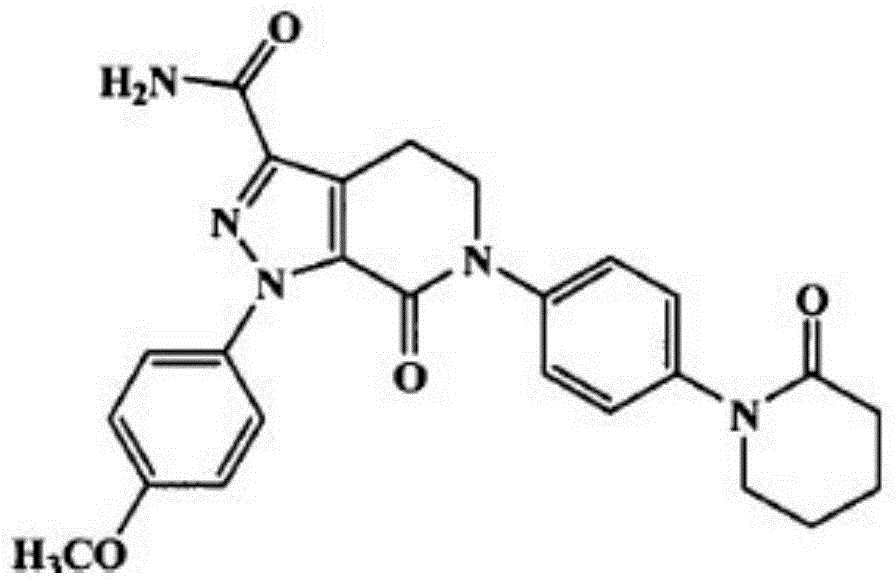
Apixaban tablet and preparation method thereof
A technology for apixaban tablets and excipients, applied in the field of apixaban tablets and its preparation, can solve the problems of low in vitro dissolution rate, low bioavailability, slow dissolution rate, etc., and achieve simple preparation process, The effect of high bioavailability and low packaging cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Components of Apixaban Tablets
[0029] components
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
10
20
30
38
76
114
70
140
210
2
4
6
2
4
6
0.2
0.4
0.6
[0030] Preparation Process:
[0031] (1) Weigh the raw and auxiliary materials of the above prescription amount, except for magnesium stearate, mix all the raw and auxiliary materials and carry out wet granulation. The soft material obtained is granulated with a 20-mesh sieve, and the obtained granules are dried and granulated. Granules, and finally collect dry particles between 20 mesh and 80 mesh.
[0032] (2) After mixing the prepared granules with magnesium stearate for 30 minutes, press into tablets, and the hardness of the tablets is 50-60N.
Embodiment 2
[0034] Apixaban Tablet Ingredients
[0035] components
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
Apixaban
10
20
40
powdered sugar
60
120
250
22
40
88
Crospovidone
3
6
16
2
4
6
Magnesium stearate
0.2
0.4
0.6
[0036] Preparation Process:
[0037] (1) Weigh the raw and auxiliary materials of the above prescription amount, except for magnesium stearate, mix all the raw and auxiliary materials and carry out wet granulation. The soft material obtained is granulated with a 20-mesh sieve, and the obtained granules are dried and granulated. Granules, and finally collect dry particles between 20 mesh and 80 mesh.
[0038] (2) After mixing the prepared granules with magnesium stearate for 30 minutes, press into tablets, and the hardness of the tablets is 50-60N.
Embodiment 3
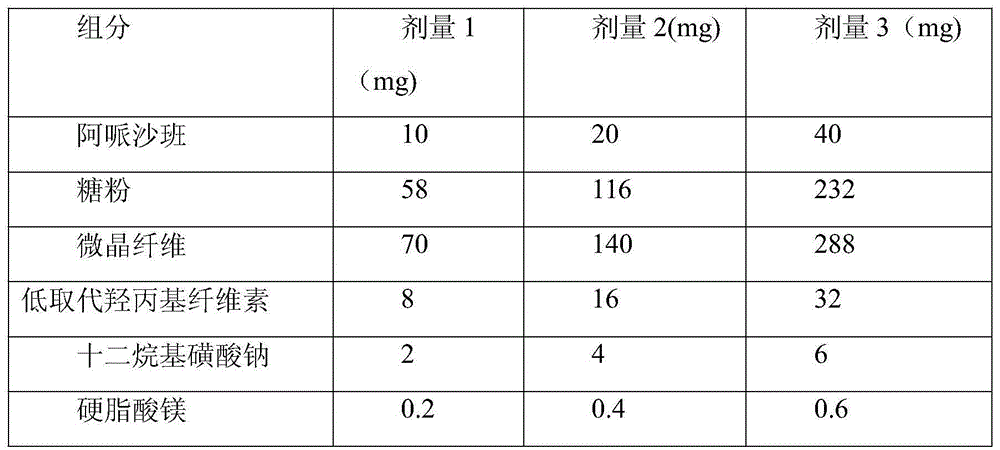
[0040] Components of Apixaban Tablets
[0041]
[0042] Preparation Process:
[0043] (1) Weigh the raw and auxiliary materials of the above prescription amount, except for magnesium stearate, mix all the raw and auxiliary materials and carry out wet granulation. The soft material obtained is granulated with a 20-mesh sieve, and the obtained granules are dried and granulated. Granules, and finally collect dry particles between 20 mesh and 80 mesh.
[0044] (2) After mixing the prepared granules with magnesium stearate for 30 minutes, press into tablets, and the hardness of the tablets is 50-60N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com