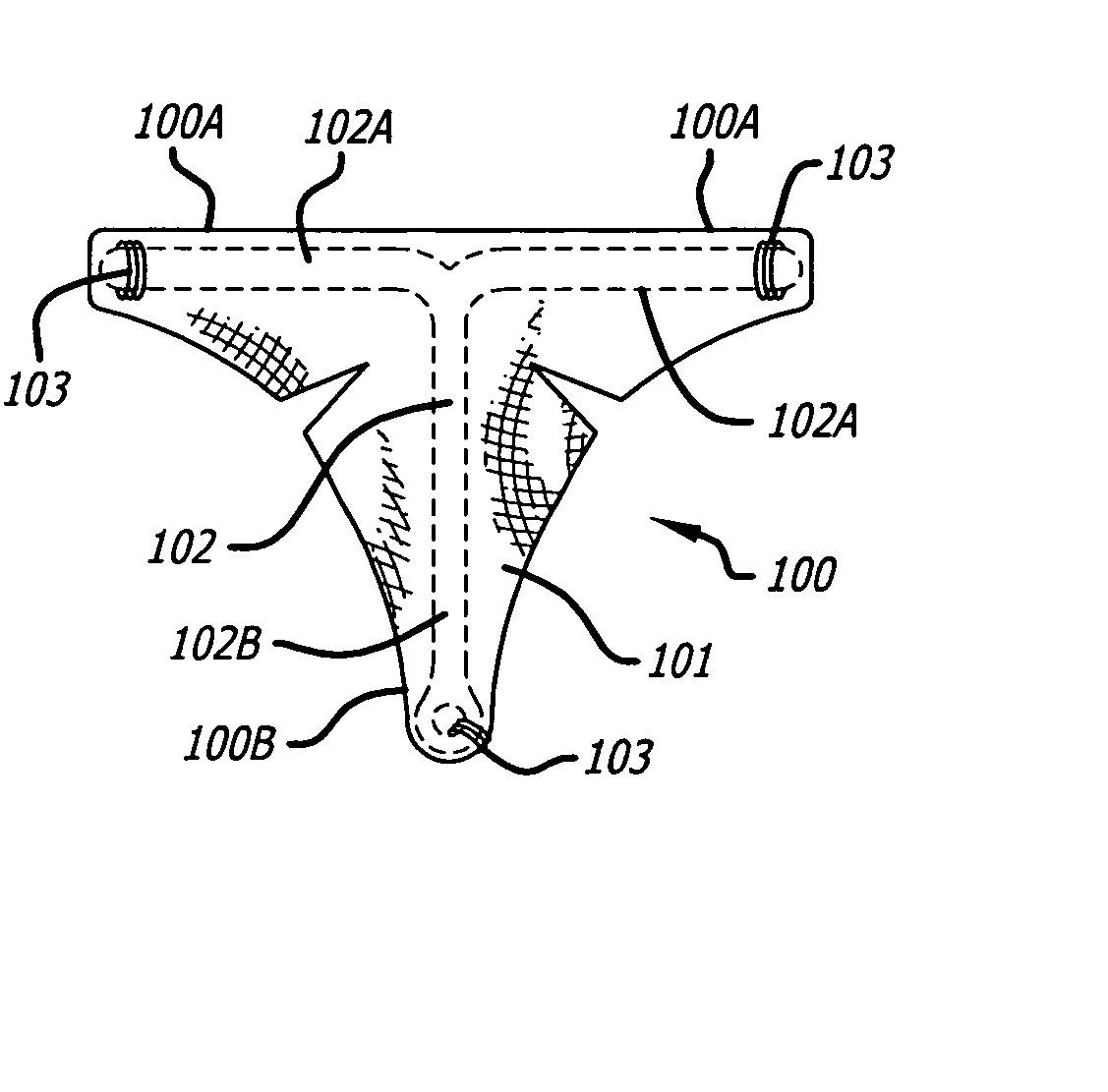
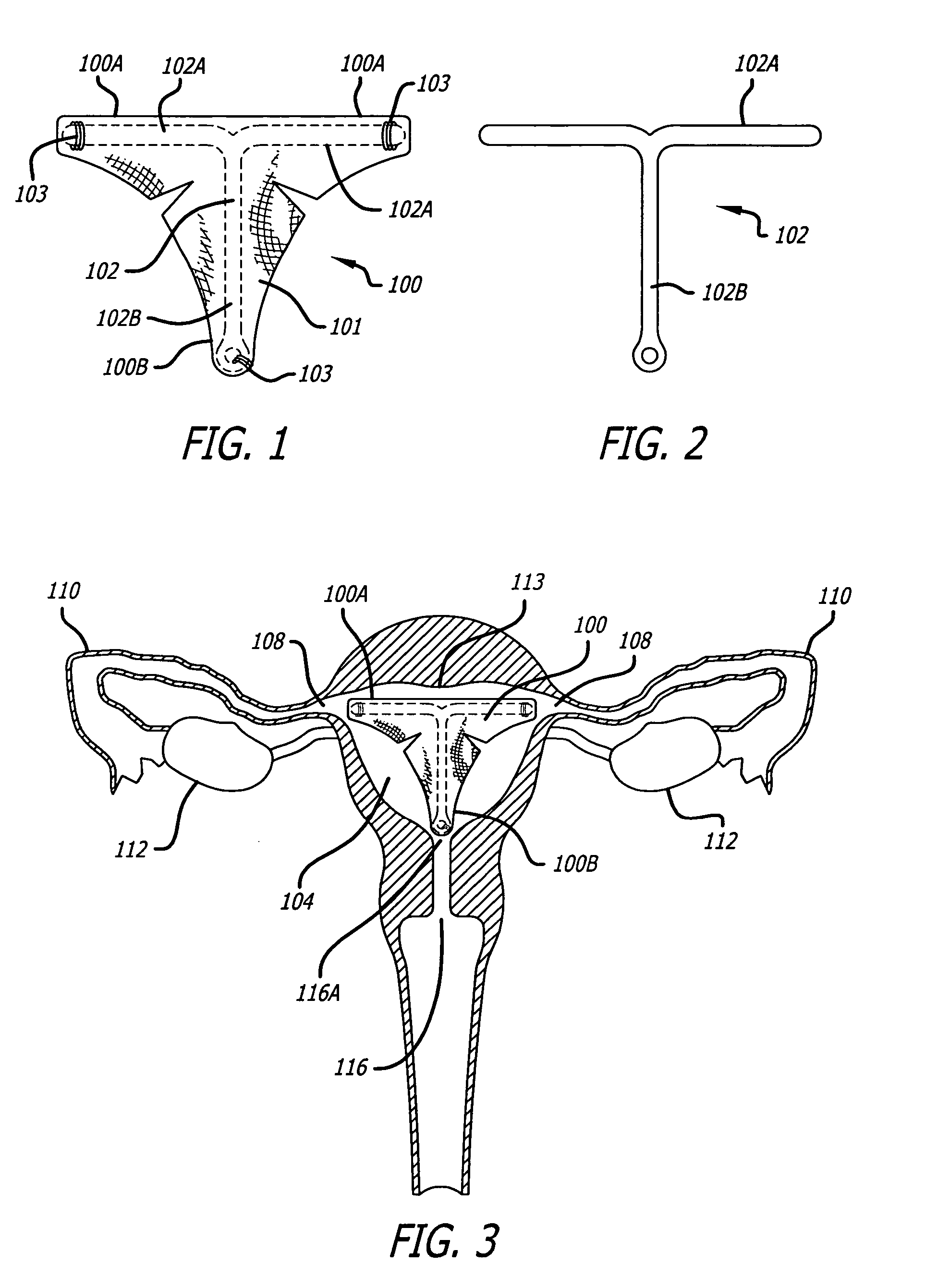
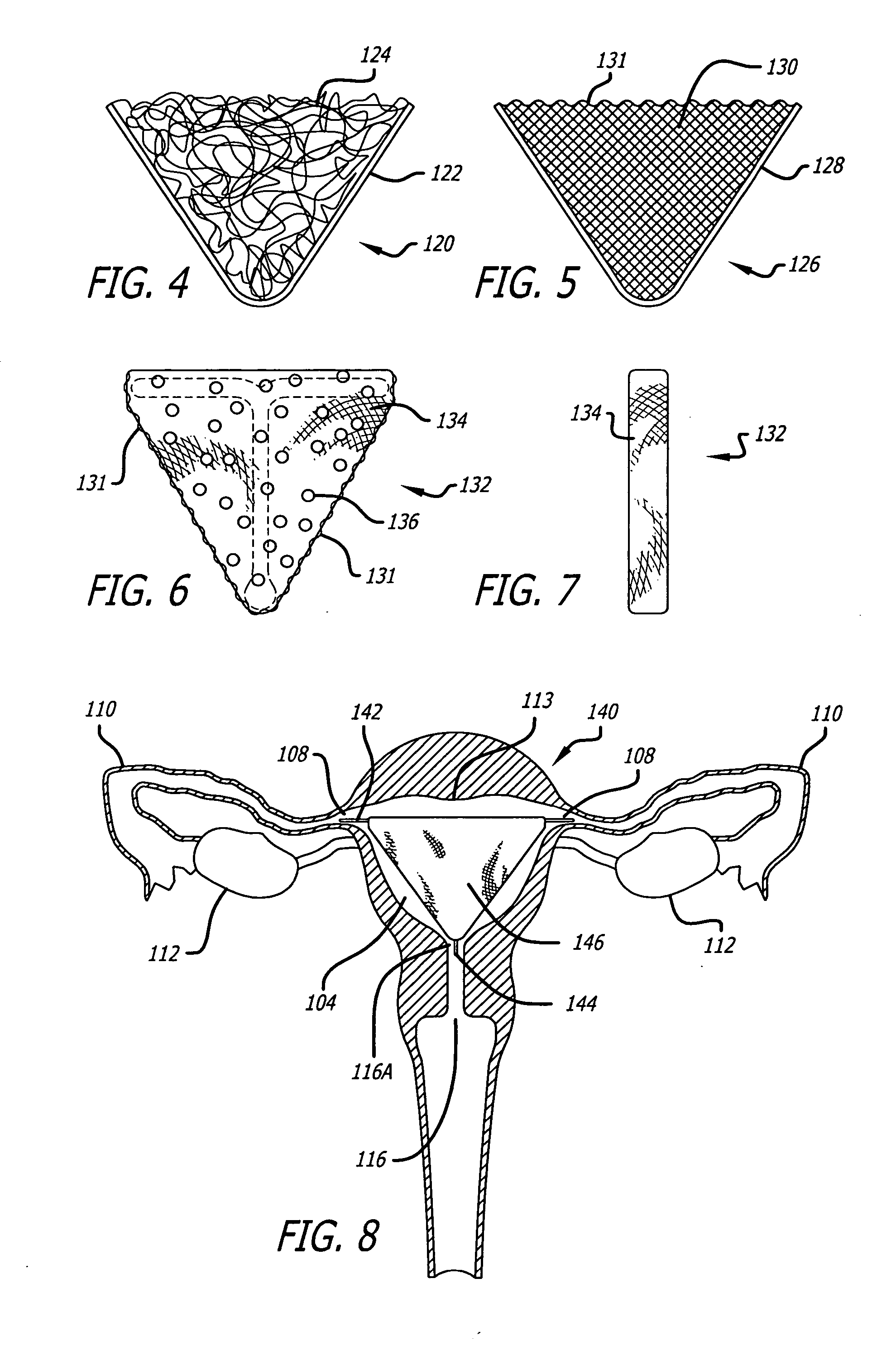
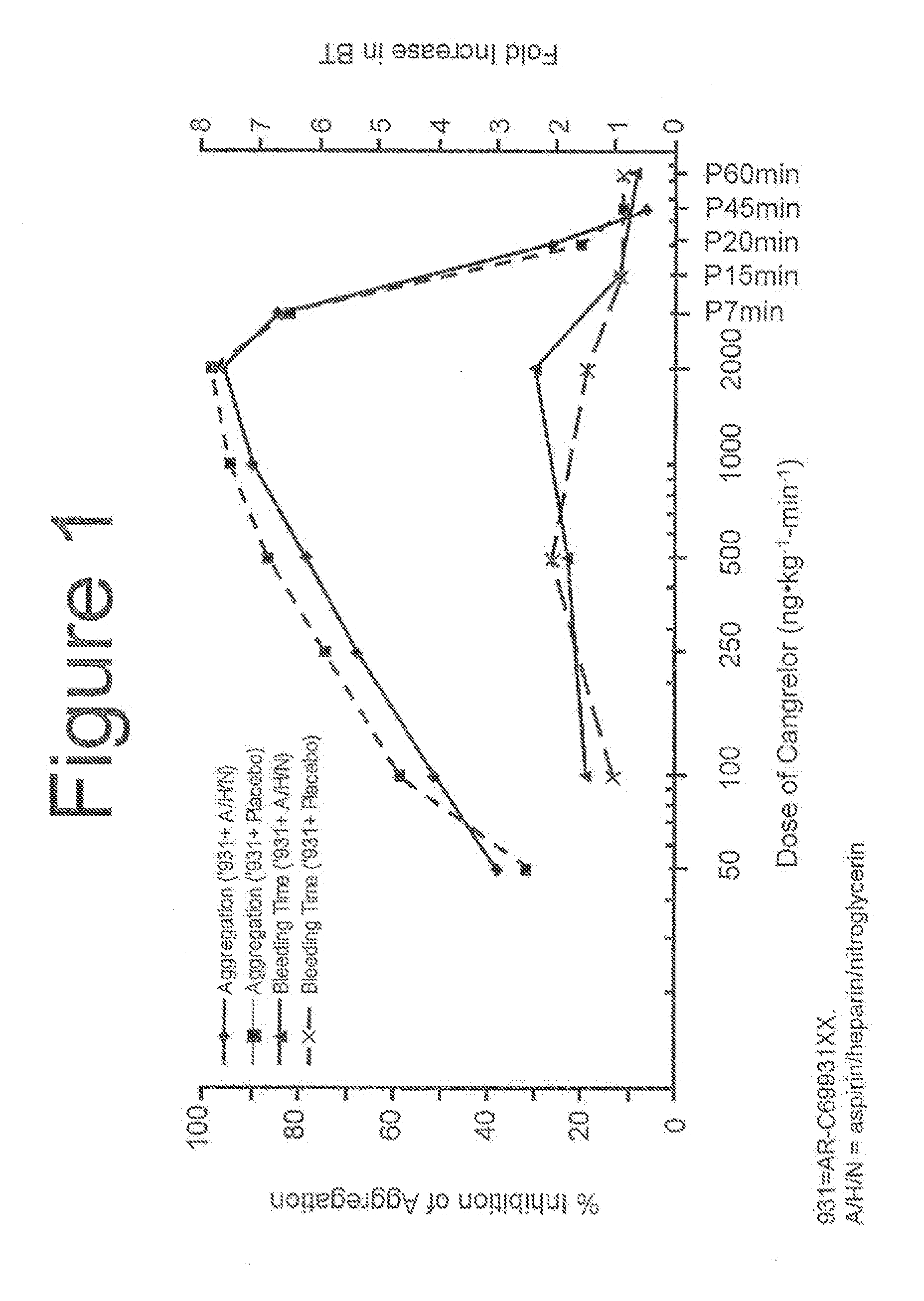
Patents
Literature
91 results about "Excessive Bleeding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Abnormally heavy or prolonged loss of blood.
Method and apparatus for creating intrauterine adhesions
InactiveUS20050171569A1Better treat excessive bleedingPromote tissue growthUltrasonic/sonic/infrasonic diagnosticsSuture equipmentsExcessive BleedingGynecology
In general, the present invention contemplates an implantable device for treating excessive bleeding in a body cavity. The device comprises a biocompatible material, for example polyethylene teraphathalate (PET), which is deliverable into the body cavity. The biocompatible material contains an attribute(s) that promotes tissue reaction or growth that results in a tissue response and / or adhesion formation within the body cavity to reduce or stop the excessive bleeding.
Owner:AUB HLDG
Compositions and methods for treating lacerations, abrasions, avulsions, burns, ulcers, and cases of excessive bleeding
InactiveUS20090148502A1Reducing and ameliorating excessive bleedingReduce controlAntibacterial agentsPowder deliveryExcessive BleedingMedicine
Described herein are compositions and methods related to wound treatment. Compositions are multi-components admixed in amounts and ratios to meet specific objectives for optimally treating various types of wound injury.
Owner:HEMO NANOSCI
Liquid, aqueous, pharmaceutical compositions of factor VII polypeptides
InactiveUS20060063714A1Good storage stabilityPeptide/protein ingredientsInorganic non-active ingredientsFactor VIIaClotting factor deficiency
The invention relates to a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (e.g. human Factor VIIa) and a buffering agent; wherein the molar ratio of non-complexed calcium ions (Ca2+) to the Factor VII polypeptide is lower than 0.5. The composition may further comprise a stabilizing agent (e.g. copper or magnesium ions, benzamidine, or guanidine), a non-ionic surfactant, a tonicity modifying agent, an antioxidant and a preservative. The composition is useful for treating a Factor VII-responsive syndrome, such as bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor XI deficiency, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy.
