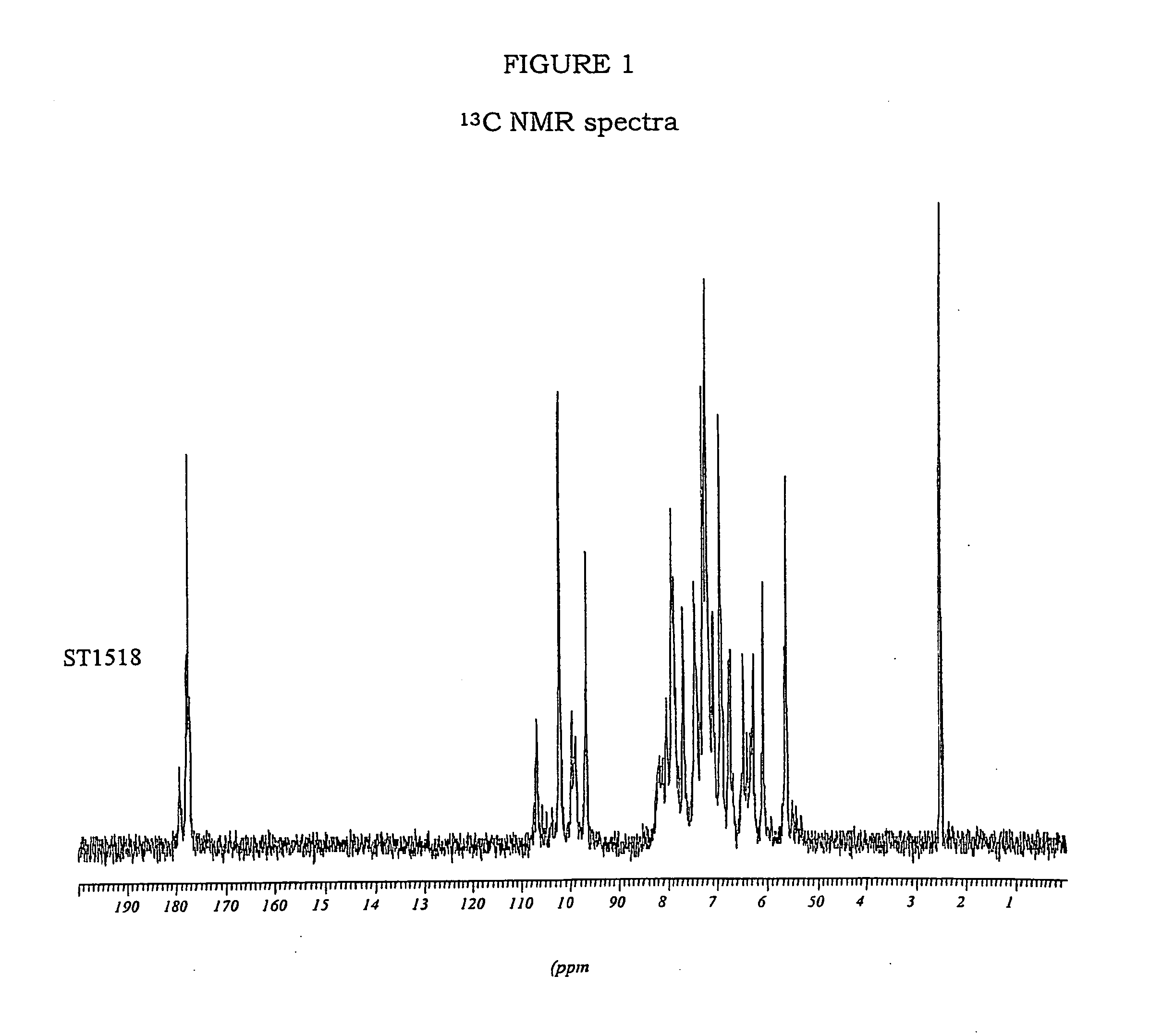
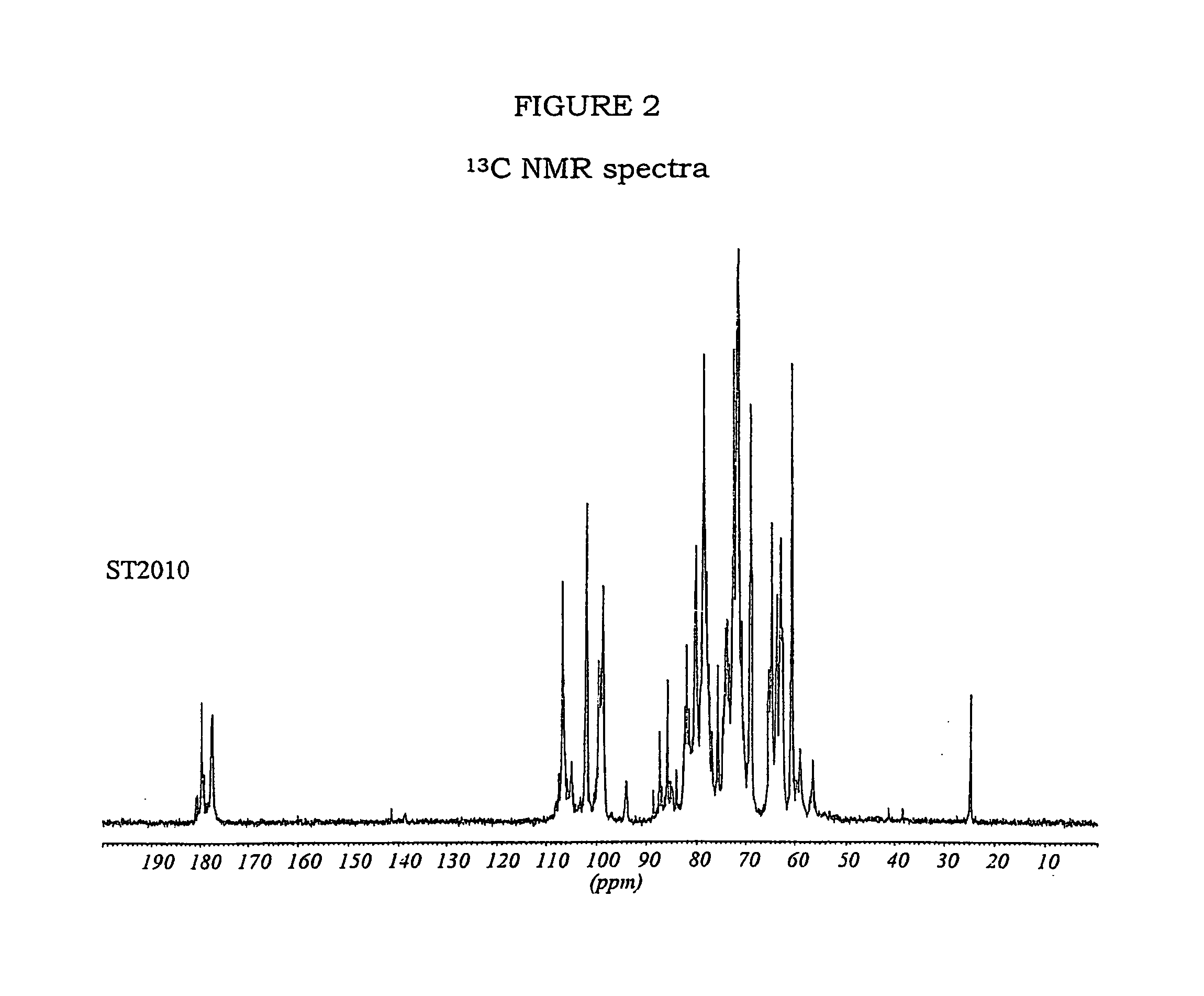
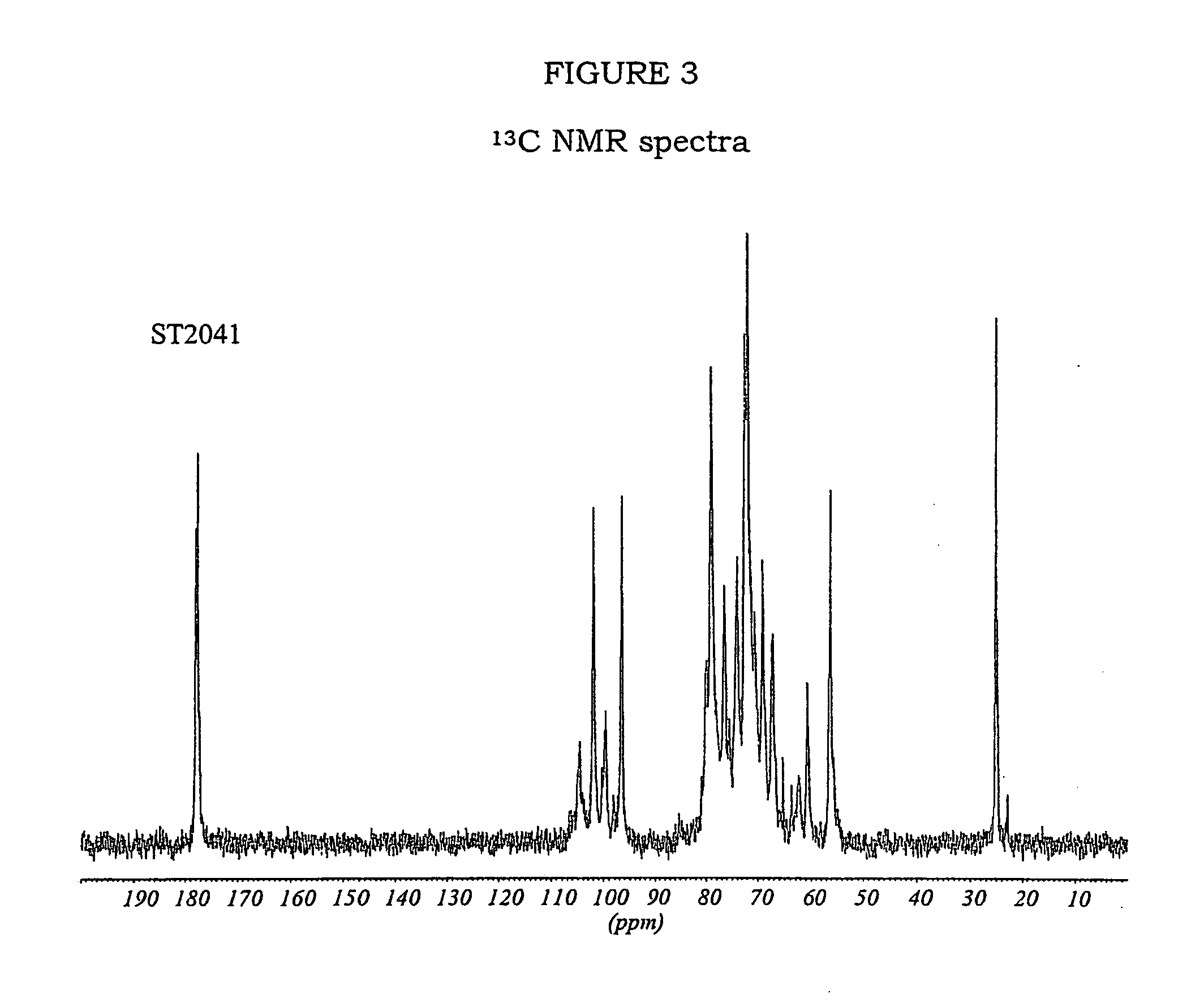
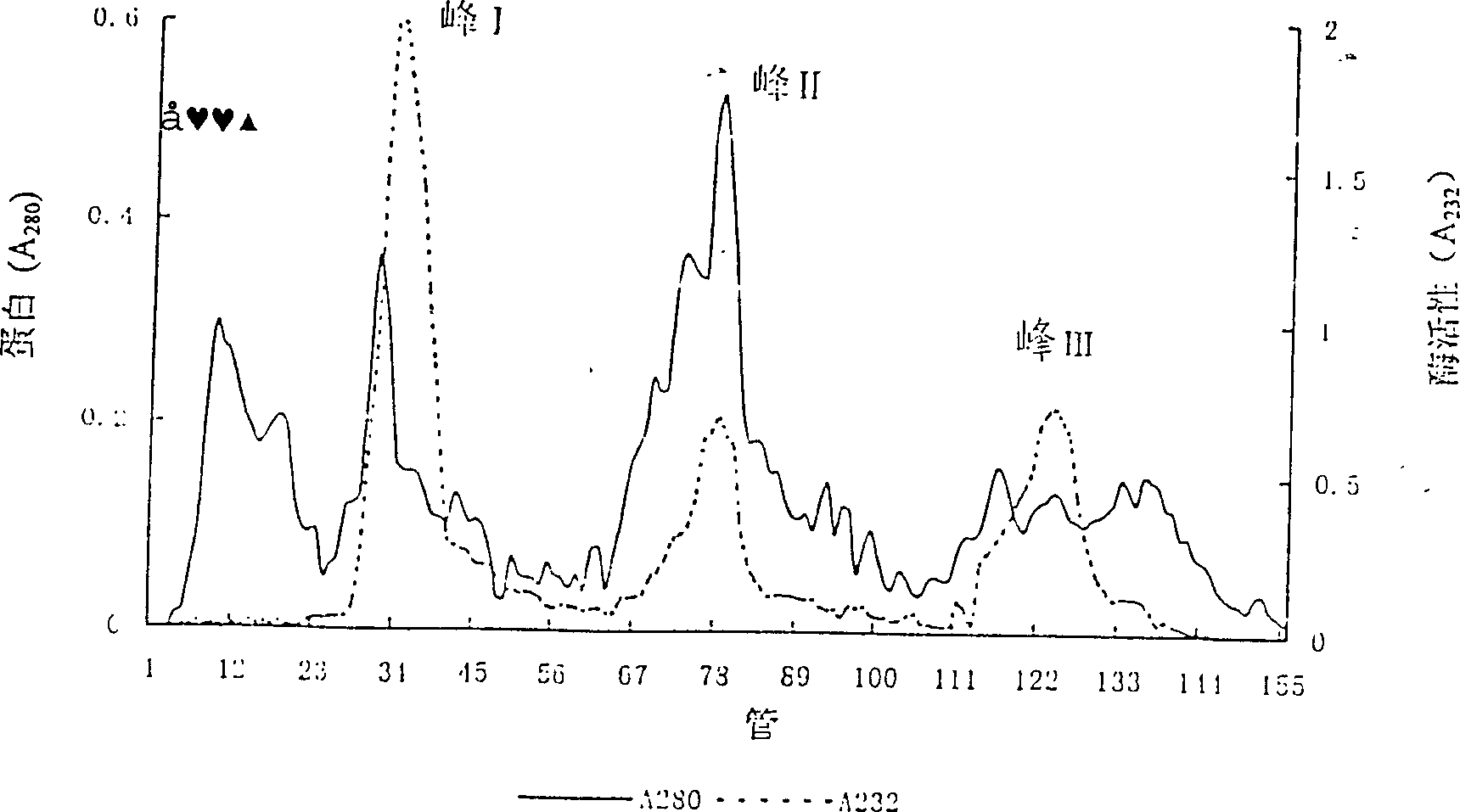
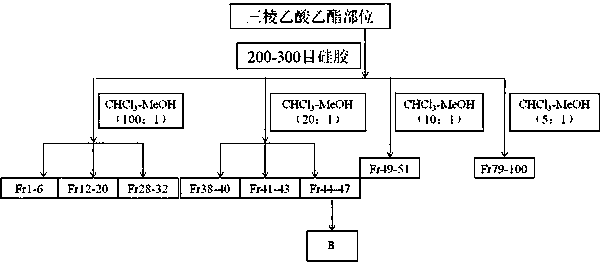
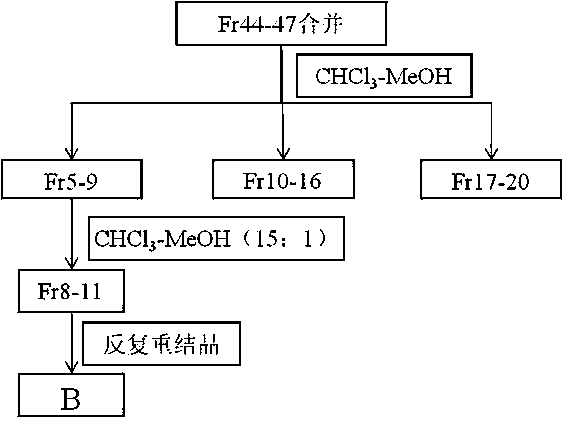
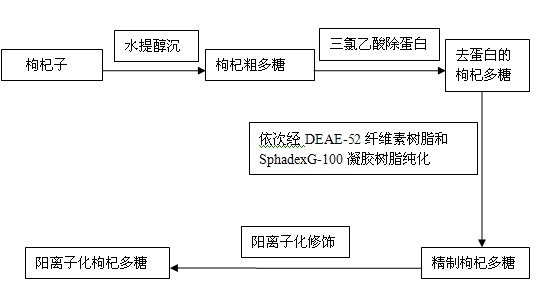
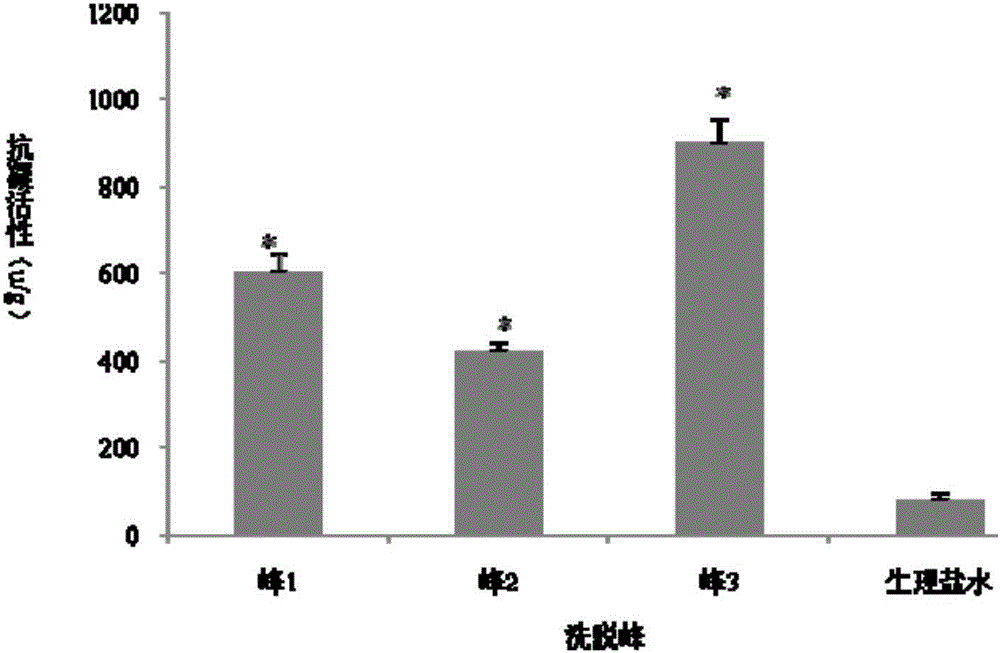
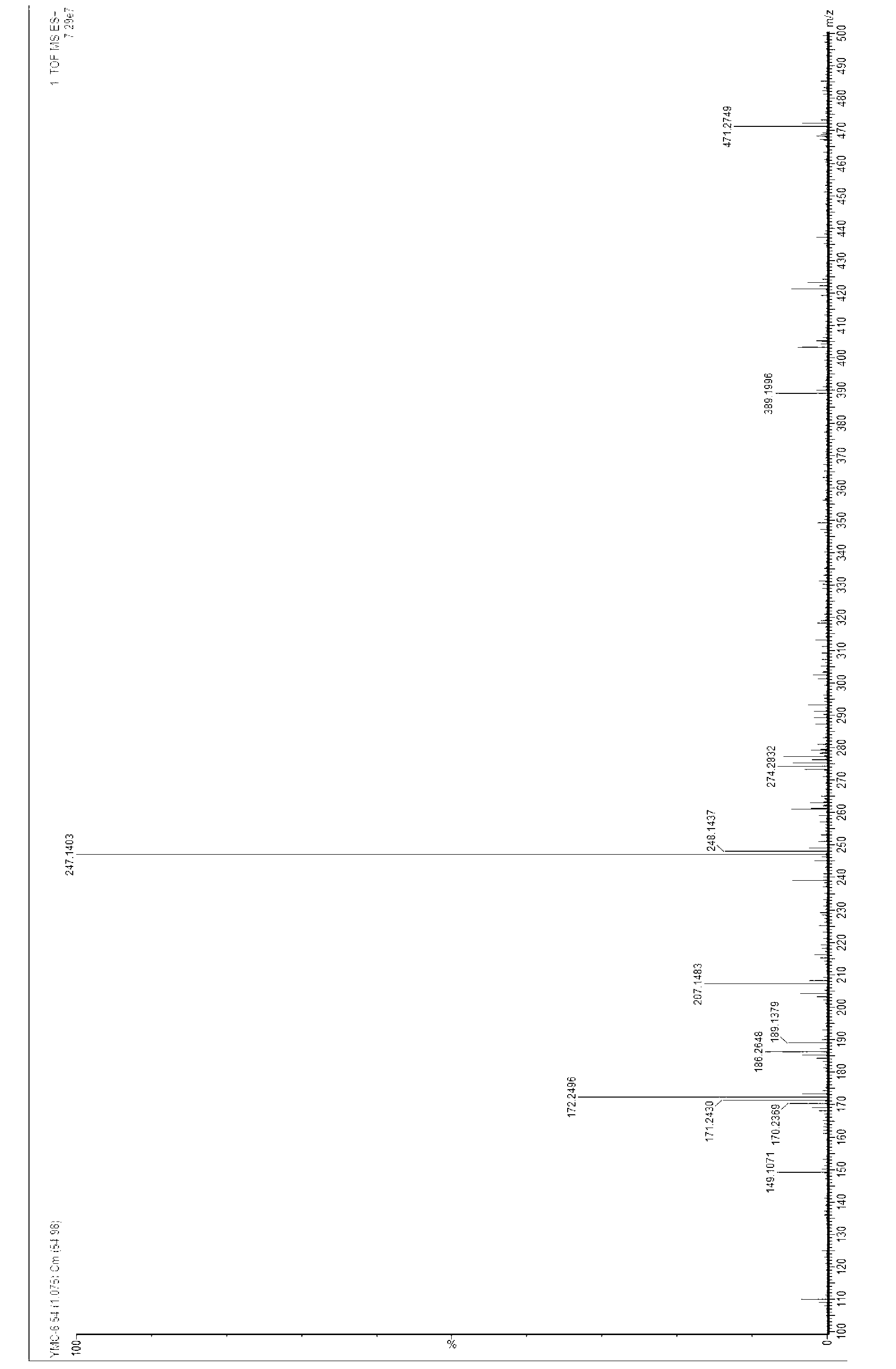
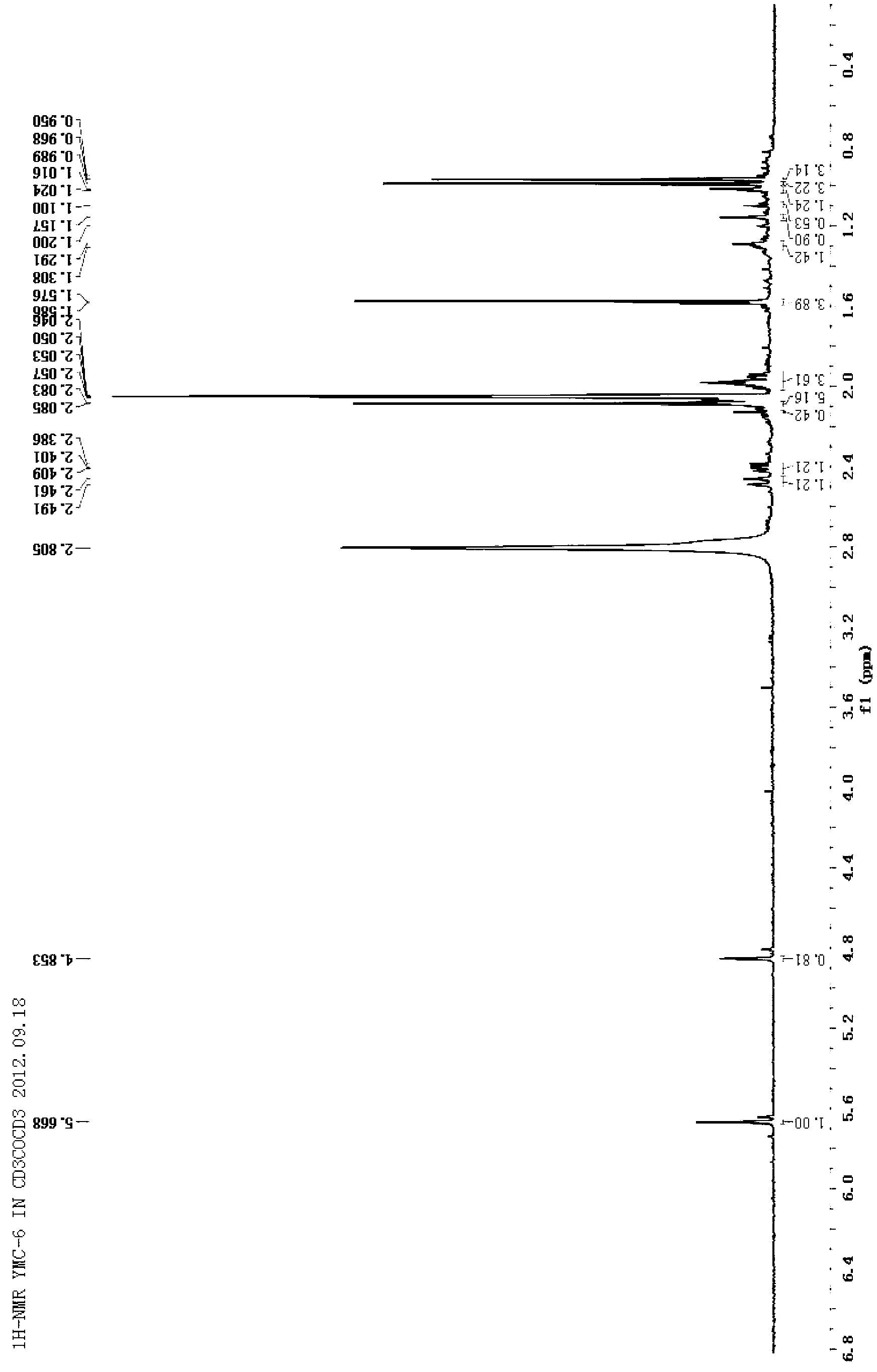
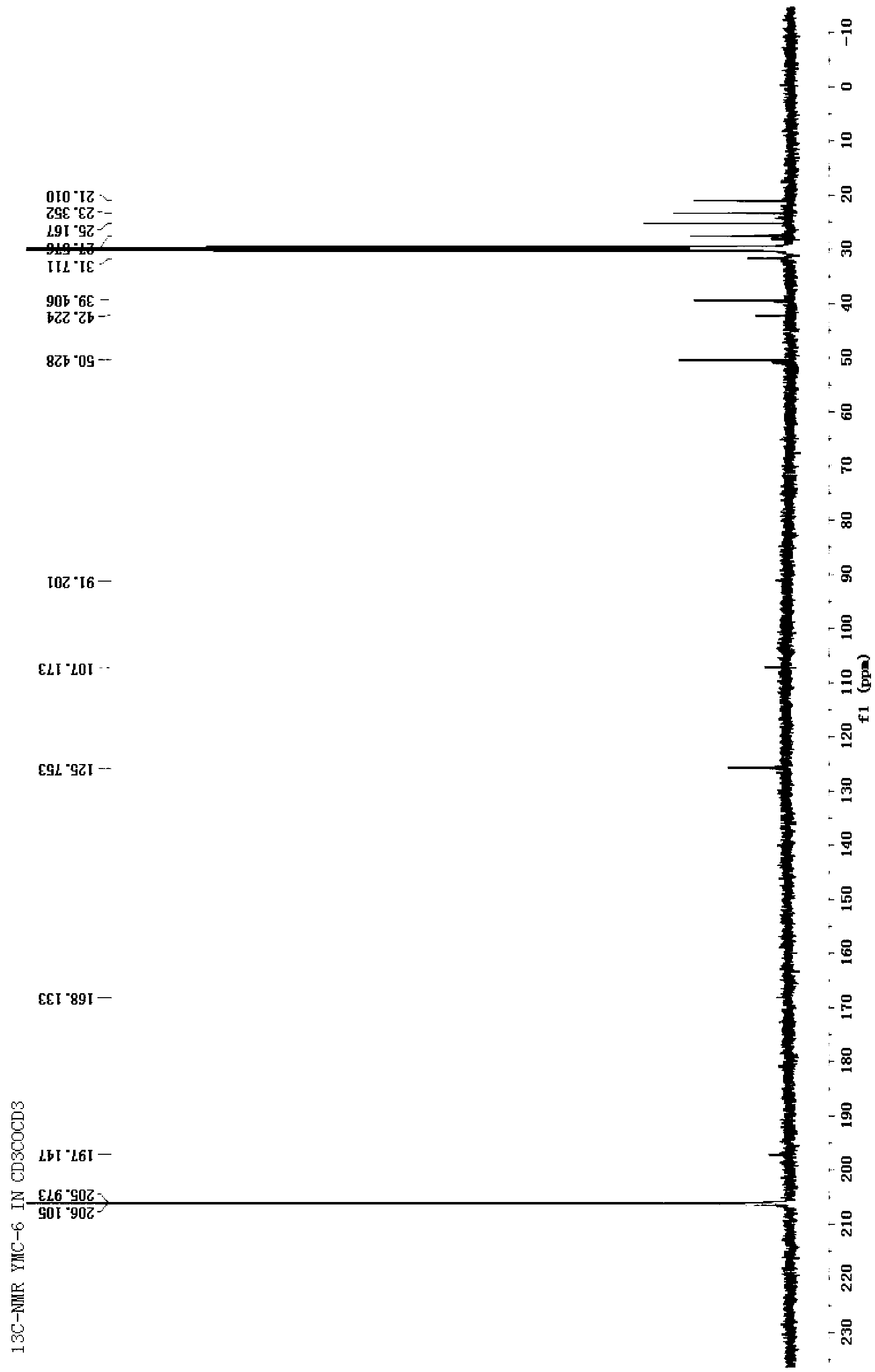
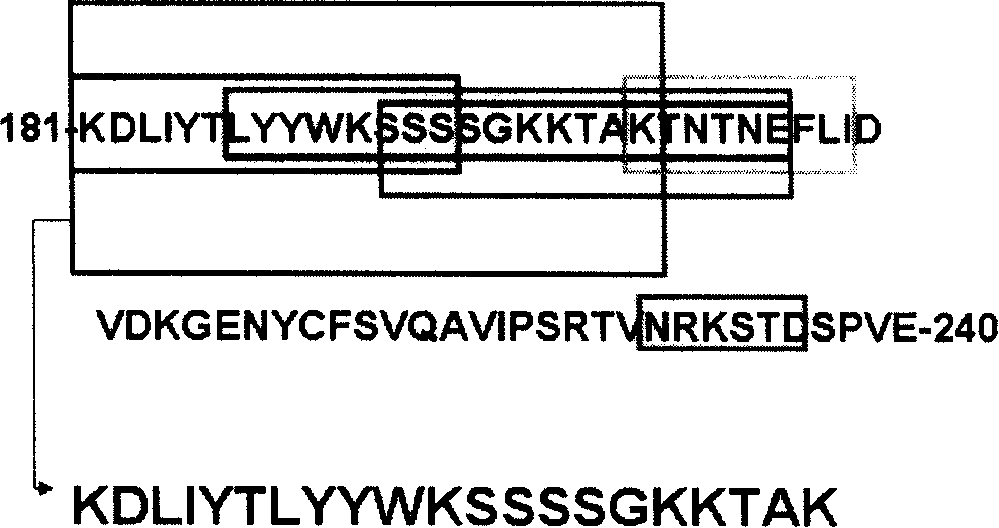
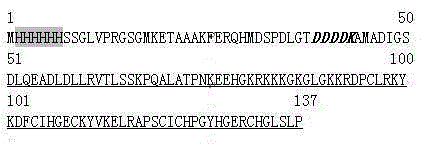Patents
Literature
73 results about "Anticoagulation Activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Derivatives of partially desulphated glycosaminoglycans as heparanase inhibitors, endowed with antiangiogenic activity and devoid of anticoagulating effect
InactiveUS20060172968A1Avoiding and reducing side effectLoss of anticoagulant activityOrganic active ingredientsBiocideMedicinal chemistryAnticoagulant activity
Partially desulphated glycosaminoglycan derivatives are described, particularly heparin, and more particularly a compound of formula (I) where the U, R and R1 groups have the meanings indicated in the description. These glycosaminoglycan derivatives have antiangiogenic and heparanase-inhibiting activity and are devoid of anticoagulant activity.
Owner:LEADIANT BIOSCI SA
Method of producing heparin oligosaccharide using heparinase
A process for preparing heparin oligose from heparinase includes such steps as culturing sphingobacterium (CGMCC No.0660), preparing non-cell coarse enzyme liquid, extracting and purifying heparinase, degradating heparin by the heparinase at 20-30 deg.C to obtain heparin oligose mixture, ultrafilter, gel filter for fractionation and evaporation concentration. The product has the activity of resisting smooth muscle hyperplasia.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Fucosylated glycosaminoglycan derivative and preparation method thereof
