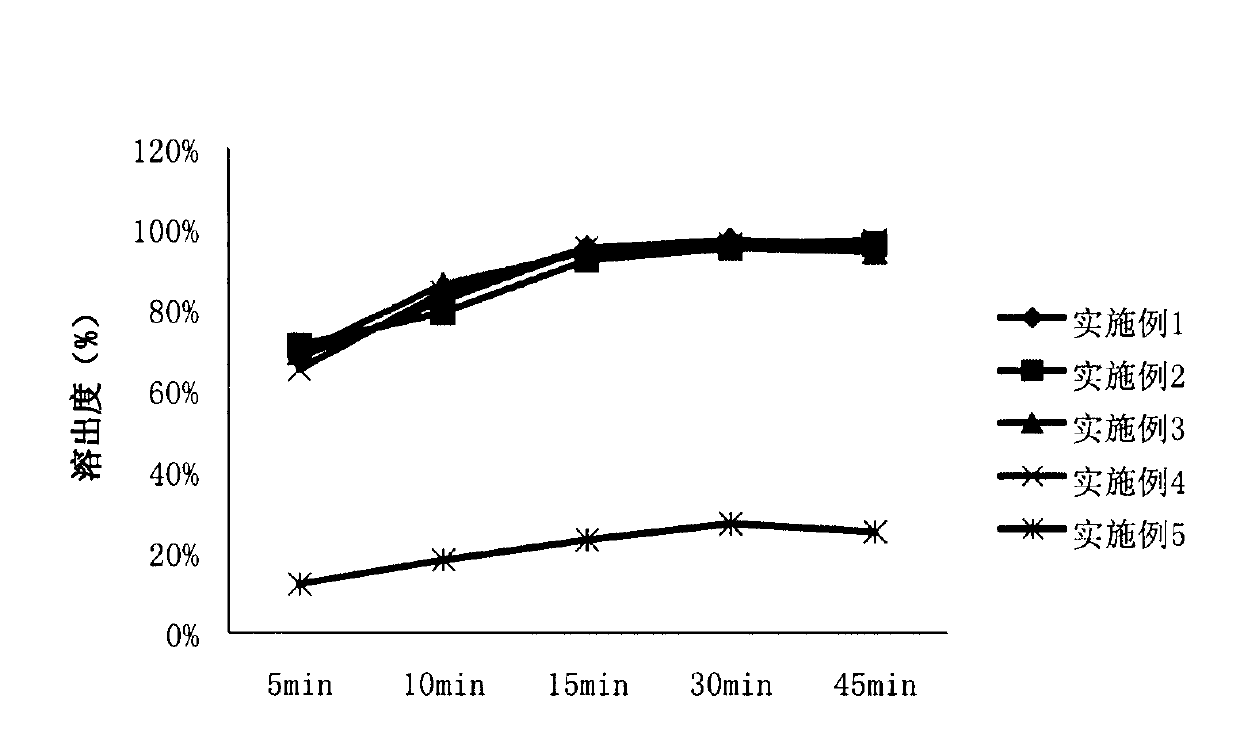
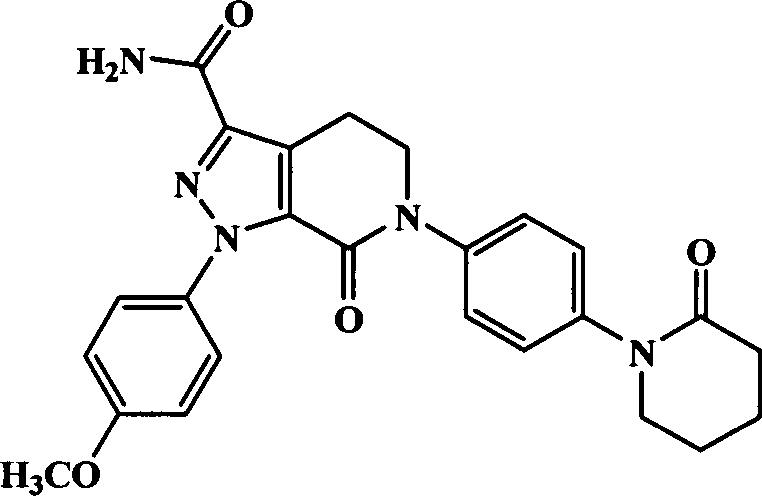
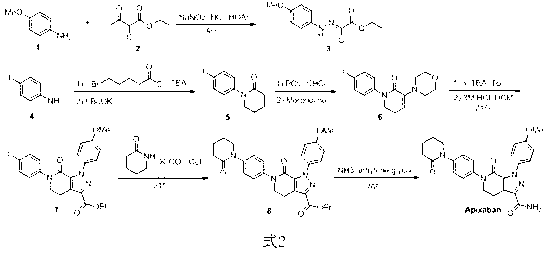
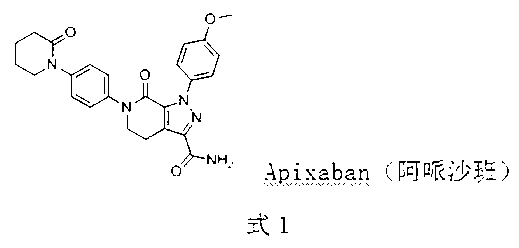
Patents
Literature
107 results about "Apixaban" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
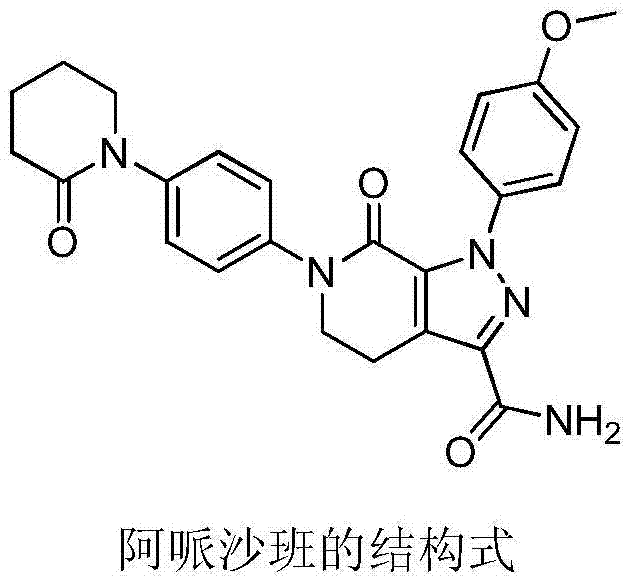
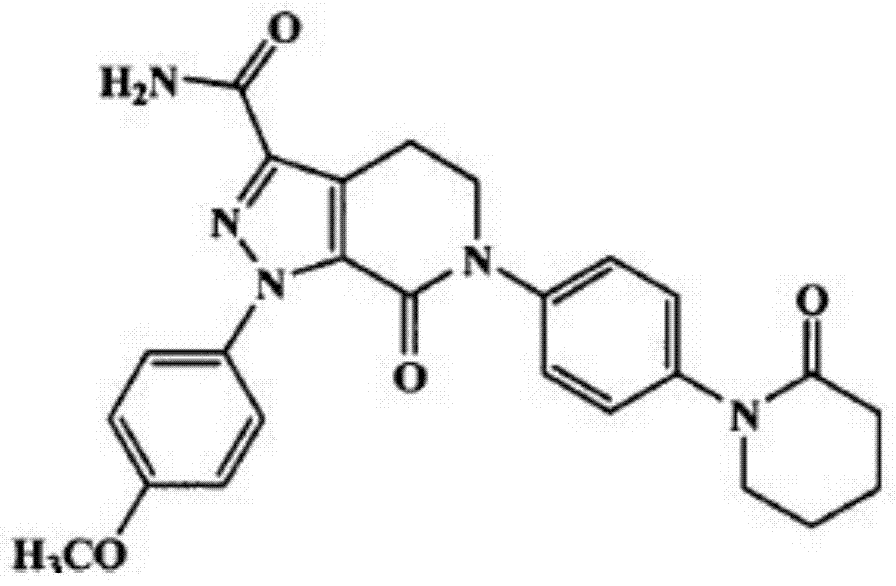
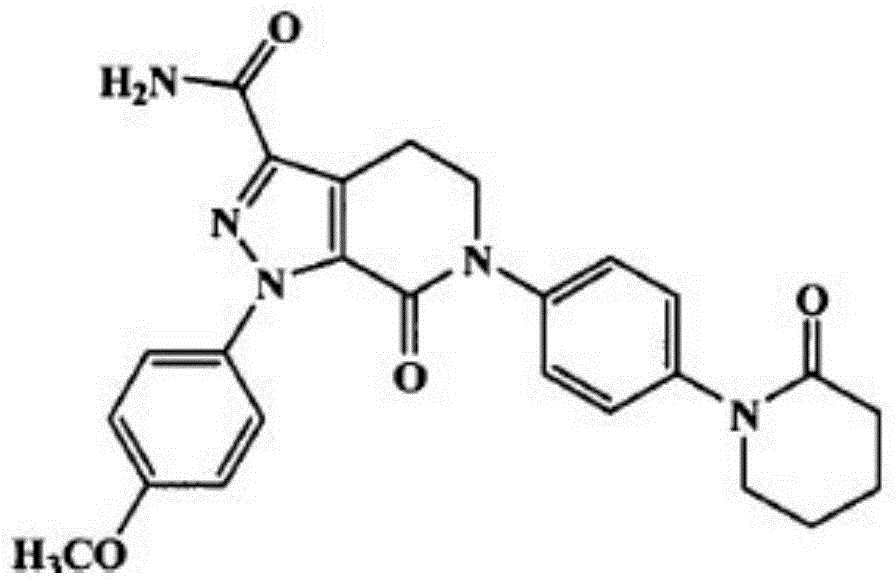
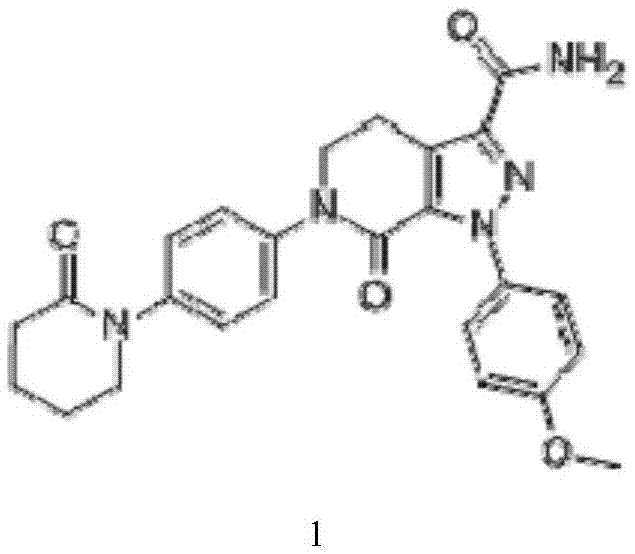
Apixaban is used to prevent serious blood clots from forming due to a certain irregular heartbeat (atrial fibrillation) or after hip/knee replacement surgery.
