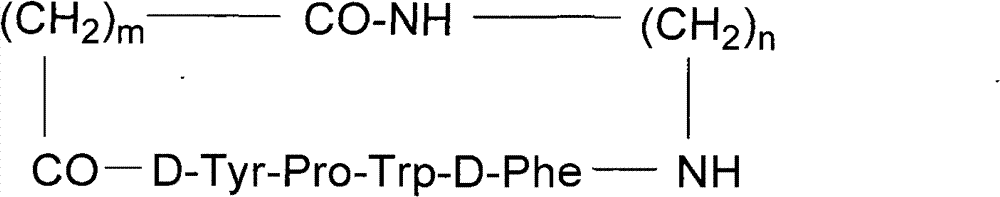Patents
Literature
45results about How to "Low hemolytic toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Micromolecule synthesized anti-microbial peptide, as well as preparation method and application thereof
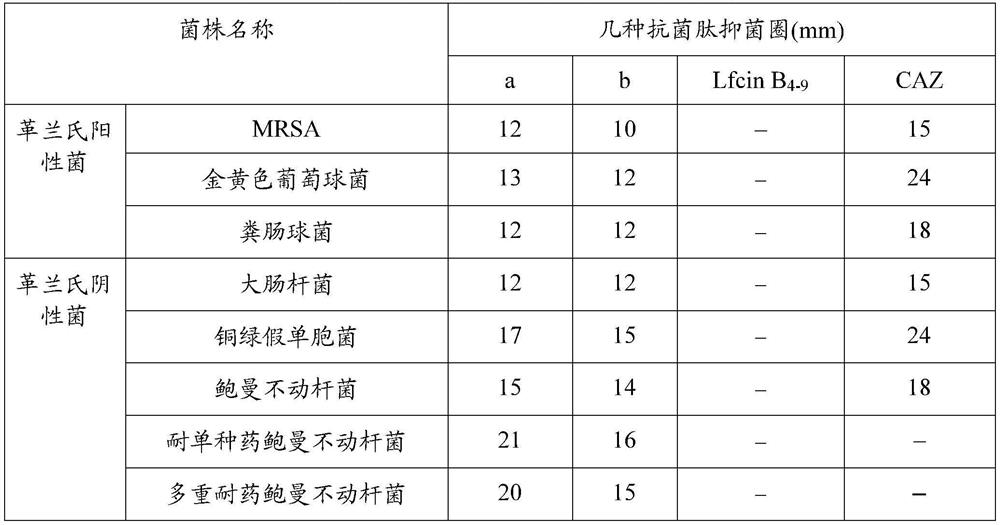
InactiveCN104292301ASimple structureReduce manufacturing costAntibacterial agentsPeptide/protein ingredientsChemical synthesisAntibiosis
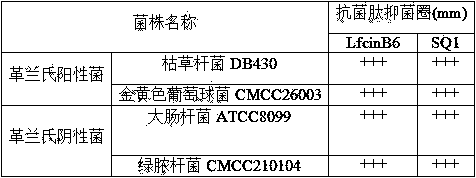
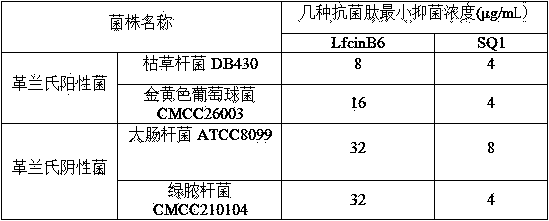
The invention discloses a micromolecule synthesized anti-microbial peptide, as well as a preparation method and application thereof. The sequence of a cationic anti-microbial peptide is SQ1: R-W-W-R-F-NH2 (Arg-Arg-Trp-Trp-Arg-NH2), and the preparation method is the solid-phase chemosynthesis method. The micromolecule synthesized anti-microbial peptide has broad-spectrum cytotoxicity to gram-positive bacteria and gram-negative bacteria, has stronger bactericidal activity than a natural anti-microbial peptide, is simple in structure, convenient for artificial synthesis without any modification and connection, has the advantages of small hemolytic toxicity and no poison effects on animal and plant cells, and has an important value in development and application of antimicrobial agents.
Owner:SOUTHWEST UNIVERSITY
Antibacterial peptide composite material and preparation method and application thereof
ActiveCN109125708AImprove antibacterial propertiesHigh antibacterial efficiencyAntibacterial agentsPeptide/protein ingredientsActivity concentrationCell membrane
The invention discloses an antibacterial peptide composite material and a preparation method and application thereof and belongs to the technical field of biomedicine. An antibacterial peptide-graphene composition material is prepared by using natural antibacterial peptide and a small-size graphene material as raw materials and adopting a chemical modification or physical adsorption method. The material has good antibacterial ability and low hemolytic toxicity within an activity concentration range. Especially, antibacterial efficiency of a melittin / graphene oxide composite material prepared by the method is higher