Patents
Literature
46 results about "Biopharmaceutics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The study of the physical and chemical properties of a drug and its dosage form as related to the onset, duration, and intensity of its action.
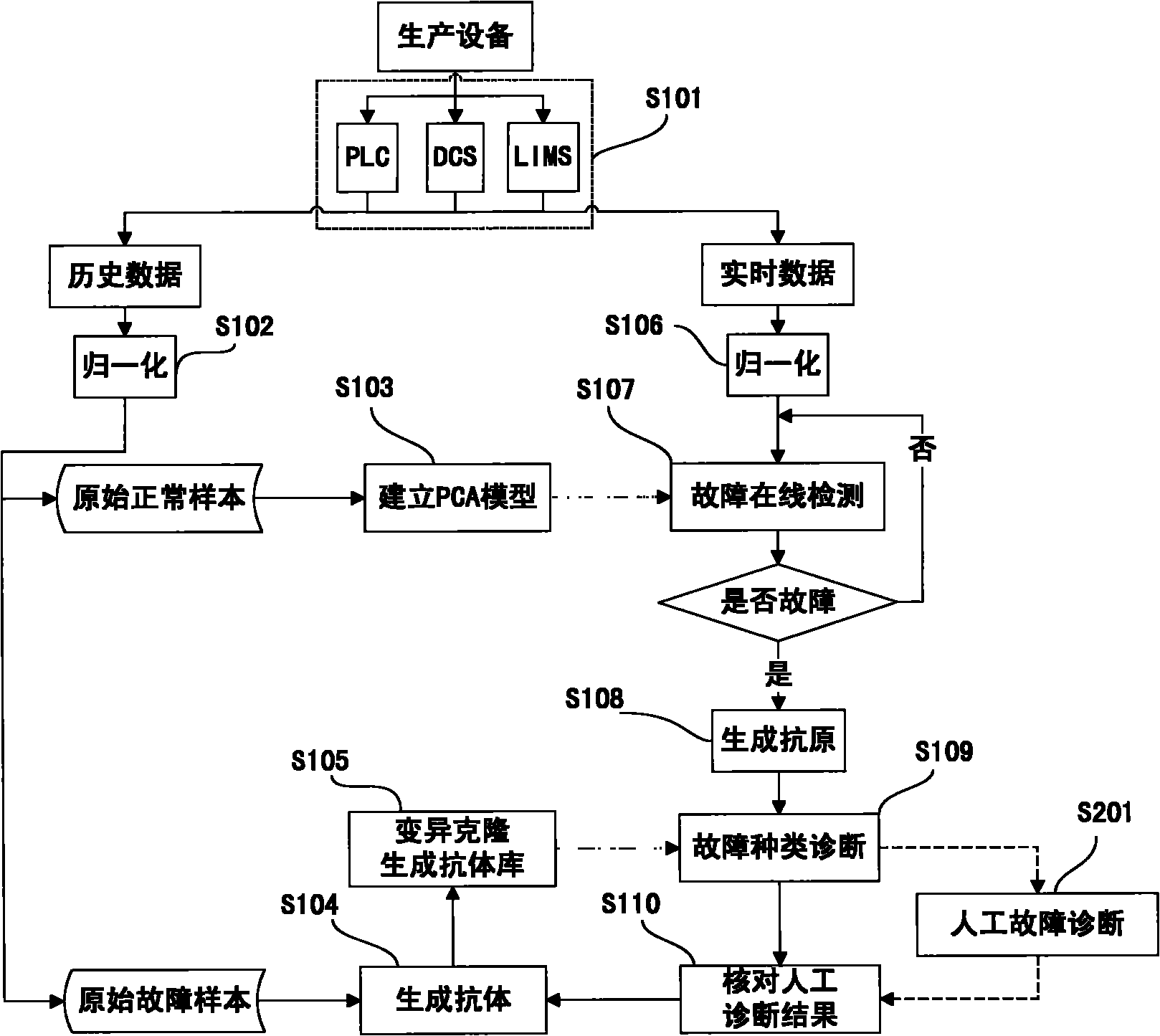
Method and system for diagnosing mixed failure based on PCA and artificial immune system
ActiveCN102110187ABiological modelsSpecial data processing applicationsChemical industryArtificial immune system
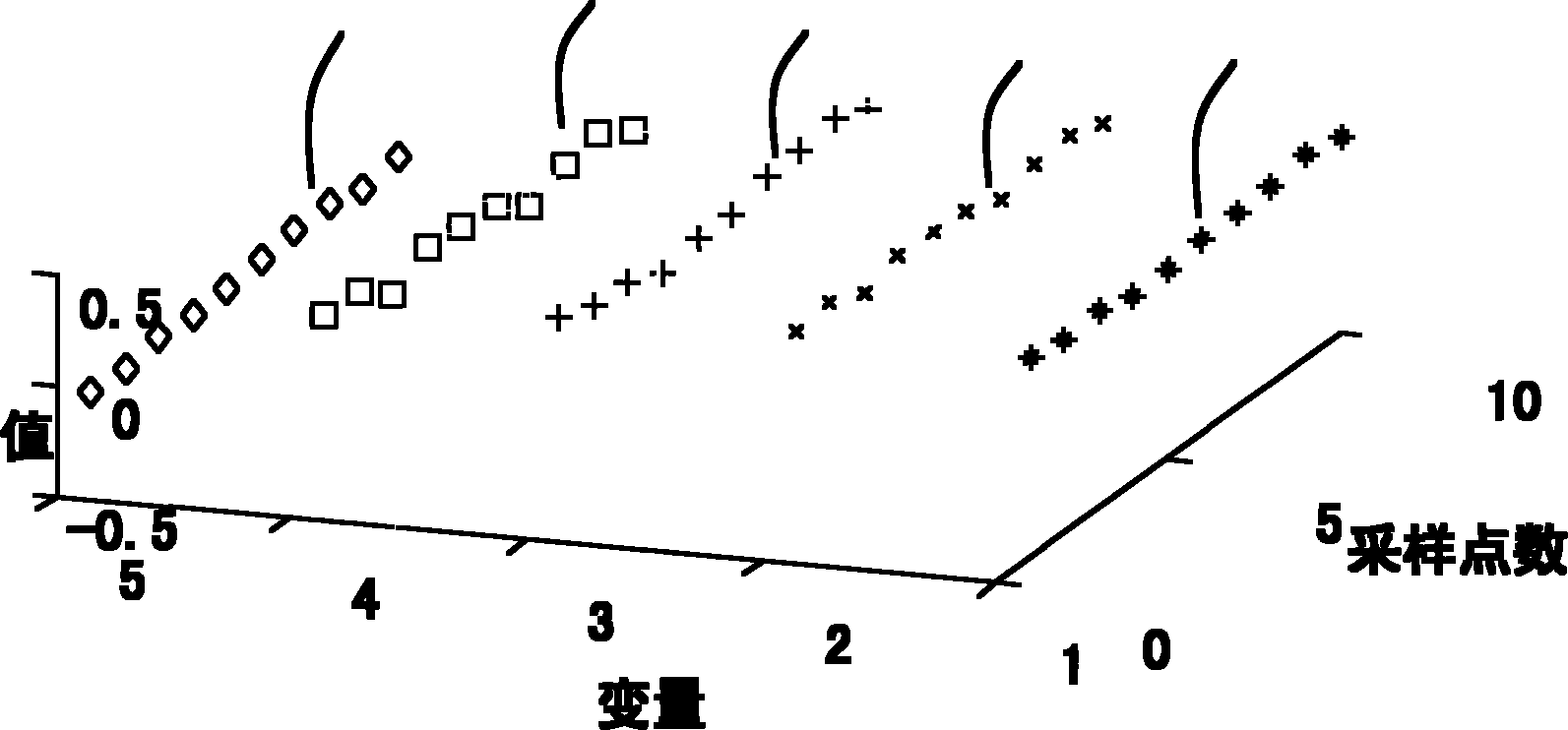
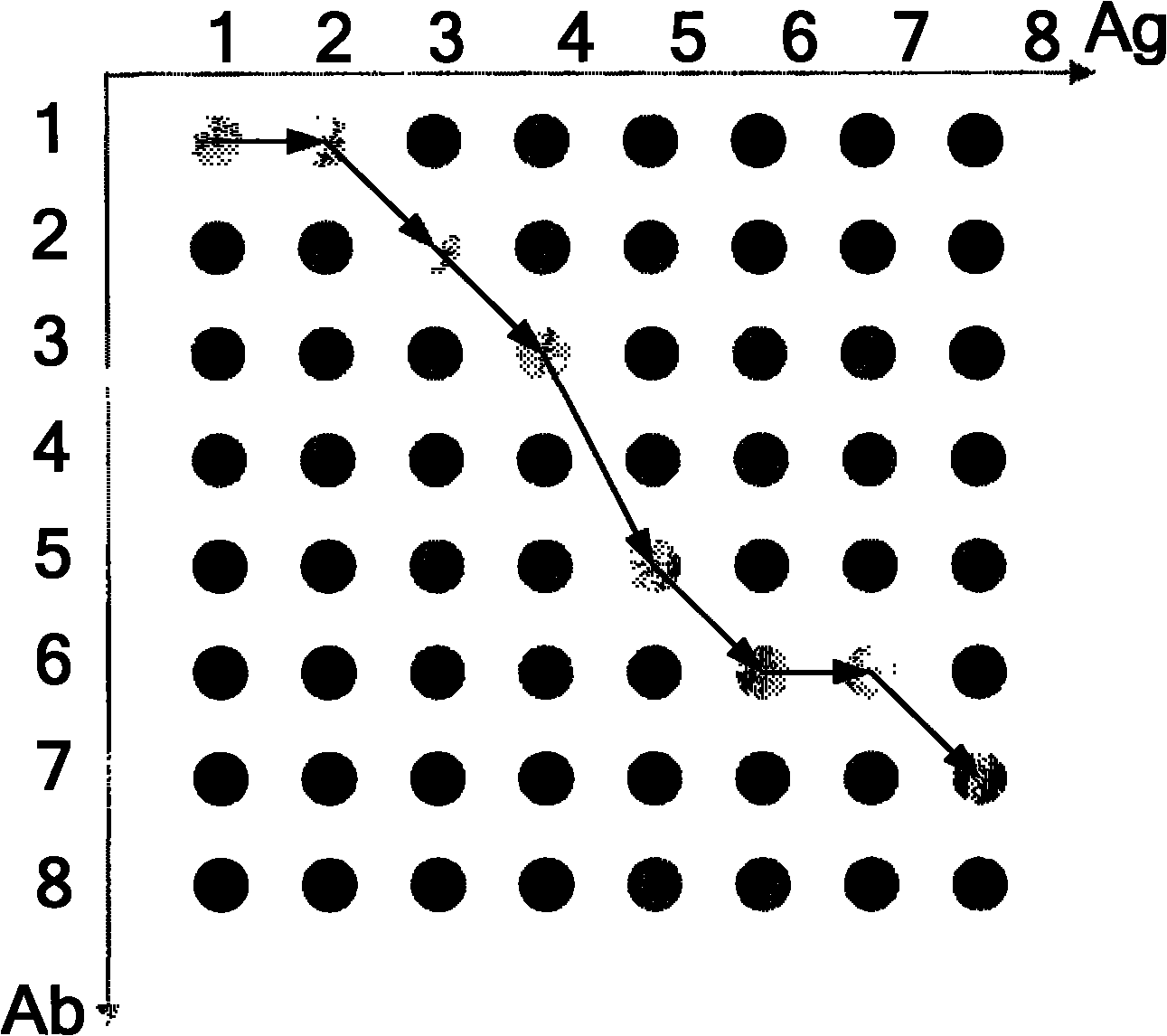
The invention provides a method and a system for diagnosing mixed failure based on a PCA and an artificial immune system, and the method and the system can be applied in industrial processes as chemical industry, oil refining, biopharmaceutics, and the like. The PCA algorithm is used for detecting failure, the artificial immune system is used for diagnose failure kinds of self and non-self judgments, and self-adaptation and self-study capabilities of the artificial immune system are used, so that the updating of a failure diagnosis system during an online operation process is realized. Antigens and the antibodies of the artificial immune system are represented as a matrix composed of data samples of time series, wherein data to be detected is generated as antigens, all types of historicaldata are generated as different antibodies, the kind of working condition is judged by computing the difference between the antigen and the antibody, so as to realize failure detection and diagnosis.A clone and variation computation is also presented, so as to produce a large amount of different antibodies from the known working condition data during the failure diagnosis process, and the antibodies are automatically updated during the diagnosis, so that the requirements of actual industrial process on adaptability are met.
Owner:TSINGHUA UNIV
Recombinant fusion protein vaccine and attenuated live vector vaccine for treating and preventing helicobacter pylori (Hp) infection
The invention relates to a recombinant fusion protein vaccine and an attenuated live vector vaccine for treating and preventing helicobacter pylori (Hp) infection, and belongs to the field of biopharmaceutics. The recombinant fusion protein vaccine and the attenuated live vector vaccine for expressing the recombinant fusion protein are characterized in that: the recombinant fusion protein is formed by connecting immune protective function fragments of helicobacter pylori cytotoxin relevant gene protein CagA from helicobacter pylori, vacuolization cytotoxin VacA and urease subunit UreB linearly, and the immunogenicity and immune protection of the recombinant fusion protein are verified through animal experiments. The live vaccine has the advantages that: 1, an Hp fusion protein gene can beexpressed stably; 2, mucosa and systemic immune response can be induced after immunity; and 3, the method is convenient, the cost is low, and the economic benefit is obvious. Therefore, the attenuated live vector vaccine can be used as candidate vaccines for treating and preventing the Hp infection.
Owner:ARMY MEDICAL UNIV
Tumor targeting polypeptide-medicine coupling derivative, and preparation method and application thereof
InactiveCN107952080AExtended half-lifeProlong the action timeOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetChemical Linkage
The invention belongs to the technical field of biological pharmacy, and relates to a tumor targeting polypeptide-medicine coupling derivative, and a preparation method and application thereof. The tumor targeting polypeptide-medicine coupling derivative comprises targeting polypeptides, long effect polypeptides and medicine molecules, wherein the targeting polypeptides are connected onto the endC of the long effect polypeptides through flexible connecting peptides; fusion peptides are used as carriers of the medicine molecules; the medicine carriers and the long effect polypeptides in the fusion peptides are connected through chemical bonds; and the long effect polypeptides are polypeptide or protein structural domains having the affine mutual effects with human serum albumin. The tumortargeting polypeptide-medicine coupling derivative can be combined with the human serum albumin; the remaining half-life period in the blood circulation system can be obviously prolonged; the long-term effectiveness in the body is realized; the targeting and seepage into the tumor tissues or cells can be realized; then, free effect molecules are released in tumor tissue micro acid environments orin cells in through an acid hydrolysis mechanism; and a better anti-tumor efficiency is achieved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Fungus culture extract and preparation method and application thereof
ActiveCN106497797ANo toxicityStrong inhibitory activityOrganic active ingredientsFungiDiseaseDipeptide
The invention is suitable for the field of biopharmaceutics, and provides a fungus culture extract and a preparation method and application thereof. The extract is a cyclic dipeptide compound which can be separated from a deep sea fungus fermentation culture of which the number is CCTCC M 2015628. The cyclic dipeptide compound has the high activity of inhibiting generation of nitric oxide (NO), has no toxicity on cells and can be applied to preparation of medicine for treating an Alzheimer disease.
