HyponicosidesA-D(1-4) as well as pharmaceutical composition thereof and application thereof
A technology of dimethylpyroglucinol and its derivatives, which is applied in the field of medicine, and can solve the problems of no dimethylpyroglucinol derivatives, no PC12 cell protection, and no reports of compound 1-4 pharmaceutical compositions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
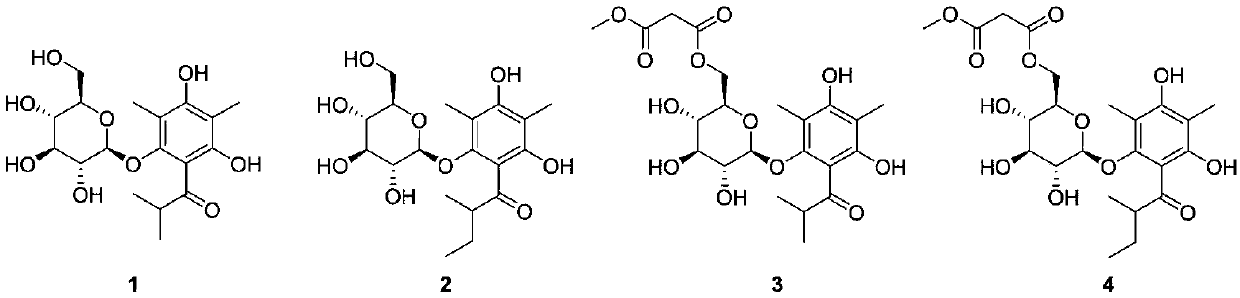
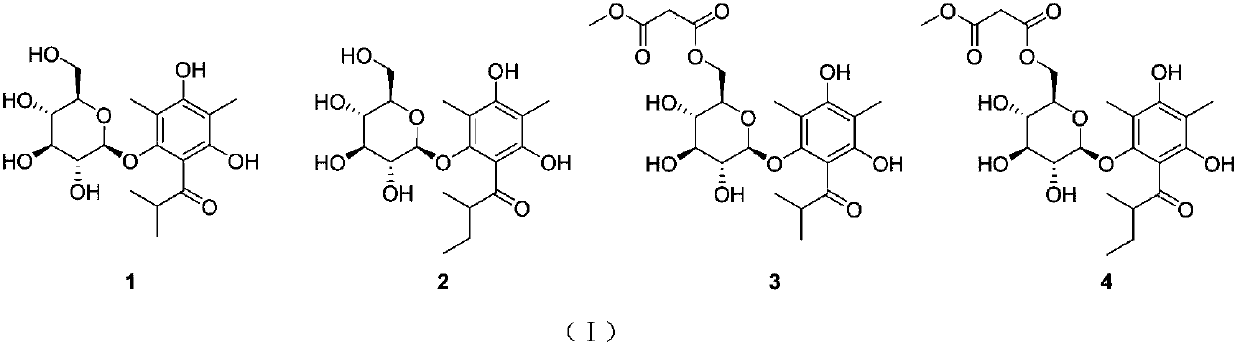
[0030] Preparation of compounds hyponicosides A-D (1-4):
[0031] Take the dried whole herb of Lymphis chinensis, crush it, soak and extract it with 95% ethanol at room temperature for three times, each time for 48 hours, combine the extracts and recover the ethanol under reduced pressure to obtain an extract, which is dissolved and adsorbed on silica gel with 95% ethanol, and left at room temperature Place and evaporate the solvent, grind and sieve, and then go through silica gel column chromatography, and then use 1:0, 10:1, 5:1, 0:1 chloroform-methanol gradient elution, and the amount of each gradient solvent is 1 / 2 of the column volume. 2 times, the 10:1 chloroform-methanol elution part continued to go through silica gel column chromatography, and was combined into four components by TLC detection. The second component was subjected to silica gel column chromatography and wasocratically eluted with 20:1 chloroform-methanol , combined into 8 subcomponents Fr2.1-Fr2.8 after ...
Embodiment 2
[0033] Structural identification of compound hyponicosidesA-D(1-4):
[0034] Compound 1
[0035] Molecular formula: C 18 h 26 o 9
[0036] Molecular weight: 386
[0037] Appearance: white amorphous powder
[0038] HRESIMS(m / z):409.1466[M+Na] + (Calcd for 409.1469)
[0039] IR(KBr)cm -1 : 3428, 2969, 2928, 2879, 1620, 1460, 1426, 1384, 1148, 1075, 907, 805, 617, 571.
[0040] UV (MeOH) λ max (log ε): 219 (5.24), 287 (4.85) nm.
[0041] 1 H-NMR and 13 See Table 1 for C-NMR data.
[0042] (c 0.003, MeOH)
[0043] Compound 2
[0044] Molecular formula: C 18 h 26 o 9
[0045] Molecular weight: 400
[0046] Appearance: white amorphous powder
[0047] HRESIMS(m / z):423.1629[M+Na] + (Calcd for 423.1630)
[0048] IR(KBr)cm -1 : 3420, 2966, 2931, 2877, 1614, 1459, 1427, 1378, 1286, 1238, 1147, 1074, 907, 818, 796, 775, 657, 631, 567, 519.
