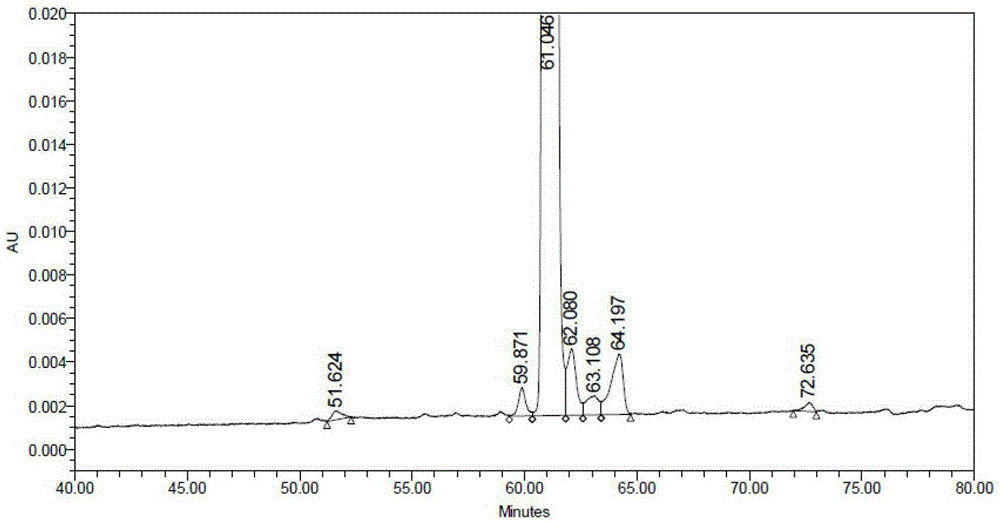
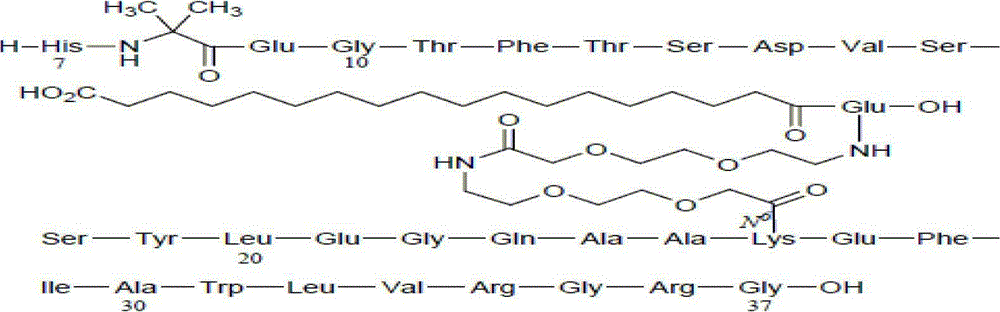
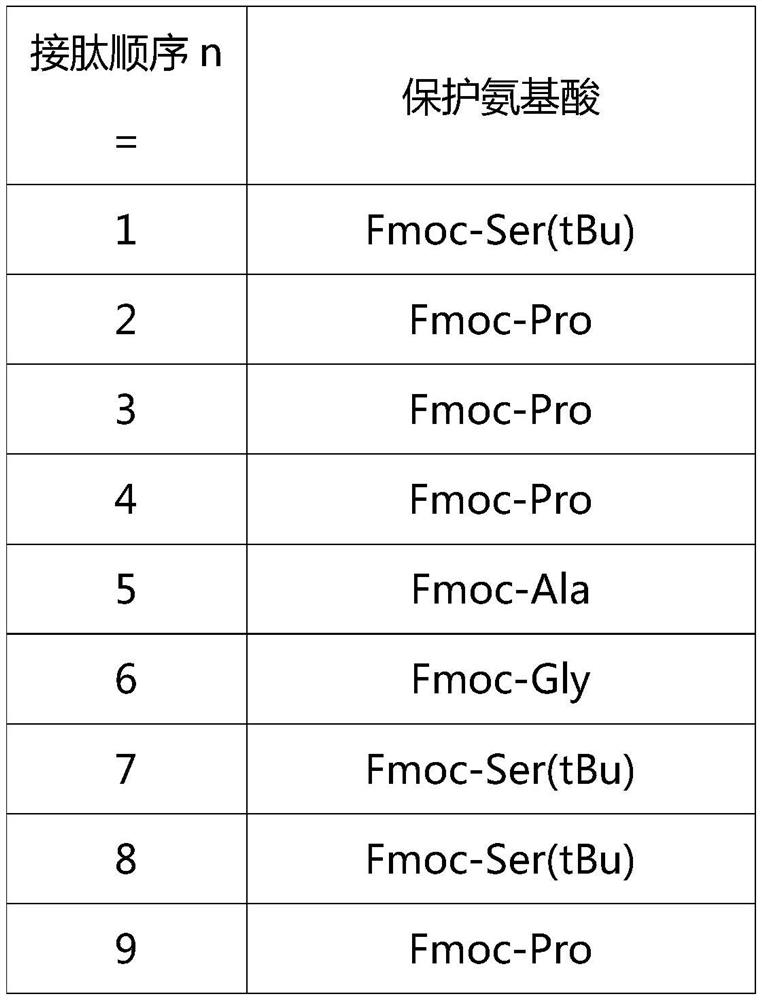
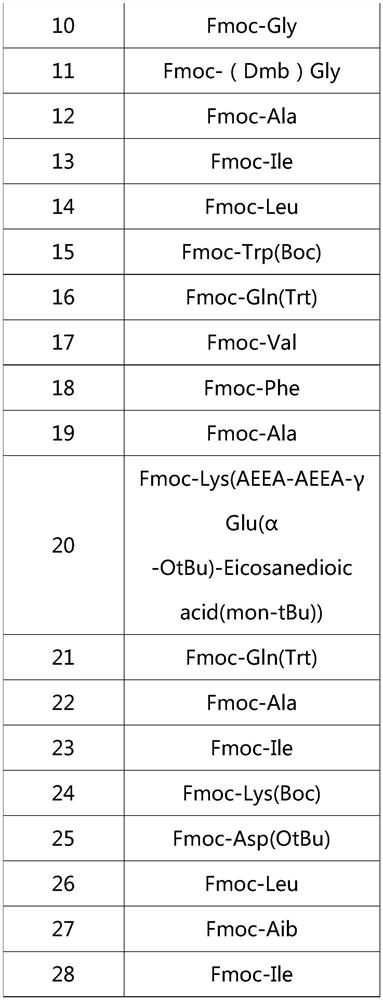
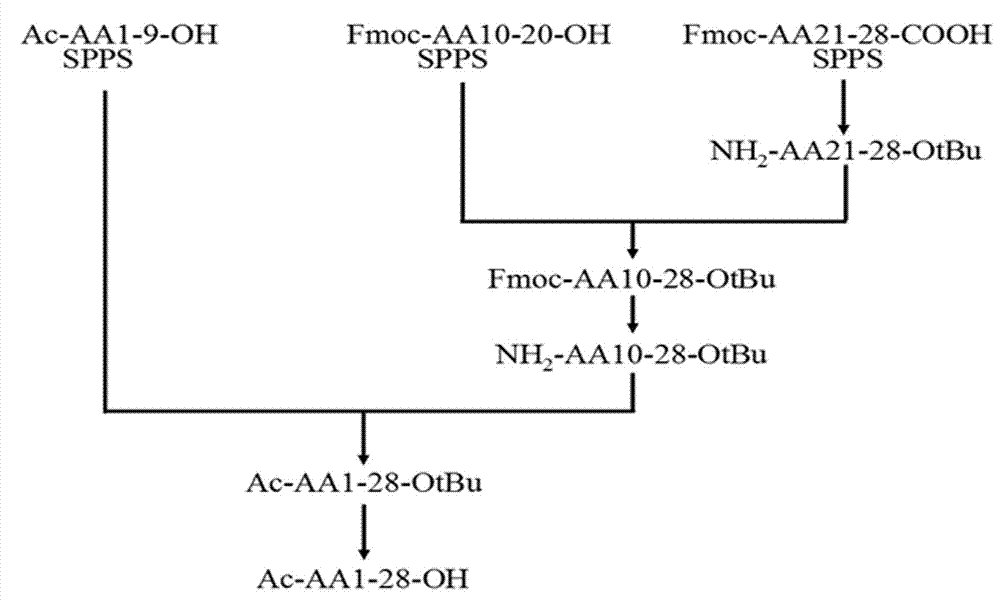
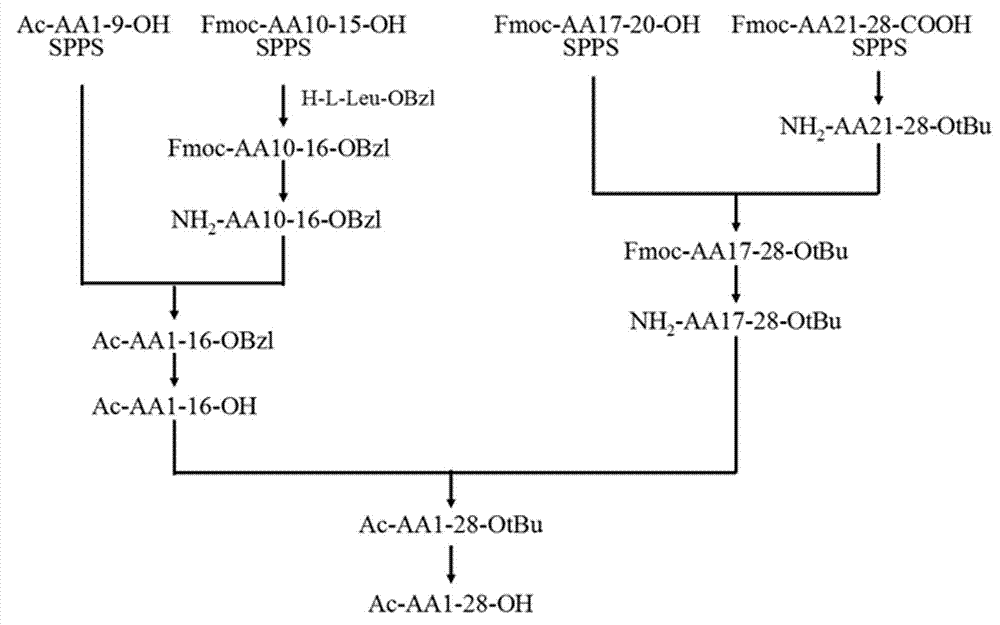
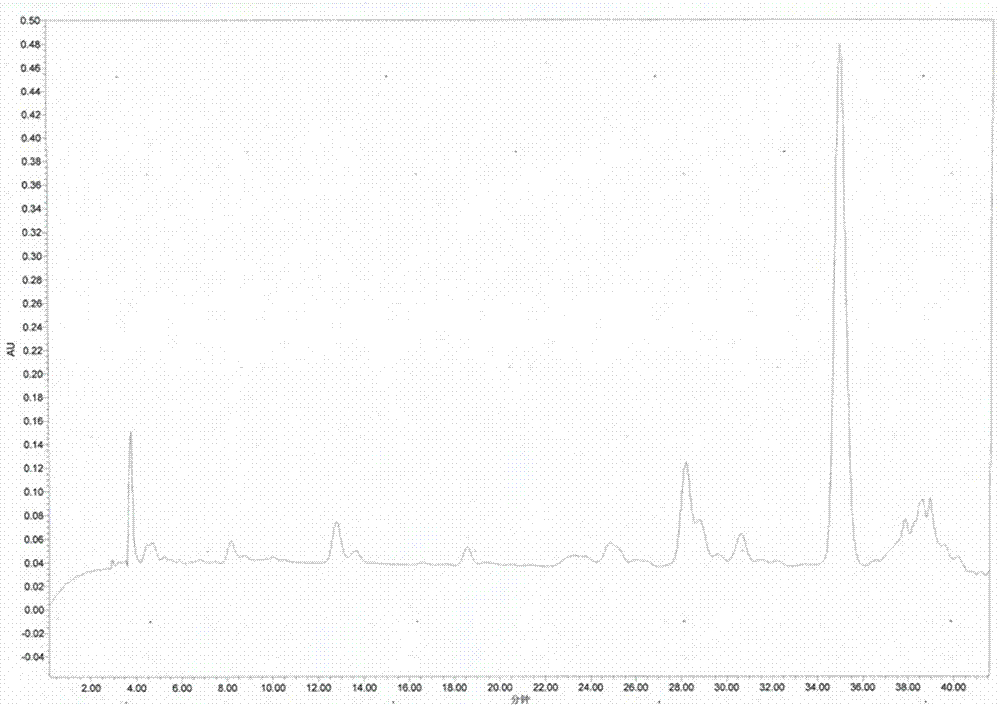
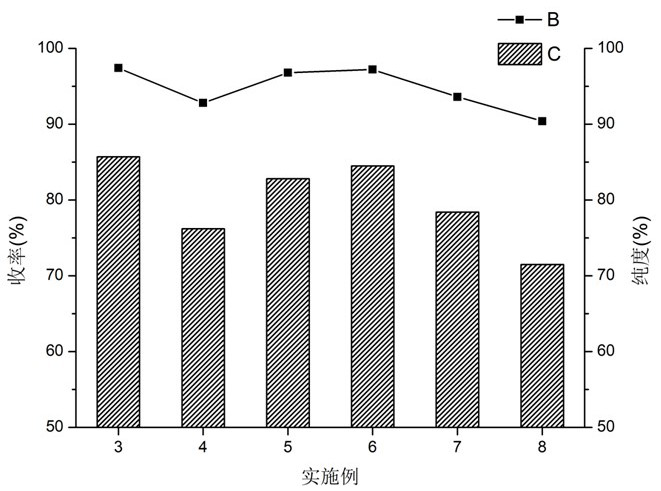
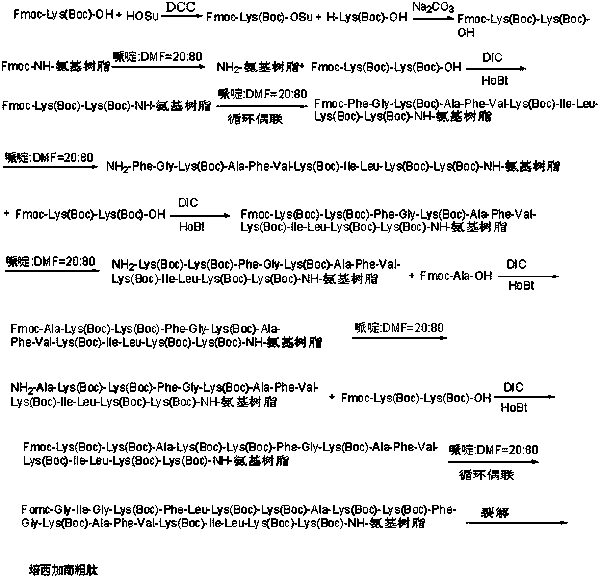
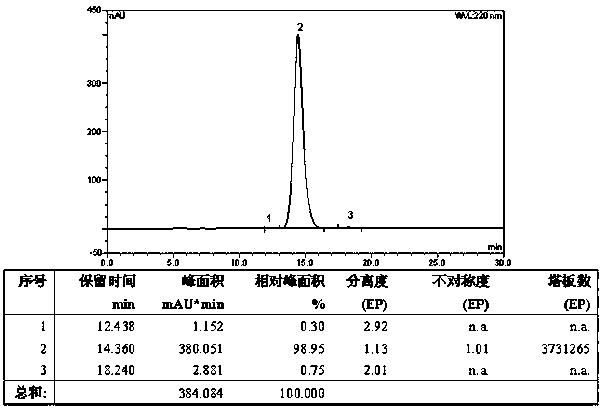
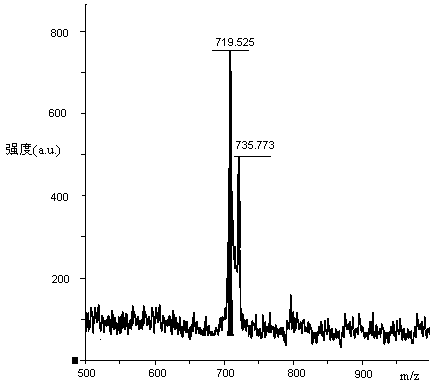
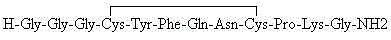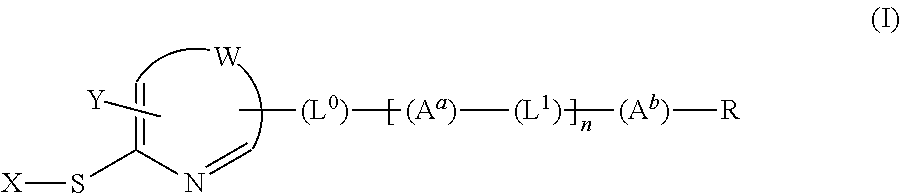Patents
Literature
44 results about "Peptide Synthesis technique" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
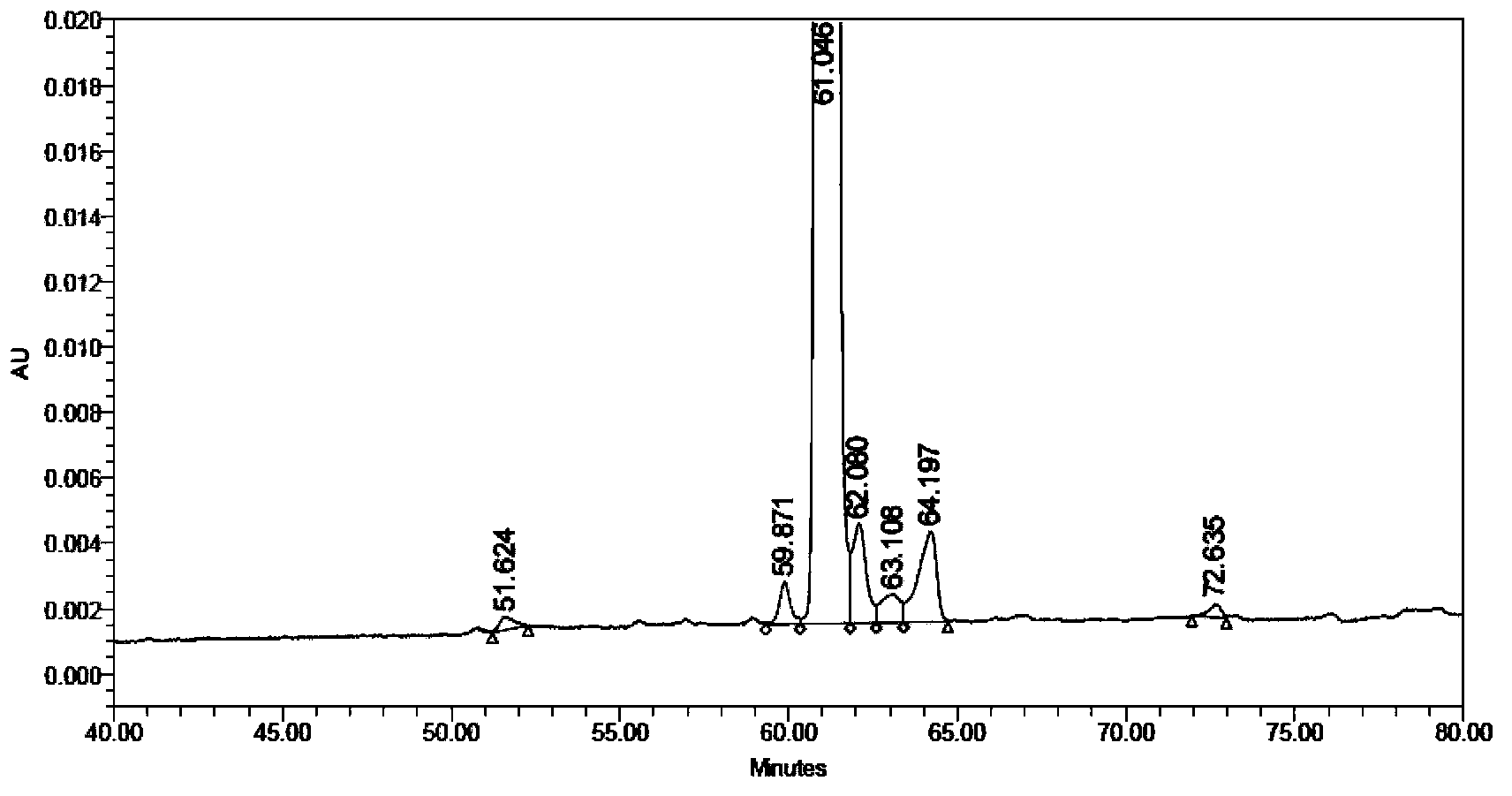
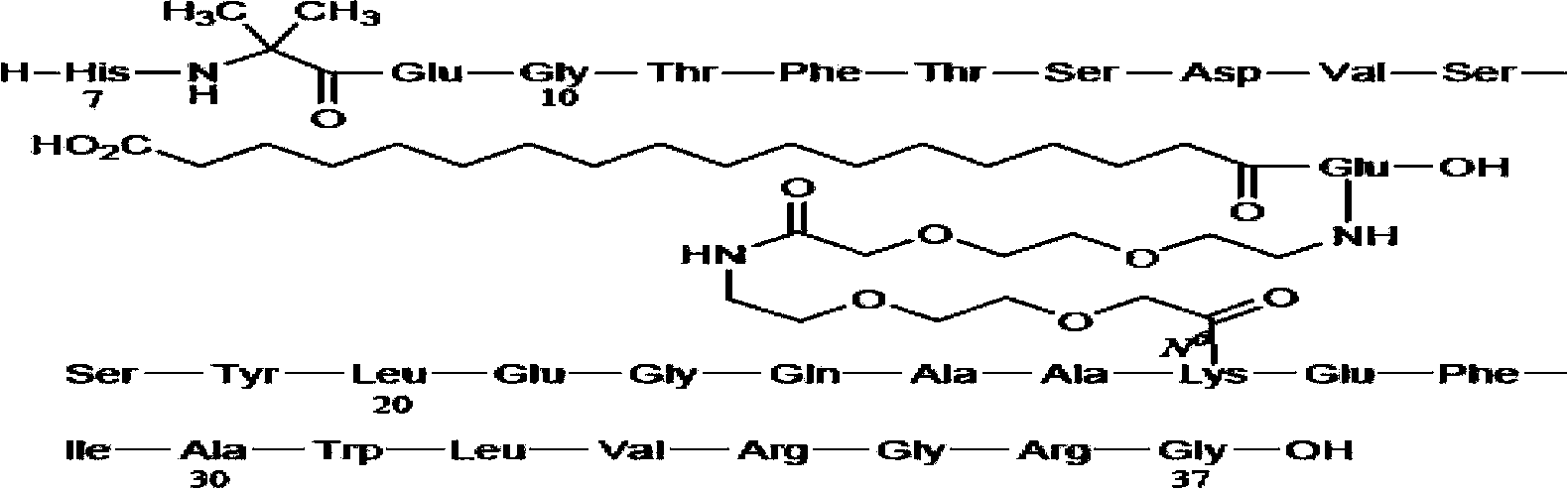
Solid synthetic method of semaglutide
ActiveCN103848910AEase of mass productionReduce generationPeptide preparation methodsAnimals/human peptidesAcetic acidSide chain
The invention relates to the technical field of polypeptide synthesis, and particularly relates to a solid synthetic method of semaglutide. The solid synthetic method includes coupling Gly and a resin to obtain a Gly-resin; coupling step by step for the first time an ammonia acid or an amino acid derivative to obtain a first peptide resin the sequence of which is shown as SEQ ID No.1; removing a side chain protective group of Lys; coupling step by step for a second time 2-(2-(2-aminoethoxy)ethoxy) acetic acid, 2-(2-(2-aminoethoxy)ethoxy) acetic acid, Glu and octadecanedioic acid to obtain a second peptide resin; performing pyrolysis; and purifying. The solid synthetic method is simplified in operation step, short in synthetic period, low in cost, reduced in production of waste liquid, low in side products and high in product yield, and is suitable for large-scale production of the semaglutide.
