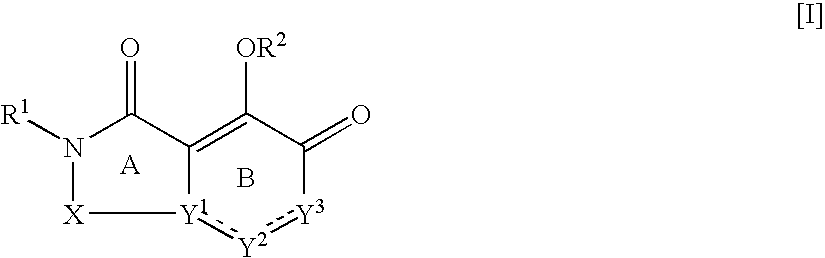Patents
Literature
216 results about "Anti hiv" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anti HCV is the antibody to chronic hepatitis C that appears after infection and disappears after recovery. Anti HCV negative denotes you are not suffering from hepatitis C infection. Anti HIV also denotes that you are free from the HIV infection.
Methods and compositions for treatment of human immunodeficiency virus infection with conjugated antibodies or antibody fragments
ActiveUS20070264265A1Avoid infectionReduce eliminateOrganic active ingredientsAntiviralsDiagnostic agentBinding site
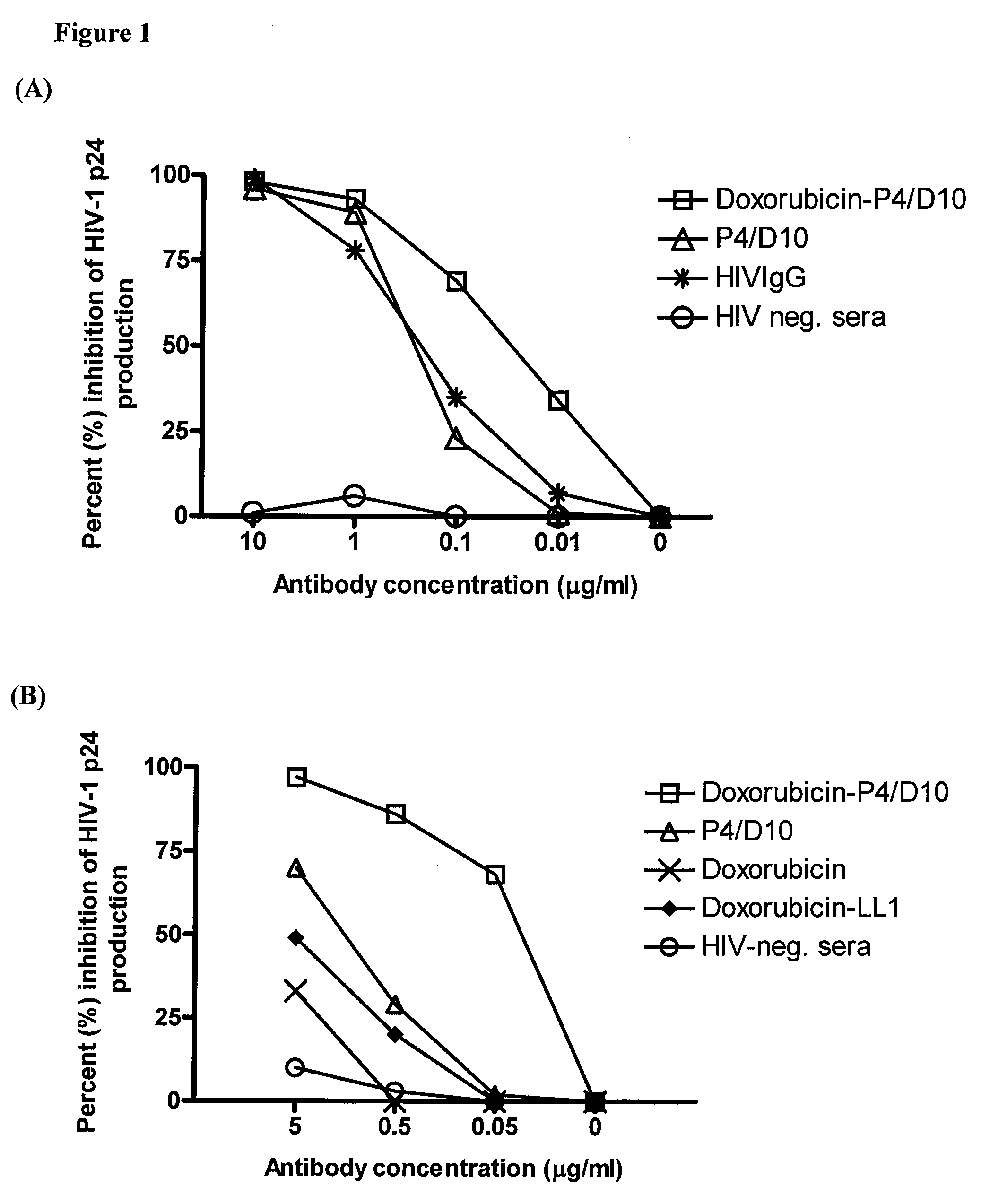
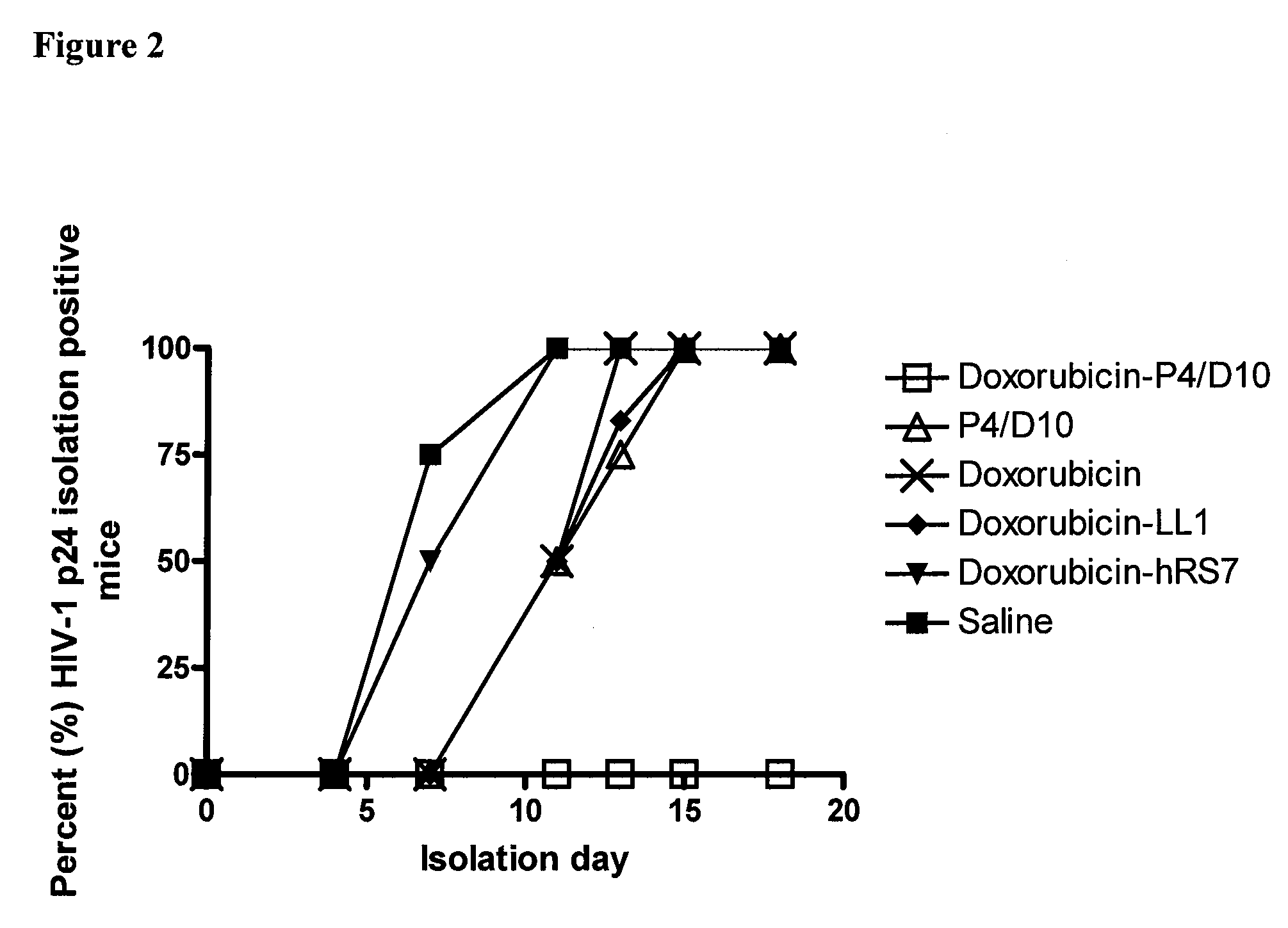
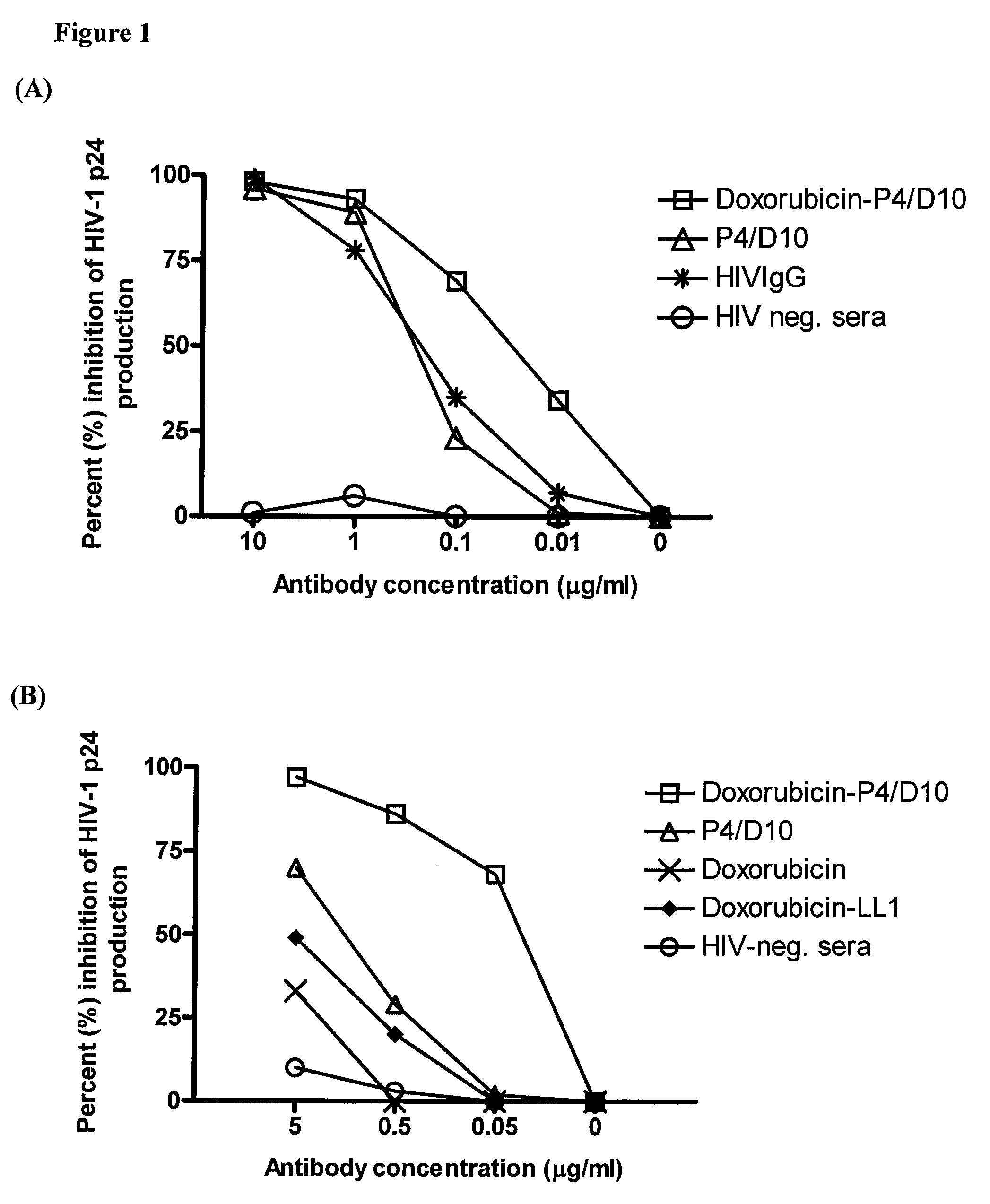
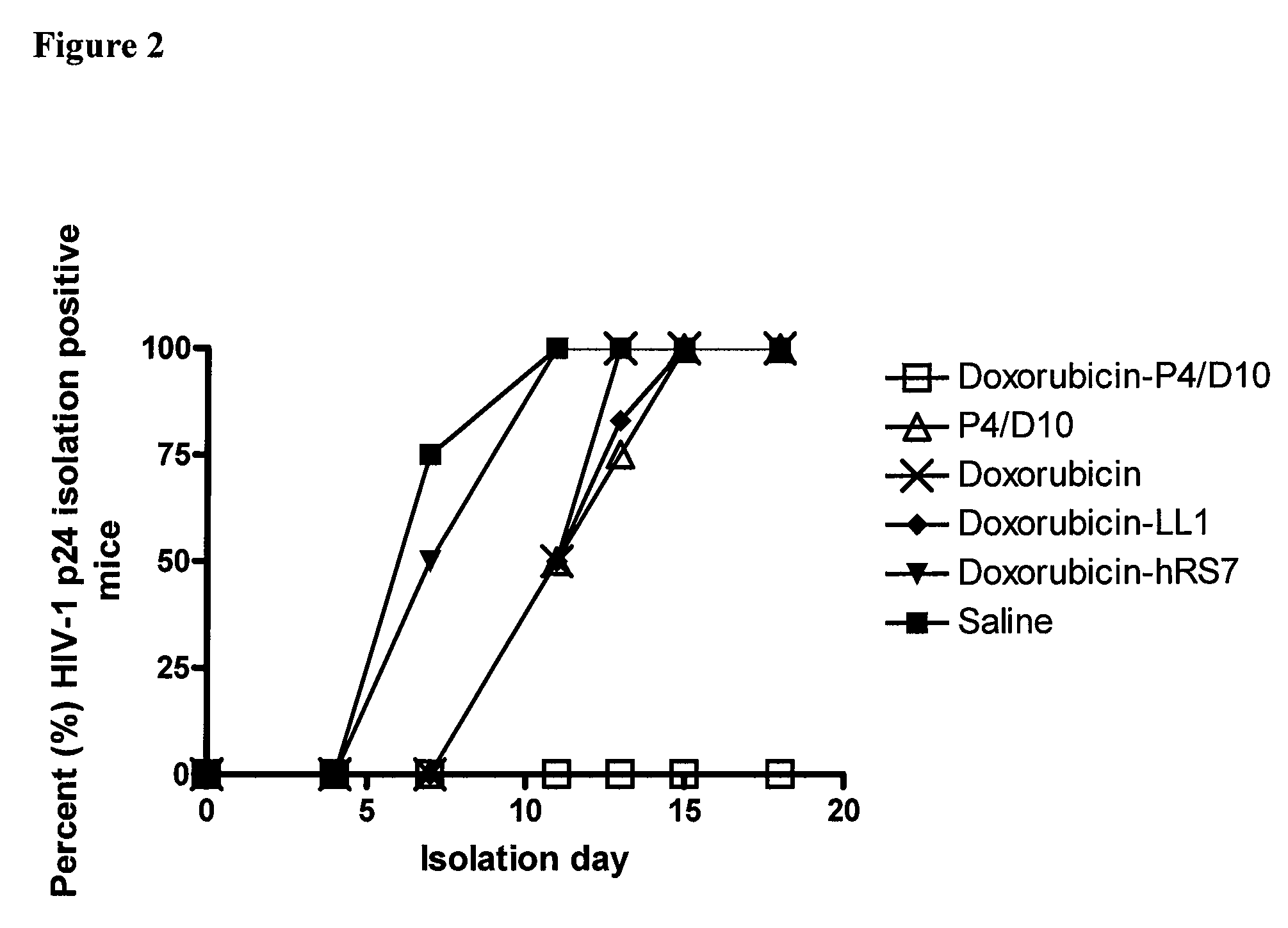
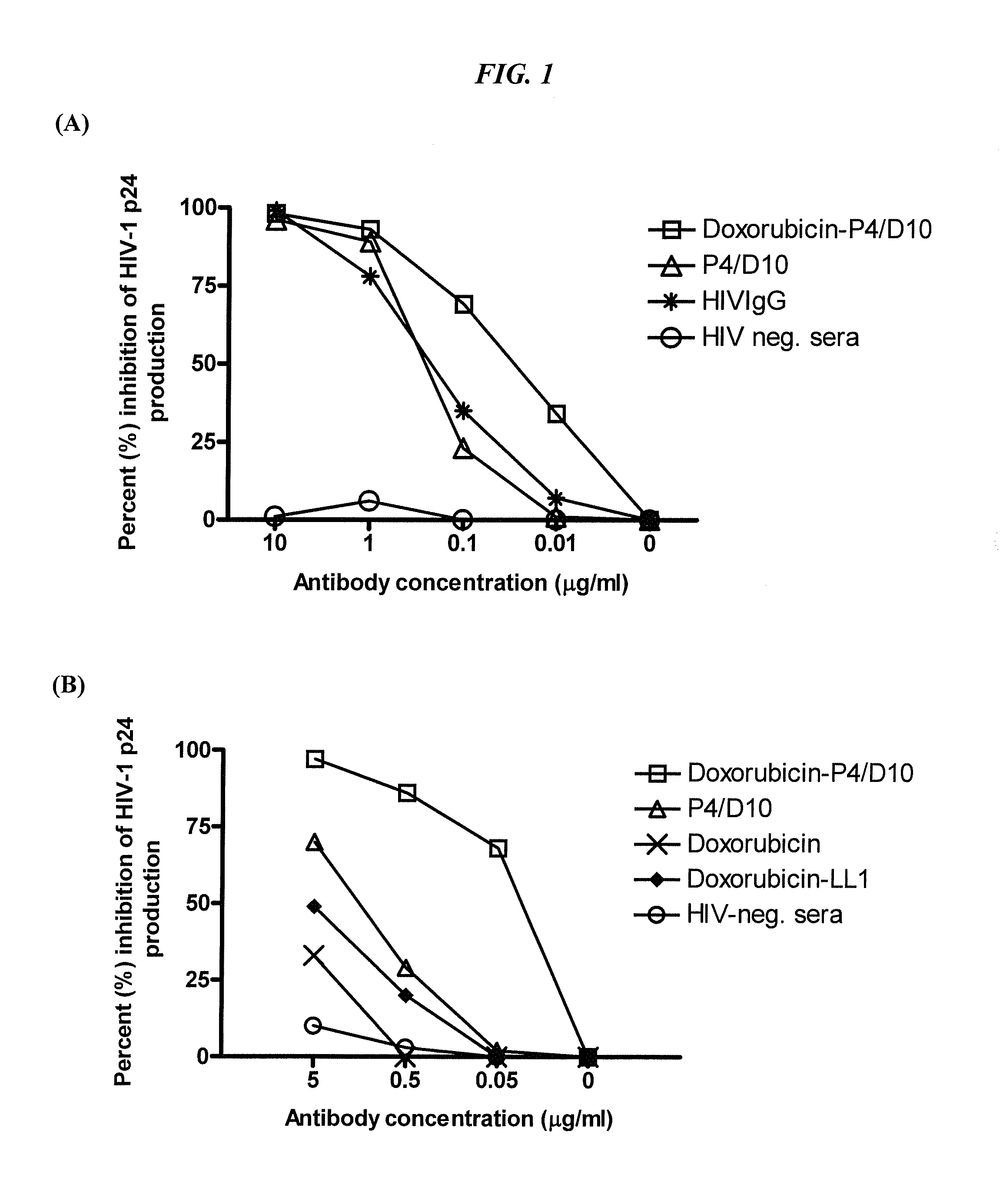
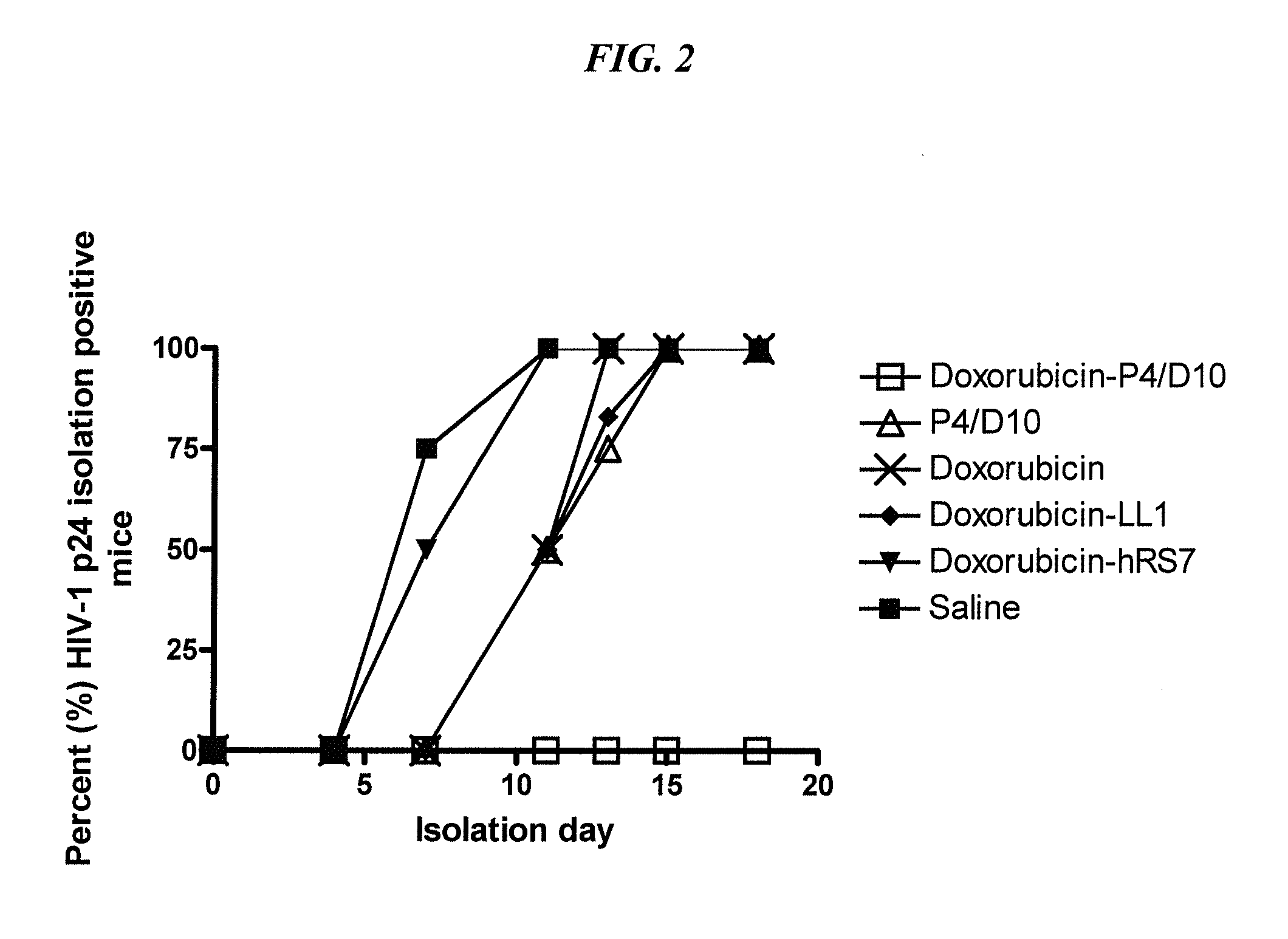
The present invention concerns methods and compositions for treatment of HIV infection in a subject. The compositions may comprise a targeting molecule against an HIV antigen, such as an anti-HIV antibody or antibody fragment. The anti-HIV antibody or fragment may be conjugated to a variety of cytotoxic agents, such as doxorubicin. In a preferred embodiment, the antibody or fragment is P4 / D10. Other embodiments may concern methods of imaging, detection or diagnosis of HIV infection in a subject using an anti-HIV antibody or fragment conjugated to a diagnostic agent. In alternative embodiments, a bispecific antibody with at least one binding site for an HIV antigen and at least one binding site for a carrier molecule may be administered, optionally followed by a clearing agent, followed by administration of a carrier molecule conjugated to a therapeutic agent.
