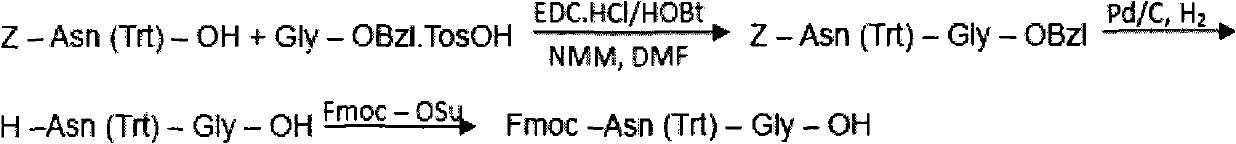Patents
Literature
114 results about "Bivalirudin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bivalirudin (Angiomax or Angiox, manufactured by The Medicines Company) is a direct thrombin inhibitor (DTI). Chemically, it is a synthetic congener of the naturally occurring drug hirudin (found in the saliva of the medicinal leech Hirudo medicinalis).
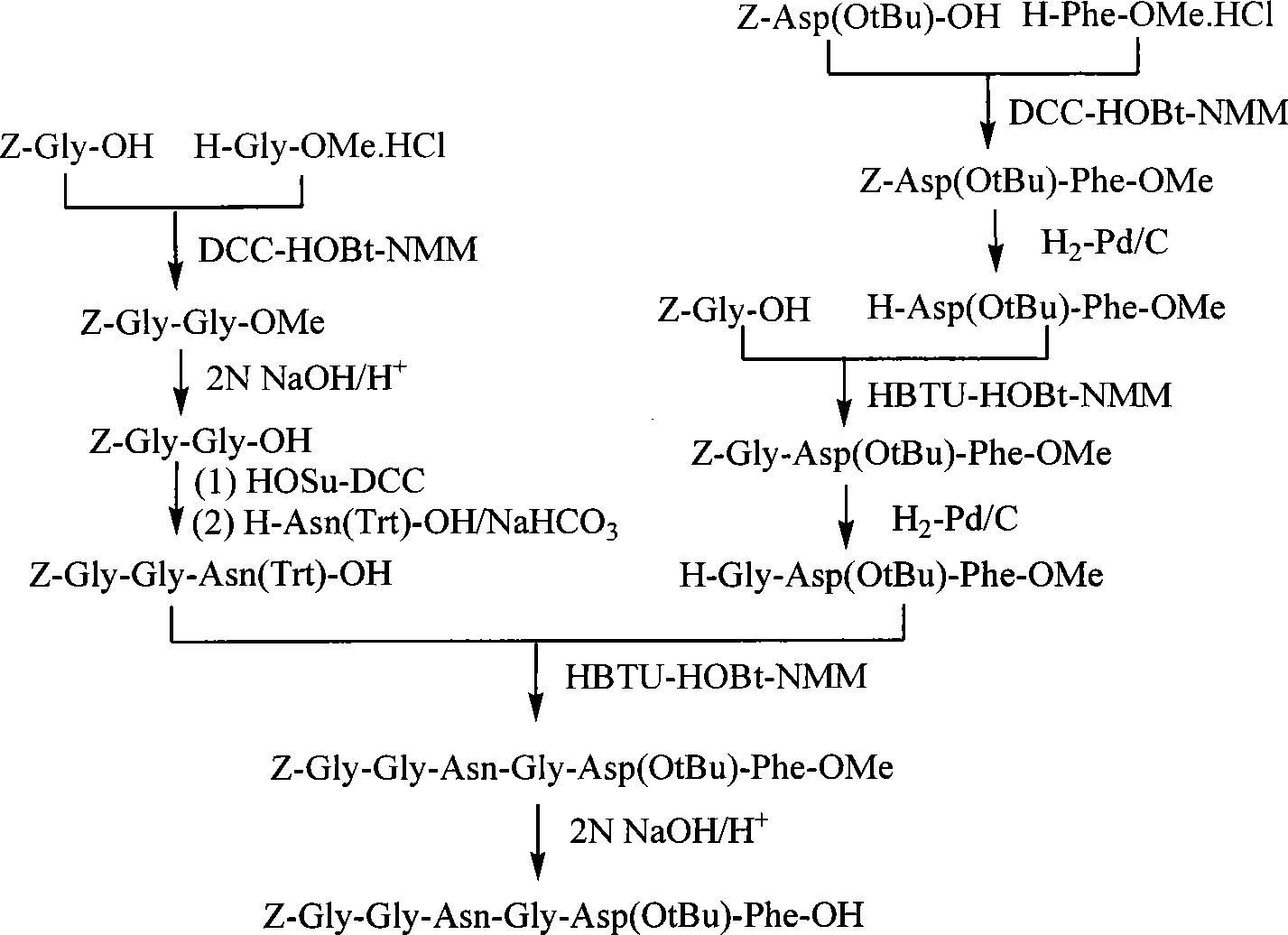
Process for production of Bivalirudin
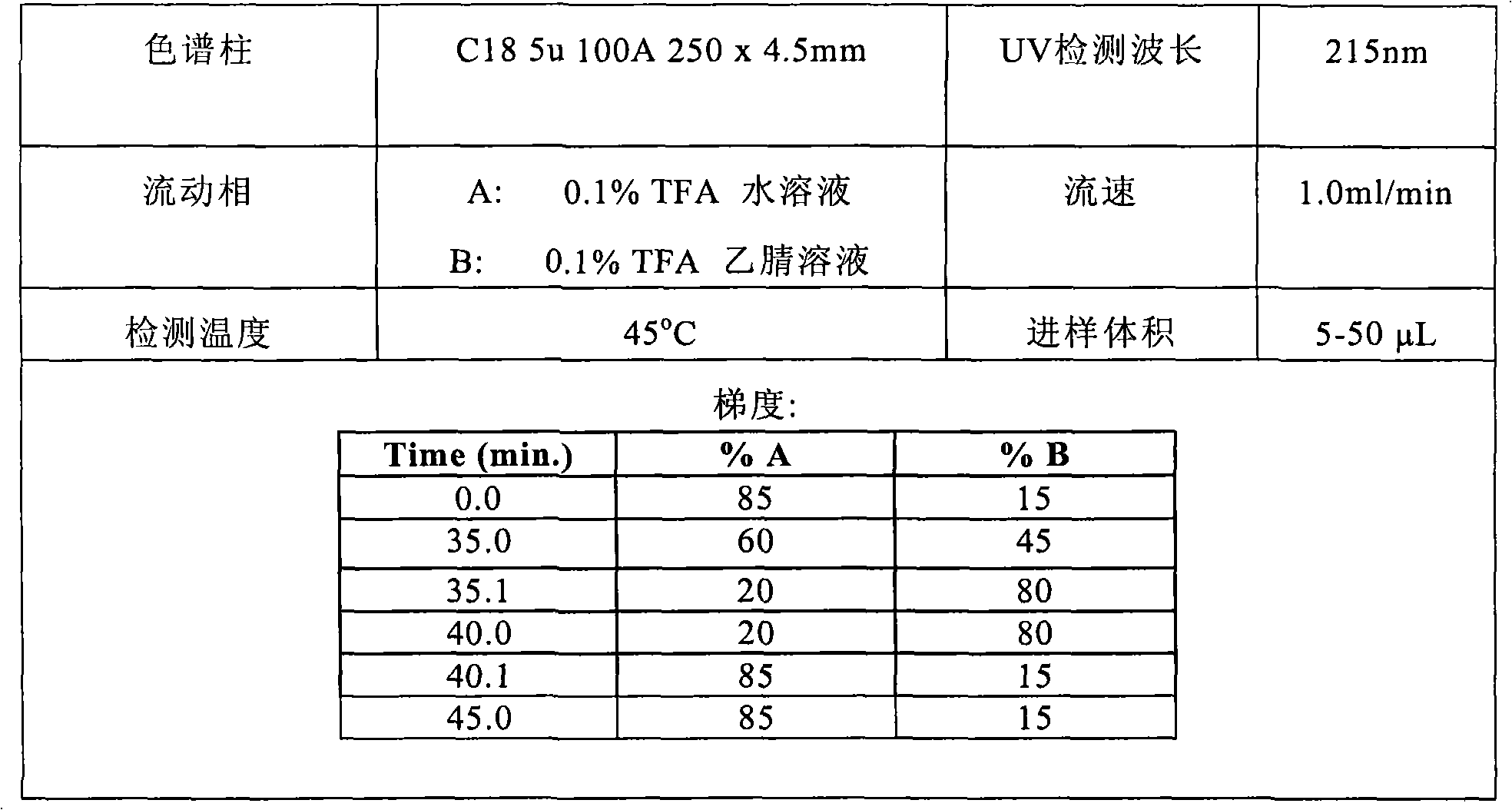
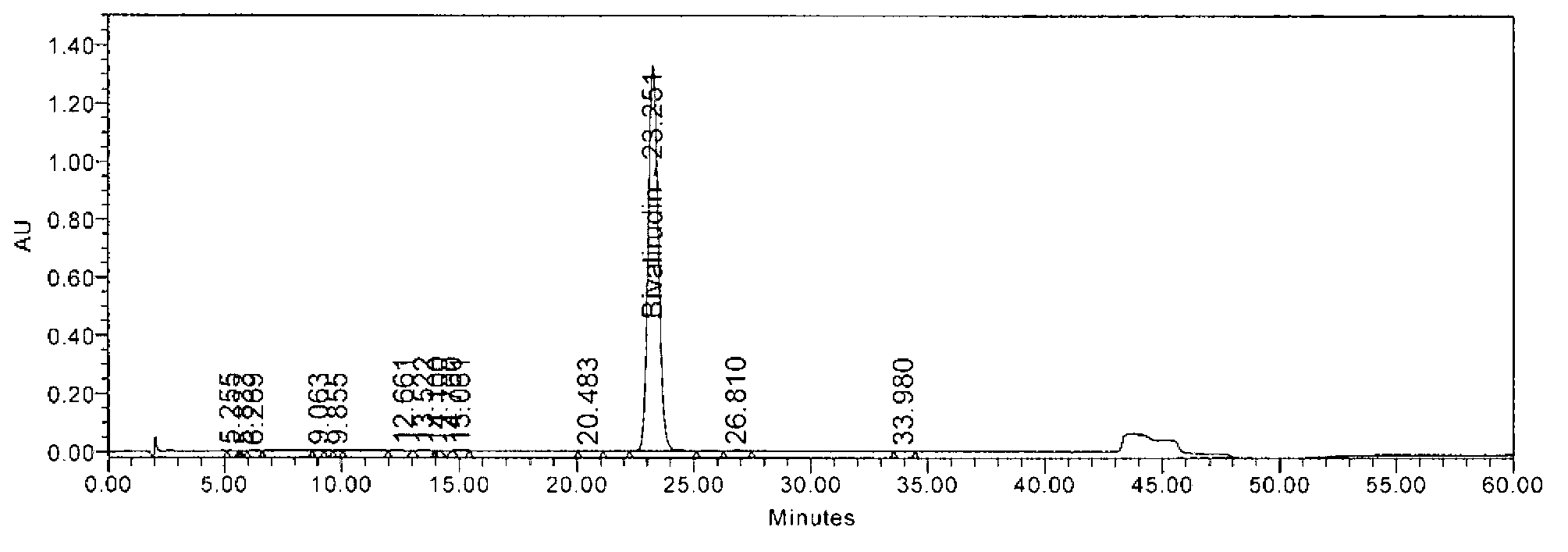
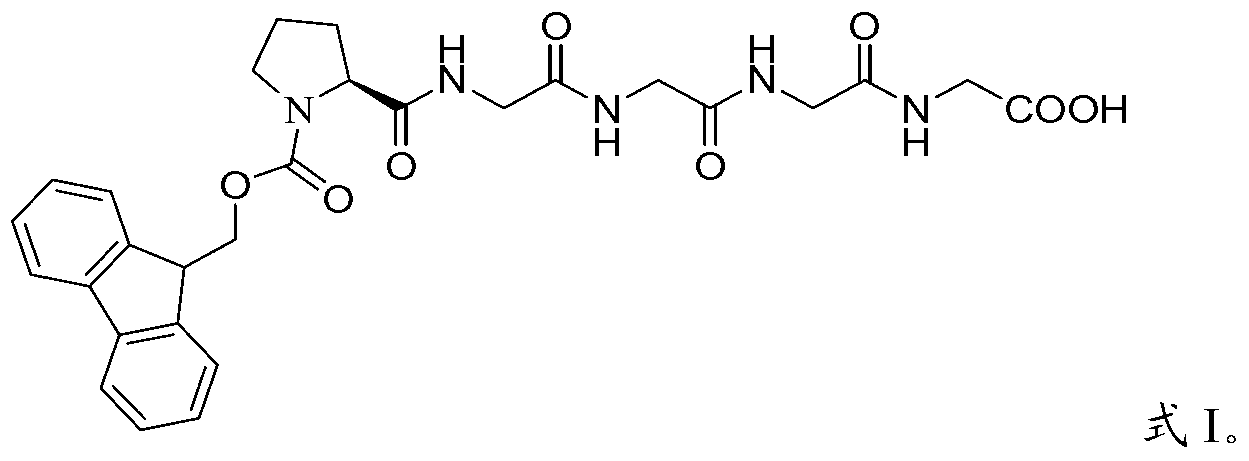
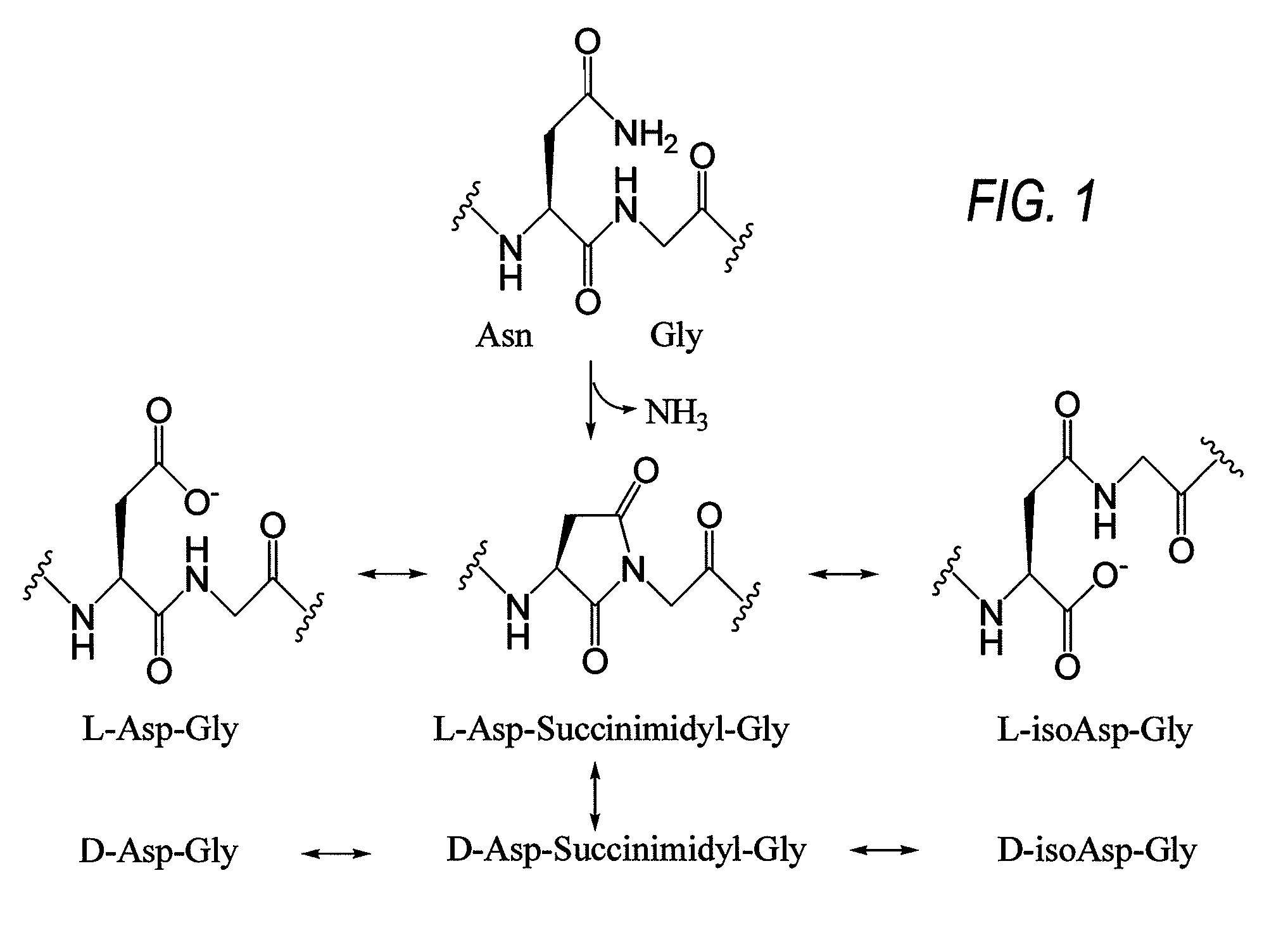
The invention relates to methods for the preparation of high purity Bivalirudin. The polypeptide is prepared in a high purity of at least 98.5% (by HPLC), wherein the total impurities amount to less than 1.5%, comprising not more than 0.5% [Asp9-Bivalirudin] and each is impurity less than 1.0%, and preferably having a purity of at least about 99.0% by HPLC, wherein the total impurities amount to less than 1.0%, comprising not more than 0.5% [Asp9-Bivalirudin] and each impurity is less than 0.5%.
