Preparation method of Cangrelor intermediate
A technology for intermediates and compounds, applied in the field of preparation of cangrelor intermediate 6-N-ethyl-2-thio)adenosine, capable of solving problems such as low yield, unfavorable environment, production operation, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
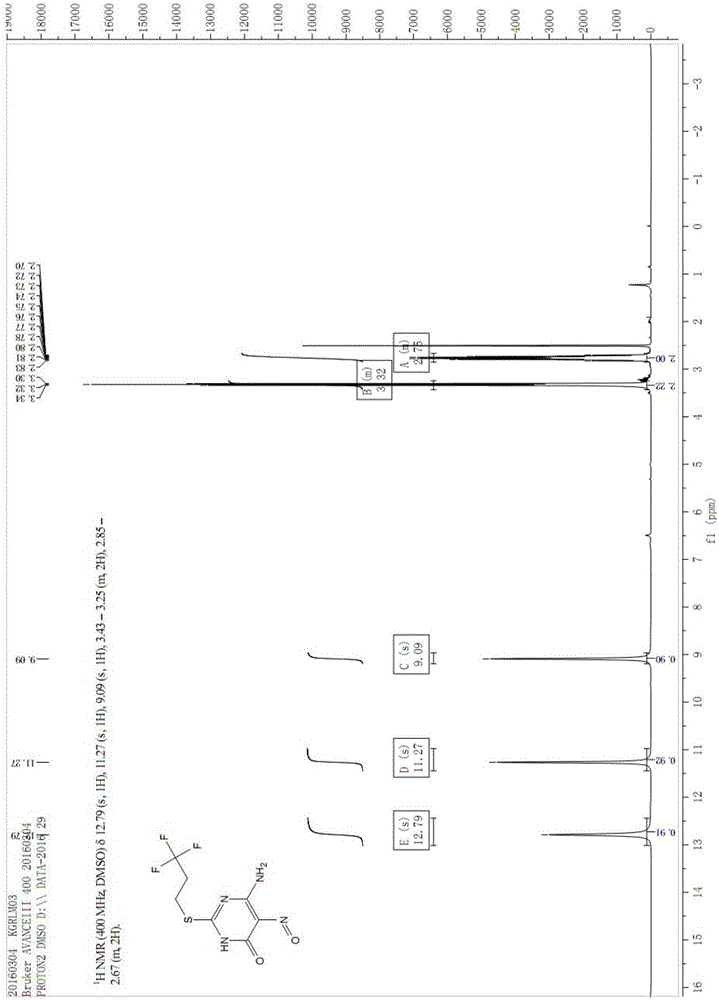
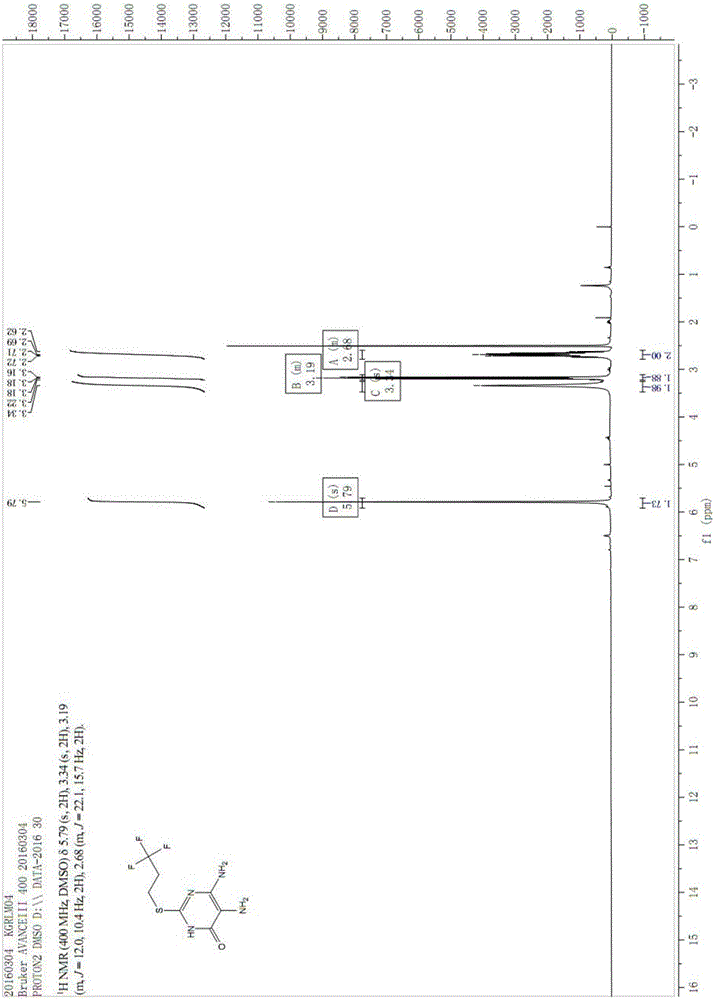
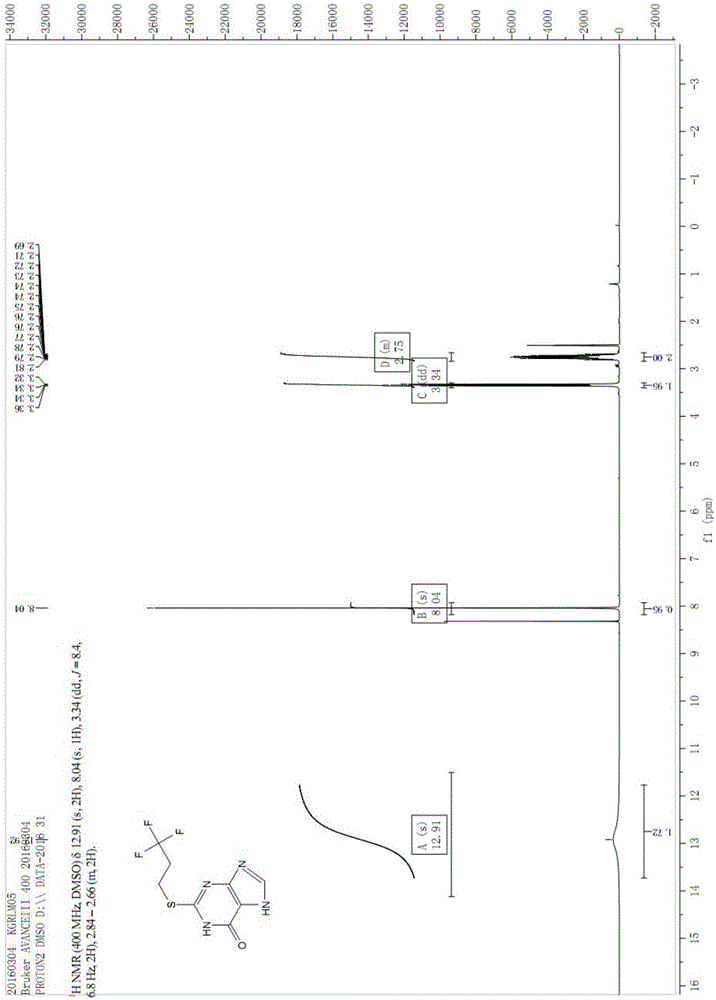
Image
Examples
Embodiment 1
[0066] A preparation method of 6-N-(2-(methylthio)ethyl-2-((3,3,3-trifluoropropyl)thio)adenosine (I), comprising the following steps:
[0067] (1) Preparation of compound 16
[0068] At room temperature, weigh 101.5g ((1.88mol) of sodium methoxide and add it to a 3L reaction bottle of 670g ethanol, stir to dissolve it completely, add 67.3g (0.88mol) of thiourea. Heat to 55°C, weigh ethyl cyanoacetate Add 100g (0.884mol) of the ester into the reaction flask and heat to reflux. After 4.5h of reflux, the TLC reaction is complete, lower to 15°C, filter, wash the filter cake once with 170g of ethanol, take out the filter cake, add it to a 3L reaction flask, and add Stir 1.3kg of water to dissolve it, add acetic acid below 20°C, adjust the pH to 4, and a large amount of solids precipitate out. Suction filtration. The filter cake is dried at 45°C overnight to obtain 115.4g of the product, with a yield of 91.7%.
[0069] (2) Preparation of compound 17
[0070] At room temperature, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com