Owner:IMMUNOMEDICS INC
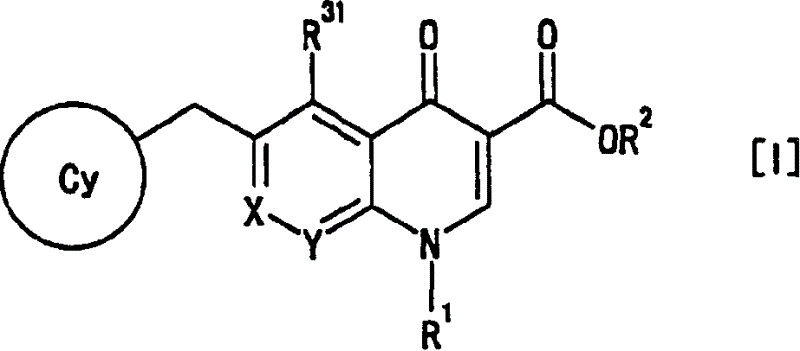
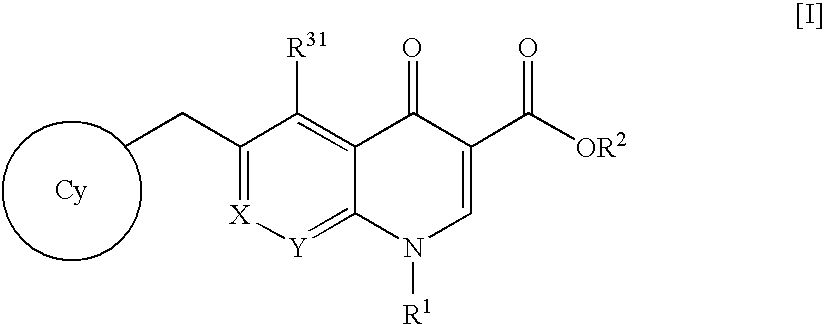
4-Oxoquinoline compounds and utilization thereof as hiv integrase inhibitors
ActiveUS20050239819A1Promote absorptionIncreased riskBiocideOrganic chemistrySide effectReverse transcriptase
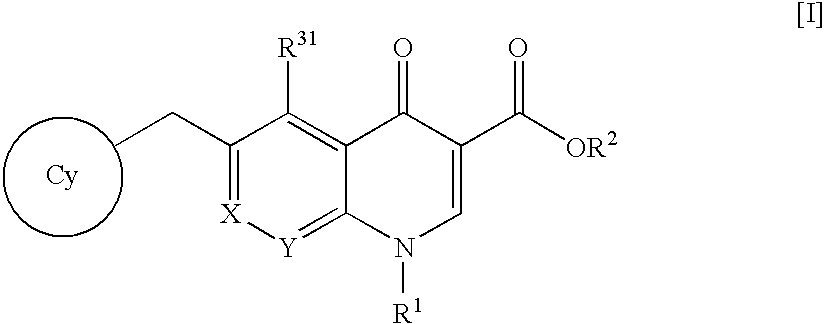
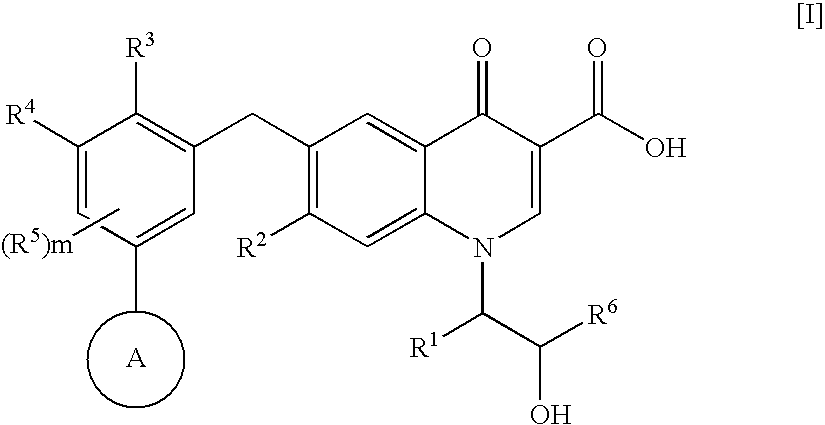
An anti-HIV agent containing, as an active ingredient, a 4-oxoquinoline compound represented by the following formula [I]wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof. The compound of the present invention has HIV integrase inhibitory action and is useful as an anti-HIV agent for the prophylaxis or therapy of AIDS. Moreover, by a combined use with other anti-HIV agents such as protease inhibitors, reverse transcriptase inhibitors and the like, the compound can become a more effective anti-HIV agent. Since the compound has high inhibitory activity specific for integrases, it can provide a safe pharmaceutical agent with a fewer side effects for human.
Owner:JAPAN TOBACCO INC
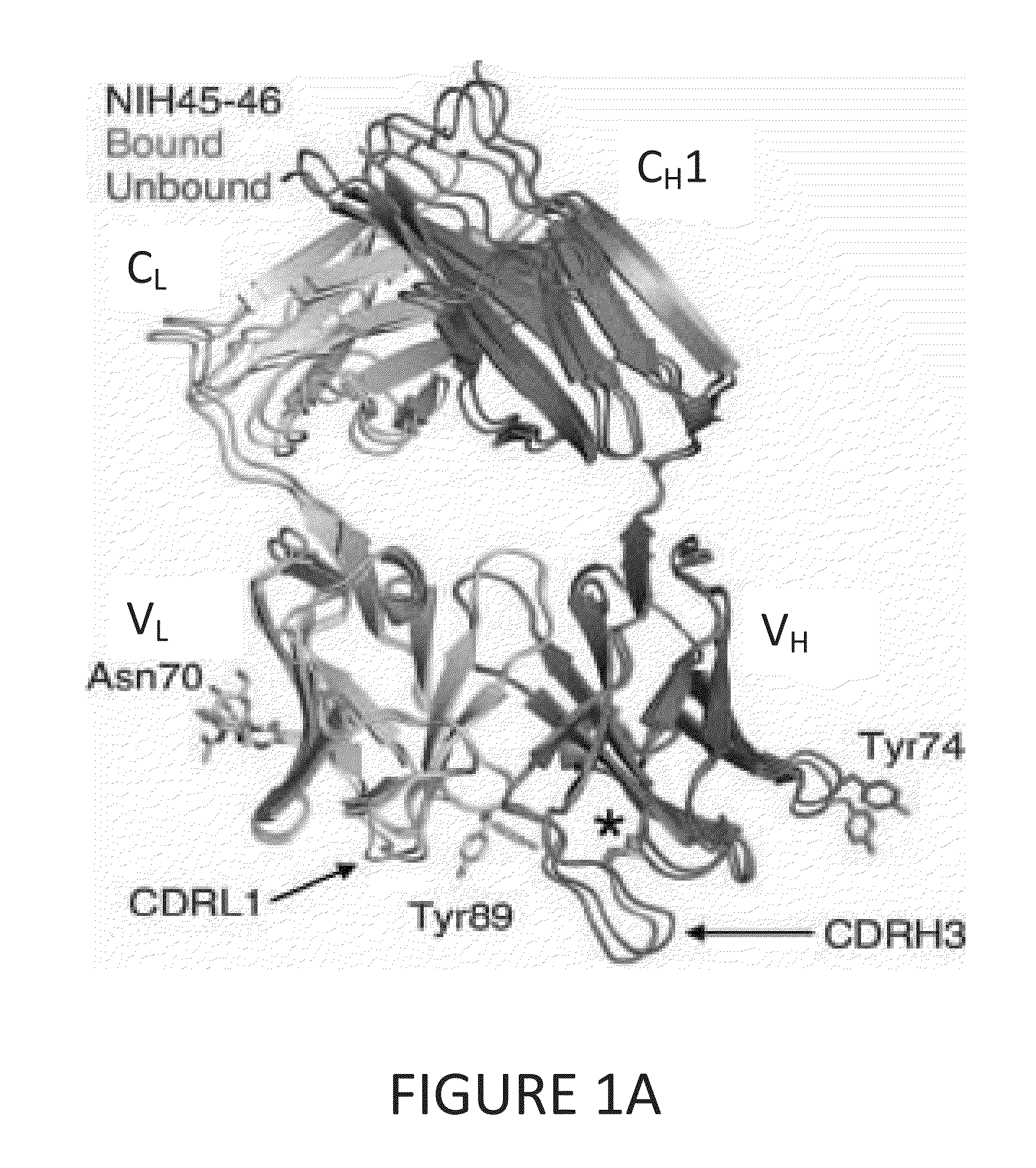
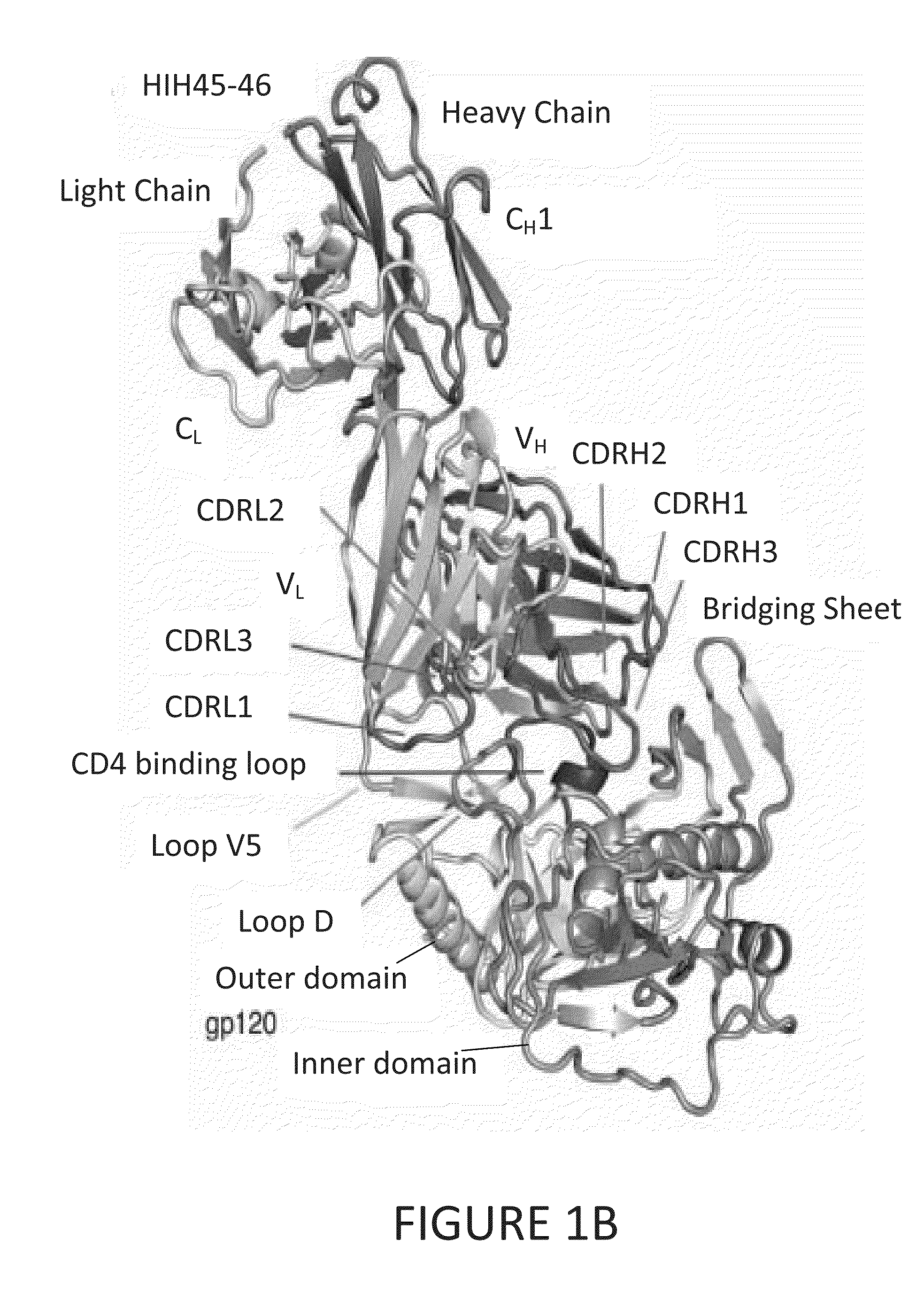
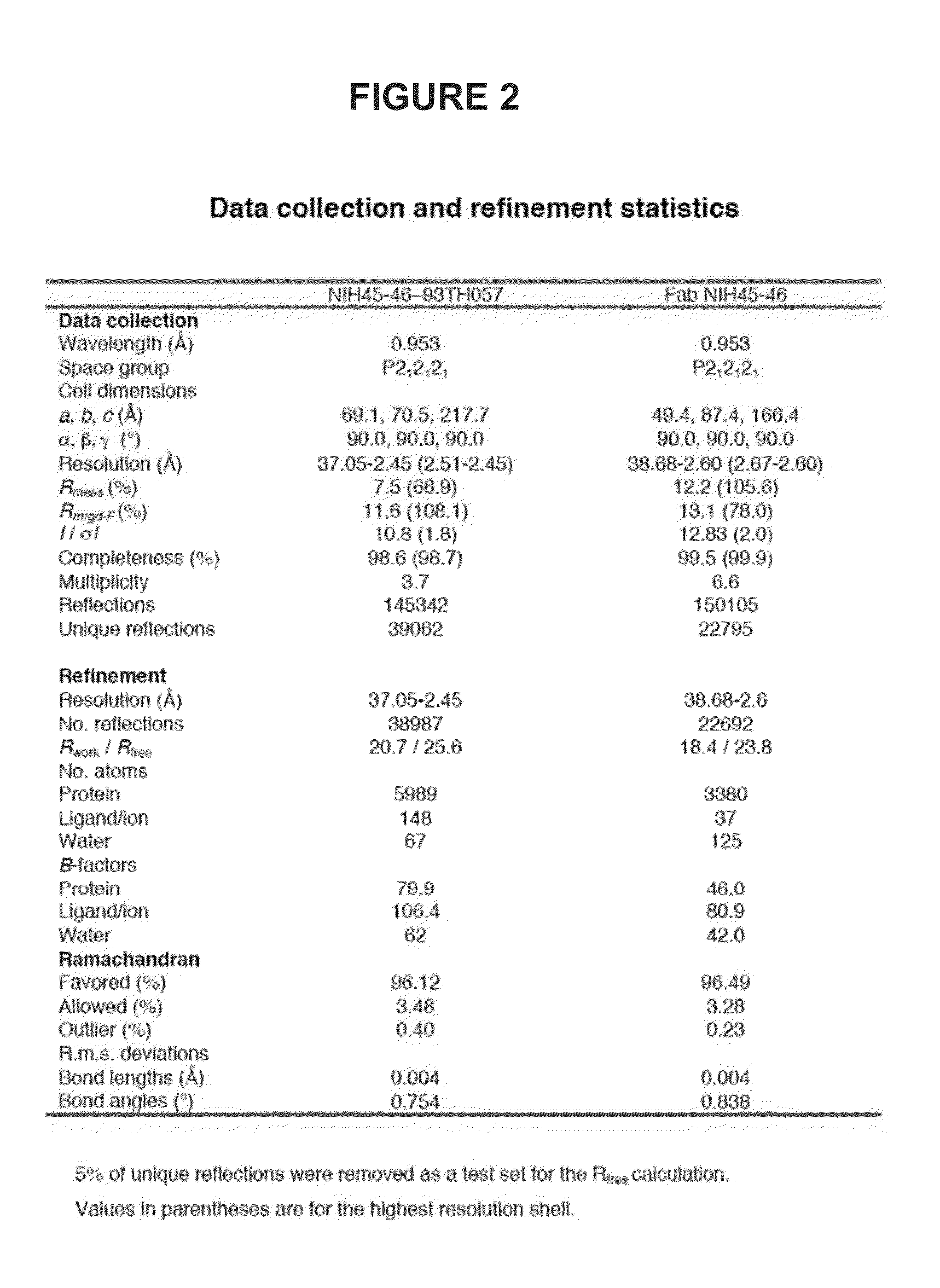
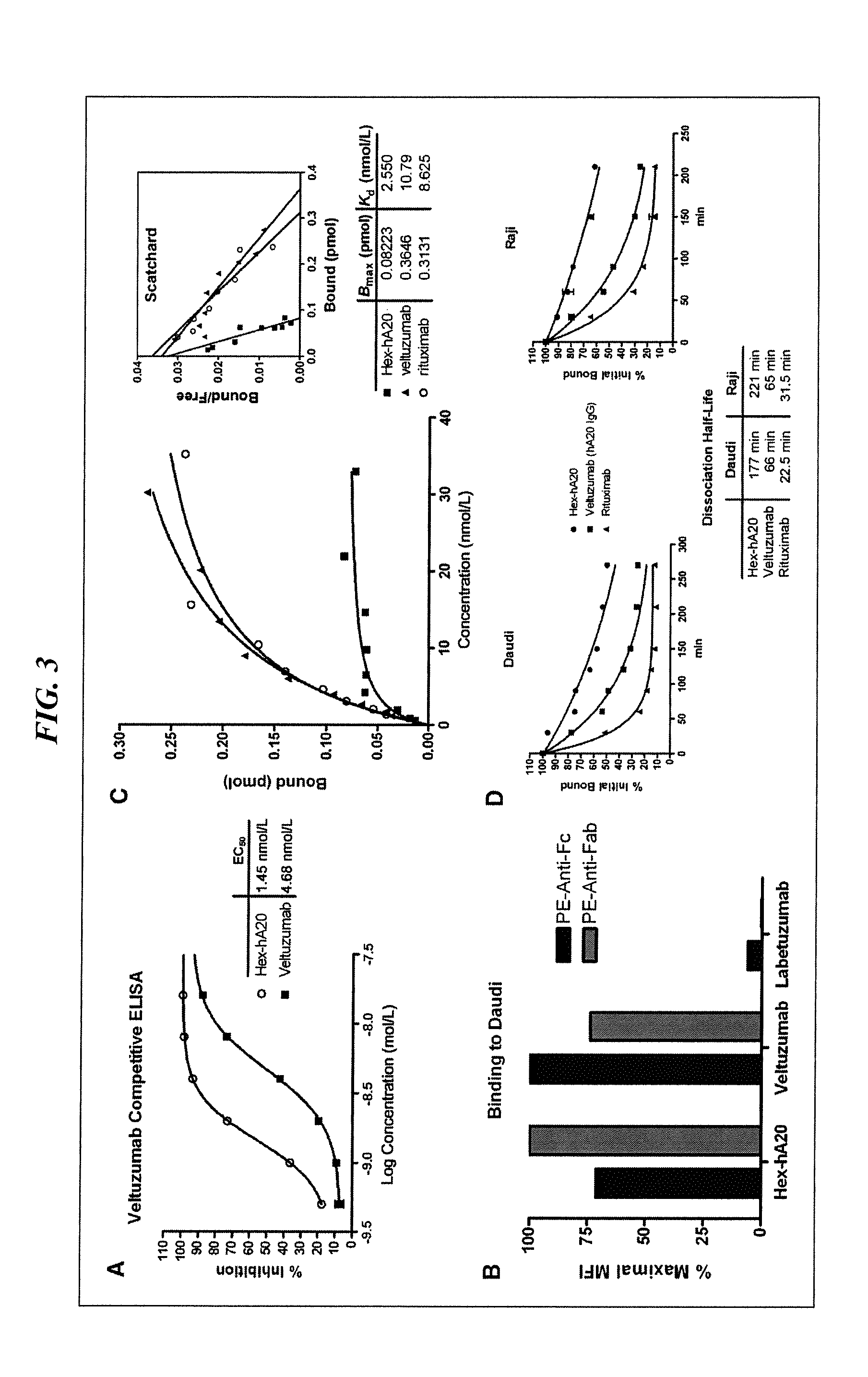
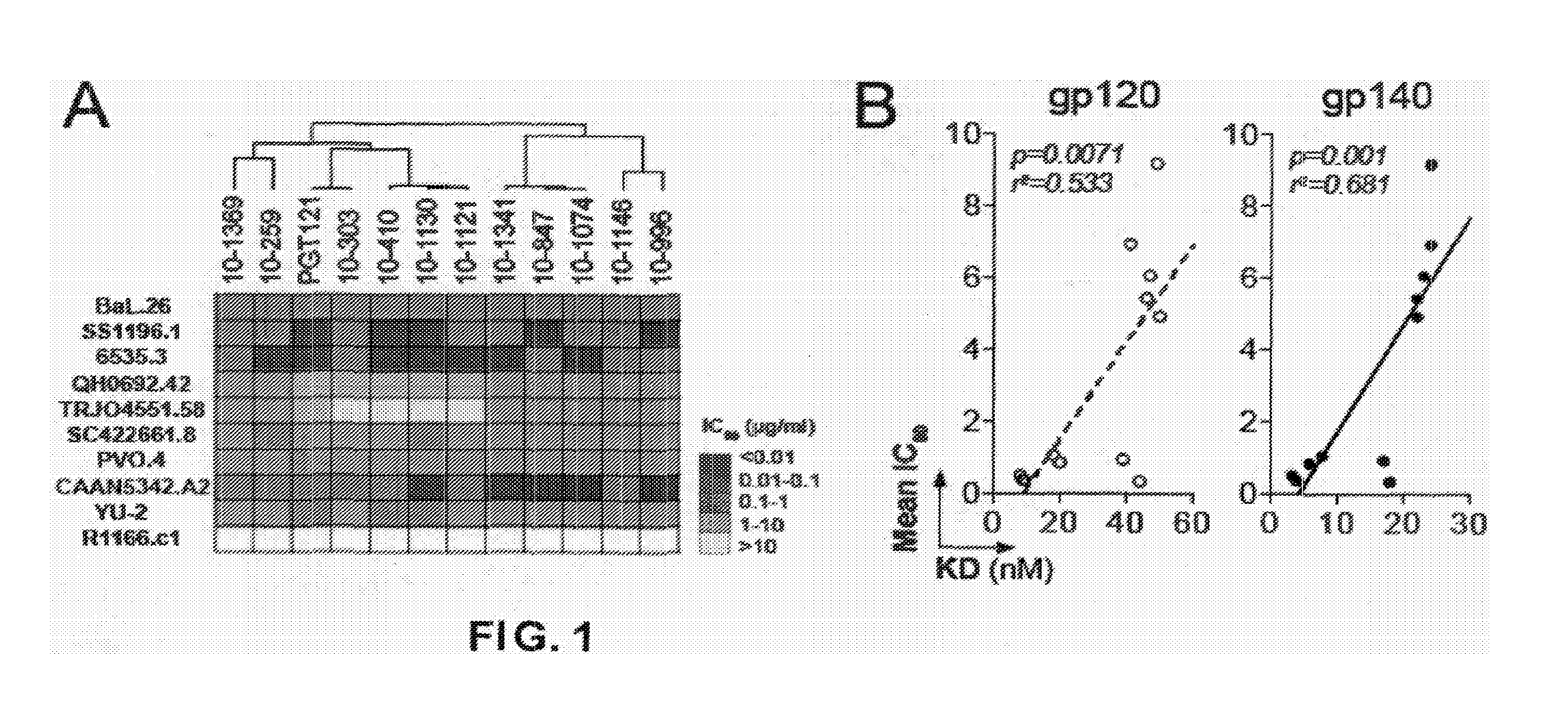
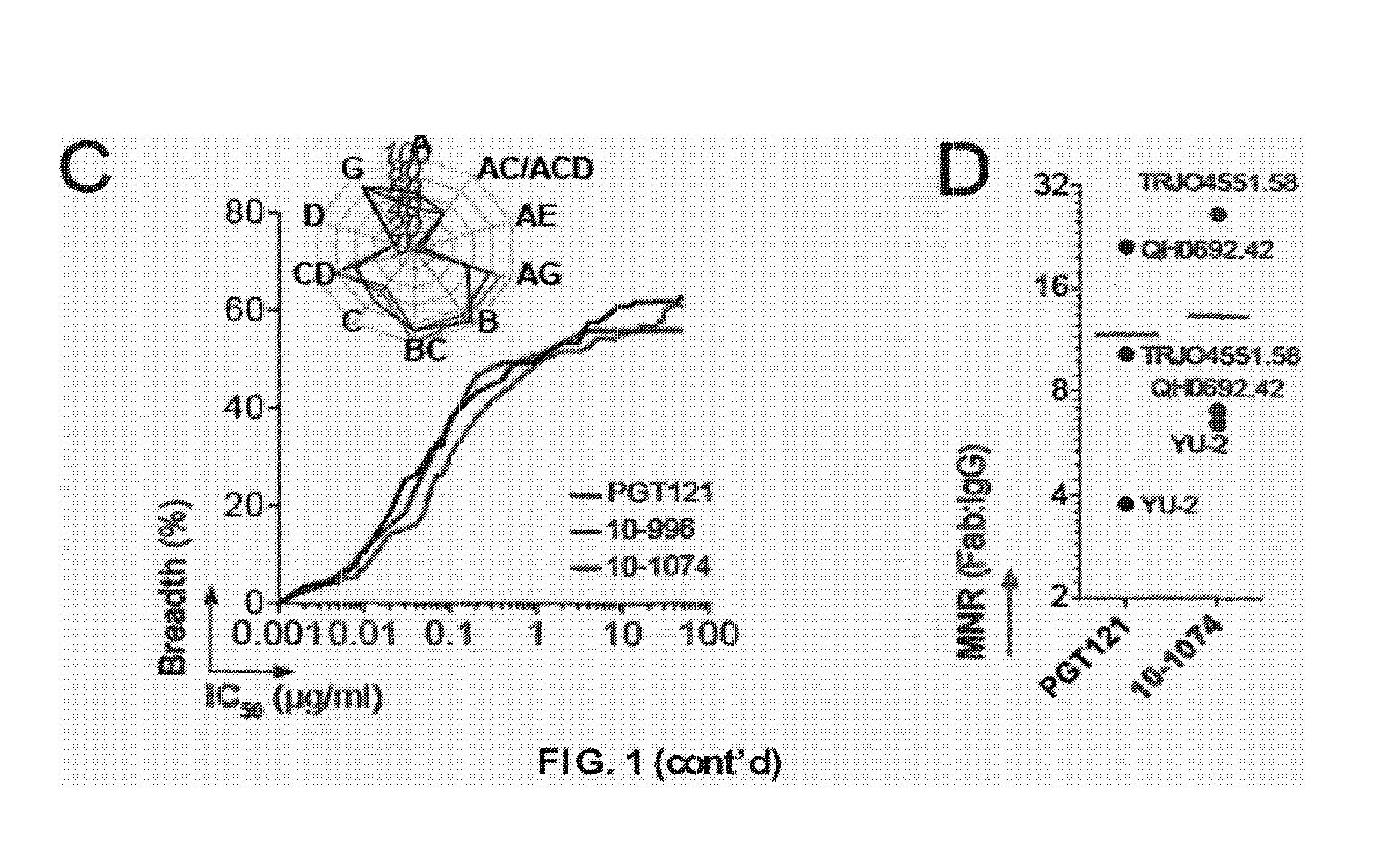
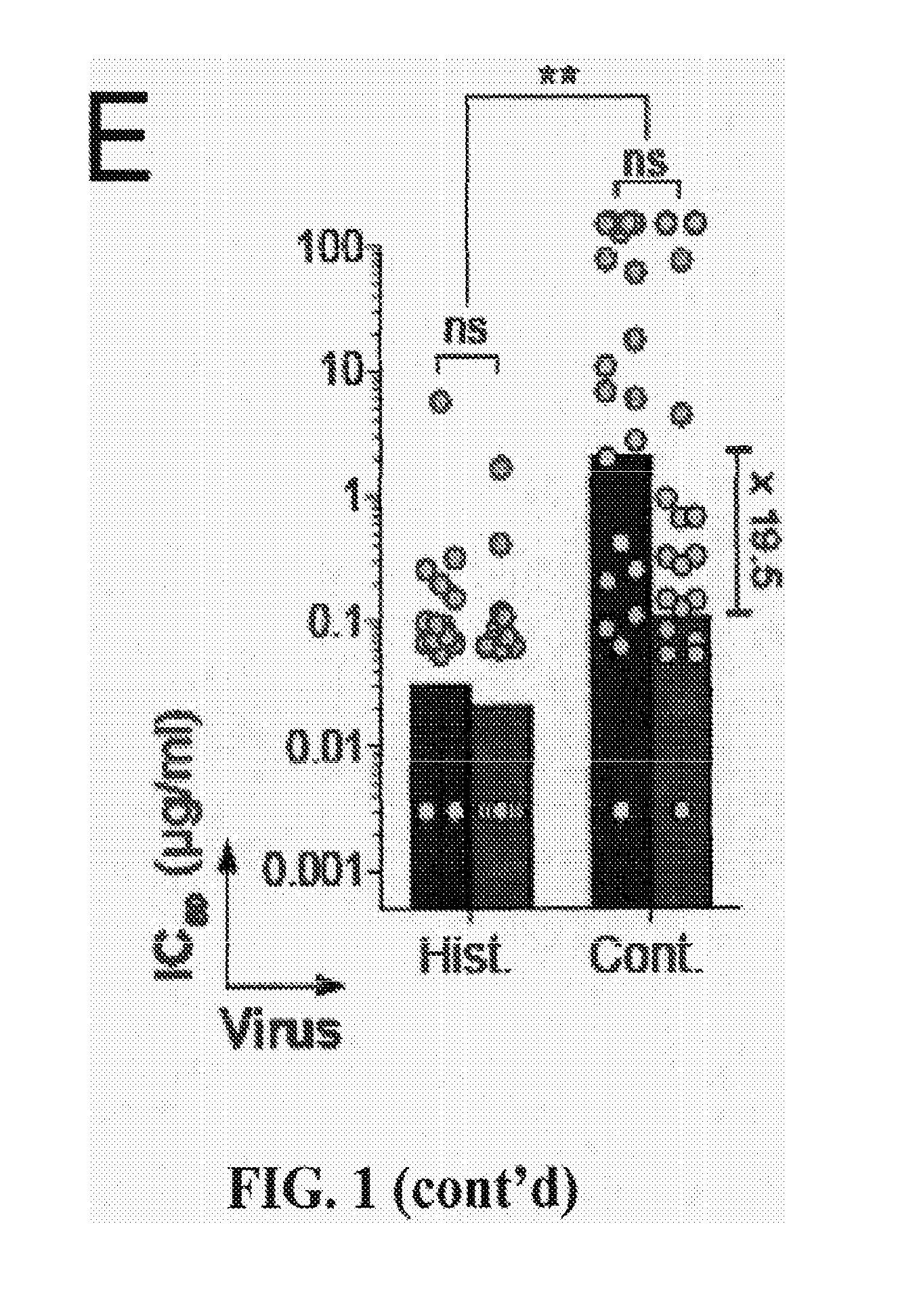
Anti-hiv antibodies having increased potency and breadth
Embodiments of the present invention are directed to compositions and methods for anti-HIV (anti-CD4 binding site) antibodies having improved potency and breadth.
Owner:CALIFORNIA INST OF TECH +1
Methods and compositions for treatment of human immunodeficiency virus infection with conjugated antibodies or antibody fragments
ActiveUS8333971B2Avoid infectionReduce eliminateOrganic active ingredientsAntiviralsDiagnostic agentBinding site
The present invention concerns methods and compositions for treatment of HIV infection in a subject. The compositions may comprise a targeting molecule against an HIV antigen, such as an anti-HIV antibody or antibody fragment. The anti-HIV antibody or fragment may be conjugated to a variety of cytotoxic agents, such as doxorubicin. In a preferred embodiment, the antibody or