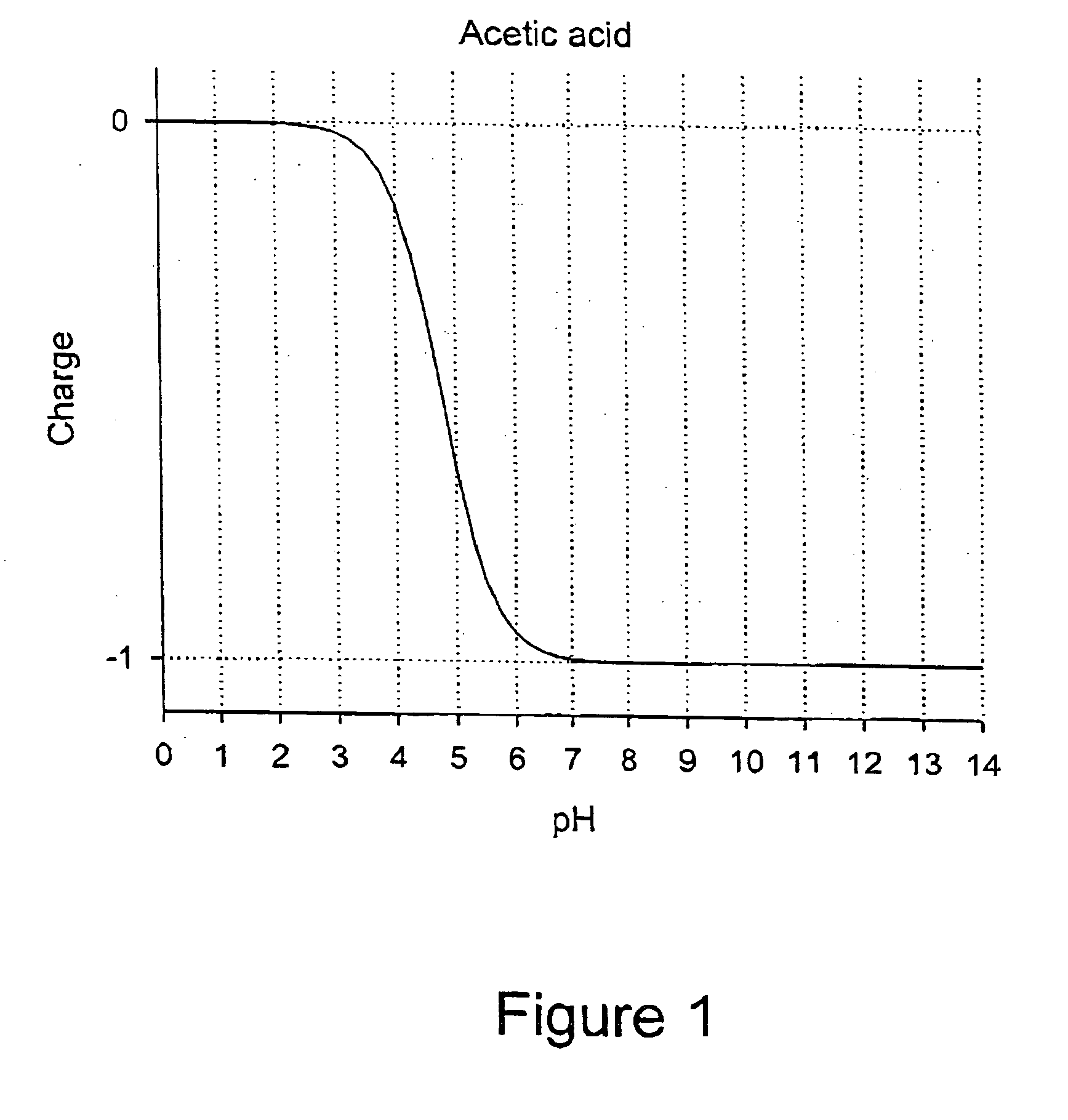
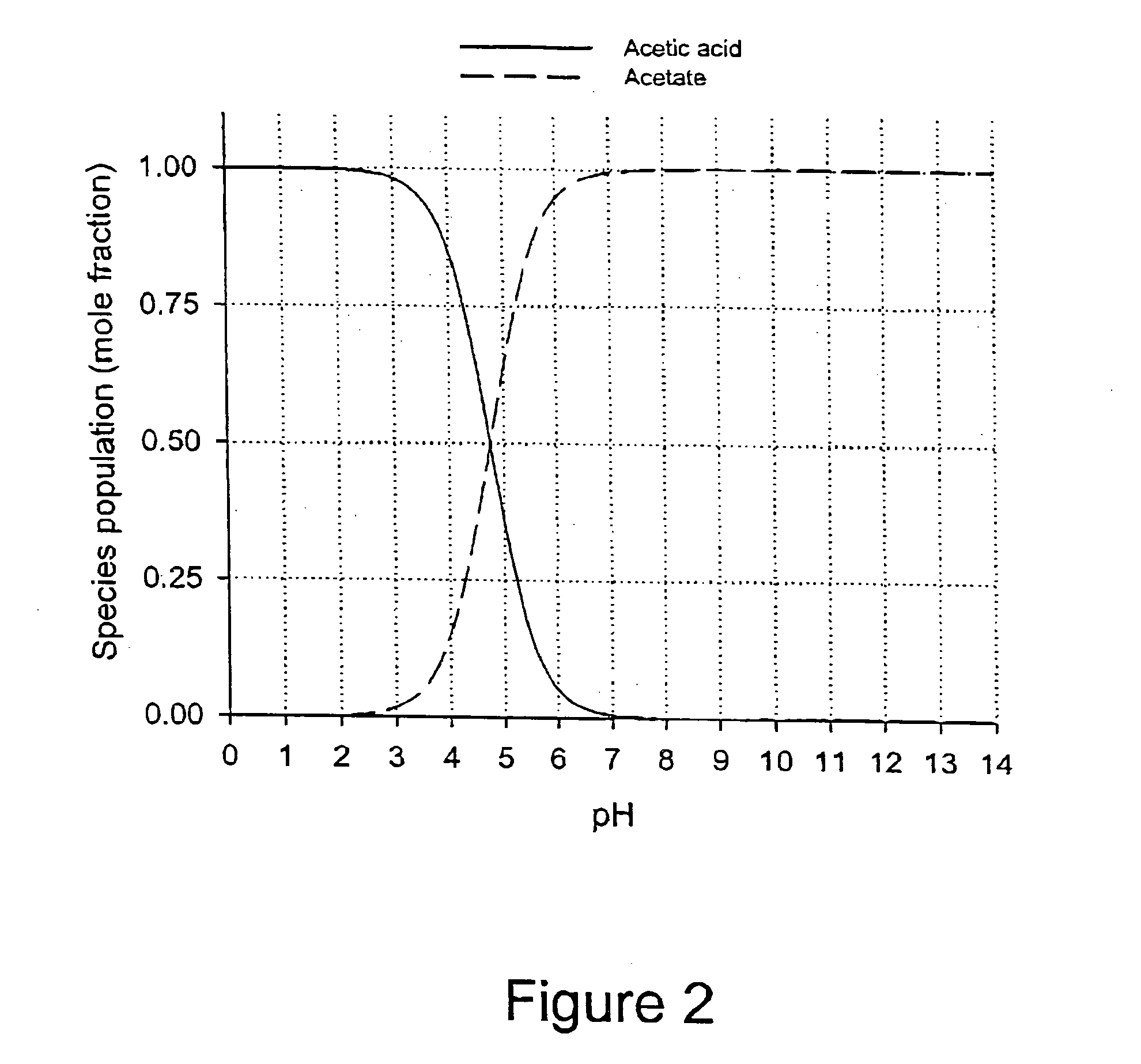
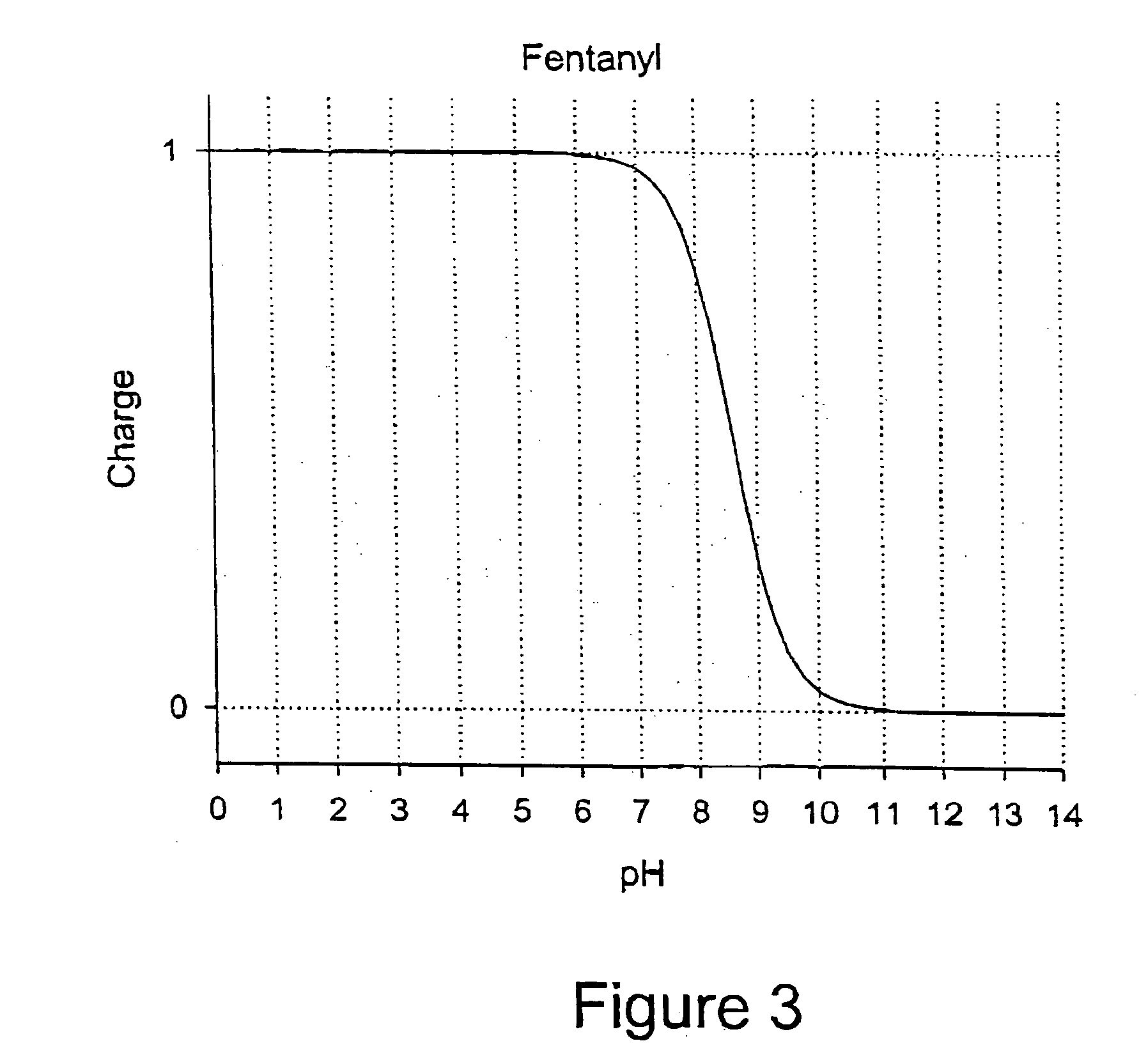
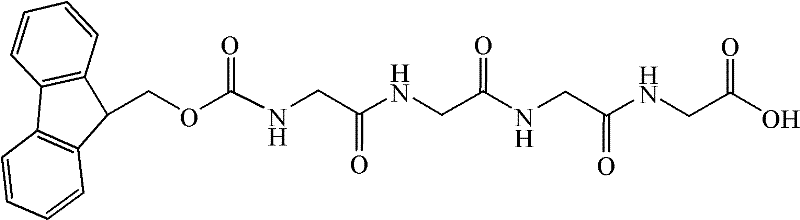
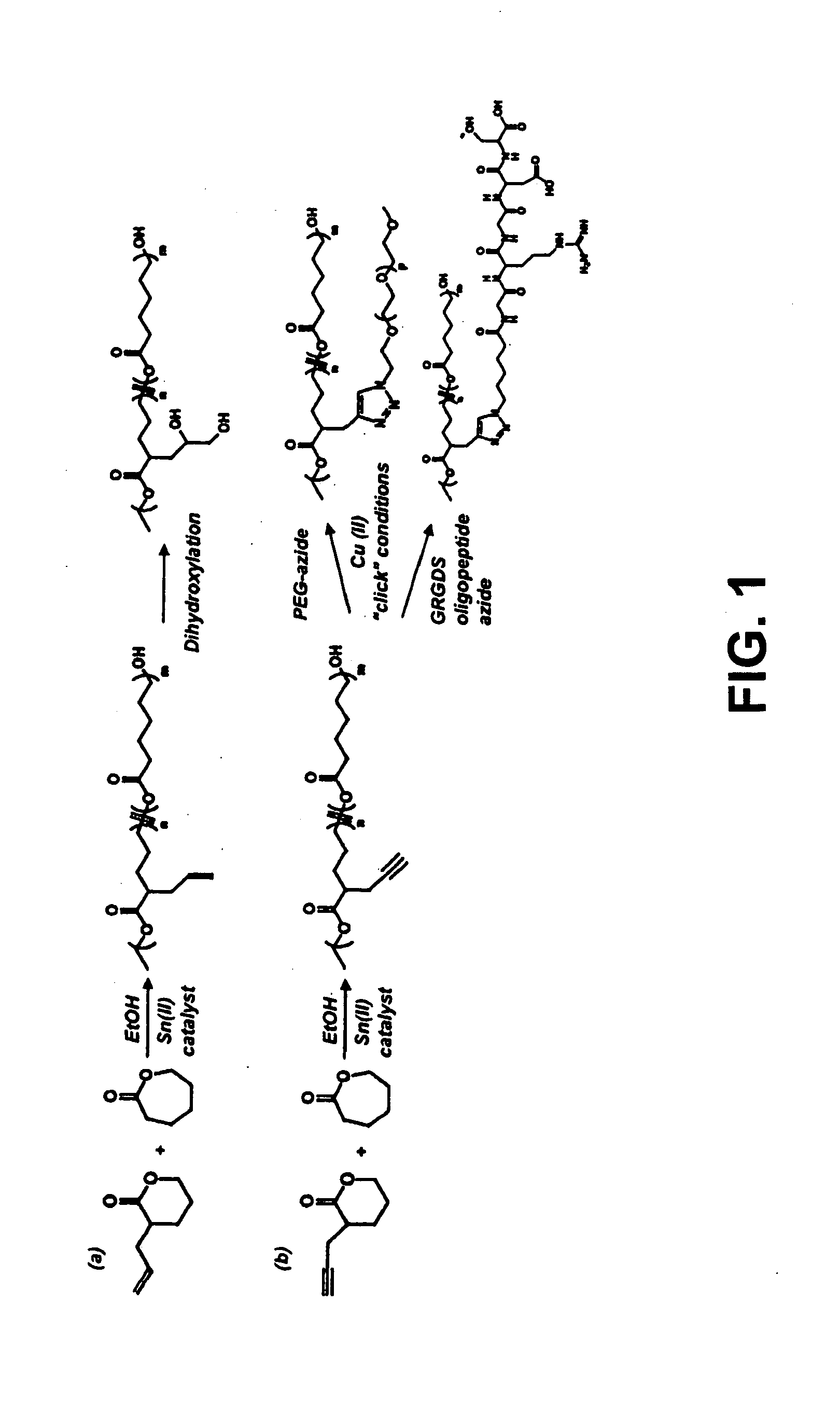
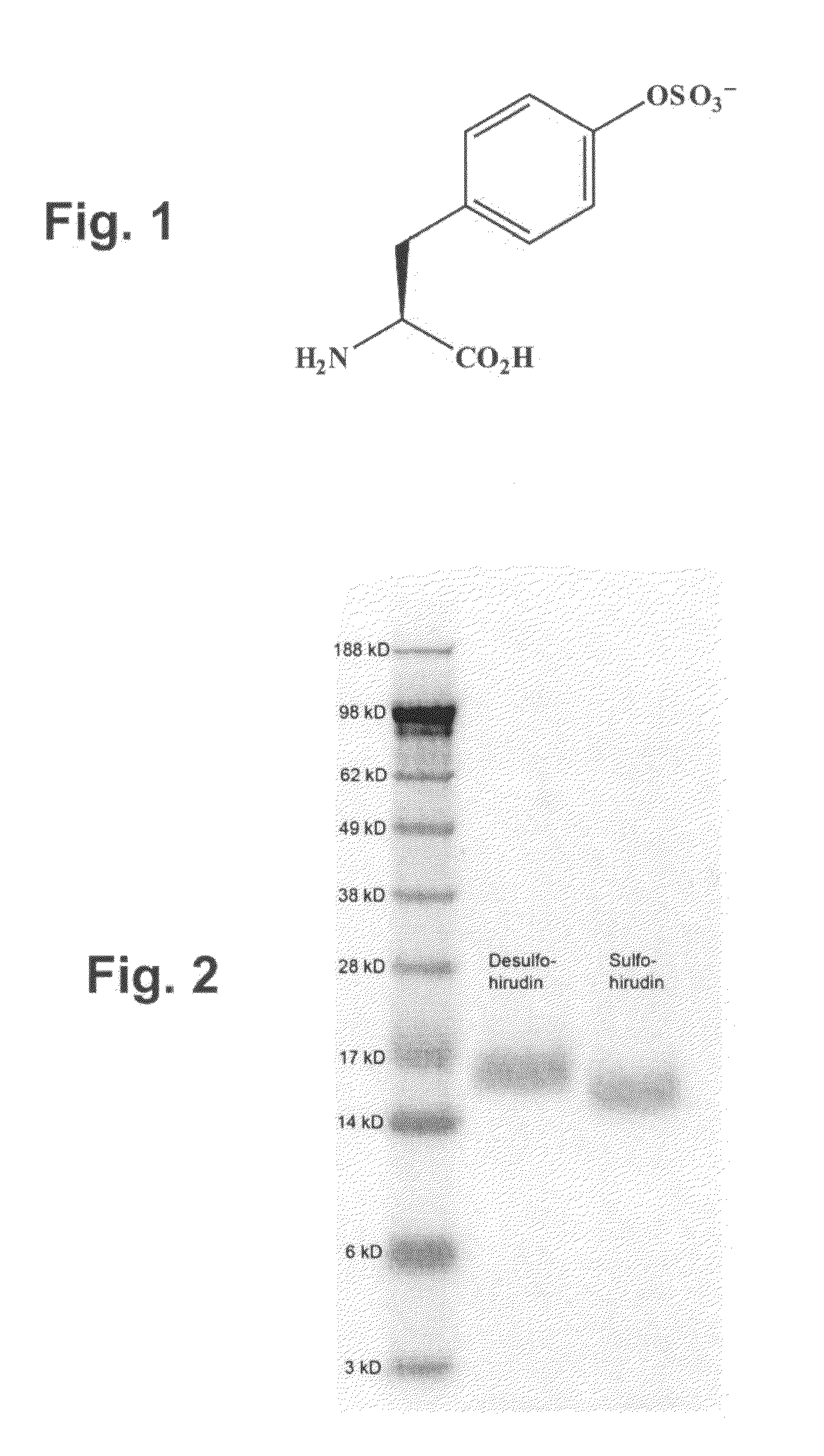
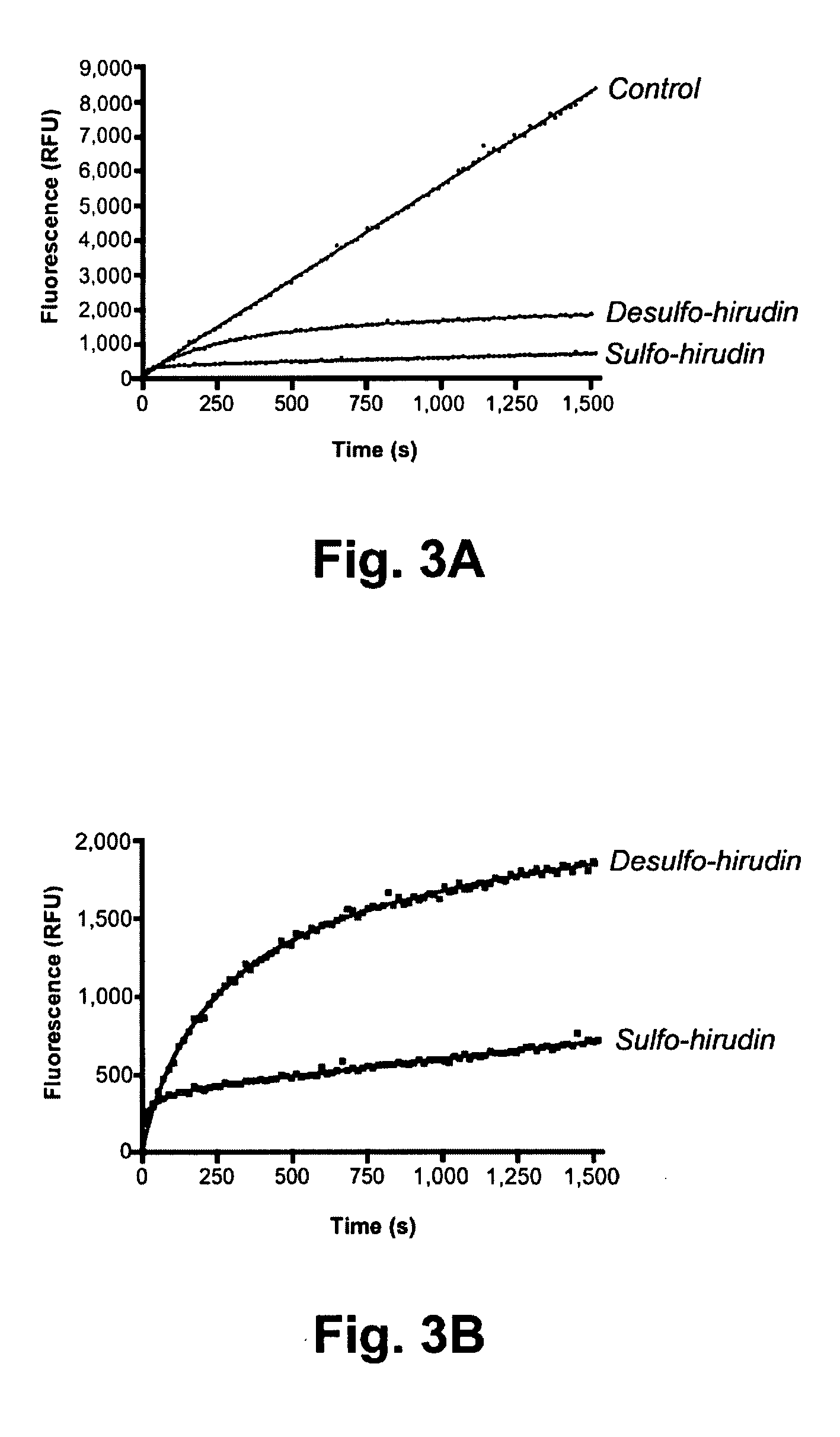
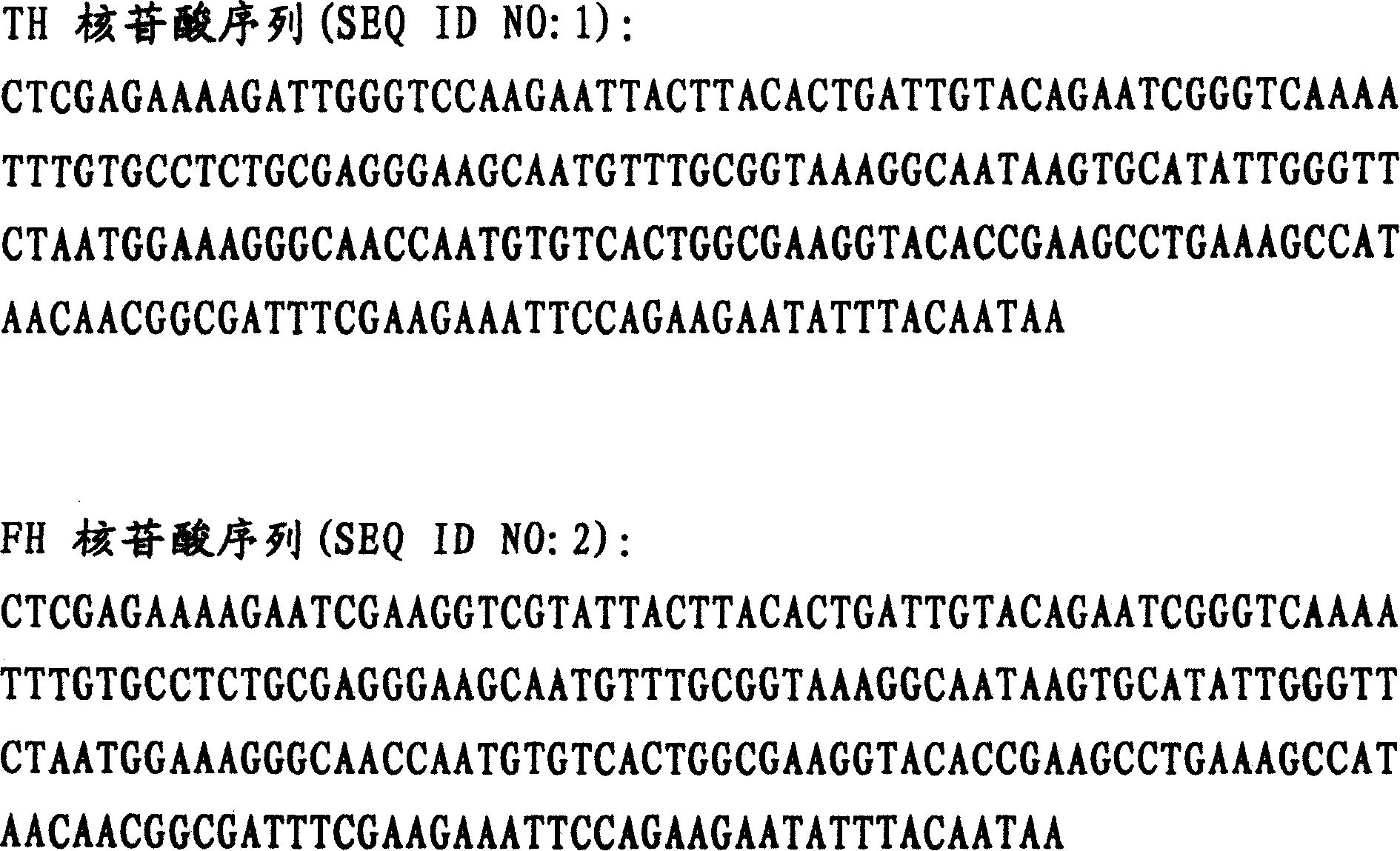
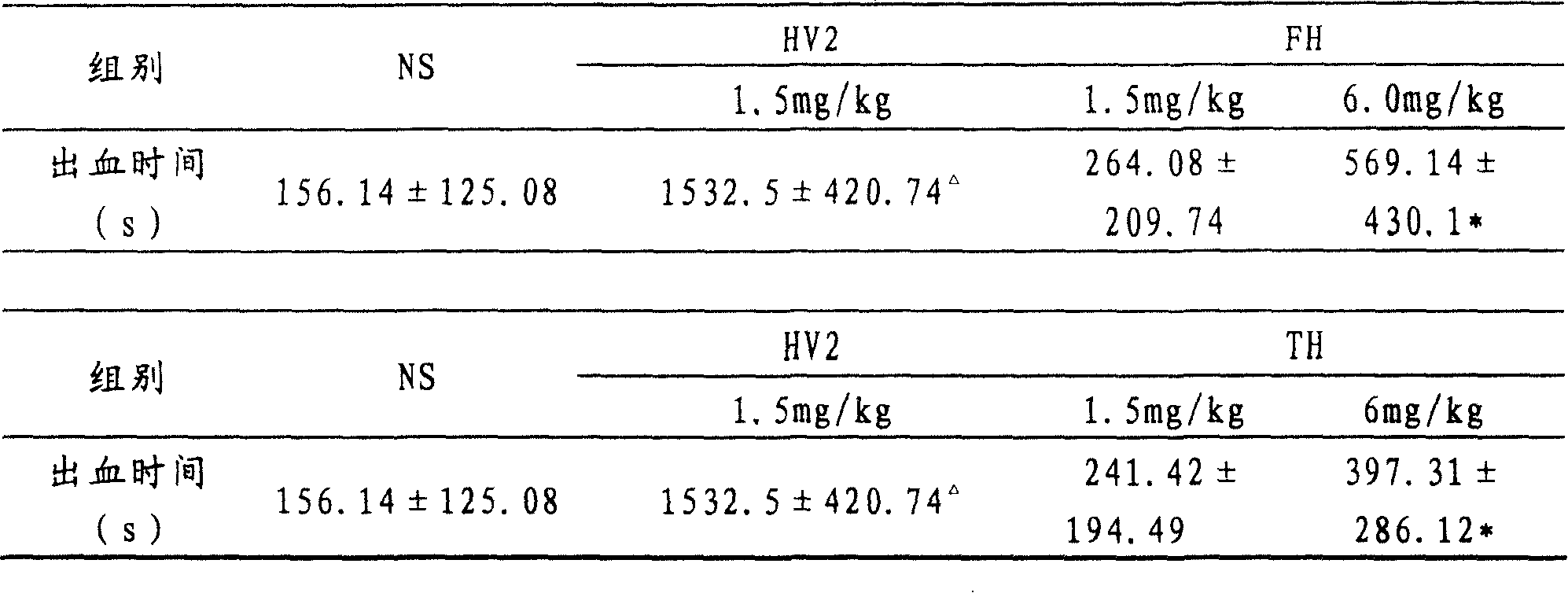
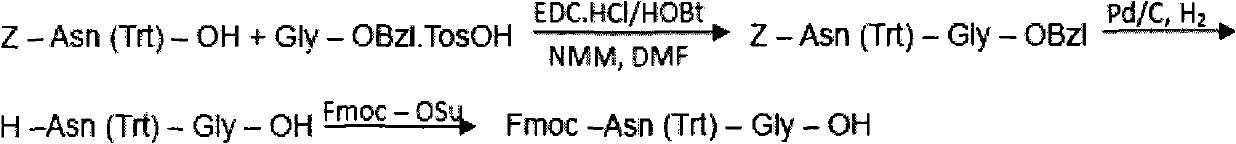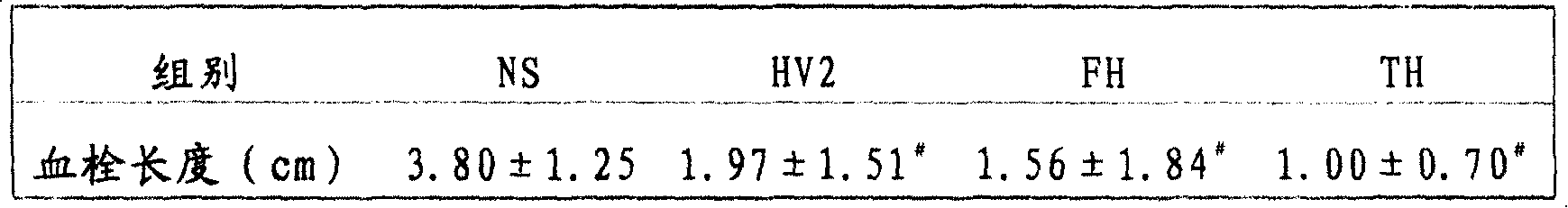Patents
Literature
201results about "Leech-based protease inhibitors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
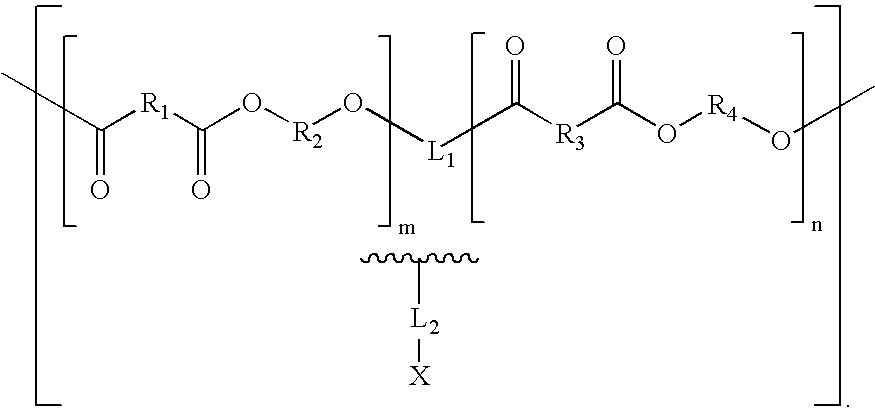
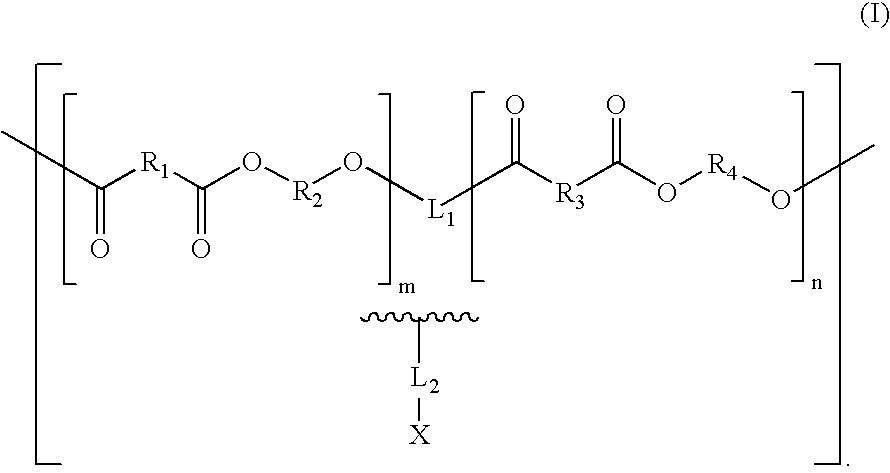
Combination Degradable and Non-Degradable Matrices for Active Agent Delivery
