Lyophilization technology for preparing bivalirudin for injection
A technology for bivalirudin and injection, which is applied in the field of freeze-drying technology for preparing bivalirudin for injection, and can solve problems such as long freeze-drying time, high energy consumption, and unqualified foreign matter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
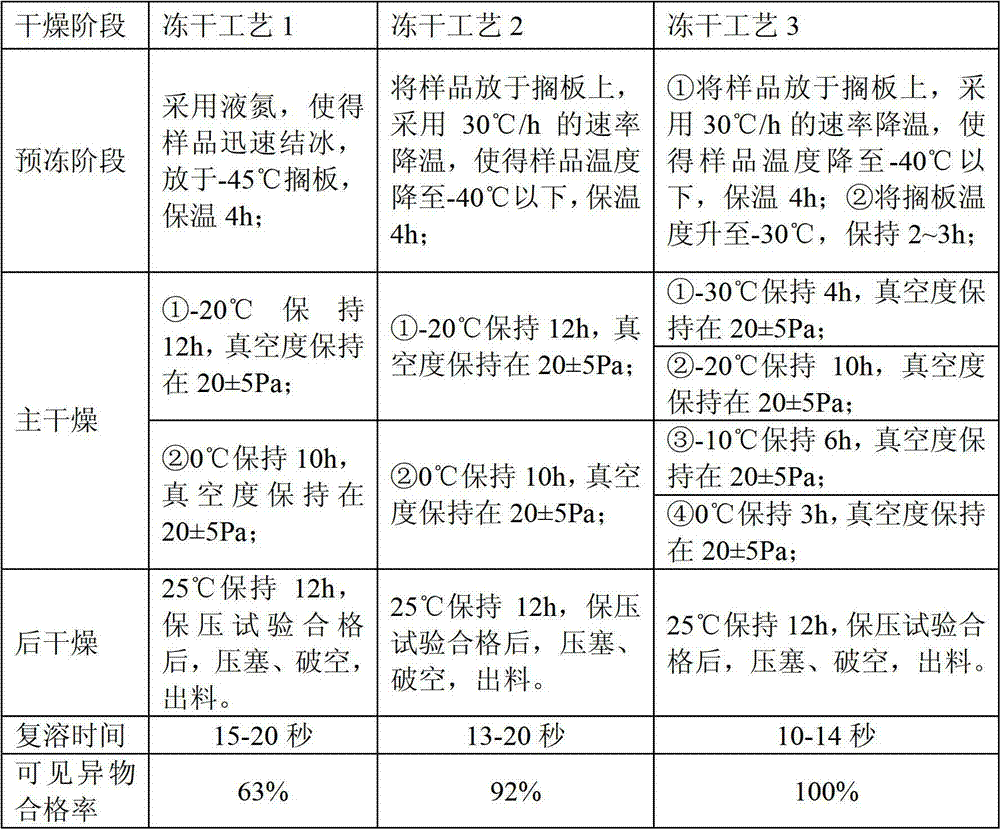
[0053] Get 17.5g of bivalirudin, dissolve it in 70ml of water for injection, add 7.5g of mannitol, and make it dissolve. Slowly add 5% (w / v) lye solution dropwise, adjust the pH value to 5.5, and add water for injection to a volume of 300ml. Under 100-level laminar flow, subpackage, 5ml / bottle, semi-stoppered, and freeze-dry using the following different freeze-drying processes, see the table below for details:
[0054]
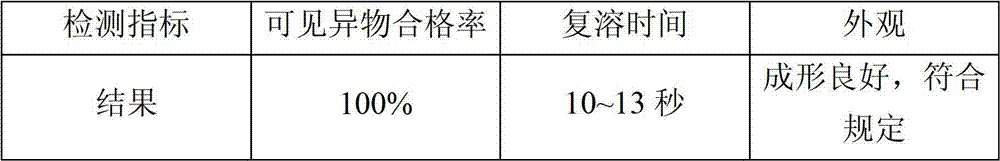
[0055] According to the freeze-drying process involved in the present invention, the quality of the prepared samples is stable, and the results of the redissolved visible foreign matter all comply with regulations, thereby improving the safety of clinical medication.
Embodiment 2
[0057] Get 11.6g of bivalirudin, be dissolved in 120ml water for injection, add the mannitol of 7.5g, make it dissolve. Slowly add 5% (w / v) lye solution dropwise, adjust the pH value to 5.5, and add water for injection to a volume of 200ml. Under 100-level laminar flow, subpackage, 5ml / bottle, half-stoppered, and freeze-dry using the following freeze-drying process:
[0058] a. Put the bottle containing the liquid medicine into the freeze dryer;
[0059] b. Reduce the shelf temperature of the freeze dryer to below -40°C at a rate of 35°C per hour;
[0060] c. When the temperature of the product drops below -40°C, keep it warm for 3 hours;
[0061] d. Raise the shelf temperature to -30°C and keep it warm for 3 hours;
[0062] e. Then lower the temperature of the shelf to -45°C and keep it warm for 2 to 4 hours;
[0063] f. Start vacuuming, when the vacuum degree is below 2Pa, start to heat up;
[0064] g. Raise the shelf temperature to -10°C and keep it for 10 hours;
[0...
Embodiment 3
[0071] Get 11.6g of bivalirudin, be dissolved in 120ml water for injection, add the mannitol of 7.5g, make it dissolve. Slowly add 5% (w / v) lye solution dropwise, adjust the pH value to 5.5, and add water for injection to a volume of 200ml. Under 100-level laminar flow, subpackage, 5ml / bottle, half-stoppered, and freeze-dry using the following freeze-drying process:
[0072] a. Put the bottle containing the liquid medicine into the freeze dryer;
[0073] b. Reduce the shelf temperature of the freeze dryer to below -40°C at a rate of 30-35°C per hour;
[0074] c. When the temperature of the product drops below -40°C, keep it warm for 2 to 4 hours;
[0075] d. Start vacuuming, when the vacuum degree reaches below 20Pa, start to heat up;
[0076] e. Raise the shelf temperature to -10°C~-20°C and keep it for 10 hours;
[0077] f. Raise the shelf temperature to 0°C and keep it for 4 hours;
[0078] g. Raise the shelf temperature to 25°C~30°C and keep it warm for 6 hours;
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com