Owner:SHENZHEN UNIV
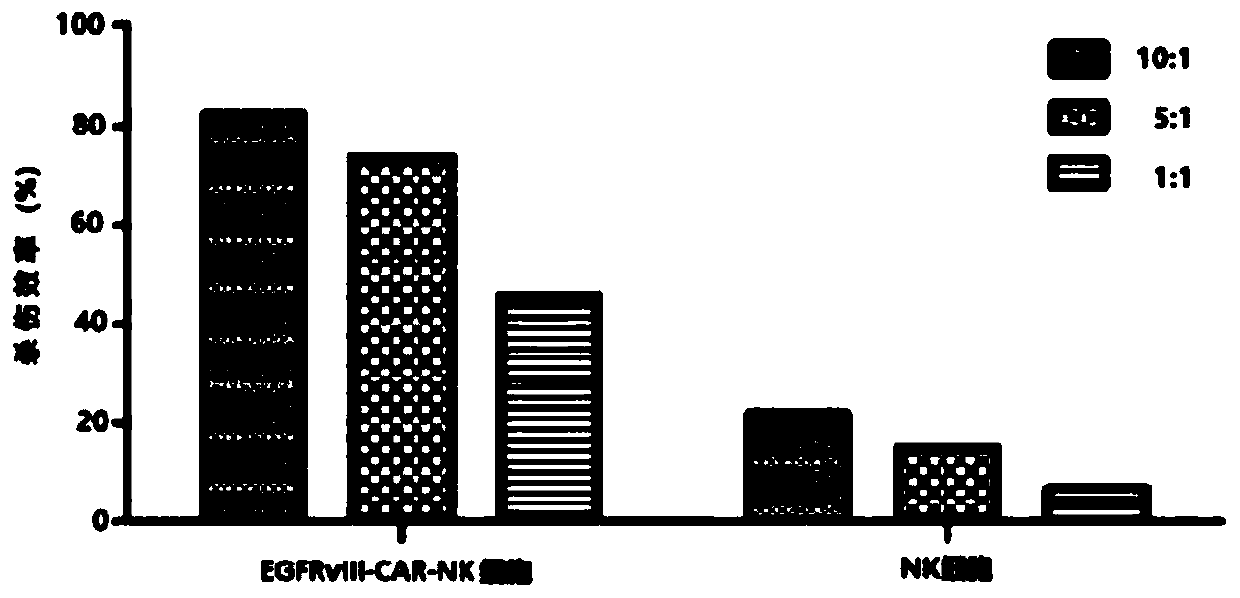
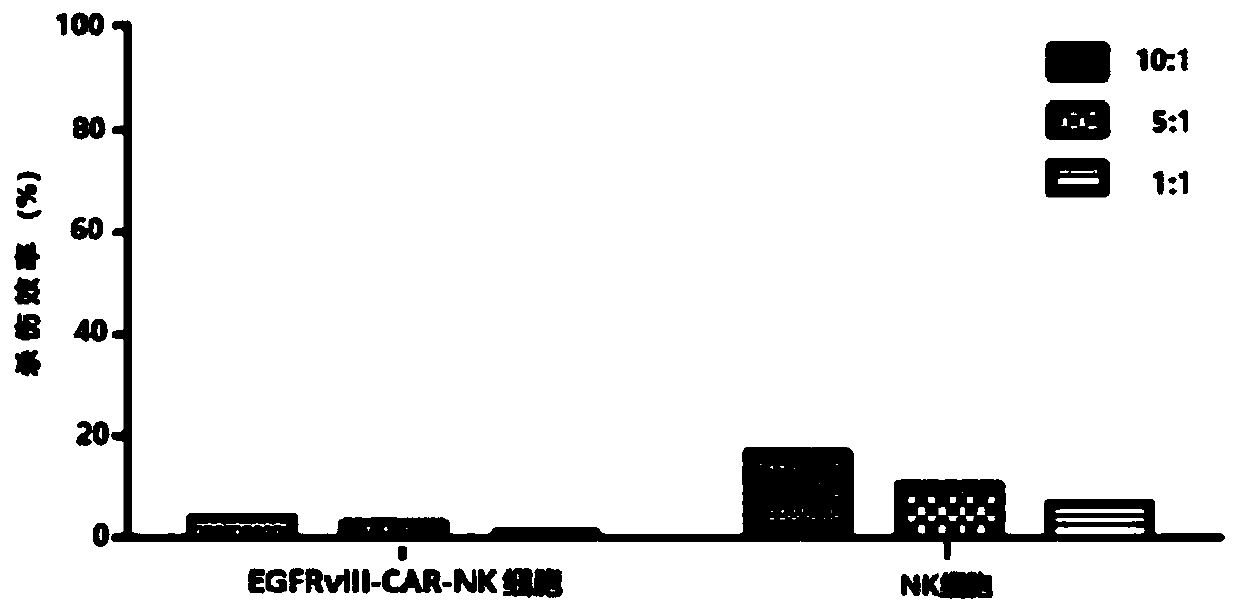
CAR-NK cell, and preparation method and application thereof
ActiveCN109536455ASolve some problemsTumor killerImmunoglobulin superfamilyGenetically modified cellsCell activityAutologous blood
The invention discloses a CAR-NK cell, and a preparation method and application thereof, and belongs to the field of cell therapy and biopharmaceutics. The method uses umbilical cord blood as a sourceof NK cells, and solves the problems of CAR-NK cells from a NK cell line, peripheral blood and autologous blood, such as complicated operation, risk of tumor formation, insufficient cell activity, asmall number of cells and GVHD caused by partial allogeneic origin cells.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Aeromonas hydrophila micro-capsular oral vaccine
InactiveCN101862308AWide variety of sourcesLow priceAntibacterial agentsPharmaceutical non-active ingredientsBacteroidesCytokine
The invention relates to an aeromonas hydrophila micro-capsular oral vaccine, which belongs to the technical field of biological pharmacy. The aeromonas hydrophila micro-capsular oral vaccine is prepared by using sodium alga acid and chitosan as wall materials, using the inactivated bacteria of aeromonas hydrophila as a core material and by using improved atomization and ion crosslinking technology. When the aeromonas hydrophila sodium alga acid-chitosan micro-capsular oral vaccine is used for immunizing a mouse and crucian, the phagocytosis activities of macrophages, the conversion efficiency of the splenic lymphocytes and the expression amount of cell factors such as IFN-gamma and Il-4 of the mouse and the crucian can be improved obviously. The relative protection rate of the vaccine for the crucian is 39.3 percent. A microcapsule is stable in a simulated gastrointestinal environment and shows high burst release and slow release; and the bacterial antigenicity of the microcapsule is well maintained and the vaccine has high safety and high storage stability.
Owner:NANJING AGRICULTURAL UNIVERSITY
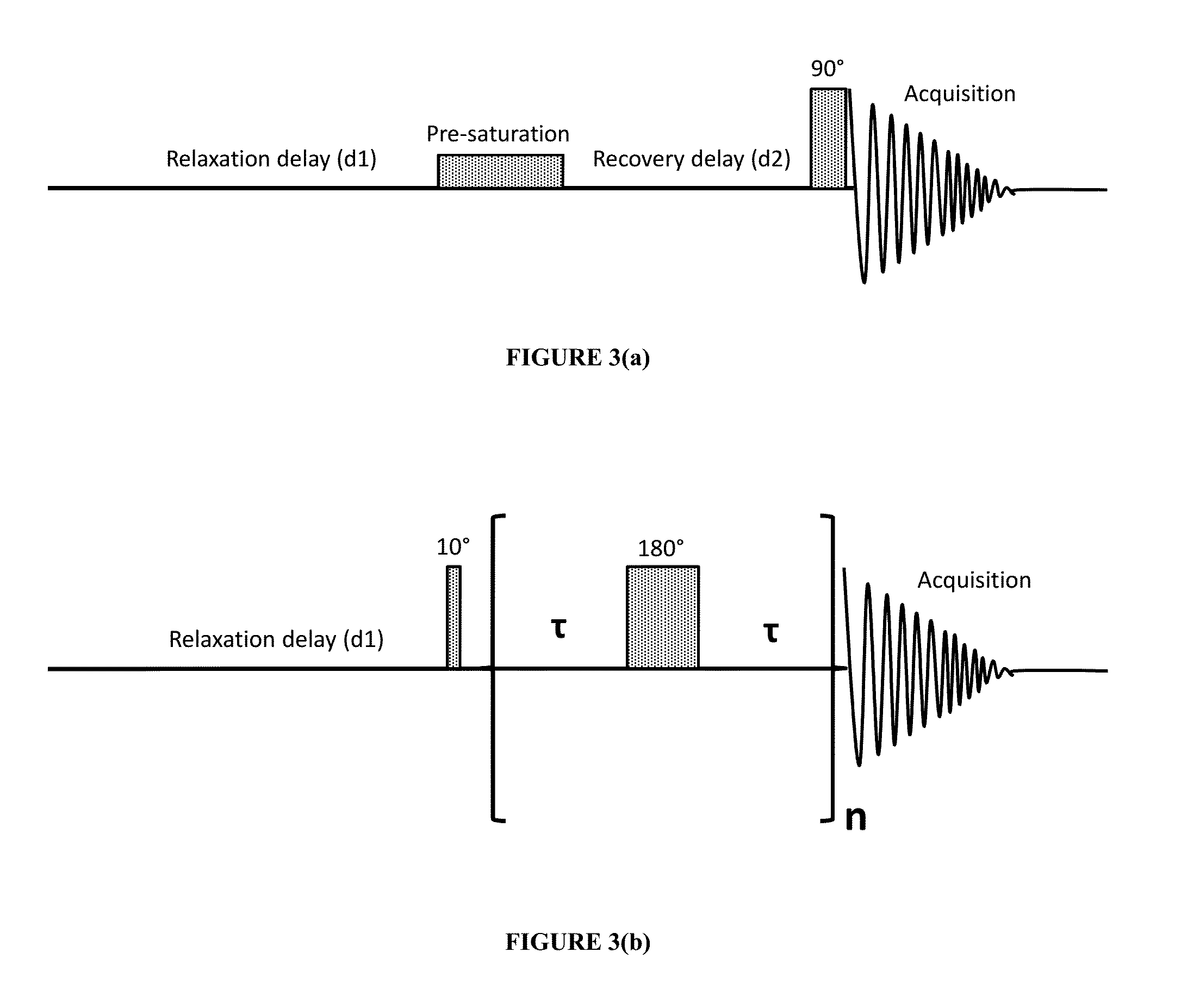
Assessing biopharmaceutical aggregation using magnetic resonance relaxometry
ActiveUS10267754B2Analysis using nuclear magnetic resonanceMeasurements using NMR imaging systemsNon destructiveResonance
The present invention generally relates to a method of using NMR relaxation rates (R2) of water molecules as an indicator of the extent of aggregation of biopharmaceutical formulations. The biopharmaceutical can be evaluated nondestructively without the vial or container being opened or protective seal compromised (i.e., broken). The method is applicable to all biopharmaceuticals and the water signal obtained by magnetic resonance relaxometry is very strong and sensitive because water is used as the solvent and is present in high (>90%) concentrations in every biopharmaceutical formulation.
Owner:UNIV OF MARYLAND BALTIMORE
Sorangiumcellulosum strain and application thereof to synthesis of epothilone
The invention belongs to the fields of bioengineering and biological pharmacy, and in particular to a Sorangiumcellulosum So2163 strain, and application of the strain to synthesis of epothilone. The Sorangiumcellulosum So2163 strain was preserved on June 10, 2012 in China Center for Type Culture Collection, and has a collection number CCTCC M 2012211. The Sorangiumcellulosum So2163 strain involved in the invention provides new strain resource for industrialization production of epothilone.
Owner:QILU UNIV OF TECH
Bletilla striata gelatin and preparation method thereof
The invention relates to a bletilla striata gelatin and a preparation method thereof, which relate to the technical field of biopharmaceutics. The preparation method of the bletilla striata gelatin comprises the steps of a smashing step, a screening step, an extracting step and a purifying step. The preparation method of the bletilla striata gelatin comprises the concrete steps of smashing bletilla striata, then screening through a saturated sodium chloride solution, adding water for ultrasonic processing after screening, and purifying through an ethanol solution. According to the method, the bletilla striata raw materials are well screened, and the prepared bletilla striata gelatin has high purity and a good pharmacological function; the extraction rate of the bletilla striata gelatin is greatly improved, the utilization ratio of the raw materials is improved, and the cost is reduced. The bletilla striata gelatin provided by the invention is prepared through the preparation method of the bletilla striata gelatin, and has high purity and a good pharmacological function.
