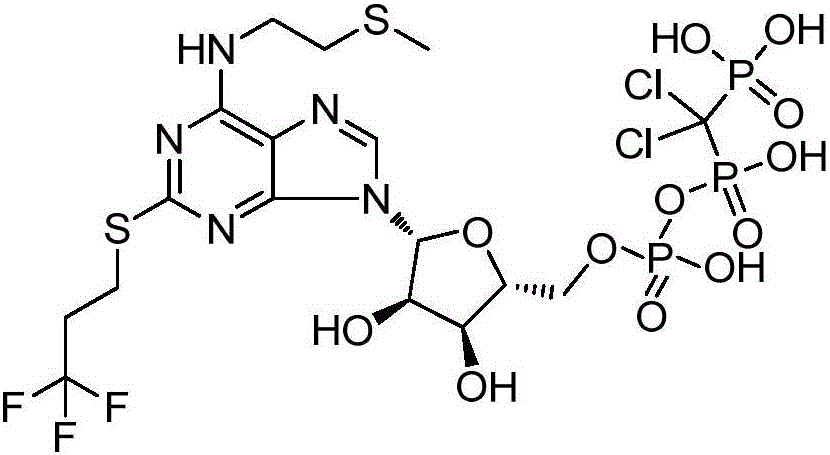
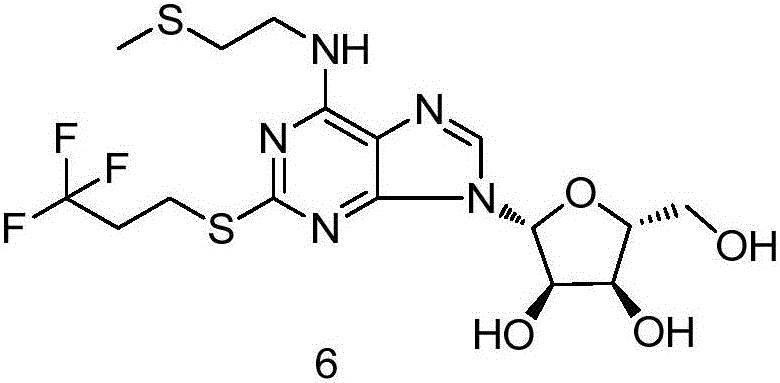
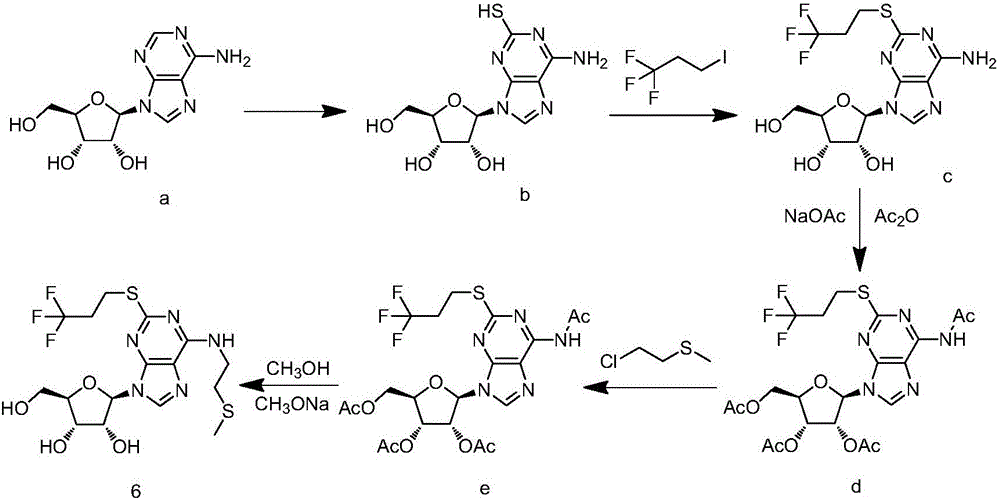
Synthesis method of cangrelor intermediate
A synthesis method and intermediate technology, applied in the field of pharmaceutical chemical synthesis, can solve problems such as unfavorable industrial production, phosphorus oxychloride is highly toxic, and increase reaction cost, so as to be beneficial to industrial production, improve utilization rate, and reduce reaction cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] Add compound 2 (50 g, 0.167 mol) and 0.5 mol / L NaOH solution (840 mL) into a 2 L three-necked flask, and stir for 10 min. Add trifluoroiodopropane (45 g, 0.2 mol) and stir the reaction at room temperature for 4-5 hours. 500 mL of ethyl acetate was added for extraction, the organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain 55.5 g of a yellow solid with a yield of 84%.
Embodiment 2
[0047]
[0048] Add compound 3 (25.3g, 0.064mol), 100mL of acetonitrile, 26g of triethylamine, 0.6g of DMAP into a 500mL three-neck flask, and slowly add 23g of acetic anhydride dropwise under stirring. After the addition, control the temperature within the range of 20-25°C Reaction 0.5h. Add 25 mL of methanol, stir for 30 min, evaporate the solvent under reduced pressure, add 100 mL of isopropanol for slurry, and filter to obtain 31.0 g of compound 4a as a white solid with a yield of 93%.
[0049] The NMR data of compound 4a are: 1H NMR (400MHz, DMSO) δ8.24(s,1H),7.53(s,2H),6.17(d,J=5.1Hz,1H),6.04–5.97(m,1H),5.54(t,J= 5.5Hz, 1H), 4.42–4.34(m, 2H), 4.24(td, J=6.9, 2.8Hz, 1H), 3.29–3.24(m, 2H), 2.79–2.64(m, 2H), 2.11(s ,3H), 2.04(s,3H), 2.00(s,3H).
Embodiment 3
[0051] Prepare compound 4a according to the method for embodiment 2, the charging amount of compound 3 is by 0.064mol, add different bases respectively, adjust the scope of temperature, and real-time monitoring reaction situation; With triethylamine in embodiment 2 as base, temperature is 20-25°C as a control, the results are shown in the following table: when a strong base is used or the reaction temperature is too high, the yield of the target product is reduced due to the formation of by-product 4a'; when the temperature is too low, the reaction time is prolonged, and The reaction is difficult to complete.
[0052]
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com