fragment is P4 / D10. Other embodiments may concern methods of imaging, detection or diagnosis of HIV infection in a subject using an anti-HIV antibody or fragment conjugated to a diagnostic agent. In alternative embodiments, a bispecific antibody with at least one binding site for an HIV antigen and at least one binding site for a carrier molecule may be administered, optionally followed by a clearing agent, followed by administration of a carrier molecule conjugated to a therapeutic agent.
Owner:IMMUNOMEDICS INC
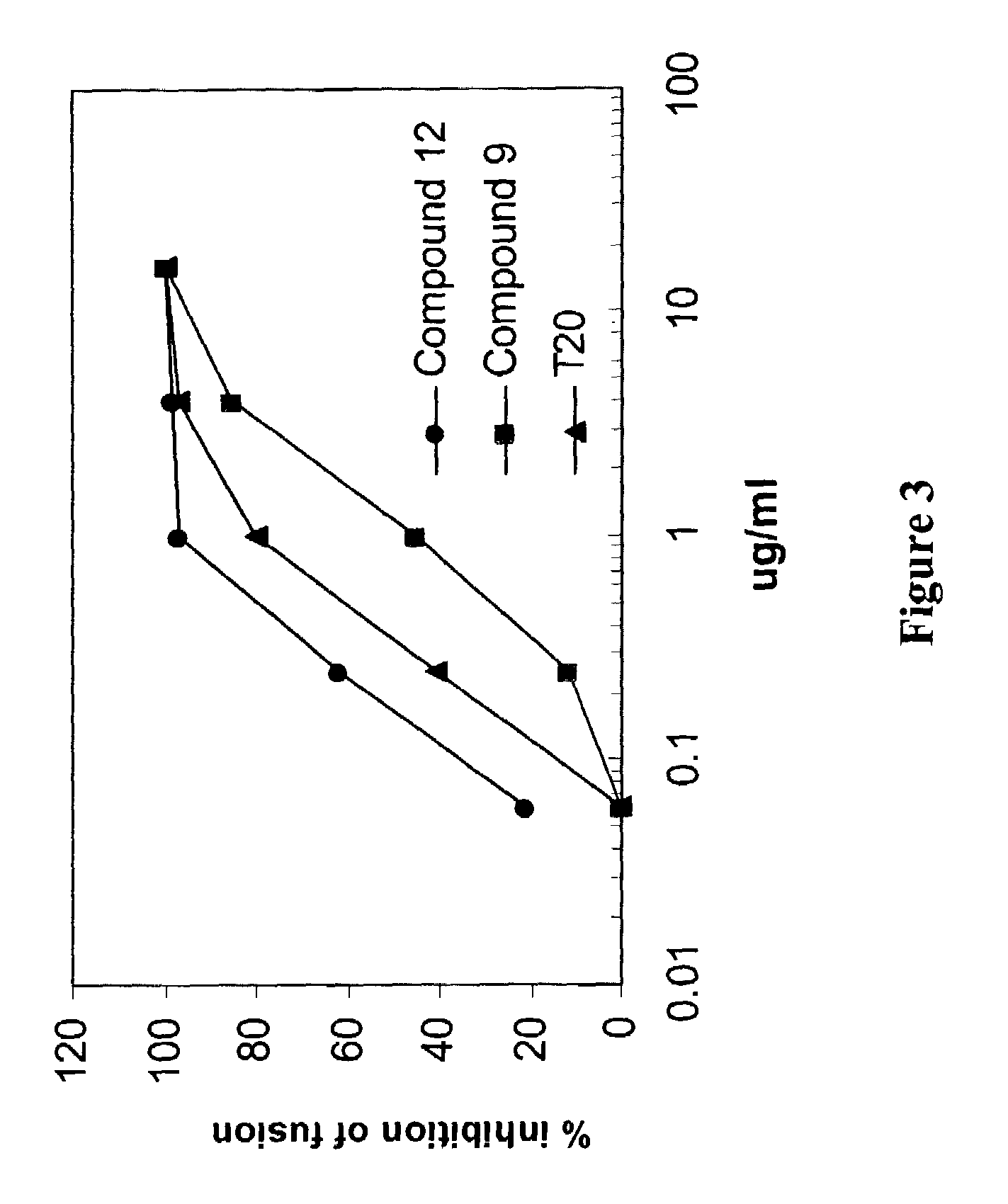
Dock-and-lock (DNL) constructs for human immunodeficiency virus (HIV) therapy
ActiveUS8481041B2Reduce eliminateReduce and eliminate focusOrganic active ingredientsImmunoglobulins against virusesAntigenAntibody fragments
The present invention concerns methods and compositions for treatment of HIV infection in a subject, utilizing a DNL complex comprising at least one anti-HIV therapeutic agent, attached to an antibody, antibody fragment or PEG. In a preferred embodiment, the antibody or fragment binds to an antigen selected from gp120, gp41, CD4 and CCR5. In a more preferred embodiment the antibody is P4 / D10 or 2G12, although other anti-HIV antibodies are known and may be utilized. In a most preferred embodiment, the anti-HIV therapeutic agent is a fusion inhibitor, such as T20, T61, T651, T1249, T2635, CP32M or T-1444, although other anti-HIV therapeutic agents are known and may be utilized. The DNL complex may be administered alone or may be co-administered with one or more additional anti-HIV therapeutic agents.
Owner:IBC PHARMACEUTICALS INC
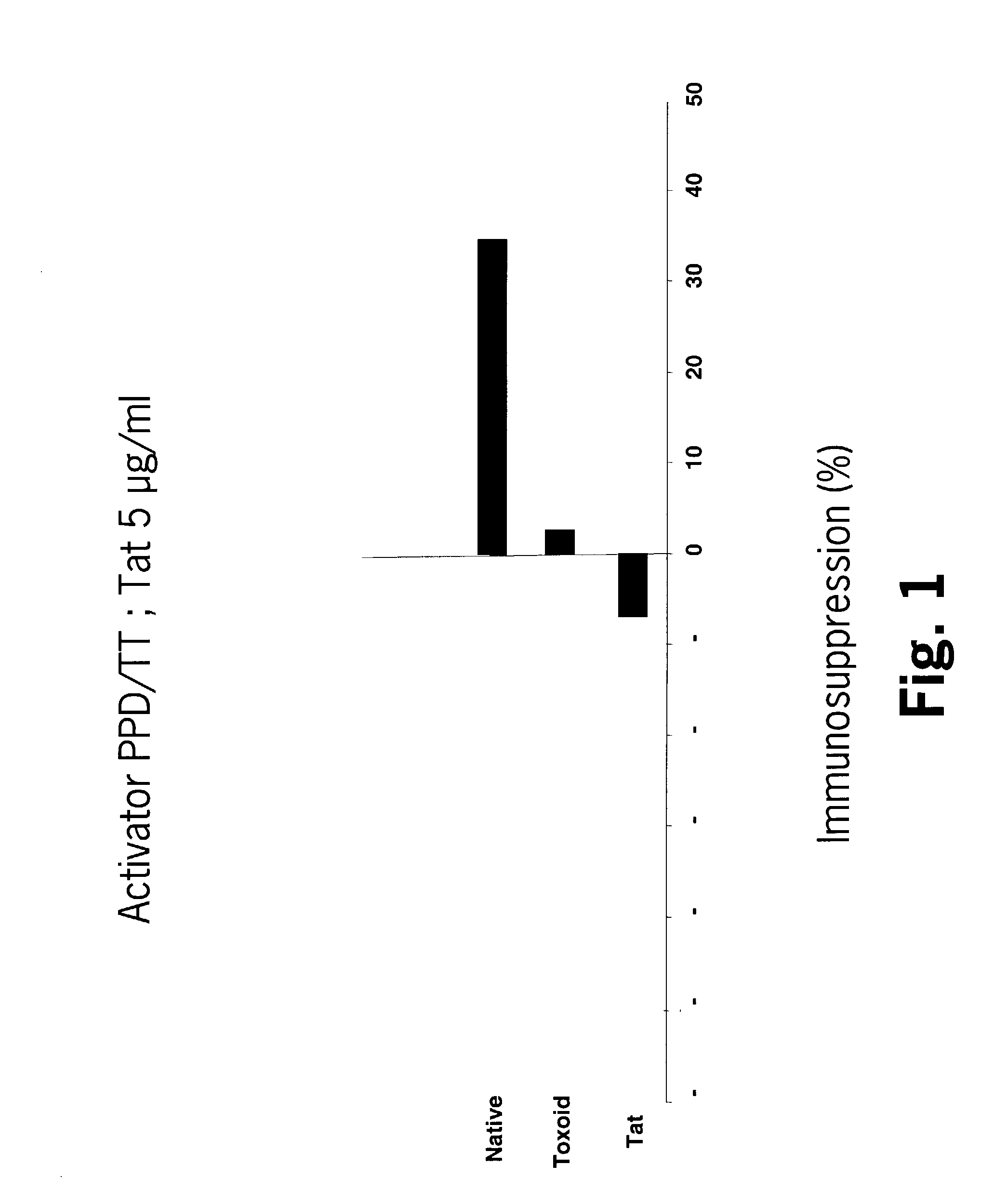
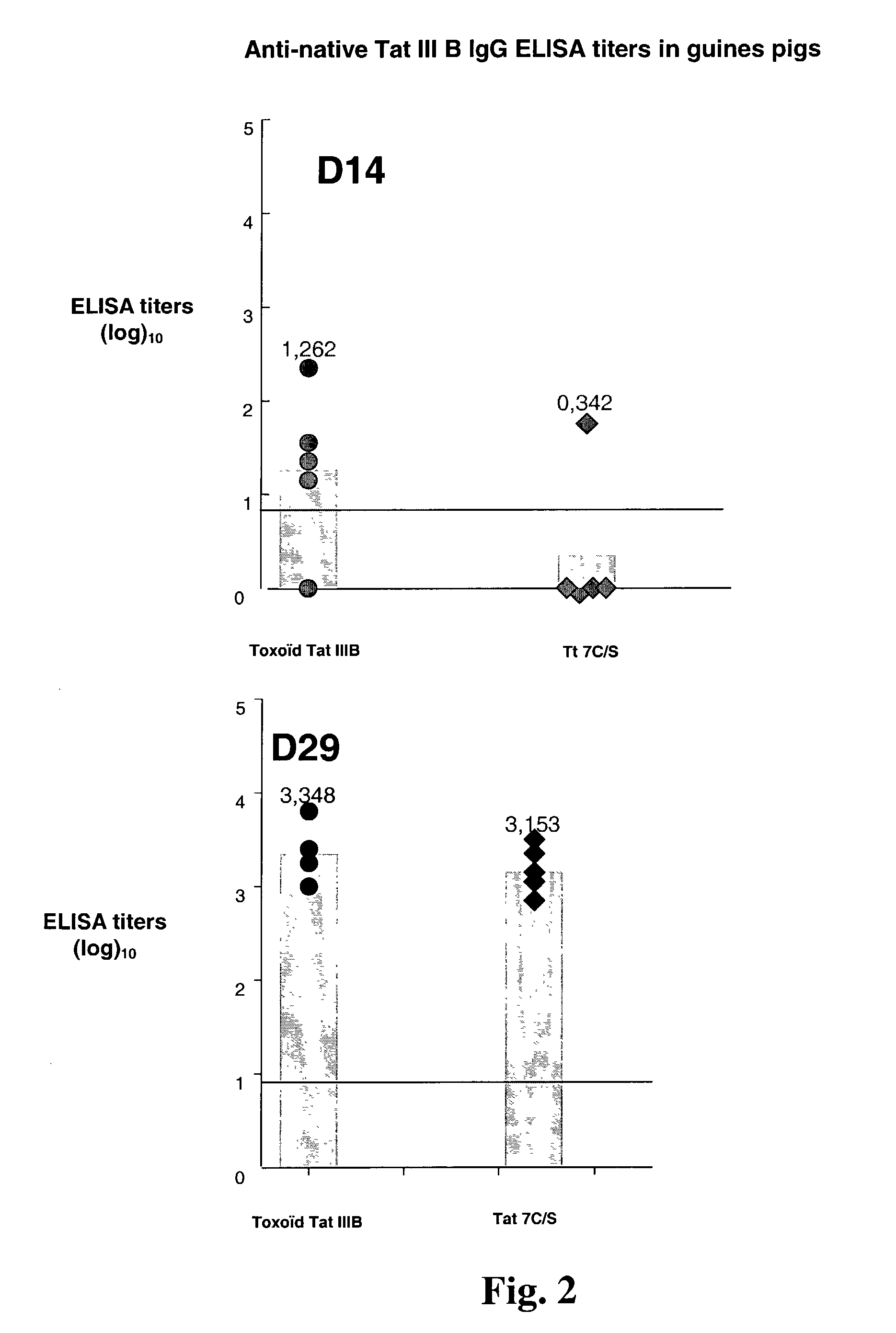
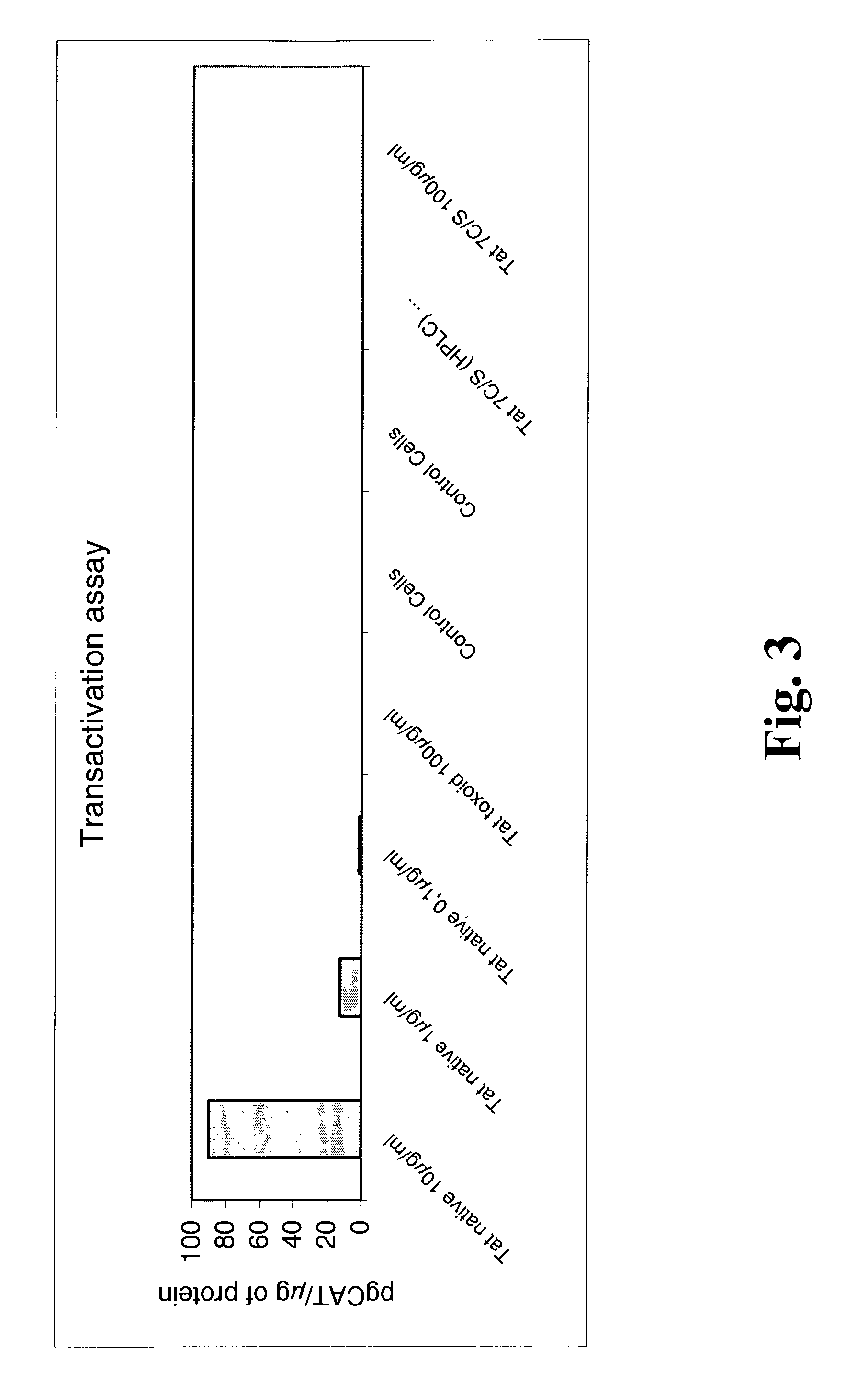
Mutated HIV Tat
The present invention provides a Tat protein wherein all the cysteine residues of the cysteine-rich domain have been replaced with another amino acid, preferably with serine, nucleic acids encoding it, and methods of using it to elicit a humoral and cellular immune responses in a mammal. The Tat protein of the invention is therefore useful, inter alia, for prophylactic and / or therapeutic anti-HIV use as well as raising anti-native Tat antibodies in mammals.