Owner:NOVO NORDISK AS
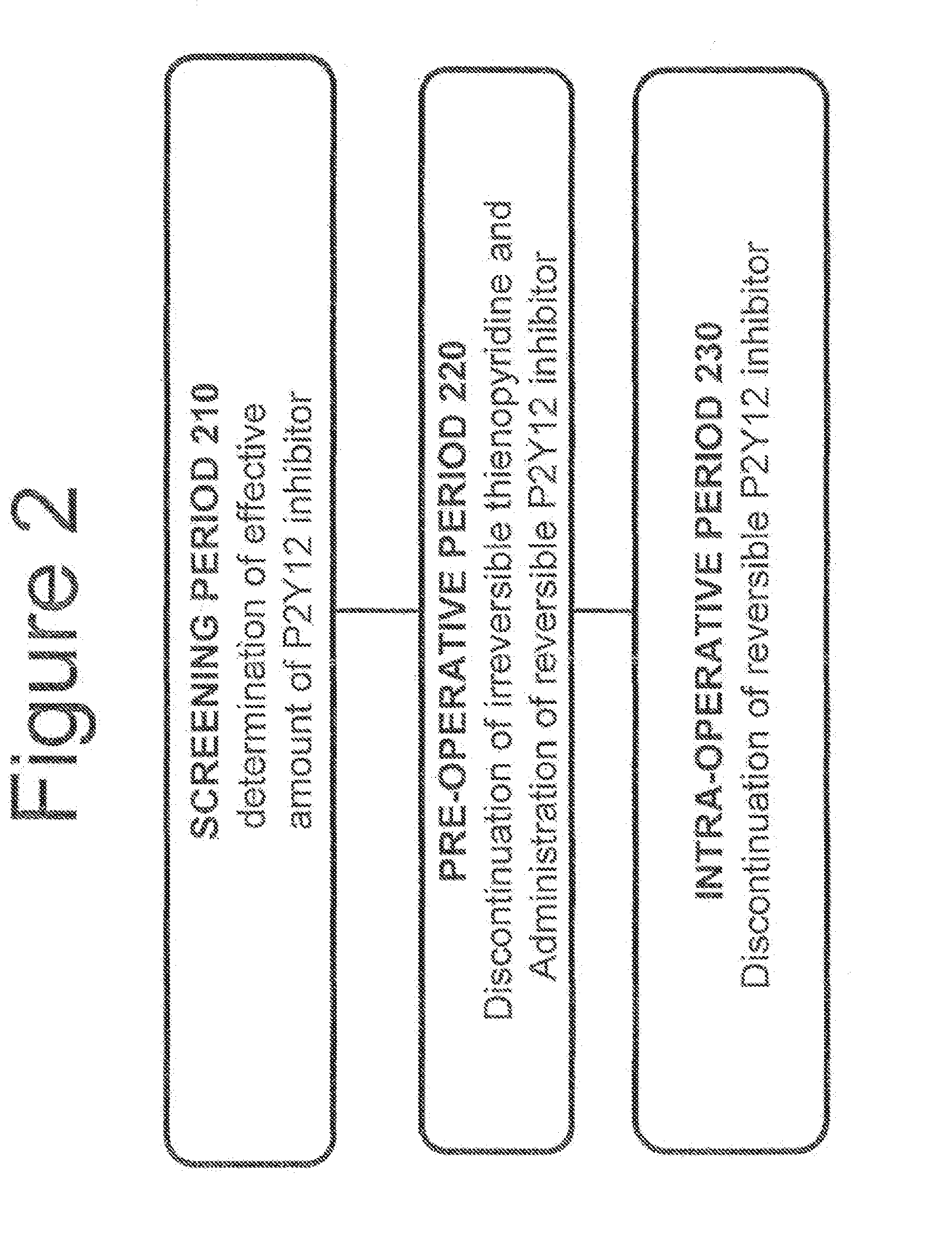
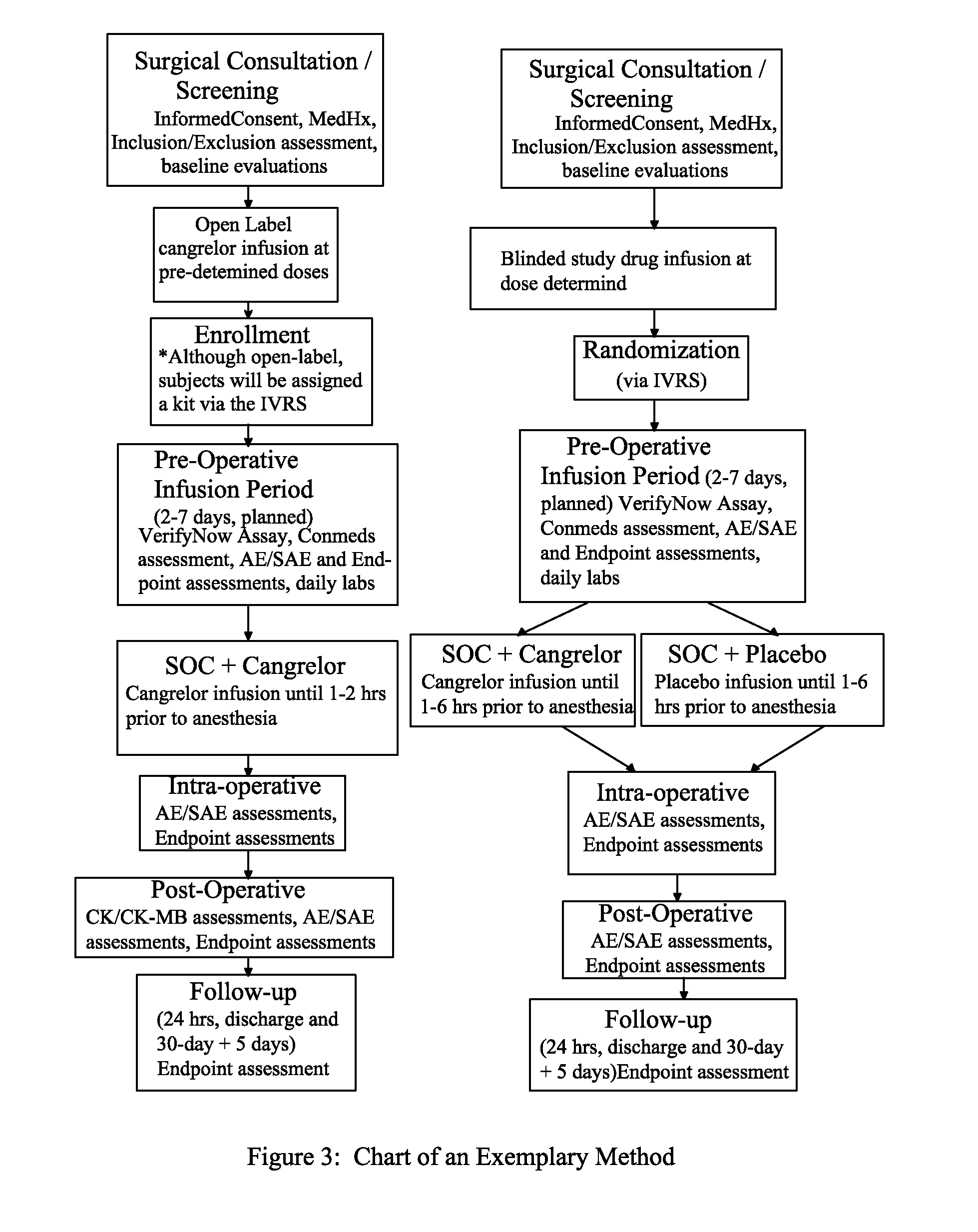
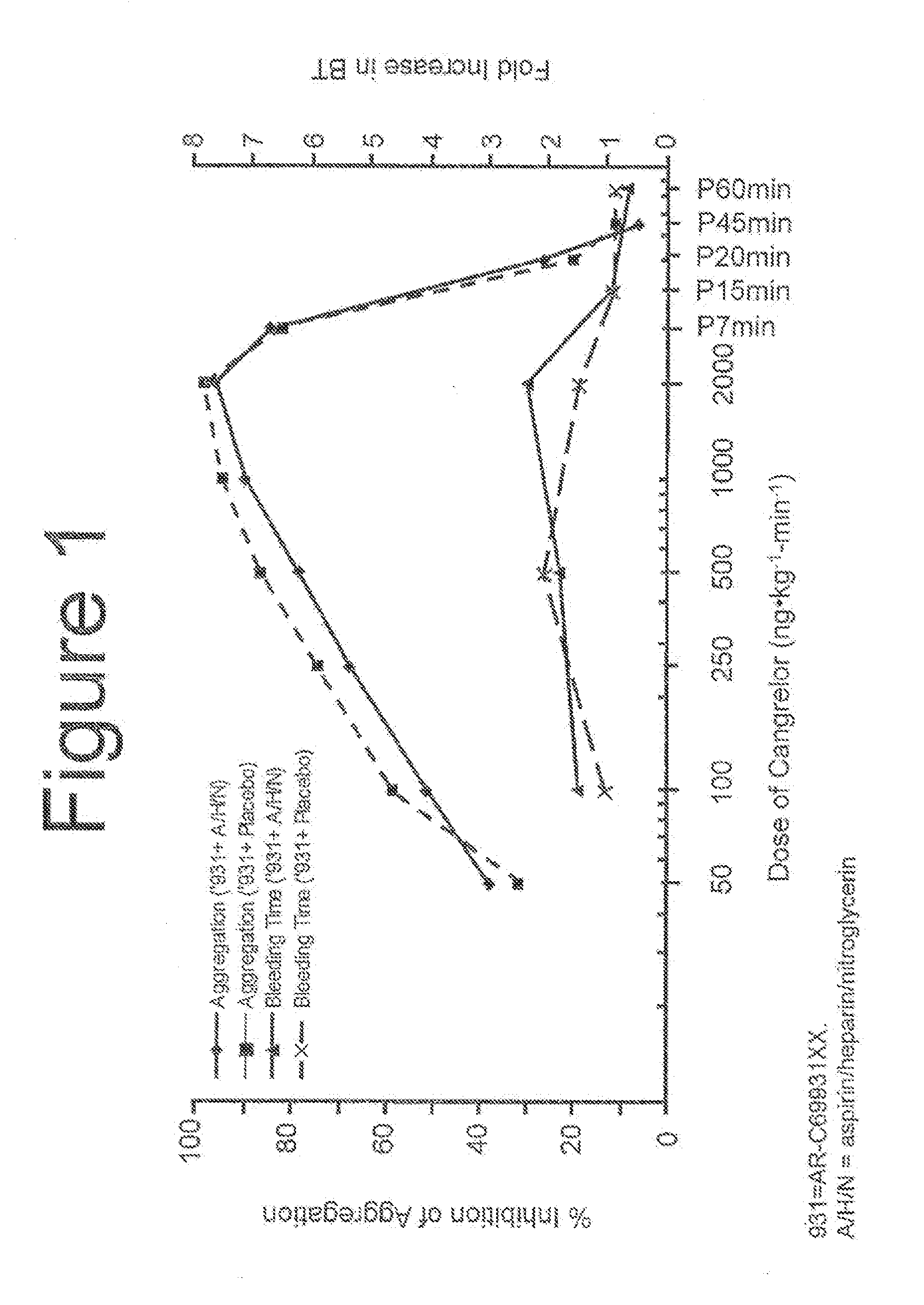
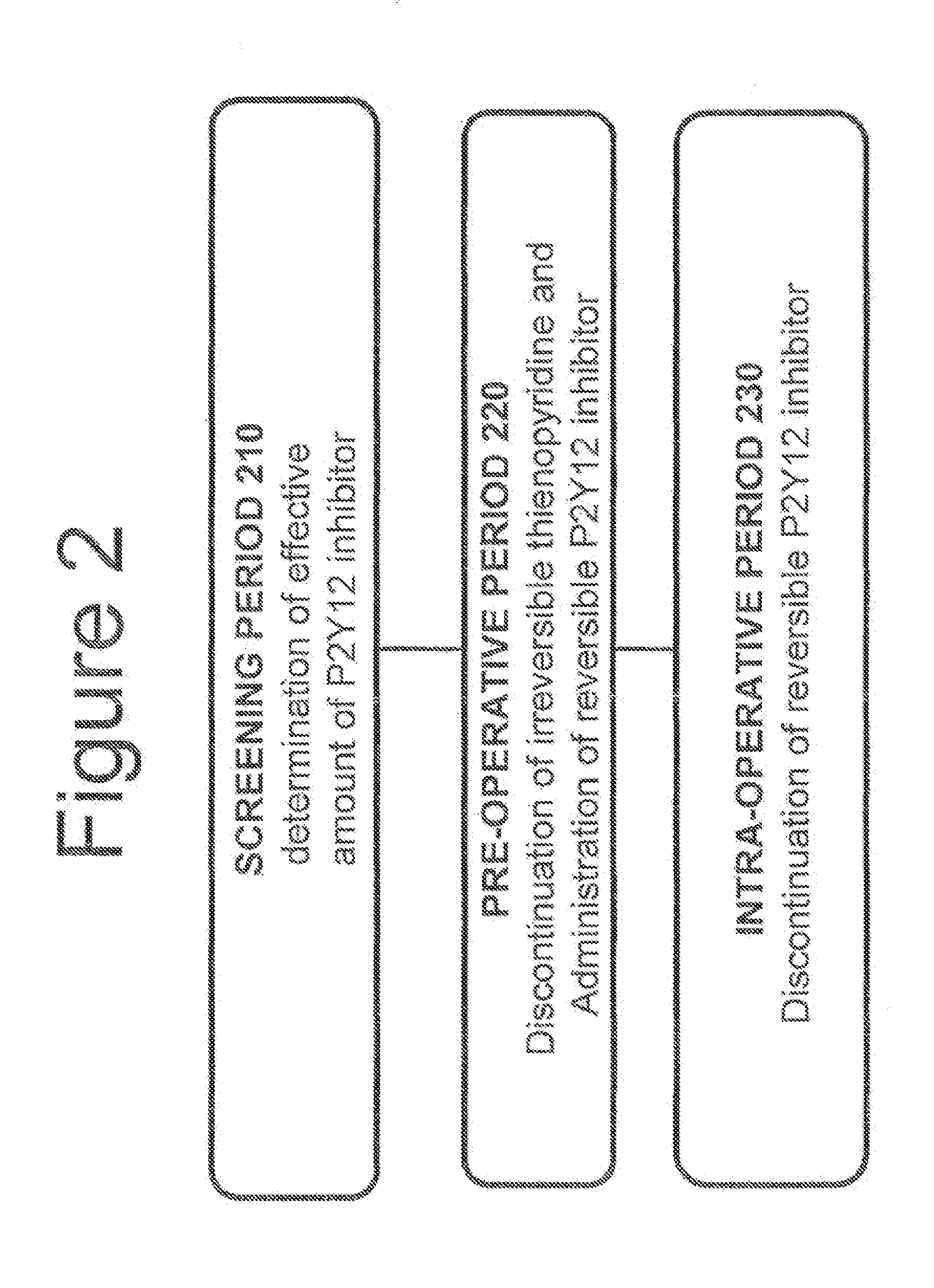
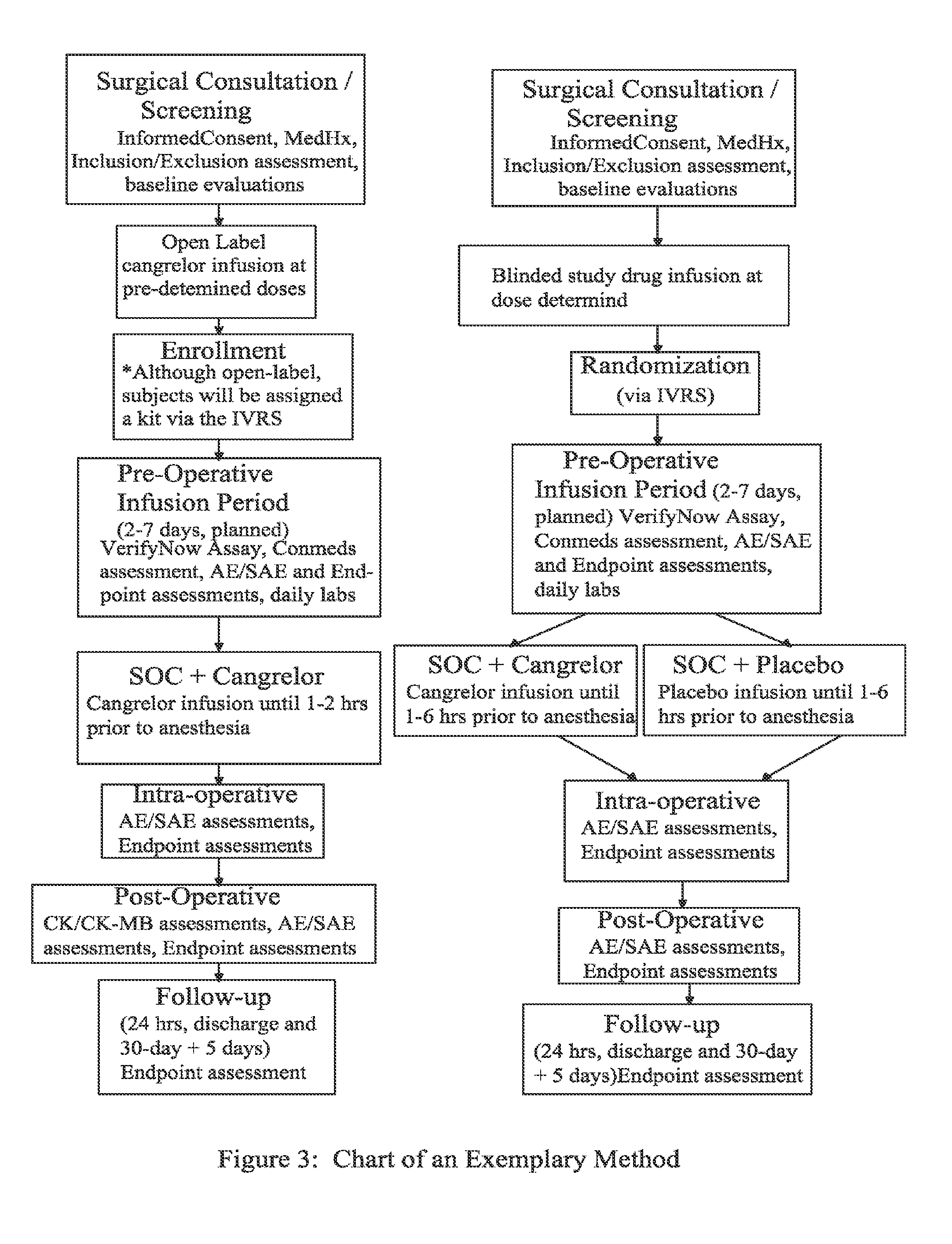
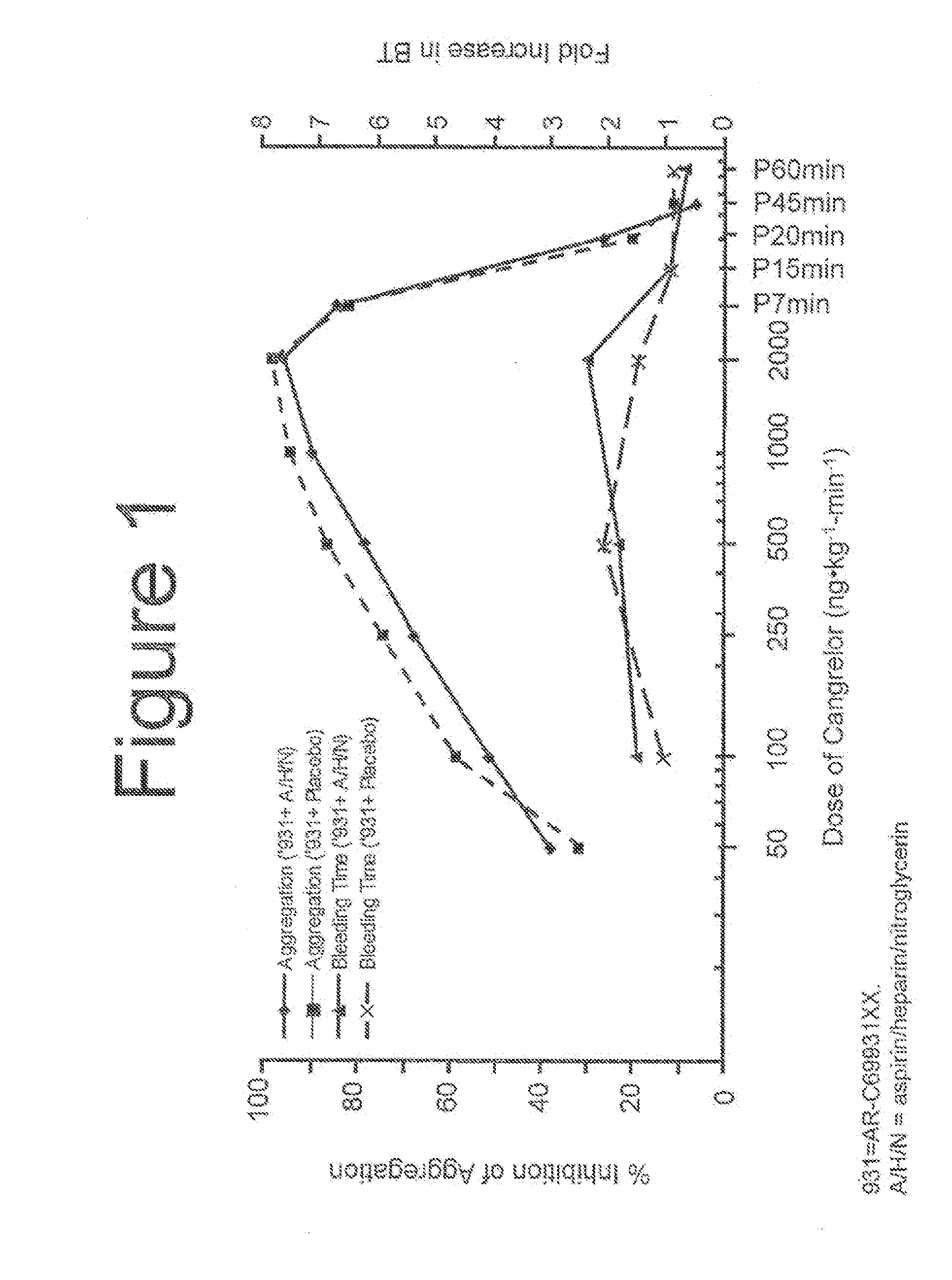
Maintenance of platelet inhibition during antiplatelet therapy
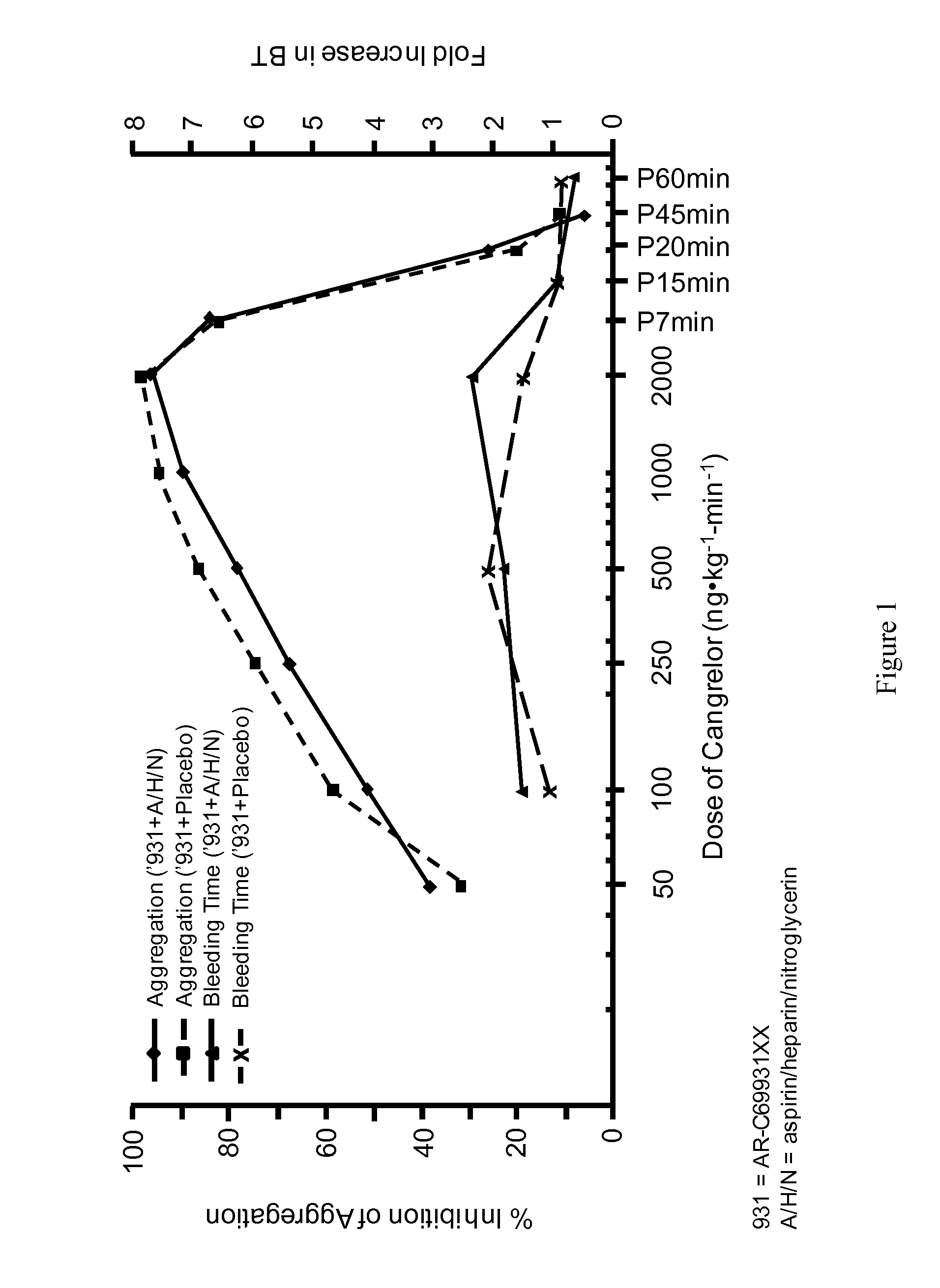
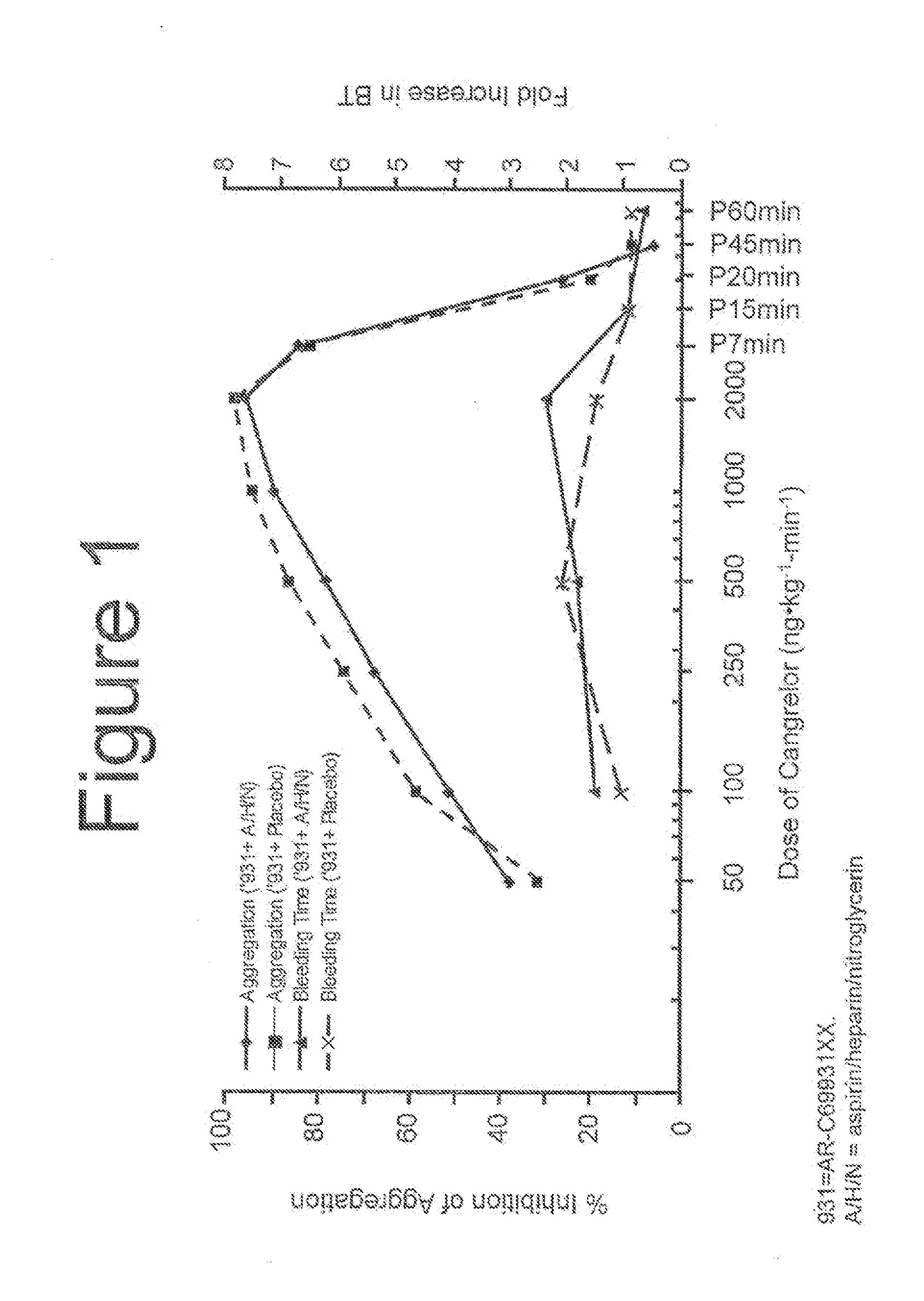
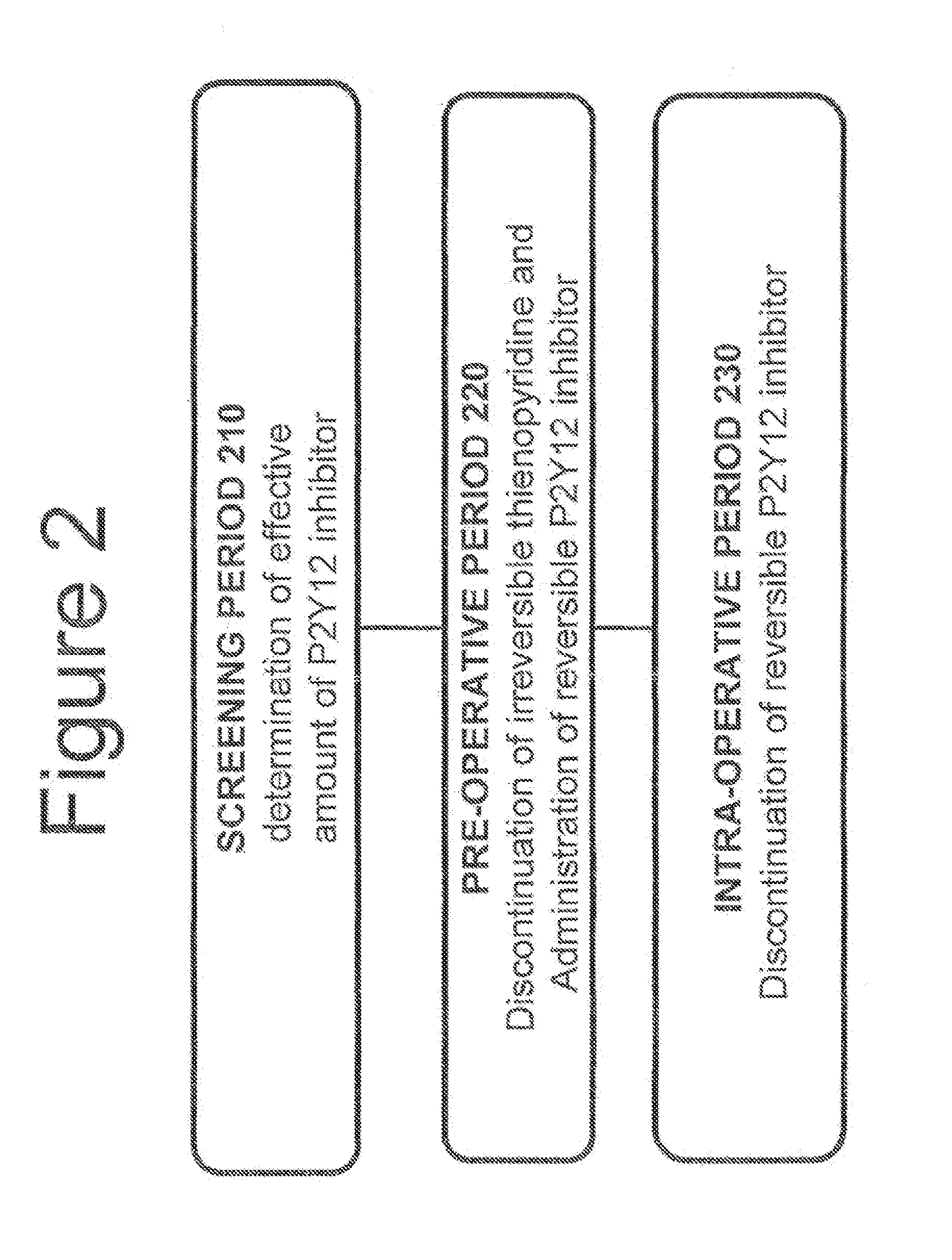
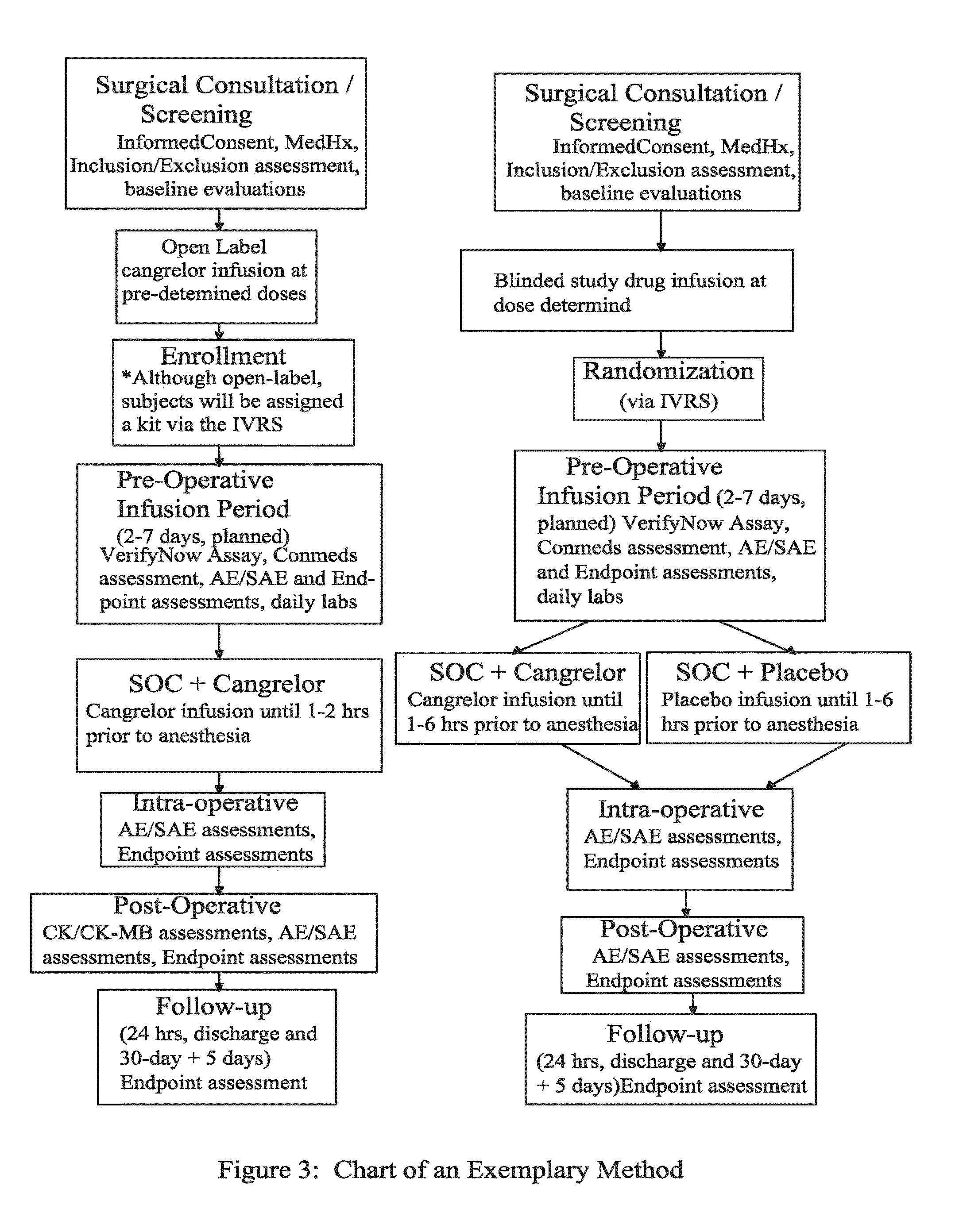
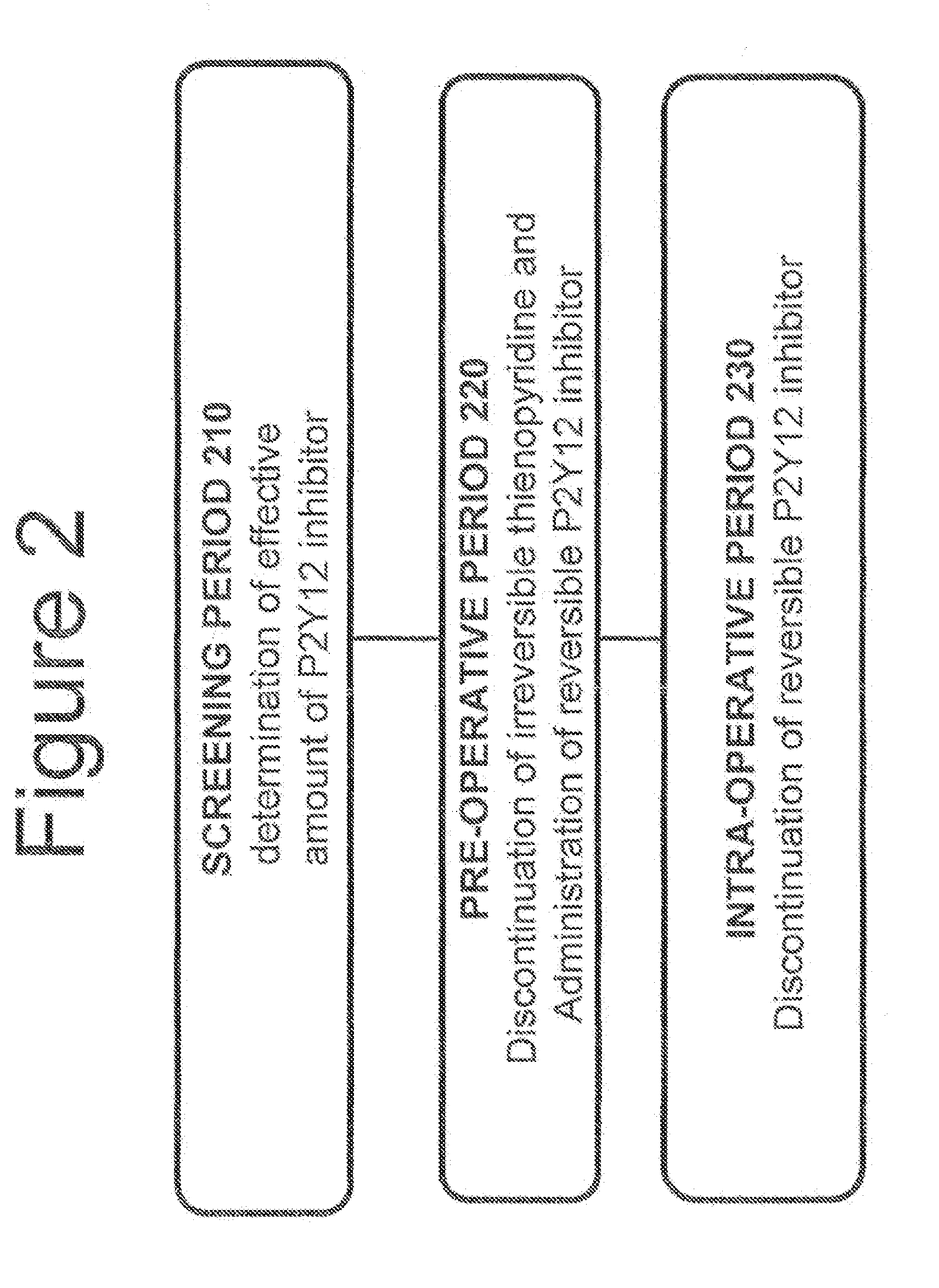
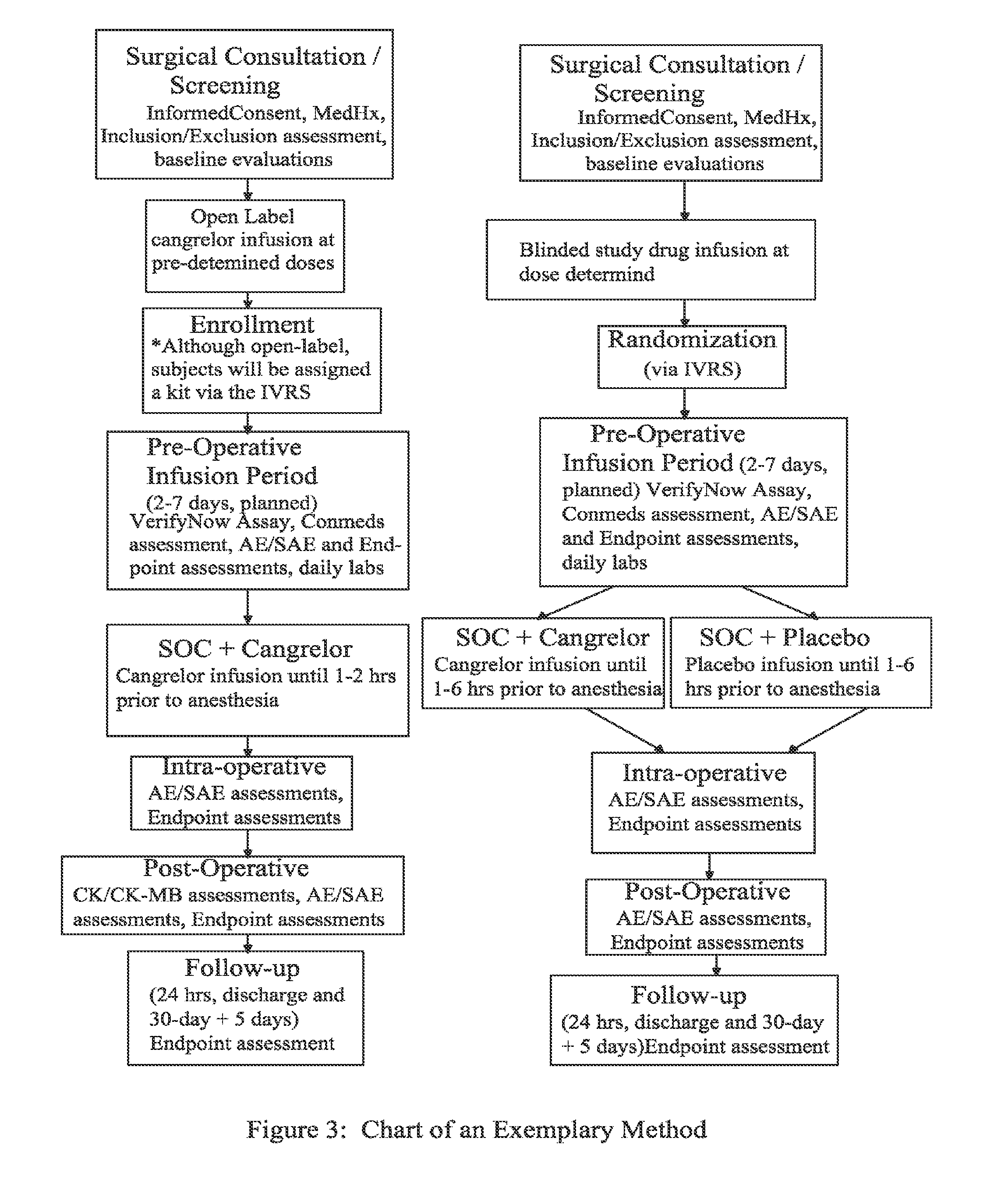
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:THE MEDICINES
Methods of diminishing permanent tissue markings and related apparatus
InactiveUS20080009774A1Efficient alterationDiminishment of permanent tissue markingsUltrasound therapyChiropractic devicesExcessive BleedingCombined use
A method of diminishing permanent tissue markings on a person caused by particles in the dermis includes applying ultrasound radiation to the tissue to generate cavitation bubbles and altering the tissue marking particles by collapse of the cavitation bubbles and transferring energy to the particles. The ultrasound radiation in one embodiment may have a frequency of about 15 KHz to 2 MHz and may be pulsed. The process may be repeated at the same location or other locations while resisting undesired, excessive bleeding of the dermis. The permanent tissue markings may be tattoos. The method may be used in conjunction with other methods of removing the permanent tissue markings, which may include laser, chemical agents, and biological agents. Related apparatus is enclosed.
