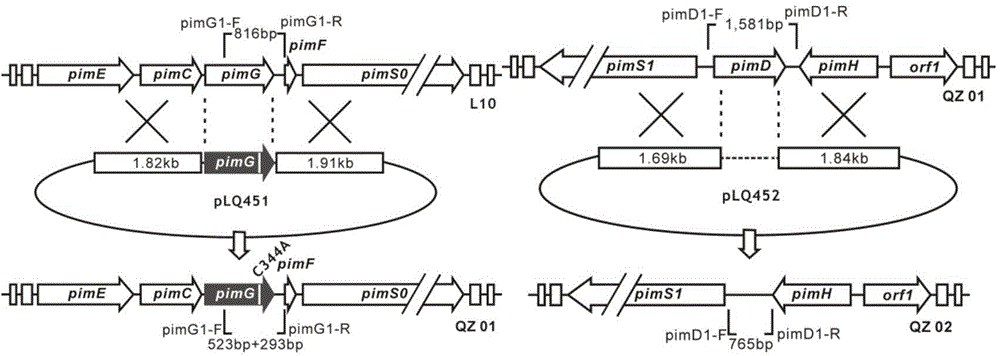
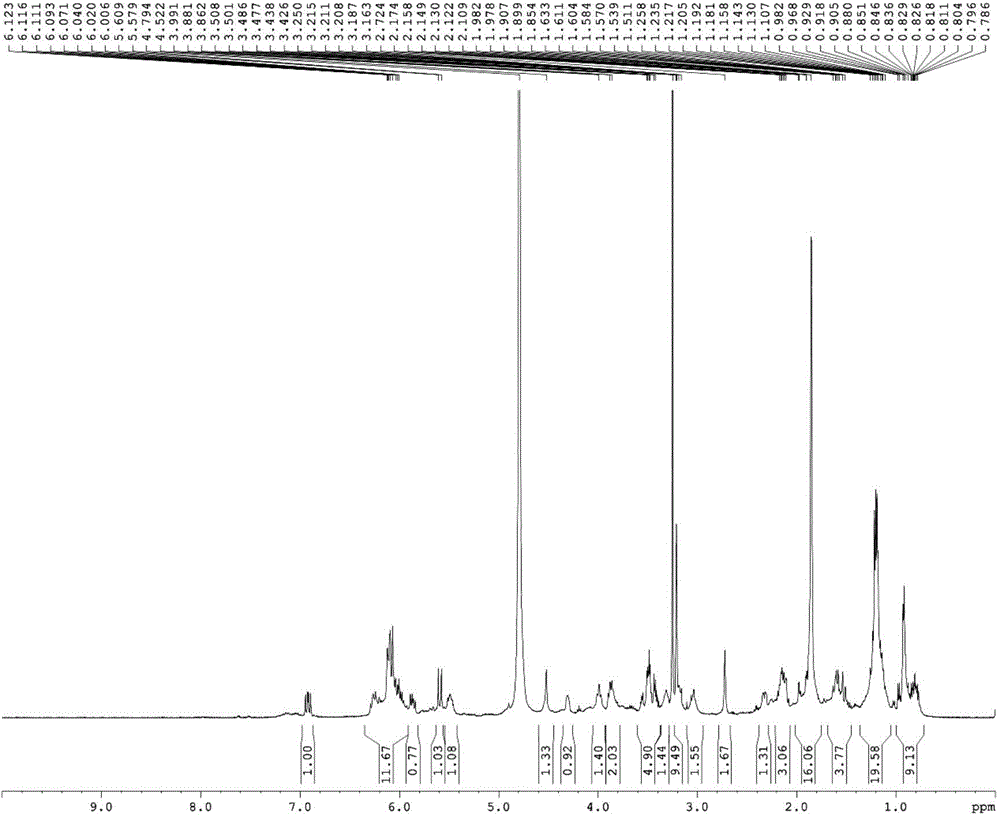
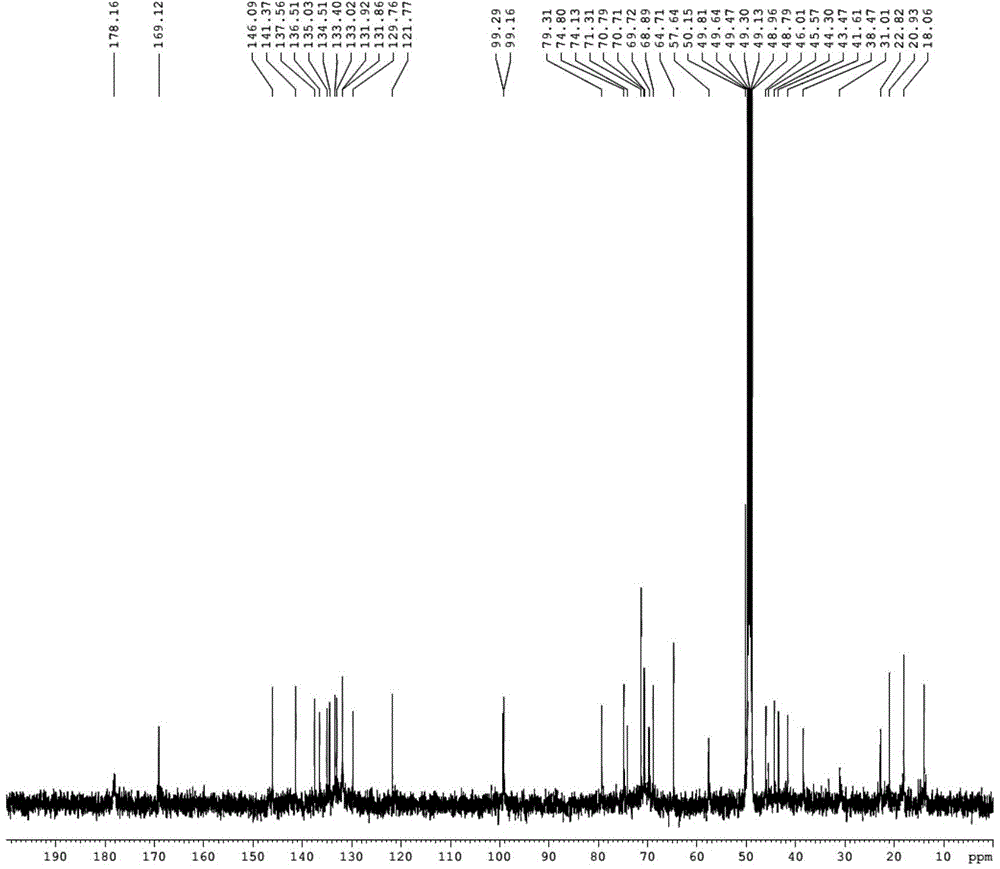
Low-toxicity pimaricin derivative as well as preparation method and application thereof
A pimamycin and derivative technology, which is applied in the field of low-toxic pimamycin derivatives and their preparation, can solve the problems of hemolytic toxicity, limiting the application value of polyene antibiotics and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Prepare 500ml of seed medium and 5L of fermentation medium according to the seed medium and fermentation culture formula, respectively, pack them in 250ml triangular bottles, fill each bottle with 50ml, sterilize at 115°C for 30min, insert the spores of the mutant strain QZ02 into the above seeds In the culture medium, at 220 rpm, at 30° C., on a shaker for 24 hours to obtain a seed culture solution. The seed culture solution was added to the fermentation medium according to 10% inoculum amount, and cultured on a shaker at 220 rpm at 30° C. for 5 days to obtain a fermentation culture of the mutant strain QZ02. Centrifuge the 5L fermentation culture obtained from the fermentation culture, collect the fermented cells, wash the cells with water for 3 times, remove the washing liquid, and freeze-dry the cells overnight; Centrifuge to obtain the extract, repeat the ultrasonic extraction 3 times, combine the extracts, filter the extracts, concentrate under reduced pressure to...
Embodiment 2
[0033]According to the seed medium and fermentation culture formula, prepare 1L seed medium and 10L fermentation medium respectively, pack them into 250ml triangular bottles, fill each bottle with 50ml, and sterilize at 115°C for 30min, insert the spores of the mutant strain