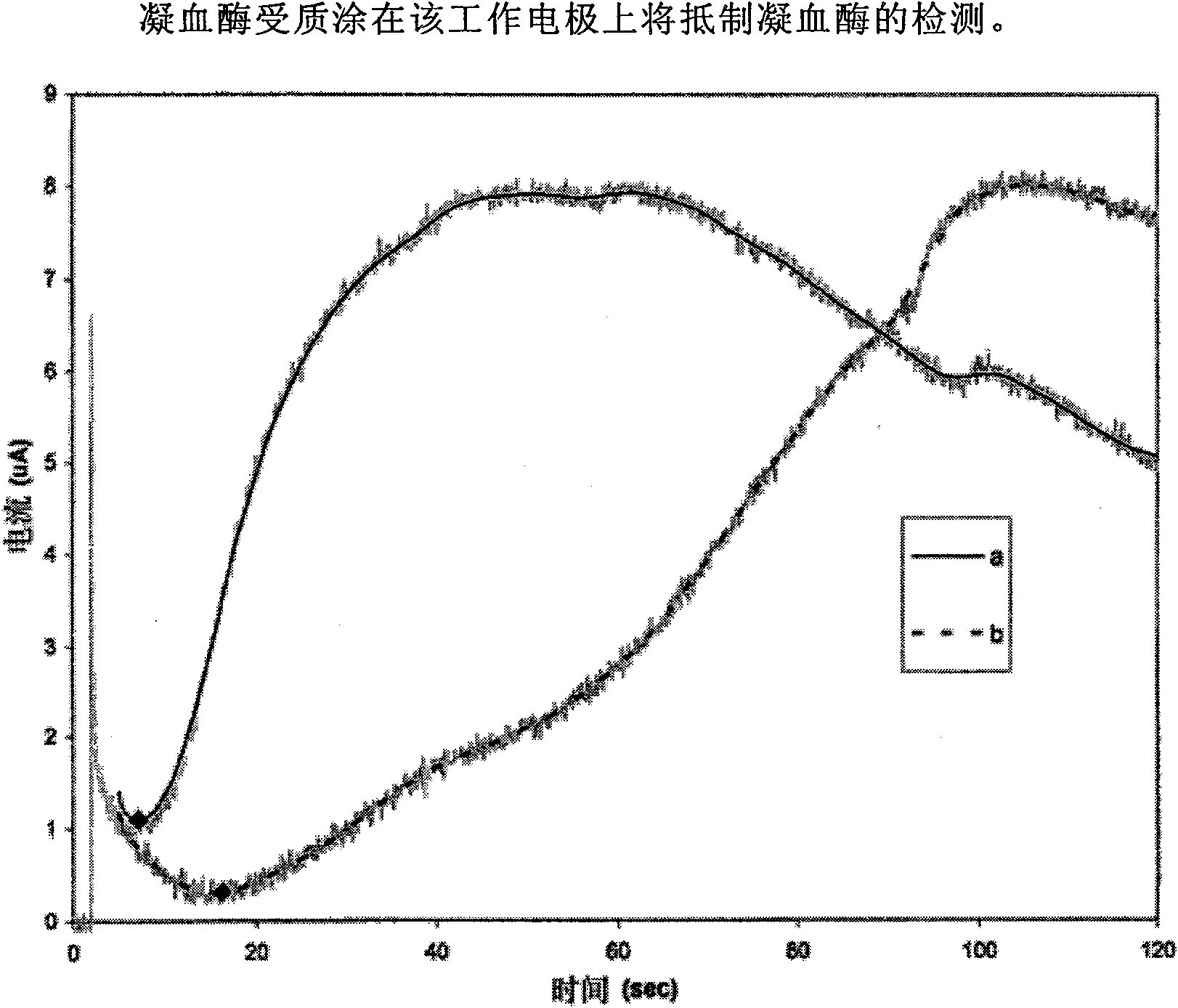
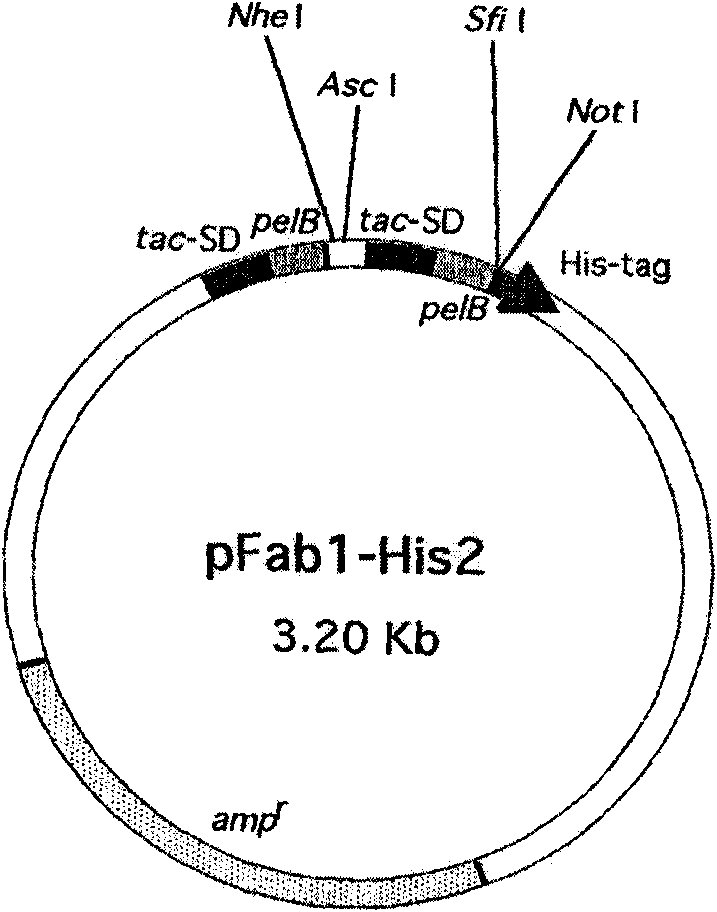
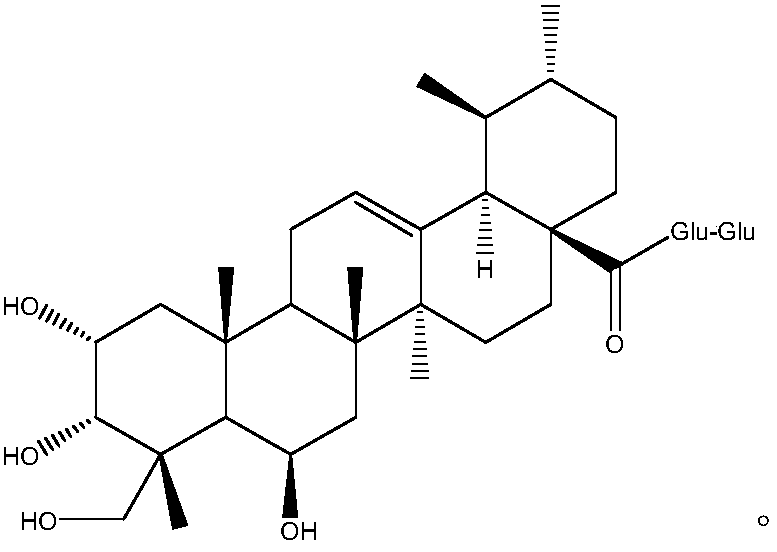
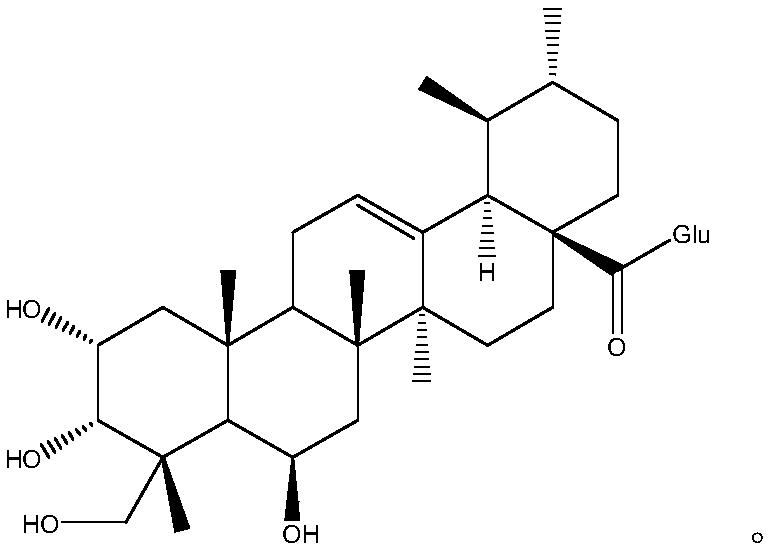
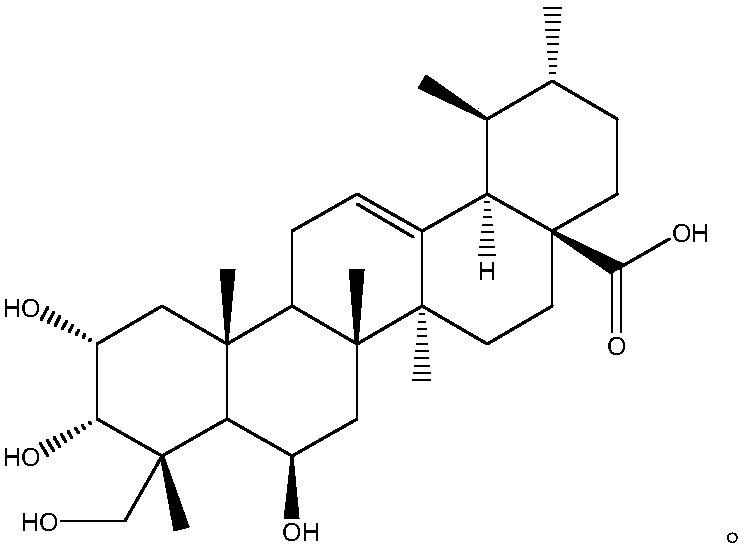
Patents
Literature
94 results about "Prothrombin time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – are assays evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X (Stuart–Prower factor).
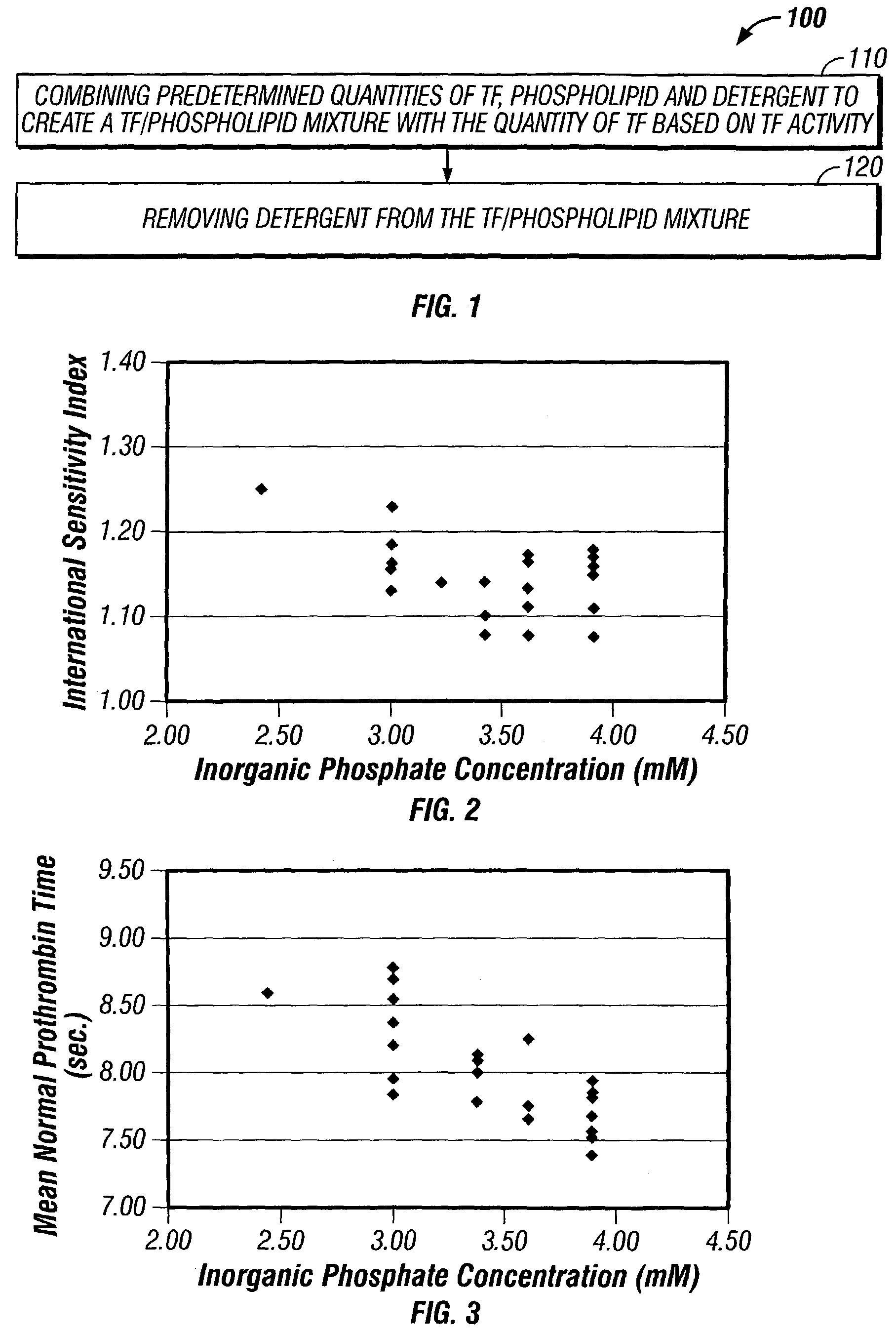
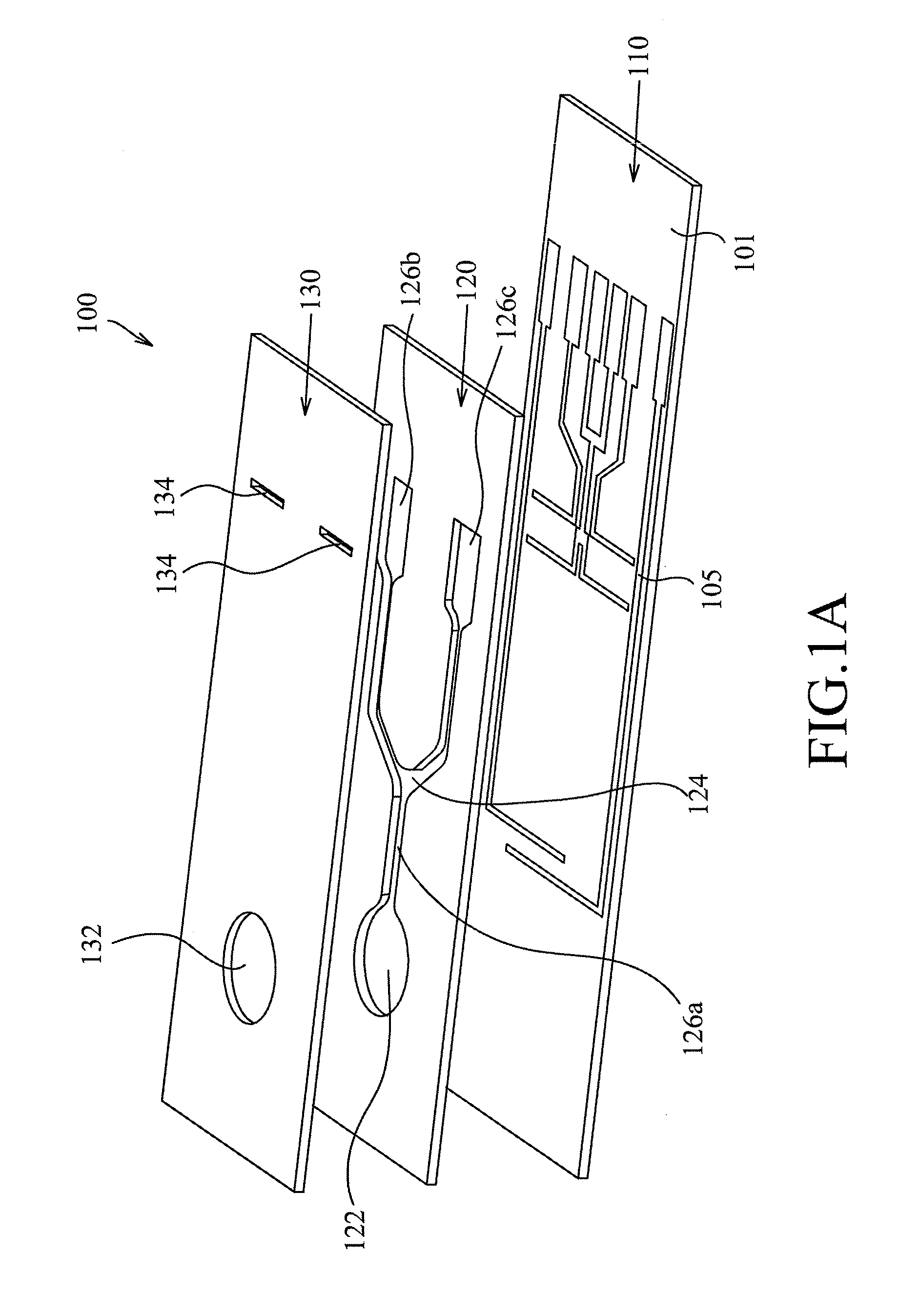
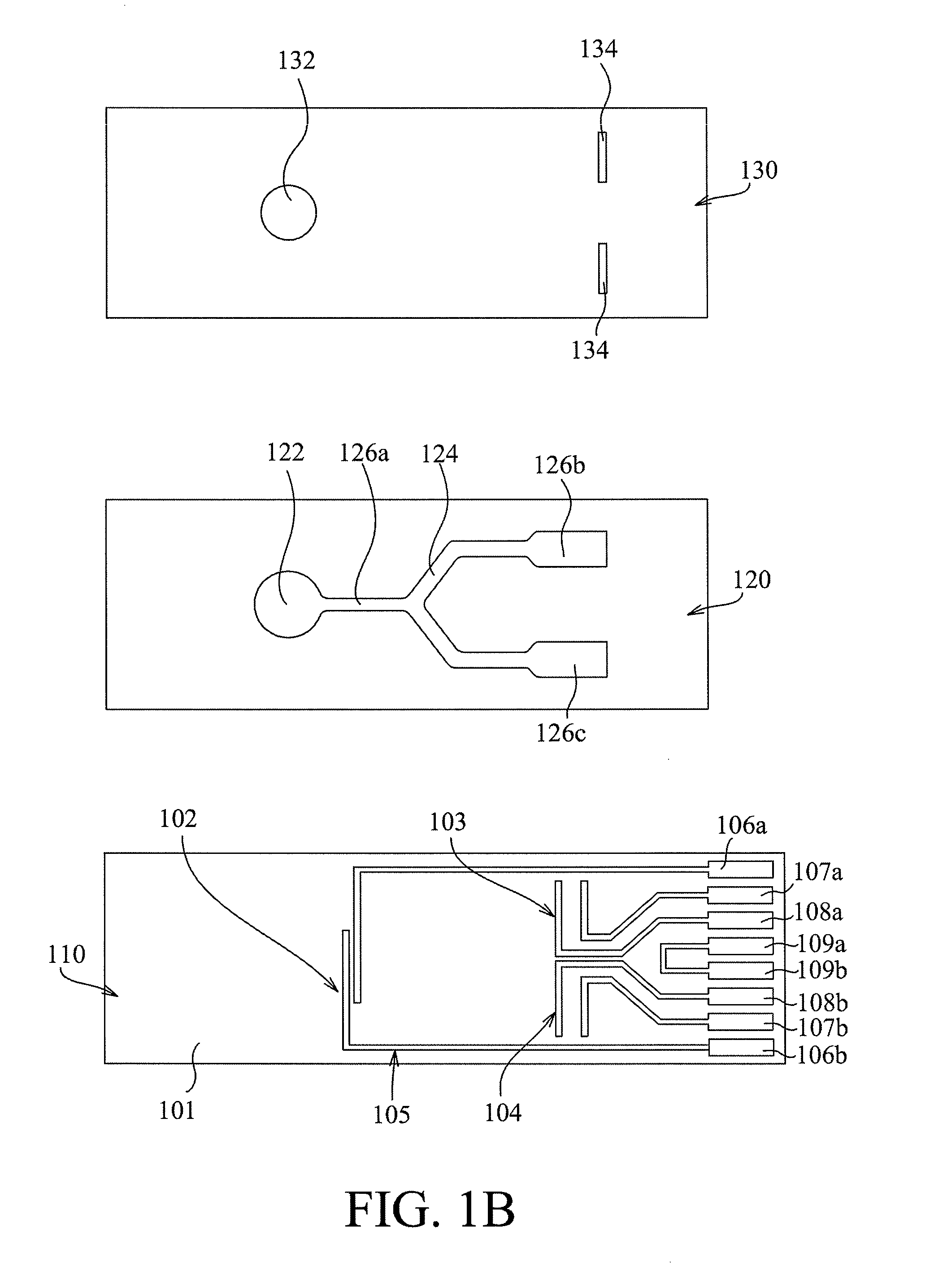
Method for manufacturing a tissue factor-based prothrombin time reagent
ActiveUS7049087B2Microbiological testing/measurementBiological material analysisTissue factorPhospholipid
Owner:LIFESCAN IP HLDG LLC
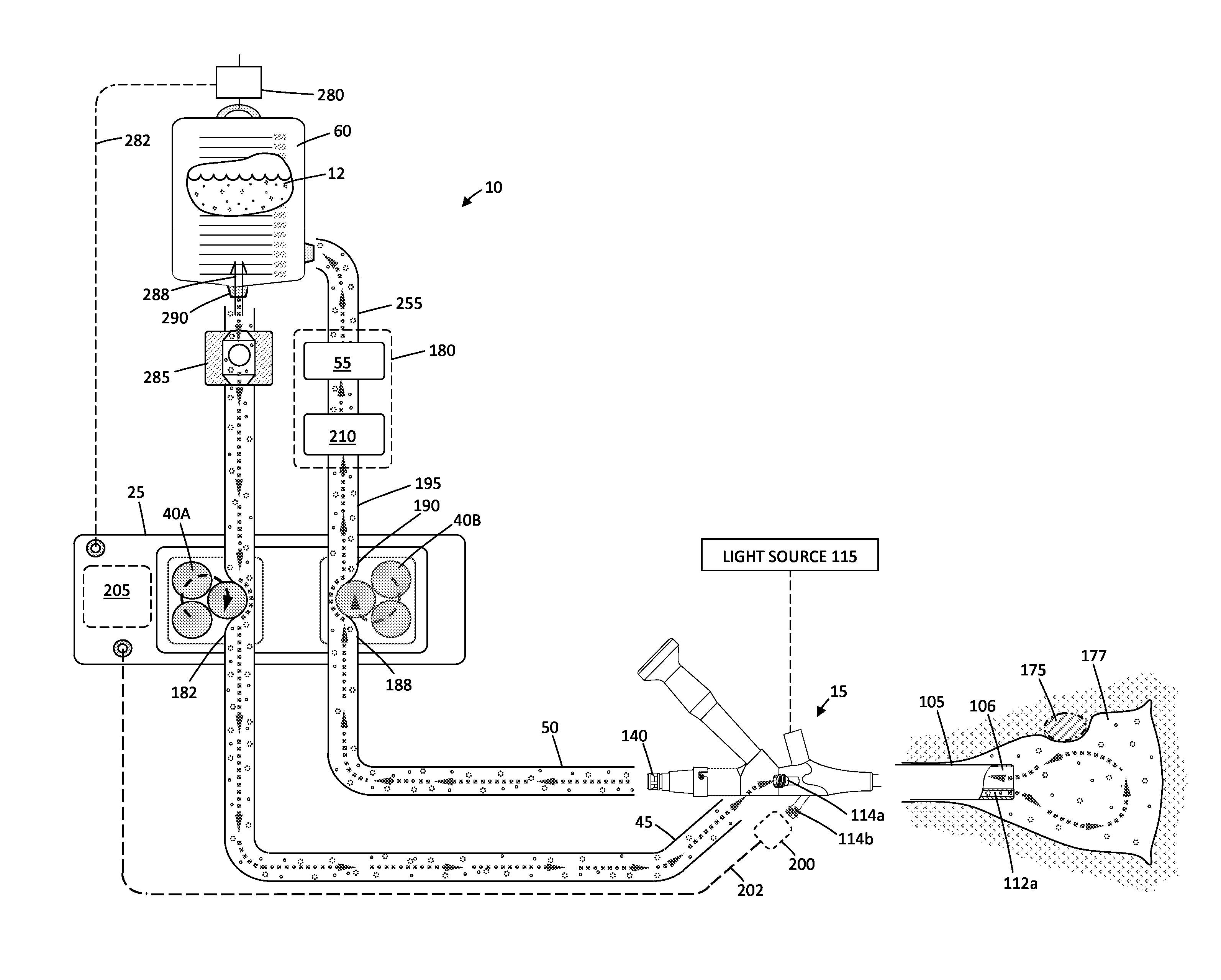
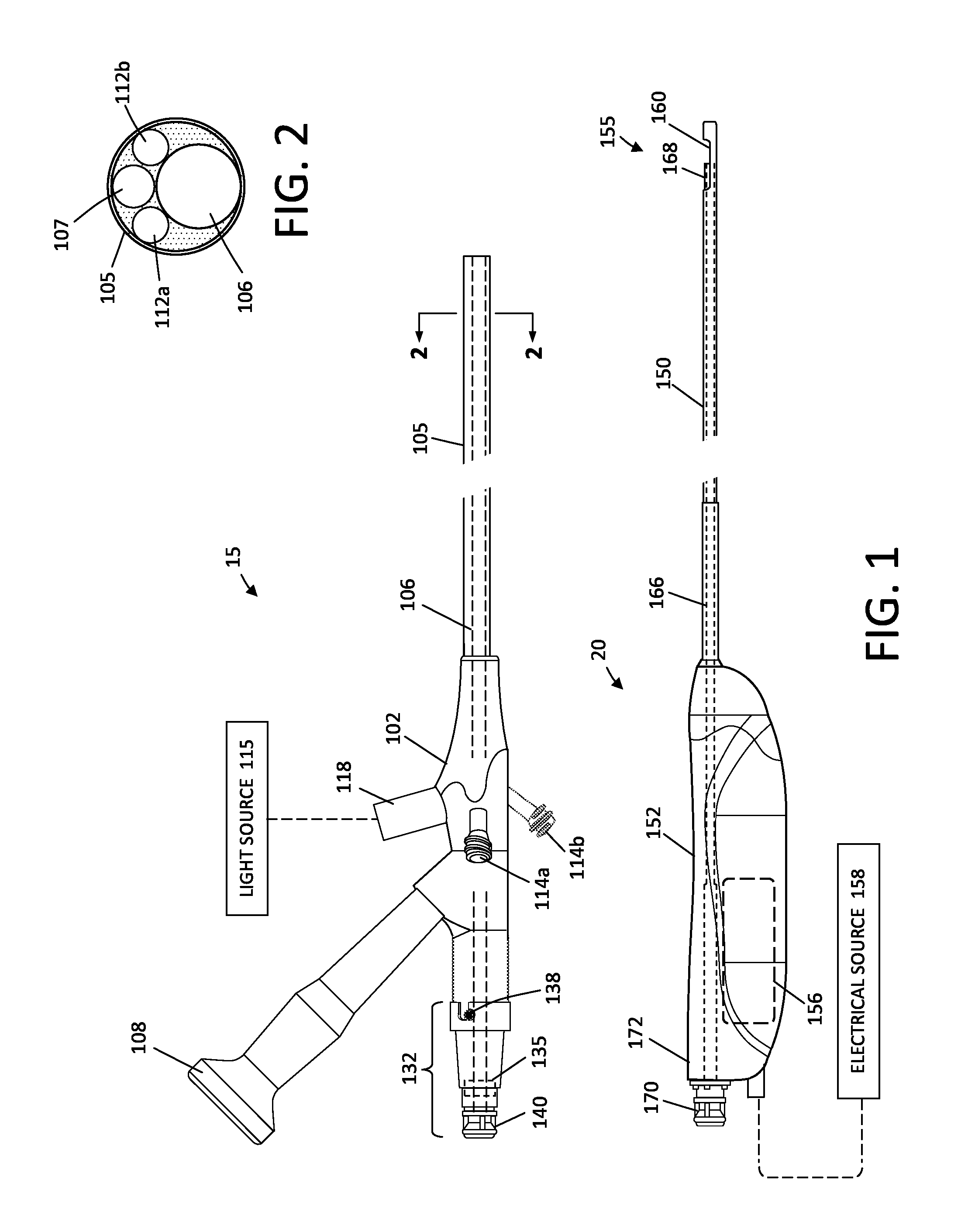
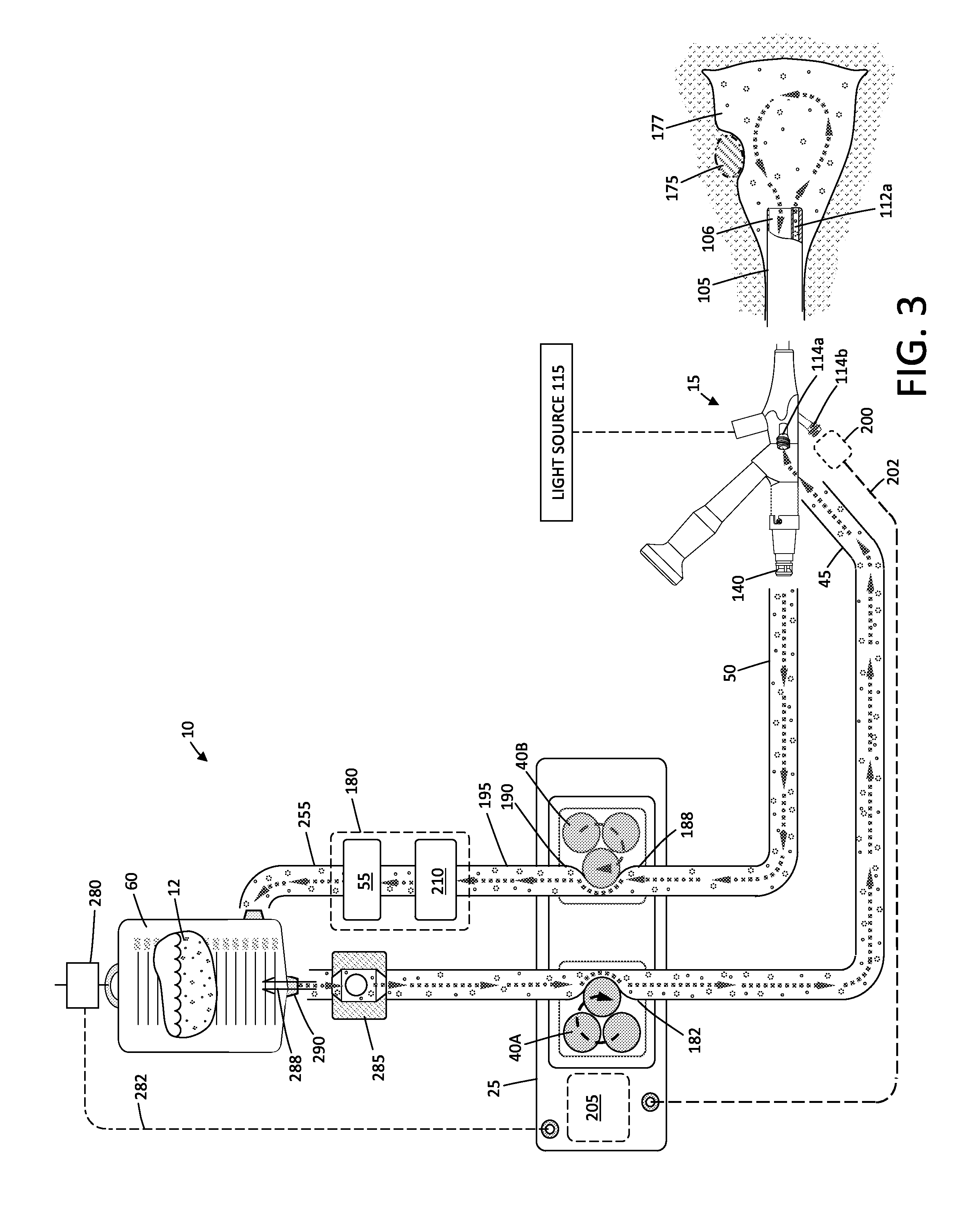
Fluid management system and methods
ActiveUS20150119795A1Avoid hemolysisControl pressureDialysis systemsMedical devicesControl flowSaline water