Owner:贵州省武陵山药用植物白芨开发有限公司
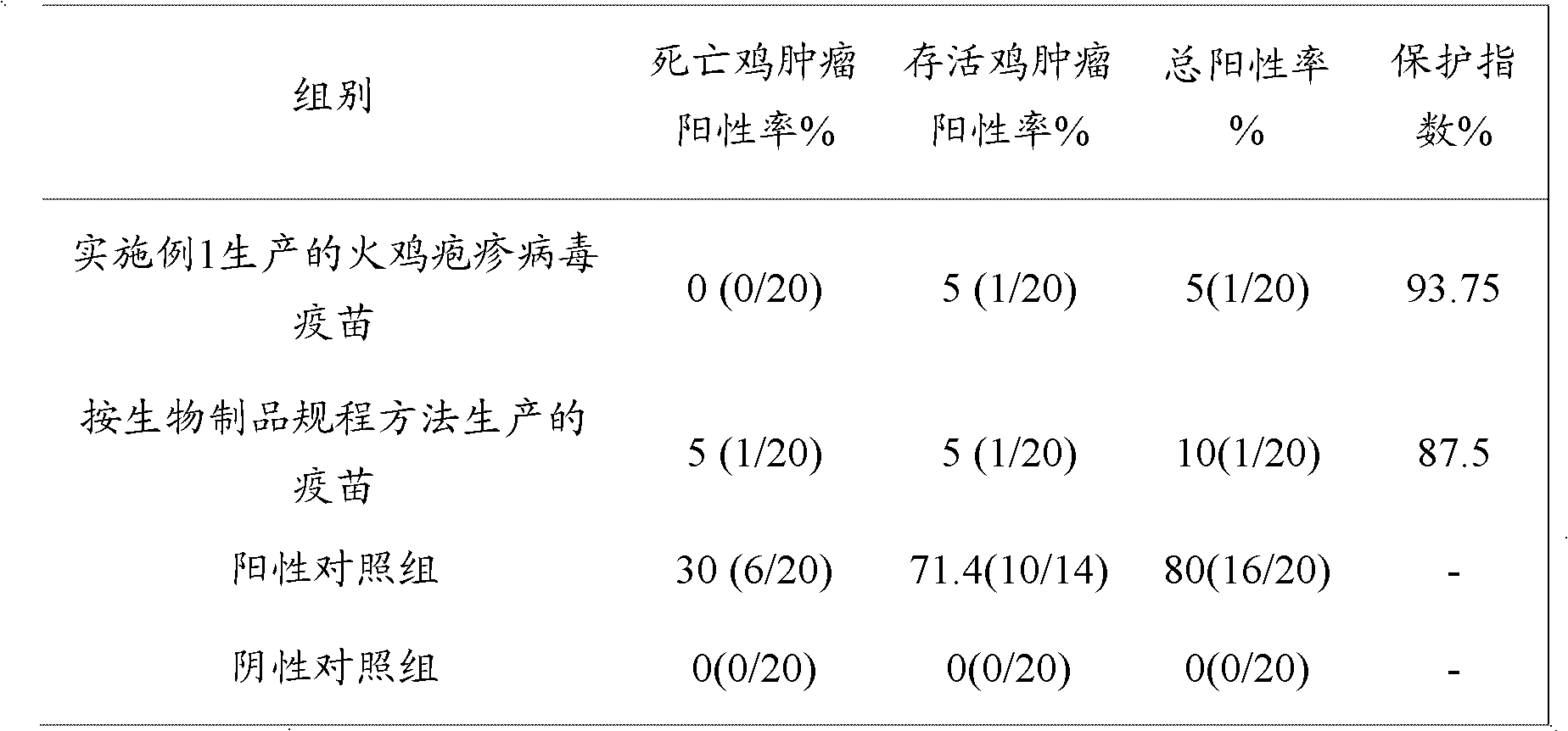
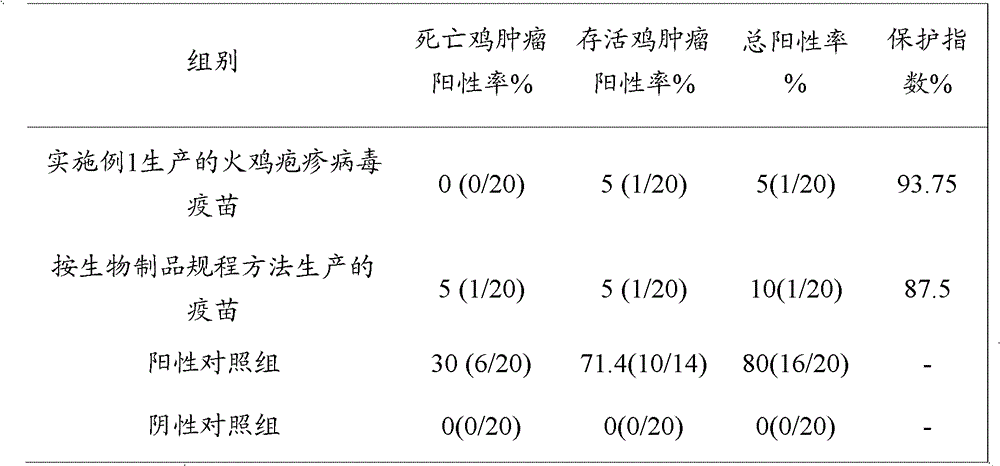
III type turkey herpes virus freeze-drying vaccine
The invention belongs to the technical field of biopharming, and discloses a method for preparing a chicken Marek's disease turkey herpes virus Fc126 strain live vaccine by utilizing a cell factory and the turkey herpes virus vaccine (Fc126 strain) prepared by utilizing the method. The turkey herpes virus vaccine (Fc126 strain) prepared in the cell factory is good in quality, high in immunization potency and stable in immunization effect. In addition, the turkey herpes virus vaccine (Fc126 strain) has the advantages of being low in generation, high in content of plaque forming unit (PFU), low in cost, simple in operation technology, easy to control and the like.
Owner:PU LIKE BIO ENG
Assessing biopharmaceutical aggregation using magnetic resonance relaxometry
ActiveUS20160047761A1Strong signalAnalysis using nuclear magnetic resonanceMeasurements using NMR imaging systemsNon destructiveResonance
The present invention generally relates to a method of using NMR relaxation rates (R2) of water molecules as an indicator of the extent of aggregation of biopharmaceutical formulations. The biopharmaceutical can be evaluated nondestructively without the vial or container being opened or protective seal compromised (i.e., broken). The method is applicable to all biopharmaceuticals and the water signal obtained by magnetic resonance relaxometry is very strong and sensitive because water is used as the solvent and is present in high (>90%) concentrations in every biopharmaceutical formulation.
Owner:UNIV OF MARYLAND BALTIMORE
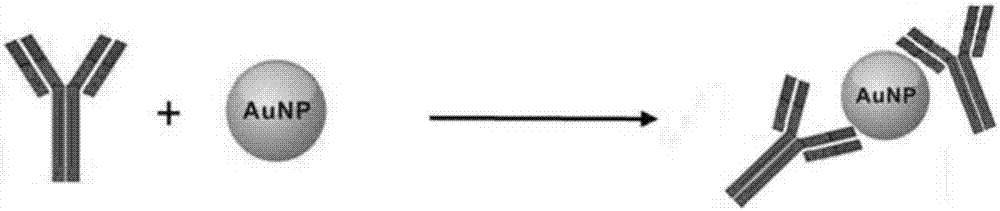
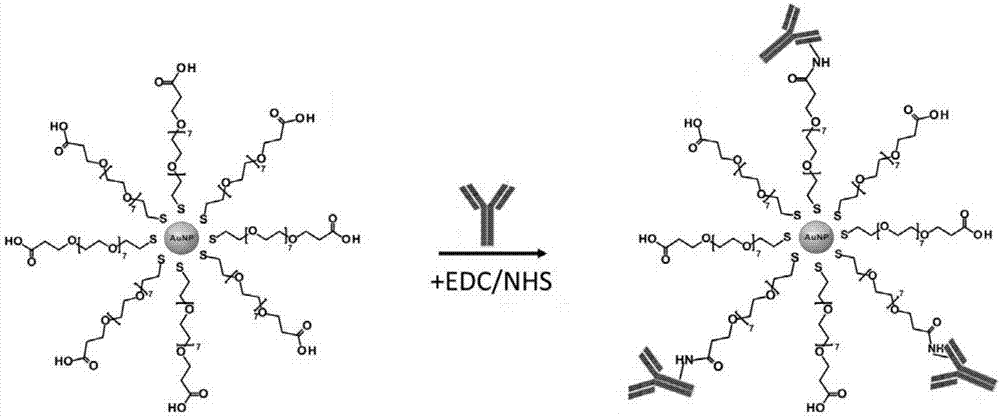
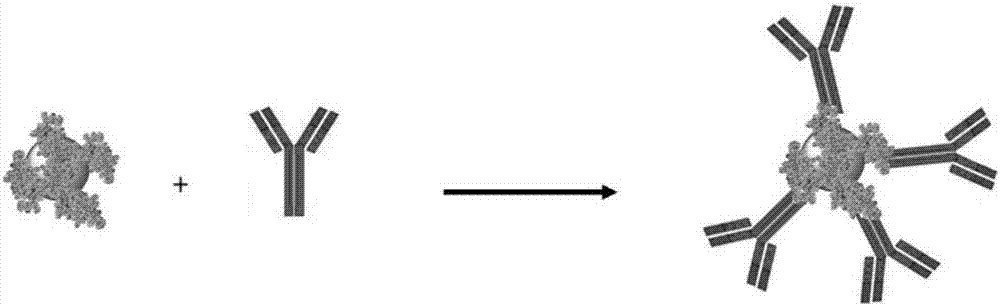
Preparation method of recombinant protein A mediated immune targeting nano-gold
ActiveCN106932567AMaintain stabilityImprove anti-interference abilityMaterial nanotechnologyMaterial analysisChemical LinkageImmune Targeting
The invention provides a preparation method of recombinant protein A mediated immune targeting nano-gold. A recombinant Protein A / G is used as an affinity ligand to enable an antibody fix on the surface of the nano-gold to form an immune nano-gold compound. The compound is composed of three parts including the antibody, the recombinant protein A and the nano-gold; the recombinant protein A contains C-terminal cysteine after being modified and can be coupled on the nano-gold through a single site, so that steric hindrance is reduced and a binding capacity between the recombinant protein A and the antibody is also increased; an Fc segment of the antibody is specifically combined through a non-covalent bond and an antibody orientation effect which is better than physical adsorption and chemical bonding is obtained. The method can be used for preparing complicated multi-component and multifunctional compound immune targeting nano-gold to meet different requirements. The immune targeting nano-gold prepared by the invention not only has reaction specificity and stability, but also has relatively high anti-interference capability and environment tolerance and has a very good application prospect in the fields including targeting tracing, biological pharmacy, accurate treatment and the like.
Owner:天津市泌尿外科研究所 +1
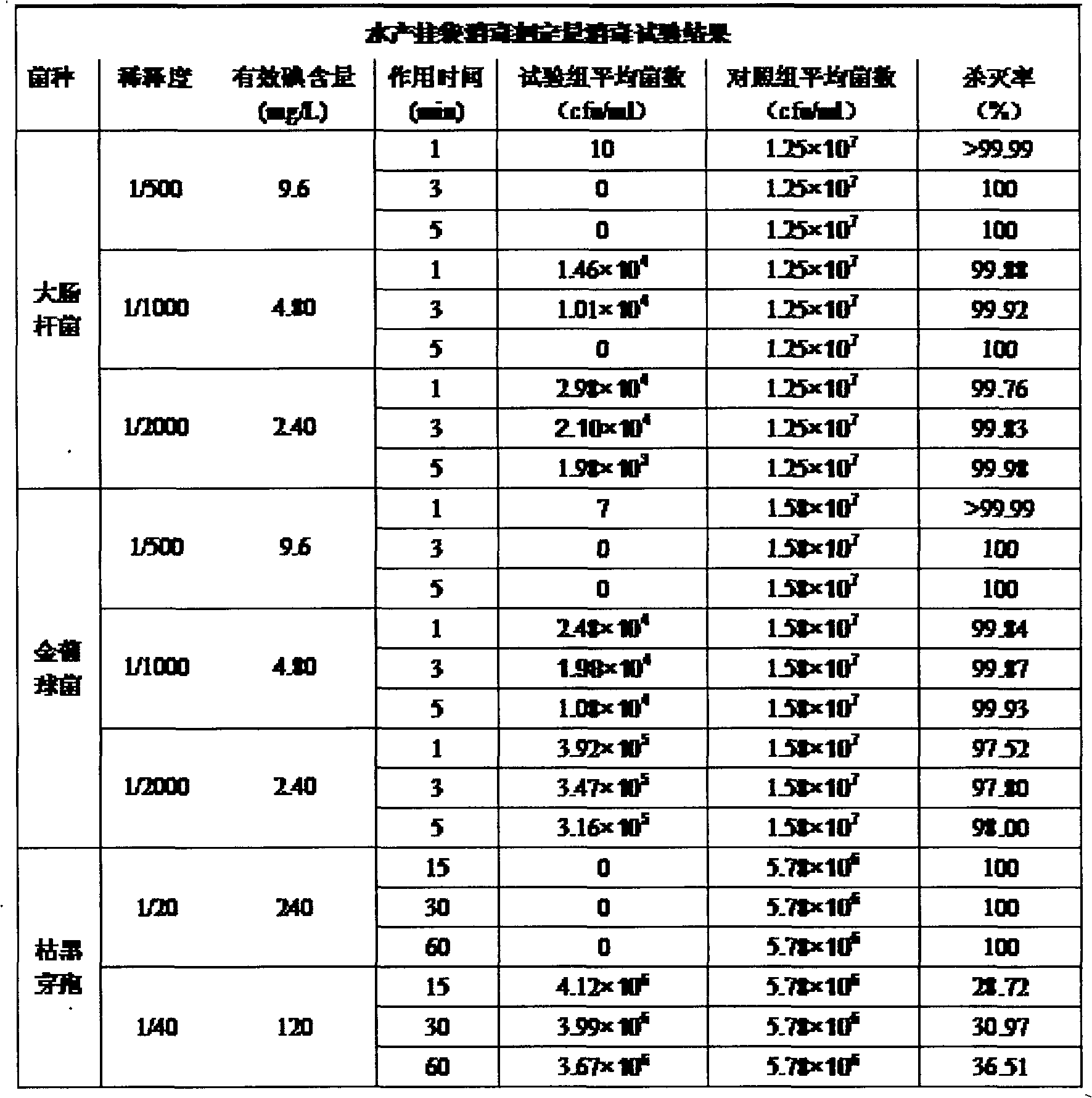
Aquatic product dedicated hanging bag colloidal iodine disinfection drug
InactiveCN103503863ANo stress effectDelay drug resistanceBiocideDisinfectantsAquatic animalAdditive ingredient
The invention discloses an aquatic product dedicated hanging bag colloidal iodine, belongs to the field of biological pharmacy, relates to the aquatic product disinfectant, and particularly relates to the disinfectant for a dedicated hanging bag for breeding fishes, shrimps, crabs, frogs, finless eels, loaches, tortoises, trionyx sinensis and other aquatic animals. The disinfectant comprises the following components: 1-5 g of a dilute alkali solution; 2-16 g of casein; 6-20 g of a gel agent; 15-30 g of polyvinylpyrrolidone iodine; 1-15 g of a softening agent; 1-20 g of a sustained-release agent; 2-12 g of sodium carboxymethylcellulose; and the balance being an auxiliary agent, wherein the sum of the weight of the components is 100 g. The disinfectant can effectively reduce the drug using amount of aquaculture sterilization, is safe, green and environment-friendly, and has the advantages of high efficiency, no toxicity, no residues, thorough sterilization, delayed sustained release, lasting drug effect, convenience and practicality.
Owner:JIANGSU TIANYIJIAN DISINFECTANT
Combination of stem cells and cytokines, and use thereof in improving sperm activity
The present invention relates to the field of biopharmaceutics and particularly relates to a use of autologous peripheral blood stem cells combined with cytokines for improving low sperm activity. Inparticular, the combination of peripheral blood stem cells and cytokines of monocyte chemoattractant protein-1 (MCP-1) and glial cell-derived neurotrophic factors (GDNF) has a synergistic treatment oran inhibition function to improve the low sperm activity, and thus the new composition or a medicinal box for the low sperm activity is provided.
Owner:SINO AMERICAN BIOTECH INC
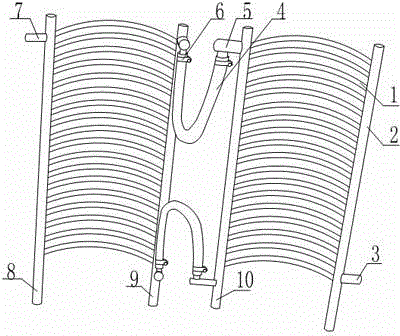
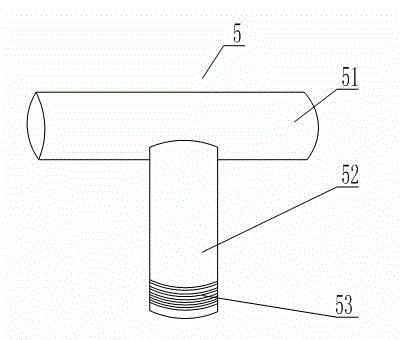
Condenser for biological pharmacy
The invention relates to a condenser for biological pharmacy. The condenser for biological pharmacy comprises a condensation column I, a condensation column II, a condensation column III and a condensation column IV. A plurality of condensation pipes shaped like semi-circles in a sunken mode are arranged between the condensation column II and the condensation column III, and a plurality of condensation pipes shaped like semi-circles in a sunken mode are arranged between the condensation column I and the condensation column IV. The condensation column III and the condensation column IV are both provided with connectors, a connection pipe is arranged between the connector on the condensation column III and the connector on the condensation column IV, fixing devices are arranged on junctions of the connection pipe and the connectors, a water inlet pipe connector is arranged at the lower end of the condensation column I, and a water outlet pipe connector is arranged at the upper end of the condensation column II. According to the condenser for biological pharmacy, as the connection pipe is arranged between the condensation column III and the condensation column IV, the defect that an existing condensation structure can not be applied to a closed cooling type and also applied to a semi-open type cooling device is overcome.
