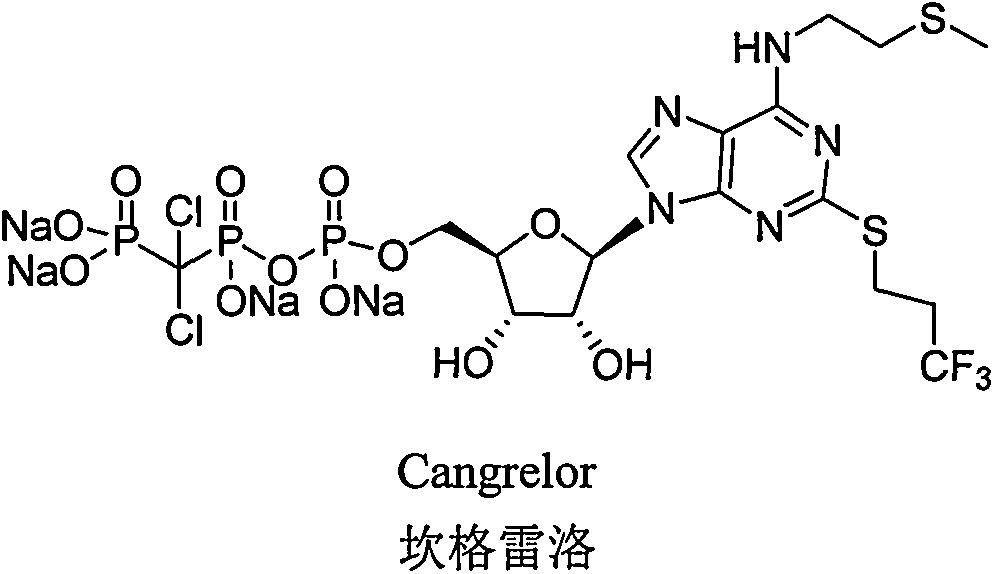
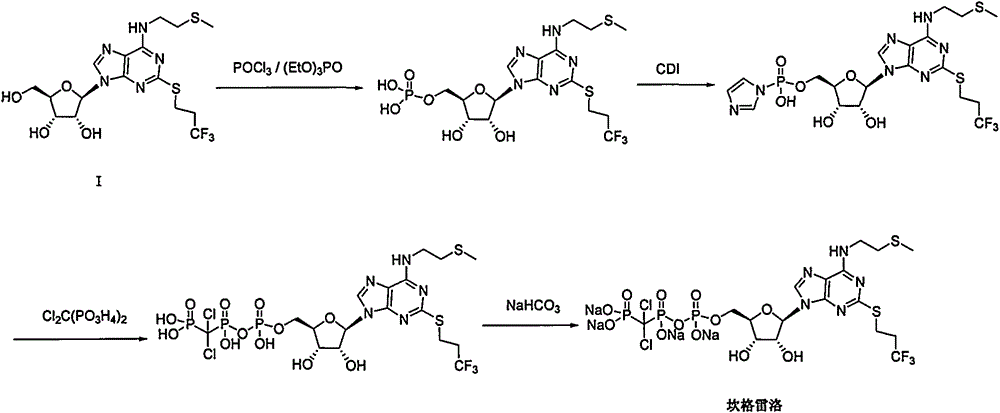
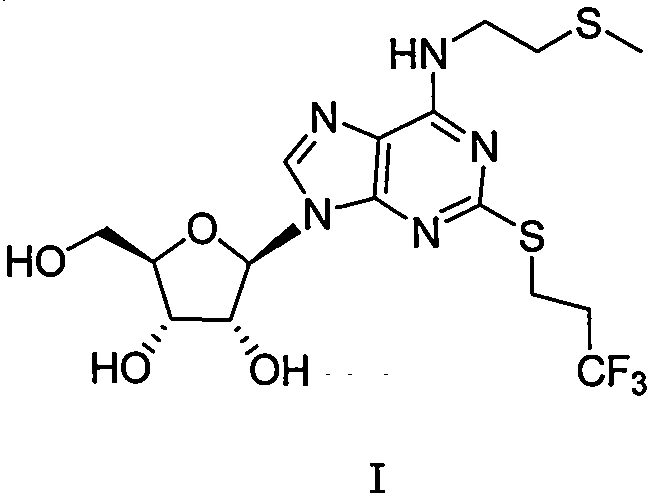
Preparation method of cangrelor intermediate
An intermediate and system technology, applied in the field of chemical preparation of cangrelor intermediates, can solve the problems of increased production cost, difficulty in separation and purification, unfavorable environmental protection and the like, and achieves mild reaction conditions, environmental friendliness, and convenient post-reaction treatment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 6-chloro-5-nitro-4-amino-2-(3,3,3-trifluoropropylthio)pyrimidine (III):
[0035]
[0036] At room temperature, the compound II 4,6-dichloro-5-nitro-2-(3,3,3-trifluoropropylthio)pyrimidine (32.2g, 100mmol) was dissolved in 80ml of absolute ethanol, and the system Add 150ml of ammonia water. The system was transferred to a closed high-pressure valve, and the system was reacted at an external temperature of 80°C for 3 hours, cooled to room temperature and stirred for 1 hour, filtered, washed with 50% ethanol, and dried to obtain 24.8 g of the light yellow title compound, with a yield of 82% . ESI-MS: [M+H] + = 303.61.
Embodiment 2
[0037] Example 2: Preparation of 6-chloro-5-nitro-4-amino-2-(3,3,3-trifluoropropylthio)pyrimidine (III):
[0038] At room temperature, dissolve compound II 4,6-dichloro-5-nitro-2-(3,3,3-trifluoropropylthio)pyrimidine (32.2g, 100mmol) in 80ml tert-butanol, and Add 150ml of ammonia water. The system was transferred to a closed high-pressure valve, and the system was reacted at an external temperature of 30°C for 6 hours, cooled to room temperature and stirred for 1 hour, filtered, washed with 50% tert-butanol, and dried to obtain 25.7 g of the light yellow title compound, yield 85%. ESI-MS: [M+H] + = 303.61.
Embodiment 3
[0039] Example 3: Preparation of 6-chloro-5-nitro-4-amino-2-(3,3,3-trifluoropropylthio)pyrimidine (III):
[0040] At room temperature, compound II 4,6-dichloro-5-nitro-2-(3,3,3-trifluoropropylthio)pyrimidine (32.2g, 100mmol) was suspended in 80ml of water, and 150ml was added to the system ammonia. The system was transferred to a closed high-pressure valve, and the system was reacted at an external temperature of 120°C for 2 hours, cooled to room temperature and stirred for 1 hour, filtered, washed with 50% ethanol, and dried to obtain 24.2 g of the light yellow title compound, with a yield of 80% . ESI-MS: [M+H] + = 303.61.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com