InactiveCN102329397APotent anticoagulant activityOrganic active ingredientsBlood disorderOrganosulfateCarboxylic ester
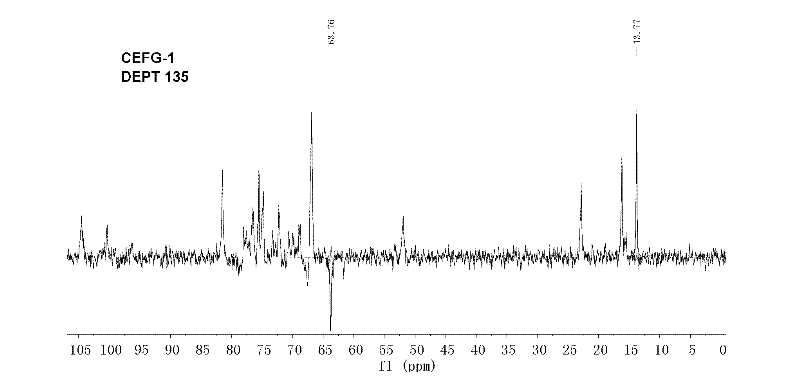
The invention discloses a carboxylic ester of fucosylated glycosaminoglycan (CEFG) with anticoagulation activity, a pharmaceutically acceptable salt thereof, a preparation method of the CEFG and the pharmaceutically acceptable salt thereof, a pharmaceutical composition containing the CEFG or the salt thereof, and application of the pharmaceutical composition in preparation of anticoagulants. The monosaccharides for preparing the CEFG comprise D-glucuronic acid or D-glucuronate (D-GlcU), D-2-deoxy-2-acetyl galactosamine sulfate (D-GalNAcS) and L-fucose sulfate (L-FucS), wherein the molar ratio of D-GlcU to D-GalNAc to L-Fuc to -OSO3<-> is 1:(1+ / -0.3):(1+ / -0.3):(3.5+ / -0.5); the esterification degree of the D-GlcU is not lower than 20%; and the weight average molecular weight of the CEFG is 3000-20000 Da. The glycosylated chondroitin sulfate esterification derivative has strong anticoagulation activity, and can be applied in preparation of drugs for preventing and / or treating thrombotic diseases.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Method for preparing small-diameter artificial blood vessels on basis of nanotechnologies
InactiveCN105079874AGood tissue compatibilityHigh porosityFilament/thread formingProsthesisFiberPorosity
The invention belongs to the field of medicine and high-polymer materials, and discloses a method for preparing small-diameter artificial blood vessels on the basis of nanotechnologies. The method includes steps of dissolving, by weight, 8% of fibroin and 5% of polycaprolactone in hexafluoroisopropanol to obtain spinning liquor; manufacturing the nano-fiber blood vessels with the wall thicknesses of 130-170 micrometers on cylindrical rod-shaped receiving screens with the diameters of 1-1.2mm by means of electrospinning by the aid of electrospinning technologies. Compared with the