Owner:CHENGDU OLYMVAX BIOPHARM
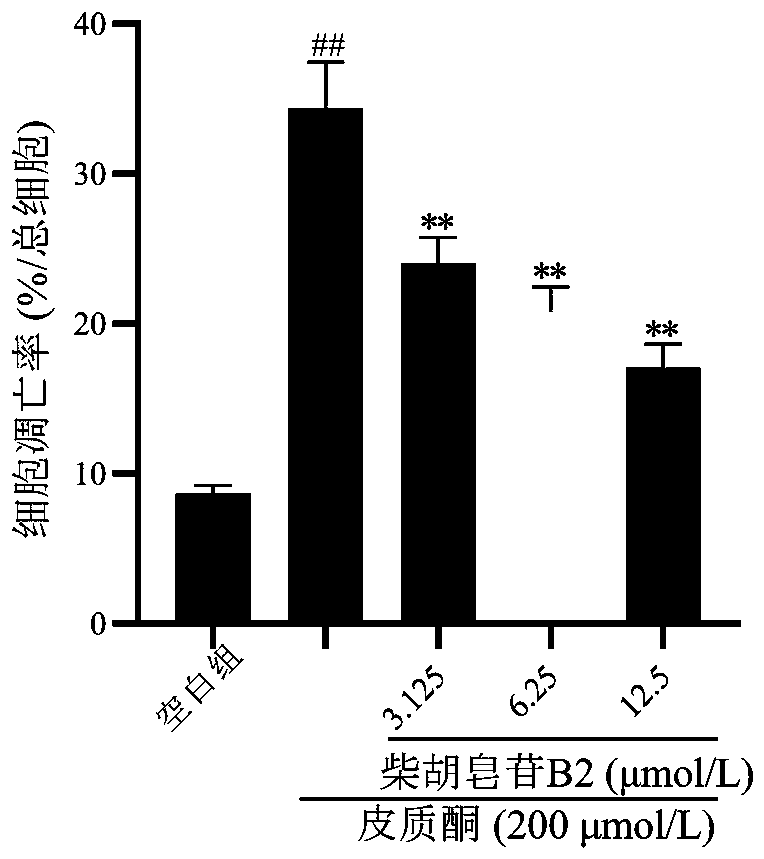
Application of saikosaponin B2 in preparation of antidepressant drug
InactiveCN110179809ARevealing the material basis of antidepressantOrganic active ingredientsNervous disorderCorticosteroneStructural formula
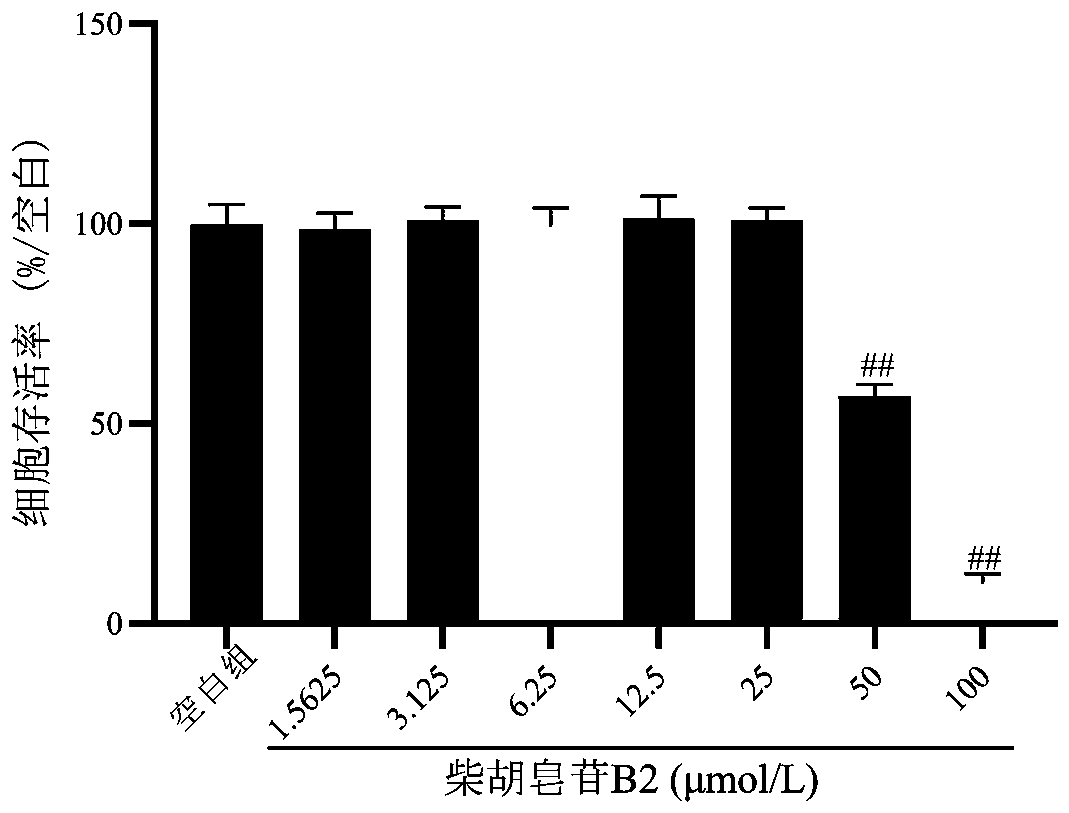
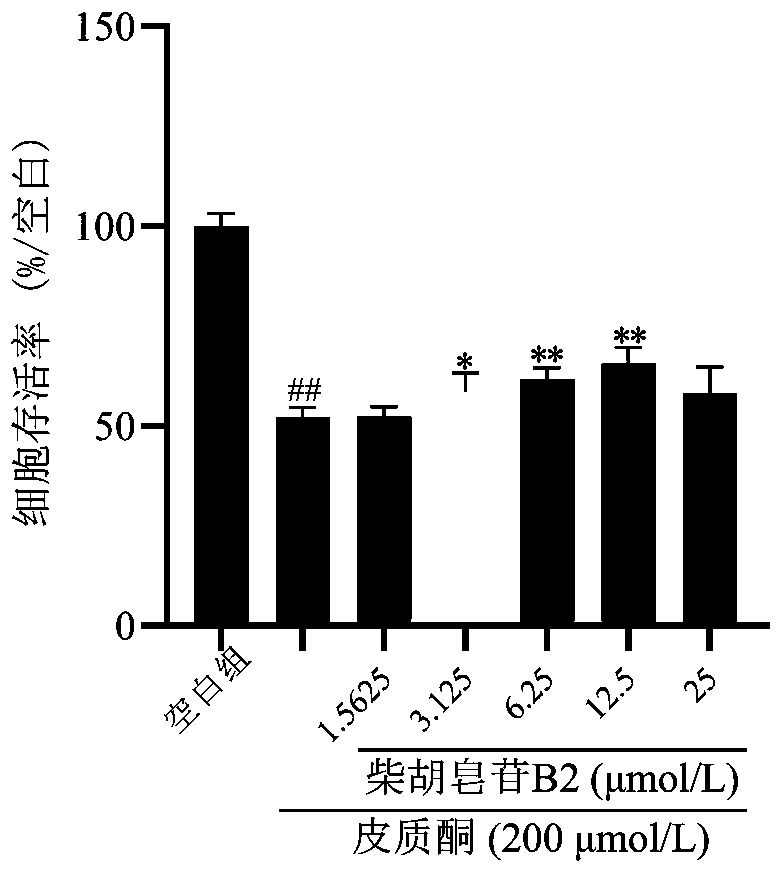
The invention belongs to the technical field of biopharmaceutics and provides an application of saikosaponin B2 in preparation of an antidepressant drug. A structural formula of saikosaponin B2 is asshown in the specification, and saikosaponin B2 is a unique active ingredient in the antidepressant drug. In-vitro activity tests prove that saikosaponin B2 has better protection activity for PC12 injured by corticosterone, and in-vivo test results show that saikosaponin B2 can significantly shorten immobile time of mice in forced swimming and tail suspension tests. Test results show that saikosaponin B2 has a significant antidepressant effect and can be used for preparing a drug for preventing and treating depression.
Owner:SHANXI UNIV
Anti-BLyS antibody
Owner:SHANGHAI JUNSHI BIOSCI
Ginseng-royal jelly nanoemulsion oral liquid and preparation method thereof
InactiveCN103735505ANo pollution in the processRealize the health care concept of green health and safetyAnthropod material medical ingredientsAntinoxious agentsFruit juiceDistilled water
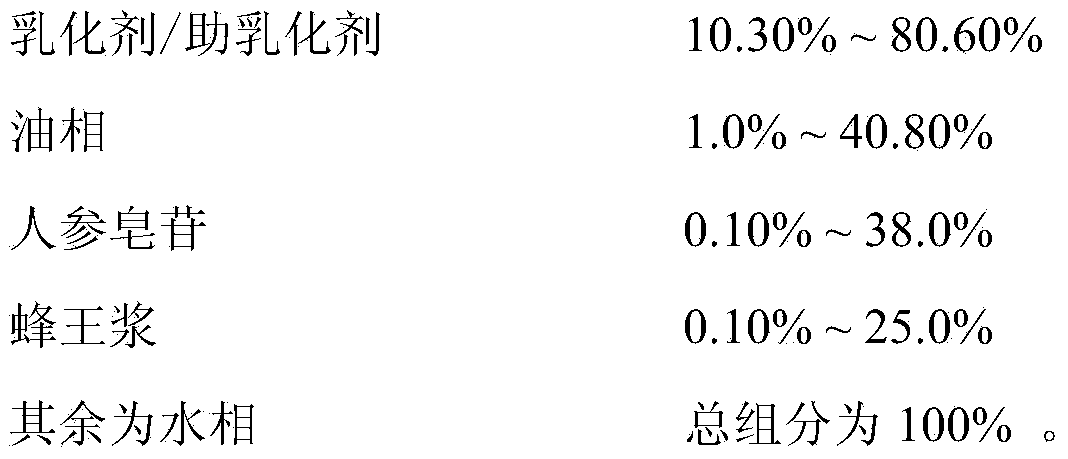
The invention relates to the technical fields of a nanoemulsion drug delivery system in the pharmaceutics and gastrointestinal transmembrane transport and absorption in the biopharmaceutics, and specifically relates to a nanoemulsion oral liquid containing ginseng and royal jelly, and a preparation method thereof. The nanoemulsion oral liquid comprises a natural or food-grade emulsifier and an oil phase, and also an aqueous phase, wherein the aqueous phase contains fruit juice water, vegetable juice water or honey water and double distilled water; the emulsifier / co-emulsifier accounts for 10.30%-80.60%, the oil phase accounts for 1.0%-40.80%, ginsenoside accounts for 0.10%-38.0% and the royal jelly accounts for 0.10%-25.0%, the balance is the aqueous phase, and the total ingredients are 100%. The results of animal experiments show that the oral liquid is good in gastrointestinal transmembrane transport and absorption effects, non-toxic, harmless and free of pollution.
Owner:JILIN UNIV
Bacillus CCPM7645 with strong anti-cancer activity and application thereof
PendingCN110295132AHigh anticancer activityLow or no side effectsBacteriaMicroorganism based processesCancer preventionHuman body
The invention relates to bacillus CCPM7645 with strong anti-cancer activity and application thereof, and belongs to the technical field of bio-pharmaceuticals. In order to solve the problem that existing anti-cancer medicines have large side effects, the bacillus CCPM7645 with strong anti-cancer activity is provided; the bacillus CCPM7645 belongs to Bacillus, represents a new bacterial species, ispreserved in China Center For Type Culture Collection and has the preservation number of CGMCC No.17887; the bacillus comes from human intestines, is new-source bacillus and can generate metaboliteswith anti-cancer activity, and cancers can be treated by the metabolites of the bacillus. Since the bacillus is found in human intestines, the bacillus has quite small side effects on a human body orhas no side effects on the human body, the problem that the existing anti-cancer medicines have large side effects and poor patient compliance is solved, and the aim of preventing cancers by adjustingthe balance of intestinal flora is expected to be realized.
