Tumor targeting polypeptide-medicine coupling derivative, and preparation method and application thereof
A tumor-targeting and targeting peptide technology, which can be used in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., to solve problems such as limitations, reduced efficacy, and weak penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Preparation method of tumor targeting, long-acting bifunctional polypeptides ABD-RGDK and ABD-RPARPAR
[0077] (1) Construct the amino acid sequence:
[0078] ABD-RGDK: CGSLAEAKVLANRELDKYGVSDFYKRLINKAKTVEGVEALKLHILAALPGGGSGGGSGGGSSRGDK
[0079] ABD-RPARPAR: CGSLAEAKVLANRELDKYGVSDFYKRLINKAKTVEGVEALKLHILAALPGGGSGGGSGGGSSRPARPAR
[0080] (2) Nucleotide sequence:
[0081] ABD-RGDK: GGTTGCGGTTCTCTGGCTGAAGCTAAAGTCCTGGCAAATCGTGAGCTGGACAAATATGGTGTGTCCGATTTCTACAAACGCCTGATCAACAAAGCGAAGACCGTTGAAGGTGTAGAAGCACTGAAACTGCACATTCTGGCCGCGCTGCCGGGTGGCGGTTCCGGTGGCGCAGCGGCGGCGGTAGCTCTCGTGGCGACAAA
[0082]ABD-RPARPAR: GGTTGCGGTTCTCTGGCTGAAGCTAAAGTCCTGGCAAATCGCGAGCTGGACAAGTATGGCGTGTCCGATTTCTACAAACGTCTGATCAACAAAGCGAAAACCGTAGAAGGTGTTGAAGCGCTGAAACTGCACATTCTGGCCGCGCTGCCTGGCGGTGGCTCCGTCGGCGTAGCGGTGGCGGTTCTAGCCGTCACCGGCACG;
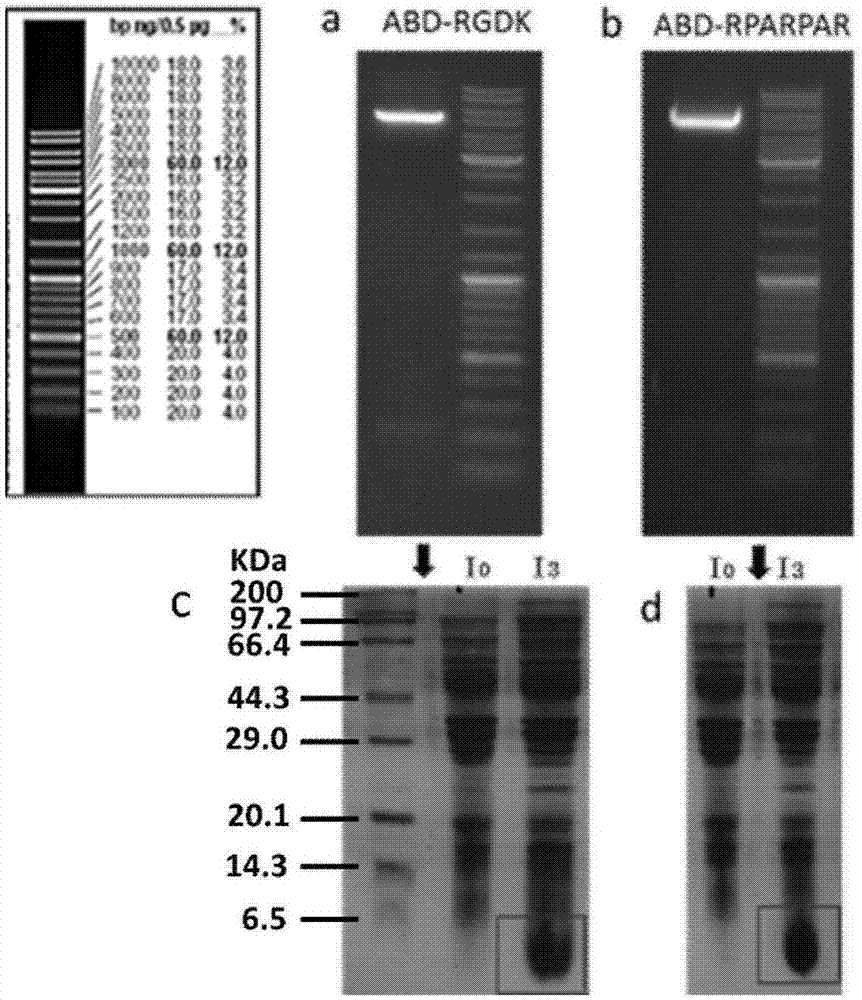
[0083] The electrophoresis of the fusion peptide is shown in figure 1 (a)-(b);
[0084] (3) The plasmid type is selected as pET-30a, and the host bacteria is E....