Owner:TEVA PHARM USA INC
Methods of treatment of patients at increased risk of development of ischemic events and compounds hereof
InactiveUS20130040898A1Impair thrombus formationIncreased riskElcosanoid active ingredientsInorganic active ingredientsBeta blockerPlatelet inhibitors
The present invention relates to compounds for treatment that protects the endothelium, prevents pathologic thrombus formation in the microcirculation and preserves platelet number and function and thus may be related to treatment or prevention of ischemic events in patients with cardiovascular disease. The present invention is particularly useful for patients having or being at increased risk of development of an ischemic event such as an acute myocardial infarction and / or no-reflow phenomena and / or ischemia-reperfusion injury by administration of agent(s) modulating and / or preserving endothelial integrity. The compounds may be administered in combination with standard treatment of acute cardiovascular ischemic events such as Platelet inhibitors such as aspirin (ASA), Thienopyridins, GPIIb / IIIa inhibitors), Parenteral anticoagulants such as unfractioned heparin (UFH), bivalirudin, enoxaparin, and fondaparinux, Verapamil, Adenosine, Sodium nitroprusside, Nitroglycerin, Epinephrine, Beta-blockers and surgical methods such as percutaneous coronary intervention (PCI), PCI with thrombus aspiration, PCI with stents.
Owner:THROMBOLOGIC
Preparation method for bivalirudin
ActiveCN102286076AEasy to operateReaction temperaturePeptide preparation methodsLeech-based protease inhibitorsSynthesis methodsAcid hydrolysis
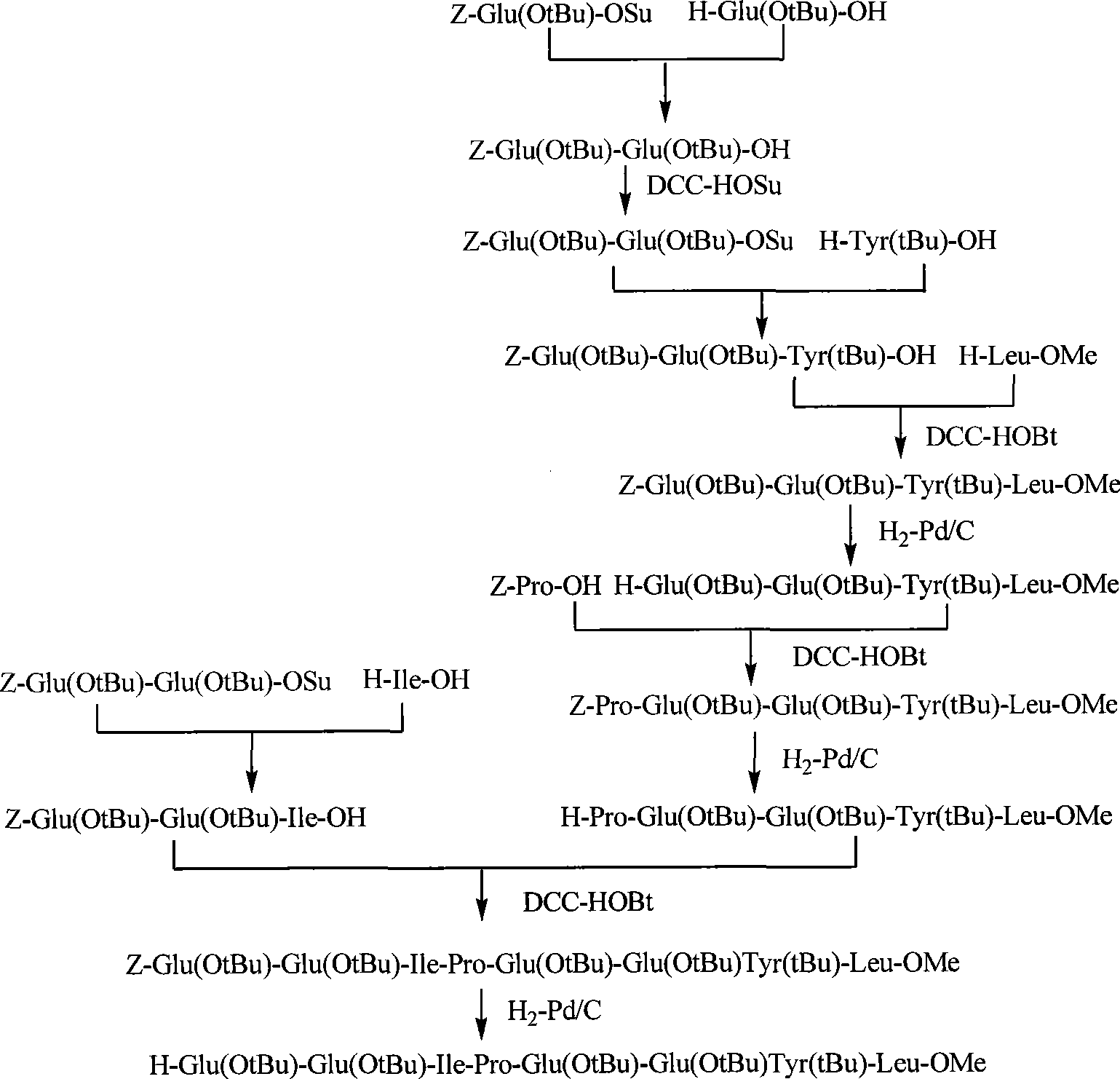
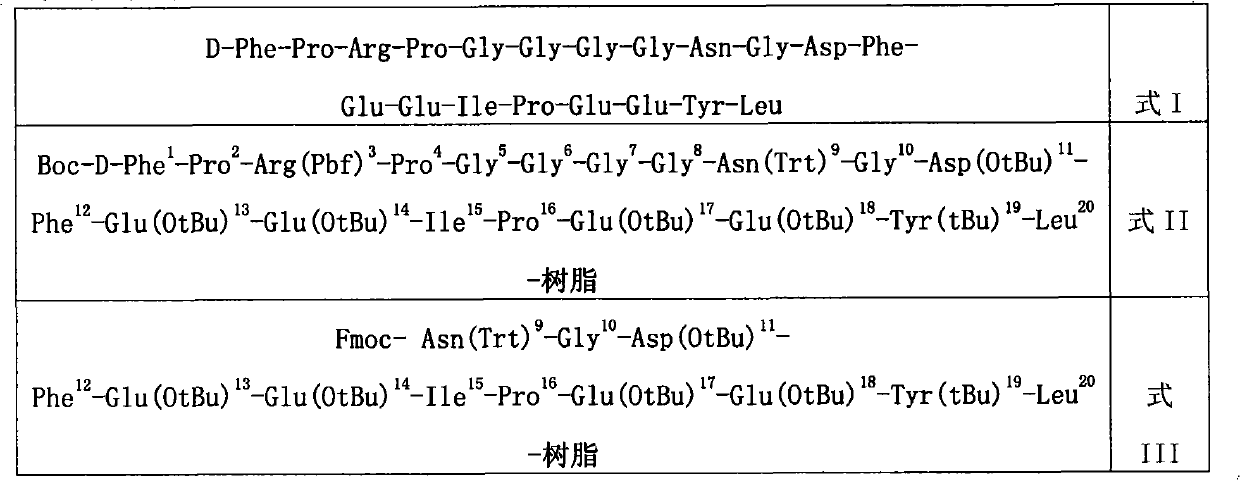
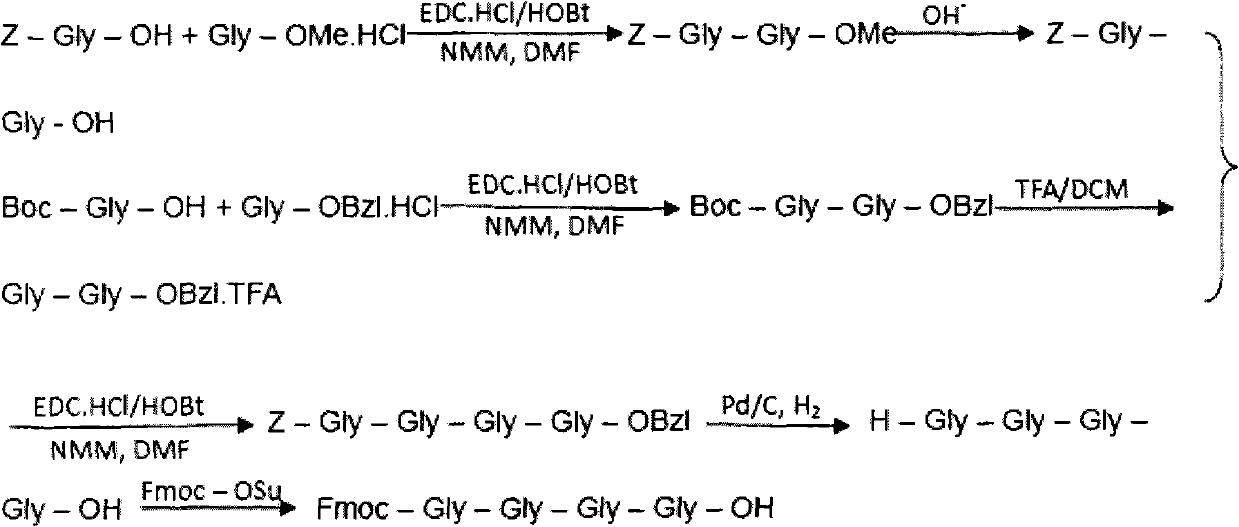
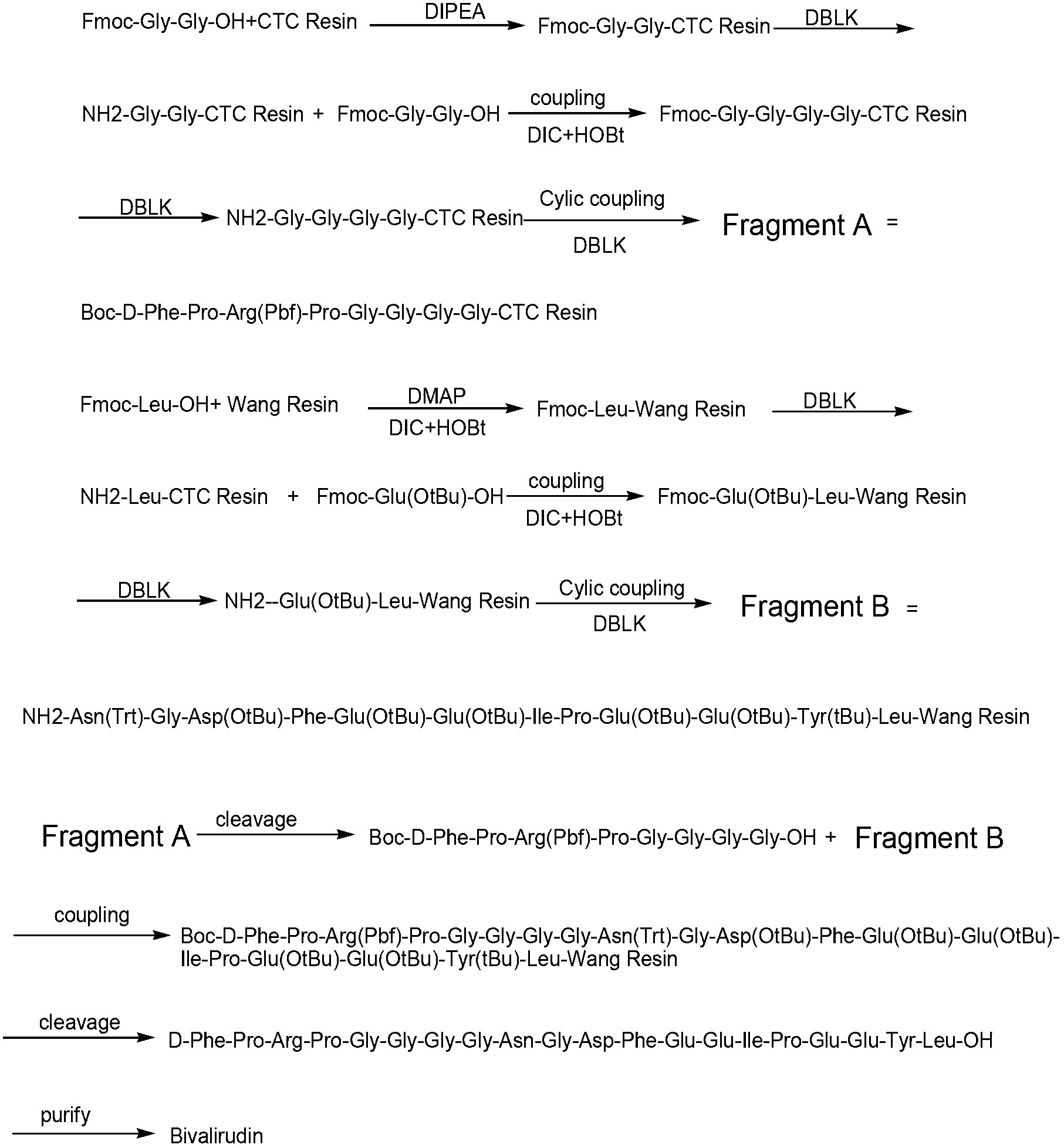
The invention belongs to the technical field of polypeptide medicament preparation methods, and in particular relates to a preparation method for bivalirudin. The preparation method for the bivalirudin comprises solid phase polypeptide synthesis for preparing bivalirudin resin, acid hydrolysis of the bivalirudin resin to obtain a crude bivalirudin product, and purification of the crude bivalirudin product to obtain a purified bivalirudin product, wherein the solid phase polypeptide synthesis for preparing the bivalirudin resin comprises the following steps of: sequentially connecting corresponding Fmoc- protected amino acids in the following sequences to Fmoc-Leu-carrier resin by a solid phase coupling synthesis method: R1-D-Phe-Pro-Arg(Pbf)-Pro-X-Asn(R2)-Gly-Asp(OtBu)-, Phe-Glu(OtBu)-Glu(OtBu)-Ile-Pro-Glu(OtBu)-Glu(OtBu)- and Tyr(tBu)-Leu-resin, and thus obtaining the bivalirudin resin; and when the X fragment is connected, only one times of solid phase coupling synthesis reaction is used, and the corresponding Fmoc- protected amino acid is Fmoc-Gly-Gly-Gly-Gly-OH. The purity of the bivalirudin is more than 99.5 percent, and the single impurity is less than 0.2 percent.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Process for the preparation of bivalirudin and its pharmaceutical compositions