Owner:HYBIO PHARMA
Method used for preparing semaglutide
InactiveCN108203462AScale upPrevent polycondensationPeptide preparation methodsBulk chemical productionDipeptideHigh volume manufacturing
The invention belongs to the technical field of polypeptide synthesis, and especially relates to a synthesis method of semaglutide. The synthesis method comprises following steps; 1, prior coupling oflys26 side chain is carried out; and 2, O-iso-acylated dipeptide is adopted to break the secondary structure of beta-sheet in semaglutide synthesis process effectively so as to avoid polycondensationin polypeptide synthesis process, and then O to N transfer reaction is adopted to convert esterified semaglutide into semaglutide. The method is capable of reducing product synthesis difficulty, avoiding generation of a plurality of impurities, ensuring the product quality of semaglutide bulk drugs, increasing product yield, increasing the scale of single batch semaglutide production, and realizing mass production of semaglutide.
Owner:上海脉凯生物科技有限公司
Method for preparing ularitide
ActiveCN106519009AReduce the difficulty of synthesisHigh yieldPeptide preparation methodsDepsipeptidesFreeze-dryingPeptide Synthesis technique
The invention relates to the technical field of polypeptide synthesis, and discloses a method for preparing ularitide. The method includes the following steps that (1) full-protection linear peptide resin is synthesized; (2) splitting is performed to obtain linear peptide; (3) oxidation is conducted to obtain the ularitide; and (4) purification, salt rotation and freeze-drying are carried out to obtain the ularitide competitive product. By the adoption of the method, the process is simple, synthesis difficulty of the ularitide can be lowered, the yield of the synthesized ularitide is high, cost is low, the purity is 98% or above, and popularization is facilitated.
Owner:HANGZHOU GOTOP BIOTECH
High-purity bivalirudin and industrial preparation method thereof
ActiveCN103965293ALow costEasy to recyclePeptide preparation methodsEthyl acetatePeptide Synthesis technique
The invention provides high-purity bivalirudin and an industrial preparation method thereof. The preparation method employs solid phase peptide synthesis technology and uses the solvent--ethyl acetate which is convenient to use and recover and has a low price as a washing agent, so reaction yield and product quality are improved, production cost is effectively reduced, and discharge of waste is reduced.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
A kind of solid-phase synthesis method of Samolute
ActiveCN103848910BEase of mass productionReduce generationPeptide preparation methodsAnimals/human peptidesAcetic acidSide chain
The invention relates to the technical field of polypeptide synthesis, and particularly relates to a solid synthetic method of semaglutide. The solid synthetic method includes coupling Gly and a resin to obtain a Gly-resin; coupling step by step for the first time an ammonia acid or an amino acid derivative to obtain a first peptide resin the sequence of which is shown as SEQ ID No.1; removing a side chain protective group of Lys; coupling step by step for a second time 2-(2-(2-aminoethoxy)ethoxy) acetic acid, 2-(2-(2-aminoethoxy)ethoxy) acetic acid, Glu and octadecanedioic acid to obtain a second peptide resin; performing pyrolysis; and purifying. The solid synthetic method is simplified in operation step, short in synthetic period, low in cost, reduced in production of waste liquid, low in side products and high in product yield, and is suitable for large-scale production of the semaglutide.
Owner:HYBIO PHARMA
Preparation method of Tirzepatide
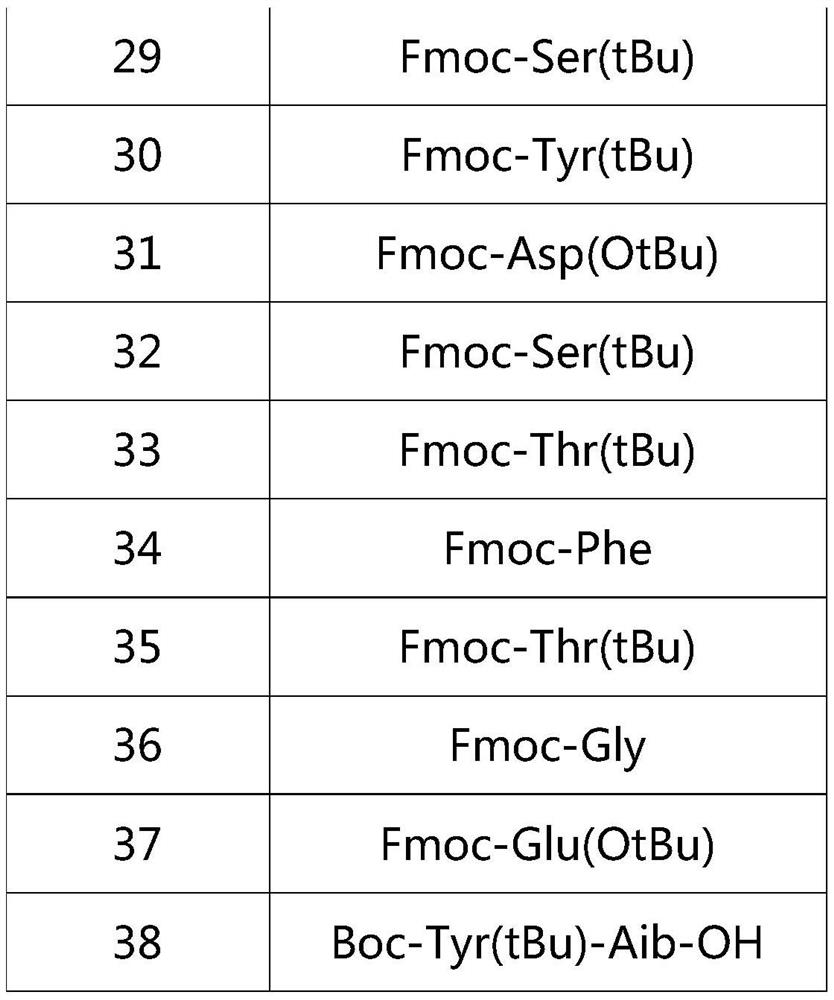
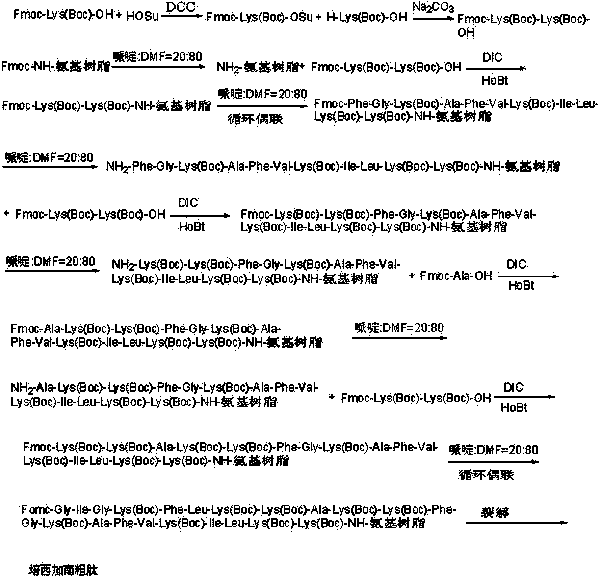
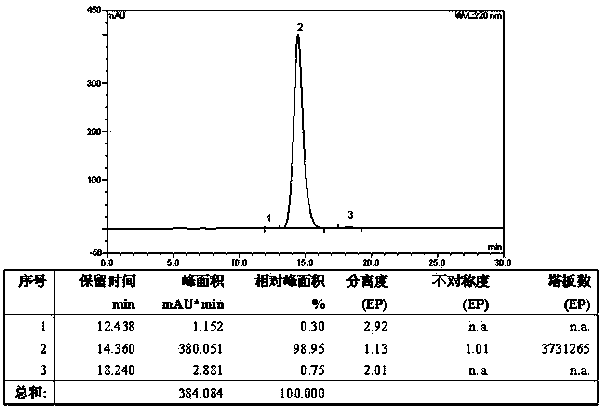
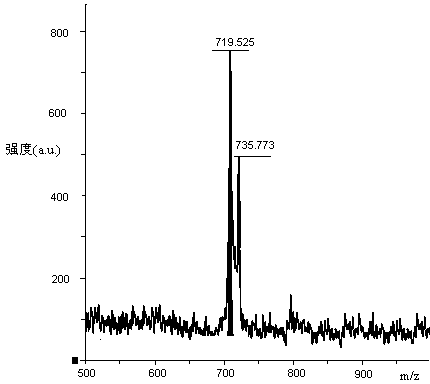
ActiveCN112592387ALow costReduce purification costsPeptide-nucleic acidsDepsipeptidesBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method of Tirzepatide, and belongs to the technical field of peptide synthesis. According to the method, amino resin is adopted as starting resin, a solid-phase polypeptide synthesis method is adopted for preparation, Tirzepatide peptide resin is obtained by a polypeptide solid-phase synthesis method, then Tirzepatide resin is subjected to acidolysis to obtaina Tirzepatide crude product, and finally, the Tirzepatide crude product is purified to obtain a Tirzepatide pure product. According to the method disclosed by the invention, special protective aminoacids, namely Boc-Tyr (tBu)-Aib-OH and Fmoc-Lys (AEEA-AEEA-gamma Glu (alpha-OtBu)-Eicosanedioicacid (mon-tBu))-OH, Fmoc-Tyr-Ser (pro-me-me)-OH and Fmoc-(Dmb)Gly-OH, are adopted. According to the method, the purity of the crude peptide is improved, the material cost and the purification cost are greatly reduced, and industrial large-scale production is facilitated.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Preparation method of pexiganan
ActiveCN104356221AImprove efficiencyResolving incomplete couplingPeptide preparation methodsAnimals/human peptidesDipeptideFreeze-drying
The invention relates to the technical field of polypeptide synthesis, in particular to a preparation method of pexiganan. The preparation method comprises the following specific steps: A) synthesizing a dipeptide fragment FmoC-Lys (BoC)-Lys (BoC)-OH by a liquid phase method; B) by a solid-phase synthesis method, with amino resin as starting resin, sequentially coupling amino acids with FmoC protections at N terminals and side chain protections according to a peptide sequence of a main chain of the pexiganan, wherein the amino acids at 7 and 8 positions, the amino acids 10 and 11 positions and the amino acids at 21 and 22 positions are coupled by the dipeptide fragment FmoC-Lys (BoC)-Lys (BoC)-OH; C) cracking, purifying and freeze-drying peptide resin to obtain the pexiganan. The invention provides a synthesis process of the pexiganan, which is high in synthesis efficiency and low in cost, can avoid deleted peptide impurities caused by incomplete coupling of two lysines and is suitable for mass production.