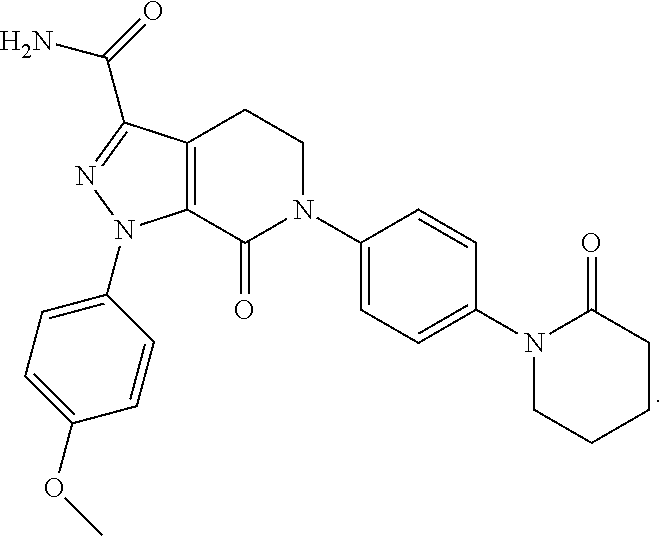
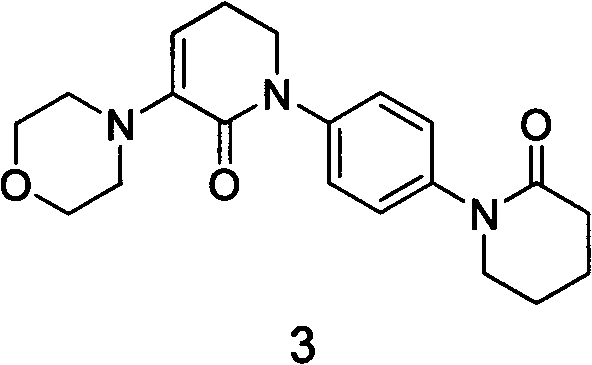
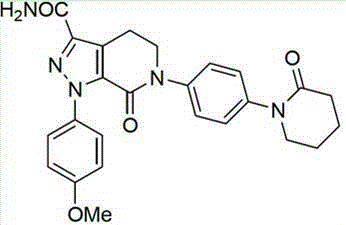
Apixaban tablet
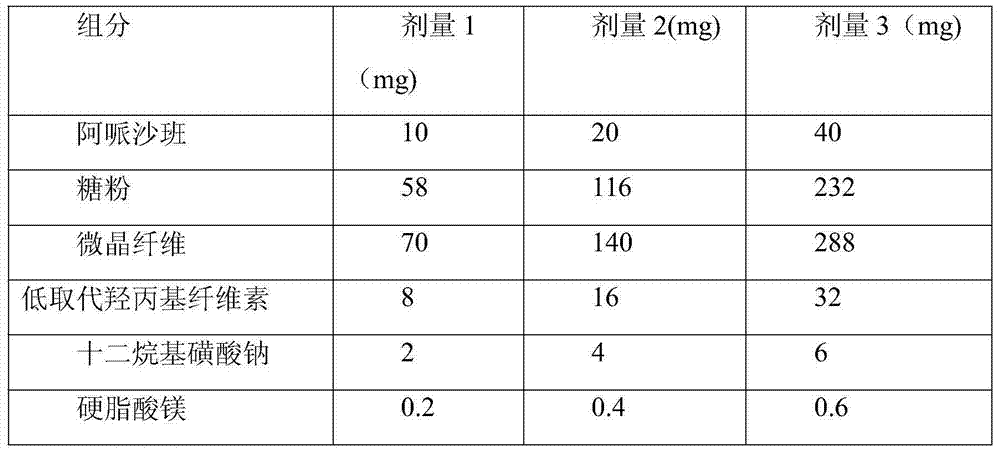
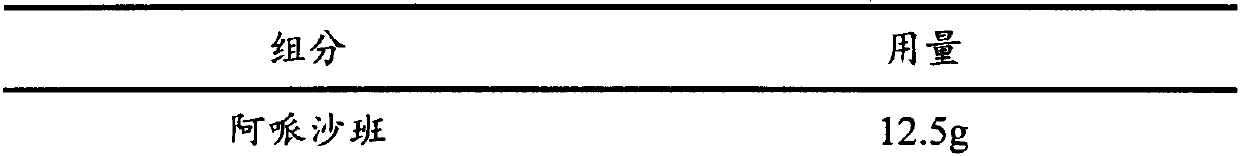
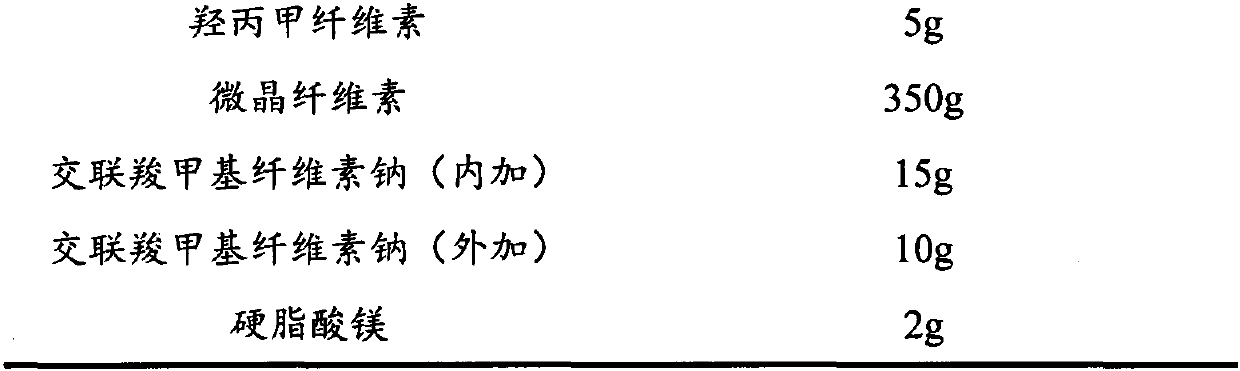
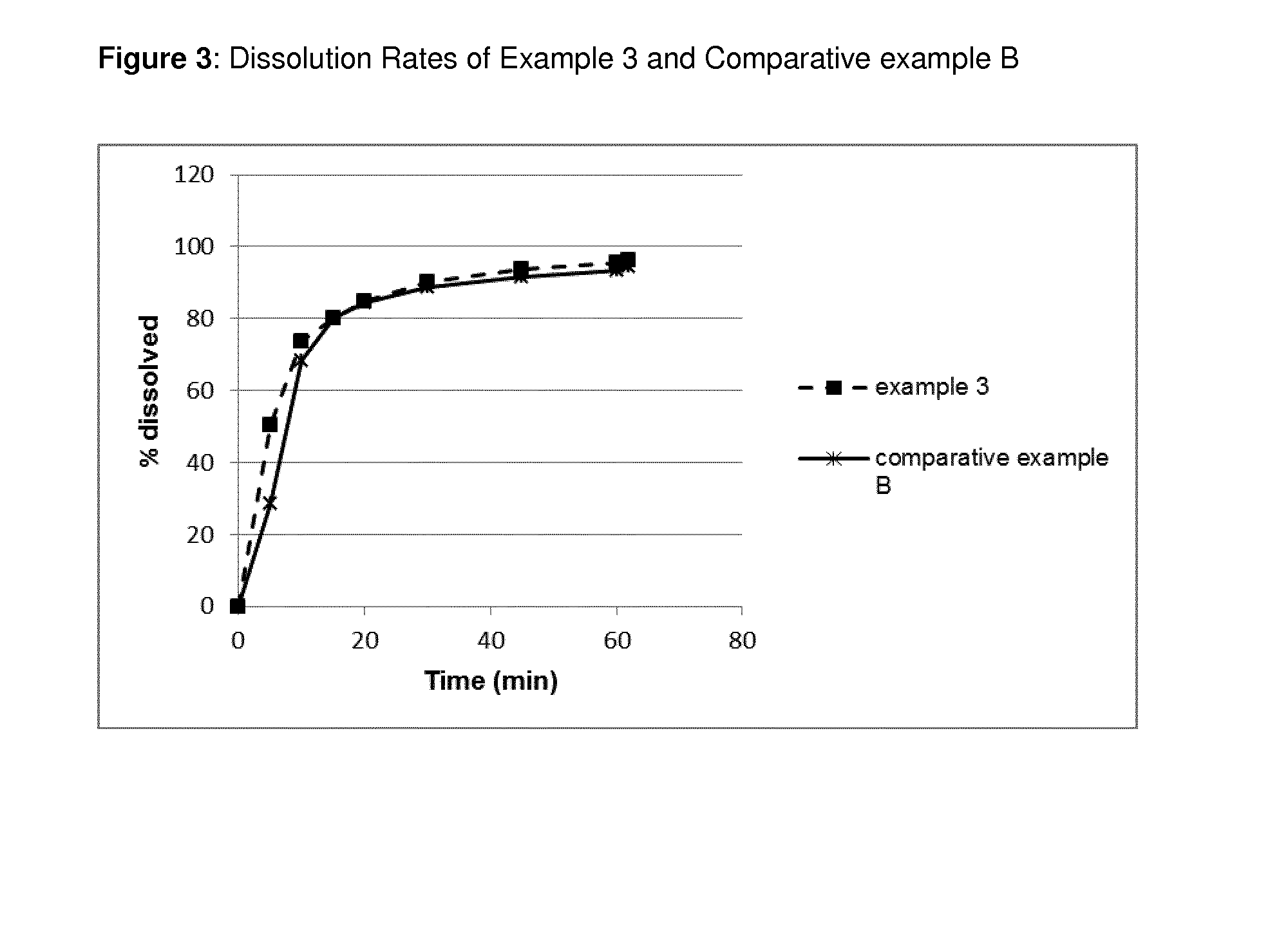
InactiveCN102908324ASmall particle sizeIncrease dissolution rateOrganic active ingredientsPill deliveryMedicineDissolution
The invention discloses an Apixaban tablet, which comprises the following components in parts: 1 part of Apixaban, 10-20 parts of water-soluble carrier material, 30-60 parts of filling agent, 5-10 parts of disintegrating agent and 5-10 parts of glidant. The Apixaban tablet provided by the invention improves the deficiencies in the prior art that the extracorporeal dissolution rate and the bioavailability is low. The preparation method of the Apixaban tablet disclosed by the invention is simple to operate, has good reproducibility, and is suitable for large-scale production. The Apixaban tablet is mainly used for the anti-thrombosis purpose.
Owner:NANJING ZENKOM PHARMA
Preparation method of Apixaban as anti-thrombotic drug
ActiveCN103342704AEasy to synthesizeMethod steps are shortOrganic chemistryBulk chemical productionAntithrombotic AgentAniline
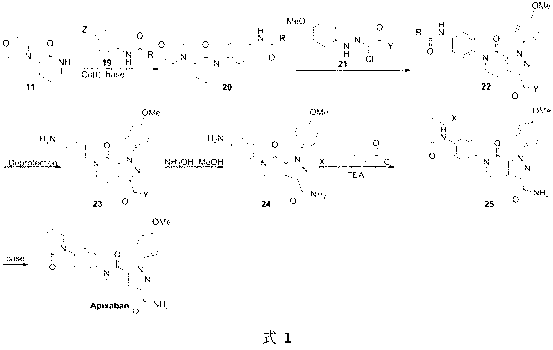
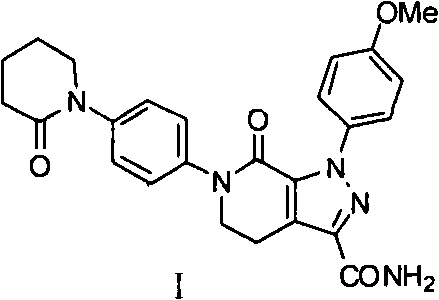
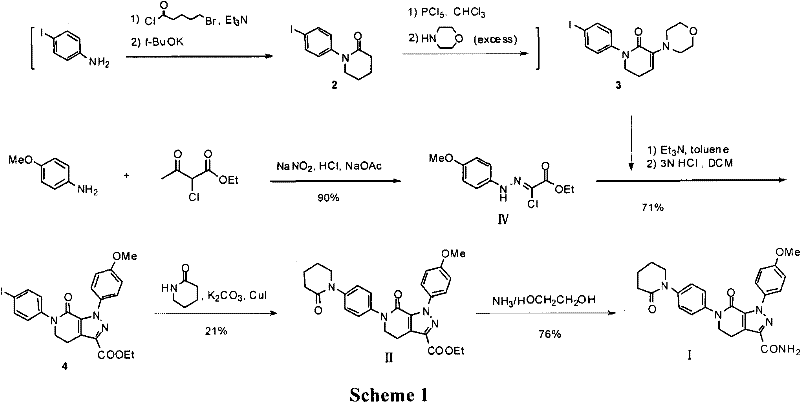
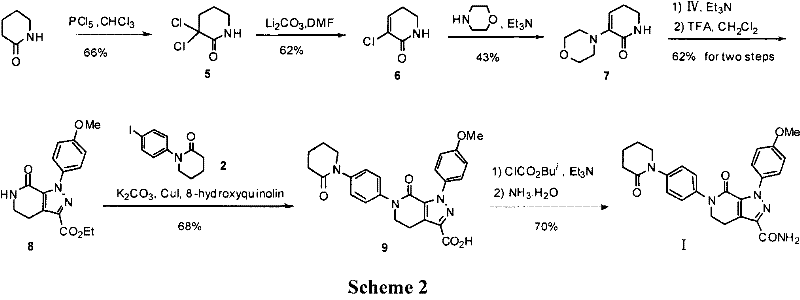
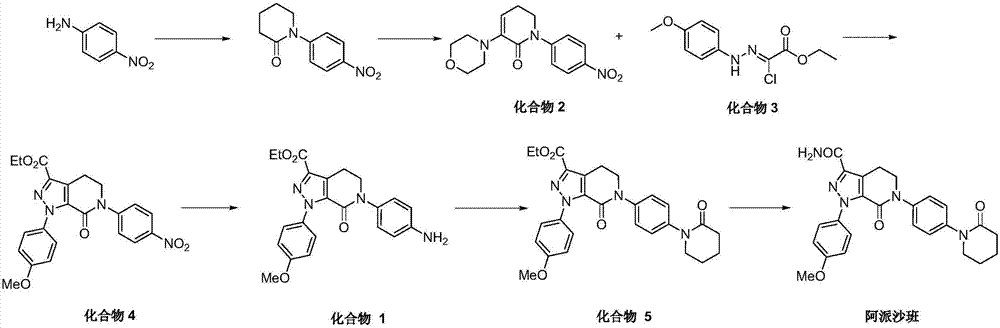
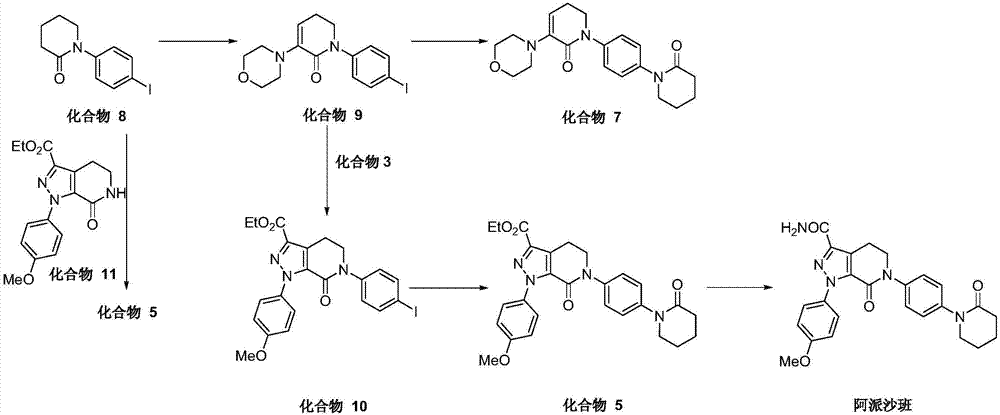
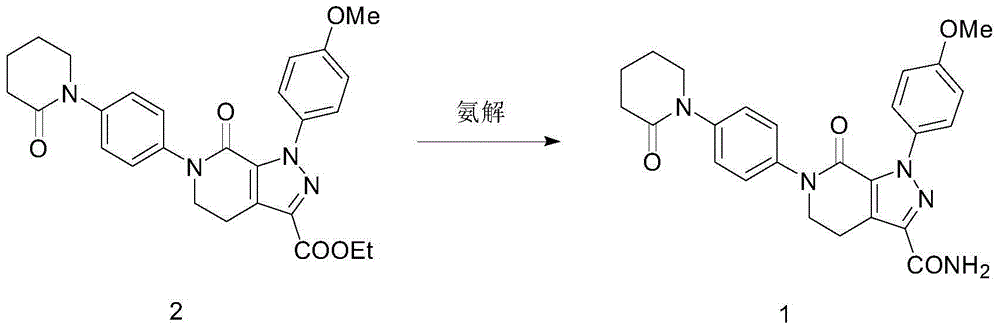
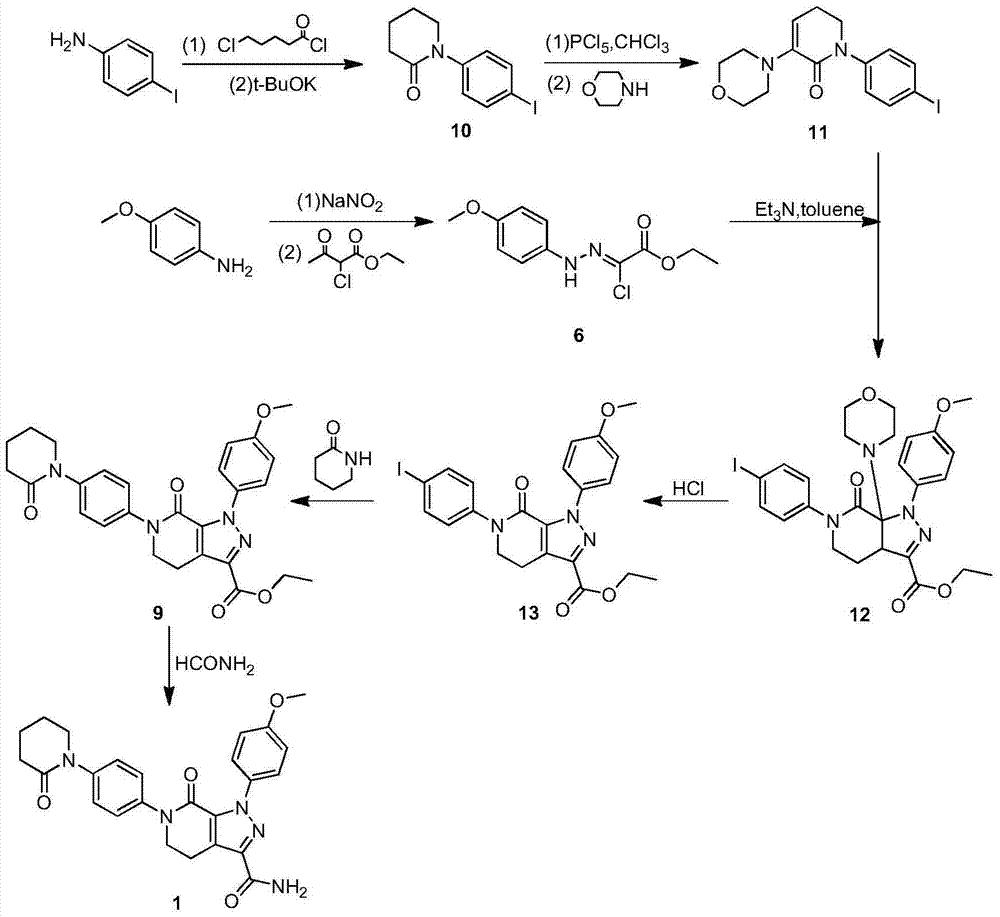
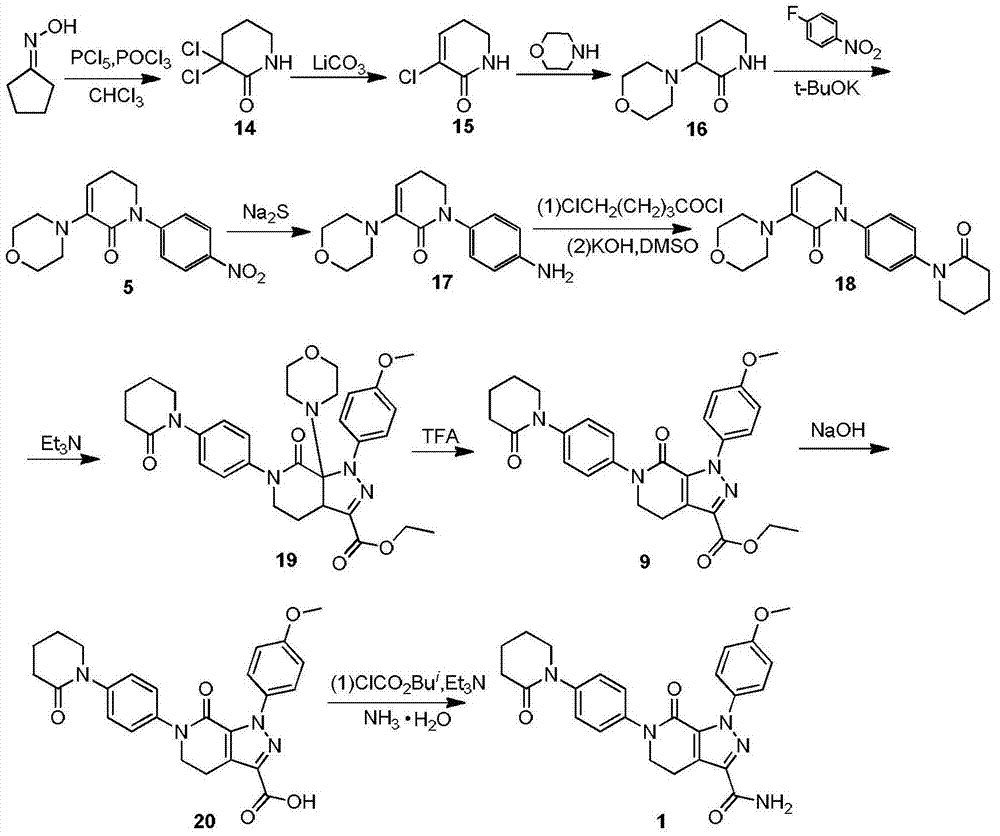
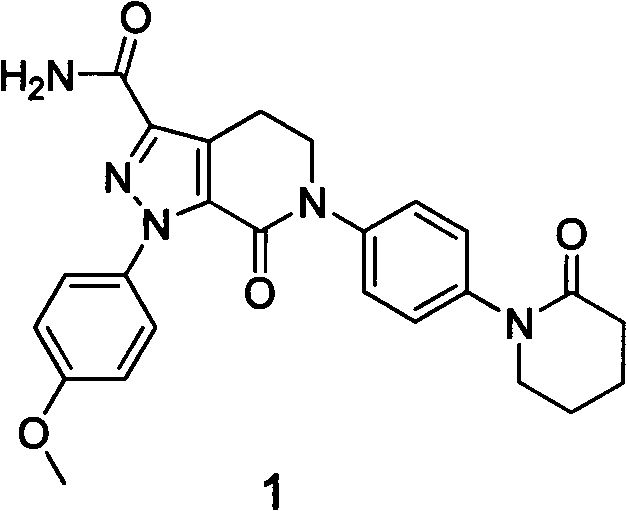
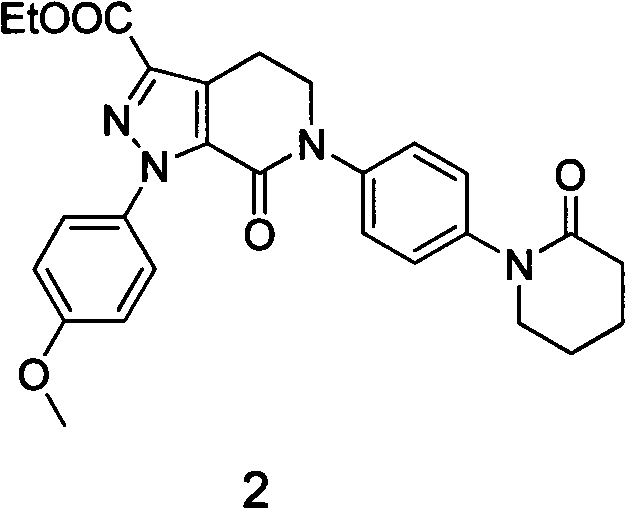
