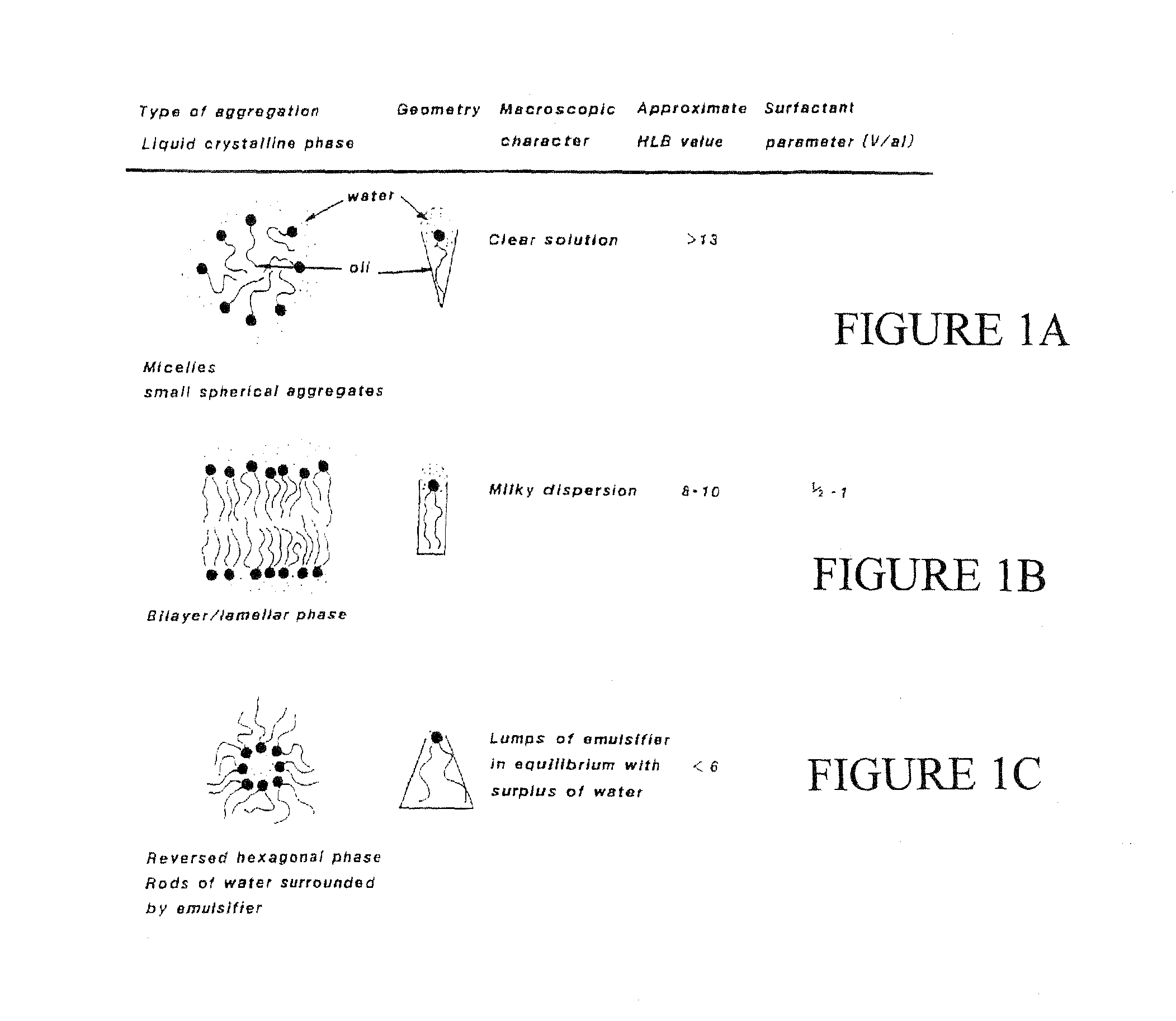
A composition for treating damaged tissue and promoting healthy tissue growth, healing and tissue regeneration, wherein said composition comprises an extracellular matrix compound, a surface-active lipid, one or more enantioinerically pure L-amino acids or glycine, a hydrophilic surfactant with a high HLB, as well as vitamins, minerals or trace elements. Not only does it maintain good health, but the components are non-intrusive, bio-safe, non-coalescent and can mimic normally occurring stem-cells within a body. When applied to diseased tissues, the subject compositions can stimulate, facilitate, and accelerate protein synthesis for the regeneration of diseased organs and tissues. The healing efficacy of these tissue components gives us further appreciation of the protective action of human tissue over and above and other than the immune protective system or perhaps an integral component part of the immune system.