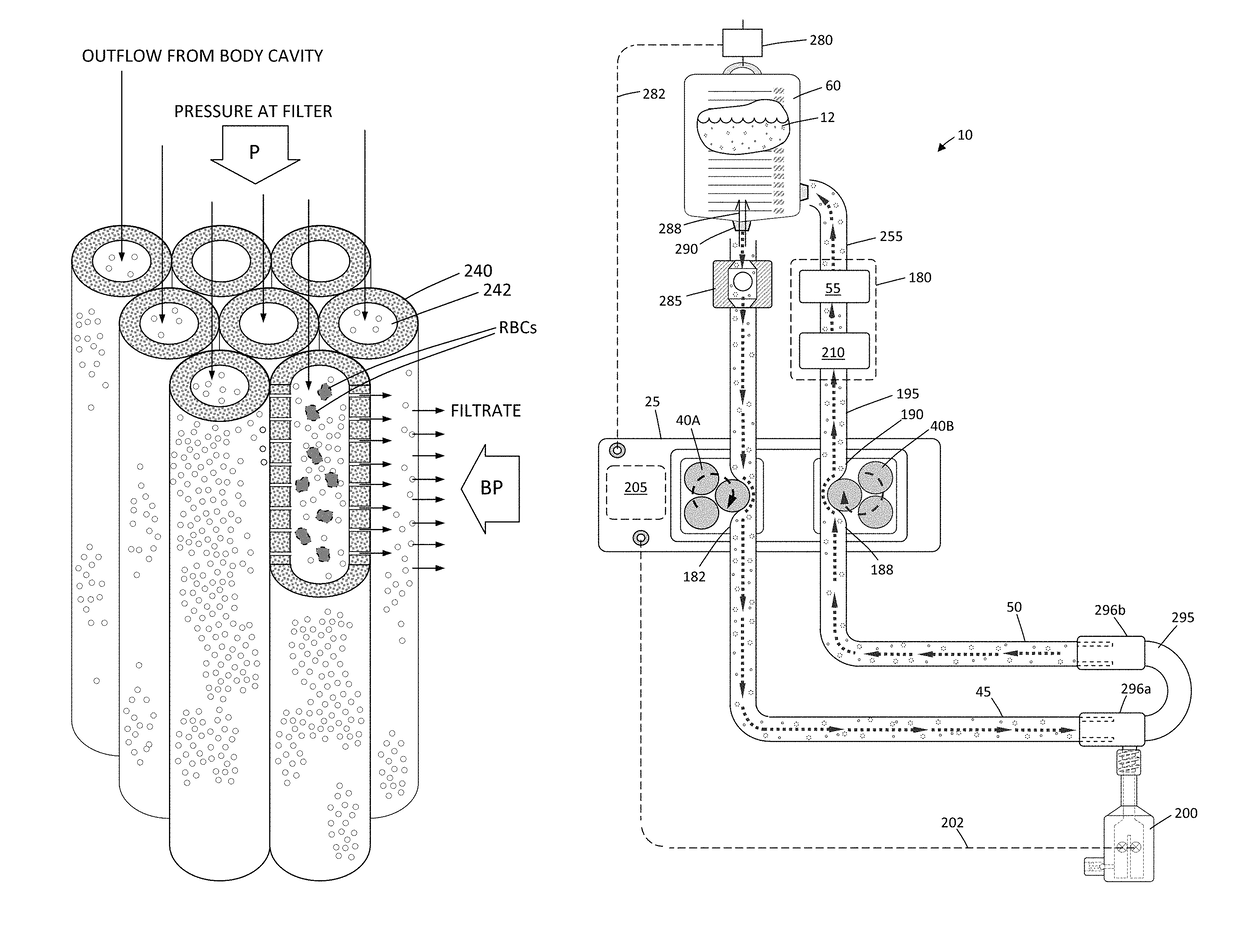
A hysteroscopic fluid management system includes a saline source with an electrolyte concentration, at least one pressure mechanism for circulating saline to and from a targeted site and through a filter having filter characteristics back to the source, and a controller. The controller provides a saline inflow in a first flow path to the site and a saline outflow in a second flow path from the site through the filter and back to the source at a controlled flow rate. A diagnostic or therapeutic procedure is performed at the site in the presence of the saline. The filter characteristics and the controlled flow rate are selected to (1) cause substantially no change in the electrolyte concentration in the saline, (2) to prevent hemolysis of greater than 5% of filtered red blood cells exposed to the saline, and / or (3) to minimize effect on prothrombin time of plasma exposed to the filter.
