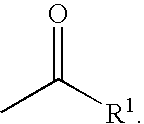
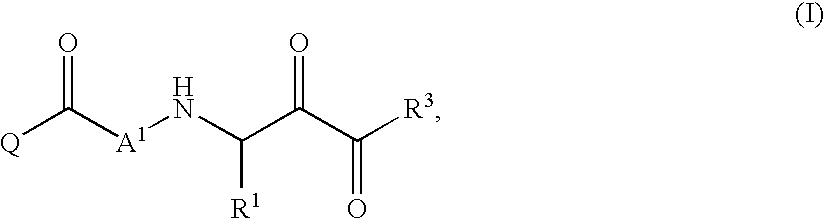
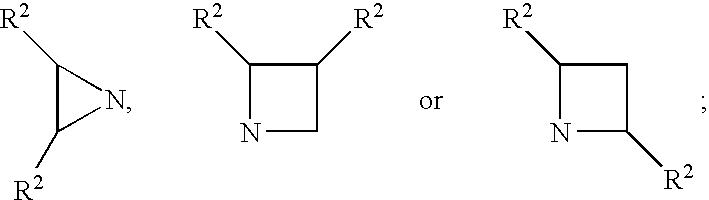
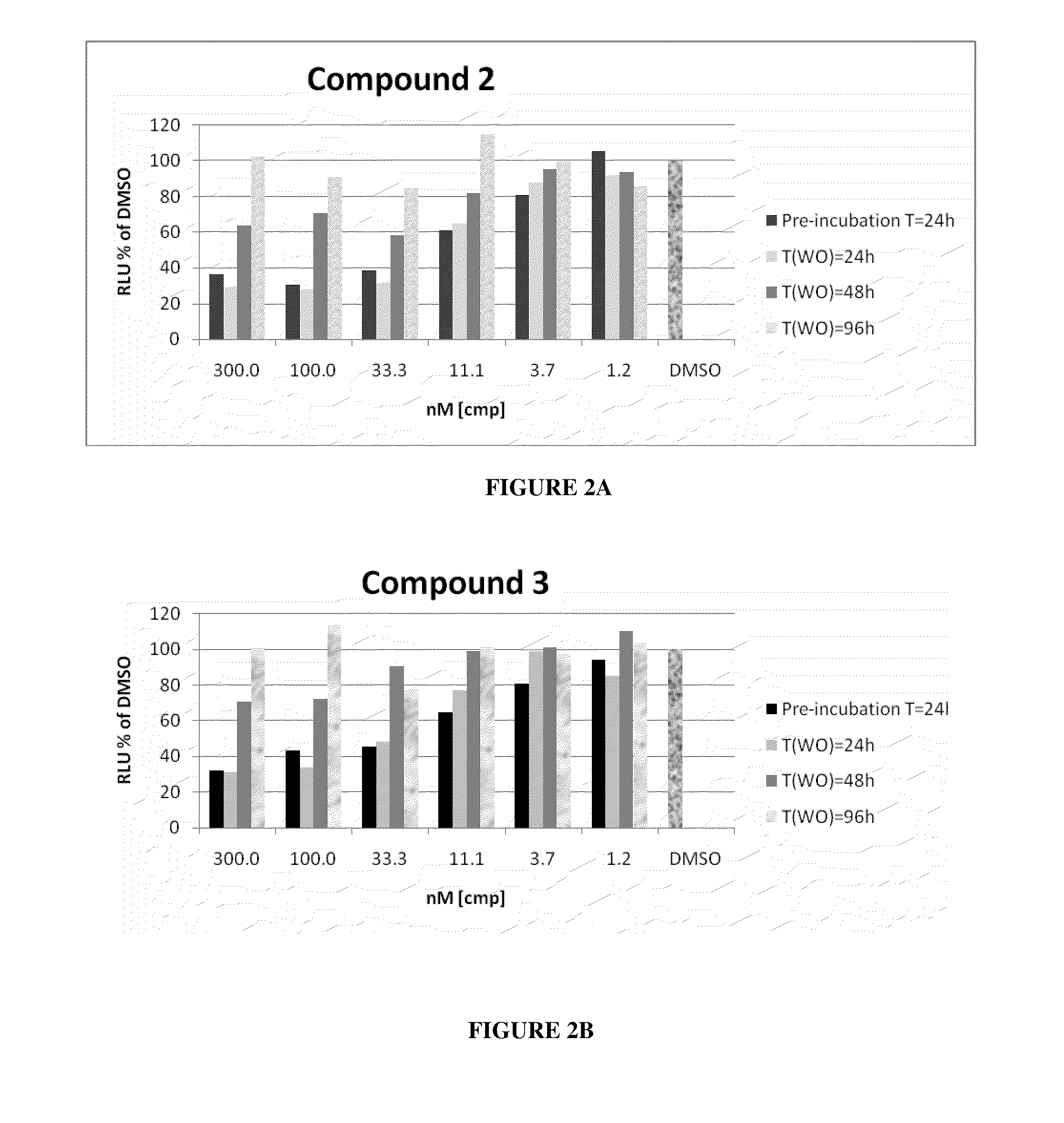
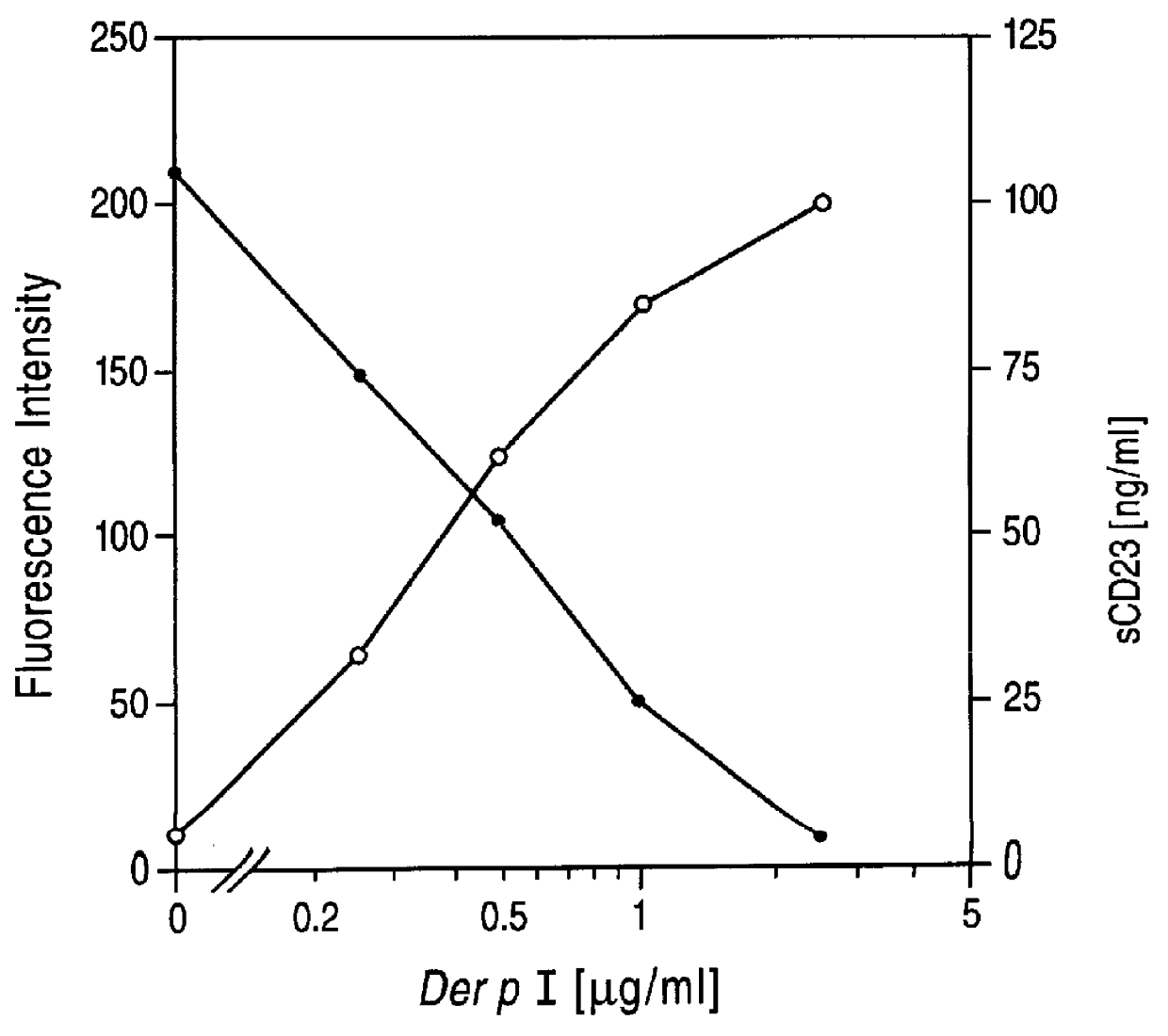
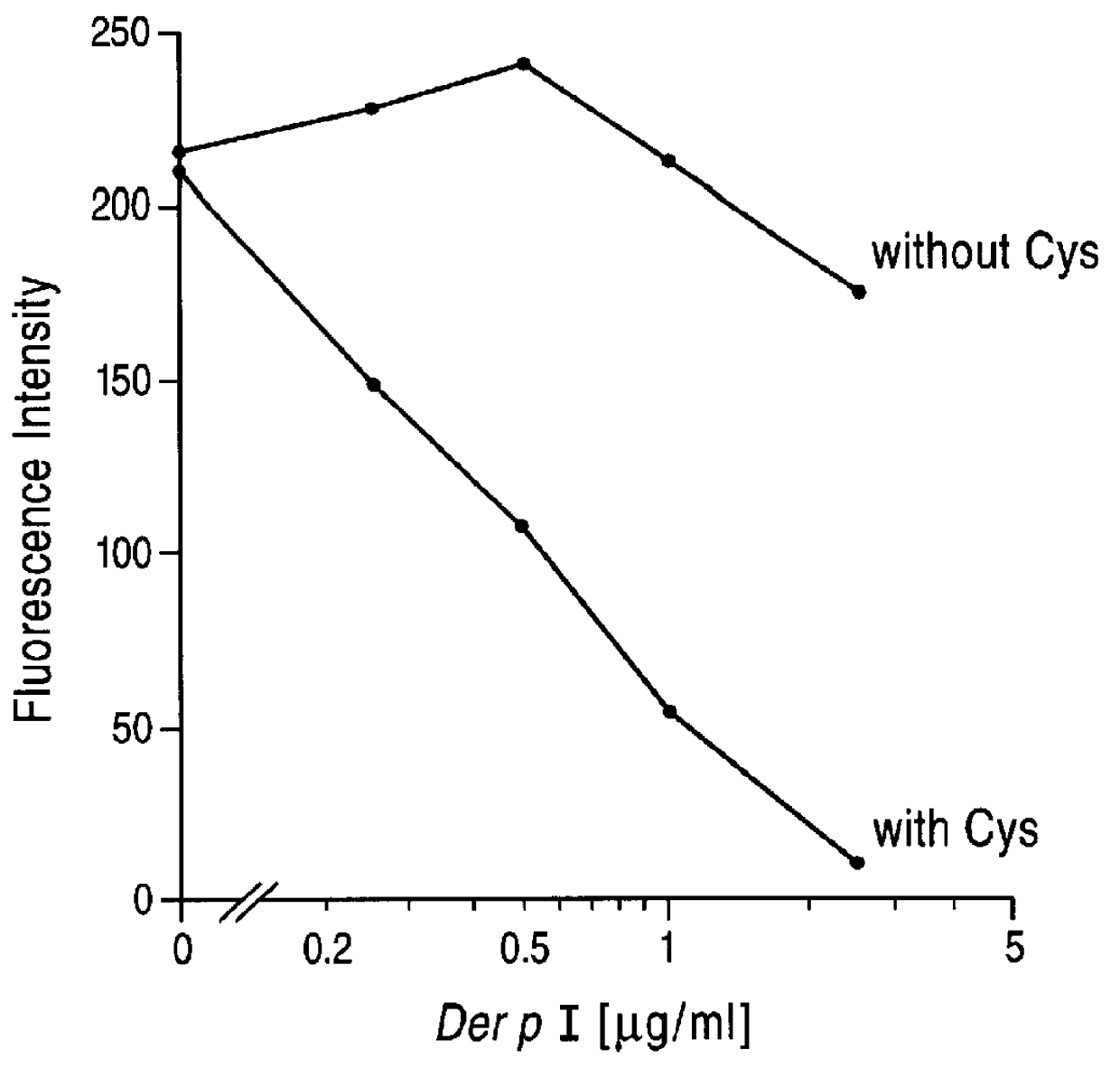
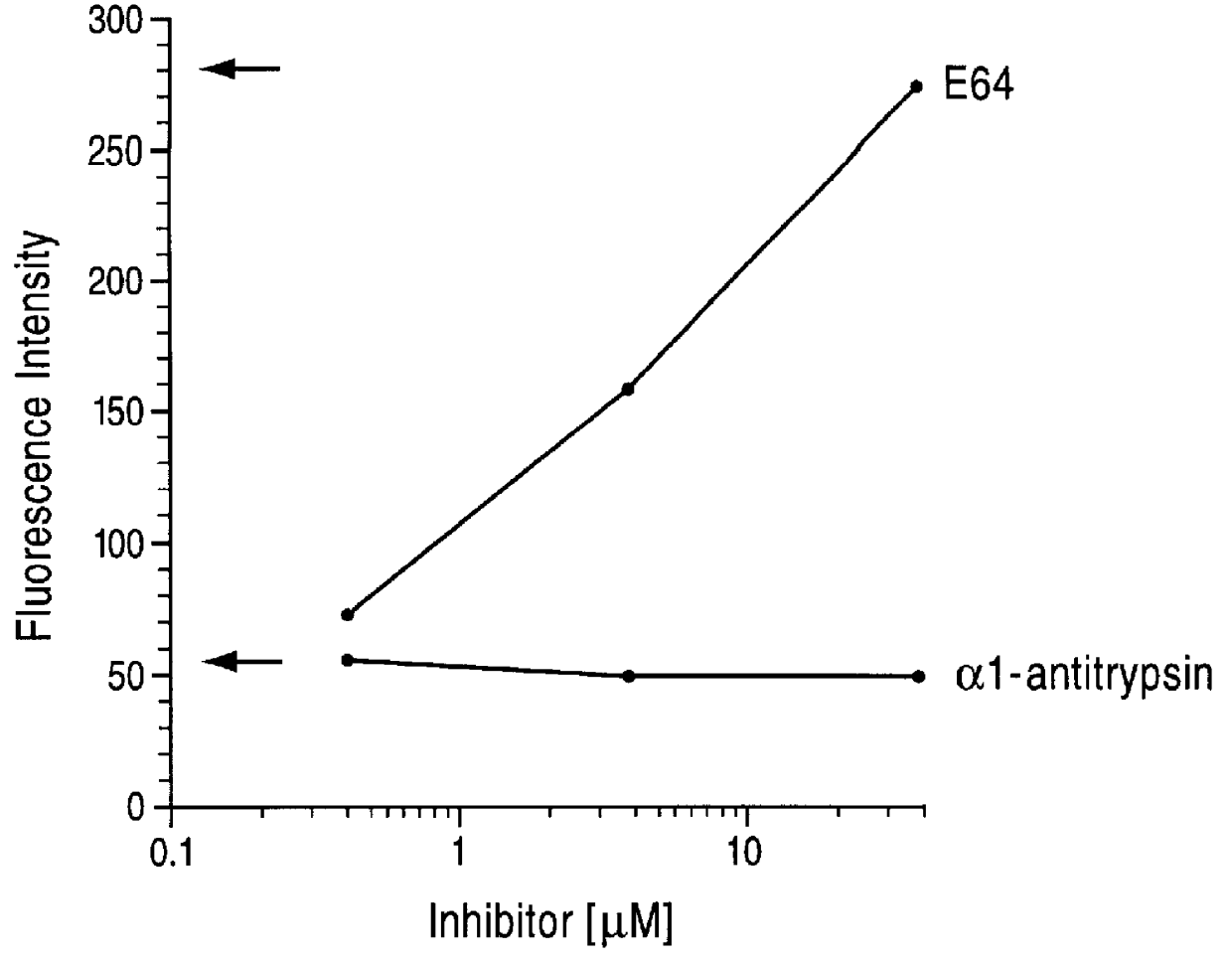
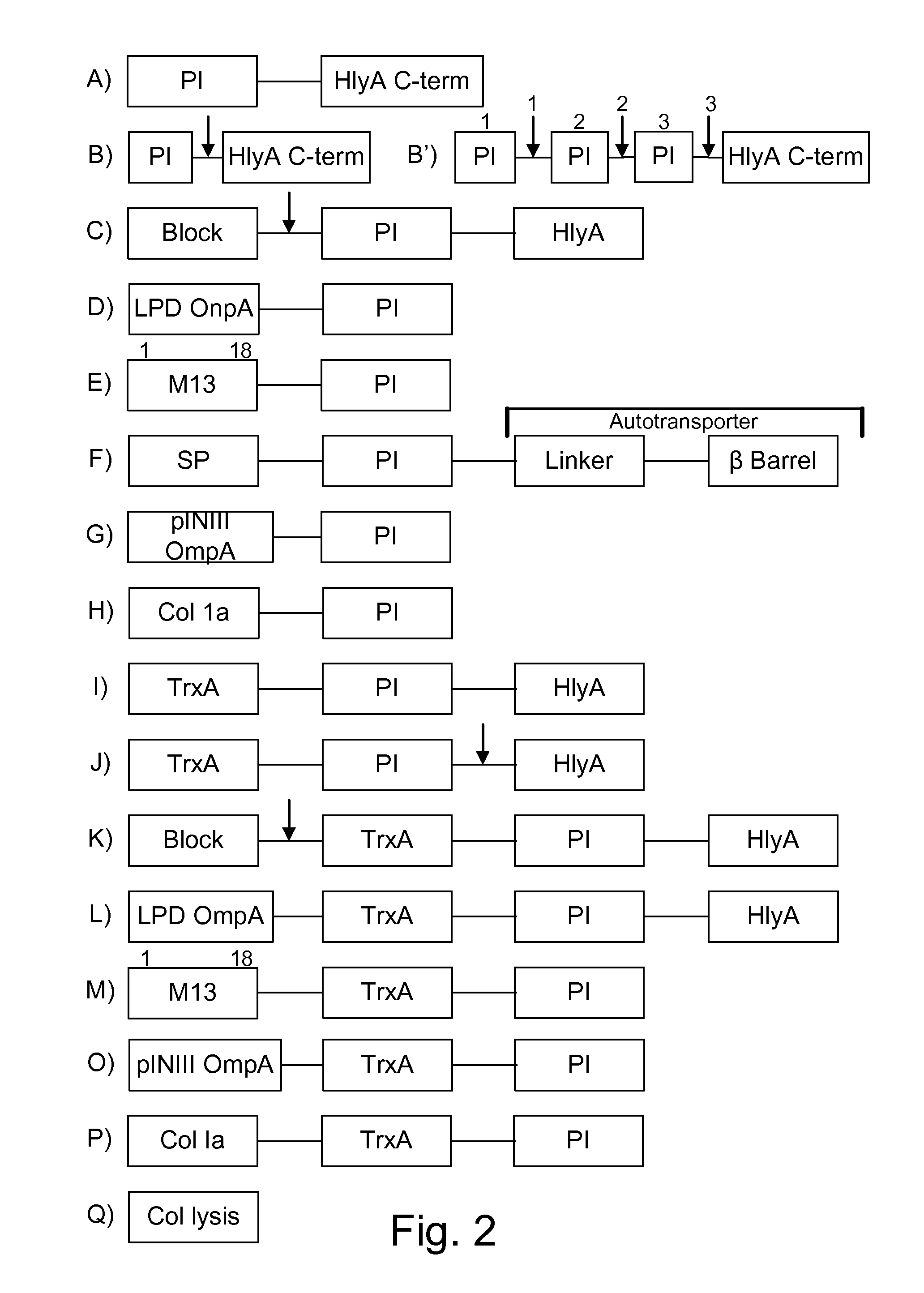
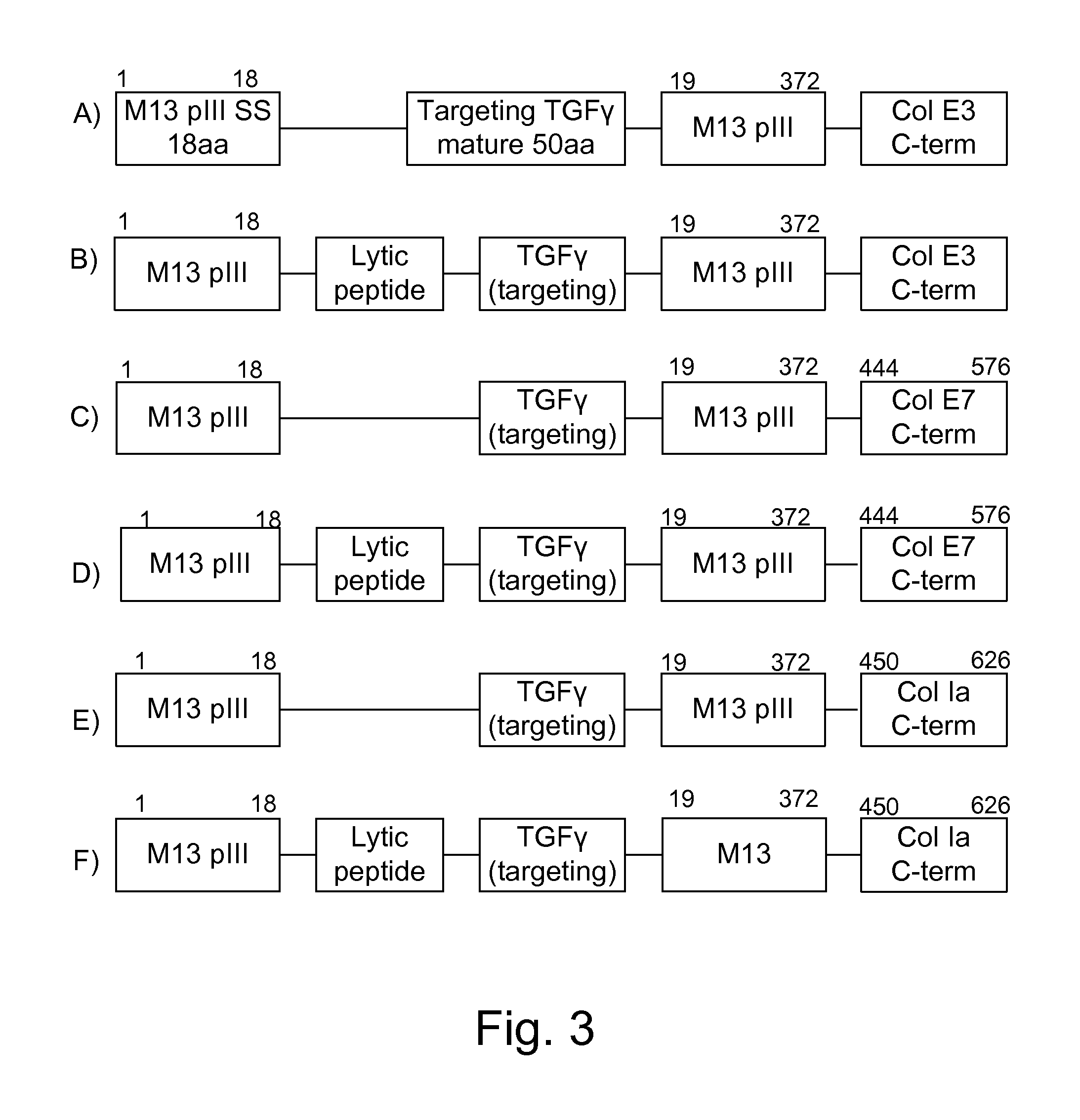