Owner:IMMUNOPATH PROFILE INC A CORP OF PA
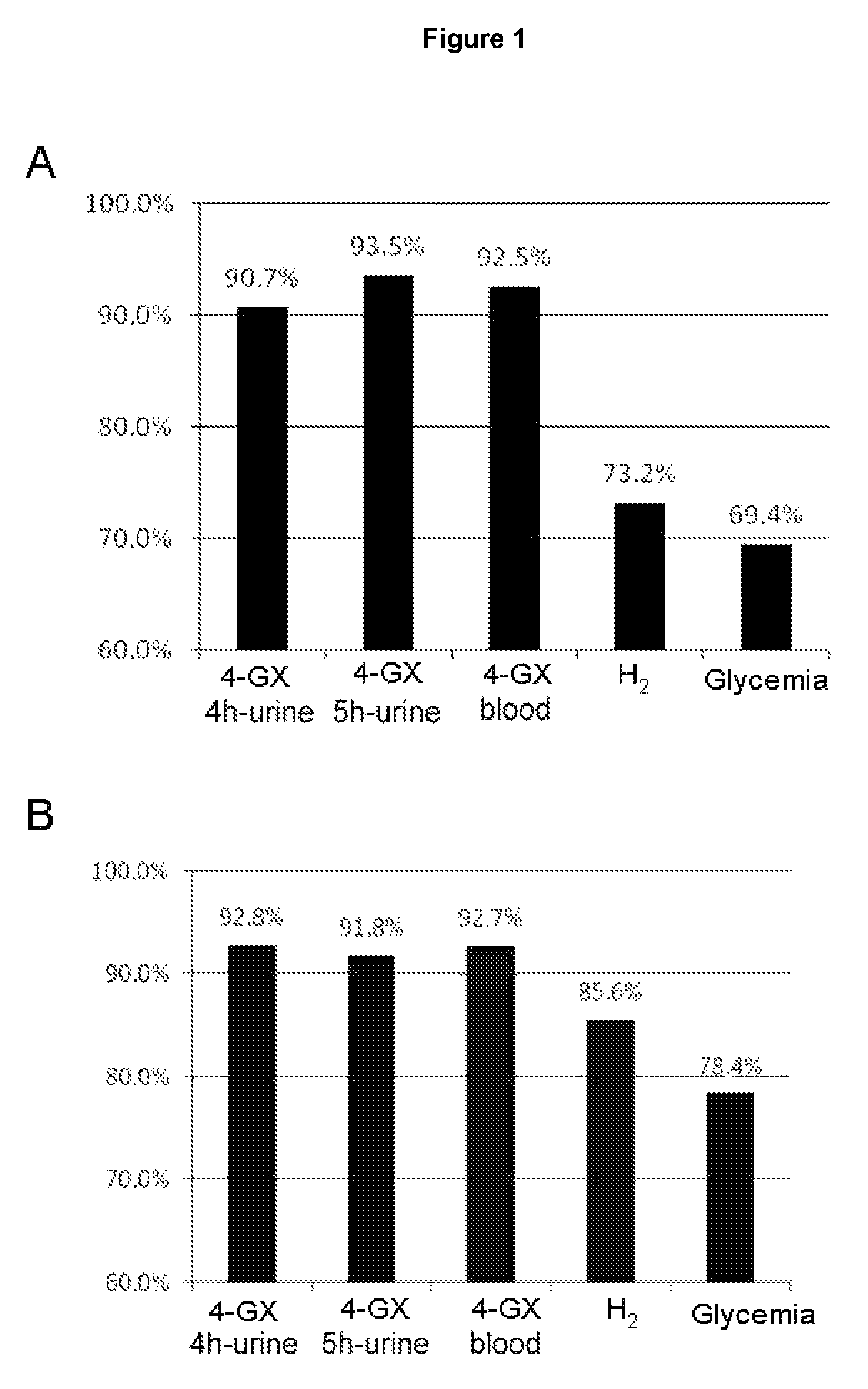
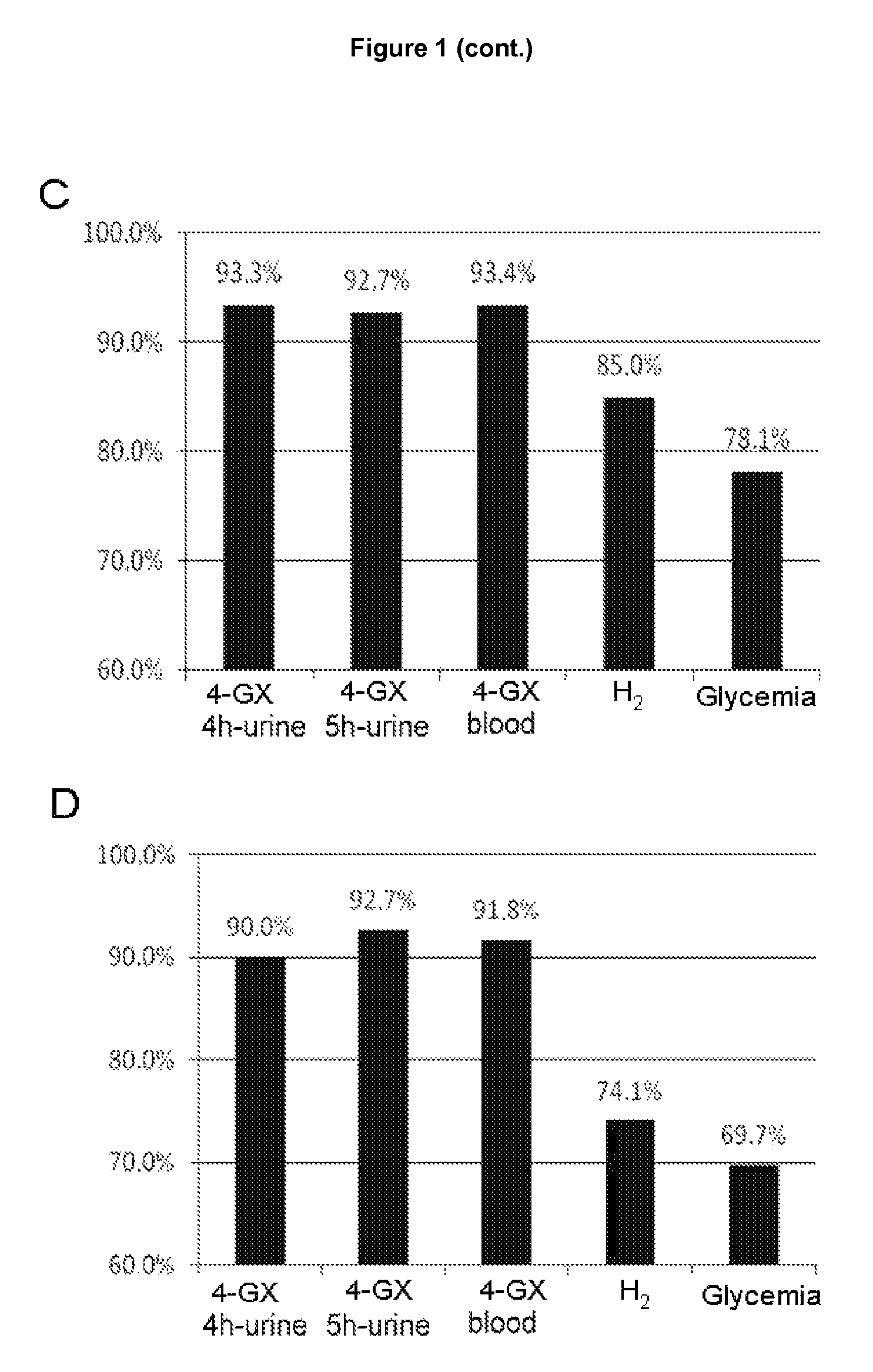
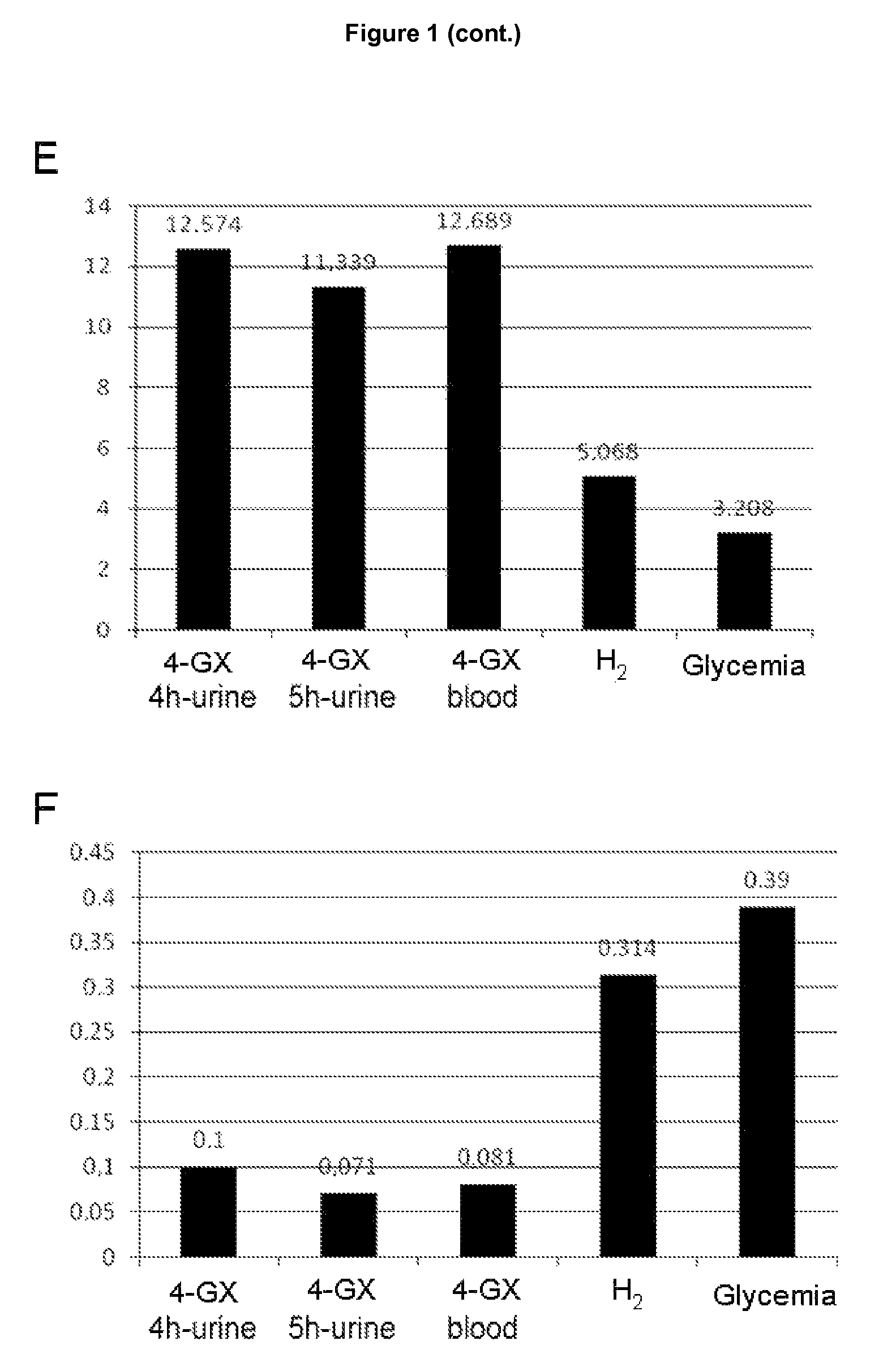
Non-invasive diagnostic method for the evaluation of intestinal lactase deficiency (hypolactasia)
InactiveUS9128100B2Absence of adverseFunction increaseMicrobiological testing/measurementDisease diagnosisDiagnostic testDirect evaluation
The test of the invention comprises the measuring the total amount of xylose in urine and / or its concentration in blood following oral administration of 4-O-β-D-galactopyranosyl-D-xylose (4-GX) to the patient. It is a non-invasive test that is based on the direct evaluation of the global enzyme activity in the whole individual, not on measuring the metabolic consequences derived from its deficiency. It does not require specialized equipment, does not cause apparent discomfort in patients with lactase deficiency and is very reliable, thus overcoming the drawbacks of the diagnostic tests currently in use and is a statistically significantly better test in terms of its reliability; consequently it should become the reference or gold standard test for the indication of hypolactasia.