Owner:BOSTON SCI SCIMED INC
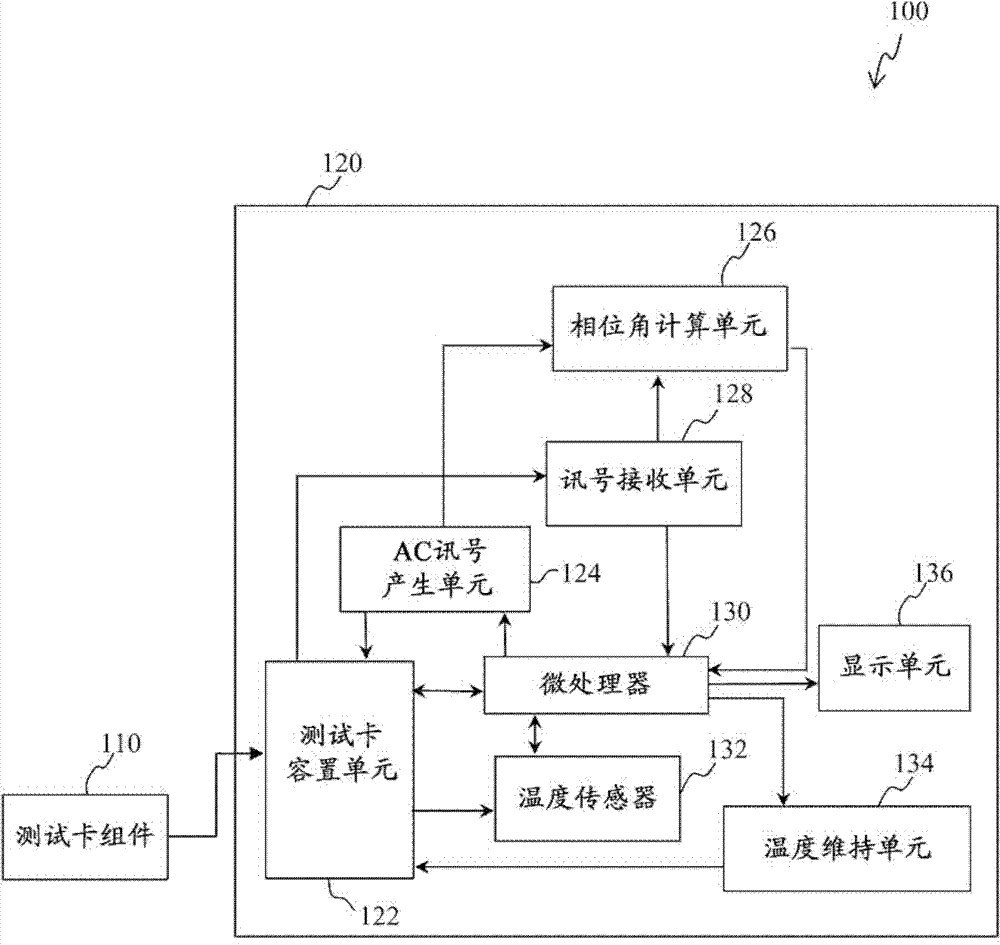
Device and method for measuring prothrombin time and hematocrit (HCT%) by analyzing change in reactance in a sample
ActiveCN102818822AAccurate blood property analysisReduce test errorMaterial impedanceMaterial capacitanceElectricityBlood test
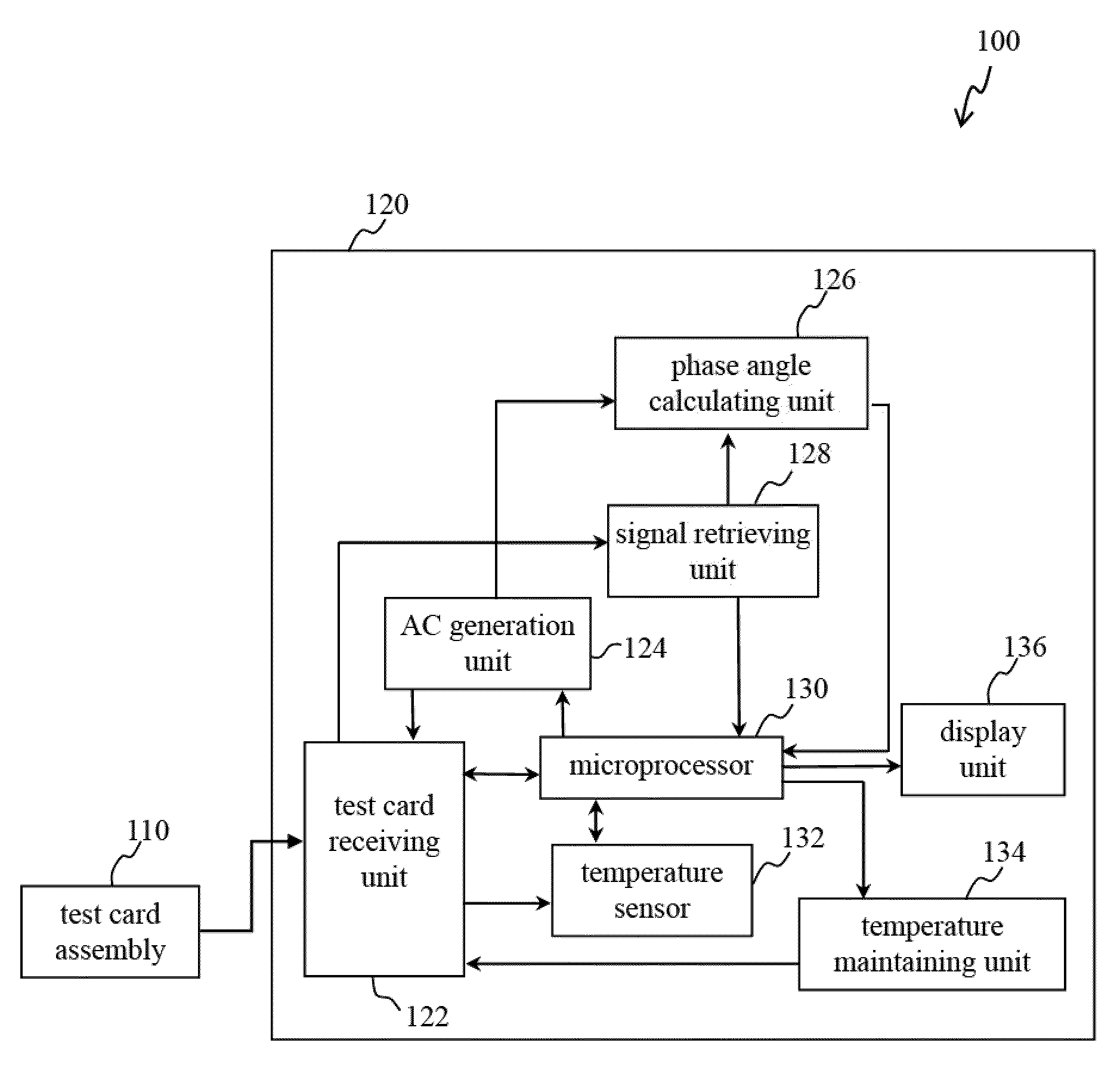
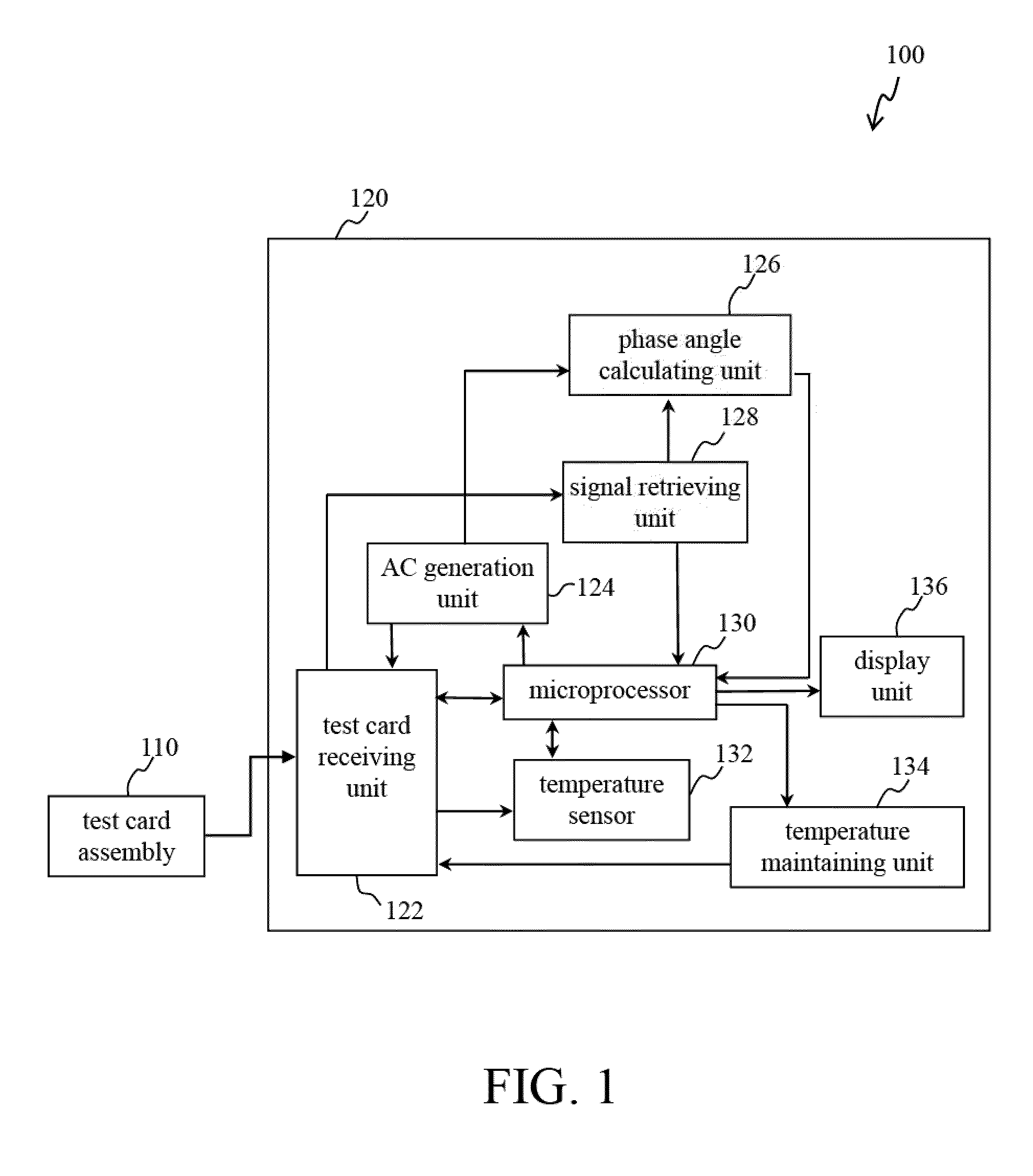
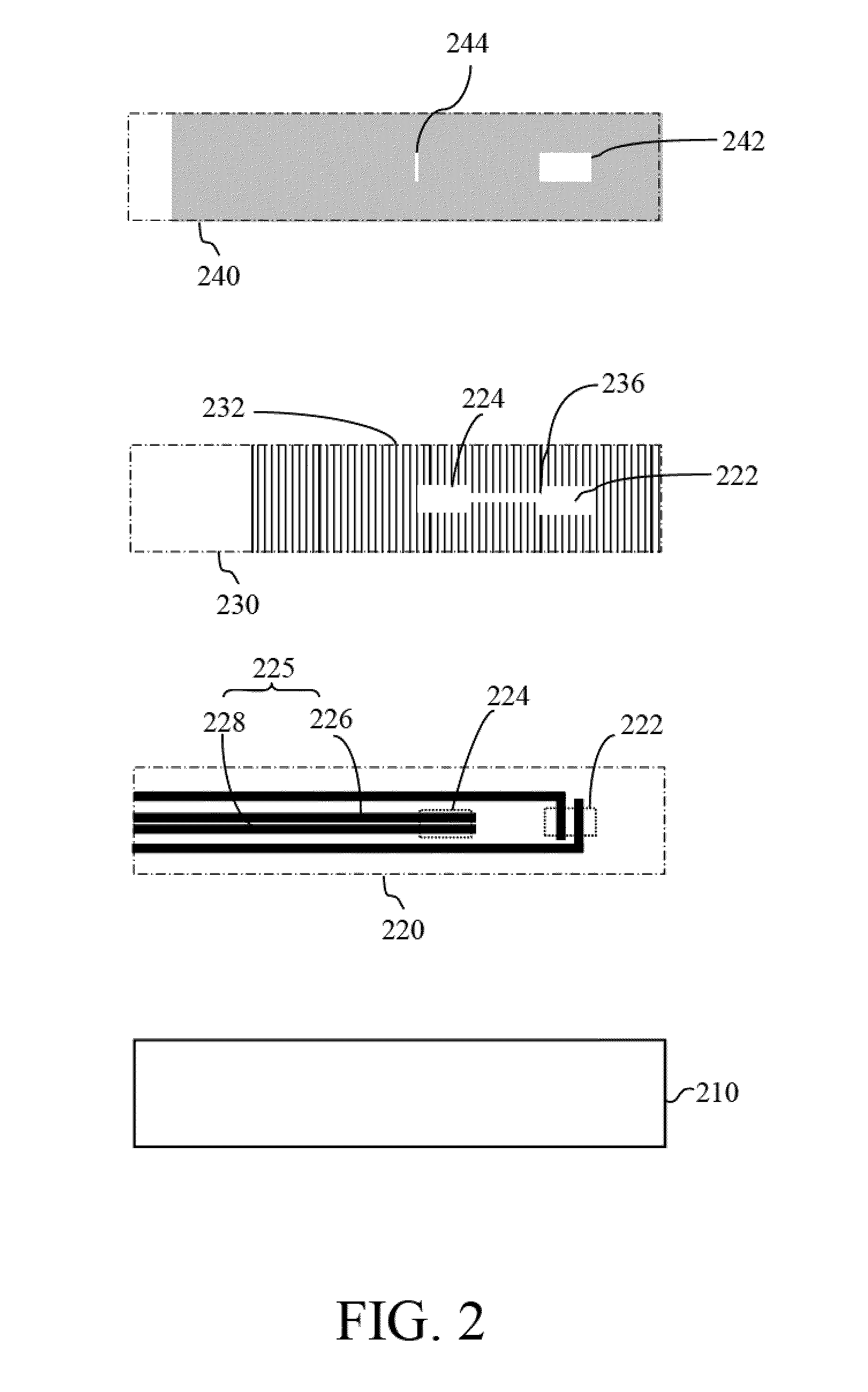
Devices and methods for measuring prothrombin time and hematocrit (HCT%) by analyzing the change in reactance in a sample are presented. A diagnostic device for measuring HCT and prothrombin time of a fluid includes a relative electrode-type sensor device and a blood test card assembly including one or more pairs of electrodes, wherein alternating current (AC) provided by the sensor device is used to measure and calculate prothrombin time and HCT of blood test using the reactance analysis.
