HPLC detection method of cangrelor related substances
A detection method and technology of detection conditions, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of affecting the active site of the stationary phase, failing to achieve separation effect, long elution separation time, etc., and achieving a simple and fast analysis method. , Improve drug safety, the effect of accurate quantitative measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
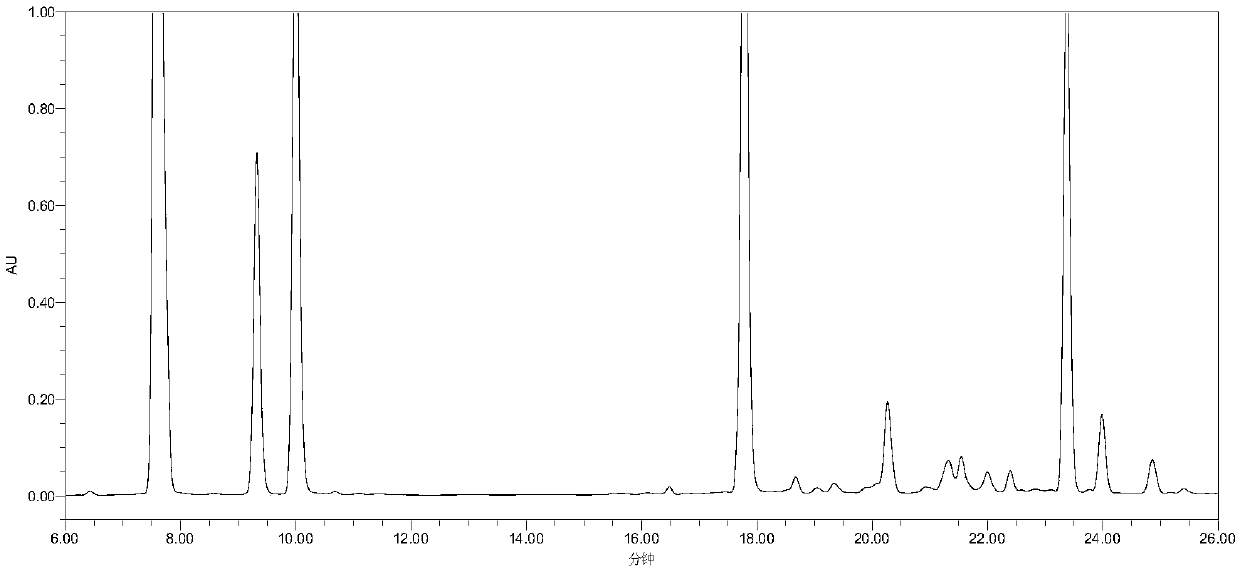
[0048] Example 1: Detection of related substances of cangrelor
[0049] Instruments and equipment: high performance liquid chromatography: Agilent 1260 high performance liquid chromatography, chromatographic column: WatersSymmetry C18 (4.6mm × 250mm, 5μm).
[0050] Chromatographic conditions: mobile phase: mobile phase A: 15mmol L -1 Diammonium hydrogen phosphate sodium perchlorate solution (take 1.98g of diammonium hydrogen phosphate and 2.11g of sodium perchlorate, add purified water to dilute to 1000mL, adjust pH to 7.0 with phosphoric acid); mobile phase B: acetonitrile, use gradient elution . Detection wavelength: 242nm, column temperature: 30°C; injection volume: 20μL; flow rate: 1.0mL min -1 .
[0051] The specific gradient elution process is as follows:
[0052]
[0053]
[0054] Experimental method: Take 1 part of the mixed solution to be used for sample analysis under the above-mentioned chromatographic conditions, and record the chromatogram (see figure ...