than more than 20 times than that of original melittin or graphene oxide. The characteristic that antibacterial peptide and graphene material have similar mechanism of damagingcell membranes is utilized, the antibacterial peptide and the graphene material are synergistic to realize quick and efficient inserting and pore-forming of the cell membranes so as to cause leakage of bacterial content, and effective bacteria killing at extremely low drug concentration is realized.
Owner:SUZHOU UNIV
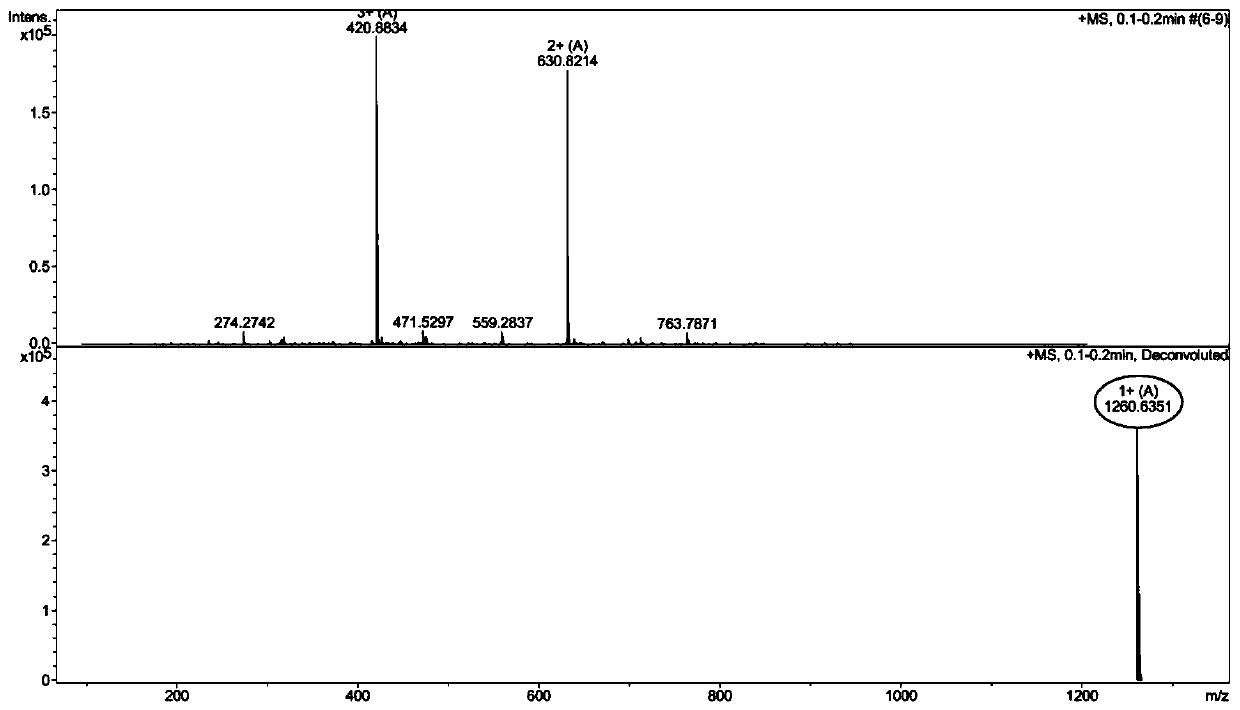
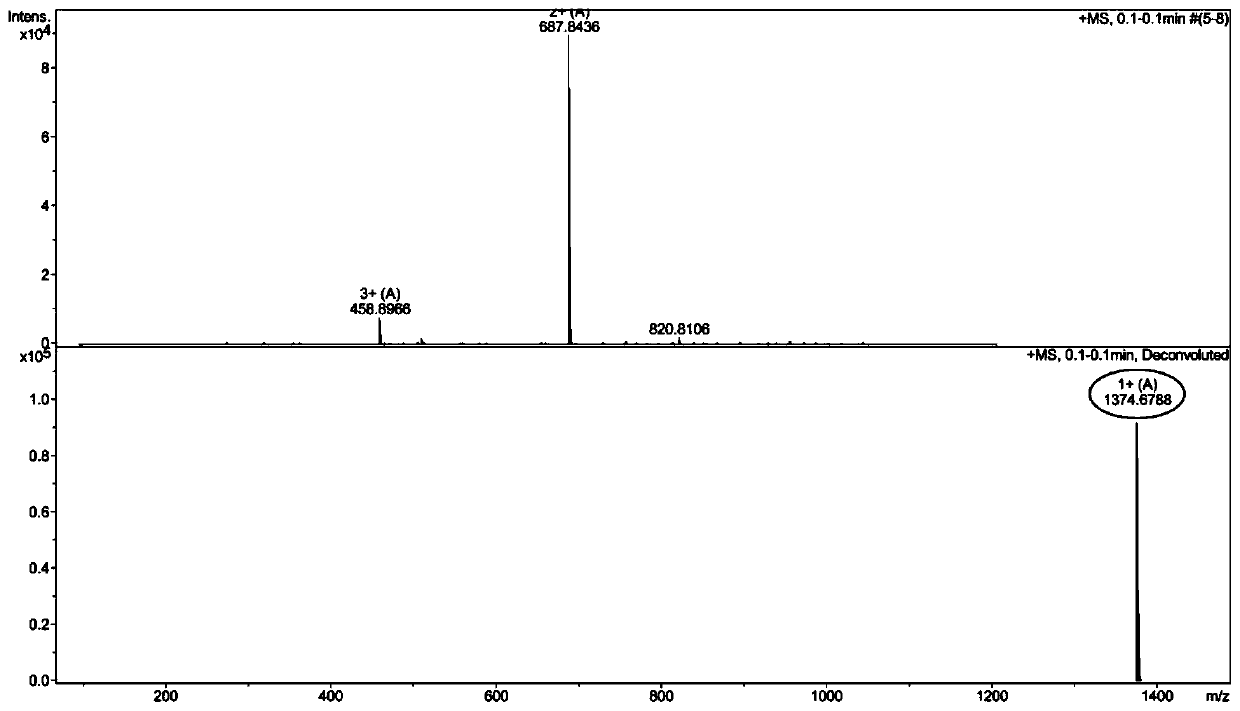
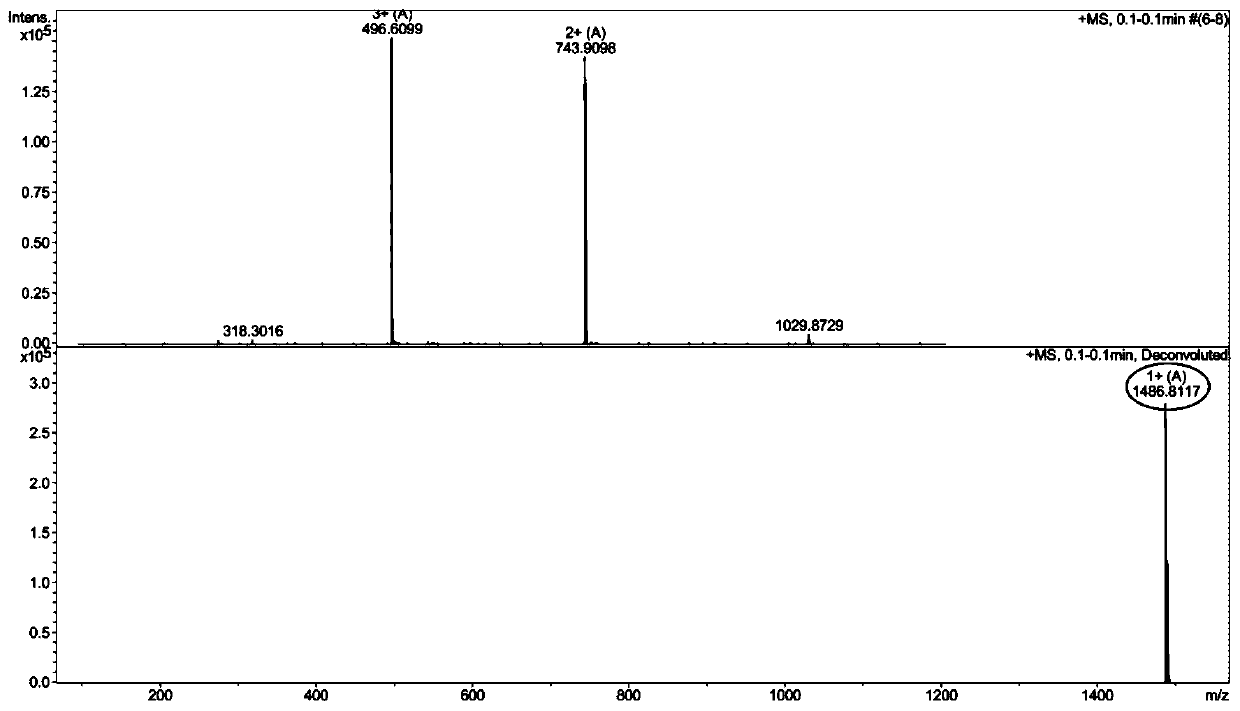
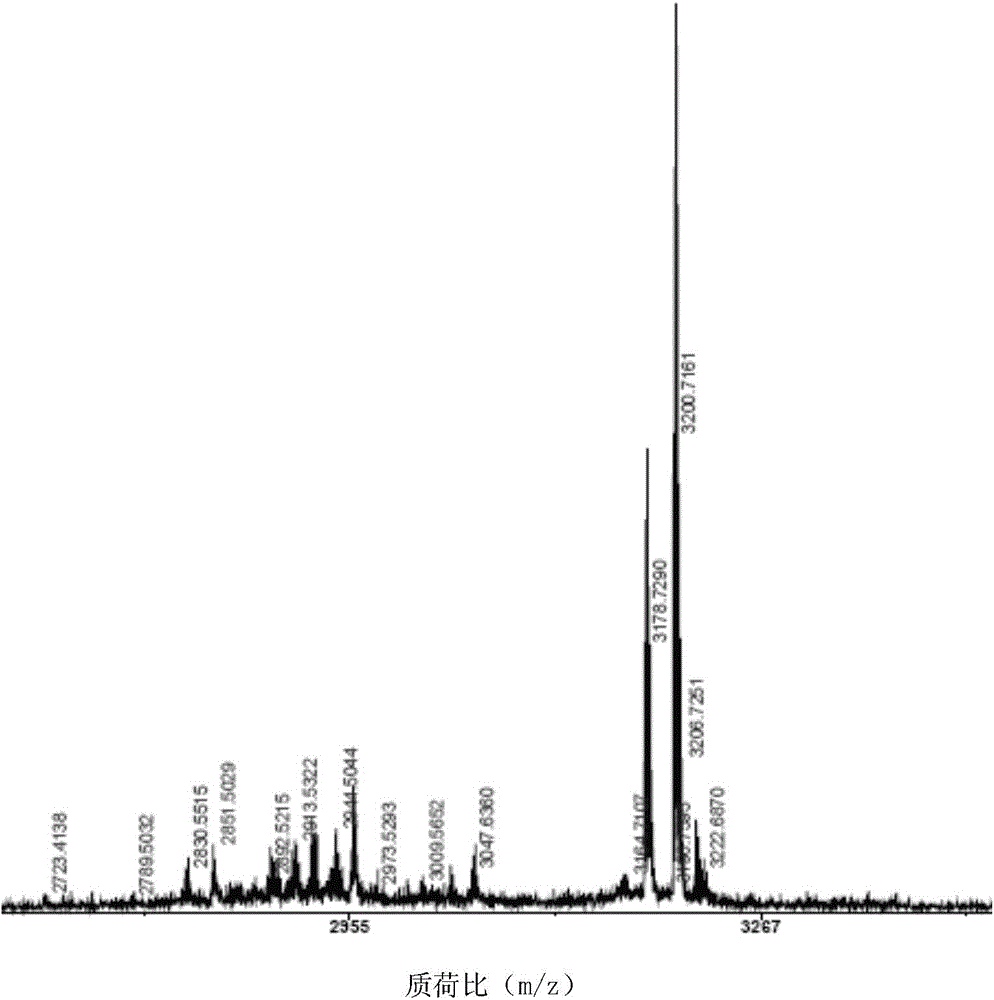
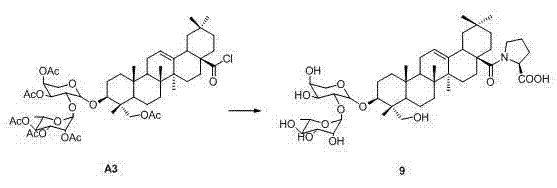
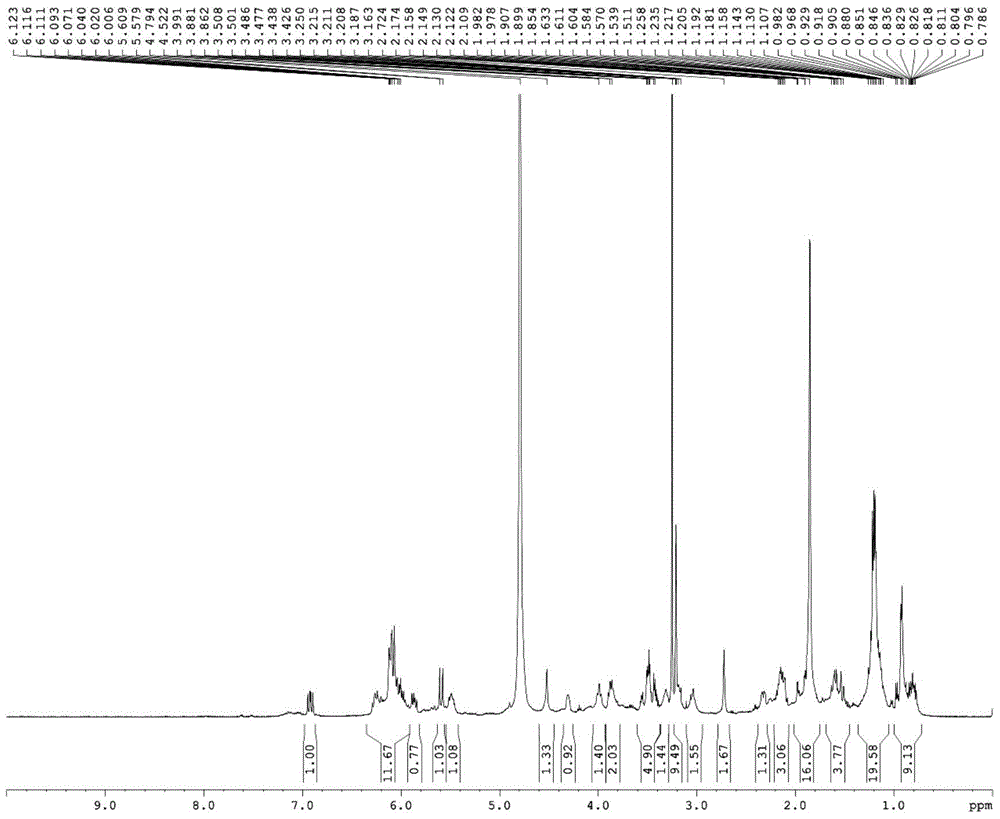
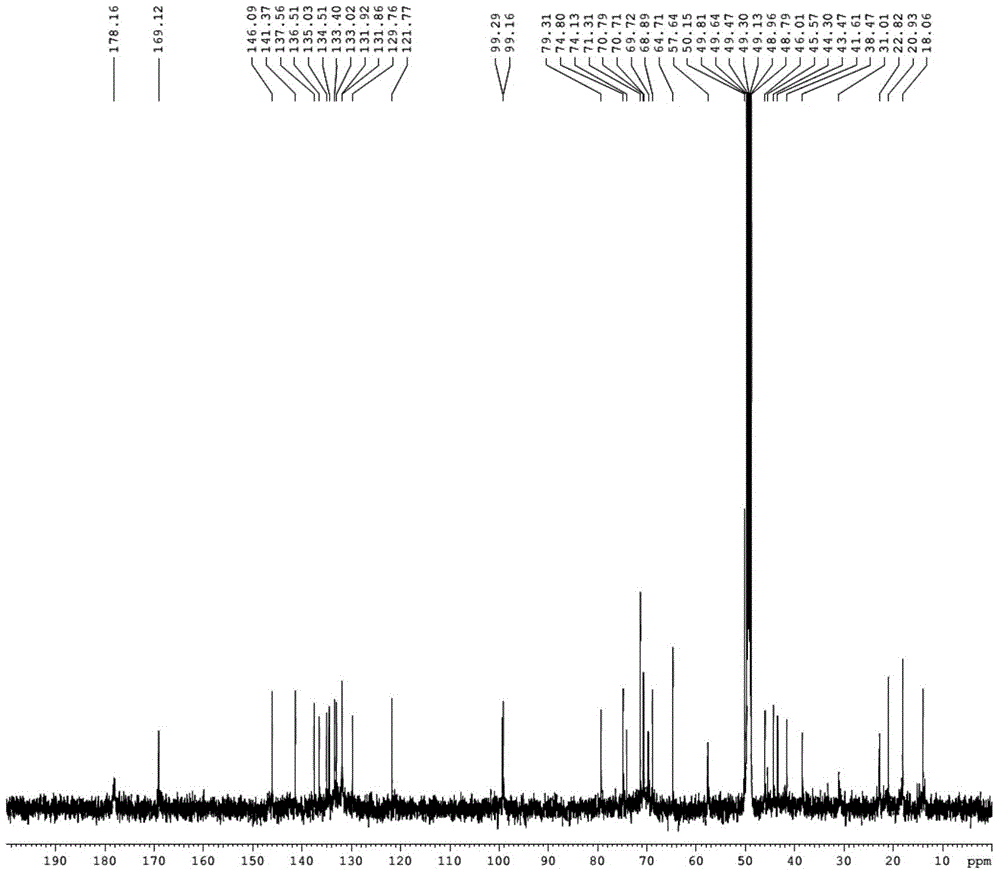
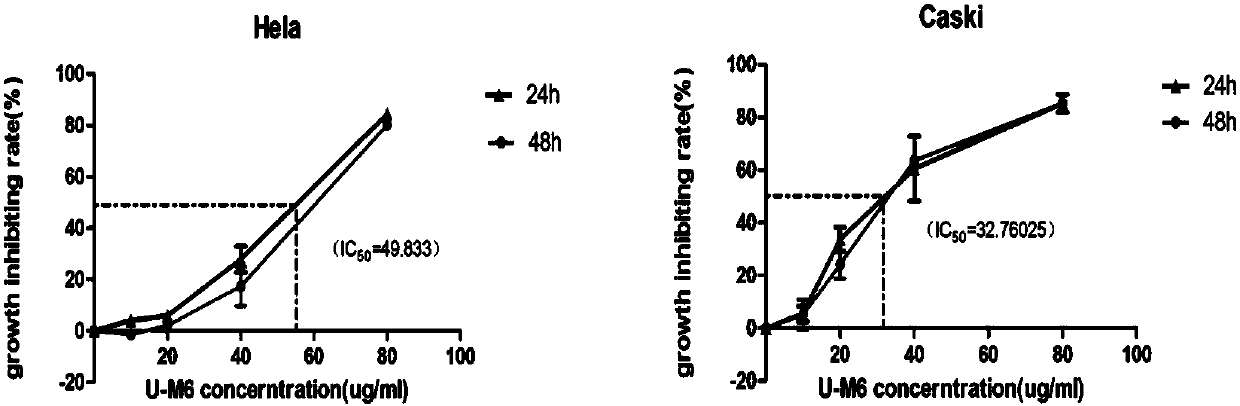
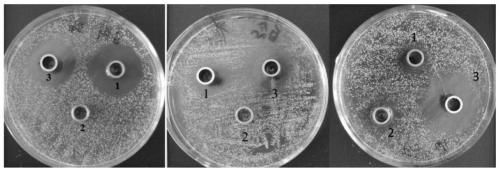
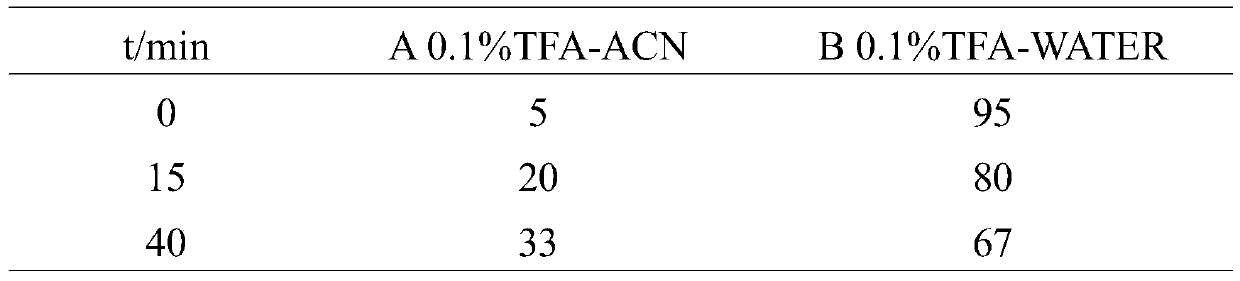
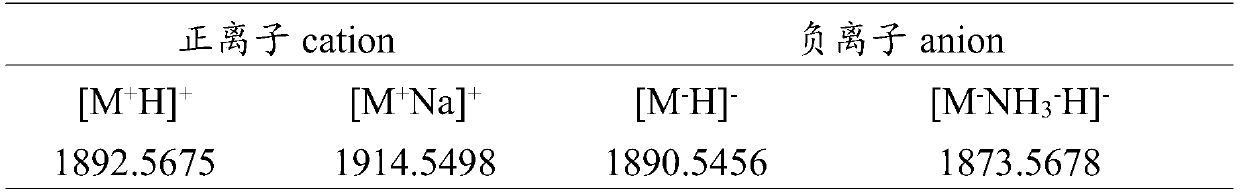
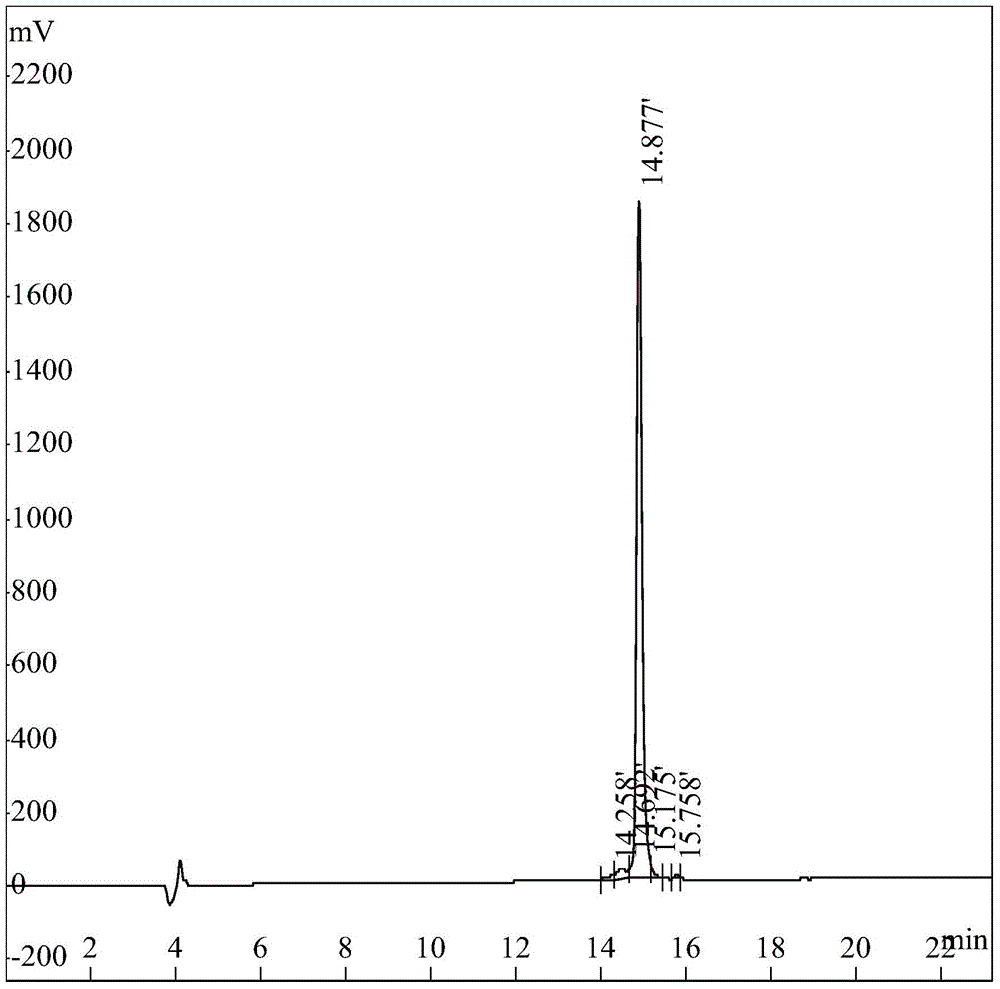
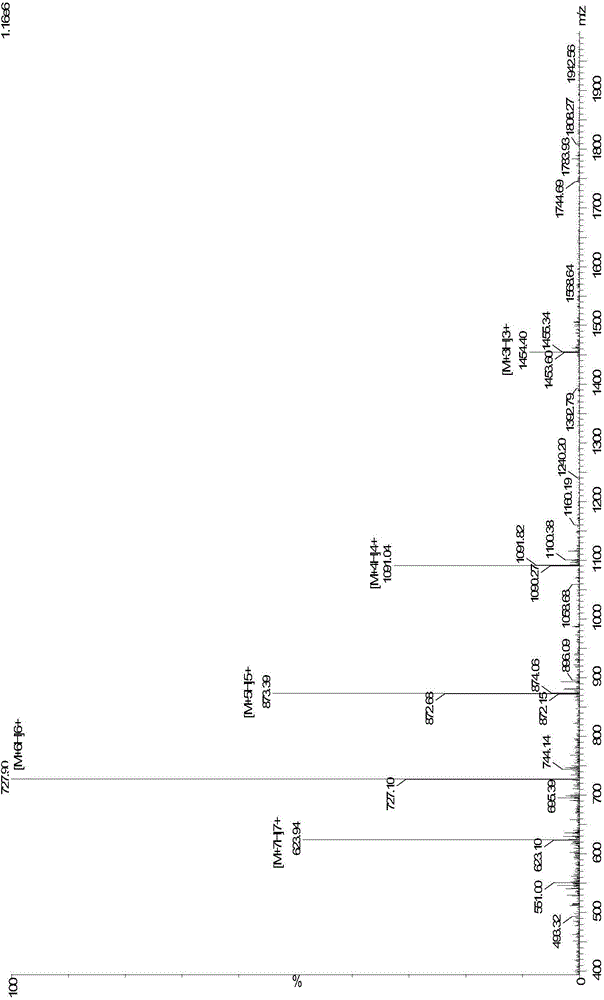
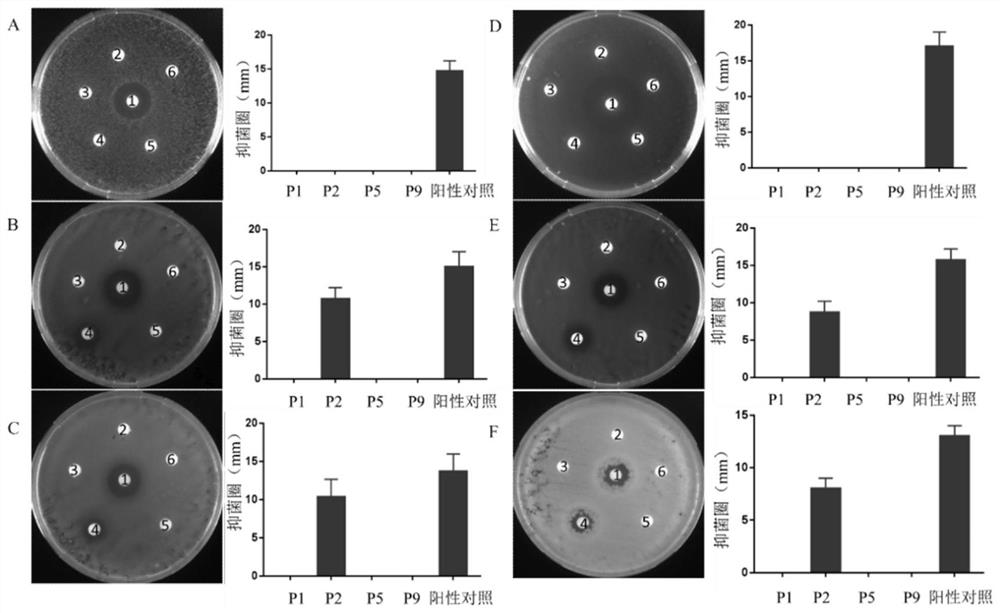
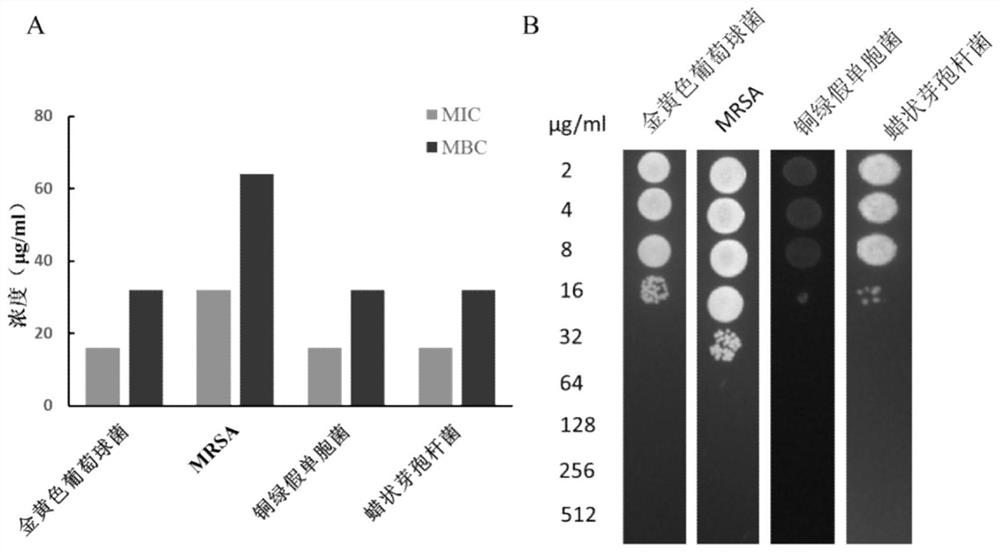
Pillar[5]arene artificial transmembrane channel having antibacterial activity, and production method and application thereof
ActiveCN108017690AEfficient embeddingImprove antibacterial propertiesAntibacterial agentsPeptide/protein ingredientsDiseaseHemolysis
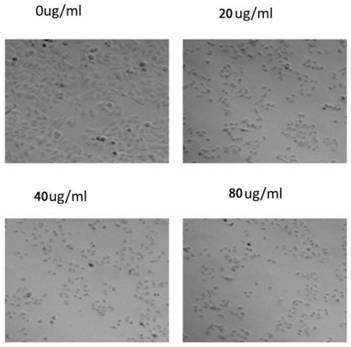
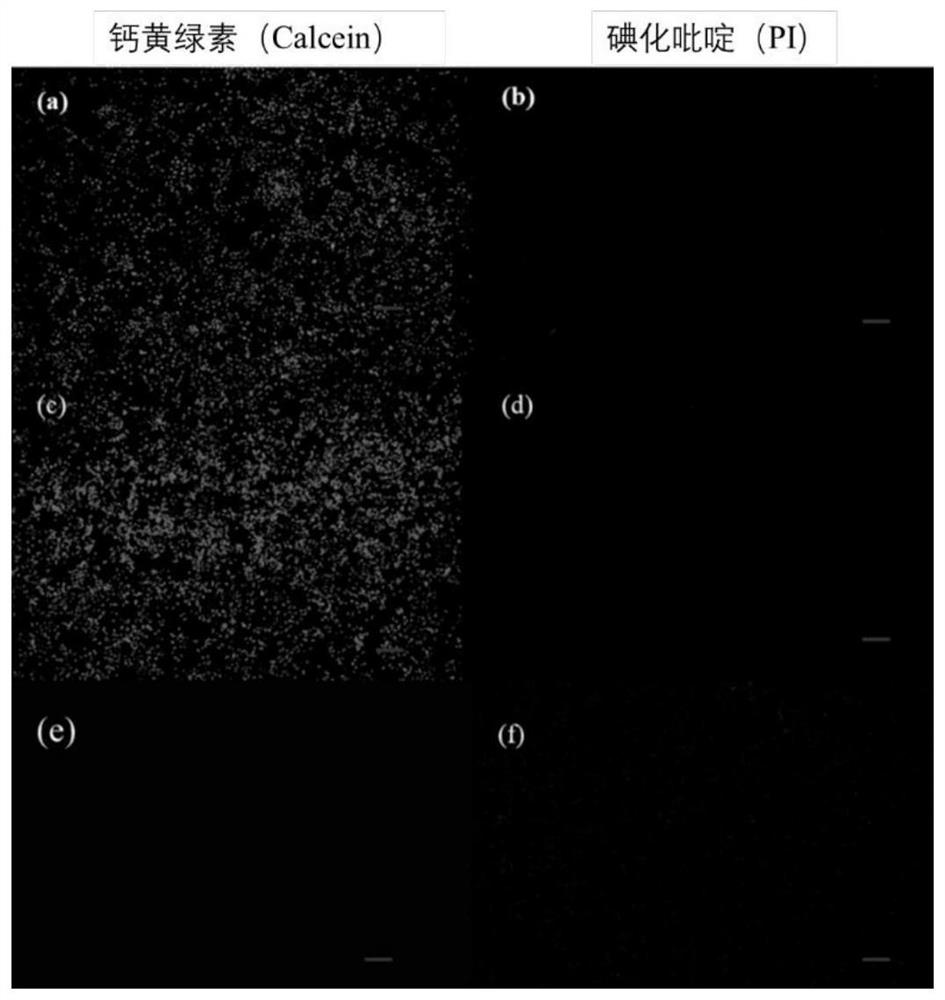
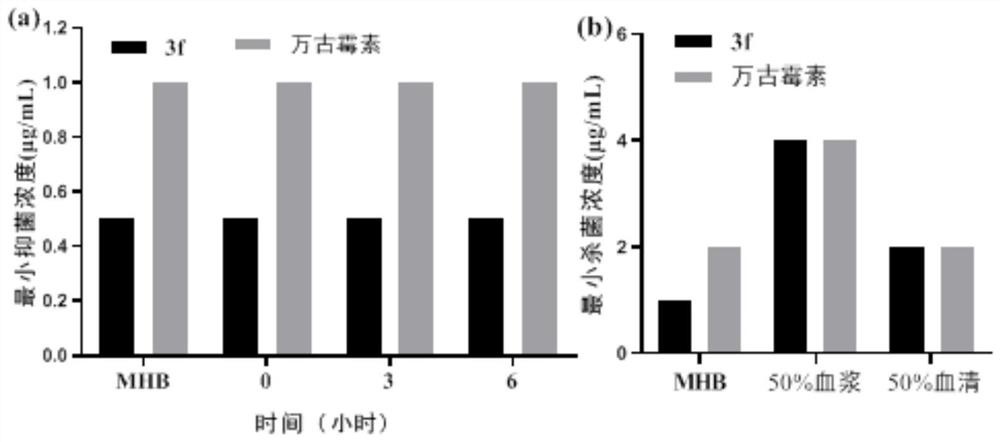
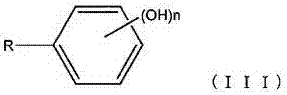
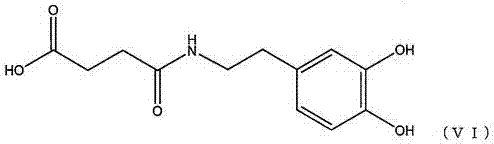
The invention discloses a pillar[5]arene artificial transmembrane channel having an antibacterial activity, and a production method and an application thereof, and belongs to the technical field of compounds having an antibacterial activity. The structural general formula of the pillar[5]arene artificial transmembrane channel having the antibacterial activity is shown in the description. The invention also discloses the production method of the pillar[5]arene artificial transmembrane channel having the antibacterial activity, and an application of the pillar[5]arene artificial transmembrane channel in the preparation of medicines