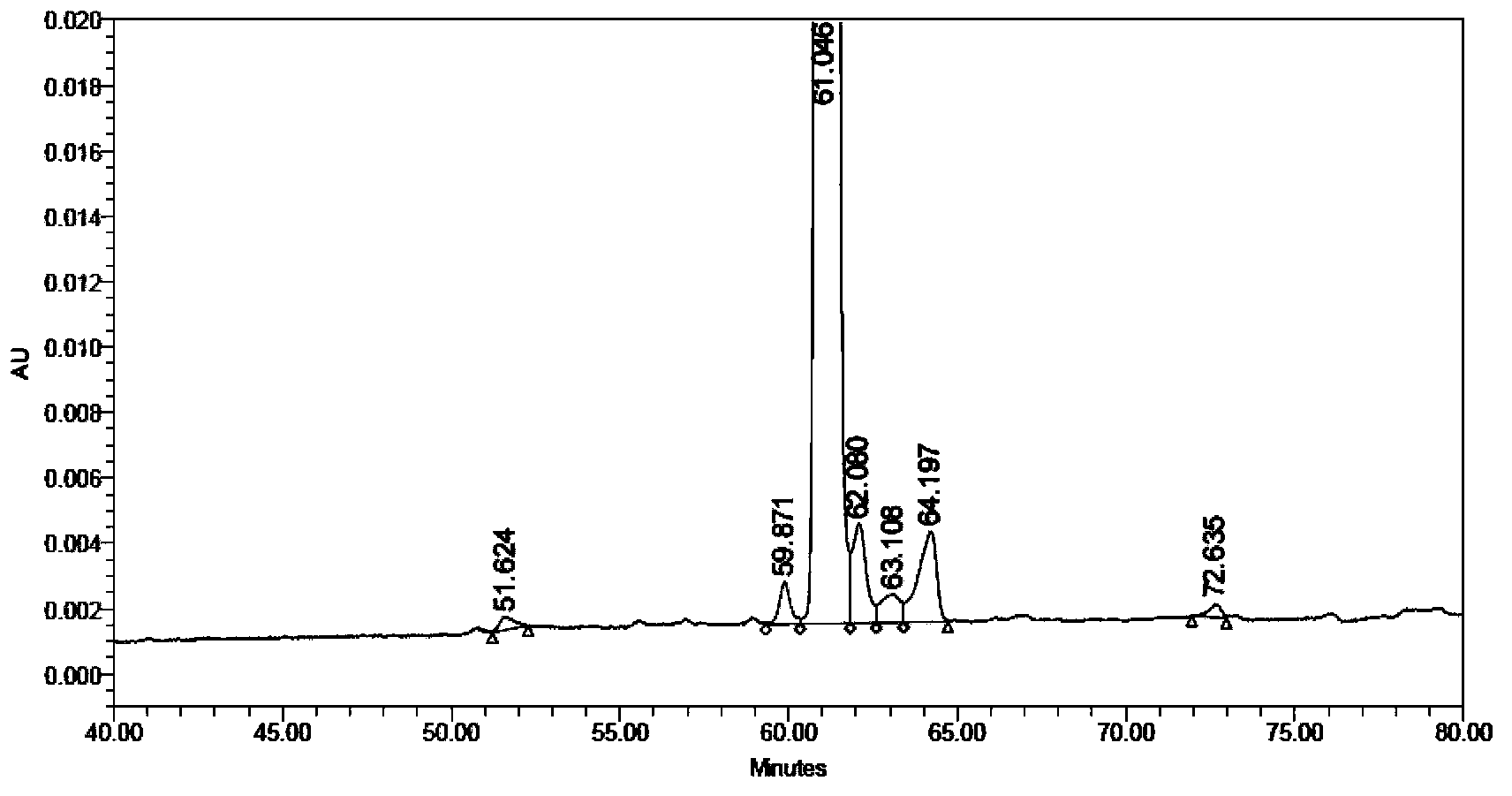
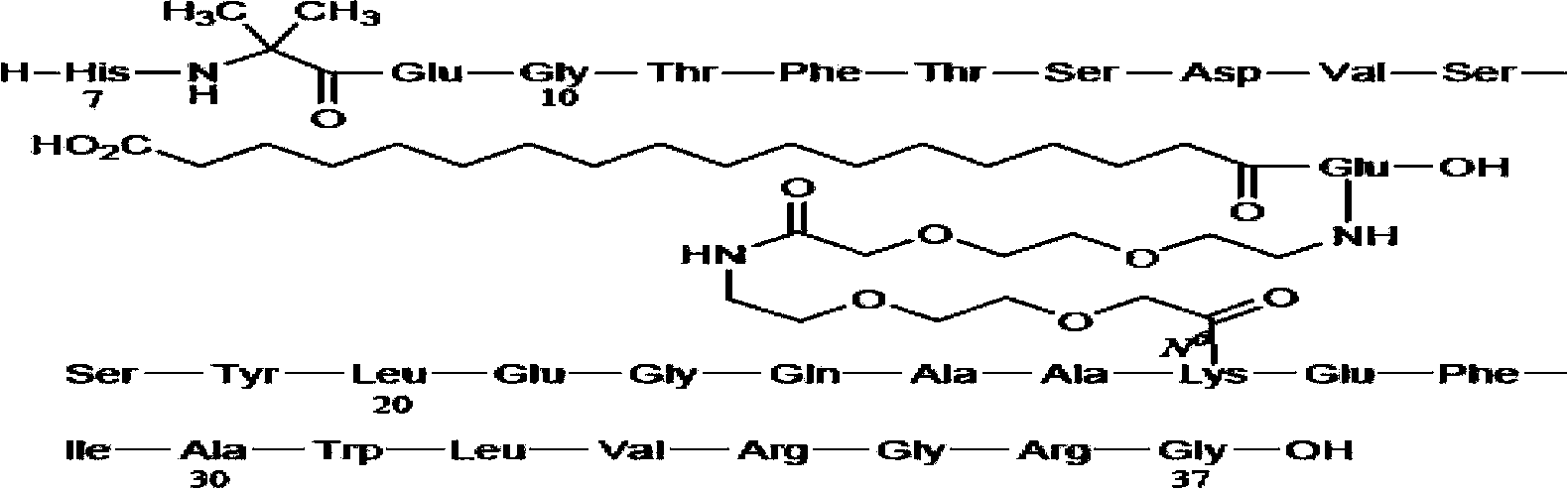
Solid synthetic method of semaglutide
A solid-phase synthesis, Samolu technology, applied in the preparation methods of peptides, chemical instruments and methods, animal/human proteins, etc., can solve the problems of strong steric hindrance effect, low product yield, difficult coupling reaction, etc. Achieve the effect of short synthesis cycle, high product yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The invention provides a solid-phase synthesis method of samoglutide, comprising the following steps:
[0067] Step A: coupling Gly with resin to obtain Gly-resin;
[0068] Step B: Gly-resin undergoes the first stepwise coupling of amino acids or amino acid derivatives to obtain the first peptide resin whose sequence is shown in SEQ ID No.1;
[0069] Step C: removing the side chain protecting group of Lys in the first peptide resin whose sequence is shown in SEQ ID No.1, and coupling 2-(2-(2-aminoethoxy)ethoxy) through the second stepwise coupling ) acetic acid, 2-(2-(2-aminoethoxy) ethoxy) acetic acid, Glu and octadecanedioic acid to obtain a second peptide resin;
[0070] Step D: The second peptide resin is obtained by cleavage, purification and freeze-drying.
[0071] In some embodiments provided by the present invention, the resin in step A is 2-CTC resin, and the activator used for the coupling of 2-CTC resin and N-terminal Fmoc-protected Gly is selected from DIE...