Owner:上海飞腾医药科技有限公司
Method for preparing thymosin alpha 1 by liquid phase fragment condensation
ActiveCN103665144AHigh purityHigh yieldThymosin peptidesPeptide preparation methodsFluid phaseTarget peptide
The invention provides a method for preparing thymosin alpha 1 by liquid phase fragment condensation, belonging to the technical field of biochemistry. According to the method, high capacity value (not less than 0.8mmol / g) resin is used as a starting material, firstly, a standard solid phase polypeptide synthesis (SPPS) technology is adopted for synthesizing a high-purity peptide fragments with selected structures, then, a liquid phase condensation technology is adopted for connecting the peptide fragments, and thus, a high-purity (more than 99%) target peptide is obtained. Compared with the solid phase thymosin alpha 1 synthesis technology, the method provided by the invention avoids the problem of low coupling ratio of amino acids behind the 12th position, and greatly improves yield (up to 25-30%) of the thymosin alpha 1; meanwhile, the peptide fragment formed by solid phase synthesis is free from purification, post-treatment technology is simplified, finally, the thymosin alpha 1 is purified by means of high performance liquid chromatography, preparation difficulty is reduced, preparation times is decreased, synthesis cost of the thymosin alpha 1 is lowered, and implementation of large-scale and industrialized production is facilitated.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Method for preparing terlipressin
InactiveCN105367627AReduce generationLow costOxytocins/vasopressinsPeptide preparation methodsGlycineBiochemical engineering
The invention relates to a method for preparing terlipressin, and belongs to the technical field of polypeptide synthesis. The method comprises the steps that terlipressin peptide resin is synthesized through a fragment condensation method, 3-12 fragments are synthesized through a solid phase method, 1-2 fragments are connected to 3-12 solid phase amino-acid resin, full-peptide resin is obtained, and the terlipressin is obtained after cracking and cyclizing. Due to the fact that Boc-Gly-Cly-OH is adopted as a raw material, lack of glycine impurities is reduced, the operation step of removing glycine protection groups at an N terminal is omitted, product purity is improved, and the total cost is obviously reduced.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Method for synthesizing phenylalanyl-lysine dipeptide
InactiveCN107936089AMild conditionsStrong specificityPeptide preparation methodsFermentationDipeptideFreeze-drying
A method for synthesizing phenylalanyl-lysine dipeptide belongs to the technical field of peptide synthesis. The method comprises the following steps: preparing an aqueous solution of phenylalanine and lysine from commercially available phenylalanine and lysine, preparing a non-aqueous reaction medium of ethylene glycol, preparing a phenylalanine-lysine dipeptide reaction solution, preparing a resin column for purifying loaded phenylalanine-lysine dipeptide, preparing a phenylalanyl-lysine dipeptide eluent, preparing phenylalanyl-lysine dipeptide freeze-dried powder, preparing an aqueous solution of recovered phenylalanine and lysine, and preparing a regenerated resin column in order to prepare the phenylalanyl-lysine dipeptide with the content being 95-98%. The method using chymotrypsin as a catalyst in the non-aqueous medium of ethylene glycol to synthesize the phenylalanyl-lysine dipeptide has the advantages of mild conditions, high specificity and high synthesis efficiency, and isa typical green production technology; and the phenylalanyl-lysine dipeptide can be widely applied to the fields of medicines, textiles, foods and the like, and has considerable social values.
Owner:吴?基
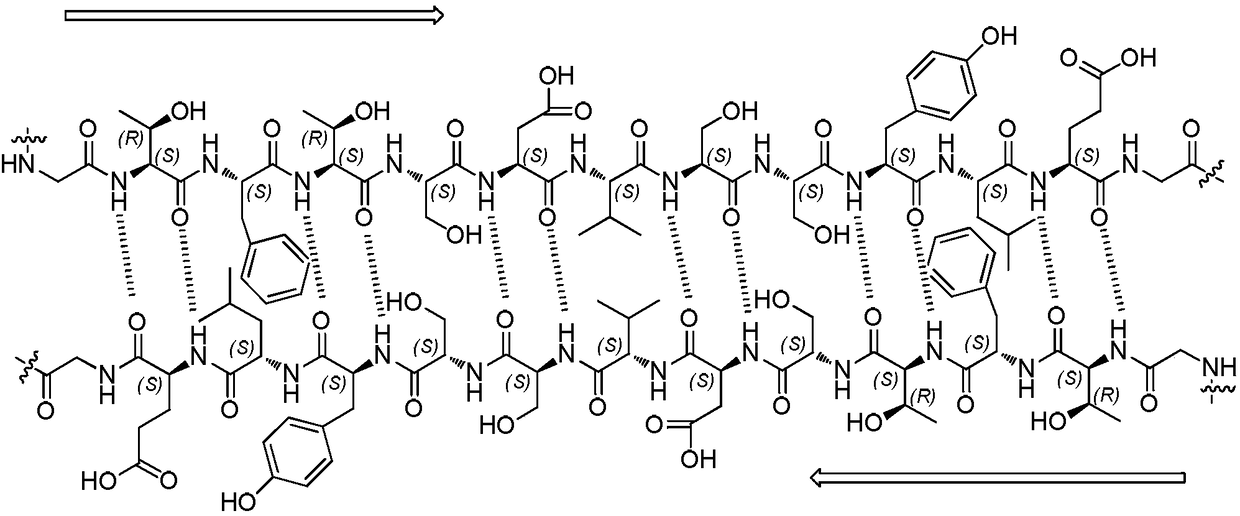
Preparation method of plecanatide
The invention provides a preparation method of plecanatide, and relates to the technical field of polypeptide synthesis. AM resin or MBHA resin is modified through FMPB, H-Leu-OtBu serves as first amino acid to be coupled to the modified resin, amino acid at sites difficult to connect is modified and then is gradually coupled to the modified resin according to the sequence of plecanatide main chain peptide, so that the synthesis difficulty of plecanatide linear peptide is greatly reduced, and the problems of many impurities, low purity, low yield, amino acid racemization and the like in the existing synthesis are solved. The Cys at sites of 4 and 12 in the sequence are subjected to first oxidation bonding and then subjected to column chromatography coarse purification, so that the purity of the obtained plecanatide is improved; the purification liquid is diluted, and the Cys at the 7-site and the 15-site are subjected to the second oxidation bonding, such that the concentration of thepre-oxidized plecanatide line peptide can be reduced so as to reduce the intermolecular disulfide bond during the second oxidation bonding process, and improve the yield of plecanatide; and further, the operation is simple and convenient, and the method is suitable for industrial production.