InactiveUS20090062511A1Superior mechanical and swelling propertyImprove stabilityPeptide/protein ingredientsImmunoglobulinsBivalirudinStereochemistry
The present application provides an improved process for the preparation of Bivalirudin and its pharmaceutical compositions.The present application also provides an improved process for the purification of Bivalirudin.
Owner:DR REDDYS LAB LTD +1
Liquid phase synthesizing method for bivalirudin
ActiveCN101475631ASimple equipmentEasy to achieve large-scale productionPeptide preparation methodsBlood disorderSynthesis methodsCombinatorial chemistry
The invention provides a liquid phase synthesis method for bivalirudin. Firstly, three all-protected fragments, namely 6 peptide with an all-protected N-terminal, 6 peptide with an all-protected middle segment and 8 peptide with an all-protected C-terminal are gradually synthesized, then all-protected bivalirudin is obtained by orderly condensing the three fragments, finally all protective genes are removed to obtain a crude product of the bivalirudin, and then a pure product of the bivalirudin is obtained through high-efficiency liquid chromatography purification. The method does not need a resin, excessive protected amino acid and a condensing agent, is easy to realize mass production, and has relatively lower production cost.
Owner:苏州天马医药集团天吉生物制药有限公司
Preparation method of synthesizing bivalirudin from solid phase polypeptide
ActiveCN101033249AConvenient sourceReduce usagePeptide-nucleic acidsPeptide preparation methodsSide chainWang resin
This invention discloses a preparation method of solid-phase peptide synthesizing bivalirudin. It includes the following steps: taking any one of triphenyl methyl chloride resin, 4-methyl-triphenyl methyl chloride resin, 4-methoxy-triphenyl methyl chloride resin, 2-chlorine-triphenyl methyl chloride resin, or Wang resin as the starting raw materials, connecting amino acids in turn according to the method of solid-phase synthesis, to get a protective 28-peptide resin, removing Fmoc-protective group in turn, side-chain protecting group and cutting the peptide to get a crude, then purifying the crude through C18 (or C8) high-pressure column to get bivalirudin exquisite article. In this invention, the peptide yield of every step is more than 99%, and the total yield is 14%.
Owner:SHANGHAI SOHO YIMING PHARMA
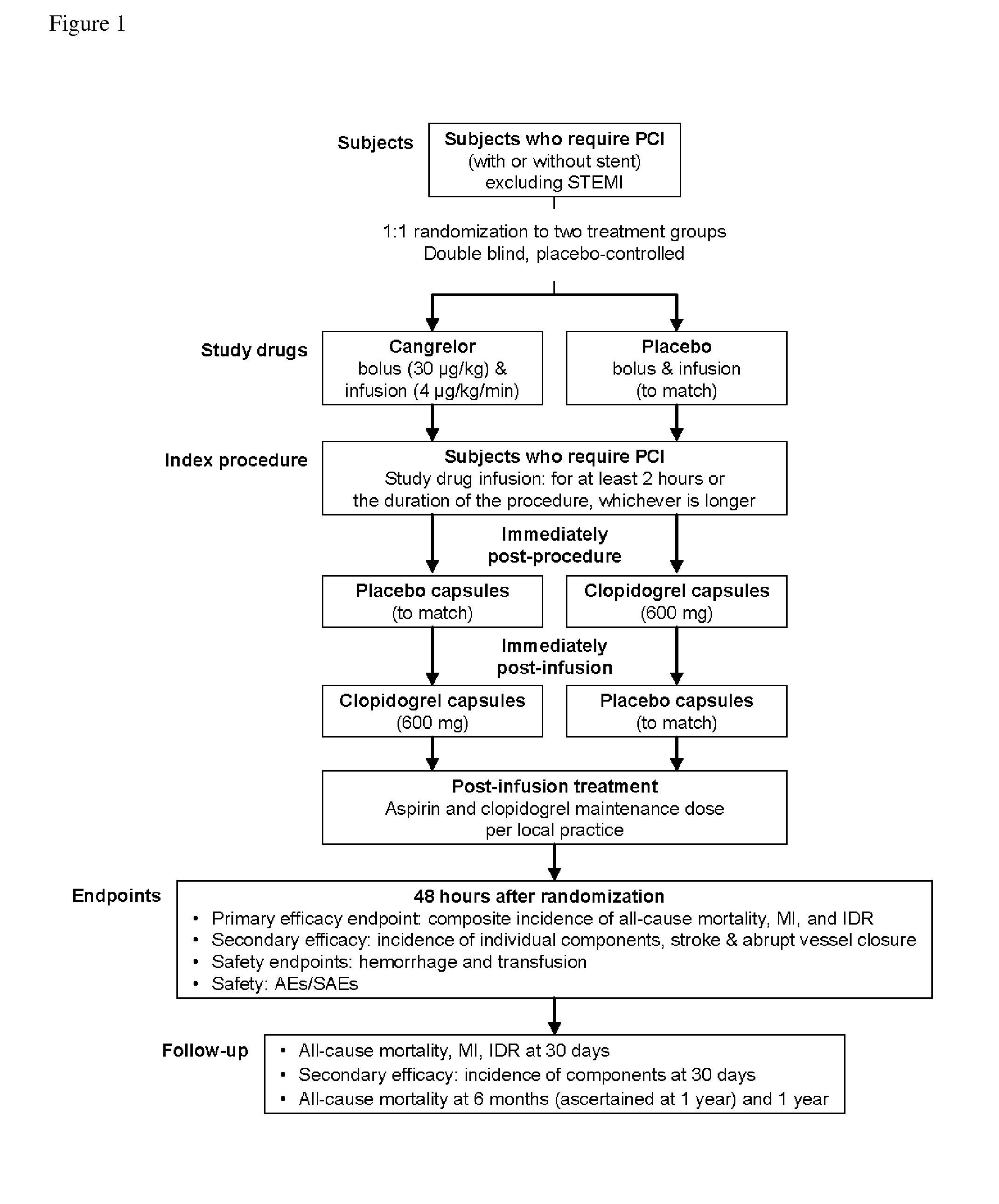
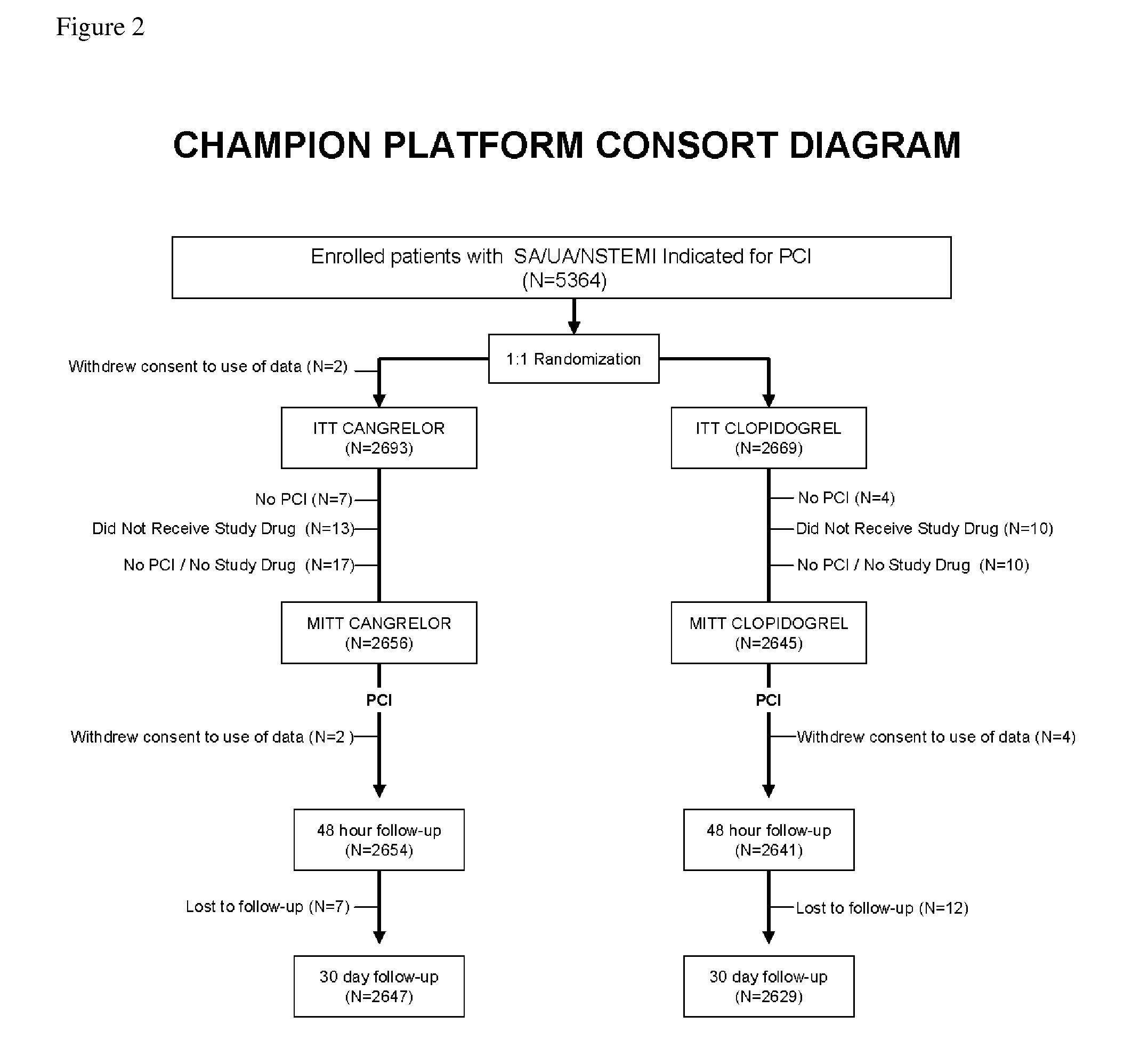
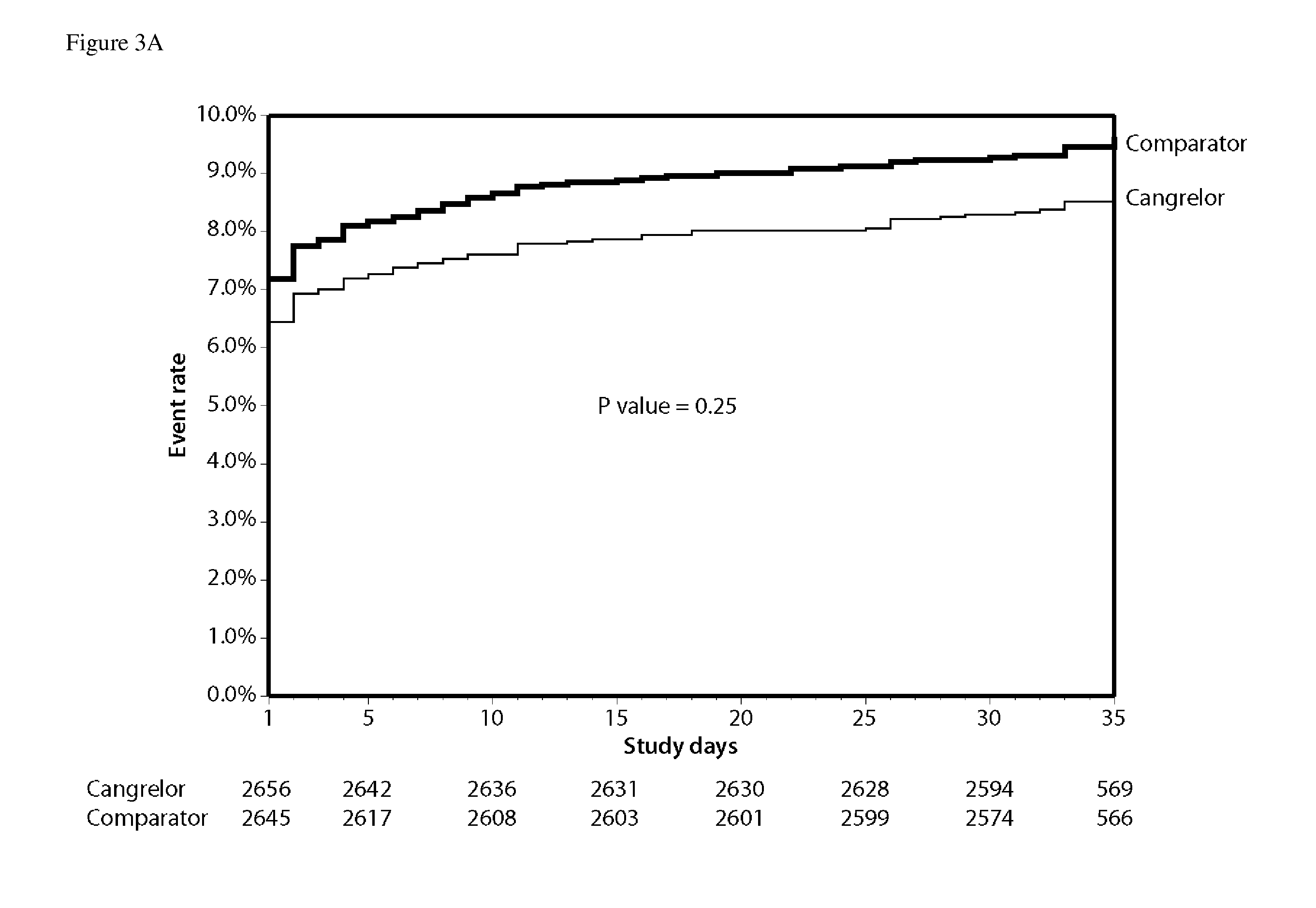
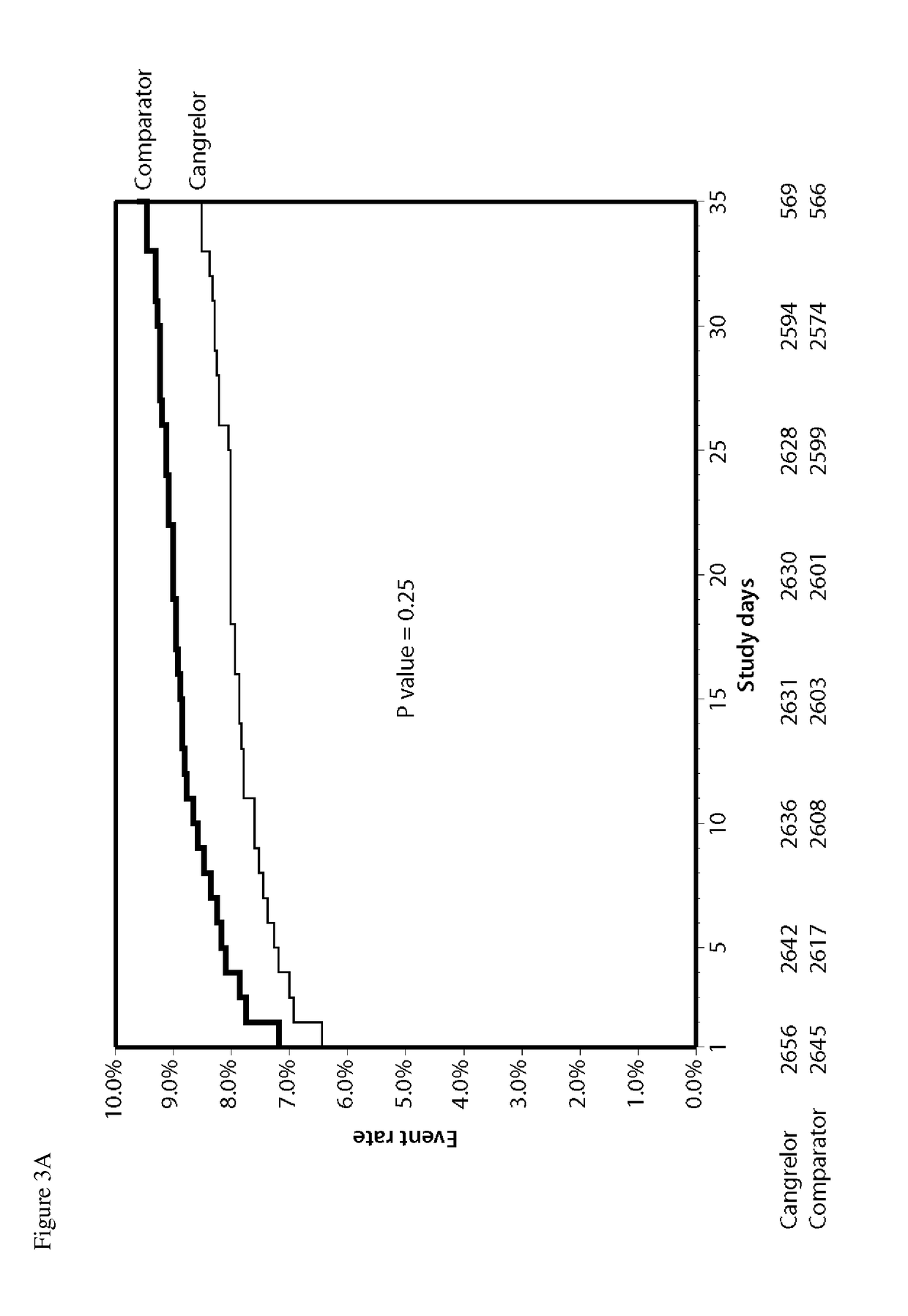
Methods of treating or preventing stent thrombosis
ActiveUS20110112030A1Reduce mortalityPrevent myocardial infarctionOrganic active ingredientsBiocideMortality rateStent implantation
