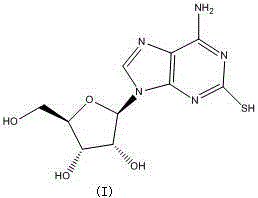
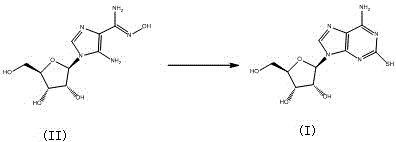
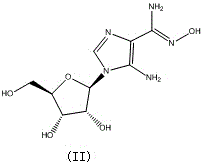
Preparation method of Cangrelor intermediate
A technology of intermediates and specific structures, applied in the field of pharmaceutical intermediate synthesis, can solve problems such as hidden dangers in production safety, high requirements for production equipment, etc., and achieve the effects of low production equipment, low cost and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Thoroughly stir tetrahydrofuran (5ml), (II) (27g, 100mmol), methanol (100ml), carbon disulfide (100ml), and dimethyl sulfoxide (500ml) in a reaction flask, and stir the reactants at 140-150°C for 12 Hour. After the reaction was completed, the solvent was distilled off under reduced pressure, washed with ethanol, water and saturated aqueous sodium chloride solution, and dried to obtain 24 g of the product with a yield of 80%.
[0042] Spectral data:
[0043] 1 HNMR(500MHz,DMSO)δ12.15(s,1H),8.35(s,1H),6.99(s,2H),6.16(m,1H),4.75-4.40(m,sH),3.79(d,2H ), 3.65(s,1H), 3.58(s,2H);
[0044] MS(ESI):299.07
Embodiment 2
[0046]
[0047] Diethyl ether (5ml), (II) (27g, 100mmol), methanol (100ml), carbon disulfide (100ml), and dimethyl sulfoxide (500ml) were fully stirred in a reaction flask, and the reactants were stirred at 140-150°C for 12 Hour. After the reaction was completed, the solvent was distilled off under reduced pressure, washed with ethanol, water and saturated aqueous sodium chloride solution, and dried to obtain 23 g of the product with a yield of 76.7%.
[0048] Spectral data:
[0049]1 HNMR(500MHz,DMSO)δ12.15(s,1H),8.35(s,1H),6.99(s,2H),6.16(m,1H),4.75-4.40(m,sH),3.79(d,2H ), 3.65(s,1H), 3.58(s,2H);
[0050] MS(ESI):299.07
Embodiment 3
[0052]
[0053] Thoroughly stir tetrahydrofuran and diethyl ether (5ml), (II) (27g, 100mmol), methanol (100ml), carbon disulfide (100ml), and dimethyl sulfoxide (500ml) in a reaction flask. Stir for 12 hours. After the reaction was completed, the solvent was distilled off under reduced pressure, washed with ethanol, water and saturated aqueous sodium chloride solution, and dried to obtain 23.5 g of the product with a yield of 78.3%.
[0054] Spectral data:
[0055] 1 HNMR(500MHz,DMSO)δ12.15(s,1H),8.35(s,1H),6.99(s,2H),6.16(m,1H),4.75-4.40(m,sH),3.79(d,2H ), 3.65(s,1H), 3.58(s,2H);
[0056] MS(ESI):299.07
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com