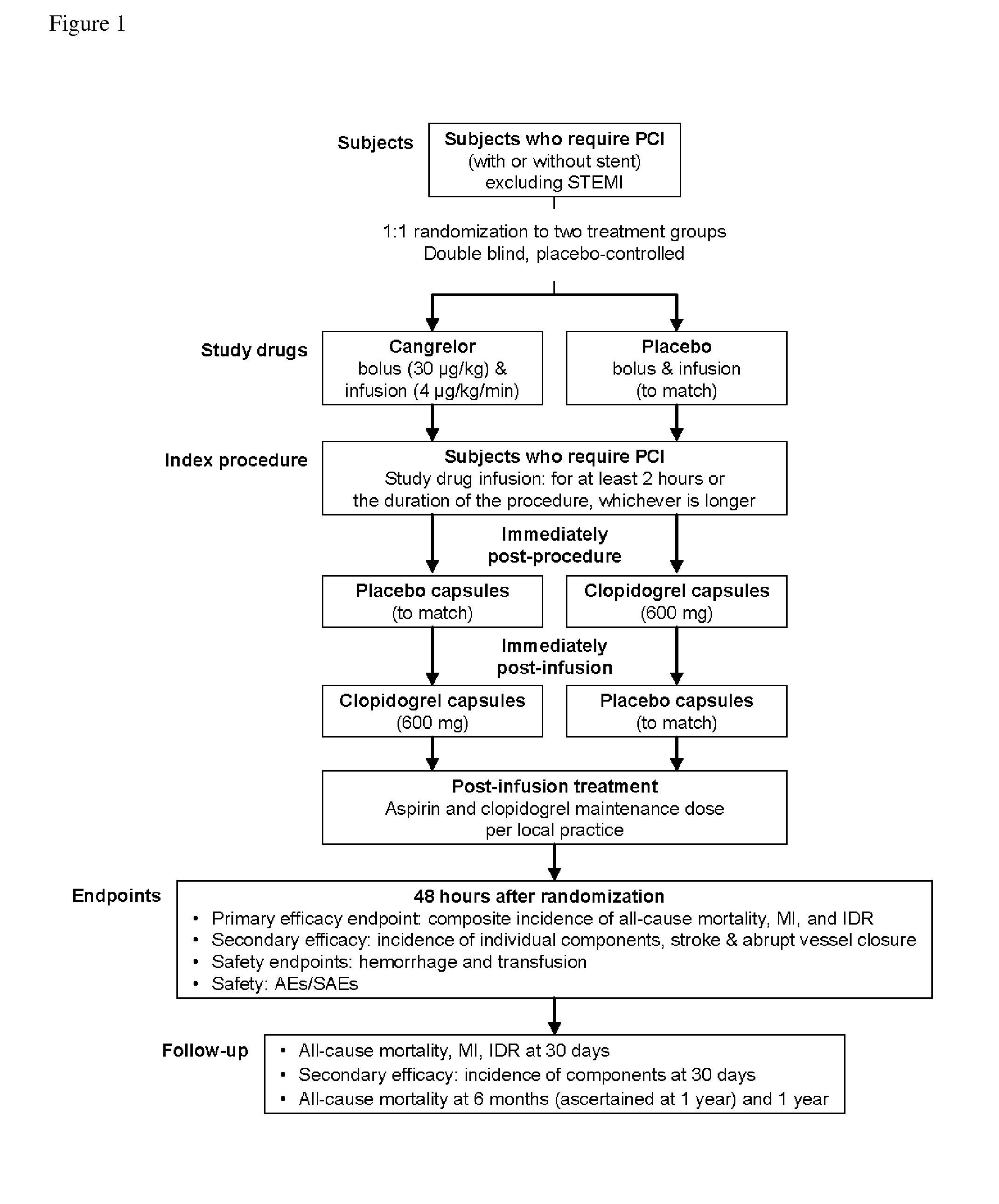
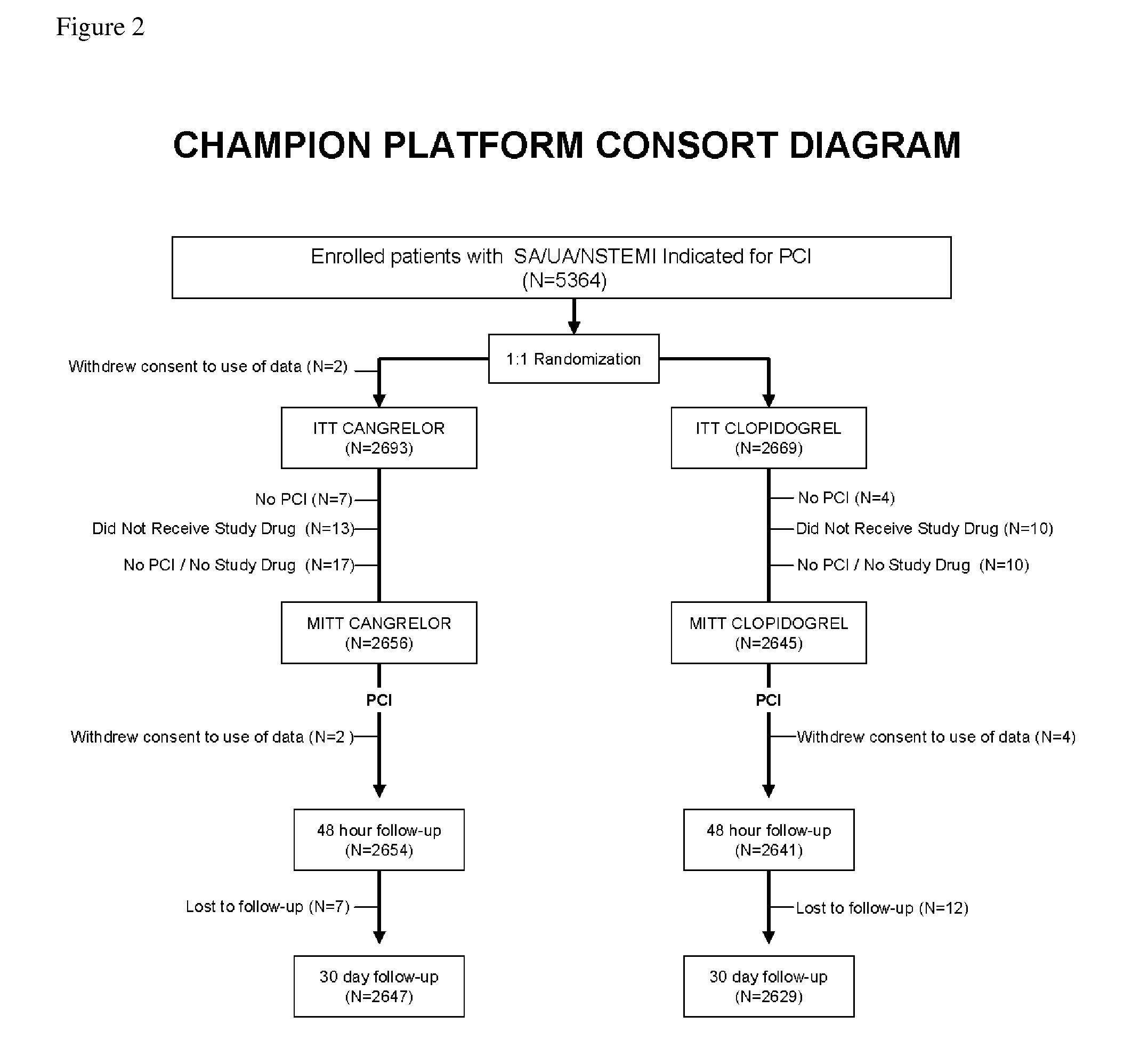
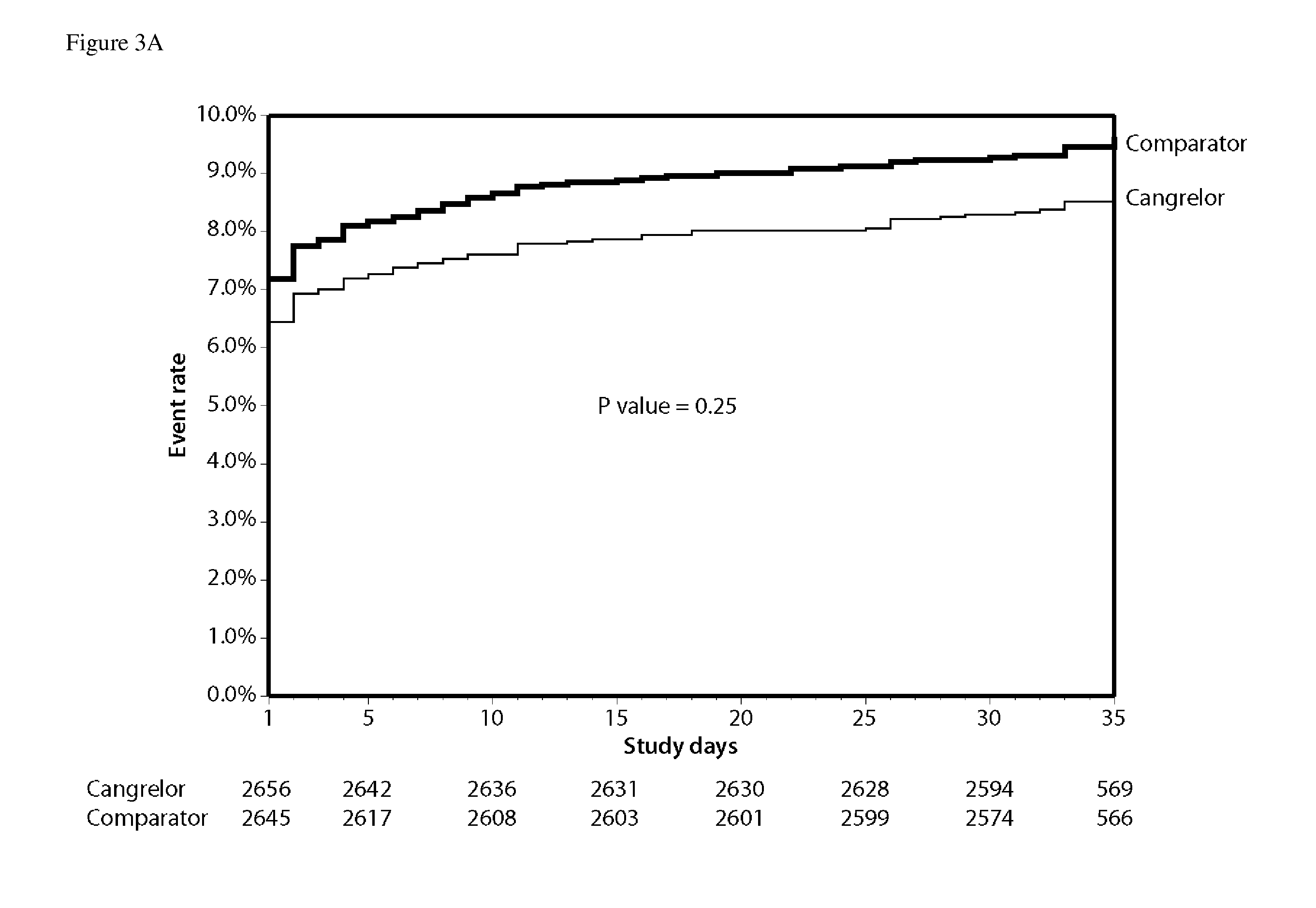
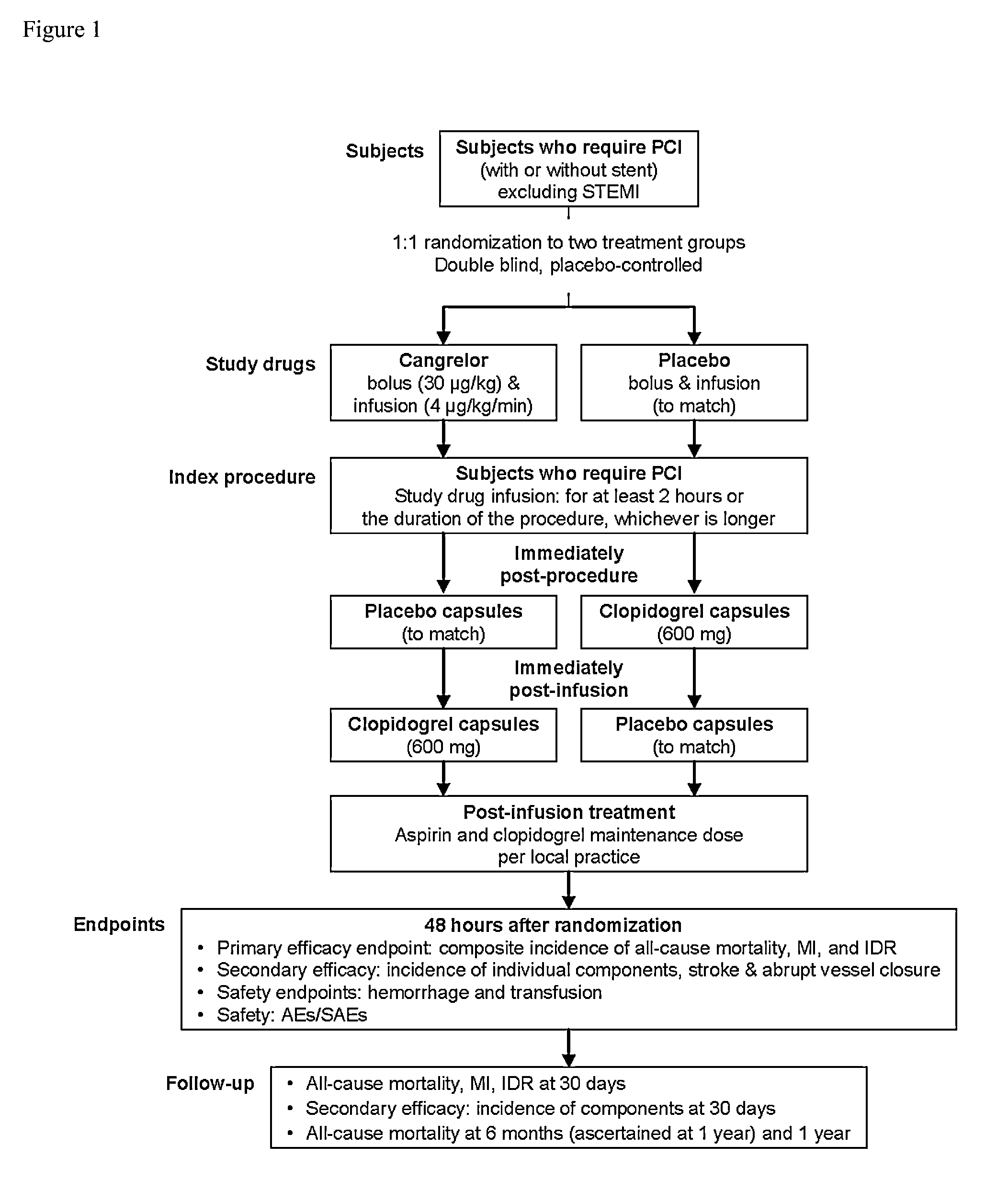
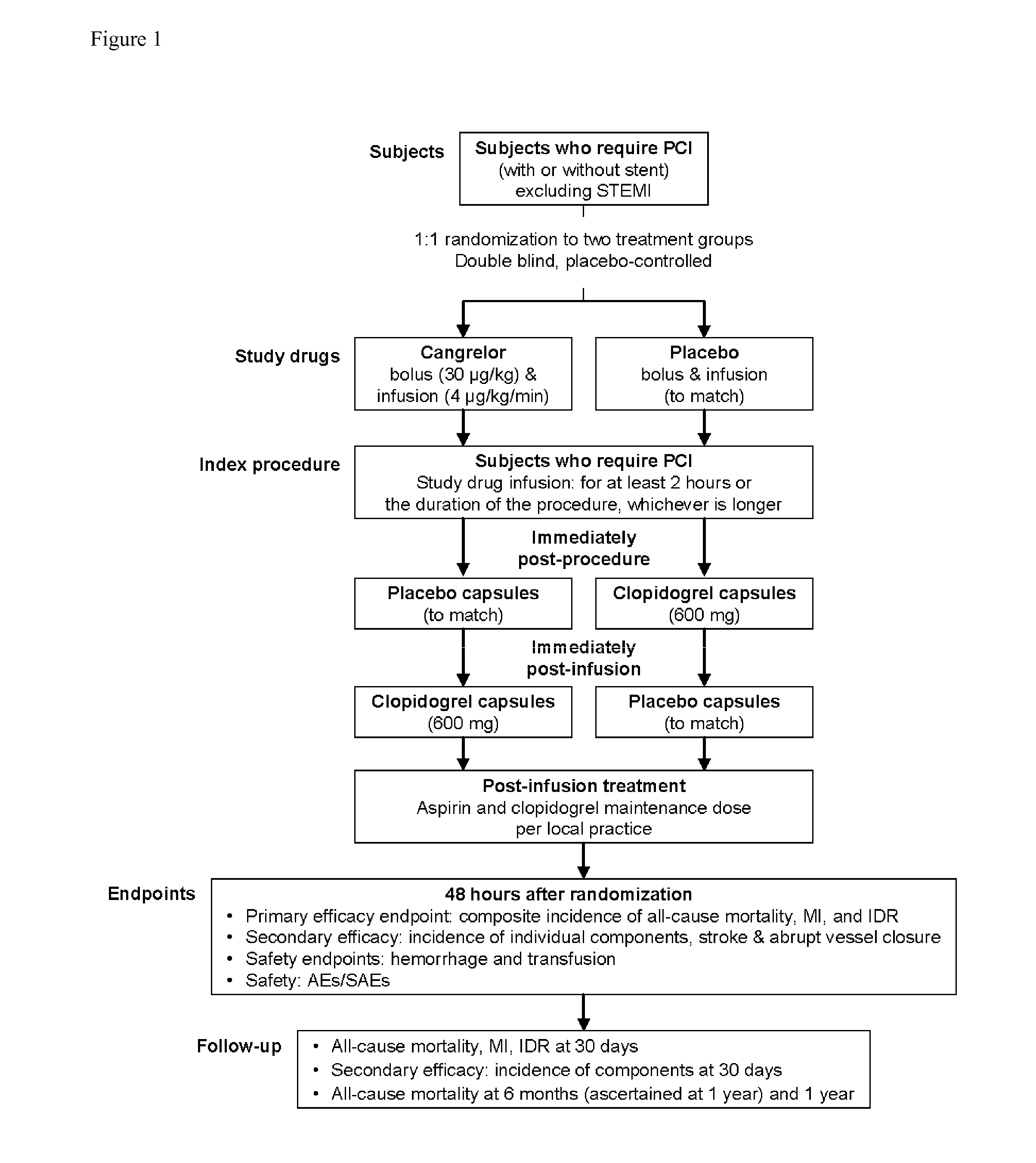
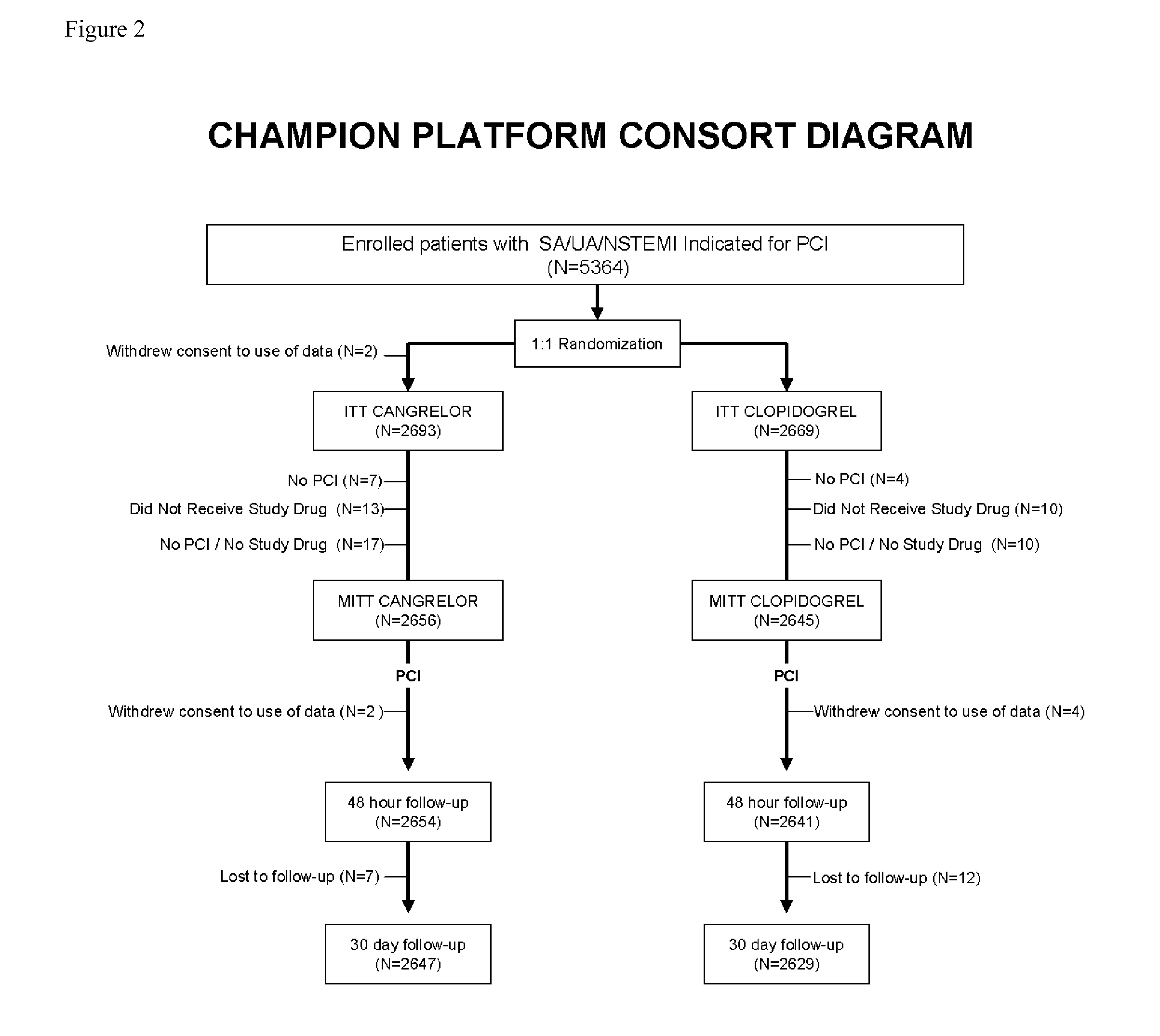
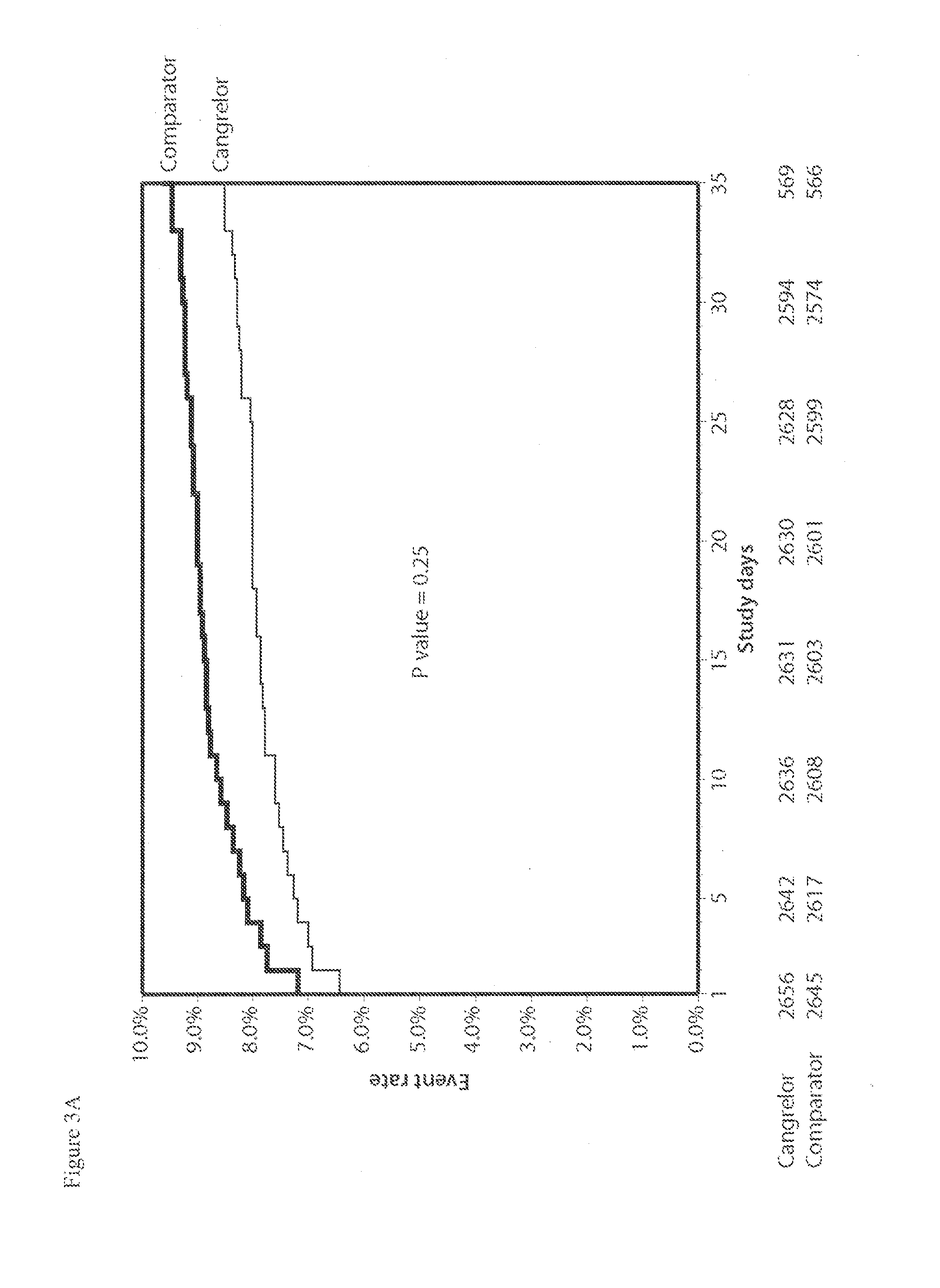
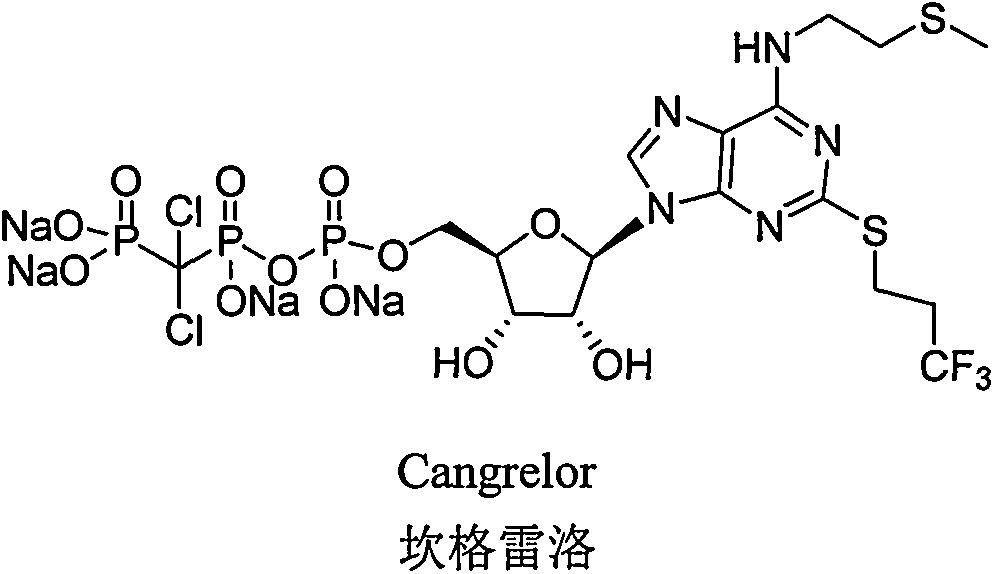
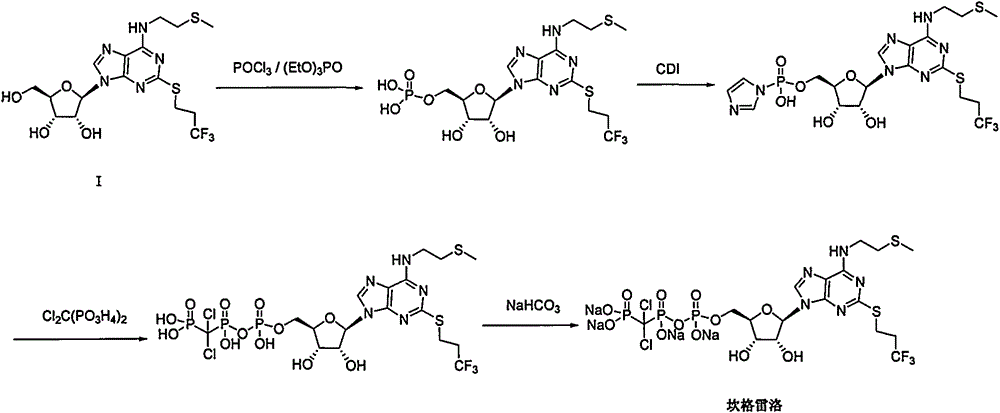
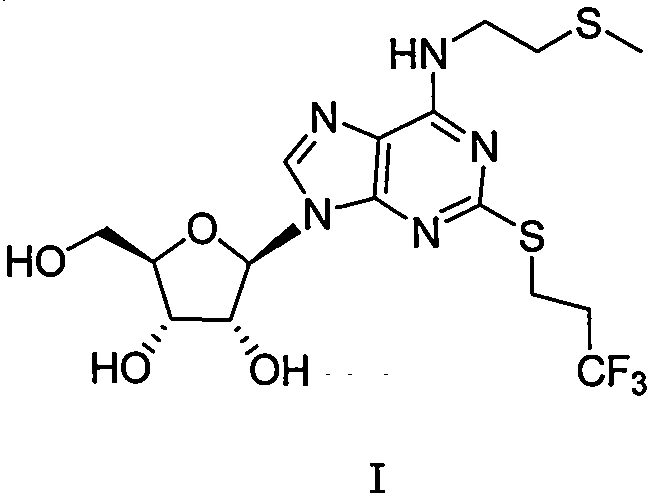
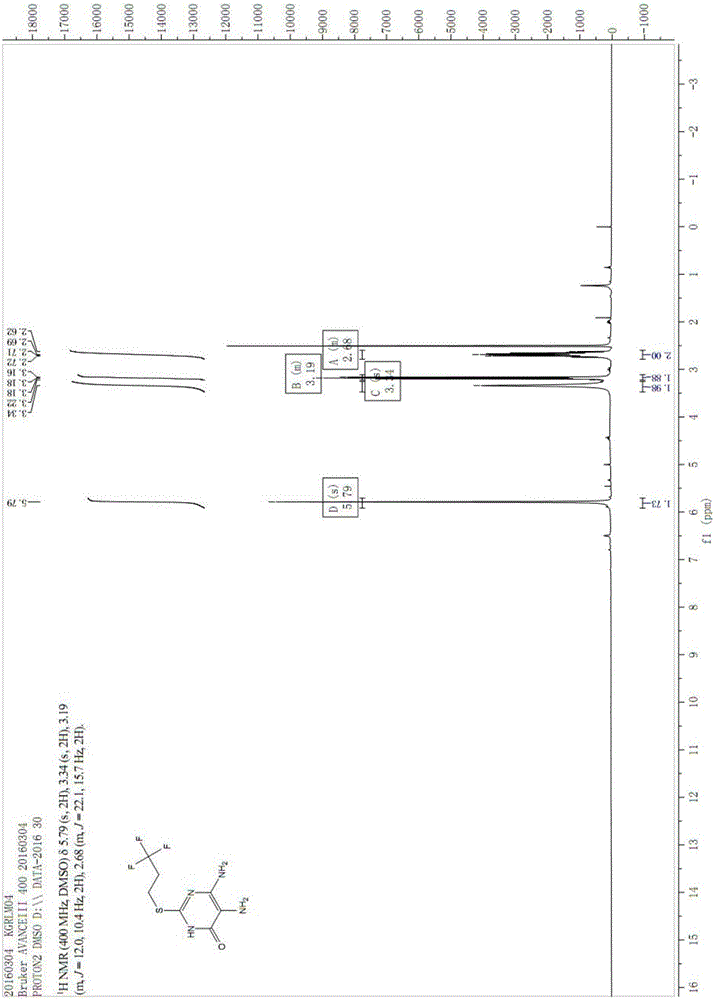
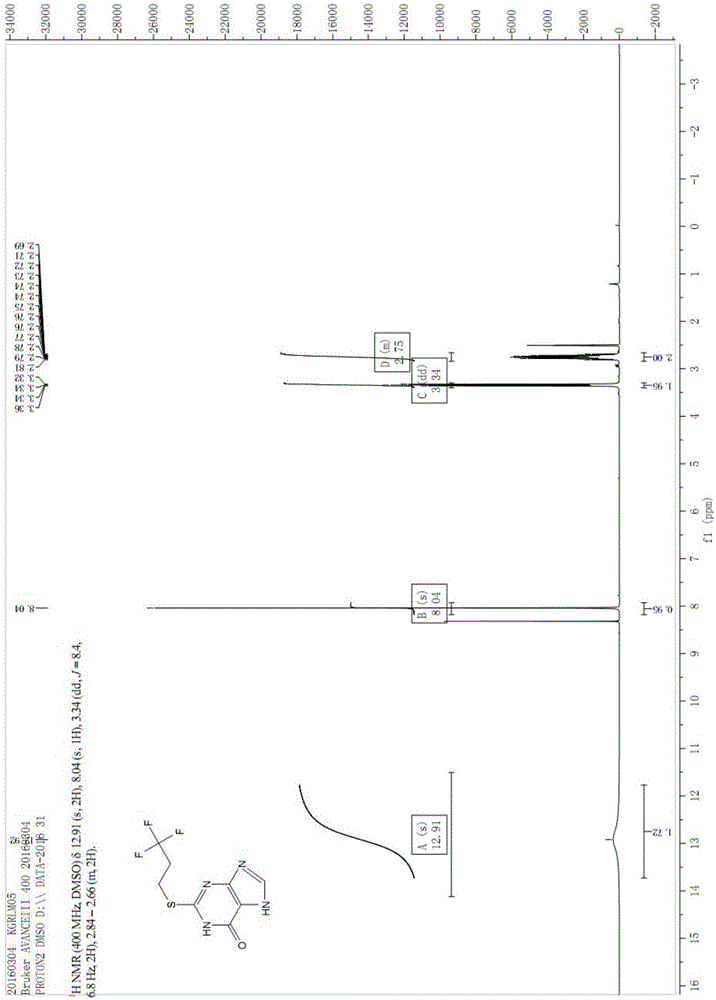
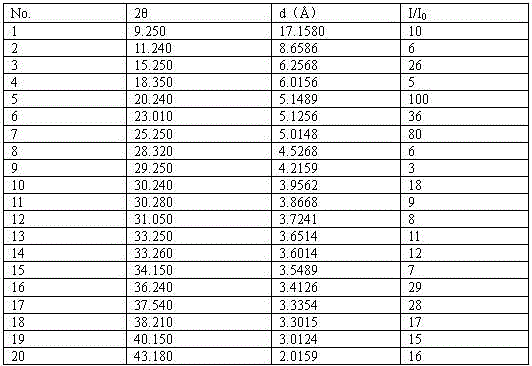