Owner:APEX BIOTECH
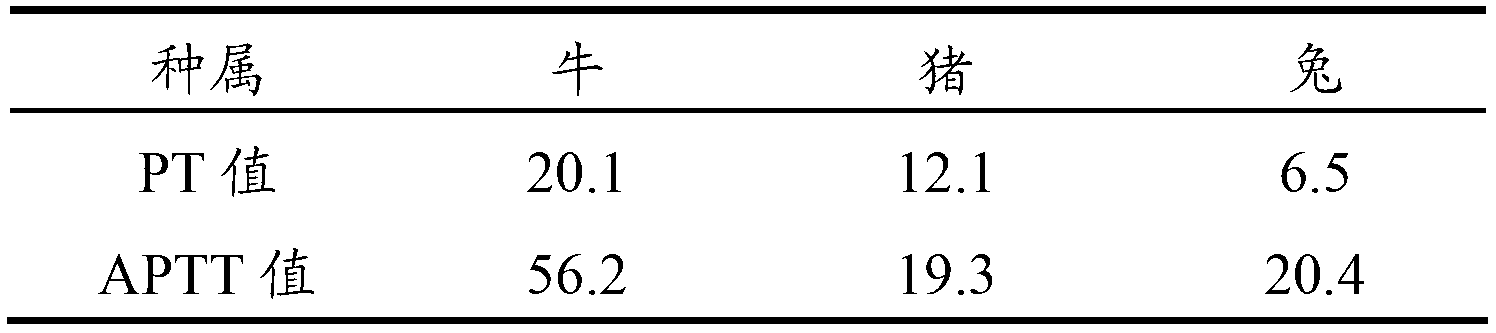
Blood coagulation quality control product and preparing method thereof
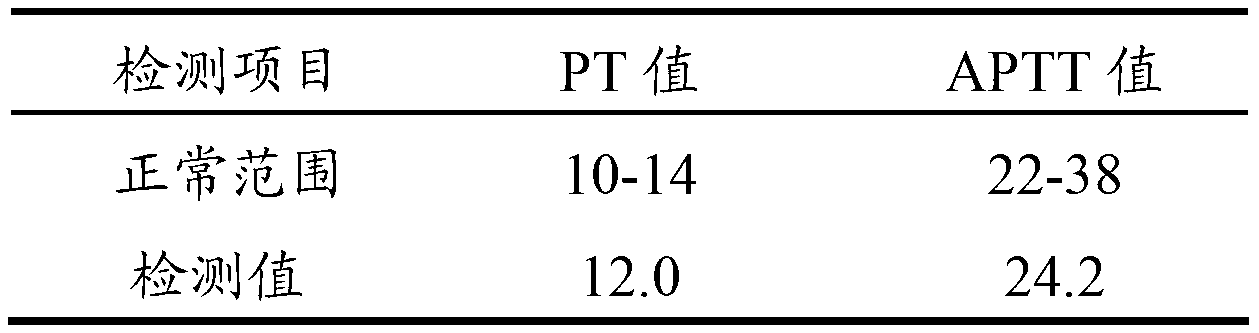
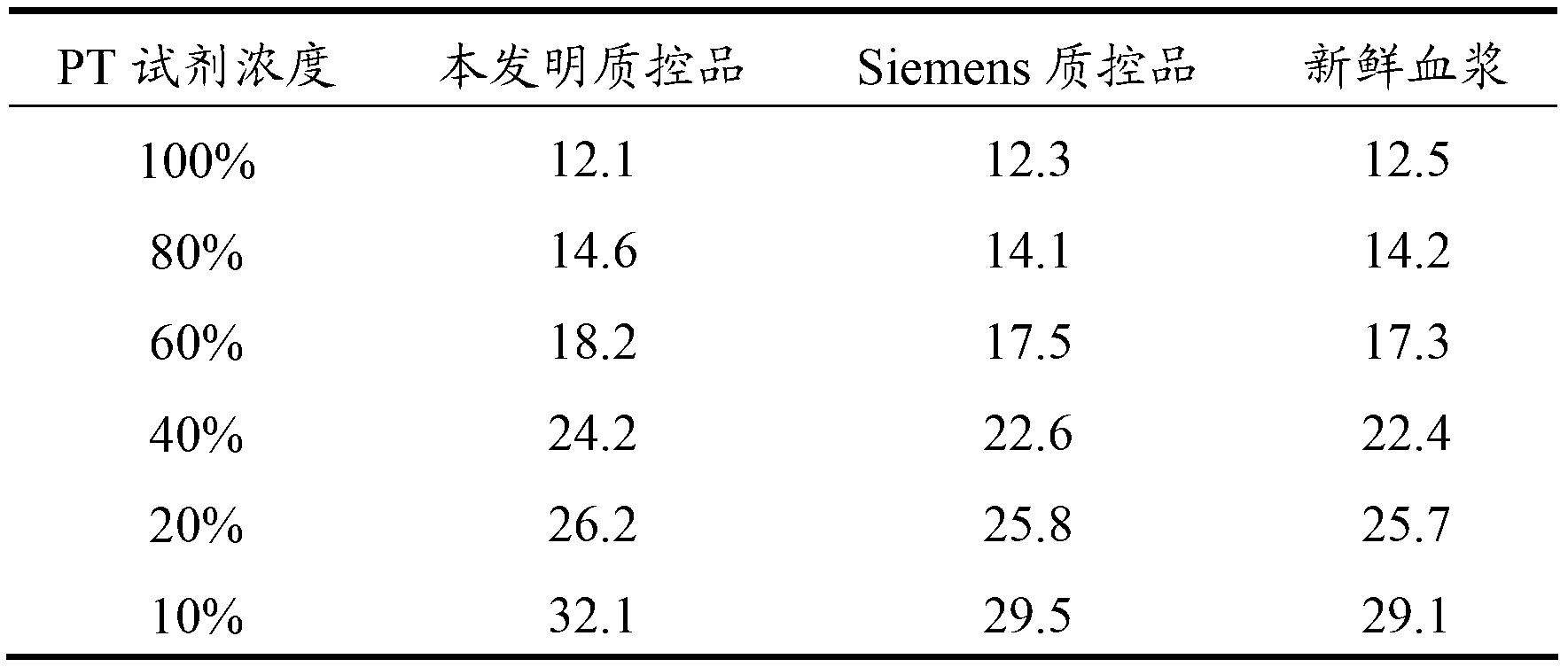
The invention relates to the field of quality control of a clinical blood coagulation inspection item, and in particular provides to a method for preparing a quality control product which can be used for simultaneously carrying out PT (Prothrombin Time), APTT (activated partial thromboplastin time) and FIB (fibrinogen) blood coagulation inspection items through animal blood plasma. The method comprises the steps of mixing the animal blood plasma according to an appropriate rate, adding or removing the FIB, adding proper amount of blood plasma buffer solution contacting a stabilizing agent so as to enable detecting results of mixed blood plasma PT, APTT and FIB to be in the range of a blood coagulation indicator of a normal person, drying and cooling, thus obtaining the product. The blood coagulation quality control product prepared by the method provided by the invention has good sensibility to variation of a detection reagent, has high stability and meets the quality control requirement of the clinical blood coagulation inspection item.
Owner:SHANGHAI SUNBIO TECH
Method for evaluating chemical composition of Rosa xanthina on basis of antithrombotic spectrum-effect relationship
ActiveCN108195989AComprehensive and accurate spectrum effect basisClear chemical compositionComponent separationMathematical modelSeparation technology
The invention discloses a method for evaluating chemical composition of Rosa xanthina on the basis of antithrombotic spectrum-effect relationship. The method comprises the following steps: preparing extract of different polar components of the Rosa xanthina with a modern separation technology; establishing fingerprint of extract of each component with high-performance liquid chromatography, and calibrating characteristic peaks; evaluating antithrombotic activity of different extract on the basis of platelet aggregation inhibition rate, prothrombin time, thrombin time and activated partial thromboplastin time as indexes; substituting fingerprint characteristic peak data and pharmacodynamical activity data into a mathematical model for spectrum-effect correlation analysis, and evaluating pharmacodynamical activity of the characteristic peaks. With adoption of the method for evaluating the chemical composition of the Rosa xanthina on the basis of the antithrombotic spectrum-effect relationship, the antithrombotic chemical composition in the Rosa xanthina can be evaluated rapidly and accurately, a scientific and effective method is provided for research of pharmacodynamic material basis and quality control of the Rosa xanthina, and reference is provided for further development of Rosa xanthina drugs or health care products for treating thrombotic diseases.
Owner:山西省医药与生命科学研究院
In-vitro detection diagnosis kit for clinical examination of prothrombin time (PT)
ActiveCN1952169AGood repeatabilityImprove stabilityMicrobiological testing/measurementBiological testingBasic researchFactor ii
The invention concerns a diagnostic kit for clinical testing the prothrombin time (PT) in vitro. It is composed by thromboplastin and buffer system. And it is used for monitoring blood coagulation factor absence screening experiment and oral administration of decoagulant therapy. The advantages of the kit are good safety, simple operation, good reproducibility and good stability. The kit provides reliable experimental data for clinical diagnosis and treatment reduces costs and burden on patients and improves the efficiency and level of thrombosis and haemostasis basic research.
Owner:SHANGHAI SUNBIO TECH
A starch composite hemostatic dressing of mesoporous silica microspheres
InactiveCN106806931AHas a hemostatic effectThe hemostatic effect is achievedAbsorbent padsBandagesWound dressingMicrosphere
The invention relates to a starch composite hemostatic dressing of mesoporous silica microspheres, which adopts a sol-gel method and uses a three-block copolymer pluronic as a template agent to synthesize mesoporous silica microspheres with a pore diameter of about 5 nm. It is compounded with starch to prepare a mesoporous silicon oxide microsphere / starch composite hemostatic dressing, and the hemostatic performance of the composite material is observed through animal experiments. The mesoporous silica microsphere / starch composite material not only has strong water absorption performance, but also has obvious in vitro coagulation performance, which can significantly shorten the partial thromboplastin time and prothrombin time. The mesoporous silica microsphere / starch composite material can prevent the bleeding of the rabbit's back skin and liver injury and shorten the bleeding time, and has obvious hemostatic effect.
Owner:TIANJIN YIYAO SCI & TECH
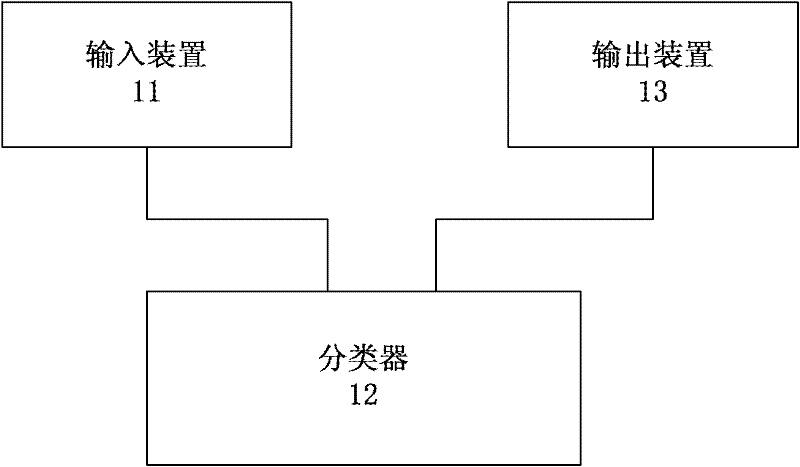
Hepatic fibrosis detection equipment and system
ActiveCN102302358AGet the most out of your test resultsDiagnostic recording/measuringSensorsSerum igeSerum direct bilirubin
The invention discloses hepatic fibrosis detection system, relating to the technical field of hepatic fibrosis research. The hepatic fibrosis detection system comprises a transient elastography device an input device, a classifier and an output device, wherein the input device is used for receiving ages and serum biochemical indicators, and the serum biochemical indicators at least comprise blood platelet, hyaluronic acid (HA), serum direct bilirubin (DBIL), prothrombin time (PT), alanine aminotransferase / serum glutamic pyruvic transaminase (ALT; GPT), and aspartate transaminase / serum glutamic oxalacetic transaminase (AST; GOT); the classifier is used for performing hepatic fibrosis staging according to the ages and the serum biochemical indicators and received by the input device and transient elasticity imaging data; and the output device is used for outputting of hepatic fibrosis staging results of the classifier. The hepatic fibrosis detection system of the embodiment disclosed by the invention has the advantages of noninvasiveness, strong practicability, simple and convenient method, low price, good safety and the like.
Owner:INNER MONGOLIA FURUI MEDICAL SCI
Microfabricated device with micro-environment sensors for assaying coagulation in fluid samples
ActiveUS20160091508A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careEngineering
The present invention relates to sample analysis cartridges comprising micro-environment sensors and methods for assaying coagulation in a fluid sample applied to the micro-environment sensors, and in particular, to performing coagulation assays using micro-environment sensors in a point of care sample analysis cartridge. For example, the present invention may be directed to a sample analysis cartridge including an inlet chamber configured to receive a biological sample, and a conduit fluidically connected to the inlet chamber and configured to receive the biological sample from the inlet chamber. The conduit may include a micro-environment prothrombin time (PT) sensor, and a micro-environment activated partial thromboplastin time (aPTT) sensor.
Owner:ABBOTT POINT CARE
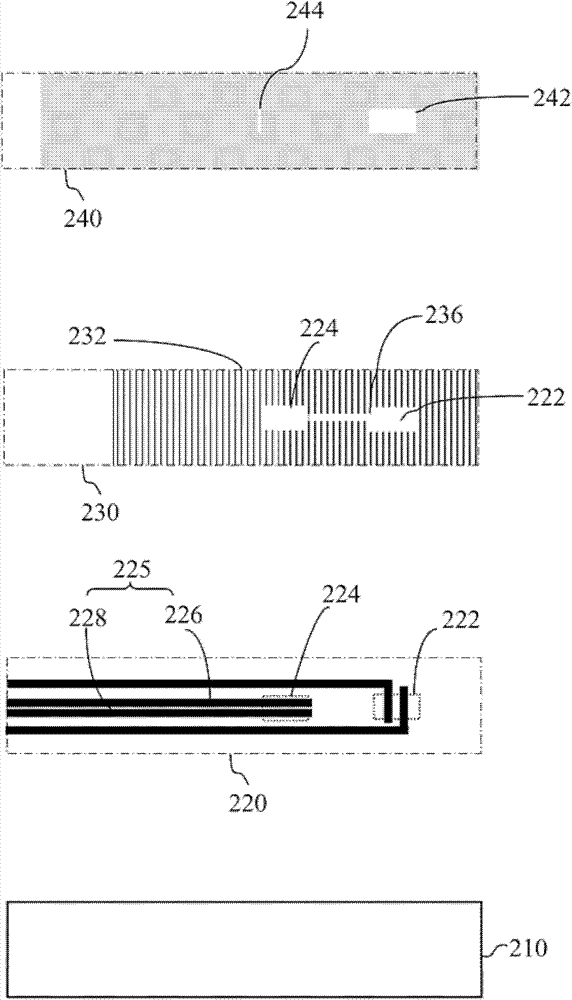
Electrochemical blood test strips and diagnosis systems using the same
ActiveUS20130118899A1Reduce chanceHigh measurement accuracyImmobilised enzymesBioreactor/fermenter combinationsBlood testEngineering
Electrochemical blood test strips and diagnosis systems are presented. An electrochemical blood test strip for measuring HCT % and prothrombin time includes an electrode plate having electrode circuit patterns on an insulator substrate; a separation plate disposed on the electrode plate defining a blood sample region, a channel and three reaction regions; and a cover plate disposed on the separation plate having a blood sample inlet and vents. In measuring, one of the three reaction regions is used for detecting hemoglobin and hematocrit and the other two reaction regions are biochemical reaction regions and used for detecting prothrombin time.