for treating or preventing bacterium induced diseases. The artificial transmembrane channel produced in the invention has an excellent antibacterial performance,so the artificial transmembrane channel can be well applied to the prevention and treatment of bacterium induced animal and plant diseases; and the produced artificial transmembrane channel has very low hemolysis toxicity, so the artificial transmembrane channel can be used as a whole-body ion channel type antibiotic candidate medicine.
Owner:HENAN NORMAL UNIV
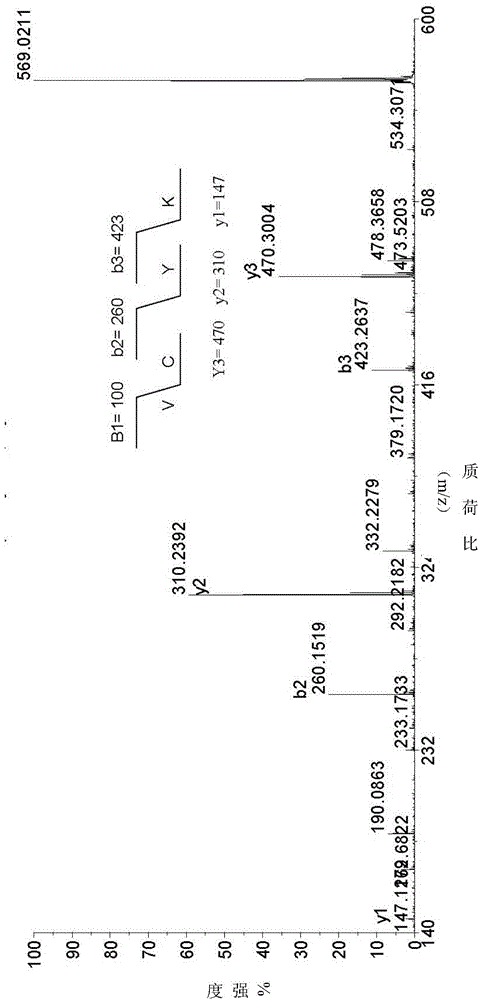
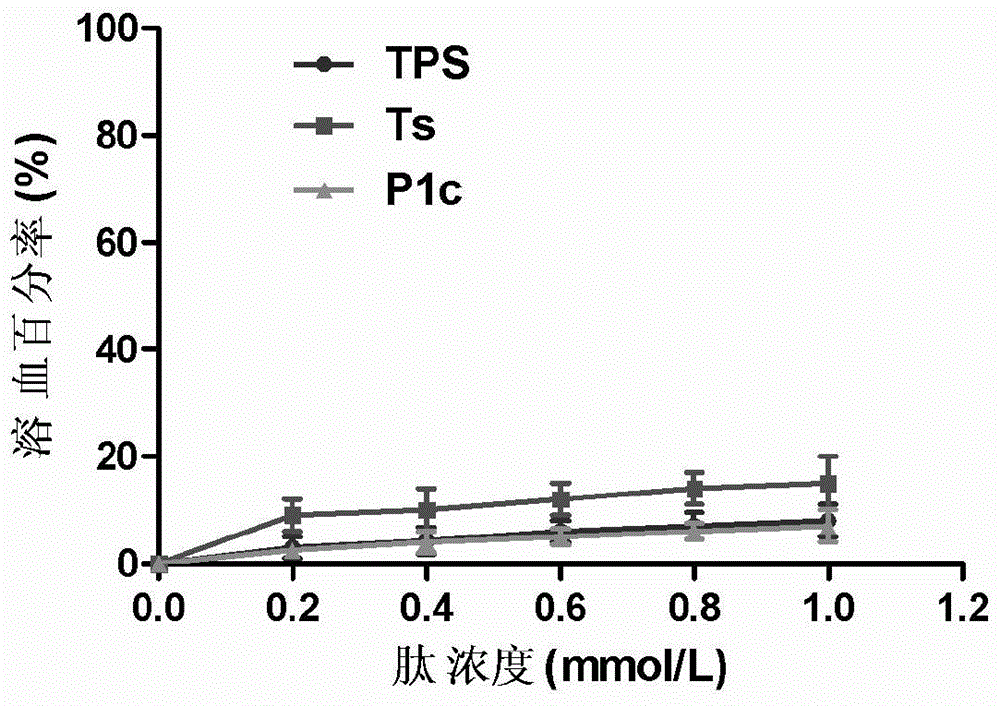
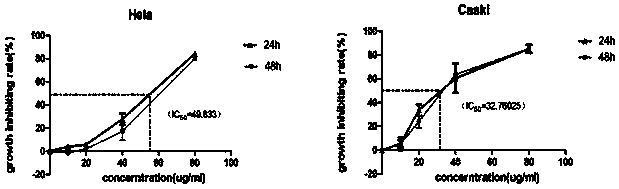
Symmetric short-sequence antimicrobial peptide analogues and application thereof
ActiveCN111363010ANovel design strategySimple designAntibacterial agentsPeptide/protein ingredientsHemolysisAntimicrobial drug
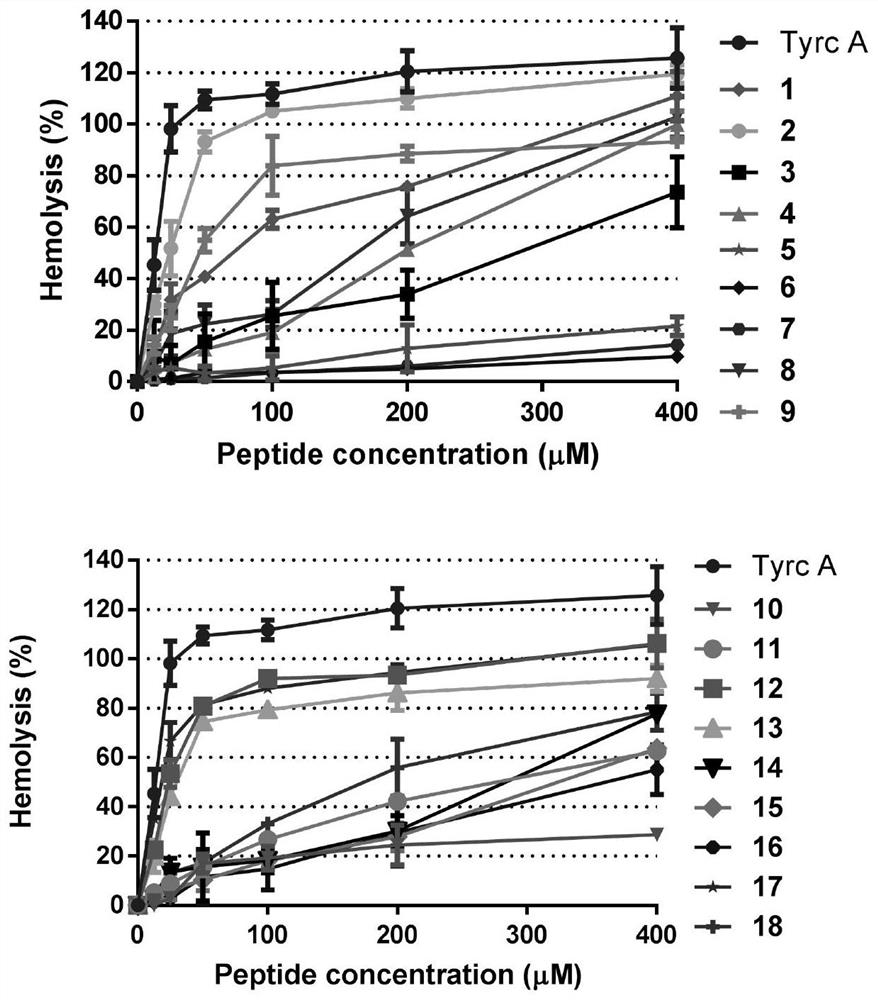
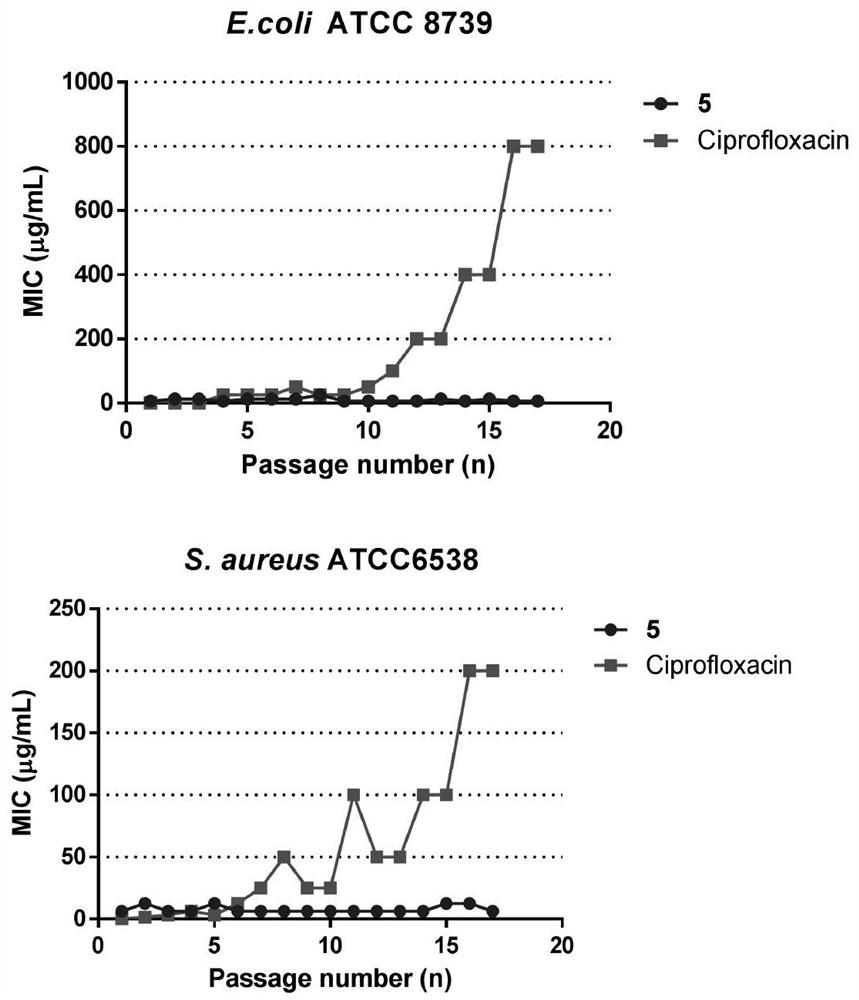
The invention designs and synthesizes a class of symmetric short-sequence antibacterial peptide analogues. The symmetric short-sequence antibacterial peptide analogues are obtained by taking tryptophan ''WWW'' as a mirror symmetry center by introducing arginine and hydrophobic amino acid of positive charges to two sides respectively, and then, carrying out C terminal amidation, and structural formulas of the symmetric short-sequence antibacterial peptide analogues are XYWWWYX-NH 2, XWYWWWYWX-NH 2 and XYYWWWYYX-NH2 respectively, wherein X is equal to G, I, L, F, W, V, A, and Y is equal to R. The antibacterial peptide analogues are simple in structural design, and low in manufacturing cost. In vitro bacteriostasis experiments, hemolysis experiments and induced resistance experiments show that the symmetric short-sequence antimicrobial peptide analogues have high antibacterial activity on strains of common gram-positive bacteria and gram negative bacteria, and have low hemolytic toxicity,cannot easily induce bacteria to produce drug resistance, and have good application prospects in the preparation of clinical antibacterial drugs.
Owner:倪京满 +1
Antibacterial pentapeptide derivative and application thereof