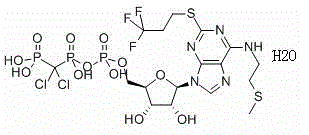
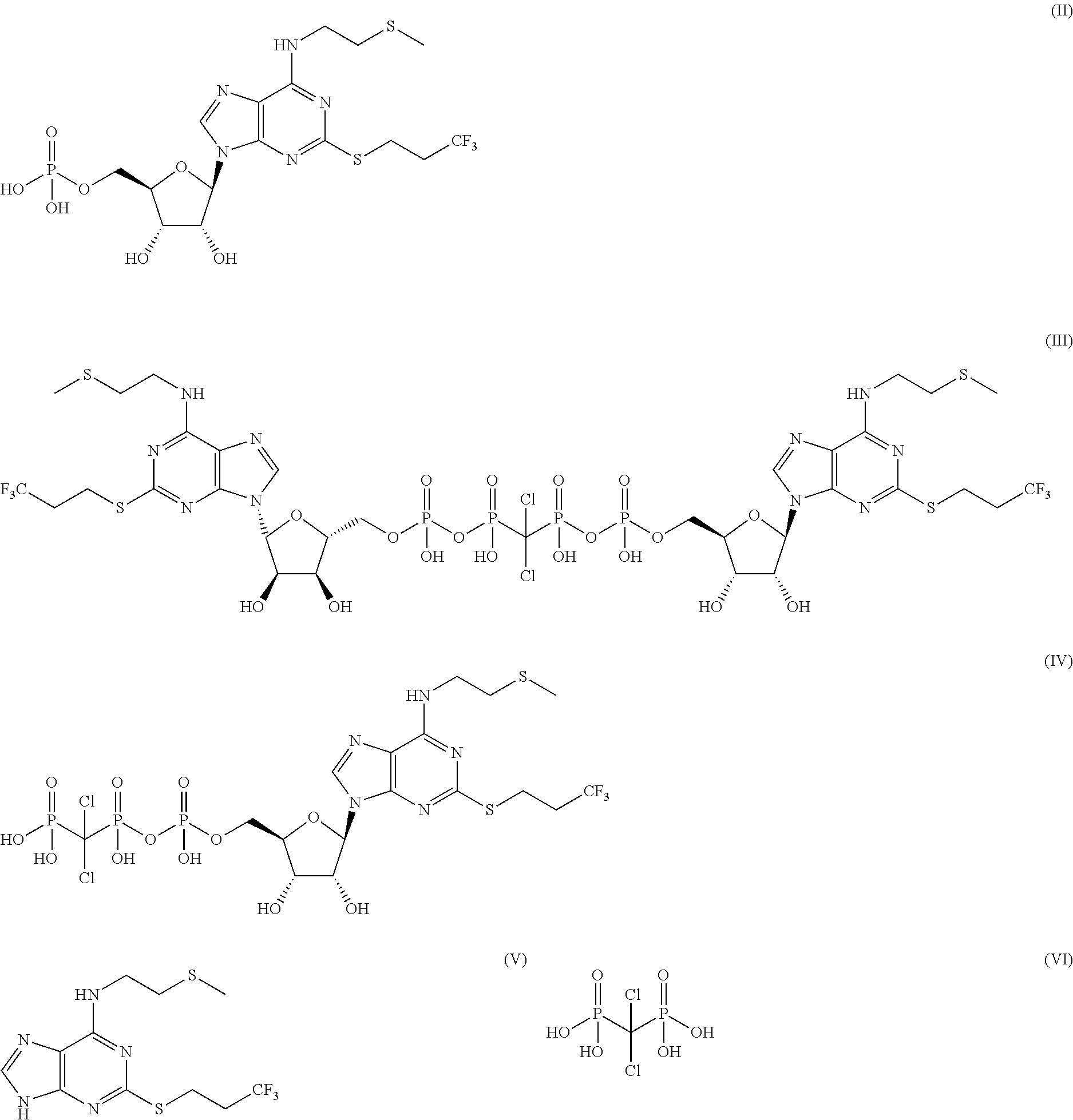Patents
Literature
55 results about "Cangrelor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
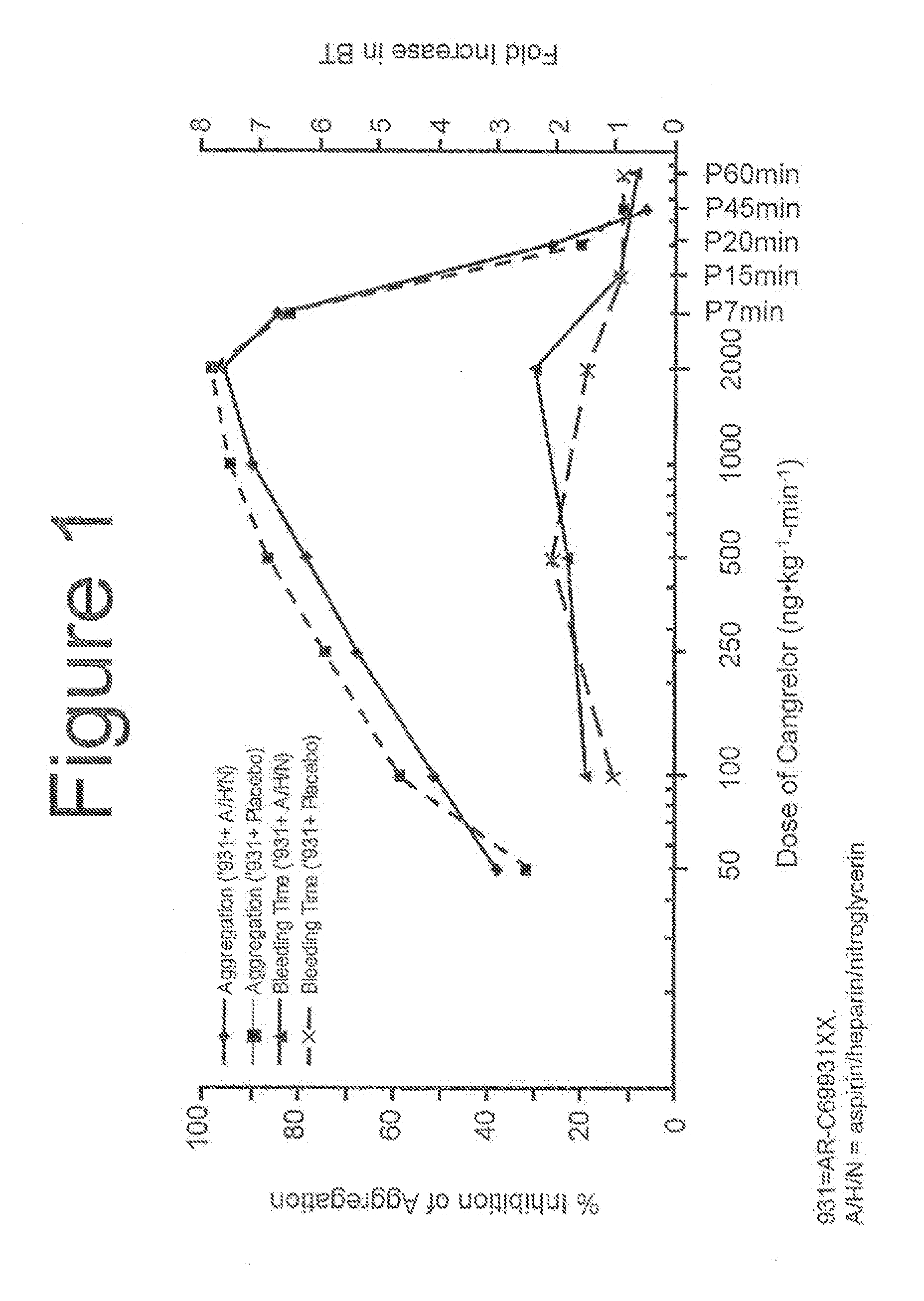
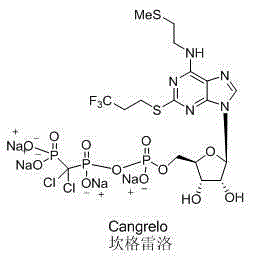
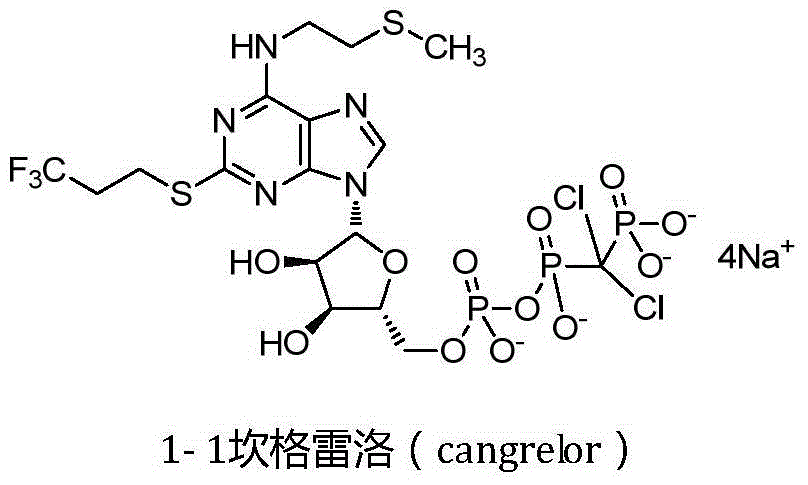
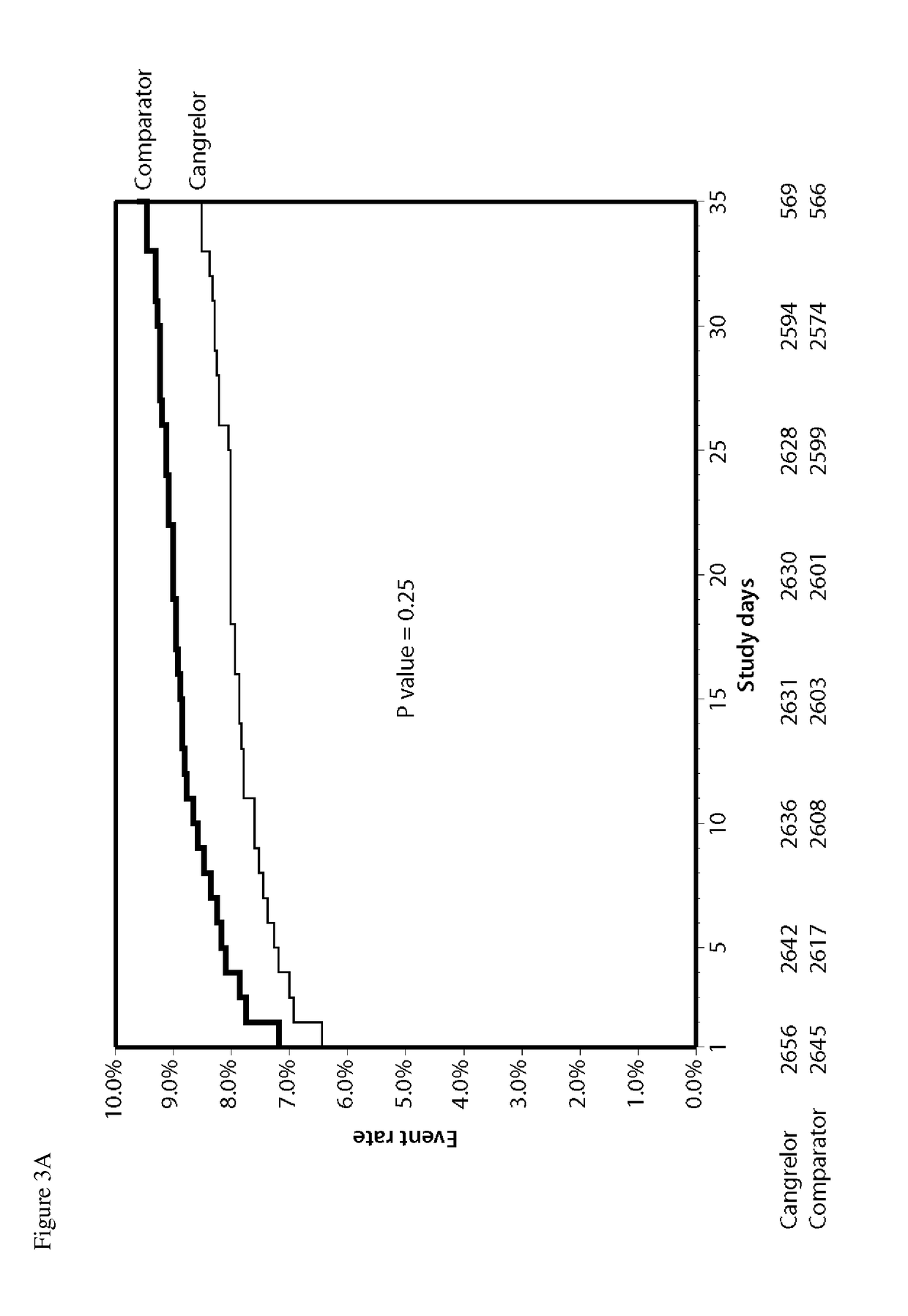
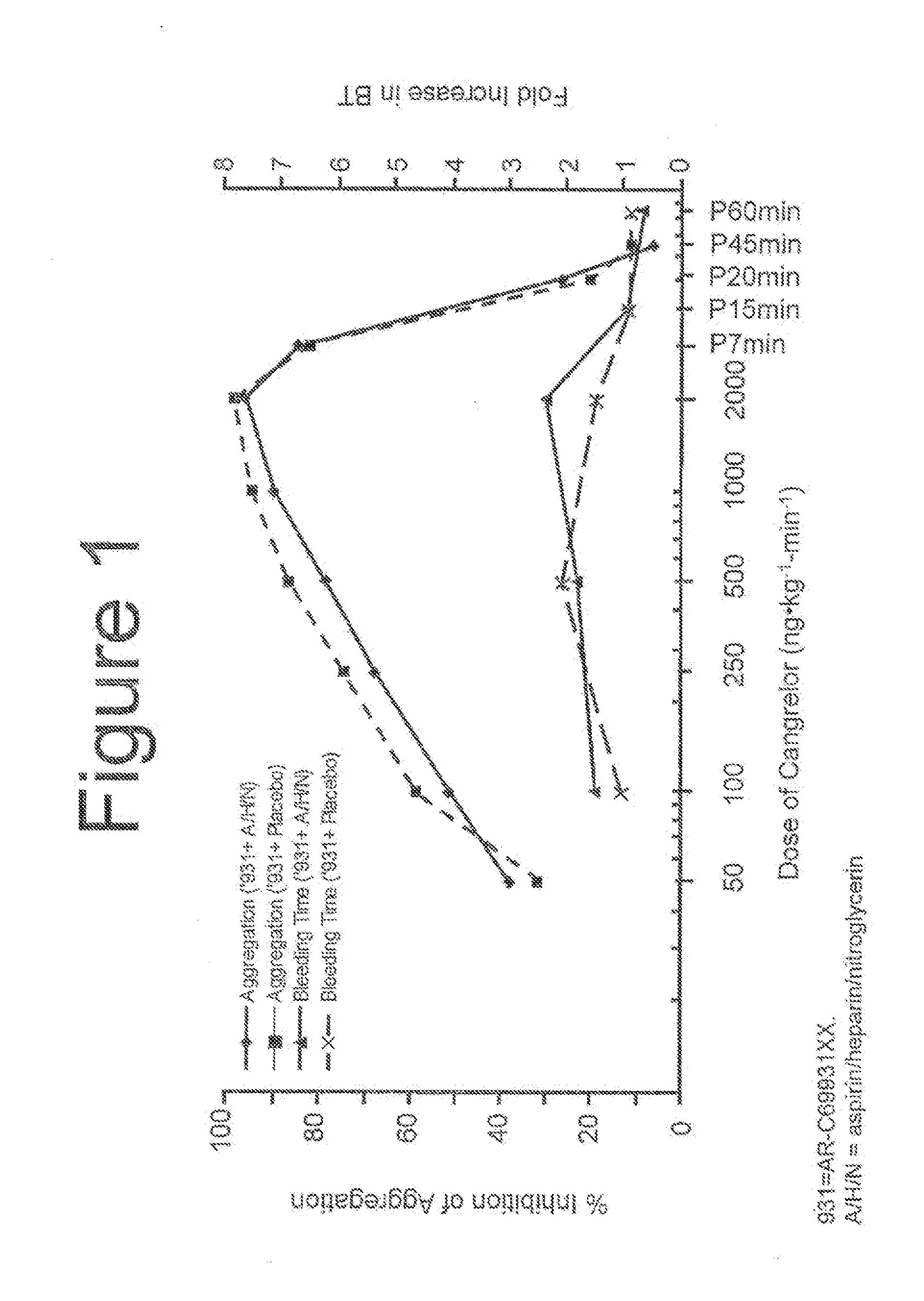
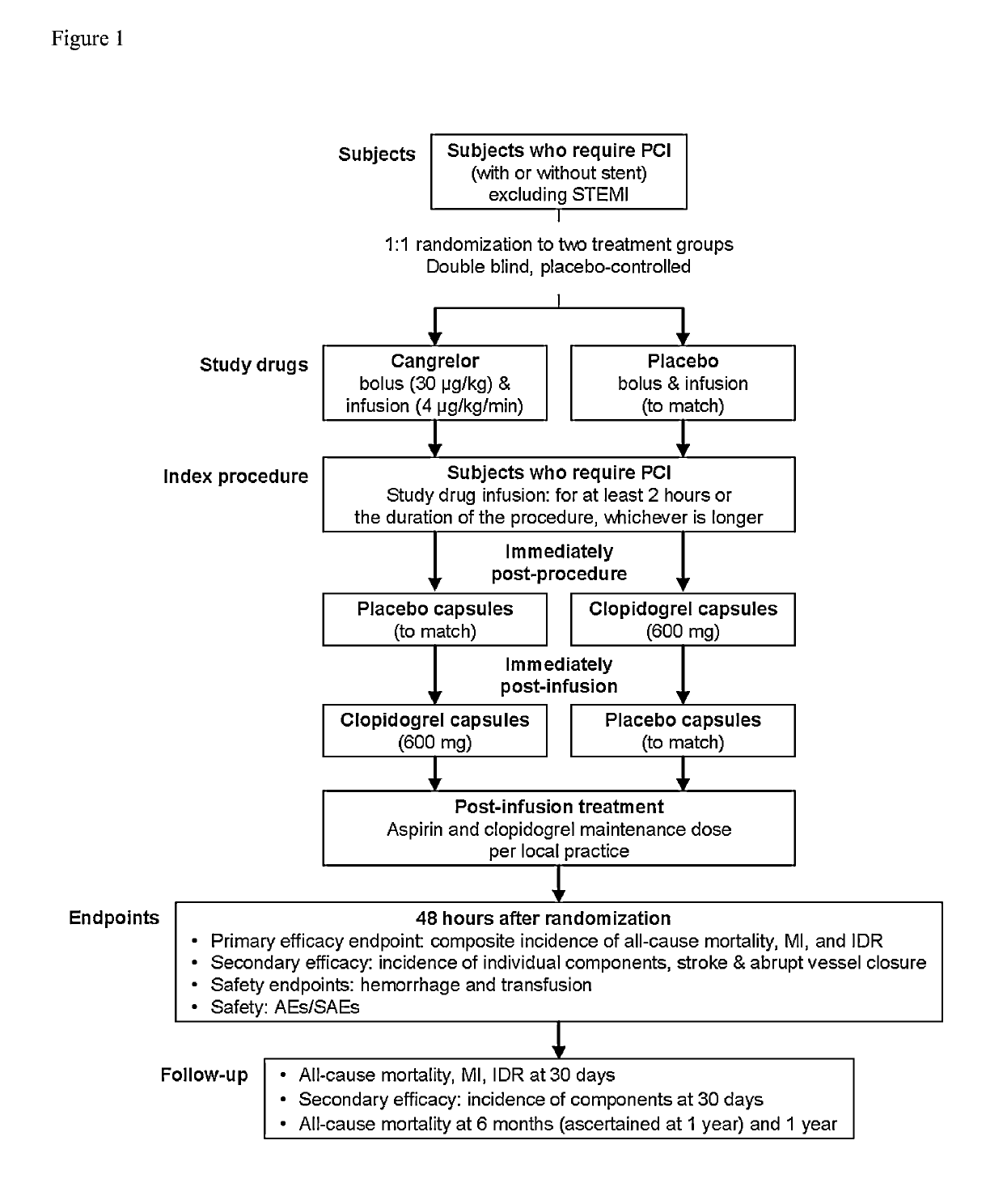
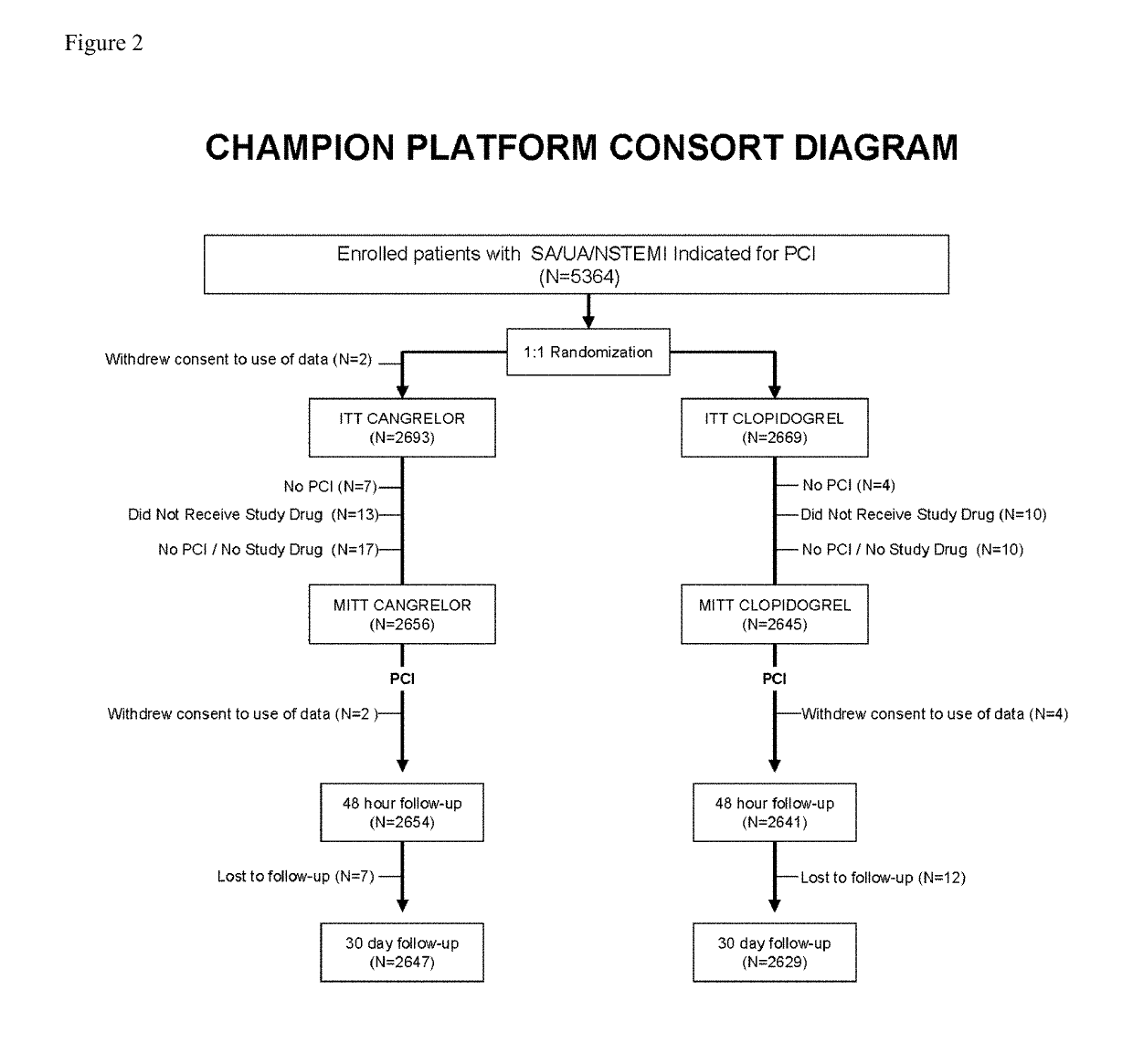
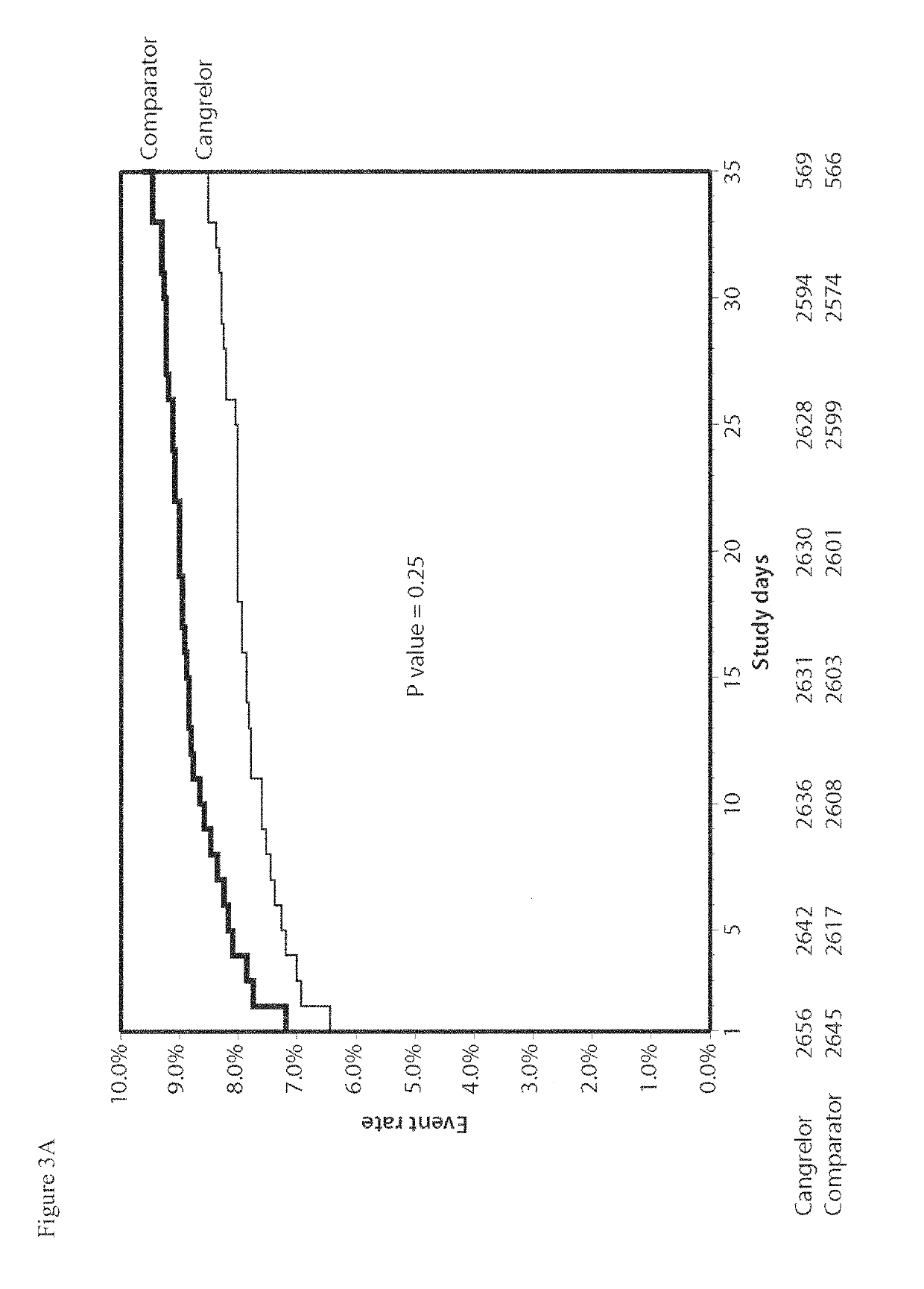
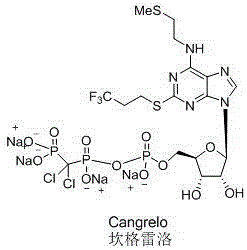
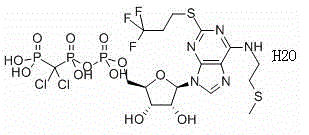
Cangrelor (trade name Kengreal in the US and Kengrexal in Europe) is a P2Y₁₂ inhibitor FDA approved as of June 2015 as an antiplatelet drug for intravenous application. Some P2Y₁₂ inhibitors are used clinically as effective inhibitors of adenosine diphosphate-mediated platelet activation and aggregation. Unlike clopidogrel (Plavix), which is a prodrug, cangrelor is an active drug not requiring metabolic conversion.