prior art, the method has the advantages that requirements of tissue engineering on high tissue compatibility, high porosity, plasticity and degradability can be met owing to the electrospinning technologies, vascular stents can be modified by cell activity factors (mechanical growth factors) and medicine (heparin) with anticoagulant activity, and accordingly shortcomings of technological complexity and poor tissue compatibility of existing cell-modified vascular stents can be overcome by the aid of the method.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Substrate and method for production thereof
InactiveUS20100176048A1Good anticoagulant effectEasy to useSurgeryGlovesAnticoagulation ActivityFluence
The present invention relates to a substrate including a compound having blood anticoagulation activity and a hydrophilic polymer compound, wherein the amount of elution of the compound having blood anticoagulation activity is less than 0.6 μg / ml, and a manufacturing method of a substrate, wherein after a compound having blood anticoagulation activity and a hydrophilic compound brought in contact with a substrate are irradiated with radiation, unreacted components are washed out.
Owner:TORAY IND INC
Ancylostoma caninum anticoagulant peptide and its preparation and application
InactiveCN101260150APeptide/protein ingredientsFermentationAntithrombotic AgentAnticoagulation Activity
The invention discloses a novel ancylostoma caninum anticoagulation peptide and an encoded sequence thereof and a preparation method for the anticoagulation peptide. The anticoagulation peptide acquired by the method possesses of anticoagulation activity, can markedly prolong the plasma prothrombin time (PT) and the activation part thrombozyme time(aPTT) of people and has obvious anti thrombosis effects. The invention also relates to applications of the anticoagulation peptide on aspects of anticoagulation drugs, antithrombotic drugs or anticoagulation preparations.