Owner:AVENTIS PASTUER LTD
Nitrogen-containing fused ring compound and use thereof as HIV integrase inhibitor
ActiveUS20060052361A1Effective anti-HIV agentStrong inhibitory activityBiocideOrganic chemistryAnti-HIV AgentSide effect
Owner:JAPAN TOBACCO INC
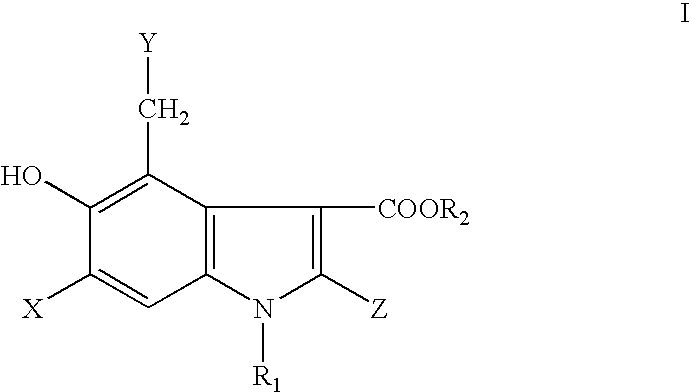
5-Hydroxyindole-3-Carboxylate Derivatives and Uses Thereof
InactiveUS20080249155A1Treatment and/or prophylaxisBiocideOrganic chemistry methodsImmunodeficiency virusMedicine
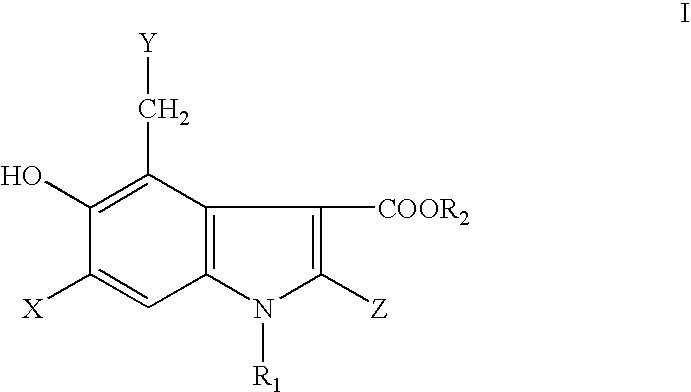
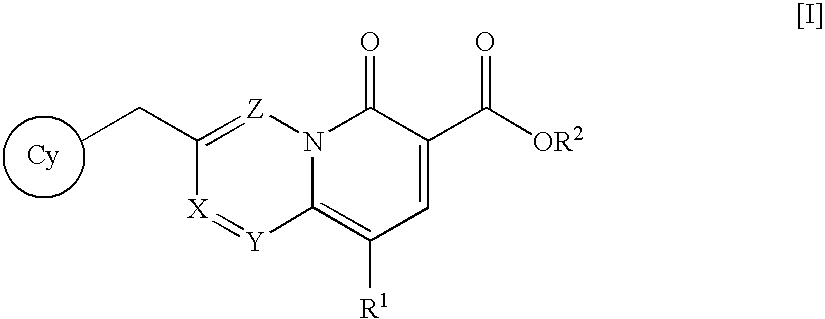
The present invention relates to 5-hydroxy-indole-3-carboxylate derivatives of formula I, or racemic mixture or optical isomers or pharmaceutically acceptable salts and / or hydrates thereof,wherein: substituents R1, R2, Z, X and Y are as defined in the description. The compounds of formula I can be useful for preparation of medicament for treatment and / or prophylaxis of virus infections, especially for preparation of medicament for anti-HBV (Hepatitis B virus) and anti-HIV (Human immunodeficiency virus).
Owner:SHENYANG PHARMA UNIVERSITY
Vaccines and methods for using the same
ActiveUS20100166787A1SsRNA viruses negative-senseOrganic active ingredientsRecombinant vaccinesBioinformatics
Improved anti-HIV immunogens and nucleic acid molecules that encode them are disclosed, Immunogens disclosed include those having consensus sequences for HIV Subtype A Envelope protein, those having consensus sequences for HIV Subtype B Envelope protein, those having consensus sequences for HIV Subtype C Envelope protein, those having consensus sequences for HIV Subtype D Envelope protein, those having consensus sequences for HIV Subtype B consensus Nef-Rev protein, and those having consensus sequences form HIV Gag protein subtypes A, B, C and D. Improved anti-HPV immunogens and nucleic acid molecules that encode them; improved anti-HCV immunogens and nucleic acid molecules that encode them; improved hTERT immunogens and nucleic acid molecules that encode them; and improved anti-Influenza immunogens and nucleic acid molecules that encode them are disclosed. Pharmaceutical composition, recombinant vaccines comprising and live attenuated pathogens are disclosed as well methods of inducing an immune response in an individual against HIV, HPV, HCV, hTERT and Influenza are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Immunoconjugates with improved efficacy for the treatment of diseases
InactiveUS20070196274A1Improve therapeutic efficacyHigh detection sensitivityIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntibody conjugateCD11a
The invention provides therapeutic or diagnostic antibodies with modified N— or C-terminal sequences that are enriched with lysine or tyrosine residues. These lysine or tyfosine residues can be used to couple radioisotopes, cytotoxic agents, or detectable labels. The increased stoichiometric ratios of these agents in the antibody conjugates lead to improved therapeutic efficacy or enhanced detection sensitivity. Non-limiting examples of antibodies suitable for the present invention include anti-CD22, anti-ErbB2, anti-VEGF, anti-EGFR, anti-VEGFR, anti-Her-3, anti-Her-4, anti-CEA, anti-CTLA-4, anti-CD4, anti-CD3, anti-CD20, anti-TNF-a, anti-CD11a, anti-Lewis Y antigen, anti-TrailR, anti-IL2R, anti-CD30, anti-CD146, anti-CD147, anti-alpha V integrin beta, anti-CD19, anti-GD2, anti-3H11, anti-EBV, anti-HIV, anti-HBV, anti-HCV, and other disease-specific antibodies.
Owner:WELSON PHARMA
Combination therapy
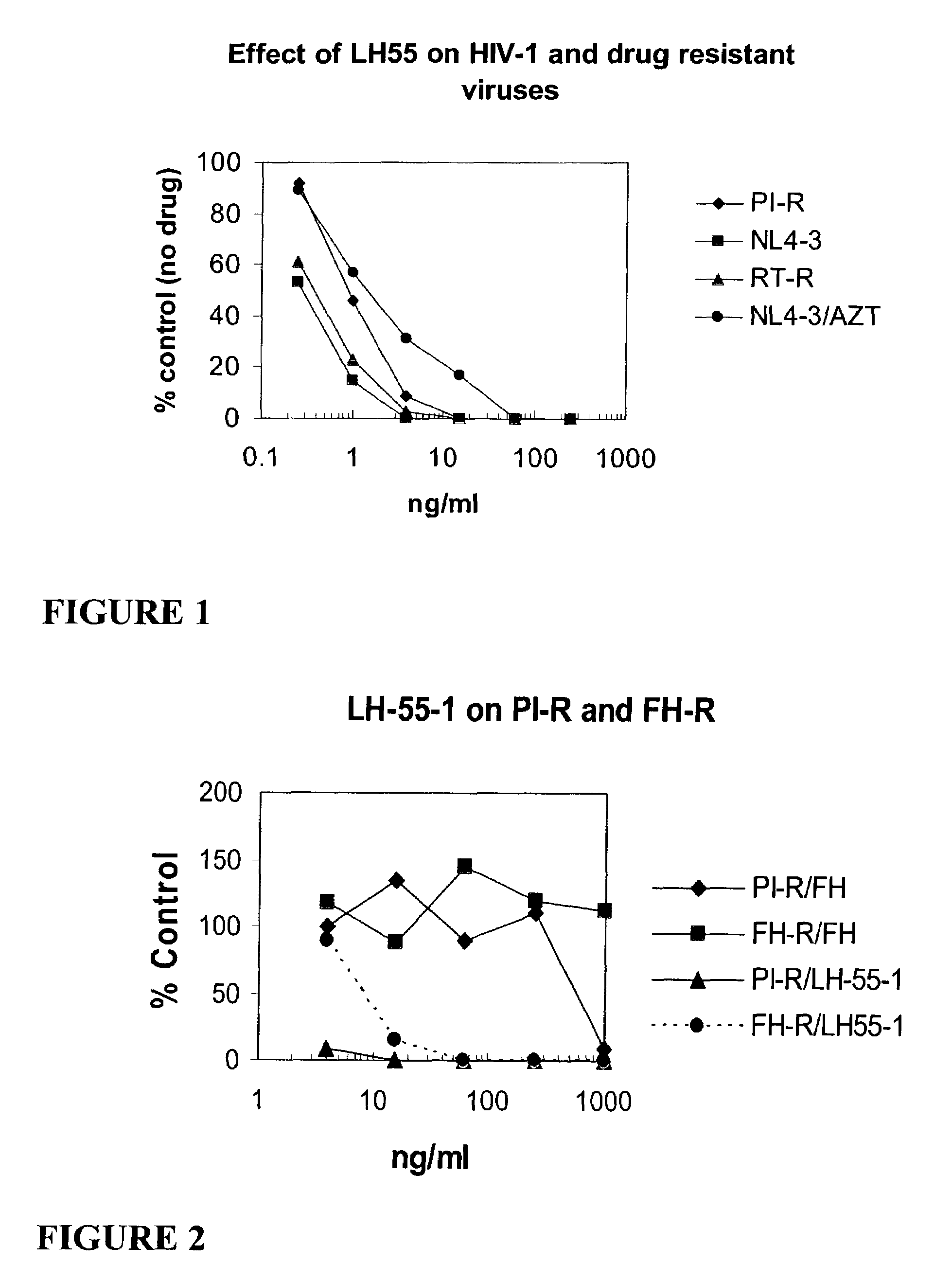
ActiveUS20050288326A1Low effective doseLow cytotoxicityBiocideAntiviralsSide effectReverse transcriptase
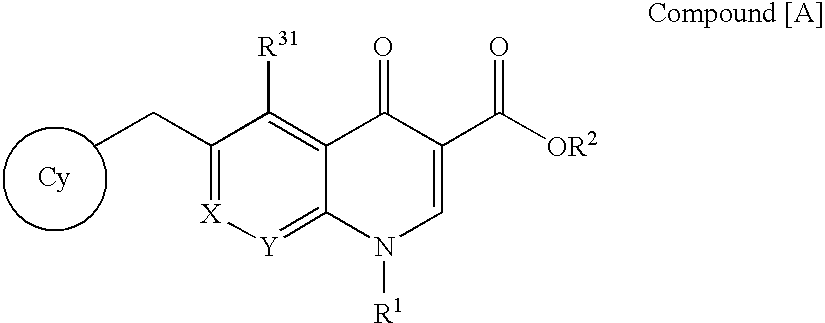
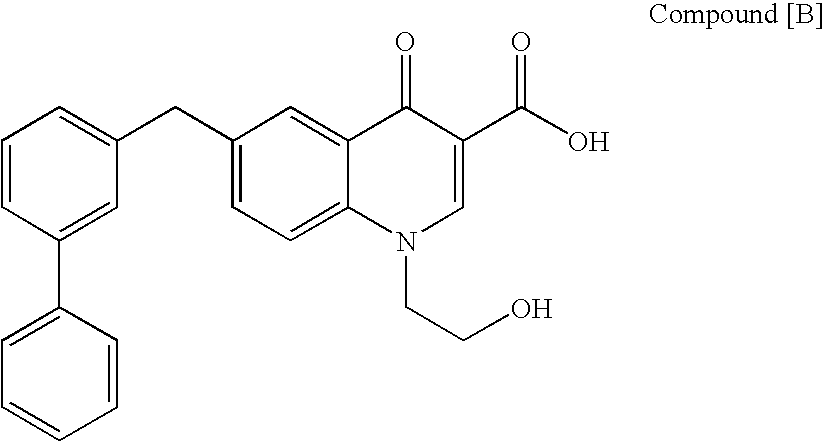
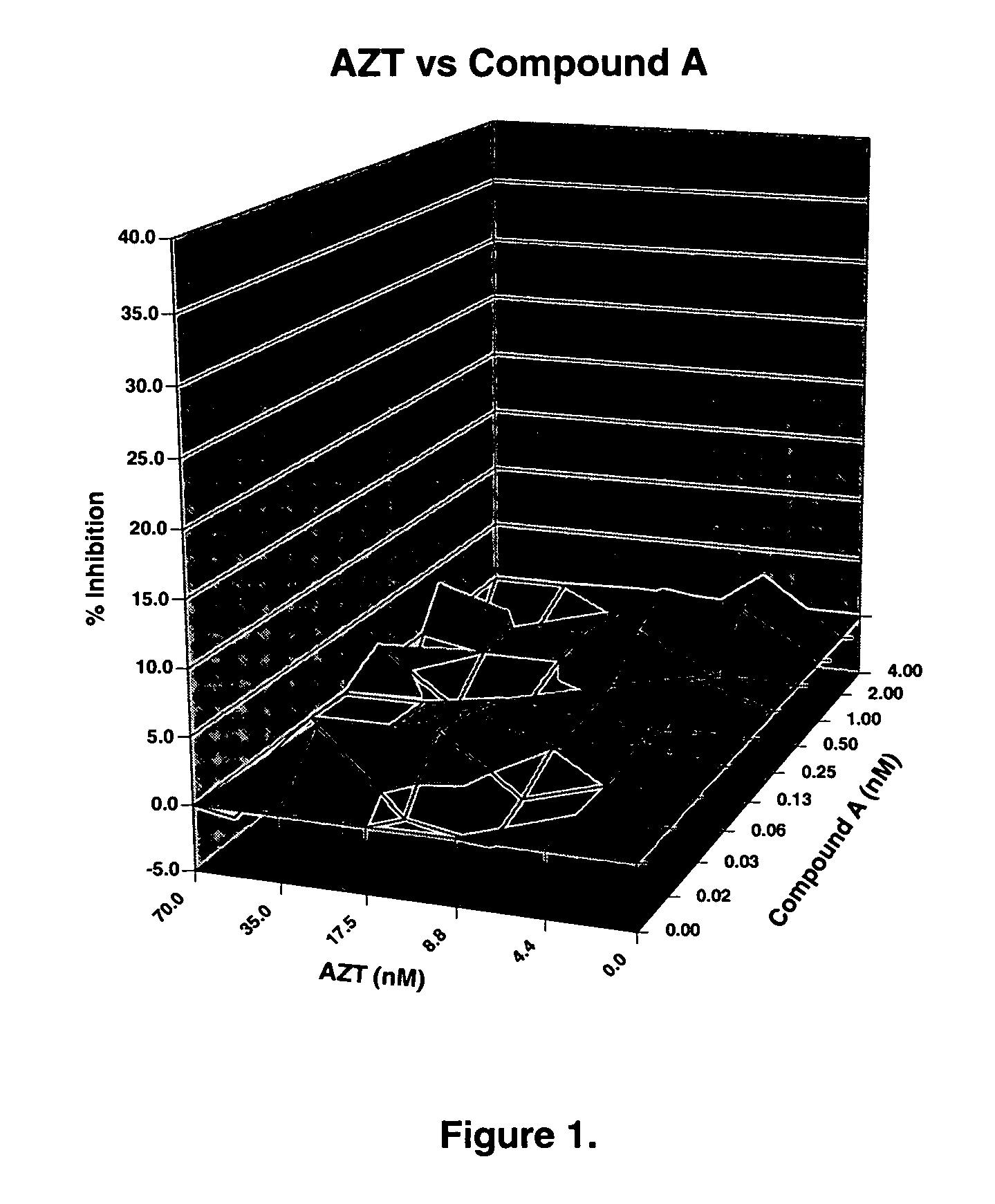
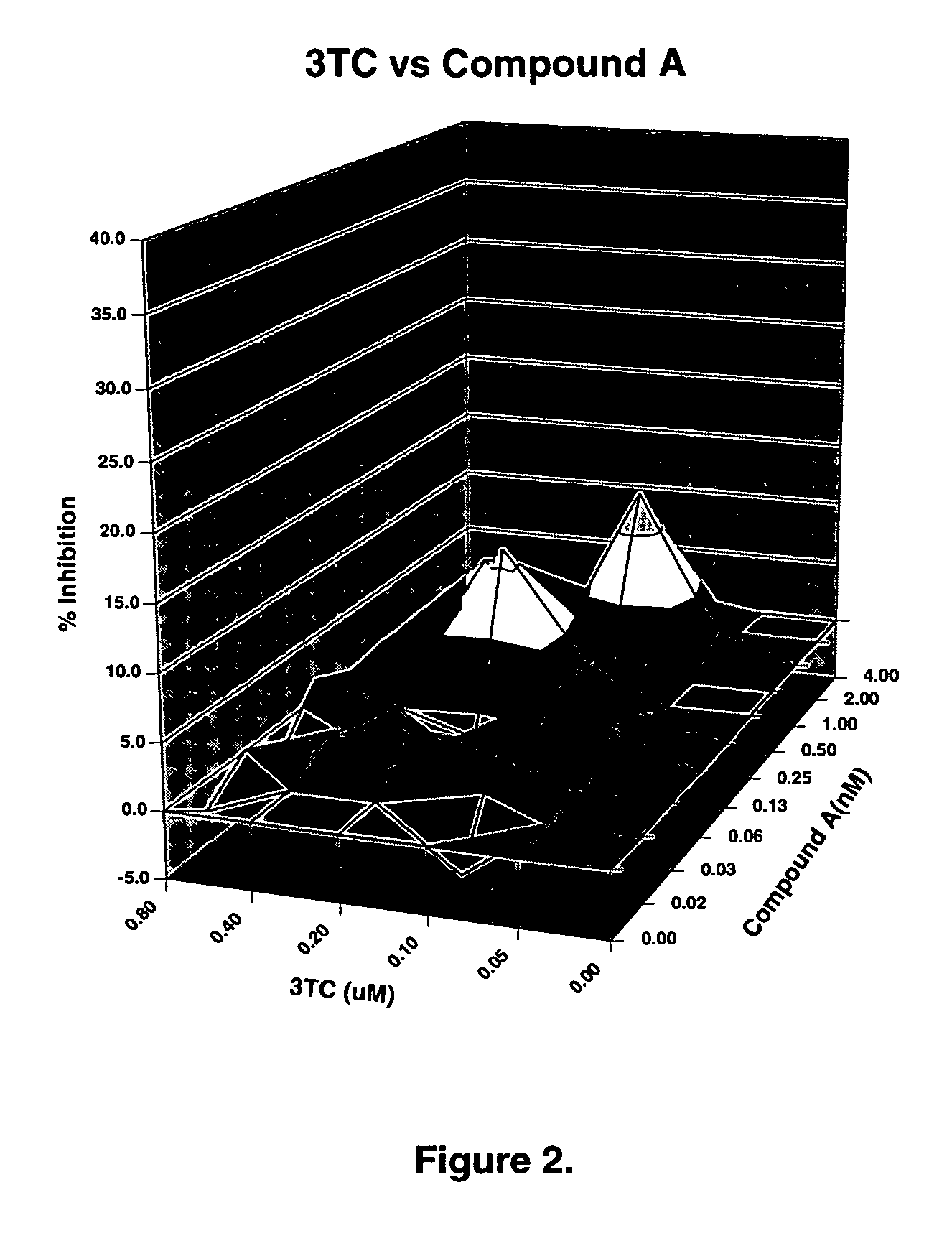
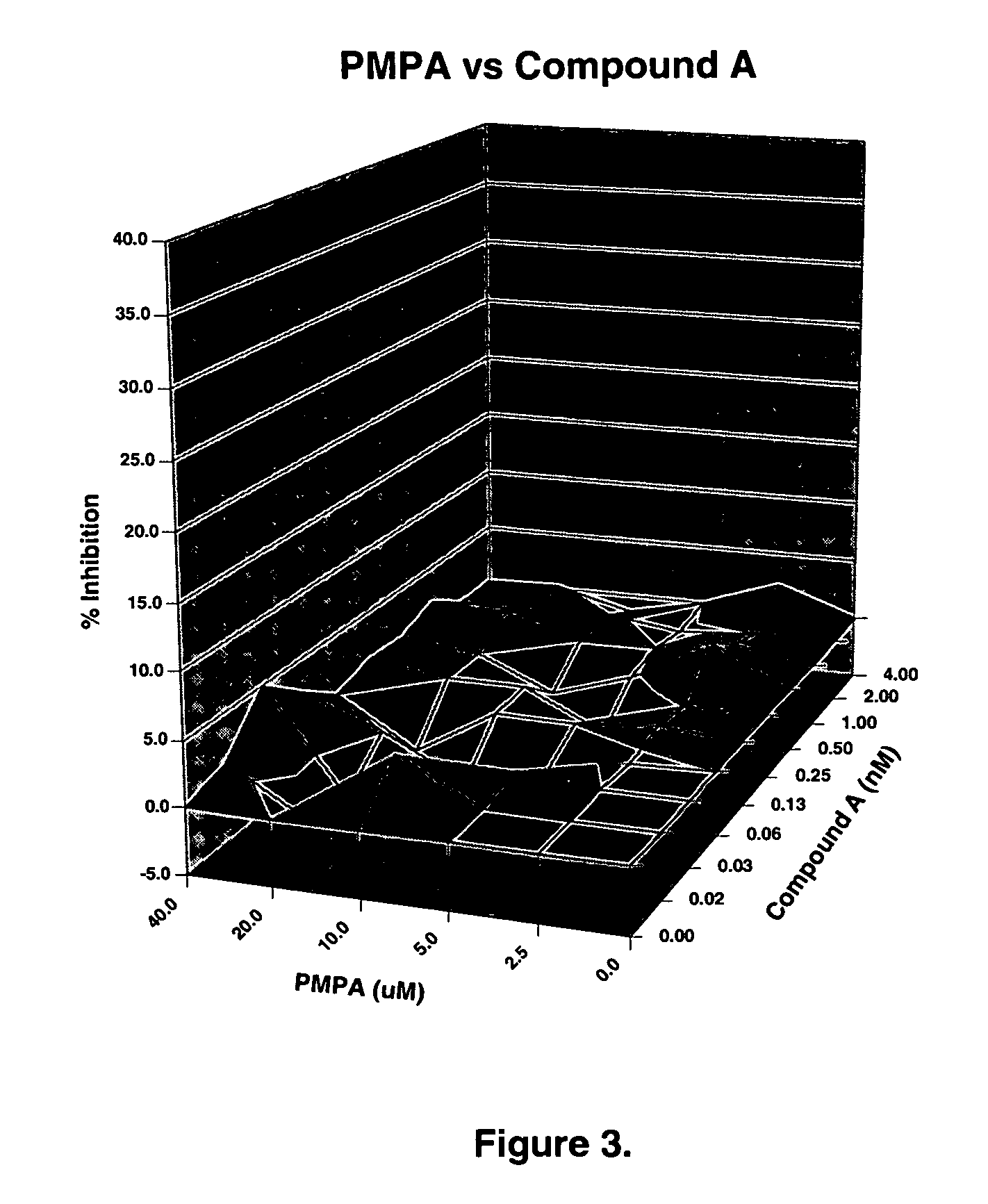
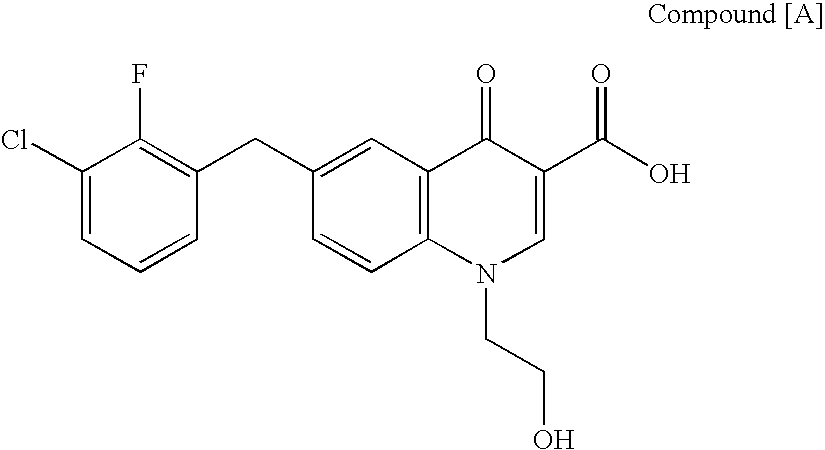
The present invention relates to a combination therapy for treating an HIV infection or inhibiting integrase comprising (S)-6-(3-Chloro-2-fluorobenzyl)-1-(1-hydroxymethyl-2-methylpropyl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (“Compound A”) or a pharmaceutically acceptable solvate or salt thereof in combination with at least one other anti-HIV agent. In some embodiments of the present invention, the other anti-HIV agents are chosen from reverse transcriptase inhibitors and protease inhibitors. In certain embodiments of the present invention, the other anti-HIV agents are chosen from AZT, 3TC, PMPA, efavirenz, indinavir, nelfinavir, a combination of AZT / 3TC, and a combination of PMPA / 3TC. Since Compound A has a high inhibitory activity specific for integrases, when used in combinations with other anti-HIV agents it can provide a combination therapy with fewer side effects for humans.