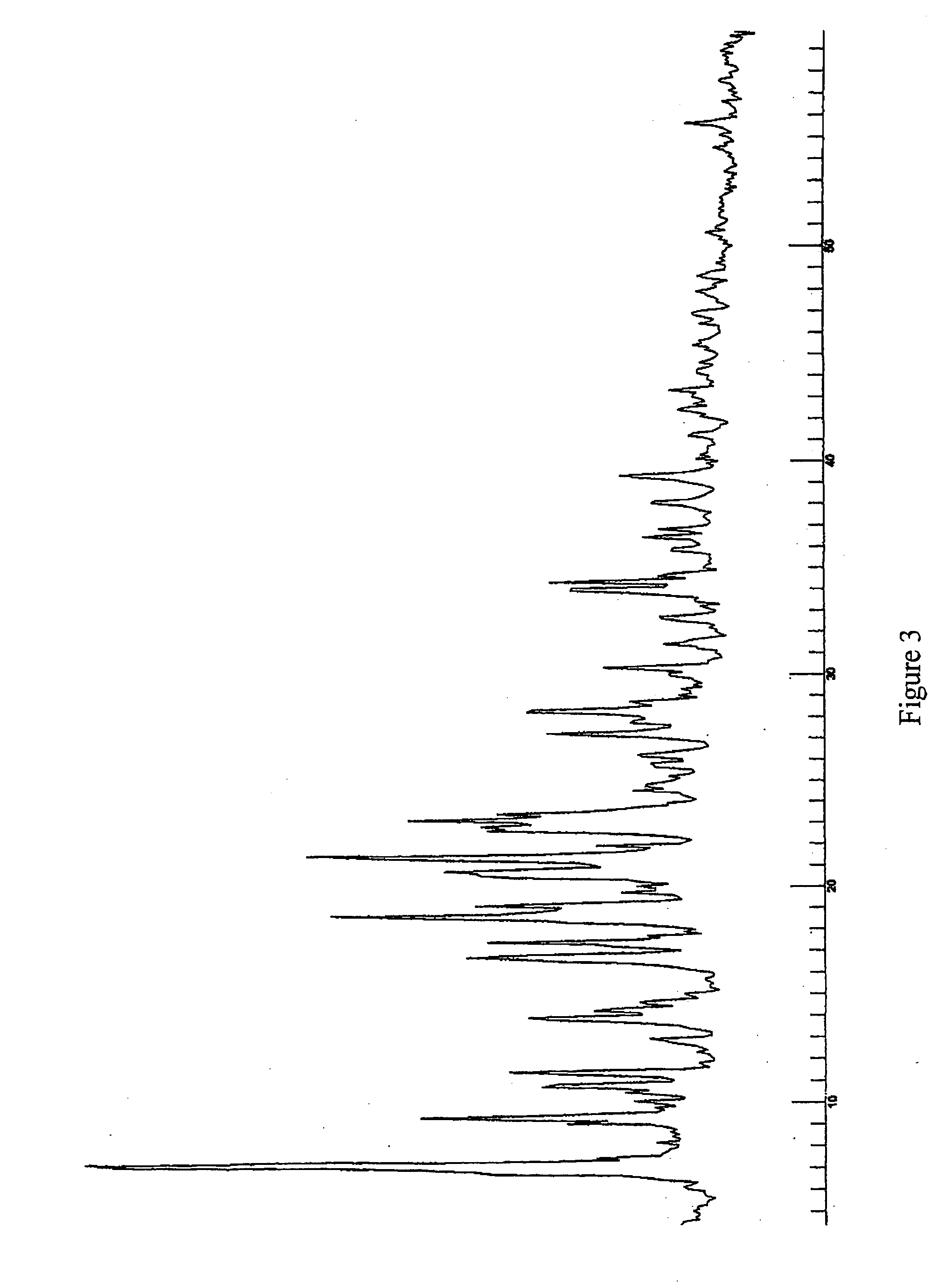
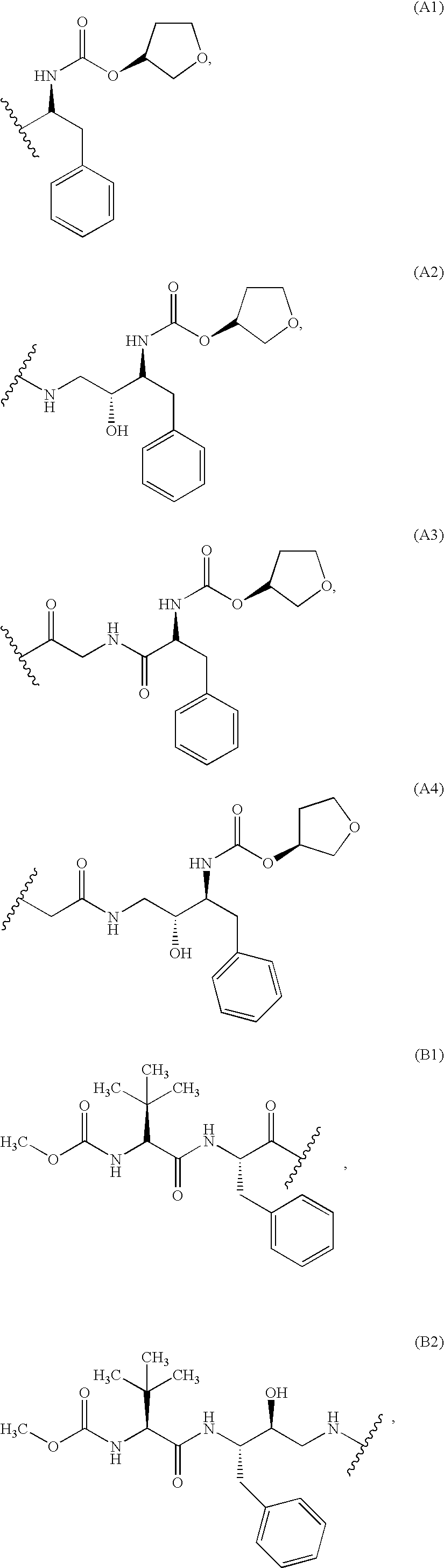
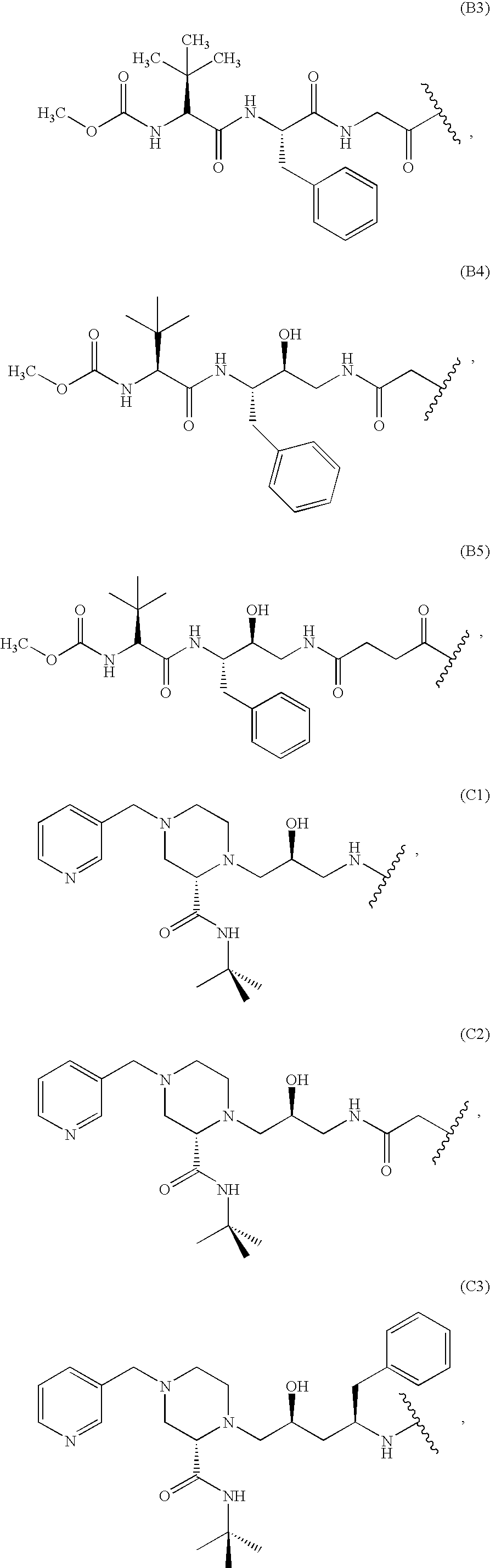
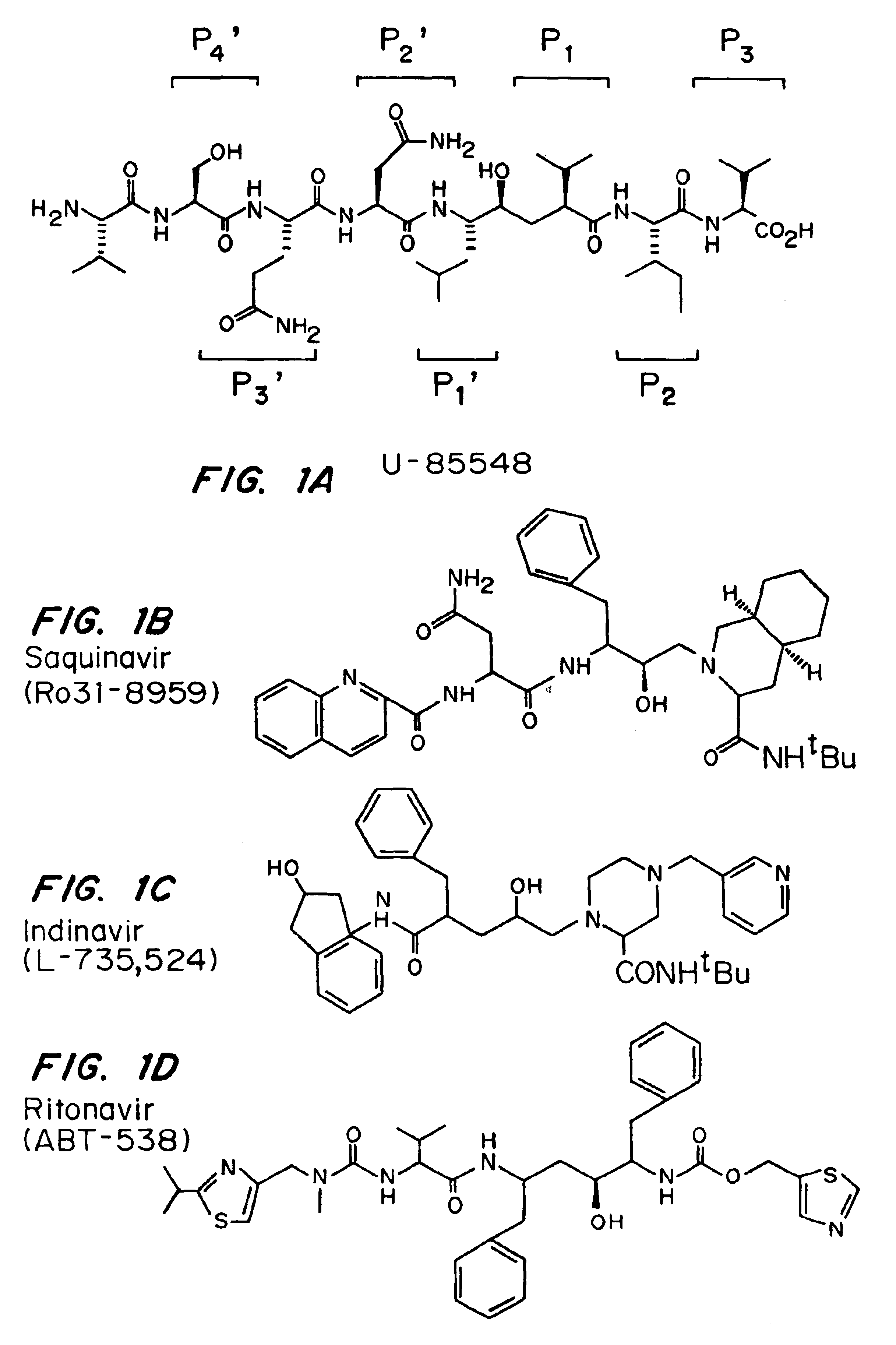
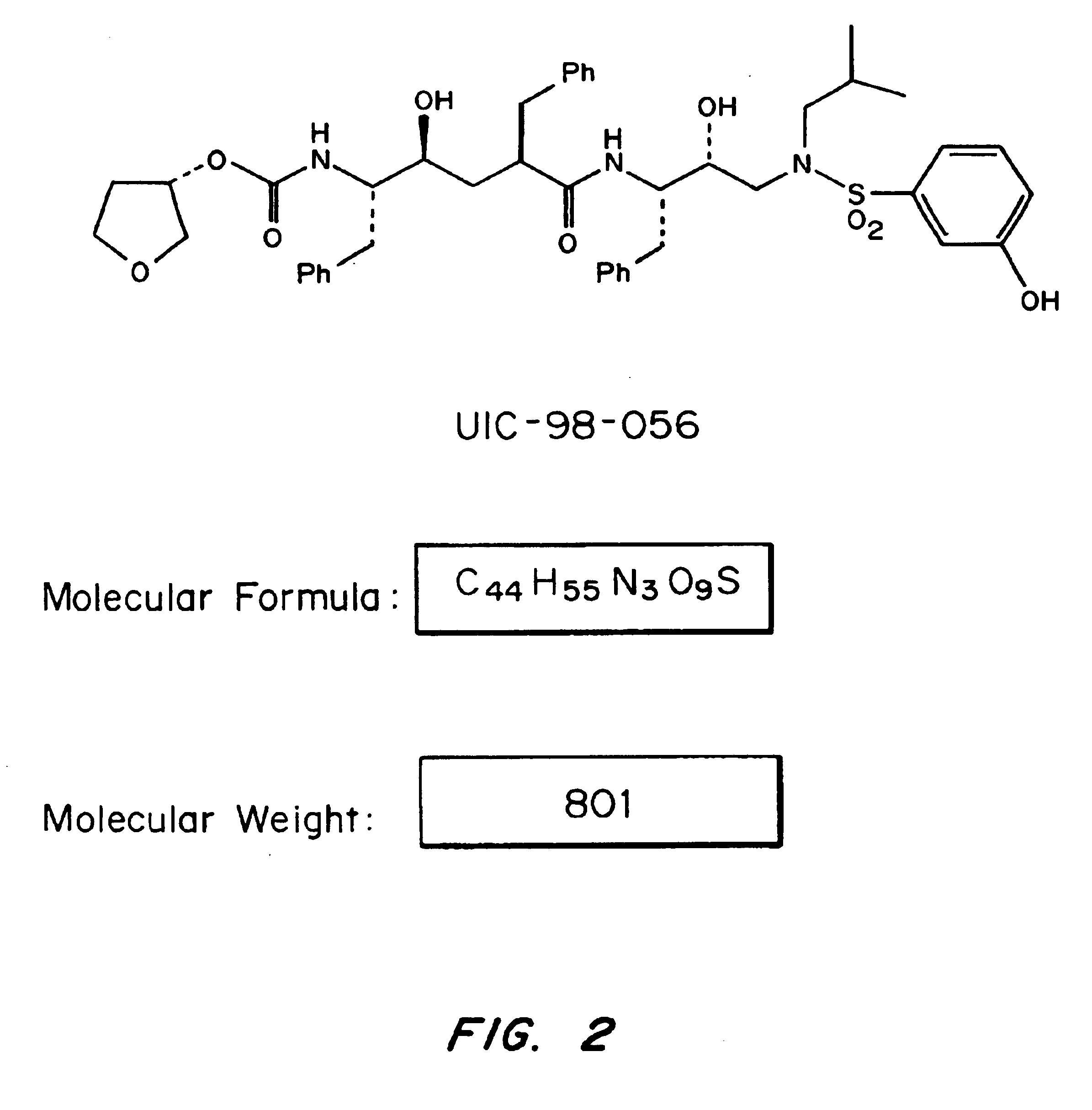
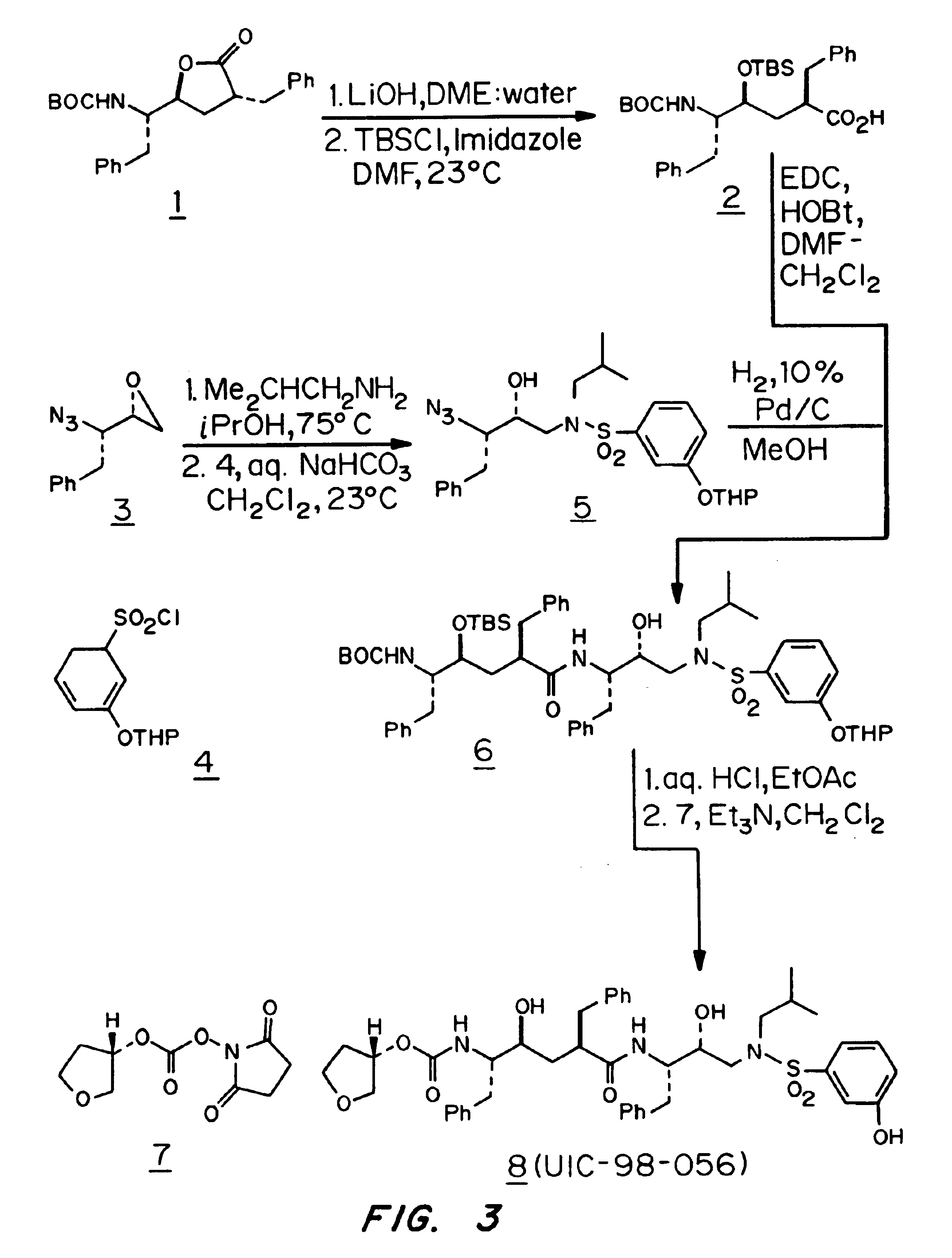
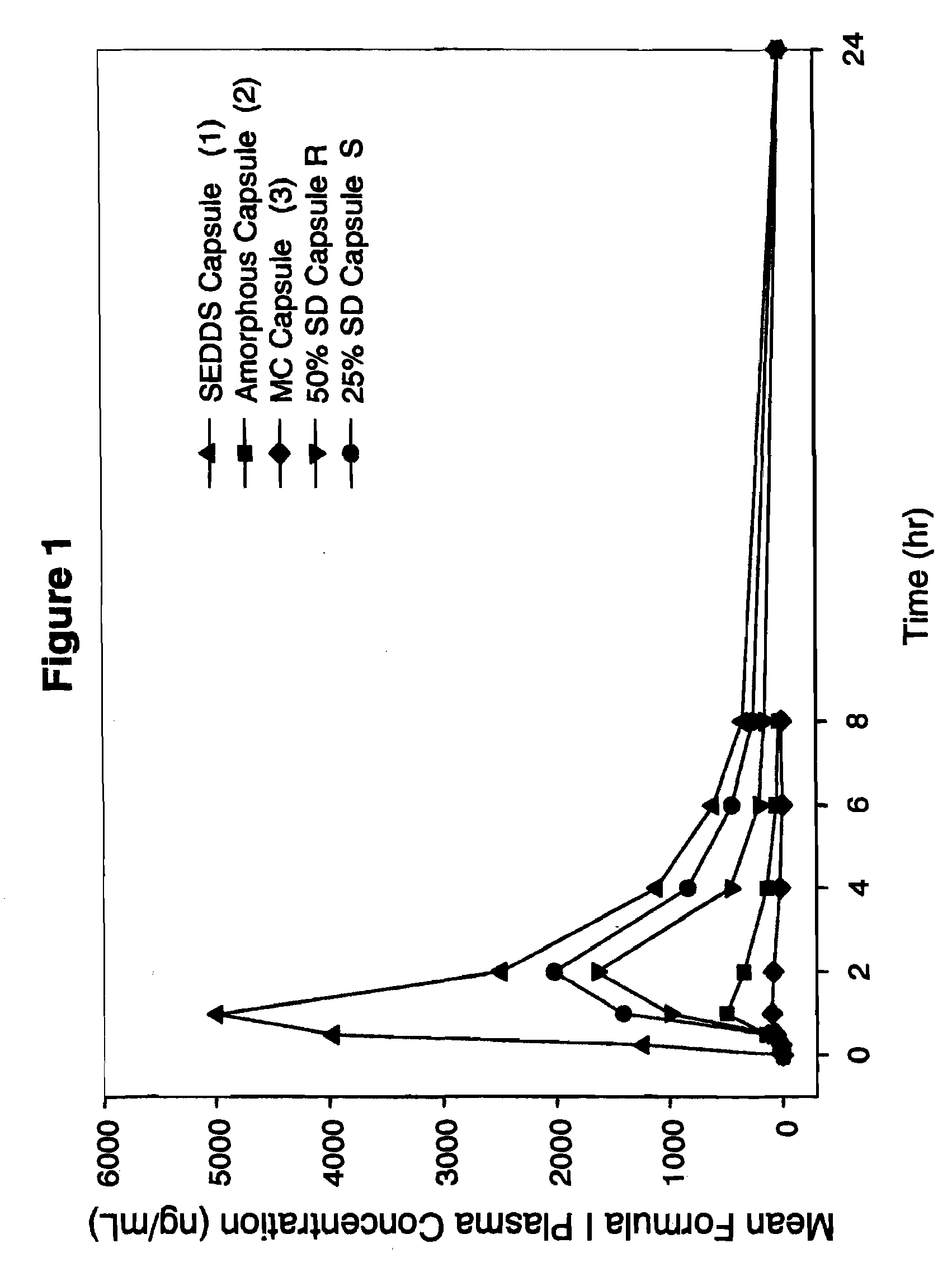
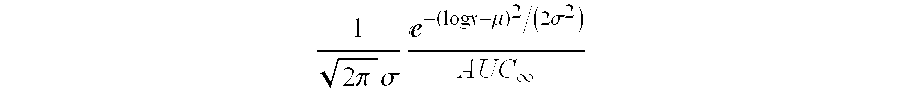Patents
Literature
225 results about "HIV Protease Inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A protease inhibitor that is designed to target the human immunodeficiency virus (HIV) protease while sparing other host cell proteases. HIV protease mediates the cleavage of viral Gag, Gag-Pol and Nef precursor polypeptides into their mature proteins. Inhibition of HIV protease results in production of noninfectious viral particles.