Embodiment 2
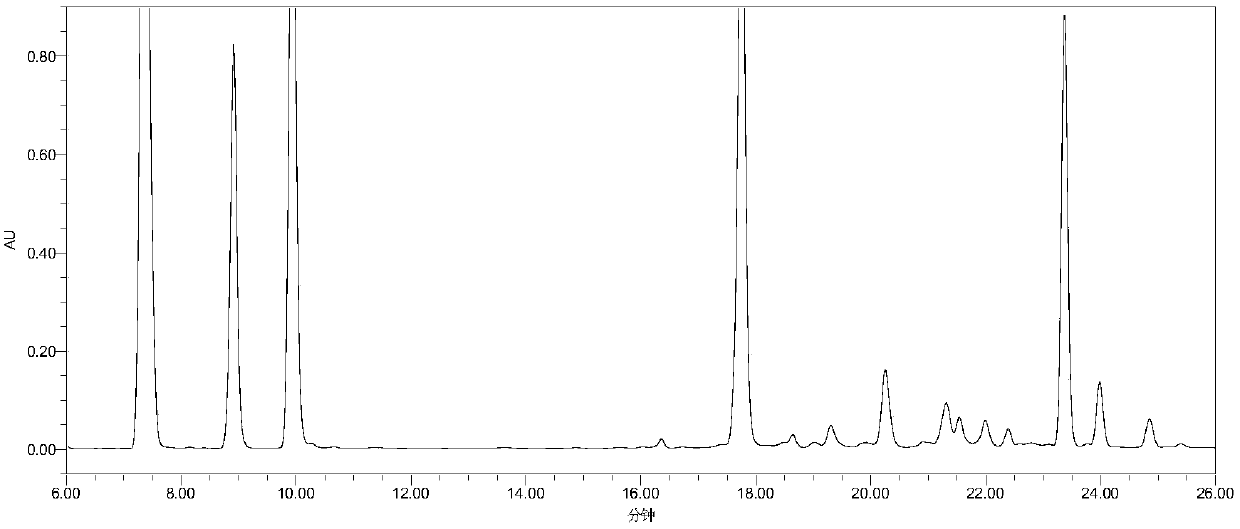
[0056] Example 2: Detection of related substances of cangrelor
[0057] Instruments and equipment: high performance liquid chromatography: Agilent 1260 high performance liquid chromatography, chromatographic column: WatersSymmetry C18 (4.6mm × 250mm, 5μm).
[0058] Chromatographic conditions: mobile phase: mobile phase A: 15mmol L -1 Dipotassium hydrogen phosphate sodium perchlorate solution (take 3.42g of dipotassium hydrogen phosphate and 2.11g of sodium perchlorate, add purified water to dilute to 1000mL, adjust pH to 7.0 with phosphoric acid); mobile phase B: acetonitrile, using gradient elution . Detection wavelength: 242nm, column temperature: 30°C; injection volume: 20μL; flow rate: 1.0mL min -1 .
[0059] The specific gradient elution process is as follows:
[0060]
[0061] Experimental method: Take 1 portion of the mixed solution to be used for sample analysis under the above-mentioned chromatographic conditions, and record the chromatogram.
[0062] Experime...
Embodiment 3
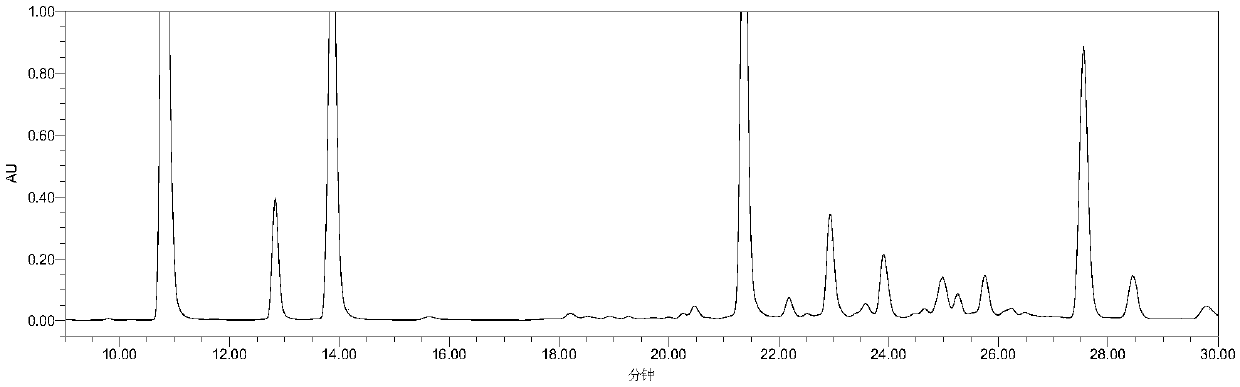
[0063] Example 3: Detection of related substances of cangrelor
[0064] Instruments and equipment: high performance liquid chromatography: Agilent 1260 high performance liquid chromatography, chromatographic column: CAPCELLPAK C18 (4.6mm×250mm, 5μm).
[0065] Chromatographic conditions: mobile phase: mobile phase A: 15mmol L -1 Diammonium hydrogen phosphate sodium perchlorate solution (take 1.98g of diammonium hydrogen phosphate and 2.11g of sodium perchlorate, add purified water to dilute to 1000mL, adjust pH to 7.0 with phosphoric acid); mobile phase B: acetonitrile, use gradient elution . Detection wavelength: 242nm, column temperature: 30°C; injection volume: 20μL; flow rate: 1.0mL min -1 .
[0066] The specific gradient elution process is as in Example 1.
[0067] Experimental method: Take 1 portion of the mixed solution to be used for sample analysis under the above-mentioned chromatographic conditions, and record the chromatogram.
[0068] Experimental results: eac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com