The present invention is directed to the following: methods of treating or preventing stent thrombosis using pharmaceutical compositions comprising cangrelor and optionally bivalirudin; methods of reducing mortality in a subject undergoing stent implantation using pharmaceutical compositions comprising cangrelor and optionally bivalirudin; medicaments comprising cangrelor and optionally bivalirudin useful for treating or preventing stent thrombosis, or useful for reducing mortality in a subject undergoing stent implantation; pharmaceutical compositions comprising cangrelor and bivalirudin; and methods of preparing a medicament comprising cangrelor and optionally bivalirudin useful for treating or preventing stent thrombosis, or useful for reducing mortality in a subject undergoing stent implantation.
Owner:CHIESI FARM SPA
Preparation method of polypeptide solid-phase synthesis bivalirudin crude product
ActiveCN101555274ALow costHigh purityPeptide preparation methodsBlood disorderProtecting groupAmino acid supplementation
The invention discloses a preparation method of polypeptide solid-phase synthesis bivalirudin crude product. The preparation method comprises the following steps of: (1) mixing Fmoc-amino-acid resin or Fmoc-polypeptide resin and an unprotecting agent and removing Fmoc protecting group; (2) in the existence of a condensing agent, leading amino acid with Fmoc or Boc and amino acid or polypeptide onthe resin to carry out condensation; (3) repeating the step (1) and step (2) and obtaining polypeptide resin shown as formula II; and (4) in the existence of a cutting agent, leading the polypeptide and the resin on the polypeptide resin to be separated, and obtaining bivalirudin shown as formula I. The unprotecting agent contains 3 to 20 percent of piperidine and 0.5 to 10 percent of bicyclic amidine by total volume.
Owner:HAINAN SHUANGCHENG PHARMA
Preparation method of bivalirudin
ActiveCN103242431AEasy to operateMild conditionsPeptide preparation methodsBulk chemical productionSide chainCombinatorial chemistry
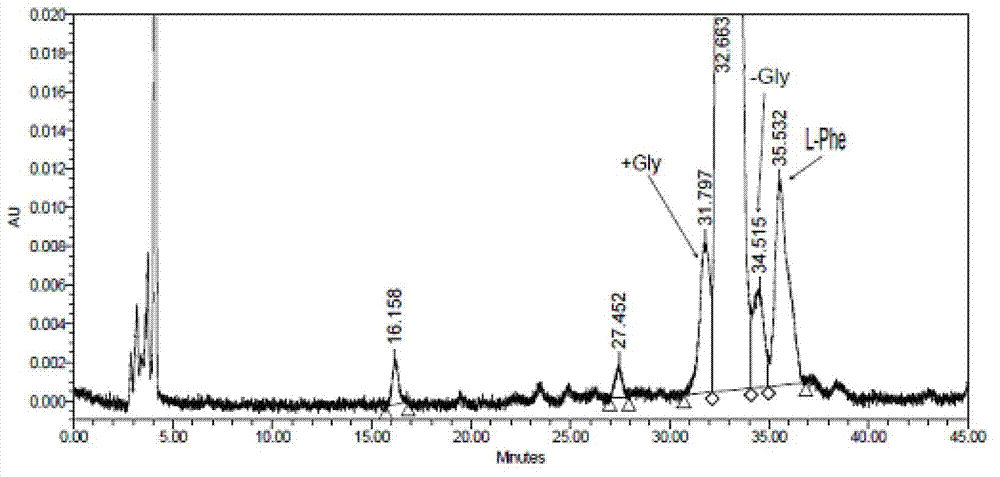
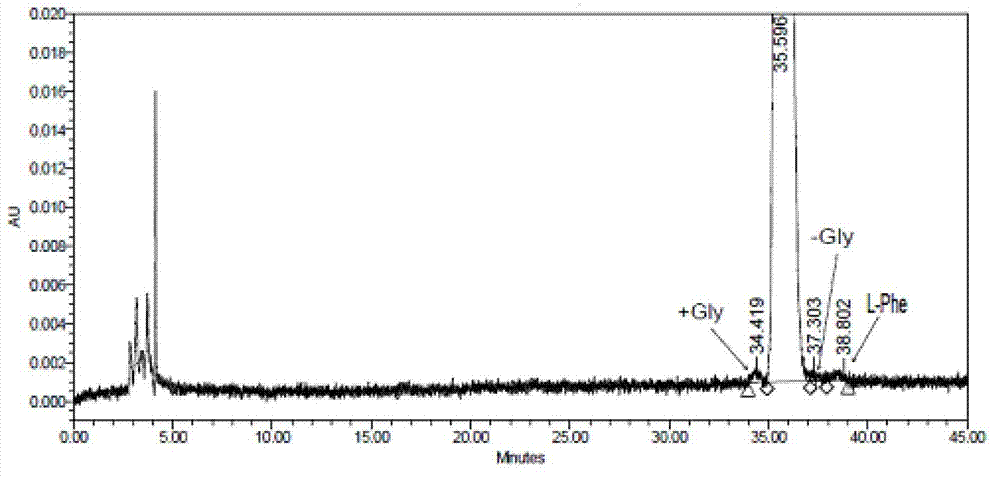
The invention relates to a preparation method of bivalirudin, which is used for avoiding generation of impurities. The method comprises the following steps of: removing an Fmoc protecting group by using Fmoc-Leu-Wang resins or Fmoc-Leu-2-chlorotrityl resin as a raw material by means of solid phase coupling; coupling amino acid protected by the Fmoc one by one to obtain side-chain full-protection bivalirudin [9-20] peptide-resin; removing the protection of the Fmoc and performing solid phase coupling on Fmoc-Pro-Gly-Gly-Gly-Gly-OH pentapeptide segments to obtain a side-chain full-protection bivalirudin [4-20] peptide-resin; coupling the other coupled amino acid one by one to obtain a side-chain full-protection bivalirudin-resin; and splitting and removing resin and the protecting groups on the side-chain full-protection bivalirudin peptide-resins, and precipitating to obtain bivalirudin. According to the method, the impurities of bivalirudin [-Gly] and [+Gly] are reduced, and the yield and the purity of the product are improved.