Owner:哈尔滨天齐人类第二基因组技术开发应用科技有限责任公司
Rapid analysis method for monoclonal antibody N sugar glycoform
ActiveCN109358131AHigh sensitivitySimplified processing requirementsComponent separationDigestionOrganism
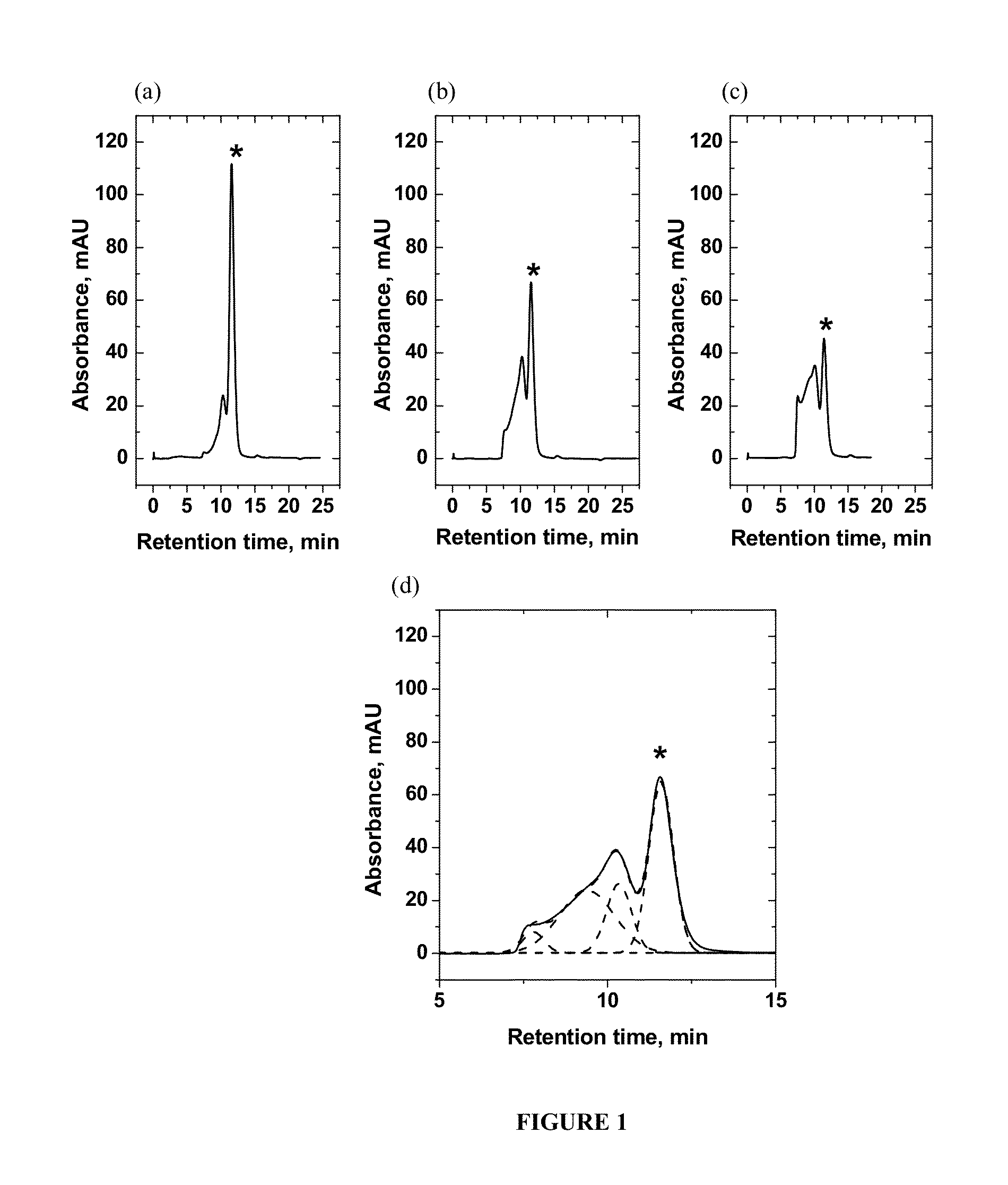
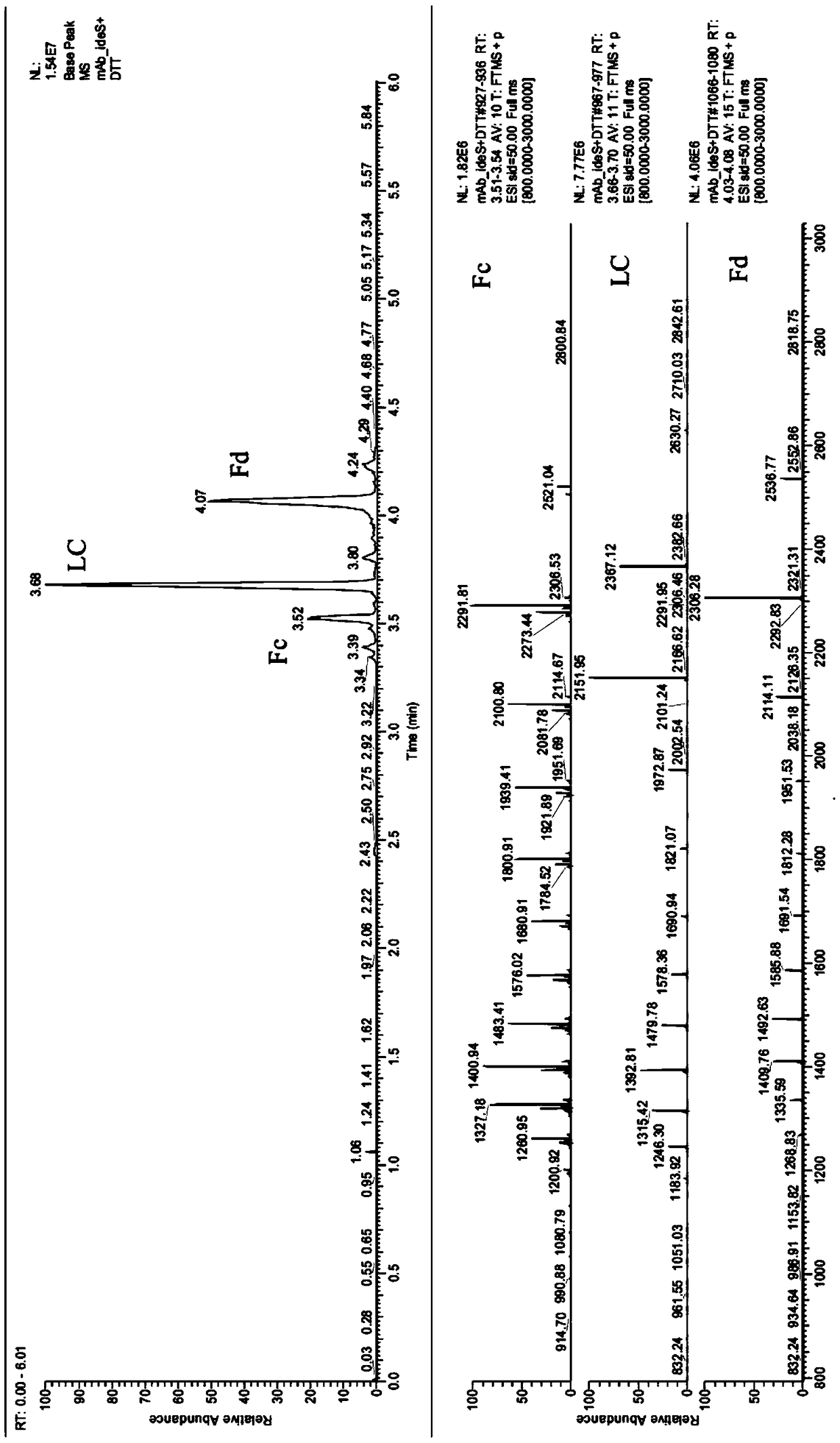
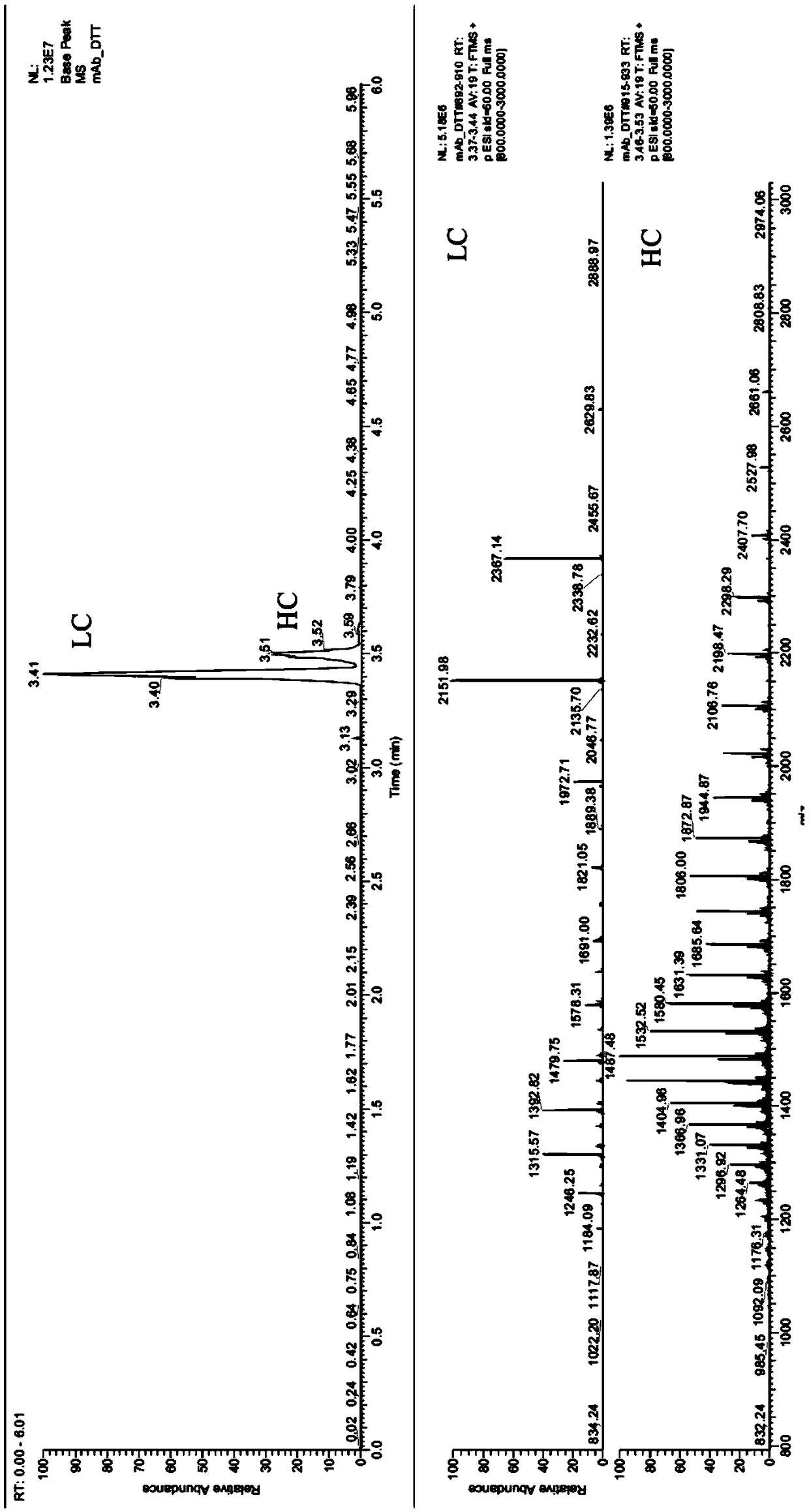
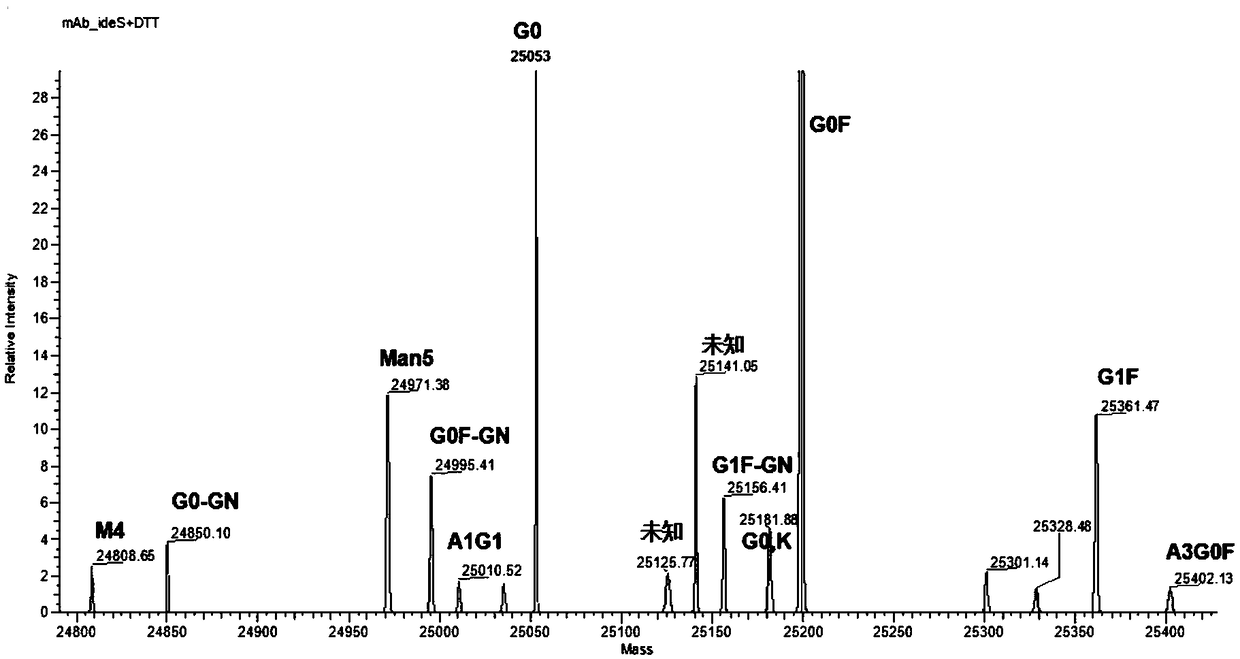
The invention provides a rapid analysis method for monoclonal antibody N sugar glycoform. The analysis method comprises the following steps: after centrifuging a to-be-tested cell culture, taking thesupernatant, diluting, adding an IdeS enzyme and a reducing agent to perform enzymatic hydrolysis reaction and reduction reaction, centrifuging, and taking the supernatant; detecting an antibody digestion and a reducing subunit solution by using capillary electrophoresis mass spectrometry to obtain the intensity and molecular weight of different glycoform molecules Ai in the antibody digestion andthe reduction subunit solution; determining the glycoforms corresponding to the molecular weights of the different glycoform molecules Ai by querying a database containing molecular weights of various glycoform molecules, and taking the ratio of the total strength of the subunits to which the intensity of each glycoform molecule Ai belongs as its glycoform ratio. Compared with the traditional polysaccharide labeling method, the rapid analysis method for the monoclonal antibody N sugar glycoform has advantages of rapid, high efficiency and sensitivity, provides strong support for the development of cell strains in the biopharmaceutical industry, and will greatly assist in speeding up the development progress of cell strain screening.
Owner:HJB HANGZHOU CO LTD
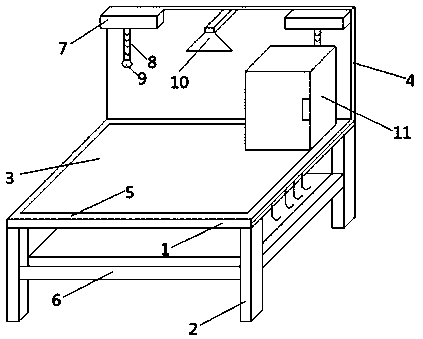
Special table for biopharmaceutical experiments
InactiveCN109908991AAvoid Waterlogging ProblemsMeet cleanliness requirementsLaboratory benches/tablesRadiationBody right sideWater leakage
The invention relates to a special table for biopharmaceutical experiments, and belongs to the field of experimental appliances. The special table mainly comprises a table body, table legs, a table surface, a baffle, water leakage grooves, water receiving grooves, a lampshade, a gooseneck pipe, an ultraviolet lamp, a spraying head, an object storage case, a case door, object storage boxes, concavegrooves, card grooves, a table body right side surface, a cross beam and hooks, wherein the water leakage grooves are arranged on the surface of the table body, the bottom portions of the water leakage grooves are provided with water outlet holes, and the middle positions of the table legs are provided with the water receiving grooves corresponding to the water leakage grooves. According to the present invention, the spraying head sprays water so as to completely clean the table surface, and the cleaning water flow enters the water receiving grooves through the water leakage grooves so as toavoid the water accumulating on the table surface, such that the operation is convenient and rapid; the ultraviolet lamp is arranged on the surface of the baffle, is connected to the lampshade throughthe gooseneck pipe, has disinfecting and sterilizing effect, and can move by moving the gooseneck pipe so as to sterilize and disinfect the table surface; and the special table has advantages of simple structure, novel design, convenience and practicality.