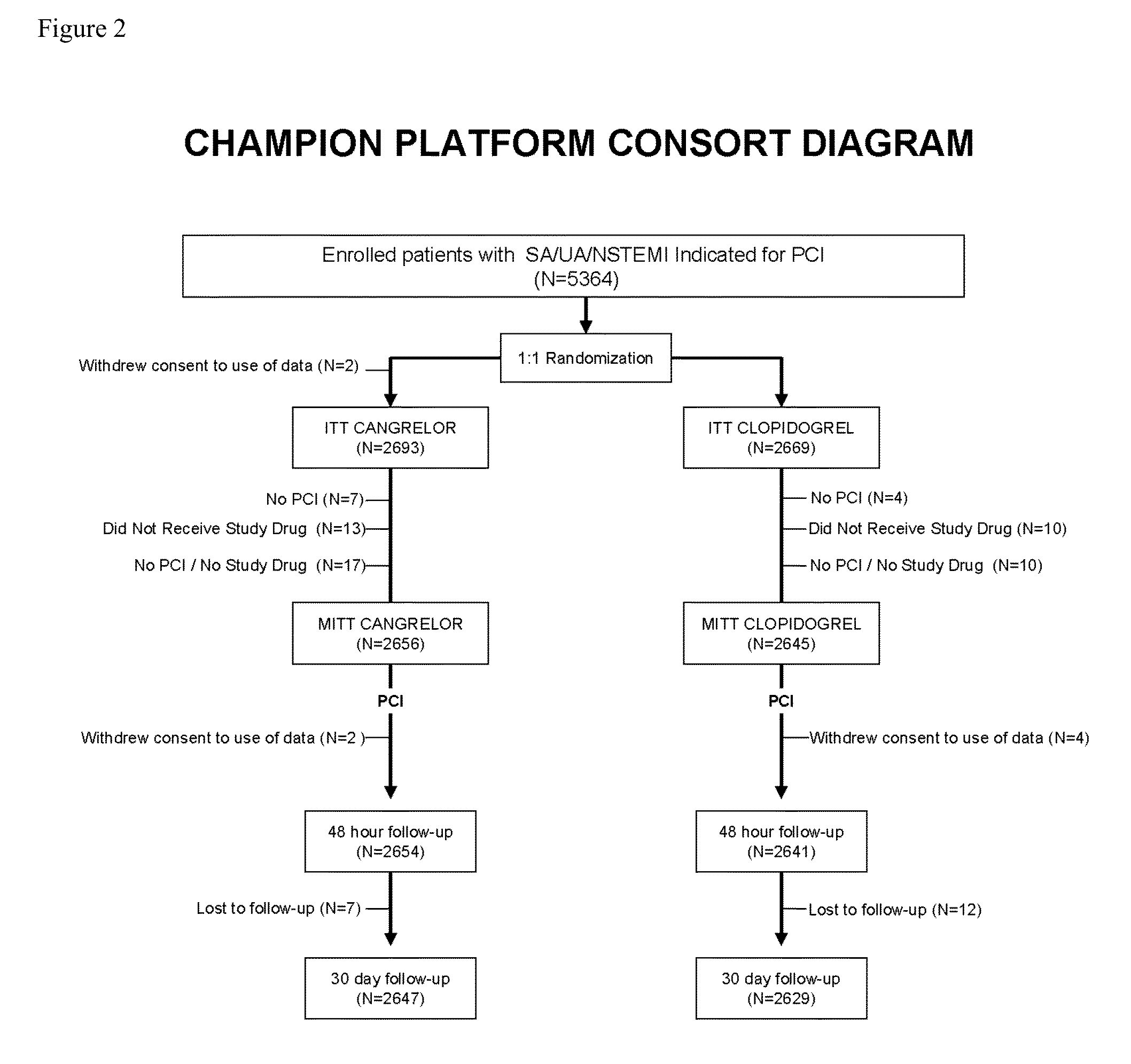
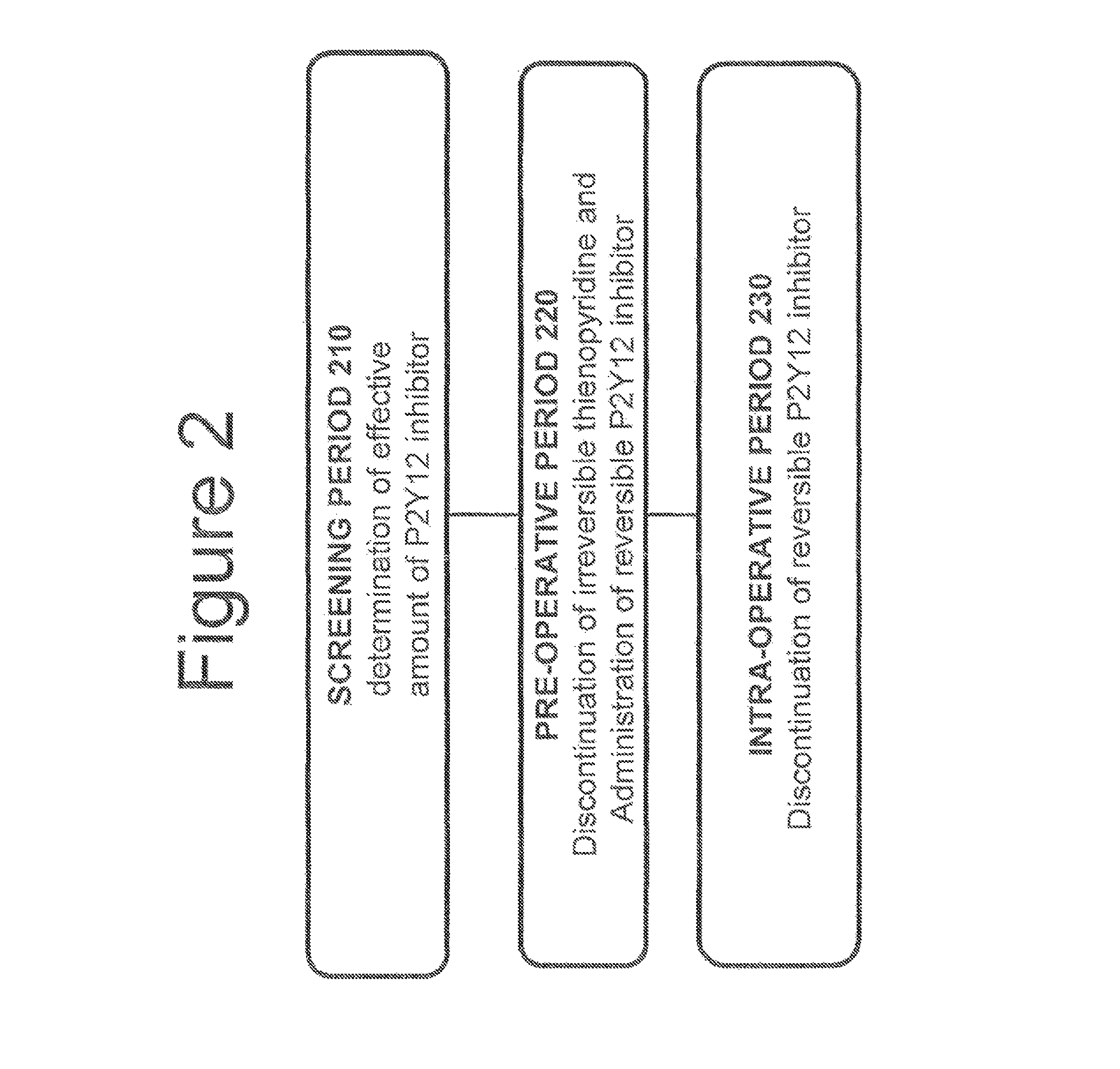
Methods of treating or preventing stent thrombosis
ActiveUS20110112030A1Reduce mortalityPrevent myocardial infarctionOrganic active ingredientsBiocideMortality rateStent implantation
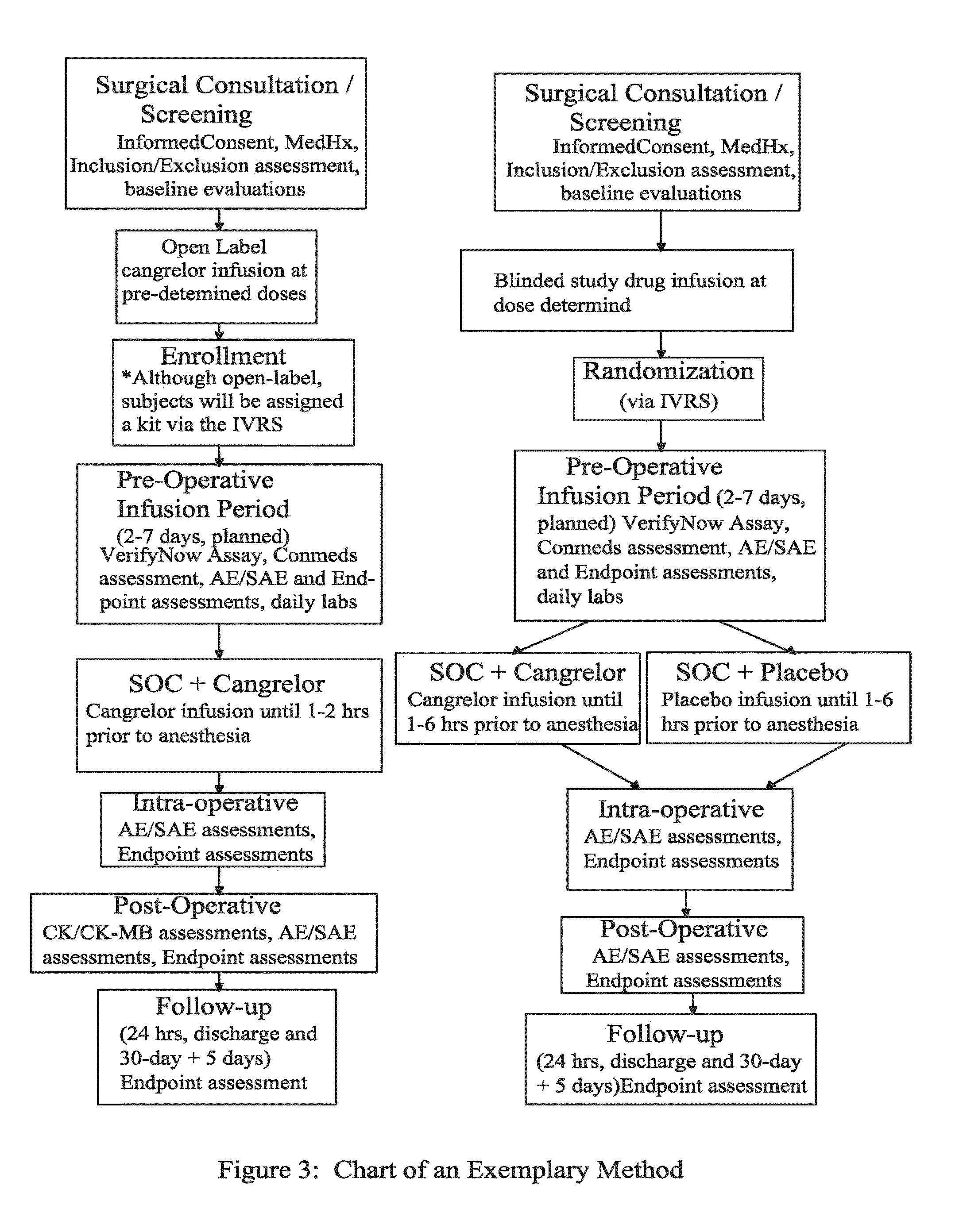
The present invention is directed to the following: methods of treating or preventing stent thrombosis using pharmaceutical compositions comprising cangrelor and optionally bivalirudin; methods of reducing mortality in a subject undergoing stent implantation using pharmaceutical compositions comprising cangrelor and optionally bivalirudin; medicaments comprising cangrelor and optionally bivalirudin useful for treating or preventing stent thrombosis, or useful for reducing mortality in a subject undergoing stent implantation; pharmaceutical compositions comprising cangrelor and bivalirudin; and methods of preparing a medicament comprising cangrelor and optionally bivalirudin useful for treating or preventing stent thrombosis, or useful for reducing mortality in a subject undergoing stent implantation.
Owner:CHIESI FARM SPA
Methods of treating, reducing the incidence of, and/or preventing ischemic events
ActiveUS8680052B1Reduce morbidityPreventing an ischemic eventOrganic active ingredientsPeptide/protein ingredientsThrombusPharmaceutical medicine
Methods of treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing percutaneous coronary intervention (PCI), comprising administering to the patient a pharmaceutical composition comprising cangrelor. The method may further comprise administering an additional therapeutic agent to the patient, the additional therapeutic agent comprising a P2Y12 inhibitor. Pharmaceutical compositions useful for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI. The pharmaceutical compositions comprise cangrelor. Methods of preparing a pharmaceutical composition for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI, comprising admixing cangrelor with one or more pharmaceutically acceptable excipients. An ischemic event may include stent thrombosis, myocardial infarction, ischemia-driven revascularization, and mortality.