Owner:QILU PHARMA HAINAN +1
Method for improving stability of polypeptide active pharmaceutical ingredients
ActiveCN106188218AChange particle shapeChange moistureOxytocins/vasopressinsThymosin peptidesOxytocinBivalirudin
The invention discloses a method for improving the stability of polypeptide active pharmaceutical ingredients. The polypeptide active pharmaceutical ingredients comprise but are not limited to bivalirudin, octreotide acetate, lanreotide acetate, eptifibatide or cetrorelix acetate, ganirelix acetate, degarelix, liraglutide, oxytocin, thymosin alpha1, leuprolide acetate, goserelin acetate, terlipressin or linaclotide. The method comprises the steps that after polypeptide drugs are salified, a polypeptide solution containing compensation ions is obtained, and a target polypeptide product is prepared through an ultralow temperature vacuum freeze-drying method. According to the method for improving the stability of the polypeptide active pharmaceutical ingredients, the problem that the polypeptide active pharmaceutical ingredients are prone to degradation after being placed for a long time is solved, the uniformity of the product is improved, and the drug risk is reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Stable injectable composition of bivalirudin and process for its preparation
InactiveUS20170224789A1Peptide/protein ingredientsPharmaceutical delivery mechanismMedicineReady to use
The present invention relates to a non-aqueous, stable and ready-to-use injectable composition of bivalirudin or pharmaceutically acceptable salt(s) or co-crystals thereof; and processes for its preparation. It is not required to reconstitute the injectable composition of bivalirudin with water prior to administration, thereby rendering it an easy-to-use injectable composition.
Owner:PIRAMAL ENTERPRISES LTD
Method for preparing bivalirudin
ActiveCN102532274AAvoid it happening againReduce the difficulty of purificationPeptide preparation methodsBulk chemical productionSynthesis methodsCombinatorial chemistry
The invention belongs to the technical field of preparation methods for polypeptide medicines, and particularly relates to a method for preparing bivalirudin. The method for preparing the bivalirudin comprises the following steps of: preparing a bivalirudin resin by a solid phase polypeptide synthesis method, performing acidolysis on the bivalirudin resin to obtain a bivalirudin crude product, and purifying the bivalirudin crude product to obtain a bivalirudin pure product, wherein the step of preparing the bivalirudin resin by the solid phase polypeptide synthesis method comprises the following substeps of: sequentially accessing the corresponding protection amino acid or fragments on an Fmoc-Leu-carrier resin by a solid phase coupling and synthesis method to obtain the bivalirudin resin, wherein the corresponding protection amino acid or fragments have the following sequences: R1-D-Phe-Pro-X-Y-Phe-Glu(OtBu)-Glu(OtBu)-Ile-Pro-Glu(OtBu)-Glu(OtBu)-Tyr(tBu)-Leu-resin, R1 is Fmoc, Boc or H; X is Arg(Pbf)-Pro; Y is Gly-Gly-Gly-Asn(R2)-Gly-Asp-(OtBu); and R2 is Trt or H. The purity of the product can reach above 99.5 percent.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for preparing bivalirudin through solid-liquid combination
ActiveCN104031127ASolve difficult coupling problemsReduce contentPeptide preparation methodsFluid phaseEngineering
The invention belongs to the field of polypeptide synthesis and relates to a method for preparing bivalirudin through solid-liquid combination. The bivalirudin is prepared by adopting a method for combining a liquid phase and a solid phase, impurity peptides can be well avoided, the purity of crude peptides is improved, and the production cost is reduced. The method comprises the following steps: synthesizing a fragmental hexapeptide Fmoc-Arg(pbf)-Pro-Gly-Gly-Gly-Gly-OH by adopting a liquid phase method, and connecting peptides onto the solid phase. By utilizing the method, impurity peptides Biv+ / -Gly and Biv+ / -2Gly can be avoided, and the problem that the peptides are difficultly completely coupled during solid phase Arg connection is avoided. By utilizing the synthesis process, the purity of the crude peptides can be over 90 percent, and the purification difficulty is reduced, so that the purity of the final product exceeds 99.5 percent, and the production cost is further reduced. Compared with the prior art, the method disclosed by the invention is simple in operation and low in synthesis cost, and is conductive to large-scale industrial production.
Owner:JINAN KANGHE MEDICAL TECH
The method for preparing bivalirudin
Owner:POLYPEPTIDE LAB HLDG PPL AB
Preparation method of Bivalirudin
ActiveCN101906150AHigh purityEfficient removalPeptide preparation methodsBulk chemical productionCombinatorial chemistryBivalirudin
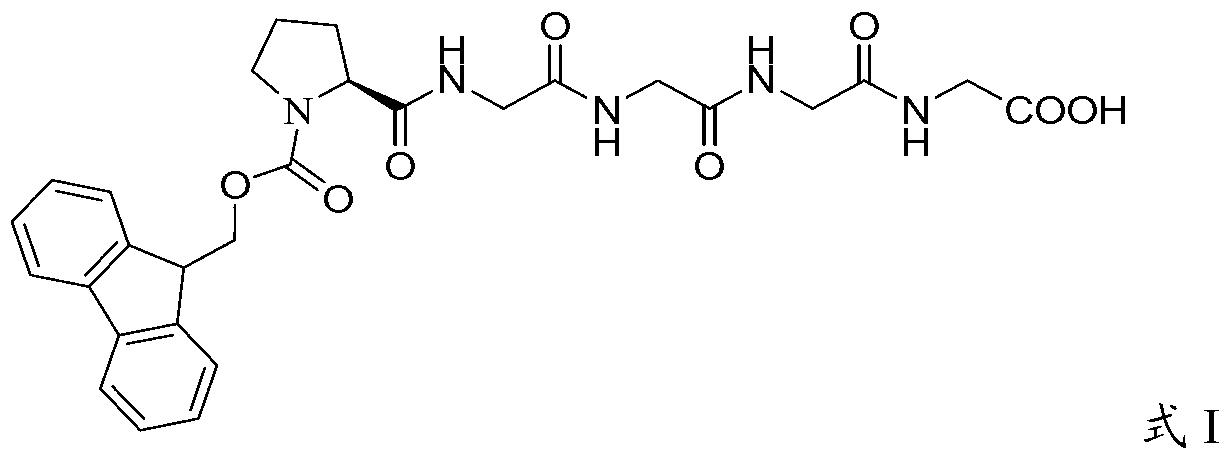
The invention discloses a preparation method of polypeptide solid-phase synthesis Bivalirudin, comprising the following steps of: (1) condensing Fmoc-Asn(Trt)-Gly-OH with a polypeptide resin shown as the formula V in the presence of a condensing agent to obtain a polypeptide resin shown as the formula III; (2) mixing the polypeptide resin shown as the formula III with a deprotection agent to remove a Fmoc protective group; (3) condensing Fmoc-Gly-Gly-Gly-Gly-OH with a polypeptide resin shown as the formula VI in the presence of the condensing agent to obtain a polypeptide resin shown as the formula IV; (4) sequentially condensing polypeptide of the polypeptide resin shown as the formula IV with amino acids from the end C to the end N according to the sequence from Pro to Arg, Pro and to D-Phe by a method of solid-phase synthesis so as to prepare a polypeptide resin shown as the formula II; and (5) separating polypeptide and resin on the polypeptide resin shown as the formula II in thepresence of a separating agent to obtain Bivalirudin shown as the formula I.
Owner:SHANGHAI AMBIOPHARM
Method of preparing bivalirudin
InactiveUS20080051558A1Improve availabilityQuality improvementPeptide-nucleic acidsPeptide/protein ingredientsSide chainHigh pressure
The present invention relates to a novel solid phase peptide synthesis method for Bivalirudin. This method contains following steps: serving Trityl Chloride Resin, 4-Methyltrityl Chloride Resin, 4-Methoxytrityl Chloride Resin, or 2-Cl Trityl Chloride Resin, or attaching of Wang Resin as a start raw material); according to general solid phase peptide synthesis rules, coupling protected amino acids after deprotection of Fmoc-protection group and then deprotecting side chain protection group; cleaving peptides from resin; and then obtaining crude Bivalirudin product. C18 high pressure liquid chromatography (HPLC) column is applied to purify the product of Bivalirudin. This method is suitable and effective for mass production, in addition to its features of high quality, low production cost, high synthetic yield, avoidance of usage of fatal toxic chemical such as HF, and less environmental pollution. The high yield rate of 99% is achieved for each synthetic step and total yield rate is 14%.
Owner:ZHOU YIMING +1
Methods of Treating, Reducing the Incidence of, and/or Preventing Ischemic Events
ActiveUS20140107032A1Reduce morbidityPreventing an ischemic eventOrganic active ingredientsPeptide/protein ingredientsMedicineThrombus
Methods of treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing percutaneous coronary intervention (PCI), comprising administering to the patient a pharmaceutical composition comprising cangrelor. The method may further comprise administering an additional therapeutic agent to the patient, the additional therapeutic agent comprising bivalirudin or a P2Y12 inhibitor. Pharmaceutical compositions useful for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI. The pharmaceutical compositions comprise cangrelor, and optionally bivalirudin. Methods of preparing a pharmaceutical composition for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI, comprising admixing cangrelor with one or more pharmaceutically acceptable excipients. An ischemic event may include stent thrombosis, myocardial infarction, ischemia-driven revascularization, and mortality.
Owner:CHIESI FARM SPA
Pharmaceutical formulations of bivalirudin and processes of making the same
ActiveUS7582727B1Efficient mixingPeptide/protein ingredientsPeptide preparation methodsBULK ACTIVE INGREDIENTSolvent
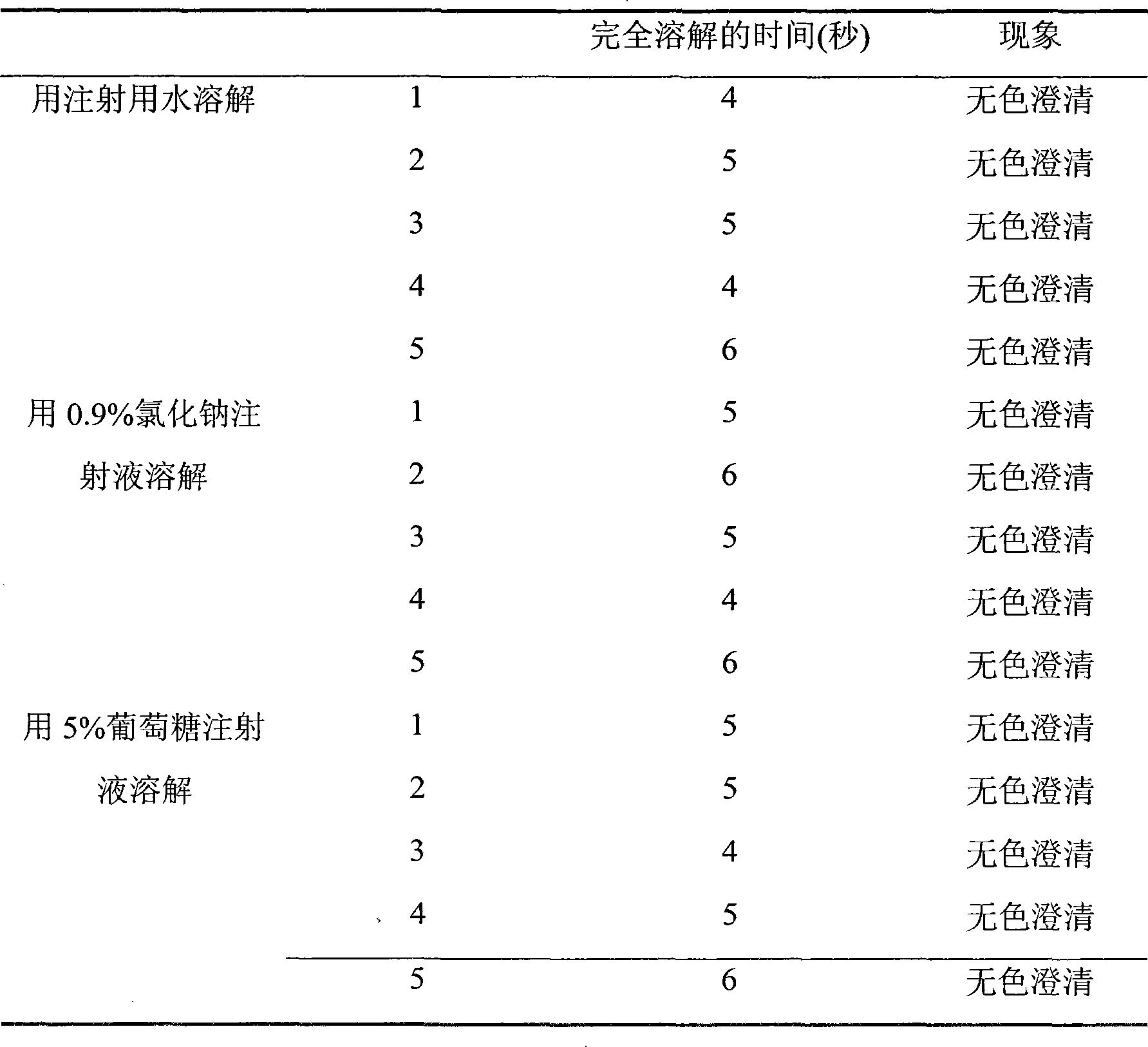
Pharmaceutical batch(es) or pharmaceutical formulation(s) comprising bivalirudin as the active ingredient, and a method of preparing the pharmaceutical batch(es) or pharmaceutical formulation(s). The pharmaceutical batch(es) or pharmaceutical formulation(s) may have a maximum impurity level of Asp9-bivalirudin that does not exceed about 0.6%. Also, the pharmaceutical batch(es) or pharmaceutical formulation(s) may have a reconstitution time that does not exceed about 42 seconds. The method of preparing the pharmaceutical batch(es) or pharmaceutical formulation(s) may comprise dissolving bivalirudin in a solvent to form a first solution, efficiently mixing a pH-adjusting solution with the first solution to form a second solution in which the pH-adjusting solution may comprise a pH-adjusting solution solvent, and removing the solvent and the pH-adjusting solution solvent from the second solution.