QZ02 into the above seeds In the culture medium, at 220 rpm, at 30° C., on a shaker for 24 hours to obtain a seed culture solution. The seed culture solution was added to the fermentation medium according to 10% inoculum amount, and cultured on a shaker at 220 rpm at 30° C. for 5 days to obtain a fermentation culture of the mutant strain QZ02. Centrifuge the 10L fermentation culture obtained from the fermentation culture, collect the fermented cells, wash the cells with water for 3 times, remove the washing liquid, and freeze-dry the cells overnight; Centrifuge to obtain the extract, repeat the ultrasonic extraction 3 times, combine the extracts, filter the extracts, concentrate under reduced pressure to o...
Embodiment 3
[0035] Determination of Antifungal Activity and Hemolytic Toxicity of Pimaricin Derivatives in Vitro
[0036] The determination of antifungal activity in vitro is based on Candida albicans as the indicator bacteria. By measuring the growth of Candida albicans at different concentrations of antibiotics, the MIC of the corresponding antibiotics can be obtained. 50 and MIC 90 value. First, the overnight culture solution of Candida albicans was inoculated in LB medium at a ratio of 1 / 10000, and distributed in 96-well plates at 200 μl / well. The DMSO solutions of the Pimaricin derivatives and Pimaricin at different concentrations (0-800 μg / ml) were prepared, and added to the above LB medium at a ratio of 2%. Place the 96-well plate in a 34°C incubator, culture it statically for 12 hours, and use a microplate reader to measure the OD under different concentrations of antibiotics 660 . MIC can be obtained by drawing the inhibition curve of the measured data against the concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com