Owner:CHIESI FARM SPA
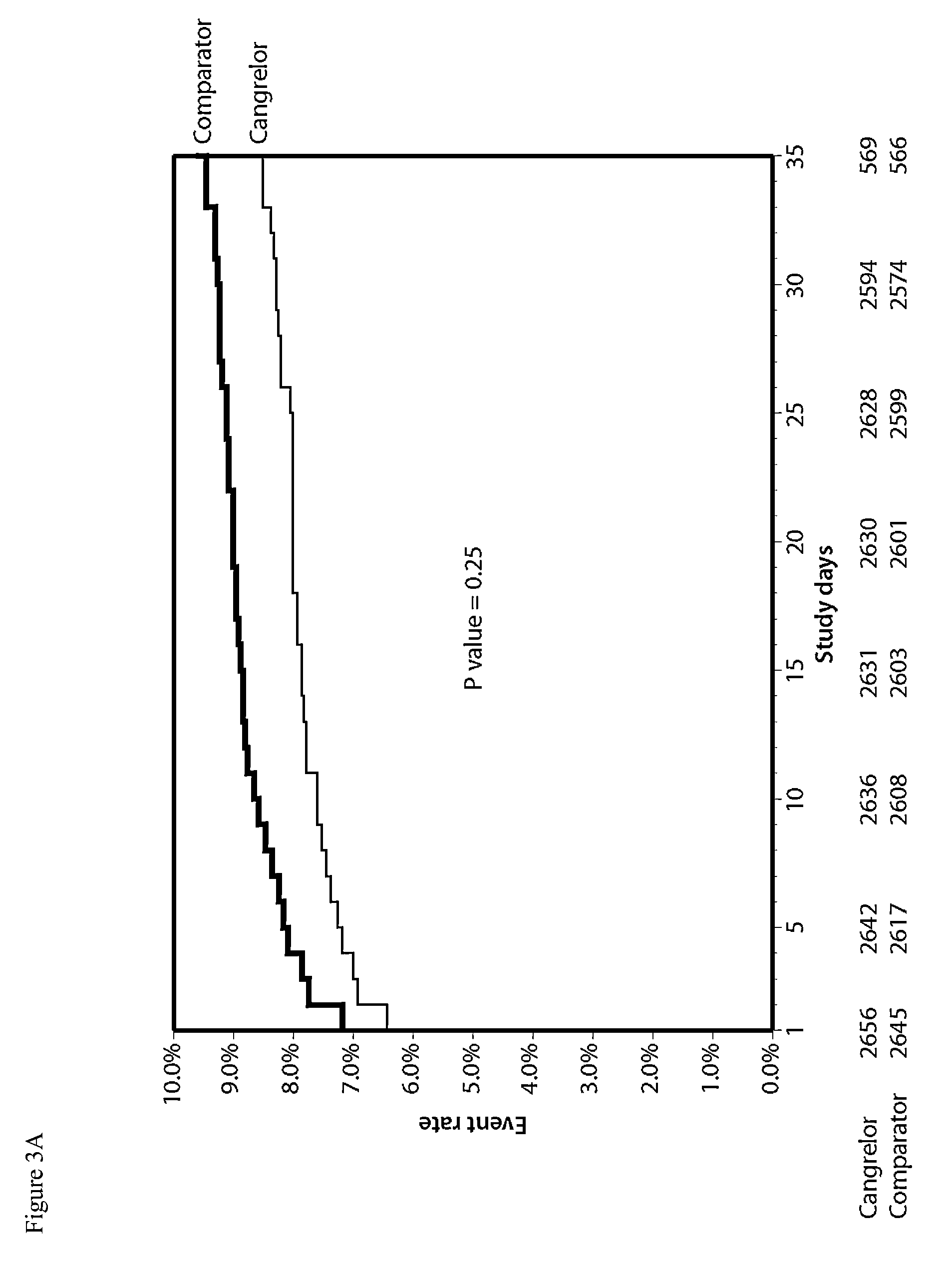
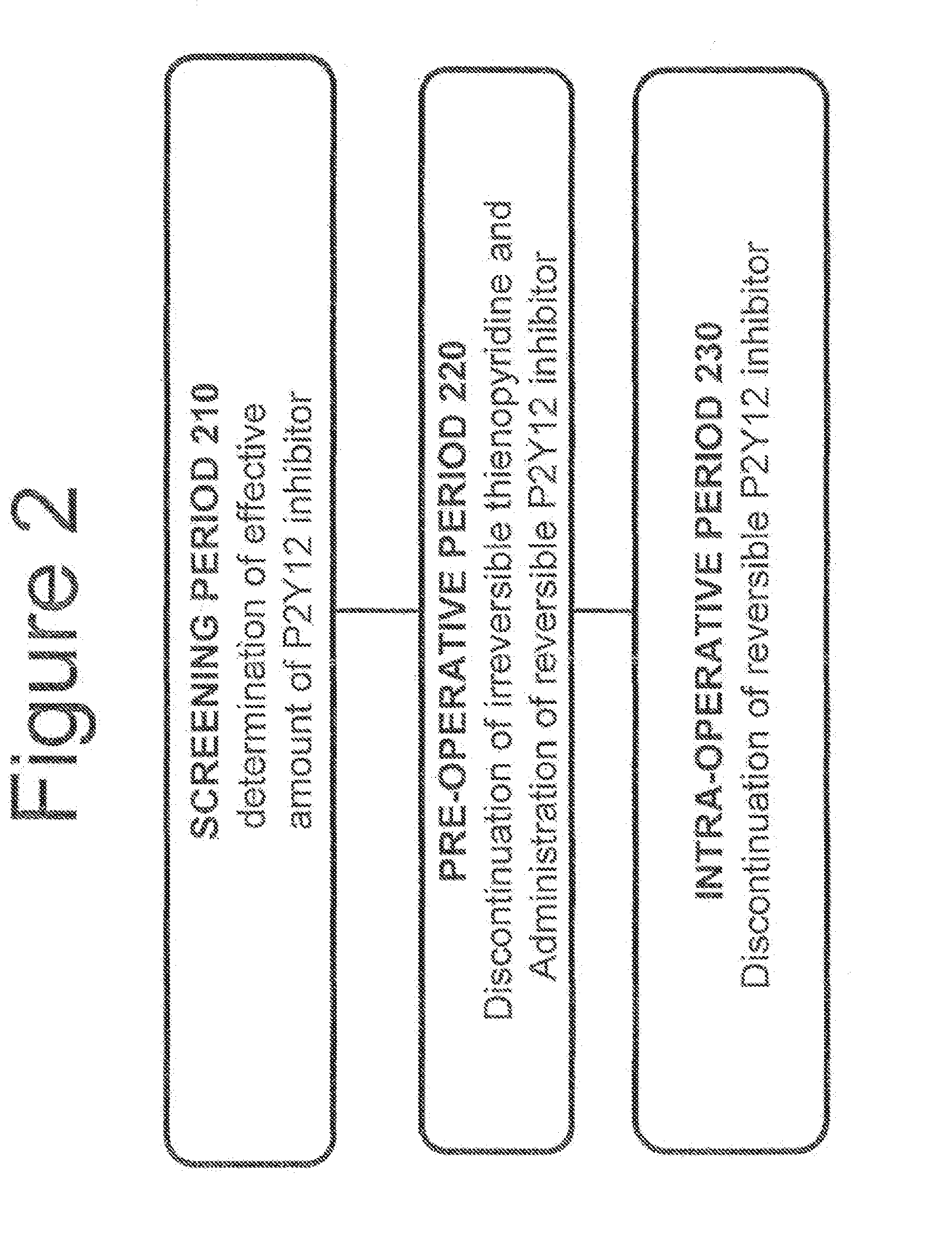
Maintenance of platelet inhibition during antiplatelet therapy
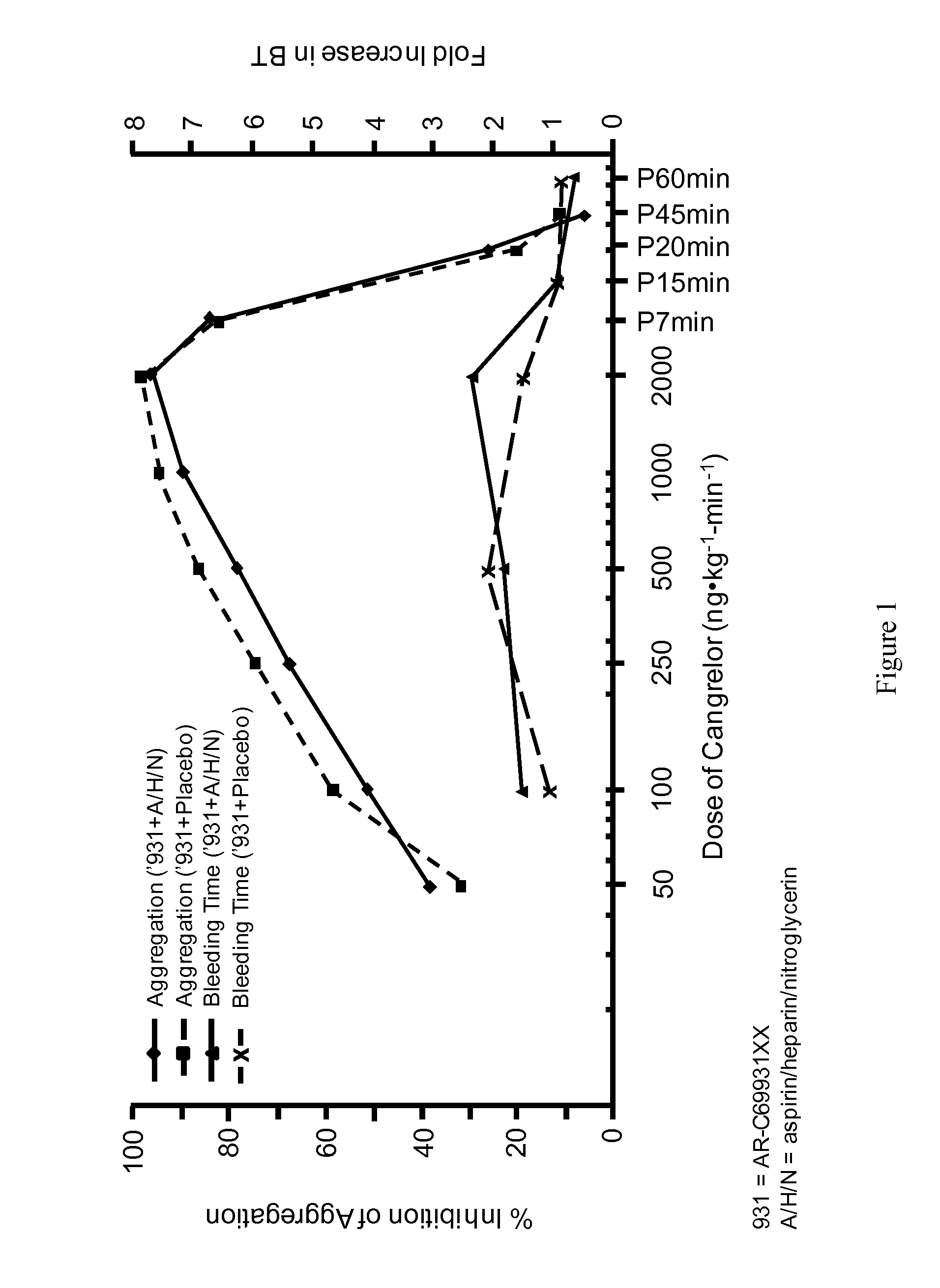
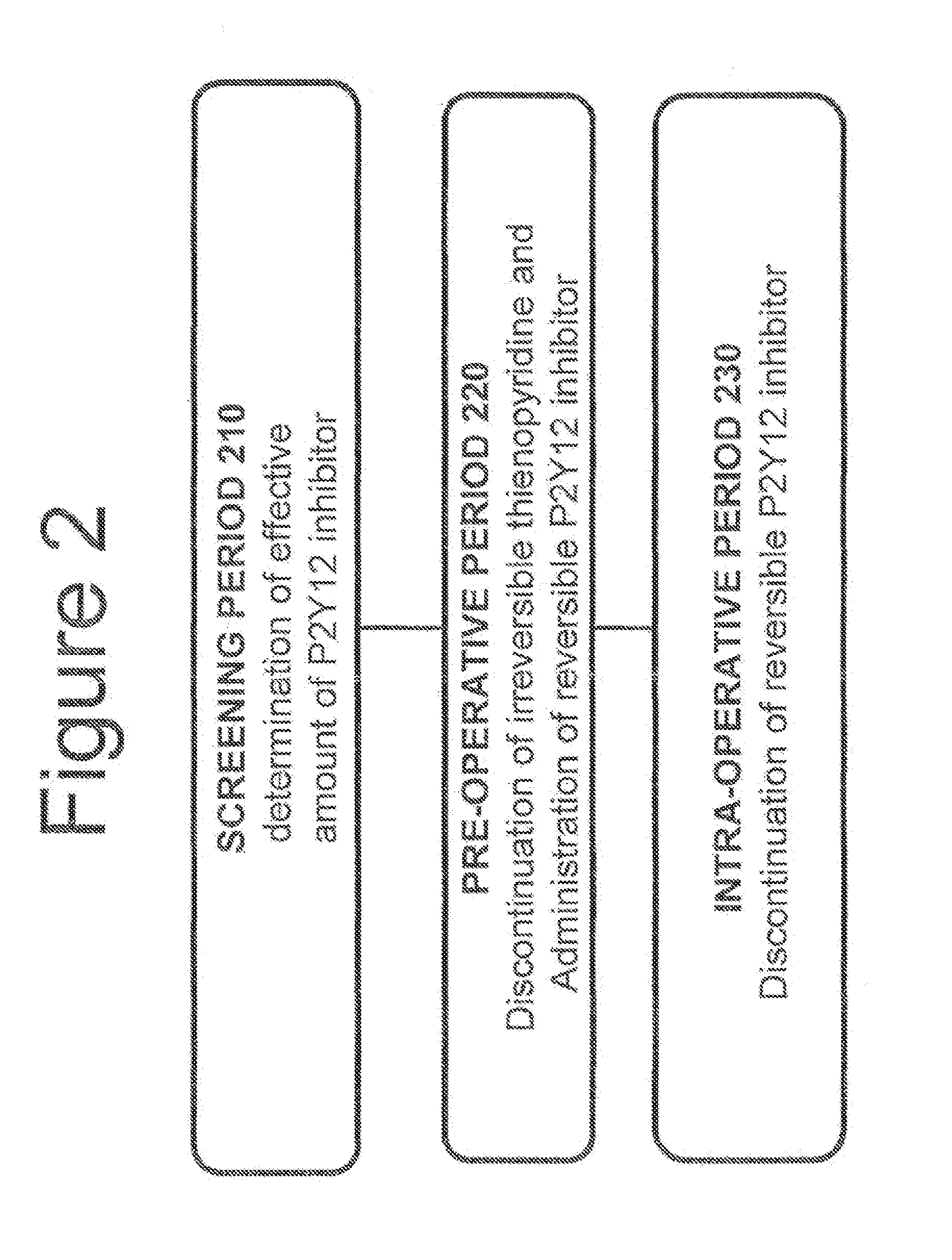
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:THE MEDICINES
Methods of Treating, Reducing the Incidence of, and/or Preventing Ischemic Events
ActiveUS20130190265A1Reduce morbidityPreventing an ischemic eventBiocideInorganic non-active ingredientsThrombusCangrelor
Methods of treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing percutaneous coronary intervention (PCI), comprising administering to the patient a pharmaceutical composition comprising cangrelor. The method may further comprise administering an additional therapeutic agent to the patient, the additional therapeutic agent comprising a P2Y12 inhibitor. Pharmaceutical compositions useful for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI. The pharmaceutical compositions comprise cangrelor. Methods of preparing a pharmaceutical composition for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI, comprising admixing cangrelor with one or more pharmaceutically acceptable excipients. An ischemic event may include stent thrombosis, myocardial infarction, ischemia-driven revascularization, and mortality.
Owner:CHIESI FARM SPA
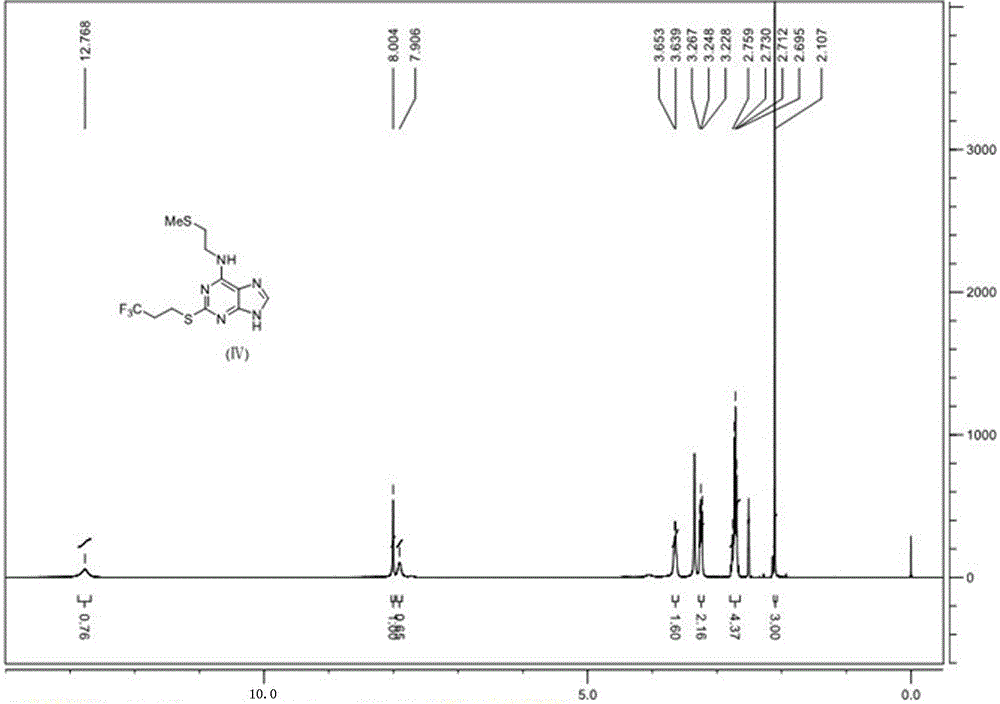
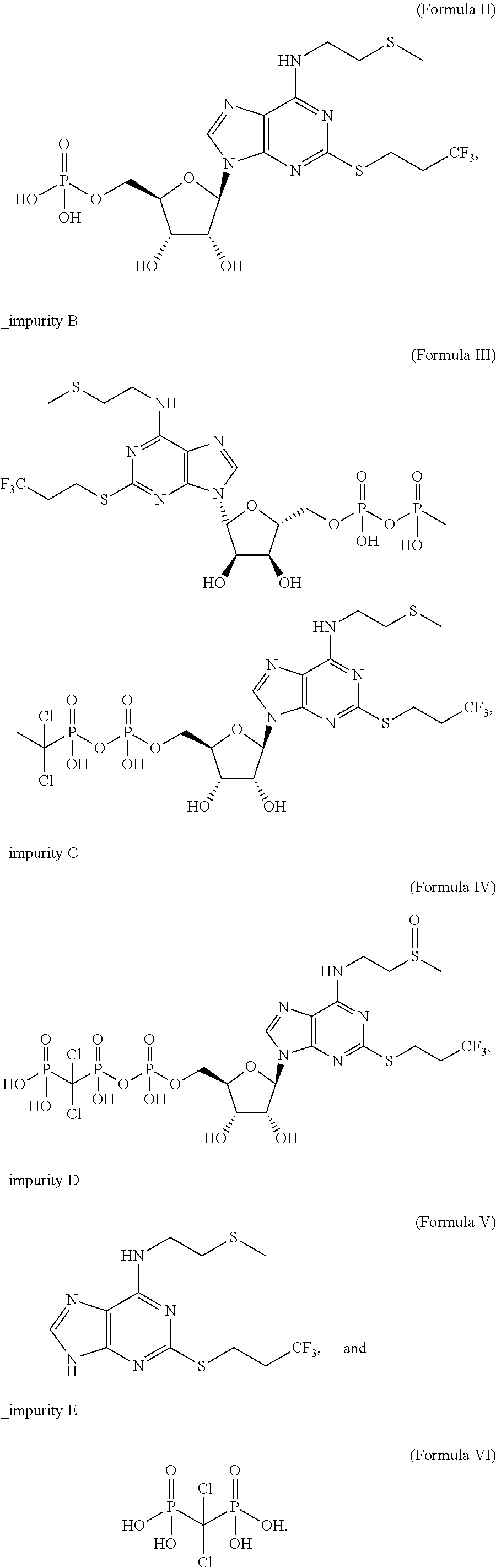
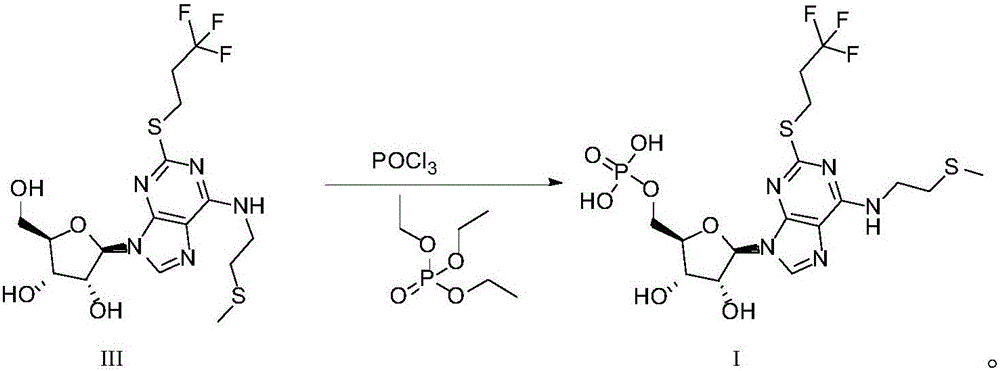
6-N-(2-(methylthio)ethyl)-2-((3,3,3-trifluoropropyl)sulfo)-9H-purine, and preparation method and application thereof
ActiveCN105061431AWide variety of sourcesMild reaction conditionsSugar derivativesSugar derivatives preparationThio-Purine
The invention relates to a novel compound 6-N-(2-(methylthio)ethyl)-2-((3,3,3-trifluoropropyl)sulfo)-9H-purine and a preparation method for the novel compound and further provides application of the novel compound as an intermediate in synthesis of cangrelor directed at the limitation of conventional synthetic methods for cangrelor. The compound is used as the intermediate for synthesis of cangrelor; and such a route has the advantages of wide sources of raw materials, mild reaction conditions, simple after-treatment, environment friendliness and improved product yield and provides a novel synthetic method for laboratory and industrialization production of cangrelor.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Maintenance of platelet inhibition during antiplatelet therapy
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:CHIESI FARM SPA
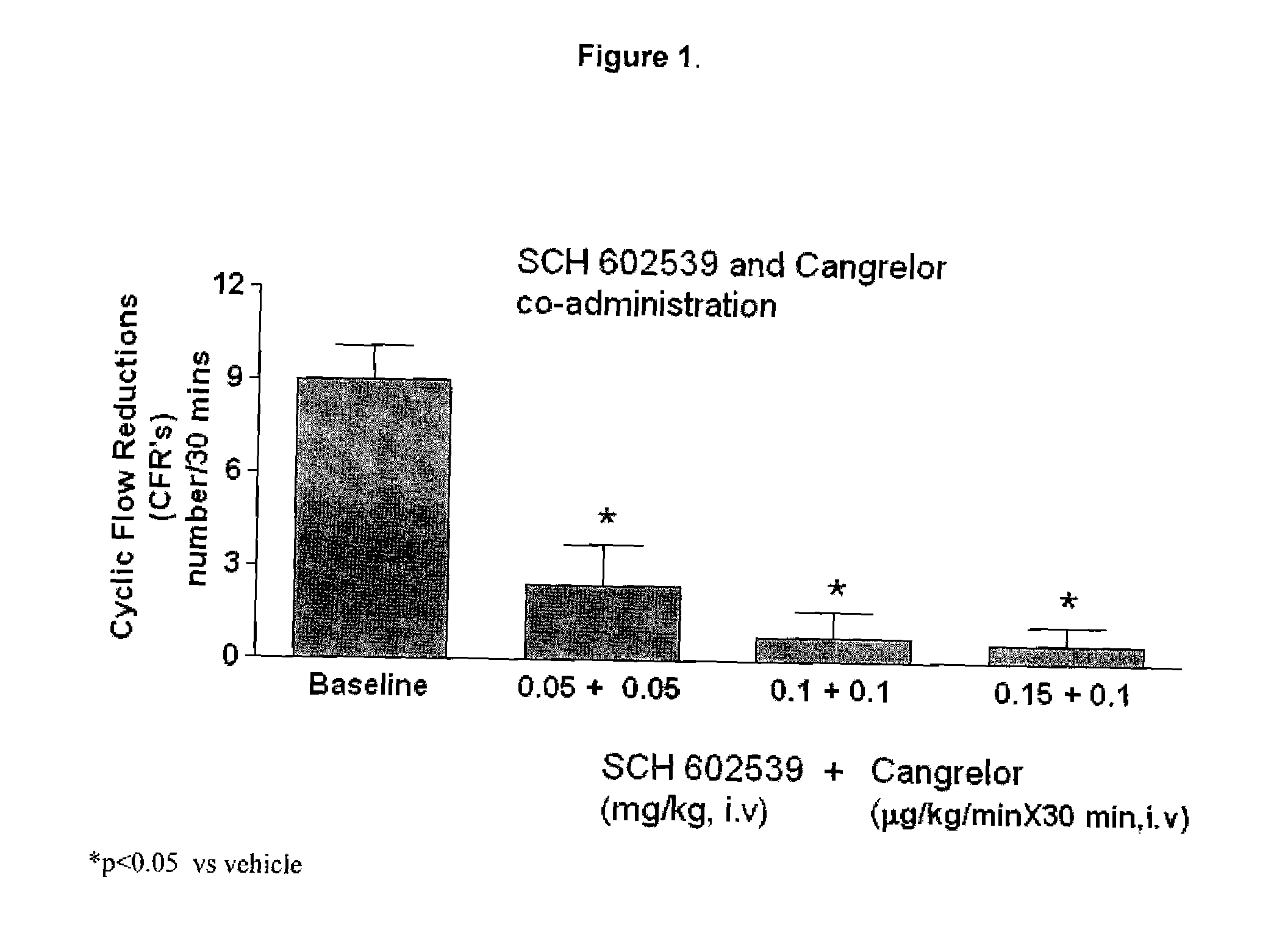
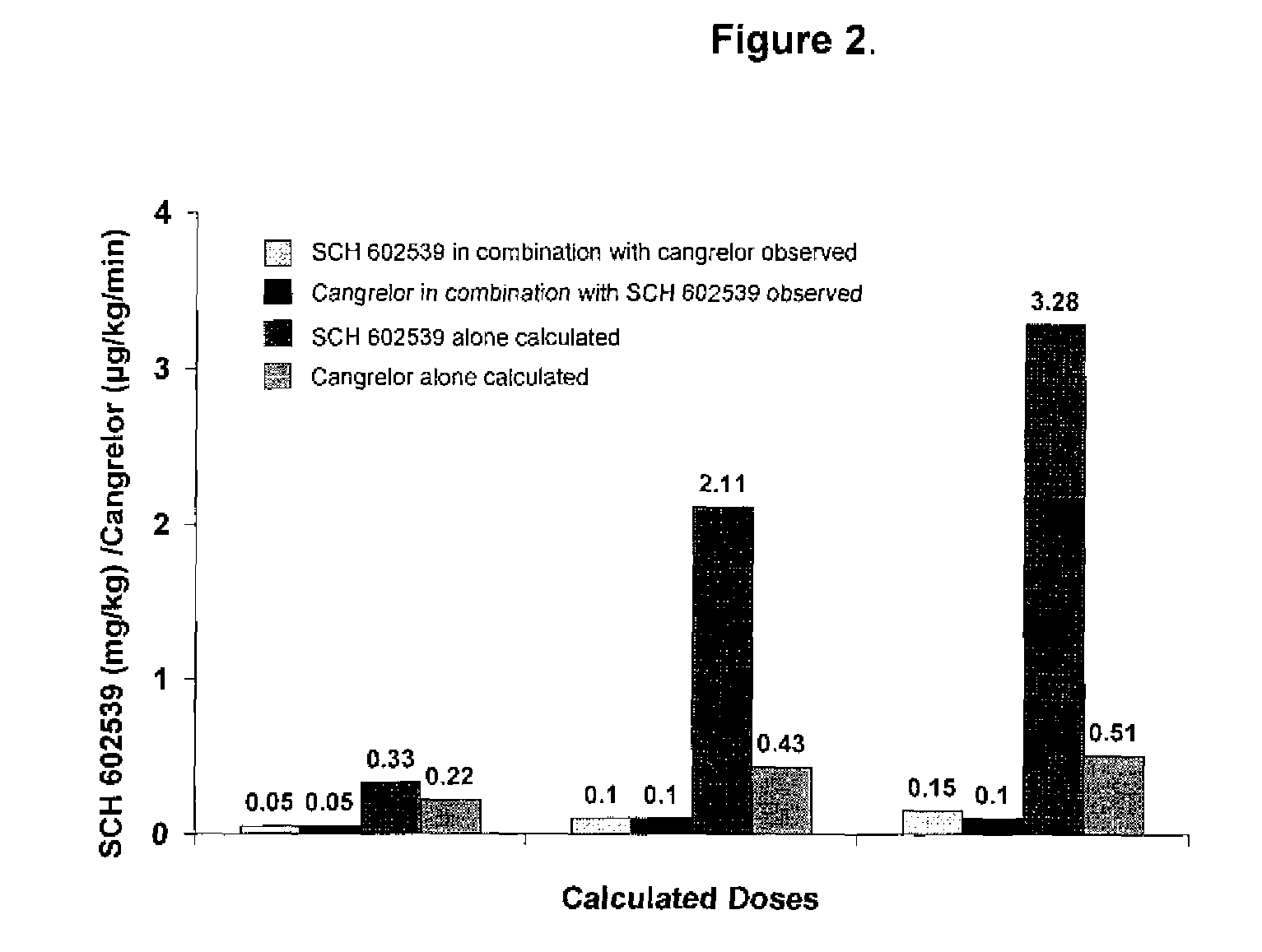
"The Use of a PAR-1 Antagonist in Combination with a P2Y12 ADP Receptor Antagonist for Inhibition of Thrombosis"
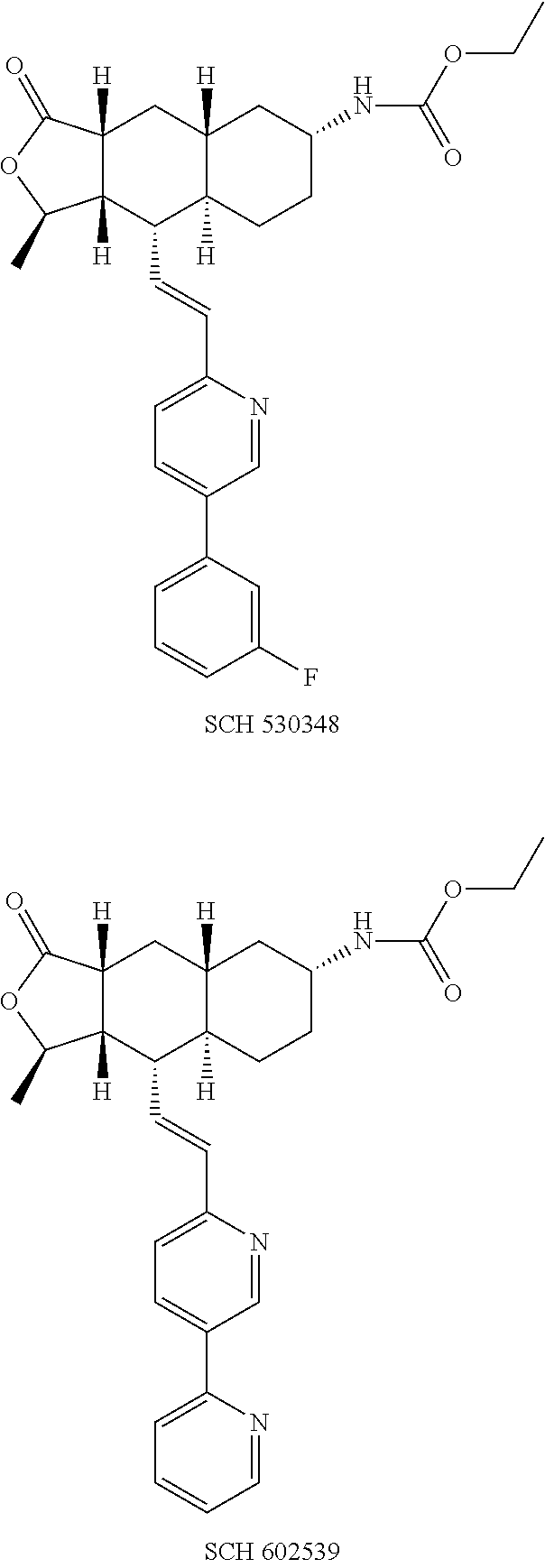
The treatment and prevention of thrombotic events are provided through co-administration of PAR-1 and the P2Y12 ADP receptor antagonists. Combined inhibition of the PAR-1 and the P2Y12 ADP platelet activation pathways had synergistic antithrombotic and antiplatelet effects, as demonstrated in co-administration of SCH 602539 and cangrelor.
Owner:MERCK SHARP & DOHME CORP
Intermediate for preparing cangrelor and preparation method and application thereof
ActiveCN105273025AHigh purityImprove stabilitySugar derivativesSugar derivatives preparationState of artCangrelor
The invention relates to the technical field of preparation method of cangrelor. The invention discloses a new intermediate compound 4 for preparing cangrelor and a preparation method thereof. By changing an N- protection group, and adopting weaker-activity formyl as an N protection group, the new N-formyl protective intermediate 4 is obtained successfully; the intermediate has the advantages of high purity, good stability, easy operation of preparation method and the like. The difficulties that the yield is low and the product is difficult to purify and separate in the prior art are solved.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Methods of Treating, Reducing the Incidence of, and/or Preventing Ischemic Events
ActiveUS20140107032A1Reduce morbidityPreventing an ischemic eventOrganic active ingredientsPeptide/protein ingredientsMedicineThrombus
Methods of treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing percutaneous coronary intervention (PCI), comprising administering to the patient a pharmaceutical composition comprising cangrelor. The method may further comprise administering an additional therapeutic agent to the patient, the additional therapeutic agent comprising bivalirudin or a P2Y12 inhibitor. Pharmaceutical compositions useful for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI. The pharmaceutical compositions comprise cangrelor, and optionally bivalirudin. Methods of preparing a pharmaceutical composition for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI, comprising admixing cangrelor with one or more pharmaceutically acceptable excipients. An ischemic event may include stent thrombosis, myocardial infarction, ischemia-driven revascularization, and mortality.
Owner:CHIESI FARM SPA
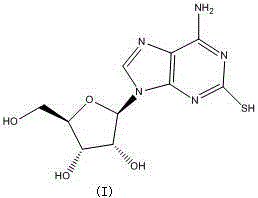
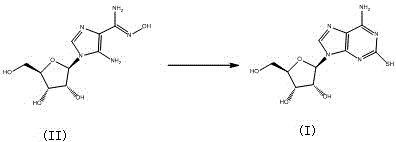
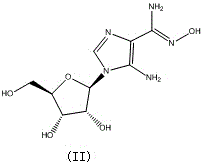
Preparation method of cangrelor intermediate
ActiveCN105481922AMild reaction conditionsRaw materials are easy to getSugar derivativesSugar derivatives preparationState of artNitro compound
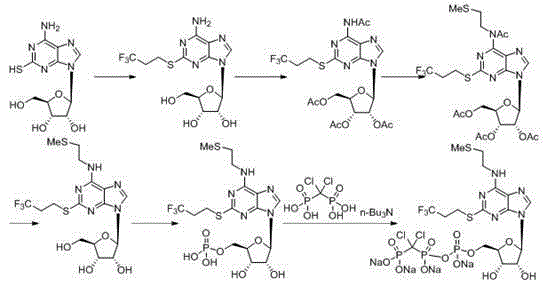
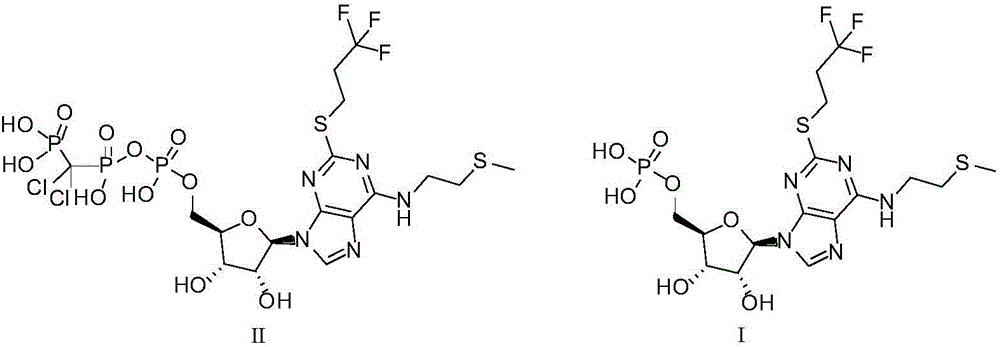
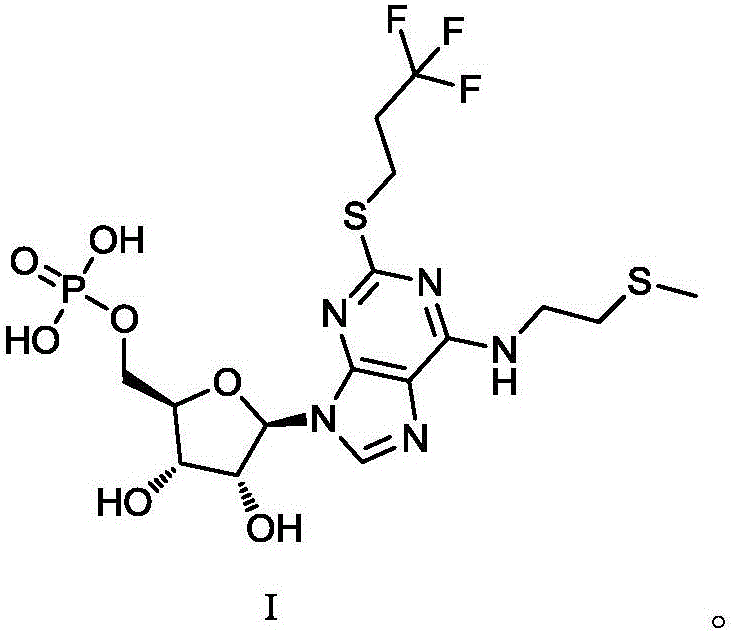