Owner:SANDOZ INC
Method for synthesis of bivalirudin in solid-phase fragment approach
ActiveCN102731624AHigh yieldIncrease costPeptide preparation methodsBulk chemical productionFreeze-dryingWang resin
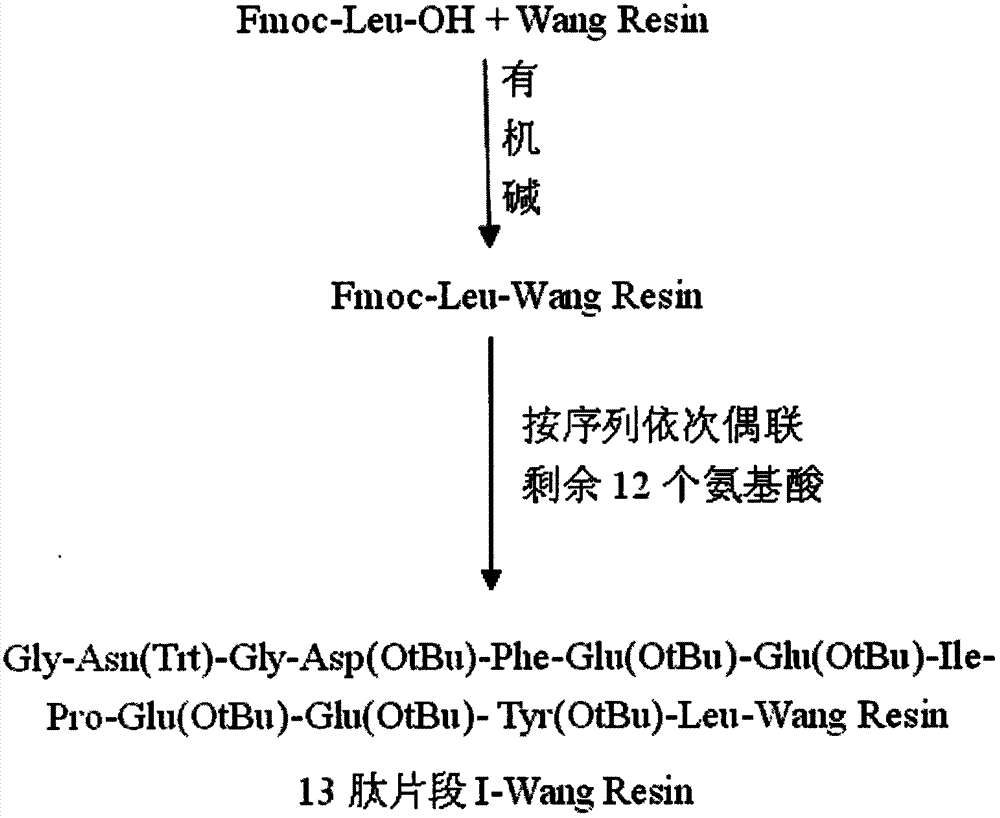
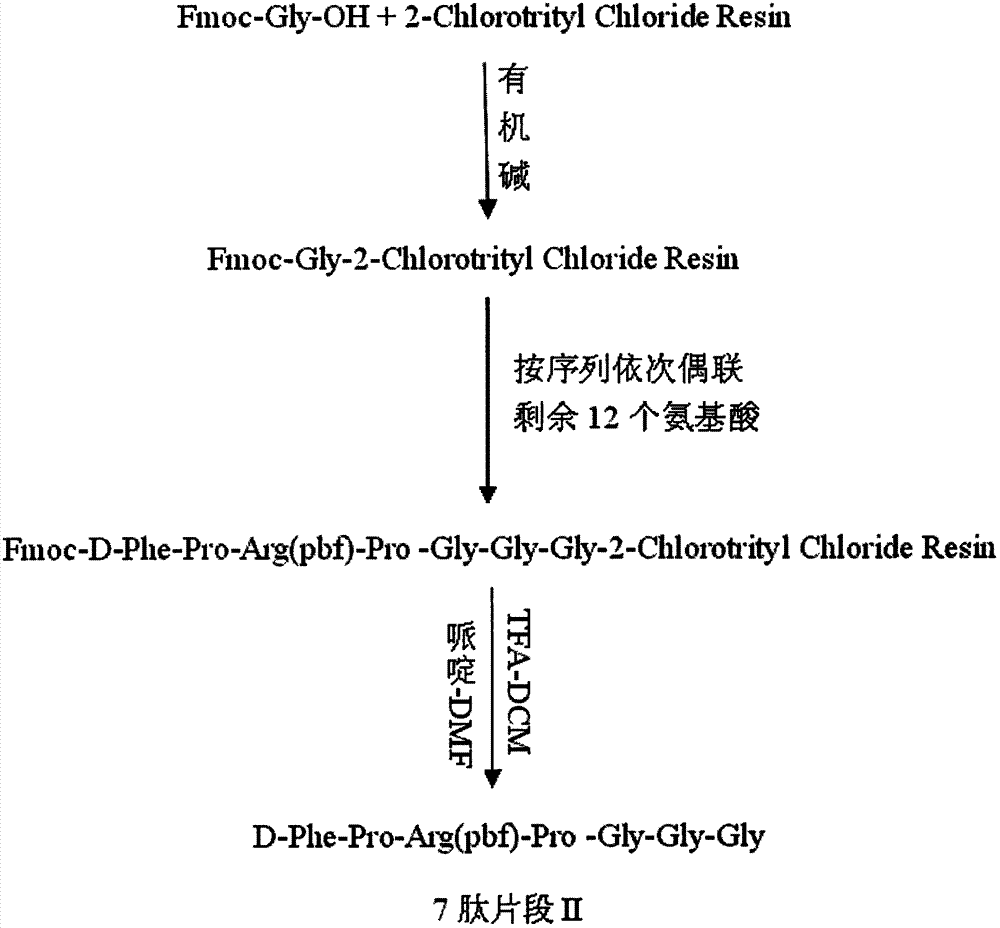
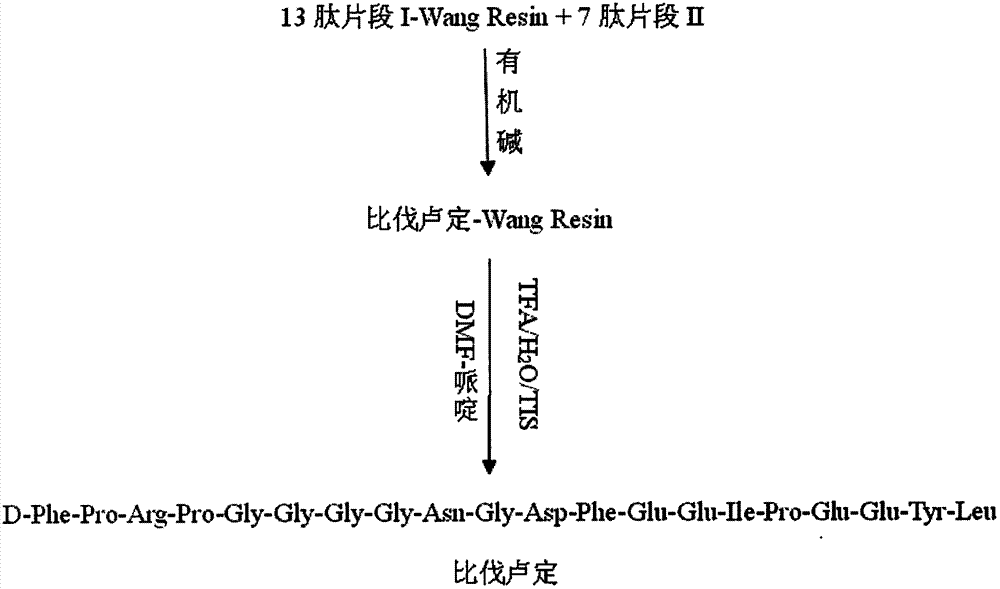
The invention provides a method for synthesis of bivalirudin in a solid-phase fragment approach. The method comprises: first employing a solid phase stepwise method to synthesize a 13-peptide fragment I-Wang Resin, and a 7-peptide fragment II with the terminal N protected by Fmoc, coupling the two fragments in the solid-phase fragment approach to synthesize bivalirudin -Wang Resin; and finally conducting deprotection, cracking, purification, and freezing drying so as to obtain pure bivalirudin. Compared with existing solid phase stepwise method or liquid phase fragment method, the method of the invention has the advantages of simple technological operation, high yield and purity, small environmental pollution, and easy large-scale production, etc.
Owner:YANCHENG KAILI PHARMA
Bivalirudin freeze-dried injection and preparation thereof
ActiveCN101244043AImprove stabilityImprove applicabilityPowder deliveryPeptide/protein ingredientsSodium bicarbonateActive component
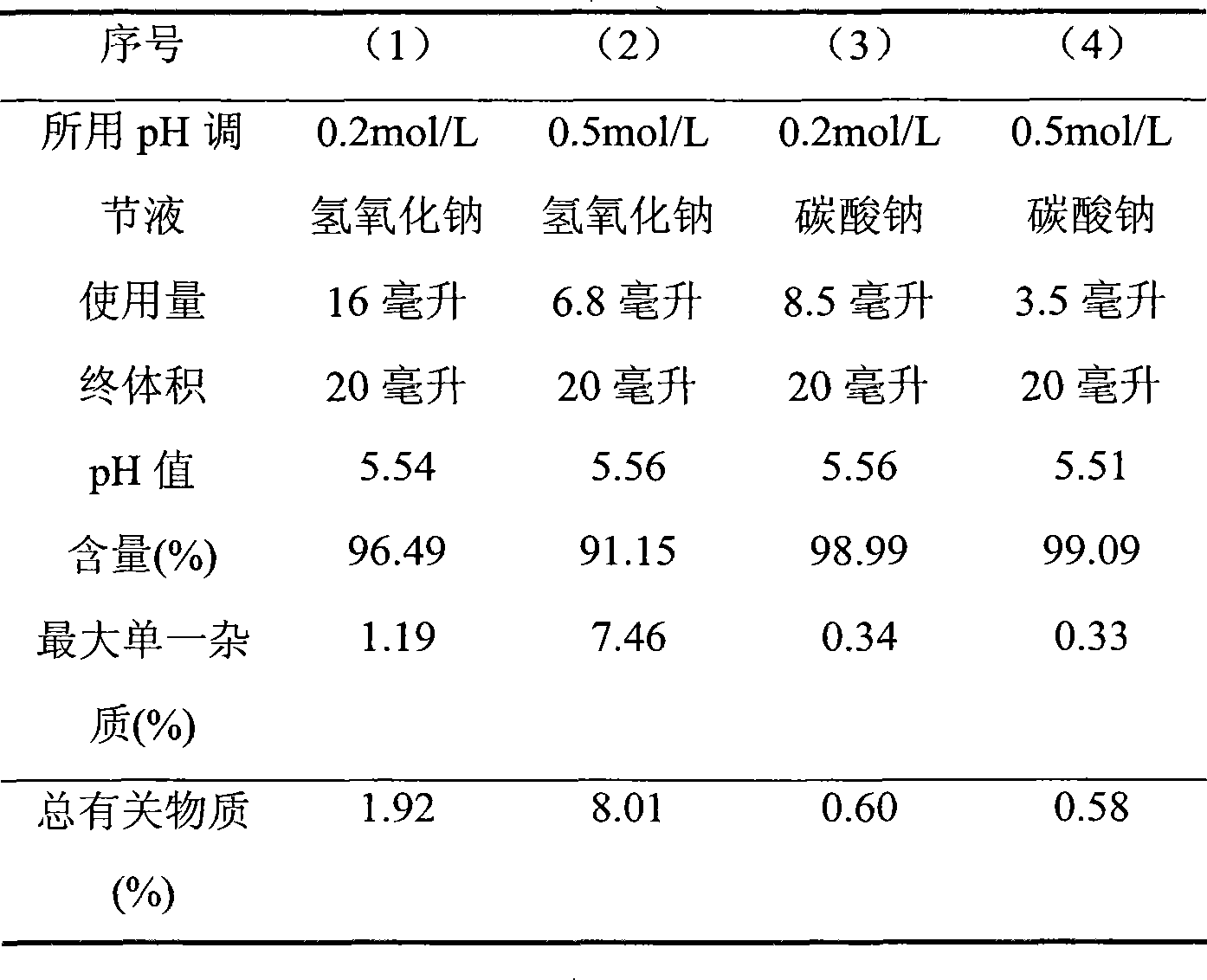
The invention discloses bivalirudin bacteria-free lyophilized injection and the preparation method, which adopts sodium carbonate or sodium bicarbonate to adjust pH. The bivalirudin bacteria-free lyophilized injection and the preparation method has the advantages of overcoming the drawback of poor stability for bivalirudin preparation of prior art, significantly lowering degradation of active components for products, having low impurity content and good stability and improving use safety and effectiveness for clinical application of preparation.