Owner:JAPAN TOBACCO INC
Human immunodeficiency virus (HIV)-neutralizing antibodies
ActiveUS20110044994A1Sugar derivativesImmunoglobulins against virusesVaccinationImmunodeficiency virus
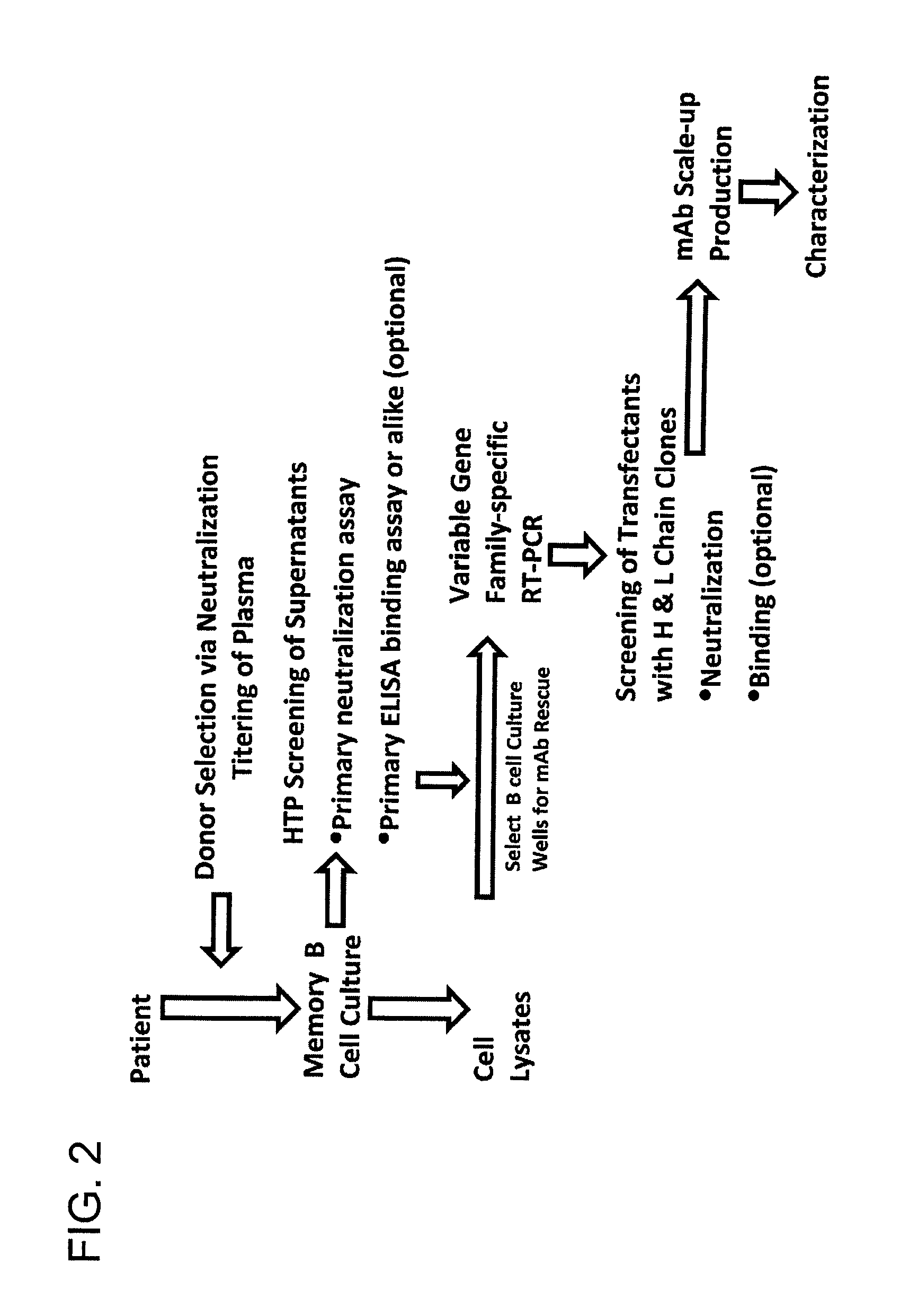
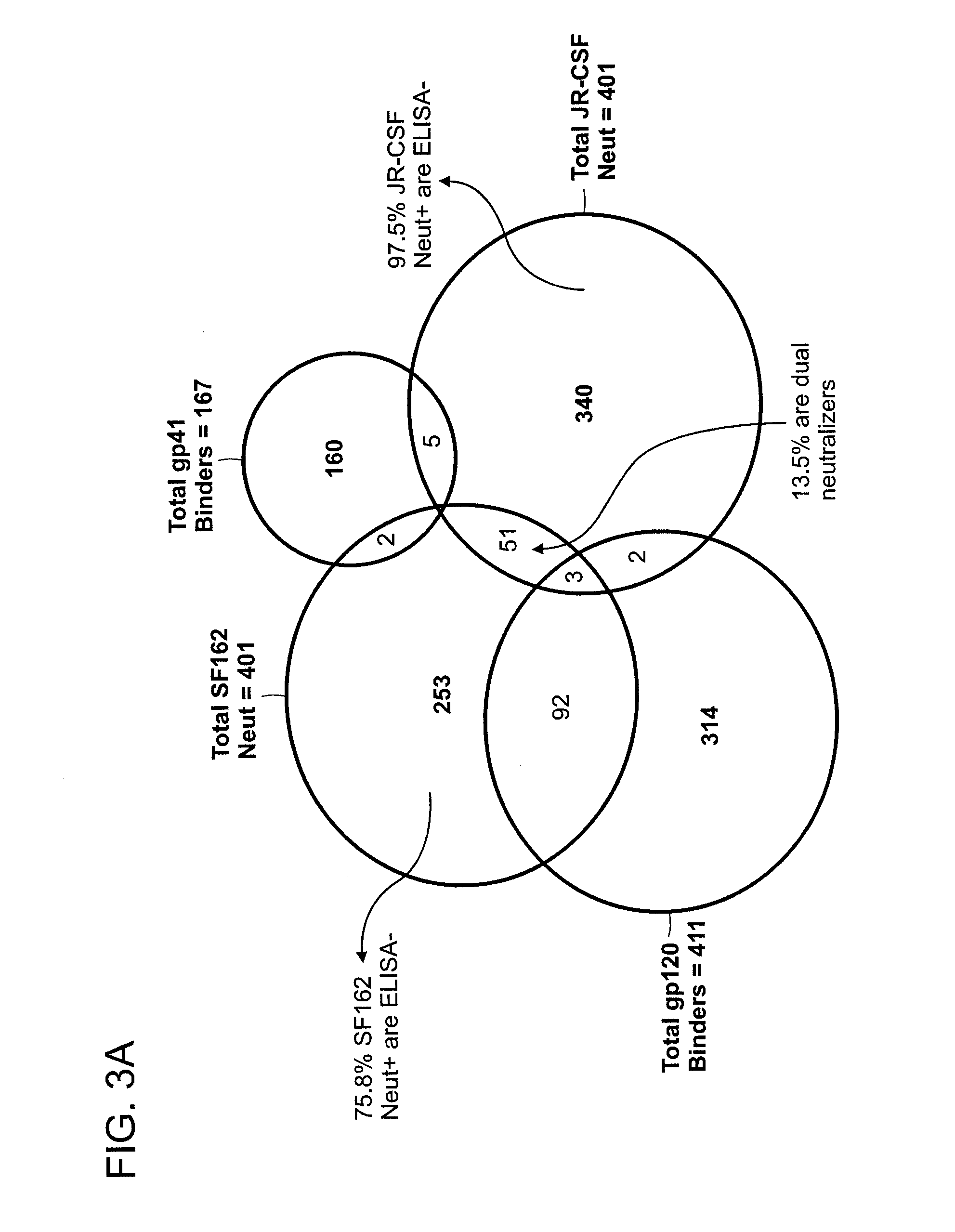
The invention provides a method for obtaining a broadly neutralizing antibody (bNab), including screening memory B cell cultures from a donor PBMC sample for neutralization activity against a plurality of HIV-1 species, cloning a memory B cell that exhibits broad neutralization activity; and rescuing a monoclonal antibody from that memory B cell culture. The resultant monoclonal antibodies are characterized by their ability to selectively bind epitopes from the Env proteins in native or monomeric form, as well as to inhibit infection of HIV-1 species from a plurality of clades. Compositions containing human monoclonal anti-HIV antibodies used for prophylaxis, diagnosis and treatment of HIV infection are provided. Methods for generating such antibodies by immunization using epitopes from conserved regions within the variable loops of gp120 are provided. Immunogens for generating anti-HIV1 bNAbs are also provided. Furthermore, methods for vaccination using suitable epitopes are provided.
Owner:THE SCRIPPS RES INST +2
6- (Heterocyclyl-substituted Benzyl) -4-Oxoquinoline Compound and Use Thereof as HIV Integrase Inhibitor
InactiveUS20080207618A1Strong inhibitory activityEffective anti-HIV agentsBiocideOrganic chemistryAnti-HIV AgentSide effect
The present invention relates to a compound represented by the following formula [I]wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof, or a solvate thereof, and a pharmaceutical composition, an anti-HIV agent and an HIV integrase inhibitor containing such compound. The compound of the present invention has an HIV integrase inhibitory activity, and is useful as an anti-HIV agent, or as an agent for the prophylaxis or treatment of AIDS. In addition, by the combined use with other anti-HIV agents such as a protease inhibitor, a reverse transcriptase inhibitor and the like, it can be a more effective anti-HIV agent. Because it shows integrase-specific high inhibitory activity, the compound can be a pharmaceutical agent safe on human body, which causes only a fewer side effects.
Owner:JAPAN TOBACCO INC
Combination therapy
ActiveUS8633219B2Low effective doseEffective treatmentBiocideOrganic chemistrySide effectCombined Modality Therapy
Owner:JAPAN TOBACCO INC
Immunogen comprising an HIV envelope protein, a ligand and H2 peptide
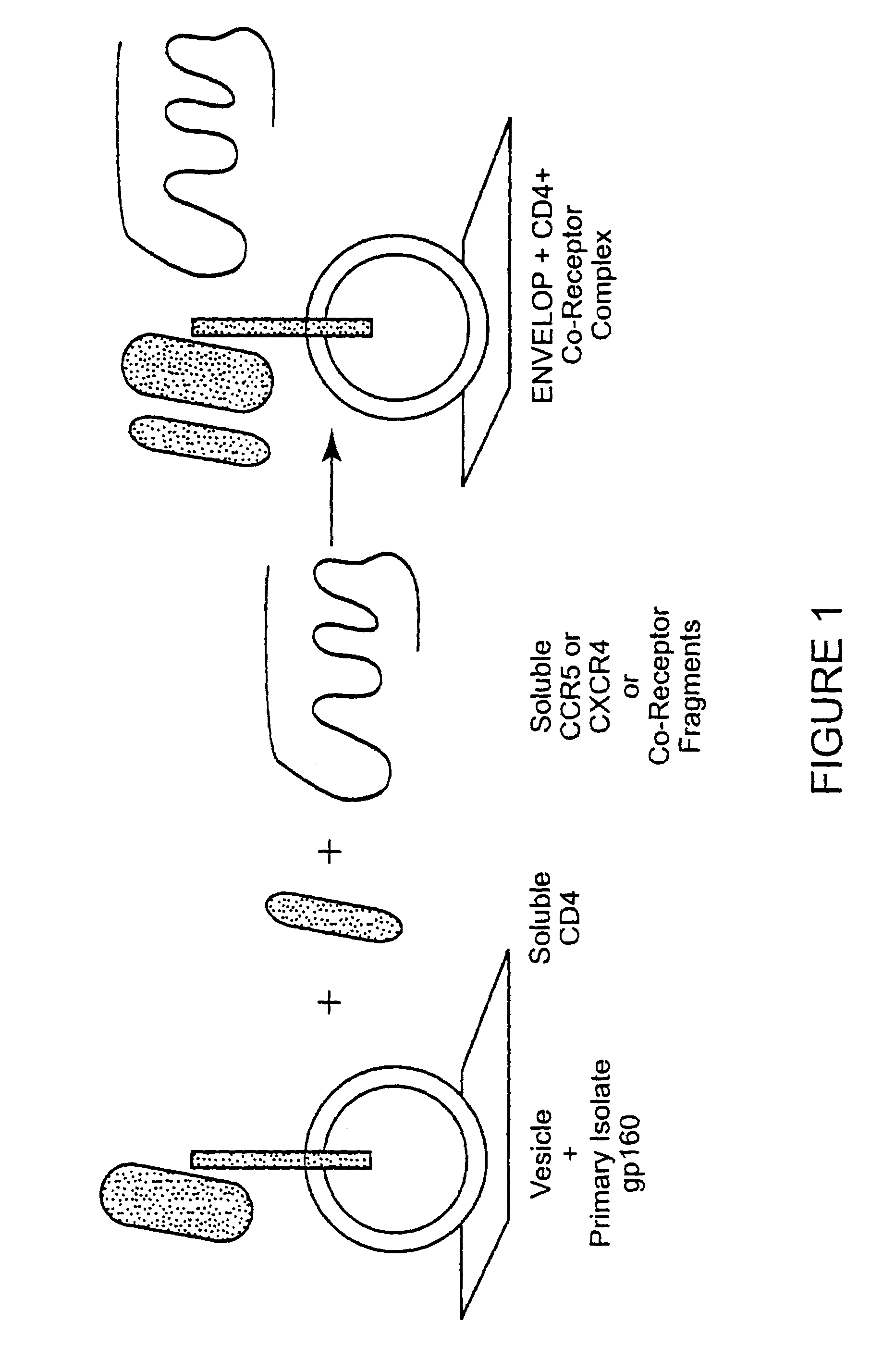
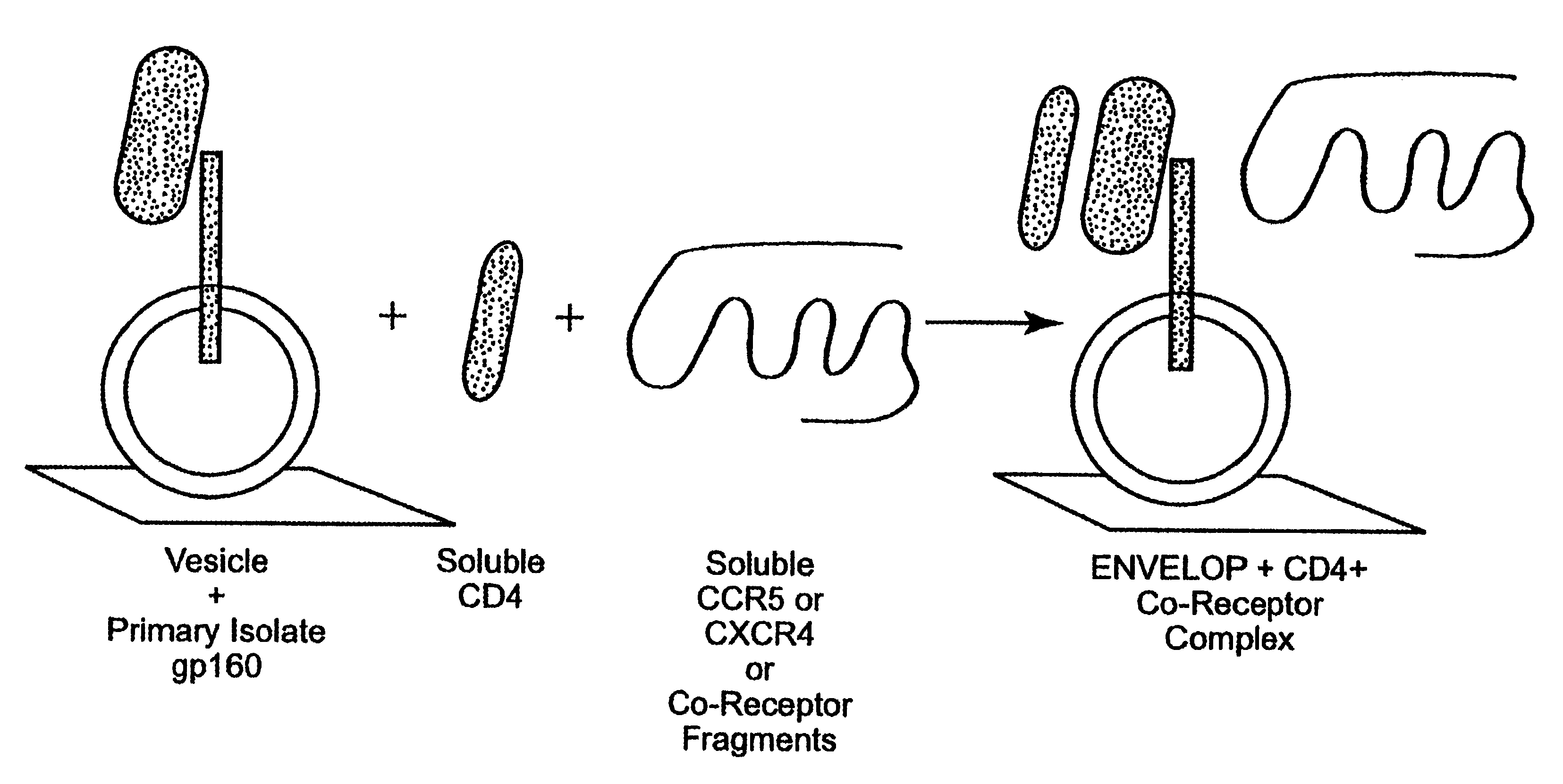
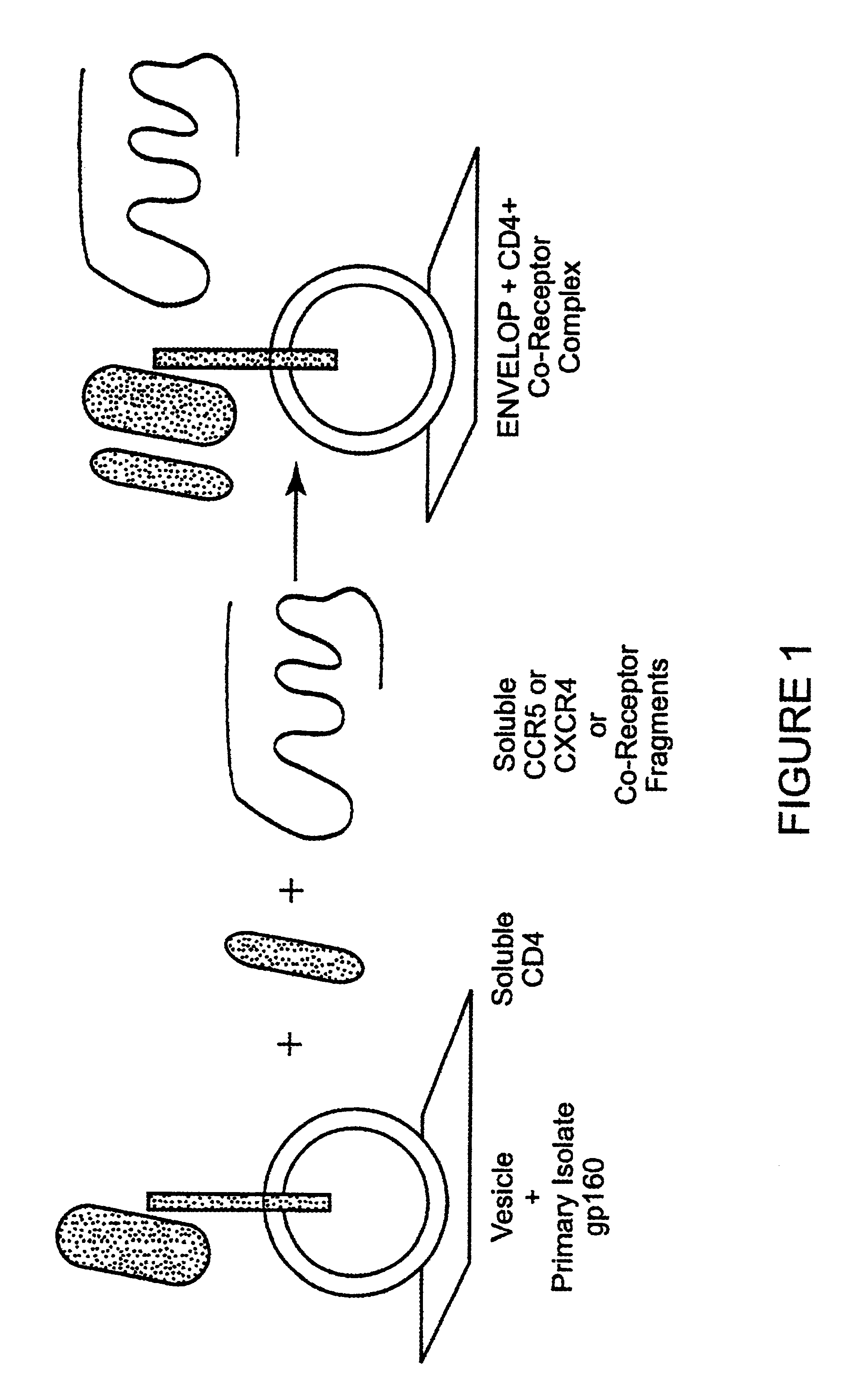
The invention relates to an immunogen comprising an HIV envelope protein bound to a ligand, which ligand upregulates at least one of the CD4 binding site and the CCR5 binding site on the protein, and bound to an HR-2 peptide. The invention also relates to a method of inducing anti-HIV antibodies using such an immunogen.