The purpose of the invention is to solve defects in the prior art by providing a new preparation method of a cangrelor intermediate (represented by formula I). The preparation method comprises the following steps: carrying out a sealed tube reaction on a compound represented by formula II, and ammonifying the above obtained material to form a compound represented by formula III; coupling the amino compound III with tetraacetylribofuranose under the action of a catalyst to obtain a compound represented by formula V; reducing the nitro compound V through a reducing system to obtain a compound represented by formula VI; carrying out a cyclization reaction on the amino compound VI and ortho-formate to obtain a compound represented by VII; coupling the compound VII with 2-methylthioethylamine (represented by formula VIII) in the presence of an alkali to obtain a compound represented by IX; and removing protection groups from the compound IX in the presence of the alkali to obtain the cangrelor intermediate (represented by the formula I). The preparation method adopting the above technical route has the advantages of mild reaction conditions, high yield, wide sources of raw materials, and environmental protection.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
Methods of treating, reducing the incidence of, and/or preventing ischemic events
ActiveUS9427448B2Reduce morbidityPreventing an ischemic eventPowder deliveryBiocideThrombusCangrelor
Methods of treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing percutaneous coronary intervention (PCI), comprising administering to the patient a pharmaceutical composition comprising cangrelor. The method may further comprise administering an additional therapeutic agent to the patient, the additional therapeutic agent comprising a P2Y12 inhibitor. Pharmaceutical compositions useful for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI. The pharmaceutical compositions comprise cangrelor. Methods of preparing a pharmaceutical composition for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI, comprising admixing cangrelor with one or more pharmaceutically acceptable excipients. An ischemic event may include stent thrombosis, myocardial infarction, ischemia-driven revascularization, and mortality.
Owner:CHIESI FARM SPA
Maintenance of Platelet Inhibition During Antiplatelet Therapy
InactiveUS20130303477A1Reduce bleeding riskMaintain and reduce platelet activityBiocidePharmaceutical delivery mechanismExcessive BleedingPlatelet inhibitors
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:THE MEDICINES
Maintenance of Platelet Inhibition During Antiplatelet Therapy
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:THE MEDICINES
Pharmaceutical formulations comprising high purity cangrelor and methods for preparing and using the same
ActiveUS9295687B1Blood lossMinimizing transfer of moisturePharmaceutical containersMedical packagingMedicineCangrelor
The present invention relates to high purity cangrelor, pharmaceutical formulations comprising high purity cangrelor as an active ingredient, methods for preparing such compounds and formulations, and methods for using the pharmaceutical formulations in the inhibition of platelet activation and aggregation.
Owner:CHIESI FARM SPA
Methods of treating or preventing stent thrombosis
ActiveUS9925265B2Reduce mortalityPrevent myocardial infarctionOrganic active ingredientsBiocideStent implantationThrombus
The present invention is directed to the following: methods of treating or preventing stent thrombosis using pharmaceutical compositions comprising cangrelor and optionally bivalirudin; methods of reducing mortality in a subject undergoing stent implantation using pharmaceutical compositions comprising cangrelor and optionally bivalirudin; medicaments comprising cangrelor and optionally bivalirudin useful for treating or preventing stent thrombosis, or useful for reducing mortality in a subject undergoing stent implantation; pharmaceutical compositions comprising cangrelor and bivalirudin; and methods of preparing a medicament comprising cangrelor and optionally bivalirudin useful for treating or preventing stent thrombosis, or useful for reducing mortality in a subject undergoing stent implantation.
Owner:CHIESI FARM SPA
Maintenance of Platelet Inhibition During Antiplatelet Therapy
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:CHIESI FARM SPA
Use of Par-1/Par-4 Inhibitors for Treating or Preventing Vascular Diseases
InactiveUS20080214599A1Reducing platelet activationReducing thrombin generationBiocideMetabolism disorderVascular diseaseTiclopidine
Use of a compound of formula (I) or a compound selected from the group consisting of a prasugrel metabolite, clopidogrel, a clopidogrel metabolite, ticlopidine, a ticlopidine metabolite, cangrelor, a cangrelor metabolite, AZD-6140, and an AZD-6140 metabolite for the treatment of and / or prevention of coagulation induced vascular diseases and recurrence thereof in a patient in need thereof.
Owner:ELI LILLY & CO
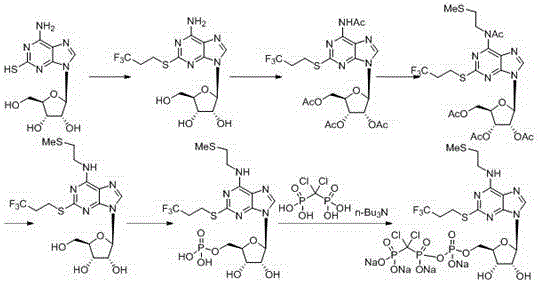
Synthesis method of cangrelor intermediate
ActiveCN105949258AReduce dosageIncrease profitSugar derivativesSugar derivatives preparationChemical synthesisSynthesis methods
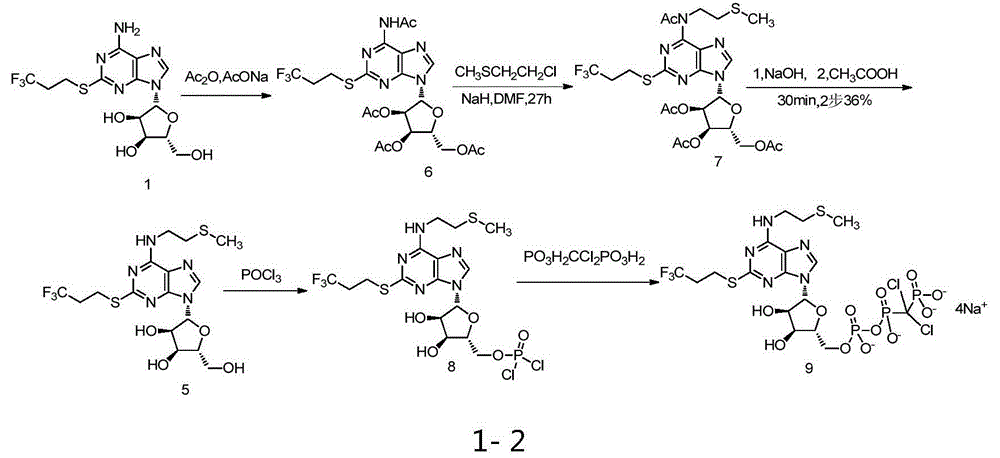
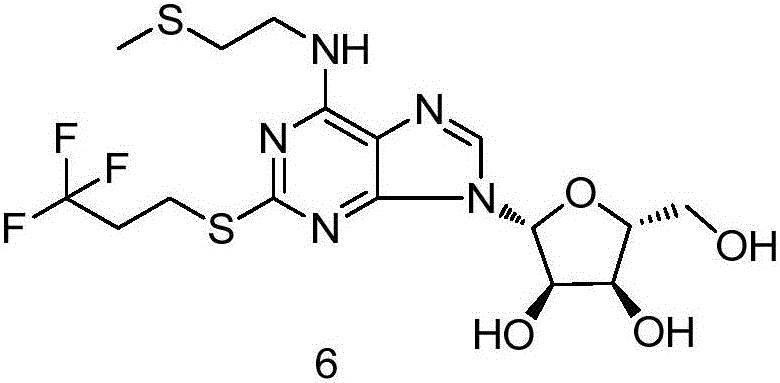
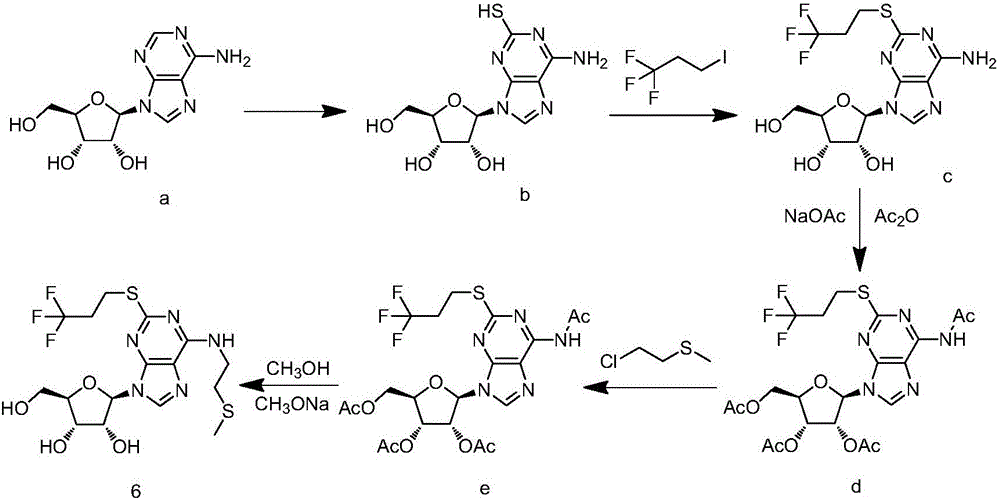
Belonging to the field of chemical synthesis of drugs, the invention in particular relates to a synthesis method of a compound cangrelor intermediate shown as formula (6). The invention provides a new synthesis method of the cangrelor intermediate (compound 6). The method includes: taking thioadenosine as the raw material, firstly carrying out reaction with a diazotization reagent and a halogenated reagent, then carrying out reaction with 2-(methylthio)ethylamine, and finally conducting hydrolysis to obtain a compound 6. The method has the advantages of cheap raw materials, efficient reaction, safety and easy operation, and is conducive to industrial production. (formula 6).
