Ancylostoma caninum anticoagulant peptide and its preparation and application
A technology of anticoagulant peptide and hookworm canis, which can be applied in the fields of medicine and biology, and can solve problems such as the need for special monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Cloning of hookworm anticoagulant peptide gene
[0060] 1. Primer Design
[0061] According to the nucleotide sequences of AcaNAP7 (GenBank entry number: DQ435781), AcAPc4 (GenBank entry number: AY253915), AcAPc3 (GenBank entry number: AY232998) and AcAPc2 (GenBank entry number: U30793), the downstream primer N2 was designed; The guide sequence design upstream primer NSL1, the sequence is as follows:
[0062] NSL1: 5'-GGTTTAATTACCCAAGTTTGAG-3'
[0063] N2: 5'-TTTGGTCATTTTTCTGTTAGG-3'
[0064] 2. RNA isolation and reverse transcription
[0065] Take 20 adult hookworms of hookworm (the host dogs are from Zhanjiang and Guangxi, Guangdong), and use TRIzol Reagent (Invitrogen) to extract total RNA according to the operating instructions. cDNA was obtained by reverse-transcribing the extracted total RNA of H. caninum with 3'-Full RACECore Set (Takara, Dalian) according to the operating instructions.
[0066] 3. Amplification, cloning and sequencing of anticoag...
Embodiment 2
[0080] Example 2: Sequence information and homology analysis of the anticoagulant peptide AcaNAP9 of hookworm hookworm
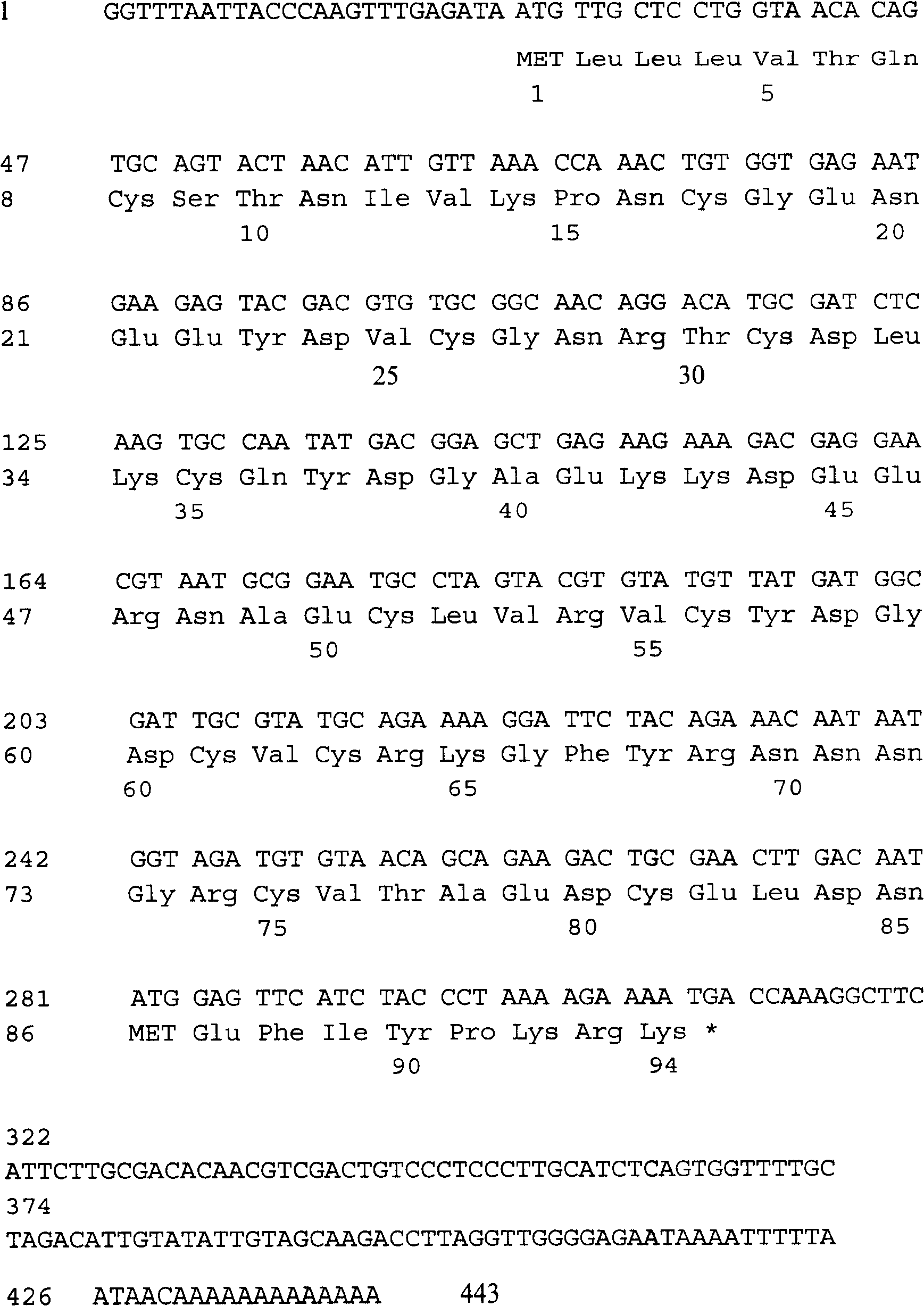
[0081] The full-length cDNA sequence of the isolated hookworm anticoagulant peptide AcaNAP9 is 443 bases (including 13 adenosine A at the 3' end) (SEQ ID NO.11), and its open reading frame (26-310) encodes Peptides (SEQ ID NO.1) consisting of 94 amino acids were obtained, of which the mature peptide consisted of 81 amino acid residues (SEQ ID NO.4), and there was also a signal peptide consisting of 13 amino acid residues. ( figure 1 ).