Peptide inhibitors of Hepatitis C virus NS3 protease
InactiveUS6846806B2Peptide/protein ingredientsOrganic compound preparationSerine Protease InhibitorsProteinase activity
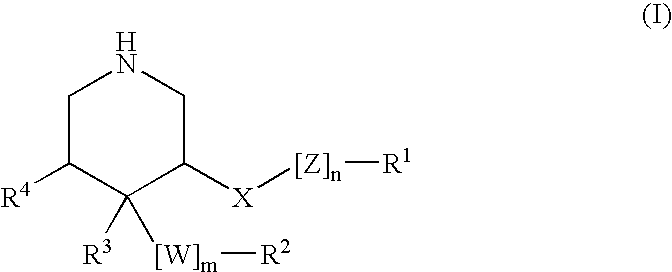
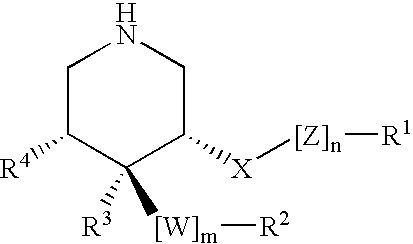
This invention relates to a novel class of peptides having the Formula (I): which are useful as serine protease inhibitors, and more particularly as Hepatitis C virus(HCV) NS3 protease inhibitors. This invention also relates to pharmaceutical compositions comprising these compounds and methods of using the same in the treatment of HCV infection.
Owner:BRISTOL MYERS SQUIBB CO
Peptide inhibitors of hepatitis C virus NS3 protease
Owner:BRISTOL MYERS SQUIBB PHARMA CO
4-Oxoquinoline compounds and utilization thereof as hiv integrase inhibitors
ActiveUS20050239819A1Promote absorptionIncreased riskBiocideOrganic chemistrySide effectReverse transcriptase
An anti-HIV agent containing, as an active ingredient, a 4-oxoquinoline compound represented by the following formula [I]wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof. The compound of the present invention has HIV integrase inhibitory action and is useful as an anti-HIV agent for the prophylaxis or therapy of AIDS. Moreover, by a combined use with other anti-HIV agents such as protease inhibitors, reverse transcriptase inhibitors and the like, the compound can become a more effective anti-HIV agent. Since the compound has high inhibitory activity specific for integrases, it can provide a safe pharmaceutical agent with a fewer side effects for human.
Owner:JAPAN TOBACCO INC
Solid pharmaceutical dosage formulation
InactiveUS20050143404A1Reduce the burden onEase complicated treatment regimenBiocideOrganic active ingredientsHIV Protease InhibitorDrug doses
The present invention provides a pharmaceutical dosage formulation, and more particularly, to a pharmaceutical dosage formulation comprising an HIV protease inhibitor.
Owner:ABBVIE INC
Process for preparing atazanavir bisulfate and novel forms
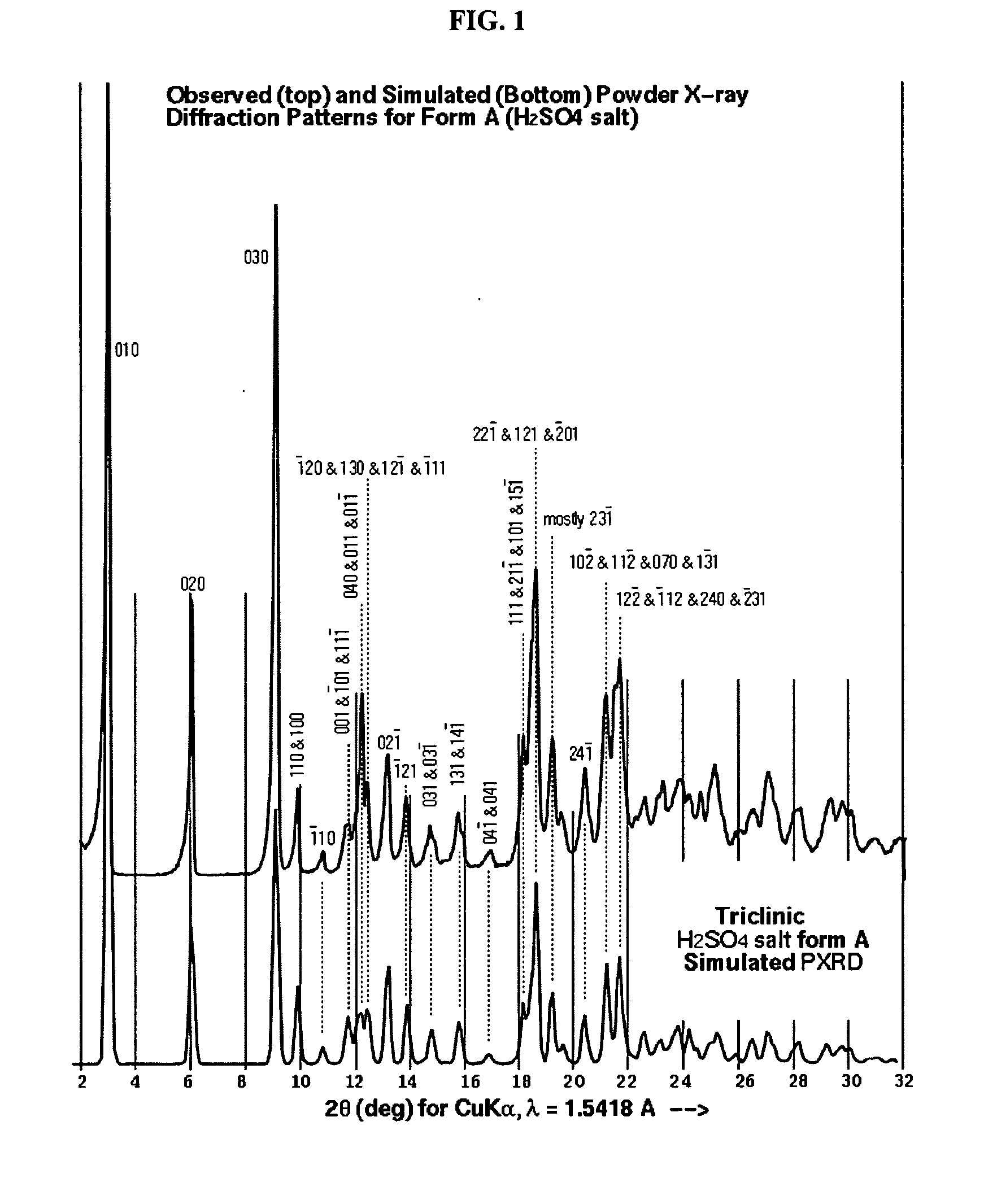
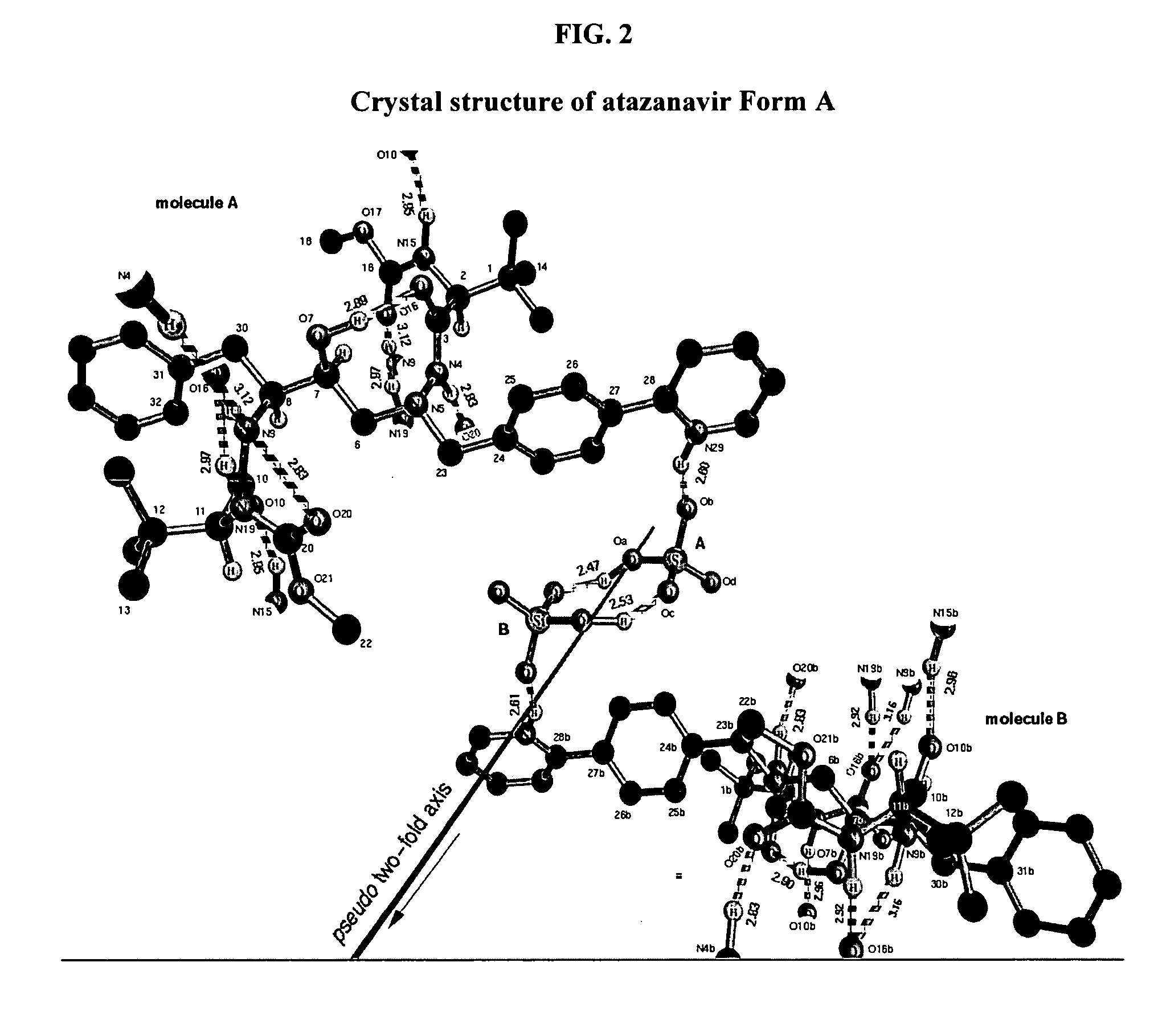
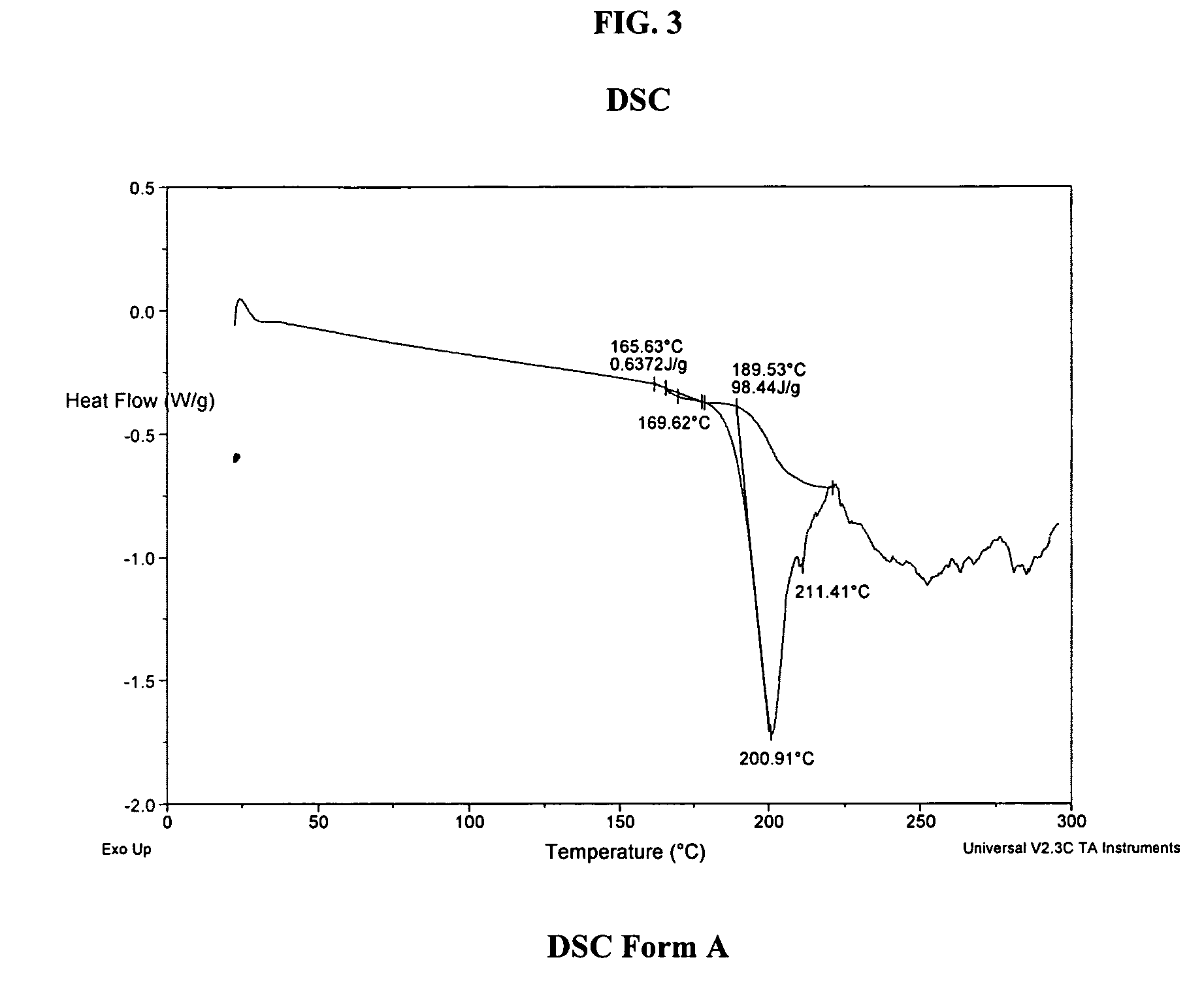
A process is provided for preparing the HIV protease inhibitor atazanavir bisulfate wherein a solution of atazanavir free base is reacted with concentrated sulfuric acid in an amount to react with less than about 15% by weight of the free base, seeds of Form A crystals of atazanavir bisulfate are added to the reaction mixture, and as crystals of the bisulfate form, additional concentrated sulfuric acid is added in multiple stages at increasing rates according to a cubic equation, to effect formation of Form A crystals of atazanavir bisulfate. A process is also provided for preparing atazanavir bisulfate as Pattern C material. A novel form of atazanavir bisulfate is also provided which is Form E3 which is a highly crystalline triethanolate solvate of the bisulfate salt from ethanol.