Owner:江苏龙之鼎生物科技有限公司
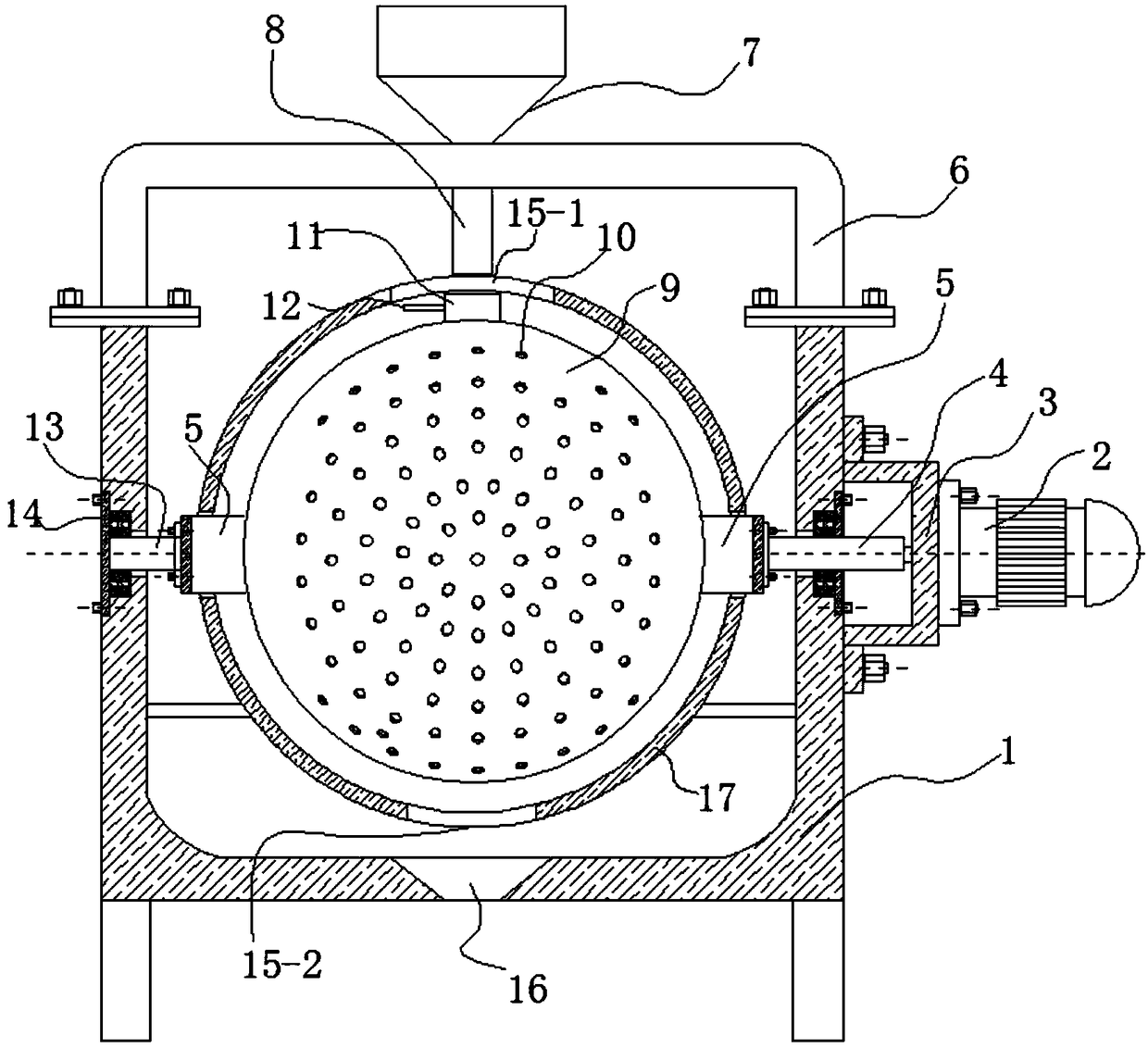
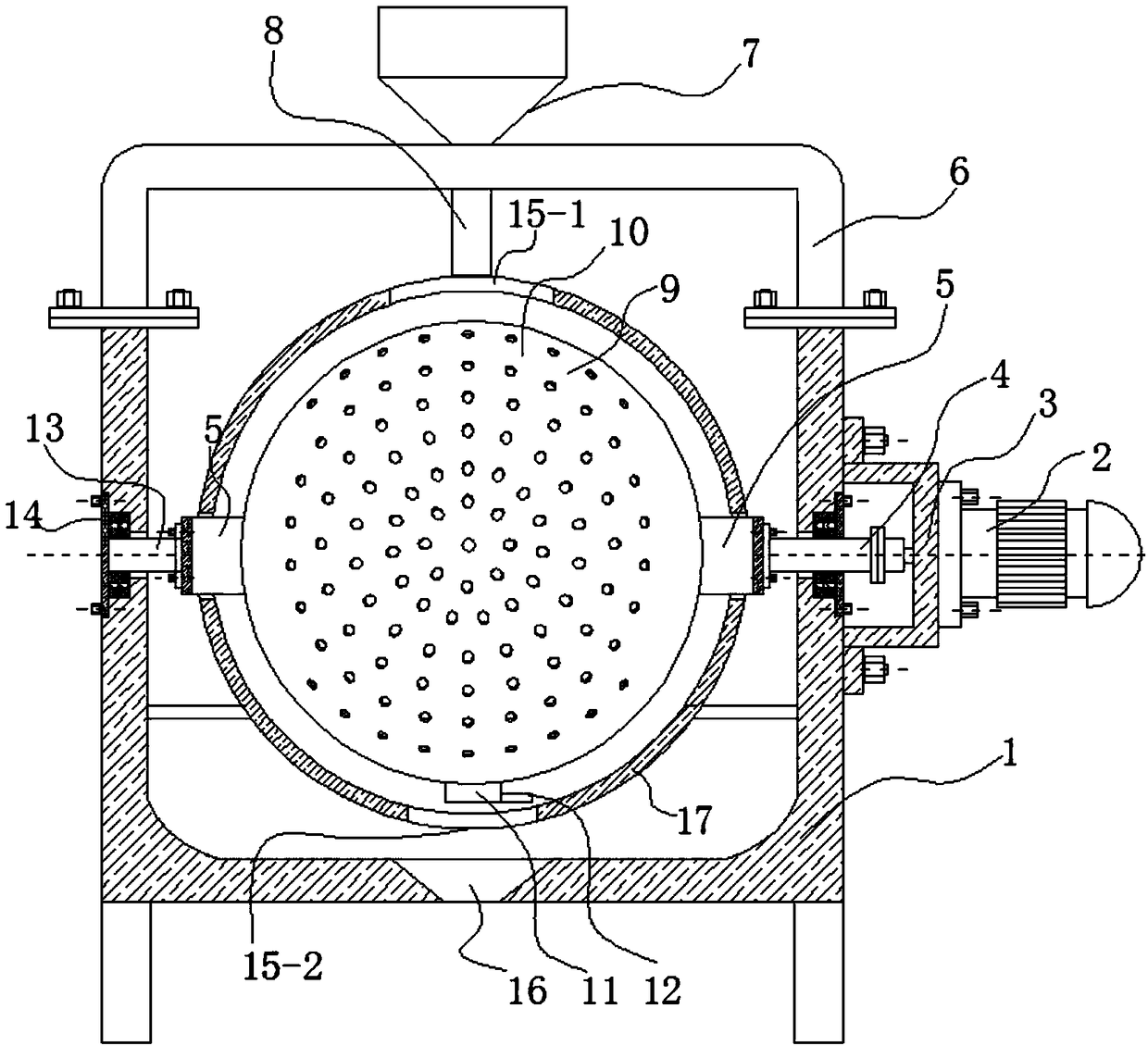
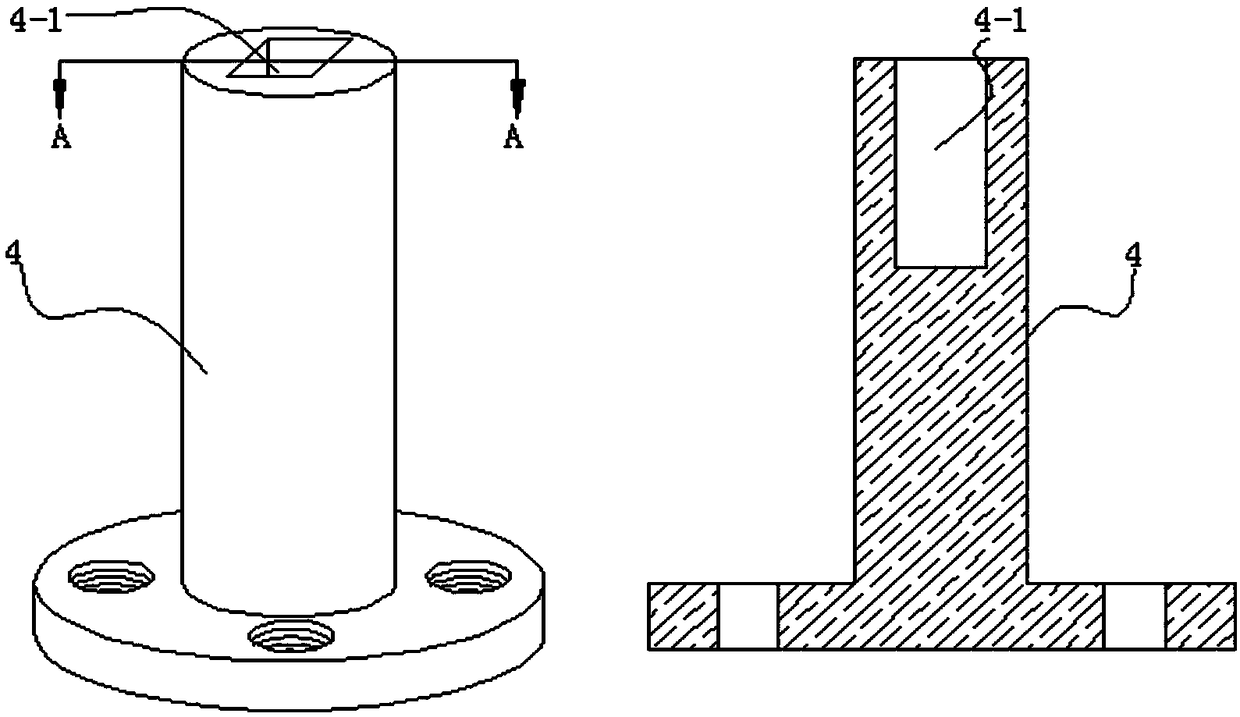
Membrane separating equipment for biological pharmacy
InactiveCN108079793AReduce cloggingGuaranteed smooth productionSemi-permeable membranesMembranesPharmacyArchitectural engineering
The invention discloses membrane separating equipment for biological pharmacy. The membrane separating equipment for the biological pharmacy comprises a filtering frame; a metal bucket is fixed to theouter wall of the bottom of the filtering frame through bolts; a metal cylinder which is placed horizontally is welded at the bottom of the metal bucket; a motor is fixed to the central position of an outer side wall of the metal cylinder through bolts; four supporting legs are welded to the outer wall of the bottom of the metal cylinder; a through hole is formed in the centre position, close tothe inner wall of one side of the motor, of the metal cylinder; an output shaft of the motor penetrates through the through hole and is fixed with a metal disc which is placed vertically through bolts; first membrane separating pipes which are distributed at equal distance and placed horizontally are fixed to the position, far away from the edge of the outer wall of one side of the motor, of the metal disc through bolts. The membrane separating equipment for biological pharmacy can separate while rotating and can stir materials so as to reduce blockage and ensure smooth production, is beneficial to improving the work efficiency and beneficial to improving the yield, separates more thoroughly, reduces material waste, reduces using cost, and improves separating quality.
Owner:蚌埠精工制药机械有限公司
Medicine screening mechanism for biological pharmacy
InactiveCN108993886ANo splashingSimple structureGas current separationPharmaceutical product form changePharmacyEngineering
The invention relates to the technical field of screening equipment, in particular to a medicine screening mechanism for biological pharmacy. The medicine screening mechanism comprises a bracket and amedicine screening mechanism body arranged on the bracket, wherein the medicine screening mechanism body is composed of a motor and a screening assembly, the motor is installed at the side end of thebracket, the screening assembly is composed of a first short shaft, a second short shaft, an outer sphere and an inner sphere, the outer sphere is formed by splicing two hemispheres, moreover, the outer sphere is installed inside the bracket through a positioning frame in a fastening mode, a feeding through groove is formed in the upper end of the outer sphere, the lower end of the outer sphere is provided with a discharging through groove, the inner sphere is arranged inside the outer sphere, a material pipe communicating with the interior of the inner sphere is arranged on the inner sphere,a material valve is arranged on the material pipe, and screen holes with the interiors communicating with the exterior are formed in the side wall of the inner sphere. According to the medicine screening mechanism, the structure is simple, in the medicine screening process, the medicine in the inner sphere can be continuously screened, due to the fact that the outer sphere is arranged, the phenomenon of splashing of the medicine is avoided, and collection is facilitated.
Owner:WUHU KANGQI PHARMA
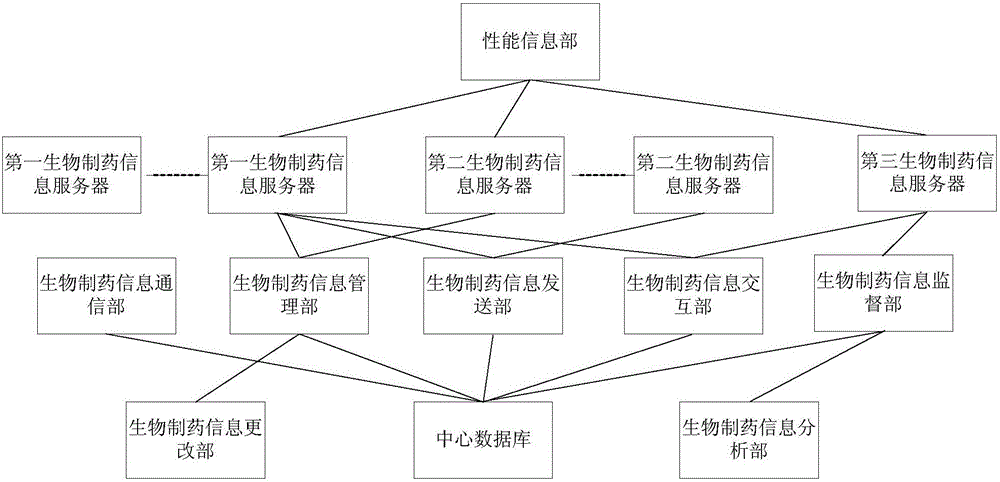
Biological pharmacy information management system
ActiveCN105844565AImprove access efficiencyImprove management efficiencyData processing applicationsPharmacyInformation analysis
The invention discloses a biological pharmacy information management system. The biological pharmacy management system includes a plurality of biological pharmacy information servers which manage biological pharmacy project information of the biological pharmacy information management system, a biological pharmacy information management part which manages the information of biological pharmacy, a biological pharmacy information supervision part which is used by a person in charge of management of the trustee of a biological pharmacy project to supervise and manage the information of the biological pharmacy project, a biological pharmacy information analysis part which is used by analysis personnel in the biological pharmacy project to store and transmit analysis results of analyzed samples in the biological pharmacy project, and a biological pharmacy information transmitting part which selects, accesses and query the complete information of the biological pharmacy project in the biological pharmacy information management part. With the biological pharmacy information management system of the invention adopted, supervision and management on a whole biological pharmacy process can be facilitated for the management level of a pharmaceutical company, and loopholes in pharmaceutical company such as information leakage and external use of the instruments of the pharmaceutical company can be eliminated.