Owner:APEX BIOTECH
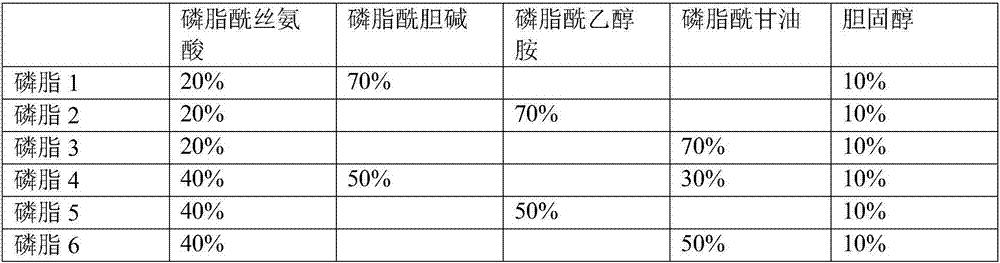
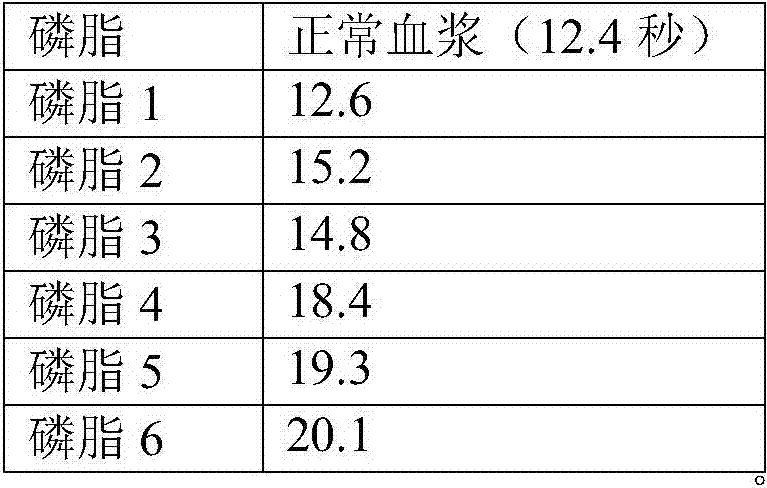
Liquid ready-to-use prothrombin time detection reagent
InactiveCN107356768AOvercome the difference between bottlesOvercome the defect of large batch differenceBiological testingTissue factorCholesterol
The invention discloses a liquid ready-to-use prothrombin time detection reagent, which includes a buffer, a synthetic phospholipid, a recombinant rabbit tissue factor, a surfactant and a stabilizer. The synthetic phospholipid is composed of phosphatidylserine, phosphatidylcholine and Cholesterol composition. The present invention uses rabbit recombinant factors and synthetic phospholipids to prepare prothrombin time detection reagents by selecting synthetic phospholipid components and optimizing stabilizers. It does not need to be reconstituted during use and can be used immediately after opening the bottle. The reagent overcomes the problem of difficult-to-control batch-to-batch variation of existing prothrombin time detection reagents and has high sensitivity, good stability, small batch-to-batch variation, easy quality control, and easy production.
Owner:NINGBO ACCUTECH BIOSCI LTD
Method for detecting whole-blood sample coagulation item using magnetic-bead method
InactiveCN1936580AEasy to masterAccurate reflectionBiological testingMagnetic beadBlood coagulations
One kind blood samples detection method using magnetic beads, completed with the following steps: (1) Get fresh clinical samples; (2)Put these specimen on coagulation analyzer, use magnetic beads and special adjustment reagents aiming at the whole blood specimens, measure four coagulations: Activated partial thromboplastin time (APTT), Prothrombin time (PT), Prothrombin time (TT), Fibrinogen (FIB). The advantages of this invention are: 1.The method is simple, time-saving and easy to technical staff. 2. Reduce costs and save centrifuges and other equipment, reduce errors; 3. It is efficient and suitable for the battlefield, natural disasters and remote mountainous areas, medical teams. Particularly, it's suitable for hemorrhagic diseases and natural disasters (such as hemophilia, etc.). 4. Save specimen volume. 5. Reflect the level of specimens of blood coagulation preciously and accurately.
Owner:MEIDE TAIPINGYANG PRECISION INSTR MFG
Ancylostoma caninum anticoagulant peptide and its preparation and application
InactiveCN101260150APeptide/protein ingredientsFermentationAntithrombotic AgentAnticoagulation Activity
The invention discloses a novel ancylostoma caninum anticoagulation peptide and an encoded sequence thereof and a preparation method for the anticoagulation peptide. The anticoagulation peptide acquired by the method possesses of anticoagulation activity, can markedly prolong the plasma prothrombin time (PT) and the activation part thrombozyme time(aPTT) of people and has obvious anti thrombosis effects. The invention also relates to applications of the anticoagulation peptide on aspects of anticoagulation drugs, antithrombotic drugs or anticoagulation preparations.
Owner:GUANGDONG MEDICAL UNIV
Coagulation tests at ambient temperature
InactiveUS20060281140A1Enabling detectionAbolish needMicrobiological testing/measurementBiological testingBlood plasmaPathology
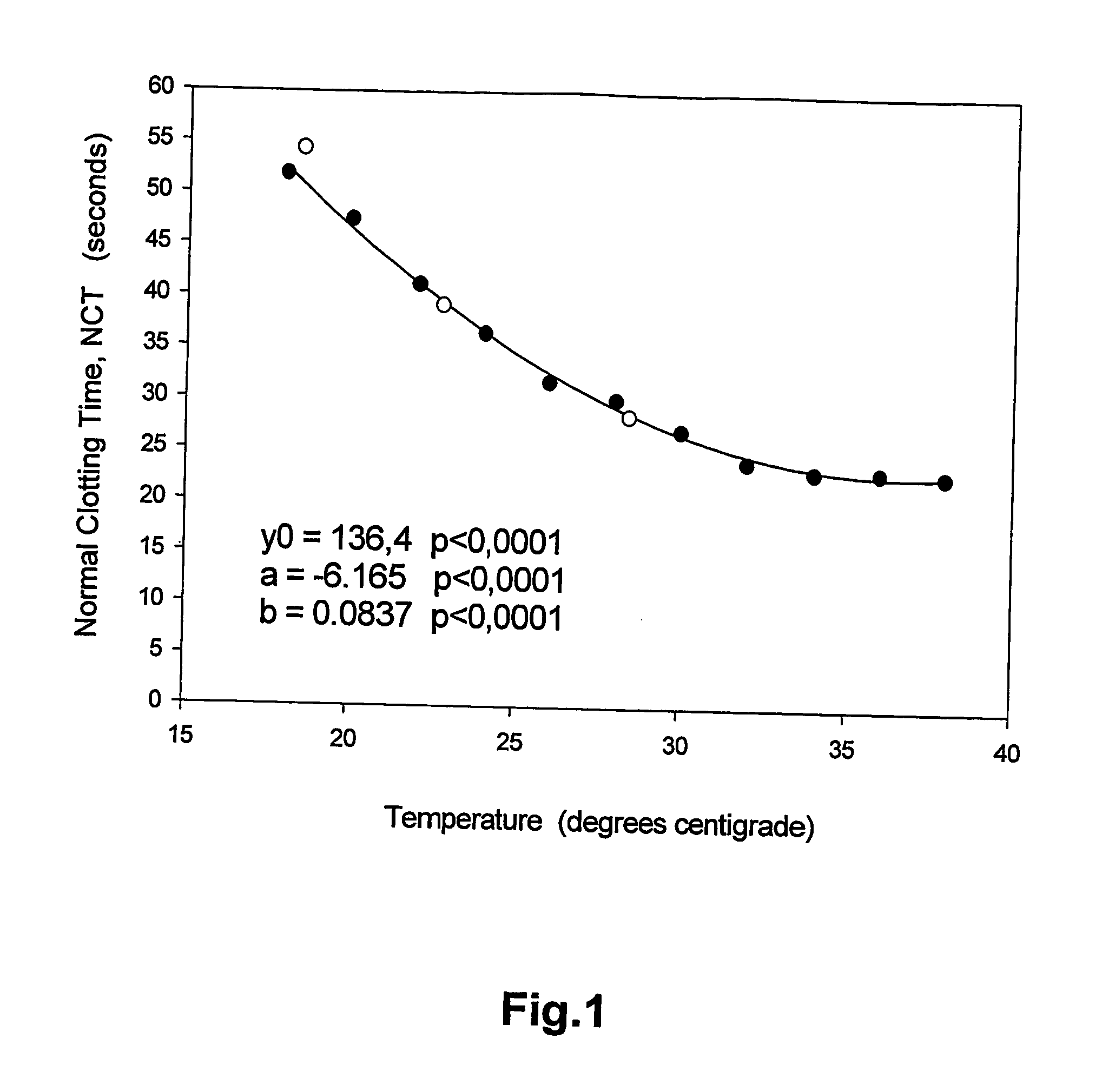
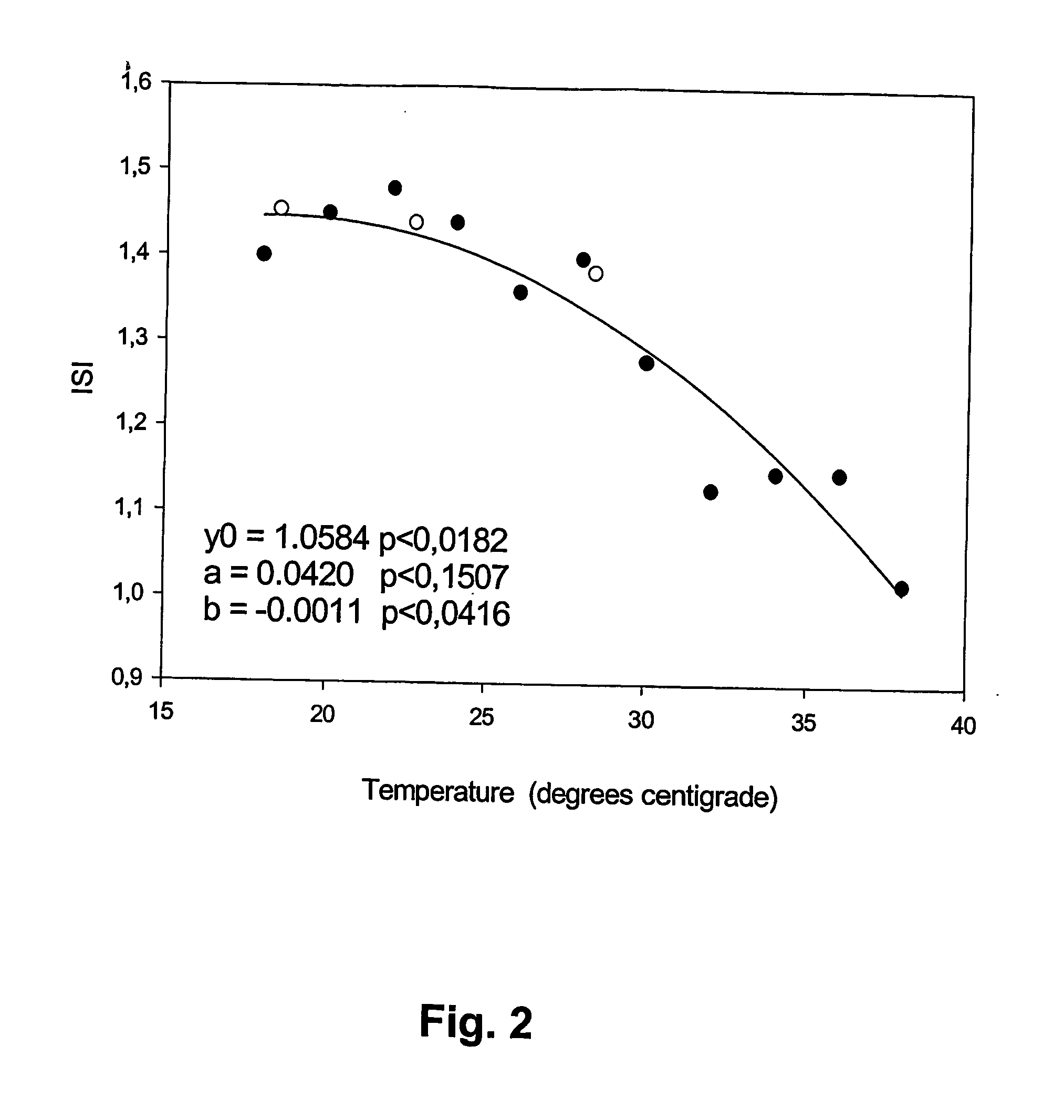
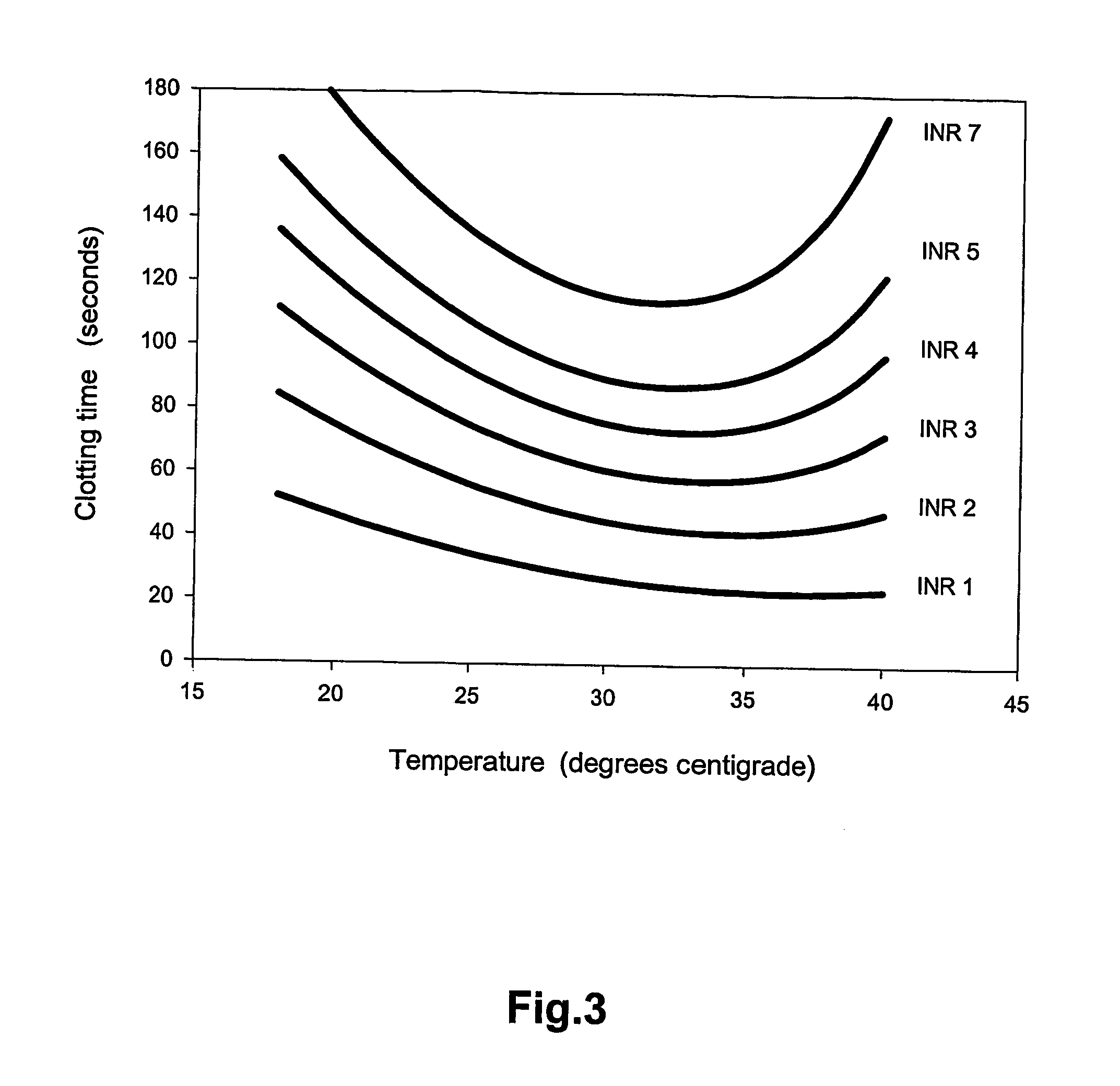
A method of determining prothrombin time (PT) in a whole blood, anti-coagulated blood, blood plasma or anti-coagulated blood plasma sample at an ambient temperature in the range of 15° C. to 45° C. is described. The method is performed with liquid reagents and the PT, preferably expressed as International Normalized Ratio (INR), is calculated based on said temperature and the clotting time (CT). A test kit is also described for analysis of PT which comprises temperature recoding means, and one or several separate sealed vessels containing reagents, optionally in lyophilized form for reconstitution prior to use, for clotting one or more defined volumes of whole blood, anti-coagulated blood, blood plasma or anti-coagulated blood plasma sample, and optionally time registration means and volume determining means.
