Method for preparing thymosin alpha 1 by liquid phase fragment condensation
A technology of thymosin and fragments, applied in the field of biochemistry, can solve the problems of time-consuming, difficult to obtain high-purity thymosin α1, and high production cost, and achieve the effects of simplifying the post-processing process, reducing the number of preparations, and reducing the cost of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
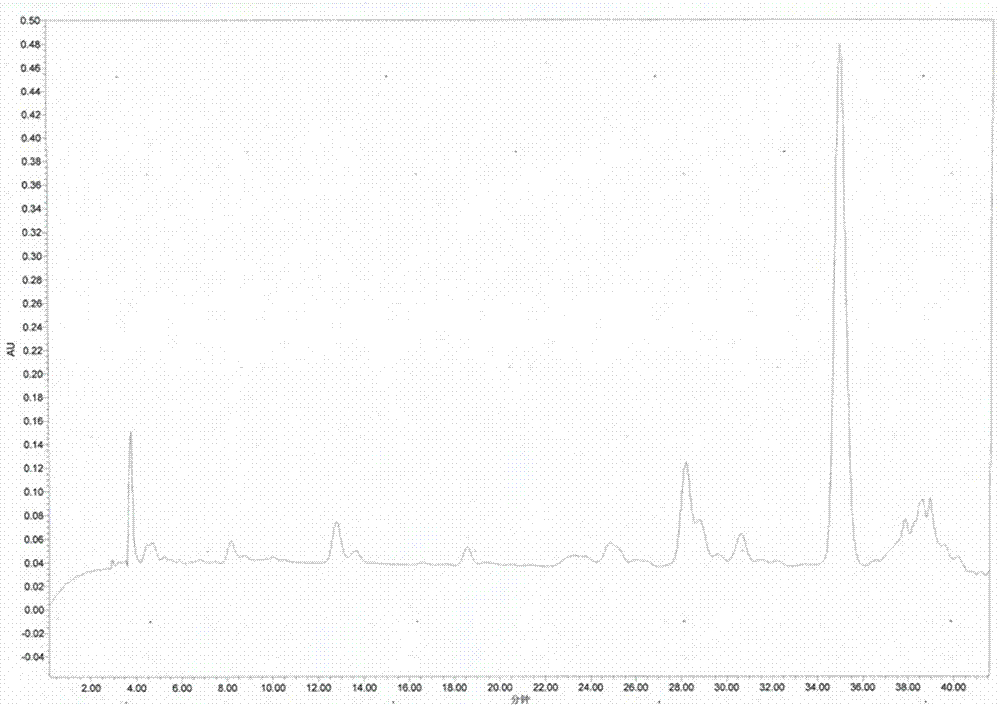
Examples
Embodiment 1 2
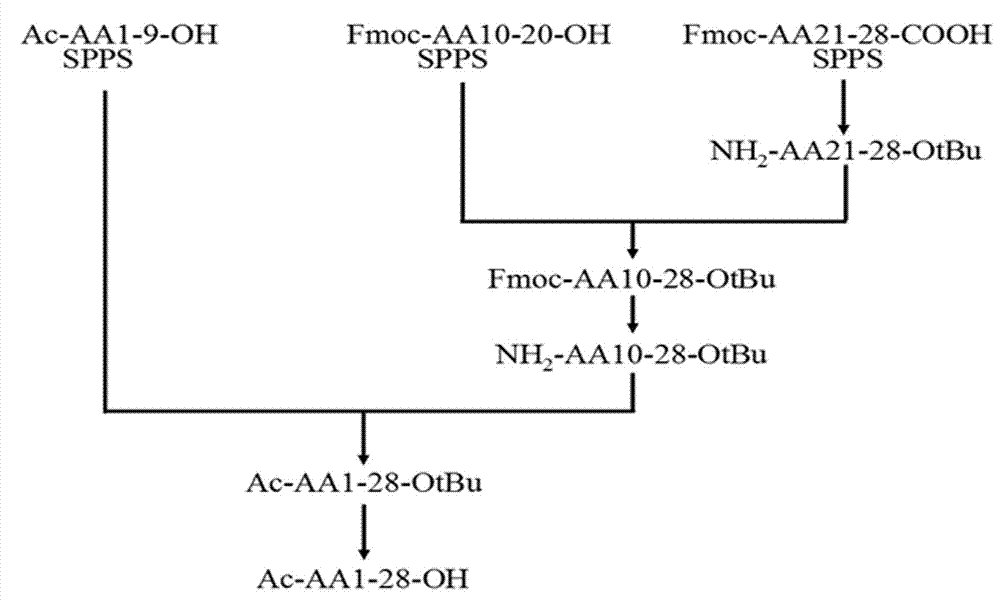
[0035] Example 1. Preparation of thymosin α1 by 2+2 fragment method
[0036] 1. Resin preparation
[0037] 1.1 Preparation of Fmoc-Asn(Trt)-2-chloro-trityl resin: add 2-chloro-trityl chloride resin (5g, substitution value 0.8mmol / g resin, 1 eq.) to 150 mL peptide synthesis Wash the swollen resin with 60 mL of DCM for 30 minutes. The solvent was drained and a solution of Fmoc-Asn(Trt)-OH (1.2 eq.) and DIEA (2.5 eq.) in 30 mL DCM was added. The mixture was mechanically stirred under an argon atmosphere for 1 hour. Add 10 mL of chromatographic grade methanol (2ml / g resin) to block the active part on the resin for 30 minutes. Drain the solvent, wash with 3×60 mL DMF, 3×60 mL DCM, 3×60 mL MeOH, vacuum filter and dry to constant weight to obtain 6.39g Fmoc-Asn(Trt)-2-chloro-trityl resin. The amount of Fmoc in the piperidine deprotection solution was measured by ultraviolet spectrophotometry, and the loading amount of the resin was 0.39 mmol / g.