Owner:ZHEJIANG YONGNING PHARMA
Maintenance of Platelet Inhibition During Antiplatelet Therapy
ActiveUS20150038449A1Reduced activityBiocideCarbohydrate active ingredientsExcessive BleedingPlatelet inhibitors
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:CHIESI FARM SPA
Cangrelor intermediate and preparation method and application thereof
ActiveCN105273027AHigh purityImprove stabilitySugar derivativesSugar derivatives preparationChemistryBiochemical engineering
The invention relates to the technical field of preparation method of cangrelor. The invention discloses a new intermediate compound 3 for preparing cangrelor. The invention discloses the new intermediate compound 3 for preparing cangrelor and a preparation method thereof. By changing an N- protection group, and adopting weaker-activity formyl as an N protection group, the new N-formyl protective intermediate 3 is obtained successfully; the intermediate has the advantages of high purity, good stability, easy operation of preparation method and the like. The difficulties in the prior art that the yield is low and the product is difficult to purify and separate are solved.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Methods of treating, reducing the incidence of, and/or preventing ischemic events
ActiveUS10376532B2Reduce morbidityPreventing an ischemic eventOrganic active ingredientsPowder deliveryThrombusMortality rate
Methods of treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing percutaneous coronary intervention (PCI), comprising administering to the patient a pharmaceutical composition comprising cangrelor. The method may further comprise administering an additional therapeutic agent to the patient, the additional therapeutic agent comprising bivalirudin or a P2Y12 inhibitor. Pharmaceutical compositions useful for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI. The pharmaceutical compositions comprise cangrelor, and optionally bivalirudin. Methods of preparing a pharmaceutical composition for treating, reducing the incidence of, and / or preventing an ischemic event in a patient undergoing PCI, comprising admixing cangrelor with one or more pharmaceutically acceptable excipients. An ischemic event may include stent thrombosis, myocardial infarction, ischemia-driven revascularization, and mortality.
Owner:CHIESI FARM SPA
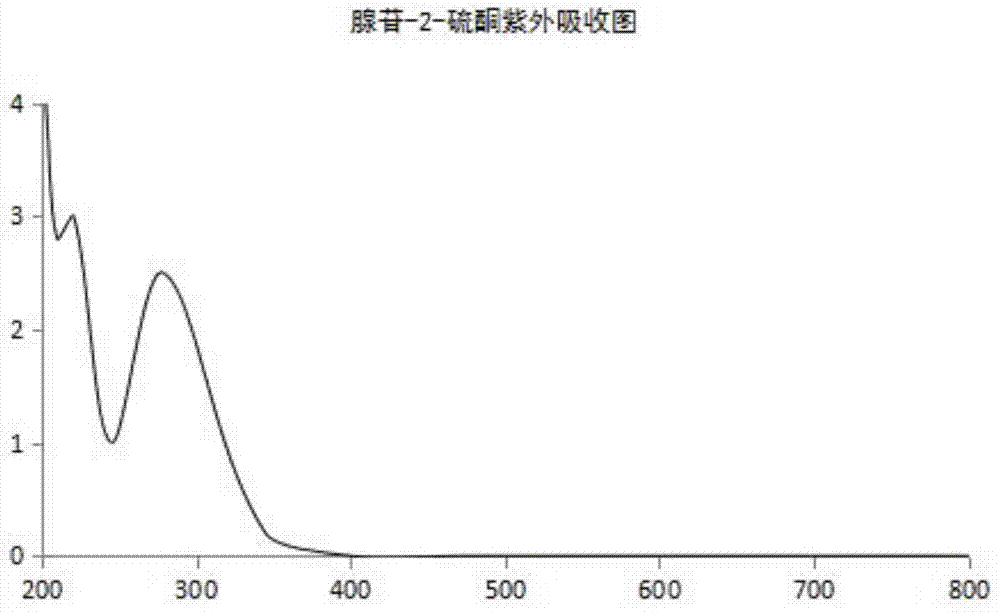
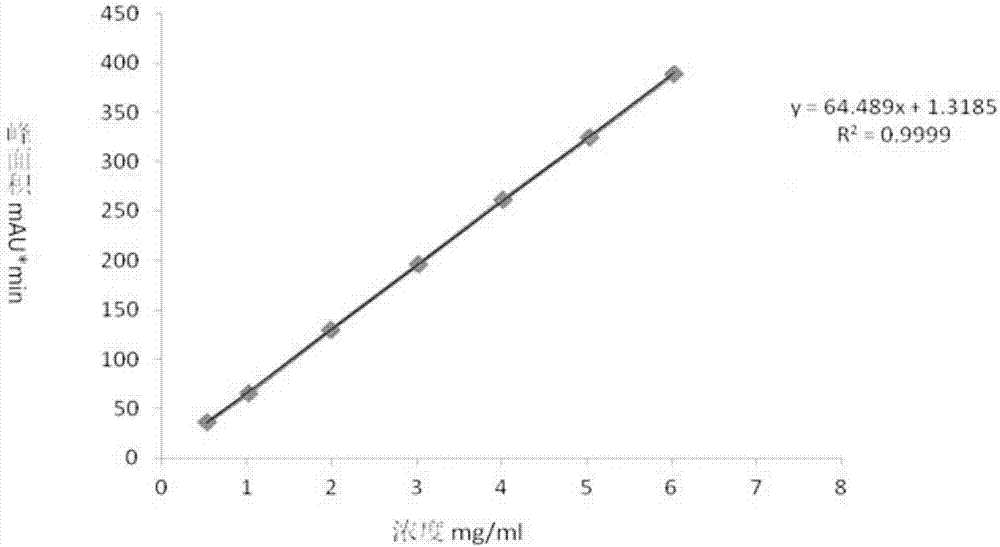
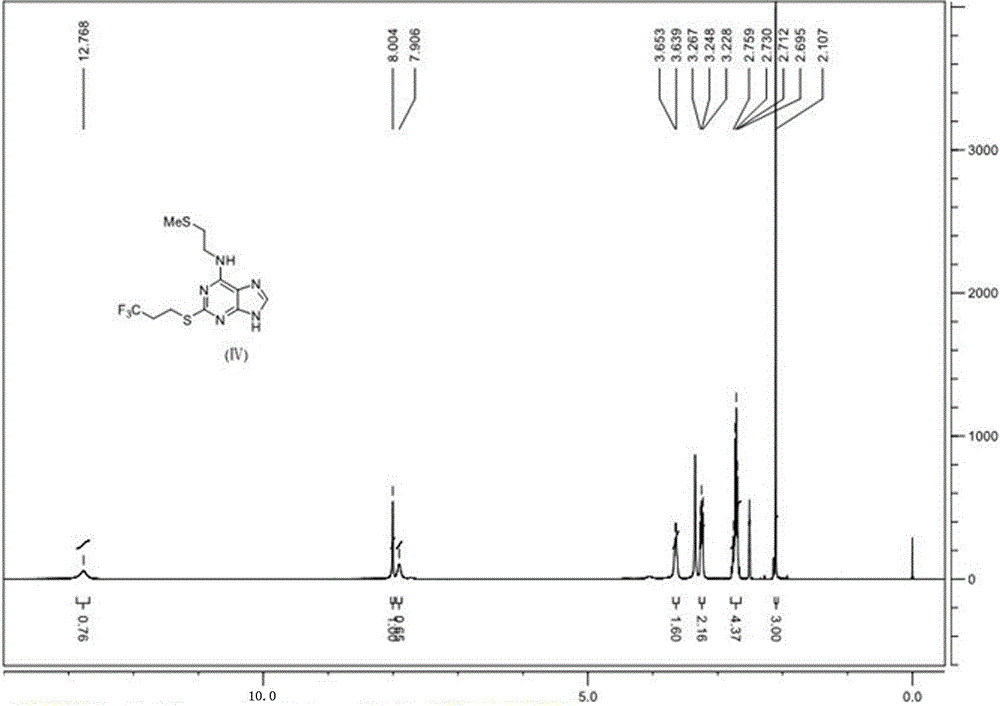
High performance liquid chromatography detection method of Cangrelor intermediate adenosine-2-thione
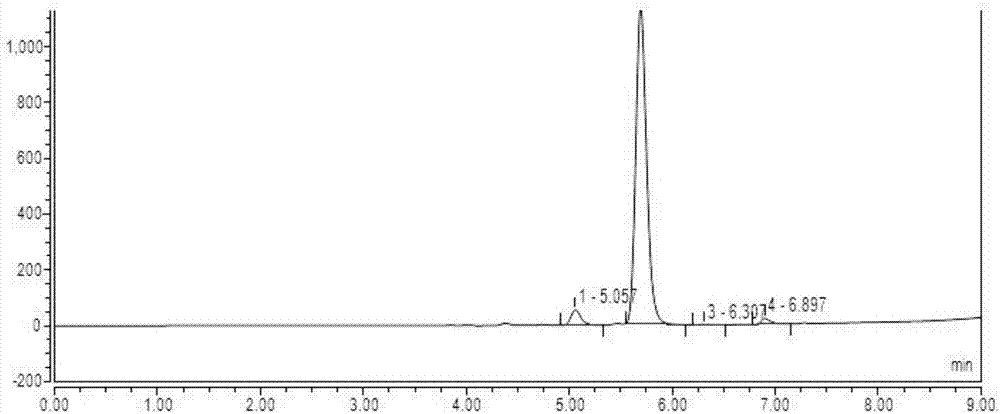
The invention discloses a high performance liquid chromatography detection method of a Cangrelor intermediate adenosine-2-thione. The high performance liquid chromatography detection method comprises the following steps: a, preparing a standard solution; b, detecting the standard solution by adopting reversed-phase high performance liquid chromatography; c, calculating the content of the adenosine-2-thione in a sample by peak area according to an external standard method. According to the detection method disclosed by the invention, the adenosine-2-thione, raw materials and other impurities can be separated well on a high performance liquid chromatograph; a linear relation is good within a range of 0.5mg / ml to 6.0mg / ml, R2 is equal to 0.9999, the average recovery rate is 102.11 percent and the RSD (Relative Standard Deviation) is 2.70 percent.
Owner:盐城锦明药业有限公司
Method for purifying cangrelor intermediate
ActiveCN106674322AEasy to operateShort reaction timeSugar derivativesOrganic chemistry methodsPurification methodsSilica gel
The invention discloses a method for purifying a cangrelor intermediate. The invention provides a method for purifying a cangrelor intermediate I; the method comprises the steps of 1, mixing a cangrelor intermediate I crude product with an organic solvent to form a mixture, enabling the mixture to react with basic salt or ammonia water, and then extracting to obtain a water phase; 2, removing water in the water phase, then adding an alcohol solvent into the water phase, and filtering or centrifuging to obtain a filter cake; 3, adjusting the pH of the obtained filter cake to 2-3 in water, and filtering or centrifuging to obtain another filter cake; 4, adjusting the pH of the obtained filter cake to 7-8 in the alcohol solvent, and filtering to obtain the cangrelor intermediate I. The method for purifying the cangrelor intermediate does not adopt a reverse silica gel separation method and cation exchange resin which is expensive, cumbersome in operation and long in purification time; the method is simple in operation, short in reaction time, high in yield and high in purity of a prepared product; after the method is used, the high performance liquid chromatography (HPLC) purity is more than 99.0%; the method is low in production cost and suitable for industrial production. The structural formula of the cangrelor intermediate I is described in the description.
Owner:上海云晟研新生物科技有限公司
6-n-(2-(methylthio)ethyl)-2-((3,3,3-trifluoropropyl)thio)-9h-purine and its preparation method and application
ActiveCN105061431BWide variety of sourcesMild reaction conditionsSugar derivativesSugar derivatives preparationThio-Purine
The invention relates to a novel compound 6-N-(2-(methylthio)ethyl)-2-((3,3,3-trifluoropropyl)sulfo)-9H-purine and a preparation method for the novel compound and further provides application of the novel compound as an intermediate in synthesis of cangrelor directed at the limitation of conventional synthetic methods for cangrelor. The compound is used as the intermediate for synthesis of cangrelor; and such a route has the advantages of wide sources of raw materials, mild reaction conditions, simple after-treatment, environment friendliness and improved product yield and provides a novel synthetic method for laboratory and industrialization production of cangrelor.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
HPLC detection method of cangrelor related substances
ActiveCN109781920AEasy to separateImprove securityComponent separationAgainst vector-borne diseasesGradient elutionSilica gel
The invention provides a high performance liquid chromatography detection method of a cangrelor related substance. The method comprises the following steps: adopting a reversed-phase chromatographic column; the filler being octadecyl bonded silica gel; and carrying out gradient elution by taking a phosphate sodium perchlorate buffer solution as a mobile phase A and acetonitrile as a mobile phase B. The method has the advantages of strong specificity, high sensitivity and accurate quantitative determination result; the analysis method is simple, convenient and rapid, can better separate all impurities in a short time, has a separation degree between each chromatographic peak of more than 1.5, can comprehensively detect the cangrelor related substance and the cangrelor related substance in the preparation of the cangrelor, improves the safety of the medicine, and provides a basis for formulating the quality standards of the cangrelor bulk drug and the preparation of the cangrelor.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Preparation method of Cangrelor intermediate
InactiveCN106008633AReduce processing costsLow costSugar derivativesSugar derivatives preparationThioureaNitration
The invention discloses a preparation method of a Cangrelor intermediate. The preparation method comprises the following steps of enabling ethyl cyanoacetate and thiourea to perform closed-loop reaction to generate a product under the alkaline condition, reacting the product and trifluoropropane under the alkaline condition, performing nitration reaction and reduction reaction, and performing closed-loop reaction with formic acid; chlorinating the generated product, and performing condensation reaction on the product and 2-(thiomethyl)ethylamine under the alkaline condition; reacting the obtained product and 1,2,3,5-tetraacetyl-beta-D-ribofuranose under the actions of alkylating agent and TMSOTF (trimethylsilyl trifluoromethanesulfonate), and hydrolyzing the product under the alkaline condition, so as to obtain the Cangrelor intermediate. The preparation method has the advantages that the silica-gel column chromatography is not needed, so that the technology cost is greatly reduced; the carbon disulfide, fuming nitric acid or concentrated sulfuric acid is not used in the preparation process, so that any danger is avoided; the noble metal hydrogenating reducing agent is not needed, so that the cost is reduced, and the operation danger is decreased; the operation is easy, safe and reliable, and the preparation method is suitable for large-scale industrial production.
Owner:北京广博德赛医药技术开发有限责任公司 +1
Cangrelor monohydrate crystal and preparation method thereof
InactiveCN104447927AHigh purityImprove stabilityOrganic active ingredientsSugar derivativesHigh humidityMedicine
Belonging to the field of medical technologies, the invention in particular relates to a cangrelor monohydrate crystal and a preparation method thereof. The cangrelor monohydrate obtained by the invention has the advantage of: high purity and good stability, unobvious moisture absorption weight gain even under a high humidity condition. The invention also relates to application of compositions using the hydrate to treatment of cardiovascular and cerebrovascular system diseases.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of Cangrelor intermediate
InactiveCN105693800AHigh purityLow costSugar derivativesSugar derivatives preparationCangrelorSolvent
The present invention relates to a formula (I): The preparation method of the shown cangrelor intermediate, the preparation method uses the structural compound shown in the formula (II) as a raw material, in the presence of a solvent and a reaction reagent, through a specific reaction, and the product after the reaction is purified to obtain the formula ( I) Cangrelor intermediate shown; wherein, the specific structure of formula (II) is: The advantages of the present invention are: the preparation method of the present invention has the advantages of simple operation, high product purity, high safety in the reaction process, suitable for industrial production, and low cost.