Owner:GUANGDONG MEDICAL UNIV
Application of peptide compound in rhizoma sparganii
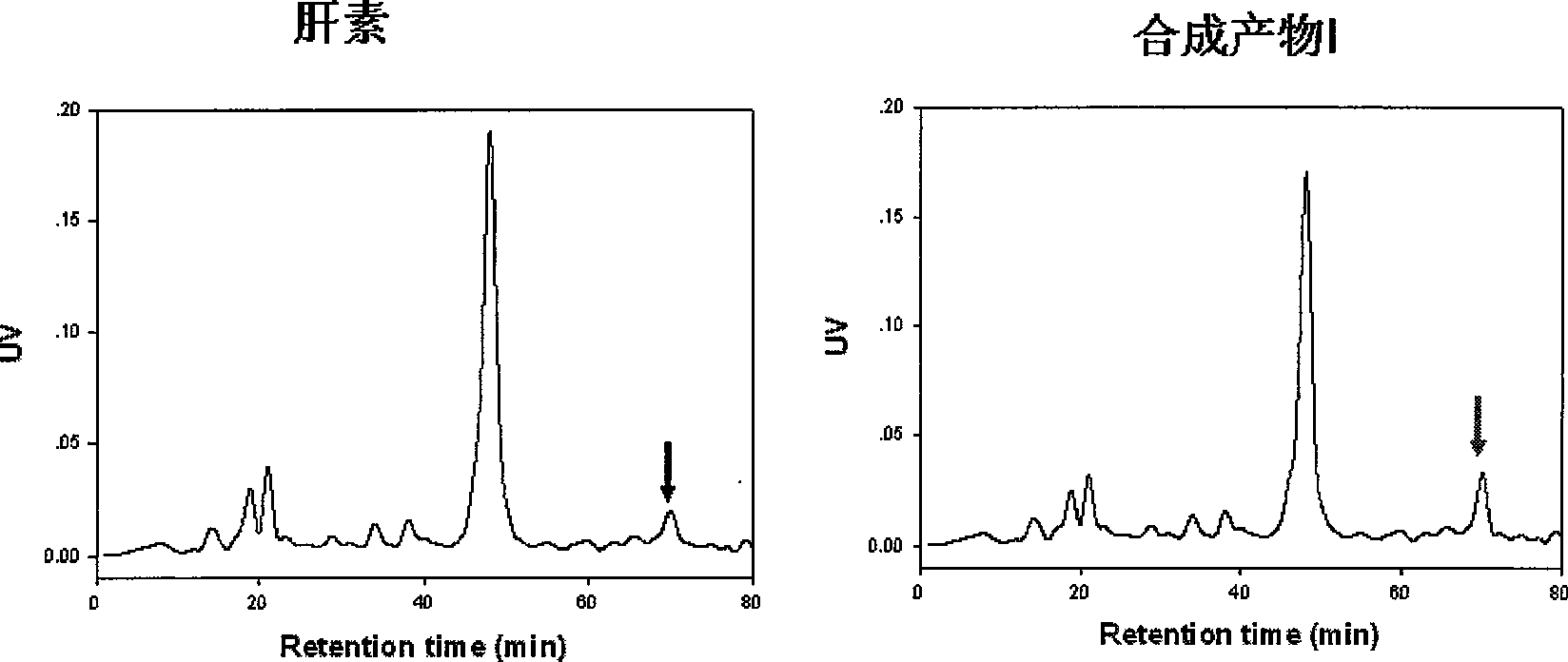
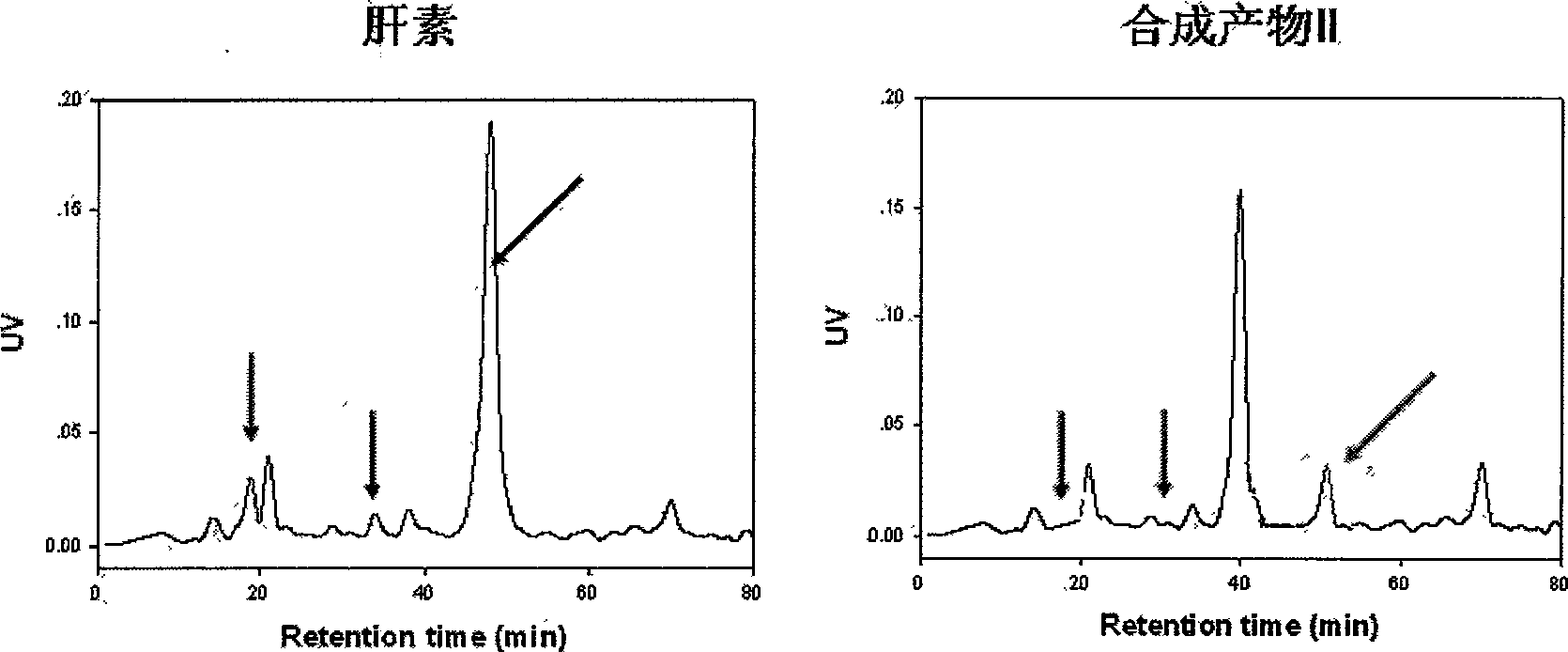
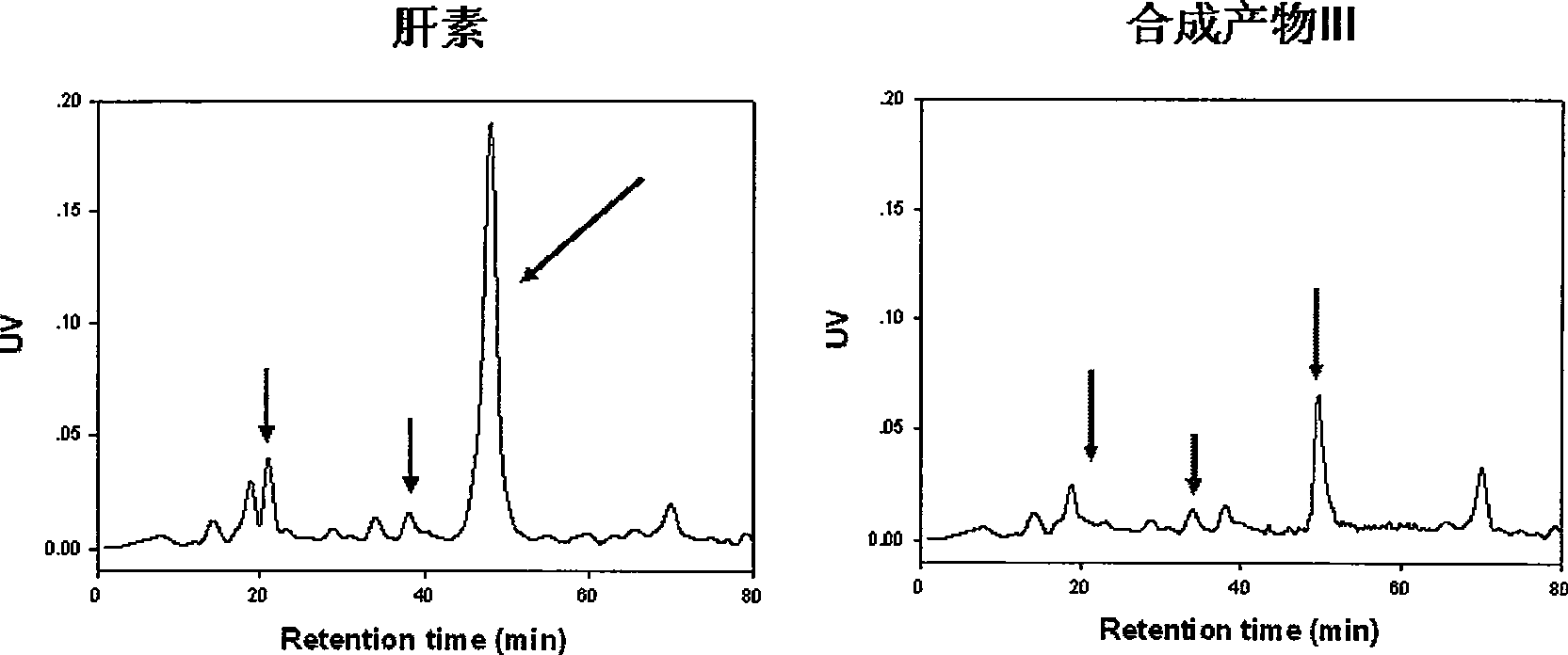
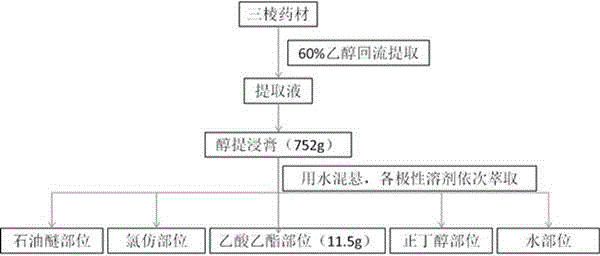
ActiveCN103520160AHigh anticoagulant activityOrganic active ingredientsOrganic chemistryPartial prothrombin timeThrombus
The invention discloses application of a peptide compound in rhizoma sparganii in the preparation of medicines for resisting blood coagulation and / or thrombus. The structural formula of the peptide compound is as shown in a formula (I). The invention provides the peptide compound obtained from the rhizoma sparganii through the separation and the purification and the application thereof for the first time. The peptide compound has an elongation tendency effect on prothrombin time (PT), activated partial thromboplastin time (APPT) and thrombin time (TT) and very good anticoagulation activity and provides powerful basis for the development of antithrombus natural medicines.
Owner:GUANGDONG PHARMA UNIV
Cationic angelica polysaccharide nanoparticle gene delivery system and preparation method thereof
ActiveCN102154351AImmunomodulatoryAnti-agingNanomedicineVector-based foreign material introductionGene deliveryImmunogenicity
The invention discloses a cationic angelica polysaccharide nanoparticle gene delivery system. The system is a gene delivery system of angelica polysaccharide combined DNA (Deoxyribonucleic Acid) plasmids modified by amine compounds, wherein molecular weight distribution of angelica polysaccharides is 30 to 50KD and 80 to 100KD; the mass ratio of the cationic angelica polysaccharide to the DNA plasmids is (1-200):1; and the particle diameter of the cationic angelica polysaccharide-DNA plasmid nancomposite is 21 to 77nm. The system has the characteristics that: 1, the angelica polysaccharide has various biological activities such as immune regulation activity, anti-aging activity, anticoagulation activity and the like, is safe and biologically degradable, does not have immunogenicity and isprepared with a simple, economic and convenient process; and 2, all the three cationic angelica polysaccharides have good DNA plasmid combination effect and gene delivery expression effect. Positive charges carried by primary amine, secondary amine and tertiary amine groups combined with saccharide chains can be effectively combined with the DNA plasmids with negative charges through an electrostatic effect, so that the plasmids are protected from being degraded by various enzymes inside and outside cells.
