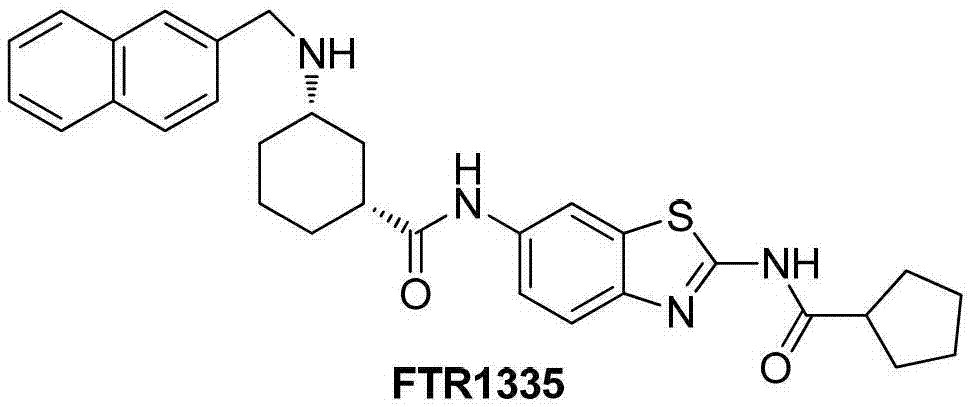
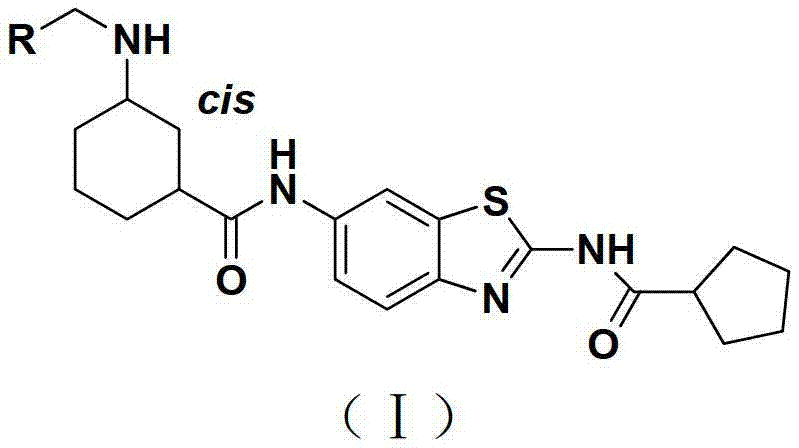
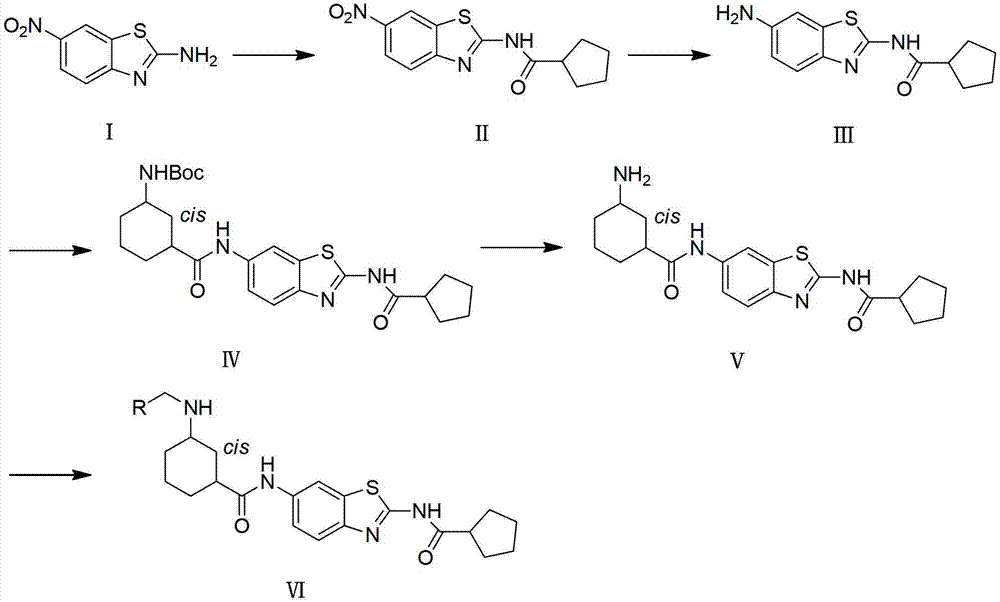
Substituted benzothiazole compound as well as preparation method and application thereof
A technology of benzothiazole and compound, applied in the application field as antifungal drug, can solve problems such as narrow antibacterial spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of N-(6-nitrobenzothiazol-2-yl) cyclopentanamide (II)
[0051] Dissolve 2-amino-6-nitrobenzothiazole (4.00g, 0.02mol, 1equiv) and cyclopentanecarboxylic acid (2.28g, 0.02mol, 1equiv) in 50mL of dichloromethane, add 1-ethyl-( 3-Dimethylaminopropyl) carbodiimide hydrochloride (5.75g, 0.03mol, 1.5equiv) and 4-dimethylaminopyridine (0.40g, 2mmol, 0.1equiv), stirred at room temperature for 16h. After the reaction, the reaction solution was washed with 5% hydrochloric acid (3×30mL), 5% sodium bicarbonate (3×30mL), saturated sodium chloride solution (3×30mL), and washed with anhydrous Na 2 SO 4 Drying. Filtration, evaporation of the solvent under reduced pressure, and purification of the residue by silica gel column chromatography (developing solvent: hexane:EtOAc=10:1, v / v) to obtain 5.66 g of a light yellow solid with a yield of 97.3%. 1 H-NMR (300MHz, CDCl 3 ):9.15(brs,1H),8.76(d,1H,J=2.4Hz),8.34(dd,1H,J=9.0,2.4Hz),7.82(d,1H,J=9.0Hz),2.80-2.96 ...
Embodiment 2
[0052] Example 2: Preparation of N-(6-aminobenzothiazol-2-yl)cyclopentamide (Ⅲ)