Owner:NANTONG HONGCI PHARMA
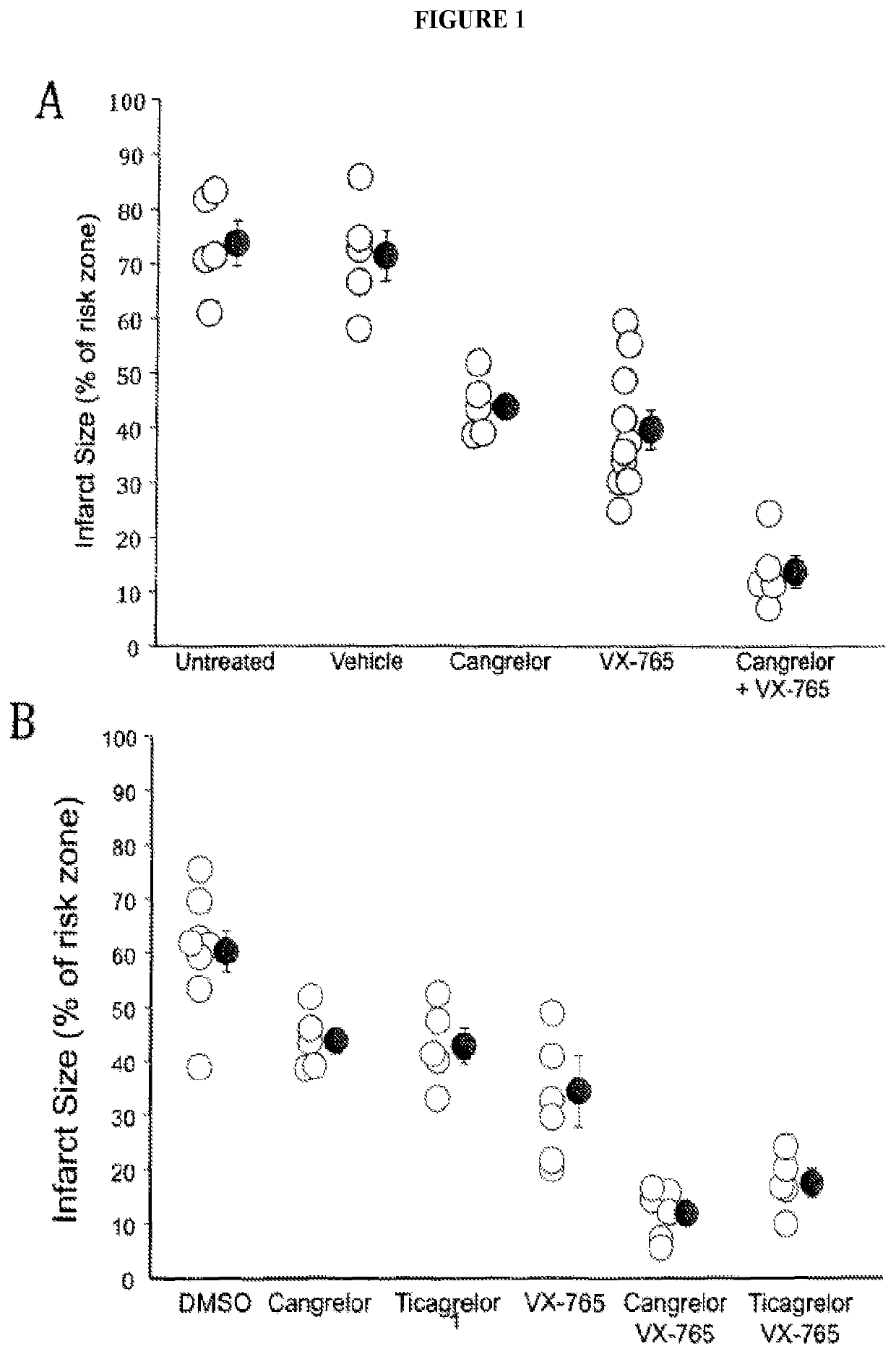
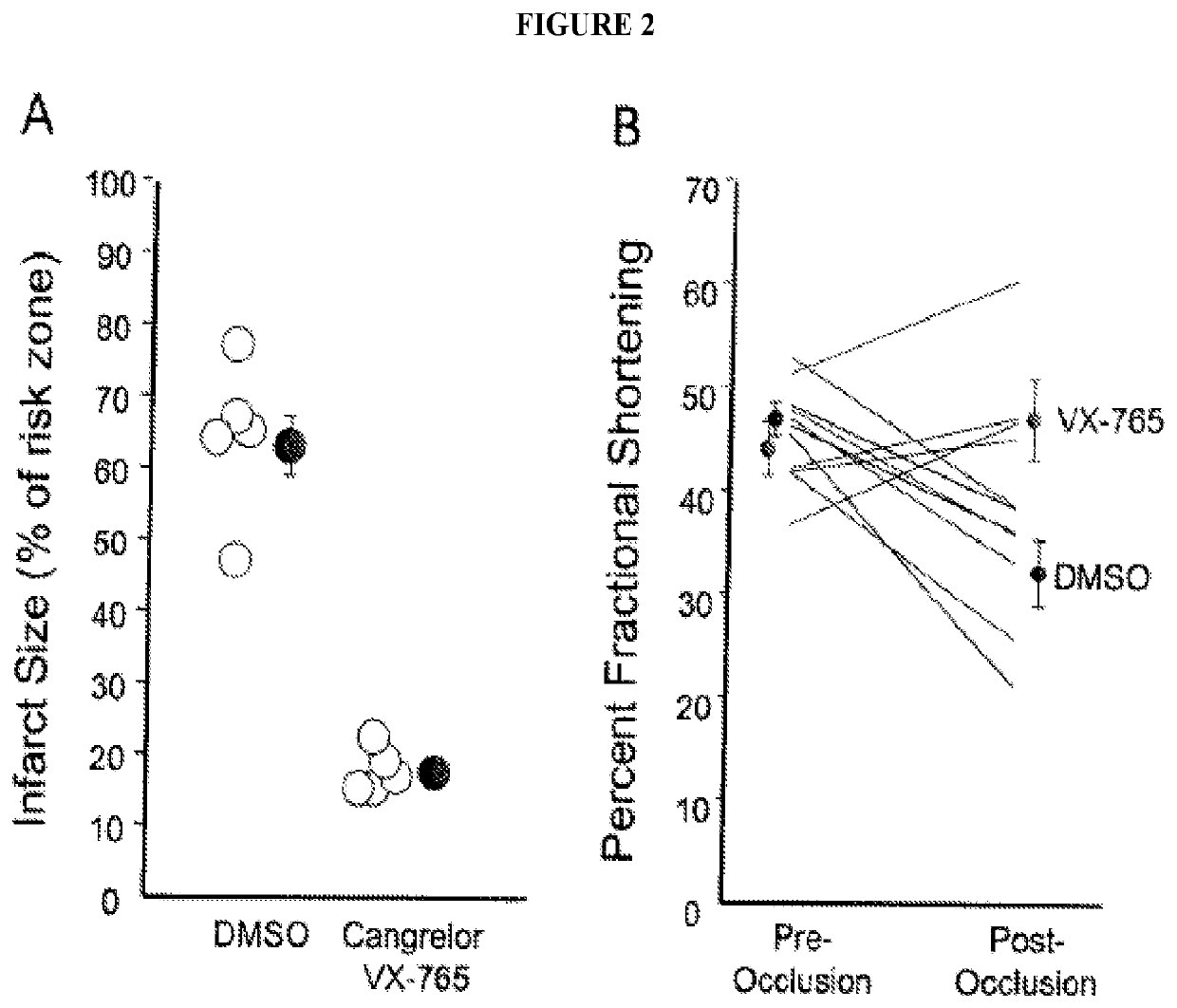
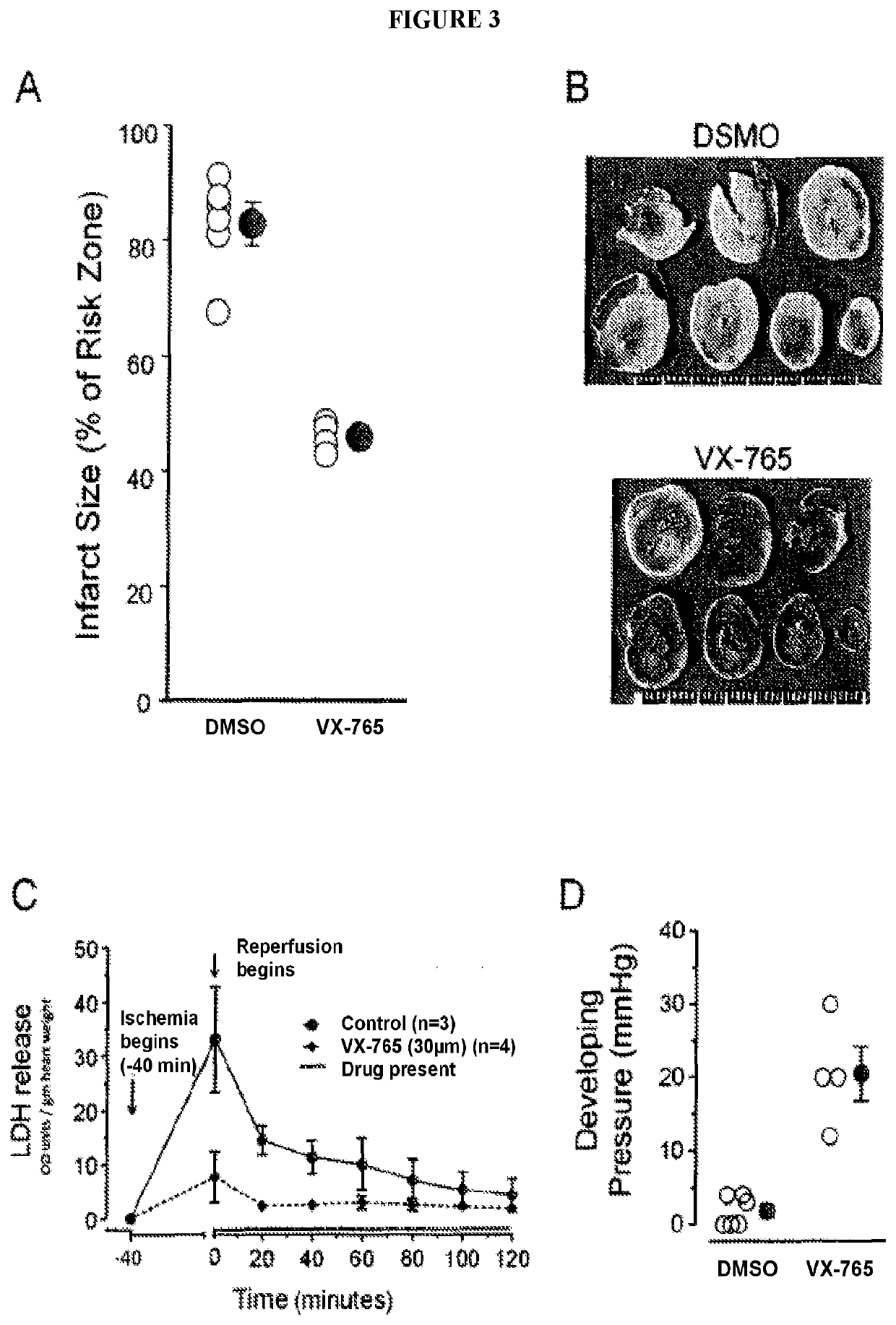
Combined administration of cysteine-aspartic protease inhibitors with p2y12 receptor antagonists protects the heart against myocardial infarction
The present disclosure generally pertains to methods of treating myocardial infarct involving administering a platelet anti-aggregate, a cysteine-aspartic protease inhibitor, and reperfusion therapy. In certain embodiments, the platelet anti-aggregate is at least one P2Y12 receptor antagonist or Glycoprotein IIb / IIIa inhibitor, the cysteine-aspartic protease inhibitor is selected from the group consisting of Caspase-1, 4, 5, 11 and 12 inhibitors, and reperfusion therapy is percutaneous coronary intervention. In certain embodiments, the at least one P2Y12 receptor antagonist is selected from the group consisting of cangrelor, ticagrelor, clopidogrel and prasugrel. The disclosed methods provide an improved cardioprotective effect against infarction when compared with the current standard of care.
Owner:UNIV OF SOUTH ALABAMA
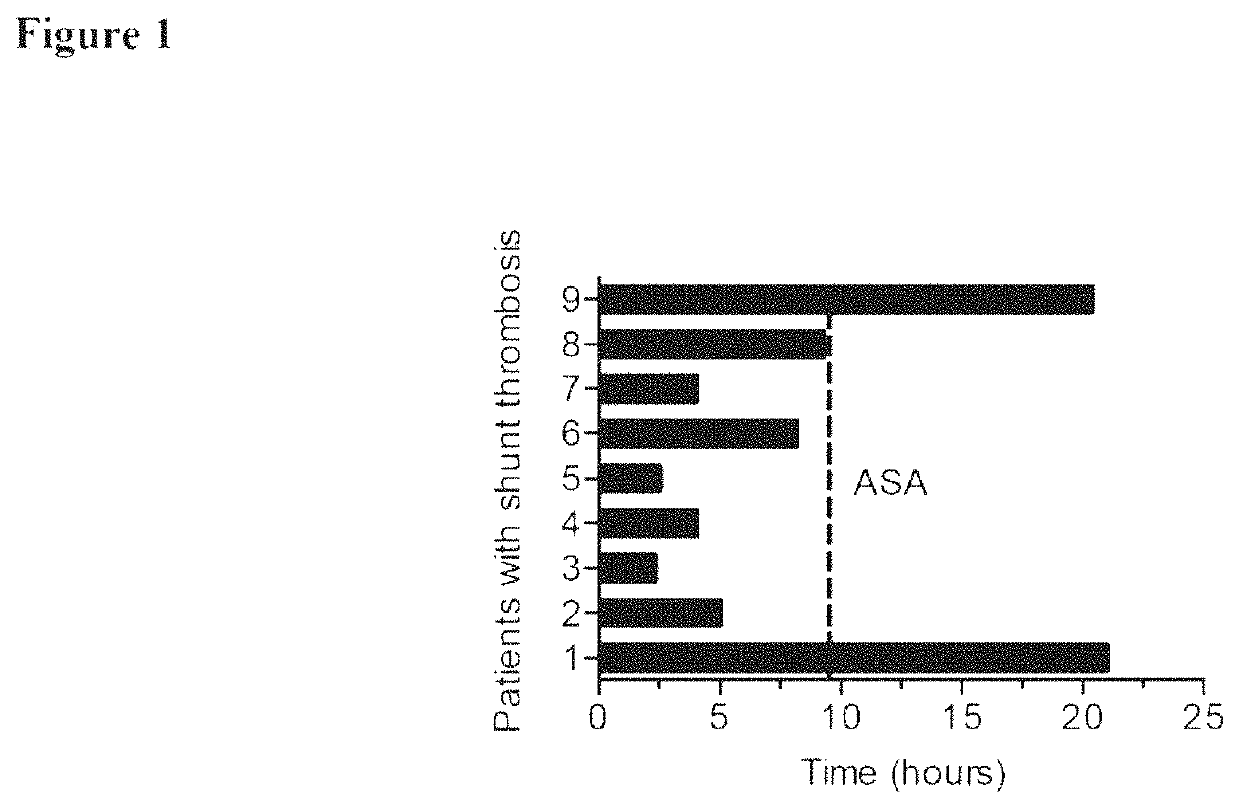
Method of preventing of systemic-to-pulmonary-artery shunt thrombosis
ActiveUS11260071B2Preventing shunt thrombosisBiocidePharmaceutical delivery mechanismPediatric patientThrombus
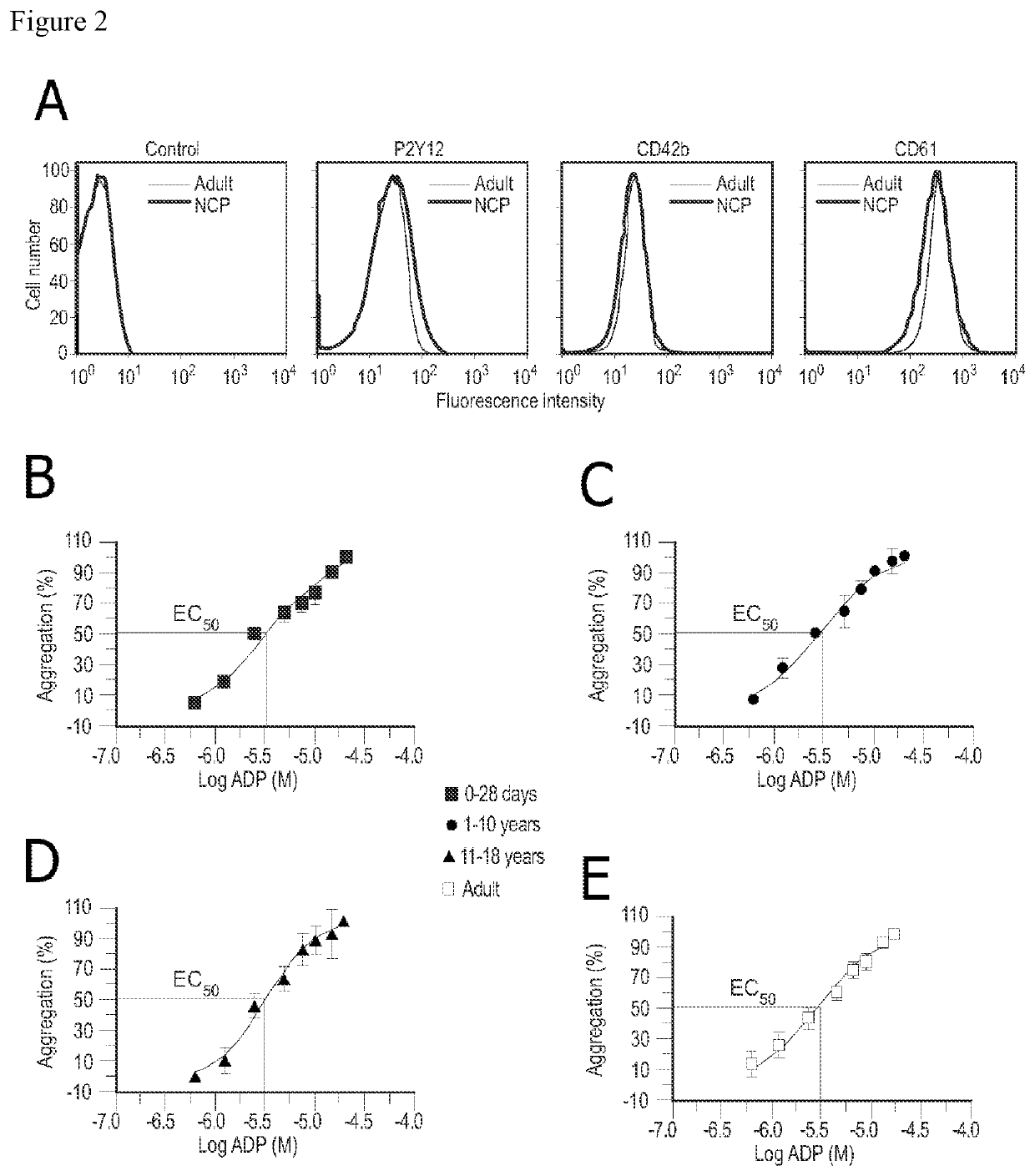
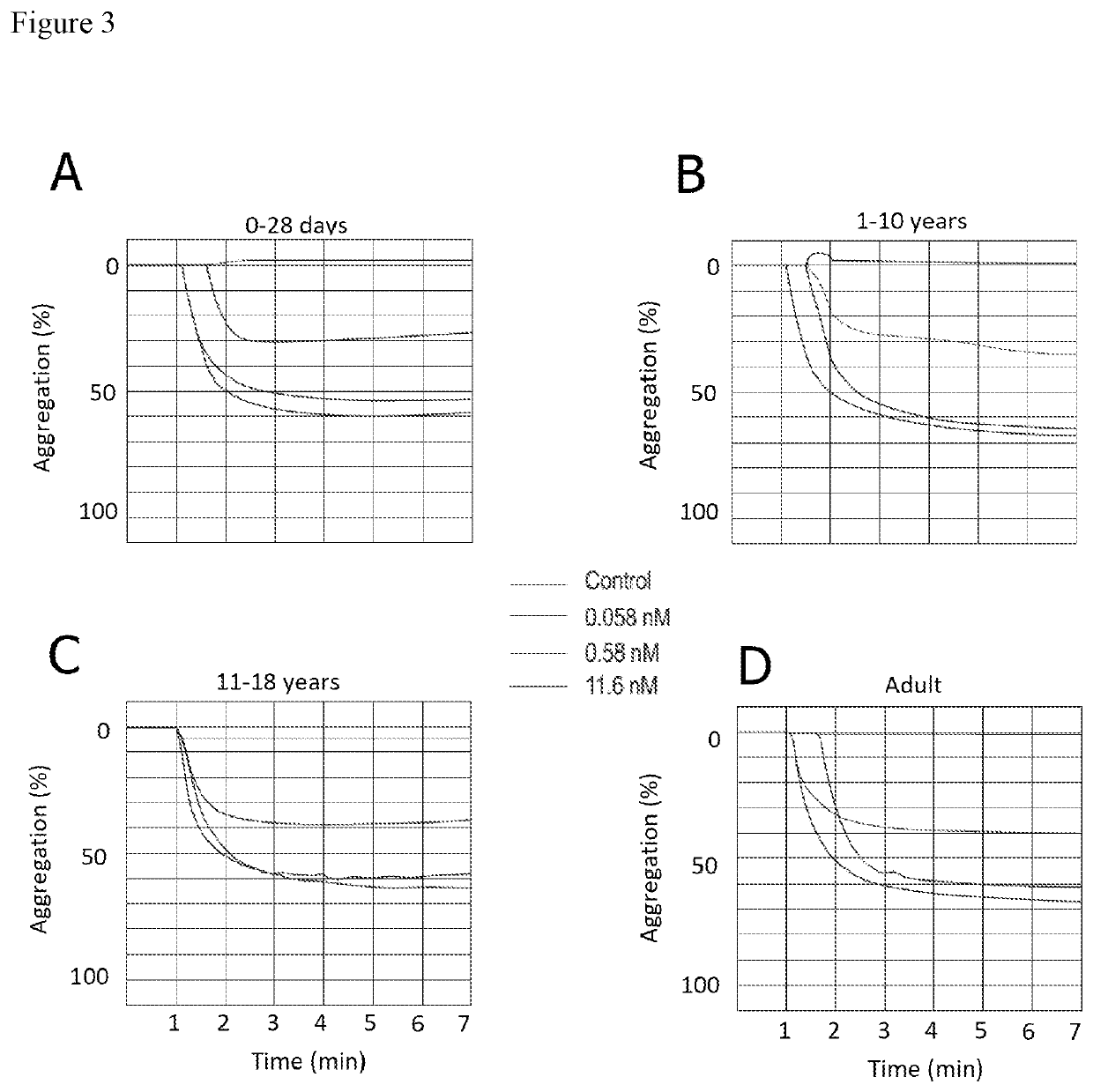
The present invention is directed to the use of cangrelor for the treatment and / or prevention of shunt thrombosis in patients suffering congenital heart diseases undergoing shunt surgery. The invention is also directed to the use of cangrelor for the treatment and / or prevention of stent thrombosis in pediatric patients undergoing stent implantation.
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com