Hornet phallotoxin antitone analog WVD-II and preparation method and application thereof
A technology of WVD-II and wasp venom, which is applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of limiting clinical drug applications, and achieve significant bactericidal effect, good stability, and low hemolytic toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The present invention also provides a preparation method of the venomin reverse analog WVD-II of the above scheme, which comprises the following steps:
[0023] 1) According to the amino acid sequence of the reverse analog WVD-II of the venomin peptide, the amino acids are sequentially coupled to the resin to obtain a peptide resin;
[0024] 2) The peptide resin described in step 1) is cleaved, a side chain protecting group scavenger is added, the target polypeptide is precipitated, and the precipitate is collected by centrifugation to obtain the venetin reverse analog WVD-II.
[0025] In the present invention, before the amino acids are sequentially coupled to the resin according to the amino acid sequence of the venetin reverse analog WVD-II, it preferably also includes the processes of washing the resin, soaking the resin, removing the amino protective group and washing the resin again The resin preferably includes Rink Amide MBHA resin (4-(2′,4′-dimethoxyphenyl-fluorenylme...
Embodiment 1
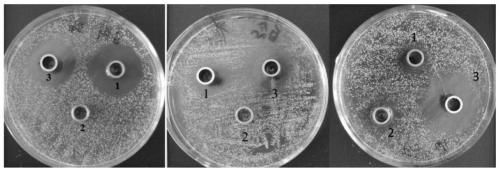
[0034] Using Fmoc protected amino acid for solid-phase synthesis, using RinkAmide MBHA resin as a carrier, 1-hydroxybenzotriazole and O-benzotriazole-N,N,N',N'-tetramethylurea hexafluorophosphate Salt is used as coupling reagent. The cleavage of peptide from resin uses lysing solution trifluoroacetic acid and side chain protecting group scavenger (phenol / water / anisyl sulfide / 1,2-dimercaptoethanol), react at room temperature for 4~6h After removing most of the trifluoroacetic acid by rotary evaporation, ether was added dropwise at 0℃ to obtain a flocculent precipitate. After centrifugation, the crude peptide was obtained. The crude peptide was initially separated with a Sephadex G-15 column. The column was equilibrated with 5% acetic acid. 1mL·min -1 Next, eluted with 5% acetic acid, detected at 280nm, collected the first peak, and freeze-dried. A semi-preparative high performance liquid chromatograph was used for purification, and the most abundant peak was collected. The puri...
Embodiment 2
[0048] Example 2 Reverse High Performance Liquid Chromatography Purification and Mass Spectrometry Identification of Vespidin Reverse Analog WVD-Ⅱ
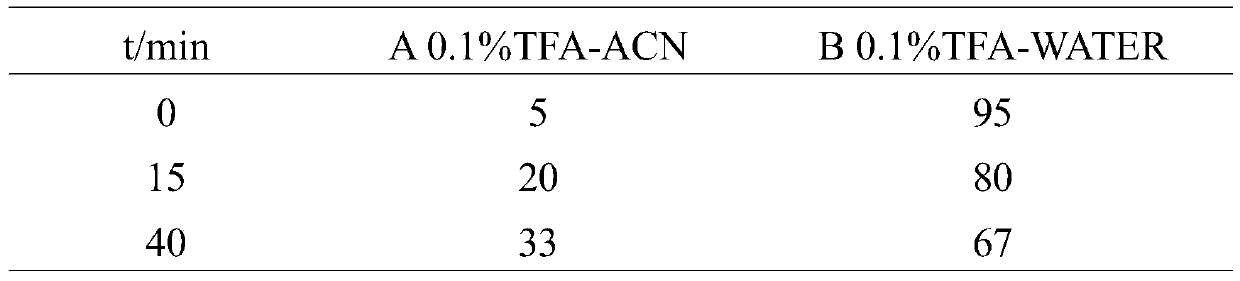
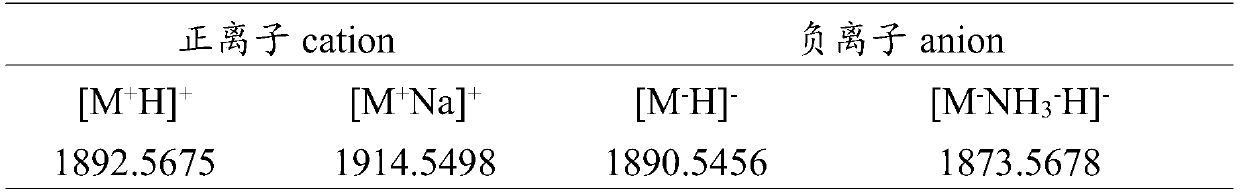
[0049] Purification was performed on a high performance liquid chromatograph, using Sepax Bio-C18 reverse phase column (10.0×250mm, 10μm, ). The oxidized crude peptide sample was dissolved in 1 mL of ultrapure water, centrifuged at 10,000 rpm for 5 min, and detected by W2998 detector with detection wavelengths of 215, 254, and 280 nm. The mobile phase is a four-phase elution system with a flow rate of 3mL·min -1 . The eluents were 0.1% TFA aqueous solution (A) and 0.1% TFA acetonitrile solution (B). The column temperature was 25°C. The elution gradient of reversed-phase high performance liquid chromatography is shown in Table 1. Collect the elution peaks and use Q- TOF mass spectrometry analysis, the identification results are shown in Table 2. The molecular weight of the mutants analyzed by mass spectrometry is consistent with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com