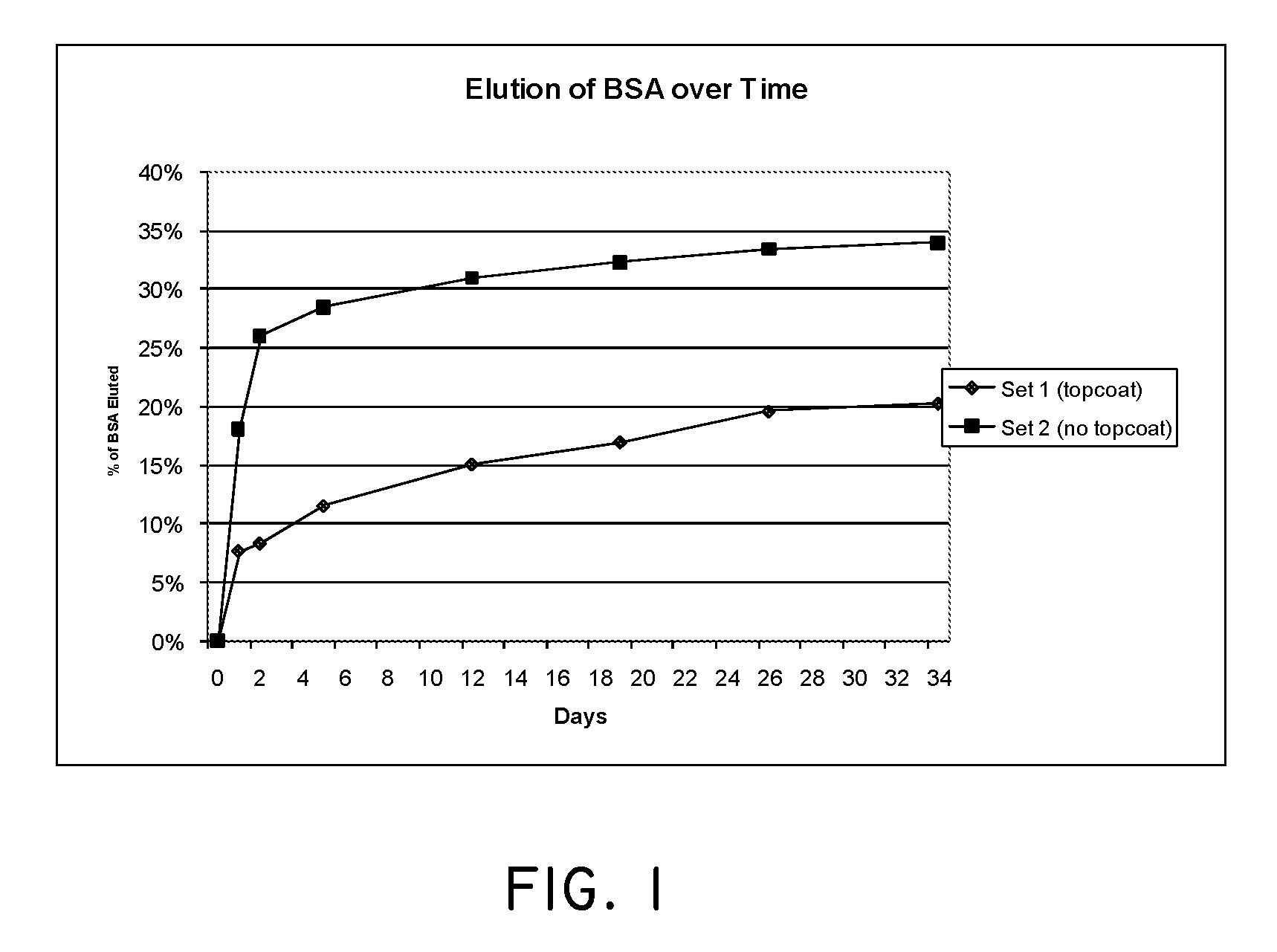
The present invention relates to relates to combination degradable and non-degradable matrices and related methods. In an embodiment, the invention includes an active agent delivery matrix including a degradable polymer network, a non-degradable polymer network, the non-degradable polymer network interspersed within the degradable polymer network, and an active agent. In an embodiment, the invention includes an active agent elution control matrix including a degradable polymer; and a non-degradable polymer interspersed with the degradable polymer. In an embodiment, the invention includes a method of making an active agent delivery matrix including mixing a degradable polymer with a first solvent to