Owner:ZAFENA
Preparation method for prothrombin time determination reagent
The invention relates to a preparation method for prothrombin time determination reagent (PT reagent), comprising the following steps. Cow or sheep tissue factor with the purity of more than 90% is prepared by the genetic engineering method, phospholipid is dispersed in phosphate buffer solution containing surfactant, and then the tissue factor and the phospholipid are mixed and sufficiently combined, and finally, C18 resin is adopted to adsorb and separate the surfactant, and the PT reagent product is obtained after freeze-drying. The preparation method is stable in technique and strong in operability, and further, the obtained PT reagent has better stability, sensitiveness and fewer impurities, and the quality can be easily ensured.
Owner:苏州良辰生物医药科技有限公司
Liquid-type prothrombin time detection reagent and preparation method thereof
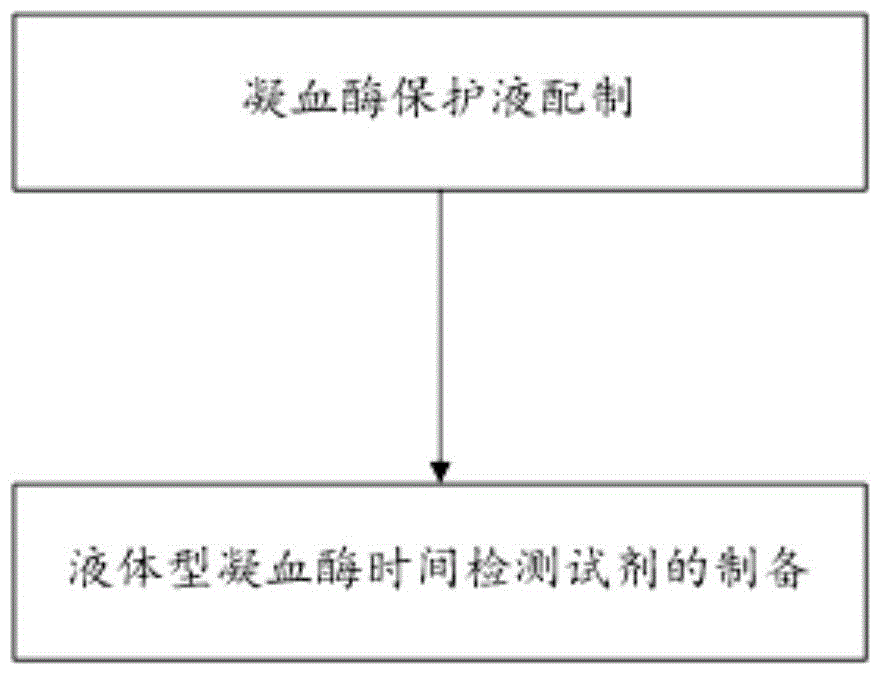
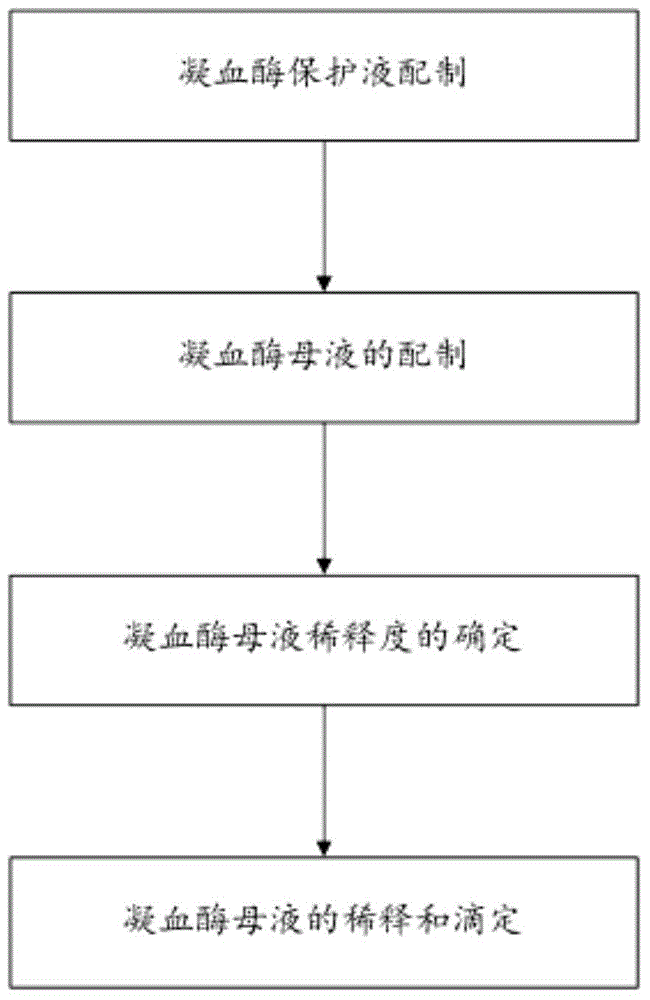
InactiveCN104569446AEasy to produceSimple production equipmentBiological testingFreeze-dryingPolyethylene glycol
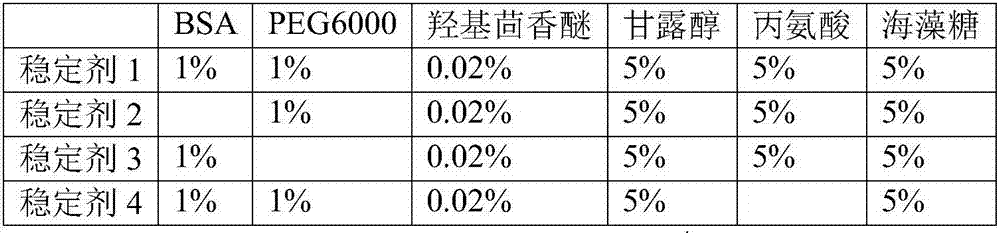
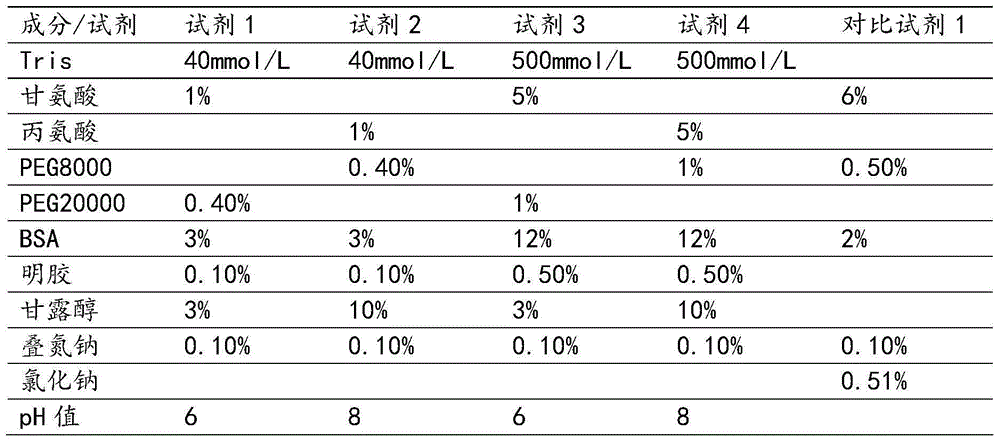
The invention discloses a liquid-type prothrombin time detection reagent and a preparation method thereof. The preparation method comprises the following steps: dissolving amino acid, polyethylene glycol, mannitol, bovine serum albumin, and gelatin in water so as to obtain a prothrombin protective fluid, and adjusting the pH value of the prothrombin protective fluid to 6.0-8.0; and dissolving prothrombin freeze-dried powder in the prothrombin protective fluid so as to obtain the liquid-type prothrombin time detection reagent, wherein the enzymatic activity of the liquid-type prothrombin time detection reagent is 5-80 IU / mL. The liquid-type prothrombin time detection reagent contains prothrombin, amino acid, polyethylene glycol, mannitol, bovine serum albumin and gelatin, and takes water as a solvent. The production process of the reagent disclosed by the invention is simplified, production facilities are simple, no freeze-drying process is performed, and the production cost is low; the reagent is used in an out-of-the-box mode; the variation among bottles is small; and the reagent is high in stability.
Owner:SHANGHAI LONG ISLAND BIOTEC CO LTD
Device and method for measuring prothrombin time and hematocrit by analyzing change in reactance in a sample
ActiveUS20110303556A1Reduce chanceHigh measurement accuracyImmobilised enzymesBioreactor/fermenter combinationsElectricityBlood test
Devices and methods for measuring prothrombin time (PT) and hematocrit (HCT) by analyzing the change in reactance in a sample are presented. A diagnostic device for measuring HCT and PT of a fluid includes a relative electrode-type sensor device and a blood test card assembly including one or more pairs of electrodes, wherein alternating current (AC) provided by the sensor device is used to measure and calculate HCT and PT of blood test using the reactance analysis.
Owner:APEX BIOTECH
Fluid management system and methods
ActiveUS9943639B2Avoid hemolysisControl pressureEnemata/irrigatorsHaemofiltrationSaline waterControl flow
Owner:BOSTON SCI SCIMED INC
Electrochemical detection chip and electrochemical sensor, as well as preparation methods and application thereof
PendingCN109507260AEasy to makeHigh detection sensitivityMaterial analysis by electric/magnetic meansReaction layerElectricity
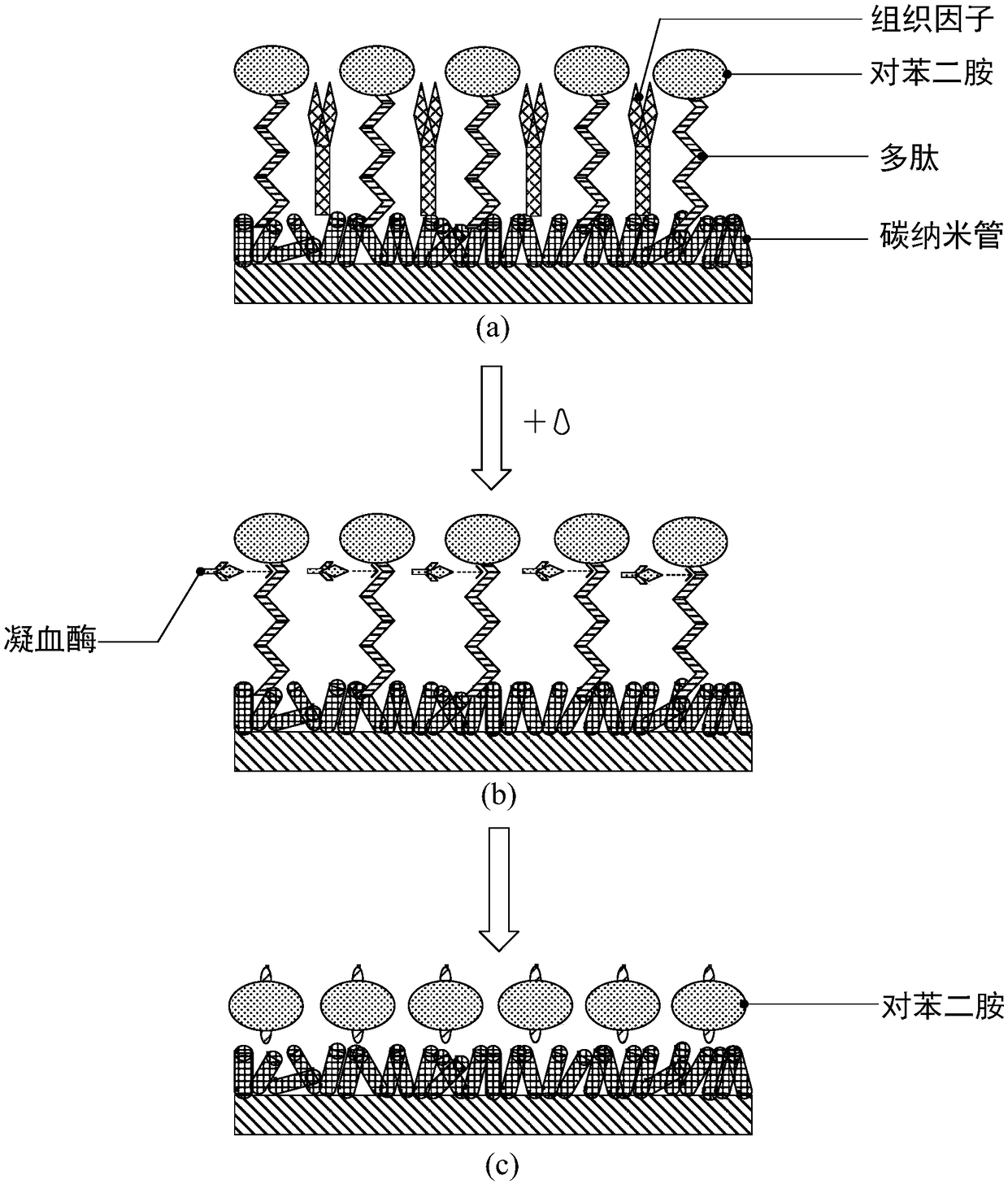
The invention discloses an electrochemical detection chip. The electrochemical detection chip comprises an electrode layer, wherein a working electrode of the electrode layer comprises a conducting layer, a nanomaterial layer and a blood clotting reaction layer which are sequentially laminated, wherein the blood clotting reaction layer is used for producing an electrical signal for detecting prothrombin time; the nanomaterial layer is used for transferring and amplifying the electrical signal. By using high conductivity, large specific area, good biocompatibility and the like of the nanomaterial layer, amplification and enhancement of the electrical signal in the detection process of the prothrombin time are realized so as to improve the sensitivity of chip detection and shorten the detection time. The invention discloses an electrochemical sensor. The electrochemical sensor comprises the electrochemical detection chip. The invention discloses a preparation method of the electrochemical sensor. The preparation method is suitable for preparing the electrochemical sensor with high sensitivity and accurate detection results.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Application of terpenoid
The invention provides an application of a terpenoid to preparation of an anticoagulation drug. The terpenoid has a structural formula I which is as shown in the description, wherein R1 represents H, C1-C5 linear alkyl, glycosyl or a formula which is as shown in the description, and R2 is selected from C1-C4 linear alkyl. According to the application of the terpenoid, an ionane type sesquiterpene compound separated and obtained from leonurus for the first time can obviously inhibit the platelet aggregation in vitro and has extension tendencies to prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT) and has a certain anticoagulation activity, so that a new choice is provided for the development of natural anti-thrombus drugs.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE +1
Apparatus and method for electrochemical detection
ActiveCN102232185AMicrobiological testing/measurementBiological material analysisPoint of careAnticoagulant
The present invention is directed to a sensor with opposing electrodes and test strips using this sensor. The invention can be used to measure blood or plasma coagulation in assays like prothrombin time (PT) and thrombin potential, for example, in point-of-care monitoring of anticoagulants.
Owner:UNIVERSAL BIOSENSORS
Surface antigen 1 of Toxoplasma gondii human antibody Fab fragment and encoded gene thereof
The present invention belongs to the field of biotechnology, and relates to a surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment, encoded gene and use thereof. According to the invention, the surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment is filtered from a base through establishing a Toxoplasma gondii human immunoglobulin, ELISA, diluting the prothrombin time, sequencing analysis, etc. Through expression purifying and authenticating, the human antigen Fab fragment is authenticated to specifically identify the tachyzoite-bradyzoite recombination SAG1 of Toxoplasma gondii and have higher affinity with the tachyzoite-bradyzoite recombination SAG1 of Toxoplasma gondii, for being identified with the specificity of Toxoplasma gondii tachyzoite-bradyzoite. The human antigen Fab fragment of the invention does not contain Fc segment and does not activate the alexin or cause the histopathological damages of human immune response, etc. when the function of restricting the invasion of Toxoplasma gondii to the host cell is exerted. The surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment is safe and reliable when applied for the human body. The antigen medicine for treating toxoplasmosis or the antigen targeted medicine can be prepared.