[0053] Compound Ⅱ (1.00g, 3.4mmol, 1equiv) and stannous chloride (2.30g, 10.2mmol, 3equiv) were dissolved in 50mL of ethanol, heated to reflux for 2h. After the reaction was completed, the reaction liquid was evaporated to dryness, 50 mL of 5% sodium hydroxide solution and 100 mL of ethyl acetate were added, mixed and oscillated thoroughly, and the aqueous layer was extracted with ethyl acetate (2×100 mL). The organic phases were combined, washed with saturated sodium chloride solution (3 × 30mL), anhydrous Na 2 SO 4 dry. After filtration, the solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from 20 mL of ethyl acetate to obtain 0.45 g of a white solid, with a yield of 50.6%. 1 H-NMR (300MHz, DMSO-d 6 ):11.97(s,1H),7.37(d,1H,J=8.5Hz),6.97(d,1H,J=1.5),6.68(dd,1H,J=8.5,1.5Hz),5.14(s, 2H),2.83-2.97(m,1H),1.78-1.95(m,2H),1.45-1.95(m,6H).ESI-MS(m / z):262...
Embodiment 3
[0054] Example 3: Preparation of tert-butyl-N-cis-3-[(2-[(cyclopentylcarbonyl)amino]benzothiazole-6-amino)carbonyl]cyclohexylcarbamate (IV)
[0055] Take III (1.00g, 3.83mmol, 1equiv) and cis-3-(tert-butoxycarboxyamino)cyclohexylcarboxylic acid (0.93g, 3.83mmol, 1equiv) and dissolve in 25mL of dichloromethane, add 1-ethyl-(3 -Dimethylaminopropyl) carbodiimide hydrochloride (1.10g, 5.75mmol, 1.5equiv) and 1-hydroxybenzotriazole (0.78g, 5.75mmol, 0.1equiv), stirred at room temperature for 18h. After the reaction, the white precipitate was filtered out and washed with 20 mL of dichloromethane to obtain 0.91 g of a light yellow solid with a yield of 54.2%. 1H-NMR(300MHz,DMSO-d6):12.45(s,1H),10.21(s,1H),8.50(d,1H,J=1.8Hz),7.83(d,1H,J=8.7Hz),7.68 (dd,1H,J=8.7,1.8Hz),7.04(s,1H),3.07-3.22(m,1H),2.72-2.81(m,1H),2.55-2.66(m,1H),1.67-2.17 (m,12H),1.57(s,9H),1.20-1.53(m,4H).ESI-MS(m / z):485[M-1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com