Owner:VENTER PHARMA
Pluripotent therapeutic compositions and uses thereof
InactiveUS9555063B2Avoiding and minimizing ingestionPromote recoveryOrganic active ingredientsDispersion deliverySelf-healingBiological activation
Synthetic Stem Cell-like Tissue Healing and Regeneration Medication with Anti-inflammatory, Protein Synthesis, Enzyme Deficiency Activation and Genetic Therapy, and Anti-cancer Agent derived from a series of inventions that include these products of Biomolecular Engineering, Drug Discovery from a Biologic Periodic Table of Applied Biochemistry and Biophysics. Tissue has a self healing effect promoting tissue healing and tissue regeneration. Not only does it maintain good health but also it has been observed that the patient's blood is withdrawn from the patient and applied to the ulcer has healing qualities. Cartilage placed in a wound promotes and accelerates wound healing. The anabolic biochemical and biophysical equivalent of tissue has been found in these embodiments to have the same pharmacologic qualities, when devoid of genetic DNA mismatch and other catabolic factors including the catabolic effects of microorganism overgrowth that lacks pro-biotic qualities. The healing efficacy of these tissue components gives us further appreciation of the protective action of human tissue over and above and other than the immune protective system or perhaps an integral component part of the immune system.
Owner:IMMUNOPATH PROFILE INC A CORP OF PA
Compositions and methods for treating iron overload
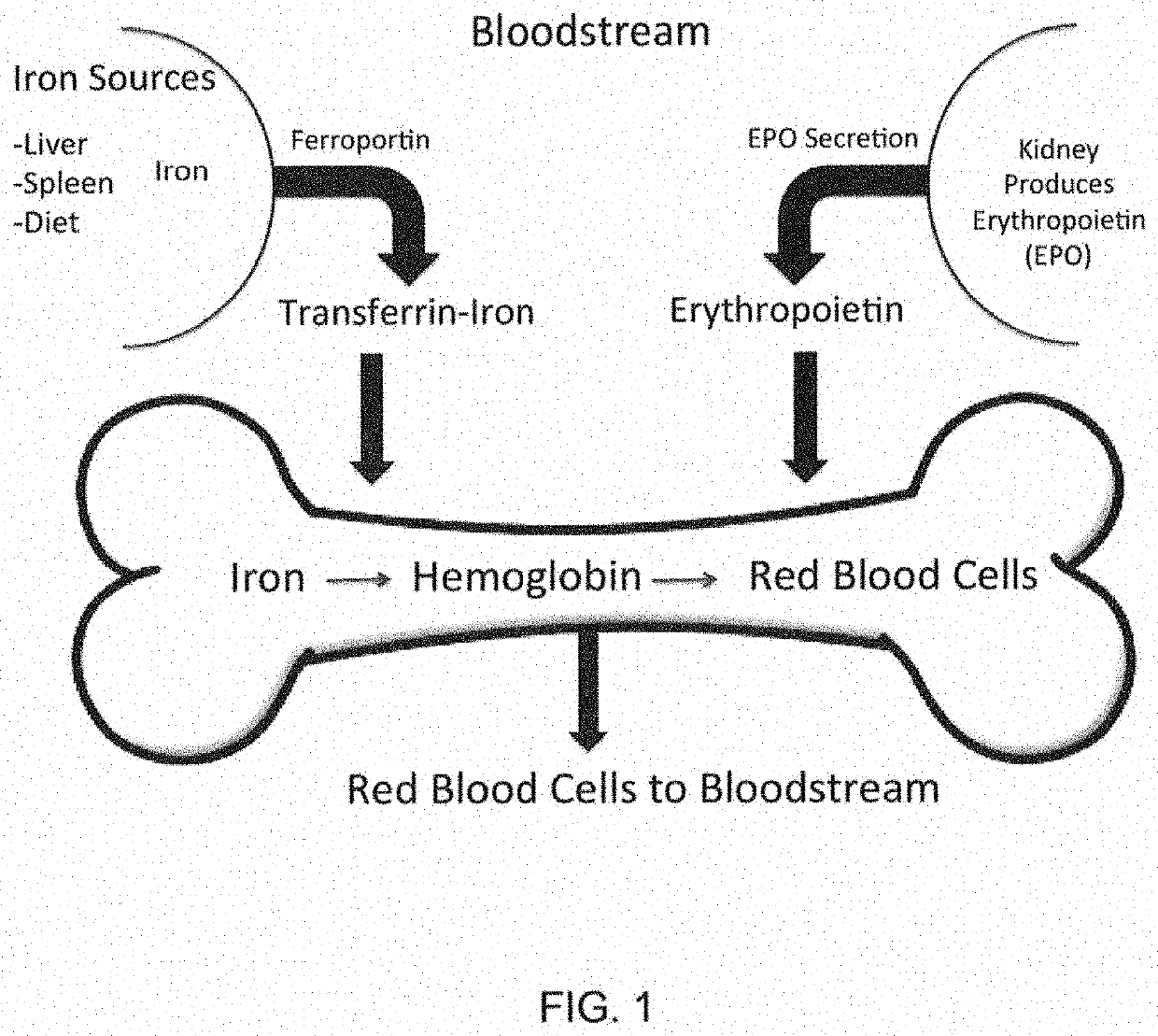
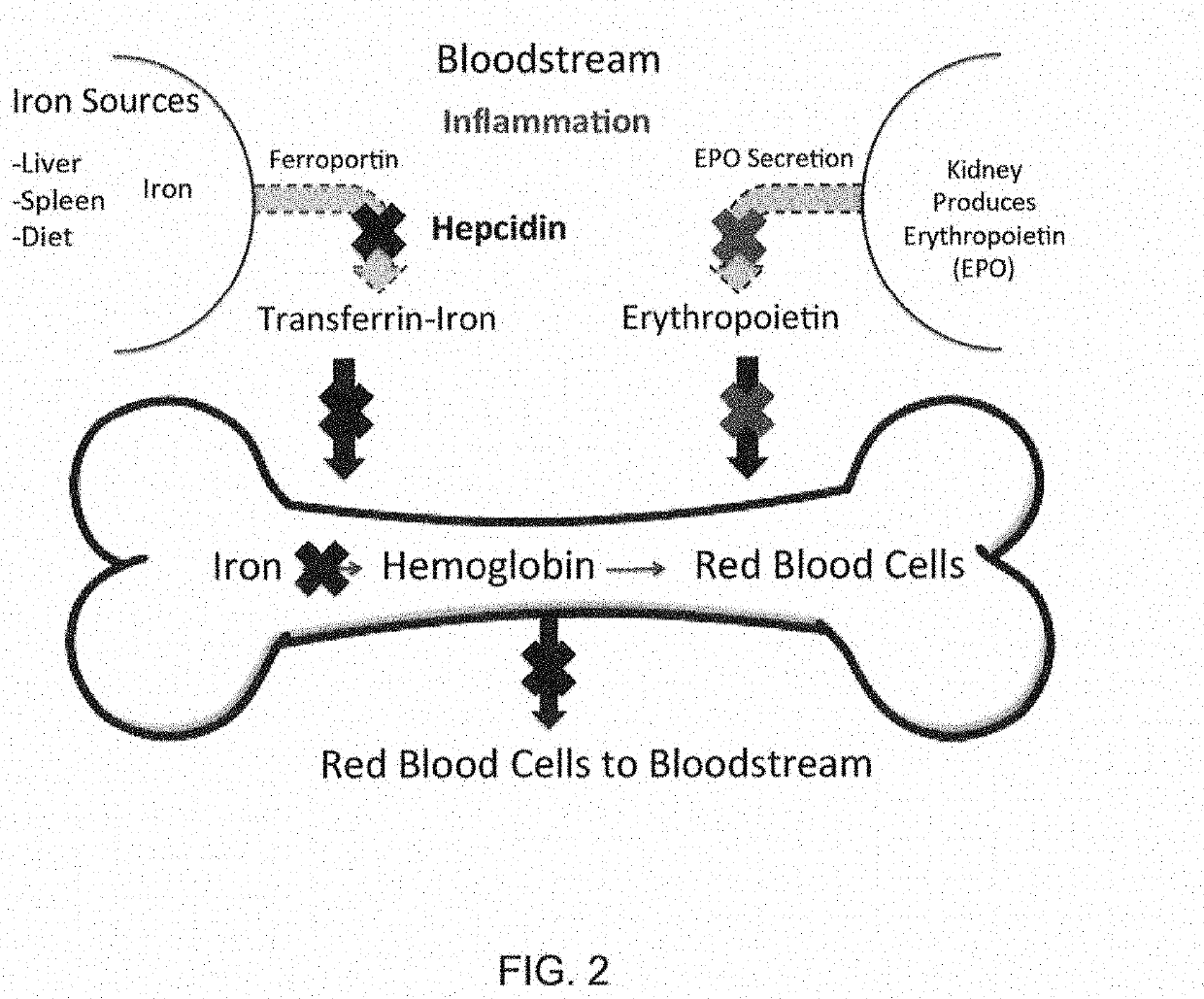
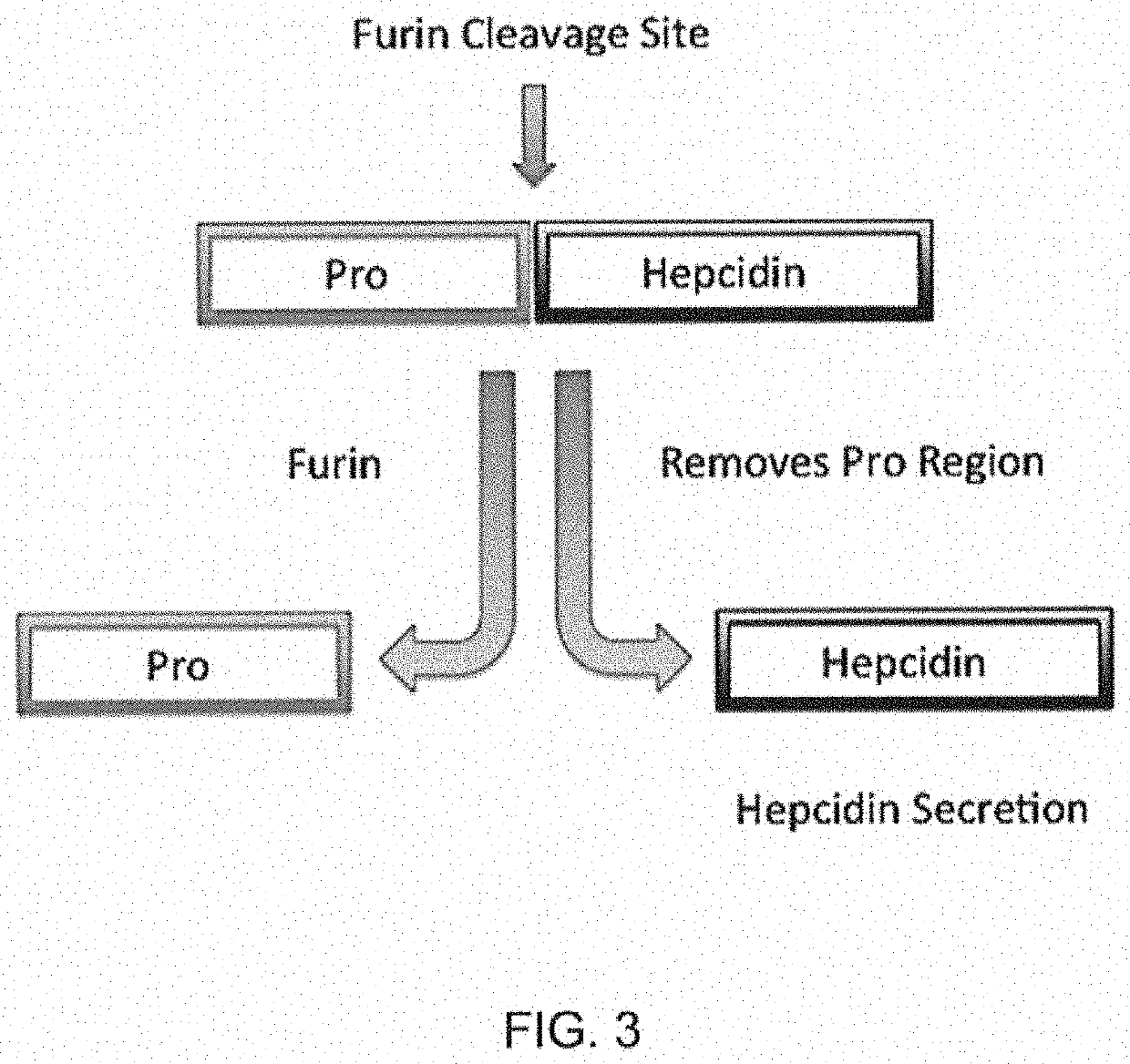
PendingUS20210338657A1Serum hepcidin concentrationConcentration of iron can be loweredAntinoxious agentsAmide active ingredientsTransferrin ironIron Chelator
The present disclosure relates to compositions and methods for treating iron overload. In particular, the methods for treating iron overload include administering to a patient an HIV protease inhibitor and an iron chelator. Iron overload may occur in patients diagnosed with anemia from inflammation or from chronic disease including hemochromatosis, sickle cell disease, thalassemia, sideroblastic anemia, an enzyme deficiency, pre-operative anemia, a cardiovascular disease, atransferrinemia, or aceruloplasminemia.
Owner:BRIGHAM YOUNG UNIV
Gene mutation detection kit for human glucose-6-phosphate dehydrogenase deficiency disease
ActiveCN111304317AHigh degree of standardizationImprove diagnostic accuracyMicrobiological testing/measurementHereditary MutationDisease
The invention relates to a gene mutation detection kit for a human glucose-6-phosphate dehydrogenase deficiency disease. The gene mutation detection kit comprises probes with sequences represented bySEQ NO.1-382. By carrying out distribution analysis on positive mutation sites of 500 G6PD genes in the China area and combining with Human Gene Mutation Database (HGMD) and gene mutation hotspot dataof the European and American areas, gene mutation hotspots are more comprehensively summarized, so that the problems of genetic mutation detection of nearly 99% of patients with a G6PD deficiency disease are solved, and rapid and effective execution of genetic diagnosis of the G6PD deficiency disease is realized.