The invention discloses a preparation method of a compound Apixaban as an anti-thrombotic drug. The method disclosed by the invention comprises the following steps of: with a compound 11 shown as the formula 1 as a starting raw material, subjecting the compound 11 and amino protected paraiodoaniline 19 to coupling reaction in the existence of a cuprous reagent and an inorganic base to obtain a compound 20; subjecting the compound 20 and a compound 21 to [3+2] cyclization-elimination reaction to obtain a compound 22; removing a protecting group of the compound 22 to obtain a compound 23, or subjecting the compound 20 and the compound 21 to [3+2] cyclization reaction, directly carrying out elimination reaction under an acidic condition, and meanwhile, removing the protecting group to obtain the compound 23; subjecting the compound 23 to ammonolysis reaction to obtain a compound 24; subjecting the compound 24 and 5-chlorovaleryl halogen to amidation reaction to obtain a compound 25; and cyclizing the compound 25 under an alkaline condition to obtain the Apixaban, or subjecting the compound 24 and 5-chlorovaleryl bromine to amidation and cyclization two-step one-pot method reaction under the alkaline condition to obtain a target product, namely the Apixaban.
Owner:甘肃皓天科技股份有限公司
Apixaban formulations
ActiveUS20130045245A1Facilitate consistent in vivo dissolutionImprove solubilityPowder deliveryBiocideThromboembolic disorderPharmacology
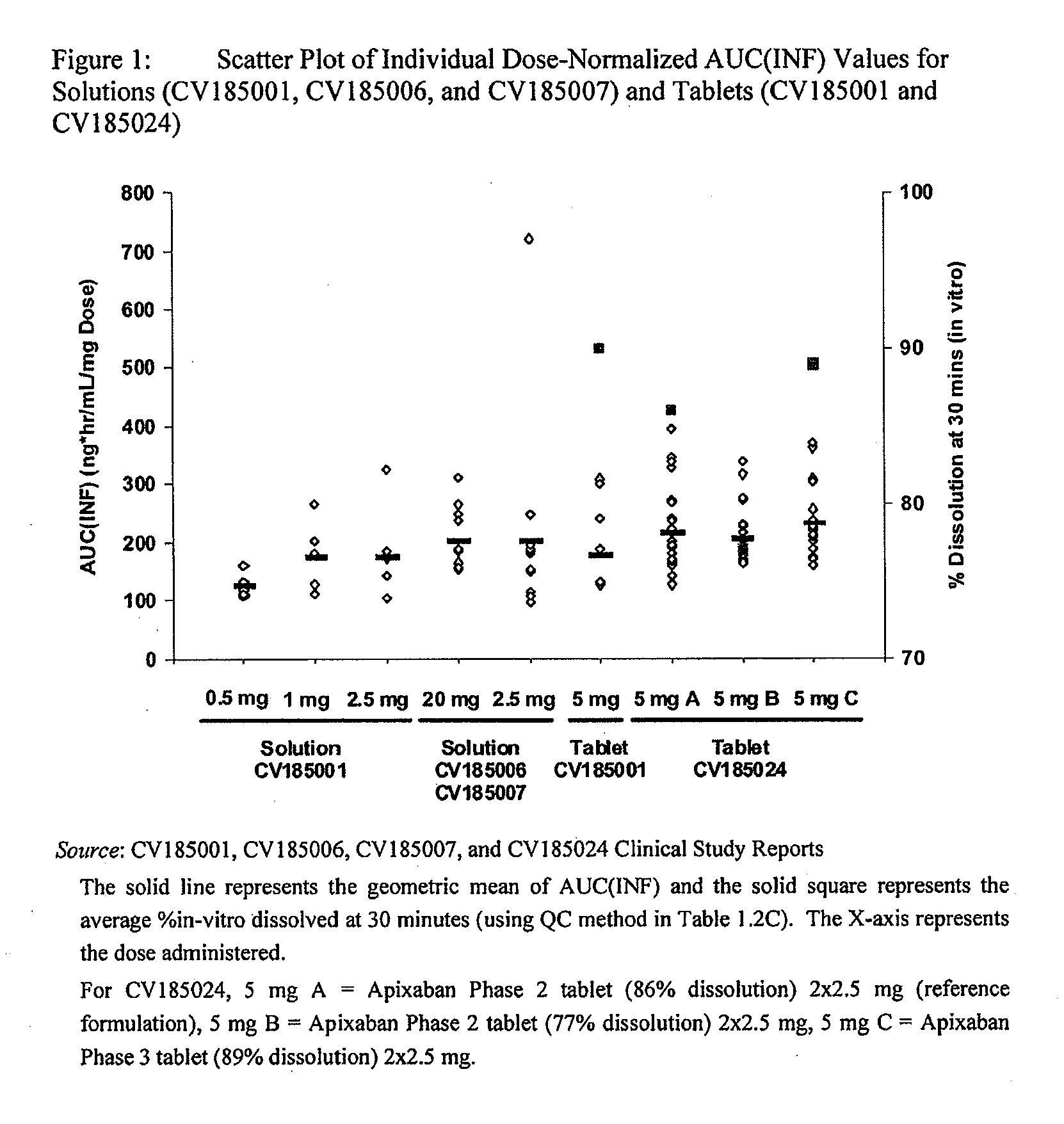
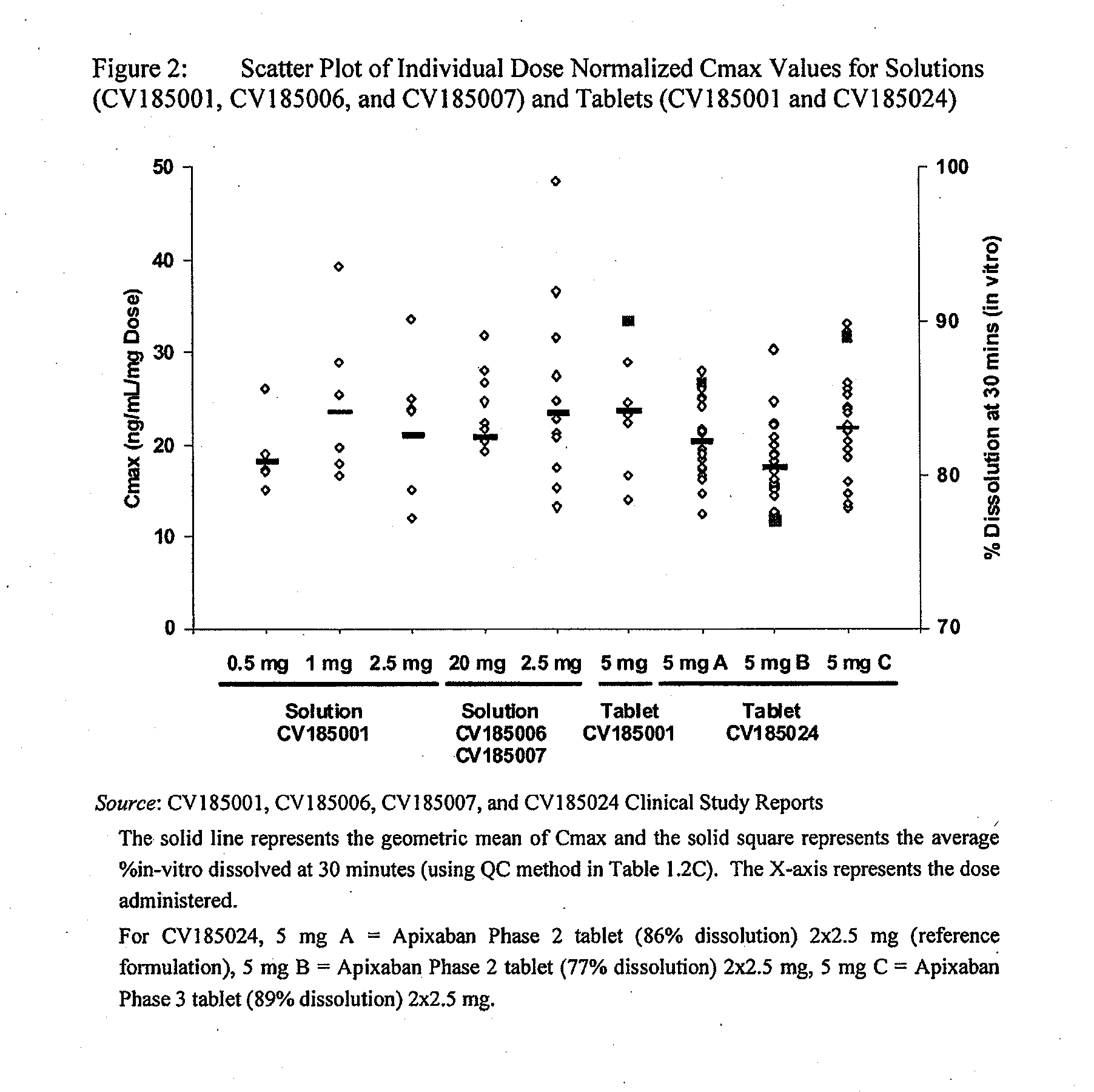
Compositions comprising crystalline apixaban particles having a D90 equal to or less than 89 μm, and a pharmaceutically acceptable carrier, are substantially bioequivalent and can be used to for the treatment and / or prophylaxis of thromboembolic disorders.
