Peptide and polypeptide inhibitors of complement C1s
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
C1s Enzyme Assay and Classical Complement Hemolysis Assay
[0159] C1s enzyme assays were performed in 96 well plates preincubated in the assay buffer for 30 minutes at room temperature. Activated human C1s (Calbiochem-Novabiochem Corporation; San Diego, Calif.); final assay concentration: 1.25 μg / ml), was incubated with samples in 50 mM Tris-HCl (pH 8) that contained 116 mM sodium chloride and 0.05% Polysorbate 80. The chromogenic substrate, Pefa-C1E (Centerchem, Inc.; Stamford, Conn.; final assay concentration: 0.4 mM), was added to the assay wells and the plate was read at 405 nm, 37° C., for 30 minutes at 20 second intervals on a SPECTRAmax PLUS plate reader (Molecular Devices Corporation; Sunnyvale, Calif.). Inhibition was determined as a decrease in Vmax, which is calculated as the maximum Δ milli-absorbance units / min over the assay period.
[0160] An assay was performed to determine the effect of BD001 on classical complement hemolysis. A pool of pre-sensitized sheep erythrocyte...
example 2
Effects of Posttranslational Modification on BD001 Activity
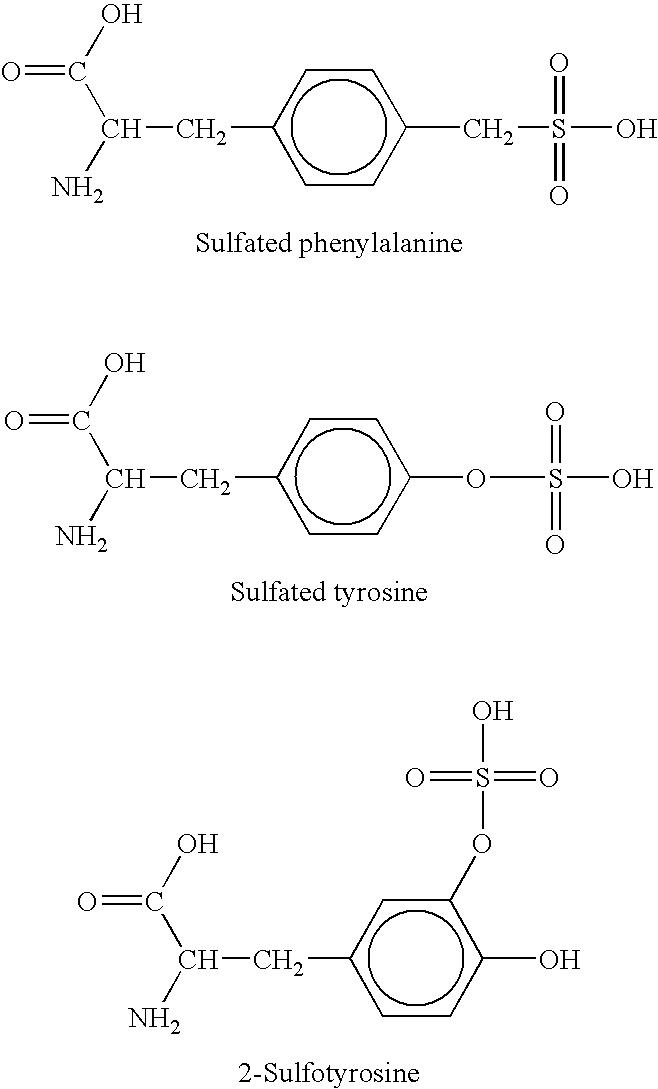
[0163] Three types of post-translational modifications have been identified in BD001: (1) glycosylation at Asn23 consisting of a fucosylated complex-type core; (2) sulfation of at least one of Tyr117, Tyr119, and Tyr121; and (3) proteolytic cleavage after Arg65. The first two modifications have been identified in native BD001 as well as recombinant BD001 expressed in Baculovirus. The third modification is seen in a fraction of the recombinant BD001 expressed in Baculovirus. To evaluate the effect of these posttranslational modifications on activity, sulfate groups were removed from recombinant BD001 using aryl sulfatase (Sigma Chemical Co.; St. Louis, Mo.) in 10 mM acetate, 120 mM NaCl (pH 5.5), recombinant BD001 proteins were deglycosylated using PNGase F (CALBIOCHEM-NOVABIOCHEM Corp.; La Jolla, Calif.) in buffer supplied by the manufacturer, and the cleaved recombinant BD001 species was separated from intact BD001 using r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com