Owner:上海源庆生物科技有限公司
Gene therapy for AADC deficiency
ActiveUS10898585B2Organic active ingredientsNervous disorderAADC DEFICIENCYAromatic amino acid decarboxylase deficiency
Owner:NAT TAIWAN UNIV
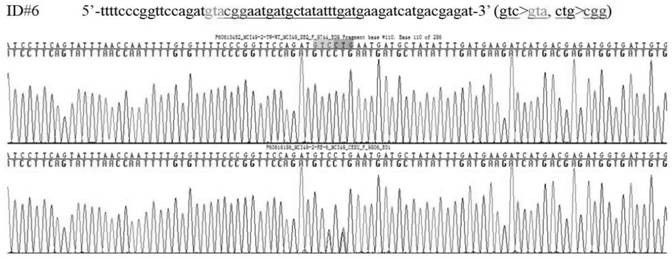
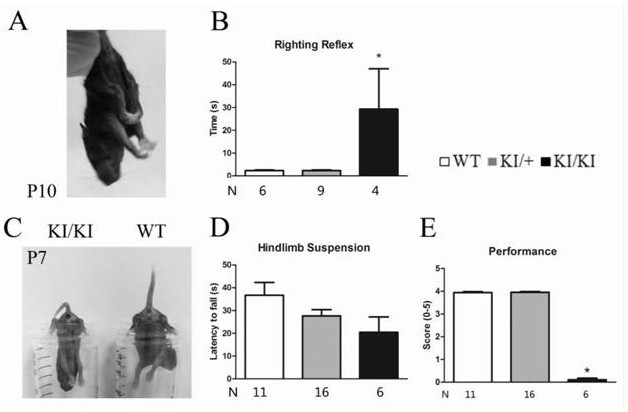
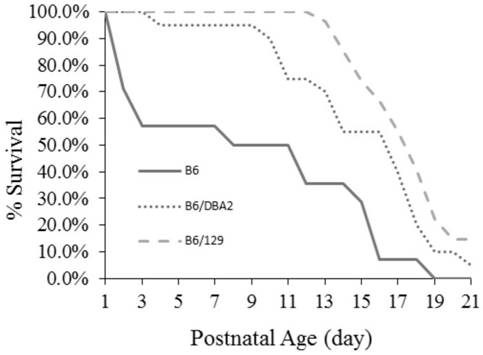
Construction method of gtpch enzyme deficiency mouse model with motor dysfunction phenotype
The invention relates to a method for constructing a motor dysfunction phenotype GTPCH enzyme deficiency mouse model. The construction includes: (1) obtaining Gch1 p.Leu108Arg heterozygous mutant mice, the leucine codon at position 108 of the Gch1 gene of the heterozygous mutant mice is mutated into an arginine codon; (2) The heterozygous mutant mice are crossed with wild-type mice of other strains to screen the F1 generation heterozygous mutant mice; (3) the F1 generation heterozygous mutant mice are selfed to screen the F2 generation homozygous mutant mice. This construction method can avoid the lethal problem during the development of GTPCH enzyme-deficient embryos, and the birth rate of homozygous mutant mice can reach or approach Mendel's expected ratio. At the same time, the crossbreeding of mice with different genetic backgrounds can also improve the survival of F2 generation mice after birth and prolong the average survival period.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Assay of arylsulfatase a enzymatic activity in dried blood spots by mass spectrometry and fluorometry
PendingUS20220348981A1Microbiological testing/measurementDisease diagnosisMultiple sulfatase deficiencyDisease
Methods, reagents, and kits for assaying for arylsulfatase A activity for diagnosing conditions associated with arylsulfatase A deficiency, such as multiple sulfatase deficiency (MSD) and metachromatic leukodystrophy (MILD).
Owner:UNIV OF WASHINGTON
Methods and compositions for increasing n-acetylglucosaminidase activity in the CNS
InactiveUS20210002379A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesReceptor
Provided herein are methods and compositions for treating a subject suffering from an enzyme deficiency in the central nervous system (CNS). The bifunctional fusion antibody provided herein comprise an antibody to an endogenous blood brain barrier (BBB) receptor and an enzyme deficient in mucopolysaccharidosis IIIB (MPS-IIIB). The fusion antibodies provided herein comprise alpha-N-acetylgulcosaminidase (NAGLU). The methods of treating an enzyme deficiency in the CNS comprise systemic administration of a fusion antibody provided herein.
Owner:ARMAGEN INC
Glucose-6-phosphate dehydrogenase deficiency detection kit and detection method
PendingCN114480606AReduced recallsReduce mental burdenMicrobiological testing/measurementDNA/RNA fragmentationMultiplexDisease
The invention provides a glucose-6-phosphate dehydrogenase deficiency disease detection kit and a primer group. The primer group is characterized in that a PCR (Polymerase Chain Reaction) primer is designed by utilizing bioinformatics knowledge and related bioinformatics software according to nucleotide sequence information of c.1376 and c.1388 loci of a glucose-6-phosphate dehydrogenase deficiency disease-causing gene G6PD, which can be retrieved in a public database, and by utilizing Primer ExpressSoftware5.0 software; the primers comprise amplification primers G6PD-F and G6PD-R and sequencing primers M13F and M13R, and the base sequence of the primers is G6PD-F: TGCCAAACGACGGTTAGTGCAGGGGTCGTCCTTA, the base sequence of the primers is TGCAAACGACGGTTAGTGCAGGGGTCGTCCTTA, and the base sequence of the primers is TGCAAACGACGGTTAGTGCAGGGTCGTCCTTA G6PD-R: AAGGTATGACCATGCACCTGCATAATATAGGGGGAT, G6PD-R: AAGGTATGACCATGCACCTGCATAATATGGGAT The M13F is TGCCAAACGACGGCCAAAGTC, and the M13F is M13R: AGCTATGACCATGAAC, M13R: Carrying out PCR amplification on the to-be-detected sample by adopting a multiplex PCR method; the method comprises the following steps: constructing an amplicon library by using a PCR amplification product, preparing an ISP template, sequencing on an IonTorrentPGM system, and analyzing a related sequencing sequence by using software. According to the invention, primers for amplifying c.1376 and c.1388 sites of the G6PD gene are designed, and a stable amplification system is constructed by adopting a PCR (Polymerase Chain Reaction) technology. The amplification efficiency can be optimal by adjusting reaction conditions such as primer concentration, annealing temperature and the like.