[0082] The full-length cDNA sequence of hookworm anticoagulant peptide AcaNAP9 and its encoded protein were searched for nucleic acid and protein homology in the Genbank+EMBL+DDBJ+PD database with the BLAST program, and it was found that the amino acid sequence homology with AcaNAP9 in the database was the largest Canine hookworm anticoagulant peptide AcAPc4 (GenBank entry number: AAP82926), their identity is 65% (62 / 94); ...
Embodiment 3
[0083] Example 3: Sequence information and homology analysis of the anticoagulant peptide AcaNAP10 of Ancylostoma canis
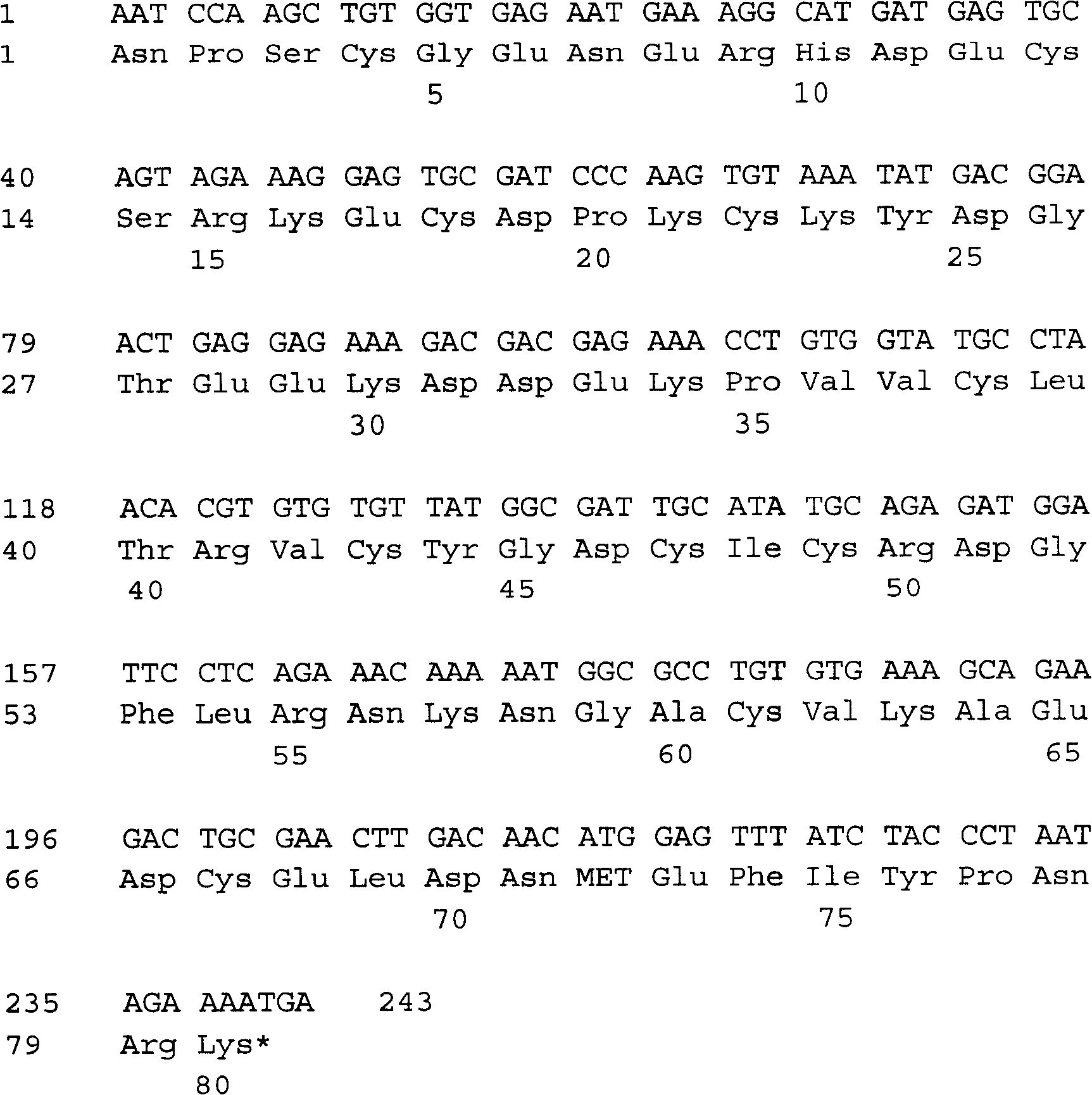
[0084] The cDNA sequence of the isolated hookworm anticoagulant peptide AcaNAP10 is 243 bases (including stop codon) (SEQ ID NO.12), and the polynucleotide sequence 1-240 encodes 80 amino acids (SEQ ID NO.2). ( figure 2 ).
[0085] Using the BLAST program to search for nucleic acid and protein homology in the Genbank+EMBL+DDBJ+PD database, it shows that the most homologous amino acid sequence of AcaNAP10 in the database is the anticoagulant peptide AcAPc4 (GenBank entry number: AAP82926). The concordance of the gene was 87%, followed by AcAPc3 (GenBank accession number: AAP57305) (76% concordance), AcaNAP7 (70% concordance) and AcAPc2 (66% concordance). The largest homology with AcaNAP10 nucleotide sequence is the anticoagulant peptide AcAPc4 gene of hookworm canis (GenBank entry number: AY253915), and their identity is 93%. The AcaNAP10 nucleotide seque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com