form a degradable polymer solution; mixing a non-degradable polymer with a second solvent to form a non-degradable polymer solution; and simultaneously depositing the degradable polymer solution and the non-degradable polymer solution onto a substrate.
Owner:SURMODICS INC
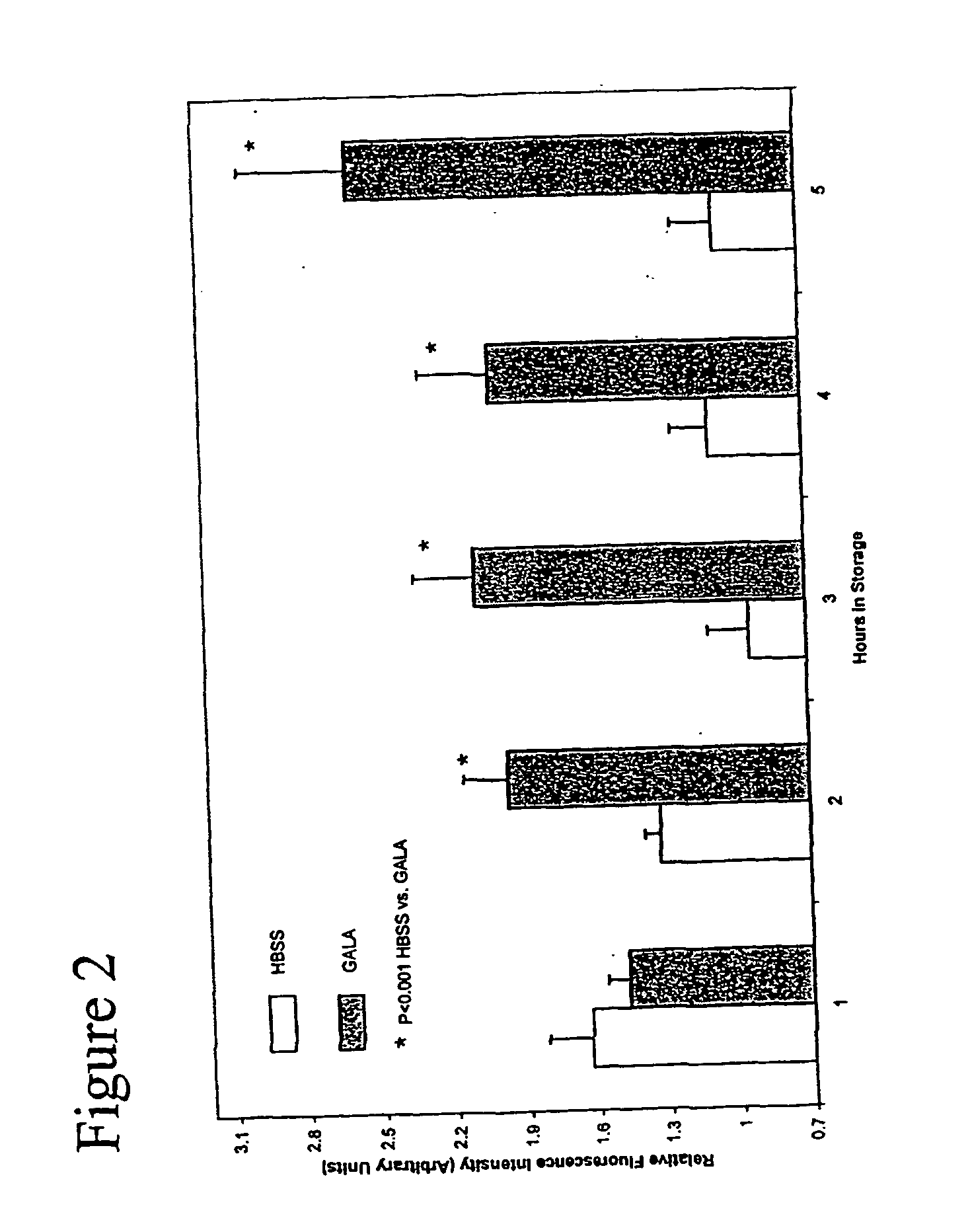
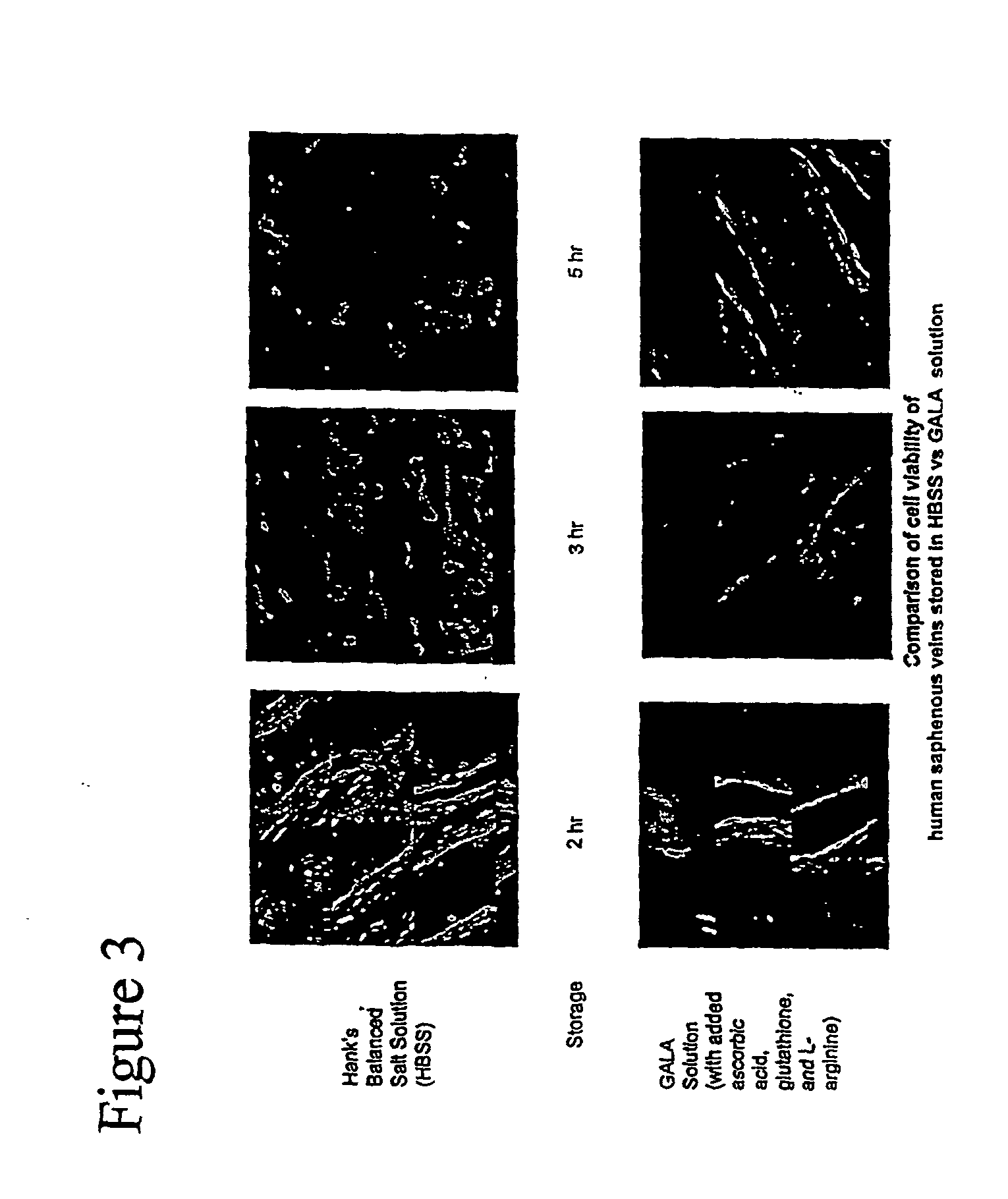
Composition and methods for tissue preservation
ActiveUS20040102415A1Preserve functionPreserve Structural IntegrityBiocideDipeptide ingredientsBiologyEx vivo
The present invention provides for compositions and methods for the preservation of tissues and organs ex vivo and in situ. In addition, the present invention provides for kits that may be used in the preparation of the solutions of the present invention. The present invention also provides a device for perfusing tissues and organs with the solutions of the present invention.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Process for production of Bivalirudin
The invention relates to methods for the preparation of high purity Bivalirudin. The polypeptide is prepared in a high purity of at least 98.5% (by HPLC), wherein the total impurities amount to less than 1.5%, comprising not more than 0.5% [Asp9-Bivalirudin] and each is impurity less than 1.0%, and preferably having a purity of at least about 99.0% by HPLC, wherein the total impurities amount to less than 1.0%, comprising not more than 0.5% [Asp9-Bivalirudin] and each impurity is less than 0.5%.