[0038] 1.2 Preparation of F...
Embodiment 2 3
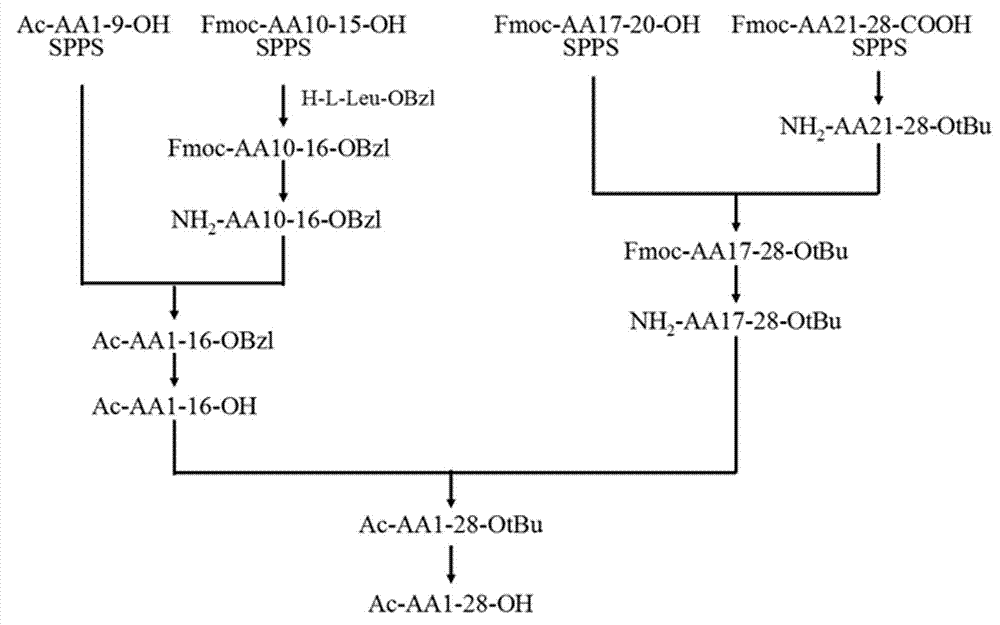
[0136] Embodiment two, three-segment method synthetic thymosin α1
[0137] 1 Resin preparation
[0138] 1.1 The synthesis of Fmoc-Asn(Trt)-2-chloro-trityl resin is the same as in Example 1.
[0139] 1.2 The synthesis of Fmoc-Ser(tBu)-2-chloro-trityl resin is the same as in Example 1.
[0140] 1.3 Preparation of Fmoc-Lys(Boc)-2-chloro-trityl resin: add 2-chloro-trityl chloride resin (5 g, substitution value 0.8 mmol / g resin, 1 eq.) to 150 mL of polypeptide Synthesizer, wash swollen resin with 60 mL DCM. Drain the resin bed, since the resin substitution value must be reduced to synthesize Fmoc-AA(10-20)-OH, add Fmoc-Lys(Boc)-OH (1.2 eq.) and DIEA (2.5 eq.) in 30 mL of DCM . The mixture was mechanically stirred under an argon atmosphere for 1 hour. Add 10 mL of chromatographic methanol (2ml / g resin) to cap the active part on the resin for 30 minutes. The resin bed was drained, washed with 3×60 mLDMF, 3×60 mLDCM, 3×60 mLMeOH, vacuum filtered and dried to constant weight to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com