ActiveCN107344958ALow hemolysis rateLow hemolytic toxicityAntibacterial agentsPeptide/protein ingredientsAntibacterial activityGram-positive bacterium
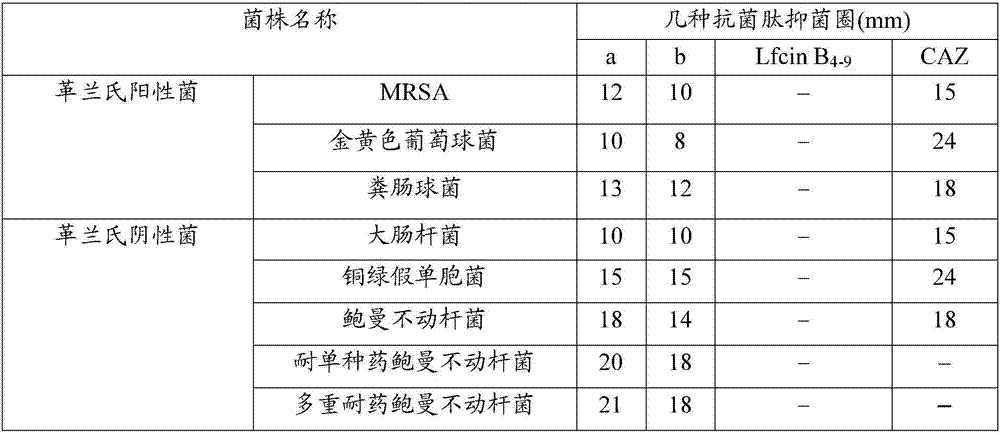
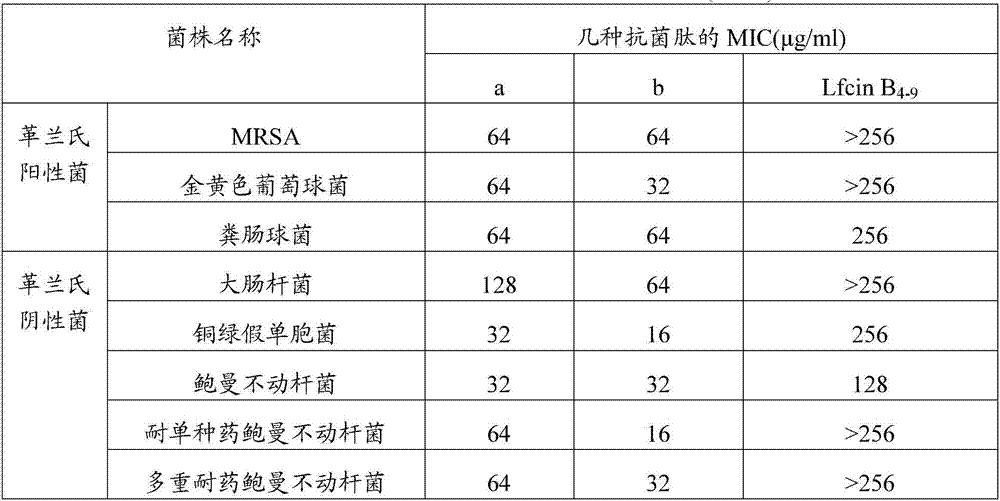
The invention discloses an antibacterial pentapeptide derivative and application thereof. The sequence of the antibacterial pentapeptide derivative is Arg-Arg-Trp-Trp-Arg-beta-phenethylamine. The antibacterial pentapeptide derivative is applied to a medicament for treating or preventing bacterial infection. The bacterium is multi-drug resistant acinetobacter baumannii. The novel antibacterial pentapeptide derivative synthesized by artificial design, provided by the invention, can be conveniently obtained by a solid-phase synthesis method. The synthesized antibacterial peptide has wide spectrum killing activity for gram-positive bacteria and gram-negative bacteria, particularly shows higher antibacterial activity for the multi-drug resistant acinetobacter baumannii, is extremely-low in hemolytic toxicity, and can be applied to medicaments for treating or preventing diseases caused by the multi-drug resistant acinetobacter baumanni.
Owner:CHONGQING UNIV OF TECH
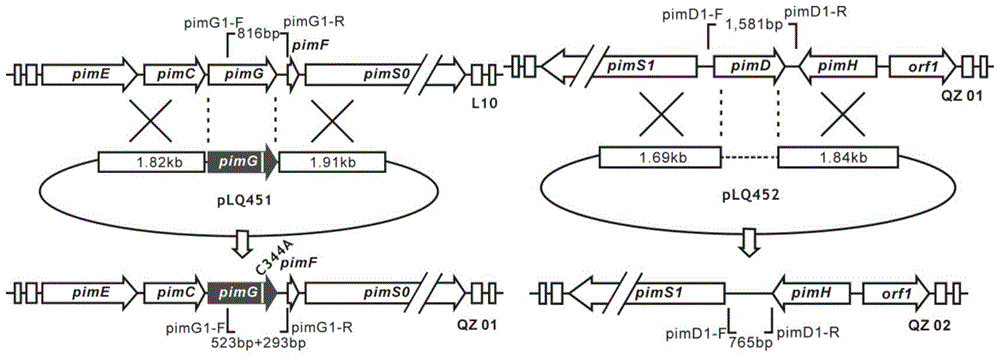
Low-toxicity pimaricin derivative as well as preparation method and application thereof
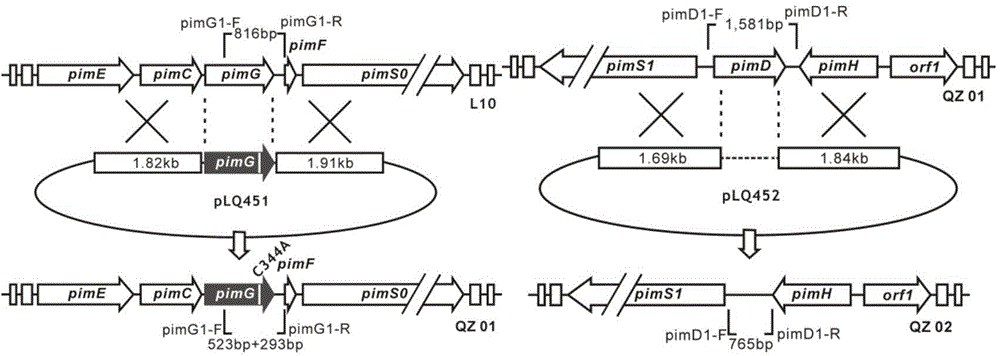
ActiveCN104447917ALow antifungal activityGood antifungal activityAntimycoticsSugar derivativesStructural formulaHigh activity
The invention discloses a low-toxicity pimaricin derivate. A molecular formula of the low-toxicity pimaricin derivative is C33H49NO10 and the chemical structural formula is shown in the specification. The low-toxicity pimaricin derivative is obtained by performing production strain (Streptomyces chattanoogensis QZ02) construction, fermentation and culture of mutant strain QZ02, thallus treatment, organic reagent extraction, reversed phase column chromatography and high performance liquid chromatography separation. The compound has the advantages of stable structure, high activity and extremely low toxicity, and can be used as an antifungal drug and a food antiseptic; therefore, the low-toxicity pimaricin derivative has good application prospect.