Owner:BRISTOL MYERS SQUIBB CO +1
Method for preparing antithrombotic medicament apixaban
InactiveCN101967145BReasonable designThe reaction steps are simpleOrganic chemistryPhysical/chemical process catalystsMorpholineP-Nitroaniline
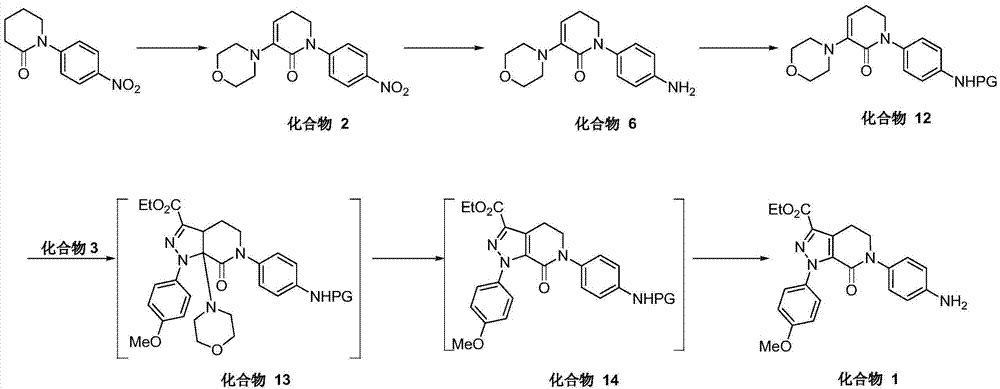
The invention discloses a method for preparing antithrombotic medicament apixaban. The method comprises the following steps of: obtaining a compound V by performing an amidation-cyclization two-step one-pot reaction on paranitroaniline serving as a raw material and a general purpose reagent 5-chlorine valeryl chloride under an alkaline condition; performing di-chlorination on the alpha-hydrogen of the V by using phosphorus pentachloride; performing a condensation-elimination reaction with excess morpholine to obtain a compound VI; reducing the VI into a compound VII by using sodium sulfide; performing the amidation-cyclization two-step one-pot reaction on the VII and the 5-chlorine valeryl chloride to obtain a key intermediate III; obtaining II by performing a [3+2] cyclization-elimination reaction on the III and another intermediate IV; and finally, obtaining I by performing aminolysis on the II. The method has the advantages that: process design is reasonable, expensive reagent is not used, reaction yield is high, raw material cost is low, and the method is simple and convenient to operate, has no harsh reaction condition, and is easy to perform large-scale production.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Synthetic method for Apixaban drug intermediate
InactiveCN104844593AHigh yieldQuality improvementOrganic chemistryBulk chemical productionMedicinal chemistryOne-pot synthesis
The present invention provides a synthetic method for an Apixaban drug intermediate. According to the invention, the reduction reaction of nitro groups in a compound 2 is conducted by sodium sulphide and amino groups in the compound 2 are protected by 5-chloro-pentanoyl. Therefore, the synthesis of Apixaban can be realized more simply and more directly. A novel one-pot synthesis method is provided by the invention and the method is mild in reaction conditions, high in safety coefficient, strong in maneuverability, simple in process and easy to industrialize. Meanwhile, a high-purity intermediate compound 14-1 can be obtained, so that the Apixaban of high purity and super-low impurity content can be obtained more easily. At the same time, the invention provides an effective and novel synthetic method for the preparation of an Apixaban key intermediate. By means of the method, the reaction efficiency is improved, and the production cost is lowered. The adoption of expensive reagents and severe reaction conditions can be avoided.
Owner:SHANGHAI TWISUN BIO PHARM
Method for preparing apixaban
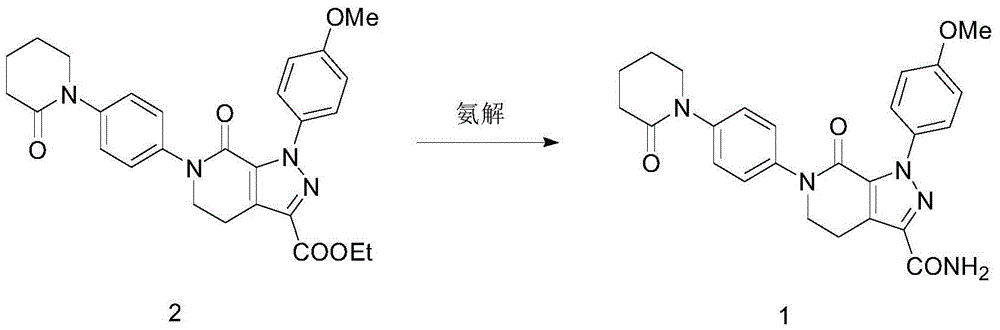
The invention relates to the field of chemical pharmacy and particularly relates to a method for preparing apixaban. The method comprises the following steps: (1) synthetic method of apixaban crude product: performing ammonolysis by an ammonia-accessing method; (2) refining method of apixaban: the refining method includes ethyl alcohol refining and isopropanol refining. The purity of the compounded apixaban is more than 97%, the byproducts are few, the operation method is simple and the reaction process is easy to control, at the same time, the yield of the ammonolysis is more than 91%; after two refining processes, the purity of the apixaban is greatly increased to more than 99.6%, and the single impurity is less than 0.1% and the yield is more than 90%. The solvent used in the refining process is low in toxicity, high in safety, cheap and easy to obtain. The refining process is simple in operation, mild in reaction condition, low in cost and is suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Preparation method of apixaban intermediate
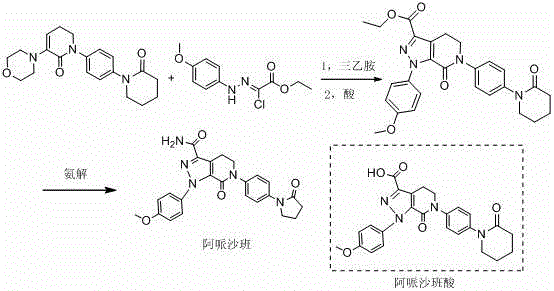
The invention provides a preparation method of an anti-thrombotic drug apixaban intermediate. The preparation method comprises the following steps: by taking paranitroaniline as a raw material, carrying out amidation, cyclization and reaction on paranitroaniline and 5-chloro valeryl chloride under an alkaline condition to obtain an intermediate (3); carrying out bicholo substation and condensation-elimination reaction on the intermediate (3) to obtain an intermediate (5); carrying out [1+3] dipolar cycloaddition reaction on the intermediate (5) and another intermediate (6) to obtain an intermediate (7); and carrying out morpholine removal and reduction reaction on the intermediate (7) to obtain the key apixaban intermediate 1-(4-methoxyl phenyl)-6-(4-amino phenyl)-7-oxo-4, 5, 6, 7-tetrahydro-1H-pyrazol[3, 4-c] pyridine-3-ethyl formate (I). The method adopting proper raw materials, solvents and catalysts is reasonable in design, mild in reaction condition, simple in posttreatment and high in reaction yield, and has certain feasibility of industrialized production.
Owner:SHENYANG J & HEALTH PHARMA
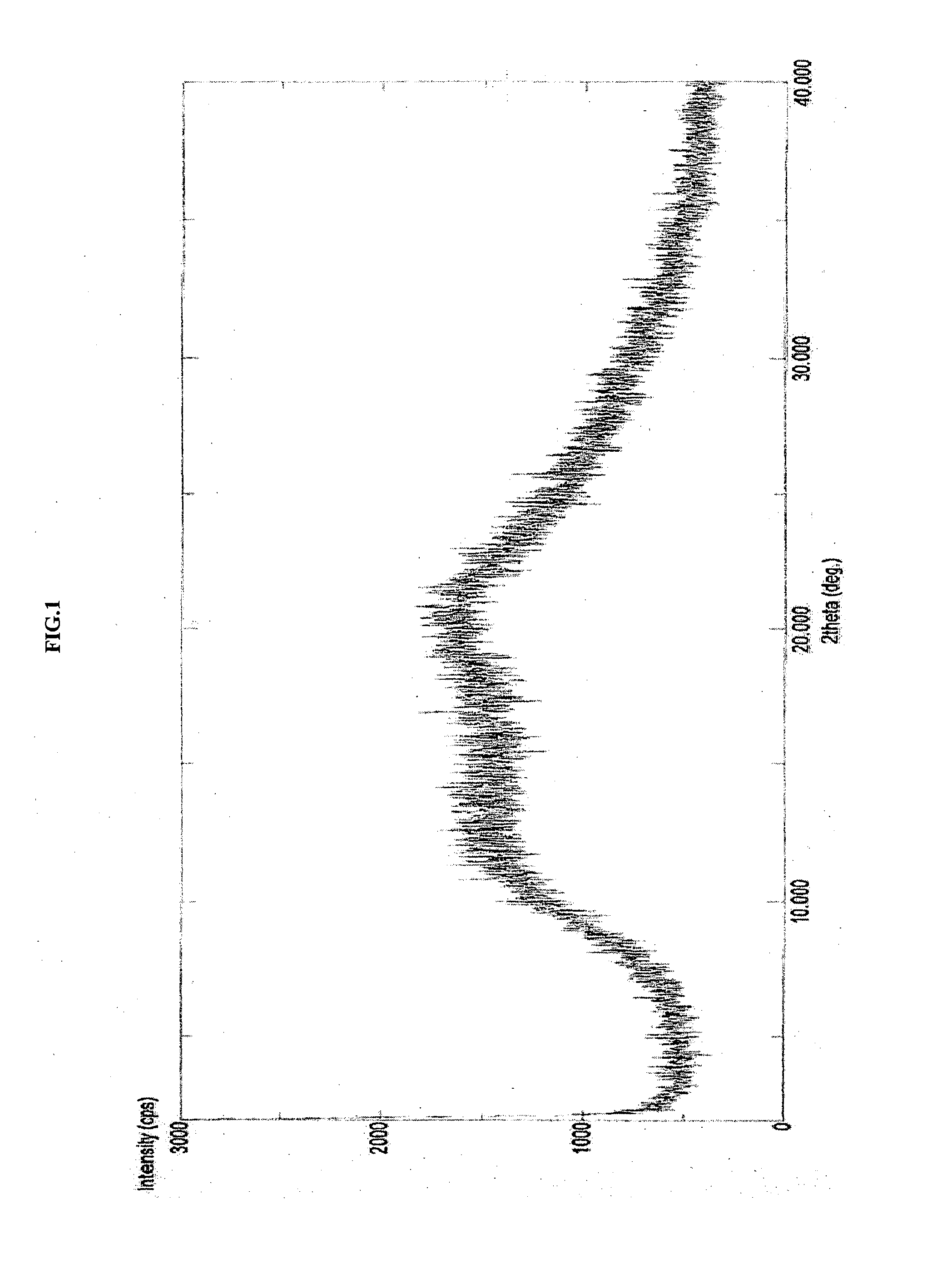
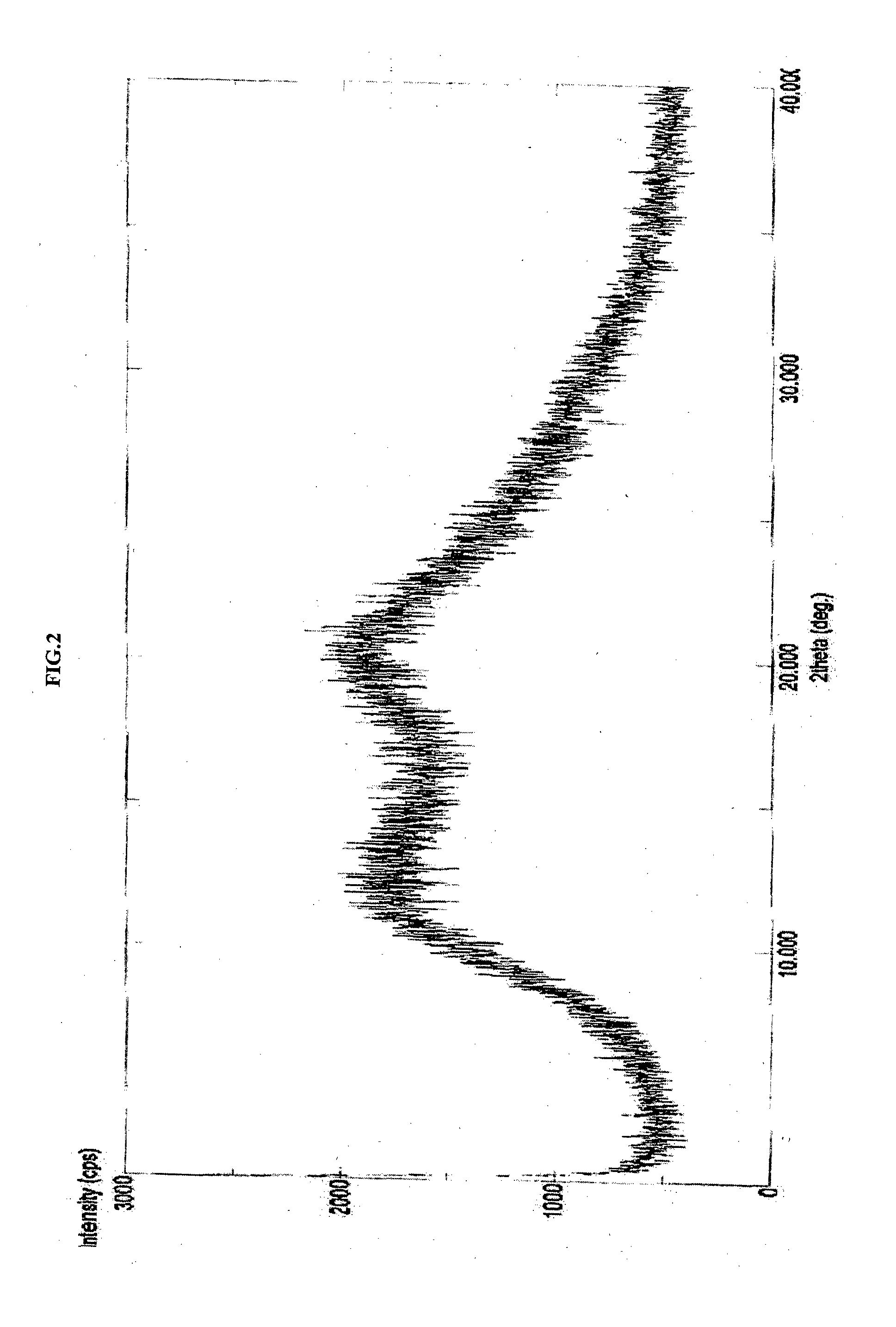
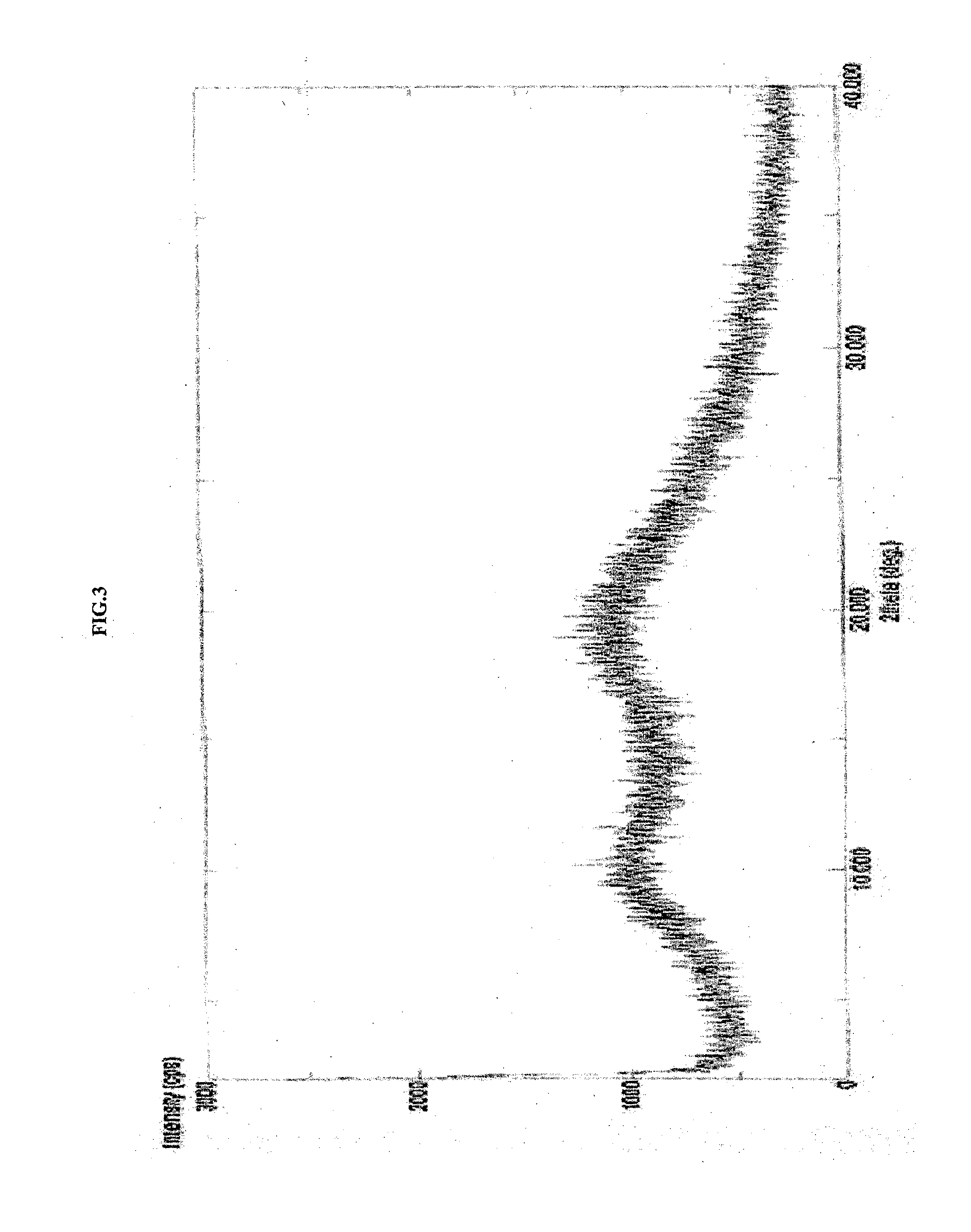
Amorphous form of apixaban, process of preparation and compositions thereof
The present invention discloses an amorphous form of apixaban, process of preparation and compositions thereof.
