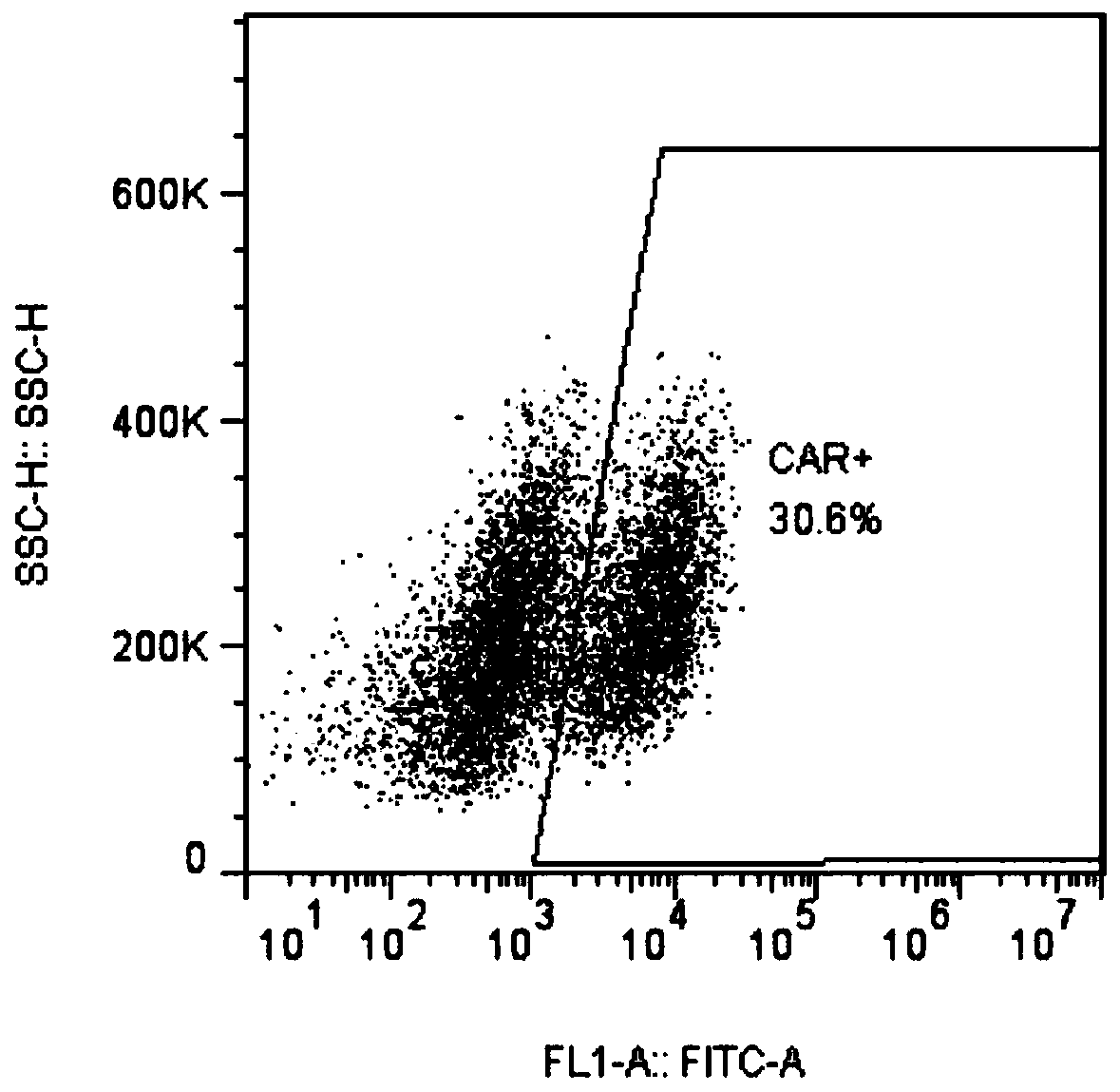
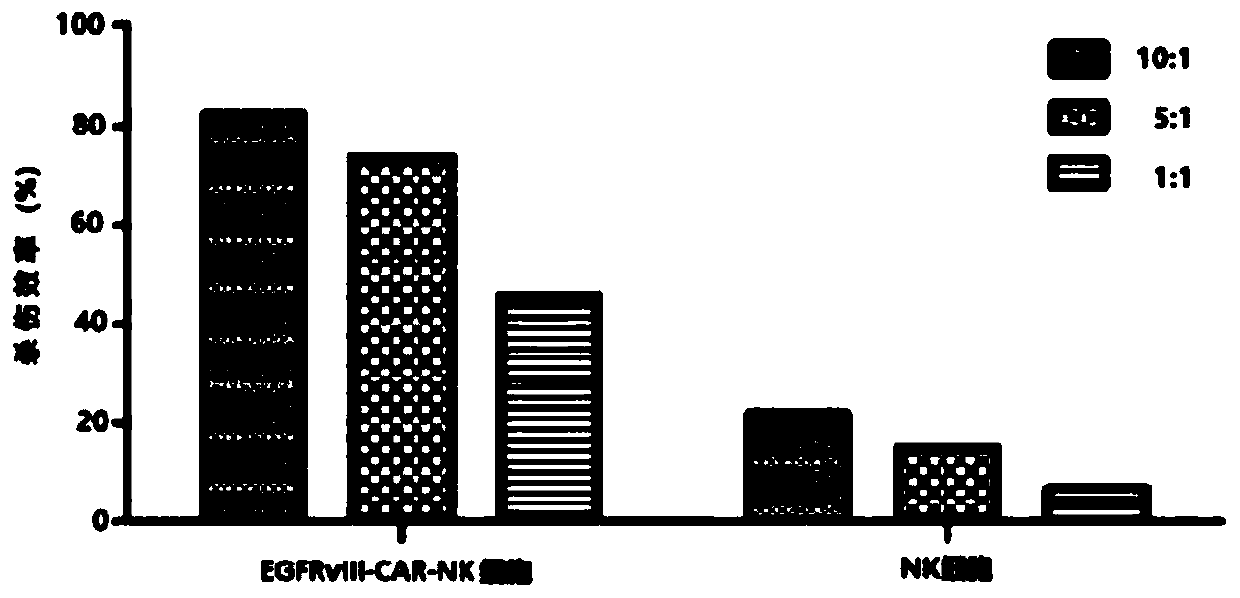
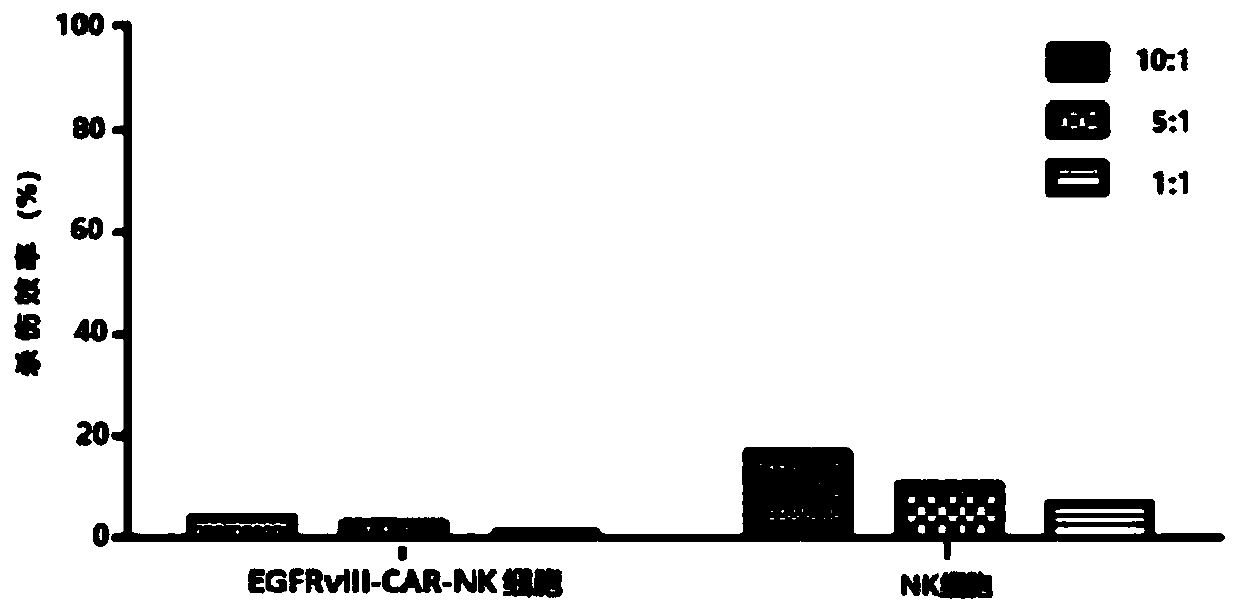
CAR-NK cell, and preparation method and application thereof
A kind of NK cell and cell technology, applied in the direction of biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve the problem that the anti-tumor function cannot be fully exerted, and achieve the effect of a healthy fetus with a smooth labor process and no history of infectious diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Cord Blood Mononuclear Cell Extraction
[0046] (1) Take 50 mL of cord blood, divide it into two 50 mL centrifuge tubes, centrifuge at 650 g for 15 min at room temperature, take the upper layer of light yellow plasma into a new 50 mL centrifuge tube (the lower layer of red liquid is used to extract mononuclear cells), The plasma was inactivated in a water bath at 56°C for 30min, then centrifuged at 900g for 10min, the supernatant was taken, and placed in an environment of -20°C for 