Anticoagulated blood polypeptides and uses thereof
An anticoagulant and application technology, applied in the field of anticoagulant polypeptide and its application, to achieve the effects of enhancing anticoagulant activity, inhibiting thrombin activity, and strong anticoagulant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
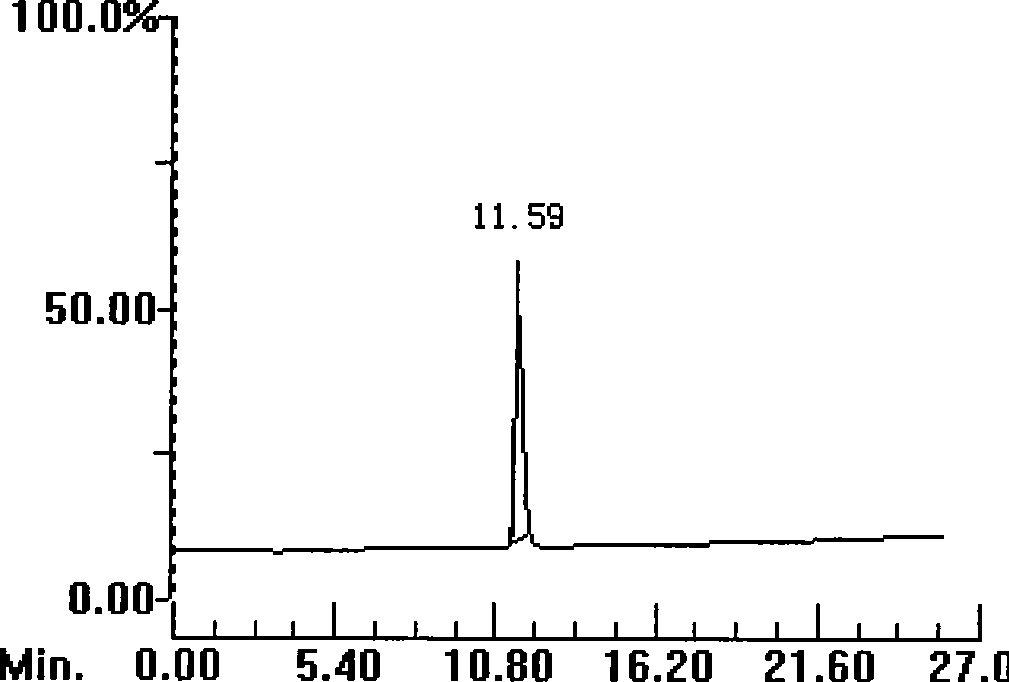
[0053] Example 1: Synthesis and purification of polypeptide 1
[0054] 1. Synthesis:
[0055] Weigh 0.17g Rink resin (0.1mmol, substitution rate 0.6g / mmol), the consumption of each Fmoc protected amino acid is 0.5mmol, with 1-hydroxybenzotriazole (HoBt) and dicyclohexylcarbodiimide (DCC, Acros company) as the condensing agent, piperidine (Piperidine, Shanghai Jill Biochemical) deprotection, according to the operating instructions of the ABI433A solid-phase synthesizer of Applied Systems Biosystems in the United States, appropriately prolong the coupling time (60-90min) and deprotection time (20- 30min), synthesize peptide-resin.
[0056] Take the above peptide-resin and put it into 10ml lysate (composition: 0.5g dimercaptothreitol (DTT), 0.5ml water, 8.8ml trifluoroacetic acid (TFA), 0.2ml triisopropylsilane (TIPS)) , lysed for 3.0 hours, filtered with a G3 glass sand core funnel, evaporated most of the filtrate with a rotary evaporator to about 2ml of residual liquid, then ...