Owner:CADILA HEALTHCARE LTD
Method for preparing apixaban tablets
InactiveCN104490834AImprove bioavailabilityHigh dissolution rateOrganic active ingredientsPill deliveryTreatment effectMedicine
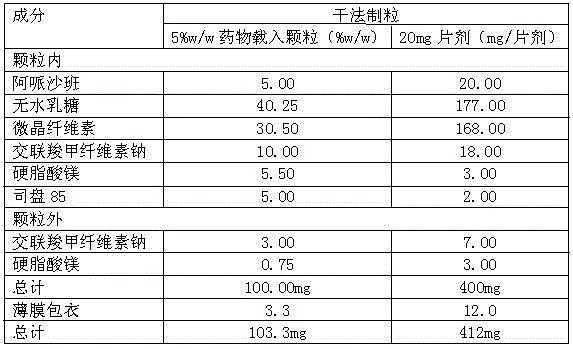
The invention relates to a method for preparing apixaban tablets. The method comprises the following steps: (1) uniformly mixing apixaban, an excipient, a disintegrating agent, a surfactant and a lubricating agent, performing wet granulation, screening the prepared soft material by using a 20-mesh sieve, granulating, drying and finishing the obtained particles, and collecting the dried particles with the particle size being between 20 meshes and 80 meshes; and (2) mixing the prepared particles with the lubricating agent for 30 minutes and tabletting, wherein the hardness of the tablets is 50-60N. In order to improve the dissolubility, a method for adding the surfactant into the prescription is adopted. According to the method, the dissolubility of the apixaban can be improved, and the problem is well solved, so that the bioavailability of the medicine is improved, and an excellent treatment effect is achieved.
Owner:TIANJIN YIYAO SCI & TECH
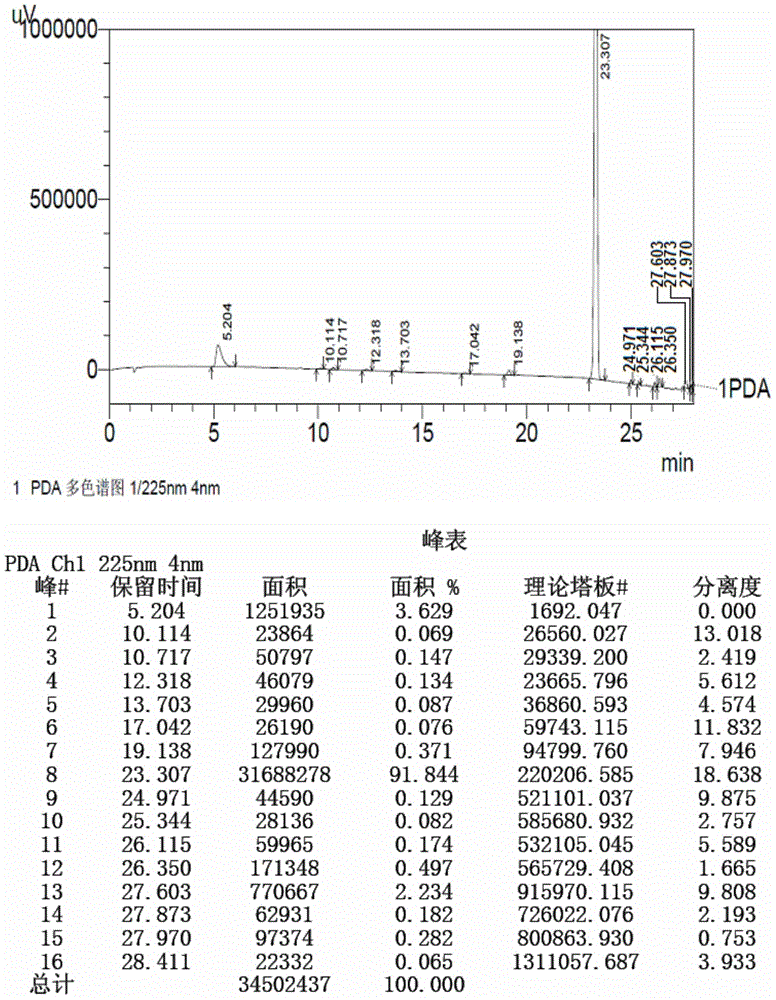
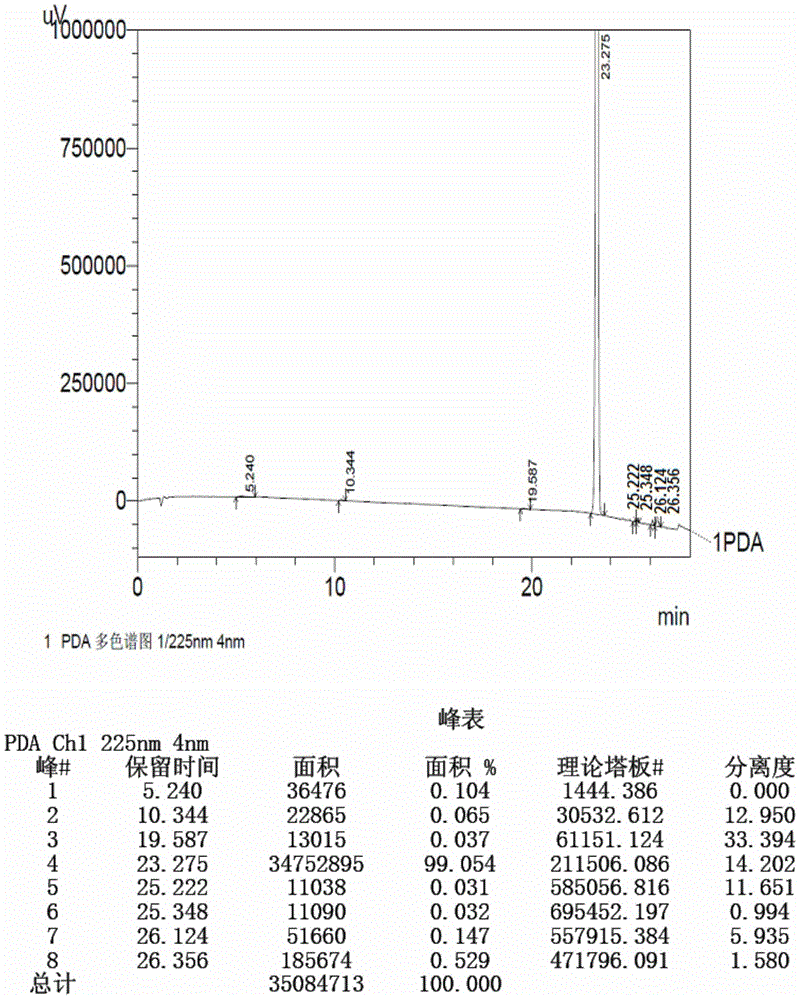
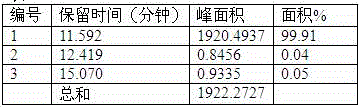
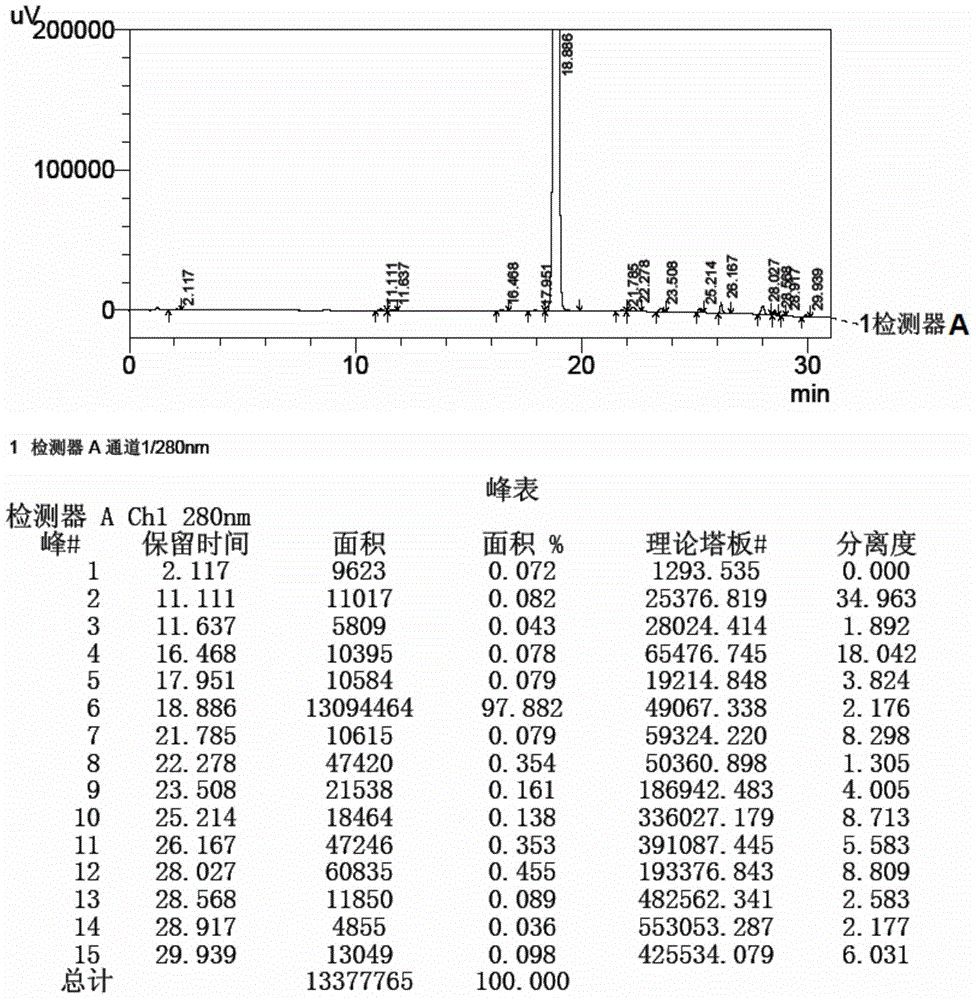
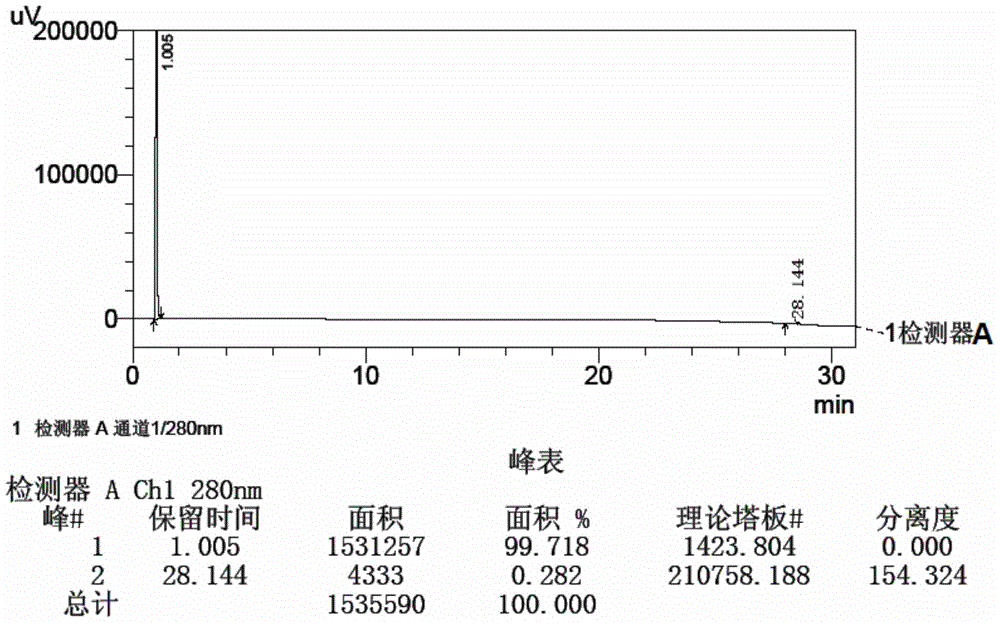
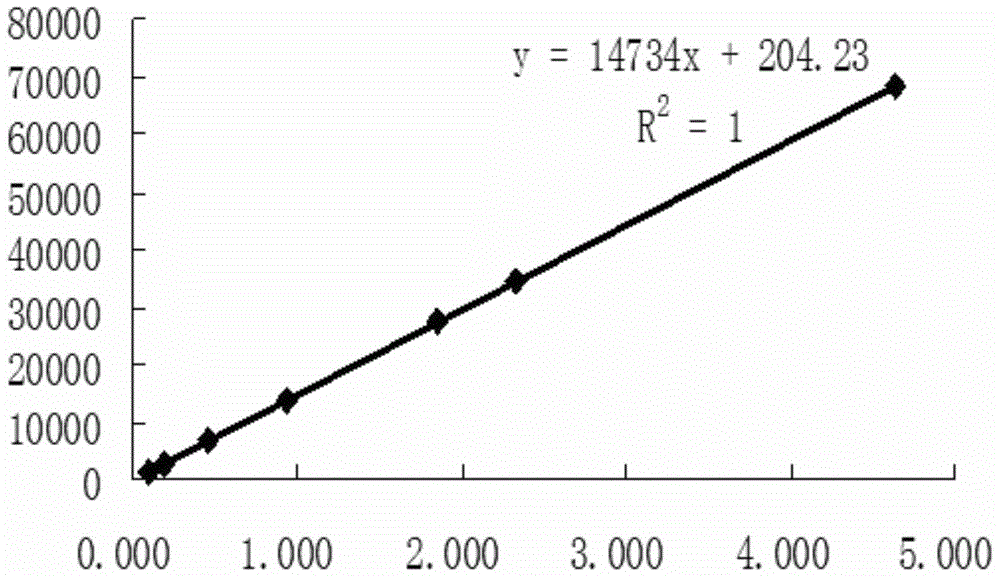
Method for detecting initial material II in apixaban through reversed-phase high performance liquid chromatography
ActiveCN105044269AEfficient separationPurpose of enhanced controlComponent separationHigh-performance Liquid Chromatography-UVImpurity
The invention discloses a method for detecting an initial material II in apixaban through reversed-phase high performance liquid chromatography. The method comprises the following steps: 1, preparing a sample solution; 2, detecting the sample through reversed-phase high performance liquid chromatography; and 3, calculating the content of every impurity and the content of total impurities in the sample through an area normalization technique. The method for detecting initial material II related substances in apixaban through reversed-phase high performance liquid chromatography realizes complete separation of the chromatographic peaks of the initial material II and all the impurities through a simple mobile phase component; and the detection result of the method is accurate and reliable, so the method makes control of the quality of the initial material in the apixaban synthesis process possible.