Owner:TEVA PHARM USA INC
Biologically absorbable coatings for implantable devices based on polyesters and methods for fabricating the same
Polymers containing polyesters and, optionally, agents for use with medical articles and methods of fabricating the same are disclosed. The medical article generally comprises an implantable substrate having a coating, and the coating contains a polymer comprising a polymeric product of a reaction comprising a polyol and a polycarboxylic acid.
Owner:ABBOTT CARDIOVASCULAR
Formulations for coated microprojections having controlled solubility
The invention provides for a formulation for coating one or more microprojections using a non-volatile counterion to improve solubility of a biologically active agent. The invention also includes formulations having a volatile counterion to reduce the solubility of a portion of the biologically active agent.
Owner:ALZA CORP
Preparation method for bivalirudin
ActiveCN102286076AEasy to operateReaction temperaturePeptide preparation methodsLeech-based protease inhibitorsSynthesis methodsAcid hydrolysis
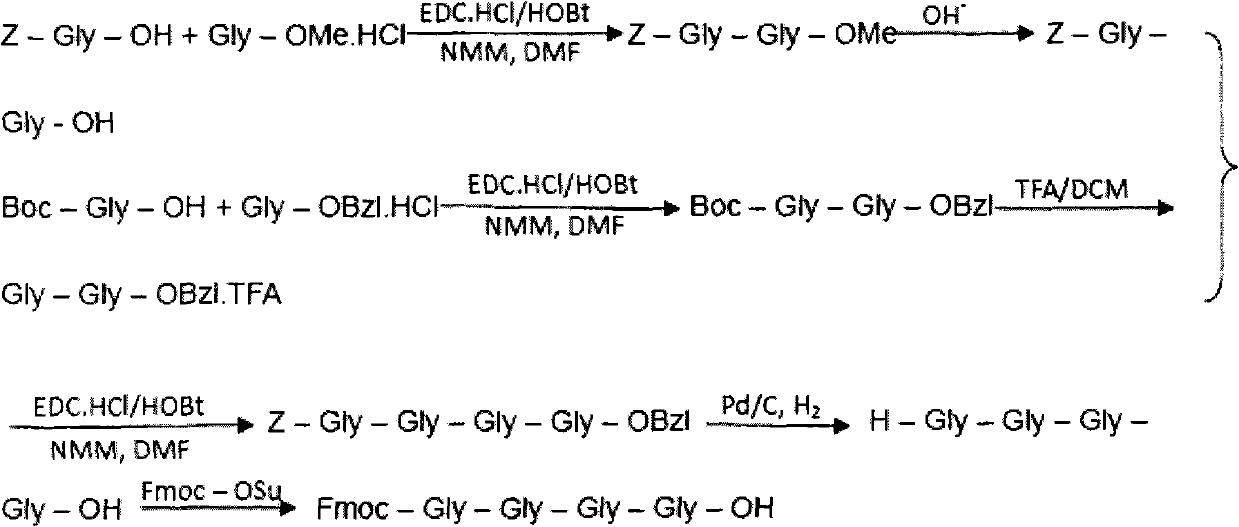
The invention belongs to the technical field of polypeptide medicament preparation methods, and in particular relates to a preparation method for bivalirudin. The preparation method for the bivalirudin comprises solid phase polypeptide synthesis for preparing bivalirudin resin, acid hydrolysis of the bivalirudin resin to obtain a crude bivalirudin product, and purification of the crude bivalirudin product to obtain a purified bivalirudin product, wherein the solid phase polypeptide synthesis for preparing the bivalirudin resin comprises the following steps of: sequentially connecting corresponding Fmoc- protected amino acids in the following sequences to Fmoc-Leu-carrier resin by a solid phase coupling synthesis method: R1-D-Phe-Pro-Arg(Pbf)-Pro-X-Asn(R2)-Gly-Asp(OtBu)-, Phe-Glu(OtBu)-Glu(OtBu)-Ile-Pro-Glu(OtBu)-Glu(OtBu)- and Tyr(tBu)-Leu-resin, and thus obtaining the bivalirudin resin; and when the X fragment is connected, only one times of solid phase coupling synthesis reaction is used, and the corresponding Fmoc- protected amino acid is Fmoc-Gly-Gly-Gly-Gly-OH. The purity of the bivalirudin is more than 99.5 percent, and the single impurity is less than 0.2 percent.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
High purity peptides
InactiveUS20080287650A1High HPLC purityThymosin peptidesPeptide preparation methodsNesiritideHPLC measurement
Owner:TEVA PHARM USA INC
Process for the preparation of bivalirudin and its pharmaceutical compositions
InactiveUS20090062511A1Superior mechanical and swelling propertyImprove stabilityPeptide/protein ingredientsImmunoglobulinsBivalirudinStereochemistry
The present application provides an improved process for the preparation of Bivalirudin and its pharmaceutical compositions.The present application also provides an improved process for the purification of Bivalirudin.
Owner:DR REDDYS LAB LTD +1
Prolonged release microspheres for injectable administration
The present invention provides microspheres intended to be administered by injection comprising a protein active ingredient and an agent coating the active ingredient intended to prolong its release, wherein they are free of any trace of organic solvent and they can be obtained according to a coating method involving bringing the active ingredient and the coating agent into contact, with stirring, in a supercritical fluid, said coating agent being soluble in this supercritical fluid.
Owner:ETHYPHARM SA
Tailored Aliphatic Polyesters for Stent Coatings
Owner:ABBOTT CARDIOVASCULAR
Pharmaceutical compositions comprising thrombin inhibitors and their use in the control of wound healing processes
There is provided the use of a thrombin inhibitor in the manufacture of a product for use in the control of wound healing processes within the body, in particular, the inhibition or prevention of fibrin-related adhesion and / or scar tissue formation, as well as products for use in the control of wound healing processes within the body comprising polysaccharides (e.g., chitosans) and low molecular weight peptide-based thrombin inhibitors.
Owner:ASTRAZENECA AB
Preparation method of polypeptide solid-phase synthesis bivalirudin crude product
ActiveCN101555274ALow costHigh purityPeptide preparation methodsBlood disorderProtecting groupAmino acid supplementation
The invention discloses a preparation method of polypeptide solid-phase synthesis bivalirudin crude product. The preparation method comprises the following steps of: (1) mixing Fmoc-amino-acid resin or Fmoc-polypeptide resin and an unprotecting agent and removing Fmoc protecting group; (2) in the existence of a condensing agent, leading amino acid with Fmoc or Boc and amino acid or polypeptide onthe resin to carry out condensation; (3) repeating the step (1) and step (2) and obtaining polypeptide resin shown as formula II; and (4) in the existence of a cutting agent, leading the polypeptide and the resin on the polypeptide resin to be separated, and obtaining bivalirudin shown as formula I. The unprotecting agent contains 3 to 20 percent of piperidine and 0.5 to 10 percent of bicyclic amidine by total volume.
Owner:HAINAN SHUANGCHENG PHARMA
Method for repeatedly extracting natural hirudin from live vampire leech
ActiveCN102964446ANon-toxic ingredientsNot corrosiveLeech-based protease inhibitorsBiotechnologyBiochemical engineering
The invention provides a method for repeatedly extracting natural hirudin from live vampire leech. The method comprises the steps of: firstly extracting crude natural hirudin from the live vampire leech, then carrying out separation and purification to obtain natural hirudin, wherein the extraction of the crude natural hirudin comprises the steps of: firstly preparing specific induction liquid and then pouring into intestinal canal to obtain specific induction liquid-containing intestinal canal; then feeding the specific inducing liquid-containing intestinal canal to the vampire leech, and extracting the spit of the vampire leech or grinding the vampire leech into slurry, thereby obtaining the crude natural hirudin; and the crude natural hirudin is separated and purified to obtain natural hirudin powder according to a conventional biochemical separation method. The method is simple in process, and high in extraction rate, the death rate of the vampire leeches can be obviously reduced after the crude natural hirudin is extracted, and biological resource utilization rate can be effectively improved, so that the production cost can be obviously lowered; therefore, the method is a convenient approach for extracting the main effective active substance (such as natural hirudin) from the leech body.