Owner:广州源古纪科技有限公司
Methods and compositions for increasing enzyme activity in the CNS
Provided herein are methods and compositions for treating a subject suffering from an enzyme deficiency in the central nervous system (CNS). The bifunctional fusion antibodies provided herein comprise an antibody to an endogenous blood brain barrier (BBB) receptor and an enzyme deficient in mucopolysaccharidosis III (MPS-III). The fusion antibodies provided herein comprise N-sulfoglucosamine sulfohydrolase (SGSH), alpha-N-acetylgulcosaminidase (NAGLU), heparin-alpha-glucosaminide N-acetyltransferase (HGSNAT), or N-acetylglucosamine-6-sulfatase (GNS). The methods of treating an enzyme deficiency in the CNS comprise systemic administration of a fusion antibody provided herein.
Owner:ARMAGEN INC
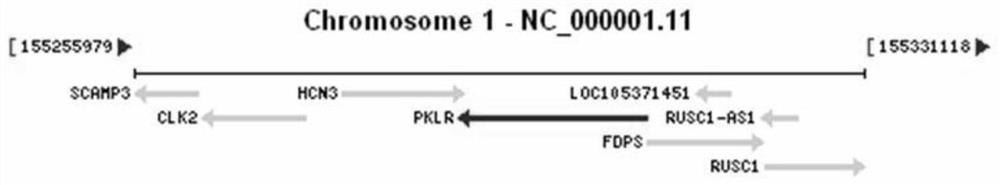
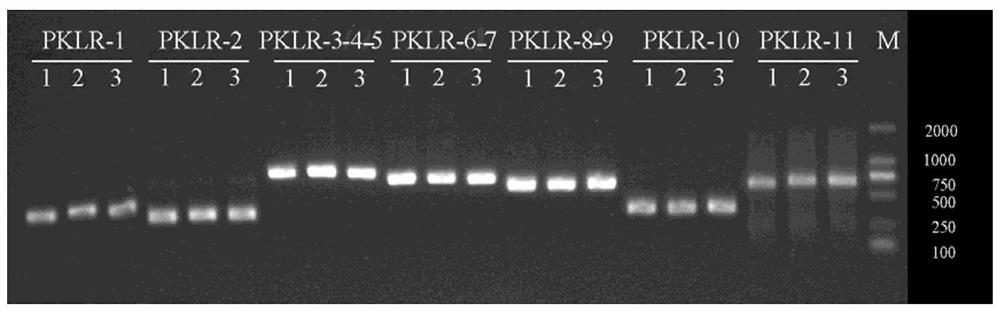
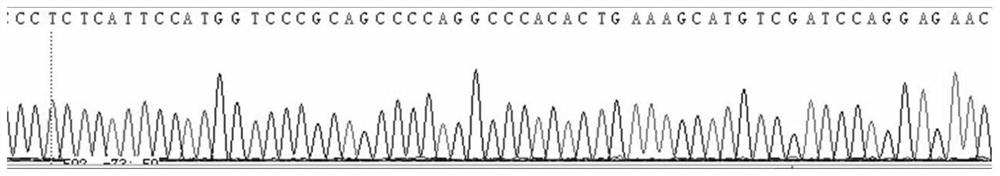
Method, kit, oligonucleotide and application thereof for detecting pklr gene mutation
ActiveCN106868184BReduce in quantityThere will be no missed detectionMicrobiological testing/measurementDNA/RNA fragmentationPrenatal diagnosisExon
The invention discloses primers and method for detecting PKLR gene mutation of autosomal recessive inherited disease pyruvate kinase deficiency patients. The primers comprise primers for amplifying all exon sequences of the PKLR gene, and the Sanger sequencing technique and sequencing primers are adopted. Mutation of all exons of the PKLR gene in pyruvate kinase deficiency patients can be detected quickly. A detection result is accurate, assistant diagnosis of pyruvate kinase deficiency can be achieved, and reference is provided for early intervention, early treatment and prenatal diagnosis.
Owner:CHANGSHA ADICON CLINICAL LAB
A gene mutation detection kit for human glucose-6-phosphate dehydrogenase deficiency
ActiveCN111304317BHigh degree of standardizationImprove diagnostic accuracyMicrobiological testing/measurementGenes mutationHereditary Mutation
The invention relates to a gene mutation detection kit for human glucose-6-phosphate dehydrogenase deficiency, which comprises probes with sequences shown in SEQ NO.1-382. The present invention analyzes the distribution of 500 G6PD gene positive mutation sites in China, and combines the human gene mutation database (Human Gene Mutation Database, HGMD) database and gene mutation hotspot data in Europe and the United States to more comprehensively summarize the G6PD gene mutation hotspots, To solve the genetic mutation detection problem of nearly 99% of patients with G6PD deficiency, and realize the rapid and effective genetic diagnosis of G6PD deficiency disease.
Owner:上海源庆生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com