Owner:上海览通生物科技有限公司
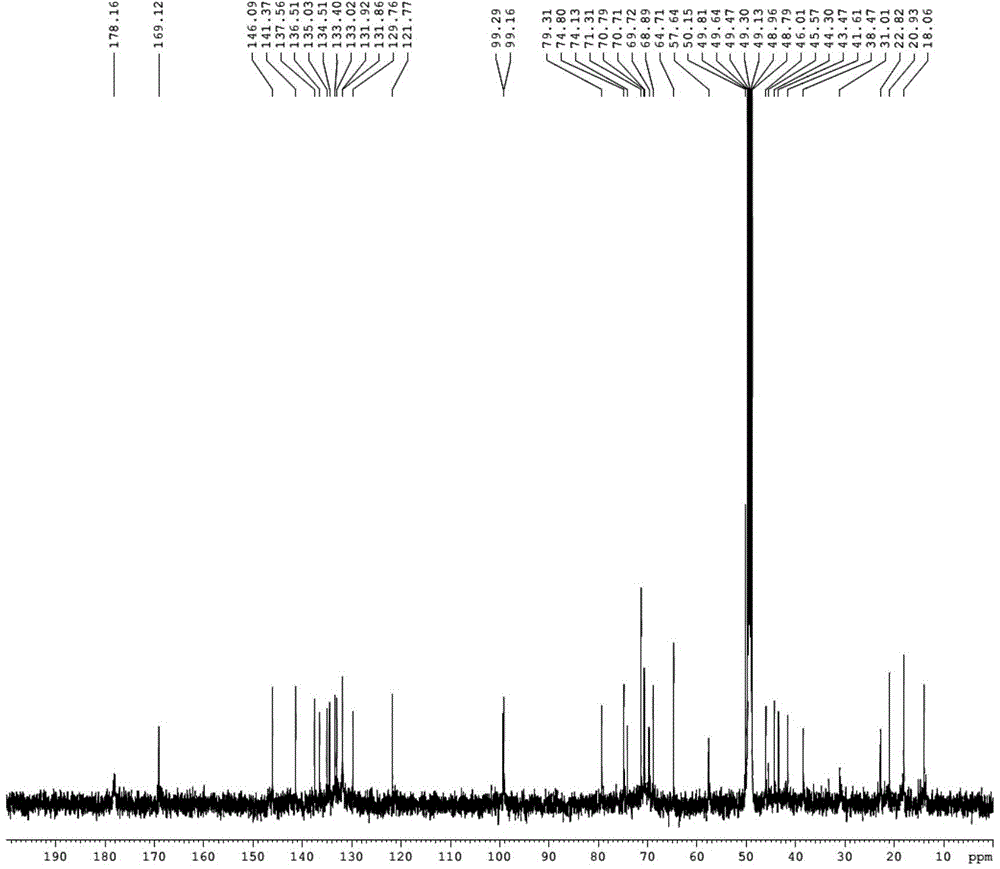
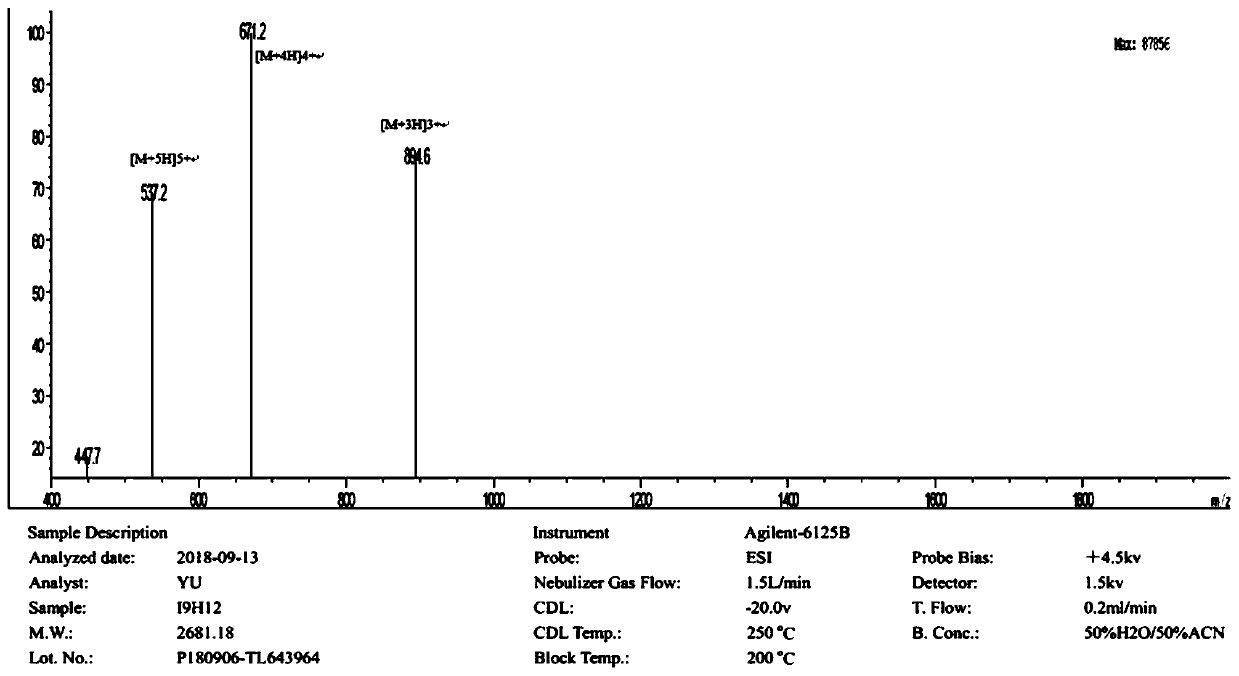
Enzymolysis-resisting antibacterial peptide I9H12 as well as preparation method and application thereof
ActiveCN109810178ASimple experimental techniqueHigh application potentialAntibacterial agentsAntimycoticsEnzyme digestionFungal Infectious Disease
The invention provides enzymolysis-resisting antibacterial peptide I9H12 as well as a preparation method and application thereof. A sequence of the antibacterial peptide I9H12 is shown as SEQ ID No. 1in a sequence table. The preparation method comprises the following steps: referring to the properties of amino acids; meanwhile, avoiding enzyme digestion sites of main proteinase in a body as muchas possible, and selecting hydrophobic amino acid isoleucine (Ile) and positive-charge amino acid histidine (His) as main amino acids to newly design the antibacterial peptide I9H12; carrying out amidation on a carboxyl terminal of the peptide to improve one positive charge and increase the stability of the peptide. The invention also provides the application of the antibacterial peptide to the preparation of targeting antibacterial medicines for treating gram-negative bacterium or fungus infectious diseases. According to the enzymolysis-resisting antibacterial peptide I9H12, the enzymolysis-resisting capability of the antibacterial peptide is effectively improved, and the application potential in actual production is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
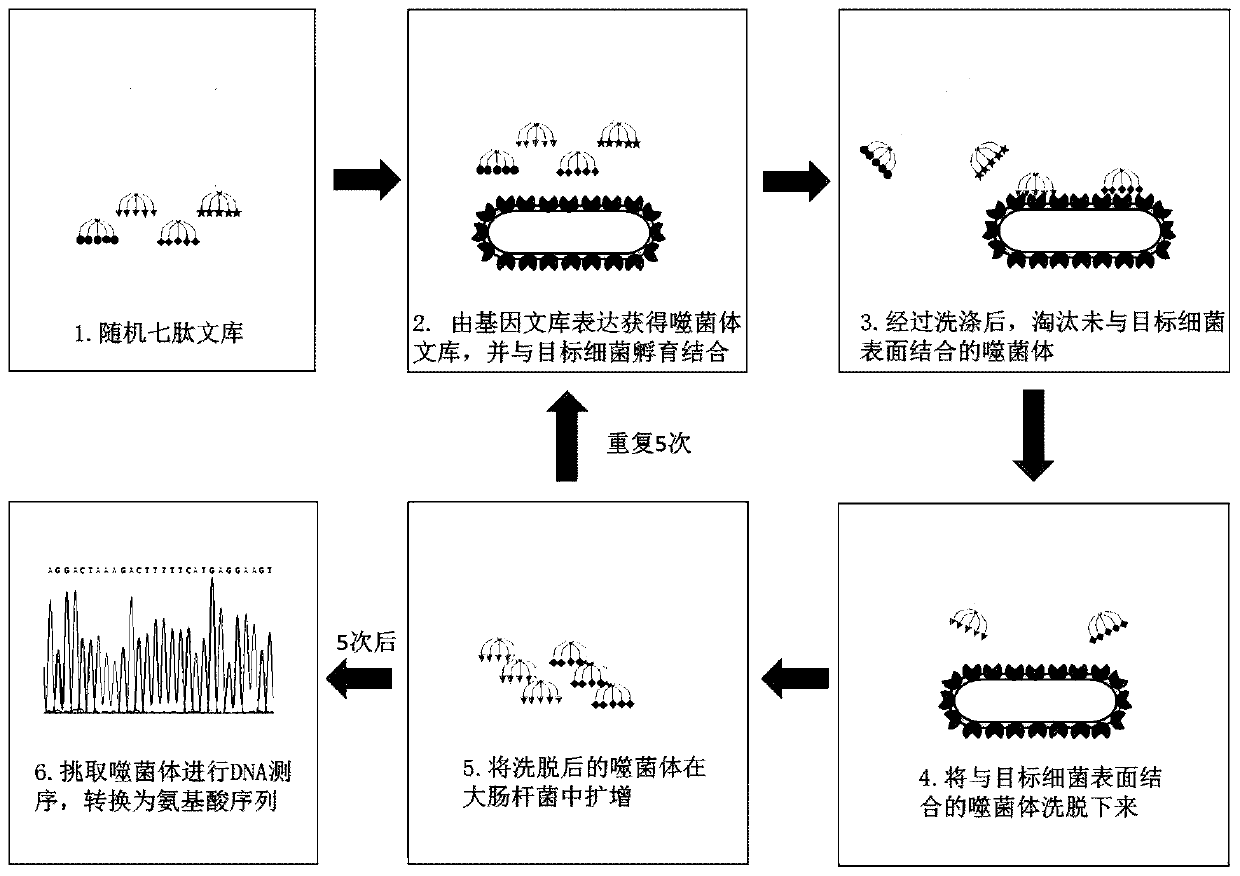
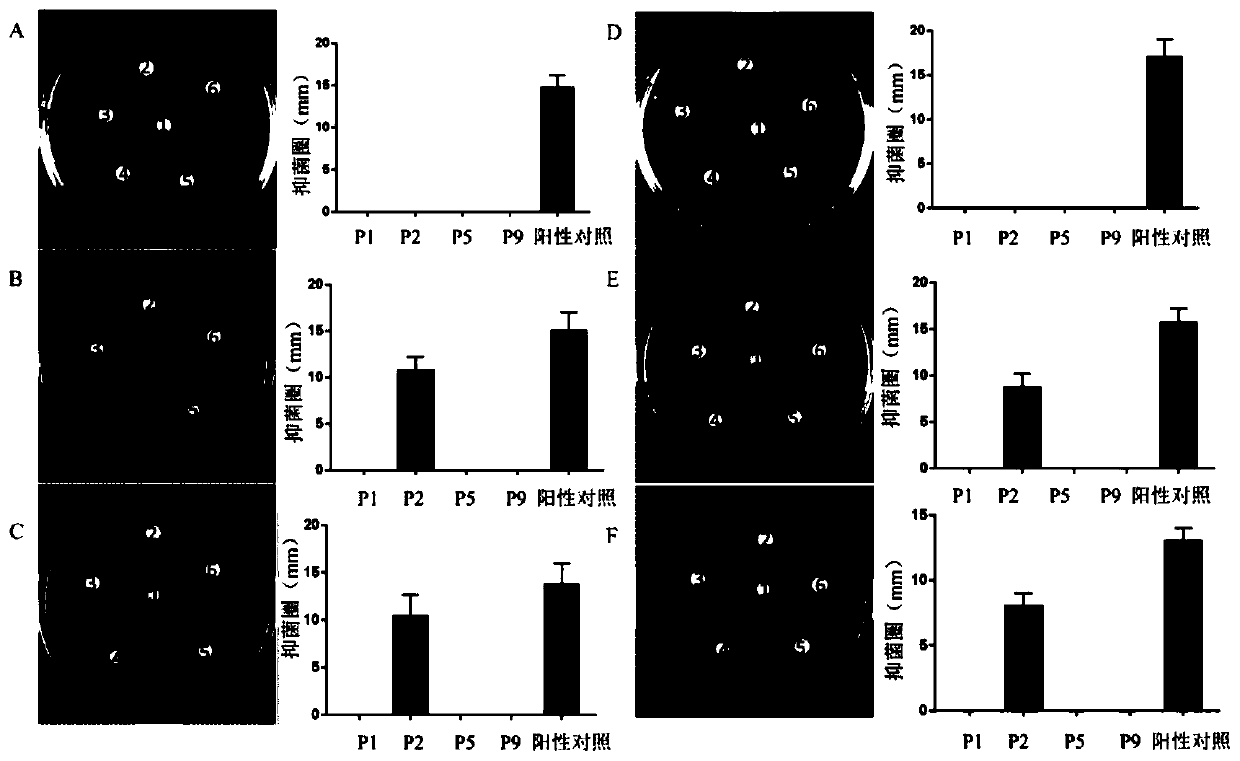
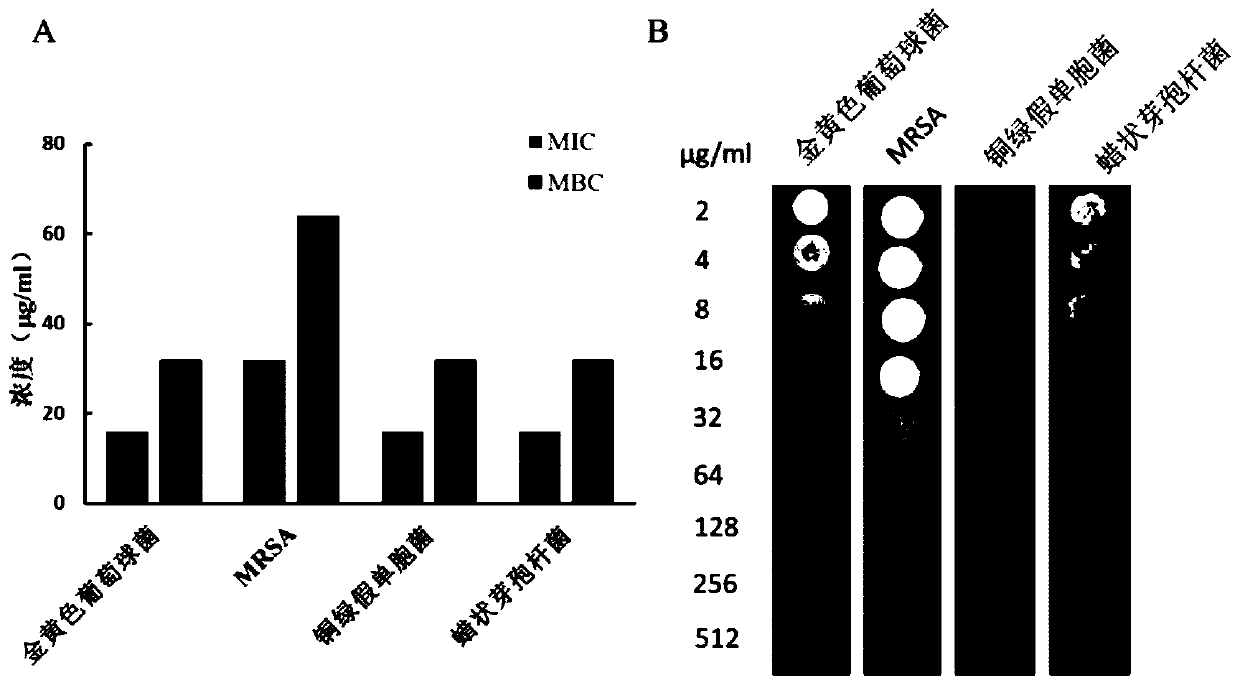
Screening method of antimicrobial peptide and application of screening method
ActiveCN110734474ASmall molecular weightReduce manufacturing costAntibacterial agentsPeptide/protein ingredientsChemical synthesisRandom Peptide Library
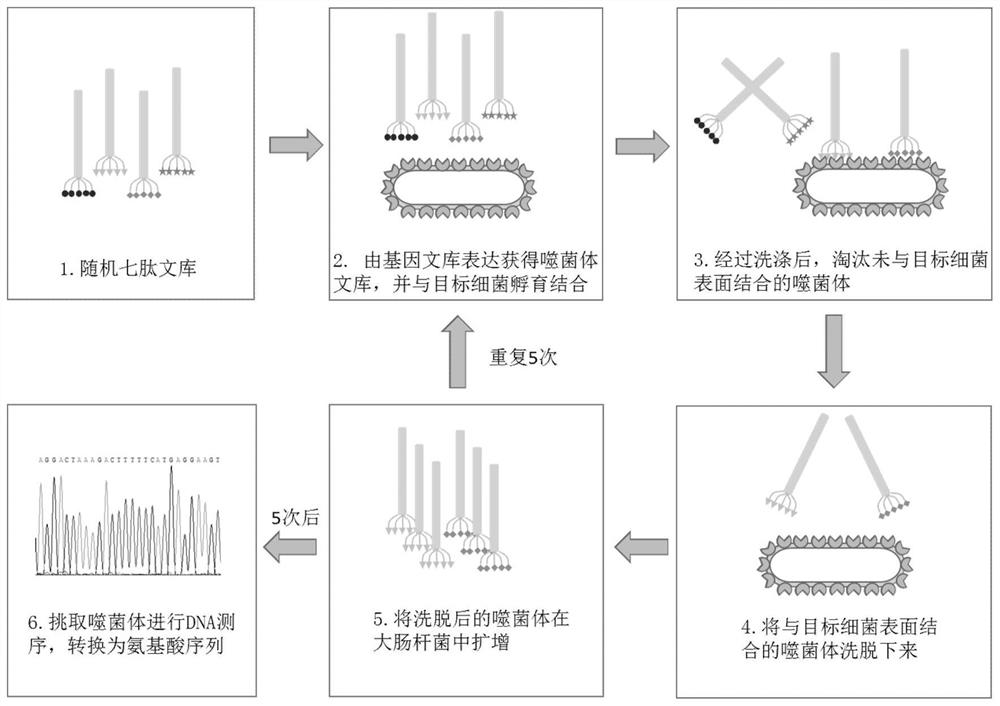
The invention relates to the technical field of antibacterial peptide, in particular to a screening method of the antimicrobial peptide and an application of the screening method, and discloses a screening method of the antimicrobial peptide. In accordance with specific pathogenic bacteria, the screening method can quickly screen the antibacterial peptide having excellent resistance. The polypeptide molecular weight in a bacteriophage random peptide library selected in the screening method is small, so that the screened antibacterial peptide is small in molecular weight, and easy to obtain bya chemosynthesis method, and the production cost of the antibacterial peptide is reduced. The antibacterial peptide screened by the screening method has high bactericidal activity for pathogenic bacteria, and the cytotoxicity and the hemolytic activity are low.