Owner:SHANGHAI KING CELL BIOTECHNOLOGY CO LTD
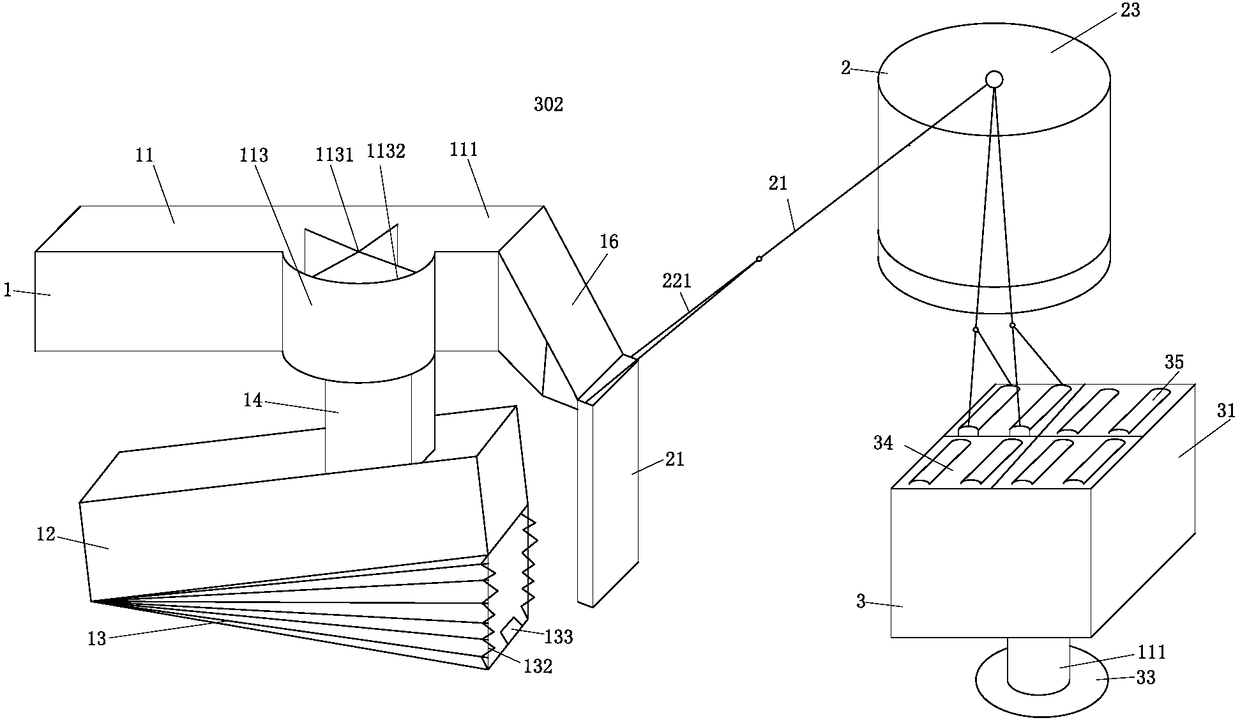
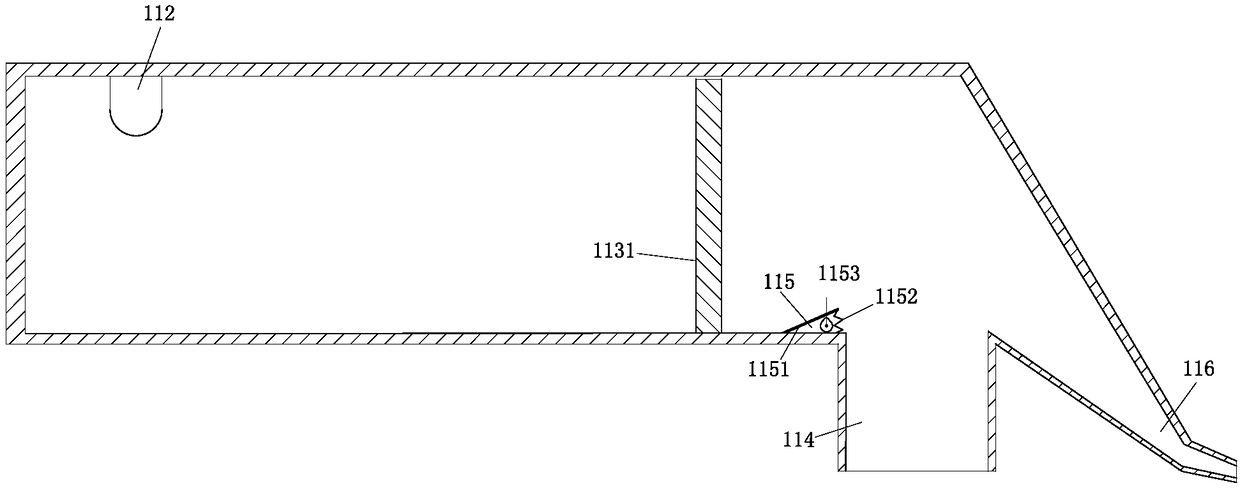
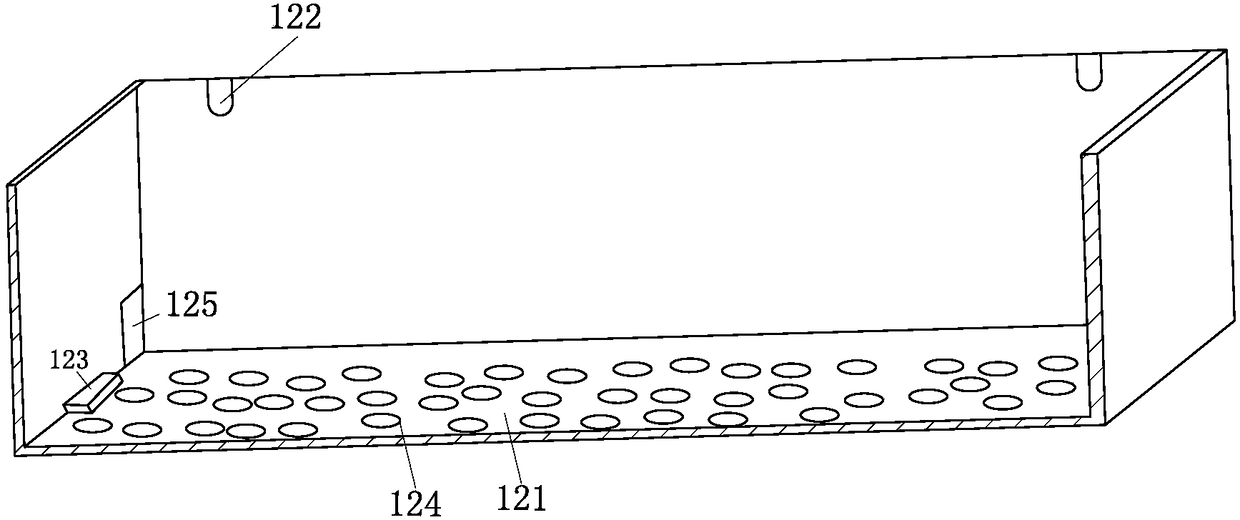
Multi-procedure deep processing system for periplaneta americana for biological pharmacy
InactiveCN108245535ADry evenlyImprove medicinal effectSievingAnthropod material medical ingredientsPharmacyMicrowave
The invention belongs to the field of drug processing equipment, in particular to a multi-procedure deep processing system for periplaneta americana for biological pharmacy. The multi-procedure deep processing system comprises a screening device, a transporting device and a drying device, wherein the screening device is arranged on the left side of the transporting device; the drying device is arranged on the right side of the transportation device; the screening device comprises a first-stage screening box, a second-stage screening box, a third-stage screening box and a transportation pipe and can be used for screening different periplaneta americana; the transportation device comprises a plurality of transportation cases, a plurality of mechanical arms and a rotary table; the transportation cases filled with male insects are transported to the drying device by the mechanical arms; the drying device comprises a square microwave drying oven, a rotary supporting column and a rotary chassis; the periplaneta americana is subjected to inactivated drying treatment by using microwave. The multi-procedure deep processing system disclosed by the invention has a simple structure and high working efficiency; the male insects needing to be processed, female insects for propagating progenies and to-be-grown larva can be effectively distinguished; the periplaneta americana can be quickly inactivated and dried by adopting the microwave; the multi-procedure deep processing system has the advantages of high drying speed, high efficiency, environment friendliness and energy conservation.
Owner:李长寿
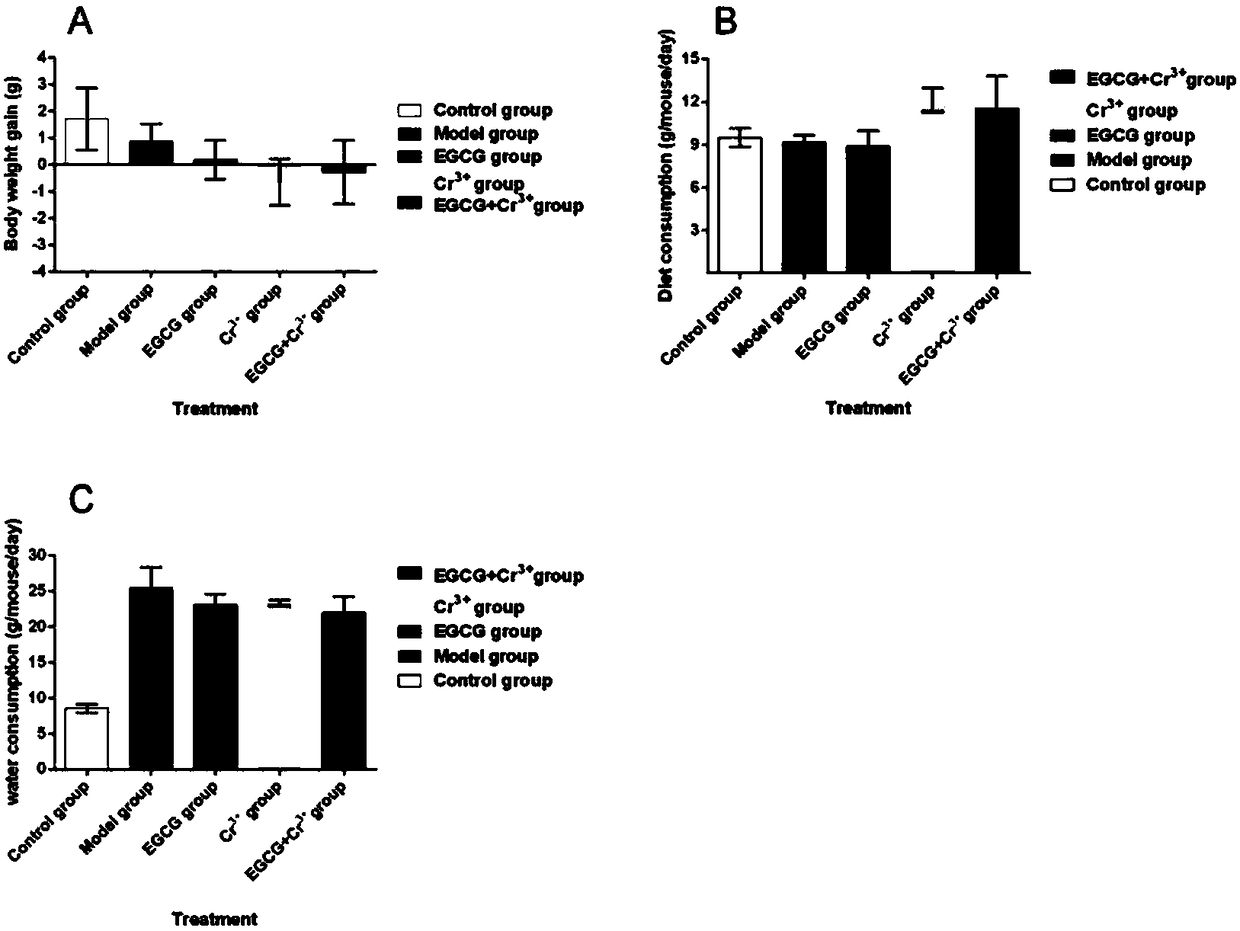
Application of tea extract composition to relief and treatment of diabetes
InactiveCN108126055AGood hypoglycemic effectImprove inflammationOrganic active ingredientsHeavy metal active ingredientsGlycemicCellular level
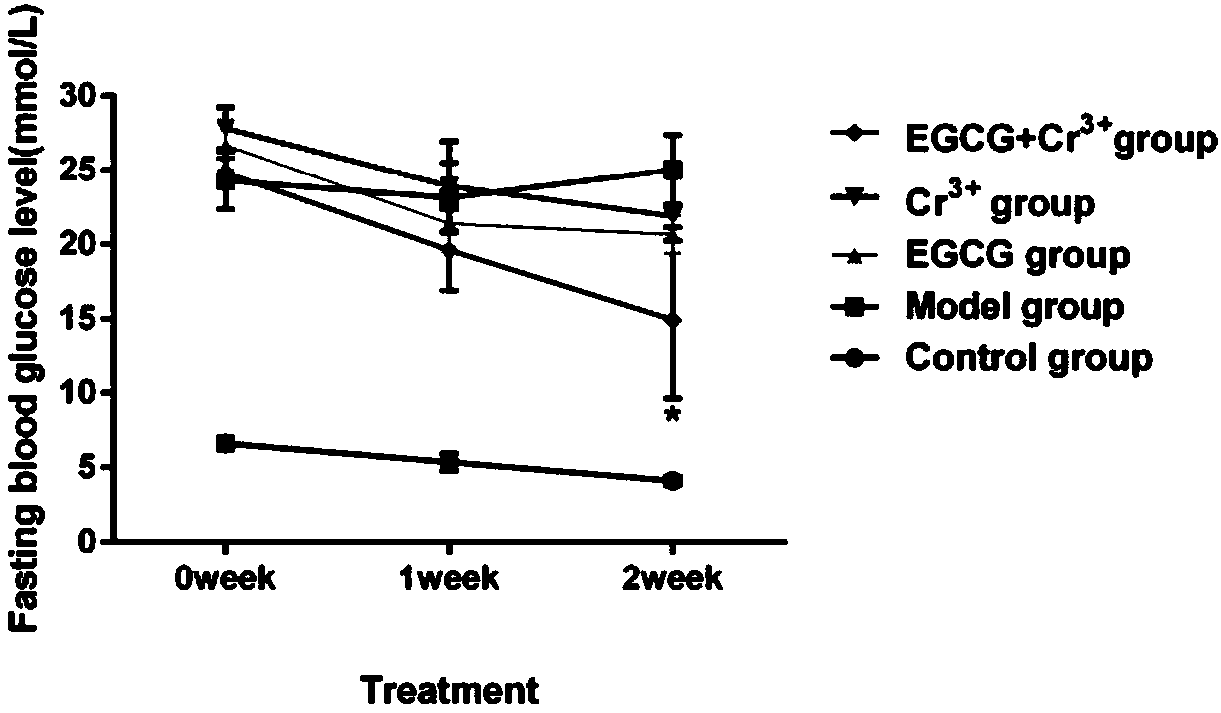
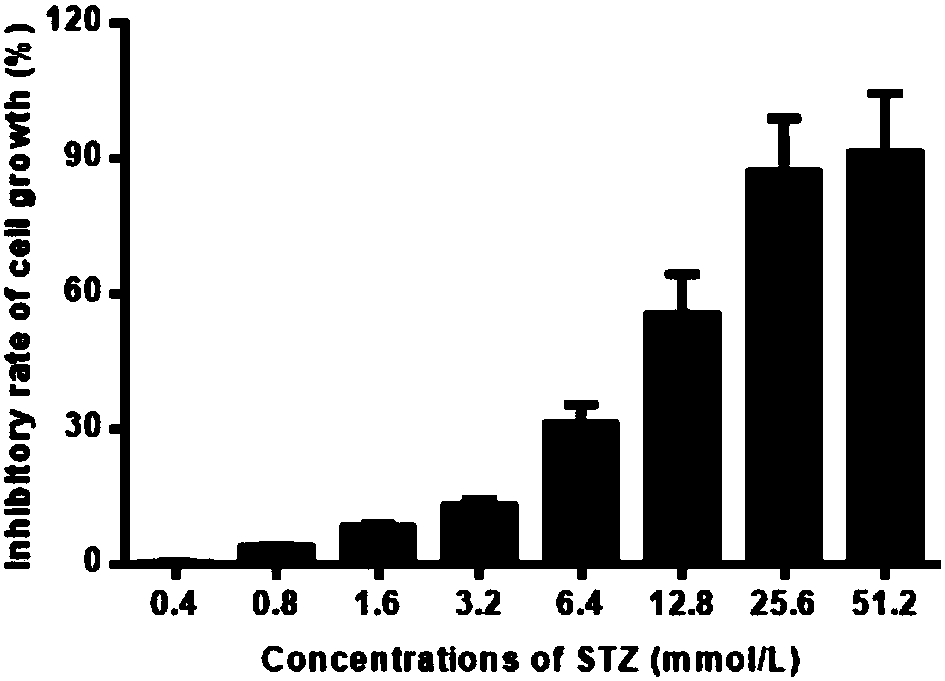
The invention belongs to the field of biopharmaceutics, and particularly relates to application of a tea extract composition to relief and treatment of diabetes. Compared with the prior art, the invention provides a novel method and a novel research theory for treating and relieving the diabetes. Studies and experiments show that a composition of EGCG extracted from tea leaves and Cr<3+> has a very good diabetes relieving effect, can effectively reduce the fasting blood glucose level of a type-2 diabetic mouse, can activate an NO signaling pathway of an NIT-1 cell, significantly improve inflammation of the NIT-1 cell and increase the insulin secretion level thereof in the cellular level, and can increase the sugar utilization rate, improve the glycogen synthesis efficiency and improve thesensitivity of an L6 cell to insulin stress by activating an AMPK pathway of the L6 cell. The tea extract composition significantly improves the hypoglycemic effect of the EGCG, which has a guiding significance and a guiding value for efficacy improvement and application of the tea extract EGCG.