Owner:CHENGDU BAIYU PHARMA CO LTD
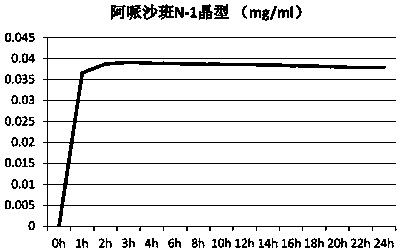
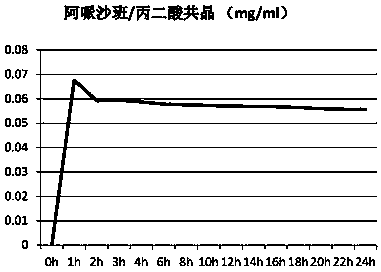
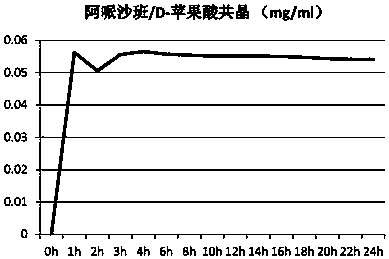
Eutectic crystal formed by apixaban and carboxylic acid and preparation method thereof
ActiveCN110922403AOrganic chemistry methodsCarboxylic compound separation/purificationMalonic acidPhysical chemistry
The invention provides an apixaban / carboxylic acid eutectic crystal which comprises an apixaban / malonic acid eutectic crystal, an apixaban / D-malic acid eutectic crystal, an apixaban / maleic acid eutectic crystal, an apixaban / L-proline eutectic crystal and an apixaban / L-tartaric acid eutectic crystal. According to the apixaban / carboxylic acid eutectic crystal, the dissolution rate of apixaban is improved while safe medication is met, the defects of low solution rate and low dissolution rate of apixaban are overcome and the bioavailability of apixaban is improved.
Owner:ZHEJIANG TIANYU PHARMA
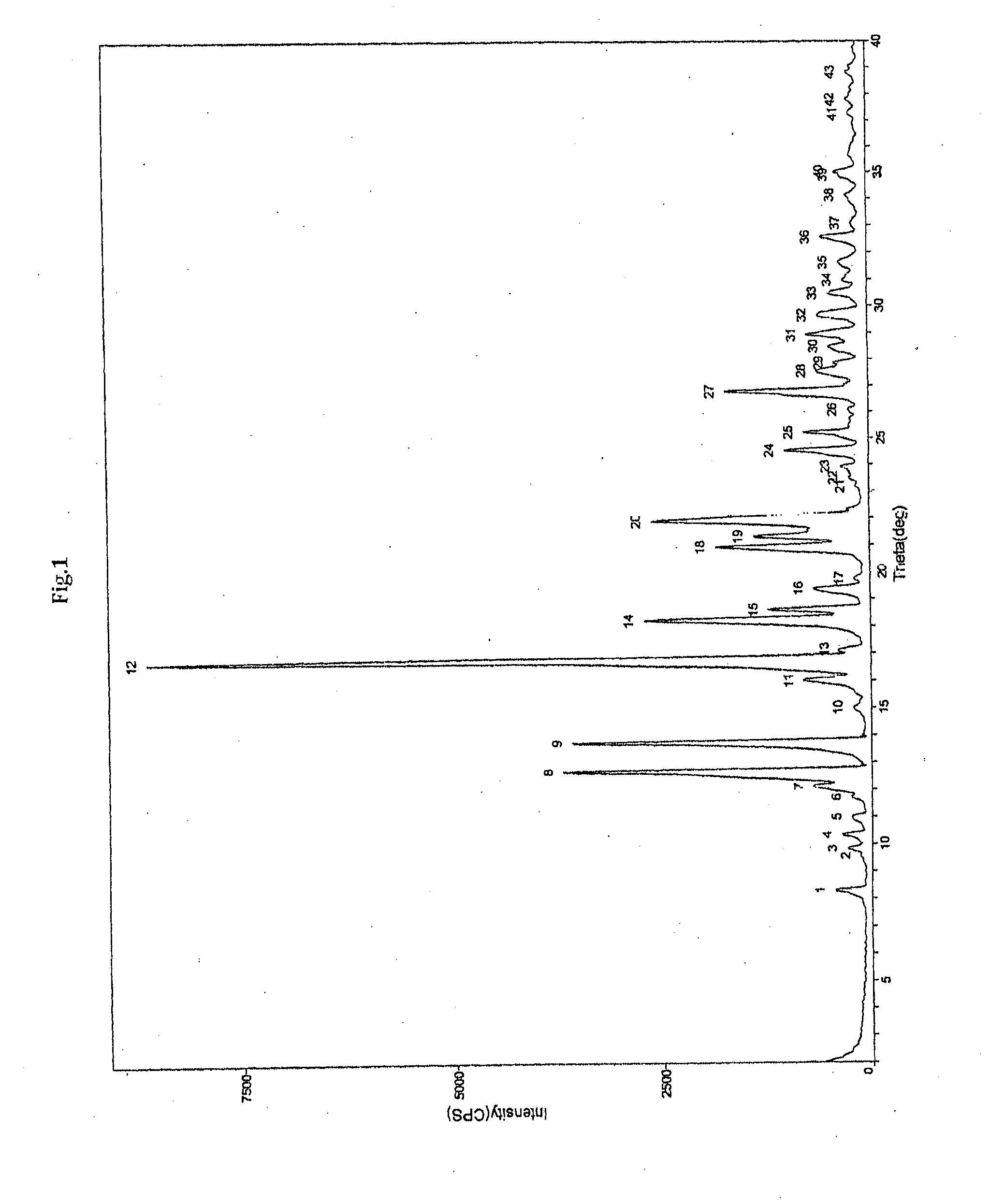
Process for the preparation of apixaban
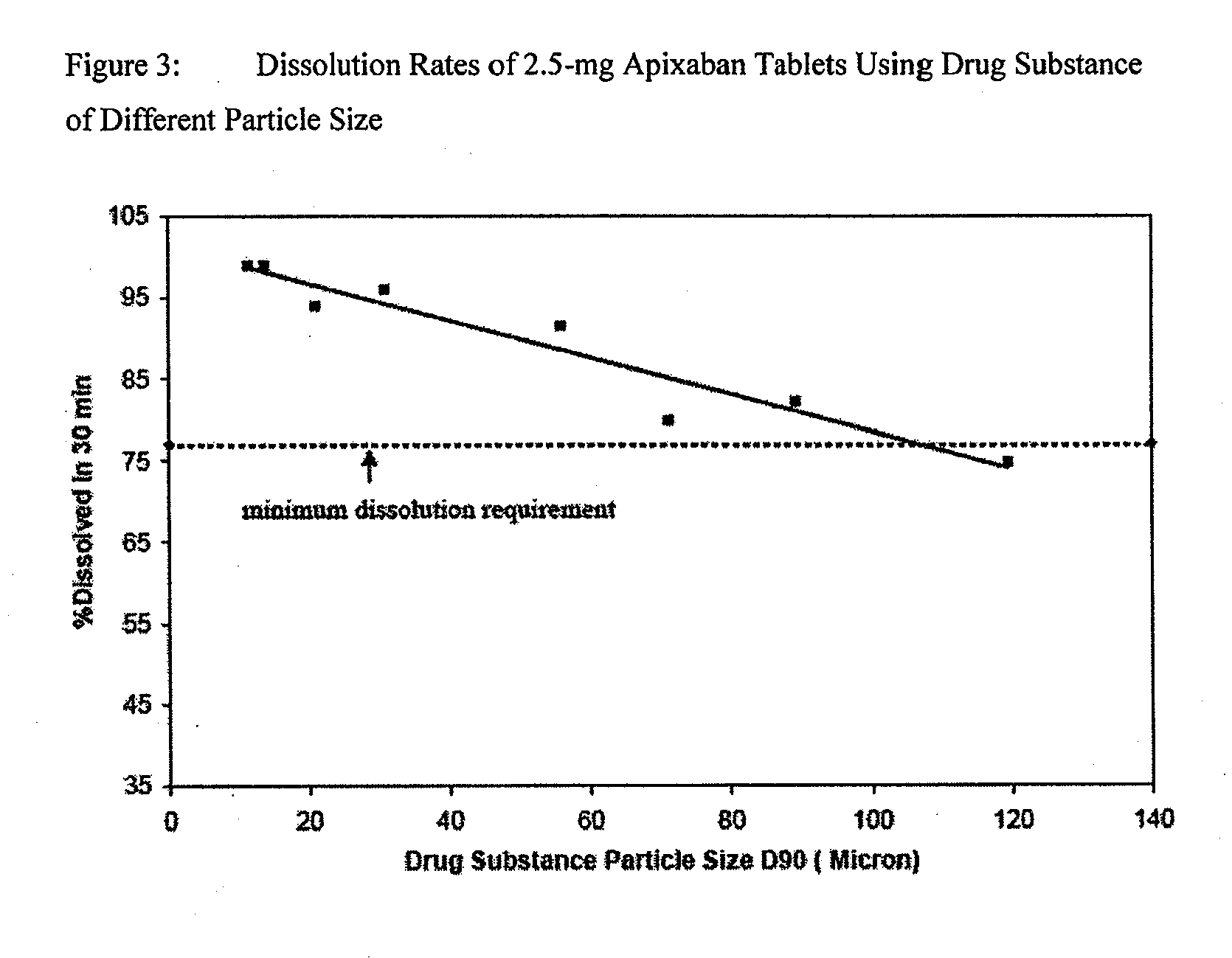
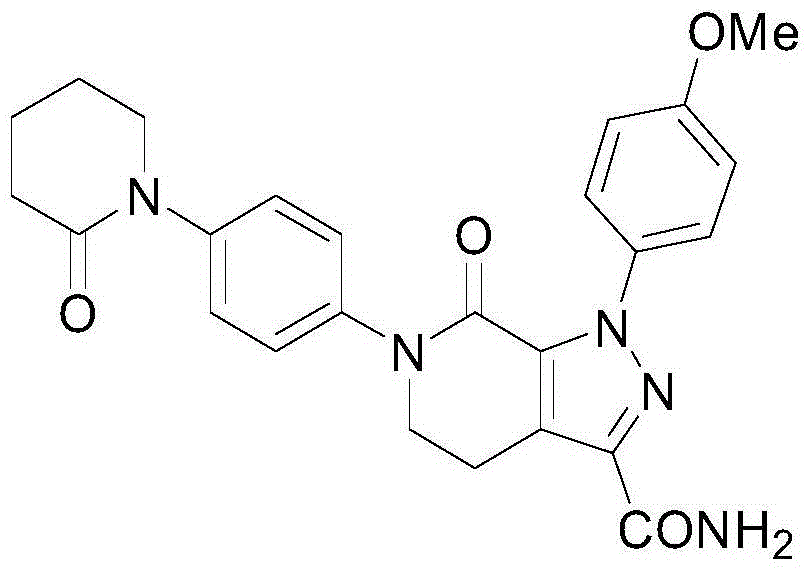
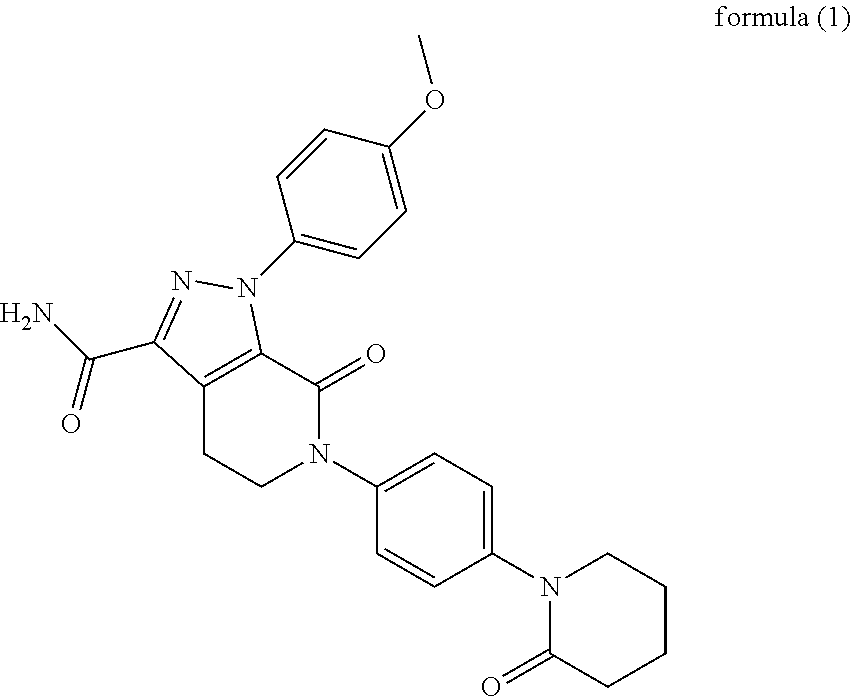
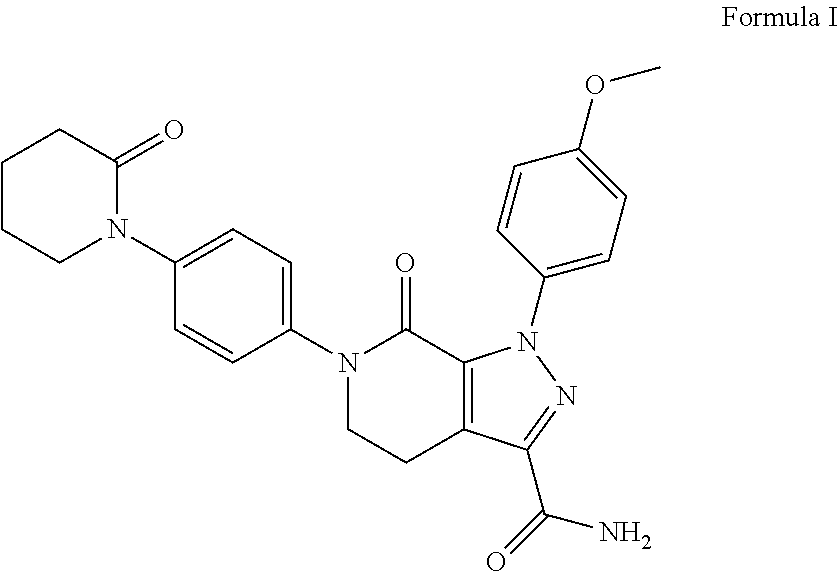
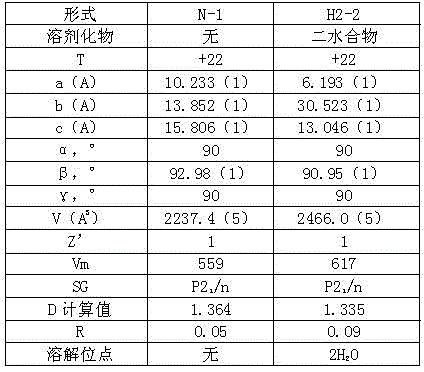
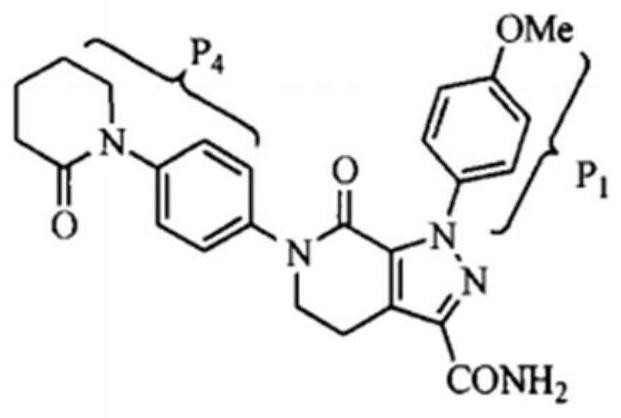
A crystalline Form N-1 of apixaban substantially free from one or more of: 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid; 7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-1-phenyl-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide; or methyl 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylate, relative to apixaban by area percentage of HPLC and having a mean particle size equal to or greater than 100 μm.
Owner:CADILA HEALTHCARE LTD
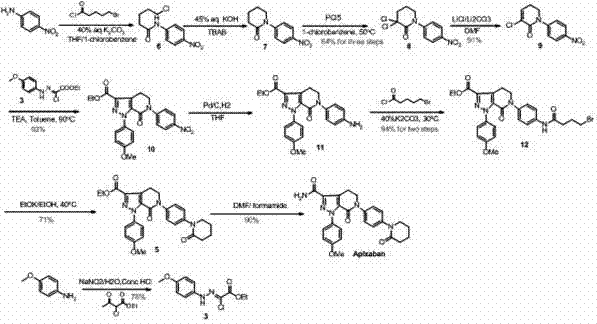
Method for preparing antithrombotic drug apixaban
