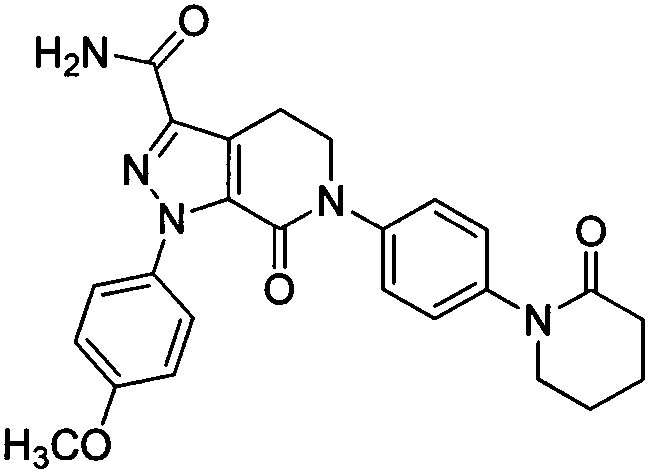
Apixaban oral solid preparation and method for preparing same
A solid preparation, apixaban technology, applied in the field of medicine, can solve problems such as being difficult to have versatility and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
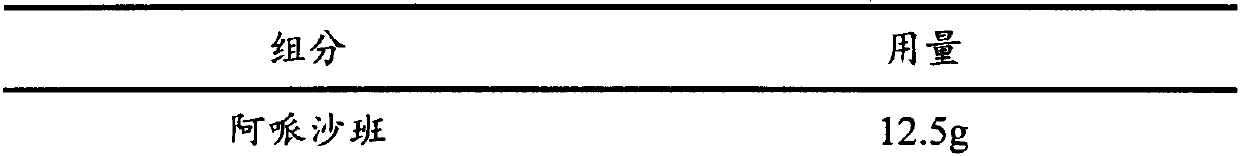
[0064] Apixaban Tablets
[0065] Components and content:
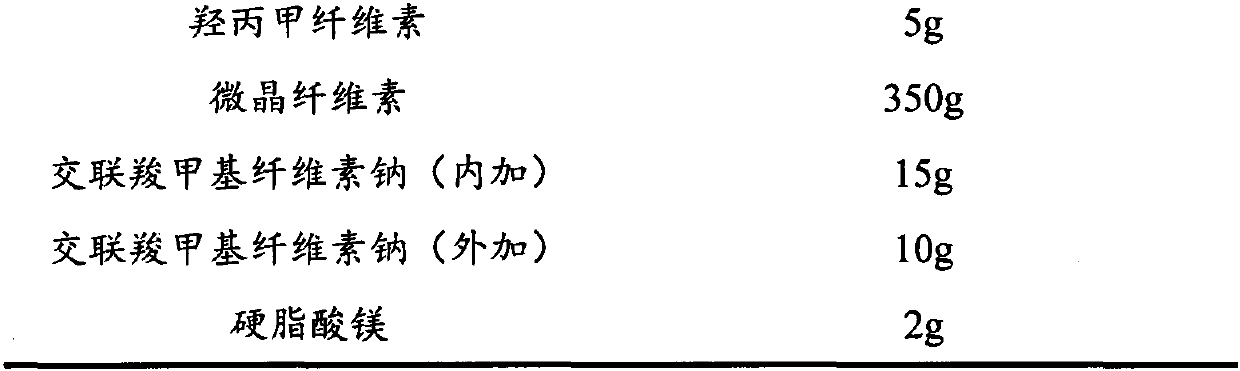
[0066]
[0067]
[0068] 1. Dissolve hypromellose in water (preparation concentration 2%) and stir to dissolve and clarify;
[0069] 2. Add apixaban under stirring conditions, and continue stirring for 5 minutes after adding to obtain a coarse suspension;
[0070] 3. Put the crude suspension in an ultrasonic instrument and sonicate for 30 minutes to obtain a suspension of apixaban and hypromellose.
[0071] 4. The prepared suspension is sprayed onto the fluidized bed chamber carrier auxiliary materials (microcrystalline cellulose, cross-linked sodium carboxymethyl cellulose) by using fluidized bed technology, and the fan frequency of the fluidized bed is set 5~20Hz, air inlet temperature 40~75℃, material temperature control 30~70℃, atomization pressure 0.1~0.3MPa, the apixaban suspension of hypromellose solution is passed through the peristaltic pump under stirring Spray into the fluidized bed at a spray speed...
Embodiment 2
[0075] Apixaban Capsules
[0076] Components and content:
[0077]
[0078]
[0079] 1. Dissolve hypromellose in water (3% preparation concentration) and stir to dissolve and clarify;
[0080] 2. Add apixaban under stirring conditions, and continue stirring for 5 minutes after adding to obtain a coarse suspension;
[0081] 3. Put the crude suspension in an ultrasonic instrument and sonicate for 30 minutes to obtain a suspension of apixaban and hypromellose.
[0082] 4. The prepared suspension is sprayed onto the fluidized bed chamber carrier auxiliary materials (microcrystalline cellulose, cross-linked sodium carboxymethyl cellulose) by using fluidized bed technology, and the fan frequency of the fluidized bed is set 5~20Hz, air inlet temperature 40~75℃, material temperature control 30~70℃, atomization pressure 0.1~0.3MPa, the apixaban suspension of hypromellose solution is passed through the peristaltic pump under stirring Spray into the fluidized bed at a spray spee...
Embodiment 3
[0086] Apixaban Granules
[0087]
[0088] 1. Dissolve hypromellose in water (preparation concentration 4%) and stir to dissolve and clarify;
[0089] 2. Add apixaban under stirring conditions, continue to stir for 5 minutes after adding to obtain a coarse suspension, and cut the coarse suspension with a high-speed shear at 10,000 rpm for 20 minutes to obtain apixaban and hypromellose solution the suspension;
[0090]3. Using a fluidized bed, spray the prepared suspension onto the fluidized bed chamber carrier auxiliary materials (microcrystalline cellulose, cross-linked sodium carboxymethyl cellulose), and set the operating parameters of the fluidized bed spray As follows: set the fan frequency of the fluidized bed at 5-20Hz, the air inlet temperature at 40-75°C, the material temperature at 30-70°C, the atomization pressure at 0.1-0.3MPa, and mix the hypromellose solution with apixaban The suspension is sprayed into the fluidized bed through a peristaltic pump in the sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com