Owner:DUKE UNIV
Anti-HIV agents with dual sites of action
Owner:MEHARRY MEDICAL COLLEGE
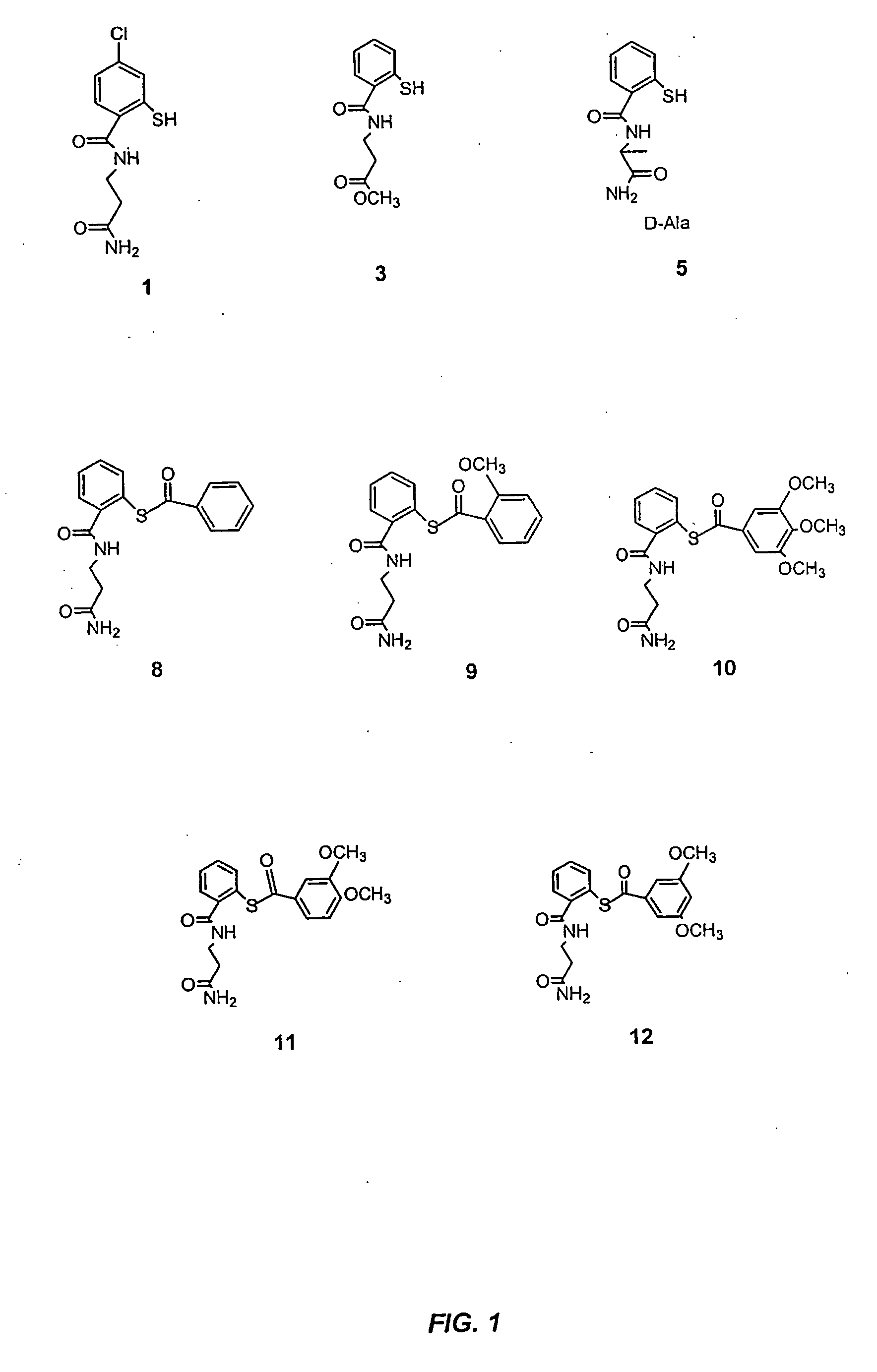
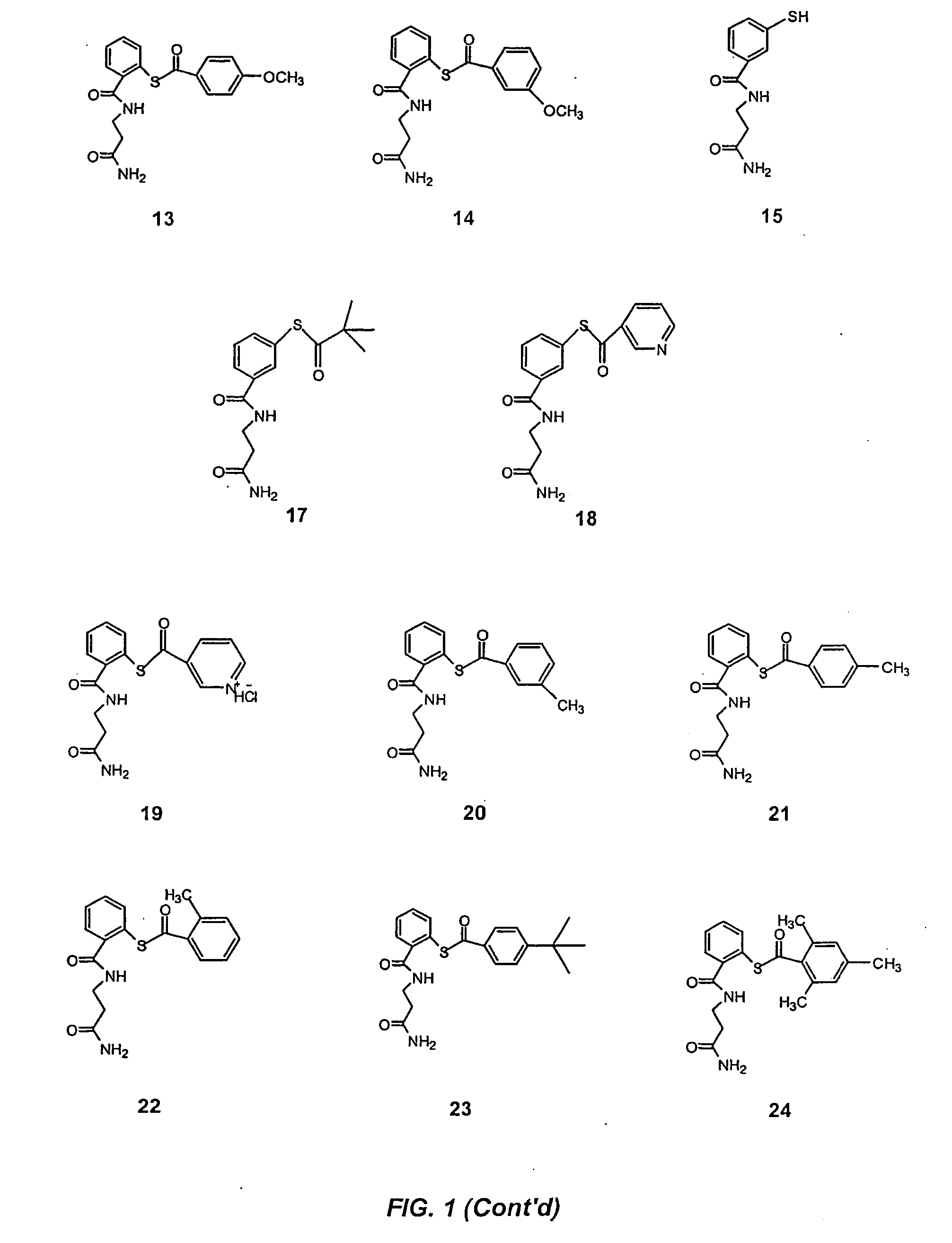
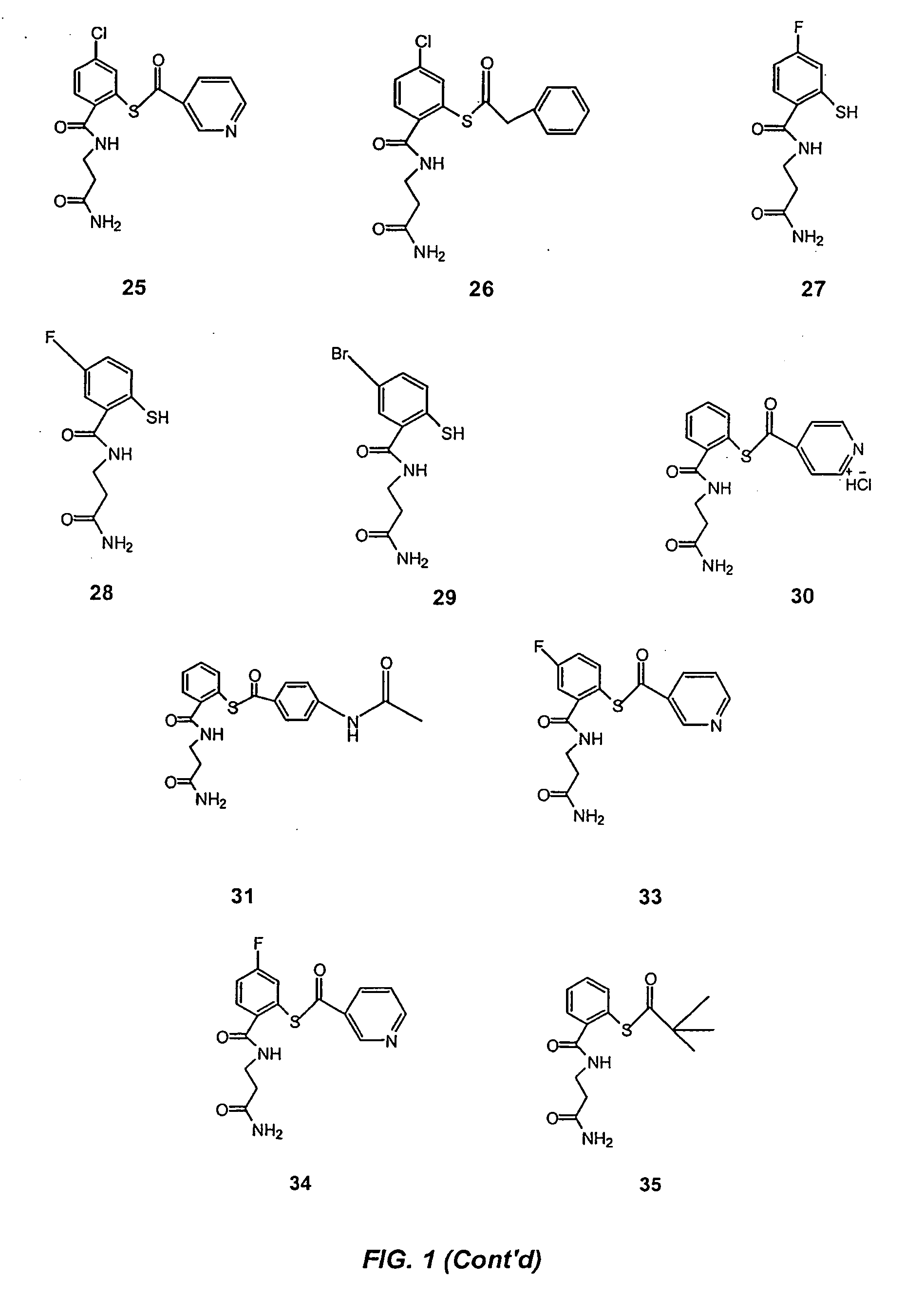
Acylthiols and component thiol compositions as anti-hiv and anti-retroviral agents
Certain thiol and acylthiol compounds inhibit retrovirus growth by attacking the highly conserved zinc finger regions of essential viral proteins. These compounds, compositions containing them, and methods of using them to treat retroviral infections such as HIV are described. These compounds are also useful for preparation of vaccines comprised of inactivated retroviruses such as HIV, prevention of the transmission of such retroviruses, and detection of retroviral proteins.