Owner:BRISTOL MYERS SQUIBB CO
Inhibitors of serine proteases, particularly hepatitis C virus NS3 protease
Owner:VERTEX PHARMA INC
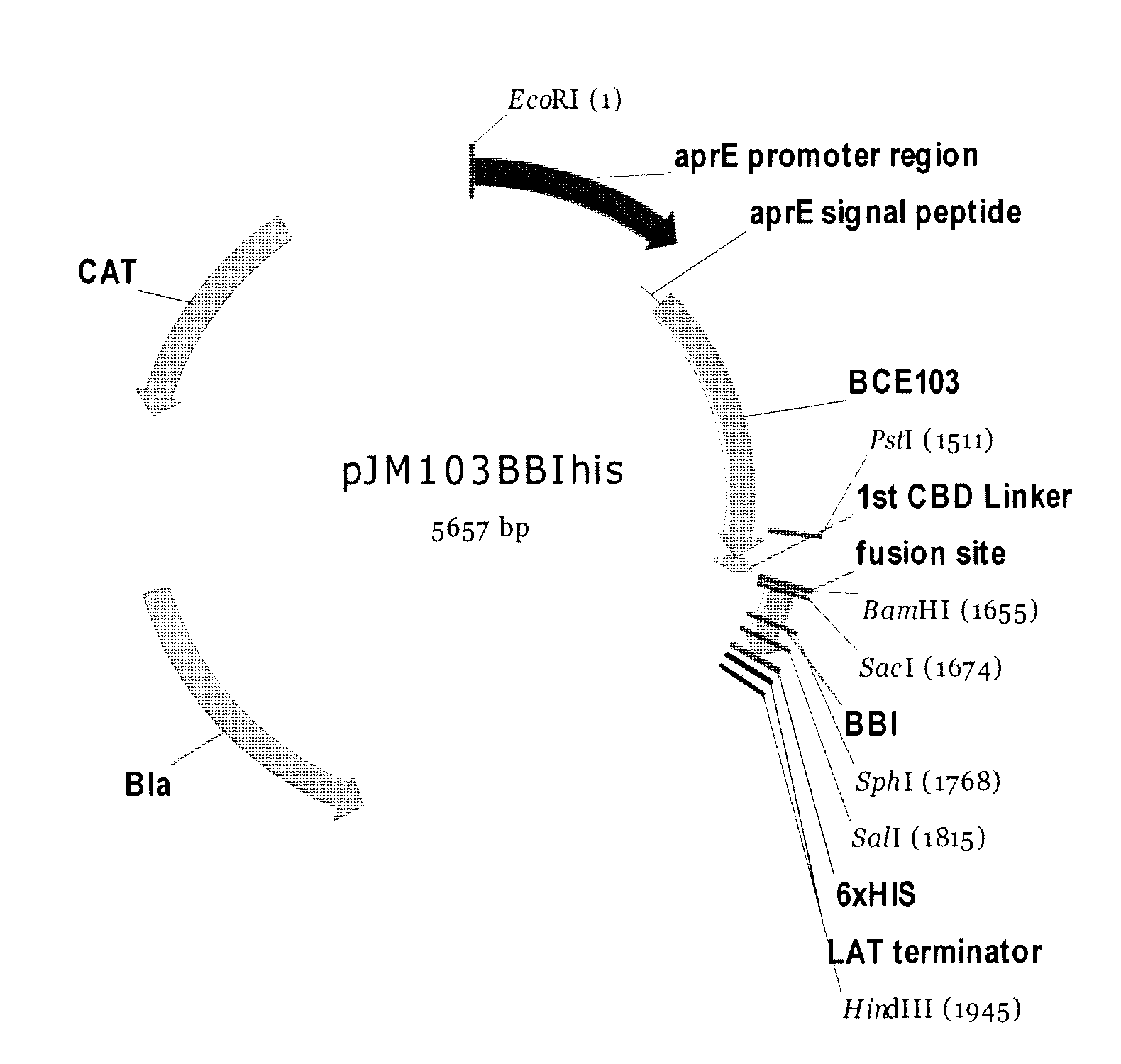
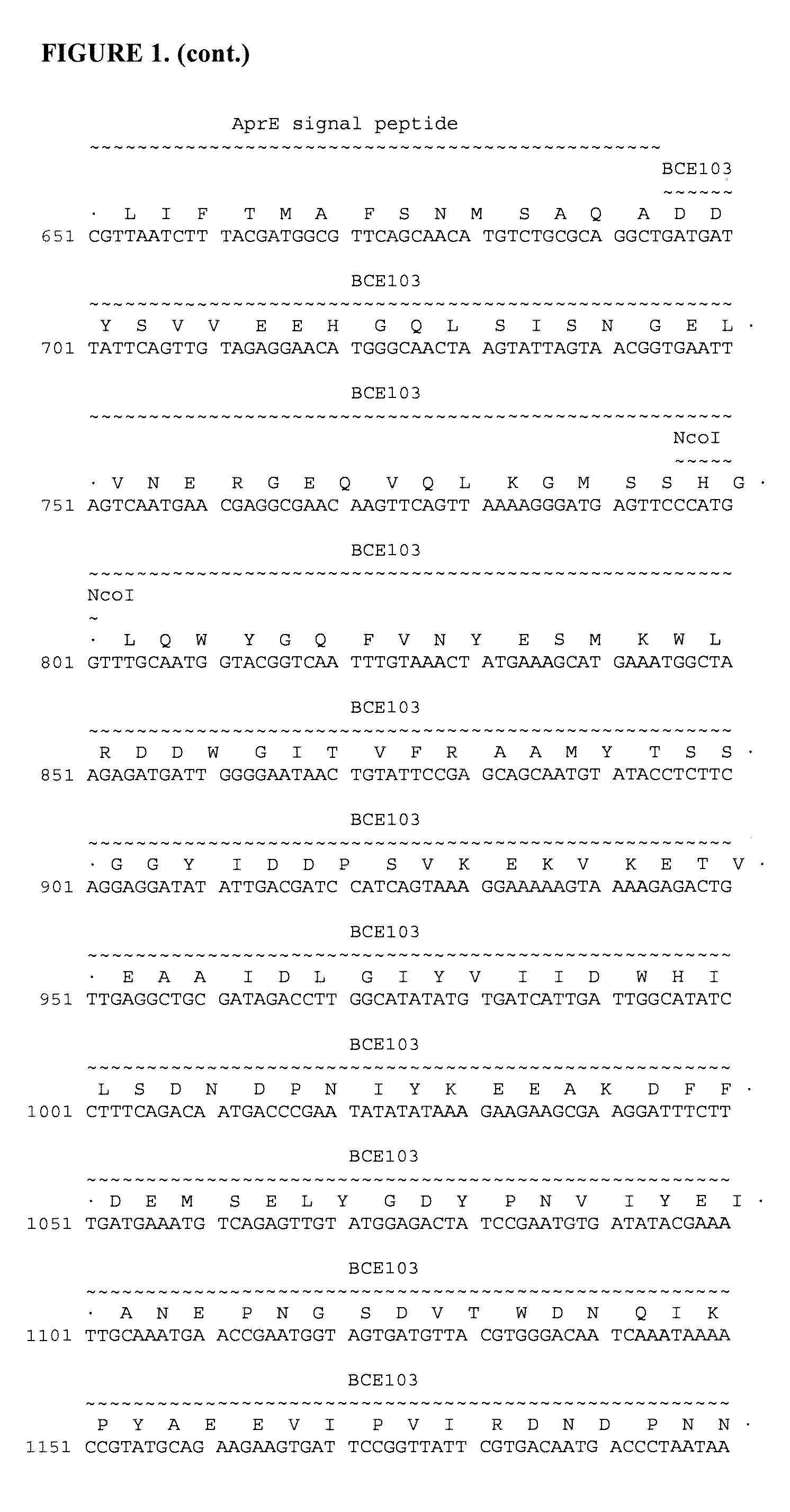
Bacterial expression of bowman-birk protease inhibitors and variants thereof
Owner:GENENCOR INT INC
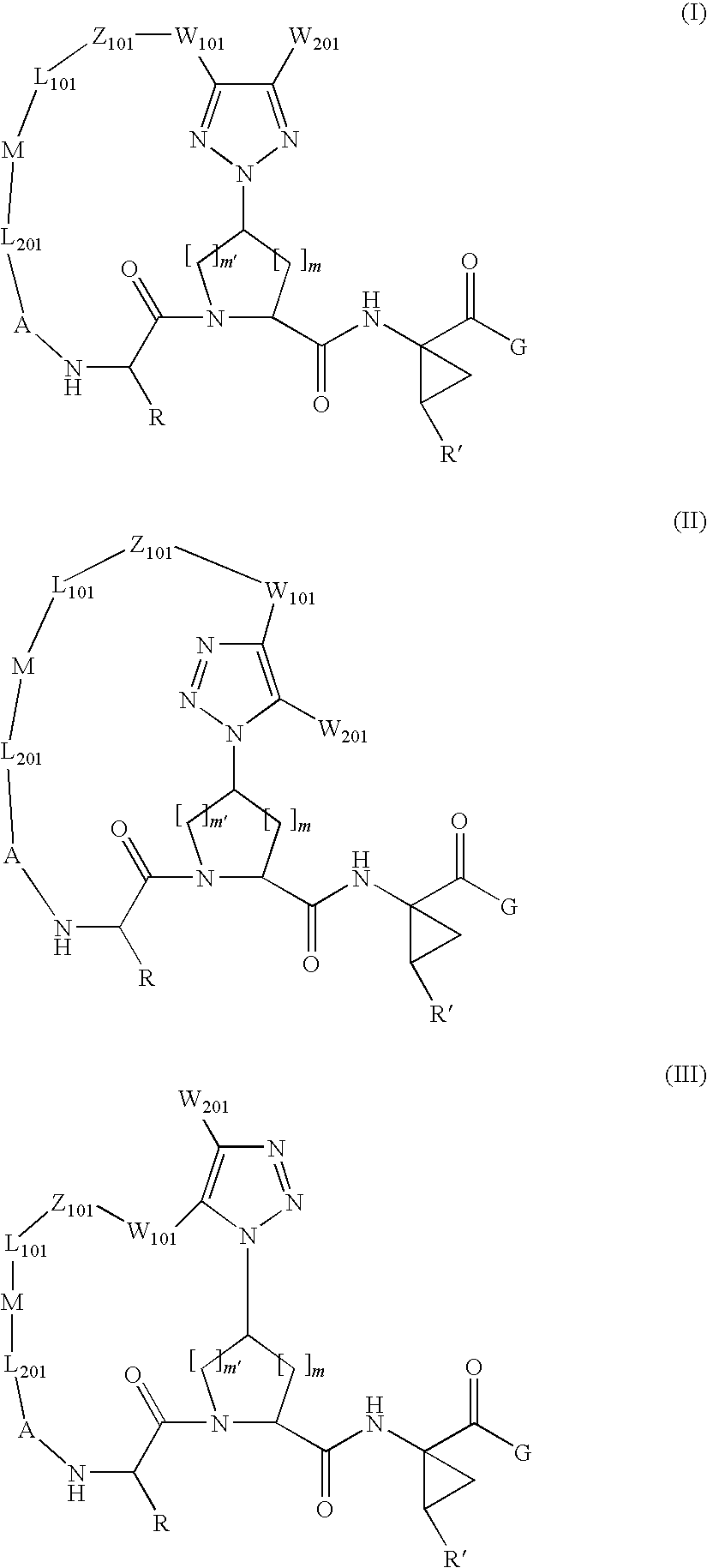
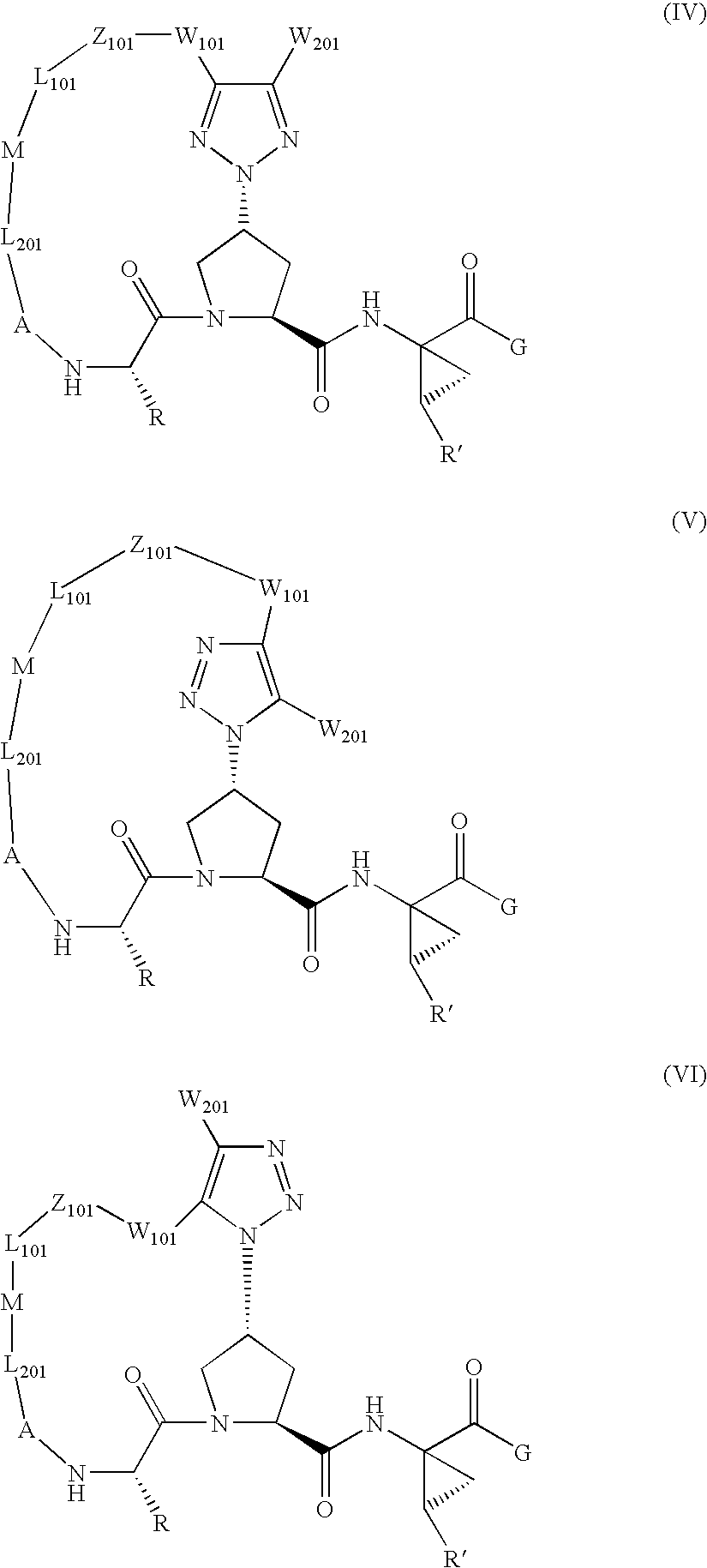
Triazole-containing macrocyclic hcv serine protease inhibitors
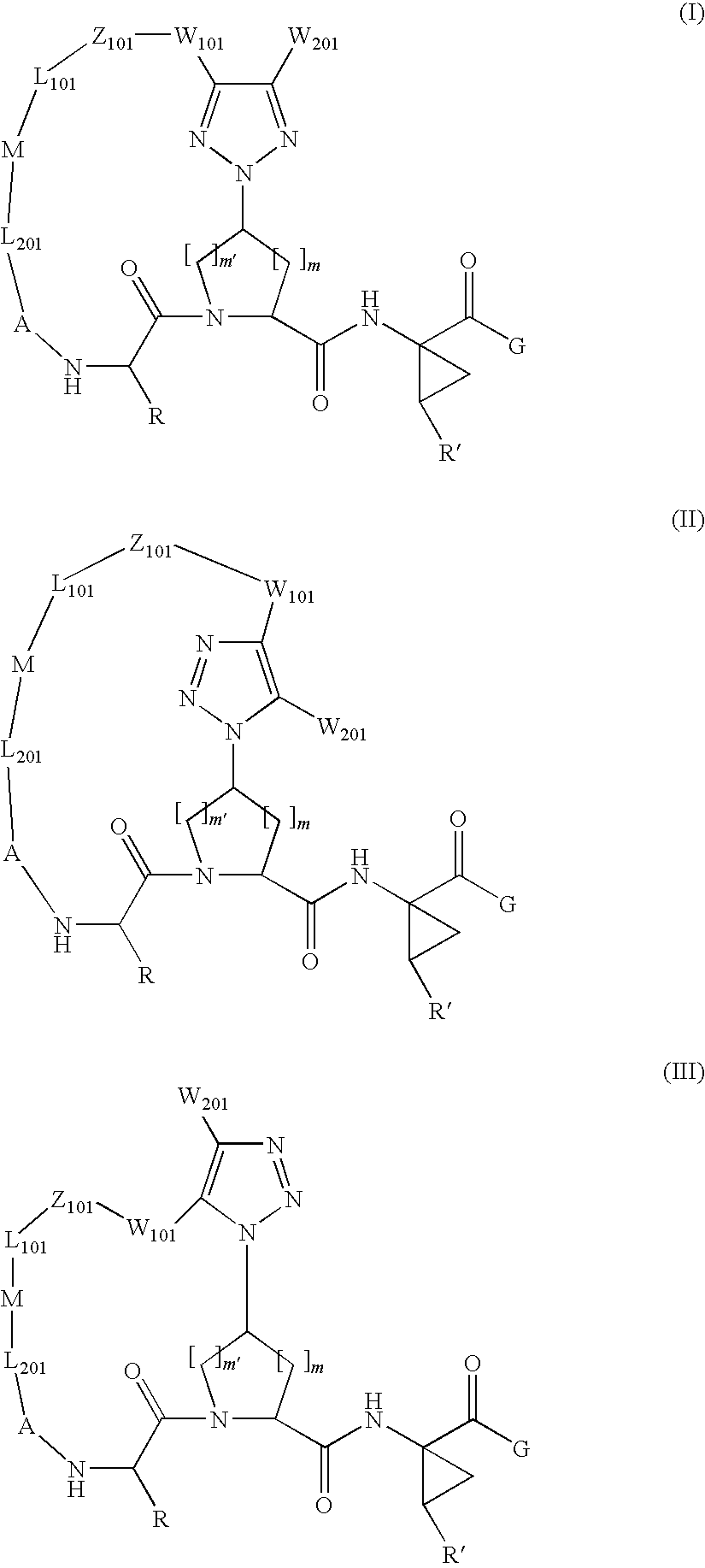
The present invention discloses compounds of formula I, II and III or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Protease inhibitor: protease sensitivity expression system composition and methods improving the therapeutic activity and specificity of proteins delivered by bacteria
Bacteria which co-express protease inhibitors and protease sensitive therapeutic agents, which are surface displayed, secreted and / or released and result in their localized production and maintenance within a target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. The bacteria may be attenuated, non-pathogenic, low pathogenic or a probiotic. Protease sensitivity may be further accomplished by engineering protease degradation sites within the therapeutic agents, further enhancing the inactivation outside of the target tissue while retaining activity within the target tissue through co-expression of a protease inhibitor. Novel chimeric proteins secreted by bacteria, including chimeric toxins targeted to neoplastic cells, tumor matrix cells and cells of the immune system, and combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria limiting exchange of genetic material, and antibody resistant bacteria are also provided.
Owner:BERMUDES DAVID GORDON
Methods for identifying hcv protease inhibitors
InactiveUS20100074890A1Useful in treatmentMicrobiological testing/measurementImmunoglobulins against virusesHIV Protease InhibitorBiochemistry
Owner:CELGENE CAR LLC
Cysteine protease inhibitors for use in treatment of IGE mediated allergic diseases
InactiveUS6034066AOrganic active ingredientsGroup 5/15 element organic compoundsOccupational allergensCysteine Proteinase Inhibitors
PCT No. PCT / GB96 / 01707 Sec. 371 Date Feb. 26, 1998 Sec. 102(e) Date Feb. 26, 1998 PCT Filed Jul. 17, 1996 PCT Pub. No. WO97 / 04004 PCT Pub. Date Feb. 6, 1997The invention provides compounds for use in the treatment of allergic diseases including juvenile asthma and eczema. The compounds can inhibit IgE mediated reaction to major environmental and occupational allergens and can also have a prophylactic effect against allergic disease by preventing allergic sensitization to environmental and occupational allergens when administered to at-risk individuals (e.g., those at genetic risk of asthma and those exposed to occupational allergens in the workplace). The compounds are also useful for inactivation or attenuation of the allergenicity of allergens in situ. The invention provides compounds and ligands per se, pharmaceutical compositions containing the compounds, processes for producing the compounds and pharmaceutical compositions, and methods for using the compounds and compositions in treatment or prophylaxis of IgE mediated allergic diseases and in inactivation or attenuation of allergens in situ. The invention also enables the reduction or destruction of the viability of allergy-causing organisms.