Owner:JIANGSU UNIV
Method for extracting high-anticoagulation-activity hirudin from natural leeches
InactiveCN101974085AIncrease profitPeptide preparation methodsLeech-based protease inhibitorsNeutral proteaseFiltration
The invention relates to a method for extracting high-anticoagulation-activity hirudin from natural leeches, which comprises the steps that two kinds or a plurality of kinds of substances in sodium chloride, acetyl cysteine hydrochloride monohydrate, lysine, neutral protease and papain are combined and dissolved in deionized water to be prepared into stimulation liquid; the weight percentage concentration of each substance in the stimulation liquid is 0.01 to 5%, wherein the enzyme concentration is 100U to 300U / ml; the living leeches are weighed and placed into a container; the stimulation liquid is added according to the weight and volume ratio of the leeches to the stimulation liquid of 1 to (1-5); the leeches secrete exudate after the stimulation is carried out for 3 hours; the exudateis separated to obtain the hirudin crude product through 16000 to 20000r / min centrifugation; the hirudin crude product is micro-filtered through a stainless steel membrane with the thickness of 0.2 micrometer; the micro-filtration is carried out through an organic membrane with the molecular weight of 10000 to 3000 Dalton; the reverse osmosis, the nano-filtration and the concentration are carriedout; starch for drugs is added according to the weight and volume ratio of the solid to the liquid of 1 to (6-10) to allocate the mixture; and the spray drying is carried out to obtain the leech finished product. Finally, the extraction yield of the hirudin is above 30%, and the activity unit of the product is stabilized between 600AT-U / g to 1000AT-U / g.
Owner:南宁市和兰记生物科技有限公司
Polypeptides with anticoagulation activity screened by phage display technique
ActiveCN105175510AHigh anticoagulant activityWide variety of sourcesPeptide/protein ingredientsPeptidesProtein targetScreening method
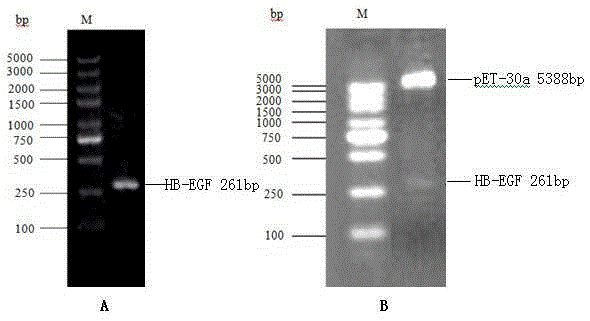
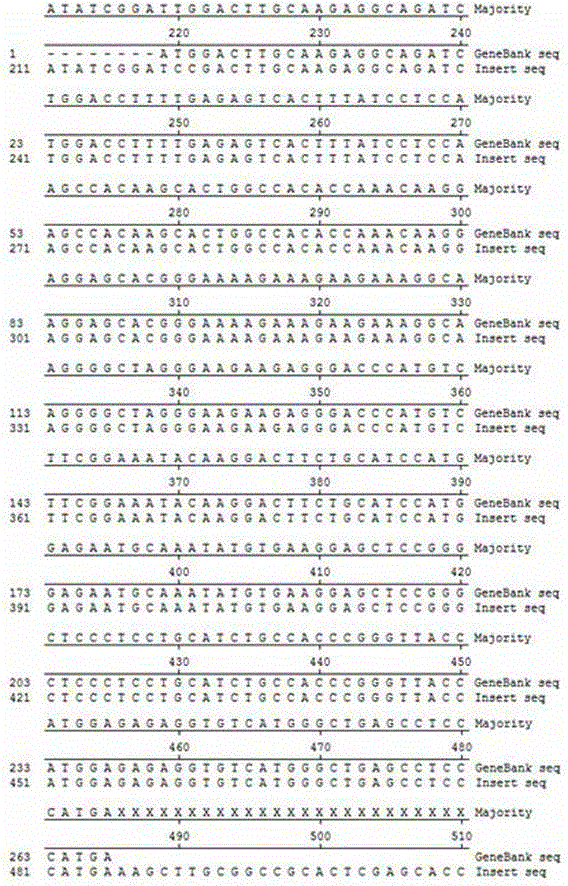
The invention relates to polypeptides with anticoagulation activity and a method for screening polypeptides with anticoagulation activity by a phage display technique, belonging to the technical field of development, research and application of anticoagulant drugs. The screening method comprises the following steps: construction of protein expression vector, expression and purification of target protein, verification of target protein, elutriation of bioactive peptide capable of specifically binding target protein by phage display, polypeptide anticoagulation action in-vivo / in-vitro detection, toxicity experimentation and the like, thereby finally obtaining the polypeptides with anticoagulation activity. The polypeptides are prepared by the following steps: by using a heparin-binding epidermal growth factor as a target molecule, carrying out elutriation three times by using a phage display technique, and eluting the specific binding target molecule phage by using heparin sodium as an effective component. The polypeptides can be used for preparing anticoagulant drugs. The polypeptides have shorter sequence, are easy for synthesis, can easily implement large-scale production, and have no obvious short-term and long-term toxicity for mice in vivo, thereby having important application value in the aspect of development and research of anticoagulant drugs.
Owner:JIANGSU UNIV
Method for preparing heparin derivatives by using biological enzyme to selectively modify heparin structure
InactiveCN101531723ALower doseEliminate side effectsBlood disorderExtracellular fluid disorderSide effectUnfractioned heparin
The invention provides a method for selectively modifying a heparin structure by using biological enzyme, which can improve anticoagulation activity of heparin, reduce combination of heparin with blood protein such as platelet factors and the like, and reduce toxic and side effects. The invention belongs to the field of biological medicine. The antithrombus and anticoagulation activities of the heparin derivatives prepared by the method are 2 to 3 times higher than common heparin medicament, the 2-O-sulfate and 6-O-sulfate contents are about 20 percent of the common heparin, and the combination capability of the heparin derivatives with the platelet factors is about 20 percent of the common heparin. The novel heparin derivatives have high anticoagulation activity and low side effect.
Owner:JIANGNAN UNIV
Applications of peptides compound in rhizome sparganii
ActiveCN103550215AHigh anticoagulant activityOrganic active ingredientsOrganic chemistryAntithrombotic AgentPartial prothrombin time
The invention discloses applications of a peptides compound in rhizome sparganii in preparing anticoagulant and / or antithrombosis medicaments. The structural formula of the peptides compound is shown in the formula (I). The peptides compound obtained by separating and purifying rhizome sparganii, and applications thereof are provided for the first time, the compound plays a role in prolonging the prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT), has good anticoagulation activity, and provides a powerful basis for developing natural antithrombosis medicaments. The formula (I) is as shown in the specification.
Owner:GUANGDONG PHARMA UNIV
A preparing method of a perinereis aibuhitensis anticoagulation peptide
ActiveCN106636273AHas anticoagulant activityHas antithrombotic effectPeptide preparation methodsFermentationUltrafiltrationPerinereis aibuhitensis
A preparing method of a perinereis aibuhitensis anticoagulation peptide is disclosed. The method includes steps of (1) sample pretreatment, (2) enzymatic hydrolysis, (3) ultrafiltration, (4) anion exchange chromatography, (5) dextran gel G-25 chromatography, and (6) reversed-phase high-performance liquid chromatography separation and purification to finally obtain the anticoagulation peptide. The method has characteristics of high safety, mild reaction conditions and easy process control. The prepared anticoagulation peptide has high purity, high anticoagulation activity, an antithrombotic function and good medical application value.
Owner:ZHEJIANG OCEAN UNIV
Application of terpenoid
The invention provides an application of a terpenoid to preparation of an anticoagulation drug. The terpenoid has a structural formula I which is as shown in the description, wherein R1 represents H, C1-C5 linear alkyl, glycosyl or a formula which is as shown in the description, and R2 is selected from C1-C4 linear alkyl. According to the application of the terpenoid, an ionane type sesquiterpene compound separated and obtained from leonurus for the first time can obviously inhibit the platelet aggregation in vitro and has extension tendencies to prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT) and has a certain anticoagulation activity, so that a new choice is provided for the development of natural anti-thrombus drugs.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE +1
Preparation method of silkworm chrysalis chitosan derivatives with anticoagulation activities
InactiveCN102086239AImprove solubilityWide variety of sourcesOrganic active ingredientsBlood disorderAnticoagulation ActivityPolymer science
The invention relates to a preparation method of silkworm chrysalis chitosan derivatives CS-B-R with anticoagulation activities, wherein the CS is a chitosan molecule which is extracted from the silkworm chrysalis and contains active groups such as -NH2, -OH and the like; B is a linking group such as a propyl group, an ethyl group and the like; R is a molecule chain section which contains -NH, -COOH and the like and can be subjected to complexing with calcium ions. The preparation method is as follows: preparing an intermediate with a halogenation activity by using corresponding amino acid and epoxy chloropropane or ethylene hydrocarbon; and reacting the intermediate with the chitosan extracted from the silkworm chrysalis so as to obtain the product. The derivatives are simple in preparation process, can be directly dissolved in water and have the advantages of good anticoagulation effect, good biocompatibility and wide medicine application prospect.
Owner:SOUTHWEST UNIVERSITY
Application of active peptide compound in rhizoma sparganii
ActiveCN103536899AHigh anticoagulant activityCyclic peptide ingredientsBlood disorderAntithrombotic AgentPartial prothrombin time
The invention discloses an application of an active peptide compound in rhizoma sparganii to the preparation of anticoagulant and / or antithrombotic drugs. The active peptide compound has the structural formula (I) shown in the specification. The invention firstly provides the peptide compound obtained from the rhizoma sparganii by separating and purifying and the application of the peptide compound. The compound has a trend prolonging effect for the prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT), has favorable anticoagulation activity and provides a powerful basis for the development of natural antithrombotic drugs.
Owner:GUANGDONG PHARMA UNIV
Medical material and method for preparing anticoagulation coating on surface of medical material
ActiveCN110585492AAvoid damageGood molecular chain stretching propertiesPharmaceutical containersMedical packagingAnticoagulation ActivityBiocompatibility Testing
The invention discloses a medical material and a method for preparing an anticoagulation coating on the surface of the medical material. The method comprises the following steps: S1, grafting an azidecompound on the surface of the medical material so as to form amino sensitive sites on the surface of the medical material; S2, grafting a polymeric amino compound on the surface of the medical material obtained in the step S1 so as to form an elongated chain with multiple amino groups on the surface of the medical material; and S3, grafting heparin on the surface of the medical material obtainedin the step S2, so as to form the anticoagulation coating on the surface of the medical material. The method disclosed by the invention is simple in operation step, small in material amount, safe inmaterial and good in biocompatibility, and the obtained medical material is high in anticoagulation activity and stable in long term use.
