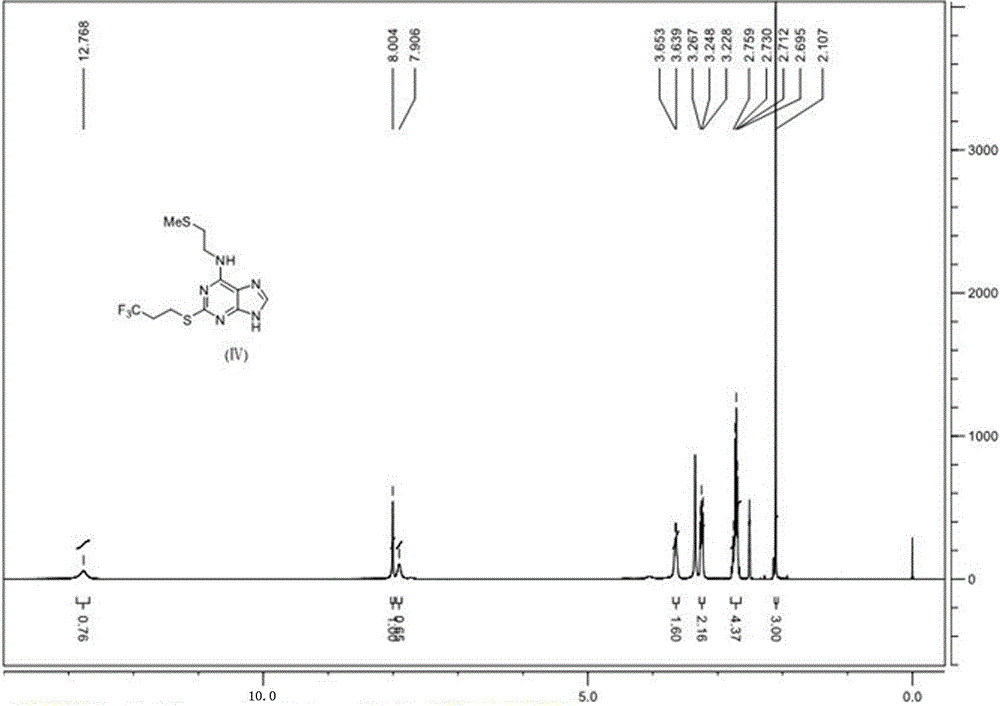
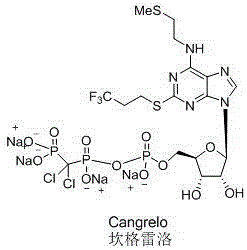
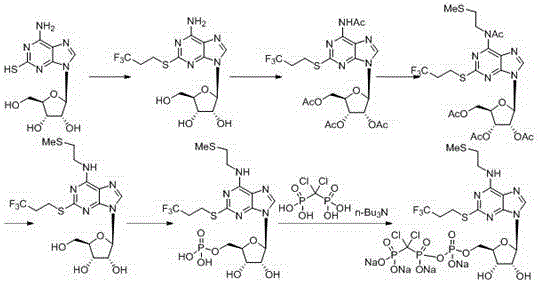
6-n-(2-(methylthio)ethyl)-2-((3,3,3-trifluoropropyl)thio)-9h-purine and its preparation method and application
A technology of trifluoropropyl group and methylthio group, applied in the application field of cangrelor synthesis, can solve the problems of human body and environmental damage, unsatisfactory acetyl group reaction effect, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Preparation method 1 of formula (X) compound 2-((3,3,3-trifluoropropyl)thio)pyrimidine-4,6-diol
[0082]At room temperature, add 110g (760mmol) of the compound of formula (Ⅺ) thiobarbituric acid into 220mL of water, the system is in a suspended state, add 152g (3.8mol) of sodium hydroxide into the system in 3 batches, the system gradually becomes clear After the addition, stir at room temperature for 0.5h, then add 220ml of nitrogen methyl pyrrolidone to the system, then add 511g (2.28mol) of 1-iodo-3,3,3-trifluoropropane into the system, after the addition, keep for 20 Stir the reaction at ℃ for 24 hours, TLC monitors that the raw materials basically disappear, the reaction is completed, stop the reaction, cool down to room temperature, adjust the pH value to about 2.5 with 2mol / L hydrochloric acid, filter, wash the filter cake with water, dry the solid, beat, and filter to obtain 166g Off-white solid, namely the compound of formula (X), yield: 91%.
[0083...
Embodiment 2
[0085] Example 2: Preparation method 2 of formula (X) compound 2-((3,3,3-trifluoropropyl)thio)pyrimidine-4,6-diol
[0086] At room temperature, add 110g (760mmol) of compound thiobarbituric acid of formula (Ⅺ) into 550mL of water, and the system is in a suspended state. Add 60.8g (1.52mol) of sodium hydroxide into the system in 3 batches, and the system gradually becomes After clarification and completion, stir at room temperature for 1h. Add 220ml of dimethyl sulfoxide to the system, then add 170g (760mmol) of 1-iodo-3,3,3-trifluoropropane into the system, after the addition is complete, raise the temperature to 80°C and stir for 6h, TLC monitors until the raw material is basically Disappeared, the reaction is complete, stop the reaction, cool down to room temperature, adjust the pH value to 2.5 with 2mol / L hydrochloric acid, filter, wash the filter cake with water, after the solid is dried, make a slurry, and filter to obtain 160.5g off-white solid, that is, the compound of ...
Embodiment 3
[0087] Example 3: Preparation method 3 of formula (X) compound 2-((3,3,3-trifluoropropyl)thio)pyrimidine-4,6-diol
[0088] At room temperature, add 110g (760mmol) of compound thiobarbituric acid of formula (Ⅺ) into 360mL of water, the system is in a suspension state, add 66.9g (1.67mol) of sodium hydroxide into the system in 3 batches, the system gradually becomes After clarification, the addition was completed and stirred at room temperature for 0.5h. Add 220ml of N,N-dimethylformamide to the system, then add 340.4g (1.52mol) of 1-iodo-3,3,3-trifluoropropane into the system, after the addition is complete, raise the temperature to 60°C and stir the reaction 8h, TLC monitoring until the raw materials basically disappeared, the reaction is complete, stop the reaction, cool down to room temperature, adjust the pH value to 2.5 with 2mol / L hydrochloric acid, filter, wash the filter cake with water, dry the solid, beat, and filter to obtain 171.5g off-white solid , namely the comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com