Owner:杭州信海医药科技有限公司
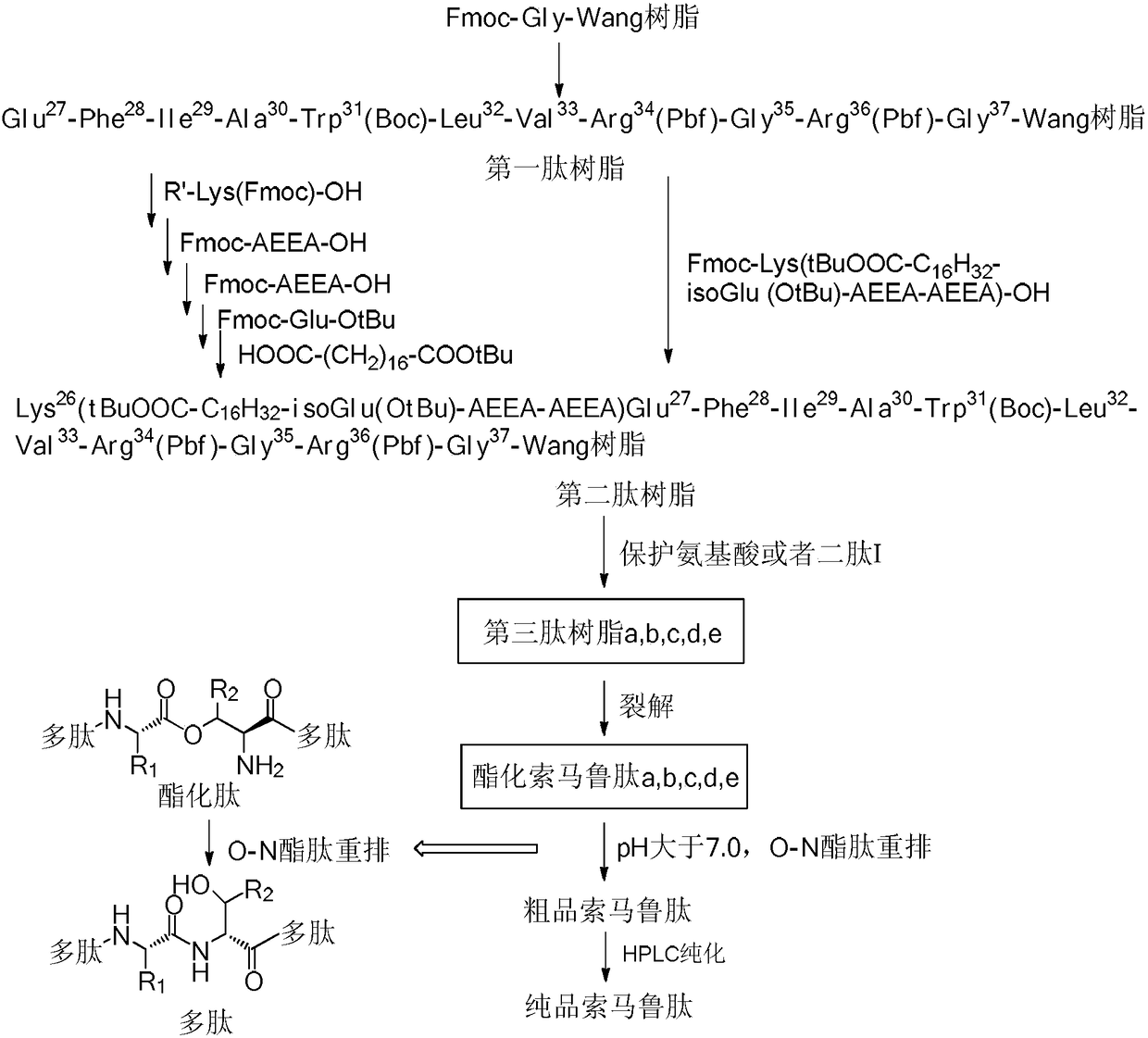
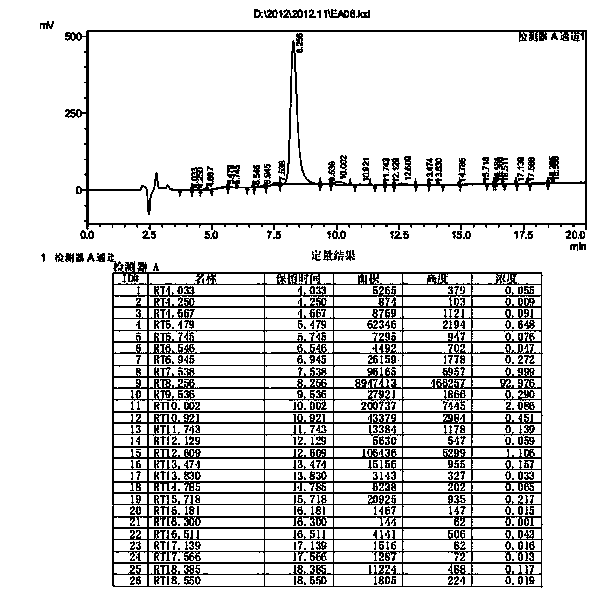
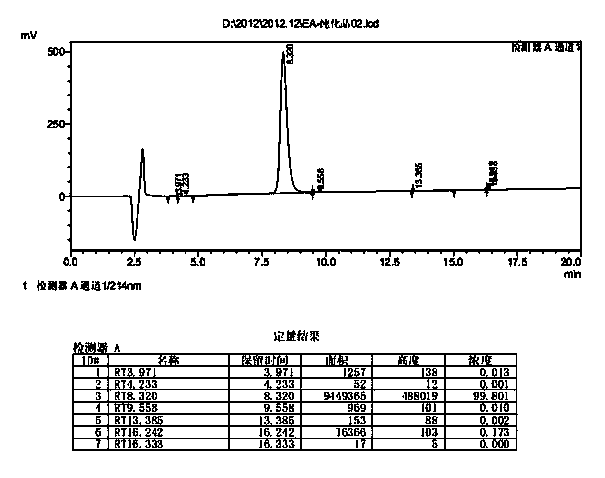
Preparation method of somaglutide
InactiveCN112250755AReduce the difficulty of synthesisAvoid it happening againPeptide preparation methodsBulk chemical productionCombinatorial chemistryBackbone chain
The invention provides a preparation method of somaglutide, and relates to the technical field of polypeptide synthesis. Firstly, 37 amino acid Fmoc-Gly-OH is coupled on initial resin, then an Fmoc protecting group is removed, then 36 to 26 amino acids in a somaglutide sequence are sequentially coupled, and side chains Fmoc-AEEA-OH, Fmoc-AEEA-OH, Fmoc-Glu-OtBu and octadecanedioic acidmono-tert-butyl ester are sequentially coupled on obtained first peptide resin; and an Lys main chain protecting group in an obtained second peptide resin is removed, the 25 to 7 amino acids in the somaglutide sequence are sequentially coupled, and an obtained third peptide resin is cracked to obtain the somaglutide. According to the preparation method, the side chain of the somaglutide isfirstly coupled, and then the main chain of the somaglutide is coupled, so that racemization impurities can be effectively prevented from being generated, the synthesis difficulty of the side chain is reduced, the yield is high, and the cost is low; and the preparation method is simple and suitable for industrial production.
Owner:浙江肽昇生物医药有限公司
Magnetic nanoparticles and application thereof in preparing magnetic solid-phase carriers
ActiveCN108376608AEasy to synthesizeSynthetic precisionPeptide preparation methodsInductances/transformers/magnets manufacturePolymer modifiedMicrosphere
The invention belongs to the technical field of solid-phase peptide synthesis, particularly relates to magnetic nanoparticles for solid-phase peptide synthesis, and further discloses a synthesis method of the magnetic nanoparticles and application of the magnetic nanoparticles in preparing magnetic solid-phase carriers. The magnetic nanoparticles are synthesized by subjecting superparamagnetic ferroferric oxide (Fe3O4) particles which are taken as a magnetic core to oleic acid modification and covering the surface of the magnetic core with polystyrene (PS) to form the magnetic nanoparticles which are of core-shell type PS@Fe3O4 structure; further, surface polymer modified magnetic nano-microspheres are obtained by introducing 1-(4-vinyl benzyloxybenzyl alcohol) in the process of covering Fe3O3 with PS. The magnetic nanoparticles have suitable size, have surface chemical adaptability capable of being functionalized, have excellent dispersibility and compatibility in media, and can be used as the magnetic solid-phase carriers in solid-phase peptide synthesis.
Owner:QINGDAO UNIV
Fluorescent probe for detecting C-reactive protein and preparation method thereof
InactiveCN109946273ALow costEasy to usePeptidesFluorescence/phosphorescenceSolid phasesFluorescence intensity
The invention provides a fluorescent probe for detecting the C-reactive protein and a preparation method thereof. With the phage display technology, the recombinant phage bound specifically is screened; and sequencing and sequence comparison are carried out by extracting the ssDNA of the recombinant to obtain a CRP-specific affinity ligand sequence. The ligand is synthesized based on the solid phase polypeptide synthesis technology and fluorescent labeling is carried out on the ligand to obtain a fluorescent probe that is specifically bound to the CRP. A fluorescent enzyme label plate is coated with a to-be-detected serum sample and a fluorescent probe with the certain concentration is added, wherein the probe can be bound to the CRP specifically; a fluorescent probe that is not bound is washed by using a proper buffer solution and a fluorescence microplate reader detects the fluorescence intensity, wherein the current fluorescence intensity is controlled by the CRP content in the to-be-detected sample strictly. According to the invention, a novel tool is provided for specific recognition and content detection of CRP. The fluorescent probe no only can qualitatively identify the CRPbut also can be used for quantitative detection.
Owner:CHANGCHUN UNIV OF SCI & TECH
Preparation method of semaglutide full-protection peptide resin and preparation method of semaglutide
ActiveCN113880935AImprove polycondensationHigh yieldPeptide preparation methodsBulk chemical productionDipeptideCombinatorial chemistry
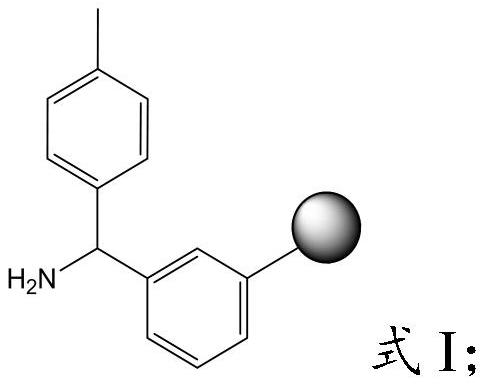
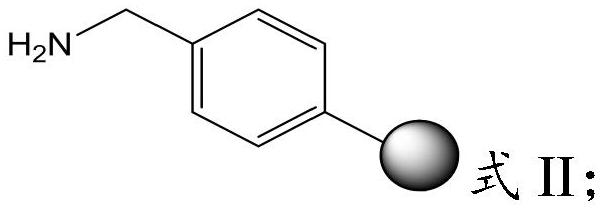
The invention provides a preparation method of semaglutide and relates to the technical field of polypeptide synthesis. According to the preparation method provided by the invention, amino acid as shown in a formula I or a dipeptide fragment as shown in a formula II is adopted, and through steric hindrance of a raw material structure, polycondensation caused by beta folding in a semaglutide full-protection peptide resin coupling process can be improved, the difficulty of coupling of semaglutide resin is lowered, so that missing impurities, inserted impurities and racemization impurities are effectively avoided. Therefore, the preparation method provided by the invention has the advantages that the quality of a semaglutide crude product can be improved, the yield of the semaglutide is increased, and the preparation method is low in cost, simple to operate and suitable for industrial production.
Owner:浙江肽昇生物医药有限公司
Heating solid-phase synthetic method of polypeptides
The invention relates to the technical field of synthesis of polypeptides, in particular to a heating solid-phase synthetic method of polypeptides. In heating solid-phase synthesis of polypeptides, all reactions are performed after temperature is raised to a constant value. Compared with traditional solid-phase synthetic methods of polypeptides, the heating solid-phase synthetic method of polypeptides has greatly shortened reaction time and increased reaction speed; compared with microwave solid-phase synthetic methods of polypeptides, the heating solid-phase synthetic method of polypeptides can gain decreased chances for side effects and increased reaction yield of long-chain polypeptides.