Owner:SHANGHAI MICROPORT MEDICAL (GROUP) CO LTD
Non-animal-derived LMW (low molecular weight) heparin, preparation method therefor and application of non-animal-derived LMW heparin
ActiveCN111154819AEasy to separateReduce the risk of contaminationOrganic active ingredientsFermentationBiotechnologyAnimal science
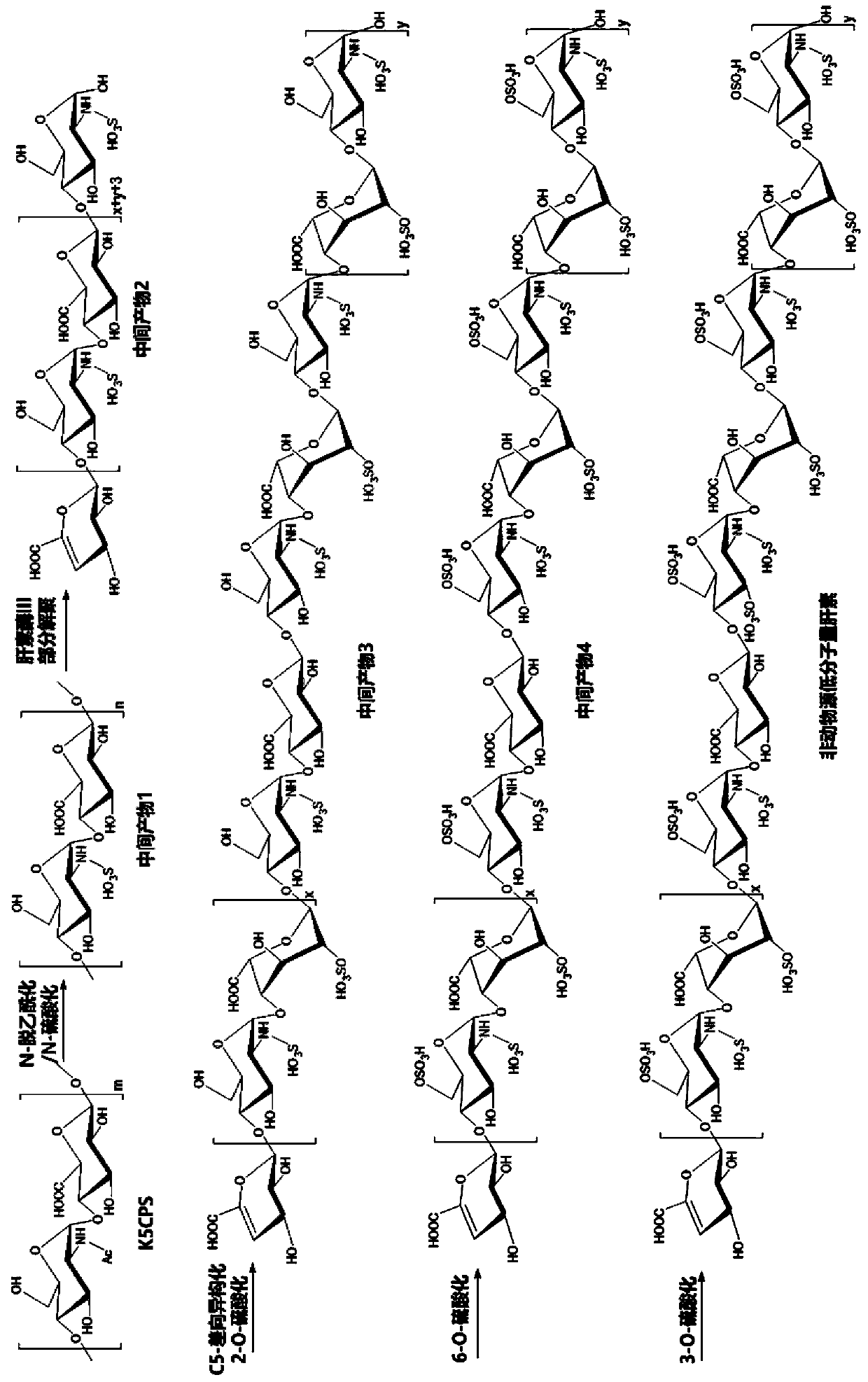
The invention relates to non-animal-derived LMW (low molecular weight) heparin, a preparation method therefor and application of the non-animal-derived LMW heparin. The preparation method disclosed bythe invention comprises the steps of performing a one-step chemical method and a variety of enzyme catalysis methods in order: extracting an exopolysaccharide K5CPS, subjecting the exopolysaccharideK5CPS to chemical-method N-deacetylation / N-sulfation modification, and then, carrying out partial depolymerization in a buffer solution through heparinase III; and subjecting obtained LMW products toenzyme-method C-5 epimerization / 2-O-sulfation modification, 6-O-sulfation modification and 3-O-sulfation modification sequentially, thereby obtaining a product with anti-FXa and anti-FIIa activity. The invention provides a novel method for preparing the LMW product with typical structure characteristics of the heparin and remarkable anticoagulation activity. According to the method, raw materialsare non-animal-derived, the quality is controllable, the risk of contamination is low, adopted reaction conditions are mild and efficient, the obtained product is easy to separate, and large-scale preparation of the LMW heparin with good structural homogeneity and high anticoagulation activity can be achieved.
Owner:SHANDONG UNIV
High anticoagulating active antihuman tissue factor monoclone antibody, preparation method and application thereof
InactiveCN101195659AHighly effective anticoagulant activityHighly effective anticoagulationImmunoglobulins against animals/humansAntibody ingredientsDiseaseBinding site
The invention belongs to the biological technical field, in particular to a high anticoagulation activity human tissue factor monoclonal antibody, a relative preparation method and application thereof. The invention process bioinformatics and antigen immunogennicity analysis at the conjugate point of tissue factor and X factor, to search amino acid sequence with better immunogenicity, which contains key amino acid sequence with conjugated tissue factor and X factor, to prepare tissue factor multiple antigenic peptide, and uses the polypeptide immunized mice to prepare hybridoma cell to obtain high anticoagulation activity mice anti-human tissue factor monoclonal antibody which can be used in thrombus treatment and relative TF disease diagnosis.
Owner:FUDAN UNIV
Method for preparing high-activity and low-molecular-weight heparin by enzymic method
InactiveCN102660610AIncrease the number ofHigh anticoagulant activityFermentationHeparin biosynthesisSulfate radicals
The invention discloses a method for preparing high-activity and low-molecular-weight heparin by an enzymic method. The method includes using heparin as substrate; degrading the heparin by HepI (histidine-tagged heparinase I) in a controlled manner; then utilizing a PAPS (3'-phosphoadenosine-5'-phosphosulfate) regeneration system; selectively decorating the heparin by heparin biological synthetase 3-O-sulfotransferase; and increasing the quantity of anticoagulation activity centers to obtain the low-molecular-weight heparin with high anticoagulation activity. The HepI, the 3-O-sulfotransferase and AST-IV which are adopted in the method can be prepared by means of high-density fermentation in high yield; the PAPS regeneration system uses PNPS (P-nitrophenol sulfonic acid potassium salt) as sulfate radical for enzyme modification reaction, and production cost is greatly reduced. The method provides a new way for enzymic method industrialization of the high-activity and low-molecular-weight heparin.
Owner:JIANGNAN UNIV
Bioactive peptide and preparation method thereof
InactiveCN107163133AHigh inhibition rateAbundant raw materialsPeptide/protein ingredientsPeptide preparation methodsFreeze-dryingReverse osmosis
The invention discloses a bioactive peptide and a preparation method thereof. The preparation method comprises the following steps: using milk or casein as a raw material, selecting an appropriate protease, carrying out enzymatic hydrolysis under certain conditions, carrying out concentration by adopting a membrane technology, desalting the obtained concentrate in a reverse osmosis manner, and drying the desalted concentrate by adopting a vacuum freeze-drying technique or a low-temperature spray drying technique in order to prepare polypeptide powder rich in bioactive peptides; and screening the polypeptides having anticoagulation activity through molecular fishing, sequencing the screened polypeptides to obtain an amino acid sequence, and synthesizing the final highly-pure polypeptide product in a solid phase synthesis manner. An identification result shows that the angiotensin converting enzyme inhibition rate and the thrombin inhibiting peptide inhibition rate of the peptide disclosed in the invention are high, the angiotensin converting enzyme inhibition rate of 1 mg of antithrombotic peptide dry powder is 30-80%, and the thrombin inhibition rate of 1 mg of the antithrombotic peptide dry powder is 70-98%. The method for preparing the bioactive peptide from the milk or casein has the advantages of richness in the raw material, low price, and meeting of large-scale industrial production.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Apixaban crystal form and preparation method therefor
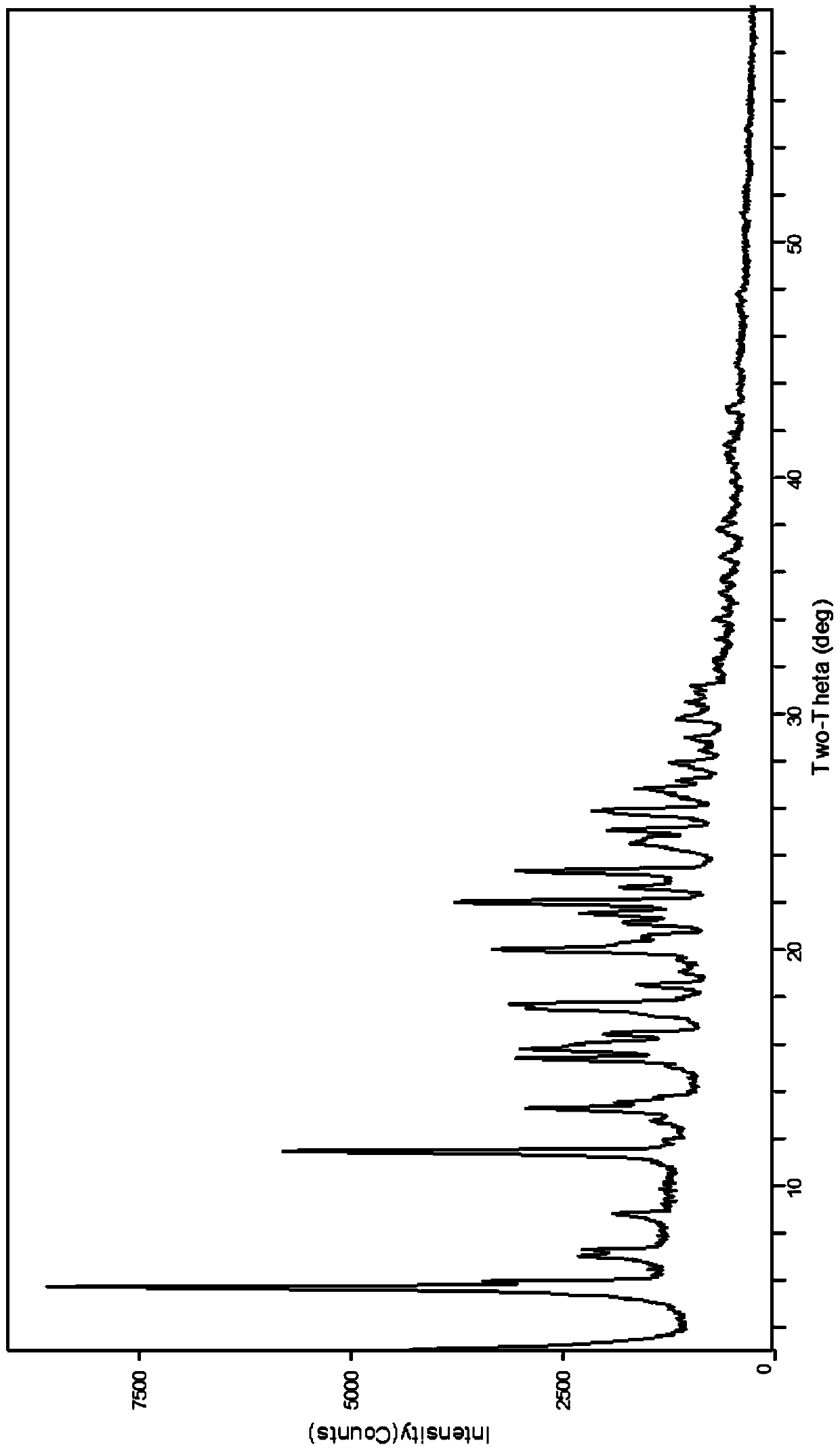
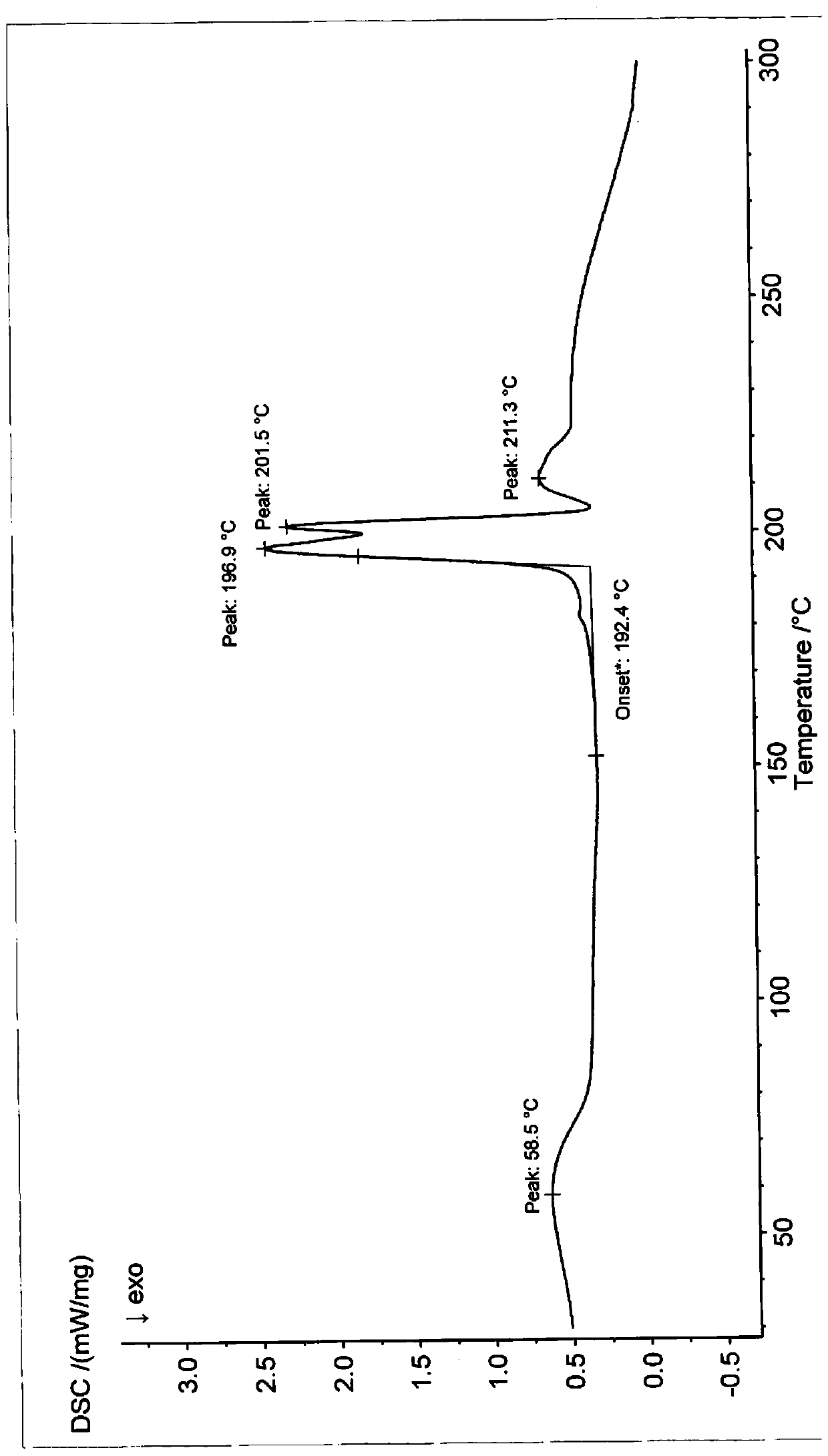
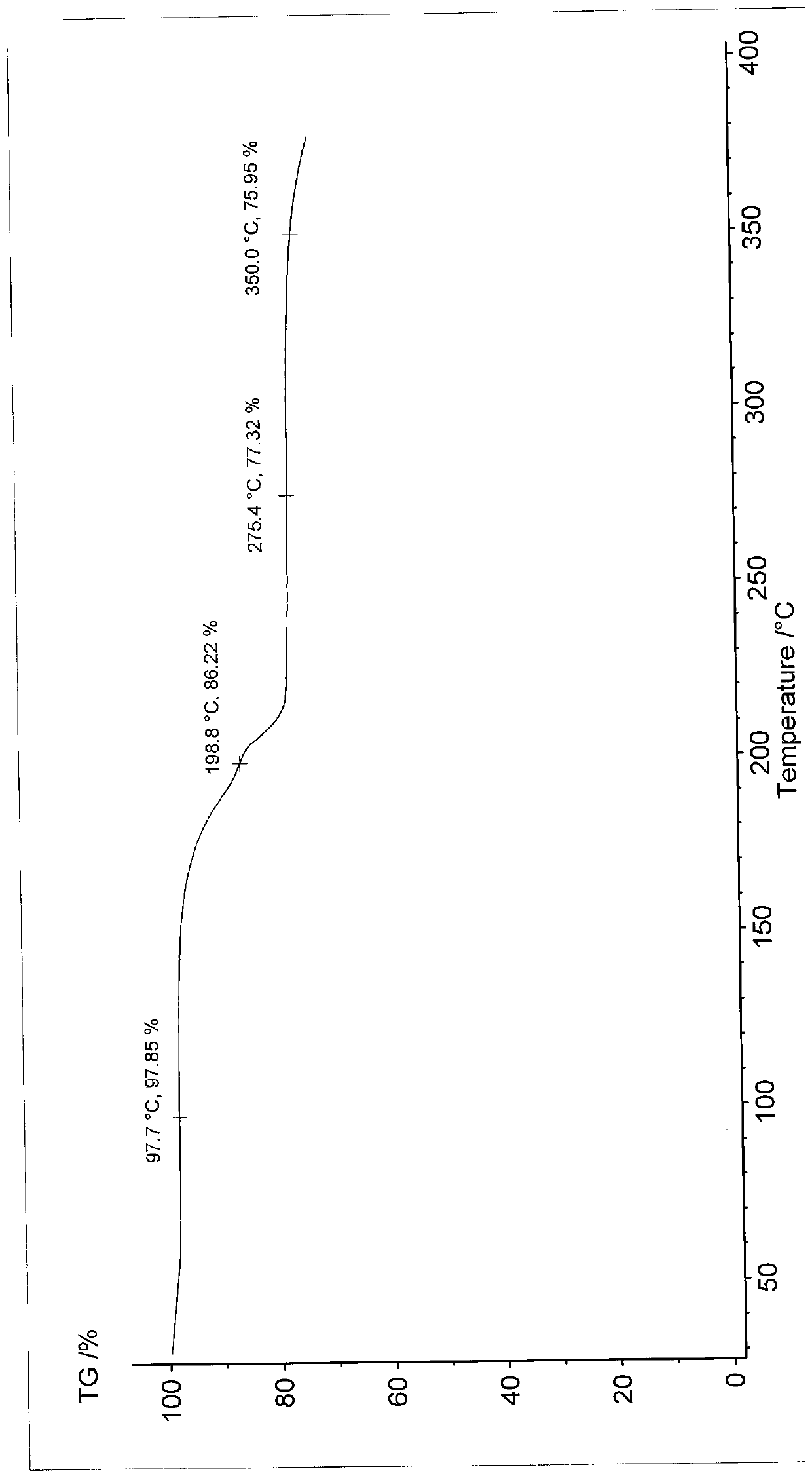
ActiveCN110452240AHigh chemical purityReduce production energy consumptionOrganic chemistry methodsBlood disorderAnticoagulation ActivityX-ray