Owner:MEDIVIR UK
Protease inhibitor: protease sensitivity expression system composition and methods improving the therapeutic activity and specificity of proteins delivered by bacteria
ActiveUS9068187B1Direct cytotoxicDirect inhibitoryBiocidePeptide/protein ingredientsBacteroidesSurface display
Bacteria which co-express protease inhibitors and protease sensitive therapeutic agents, which are surface displayed, secreted and / or released and result in their localized production and maintenance within a target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. The bacteria may be attenuated, non-pathogenic, low pathogenic or a probiotic. Protease sensitivity may be further accomplished by engineering protease degradation sites within the therapeutic agents, further enhancing the inactivation outside of the target tissue while retaining activity within the target tissue through co-expression of a protease inhibitor. Novel chimeric proteins secreted by bacteria, including chimeric toxins targeted to neoplastic cells, tumor matrix cells and cells of the immune system, and combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria limiting exchange of genetic material, and antibody resistant bacteria are also provided.
Owner:BERMUDES DAVID GORDON
Oximyl hydroxyamic analogs as hepatitis c virus protease inhibitor
InactiveUS20080274082A1Hydrocarbon active ingredientsDipeptide ingredientsProteinase activityHIV Protease Inhibitor
The present invention relates to compounds of Formula I, or a pharmaceutically acceptable salt, ester, or prodrug, thereof:which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Combination therapy comprising the use of protein kinase C modulators and Histone Deacetylase inhibitors for treating HIV-1 latency
InactiveUS20100166806A1Adverse propertyPrevent HIV-1-induced cytotoxicityBiocideOrganic chemistryReverse transcriptaseHydroxamic acid
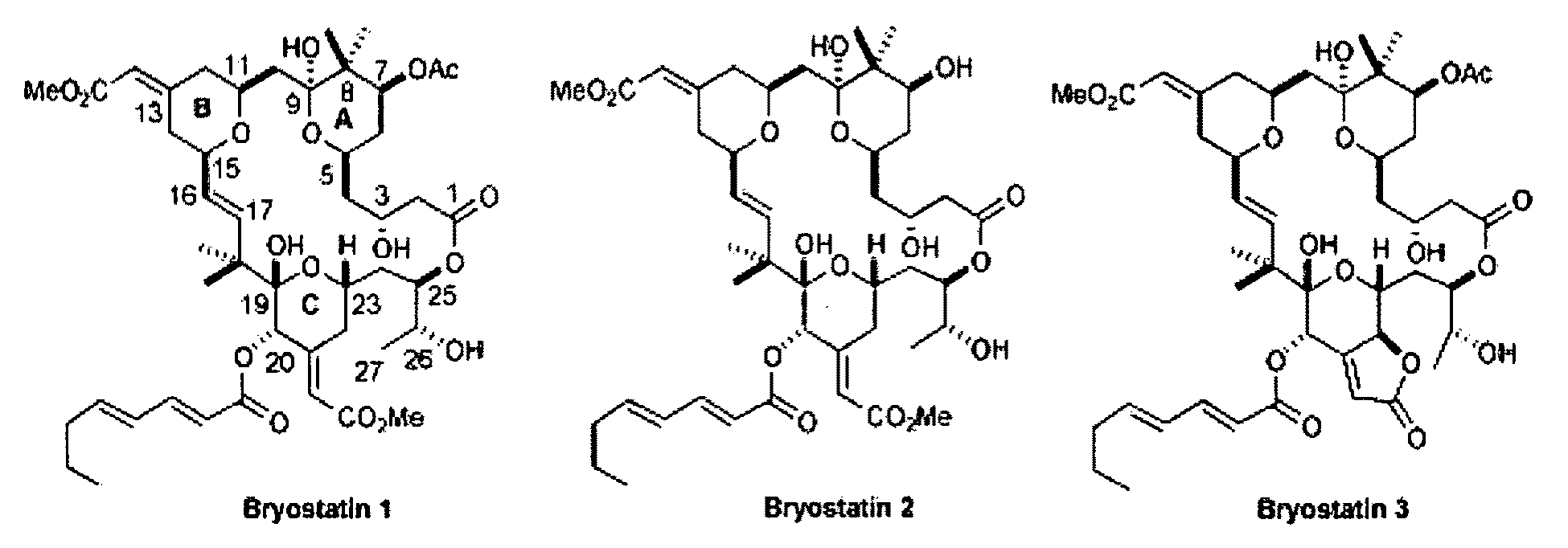
The invention relates to a combination of treatments, more particularly a combination treatment for HIV-1 infection. The present invention is directed to the use of bryostatin-1 and their natural and synthetic derivatives for AIDS therapy, in particular to the use of bryostatins in combination with other active drugs such as Histone Deacetylases (HDACs) inhibitors and anti-retrovirals, for the treatment of HIV-1 latency. According to the present invention, we provide a combination therapy for the treatment of HIV-1 latency which employs bryostatin-1 (and analogues) and one of the following HDAC inhibitors; valproic acid, butyrate derivatives, hydroxamic acids and benzamides. While HDACi can be used in continuous dosing protocol, bryostatins can be used following a cyclical dosing protocol. Bryostatins can be formulated in pharmaceutical acceptable carriers including nanoparticles, phospholipids nanosomes and / or biodegradable polymer nanospheres. This combination therapy needs to be used in patients treated with antiretroviral therapy (HIV-1 protease inhibitors, HIV-1 reverse transcriptase inhibitors, HIV-1 integrase inhibitors, CCR5 co-receptor inhibitors and fusion inhibitors).