Embodiment 2
[0087] Example 2: Preparation method of tumor targeting, long-acting bifunctional polypeptide-doxorubicin derivative
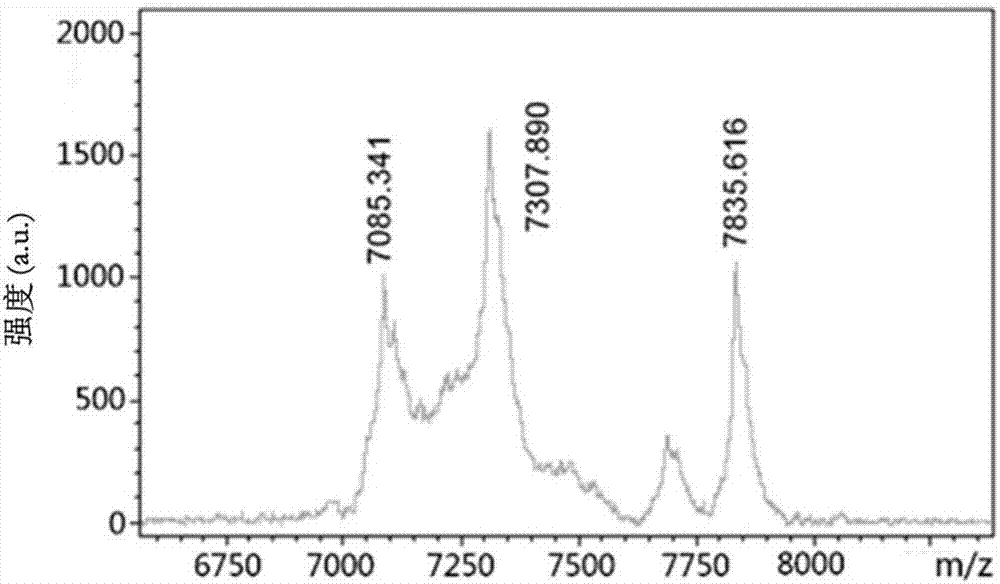
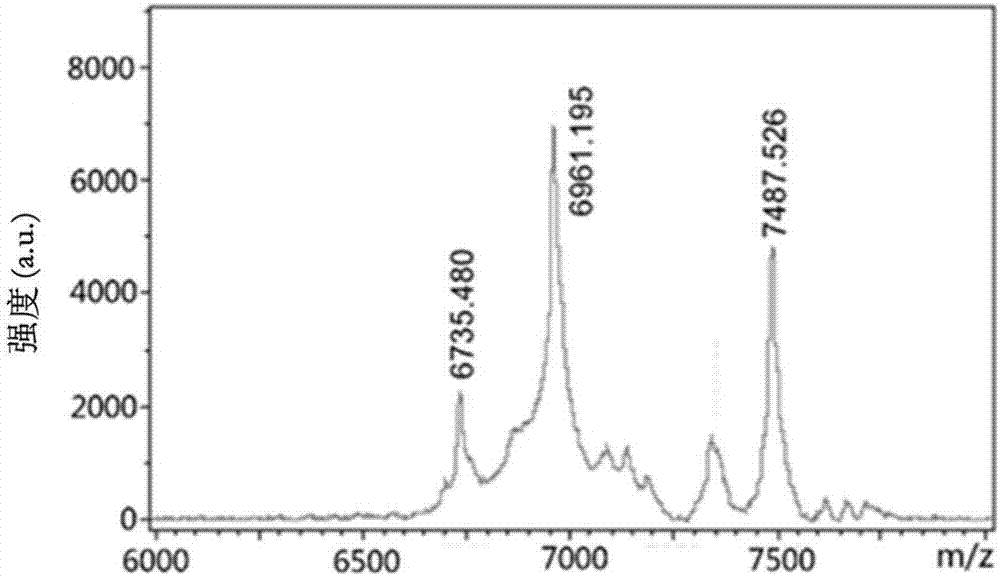
[0088] (1): Synthesis of ABD-RGDK-DOXO and ABD-RPARPAR-DOXO:
[0089] Synthesis of ABD-RGDK-DOXO: Weigh 60 mg of lyophilized powder of ABD-RGDK peptide, dissolve in 6 mL of 100 mM Na at pH 8.5-9.5 2 CO 3 -NaHCO 3 Add 320μL 50mM TCEP mother solution to the solution, reduce at room temperature for 20min, then weigh 18.57mg of DOXO-EMCH solid, fully dissolve in 4mL DMF, then add dropwise to the peptide solution at a speed of 30μL / min, stir gently, and Protect from light for 2-4h. Finally, centrifuge at 10,000 rpm at 4°C for 5 min to remove unreacted precipitates to obtain the crude product of ABD-RGDK-DOXO.
[0090] Synthesis of ABD-RPARPAR-DOXO: Weigh 60mg of lyophilized powder of ABD-RGDK peptide, dissolve in 6mL of 100Mm Na with pH8.5-9.5 2 CO 3 -NaHCO 3 Add 280μL 50mM TCEP mother solution to the solution, reduce it at room temperature for 20min, then w...
Embodiment 3
[0102] Example 3: Uptake of drugs by tumor cells
[0103] A549 cells in good growth state were selected, digested with trypsin, and plated in a 96-well plate with a plate density of 4×10 4 cells / hole. Cells were incubated at 37°C, 5% CO 2 Incubate in the incubator for 24 hours to allow the cells to adhere to the wall, then remove the medium and replace it with 100 μL drug-containing medium, the drugs are DOX, ABD-RGDK-DOXO, ABD-RPARPAR-DOXO, ABD-RGDK-DOXO+RGDK peptide , ABD-RPARPAR-DOXO+RPARPAR peptide, the administration concentration is 20uMDOXeqv and 500μM peptide, and three replicate wells are set up for each group of drugs. After the drug was applied for 1, 2, and 8 hours, the drug was removed, the wells were washed three times with PBS, and then 100 μL PBS was added to measure the absorption value of each well with a fluorescent microplate reader. The excitation wavelength was 480 nm, and the emission wavelength was 580 nm. Plot the cellular uptake fluorescence intens...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com