15min; centrifuged again at 900g, 10min, the supernatant was taken and stored at 4°C until use. (The centrifuge adjusts the speed up by 1 and the speed down by 1).
[0047] (2) Take the lower red liquid obtained in the previous step of plasma extraction and dilute it with an equal volume of normal saline to a total volume of about 20 mL, mix it upside down, and set aside. Take another 2 new 50mL centrifuge tubes, and add 20mL of lymphocyte separation solution to each tube. Add 20m...
Embodiment 2
[0049] Example 2 Induction and activation of umbilical cord blood NK cells
[0050] The mononuclear cells obtained in Example 1 were divided into 2 × 10 6 Individuals / mL inoculated with 1-10% cord blood autologous plasma, 200-1500 IU / mL IL-2, 10-50 ng / mL IL-15, 10-30 ng / mL IL-7 and 50-100 ng / mL IL-21 lymphocyte culture medium, placed at 37 °C, 5% CO 2 Cultured in an incubator, and on the 3rd day according to the cell density of 0.8~1.0×10 6 pc / mL rehydration solution.
Embodiment 3
[0051] Example 3 Detection of NK cell surface markers derived from peripheral blood and umbilical cord blood
[0052] Take 50 mL of peripheral blood, extract mononuclear cells according to the method in Example 1, and carry out activation culture according to the method in Example 2.
[0053]Take the cells in Example 2 and the activated cultured NK cells derived from peripheral blood, wash them twice with PBS, then add mouse IgG, and keep away from light for 30 minutes at 4°C; add specific antibodies CD16, CD161, NKG2A, NKG2D, NKp46, respectively. Incubate at ℃ for 30 min in the dark. The stained cells were washed twice, and the above markers were detected by flow cytometry. Statistical methods using SPSS10 statistical software. All data are expressed as x±s, and t test and Mann-Whitney test were used. The results are shown in Table 1. There was no significant difference in phenotype between the two sources of NK cells.
[0054] Table 1 Expression of NK cell surface marker...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com