Owner:周维官
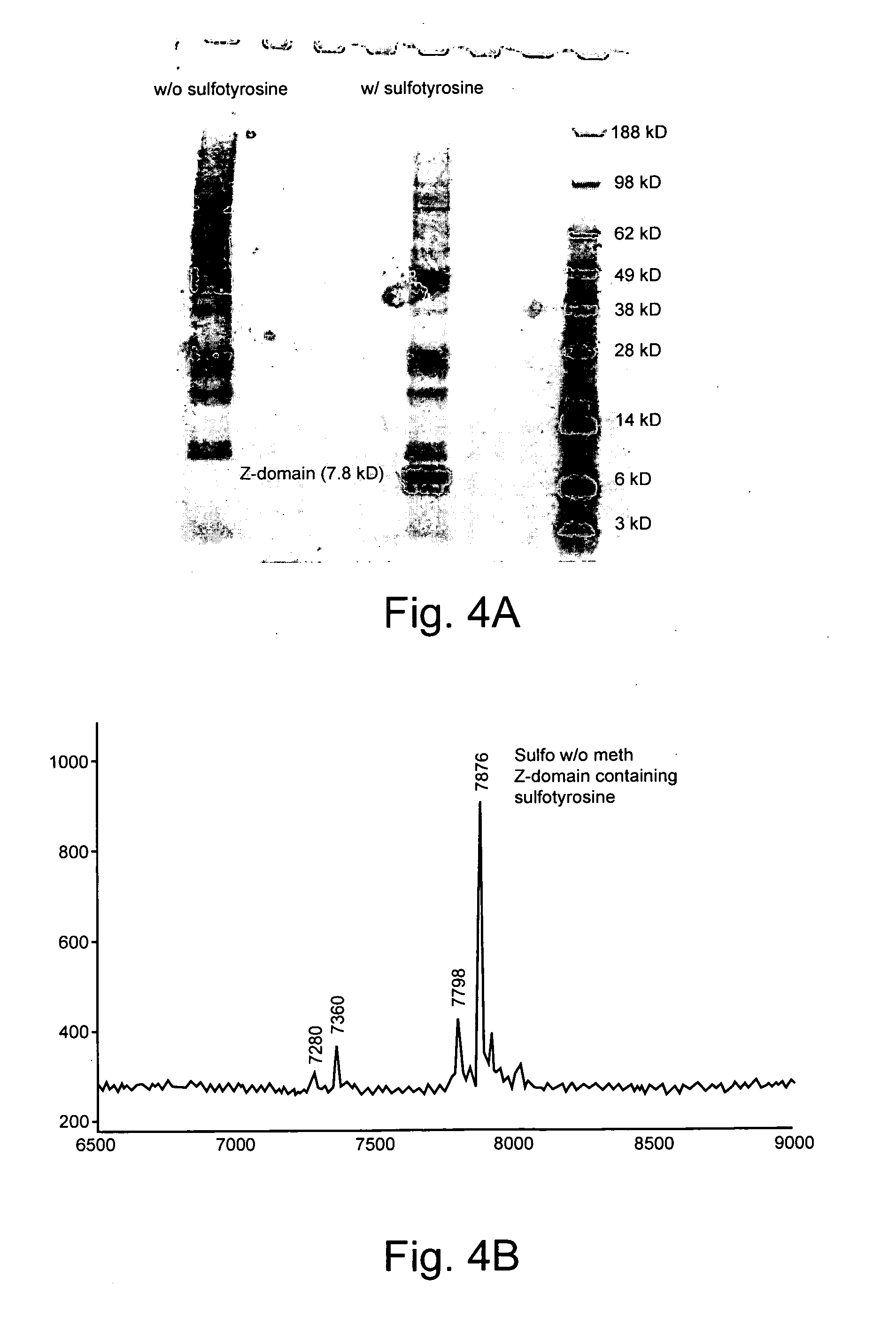
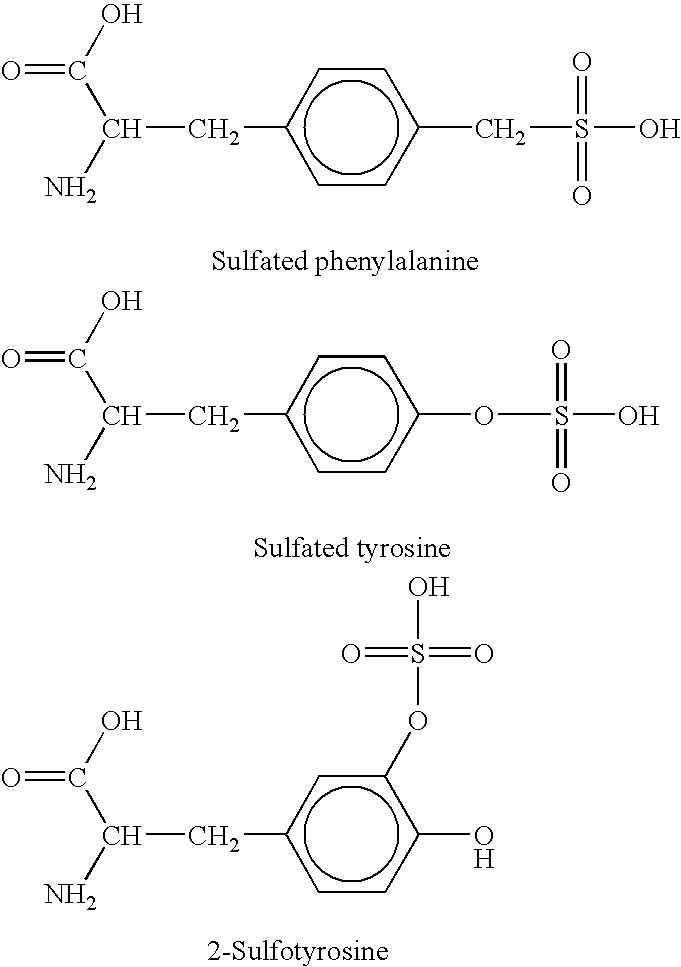
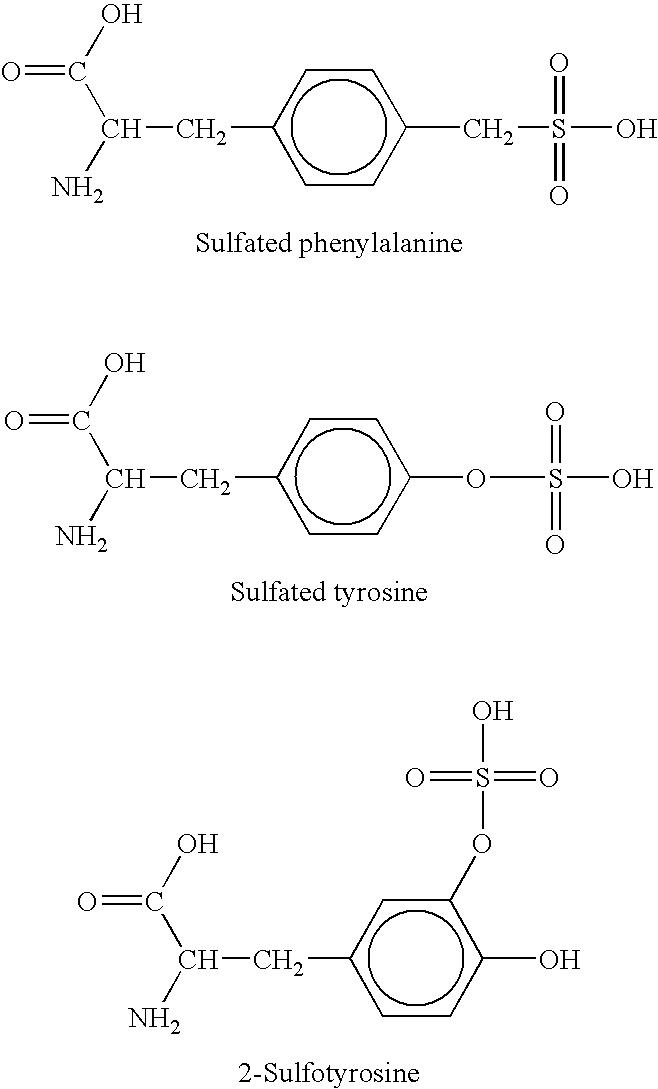
Genetically programmed expression of selectively sulfated proteins in eubacteria
The invention relates to orthogonal pairs of tRNAs and aminoacyl-tRNA synthetases that can incorporate the unnatural amino acid sulfotyrosine into proteins produced in eubacterial host cells such as E. coli. The invention provides, for example but not limited to, novel orthogonal aminoacyl-tRNA synthetases, polynucleotides encoding the novel synthetase molecules, methods for identifying and making the novel synthetases, methods for producing proteins containing the unnatural amino acid sulfotyrosine and translation systems.
Owner:THE SCRIPPS RES INST
A kind of production method of natural hirudin
ActiveCN102286098ASimple processHigh extraction ratePeptide preparation methodsLeech-based protease inhibitorsCurative effectBULK ACTIVE INGREDIENT
The invention discloses a production method of natural hirudin. The production method is characterized by comprising the steps of extracting, acid depositing, heating, dehydrating and drying natural hirudin liquid, wherein the content of active ingredients of the obtained natural hirudin powder can be detected through Markwardt direct thrombin titration so that the quality is stable and the curative effect of the product is ensured. The method has the characteristics of simple process, high extraction ratio, low production cost, high product purity, long storage period and the like and can beused for providing safe and high-quality raw materials for foods, health-care foods, medicines or cosmetics.