Owner:UNIVERSITY OF PITTSBURGH
Herbal composition and method of manufacturing such composition for the management of gynecological disorders
A herbal composition enriched with Plant coagulate for the management of Gynecological disorders is envisioned. Also disclosed is a process which involves selective solvent extraction of crude herbs in contrast to conventional aqueous extraction to improve the efficacy. The extract prepared by this method, enriched with Plant coagulate is useful in the management of Gynecological disorders and to prevent / treat anaemia due to excessive bleeding associated with menstrual disorders.The composition comprises Saraca indica, Emblica officinalis, Terminalia chebula, Terminalia belerica, Zingiber officinale, Cyperus rotundus, Pterocarpus santalinus, Berberis aristata, Cuminum cyminum, Adhatoda vasica, Nelumbo nucifera, Piper longum, Symplocos racemosa, Woodfordia fruticosa, Mangifera indica, Spinacia oleracea, Amaranthus, Trifolium alaxandrum and Vigna sinensis.
Owner:DABUR RESEARCH FOUNDATION
Hemostatic system and components for extracorporeal circuit
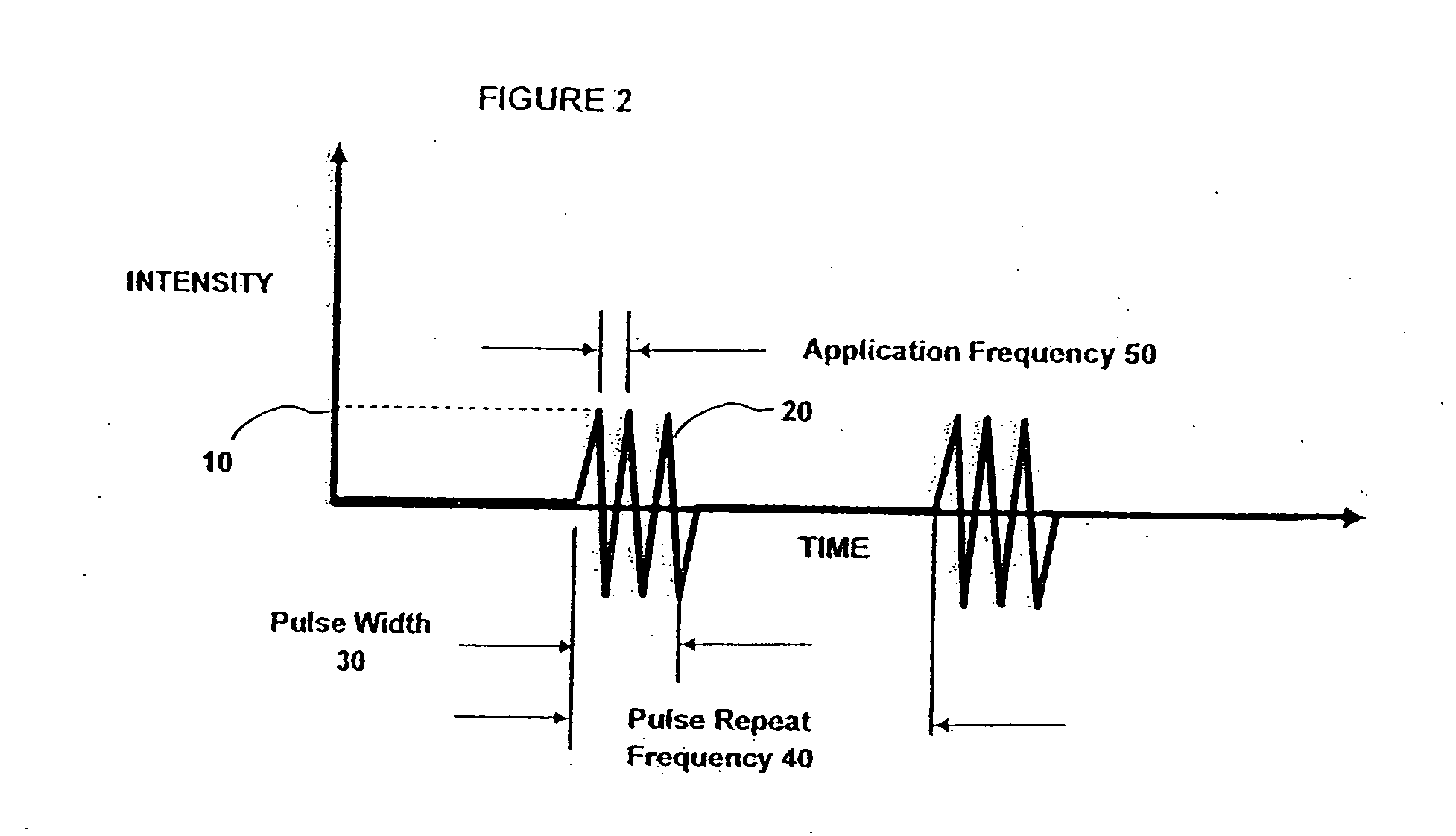
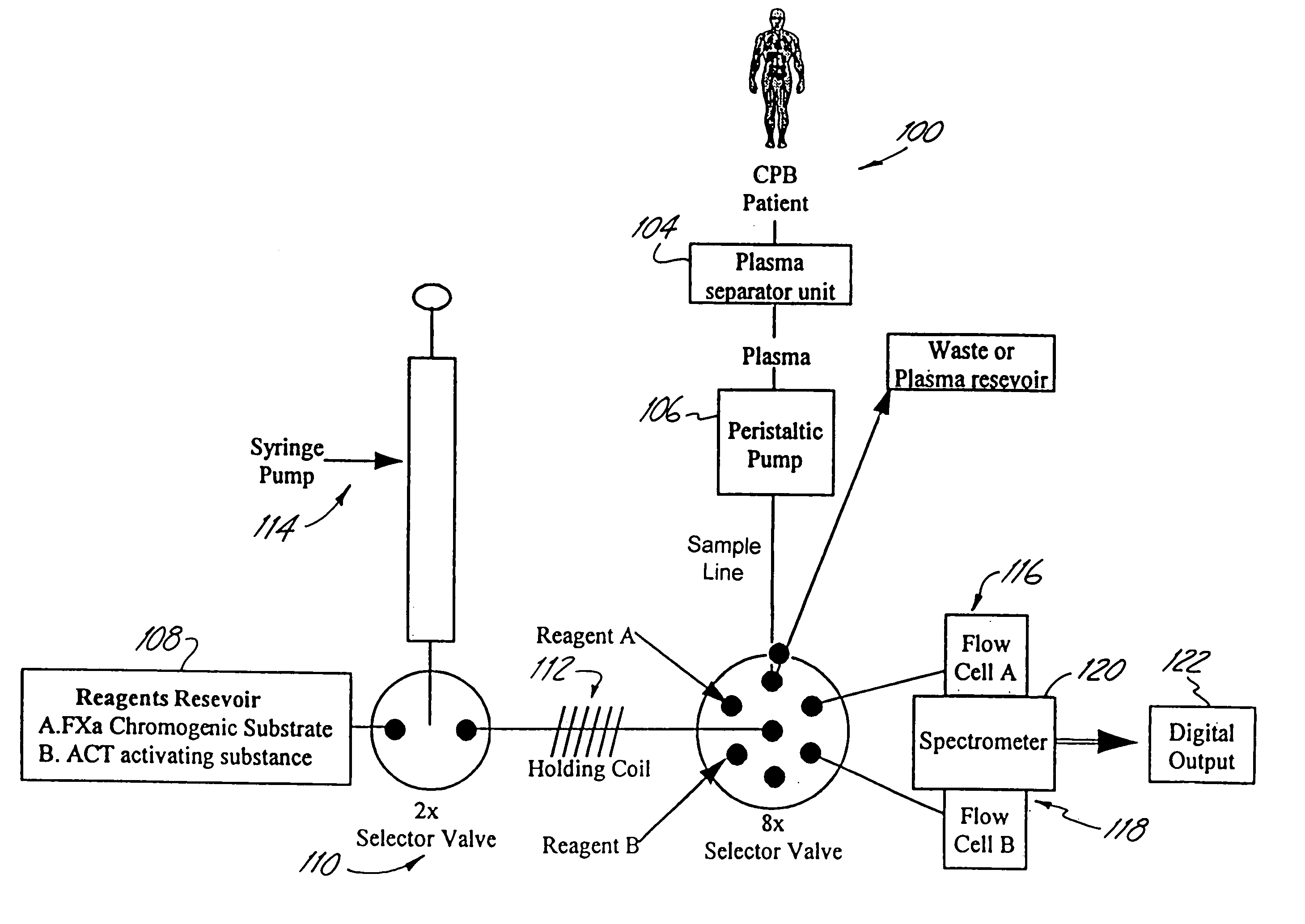
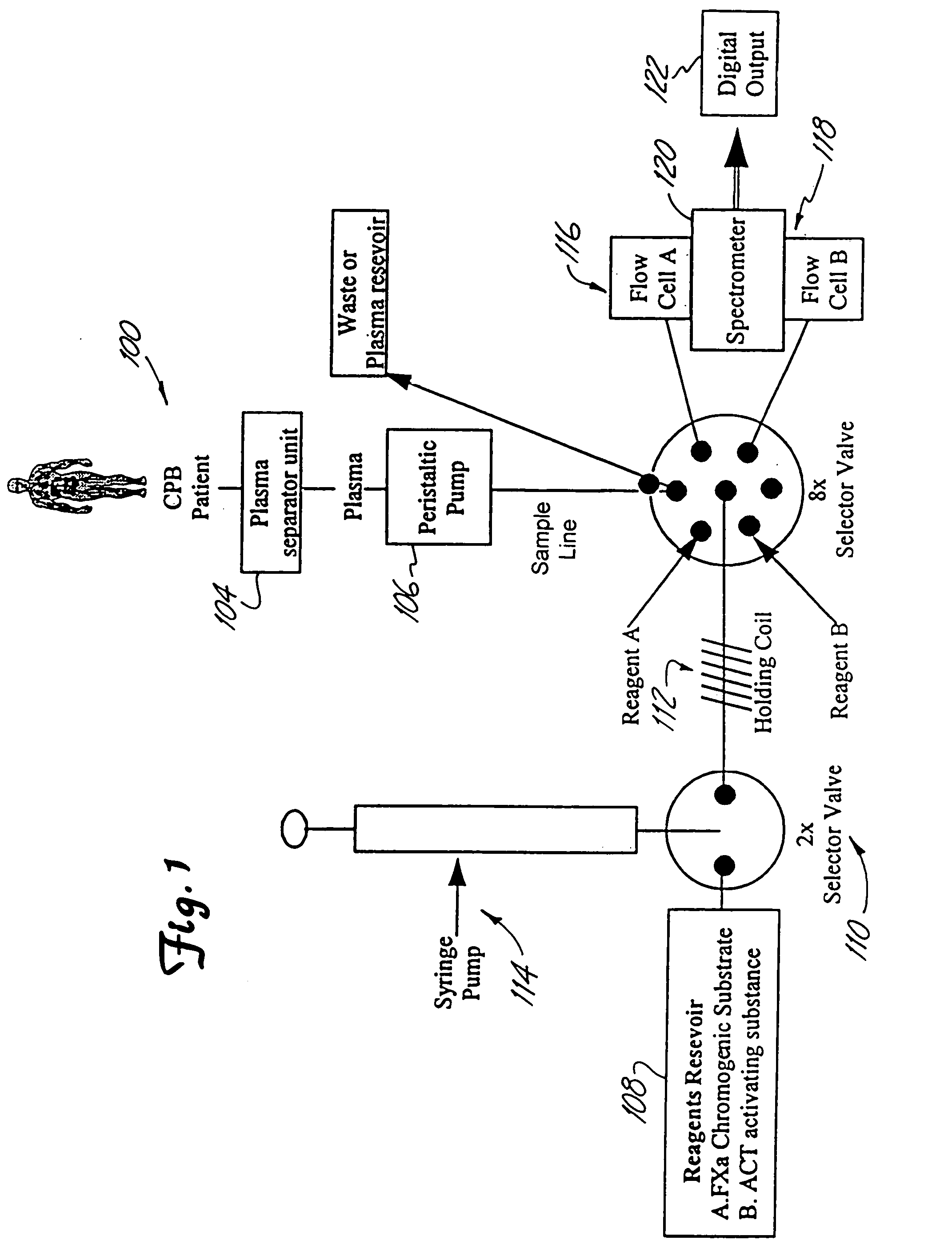
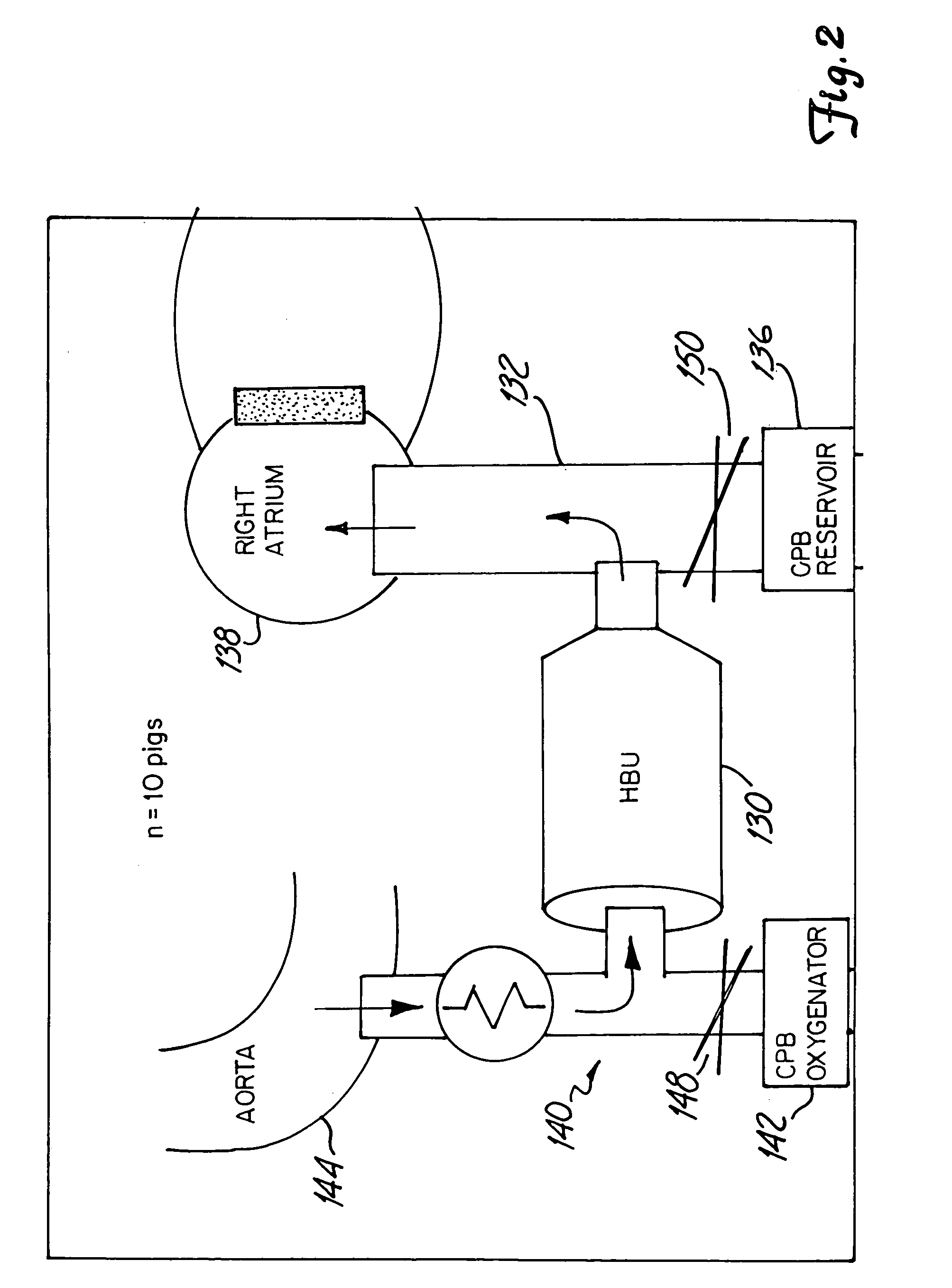
InactiveUS7153473B2Reduce or minimize inflammation, excessive bleedingEasy to adaptSolvent extractionOther blood circulation devicesCardiopulmonary bypassExtracorporeal circulation
A method and system for use in the course of extracorporeal blood flow, e.g., cardiopulmonary bypass, dialysis, and angioplasty procedures, in order to reduce or minimize inflammation, excessive bleeding, and other undesirable side effects. The system can include one or more automated blood parameter sensor modules and one or more blood parameter regulating modules. The system is particularly well suited to monitor and / or regulate blood parameters that include blood analytes (e.g., biomolecules, drugs or metabolites) as well as blood functions (e.g., clotting time, fibrinolytic activity, immune response). The system is particularly well suited for use in the management of clotting (e.g., heparing / protamine) and bleeding (e.g., aprotinin).