Owner:SUN YAT SEN UNIV
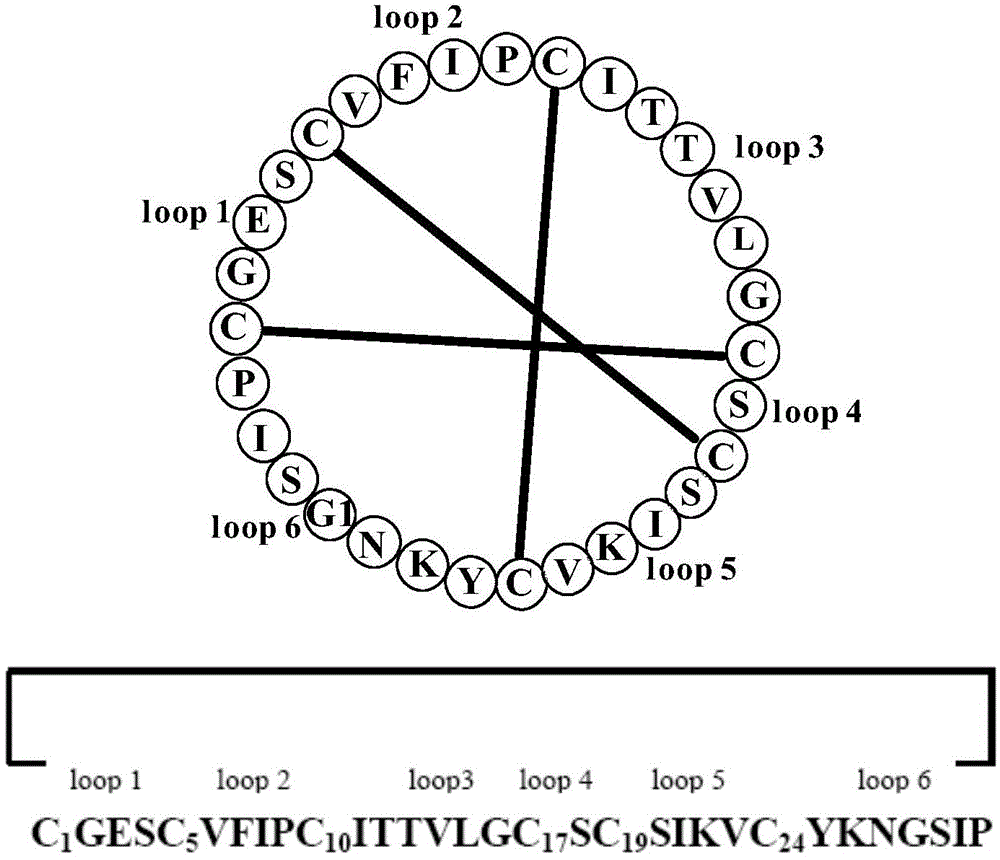
Application of novel cyclic peptide
ActiveCN104974234AMaintain stabilityLow hemolytic toxicityAntibacterial agentsCosmetic preparationsCyclic peptideDisulfide bonding
The invention relates to a cyclic peptide with antiviral, antibacterial and antifungal activity. The cyclic peptide has an amino acid residue sequence Cyclo (CGESCVFIPCITTVLGCSCSIKVCYKNGSIP) shown in SEQ ID NO:1, wherein three pairs of disulfide bonds are respectively formed by Cys in the first and the 17th sites, Cys in the fifth site and the 19th site and Cys in the 10th site and the 24th site, so that the cyclic peptide has thermotropy resistance and proteaseenzymolysis stability, and is low in hemolytic toxicity. Therefore, a pharmaceutical composition containing the cyclic peptide or salts thereof can be used for preventing or treating the diseases such as influenza viruses A, bacteria and fungi of people or livestock.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Novel alpha-hederin derivative and preparation method and use thereof
InactiveCN104761610AStrong cytotoxicityLow hemolytic toxicitySugar derivativesSteroidsPharmaceutical drugPerylene derivatives
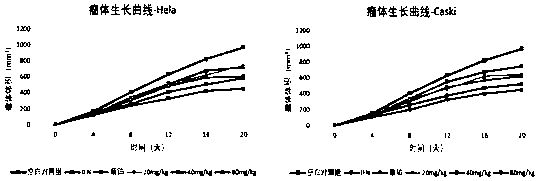
The invention belongs to the technical field of medicines, in particular relates to a novel alpha-hederin derivative and a preparation method and use thereof, and more particularly relates to a novel alpha-hederin derivative shown in general formula (I) and a preparation method and anti tumor use thereof. Compared with alpha-hederin, the novel alpha-hederin derivative has significant cytotoxicity, hemolytic toxicity is greatly reduced, and the novel alpha-hederin derivative has certain antitumor activity on a tumor-bearing mice model, and can be used for preparing anti-tumor drugs or tumor adjuvant treatment drugs.
Owner:JIANGXI HERBFINE HI TECH
Antimicrobial hexapeptide and its derivative and application
ActiveCN106916205AHigh antibacterial activityHigh bactericidal activityAntibacterial agentsPeptide/protein ingredientsAntimicrobial peptidesAntibacterial activity
The invention discloses an antimicrobial hexapeptide. The antimicrobial hexapeptide has a sequence of Arg-Arg-Trp-Trp-Arg-Trp. The antimicrobial hexapeptide can be used in preparation of drugs for treating or preventing bacterial infection. The invention also provides an antimicrobial hexapeptide derivative. The antimicrobial hexapeptide derivative has a sequence of Arg-Arg-Trp-Trp-Arg-Trp-beta-phenylethylamine. The antimicrobial hexapeptide derivative can be used in preparation of drugs for treating or preventing bacterial infection. The bacterium is drug-resistant acinetobacter baumannii. The artificially designed synthetic antimicrobial hexapeptide and its phenylethylamine-modified product can be conveniently obtained by a solid phase synthesis method. The synthetic antimicrobial peptide and its derivative have broad spectrum killing activity to Gram-positive bacteria and Gram-negative bacteria, and particularly, the derivative exhibits stronger antibacterial activity to drug-resistant acinetobacter baumannii, has very small hemolytic toxicity and can be used in drugs for treatment or prevention of diseases caused by drug-resistant acinetobacter baumannii.
Owner:湖南湃洵生物科技有限公司
Anti-enzymolysis branched antibacterial peptide Pal-CRKP as well as preparation method and application thereof
ActiveCN114805495AHigh application potentialImprove stabilityAntibacterial agentsPeptide/protein ingredientsSide chainAklanonic acid
The invention provides an anti-enzymolysis branched antibacterial peptide Pal-CRKP as well as a preparation method and application of the anti-enzymolysis branched antibacterial peptide Pal-CRKP. The amino acid sequence of the branched antibacterial peptide Pal-CRKP is as follows: Pal-GGGK (CRKPCRKP) CRKPCRKP, and Pal is n-hexadecanoic acid. According to the preparation method, by utilizing the characteristics of a lysine side chain, n-hexadecanoic acid and an anti-enzymolysis peptide sequence unit are combined by utilizing lysine to form a branched antibacterial macromolecule, and the branched antibacterial peptide with high antibacterial activity, low toxicity and high stability is designed. The anti-enzymolysis branched antibacterial peptide Pal-CRKP not only shows high-efficiency bacteriostatic activity on gram-negative bacteria, but also has a high-efficiency inhibition effect on gram-positive bacteria, meanwhile, the anti-enzymolysis branched antibacterial peptide Pal-CRKP has relatively low hemolytic toxicity, and the degradation degree of high-concentration protease on the branched antibacterial peptide Pal-CRKP is very weak, so that the anti-enzymolysis branched antibacterial peptide Pal-CRKP can be used for preparing the anti-enzymolysis branched antibacterial peptide Pal-CRKP. The anti-enzymolysis branched antibacterial peptide disclosed by the invention has relatively high development potential of replacing antibiotics.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Low toxicity pimamycin derivatives and preparation method and application thereof
ActiveCN104447917BLow antifungal activityGood antifungal activityAntimycoticsSugar derivativesStructural formulaHigh activity
The invention discloses a low-toxicity pimaricin derivate. A molecular formula of the low-toxicity pimaricin derivative is C33H49NO10 and the chemical structural formula is shown in the specification. The low-toxicity pimaricin derivative is obtained by performing production strain (Streptomyces chattanoogensis QZ02) construction, fermentation and culture of mutant strain QZ02, thallus treatment, organic reagent extraction, reversed phase column chromatography and high performance liquid chromatography separation. The compound has the advantages of stable structure, high activity and extremely low toxicity, and can be used as an antifungal drug and a food antiseptic; therefore, the low-toxicity pimaricin derivative has good application prospect.
Owner:上海览通生物科技有限公司
Novel recombinant bee venom polypeptide as well as preparation method and application of novel recombinant bee venom polypeptide
InactiveCN107722115AGood antitumor activityLow hemolytic toxicityPeptide/protein ingredientsAntiviralsPichia pastorisNucleotide
The invention relates to a novel recombinant bee venom polypeptide of which an amino acid sequence is SEQ ID No.1. A nucleotide sequence for encoding a novel recombinant bee venom protein is SEQ ID No.2. The preparation method comprises the steps: connecting a UA8 oligopeptide and a melittin gene to form a recombinant bee venom fusion gene, an eukaryotic expression vector U-melittin-pPICZalpha ofthe recombinant bee venom fusion gene is constructed, and pichia pastoris is transferred to carry out induced expression of the recombinant bee venom protein and also carry out expression and purification. The novel recombinant bee venom polypeptide has stronger antitumor activity and lower hemolytic toxicity than a melittin and is applied to the prevention and treatment of cervical cancer causedby an HPV virus.
Owner:JILIN UNIV
Hornet phallotoxin antitone analog WVD-II and preparation method and application thereof
ActiveCN111100190AImprove the bactericidal effectImprove stabilityAntibacterial agentsAntimycoticsBiotechnologyCell membrane
The invention provides a hornet phallotoxin antitone analog WVD-II and a preparation method and application thereof, and belongs to and relates to the technical field of polypeptide synthesis. The amino acid sequence of the hornet phallotoxin antitone analog WVD-II is as shown in SEQID NO:1. The N end of the hornet phallotoxin antitone analog WVD-II disclosed by the invention has positive charges,so that the N end can be combined with a cell membrane having negative electricity; an alpha helical structure is formed from hydrophobic amino acids at a C end, and can bore a hole in the surface ofthe cell membrane, so that death of bacteria is caused. The in vitro antibacterial activity determination indicates that the hornet phallotoxin antitone analog WVD-II disclosed by the invention has significant sterilizing effects on gram-positive bacteria, gram-negative bacteria and fungi, has good stability, is low in hemolytic toxicity and has the characteristics of being broad-spectrum, efficient and safe. The hornet phallotoxin antitone analog WVD-II disclosed by the invention can be used as an active component clinically for replacing traditional antibiotics for treatment.
Owner:DALI UNIV
Small peptide having tumor targeting and cell penetrating properties and application of small peptide
InactiveCN104530200ALow hemolytic toxicityDepsipeptidesMacromolecular non-active ingredientsSmall peptideTumor targeting
The invention discloses a small peptide having tumor targeting and cell penetrating properties and an application of the small peptide. The amino acid sequence of the small peptide is shown in GSKKPVPIIYCNRRSGKCQRMGSIRTPKISKPIKFELSG. The small peptide (PTS) disclosed by the invention shows relatively low hemolytic toxicity in vitro; flow cytometry shows that the FITC-PTS can be specifically combined to MDA-MB-231 breast cancer cell; upon confocal microscopy observation, the fluorescence of the FITC-PTS is mainly concentrated in cytoplasm; and based on in vivo near-infrared fluorescence imaging technique, Cy5.5-PTS probe high signal is detected in alpha-v-beta-3 positive MDA-MB-231 tumor.
Owner:SOUTHEAST UNIV
Novel recombinant bee venom polypeptide as well as preparation method and application thereof
ActiveCN108623668AGood antitumor activityLow hemolytic toxicityPeptide/protein ingredientsAntiviralsPichia pastorisDisease
The invention relates to a novel recombinant bee venom polypeptide. An amino acid sequence of the novel recombinant bee venom polypeptide is shown in SEQ ID No.1; a nucleotide sequence encoding a novel recombinant bee venom protein is shown in SEQ ID No.2; a preparation method comprises the following steps: connecting an UA8 short peptide with a melittin gene to form a recombinant bee venom fusiongene, constructing an eukaryotic expression vector U-melittin-pPICZalpha of the recombinant bee venom polypeptide fusion gene, and transferring into Pichia pastoris to induce the expression and the purification of the recombinant bee venom protein. The novel recombinant bee venom polypeptide is stronger in antitumor activity and less in hemolytic toxicity with compared with melittin, and is applied to the prevention and the treatment of cervical-related diseases and cervical cancer caused by an HPV virus.
Owner:JILIN UNIV
Cationic antibacterial peptide as well as preparation method and application thereof
InactiveCN104356202ASimple structureArtificial synthesis is convenientAntibacterial agentsPeptide/protein ingredientsAnti bacterialToxicity
The invention discloses cationic antibacterial peptide as well as a preparation method and application thereof. The sequence of the cationic antibacterial peptide is as follows: SK1:FRWWR-NH2((Phe-Arg-Trp-Trp-Arg-NH2); the preparation method is a solid phase chemical synthesis method. the cationic antibacterial peptide provided by the invention has broad-spectrum killing activity to gram-positive bacteria and gram-negative bacteria and has higher bactericidal activity when being compared with natural antibacterial peptide; besides, the cationic antibacterial peptide has the advantages that the structure is simple, artificial synthesis is convenient, no decorative connection is required, the hemolytic toxicity is small, and no toxic effect is caused to animal and plant cells; the cationic antibacterial peptide has very important values in the aspects of development and application of antibacterial medicines.
