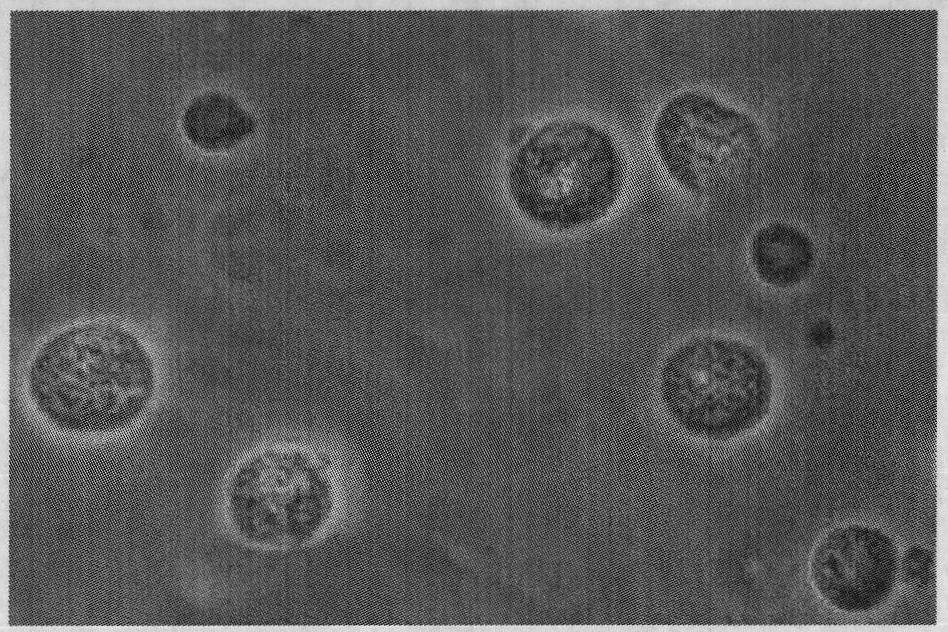
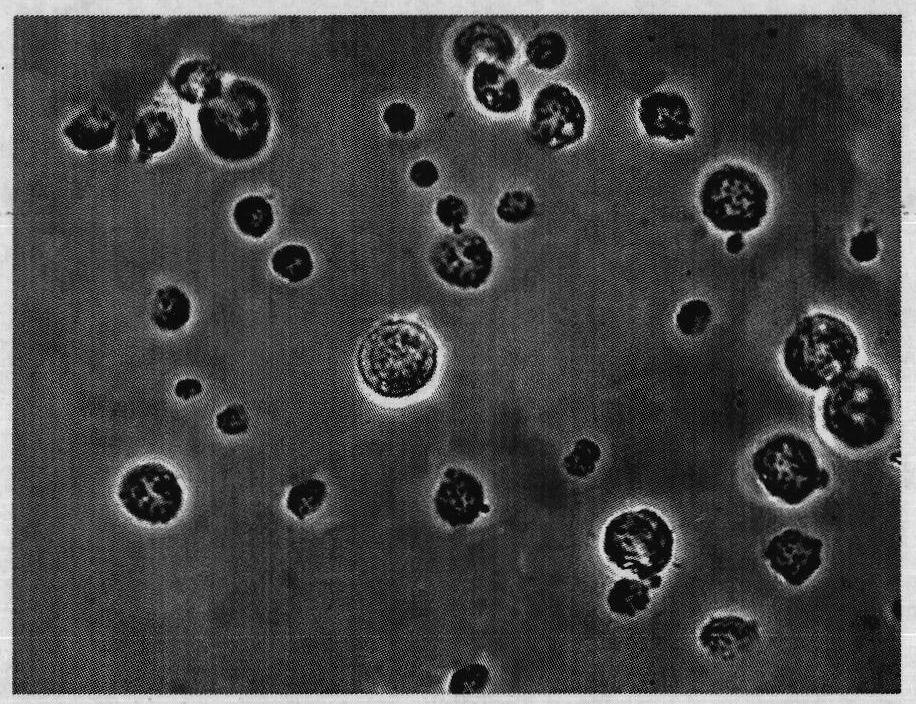
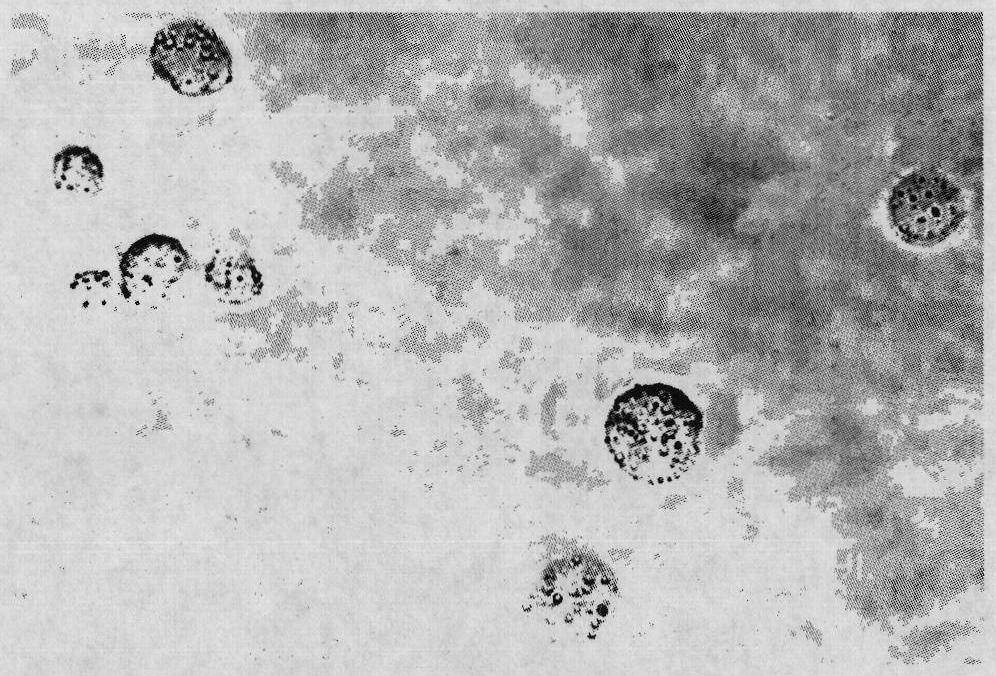
Aeromonas hydrophila micro-capsular oral vaccine
A technology of Aeromonas hydrophila and oral vaccine, which is applied in the field of biopharmaceuticals, can solve problems such as poor immune effect, and achieve the effects of safe reagents, convenient operation, convenient storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] 1.2 Preparation of inactivated vaccine
[0042] Ah J for rejuvenating infected crucian carp 1 Inoculate in a 500mL flask containing 250mL LB broth, and incubate at 28°C with shaking at 150rpm for 24h. After staining and microscopic examination, formalin with a final concentration of 0.4% was added and reacted at 28°C for 3h, then at 4°C for 24h. Then take a small amount of inactivated bacterial liquid to inoculate LB broth, and culture at 28°C with shaking for 24 hours to check whether the inactivation is complete. After the completely inactivated bacterial solution was centrifuged at 8000rpm for 10min, the bacteria were washed three times with PBS solution of pH 7.2. Adjust the final concentration of bacteria to 1.0 × 10 10 cfu / mL, stored at 4°C for later use.
[0043] 1.3 Preparation of Aeromonas hydrophila sodium alginate-chitosan microcapsules (Ah-ACMS) oral vaccine
[0044] According to the orthogonal design scheme in Table 1, 9 levels were set up to carry out...
Embodiment 3A
[0079] The immunoprotective effect of embodiment 3Ah-ACMS oral vaccine
[0080] The gibel crucian carp of 120-150 g is used as a model animal and raised in a 300L glass tank, and the gibel carp is fed with aerated tap water, and the oxygenation pump is used to continuously aerate the gibel carp. Regularly feed commercial pellet feed, change water and clean up solid metabolites. The crucian carp was acclimatized for a week, and after eating normally, the experiment began. The immune effect of the Ah-ACMS oral vaccine of the present invention on crucian carp was verified according to the following scheme.
[0081] 3.1 Immunization procedure
[0082] The test fish were divided into six groups, 40 in each group, fasted for 24 hours before immunization, and immunized according to the scheme in Table 3. The oral control group was fed feed granules containing vaccine at 8:00, 12:00, and 16:00 according to 1% of the fish body weight each time; the Ah oral and injection control grou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com