Novel compound, production method therefor, and application therefor
A compound, selected technology, applied in the preparation method of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problems that cannot be applied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
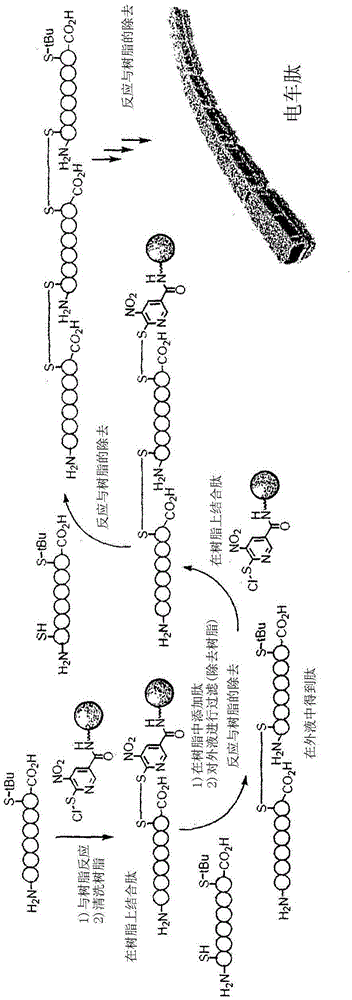
[0380] As an example of the compound of the present invention, the synthesis of compound A is shown below.
[0381] Synthesis of Compound A (6-chlorosulfenyl-5-nitronicotinic acid carboxamide resin)
[0382] Compound A was synthesized by the following route.
[0383] [chem 67]
[0384]
[0385] Resin: Cross-linked product of polyethylene glycol (MethylChemMatrix (registered trademark) resin)
[0386] (1) Synthesis of Compound 2
[0387] Compound 1 (25 g, 0.180 mol) was placed in a 500 ml eggplant-shaped bottle, and fuming nitric acid (1.52) (125 ml) was added. While stirring, it heated slowly using an oil bath, and stirred for 5 hours under the temperature condition of 50 degreeC. Heating was stopped, and after natural cooling to room temperature, the reaction solution was concentrated under reduced pressure. The obtained residue was cooled in an ice bath, recrystallized using methanol as a solvent, and the solid obtained by filtration was dried under reduced pressure ...
Embodiment 2
[0403] As an example of the compound of the present invention, the synthesis of Compound B is shown below.
[0404] Synthesis of Compound B (5-((6-(methylaminoresin)-6-oxohexyl)amino)-6-oxohexyl)carbonyl)-3-nitropyridine-2-sulfenyl chloride)
[0405] Compound B was synthesized by the following route.
[0406] [chem 68]
[0407]
[0408] Resin: Cross-linked product of polyethylene glycol (MethylChemMatrix (registered trademark) resin)
[0409] (1) Synthesis of Compound 8
[0410] 9-Fluorenylmethoxycarbonylaminocaproic acid (compound 7, 632.4 mg, 1.79 mmol), (O-(7-azabenzotriazol-1-yl)- N,N,N',N'-tetramethyluronium hexafluorophosphate) (540.6mg, 1.76mmol), DMF (16ml), diisopropylethylamine (257.0μl, 1.79mmol) for 1 min Shake to stir. Take 511.7 mg of aminomethyl-ChemMatrix resin (H in the formula) in an additionally prepared 60 ml polypropylene filter tube 2N-resin, functional group substitution rate 0.70mmol / g), to which was added the solution in the above 15ml tube at...
Embodiment 3
[0422] Modification of the octaarginine derivative of the compound captopril (compound 13) having an SH group using compound A was carried out through the following synthetic route.
[0423] [chem 69]
[0424]
[0425] Resin: Cross-linked product of polyethylene glycol (MethylChemMatrix (registered trademark) resin)
[0426] To a 10-ml glass test tube containing compound A and a stir bar, 90% formic acid aqueous solution (2 ml) was added under ice-cooling, followed by gentle stirring to replace the solvent. After removing the washing solution with a Pasteur pipette, 90% formic acid aqueous solution was added again, and the same washing was repeated five times. In an additionally prepared 30ml Erlenmeyer flask, the peptide Ac-Arg containing octa-arginine consisting of 10 residues was 8 -Acp-Cys(tBu)-NH 2 • TFA salt (143.37 mg) was dissolved in 90% formic acid (1 ml), and the resulting aqueous solution was added to the 10 ml glass test tube containing the above compound A ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com