The invention discloses a method for preparing an antithrombotic drug apixaban. The method is characterized in that a compound IV is used as a raw material to perform a hydrolysis reaction for 5-60 minutes at 10-100 DEG C in a polar organic solvent under an alkaline condition, and is transformed into a compound antithrombotic drug apixaban; the antithrombotic drug apixaban is prepared by re-crystallizing, sizing and column chromatography purification. The method has the advantage of high yield, is simple and convenient in the subsequent treatment due to extraction, drying, sizing, filtering, crystallizing, recrystallizing and other operation methods, and large-scale production is easily realized.
Owner:PHARMA SHANGHAI
Pharmaceutical composition of apixaban
ActiveUS20190358215A1Organic active ingredientsGranular deliveryExcipientPharmaceutical preservatives
The present disclosure relates to a stable, reproducible and bioequivalent apixaban compositions, wherein the composition comprising apixaban having a D90 particle size of more than 100 microns, preferably between 300 and 1000 microns, and more preferably between 350 and 800 microns, and further comprising one or more pharmaceutically acceptable excipients. The present disclosure further provides a process for preparation of a pharmaceutical composition comprising apixaban by wet granulation.
Owner:UNICHEM LAB LTD
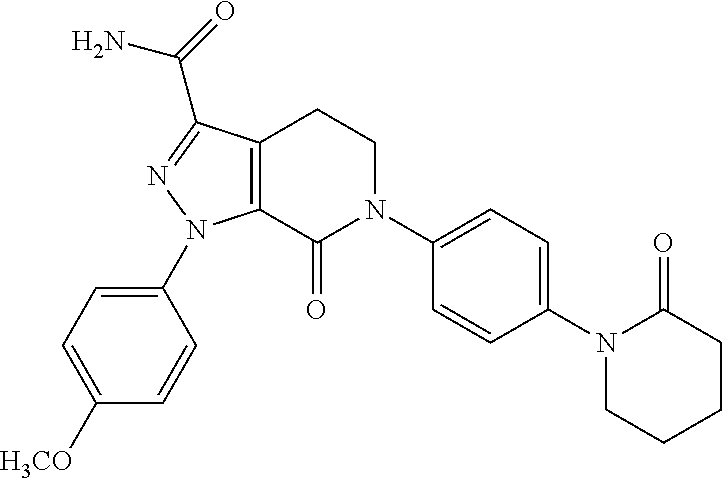
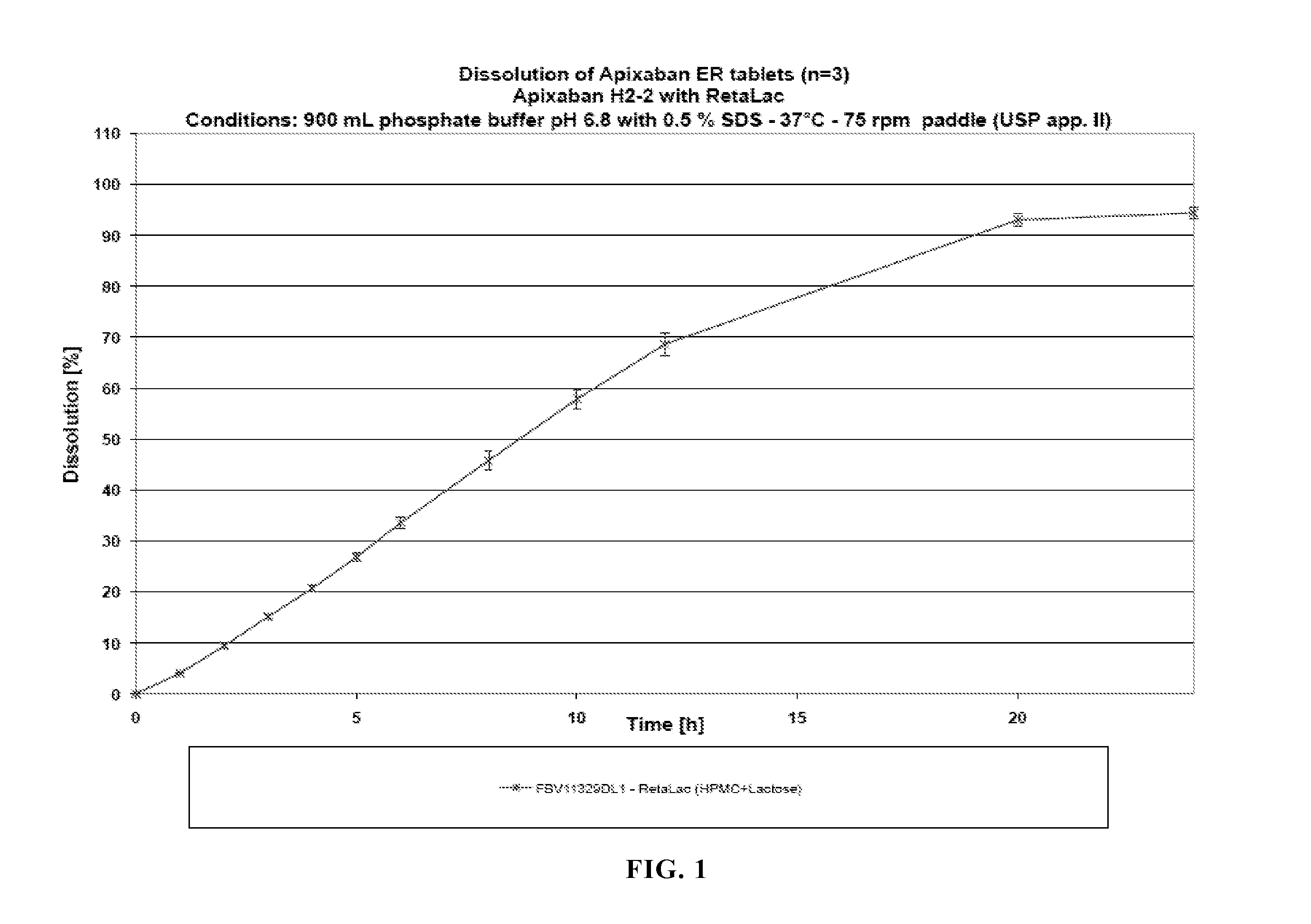
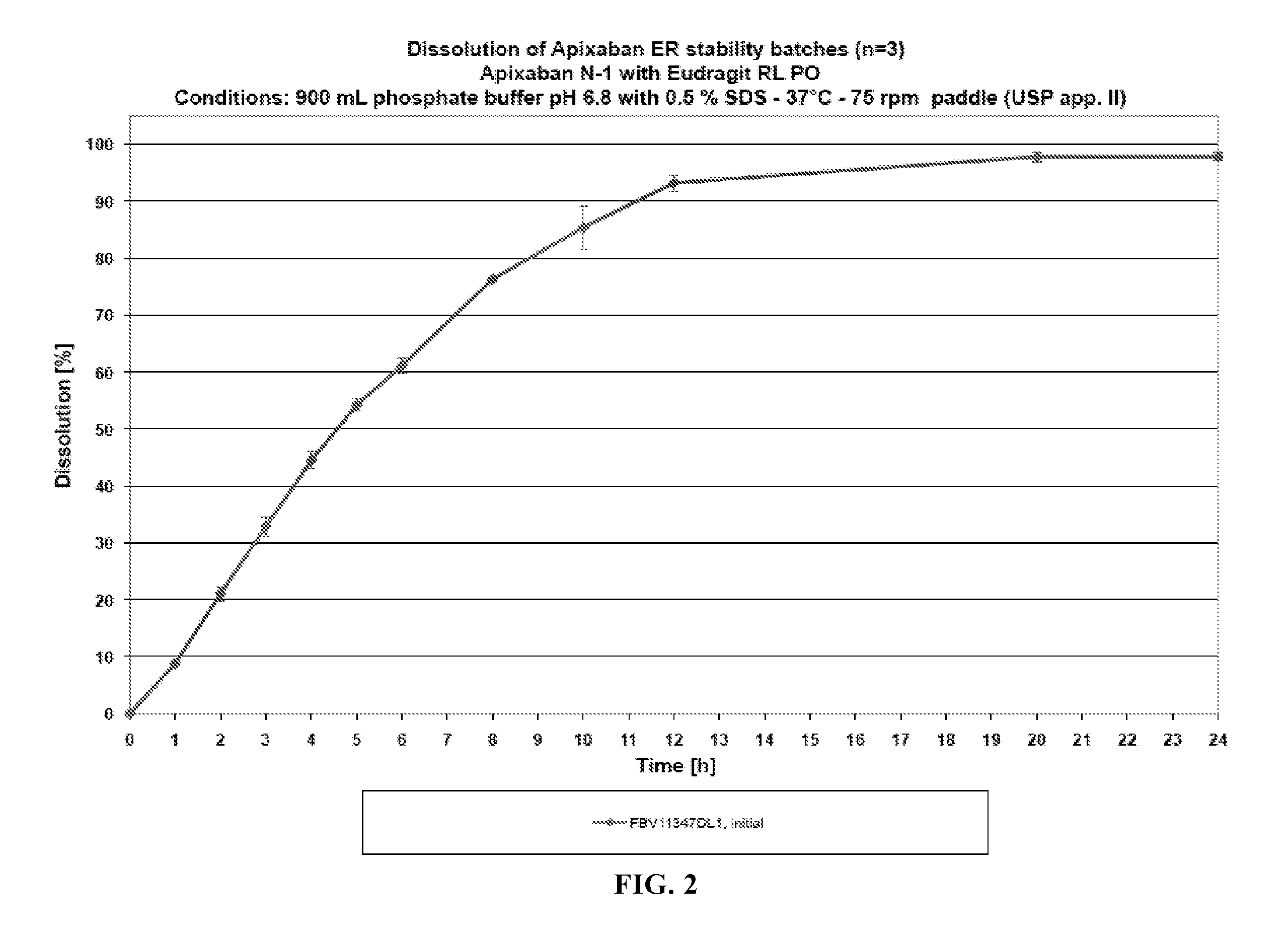
Dosage forms comprising apixaban and matrix former
The invention relates to oral dosage forms for modified release of apixaban. The invention also relates to methods of preparing said dosage forms and to an agglomerated mixture of matrix former and filler for preparing an oral dosage form for use in the treatment of venous thromboembolism.