Owner:APHIOS
Stereoselective reduction of substituted oxo-butanes
InactiveUS7083973B2High yieldExcellent diastereomeric purityOrganic chemistryBacteriaProteinase activityEnantiomer
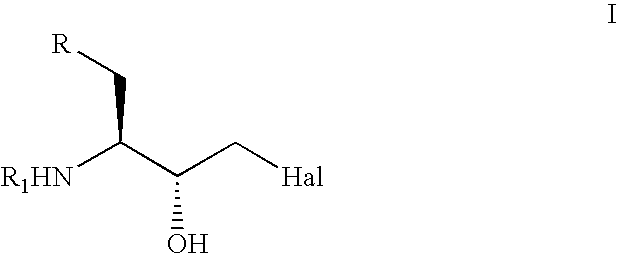
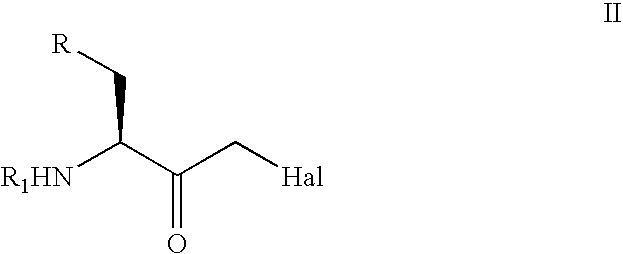
The present invention relates to a process for the stereoselective enzymatic reduction of 1-halo-2-oxo-3-(protected)amino-4-substituted-butanes utilizing certain species of Rhodococcus and Brevibacterium. The product 1-halo-2-hydroxy-3-(protected)amino-4-substituted-butanes, which are useful as intermediates in the synthesis of compounds that are inhibitors of ACE, renin and HIV proteases, are obtained in high yield and, particularly, in very high diastereomeric purity. The process is advantageously highly selective for the (3S,2R) enantiomer of the product.
Owner:BRISTOL MYERS SQUIBB CO
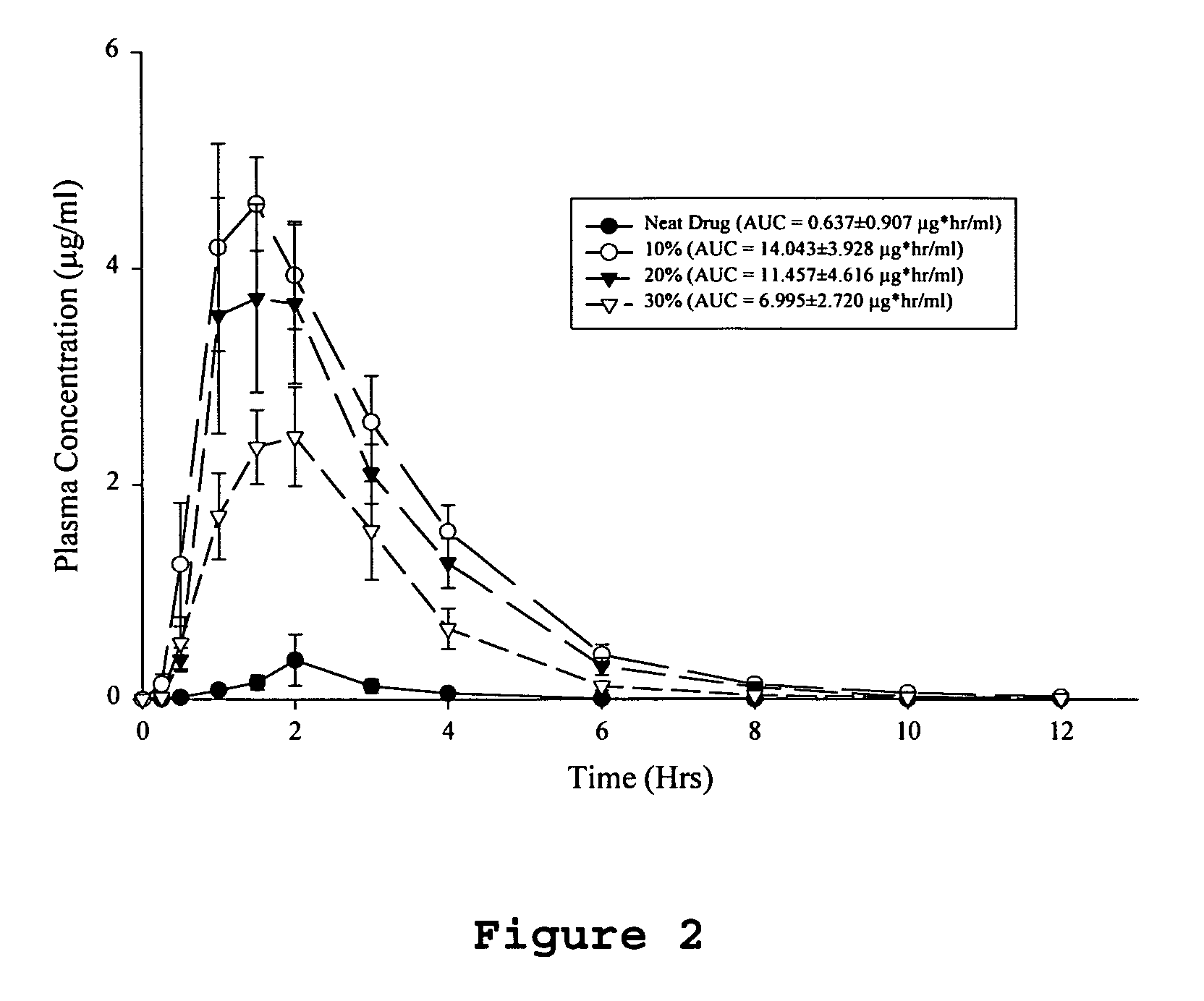
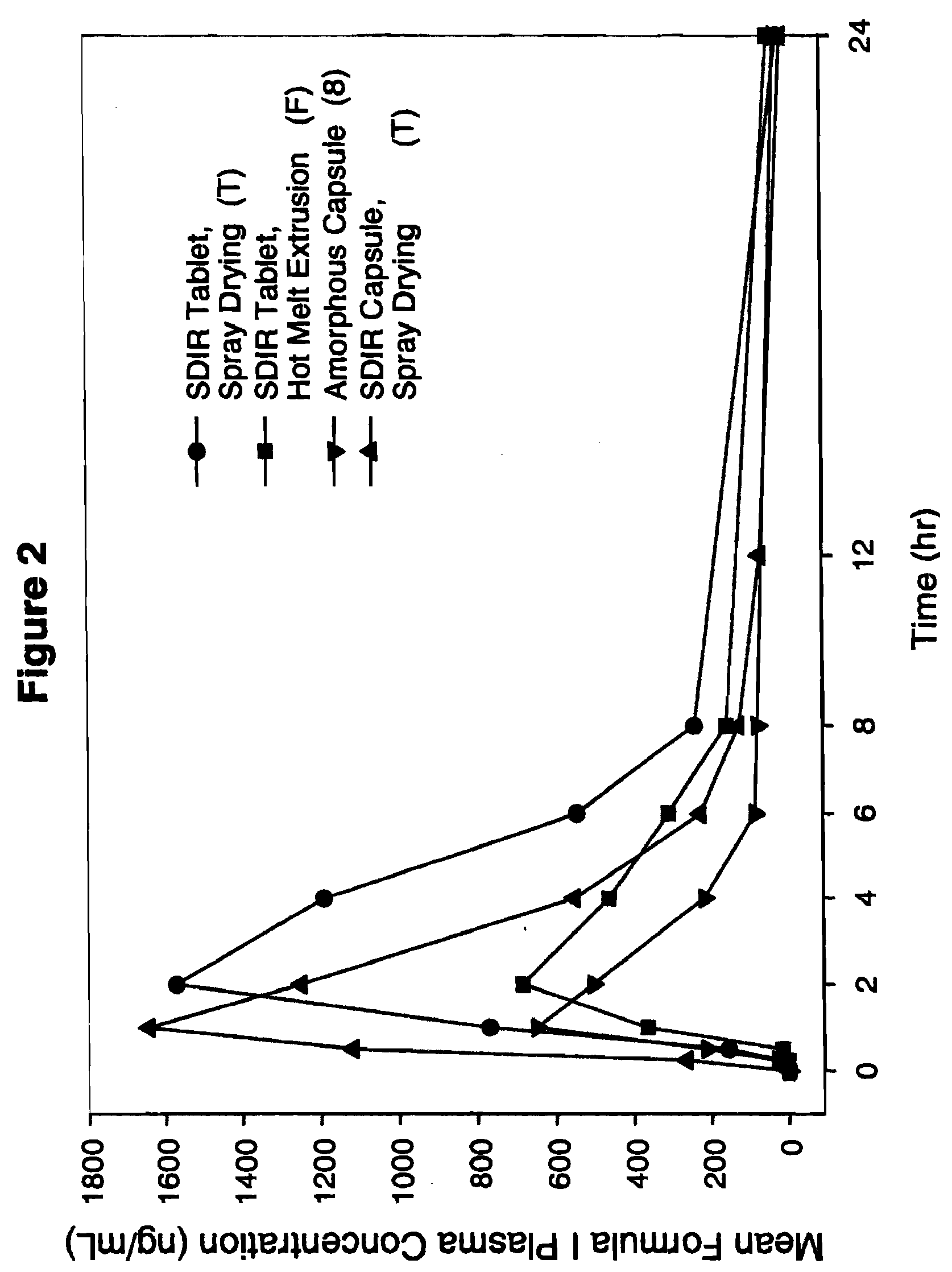
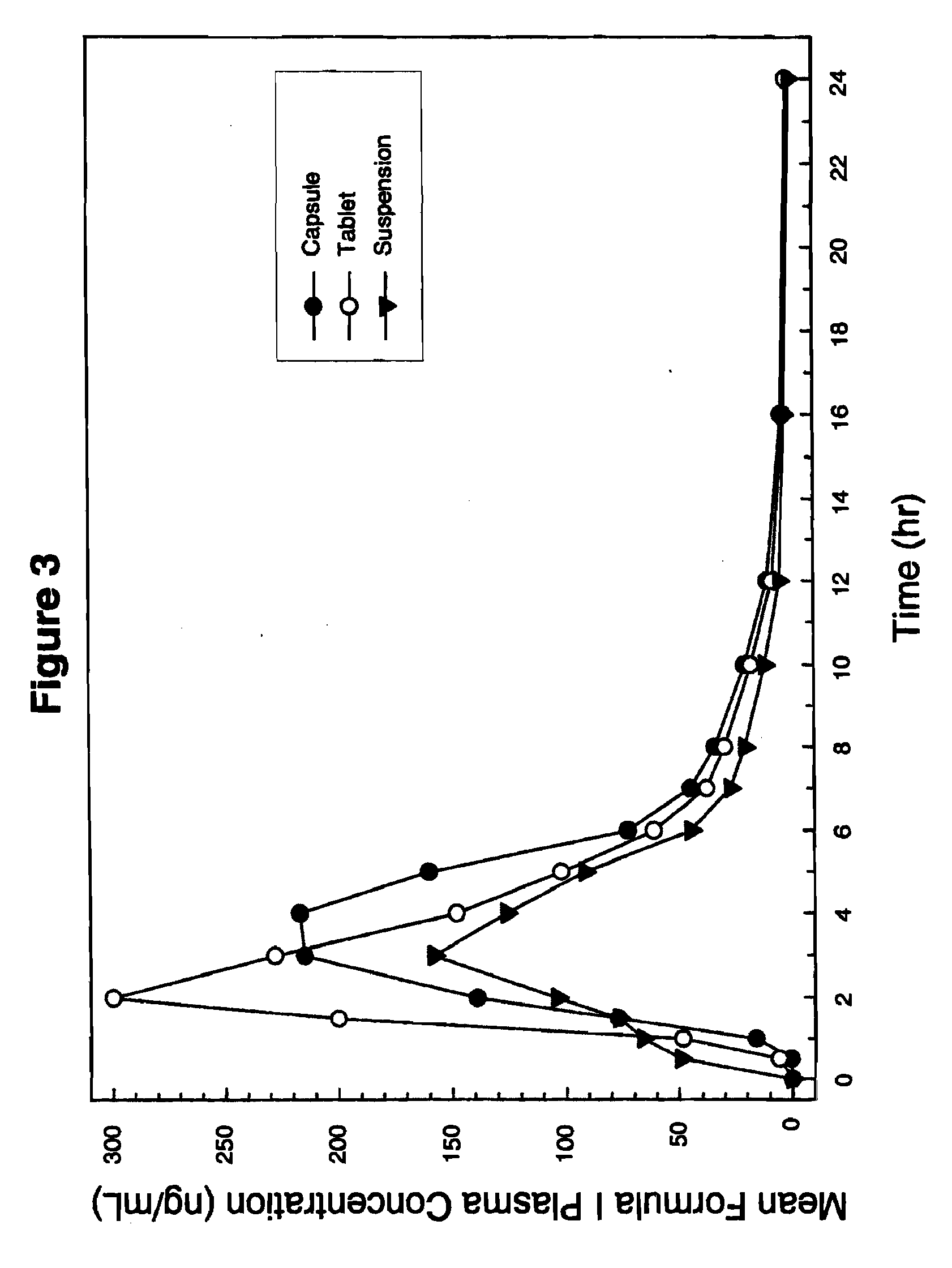
Solid dispersion pharamaceutical formulations
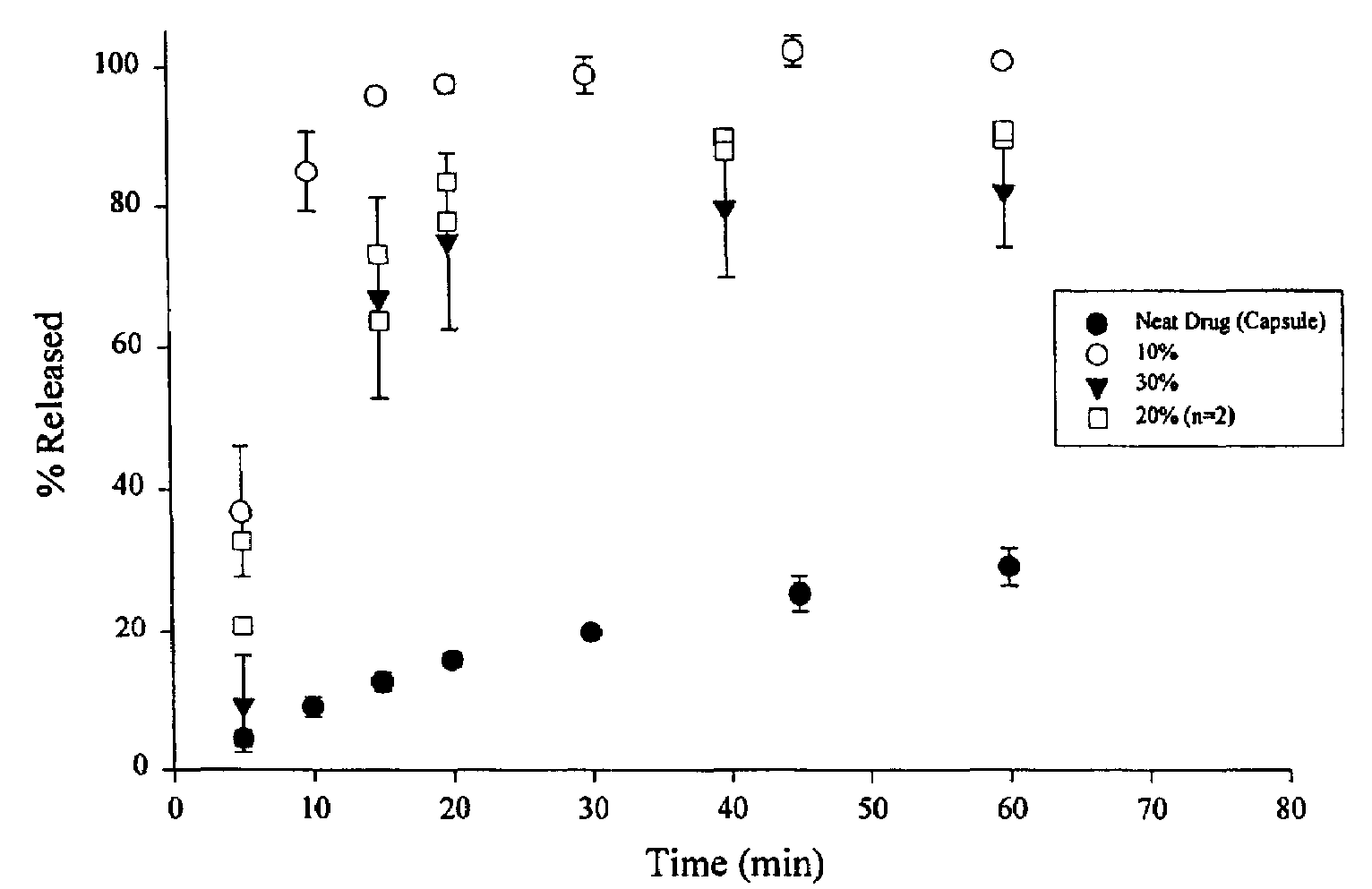
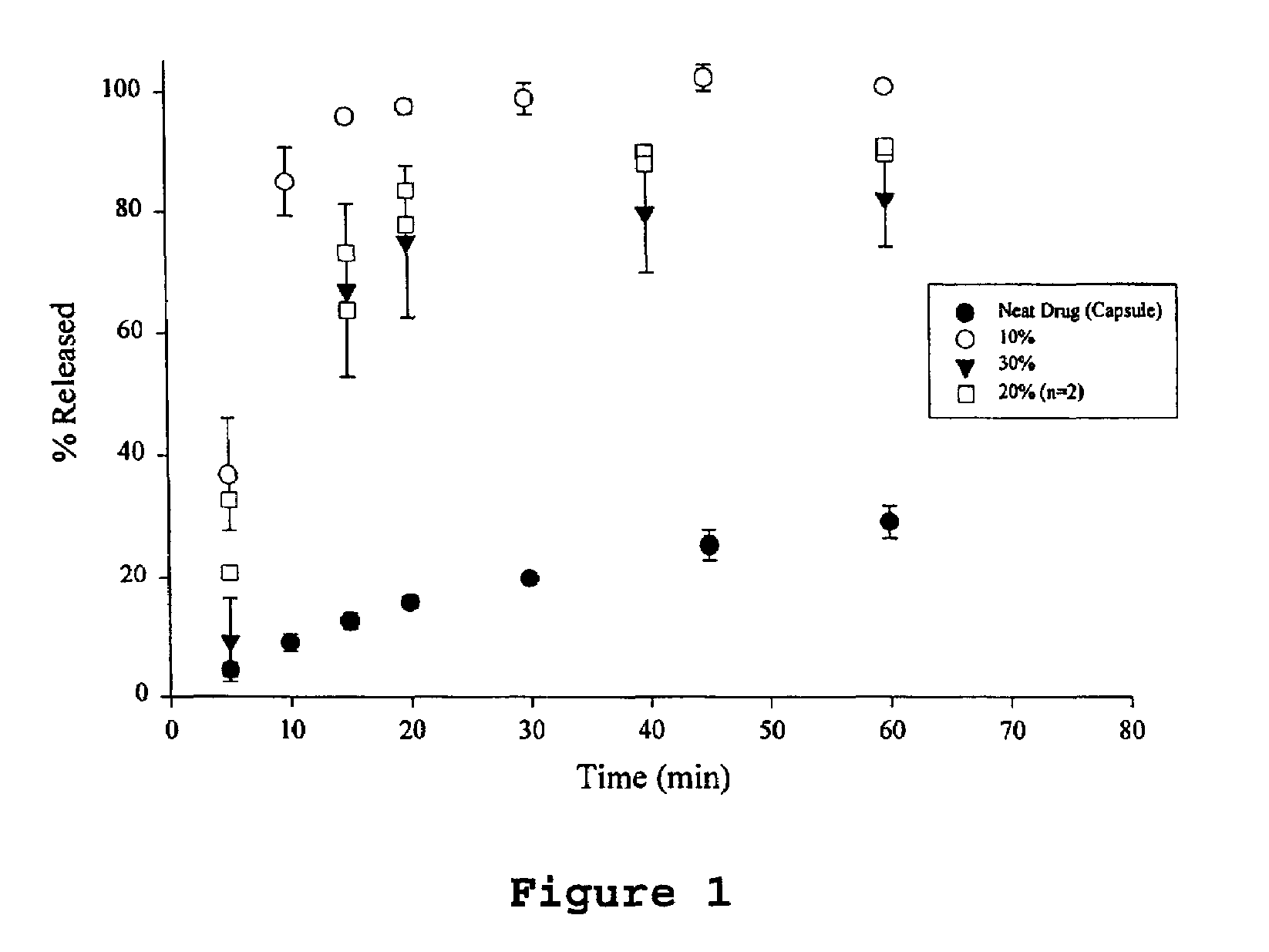
A pharmaceutical composition is disclosed which comprises a solid dispersion of an HIV protease inhibitor in a water soluble carrier, such as PEG, having enhanced bioavailability and improved dissolution properties. The solid dispersion may optionally be encapsulated in hard gelatin capsules, compressed into a tablet, or may be granulated with a pharmaceutically acceptable granulating agent. Also disclosed are methods of making said solid dispersion and methods of treating an HIV infection employing said solid dispersion.
Owner:ABBVIE INC
Targeted intracellular delivery of antiviral agents
InactiveUS20100129437A1High affinitySpecificity of bindingPowder deliveryBiocideInternalizationChemical agent
The invention relates to methods of targeted drug delivery of antiviral compounds, including, chemical agents (like nucleoside analogs or protease inhibitors) and nucleic acid based drugs (like DNA vaccines, antisense oligonucleotides, ribozymes, catalytic DNA (DNAzymes) or RNA molecules, siRNAs or plasmids encoding thereof). Furthermore, the invention relates to targeted drug delivery of antiviral compounds to intracellular target sites within cells, tissues and organs, in particular to target sites within the central nervous system (CNS), into and across the blood-brain barrier, by targeting to internalizing uptake receptors present on these cells, tissues and organs. Thereto, the antiviral compounds, or the pharmaceutical acceptable carrier thereof, are conjugated to ligands that facilitate the specific binding to and internalization by these receptors.
Owner:TO BBB HLDG
HCV protease inhibitors and uses thereof
Owner:CELGENE CAR LLC
Serine protease inhibitors
InactiveUS20050130883A1Decrease inhibit proteolytic activityDepsipeptidesImmunoglobulinsHIV Protease InhibitorSerine
The present disclosure concerns peptides and peptidomimetics having inhibitory activity against serine proteases. The disclosed peptides and peptidomimetics are particularly useful as selective inhibitors of matriptase and MTSP1. Pharmaceutical compositions comprising serine protease inhibitors and methods for administering the compositions also are disclosed.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE US SEC THE DEPT OF
Combinations of hcv protease inhibitor(s) and cyp3a4 inhibitor(s), and methods of treatment related thereto
Disclosed are medicaments, pharmaceutical compositions, pharmaceutical kits, and methods based on combinations comprising, separately or together: (a) a CYP3A4 inhibitor; and (b) a HCV protease inhibitor; for concurrent or consecutive administration in treating a human subject infected with HCV.