Owner:UNITED STATES OF AMERICA
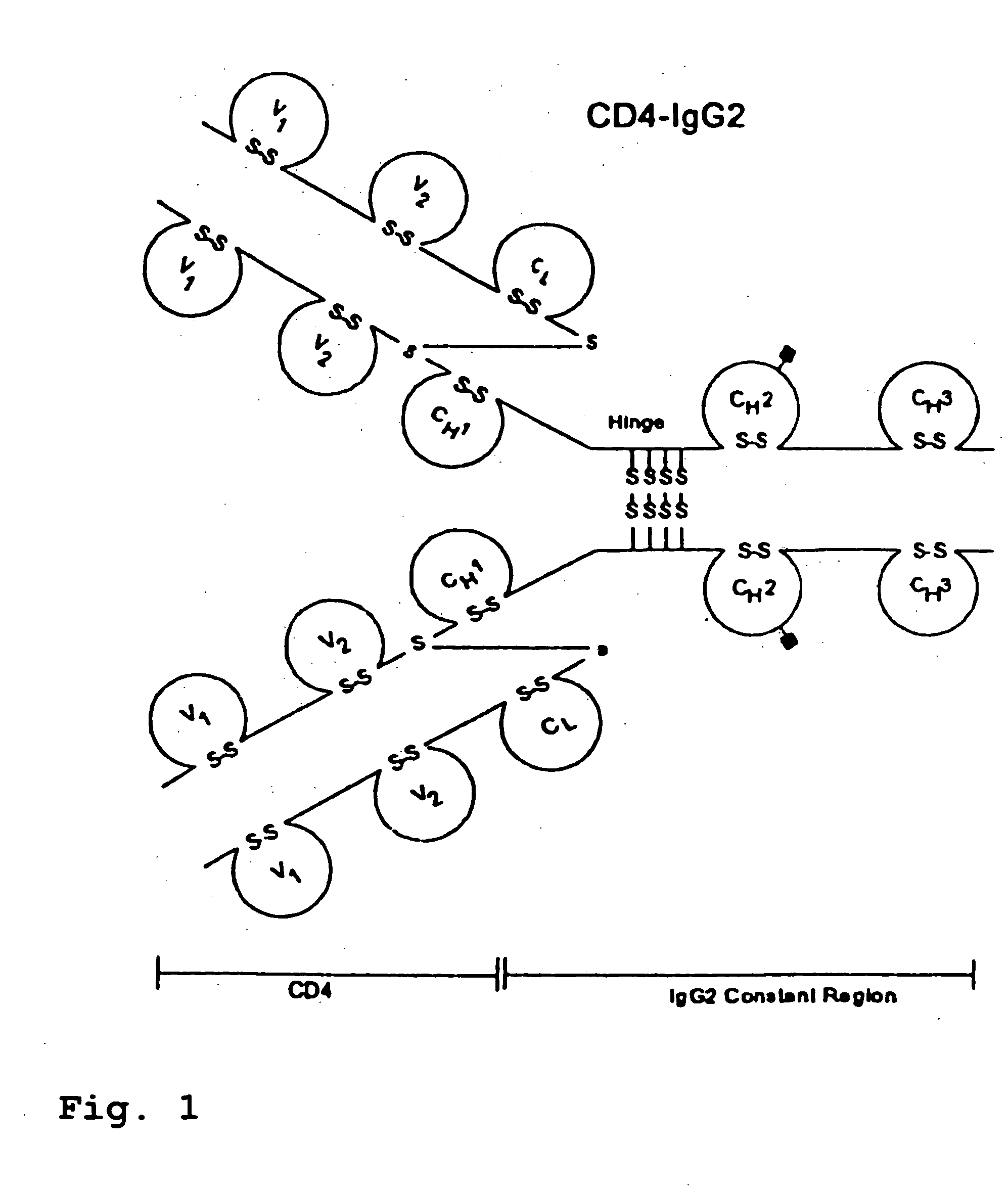
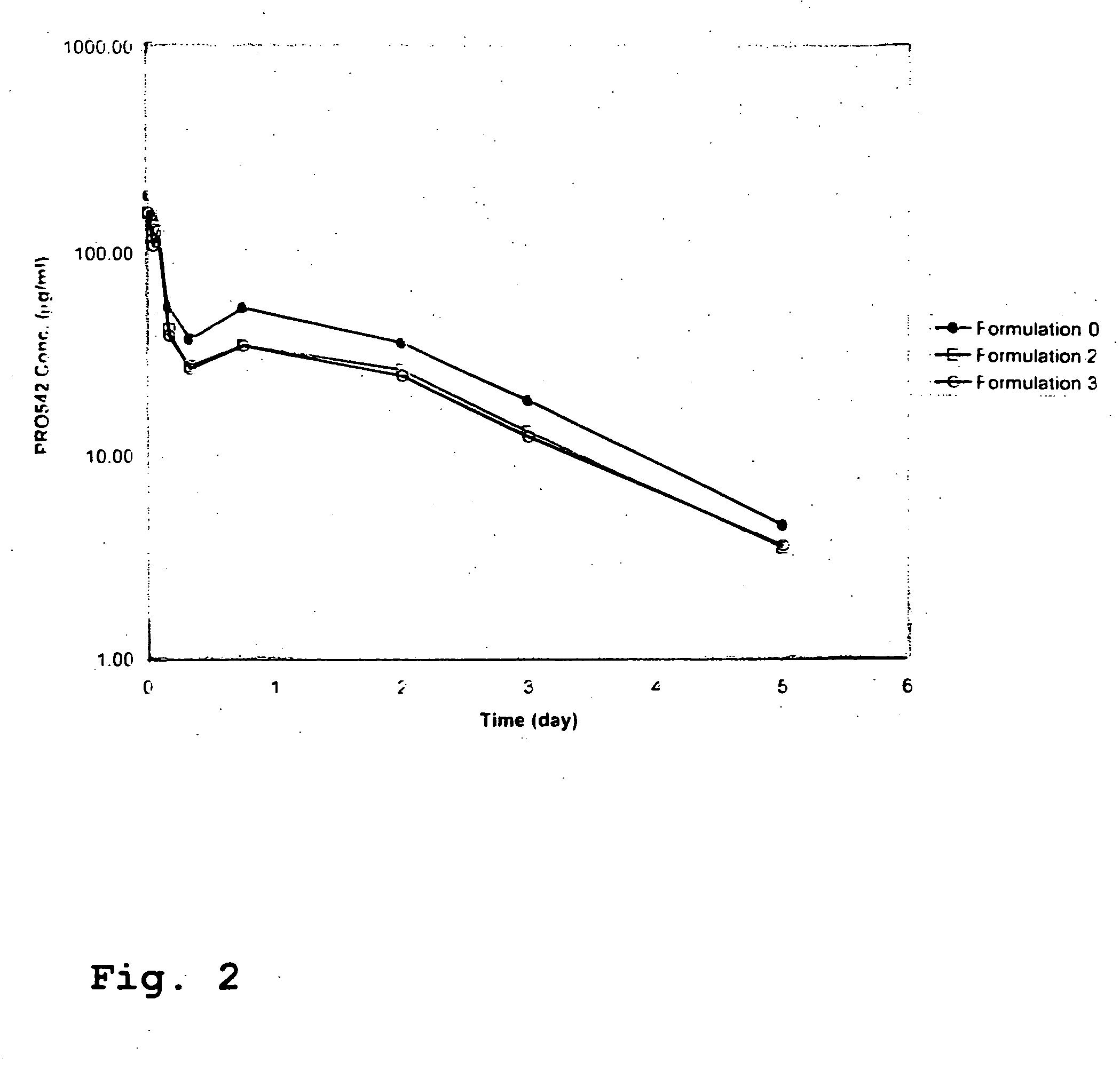
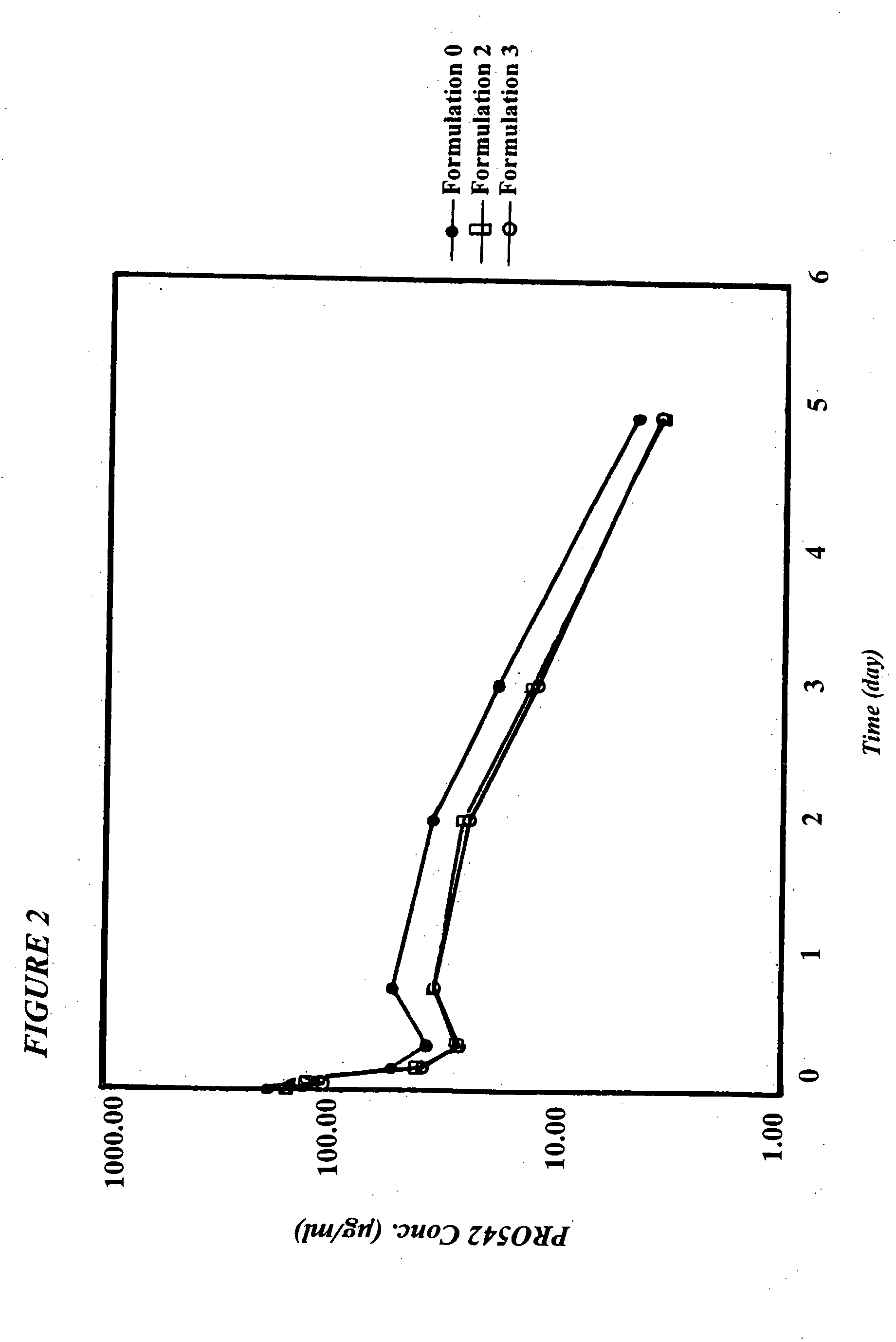
CD4-IgG2 formulations
InactiveUS20050142139A1Avoid infectionOrganic active ingredientsBiocideHigh concentrationPrevention infection
Owner:PROGENICS PHARMA INC
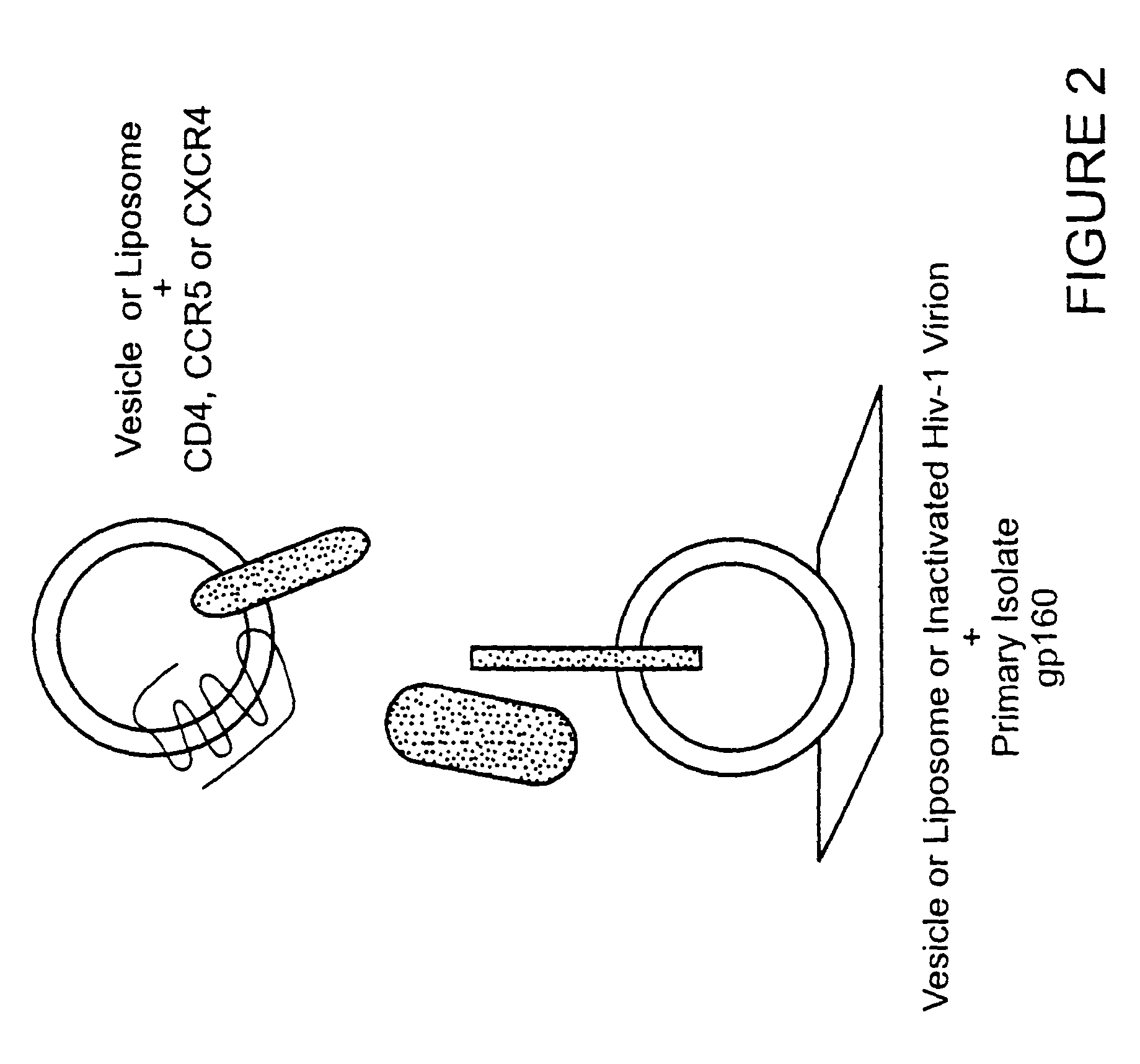
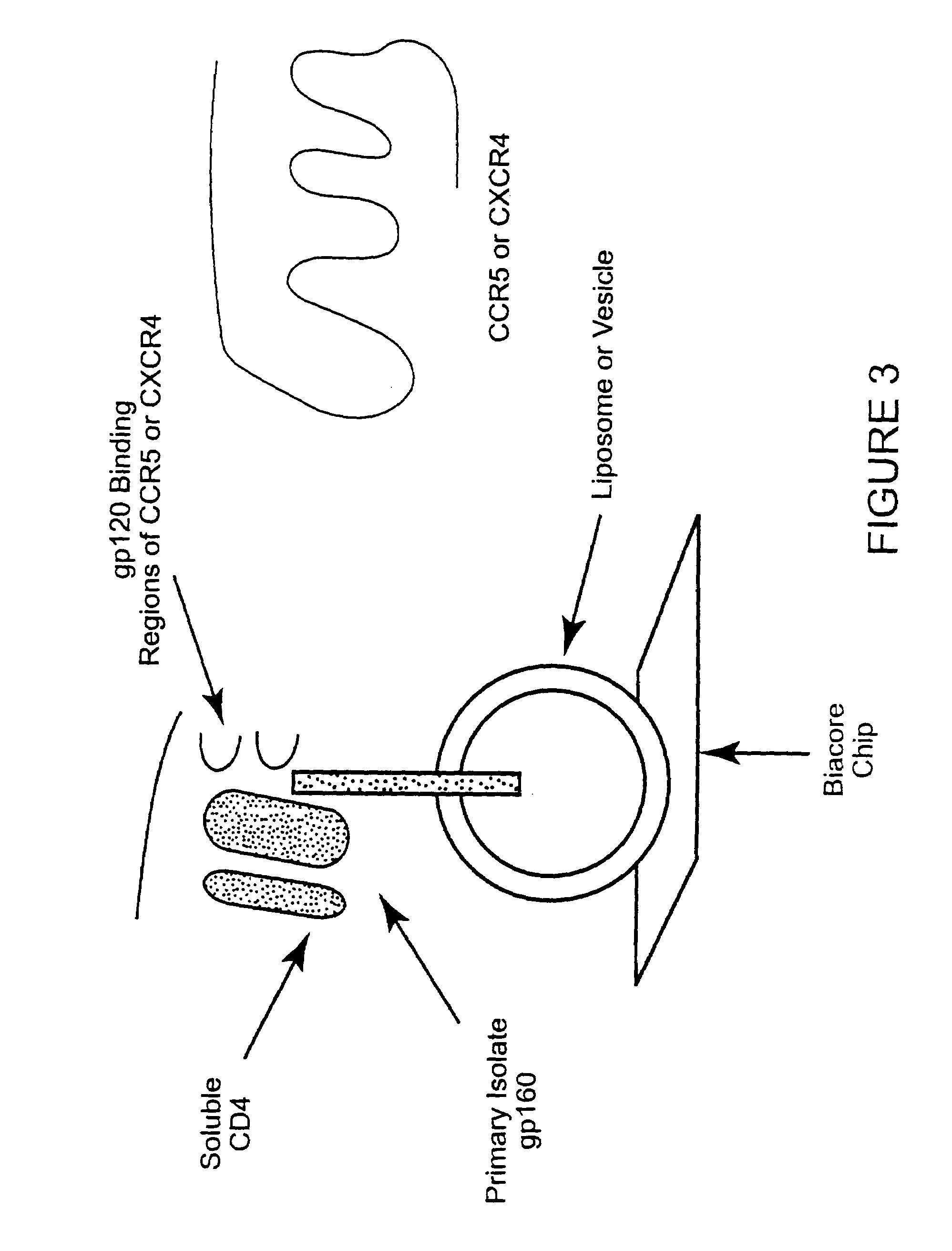
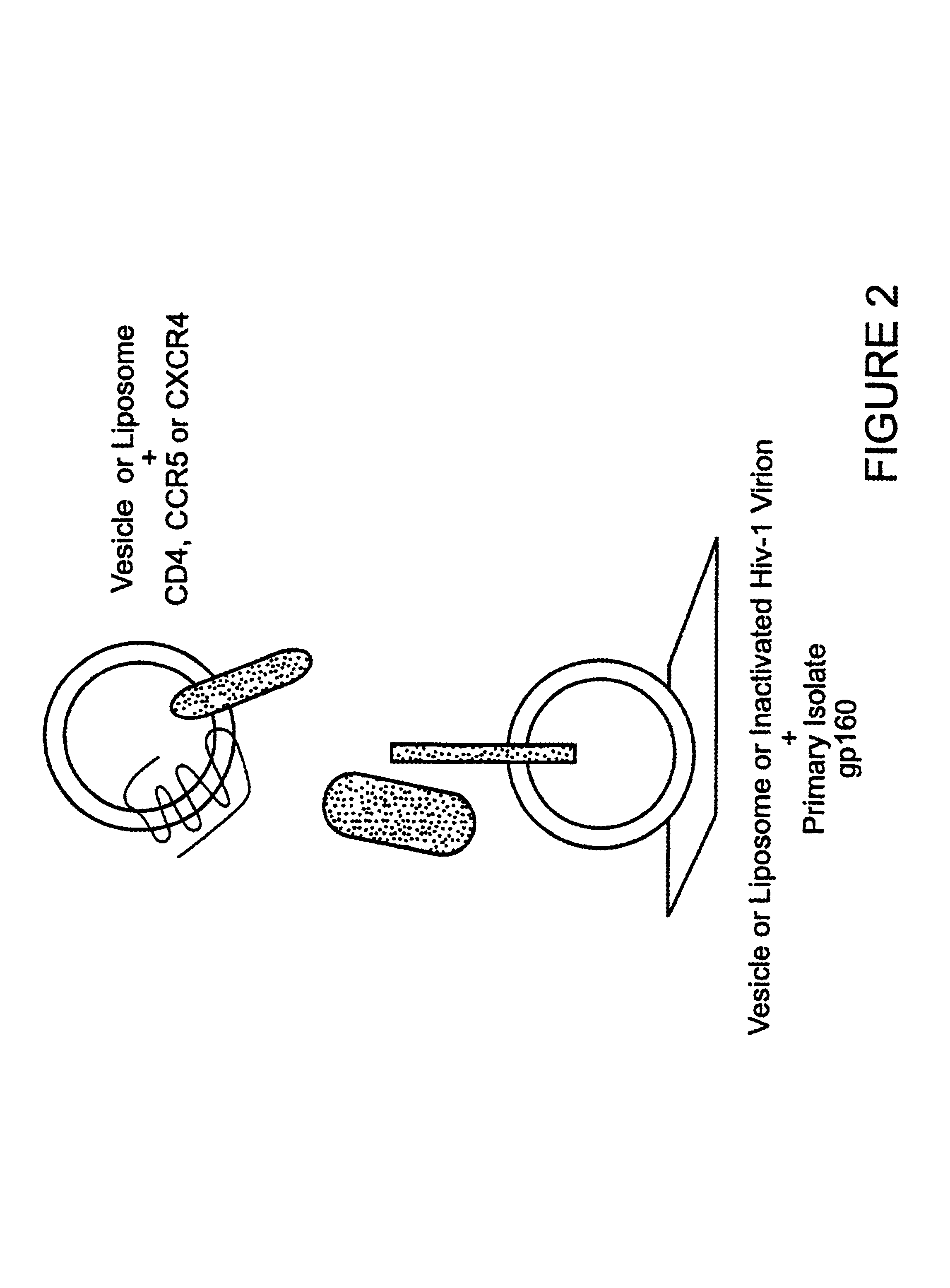
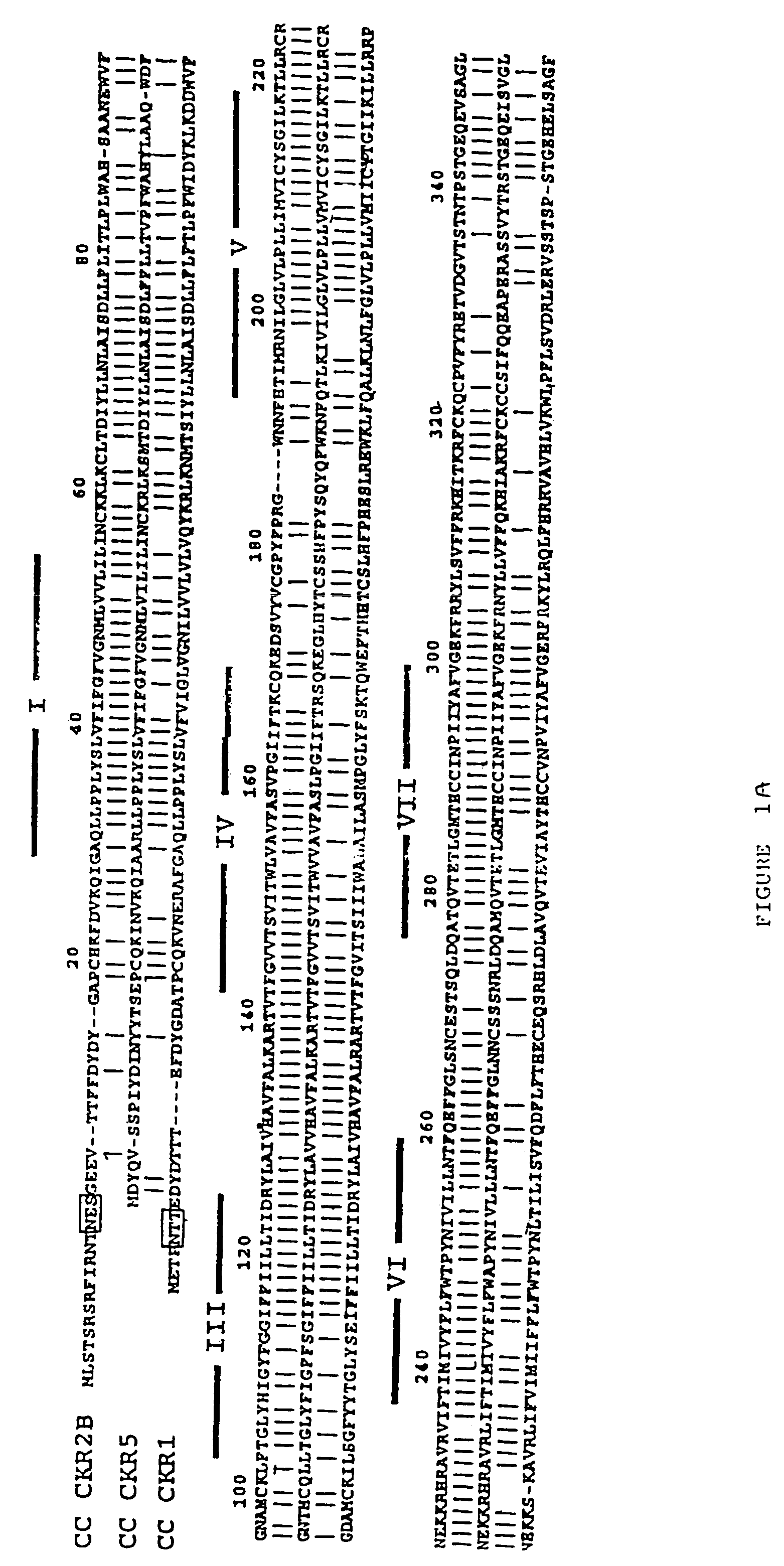
CC chemokine receptor 5 DNA, new animal models and therapeutic agents for HIV infection
InactiveUS7151087B2Effective in regulating monocyte accumulationEffective in regulating activationBiocidePeptide/protein ingredientsChemokine receptor D6Mammal
The susceptibility of human macrophages to human immunodeficiency virus (HIV) infection depends on cell surface expression of the human CD4 molecule and CC cytokine receptor 5. CCR5 is a member of the 7-transmembrane segment superfamily of G-protein-coupled cell surface molecules. CCR5 plays an essential role in the membrane fusion step of infection by some HIV isolates. The establishment of stable, nonhuman cell lines and transgenic mammals having cells that coexpress human CD4 and CCR5 provides valuable tools for the continuing research of HIV infection. In addition, antibodies which bind to CCR5, CCR5 variants, and CCR5-binding agents, capable of blocking membrane fusion between HIV and target cells represent potential anti-HIV therapeutics for macrophage-tropic strains of HIV.
Owner:UNITED STATES OF AMERICA
Quinolizinone compound and use thereof as HIV integrase inhibitor
InactiveUS20060084665A1Less side effectsStrong inhibitory activityBiocideOrganic chemistrySide effectReverse transcriptase
A pharmaceutical agent having an anti-HIV action, particularly, a pharmaceutical agent having an integrase inhibitory action, is provided. The present invention relates to a quinolizinone compound represented by the following formula [I]wherein each symbol is as defined in the specification, a pharmaceutically acceptable salt thereof, and an anti-HIV agent containing same as an active ingredient. The compound of the present invention has an HIV integrase inhibitory action and is useful as an anti-HIV agent for the prophylaxis or therapy of AIDS. Moreover, by a combined use with other anti-HIV agents such as protease inhibitors, reverse transcriptase inhibitors and the like, the compounds can become a more effective anti-HIV agent. Since the compound has a high inhibitory activity specific for integrases, the compound can provide a safe pharmaceutical agent for human with a fewer side effects.