Owner:CS BIO SHANGHAI
Hornet phallotoxin antitone analog WVD-II and preparation method and application thereof
ActiveCN111100190AImprove the bactericidal effectImprove stabilityAntibacterial agentsAntimycoticsBiotechnologyCell membrane
The invention provides a hornet phallotoxin antitone analog WVD-II and a preparation method and application thereof, and belongs to and relates to the technical field of polypeptide synthesis. The amino acid sequence of the hornet phallotoxin antitone analog WVD-II is as shown in SEQID NO:1. The N end of the hornet phallotoxin antitone analog WVD-II disclosed by the invention has positive charges,so that the N end can be combined with a cell membrane having negative electricity; an alpha helical structure is formed from hydrophobic amino acids at a C end, and can bore a hole in the surface ofthe cell membrane, so that death of bacteria is caused. The in vitro antibacterial activity determination indicates that the hornet phallotoxin antitone analog WVD-II disclosed by the invention has significant sterilizing effects on gram-positive bacteria, gram-negative bacteria and fungi, has good stability, is low in hemolytic toxicity and has the characteristics of being broad-spectrum, efficient and safe. The hornet phallotoxin antitone analog WVD-II disclosed by the invention can be used as an active component clinically for replacing traditional antibiotics for treatment.
Owner:DALI UNIV
Novel compound, production method therefor, and application therefor
InactiveUS20160304459A1High selectivityPossible to connectOxytocins/vasopressinsPeptide preparation methodsCombinatorial chemistryPeptide Synthesis technique
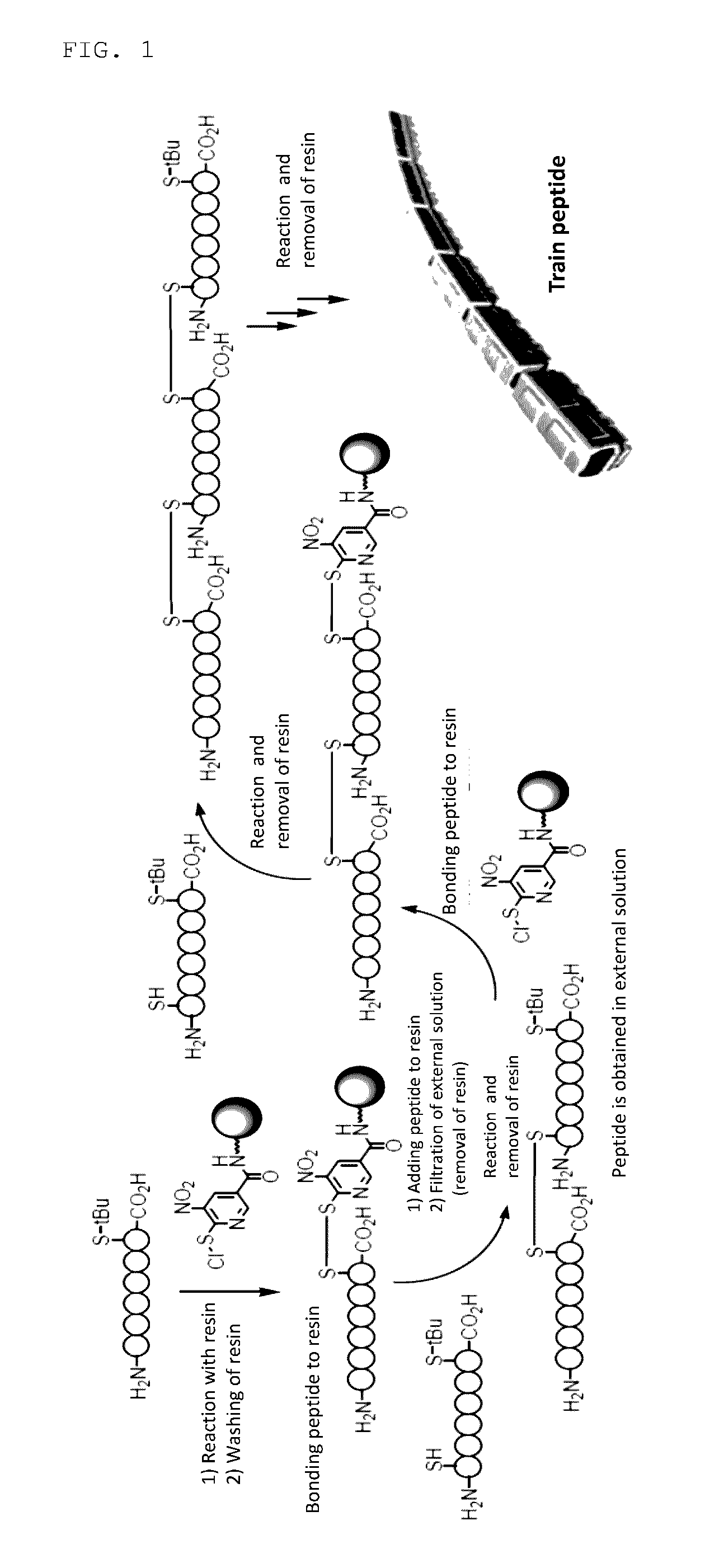
[Problems] To provide a novel peptide synthesis technique that is completely different than heretofore, and to provide a novel compound that enables the synthesis / creation of a novel artificial functional protein and the synthesis / creation of a novel functional peptide, as well as a method for producing the same. [Solution] A compound represented by formula (I) or a salt thereof.
Owner:KOKUSAN CHEM +1
Synthetic method of decarboxylated carnosine
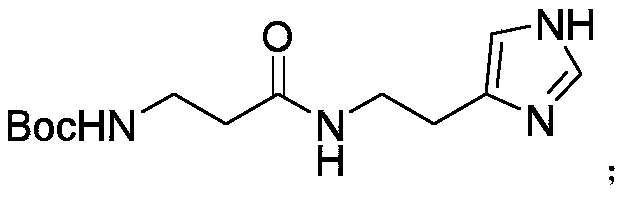
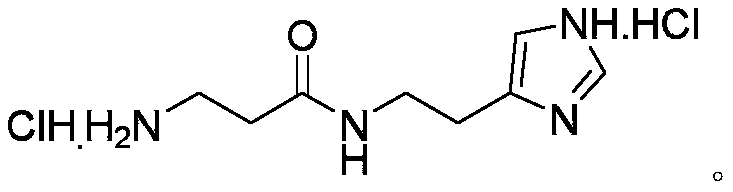
InactiveCN110981810ALow costFew reaction stepsOrganic chemistryBulk chemical productionBiotechnologySide chain
The invention discloses a synthetic method of decarboxylated carnosine, and belongs to the technical field of short peptide synthesis. The synthetic method comprises the steps: Boc-beta-Ala-OH reactswith HOSU to obtain Boc-beta-Ala-OSU, Boc-beta-Ala-OSU reacts with histamine dihydrochloride and NaHCO3 to obtain Boc-beta-Ala-histamine, and finally, a Boc protecting group is removed to obtain the finished product decarboxylated carnosine. According to the synthetic method of the decarboxylated carnosine, the adopted raw material histamine side chain does not need to be protected, the reaction steps are reduced, the cost of the protecting group is reduced, the operation is simple, byproducts are few, the purity and the total yield are high, and the synthetic method is suitable for industriallarge-scale production.
Owner:东莞市维琪科技有限公司
Method for preparing thymosin α1 by condensation of liquid phase fragments
ActiveCN103665144BHigh purityHigh yieldHormone peptidesPeptide preparation methodsFluid phaseTarget peptide
The invention provides a method for preparing thymosin alpha 1 by liquid phase fragment condensation, belonging to the technical field of biochemistry. According to the method, high capacity value (not less than 0.8mmol / g) resin is used as a starting material, firstly, a standard solid phase polypeptide synthesis (SPPS) technology is adopted for synthesizing a high-purity peptide fragments with selected structures, then, a liquid phase condensation technology is adopted for connecting the peptide fragments, and thus, a high-purity (more than 99%) target peptide is obtained. Compared with the solid phase thymosin alpha 1 synthesis technology, the method provided by the invention avoids the problem of low coupling ratio of amino acids behind the 12th position, and greatly improves yield (up to 25-30%) of the thymosin alpha 1; meanwhile, the peptide fragment formed by solid phase synthesis is free from purification, post-treatment technology is simplified, finally, the thymosin alpha 1 is purified by means of high performance liquid chromatography, preparation difficulty is reduced, preparation times is decreased, synthesis cost of the thymosin alpha 1 is lowered, and implementation of large-scale and industrialized production is facilitated.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Novel compound, production method therefor, and application therefor
ActiveCN105593268AHigh purityImprove responseOxytocins/vasopressinsPeptide preparation methodsPeptide Synthesis techniqueFunctional peptide
[Problem] To provide a heretofore completely different and novel peptide synthesis technique, and to provide a novel compound that enables the synthesis / creation of a novel artificial functional protein and the synthesis / creation of a novel functional peptide, as well as a production method for said compound. [Solution] A compound represented by formula (I) or a salt thereof.
