Application of saikosaponin B2 in preparation of antidepressant drug
A saikosaponin and antidepressant technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
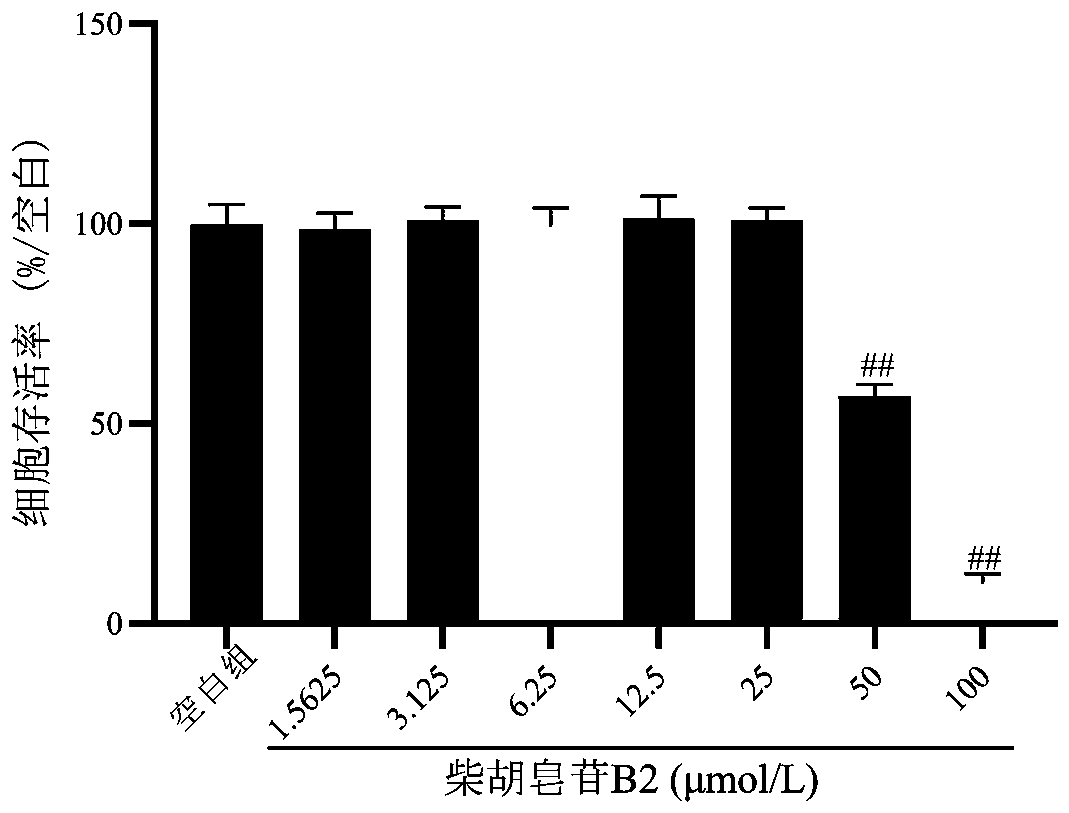
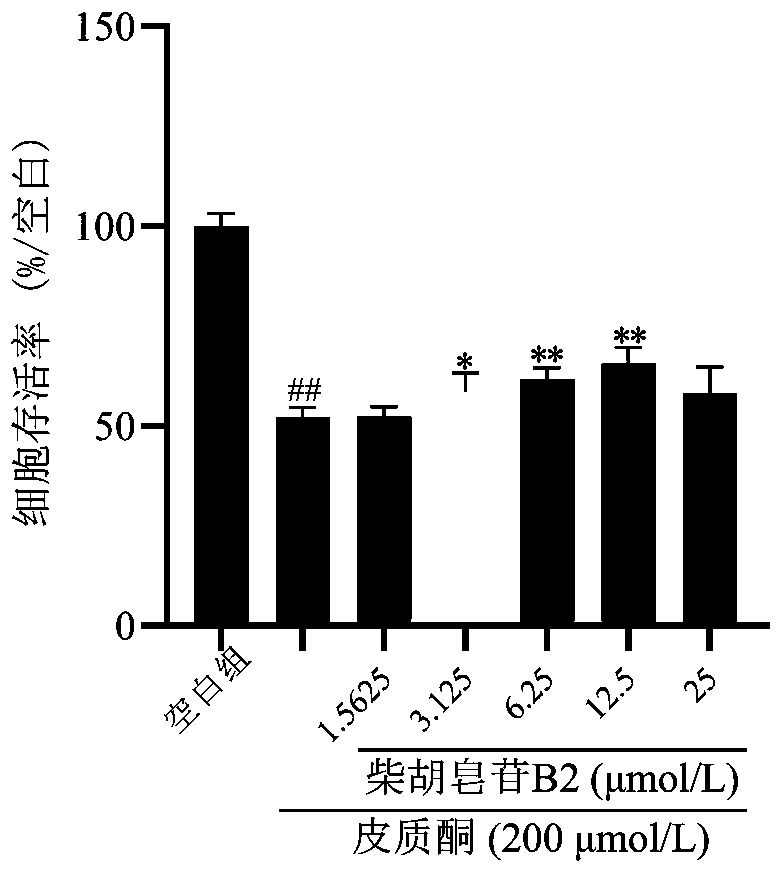
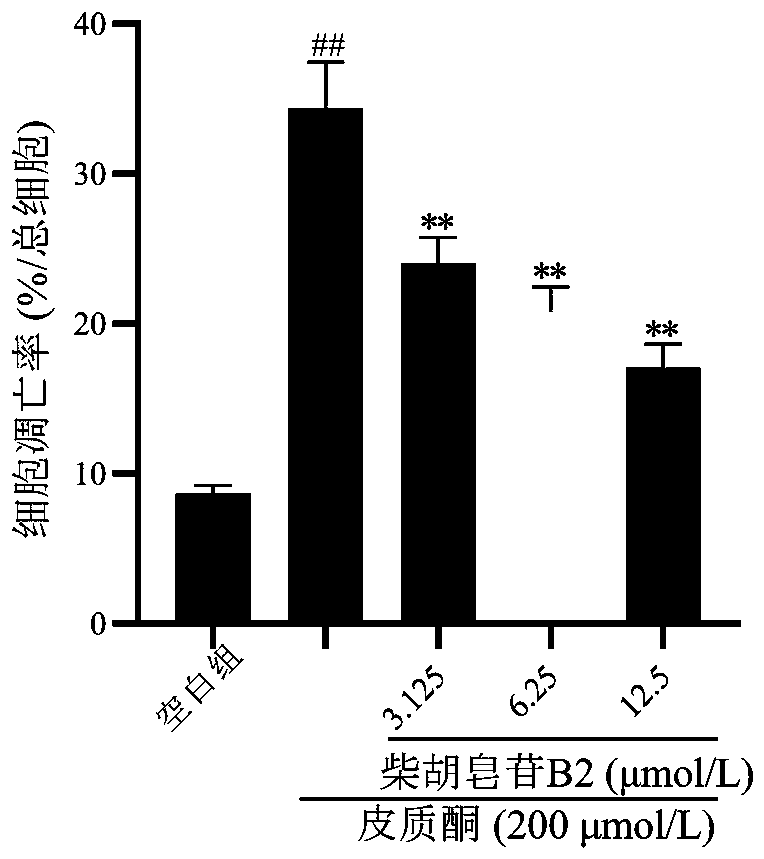
[0019] Example 1: In vitro antidepressant activity test of saikosaponin B2
[0020] In this part of the experiment, PC12 cells were injured by corticosterone to simulate nerve cell damage caused by hyperactivity of the HPA axis in depression, and the protective effect of saikosaponin B2 on the cell damage model was investigated, and then its antidepressant activity was evaluated.
[0021] 1. Experimental materials: poorly differentiated PC12 cells (purchased from the Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences). Saikosaponin B2 glycoside reference substance was purchased from Chengdu Pufeide Biotechnology Co., Ltd. (pure≧98%); RPMI-1640 medium (HyClone company); fetal bovine serum (Gibco company); MTT, poly-L-lysine Propidium iodide (PLL), DMSO (Sigma Company); Annexin V-FITC / propidium iodide (PI) apoptosis kit (BD Company); corticosterone (SIGMA Company); LDH detection kit (Nanjing Jiancheng Company).
[0022] 2. Test method...
Embodiment 2
[0030] Example 2: In vivo antidepressant efficacy test of saikosaponin B2
[0031] This part of the experiment evaluates the antidepressant effect of saikosaponin B2 in vivo through the classic mouse behavioral despair model (tail suspension model, forced swimming model).