[0049] UV (MeOH) λ max (log ε): 219 (4.89), 287 (4.12) nm.
[0050] 1 H-NMR and 13 See Table 1 for C-NMR data.
[0051] ...
Embodiment 3
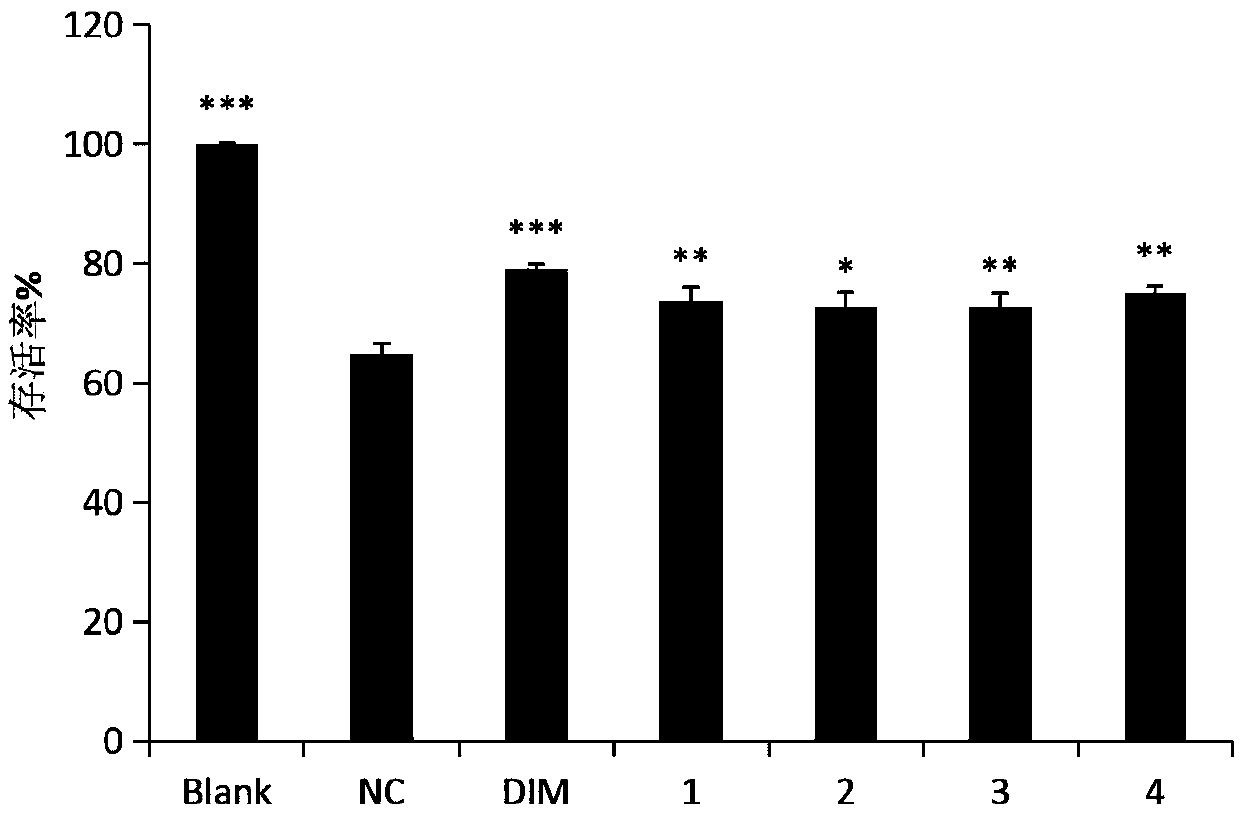
[0074] Protective effects of compounds hyponicosides A-D(1-4) against corticosterone-induced neurological injury.
[0075] In this example, corticosterone (CORT) was purchased from Sigma, high glucose medium (DMEM), fetal bovine serum (FBS), and PBS were purchased from BI, and PC12 poorly differentiated cells were purchased from the Cell Bank of Kunming Institute of Zoology, Chinese Academy of Sciences, Pancreas Protease was purchased from Gibco, USA; phosphate buffer saline (PBS) was purchased from Wuhan Boster Company; [3-(4,5-dimethylpyridin-2-yl)-5-(3-carboxymethoxyphenyl )-2-(4-sulfophenyl)-2H-tetrazole] (MTS) was purchased from Sigma, USA; desipramine (DIM) was purchased from Beijing Putin Kangli Technology Co., Ltd.
[0076] Experimental design: Each group was designed to do 3 repetitions.
[0077] Blank control group (Blank): no CORT, DIM, only cells and DMSO with a final concentration of 0.1%;
[0078] Model group (NC): DMSO with a final concentration of 120 μM CORT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com