Owner:TOKYO UNIVERSITY OF PHARMACY AND LIFE SCIENCES +1
A kind of method for preparing alendidil
ActiveCN104447963BRealize large-scale productionImprove efficiencyDepsipeptidesPeptide preparation methodsDipeptideSide chain
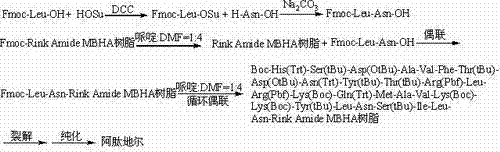
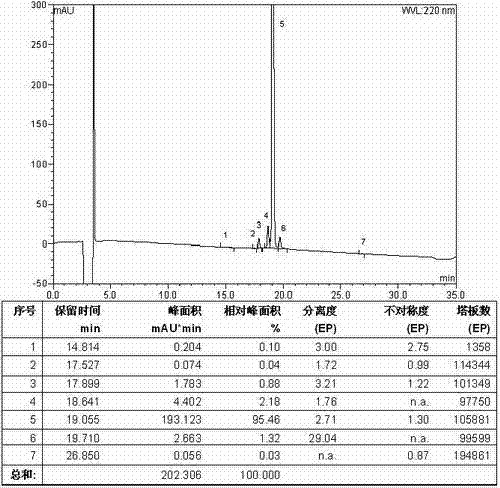
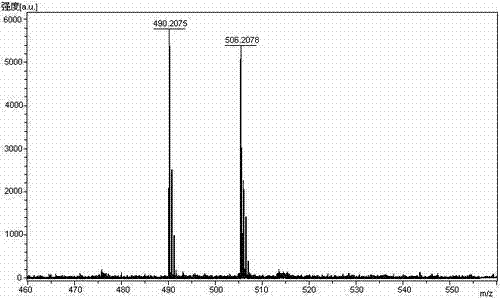
The invention relates to the technical field of polypeptide synthesis, and in particular relates to a method for preparing aviptadil. The method specifically comprises the following steps: (A) synthesizing a dipeptide fragment Fmoc-Leu-Asn-OH by virtue of a liquid phase method; (B) sequentially coupling amino acids with N end Fmoc protection and side chain protection according to an aviptadil main chain peptide sequence by adopting a solid phase synthesis method and taking Rink Amide MBHA amino resin as initial resin, wherein the amino acid coupling of 23, 24, 27 and 28 positions is realized by adopting the dipeptide fragment Fmoc-Leu-Asn-OH coupling, and the histidine coupling of a first position is realized by adopting Boc-His(Trt)-OH; and (C) performing peptide resin pyrolysis, purification and freeze-drying to obtain aviptadil. The invention provides D-His1-aviptadil which is high in synthetic efficiency and low in cost and can avoid racemization impurities, so that the method is suitable for a synthetic process of aviptadil in scale production.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
A method for synthesizing amino acid n-methylation and its product and application
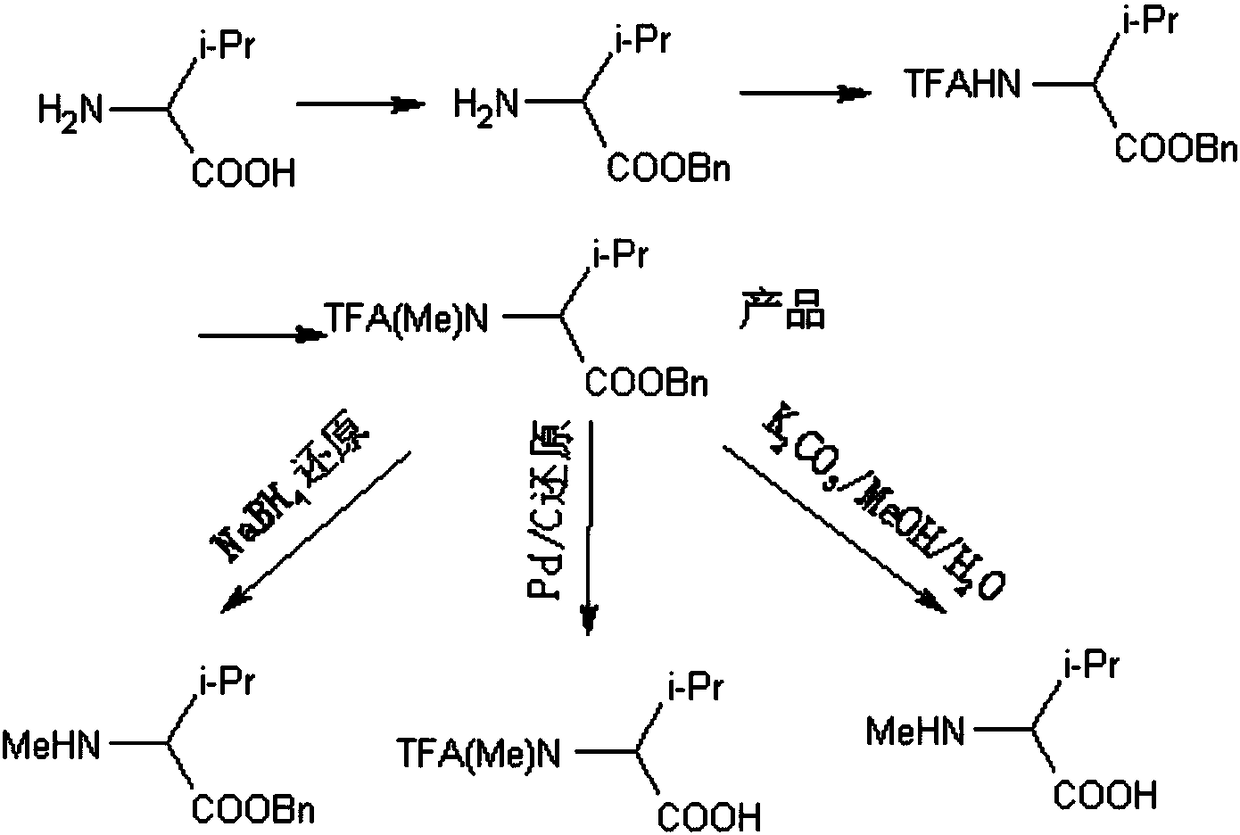
ActiveCN105949078BEasy to operateHigh yieldOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsN methylation
The invention relates to the technical field of amino acid polypeptide synthesis, and discloses an amino acid N-methylation synthesis method, and a product and an application thereof. According to the invention, through benzyl protection upon the carboxyl group of amino acid and through trifluoroacetyl protection upon the amino group, N-methylated amino acid can be obtained after methylation. The N-methyl(trifluoroacetyl)-amino acid benzyl ester prepared by the method provided by the invention can be directly used in polypeptide synthesis, such that an amino acid benzyl ester product with an N-methyl structure can be obtained. Also, the compound has good deprotection selectivity, and selective deprotection can be carried out upon the N-terminal and the C-terminal. Compared to a Ag2O / CH3I / Boc method (high toxicity and high cost) and a NaH / (CH3O)2SO2 method (high toxicity) abroad, the method provided by the invention has high yield. With the use of nontoxic reagents, the method is safe and nontoxic, and has the advantages of simple operation, common and easy-to-obtain raw material reagents, low cost, and high structural selectivity. The obtained product has low racemization possibility. The method has a good industrial prospect.
Owner:SOUTH CHINA AGRI UNIV
Polypeptide reagent for activating human immune cells
The invention provides a polypeptide reagent for activating human immune cells. The polypeptide reagent comprises 6-aminopurine, folic acid, glycyl-L-glutamine, L-glutathione, thymopentin, Pp25 and Pp21, wherein Pp25 and Pp21 are prepared by a solid-phase polypeptide synthesis technology.
Owner:定山石生物科技(北京)有限公司
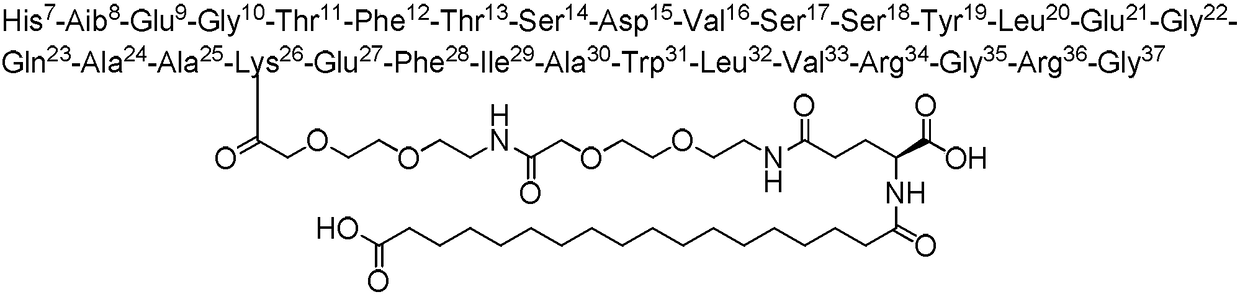
Semaglutide main peptide chain and preparation method thereof
ActiveCN114014925AMake up the priceMake up processOther chemical processesPeptide preparation methodsEpoxyFluid phase
The invention provides a semaglutide main peptide chain and a preparation method thereof, belongs to the technical field of polypeptide synthesis, and particularly relates to a method for performing condensation coupling on amino acid according to a polypeptide sequence of the semaglutide main peptide chain by taking a self-made polypeptide condensing agent and organic alkali as a condensation coupling system, which is beneficial to improving the coupling efficiency and reducing the generation of impurities. The purity and the yield of the crude peptide are improved, the synthesis cost is reduced, the purification difficulty is reduced, and the purification yield is improved; in the reversed-phase high performance liquid chromatography purification process of the main peptide chain of the semaglutide, a silica gel chromatographic filler modified by 1-pyridine-2-yl-1, 4-butanediamine and epoxy siloxane is used as a filler, so that the purification efficiency is favorably improved.
Owner:浙江湃肽生物股份有限公司南京分公司
A kind of method for preparing peregrine
ActiveCN104356221BImprove efficiencyResolving incomplete couplingPeptide preparation methodsAnimals/human peptidesDipeptideSide chain
The invention relates to the technical field of polypeptide synthesis, in particular to a preparation method of pexiganan. The preparation method comprises the following specific steps: A) synthesizing a dipeptide fragment FmoC-Lys (BoC)-Lys (BoC)-OH by a liquid phase method; B) by a solid-phase synthesis method, with amino resin as starting resin, sequentially coupling amino acids with FmoC protections at N terminals and side chain protections according to a peptide sequence of a main chain of the pexiganan, wherein the amino acids at 7 and 8 positions, the amino acids 10 and 11 positions and the amino acids at 21 and 22 positions are coupled by the dipeptide fragment FmoC-Lys (BoC)-Lys (BoC)-OH; C) cracking, purifying and freeze-drying peptide resin to obtain the pexiganan. The invention provides a synthesis process of the pexiganan, which is high in synthesis efficiency and low in cost, can avoid deleted peptide impurities caused by incomplete coupling of two lysines and is suitable for mass production.
Owner:上海飞腾医药科技有限公司
Anti-myocardial remodelling polypeptide, preparation method thereof, preparations and application thereof in preparation of anti-myocardial remodelling medicament
ActiveCN101648996BShorten the lengthMaintain and enhance activityPeptide/protein ingredientsPeptide preparation methodsPeptide sequenceC-terminus
The invention relates to a G protein competitive inhibitory peptide, which starts from a 55 peptide sequence and obtains a series of derived polypeptides by deleting amino acid residues in any numbermore than one in any point from the first amino acid residue at the amino end and at least remaining twelve amino acid residues at the amino end. The length of the polypeptide is shortened up to 78.2percent; and the polypeptide has a remarkable effect on anti-myocardial remodelling. The invention also relates to a method for preparing the series of derived polypeptides, which successfully synthesizes a target polypeptide with the purity up to 99.2 percent by utilizing advanced peptide synthesis technology. The invention further relates to the preparations containing the polypeptides and the application thereof in the preparation of an anti-myocardial remodelling medicament.