Owner:MEDTRONIC INC
Maintenance of platelet inhibition during antiplatelet therapy
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:CHIESI FARM SPA
Herbal composition and method of manufacturing such composition for the management of gynecological disorders
A herbal composition enriched with Plant coagulate for the management of Gynecological disorders is envisioned. Also disclosed is a process which involves selective solvent extraction of crude herbs in contrast to conventional aqueous extraction to improve the efficacy. The extract prepared by this method, enriched with Plant coagulate is useful in the management of Gynecological disorders and to prevent / treat anaemia due to excessive bleeding associated with menstrual disorders.
Owner:DABUR RESEARCH FOUNDATION
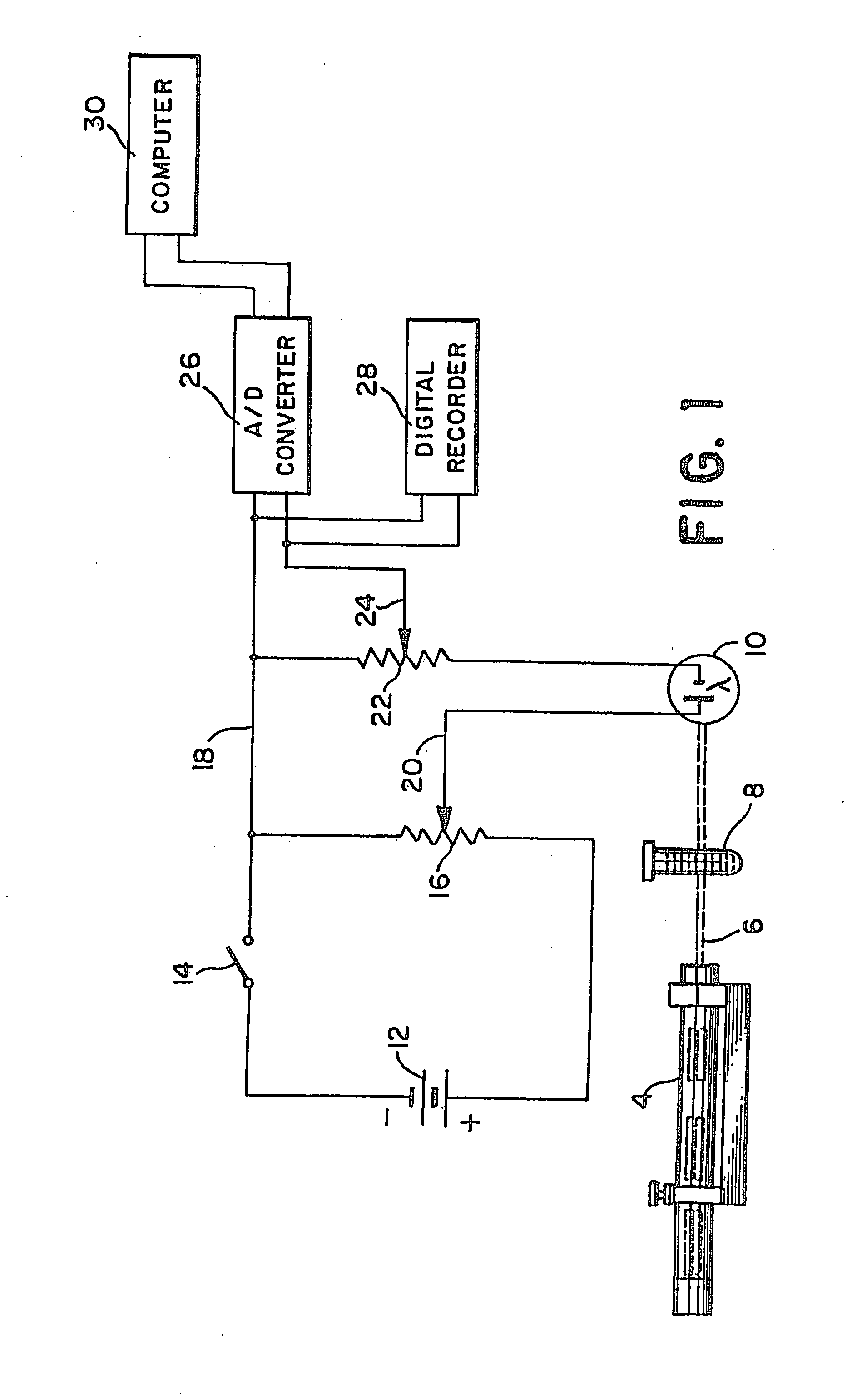
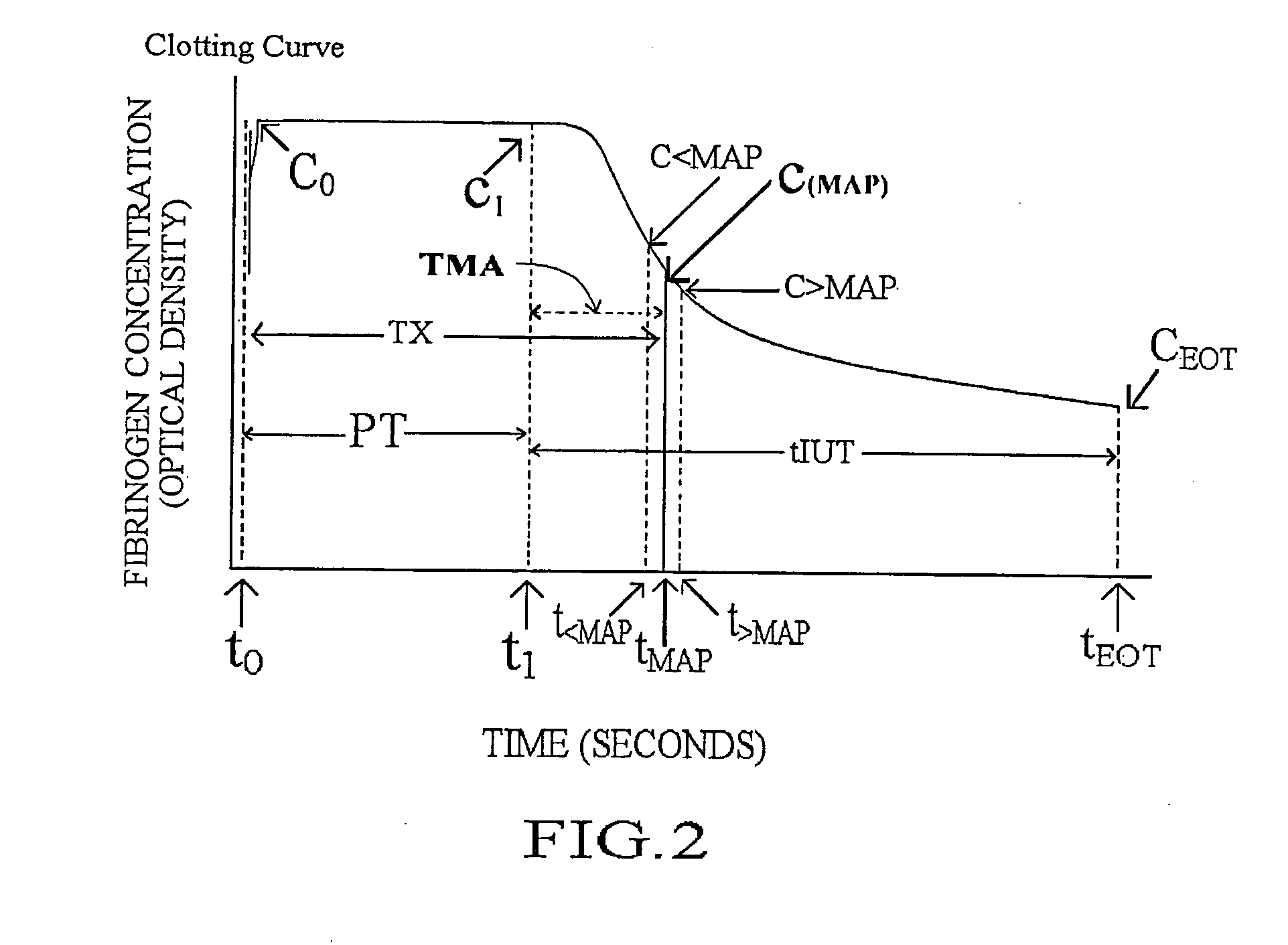
Method and apparatus for determining anticoagulant therapy factors
InactiveUS20110224292A1BiocideAnalysis by subjecting material to chemical reactionAnti coagulationCoagulation reagent
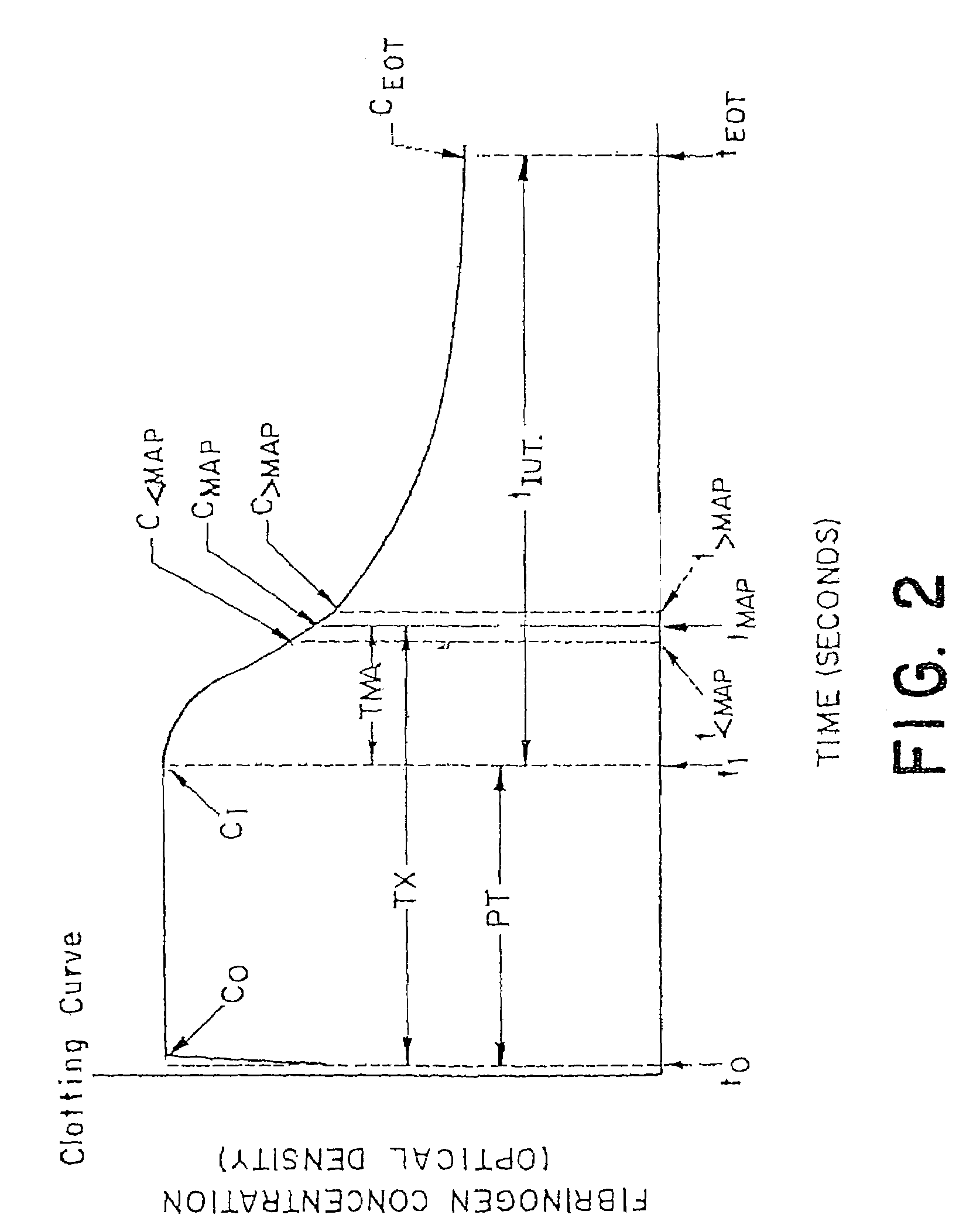
Methods and apparatus are disclosed for determining new anticoagulant therapy factors for monitoring oral anticoagulant therapy to help prevent excessive bleeding or deleterious blood clots that might otherwise occur before, during or after surgery. The inventive methods and apparatus provide an International Normalization Ratio (INR) based on a coagulation reaction with a blood sample of a living being. Embodiments include methods and apparatus for determining an anticoagulant therapy factor without requiring use of a mean normal prothrombin time determination or an ISI, and may be carried out with the patient sample and a coagulation reagent, where the coagulation reagent may be selected from a number of coagulation reagents. One embodiment provides an INRs value which is determined from a prothrombin time (PT or T1) of a patient blood sample and a theoretical end of test time (TEOT), where a theoretical clotting area is used to determine the INRs value according to the expression, INRs=T1*TEOT*MUL, where MUL is a multiplier that takes into account pixel parity and sampling times. The INRs may be used to determine a course of treatment for a patient or other living being without regard to the specific coagulation regent used to generate the coagulation data (e.g., time and optical activity values).
Owner:WADA
Method for producing aqueous polyurethane synthetic leather
ActiveCN105463867APrevent penetrationFeel goodPattern makingFibre treatmentExcessive BleedingCalcium formate
A method for producing an aqueous polyurethane synthetic leather is as below: impregnating a fabric by using a mixed liquid of calcium formate and calcium chloride, drying the rolling liquid to obtain a pretreated fabric; adding a foam coagulant, calcium formate and pigment in the aqueous polyurethane, mixing evenly to obtain an aqueous polyurethane slurry; scraping the slurry on the pretreated aqueous polyurethane fabric to obtain an aqueous polyurethane synthetic leather base; and subjecting the aqueous polyurethane synthetic leather base to aqueous surface treatment and vacuum line suction to obtain the aqueous polyurethane synthetic leather. The method employs a mixed liquid of calcium formate and calcium chloride for impregnating treatment on the fabric, so as to prevent excessive bleeding of the slurry in the adhesive layer into the fabric and influence on the handle feel of the synthetic leather; the aqueous surface treatment and vacuum line suction post-finishing process endow the aqueous polyurethane synthetic leather with good handle feel and mechanical properties. The present invention uses environmentally friendly materials and clean production process; and the obtained synthetic leather has content of any solvent less than 5ppm, and meets the requirements of ecological synthetic leather.