Owner:TEA RES INST GUANGDONG ACAD OF AGRI SCI
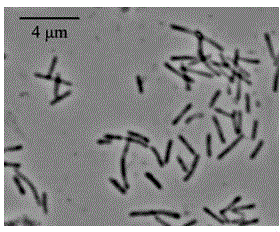
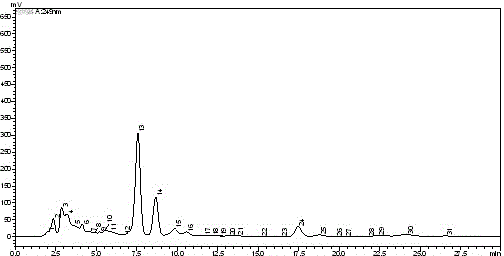
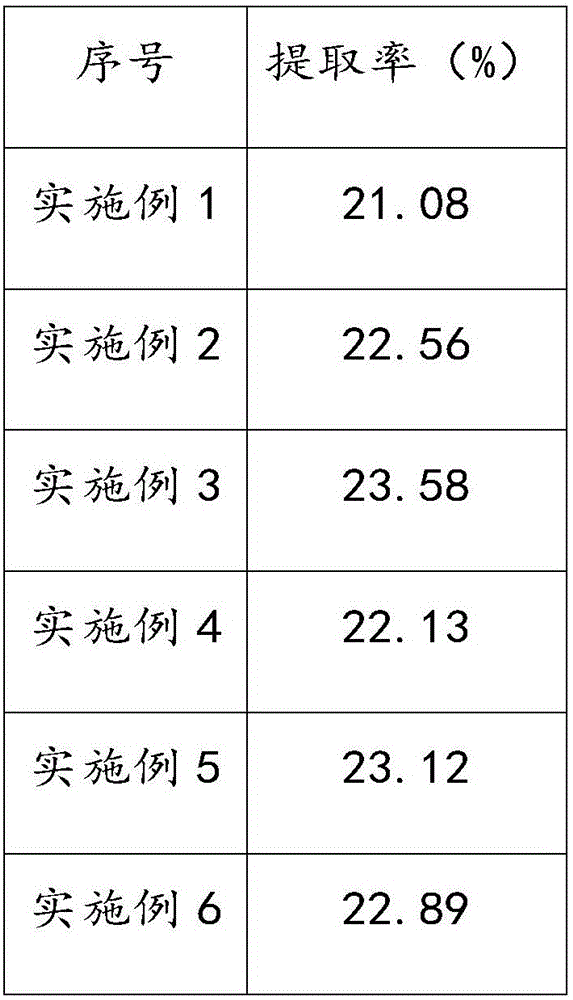
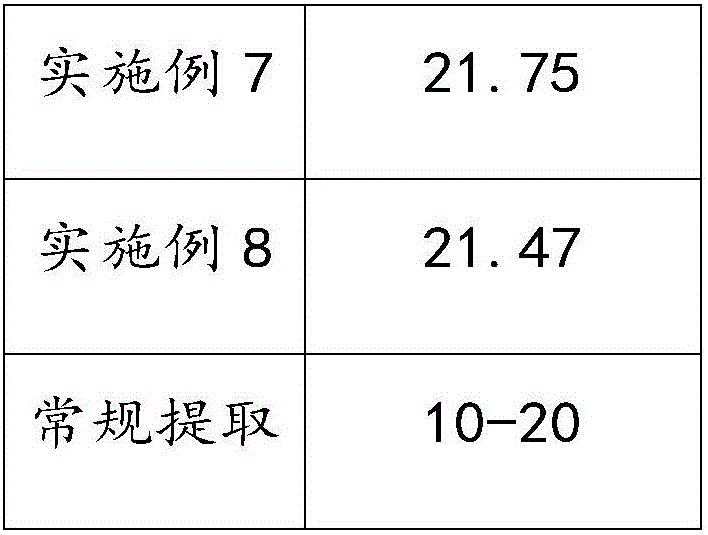
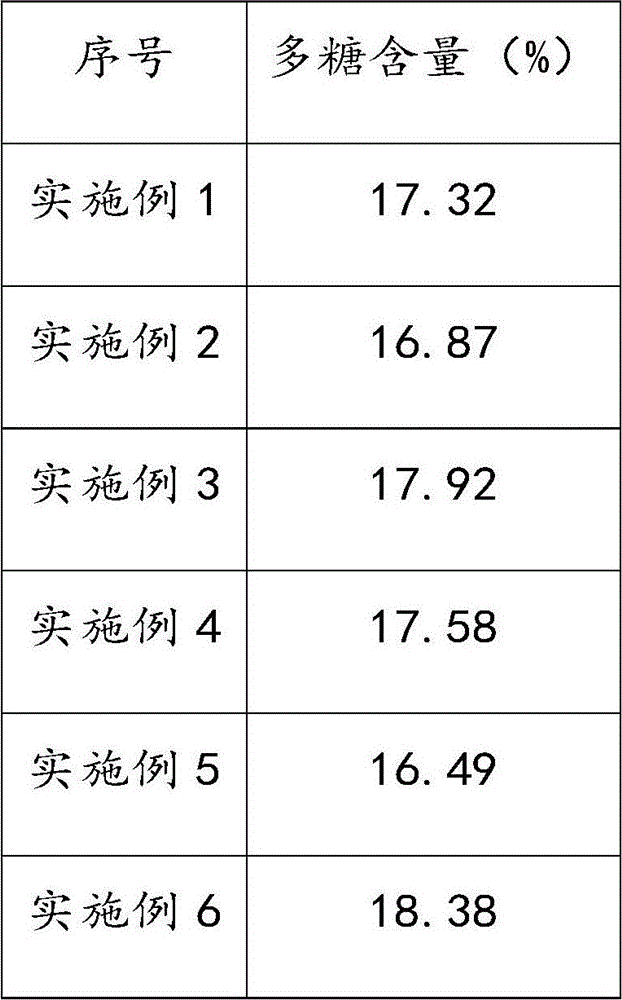
Method for stirring assisted ultrasonic extraction of Bletilla striata polysaccharides
The invention belongs to the technical field of biopharmaceutics, and concretely relates to a method for stirring assisted ultrasonic extraction of Bletilla striata polysaccharides. The method includes the following steps: 1, washing, slicing and drying fresh Bletilla striata tubers, crushing the Bletilla striata tubers by a crusher to obtain Bletilla striata powder, and performing sealing storageon the Bletilla striata powder for later use; 2, taking 1 part by weight of the Bletilla striata powder prepared in step 1, adding the Bletilla striata powder into a stirrer, adding 140-160 parts byweight of water into the stirrer, and simultaneously carrying out ultrasonic treatment and stirring for 35-45 min to obtain a mixed feed liquid, wherein the ultrasonic frequency is 53 kHz, the ultrasonic power is 2500-3000 W / m<2>, and the rotating speed of the stirrer is 250-350 r / min; and 3, centrifuging the mixed liquid obtained in step 2 to obtain an extract, and carrying out alcohol precipitation on the extract obtained by separation in order to obtain the polysaccharides. The method for stirring assisted ultrasonic intermittent extraction of Bletilla striata polysaccharides has the advantages of simplicity in extraction, fastness, high efficiency and low energy consumption.
Owner:QUJING NORMAL UNIV
Biopharma application of micell technology
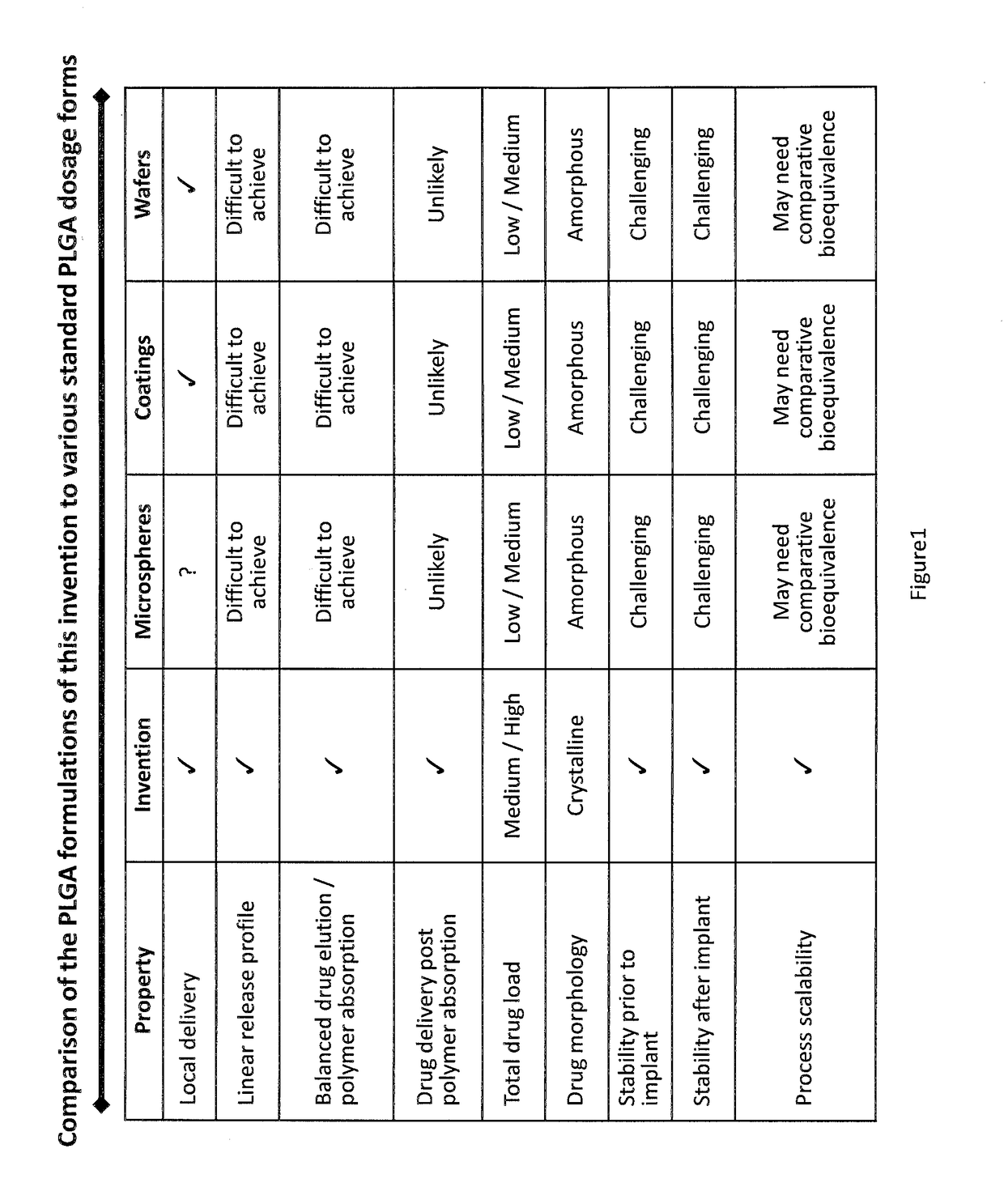
Drug delivery systems are disclosed which include a drug in dry powder form and a biodegradable or metabolizable carrier having an average particle size of less than about 1 mm for delivery of the drug to a particular location in the body and for providing for the timed elution of the drug at that location, preferably by exhibiting a linear drug elution profile for a sustained drug release period of at least 30 days. Methods for manufacturing these drug delivery systems are also disclosed.
Owner:MICELL TECH INC
Freeze-dried vaccine against turkey herpesvirus type iii
Owner:PU LIKE BIO ENG
Divided fistula powders
InactiveCN101199781AInhibit swellingReduce exudationAnthropod material medical ingredientsMammal material medical ingredientsDiseaseBiopharmaceutics
The invention discloses a special drug for specially treating anus diseases, especially the big problem- anal fistula. The drug is called <FISTULA REMOVING POWDER> and is processed by the modern biopharmaceutics process according to the hereditary secret formula. The invention avoids the pain in conventional operations and has evident cure effect.
Owner:关瑞峰
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com