Owner:SOUTHWEST UNIVERSITY
Palmitic anti-enzymolysis antibacterial peptide, and preparation method and application thereof
ActiveCN111423493ALow hemolytic toxicityHigh application potentialAntibacterial agentsPeptide/protein ingredientsSide chainMolecular biology
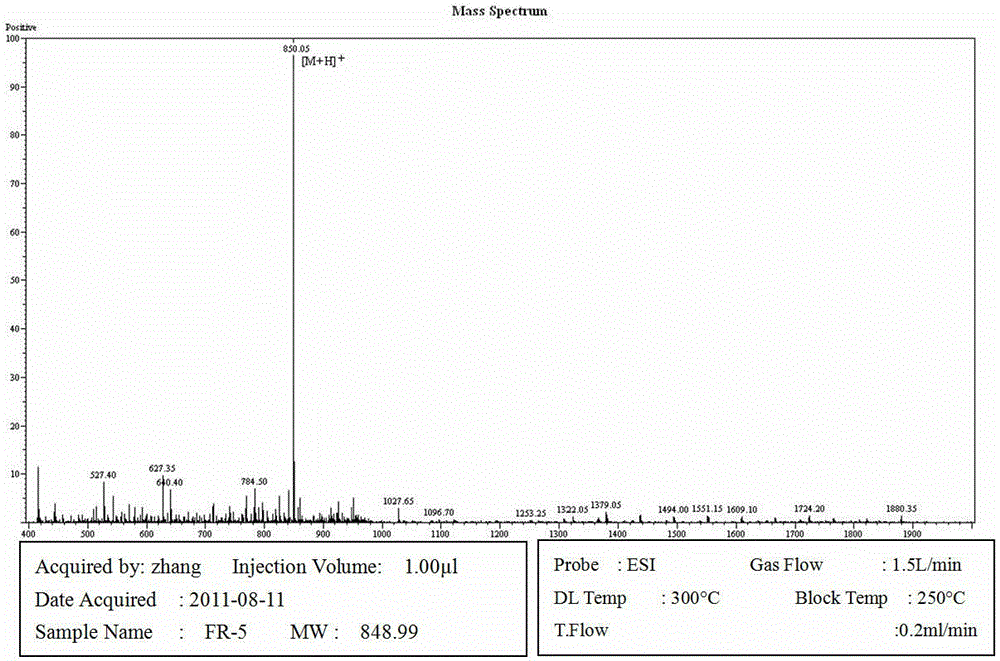
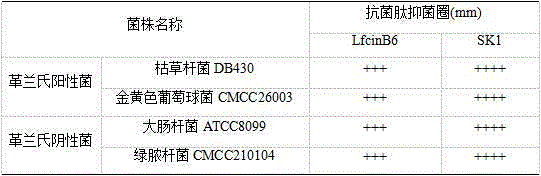
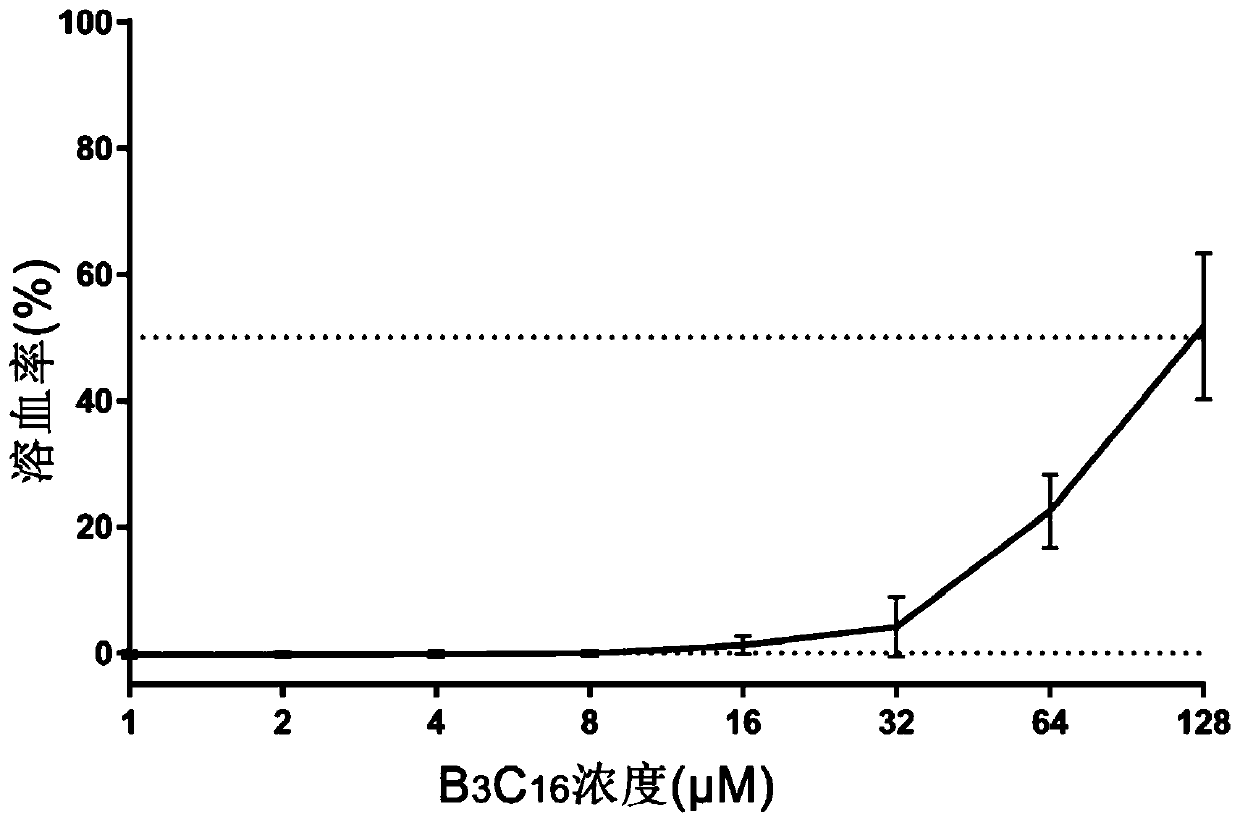
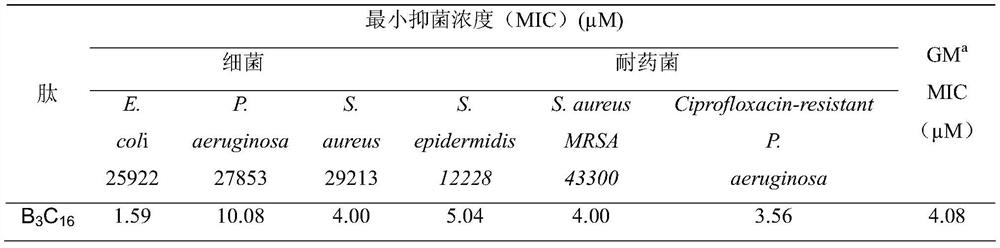
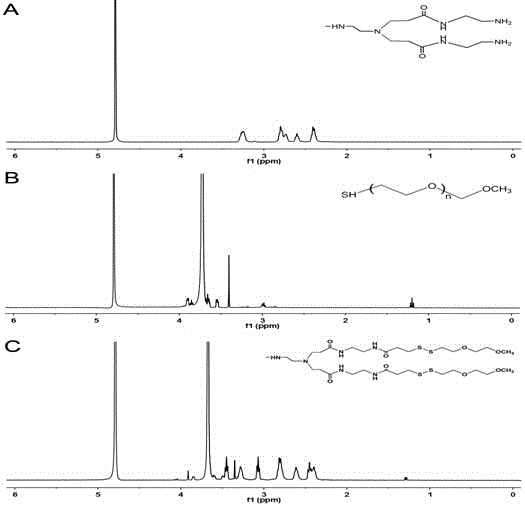
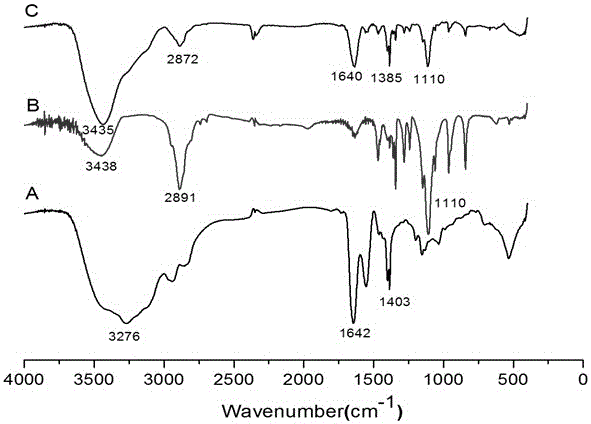
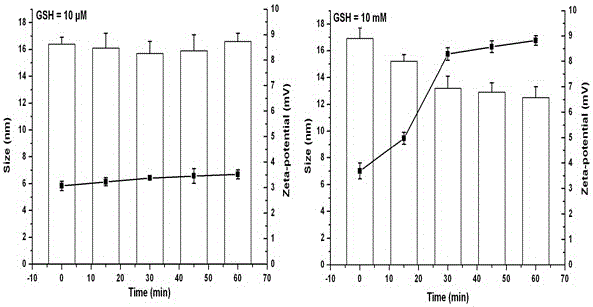
The invention provides a palmitic anti-enzymolysis antibacterial peptide, and a preparation method and application thereof. The sequence of the antibacterial peptide B3C16 is C16-GGGK(PRPR)K(PRPR)RPRP, wherein C16 is palmitic acid. The side chain of each lysine is linked with a polypeptide branched chain PRPR through an amide bond to form steric hindrance between a dendritic structure and a peptide chain; further, palmitic acid is used as a main hydrophobic source of the antibacterial peptide sequence, so that hydrolysis of hydrophobic amino acids by all proteases is perfectly avoided; and finally a flexible amino acid connector GGG is used for linking the palmitic acid with a branched peptide to form a perfect amphiphilic structure. The antibacterial peptide has high-efficiency inhibitioneffect on standard bacteria and drug-resistant bacteria such as Gram-negative bacteria, Gram-positive bacteria and the like, and has very low hemolytic toxicity, and the hydrolysis degree of high-concentration protease to the B3C16 is low.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
A kind of recombinant bee venom polypeptide and its application
ActiveCN108623668BGood antitumor activityLow hemolytic toxicityPeptide/protein ingredientsAntiviralsDiseasePichia
Owner:JILIN UNIV
Tri-cation quaternary ammonium salt antibacterial peptide simulant with antibacterial activity and preparation method thereof
ActiveCN114835594AHigh activityIncreased toxicityAntibacterial agentsOrganic compound preparationBiotechnologyEscherichia coli
The invention belongs to the technical field of medicinal chemistry, and discloses a tri-cationic quaternary ammonium salt antibacterial peptide simulant with antibacterial activity and a preparation method thereof. The compounds have the following structural general formulas I-III. In-vitro antibacterial activity experiments prove that a part of the series of compounds show good activity on gram-positive bacteria staphylococcus aureus and enterococcus faecalis and gram-negative bacteria escherichia coli and stenotrophomonas maltophilia, and the compounds have excellent broad-spectrum antibacterial activity; meanwhile, in-vitro red blood cell hemolytic data show that the compound is relatively low in hemolytic toxicity and relatively good in selectivity.
Owner:ZHENGZHOU UNIV
A kind of screening method of antimicrobial peptide and its application
ActiveCN110734474BSmall molecular weightReduce manufacturing costAntibacterial agentsPeptide/protein ingredientsChemical synthesisRandom Peptide Library
The invention relates to the technical field of antimicrobial peptides, in particular to a method for screening antimicrobial peptides and applications thereof. The invention discloses a method for screening antimicrobial peptides. The screening method can rapidly screen antimicrobial peptides with specific resistance for specific pathogenic bacteria. The polypeptides in the phage random peptide library selected in the screening method have small molecular weights, so that the antimicrobial peptides obtained by screening have small molecular weights, are easy to obtain by chemical synthesis, and reduce the production cost of the antimicrobial peptides. The antimicrobial peptide screened by the screening method has high bactericidal activity on pathogenic bacteria, and low cytotoxicity and hemolytic toxicity.
Owner:SUN YAT SEN UNIV
Antibacterial hexapeptides and their derivatives and applications
ActiveCN106916205BLow hemolysis rateLow hemolytic toxicityAntibacterial agentsPeptide/protein ingredientsDiseaseAntimicrobial peptides
The invention discloses an antimicrobial hexapeptide. The antimicrobial hexapeptide has a sequence of Arg-Arg-Trp-Trp-Arg-Trp. The antimicrobial hexapeptide can be used in preparation of drugs for treating or preventing bacterial infection. The invention also provides an antimicrobial hexapeptide derivative. The antimicrobial hexapeptide derivative has a sequence of Arg-Arg-Trp-Trp-Arg-Trp-beta-phenylethylamine. The antimicrobial hexapeptide derivative can be used in preparation of drugs for treating or preventing bacterial infection. The bacterium is drug-resistant acinetobacter baumannii. The artificially designed synthetic antimicrobial hexapeptide and its phenylethylamine-modified product can be conveniently obtained by a solid phase synthesis method. The synthetic antimicrobial peptide and its derivative have broad spectrum killing activity to Gram-positive bacteria and Gram-negative bacteria, and particularly, the derivative exhibits stronger antibacterial activity to drug-resistant acinetobacter baumannii, has very small hemolytic toxicity and can be used in drugs for treatment or prevention of diseases caused by drug-resistant acinetobacter baumannii.
Owner:湖南湃洵生物科技有限公司
Antithrombotic metallic material
ActiveCN107405431ADissolution inhibitionLow hemolytic toxicitySurgeryPharmaceutical containersPolymer scienceMetallic materials
The present invention addresses the problem of providing an antithrombotic metallic material which has improved safety against low hemolytic toxicity and can exhibit high antithrombotic properties sustainably. The present invention provides an antithrombotic metallic material comprising a metallic material of which the surface is coated with a coating material, wherein the coating material comprises a phosphonic acid derivative or a catechol derivative, a polymer containing, as a constituent monomer, a compound selected from the group consisting of an alkyleneimine, vinylamine, allylamine, lysine, protamine and diallyl dimethyl ammonium chloride and a sulfur-atom-containing compound having an anionic anticoagulant activity, the polymer is covalently bonded to the phosphonic acid derivative or the catechol derivative, the phosphonic acid derivative or the catechol derivative is bonded to the metallic material through a phosphonic acid group or a catechol group contained in the derivative, and the ratio of the amount of nitrogen atoms to the total amount of all of atoms is 4.0 to 13.0% by atom number when the surface of the antithrombotic metallic material is measured by an X-ray photoelectron spectroscopy (XPS).