Owner:江西中望实业有限公司
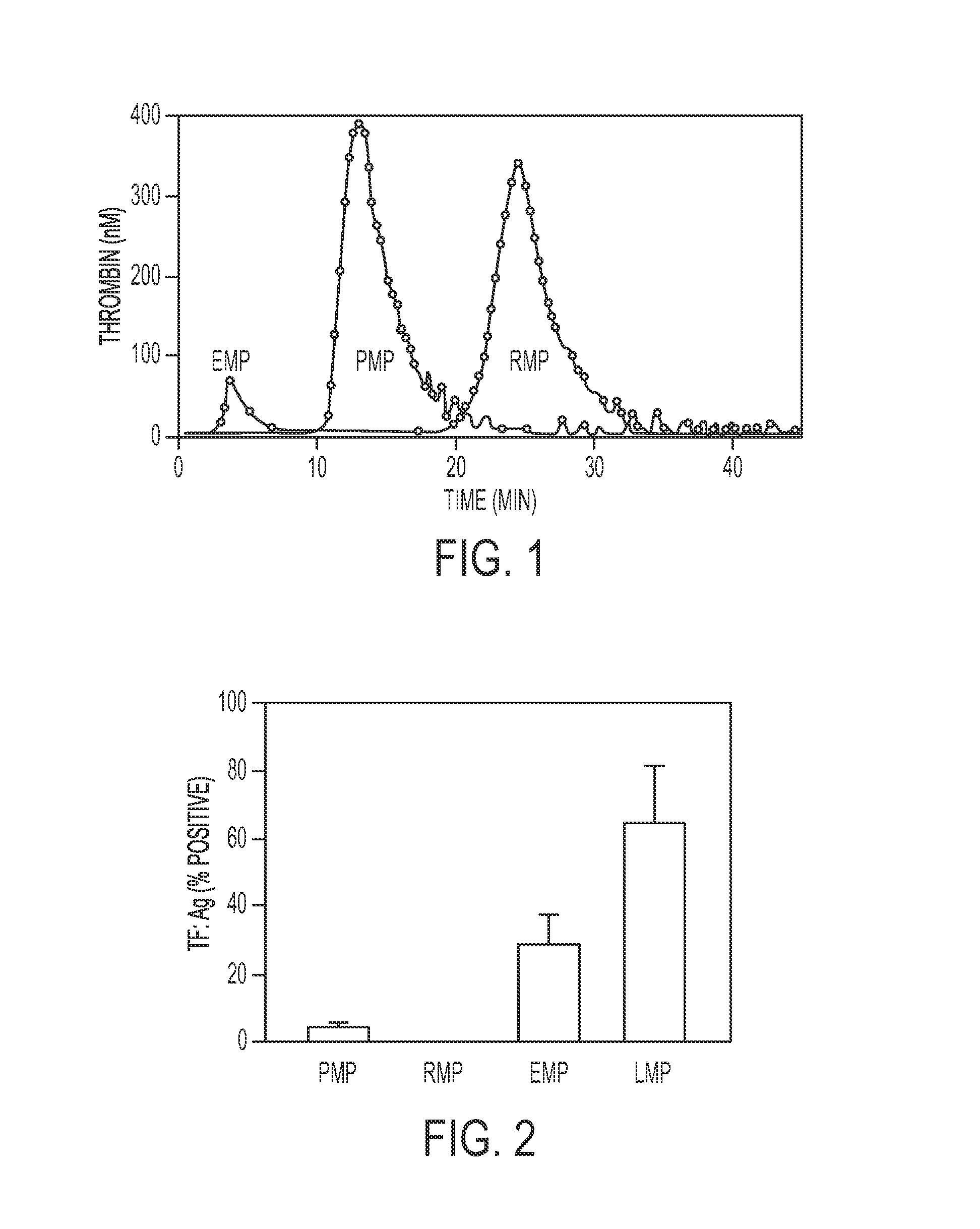
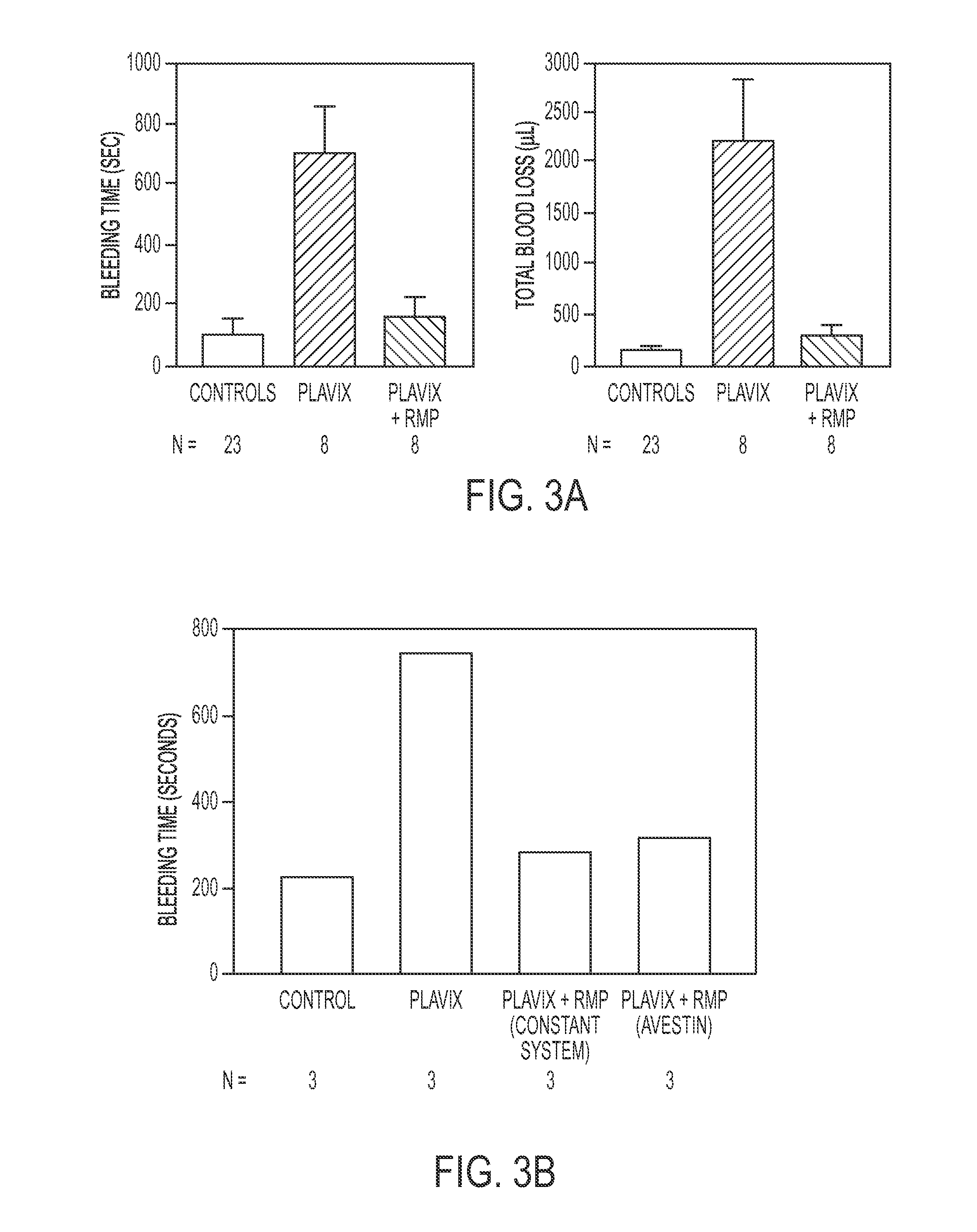
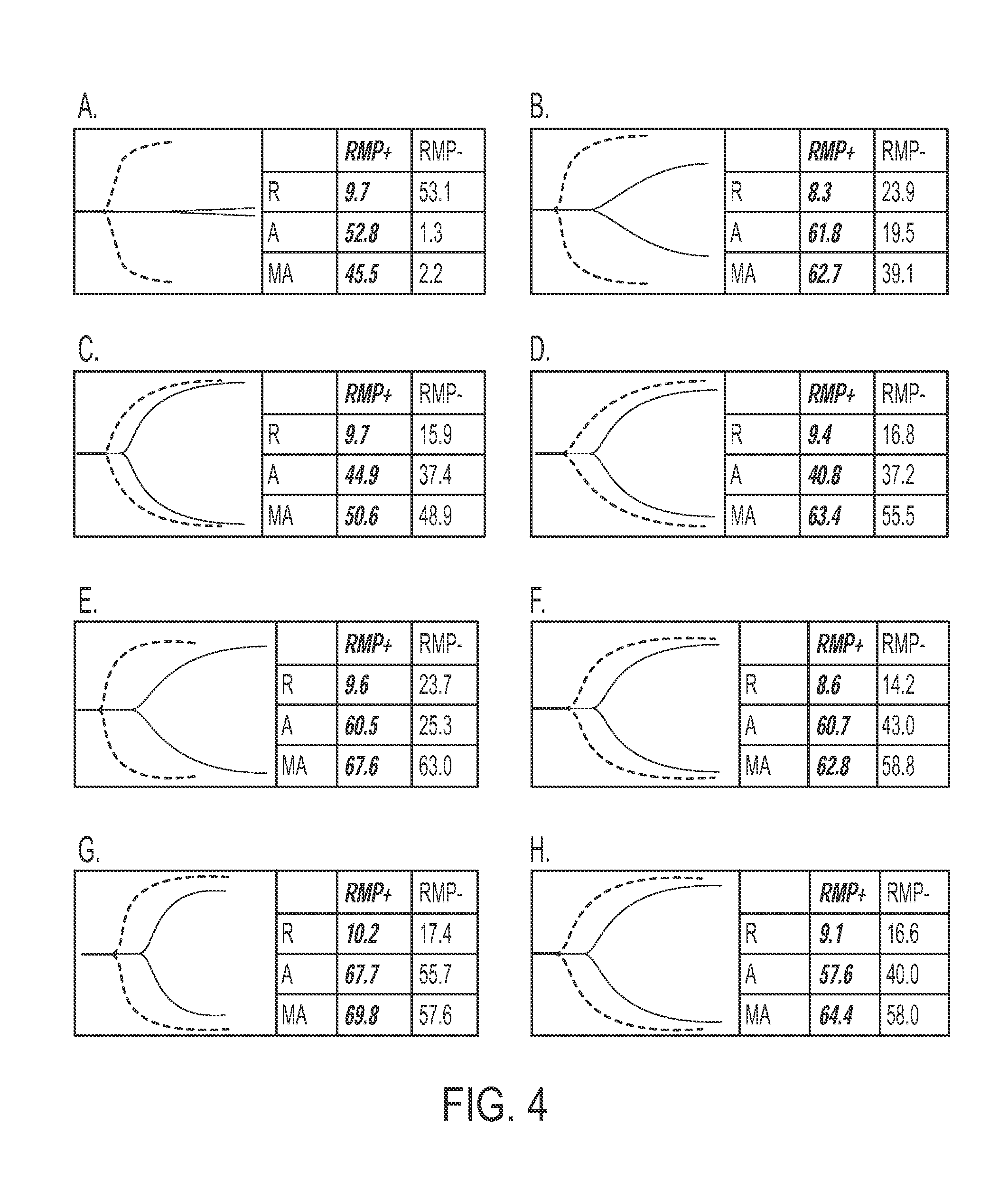
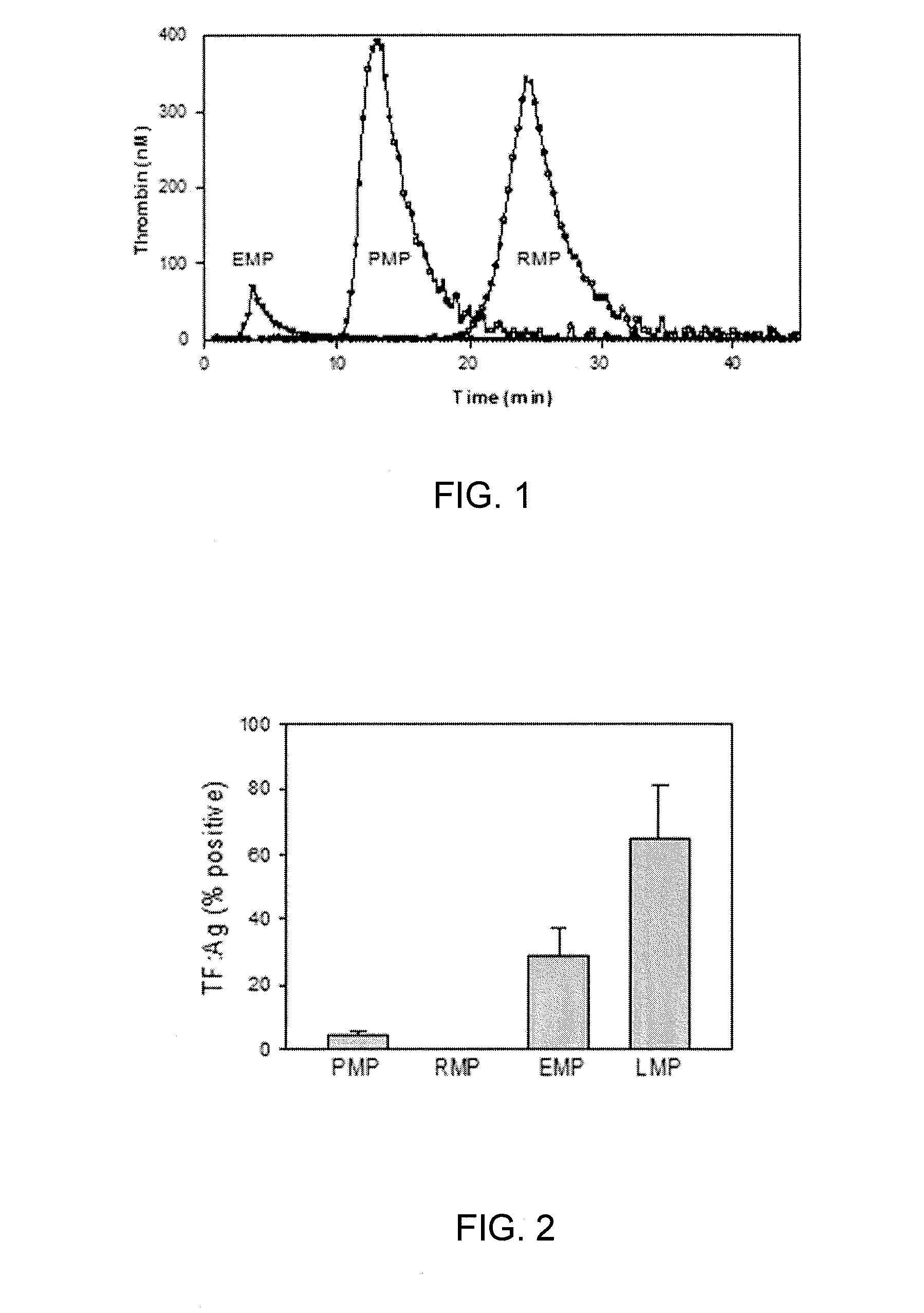
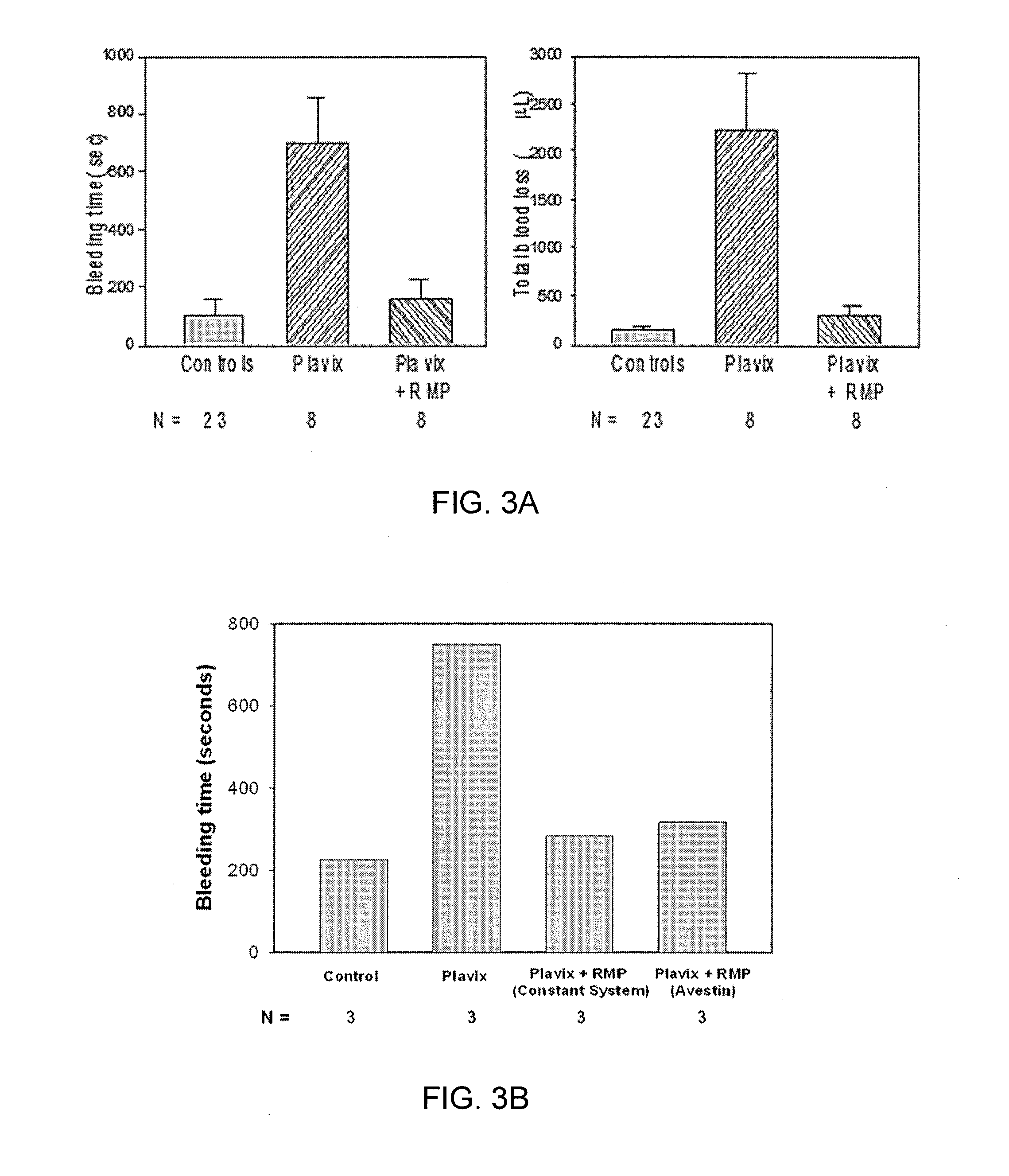
Expanded utility of red-cell derived microparticles (RMP) for treatment of bleeding
ActiveUS9155764B1Eliminate major riskRemove complicationsMammal material medical ingredientsDead animal preservationErythrocyte membranePlatelet disorder
Red blood cell membrane derived microparticles (RMP) are safe, economical, effective hemostatic agents in the treatment of a wide range of bleeding conditions and can be considered as universal hemostatic agents. RMP are produced from red blood cells using a high-pressure extrusion membrane shear process and can be lyophilized after production and retain activity even when stored at room temperature. RMP can be administered to original donors (autologous treatment), thus avoiding transfusion complications, or can be administered to blood type compatible recipients. RMP produced from type O, Rh negative red cells can be given to any person regardless of blood type. RMP can be administered to reduce excessive bleeding resulting from trauma, surgeries, invasive procedures and various bleeding disorders such as platelet disorders, either congenital or acquired, and coagulation disorders, either congenital or acquired.
Owner:UNIV OF MIAMI
Maintenance of Platelet Inhibition During Antiplatelet Therapy
InactiveUS20130303477A1Reduce bleeding riskMaintain and reduce platelet activityBiocidePharmaceutical delivery mechanismExcessive BleedingPlatelet inhibitors
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:THE MEDICINES
Rapid bleeding-stopping dressing for department of gynaecology and obstetrics and preparation method of rapid bleeding-stopping dressing
InactiveCN105288706AImprove cohesionRapid hemostasisAbsorbent padsBandagesExcessive BleedingEmergency treatment
The invention belongs to the field of medical materials for the department of gynaecology and obstetrics, in particular relates to rapid bleeding-stopping dressing for the department of gynaecology and obstetrics, and further discloses a preparation method of the rapid bleeding-stopping dressing. The rapid bleeding-stopping dressing is prepared by taking carboxymethyl chitosan, sodium carboxymethylcellulose, collagen, radix notoginseng and polyving akohol as raw materials, and the form of firstly foaming and then carrying out freeze drying in the gradient form is adopted in the preparation process; compared with the product obtained by the conventional single freeze drying form in the prior art, the prepared bleeding-stopping dressing has the advantages that on the aspects of absorption and bleeding stopping effects, the technical effect of absorbing blood is greatly promoted; the rapid bleeding-stopping dressing has the optimal bleeding stopping effect for the rapid haemorrhagic disease, can realize stopping bleeding within short time, and can be applicable to the emergency treatment for the acute excessive bleeding of the department of gynaecology and obstetrics.