Owner:MUDANJIANG YOUBO PHARMA CO LTD
Preparation method of anticoagulant polypeptide
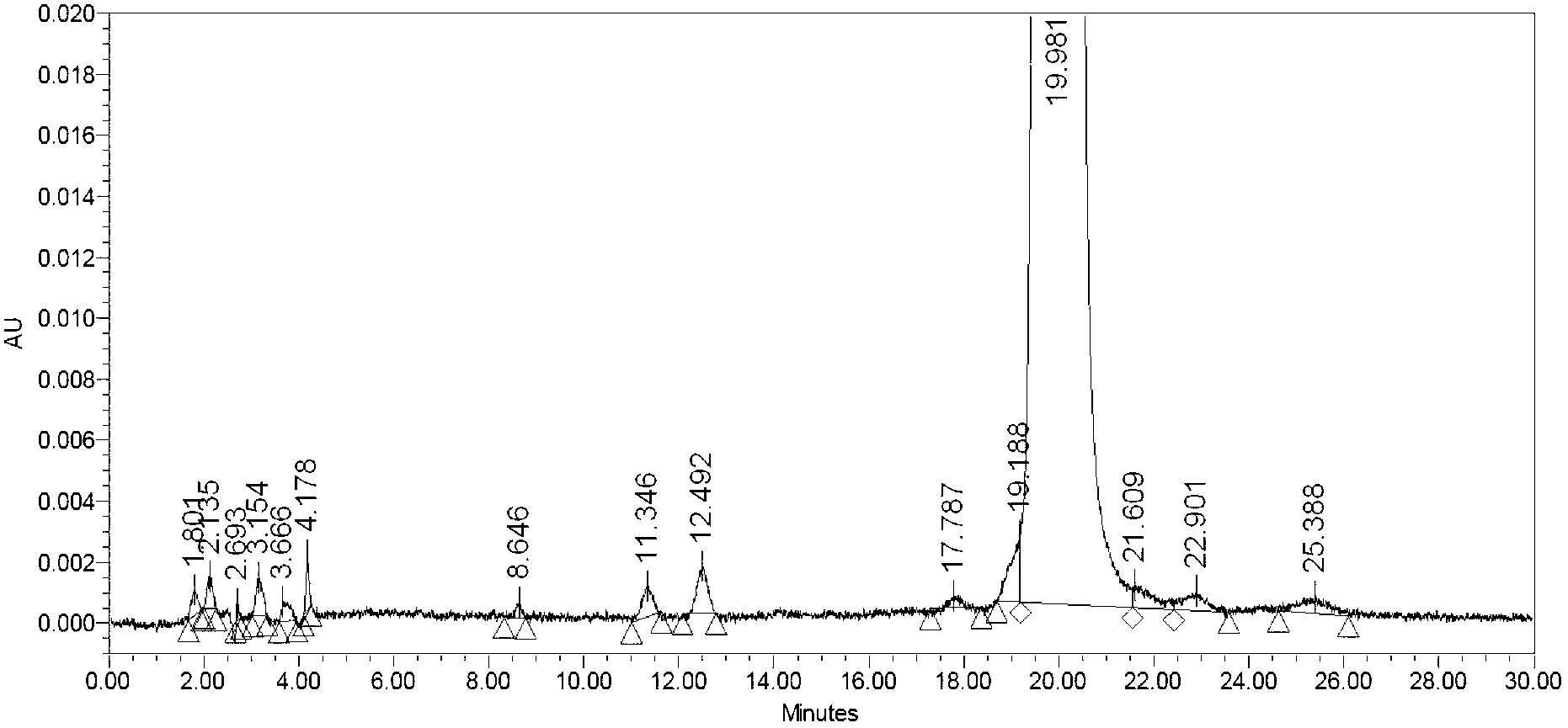
The invention relates to a preparation method of an anticoagulant polypeptide and discloses a preparation method of bivalirudin, and belongs to the technical field of pharmaceutical chemistry. The preparation method comprises the following steps: purifying a bivalirudin crude peptide by a reverse phase / weak cation exchange mixed-mode HPLC (high performance liquid chromatography) method; transferring a salt by a reverse phase HPLC method; and collecting and lyophilizing a solution to obtain the bivalirudin. The preparation method is easy to operate, low in cost and high in gram yield of the bivalirudin and is applicable for large-scale industrial production of the bivalirudin; by virtue of hydrophobicity and chargeability difference, impurities in the crude peptide can be separated and removed well at one time; and the prepared bivalirudin is high in purity and low in impurity content and has a considerable economic value and a wide application prospect.
Owner:HYBIO PHARMA
Ready-to-use bivalirudin compositions
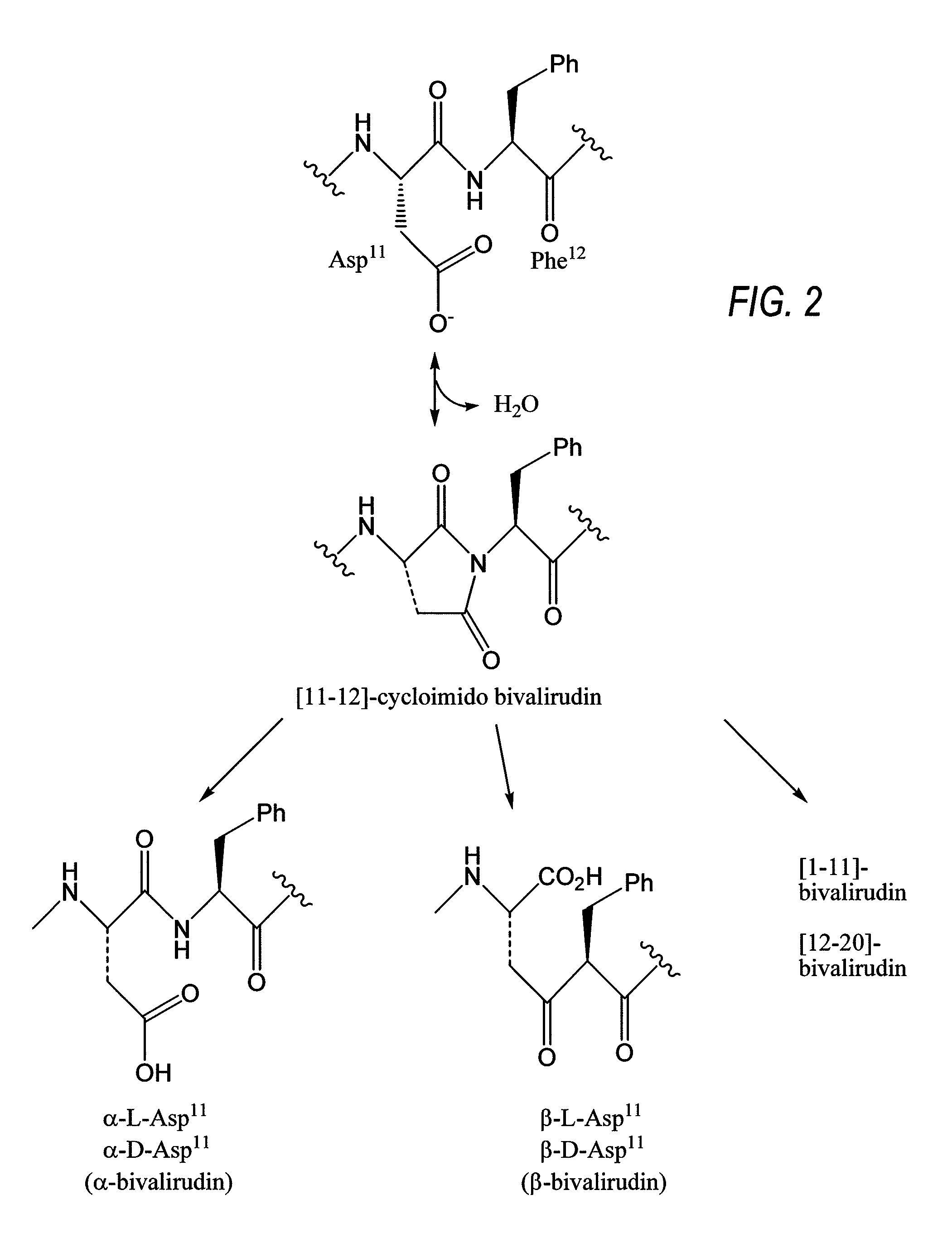
Ready-to-use bivalirudin compositions, methods of using the ready-to-use bivalirudin compositions, and methods of preparing the ready-to-use bivalirudin compositions. The ready-to-use bivalirudin compositions comprise bivalirudin and one or more stabilizing agents. The one or more stabilizing agents may be buffering agents having a pKa of about 2.5 to about 6.5, pH-adjusting agents, polymers, preservatives, antioxidants, sugars or polyols, or a combination thereof. The ready-to-use bivalirudin compositions may also comprise [9-10]-cycloimido bivalirudin, [11-12]-cycloimido bivalirudin, or a combination thereof. The method of using the ready-to-use bivalirudin compositions comprises administering the ready-to-use compositions to a patient in need thereof. Further, the method of preparing the ready-to-use bivalirudin compositions comprises mixing bivalirudin with one or more stabilizing agents.
Owner:EAGLE PHARMACEUTICALS INC +1
Preparation method of bivalirudin
InactiveCN102924575AEasy generationAvoid Bivalirudin ± 1 GlyPeptide preparation methodsBivalirudinSolid-phase synthesis
The invention relates to the field of polypeptide synthesis, and particularly relates to a preparation method of bivalirudin. In the method, a polypeptide segment Fmoc-D-Phe-Pro-Arg(Pbf)-Pro-Gly-Gly-Gly-OH obtained through solid phase synthesis is used as a raw material, thus avoiding the problems of bivalirudin+ / -1Gly and bivalirudin+ / -2Gly. Besides, by using a specific coupling reagent, the content of the isomer L-Phe is greatly reduced; and meanwhile, the yield and purity of the product are enhanced, the operation process is convenient, and the conditions are mild, thereby being beneficial to large-scale preparation.
Owner:HYBIO PHARMA
Compositions and methods for cell transplantation
ActiveUS20150037291A1Effective and safe combinationAvoid harmful effectsOrganic active ingredientsBiocideEnzyme Inhibitor AgentFactor Xa Inhibitor
The present invention relates to compositions and methods for cell transplantation. In particular, the present invention provides a composition comprising procoagulant cells and at least one factor Xa inhibitor, preferably rivaroxaban, as well as at least one thrombin inhibitor, preferably bivalirudin.