Owner:ARMY MEDICAL UNIV +2
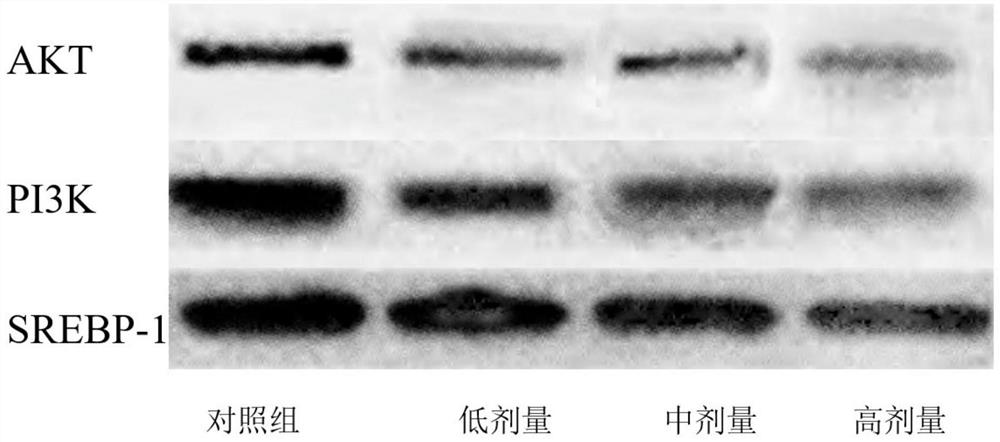
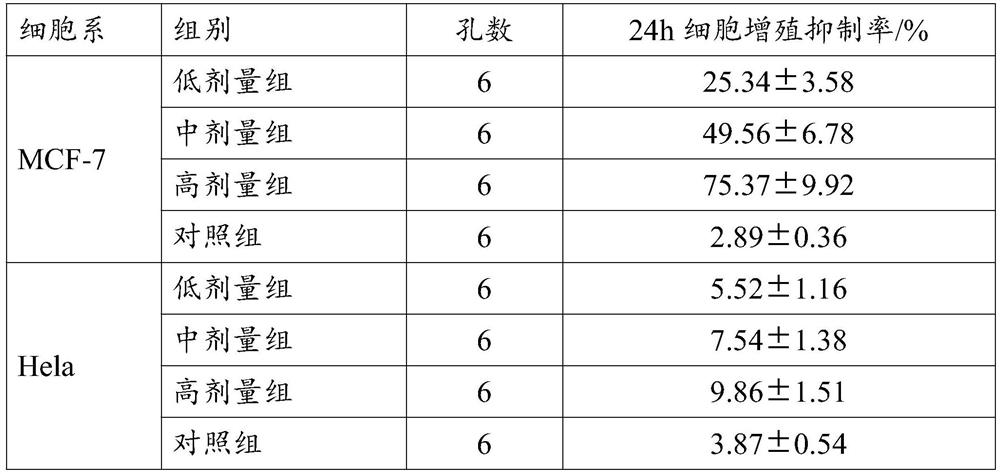
Inhibitor for regulating breast cancer proliferation and migration based on SREBP-1/PI3K/AKT signal channel and application thereof
ActiveCN114470152AInhibition of proliferation and migrationLow costPeptide/protein ingredientsPeptidesAkt signallingOncology
The invention relates to an inhibitor for regulating breast cancer proliferation and migration based on an SREBP-1 / PI3K / AKT signal pathway and application of the inhibitor, the inhibitor is a short peptide, and the short peptide has an amino acid sequence as shown in SEQ ID NO.1. The inhibitor disclosed by the invention can be generated by adopting an existing short peptide synthesis technology, and is low in cost, good in effect, high in safety and high in selectivity.
Owner:HEILONGJIANG UNIV OF CHINESE MEDICINE
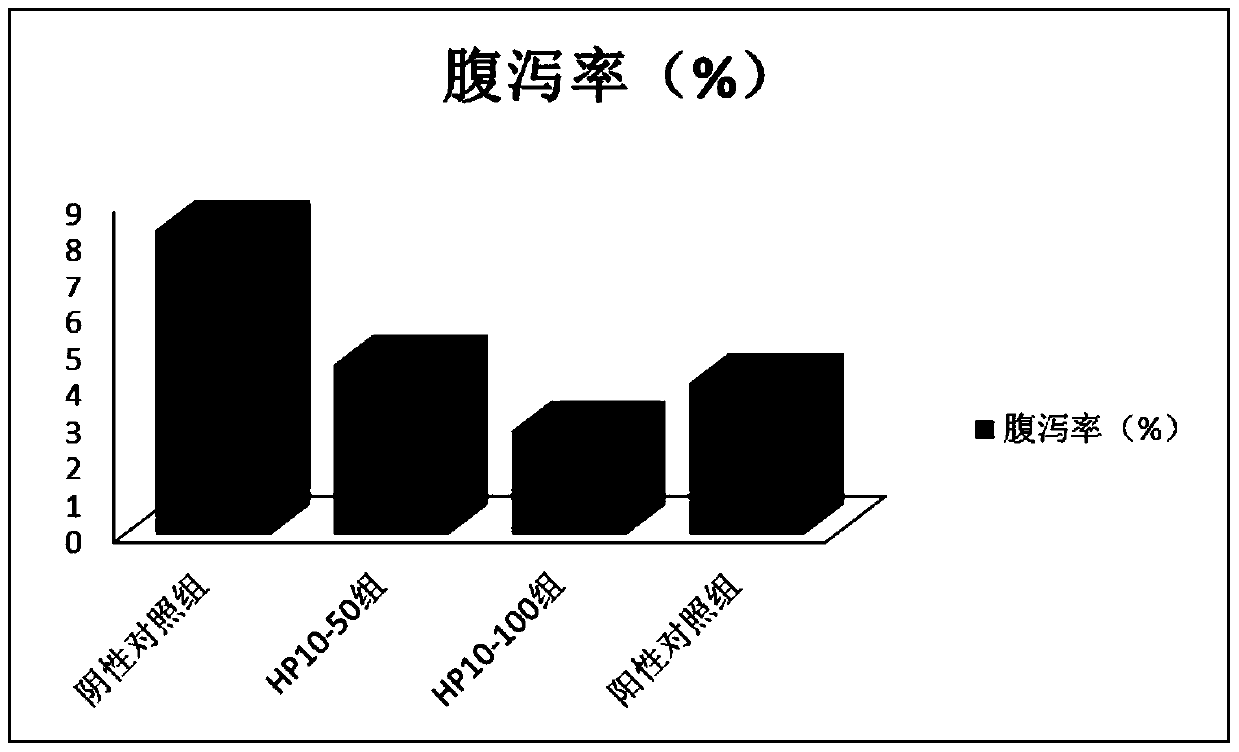
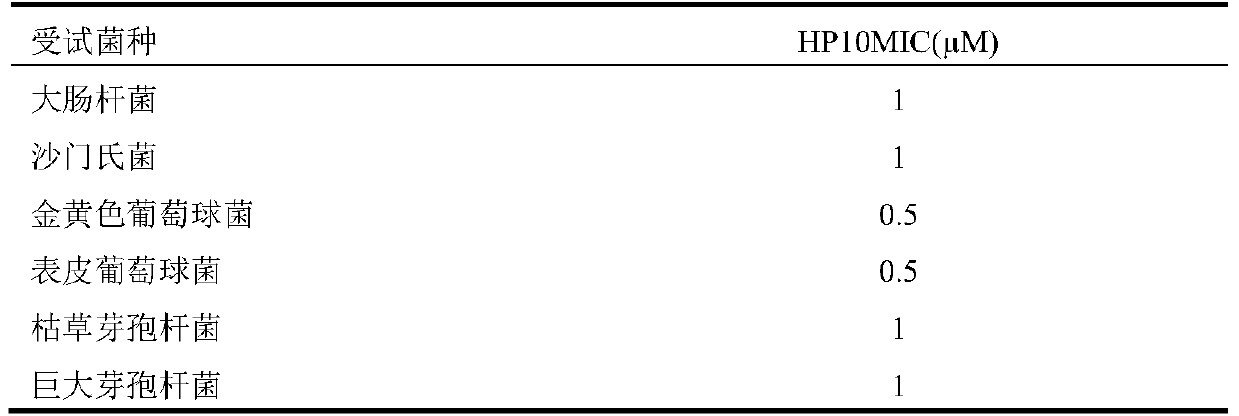
Antibacterial peptide hp10 and its preparation method and application
ActiveCN105153281BSmall molecular weightEasy to synthesizeAccessory food factorsPeptide preparation methodsAntimicrobial peptidesCombinatorial chemistry
The antibacterial peptide HP10 of the present invention and its preparation method and application belong to the technical field of peptide synthesis containing 5-20 amino acids and the specific therapeutic activity technical field of compounds and pharmaceutical preparations. The amino acid sequence of the antimicrobial peptide HP10 is HHHLHHIHKP; its molecular weight is 1292.4Da, and its isoelectric point is 8.79. Its preparation comprises the following steps: (1), the antibacterial peptide HP10 that amino acid sequence is HHHLHHIHKP is activated and cross-linked one by one from C-terminus to N-terminus by FMOC protection synthetic method; (2), the product of step (1) is subjected to HPLC Antimicrobial peptide HP10 was obtained by desalting and purification by reverse phase column chromatography. The antimicrobial peptide HP10 in the present invention has the advantages of small molecular weight, convenient artificial synthesis, strong thermal stability, strong bactericidal effect, and no drug resistance; it also has the advantages of significantly improving the intestinal health of piglets and strengthening the body when applied in piglet feed. Advantages of immunity, improving piglet growth performance.
Owner:GUANGZHOU AONONG BIOTECH
A kind of method of synthesizing phenylalanyl-lysine dipeptide
InactiveCN107936089BMild conditionsStrong specificityPeptide preparation methodsFermentationDipeptideChymotrypsin
A method for synthesizing phenylalanyl-lysine dipeptide belongs to the technical field of peptide synthesis. The invention uses commercially available phenylalanine and lysine as raw materials, prepares ethylene glycol non-aqueous reaction medium by preparing phenylalanine and lysine aqueous solution, and prepares phenylalanyl-lysine dipeptide reaction liquid , prepare a resin column for purifying loaded phenylalanyl-lysine dipeptide, prepare phenylalanyl-lysine dipeptide eluate, prepare phenylalanyl-lysine dipeptide freeze-dried powder, prepare recovery The phenylalanine and lysine aqueous solution and the step of preparing the regenerated resin column just prepare the phenylalanyl-lysine dipeptide with a content of 95-98%. The present invention uses chymotrypsin as a catalyst to synthesize phenylalanyl-lysine dipeptide in an ethylene glycol non-aqueous medium, the conditions are mild, the specificity is strong, and the synthesis efficiency is high, which is a typical green production process; the prepared Phenylalanyl-lysine dipeptide can be widely used in medicine, textile, food and other fields, and has considerable social value.
Owner:吴?基
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com