[0032] 1. Experimental materials and grouping: male ICR mice, weighing 18-22 g, provided by Beijing Weitong Lihua Animal Experiment Center. Each group of 10 animals / cage is raised at room temperature (20 ± 2) ℃, humidity 60%, receives 12 h light / 12 h dark every day, and the photoperiod is 6:00-18:00, free access to standard feed and clean drinking water, animals The experiments complied with the international ethical requirements for animal experiments.
[0033] The normal mice were randomly divided into 5 groups according to body weight, 12 in each group, namely blank control group, positive drug fluoxetine (10 mg / kg), saikosaponin B2 high (40 mg / kg), medium (20 mg / kg) kg), low (10 mg / kg) dose group. Ea...
Embodiment 3
[0047] Example 3: Preparation of saikosaponin B2 soft capsules: Take 10 g of saikosaponin B2 and 190 g of vegetable oil, stir, mix well, and use as the contents for later use. Take appropriate amount of glycerin, gelatin and water (1:3:3 ratio), add appropriate amount of water to the gelatin to make it fully absorb water and swell, mix glycerin and the rest of the water, put it into the plastic barrel and heat to 70~80 ℃ , add an appropriate amount of expanded gelatin and propyl p-hydroxybenzoate, stir, and use it as a capsule material for later use. The contents and capsule materials are pressed into soft capsules on a soft capsule machine, dried and shaped to obtain saikosaponin B2 soft capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com