Owner:李星华
Maintenance of Platelet Inhibition During Antiplatelet Therapy
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:THE MEDICINES
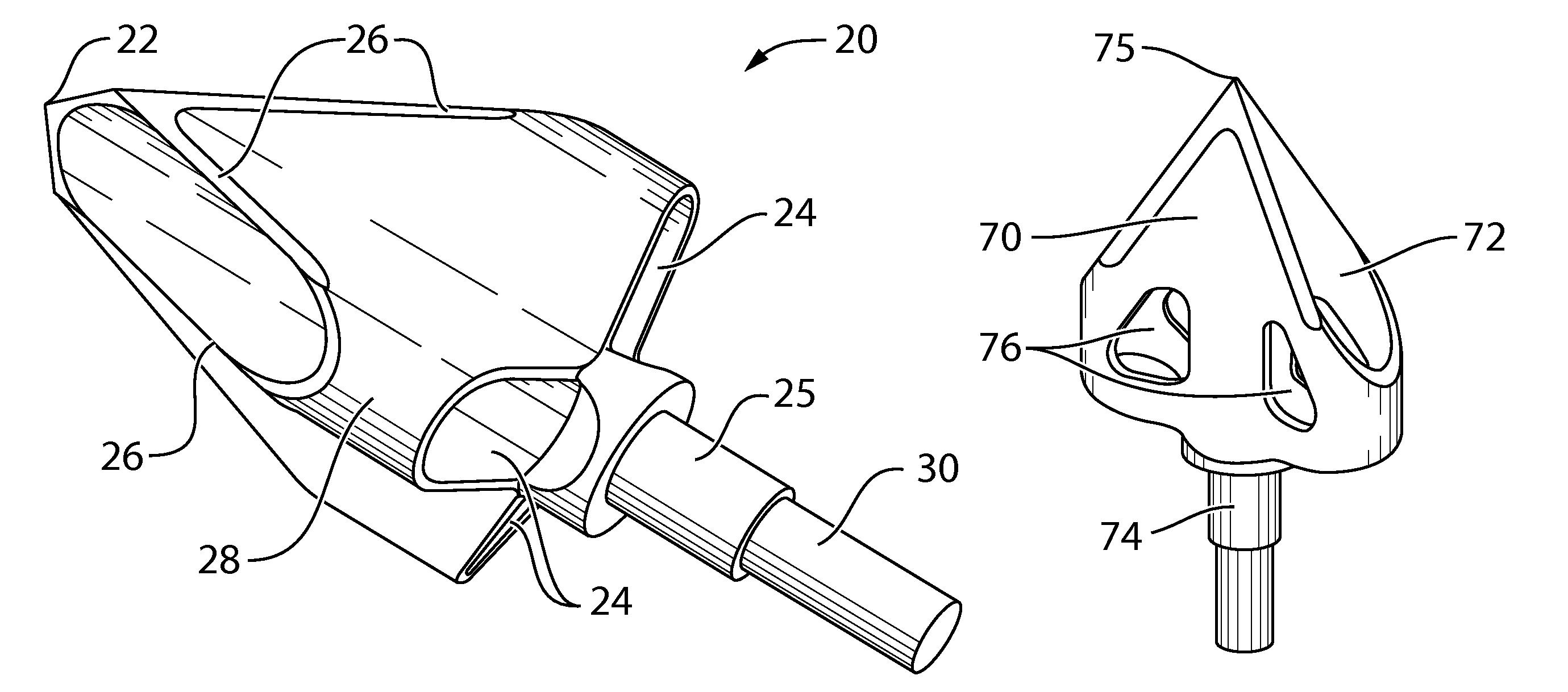
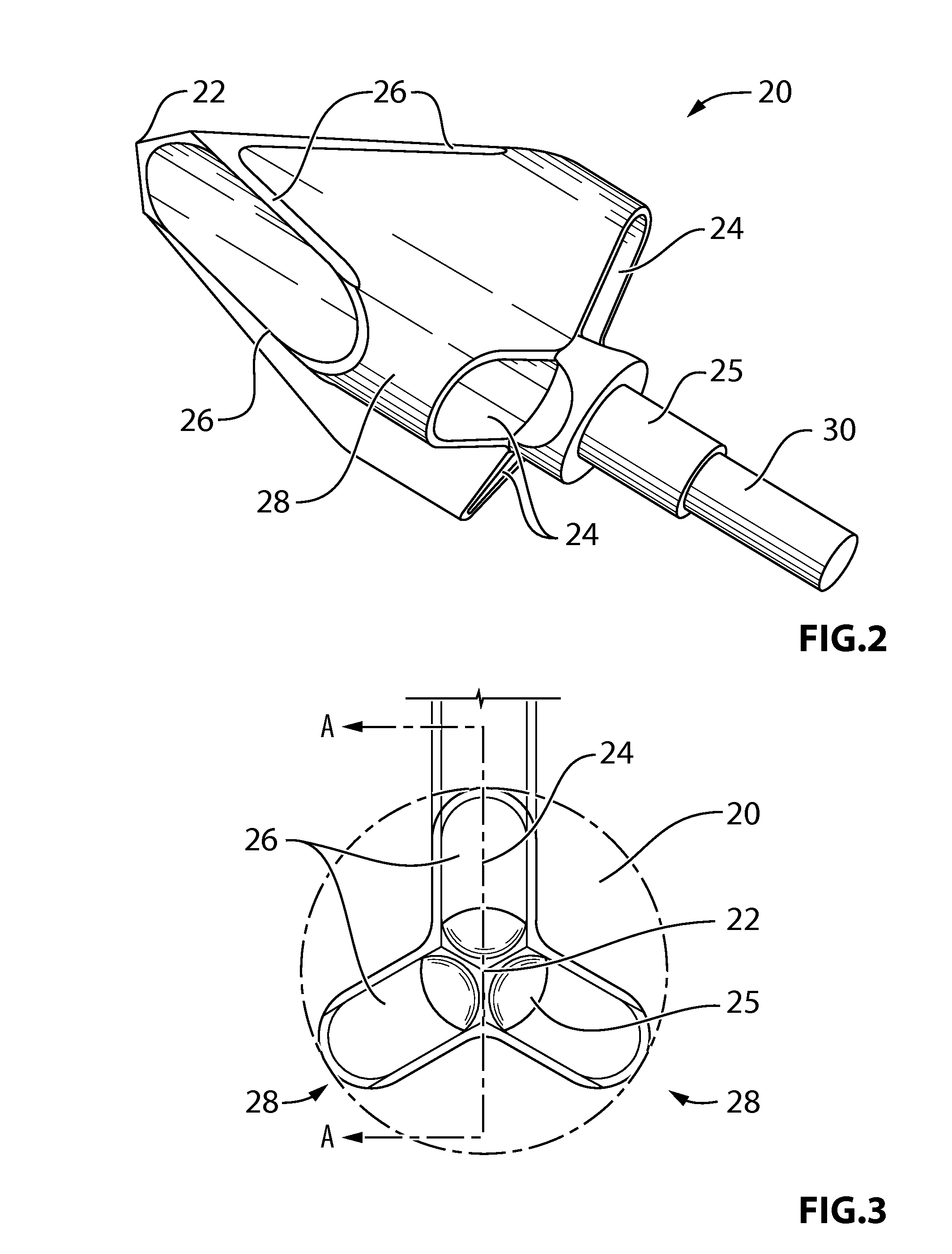
Arrowhead with improved lethal penetrating capability
The present document describes an arrow / arrowhead which depart from the conventional concepts and designs of the prior art. The arrowhead described in the present embodiments allows for higher penetration coupled with a higher ability to snatch and / or isolate tissue from the animal body to take down animals as fast and as humanly as possible. In an embodiment, the arrowhead comprises a shaft having a pointed end and at least one lobe mounted on the shaft. When the arrowhead penetrates the animal's body, the lobe snatches the tissue from the animal's body, as opposed to only making a cut therein. When the tissue is snatched from the animal's body, excessive bleeding occurs, which prevents the animal from running away and suffering longer. In an embodiment, each lobe may include a blade forming a closed loop.
Owner:COUTURE GABRIEL
Recombinant human fibrinogen for treatment of bleeding in trauma and platelet disorders
InactiveUS20100279939A1Save livesFast and efficient arrestSaccharide peptide ingredientsBlood disorderPlatelet disorderDisease
The present invention provides methods of using recombinant human fibrinogen to prevent or treat excessive bleeding in pre-hospital and hospital settings. In particular, the present invention relates to methods for treating bleeding using recombinant human fibrinogen in individuals suffering from traumatic hemorrhages in pre-hospital settings and in individuals having thrombocytopenia or qualitative platelet disorders.
Owner:FRIES DIETMAR RUDOLF +2
Chimeric proteins with phosphatidylserine binding domains
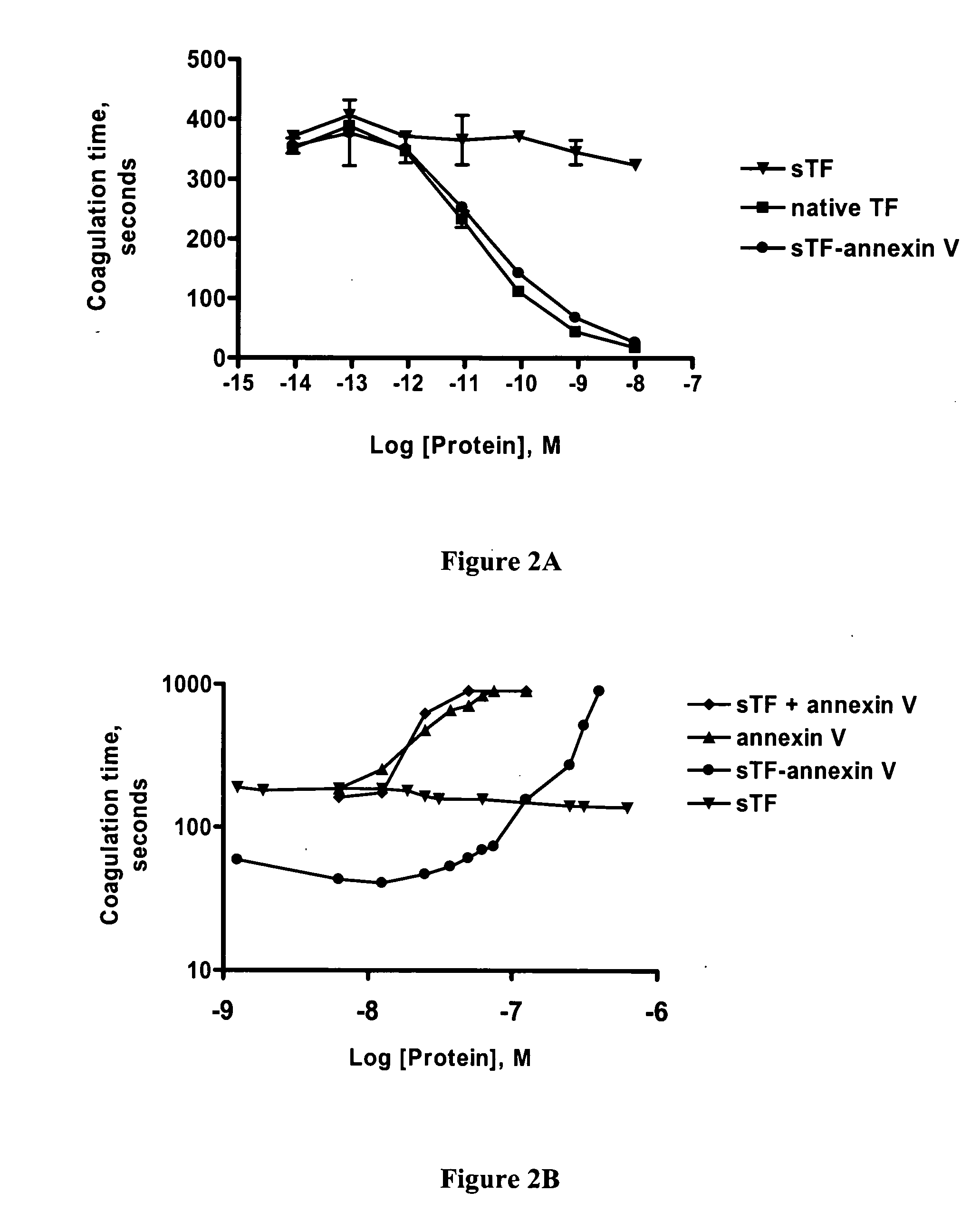
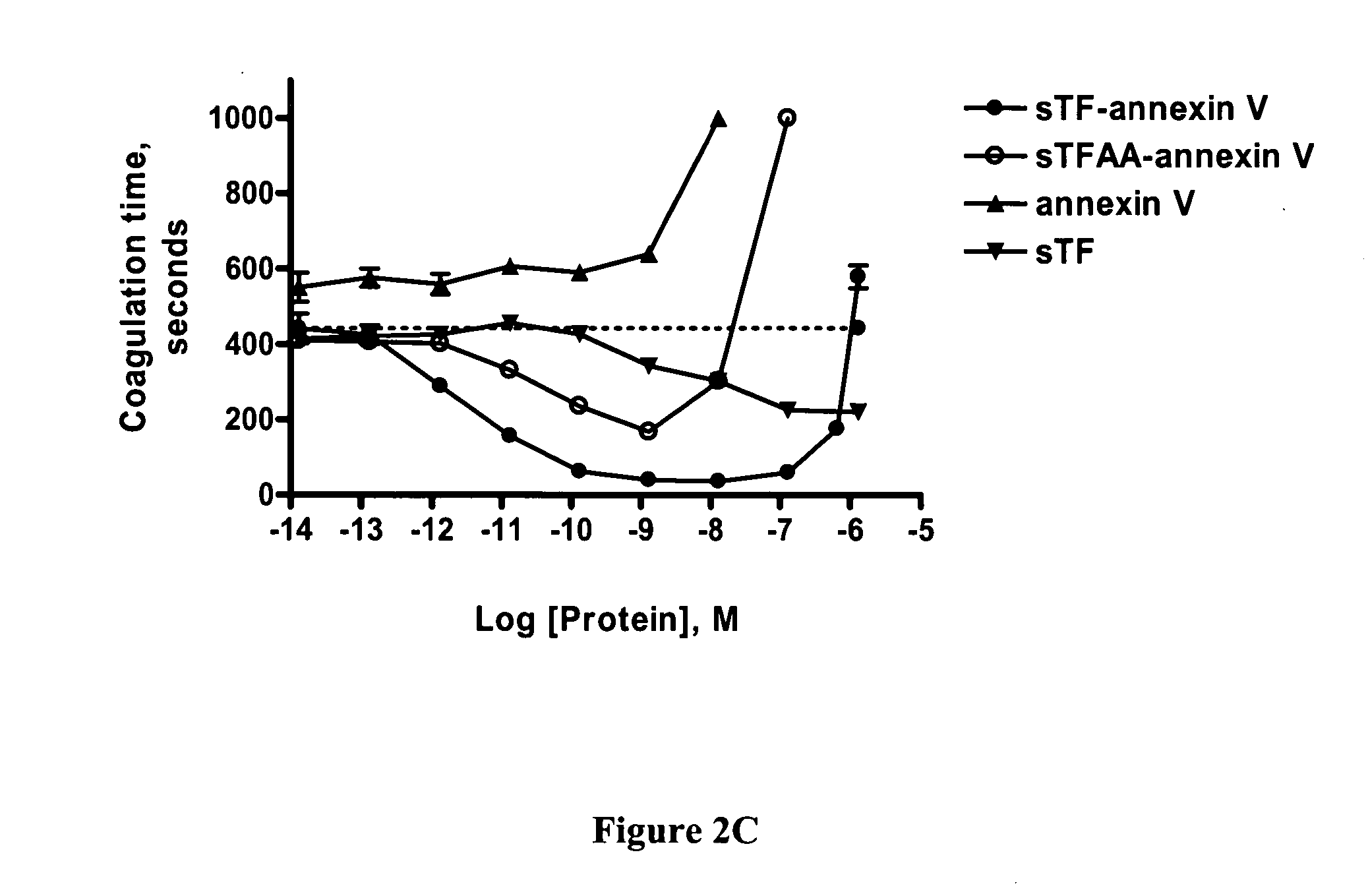
Chimeric proteins comprising soluble Tissue Factor (sTF) and another subunit (e.g., annexin V) are described. The proteins promote blood clotting and / or inhibit cancer by targeting sTF to specific receptors such as phosphatidyl serine (PS) on activated cells. These chimeric proteins are useful in treating patients with excessive bleeding due to inborn problems, drug therapy, trauma or surgery and / or as an anti-cancer therapy, for example by causing blood vessels feeding cancers to become clotted, thereby preventing adequate flow of blood to a tumor, which in turn will lead to tumor inhibition and death or may be used in a therapy to cause clotting within blood vessels that pose a threat in the subject in non-cancerous conditions.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
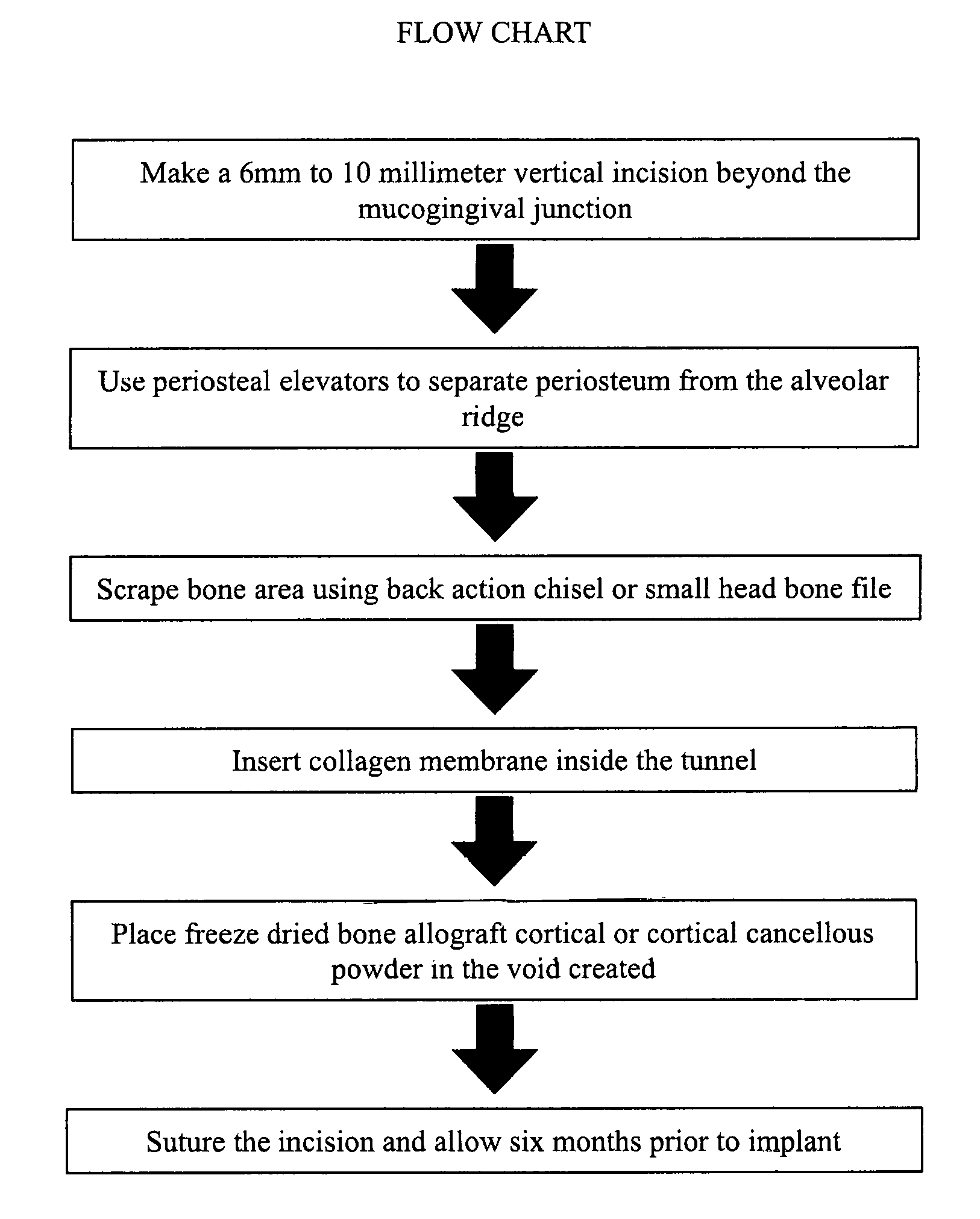
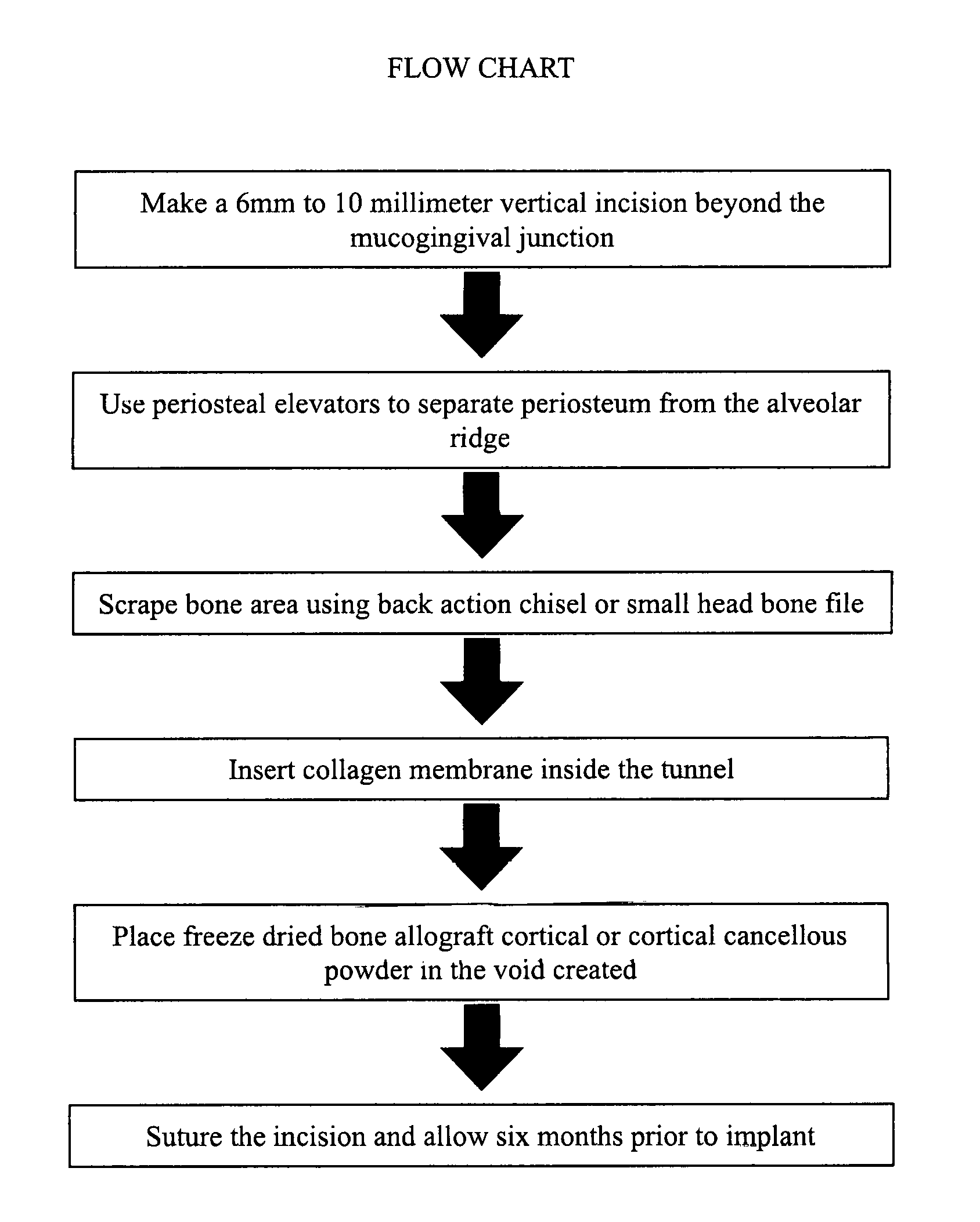
Periodontal Subperiosteal Tunnel Bone Graft Technique
Tunnel bone grafting according to the proposed method can be used when an individual has a facial bone defect due to periodontal disease, cleft palate or trauma. The proposed method can be utilized for implant placement, it reduces wrinkles and supports facial muscle in order to make the individual look younger. The method can also be used to create a thicker alveolar ridge in order to stabilize full dentures or partial dentures. Its advantages over conventional guided bone regeneration techniques is that the proposed method is minimally invasive and lessens trauma to patients, prevents soft tissue opening, and reduces surgery time. Specifically, it minimizes the incision size and thus reduces trauma to the patient. Further, this reduces the risk of complications, including excessive bleeding, due to the minimization of the incision size. There is also considerable reduction in post surgery swelling using the proposed method as opposed to conventional techniques.
