Aquilarotetrol as well as pharmaceutical composition, preparation method and application thereof
A kind of white wood fragrance and composition technology, which is applied in the field of medicine, can solve the problems of no new compound white woody tetraol, no PES-22 neurodegenerative disease and depression, and achieve significant protective activity and good neuroprotective activity , high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation process of compound Pyramidol (PES-22):
[0033] Dried Chinese medicine Aquilaria sinensis, that is, Aquilaria sinensis (Lour.) Spreng. Aquilaria sinensis (Lour.) Spreng. The heartwood (2.9 kg) containing resin was pulverized and ultrasonically extracted with 10 L of 90% ethanol for 5 times, each time for 30 min. The solvent was distilled off from the extract under reduced pressure to obtain an extract (459.3 g). Then suspend the extract in 1L of water, extract with equal volumes of petroleum ether (5×1L), ethyl acetate (5×1L) and n-butanol (5×1L) successively, and recover the solvent to obtain 0.7 g of petroleum ether, ethyl acetate The ester fraction was 374.8 g, the n-butanol fraction was 55.9 g, and the water fraction (raffinate fraction) was 6.9 g.
[0034] Take the n-butanol fraction (55.9g) and mix the sample with 80-100 mesh silica gel, pack it into a column, and carry out gradient elution with ethyl acetate-methanol (100:0→0:1, v / v) as the elue...
Embodiment 2
[0036] Spectral data and structure identification of compound PES-22:
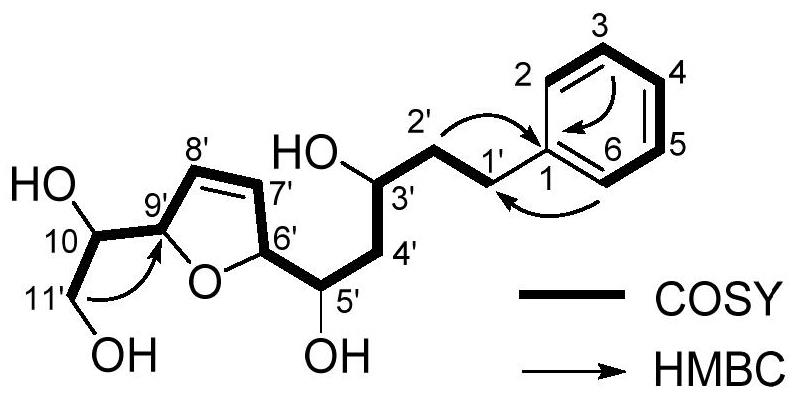
[0037] Aquilarotetrol (PES-22), colorless solid; [α] D 25 -98.1(c 0.13, MeOH); UV(MeOH)λ max (logε)250(3.20)nm; ECD(c 0.0097, MeOH)λ max (Δε)205(-19.88)nm; 1 H NMR and 13 CNMR data in Table 1; ESIMS m / z 331[M+Na] + ;HRESIMS m / z 331.1514[M+Na] + (calcd forC 17 h 24 NaO 5 , 331.1521).
[0038] According to compound PES-22 high resolution mass spectrometry (HRESIMS) and 13 C NMR data (Table 1), it is speculated that its molecular formula is C 17 h 24 o 5 , with 6 degrees of unsaturation. its 1 The H NMR signal (Table 1) indicates that there is a monosubstituted benzene ring [δ7.23(2H,m), 7.19(2H,m), 7.12(1H,m)] and a disubstituted double bond [δ5.96 (1H,m),5.92(1H,m)]. According to the COZY correlation of the compound ( figure 1 ), two fragments can be obtained, namely C-2 to C-6, and C-1' to C-11'; according to H-2 and H-6 and C-1', and H-3, H-5 and H 2 -2' is related to the HMBC of C-1, ...
Embodiment 3
[0042] The neuroprotective activity test method of compound PES-22 is as follows:
[0043] 1. PC12 poorly differentiated cells are cultured in the medium of DMEM+10%FBS+100U / mL double antibody, the temperature of the incubator is 37℃, 5%CO 2 ;
[0044] 2. When PC12 poorly differentiated cells grow to an appropriate number, take PC12 poorly differentiated cells, digest with trypsin, and make cell suspension;
[0045] 3. Pipette the cell suspension into a 15mL centrifuge tube, 800rpm, 5min;
[0046] 4. After the centrifugation is finished, the centrifuge tube is disinfected with alcohol and taken into the ultra-clean bench, and the supernatant is poured into the waste liquid tank;
[0047] 5. Add 5 mL of new complete medium, pipette ten times to blow the cells as much as possible, but not too hard;
[0048] 6. Take 0.02mL of cell suspension, add it to the cell counting plate, and count on the machine;
[0049] 7. Adjust the cell concentration to 1 x 10 5 cells / mL, add to 96...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com