Recombinant gene construction of chimeric antigen receptor (CAR) for treating HIV (human immunodeficiency virus) infection and application of chimeric antigen receptor
A technology of receptors and antigens, applied in the field of recombinant gene construction of chimeric antigen receptors, can solve the problems of low transduction efficiency of retroviral vectors, cell death, loss of CAR molecules, etc., to increase clinical effectiveness and safety , increase broad-spectrum, increase the effect of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The present invention provides a chimeric antigen receptor (CAR) recombinant gene for treating HIV infection and its construction method. The specific splicing method is: sequential splicing of signal peptide, gp120 single-chain antibody ScFv capable of recognizing the surface of HIV-infected cells , CD8hinge, leukocyte antigen differentiation group molecule transmembrane region CD28-TM+ICD, 4-1BB and CD3 (leukocyte antigen differentiation group molecule 3) ζ chain, and finally a complete chimeric antigen receptor (CAR) that can treat HIV ) molecule, its amino acid sequence is shown in SEQID NO.4, and its structure is as figure 1 shown; the nucleotide sequence of the gene encoding the chimeric antigen receptor (CAR) is shown in SEQ ID NO.3.
[0029]The amino acid sequence of the single-chain antibody ScFv derived from the chimeric antigen receptor for the treatment of HIV infection is shown in SEQ ID NO.2. The nucleotide sequence of its coding gene is shown in SEQ ID N...
Embodiment 2
[0031] Example 2: CAR Molecular Recombination Construction of PTK-EF-1α-N6 Plasmid Expression Vector
[0032] The CAR was synthesized according to the sequence shown in SEQ ID NO.4, and the gene encoding the full-length CAR was inserted into the target expression vector based on seamless recombination cloning technology (see figure 2 ). After many tests, the preferred plasmid vector is the PTK-EF1α-N6 vector (the nucleotide sequence of which is shown in SEQ ID NO.5) transformed from the PTK881 vector as the backbone, after replacing the CMV promoter with the EF1α promoter. Finally, a recombinant plasmid PTK-EF1α-N6 vector inserted into the CAR gene and capable of expressing CAR was obtained, the nucleotide sequence of which is shown in SEQ ID NO.6.
[0033] The virus packaging steps are as follows:
[0034] 1) Take two centrifuge tubes filled with 16ml DMEM culture medium, add 960μg PEI to one tube, and add 320μg premixed PTK881 vector plasmid to the other tube, vortex, and...
Embodiment 3
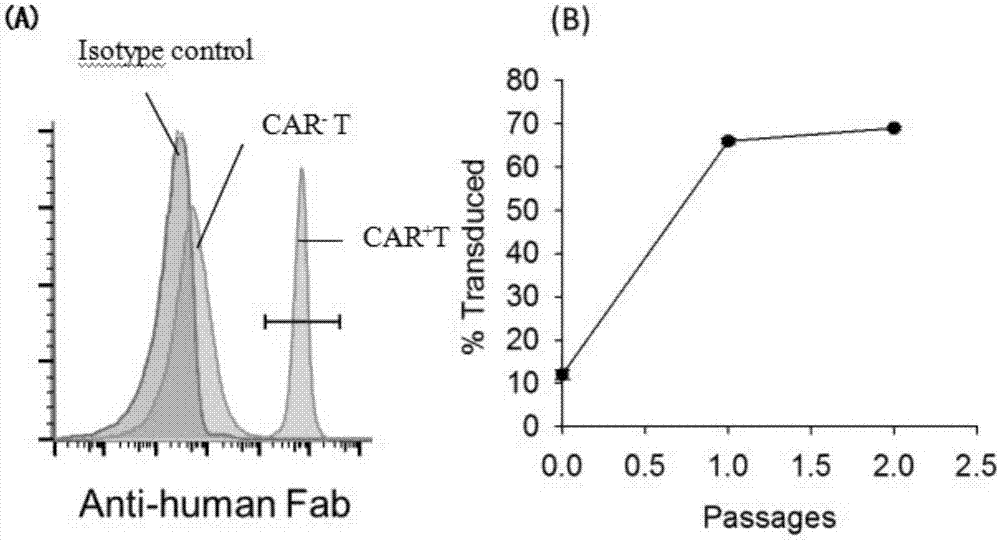
[0038] Example 3: Preparation of CD8 capable of expressing chimeric antigen receptors + T cells (CAR-T cells)
[0039] Step 1: Isolation of Patient PBMC Cells
[0040] (1) Collect 60-80ml of human peripheral blood samples, and shake while collecting to fully mix the peripheral blood with the anticoagulant;
[0041] (2) Transfer the peripheral blood into a 50ml centrifuge tube, dilute the peripheral blood 1:1 with DPBS buffer, and mix well. Slowly add the diluted blood sample into the centrifuge tube of 15ml human lymphocyte separation medium at room temperature. The method is as follows: take the blood sample with a 10ml pipette, extend it to 0.5cm above the liquid surface of the separation liquid, the blood sample will naturally slide down and spread on the surface of the separation liquid, then gently add the blood sample, be careful not to break through the liquid surface;
[0042] (3) Trim and centrifuge for 30 minutes, slowly ascend and descend slowly;
[0043] (4) Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com