The invention relates to an apixaban crystal form and a preparation method therefor, belongs to the field of medicinal chemicals and particularly relates to an apixaban nicotinate monohydrate eutecticcrystal with anticoagulation activity and a preparation method therefor. Proven by X-ray powder diffraction spectrum analysis on the disclosed crystal, a 2[theta] value of a characteristic absorptionpeak is located at 5.7+ / -0.2 degrees, 6.0+ / -0.2 degrees, 11.5+ / -0.2 degrees, 13.3+ / -0.2 degrees, 15.4+ / -0.2 degrees, 15.8+ / -0.2 degrees, 17.5+ / -0.2 degrees, 17.7+ / -0.2 degrees, 20.0+ / -0.2 degrees, 22.1+ / -0.2 degrees and 23.4+ / -0.2 degrees. The preparation method has the advantages that reagents are cheap, readily available and environmentally friendly, the preparation method is simple, the crystallization conditions are moderate, and the crystal is easy to separate; and the apixaban nicotinate monohydrate eutectic crystal disclosed by the invention can be used for treating thromboembolism.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Oral delivery of macromolecules
Polysaccharides, which are widely used as an anticoagulation drugs, especially heparin, are clinically administered only by intravenous or subcutaneous injection because of their strong hydrophilicity and high negative charge. Amphiphilic heparin derivatives were synthesized by conjugation to bile acids, sterols, and alkanoic acids, respectively. These heparin derivatives were slightly hydrophobic, exhibited good solubility in water, and have high anticoagulation activity. These slightly hydrophobic heparin derivatives are efficiently absorbed in the gastrointestinal tract and can be used in oral dosage forms. Methods of using these amphiphilic heparin derivatives and similarly modified macromolecules for oral administration are also disclosed.
Owner:(株)美大富历寿
Liposome preparation for anticoagulant thrombolytic difunctional fusion protein and preparation method thereof
InactiveCN102144974ASmall particle sizeGuaranteed Particle SizePeptide/protein ingredientsPharmaceutical non-active ingredientsCholesterolPhospholipid
The invention relates to the field of biopharmaceutics, in particular to a liposome preparation for anticoagulant thrombolytic difunctional fusion protein, namely anticoagulant thrombolytic difunctional fusion protein of 12 peptides of hirudin and reteplase (HV12p-rPA) and a preparation method thereof. The anticoagulant thrombolytic difunctional fusion protein (HV12p-rPA) constructed in the laboratory has the dual effects of anticoagulation and thrombolysis by structural identification, expression, chromatography renaturation, purification and extracorporal and intracorporal pharmacodynamic experiments. In the preparation method, the liposome preparation is prepared from the anticoagulant thrombolytic difunctional fusion protein serving as a raw material, and a liposome is prepared by an optimized membrane dispersion-probe ultrasonic method; and the optimized membrane dispersion-probe ultrasonic method is characterized in that a prescription which is most suitable for improving the envelop rate of the HV12p-rPA liposome is selected by taking a ratio of phospholipid to cholesterol, the concentration of protein medicaments, the volume of buffer solution and water-bath ultrasonic time as influence factors, and the grain diameter of the HV12p-rPA liposome is reduced and homogenized further by probe ultrasonic, so that the HV12p-rPA liposome of which the grain diameter is between 140 and 145 nanometers and which is used for intravenous injection is prepared. By the preparation method, the envelop rate of the prepared HV12p-rPA liposome is over 90 percent, and extracorporal thrombolytic activity and extracorporal anticoagulant activity are 23,810 international unit (IU) .mg<1> and 414 antithrombin unit (ATU) .mg<1> respectively.
Owner:SICHUAN UNIV
Antithrombotic biological agent prepared by adopting nereis active peptide
ActiveCN106563120AIncrease economic value addedImprove stabilityOrganic active ingredientsAnthropod material medical ingredientsAnticoagulation ActivityMedicine
The invention discloses an antithrombotic biological agent prepared by adopting nereis active peptide. The antithrombotic biological agent is prepared by mixing the following components in parts by weight: 10-20 parts of nereis anticoagulant peptide, 1-5 parts of heparin sodium, and 100 parts of a carrier. The antithrombotic biological agent is good in stability and anticoagulation activity, has the antithrombus function, and has the good medical application value, and the economic additional value of nereis is increased.
Owner:ZHEJIANG OCEAN UNIV
Substrate and method for production thereof
InactiveUS8733557B2Good anticoagulant effectEasy to useSurgeryPretreated surfacesAnticoagulation ActivityElution
Owner:TORAY IND INC
Novel method for preparing recombinant exenatide or derivative thereof
InactiveCN104894196AEasy to detectHas anticoagulant activityHormone peptidesFermentationFusion Protein ExpressionEnzyme digestion
The invention builds up a novel method for preparing recombinant exenatide or derivative thereof. According to the method, hirudin (molecular weight is 7Kd) serves as a fusion partner, exenatide or derivative thereof is spiced on the downstream of the hirudin for fusion expression, a connecting peptide is arranged between the hirudin fusion partner and the exenatide or derivative thereof and comprises a TEV protease (tobacco etching virus protease) identification cutting sequence ENLYFQH. The exenatide or derivative thereof with a natural N-terminal and biological activity is released through TEV enzyme digestion after fusion protein expression. The novel method has other advantages that (1) the hirudin fusion partner is small, so the ratio of the exenatide or derivative thereof in fusion protein is effectively increased; (2) hirudin fusion protein has anticoagulation activity, thus facilitating real-time detection and tracing; (3) fusion protein can be adsorbed by virtue of cheap macroporpous resin; and (4) hirudin fusion partner can enhance stability of exenatide or derivative thereof.