Owner:TORAY IND INC
A kind of enzymolysis-resistant α-helical antimicrobial peptide bound by full carbon and hydrogen side chains, its preparation method and application
ActiveCN113651871BHigh application potentialShorten the lengthAntibacterial agentsPeptide/protein ingredientsSide chainAntimicrobial peptides
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
A kind of palmitic acid anti-enzymolysis antimicrobial peptide and its preparation method and application
ActiveCN111423493BHigh application potentialAvoid restriction sitesAntibacterial agentsPeptide/protein ingredientsAntimikrobielle peptideResistant bacteria
The invention provides a palmitic acid anti-enzymolysis antimicrobial peptide and its preparation method and application. antimicrobial peptide B 3 C 16 The sequence is C 16 ‑GGGK(PRPR)K(PRPR)RPRP, where C 16 For palmitic acid, the side chain of each lysine is linked to a polypeptide branch chain PRPR through an amide bond, forming a dendritic structure and a steric hindrance between the peptide chains, and further using palmitic acid as the main hydrophobicity of the antimicrobial peptide sequence The source perfectly avoids the hydrolysis of hydrophobic amino acids by all proteases, and finally uses the flexible amino acid linker GGG to link palmitic acid with branched peptides to form a perfect amphiphilic structure. The antimicrobial peptide has high inhibitory effect on standard bacteria and drug-resistant bacteria such as Gram-negative bacteria and Gram-positive bacteria, and has very low hemolytic toxicity. 3 C 16 The degree of hydrolysis is very weak.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
A pamam-based targeted drug delivery carrier and preparation method thereof
InactiveCN104069501BImprove hydrophilicityGood treatment effectPharmaceutical non-active ingredientsAntineoplastic agentsSide effectHemolysis
Owner:SUZHOU UNIV
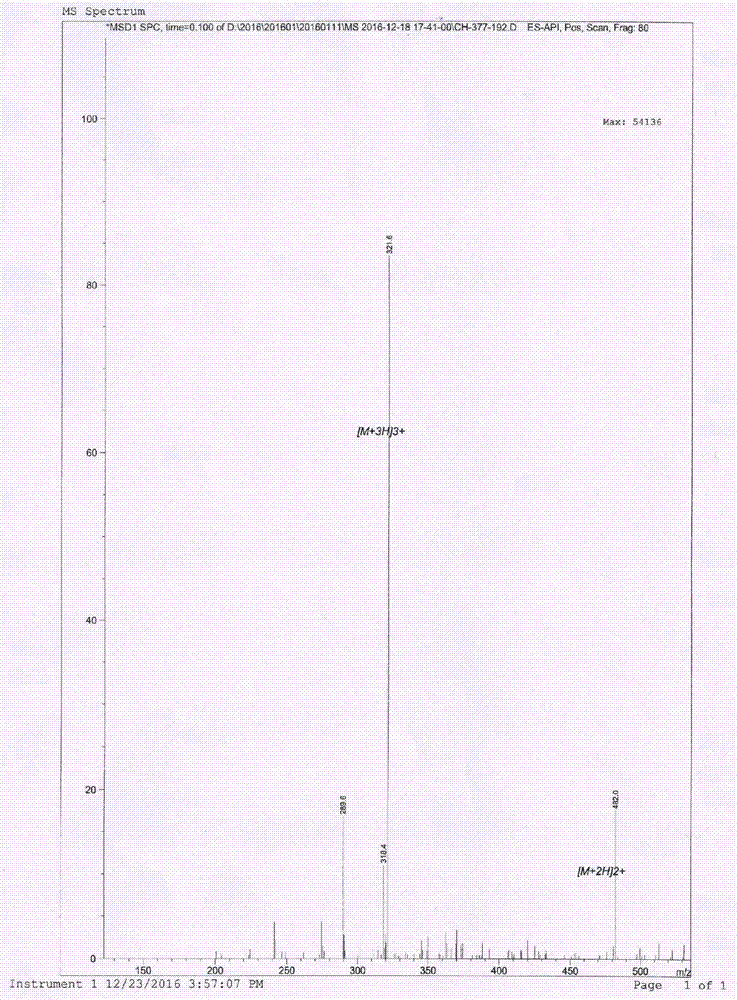
Pillar [5] aromatics artificial transmembrane channel with antibacterial activity, preparation method and application thereof
ActiveCN108017690BEfficient embeddingImprove antibacterial propertiesAntibacterial agentsPeptide/protein ingredientsBiotechnologyDisease
The invention discloses a pillar[5]arene artificial transmembrane channel having an antibacterial activity, and a production method and an application thereof, and belongs to the technical field of compounds having an antibacterial activity. The structural general formula of the pillar[5]arene artificial transmembrane channel having the antibacterial activity is shown in the description. The invention also discloses the production method of the pillar[5]arene artificial transmembrane channel having the antibacterial activity, and an application of the pillar[5]arene artificial transmembrane channel in the preparation of medicines for treating or preventing bacterium induced diseases. The artificial transmembrane channel produced in the invention has an excellent antibacterial performance,so the artificial transmembrane channel can be well applied to the prevention and treatment of bacterium induced animal and plant diseases; and the produced artificial transmembrane channel has very low hemolysis toxicity, so the artificial transmembrane channel can be used as a whole-body ion channel type antibiotic candidate medicine.
Owner:HENAN NORMAL UNIV
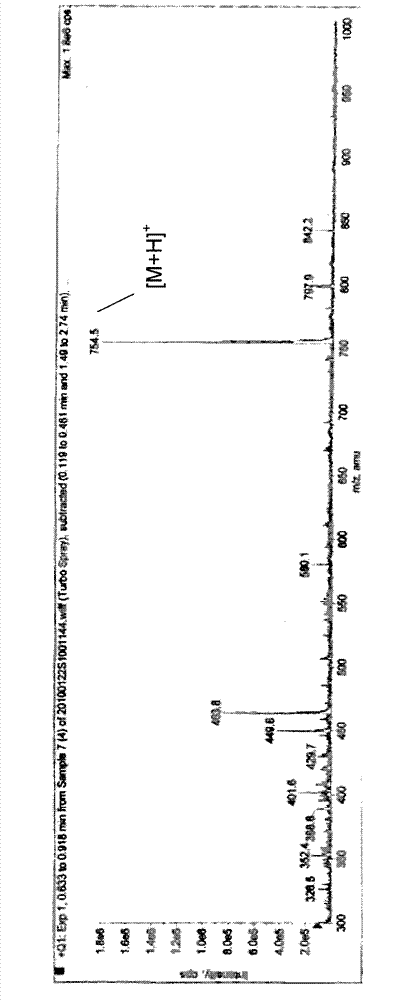
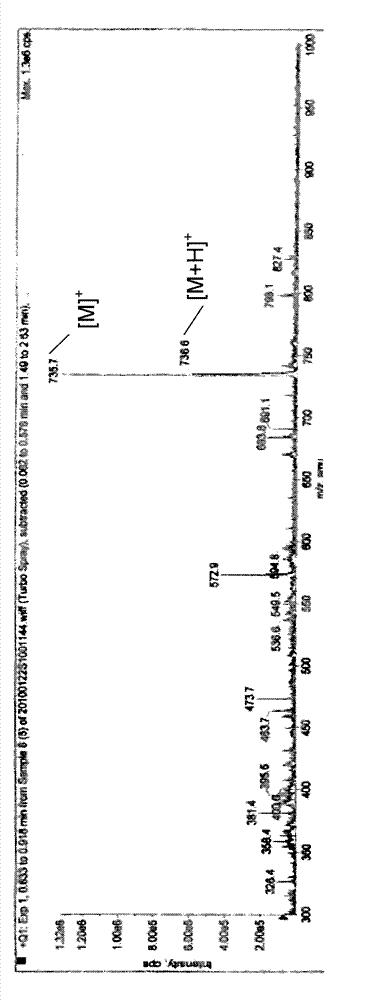
Pseudo cyclic polypeptide and synthesis method and application thereof
InactiveCN101906138BLower resistanceHigh antibacterial activityAntibacterial agentsTetrapeptide ingredientsSynthesis methodsSolid phases
The invention discloses a pseudo cyclic polypeptide and a synthesis method and application thereof. The pseudo cyclic polypeptide comprises a Loloatin C medicinal nucleus sequence structure -A1-A2-A3-A4-, namely sequence segments of Tyr, Pro, Trp and Phe, and four amino acid residues are in an L-configuration or a D-configuration. The synthesis method of the pseudo cyclic polypeptide comprises the following steps of: synthesizing structural elements by a liquid phase method; synthesizing a linear pseudo polypeptide by a solid phase method; and finally synthesizing to obtain the pseudo cyclic polypeptide by a dilute solution cyclization method. The total yield of the pseudo cyclic polypeptide is 5 to 12 percent. A method for preparing the pseudo cyclic polypeptide has the advantages of mild reaction condition, simple, convenient and safe operation, high product purity, easy automation and stable total yield. The synthesized pseudo cyclic polypeptide can be used for preparing medicaments for treating gram-positive bacterium infection or gram-negative bacterium infection.
Owner:JINAN UNIVERSITY
A kind of cyclic antimicrobial peptide analogue rich in positive charge and its application
ActiveCN112341524BHigh antibacterial activityLow hemolytic toxicityAntibacterial agentsPeptide preparation methodsMetabolic stabilityAntimicrobial drug
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Pillar[5]arene artificial transmembrane channel having antibacterial activity, and production method and application thereof Pillar[5]arene artificial transmembrane channel having antibacterial activity, and production method and application thereof](https://images-eureka.patsnap.com/patent_img/3fef2a69-58ac-45f6-a0ae-3e8ccd5ffe7f/FDA0001381899250000011.png)
![Pillar[5]arene artificial transmembrane channel having antibacterial activity, and production method and application thereof Pillar[5]arene artificial transmembrane channel having antibacterial activity, and production method and application thereof](https://images-eureka.patsnap.com/patent_img/3fef2a69-58ac-45f6-a0ae-3e8ccd5ffe7f/FDA0001381899250000012.png)
![Pillar[5]arene artificial transmembrane channel having antibacterial activity, and production method and application thereof Pillar[5]arene artificial transmembrane channel having antibacterial activity, and production method and application thereof](https://images-eureka.patsnap.com/patent_img/3fef2a69-58ac-45f6-a0ae-3e8ccd5ffe7f/BDA0001381899260000021.png)

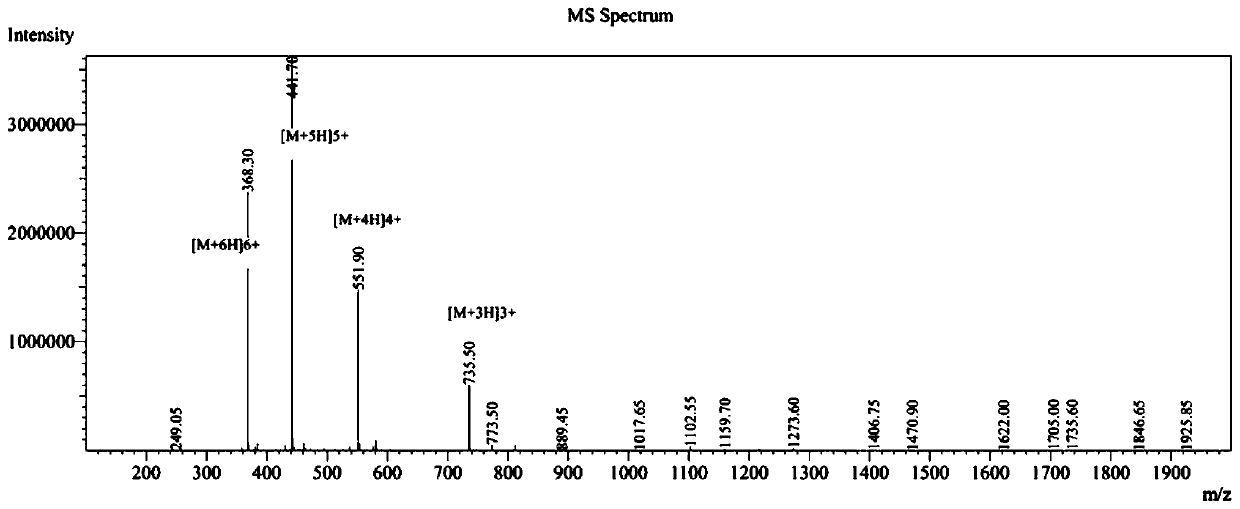
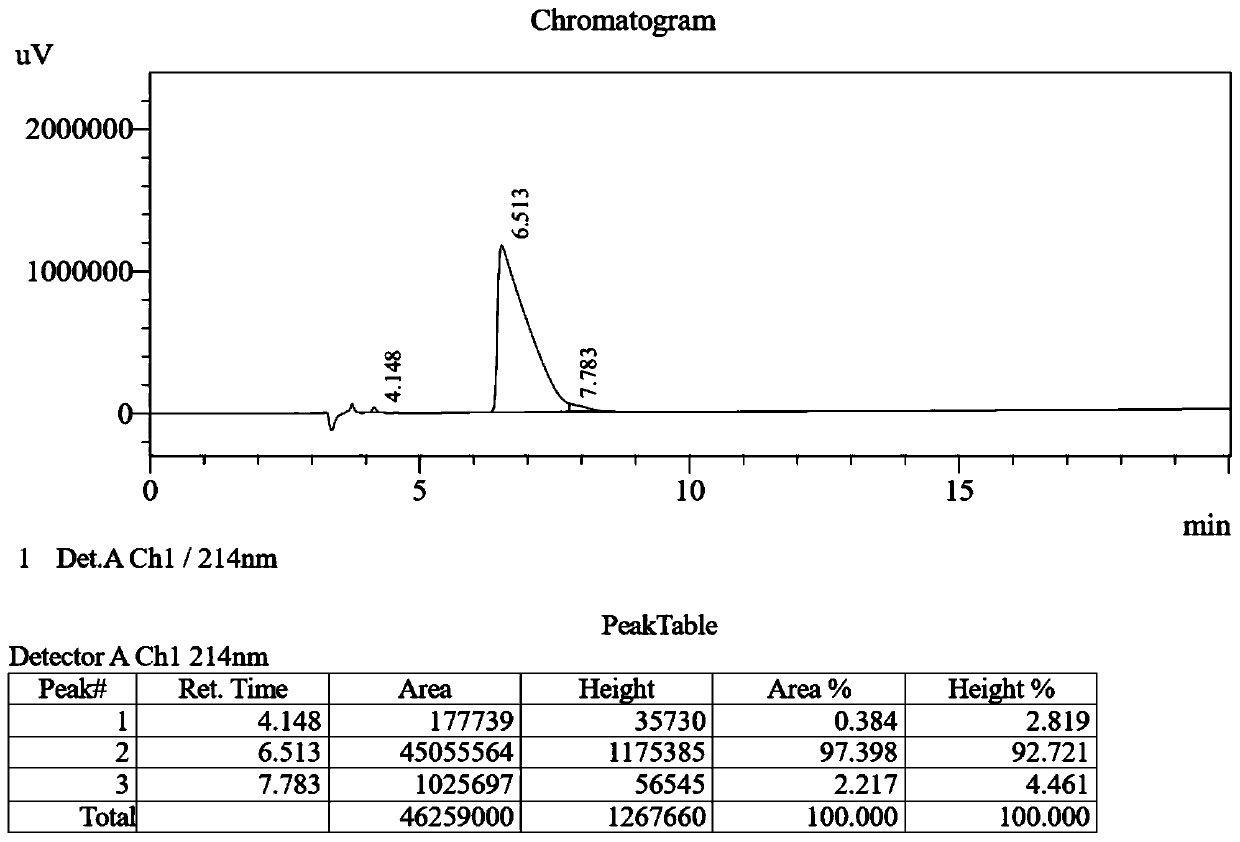


![Pillar [5] aromatics artificial transmembrane channel with antibacterial activity, preparation method and application thereof Pillar [5] aromatics artificial transmembrane channel with antibacterial activity, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/375e2392-693b-4253-84ff-71f58e106bef/FDA0002683385730000011.png)
![Pillar [5] aromatics artificial transmembrane channel with antibacterial activity, preparation method and application thereof Pillar [5] aromatics artificial transmembrane channel with antibacterial activity, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/375e2392-693b-4253-84ff-71f58e106bef/FDA0002683385730000012.png)
![Pillar [5] aromatics artificial transmembrane channel with antibacterial activity, preparation method and application thereof Pillar [5] aromatics artificial transmembrane channel with antibacterial activity, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/375e2392-693b-4253-84ff-71f58e106bef/GDA0002683385740000021.png)