Owner:广西科康科技集团有限公司
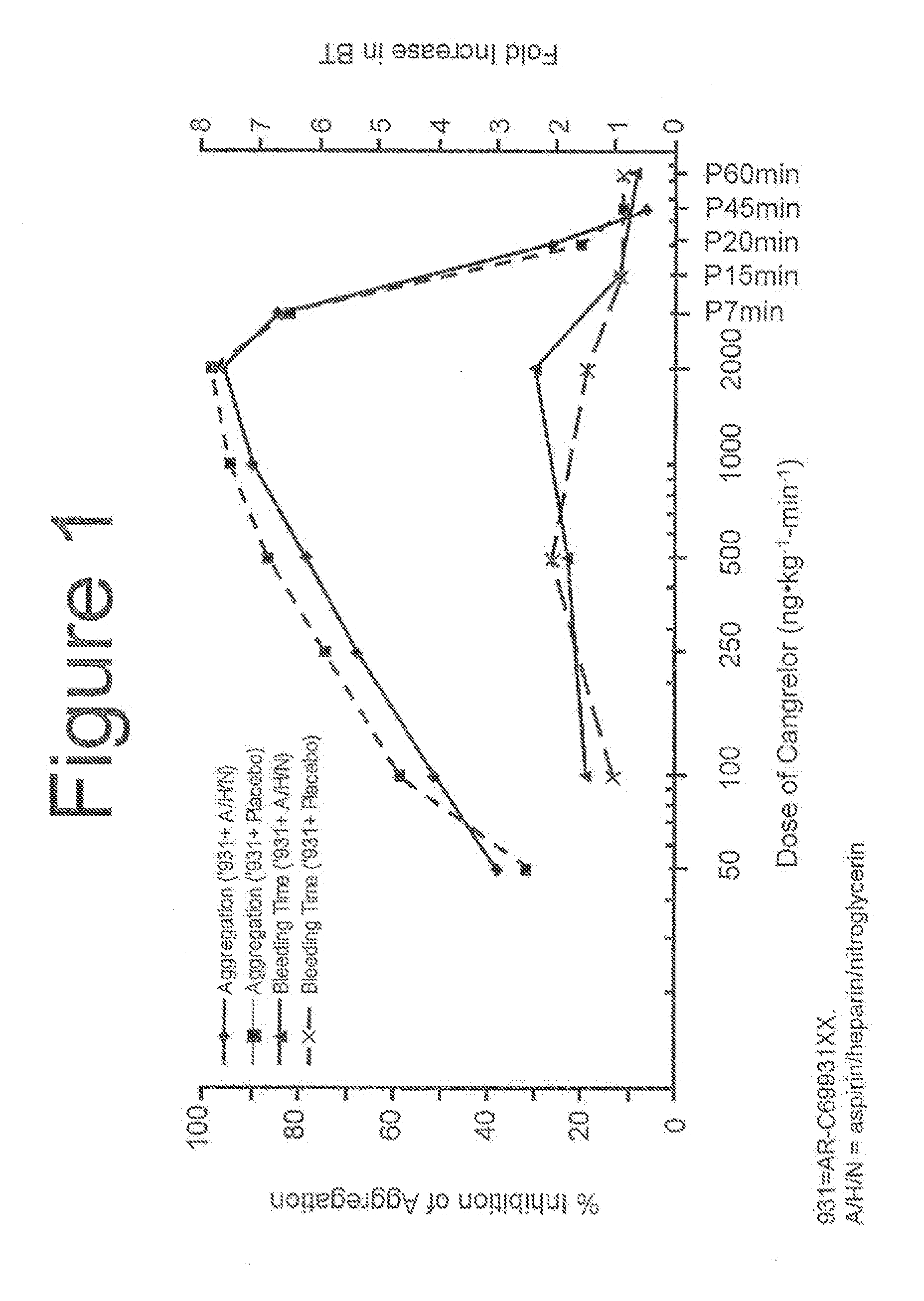
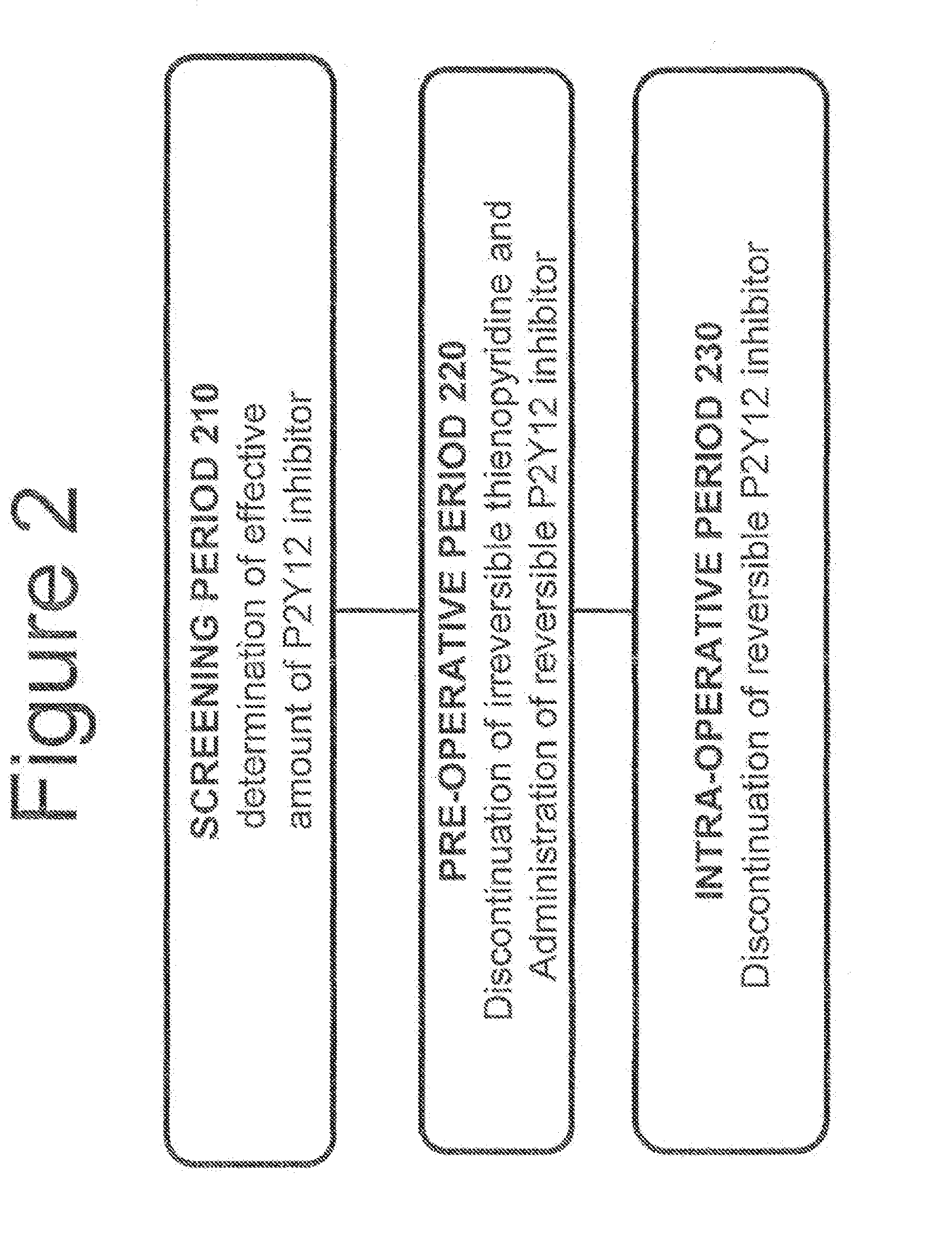
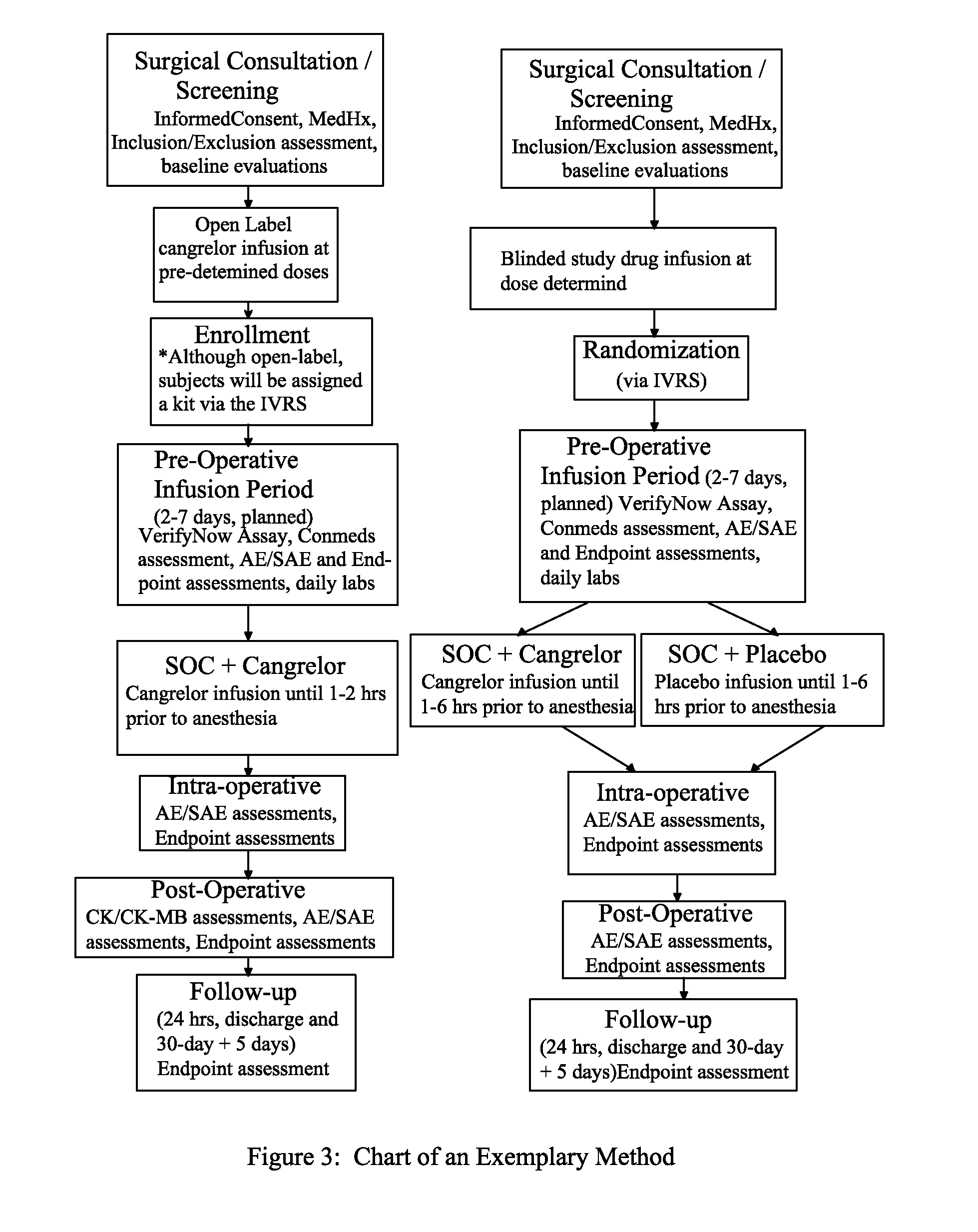
Maintenance of platelet inhibition during antiplatelet therapy
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:THE MEDICINES
Biologically absorbable coatings for implantable devices based on polyesters and methods for fabricating the same
Polymers containing polyesters and, optionally, agents for use with medical articles and methods of fabricating the same are disclosed. The medical article generally comprises an implantable substrate having a coating, and the coating contains a polymer comprising a polymeric product of a reaction comprising a polyol and a polycarboxylic acid.
Owner:ABBOTT CARDIOVASCULAR
The method for preparing bivalirudin
Owner:POLYPEPTIDE LAB HLDG PPL AB
Peptide and polypeptide inhibitors of complement C1s
The complement system plays an important role in providing resistance to infections and in the pathogenesis of tissue injury. Yet an inappropriate activation of complement can result in a variety of disorders. The present invention provides C1s catalytic site-directed moieties, C1s exosite binding moieties, and bivalent polypeptide inhibitors comprising such moieties, which can be used to treat conditions characterized by inappropriate complement activation.