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN
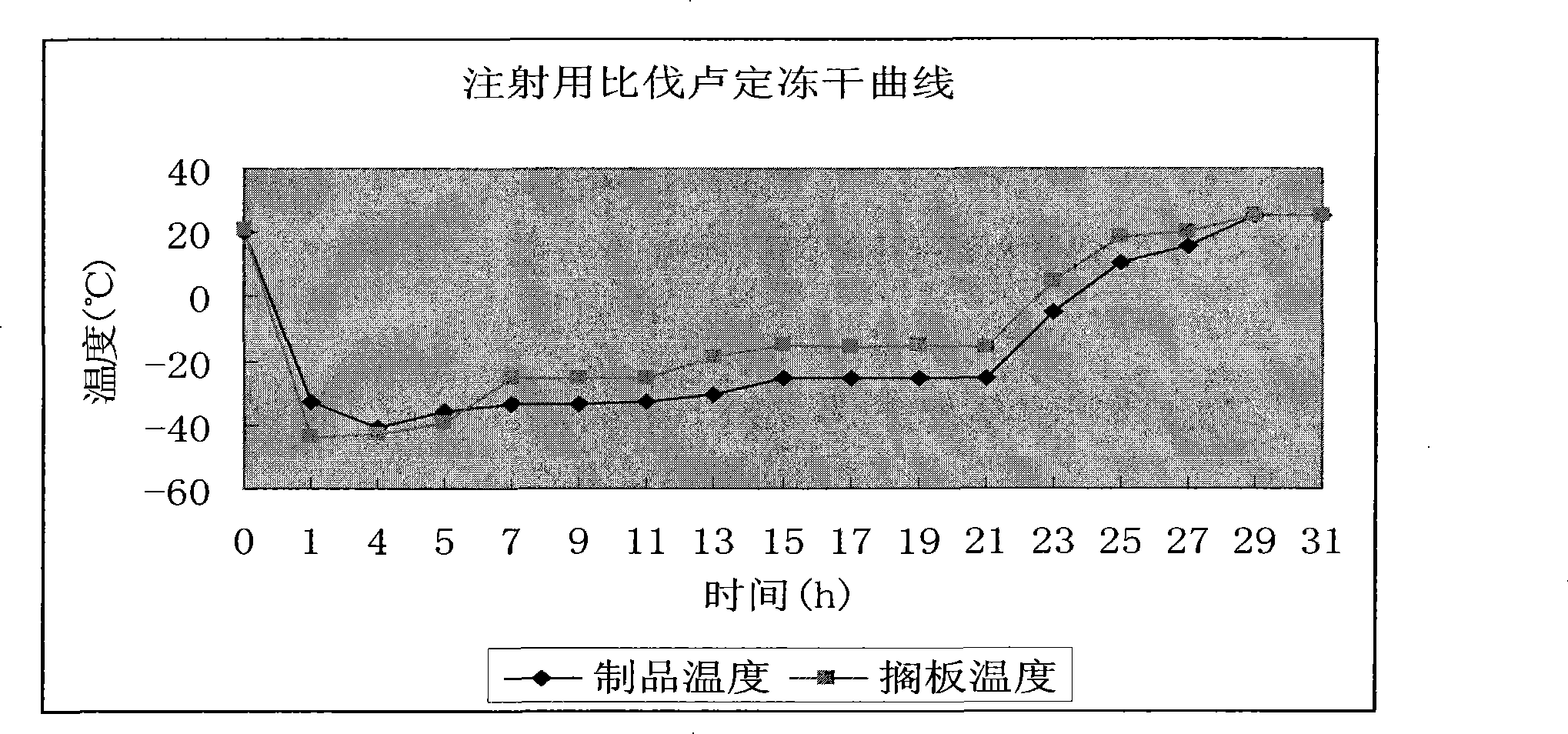
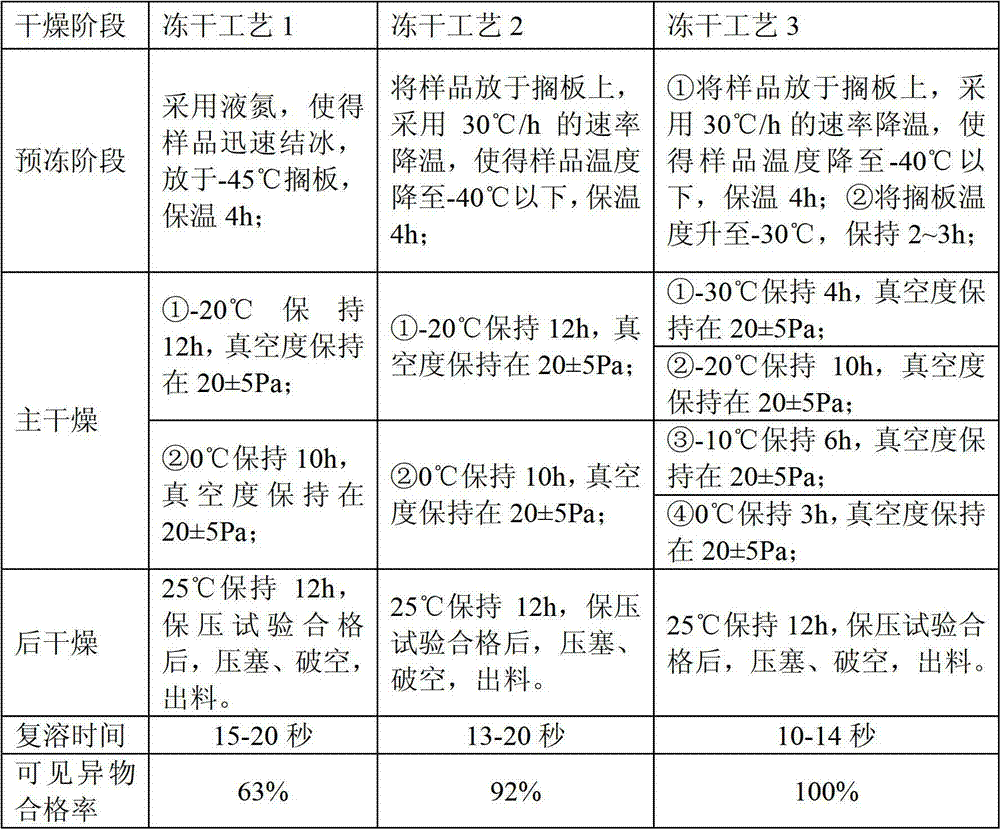
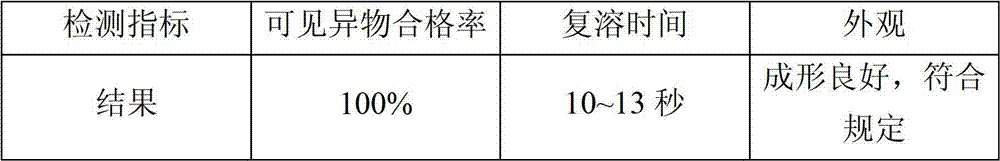
Lyophilization technology for preparing bivalirudin for injection
ActiveCN102813908AGood lookingEnsure safetyPowder deliveryPeptide/protein ingredientsMedicineMedical prescription
The invention relates to a lyophilization technology for preparing bivalirudin for injection. According to the technology, pre-freezing processes of bivalirudin are researched without changing the existing bivalirudin formula, and an annealing operation is added, so preparation products are obtained; and compared with preparation products obtained through common lyophilization technologies, the preparation products obtained through the lyophilization technology disclosed in the invention have the advantages of good appearance and stable quality.
Owner:JIANGSU HANSOH PHARMA CO LTD
Pharmaceutical formulations of bivalirudin and processes of making the same
ActiveUS7598343B1Efficient mixingPeptide/protein ingredientsInorganic non-active ingredientsBULK ACTIVE INGREDIENTPharmaceutical formulation
Pharmaceutical batch(es) or pharmaceutical formulation(s) comprising bivalirudin as the active ingredient, and a method of preparing the pharmaceutical batch(es) or pharmaceutical formulation(s). The pharmaceutical batch(es) or pharmaceutical formulation(s) may have a maximum impurity level of Asp9-bivalirudin that does not exceed about 0.6%. Also, the pharmaceutical batch(es) or pharmaceutical formulation(s) may have a reconstitution time that does not exceed about 42 seconds. The method of preparing the pharmaceutical batch(es) or pharmaceutical formulation(s) may comprise dissolving bivalirudin in a solvent to form a first solution, efficiently mixing a pH-adjusting solution with the first solution to form a second solution in which the pH-adjusting solution may comprise a pH-adjusting solution solvent, and removing the solvent and the pH-adjusting solution solvent from the second solution.
Owner:SANDOZ INC
Buffer-based method for preparing bivalirudin drug product
InactiveUS7985733B1Efficient and cost-effective processInhibition formationPeptide/protein ingredientsDepsipeptidesMedicinePharmaceutical drug
A method for preventing the formation of a bivalirudin precipitate during preparation of a pharmaceutical drug product comprising about 250 mg of bivalirudin, a dried bivalirudin drug product, and a concentrated liquid bivalirudin drug product. The method for preventing the formation of a bivalirudin precipitate comprises (i) preparing an aqueous solution comprising a buffer and a pH greater than the isoelectric point of bivalirudin; (ii) adding bivalirudin salt to the aqueous solution to form a bulk solution; (iii) transferring the bulk solution to one or more vessels; and (iv) drying the bulk solution. The buffer may have a pKa of about 4 to less than 7, and a pH greater than the isoelectric point of bivalirudin. The pH of the bulk solution may be maintained at a level greater than the isoelectric point of bivalirudin. Further, the bulk solution may have a final pH of about 4 to about 7.
Owner:THE MEDICINES
Methods of treating or preventing stent thrombosis
ActiveUS9925265B2Reduce mortalityPrevent myocardial infarctionOrganic active ingredientsBiocideStent implantationThrombus
The present invention is directed to the following: methods of treating or preventing stent thrombosis using pharmaceutical compositions comprising cangrelor and optionally bivalirudin; methods of reducing mortality in a subject undergoing stent implantation using pharmaceutical compositions comprising cangrelor and optionally bivalirudin; medicaments comprising cangrelor and optionally bivalirudin useful for treating or preventing stent thrombosis, or useful for reducing mortality in a subject undergoing stent implantation; pharmaceutical compositions comprising cangrelor and bivalirudin; and methods of preparing a medicament comprising cangrelor and optionally bivalirudin useful for treating or preventing stent thrombosis, or useful for reducing mortality in a subject undergoing stent implantation.
Owner:CHIESI FARM SPA
Anticoagulated blood polypeptides and uses thereof
ActiveCN101372512AEasy to synthesizeHigh anticoagulant activityPeptide/protein ingredientsGenetic engineeringMedicineThrombin activity
The invention discloses an anticoagulant polypeptide, belonging to the field of biomedicine. The primary structure of the anticoagulant polypeptide is shown as the general formula: (D)-FPRP-X1-X2-X3-X4-X5-QGDFEPIPEDAYDE-NH2. The invention has the advantages that the invention designs and synthesizes one type of anticoagulant polypeptide according to the structure characteristics and action mode of thrombin and Bivalirudin which is anticoagulant drug, and the polypeptide can inhibit the activity of the thrombin efficiently and specifically; furthermore, the number of amino acid in the anticoagulant polypeptide is equal to that of the Bivalirudin, thus synthesis is easy, and the activity of anticoagulation is stronger.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Method for producing bivalirudin
InactiveUS20100292436A1High purityLow costPeptide/protein ingredientsDepsipeptidesAspartic acidBivalirudin
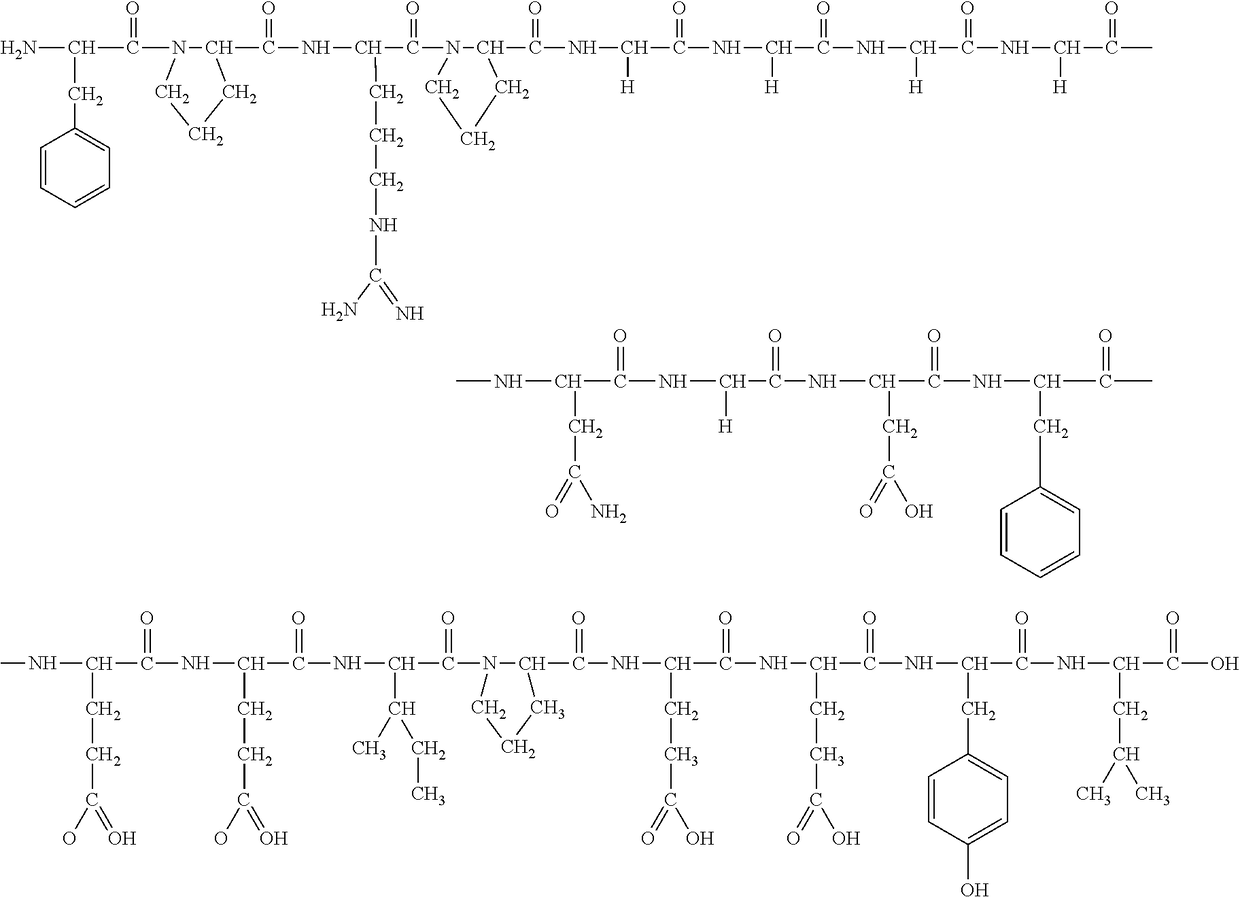
A method for producing bivalirudin using solid phase peptide synthesis by the following steps: a) mixing a Fmoc-amino acid resin or a Fmoc-peptide resin with a de-protective agent so as to remove Fmoc-; b) in the presence of a condensing agent, condensing a Fmoc- or Boc-amino acid with the amino acid or the peptide bound to the resin; c) repeating the steps a) and b) to yield a peptide resin represented by Formula I,(SEQ ID NO. 1)Boc-D-Phe1-Pro2-Arg(Pbf)3-Pro4-Gly5-Gly6-Gly7-Gly8-Asn(Trt)9-Gly10-Asp(OtBu)11-Phe12-Glu(OtBu)13-Glu(OtBu)14-Ile15-Pro16-Glu(OtBu)17-Glu(OtBu)18-Tyr(tBu)19-Leu20-Resin (I)and d) in the presence of a cleavage agent, separating the peptide from the resin to yield bivalirudin represented by Formula II (SEQ ID NO. 2).D-Phe-Pro-Arg-Pro-Gly-Gly-Gly-Gly-Asn-Gly-Asp-Phe-Glu-Glu-Ile-Pro-Glu-Glu-Tyr-Leu (II)Based on its total volume, the de-protective agent is composed of between 3 and 20% of piperidine and between 0.5 and 10% of bicyclic amidine. The method is low in cost and the resultant bivalirudin has high purity.
Owner:SHANGHAI AMBIOPHARM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com