Owner:FUDAN UNIV
Biodegradable polymer containing phosphorylcholine and polyethylene glycol and synthetic method thereof
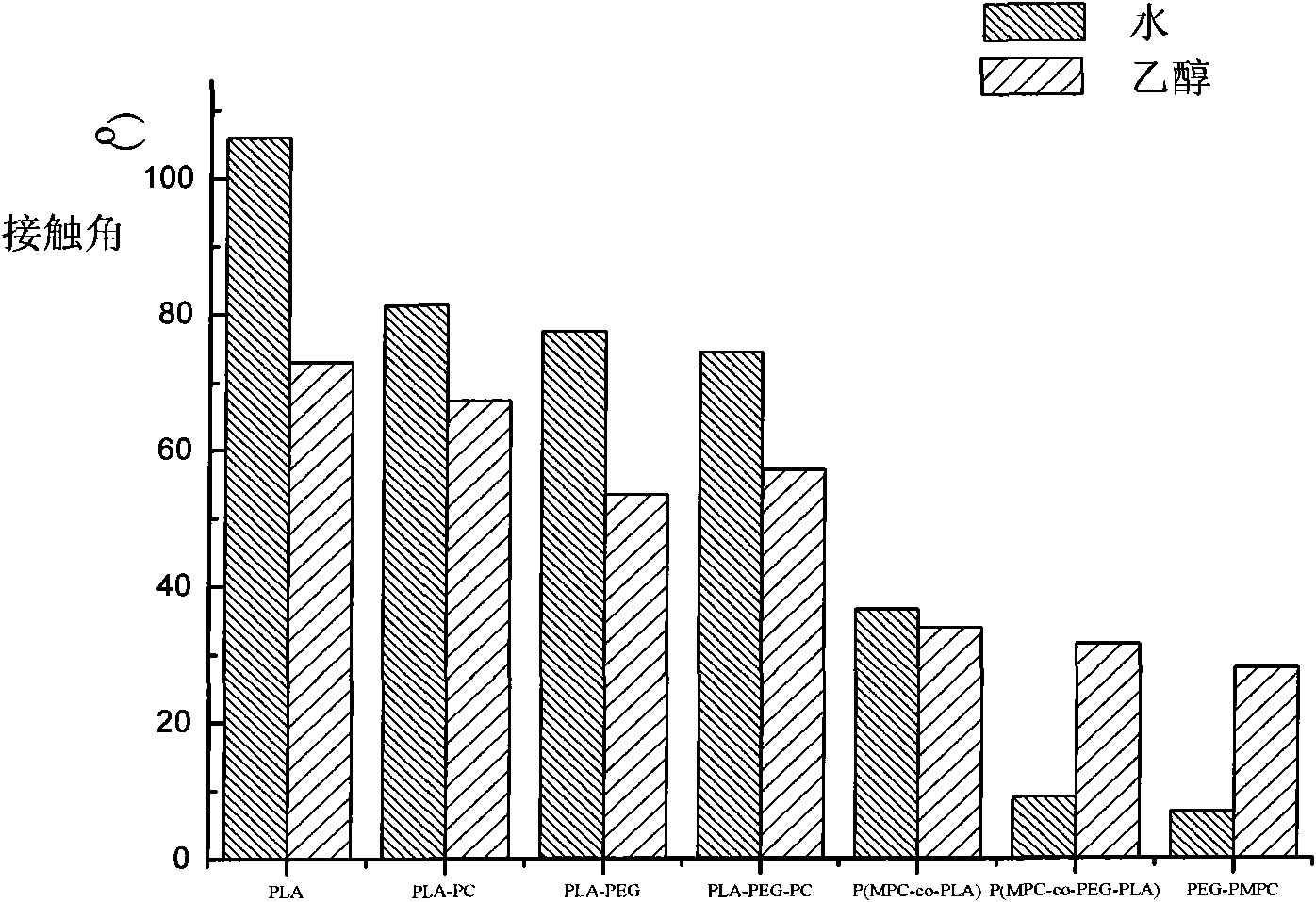
InactiveCN101538353AImprove hydrophilicityImprove anticoagulant performancePharmaceutical containersMedical packagingPolymer scienceLactide
The invention relates to a biodegradable polymer containing phosphorylcholine (PC) and polyethylene glycol (PEG) and a synthetic method thereof. The synthetic method comprises: MPC with the mass ratio of 1-99% and PEG-PLA which is connected with double linkage and has the mass ratio of 99-1% are dissolved in trichloromethane and then added with free radical polymerization initiator to react for 6-24h at the temperature of 0-80 DEG C, and the products are precipitated by methyl alcohol and dried in vacuum; the PEG-PLA which is connected with double linkage is prepared by ring opening polymerization of lactide initiated by the PEG that reacts with acryloyl chloride at a single end. The phosphorylcholine group which has positive and negative charges and is introduced on the PLA of the polymer can be seen according to the static contact angle result, and then the contact angle is obviously reduced, so that hydrophilicity is greatly improved. The higher the MPC content is, the smaller the contact angle is, and the better the wettability of the material is. Test results of anticoagulation prove that the PEG can reduce coagulation of blood platelet, affects prothrombin time (PT) and effectively avoids the activation of an extrinsic coagulation system; the high content PC group can effectively reduce the conglutination of the blood platelet and has influence on anginal partial thromboplastin time (APTT) of the activation part.
Owner:TIANJIN UNIV
Iron chelator-containing prothrombin time reagent
The present invention pertains to the field of coagulation diagnostics and relates to a prothrombin time reagent on the basis of recombinant or native tissue factor protein and phospholipids, which reagent can be stabilized by the addition of an iron chelator from the groups of siderophores and 3,5-diphenyl-1,2,4-triazoles.
Owner:德国西门子医学诊断产品有限公司
Method for determining prothrombin time
InactiveUS7767459B2Microbiological testing/measurementDiagnostic recording/measuringTest samplePhysical chemistry
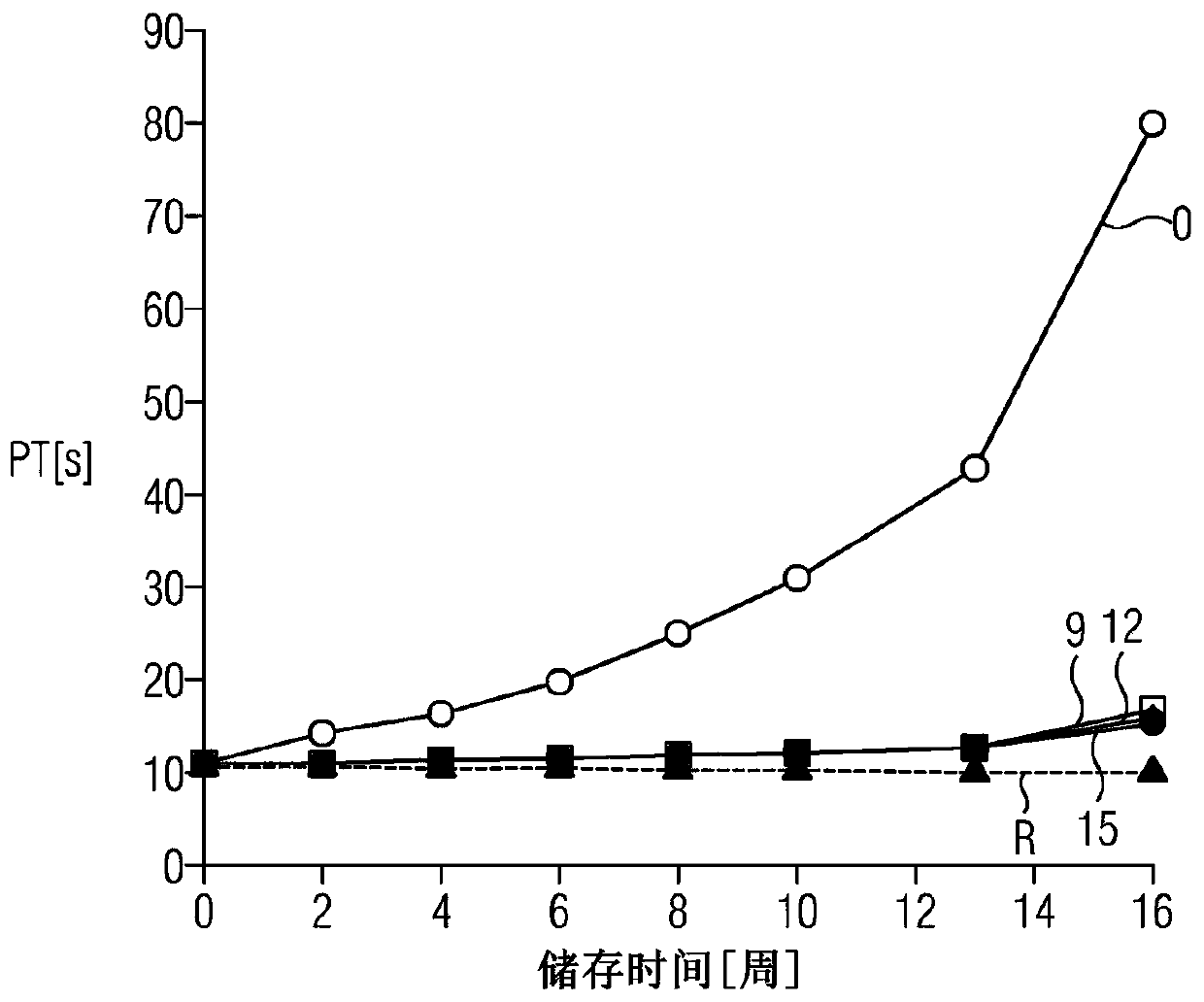
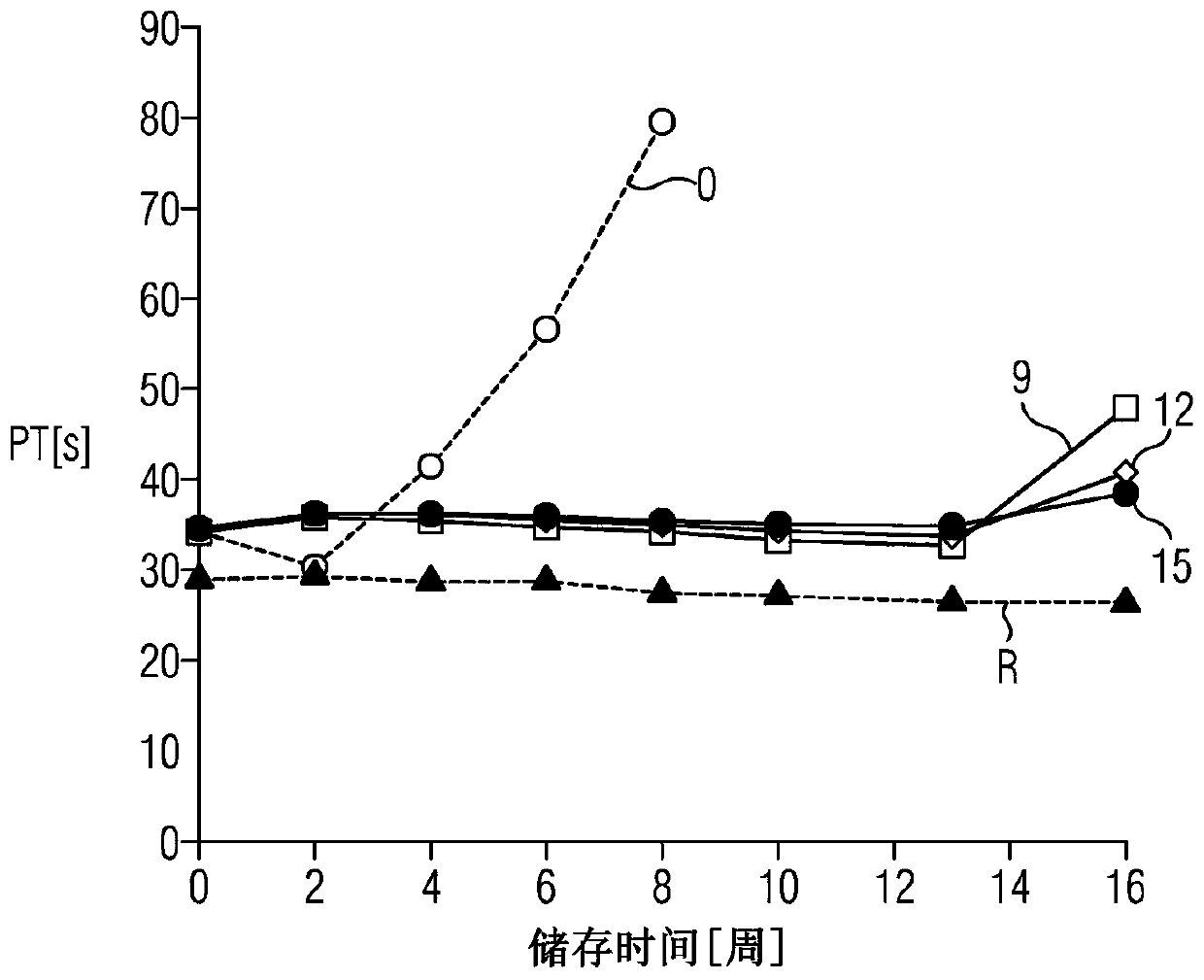
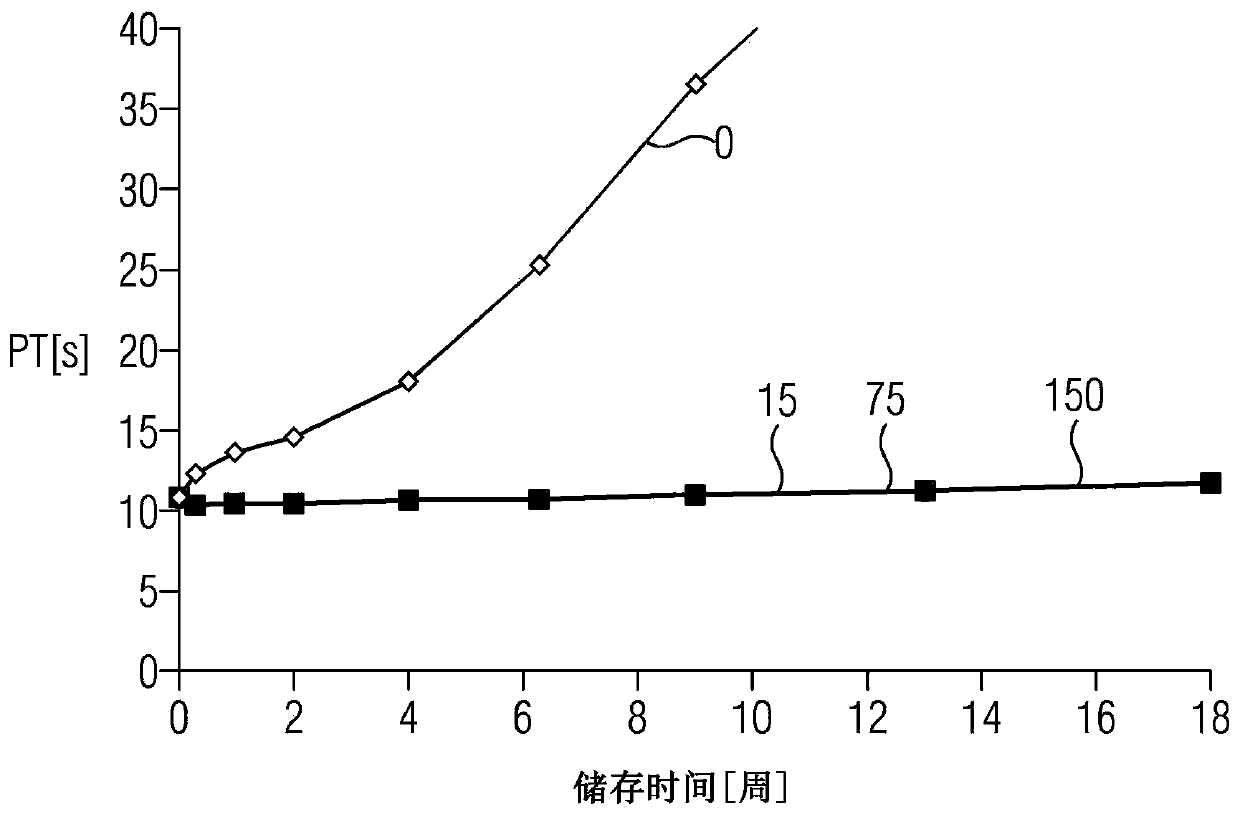
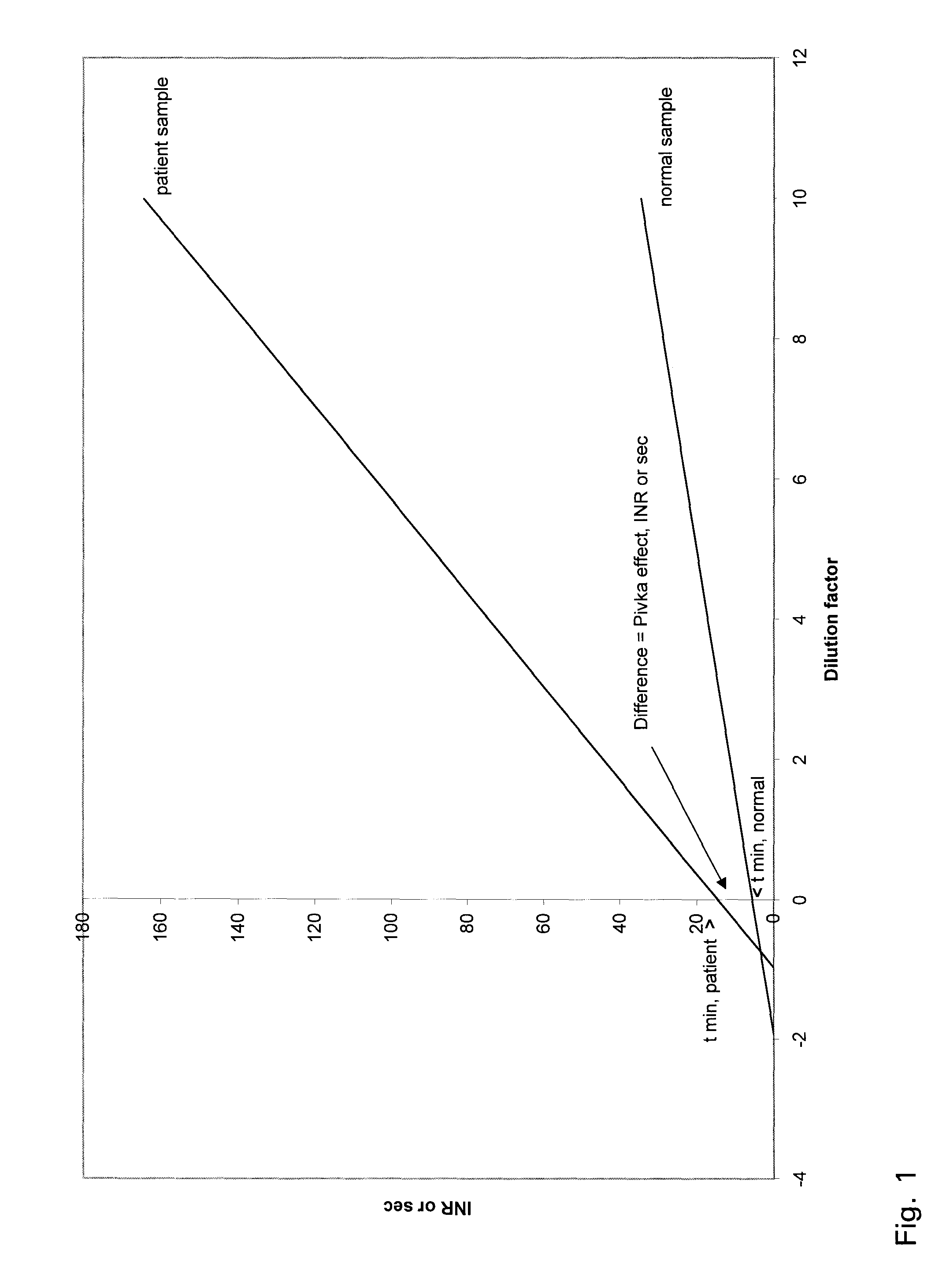
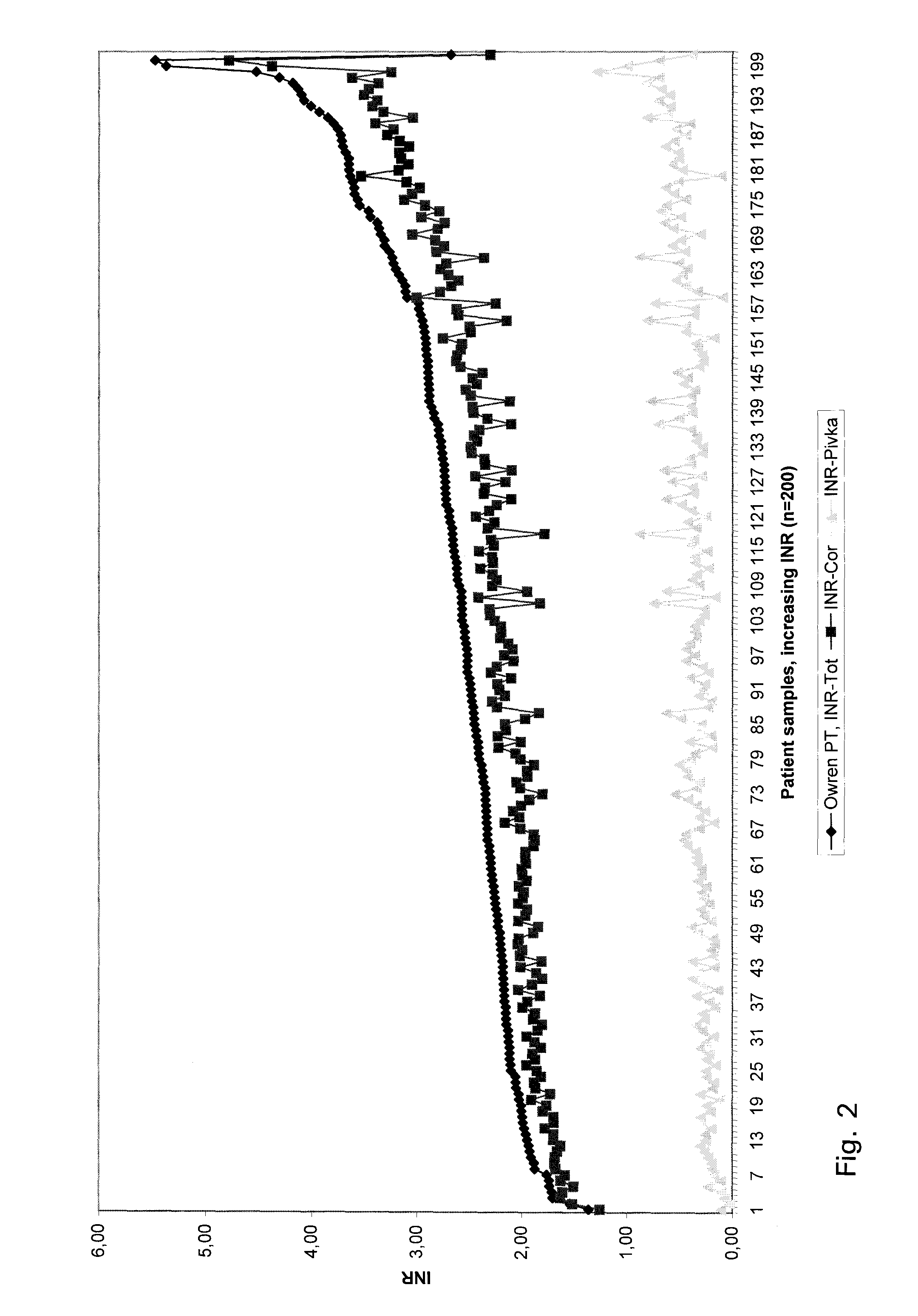
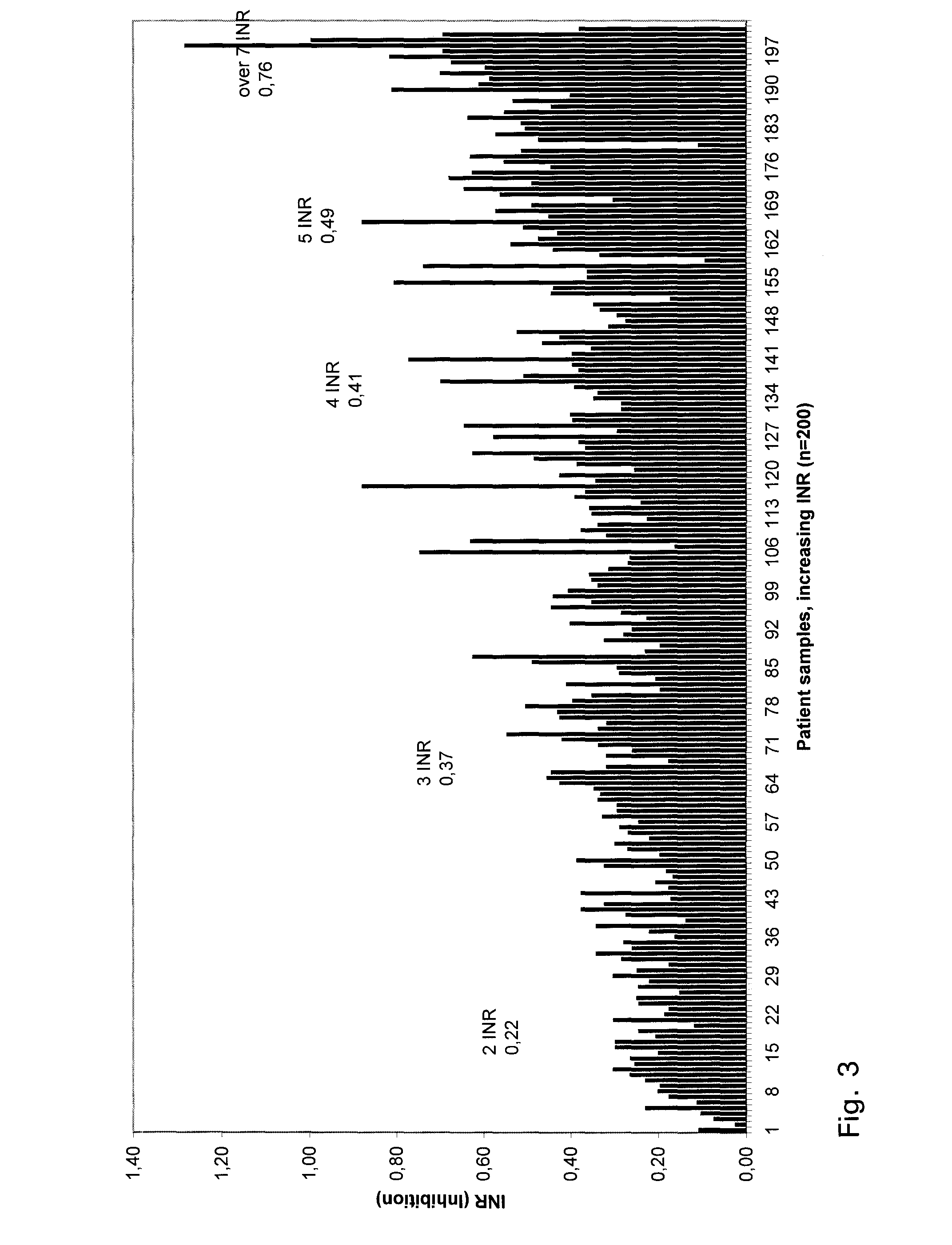
A method for determining prothrombin time of a plasma or whole blood sample includes measuring prothrombin time for at least two different dilutions for a test sample to determine tmin or INRmin. The prothrombin time for at least two different dilutions for normal plasma is measured to determine tmin or INRmin values for normal plasma. Next, tPivka or INRPivka values are calculated by subtracting the value for normal plasma from the value for the test sample. The Pivka corrected prothrombin time for the test sample is calculated by subtracting tPivka or INRPivka from the prothrombin time measured for the test sample.
Owner:HORSTI JUHA
Microfabricated device with micro-environment sensors for assaying coagulation in fluid samples
ActiveUS10247741B2Biological material analysisMaterial analysis by electric/magnetic meansPoint of careEngineering
The present invention relates to sample analysis cartridges comprising micro-environment sensors and methods for assaying coagulation in a fluid sample applied to the micro-environment sensors, and in particular, to performing coagulation assays using micro-environment sensors in a point of care sample analysis cartridge. For example, the present invention may be directed to a sample analysis cartridge including an inlet chamber configured to receive a biological sample, and a conduit fluidically connected to the inlet chamber and configured to receive the biological sample from the inlet chamber. The conduit may include a micro-environment prothrombin time (PT) sensor, and a micro-environment activated partial thromboplastin time (aPTT) sensor.
Owner:ABBOTT POINT CARE
Comb shell viscera polysaccharide, extraction method and application thereof, and drug composition thereof