Owner:RATIOPHARM GMBH
Novel forms of apixaban
The present application relate to amorphous form of Apixaban useful in making pharmaceutically acceptable dosage forms, and to processes for their preparation.
Owner:DR REDDYS LAB LTD
Apixaban tablet and preparation method thereof
InactiveCN104523619AImprove bioavailabilityHigh dissolution rateOrganic active ingredientsPill deliveryTreatment effectCurative effect
The invention discloses an apixaban tablet comprising the raw materials including apixaban, an excipient, a disintegrating agent, a surfactant and a lubricant in a weight ratio of (5-20):(0.5-2.5):(0.1-0.3):(0.1-2.0). In order to improve the dissolution degree of the apixaban tablet disclosed by the invention, a method for adding the surfactant into the formula is adopted, and the method can increase the dissolution degree of apixaban, so that the problem is well solved, the bioavailability of the medicament is improved, and a very good curative effect is achieved.
Owner:TIANJIN YIYAO SCI & TECH
Apixaban pellet and preparation method thereof
InactiveCN106913528ASimple methodEasy to operateOrganic active ingredientsGranular deliveryAdhesiveBioavailability
The invention relates to an apixaban pellet and a preparation method thereof. The apixaban pellet is composed of, from interior to exterior, a blank pellet core, a drug-containing layer and a coating layer, wherein the drug-containing layer is composed of micronized apixaban, a binder and a disintegrating agent. The apixaban pellet comprises, by weight, 60 to 84 parts of the blank pellet core, 2 to 7 parts of the micronized apixaban, 3 to 10 parts of the binder, 5 to 20 parts of the disintegrating agent and 2 to 8 parts of the coating layer. Thus, the apixaban pellet obtained in the invention has a rough and regular shape and uniform particle size; and compared with commercially available tablets, the apixaban pellet provided by the invention has better stability, faster dissolving-out rate and higher bioavailability.
Owner:WATERSTONE PHARMA WUHAN
Apixaban solid composition and preparation method thereof
ActiveUS20190022008A1Low compressibilityBad granulated capabilityOrganic active ingredientsPharmaceutical non-active ingredientsChemistryBiochemistry
The present invention provided an apixaban solid composition and a preparation method thereof. The method comprises granulating apixaban by wet granulation, wherein the apixaban has a particle size D90 more than 89 μm.
Owner:SUNSHINE LAKE PHARM CO LTD
FACTOR Xa INHIBITOR FORMULATION AND METHOD
An injectable Factor Xa inhibitor formulation is provided which includes the Factor Xa inhibitor razaxaban or apixaban, a solubilizing agent which is a substituted β-cyclodextrin, preferably, sulfobutyl ether β-cyclodextrin (SBE-CD) or hydroxypropyl-β-cyclodextrin (HPB-CD), and water. A method for preventing or treating venous thrombosis, deep venous thrombosis and acute coronary syndrome employing the above formulation is also provided.
Owner:BRISTOL MYERS SQUIBB CO
FACTOR Xa INHIBITOR FORMULATION AND METHOD
An injectable Factor Xa inhibitor formulation is provided which includes the Factor Xa inhibitor razaxaban or apixaban, a solubilizing agent which is a substituted β-cyclodextrin, preferably, sulfobutyl ether β-cyclodextrin (SBE-CD) or hydroxypropyl-β-cyclodextrin (HPB-CD), and water. A method for preventing or treating venous thrombosis, deep venous thrombosis and acute coronary syndrome employing the above formulation is also provided.
Owner:BRISTOL MYERS SQUIBB CO
Apixaban oral solid preparation and method for preparing same
ActiveCN109793715AEvenly dispersedImprove dissolution rateOrganic active ingredientsSolution deliveryHydrogenDissolution
The invention discloses an apixaban oral solid preparation. The apixaban oral solid preparation comprises apixaban, hydroxypropyl methylcelluloses and vector excipients. The vector excipients are selected from a type or a plurality of types of fillers, disintegrating agents, lubricants and glidants. The apixaban oral solid preparation does not contain surfactants. A method for preparing the apixaban oral solid preparation includes preparing suspension from the apixaban and the hydroxypropyl methylcelluloses; carrying out wet granulation to obtain the apixaban oral solid preparation. The apixaban oral solid preparation and the method have the advantages that under the condition that micronization treatment is not carried out on apixaban crude medicines, the dissolution rates of dissolutionmedia with the pH (potential of hydrogen) of 1.0-6.8 are higher than 85% in 5 min and are higher than 90% in 10 min, and the dissolution media are completely dissolved out in 15 min.
Owner:NANJING CAVENDISH BIO ENG TECH
A kind of preparation method of the intermediate of apixaban
The invention relates to a preparation method of an apixaban intermediate shown as the formula 2. The preparation method comprises steps of (1) reacting a compound 3 and a compound 4 under reflux conditions and under the action of an organic alkali, wherein dichloromethane is used as solvent; and (2) continuously reacting the reaction liquid obtained in the step (1) under the action of an inorganic acid, wherein the mole ratio of the compound 3 to the compound 4 is 1: 1.1-1:3. The preparation method is low in cost, high in product yield and high in purity, and is simple, convenient, and suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Method for determining apixaban cleaning residues by high performance liquid chromatography
The invention relates to a method for determining apixaban cleaning residues by high performance liquid chromatography, and belongs to the field of chemical pharmaceutical detection. The invention aims at providing a method for determining the apixaban cleaning residues. The method is easy and quick to operate and accurate and reliable in determination results to evaluate a cleaning effect so as to ensure medicine quality and medication safety. The high performance liquid chromatography for determining the apixaban cleaning residues, which is disclosed by the invention, is easy and quick to operate, and accurate and reliable in the determination results, so that the cleaning effect of the equipment can be evaluated effectively. According to the high performance liquid chromatography, a 280 nm flow phase is selected, so that the detection limit is reduced effectively. A chromatographic column temperature selects 40 DEG C, so that the peak type can be improved effectively, and minimal residues can be effectively detected; methanol is selected to serve as a solvent, and is not absorbed in the wavelength, so that the interference on the detection caused by the solvent is avoided, and the reliability of the detection method is improved.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Apixaban composition and preparation method thereof
InactiveCN104644593AImprove solubilityPromote dissolutionOrganic active ingredientsBlood disorderMedicineThromboembolic disease
Owner:TIANJIN HANKANG PHARMA BIOTECH
Pharmaceutical Composition Comprising Apixaban
The present invention relates to a pharmaceutical composition comprising apixaban, in particular to a pharmaceutical composition comprising apixaban and a polymer having low viscosity as binder, and to a process for its preparation. The pharmaceutical composition is particularly useful as a medicament, especially for the treatment or prevention of a thromboembolic disorder.
Owner:SANDOZ AG
Apixaban sustained-release tablets and preparation method thereof
InactiveCN104382874AGood treatment effectImprove drug stabilityOrganic active ingredientsMetabolism disorderDrug release rateProlonged-release tablet
The invention relates to apixaban sustained-release tablets. The apixaban sustained-release tablets are prepared from the raw materials in parts by weight: 5 parts of apixaban, 50-95 parts of sustained-release framework material and 3-20 parts of lubricant. A preparation method of the apixaban succinate sustained-release tablets comprises the steps of preparing materials, mixing, granulating, mixing, tabletting and carrying out aluminum-plastic packaging. According to the apixaban sustained-release tablets, postprandial hyperglycemia related symptoms of type 2 diabetes patients (only limited to the patients, of which blood sugar cannot be effectively controlled by dietotherapy and kinesitherapy, or the patients, of which the blood sugar cannot be effectively controlled yet after an alpha-glucosidase inhibitor is applied based on the dietotherapy and the kinesitherapy) can be effectively improved, novel sustained-release preparations are adopted, and sustained release means delaying the drug release rate of a drug from a dosage form, lowering the organism entering absorption rate of the drug and thus exerting a more stable treatment effect; the apixaban sustained-release tablets have the advantages of good drug stability, convenience in packaging, transportation and storage and the like; the preparation method of the apixaban sustained-release tablets is simple and easy and is applicable to industrial production.
Owner:HARBIN SHENGJI PHARMA
Preparation method of extracorporeal circulation anticoagulant modified membrane
The invention discloses a preparation method of an extracorporeal circulation anticoagulant modified membrane. The preparation method comprises the following steps: 1, synthesizing carboxylated polysulfone / polyethersulfone; 2, synthesizing apixaban modified polysulfone / polyethersulfone; 3, preparing an apixaban modified polysulfone / polyethersulfone membrane. According to the extracorporeal circulation anticoagulant modified membrane and the preparation method thereof provided by the invention, an Xa factor inhibitor apixaban is grafted to base materials such as polyethersulfone (PES) and polysulfone (PSf), the base materials are modified by the apixaban, and membrane material wall-attached thrombus is efficiently inhibited through signal amplification, so that the membrane has strong anticoagulant and antithrombotic performance. The modification process is simple in synthesis condition, high in controllability and suitable for subsequent industrial large-scale production. According to the novel extracorporeal circulation anticoagulant modified membrane, the steric hindrance effect caused by heparin / heparinoid synthesis is effectively avoided, and meanwhile, the risk of severe bleeding caused by heparin-induced thrombocytopenia (HIT) is reduced.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV +1
Refining method for apixaban
A refining method for apixaban comprises the following steps: 1) dissolving an apixaban crude product in a mixed solvent of dichloromethane and methanol, to obtain an apixaban solution; 2) adding a sodium hydroxide solution, stirring and washing the apixaban solution of the step 1), and then separating to obtain an organic layer; and 3) adding n-hexane to the organic layer of the step 2) under stirring, mixing evenly, placing to separate out a solid under natural conditions, filtering, and drying to obtain refined apixaban. The refined apixaban obtained by the refining method is from white to off-white in color, and has the advantages of high quality and high yield; the method has low required dissolution temperature, has simple operation, and is suitable for industrialized production.
Owner:LEPU PHARMACEUTICAL CO LTD
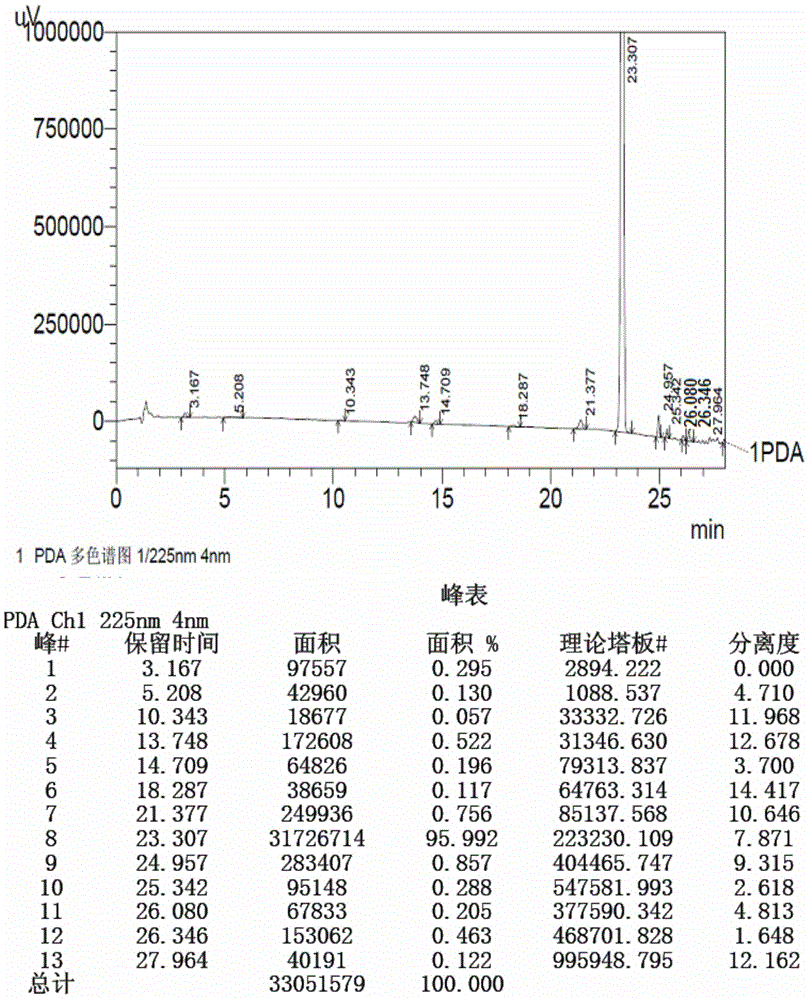
Method for detecting Apixaban intermediate II through reversed-phase high-performance liquid chromatogram
The invention belongs to the field of analysis detection, and relates to a method for detecting an Apixaban intermediate II through a reversed-phase high-performance liquid chromatogram. The method includes the following steps of firstly, preparing a test solution; secondly, detecting the test solution through the reversed-phase high-performance liquid chromatogram; thirdly, calculating the content of each single impurity and all the impurities in the test solution according to an area normalization method. By means of the method, through simple mobile-phase components, the intermediate II can be completely separated from chromatographic peaks of all the impurities; meanwhile, the method is accurate and reliable in detection result and provides possibility for controlling the quality of the intermediate product in the Apixaban synthesis process.
Owner:CHENGDU BAIYU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com