Owner:CHINA PHARM UNIV
Method for preparing alga oligosaccharide with anticoagulation activity
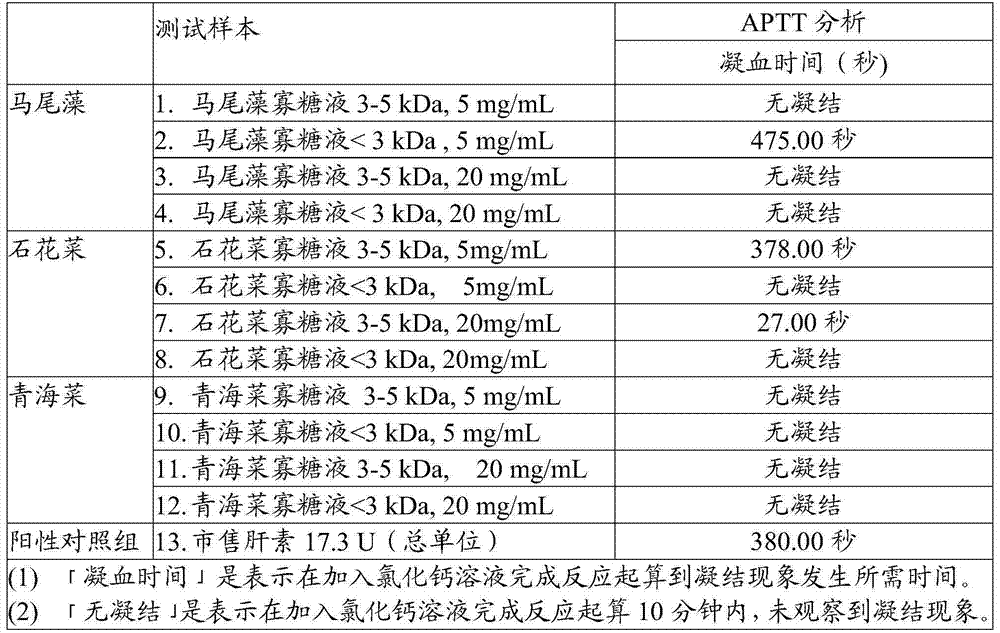
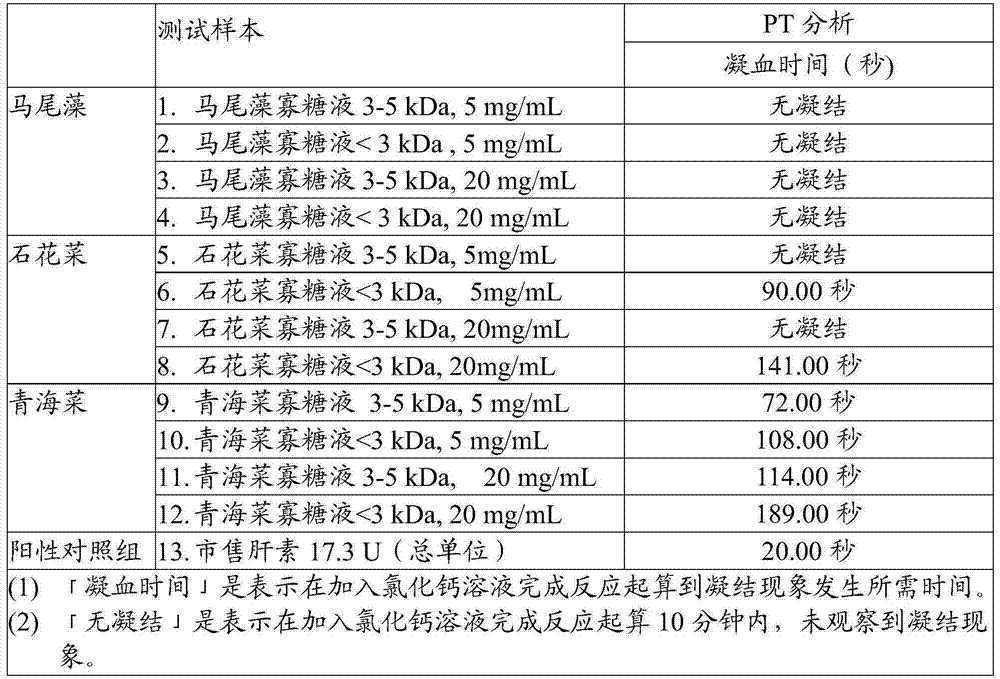
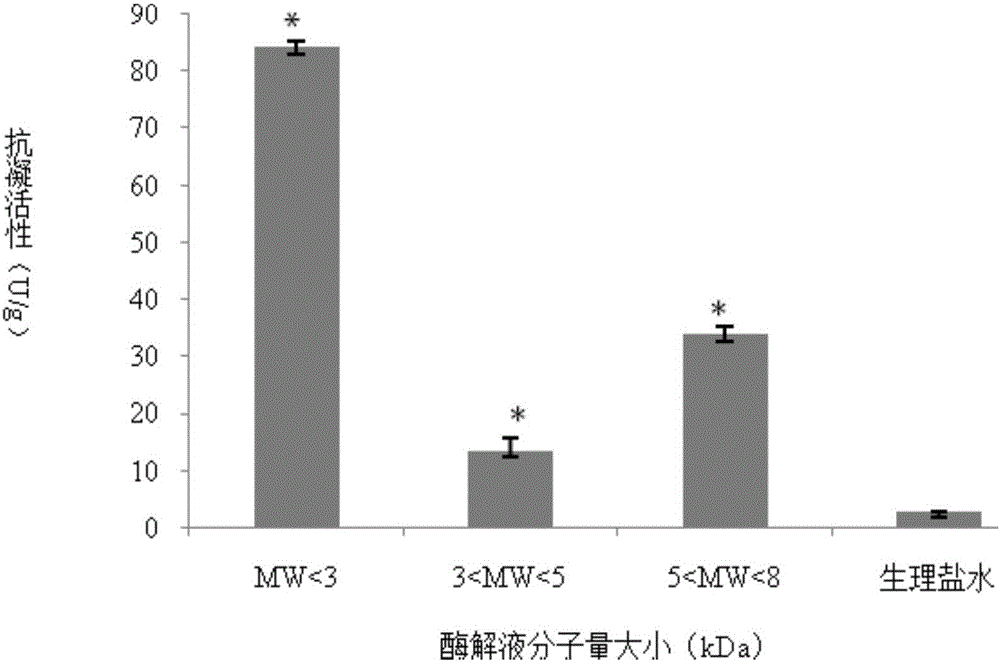
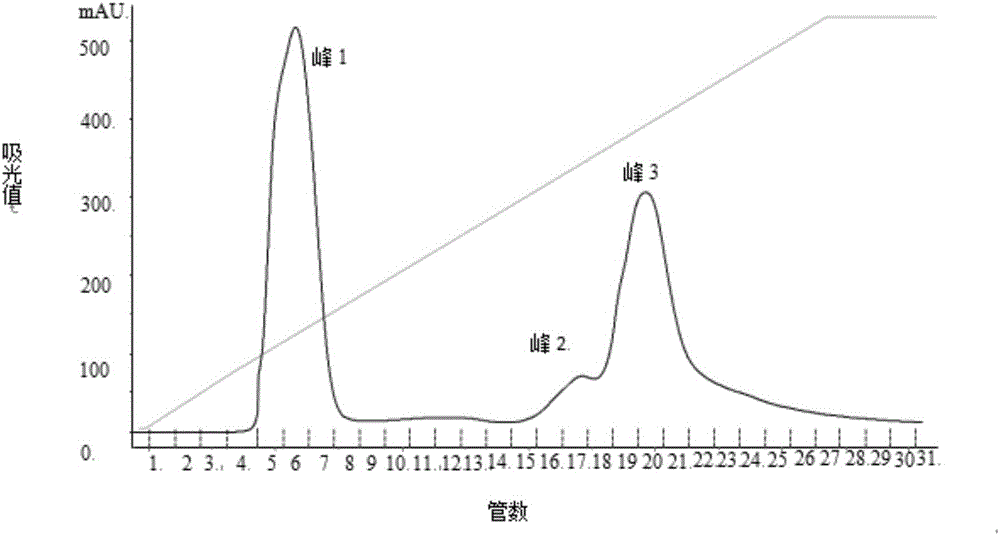
InactiveCN105441523AOrganic active ingredientsMicroorganism based processesAnticoagulation ActivityPseudomonas tolaasii
The invention discloses a method for preparing alga oligosaccharide with anticoagulation activity. Specifically, in the method, an alga oligosaccharide sample prepared from sargassum, gelidium amansii or green laver is hydrolyzed with an enzyme so as to obtain the alga oligosaccharide with anticoagulation activity, wherein the enzyme is produced by inducing an ocean strain of Pseudomonas vesicularis and / or Aeromonas salmonicida with the ocean polysaccharide from sargassum, gelidium amansii or green laver.
Owner:潘崇良
Perinereis aibuhitensis anticoagulation peptide and use thereof
ActiveCN106632602AHas anticoagulant activityHas antithrombotic effectPeptide/protein ingredientsPeptide preparation methodsAnticoagulation ActivityPerinereis aibuhitensis
The invention discloses a perinereis aibuhitensis anticoagulation peptide and use thereof. The amino acid sequence of the perinereis aibuhitensis anticoagulation peptide is Pro-Val-Glu-Arg-Lys. The perinereis aibuhitensis anticoagulation peptide is high in anticoagulation activity, has an antithrombus effect, and has a preferable medical application value.
Owner:ZHEJIANG OCEAN UNIV
Expression preparation method and application of novel anticoagulant Aedes albopictus salivary gland aegyptin-like protein ALP
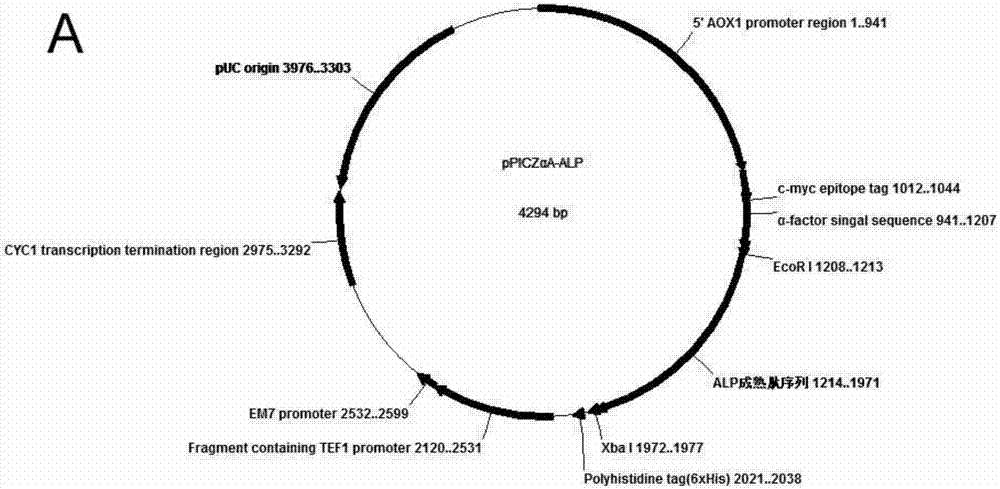
InactiveCN104711283AHas anticoagulant activityProlonged prothrombin timeMicroorganism based processesFermentationPichiaThrombotic disease
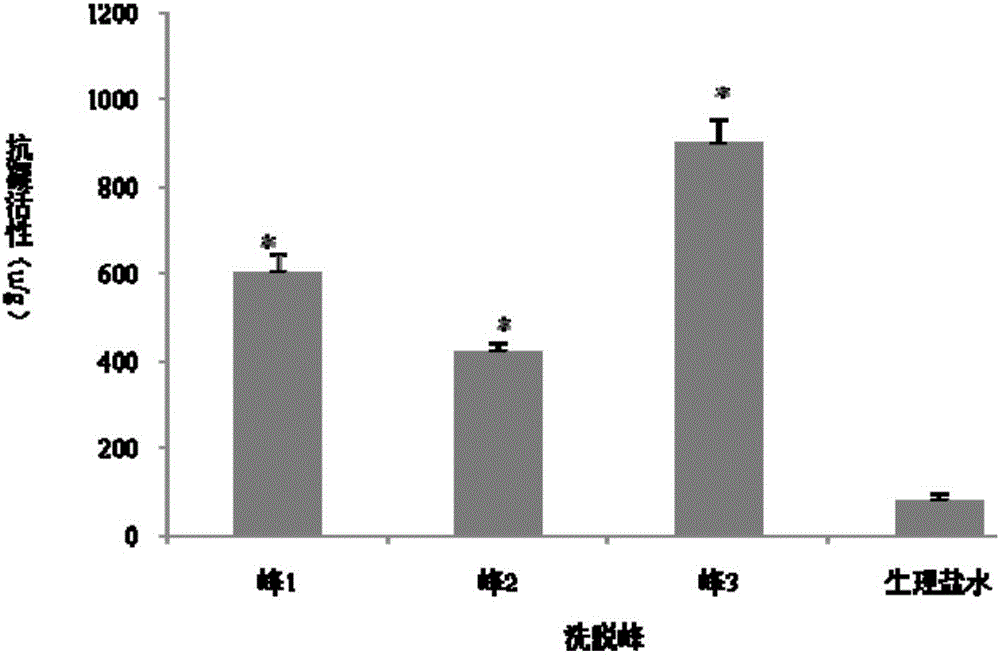
The invention relates to an expression preparation method and application of a novel anticoagulant Aedes albopictus salivary gland aegyptin-like protein ALP. The method comprises the following step: 1, carrying out optimized synthesis of ALP mature peptide DNA; 2, constructing a recombinant plasmid pPICZalphaA-ALP; and 3, expressing and purifying a recombinant protein ALP. The method uses the ALP protein expressed by a pichia yeast expression system (pPICZalphaA-ALP X33) of a pPICZalphaA vector in order to realize correct secretion and expression, and the purified ALP protein has anticoagulation activity, can substantially prolong the prothrombin time (PT), the thrombin time (TT) and the activated partial thromboplastin time (APTT) in vitro, can be used as a novel anticoagulant, and lays a research foundation for research of the feasibility of the purified ALP protein as a novel anticoagulant for preventing and treating thrombotic diseases and other vascular diseases, so the method has positive social benefit and substantial economic benefit if the method is widely proved and clinically used.
Owner:WENZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com