Embodiment 1
[0084] Embodiment 1 degree of substitution is the synthesis of the Fmoc-Gly-CTC resin of 0.1mmol / g
[0085] Weigh 20 g of 2-CTC resin with a substitution degree of 0.5 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then take 3.27 g of Fmoc-Gly-OH and dissolve it in DMF. Add 4.78mL DIEA to activate under ice-water bath, add to the above-mentioned reaction column filled with resin, react for 2 hours, add 8mL of anhydrous methanol to block for 1 hour. Wash 3 times with DMF and 3 times with DCM, block with anhydrous methanol for 30 minutes, shrink and dry with methanol to obtain Fmoc-Gly-CTC resin, and the detected substitution degree is 0.104mmol / g.
Embodiment 2
[0086] Embodiment 2 degree of substitution is the synthesis of the Fmoc-Gly-CTC resin of 0.25mmol / g
[0087] Weigh 20 g of 2-CTC resin with a substitution degree of 0.5 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and use DMF to swell the resin for 30 minutes, then take 8.18 g of Fmoc-Gly-OH and dissolve it in DMF. Add 11.95mL DIEA to activate under ice-water bath, then add to the above-mentioned reaction column filled with resin, react for 2 hours, add 8mL of anhydrous methanol to block for 1 hour. Washed 3 times with DMF and 3 times with DCM, blocked with anhydrous methanol for 30 minutes, shrank and dried with methanol to obtain Fmoc-Gly-CTC resin, and the detected substitution degree was 0.255mmol / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com