Owner:MERCK SHARP & DOHME CORP
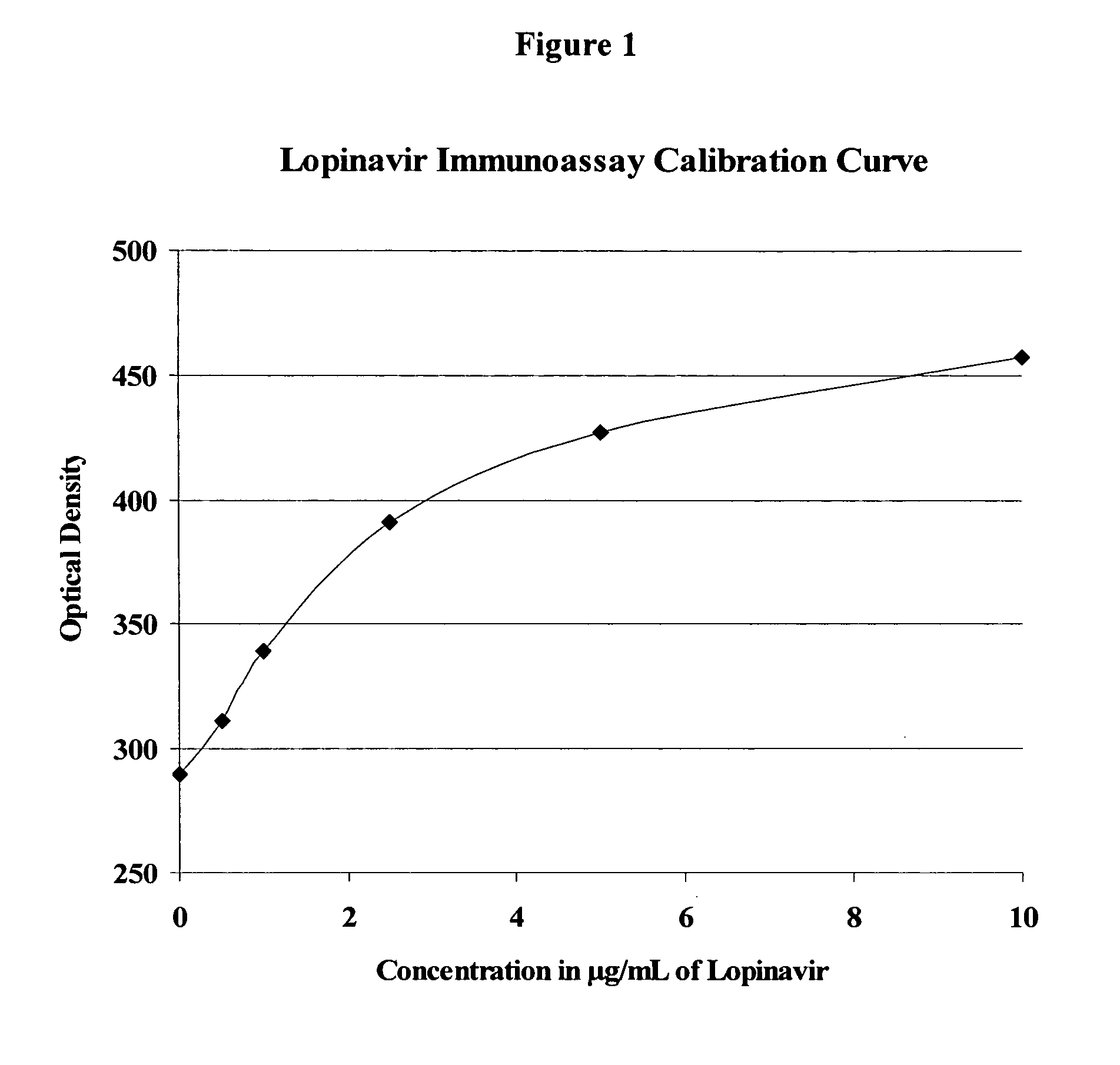
Immunoassays, haptens, immunogens and antibodies for anti-HIV therapeutics
InactiveUS20050244816A1BiocideMicrobiological testing/measurementNucleoside Reverse Transcriptase InhibitorAntiendomysial antibodies
This invention provides compounds, methods, immunoassays, and kits relating to active, metabolically sensitive (“met-sensitive”) moieties of anti-HIV therapeutics, such as HIV protease inhibitors (PI) and HIV non-nucleoside reverse transcriptase inhibitors (NNRTI).
Owner:ARK DIAGNOSTICS
Treatment of chronic obstructive pulmonary disease by low dose inhalation of protease inhibitor
The present invention encompasses methods and compositions for the treatment and prevention of chronic obstructive pulmonary disease (COPD) or emphysema, typically smoking-induced emphysema. More specifically, the present invention relates to the treatment and prevention of COPD or emphysema by inhalation of alpha one-antitrypsin (AAT).
Owner:ARRIVA PHARMA
Processes for preparing protease inhibitors of hepatitis c virus
The present invention relates to synthetic processes useful in the preparation of macrocyclic compounds of Formula (I) that are useful as inhibitors of the hepatitis C virus NS3 protease and have application in the treatment of conditions caused by the hepatitis C virus. The present invention also encompasses intermediates useful in the disclosed synthetic processes and the methods of their preparation.
Owner:MERCK SHARP & DOHME CORP
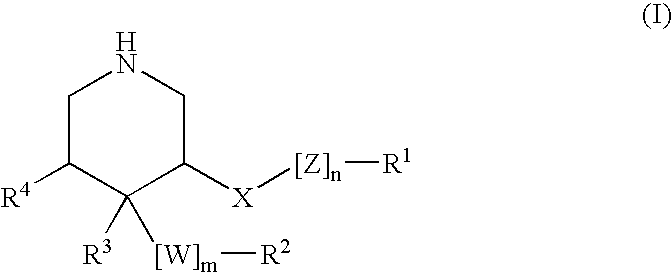
HIV-1 Protease Inhibitors
Described are novel protease inhibitors and methods for using said protease inhibitors in the treatment of human immunodeficiency virus (HIV) infection.
Owner:MASSACHUSETTS INST OF TECH +2
Protease inhibitors that overcome drug resistance
InactiveUS6969731B1ActivityLess-prone to development of resistanceBiocideOrganic chemistryMutated proteinProteinase activity
HIV protease inhibitors are among the most powerful drugs in suppressing HIV in human patients. However, HIV developed resistance to all protease inhibitor drugs so far marketed or used in clinical trials. HIV generates resistance by mutating its protease. The strains of HIV containing mutant proteases less vulnerable to inhibitor drug are able to replicate better and maintain the infection. No effective principle exists for the design of resistance-proof HIV protease inhibitors (HIVPr). A new inhibitor has been developed based on a new concept for designing resistance invulnerable HIVPr inhibitors. In vitro data have shown that this inhibitor is effective against many known HIVPr mutants resistant to other HIVPr inhibitor drugs. The new concept is, therefore, generally applicable for the design of other resistance invulnerable HIVPr inhibitor drugs.
Owner:ILLINOIS UNIV OF BOARD OF TRUSTEES OF THE THE +1
Pharmaceutical formulations of an hcv protease inhibitor in a solid molecular dispersion
InactiveUS20110207660A1Improve bioavailabilityBetter pharmacokinetic profileBiocideDigestive systemDepressantHIV Protease Inhibitor
The present invention provides pharmaceutical formulations of an HCV protease inhibitor in a solid dispersion with an excipient which provided advantageous pharmacokinetic properties for inhibiting or treating HCV infection. In preferred embodiments, the excipient is at least one polymer. The present invention also provides processes for manufacturing such formulations as well as uses of said composition for the manufacture of a medicament for treating or ameliorating one or more symptoms of HCV or disorders associated with HCV in a subject in need thereof using said formulations.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com