Owner:JEONG SUNG JOO
Maintenance of Platelet Inhibition During Antiplatelet Therapy
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:CHIESI FARM SPA
Poultry beak treatment device
The invention discloses a poultry beak treatment device, and relates to the improvement of the poultry beak treatment device structure. The invention provides the poultry beak treatment device which can reduce harmful stimulation to chickens and avoid dying caused by excessive bleeding after the beak breaks, and is high in beak breaking efficiency and accurate in beak breaking. The poultry beak treatment device comprises a base, and is characterized in that an electric push rod is arranged on the base, the end part of the electric push rod is provided with a heating source, a chicken fixing device is arranged corresponding to the heating source, the chicken fixing device is provided with a first groove corresponding to the shape of the chicken head, and a beak hole corresponding to the heating source is arranged in the first groove.
Owner:SHENZHEN ZPWTECHNOLOGY CO LTD
Liquid, aqueous pharmaceutical composition of Factor VII polypeptides
InactiveUS20060166882A1Improve stabilityHeavy metal active ingredientsBiocideClotting factor deficiencyOxidation state
Owner:NOVO NORDISK HEALTH CARE AG
Compositions and methods for treating excessive bleeding
InactiveUS20100047352A1Augment and accelerate natural clotting processesExtended shelf lifePowder deliveryBiocideSilica particleExcessive Bleeding
The inventive material is a unique family of externally used wound sealants based upon a binding agent of reactive submicron silica particles that, when hydrated, agglomerate in the form of a supramolecular cross-linked network serving as the structural framework facilitating clot formation. A thrombolytic cascade accelerant can be provided, optionally with additional clotting factors, to further accelerate the clotting process.
Owner:HEMO NANOSCI
Expanded Utility of Red Cell-Derived Microparticles (RMP) for Treatment of Bleeding
InactiveUS20130316011A1Relieve pressureReduce the burden onMammal material medical ingredientsTissue cultureErythrocyte membranePlatelet disorder
Red blood cell membrane derived microparticles (RMP) are safe, economical, effective hemostatic agents in the treatment of a wide range of bleeding conditions and can, therefore, be considered as universal hemostatic agents. Effective RMP are produced from red blood cells using a high-pressure extrusion membrane shear process. The RMP can be lyophilized after production and retain activity even when stored at room temperature. RMP can be administered to original donors (autologous treatment), thus avoiding transfusion complications, or can be administered to blood type compatible recipients. RMP produced from type O, Rh negative red cells can be given to any person regardless of blood type. RMP can be administered to reduce excessive bleeding resulting from trauma, surgeries, invasive procedures and various bleeding disorders such as platelet disorders, either congenital or acquired, and coagulation disorders, either congenital or acquired. Administration of RMP prepared according to the invention demonstrates effectiveness in safely reducing bleeding.
Owner:UNIV OF MIAMI
Maintenance of Platelet Inhibition During Antiplatelet Therapy
ActiveUS20150038449A1Reduced activityBiocideCarbohydrate active ingredientsExcessive BleedingPlatelet inhibitors
A method for reducing or maintaining platelet inhibition in a patient by administering cangrelor prior to an invasive procedure is described. The method of this invention can be used for patients in need of antiplatelet therapy or at risk of thrombosis. The method can further be used in patients who were previously treated with long-acting platelet inhibitors without increasing the risk of excessive bleeding.
Owner:CHIESI FARM SPA
Method for grafting apricot tree to peach tree
The invention relates to the field of tree seedling grafting, in particular to a method for grafting an apricot tree to a peach tree. Before grafting, rootstocks are watered and disinfected, and are coated with a healing agent, so that damage to scions caused by excessive bleeding sap of the rootstocks is avoided; in addition, after grafting, temperature and humidity are timely controlled, and grafted parts are banded, so that the survival rate after grafting is greatly increased; moreover, the method is simple, convenient, high in survival rate and surviving speed and easy to popularize.
Owner:陈兴庆
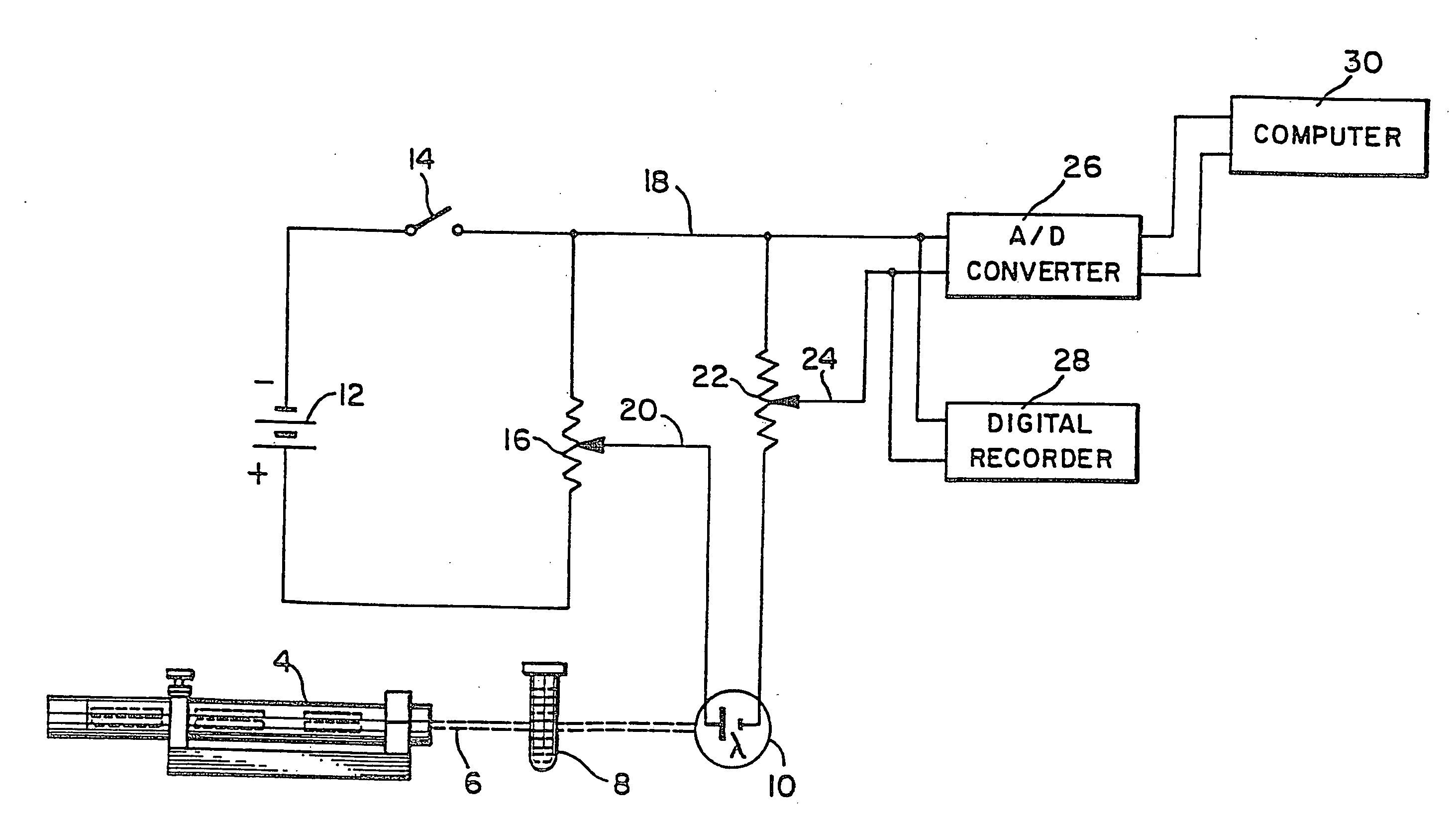
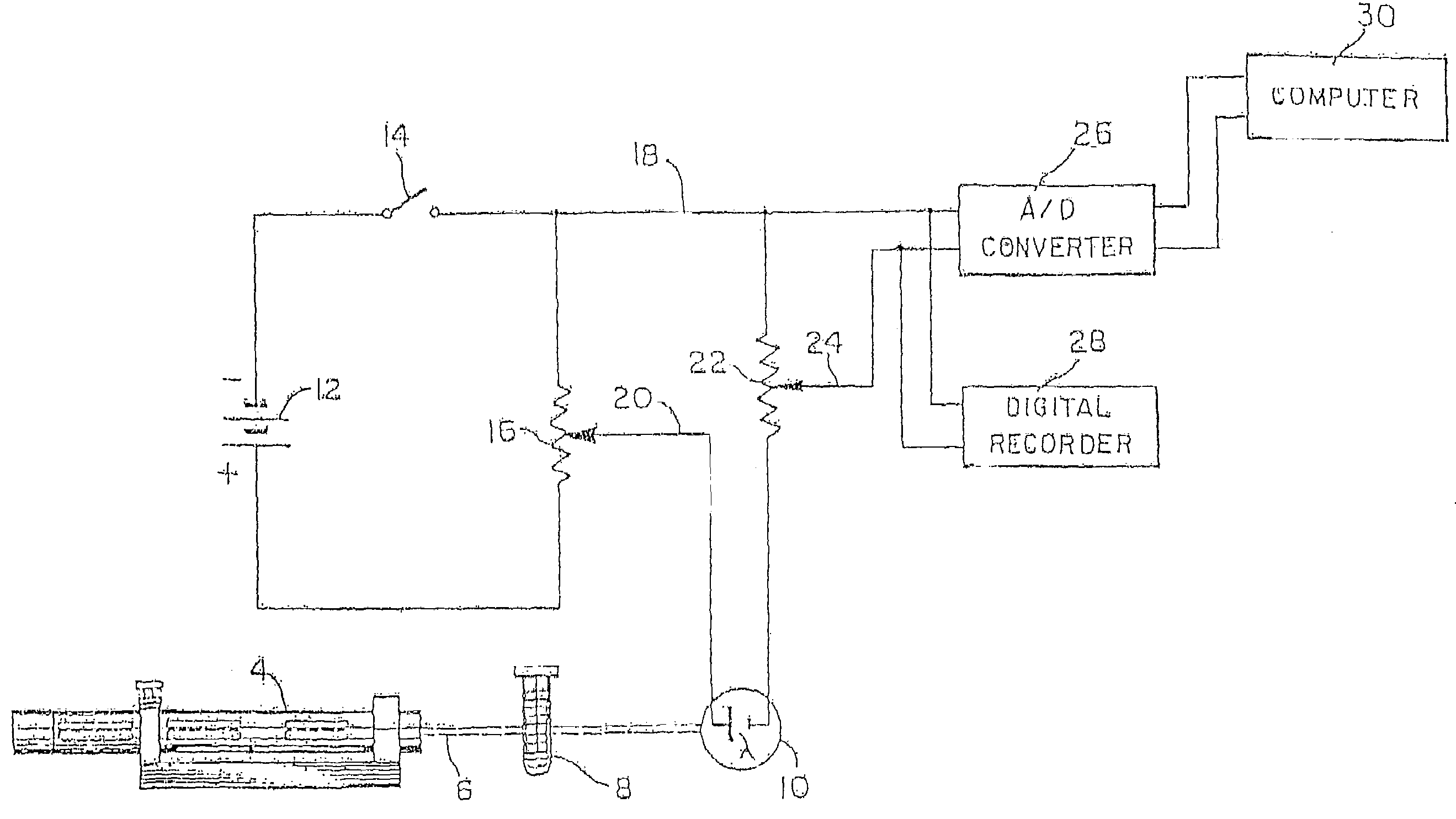
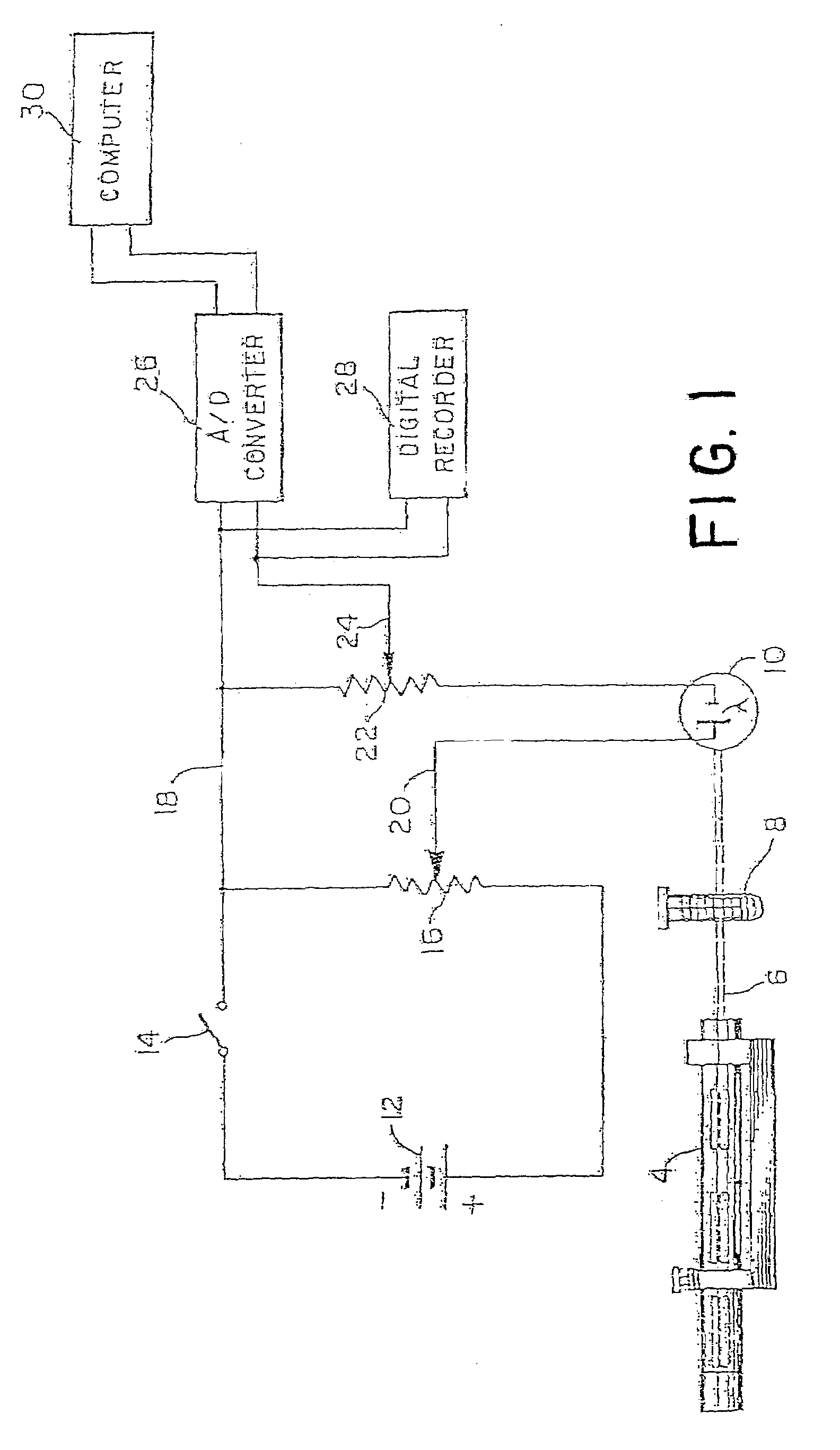
In-vivo platelet function test by online bleeding volume measurement
InactiveUS20110244589A1Achieve effectCharacter and pattern recognitionAnalysis by subjecting material to chemical reactionExcessive BleedingPlatelet
A method for remotely determining a patient's excessive bleeding tendency and a patient's resistance to blood thinning medication is disclosed. An incision is made in the patient's forearm. Blood oozing out of the incision is absorbed into a blotter paper until the bleeding stops. Blotches of blood formed on the blotter paper are captured as an image and sent to a service provider who calculates a value associated with the bleeding volume of the patient. The service provider retransmits a value associated with the bleeding volume back to the medical professional. To determine the resistance to blood thinning medication, one incision is made in the patient prior to administration of blood thinning medication. Blood oozing out of the incision is collected on blotter paper until the patient stops bleeding. A second incision is made in the patient. A second set of blotter paper is used to collect the blood oozing out of the incision until the bleeding stops. Both sets of blotter paper are sent to a service provider to calculate a value associated with the difference in bleeding volume. The service provider then retransmits the value associated with the difference in bleeding volume to the medical professional.
Owner:KLEIN JEFFREY A
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
Method and apparatus for determining anticoagulant therapy factors
InactiveUS7276377B2Material thermal conductivityMaterial analysis by observing effect on chemical indicatorBlood plasmaOral anticoagulant
Methods and apparatus are disclosed for determining a new anticoagulant therapy factor (nATF) for monitoring oral anticoagulant therapy to help prevent excessive bleeding or deleterious blood clots that might otherwise occur before, during or after surgery. In one embodiment, the new anticoagulant therapy factor is based upon a determination of a new fibrinogen transformation rate (nFTR) which, in turn, is dependent on a maximum acceleration point (MAP) for fibrinogen (FBG) conversion. The new anticoagulant therapy factor quantity is also based upon the time to maximum acceleration from the time of reagent injection (TX) into a plasma sample, but does not require the difficulty of obtaining prior art International Normalized Ratio (INR) and International Sensitivity Index (ISI) parameters. Other embodiments provide methods and apparatus for determining an anticoagulant therapy factor without requiring use of a mean normal prothrombin time determination or ISI.
Owner:WADA
Liquid, aqueous, pharmaceutical compositions of factor VII polypeptides
The present invention relates to an aqueous liquid pharmaceutical composition comprising a factor VII polypeptide (eg human factor VIIa) and a buffer; wherein the molar concentration ratio of uncomplexed calcium ions (Ca2+) to factor VII polypeptide is less than 0.5. The composition may further include stabilizers (such as copper or magnesium ions, benzamidine or guanidine), nonionic surfactants, tonicity regulators, antioxidants and preservatives. The composition is useful in the treatment of factor VII-responsive syndromes, such as bleeding disorders, including: those disorders resulting from deficiencies of clotting factors (e.g., hemophilia A, hemophilia B, factor XI deficiency, factor VII deficiency), thrombocytopenia or Willebrand disease or bleeding disorders due to clotting factor inhibitors; and intracerebral hemorrhage or excessive bleeding from any cause. These formulations may also be administered to patients in conjunction with surgery or other trauma therapy or to patients receiving anticoagulant therapy.
Owner:NOVO NORDISK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com