Embodiment 2
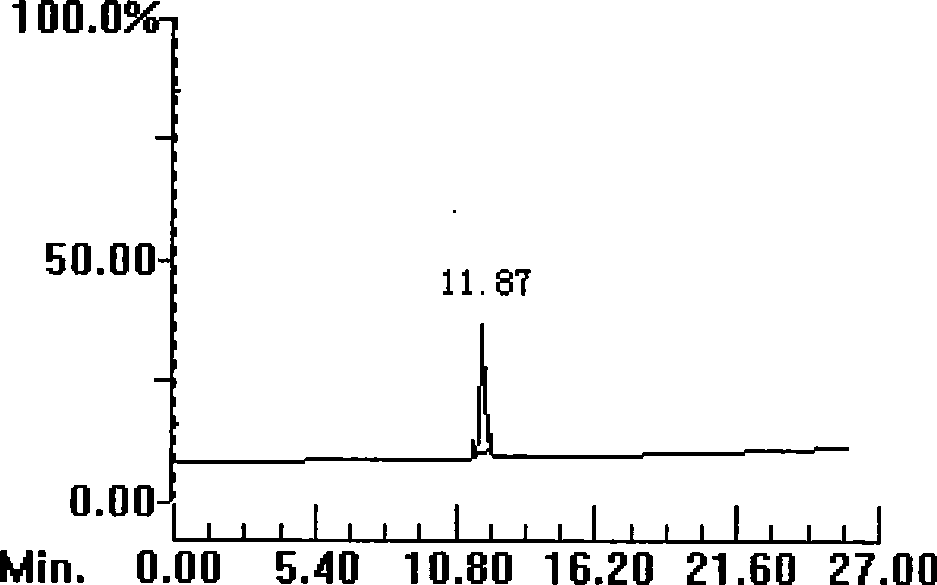
[0070] Example 2: Synthesis and purification of polypeptide 2
[0071] 1. Synthesis:
[0072] Weigh 0.17g Rink resin (0.1mmol, substitution rate 0.6g / mmol), the consumption of each Fmoc protected amino acid is 0.5mmol, with 1-hydroxybenzotriazole (HoBt) and dicyclohexylcarbodiimide (DCC, Acros company) as the condensing agent, piperidine (Piperidine, Shanghai Jill Biochemical) deprotection, according to the operating instructions of the ABI433A solid-phase synthesizer of Applied Systems Biosystems in the United States, appropriately prolong the coupling time (60-90min) and deprotection time (20- 30min), synthesize peptide-resin.
[0073]Take the above peptide-resin and put it into 10ml lysate (composition: 0.5g dimercaptothreitol (DTT), 0.5ml water, 8.8ml trifluoroacetic acid (TFA), 0.2ml triisopropylsilane (TIPS)) , lysed for 3.0 hours, filtered with a G3 glass sand core funnel, evaporated most of the filtrate with a rotary evaporator to about 2ml of residual liquid, then p...
Embodiment 3
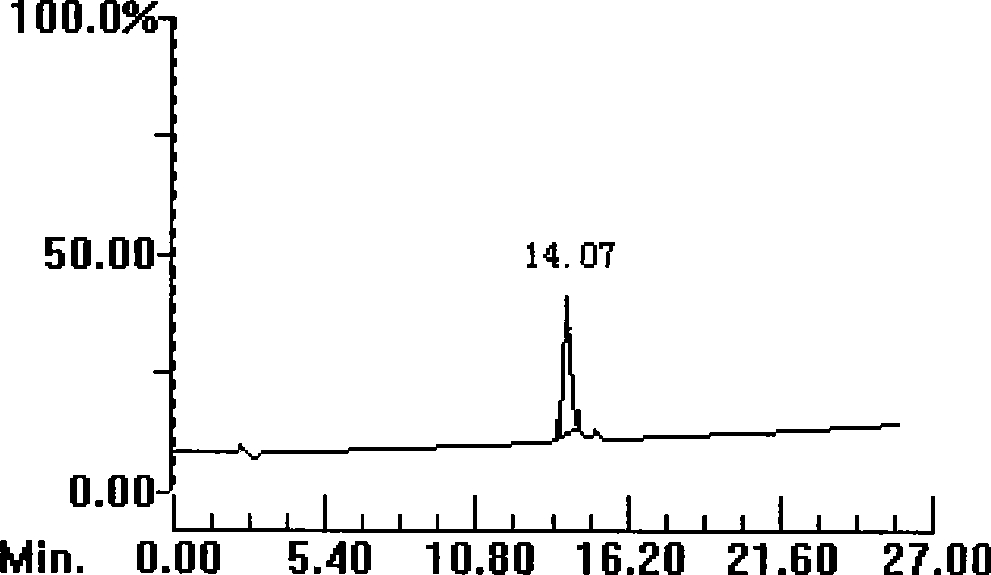
[0087] Example 3: Synthesis and purification of polypeptide 3
[0088] 1. Synthesis:
[0089] Weigh 0.17g Rink resin (0.1mmol, substitution rate 0.6g / mmol), the consumption of each Fmoc protected amino acid is 0.5mmol, with 1-hydroxybenzotriazole (HoBt) and dicyclohexylcarbodiimide (DCC, Acros company) as the condensing agent, piperidine (Piperidine, Shanghai Jill Biochemical) deprotection, according to the operating instructions of the ABI433A solid-phase synthesizer of Applied Systems Biosystems in the United States, appropriately prolong the coupling time (60-90min) and deprotection time (20- 30min), synthesize peptide-resin.
[0090] Take the above peptide-resin and put it into 10ml lysate (composition: 0.5g dimercaptothreitol (DTT), 0.5ml water, 8.8ml trifluoroacetic acid (TFA), 0.2ml triisopropylsilane (TIPS)) , lysed for 3.0 hours, filtered with a G3 glass sand core funnel, evaporated most of the filtrate with a rotary evaporator to about 2ml of residual liquid, then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com