Owner:JAPAN TOBACCO INC
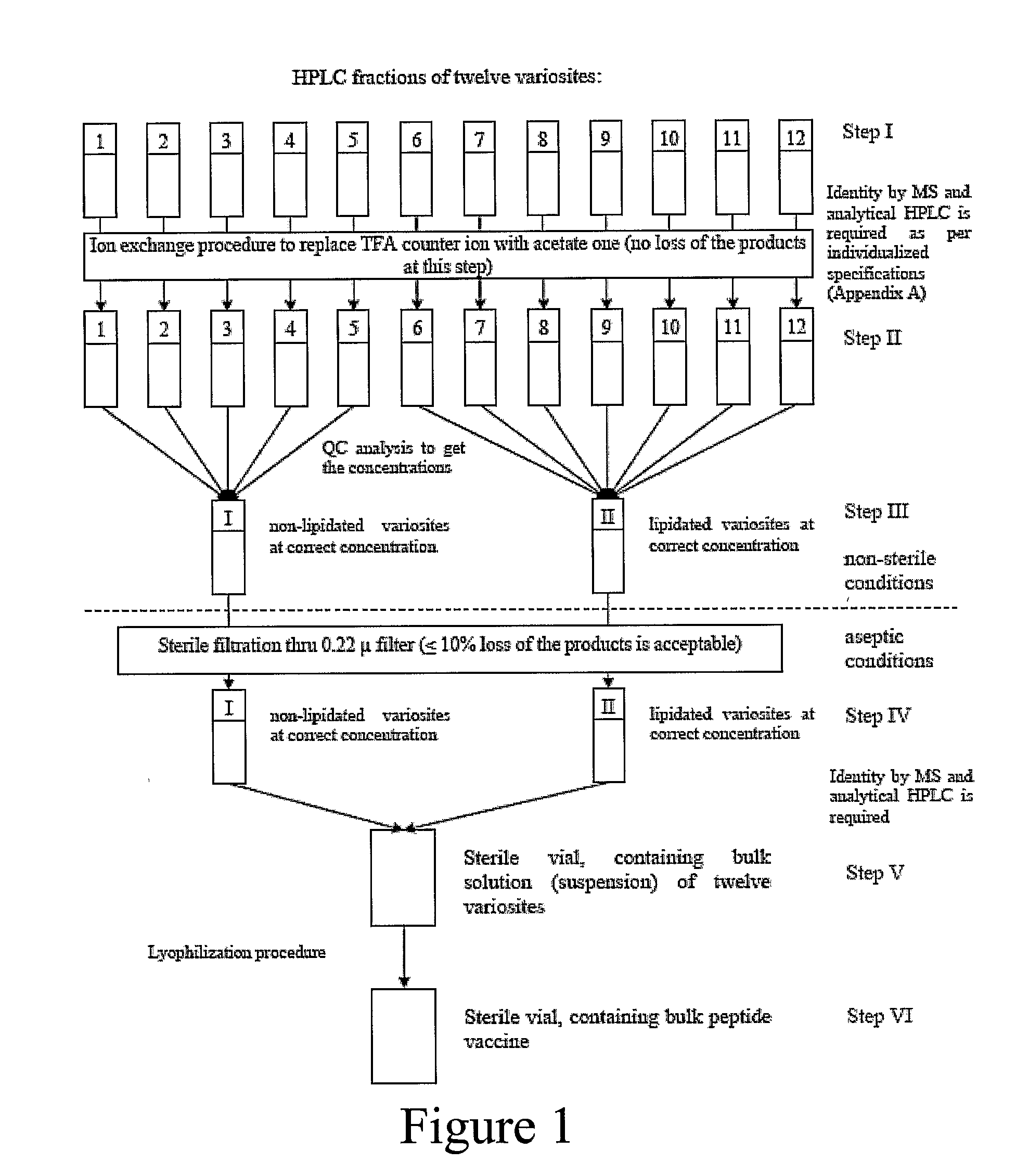
HIV vaccine composition
An anti-HIV vaccine composition is disclosed. The vaccine comprises an combination of immunogenic peptide mixtures, which mixtures may be prepared in a single synthesis. The composition collectively represents the in vivo variability seen in immunogenic epitopes from highly variable regions of HIV. Immunization with the vaccine elicits broadly reactive immunity (CTL and T helper cell responses) against the divergent strains of HIV upon which it is based. The vaccine may be formulated to target regionally distinct variability based on an HIV clade predominant in a geographical region.
Owner:VARIATION BIOTECHNOLOGIES INC
Green prune extract of anti acquired immuno-deficiency syndrome and bacterium
InactiveCN101254224AImprove bioavailabilityGood effectAntibacterial agentsAntiviralsOrganic solventMedicine
The invention relates to the application of a prunus mume extract, and belongs to the field of biomedicines. The extract is obtained by extracting prunus mume with organic solvent, performing liquid extraction with organic solvent, and optionally refining; and can be used for preparing anti-HIV and antibacterial drugs. The inventive drugs have the advantages of high bioavailability of plant, good therapeutic effect, easily accessible raw material, and good safety. Researches and developments on the pharmacological activity of the extract are of great significance, and the extract has wide application prospect.
Owner:陈光英 +2
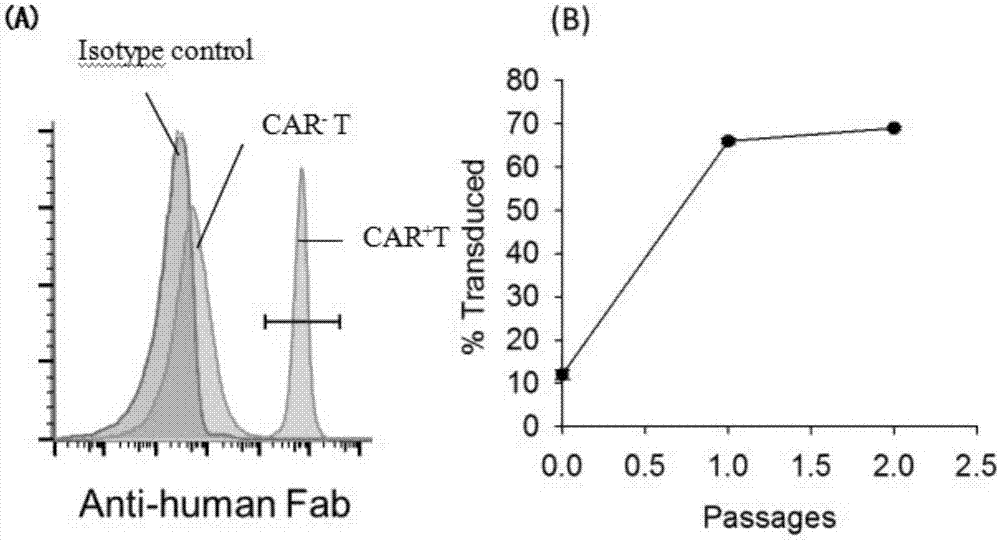
Recombinant gene construction of chimeric antigen receptor (CAR) for treating HIV (human immunodeficiency virus) infection and application of chimeric antigen receptor
ActiveCN107098969AHigh broad spectrumIncrease heightPeptide/protein ingredientsAntibody mimetics/scaffoldsSingle-Chain AntibodiesT lymphocyte
The invention relates to the technical field of immunotherapy of infectious diseases, in particular to recombinant gene construction of chimeric antigen receptor (CAR) for treating HIV (human immunodeficiency virus) infection and an application of the CAR. A single-chain antibody (ScFv) can identify gp120 of surfaces of HIV infected cells through series connection of antibody light chain and heavy chain variable zones of the gp120 of the surfaces of the HIV infected cells; the ScFv is prepared into the CAR, CAR coding genes are shifted to plasimid vectors, and lentiviral vectors shifted with the CAR coding genes are transduced to CD8+T lymphocytes. The CD8+T lymphocytes are discovered to be remarkable in restraining and killing activities of the HIV infected cells in both in-vitro and in-vivo experiments and can serve as active ingredients to prepare anti-HIV infection drugs, and good application prospect is achieved.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Combination of Anti-hiv reverse transcriptase and protease inhibitors
Owner:HOETELMANS RICHARD MARINUS WILHELMUS
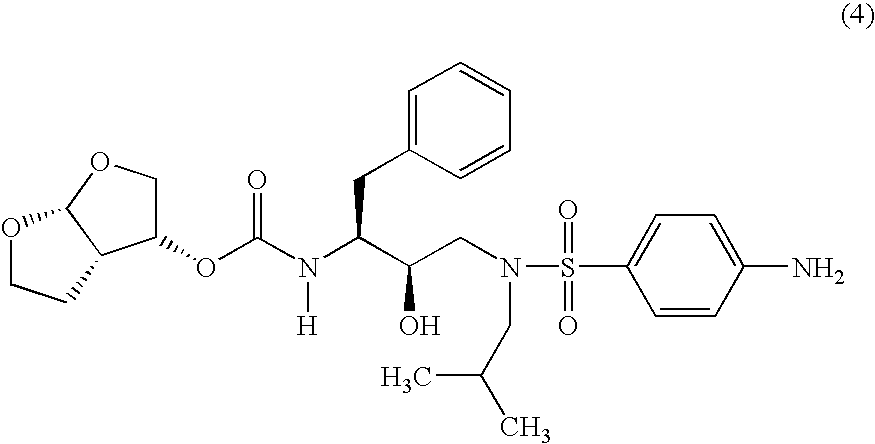
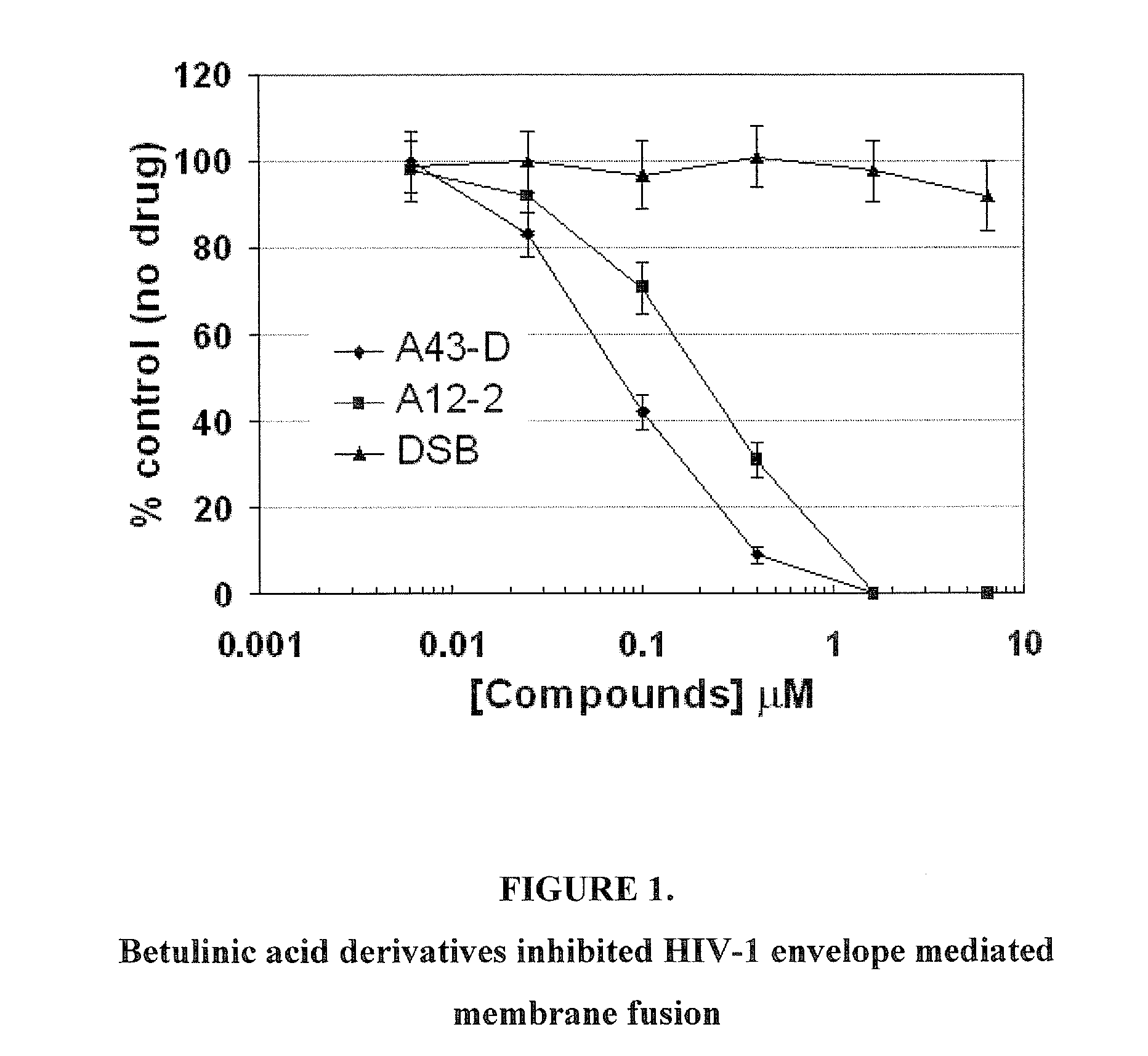
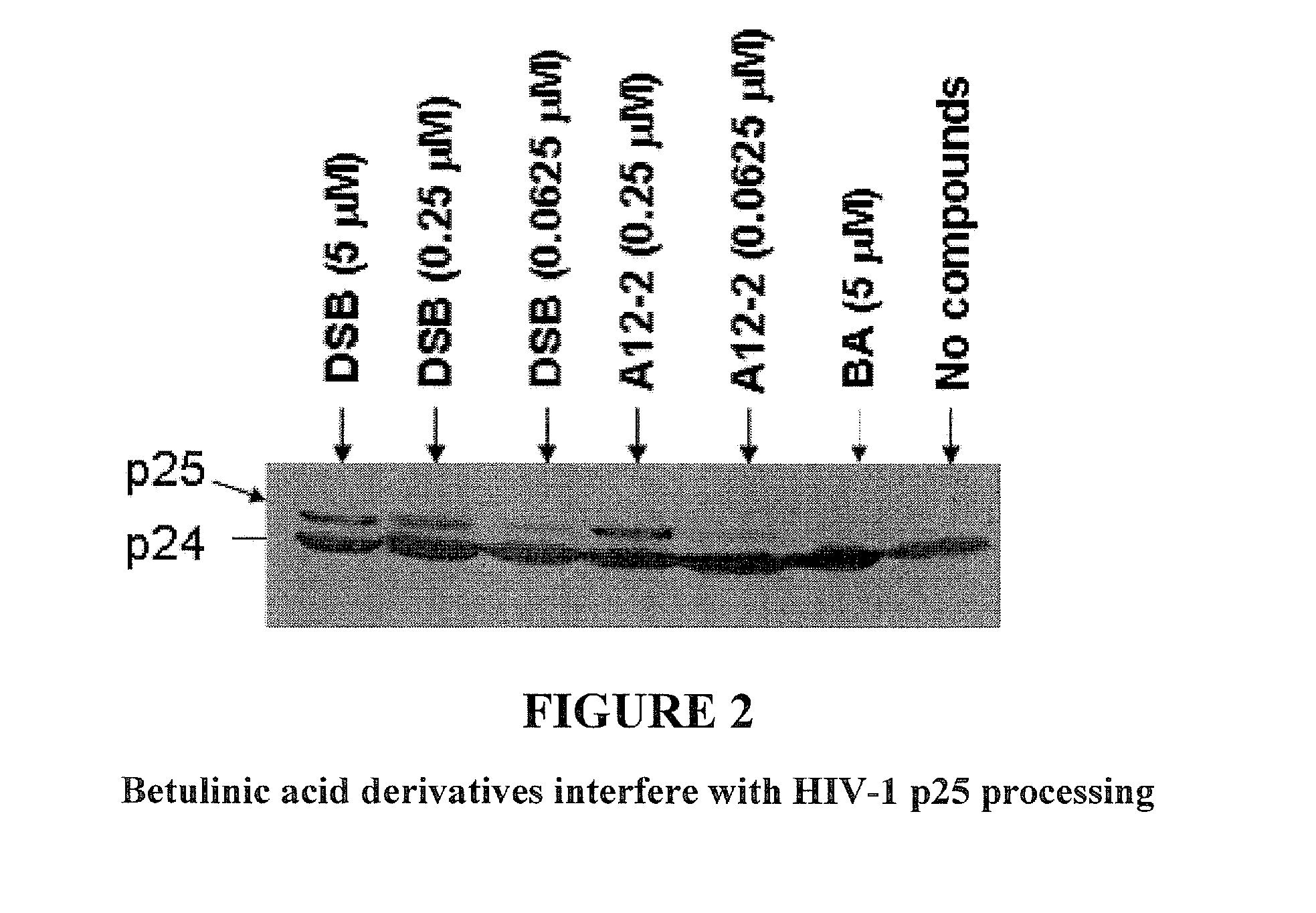
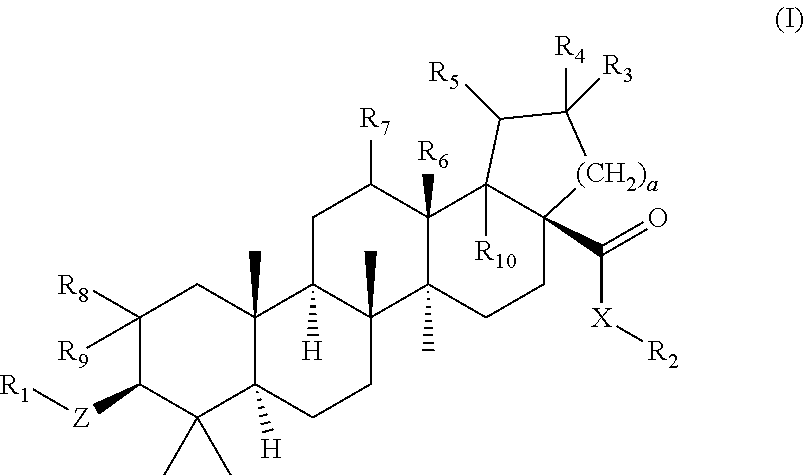
Betulinic acid derivatives as Anti-hiv agents
InactiveUS20110152229A1Useful in treatmentOrganic active ingredientsAntiviralsAnti-HIV AgentMedicine
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
4-oxoquinoline compounds and utilization thereof as HIV integrase inhibitors
ActiveCN1692101ALittle side effectsHighly specific inhibitory activityOrganic chemistryAntiviralsSide effectReverse transcriptase
An anti-HIV agent containing, as an active ingredient, a 4-oxoquinoline compound represented by the following formula, wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof. The compound of the present invention has HIV integrase inhibitory action and is useful as an anti-HIV agent for the prophylaxis or therapy of AIDS. Moreover, by a combined use with other anti-HIV agents such as protease inhibitors, reverse transcriptase inhibitors and the like, the compound can become a more effective anti-HIV agent. Since the compound has high inhibitory activity specific for integrases, it can provide a safe pharmaceutical agent with a fewer side effects for human.
Owner:JAPAN TOBACCO INC
Cells expressing both human CD4 and a human fusion accessory factor associated with HIV infection
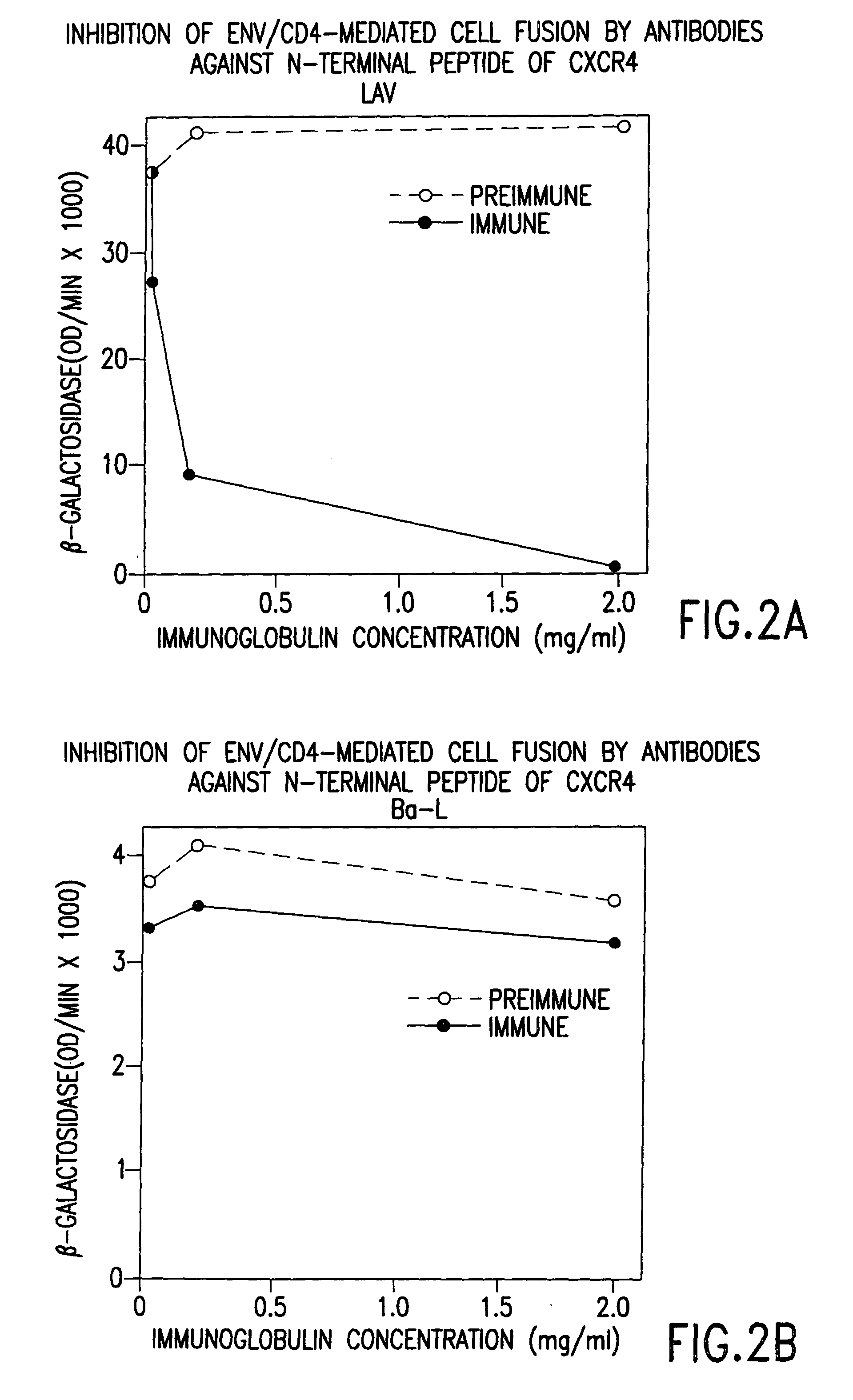
The susceptibility to human immunodeficiency virus (HIV) infection depends on the cell surface expression of the human CD4 molecule and a human fusion accessory factor associated with HIV infection (CXCR4). CXCR4 is a member of the 7-transmembrane segment superfamily of G-protein-coupled cell surface molecules. CXCR4 plays an essential role in the membrane fusion step of HIV infection. The establishment of stable, nonhuman cell lines and transgenic mammals having cells that coexpress human CD4 and CXCR4 provides valuable tools for the continuing research of HIV infection and the development of more effective anti-HIV therapeutics. In addition, antibodies against CXCR4, isolated and purified peptide fragments of CXCR4, and CXCR4-binding biologic agents, capable of blocking membrane fusion between HIV and target cells represent potential anti-HIV therapeutics.
Owner:BERGER EDWARD +3
4-Oxoquinoline compound and use thereof as HIV integrase inhibitor
ActiveUS20060217413A1Promote absorptionIncreased riskBiocideOrganic chemistrySide effectReverse transcriptase
Owner:JAPAN TOBACCO INC
Broadly-Neutralizing Anti-HIV Antibodies
The present invention relates to anti-HIV antibodies. Also disclosed are related methods and compositions. HIV causes acquired immunodeficiency syndrome (AIDS), a condition in humans characterized by clinical features including wasting syndromes, central nervous system degeneration and profound immunosuppression that results in life-threatening opportunistic infections and malignancies. Since its discovery in 1981, HIV type 1 (HIV-1) has led to the death of at least 25 million people worldwide.
Owner:CALIFORNIA INST OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com