The invention relates to comb shell viscera polysaccharide, an extraction method and application thereof, and a drug composition. The main chain of the comb shell viscera polysaccharide is -6)Manp(1-3)Galp(1-, wherein -SO4 exists in C-4 of Man (1- and / or C-4 site of -3Gal(1-. The polysaccharide has the effect of inhibiting thrombin to catalyze the activity of thrombin fibrinogen, can prolong activated partial thromboplastin time (APTT) and thrombin time (TT), and has no obvious influence on the prothrombin time (PT).
Owner:DALIAN POLYTECHNIC UNIVERSITY
Oral caring composition and application thereof in inhibiting gum bleeding
InactiveCN110870870AResolve the bleedingAvoid bleedingCosmetic preparationsOrganic active ingredientsBleeding gumPlatelet aggregation ratio
The invention discloses an oral caring composition which comprises asiatic centella triterpenoids and an orally acceptable carrier, wherein the triterpenoids comprise one of or a combination of more of asiaticoside, asiaticoside degradation products, hydroxy asiaticoside and hydroxyl asiaticoside degradation products. The invention further discloses application of the asiatic centella triterpenoids in inhibiting gum bleeding. Four blood coagulation indexes of the oral caring composition disclosed by the invention meet standards that the prothrombin time (PT) is less than or equal to 14.5 seconds, the thrombin time (TT) is less than or equal to 42.5 seconds, the activated part thromboplastin time (APTT) is less than or equal to 27.0 seconds, and the platelet aggregation rate is greater thanor equal to 53.5. The problem of gum bleeding can be effectively solved.
Owner:HAWLEY & HAZEL BVI
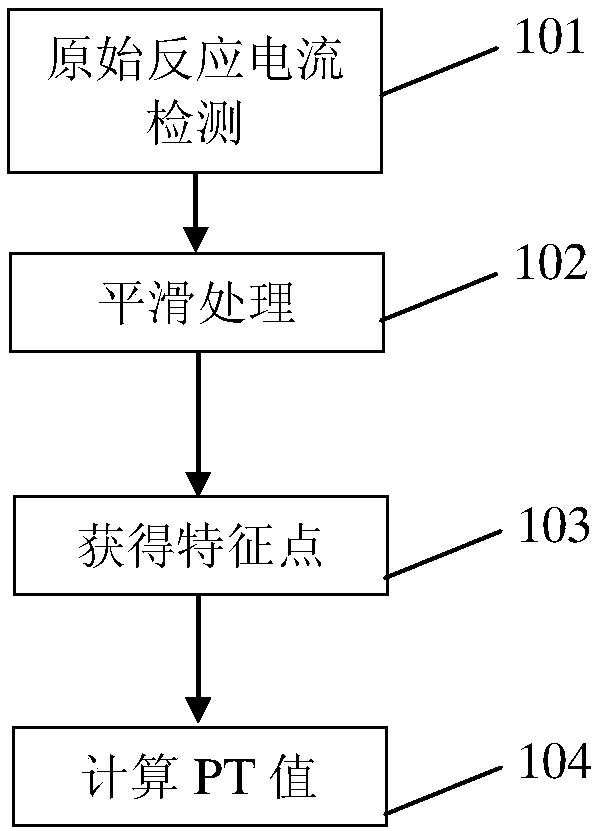
Prothrombin time examination method and device
ActiveCN108614017AImprove accuracyReliable resultsMaterial electrochemical variablesBlood coagulationsSignal-to-quantization-noise ratio
The invention belongs to the medical field and discloses a prothrombin time examination method and device. The method includes the steps of triggering a blood coagulation reaction of blood samples under the functions of a drying reagent and generating oxidation current; generating a current-time curve representing the change of the oxidation current along with time; obtaining multiple feature points on the processed current-time curve; according to the feature points, calculating and outputting a prothrombin time (PT) value. Through smoothing treatment of examination current, the signal to noise ratio of the examination current is increased, correspondingly the influences of current noise on prothrombin time examination accuracy and reliability are reduced, and the accuracy of a testing result is increased. Additionally, by obtaining the feature points on the processed current-time curve and calculating the prothrombin time (PT) value according to the feature points, the accuracy of the testing result is increased.
Owner:HELIXGEN GUANGZHOU
Application of four aspects of coagulation detection after hirudin is inactivated under specific conditions
ActiveCN102262161ASolve the problem that the four coagulation items cannot be detectedDelay in treatmentBiological testingSubtilisinsEnzyme protein
The invention discloses application of hirudin for detecting blood clotting tetrachoric aspects after inactivation under specific conditions. An inactivator for preparing inactivated hirudin is mainly papain or subtilisin or enzyme protease, wherein the weight fraction ratio of the hirudin to the papain is (1:1)-(1:5), the weight fraction ratio of the hirudin to the subtilisin is (1:1)-(1:5), andthe weight fraction ratio of the hirudin to the enzyme protease is (1:1)-(1:5). In the invention, the inactivated hirudin is adopted for the first time to detect blood clotting tetrachoric aspects, thereby solving the problem that the blood clotting tetrachoric aspects can not be detected by only using the hirudin. The invention can avoid the conditions of misdiagnosis, delay of the state of an illness, influence on treatment and the like because of improper prothrombin time (PT) caused by improperly adding the traditional anticoagulant sodium citrate.
Owner:天津百新生物技术研发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com