Owner:ZYMOGENETICS INC
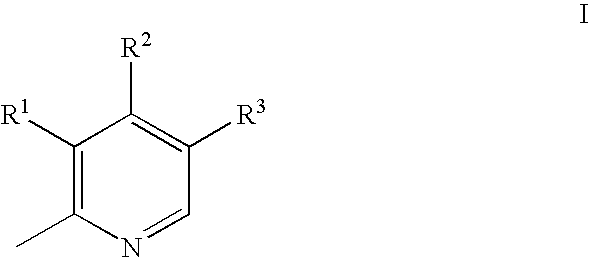
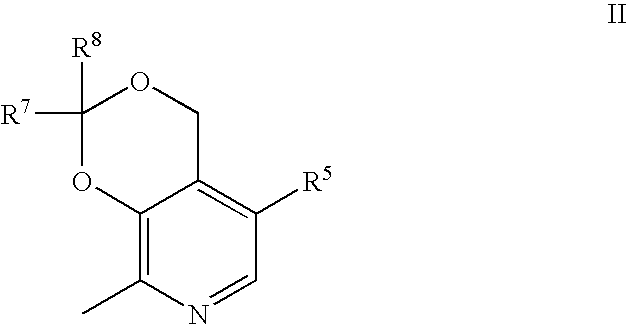
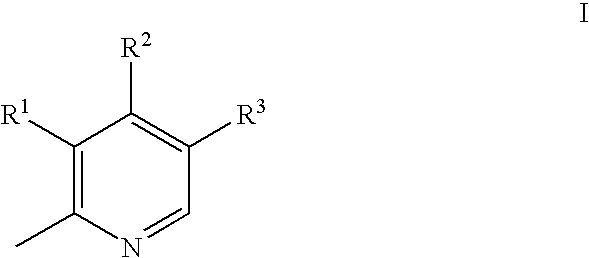
Aryl Sulfonic Pyridoxines as Antiplatelet Agents
Aryl sulfonic pyridoxine compounds with inhibition of serine protease activity and antiplatelet aggregation characteristics for the treatment of cardiovascular and cardiovascular related diseases are described. The methods are directed to administering pharmaceutical compositions comprising aryl sulfonic pyridoxines.
Owner:HAQUE WASIMUL +1
Preparation method of Bivalirudin
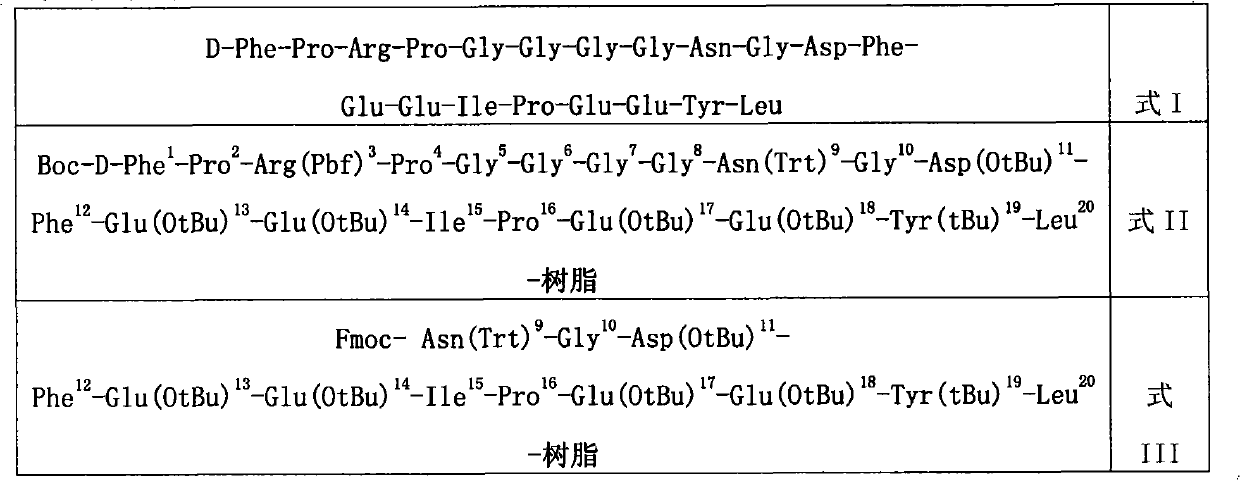
ActiveCN101906150AHigh purityEfficient removalPeptide preparation methodsBulk chemical productionCombinatorial chemistryBivalirudin
The invention discloses a preparation method of polypeptide solid-phase synthesis Bivalirudin, comprising the following steps of: (1) condensing Fmoc-Asn(Trt)-Gly-OH with a polypeptide resin shown as the formula V in the presence of a condensing agent to obtain a polypeptide resin shown as the formula III; (2) mixing the polypeptide resin shown as the formula III with a deprotection agent to remove a Fmoc protective group; (3) condensing Fmoc-Gly-Gly-Gly-Gly-OH with a polypeptide resin shown as the formula VI in the presence of the condensing agent to obtain a polypeptide resin shown as the formula IV; (4) sequentially condensing polypeptide of the polypeptide resin shown as the formula IV with amino acids from the end C to the end N according to the sequence from Pro to Arg, Pro and to D-Phe by a method of solid-phase synthesis so as to prepare a polypeptide resin shown as the formula II; and (5) separating polypeptide and resin on the polypeptide resin shown as the formula II in thepresence of a separating agent to obtain Bivalirudin shown as the formula I.
Owner:SHANGHAI AMBIOPHARM
Peptide and polypeptide inhibitors of complement C1s
The complement system plays an important role in providing resistance to infections and in the pathogenesis of tissue injury. Yet an inappropriate activation of complement can result in a variety of disorders. The present invention provides C1s catalytic site-directed moieties, C1s exosite binding moieties, and bivalent polypeptide inhibitors comprising such moieties, which can be used to treat conditions characterized by inappropriate complement activation.
Owner:ZYMOGENETICS INC
Method for extracting active ingredient of natural leech essence
InactiveCN101108879AHighlight substantive featuresSignificant progressLeech/worm material medical ingredientsLeech-based protease inhibitorsRetention periodAqueous acetone
The invention discloses an extract method of the effective constituent of the Natural hirudin. The method is that the blood-sucking leech is washed cleanly by water and is added with the ethanol water with content of 15 per cent to 25 per cent to soak for 30h to 50h, and then is filtered to gain the filter liquor and dregs of a decoction; the Poecilobdella manillensis waste residue is broken into pieces and is added with aqueous acetone solution with content of 15 per cent to 25 per cent to soak, and then is filtered to gain the filter liquor; after recovering ethanol and acetone from the two-time filter liquor in the vacuum, the filter liquor is combined and frozen and dried in the vacuum conventionally to gain the product. The invention is simple in process, low in cost, higher in yield, good in product quality and long in retention period. The ''three wastes'' are not discharged in the whole production process, and the product can work as the raw material in such three fields as medicine, hairdressing and health food.
Owner:滕海英 +2
Prohealing piezoelectric coatings
Owner:ABBOTT CARDIOVASCULAR
Method for extracting hirudin from leech saliva
InactiveCN104926937ANot corrosiveEasy extractionAnimal husbandryLeech-based protease inhibitorsFiltrationLiving body
The invention discloses a method for extracting hirudin form leech saliva. The method includes the following steps of a), placing leeches in a container to allow the leeches to be hungered sufficiently, inputting inductive substances to the leeches to allow the same to absorb fully, adding vomitives to allow the leeches to spit the saliva in bodies, taking out blood-sucking leech living bodies to return to a rearing pond to feed continuously, and meanwhile, collecting hirudin crude product liquid in the container; b), freezing above hirudin crude products, adding into precooled cold acetone, stirring prior to placing in a refrigerator for setting overnight, sucking supernatant acetone the next day prior to centrifuging, adding a trichloroacetic acid solution, and centrifuging to remove residues to obtain concentrated liquid; c), eluting the above concentrated liquid with an anion exchange column chromatography method, and performing gel filtration chromatography to obtain finished products. The method has the advantages of reasonable process, convenience and practicability in operation, good quality stability of the finished hirudin products and high yield, one-time pillage on the leeches with a traditional method can be avoided, wild resources of the leeches are protected, and development of leech farming is driven.
Owner:广西复鑫益生物科技有限公司
Pharmaceutical formulations of bivalirudin and processes of making the same
ActiveUS7582727B1Efficient mixingPeptide/protein ingredientsPeptide preparation methodsBULK ACTIVE INGREDIENTSolvent
Pharmaceutical batch(es) or pharmaceutical formulation(s) comprising bivalirudin as the active ingredient, and a method of preparing the pharmaceutical batch(es) or pharmaceutical formulation(s). The pharmaceutical batch(es) or pharmaceutical formulation(s) may have a maximum impurity level of Asp9-bivalirudin that does not exceed about 0.6%. Also, the pharmaceutical batch(es) or pharmaceutical formulation(s) may have a reconstitution time that does not exceed about 42 seconds. The method of preparing the pharmaceutical batch(es) or pharmaceutical formulation(s) may comprise dissolving bivalirudin in a solvent to form a first solution, efficiently mixing a pH-adjusting solution with the first solution to form a second solution in which the pH-adjusting solution may comprise a pH-adjusting solution solvent, and removing the solvent and the pH-adjusting solution solvent from the second solution.
Owner:SANDOZ INC
Eglin c based drugs for treatment of disease
The present invention relates to eglin c variants which inhibit proteases, and in particular to eglin c mutants at adventitious contact sites. The present invention also relates to eglin c variants which comprise mutations in both adventitious contact sites and at reactive loop sites. The present invention further relates to methods of preparing the eglin c variants, and methods of using the eglin c variants for treatment of diseases including acute bacterial, viral, and fungal infections.
Owner:RGT UNIV OF MICHIGAN
Production technology capable of efficiently separating high-activity hirudin based on anion exchange column
ActiveCN103509105ASimple processHigh extraction ratePeptide preparation methodsLeech-based protease inhibitorsPoecilobdella manillensisBiochemical engineering
The invention discloses a production technology capable of efficiently separating and purifying hirudin based on a novel anion exchange column (DEAE-silica gel separation column). The production technology taking leech powder prepared from Guangxi poecilobdella manillensis as a raw material belongs to the field of traditional Chinese medicine production, relates to extraction of animal effective ingredients and aims at solving the problems that hirudin is complicated to extract, high in cost and difficult to produce industrially. The invention is characterized in that a new hirudin separating and purifying technology is designed aiming at a separating and purifying system of the new DEAE-silica gel separation column with low cost. The technology is simple and speedy and low in cost; besides, the freeze-dried powder of the obtained hirudin is high in activity, recovery rate and purity, and long in storage life, so that safe and good-quality raw materials can be offered for industries of foods, health-care foods, medicines or cosmetics and the like.
Owner:科康生物医药(深圳)有限公司
Preparation for specificity anticoagulant substance and application thereof
InactiveCN101190945APeptide/protein ingredientsHybrid peptidesThrombin activityCoagulation Factor Xa
The invention relates to an anticoagulant protein containing an oligopeptide which is identified and cracked by thrombin or blood coagulation factor Xa; more particularly, the invention relates to a new anticoagulant substance connected by an anticoagulant substance and an amino acid sequence which can be identified and cracked by thrombin or blood coagulation factor Xa, or a new substance connected by an anticoagulant substance and other substances through taking an amino acid sequence which can be identified and cracked by thrombin or blood coagulation factor Xa as oligopeptide, as well as medicine science application of these new anticoagulant substances.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com